Simple Summary
We studied real-world patient trends and cancer survival in adult patients in a European registry, EUMelaReg. We included 200 patients with stage III melanoma with lymph node involvement who had complete resection and received adjuvant treatment with pembrolizumab. Patients initiated treatment with adjuvant pembrolizumab from 1 January 2019 to 17 April 2021 with a median follow-up of 16.5 months. Comparison with previously published real-world data showed that patients were older and more likely to have stage IIIC and IIID disease than those in the Keynote 054 clinical trial.
Keywords: melanoma, cutaneous melanoma, stage III, adjuvant pembrolizumab, EUMelaReg, real-world
Abstract
Background: Although data on patients treated with pembrolizumab are available from clinical trials and single-country real-world reports, to our knowledge no multi-country real-world studies have investigated the use of pembrolizumab as an adjuvant treatment for stage III melanoma. Methods: We used the European Melanoma Registry (EUMelaReg), a disease entity-based registry specific for melanoma, to examine treatment and outcomes for adult patients with stage III melanoma with lymph node involvement who had complete resection and received adjuvant treatment with pembrolizumab. The primary objectives were to describe the demographic and clinical characteristics of the included patients as well as time on adjuvant pembrolizumab treatment (TOT), real-world recurrence-free survival (RFS) and distant metastasis-free survival (DMFS) from adjuvant pembrolizumab initiation. Secondary objectives were time to next treatment (TTNT) after adjuvant use of pembrolizumab, next-line therapy for stage III and unresectable stage IV melanoma and overall survival (OS) from initiation of pembrolizumab. Results: Patients were stratified according to age, sex, BRAF status, number of positive lymph nodes and disease substage. Median TOT was 11.1 (9.2–11.5) months, median RFS was 29.6 [18.7–not reached (NR)] months and median DMFS was 32.4 (22.7–NR) months. TTNT was 29.9 (22.2–NR) months, while median OS was not reached. Conclusions: The results of this study offer insights into the real-world use of pembrolizumab as an adjuvant therapy for melanoma in Europe.
1. Introduction
Cutaneous melanoma is among the most frequently diagnosed cancer types in the majority of countries with fair-skinned populations, including Canada, the United States (US), Europe and Australia. In Europe, skin melanoma has an age-standardized incidence of 8.1 to 14.6 per 100,000 individuals [1]. While most melanomas are cured by simple excision, metastasis occurs in approximately 15% to 40% of cases, and despite recent improvements in systemic therapy, leads to death in at least 50% of these cases [2,3,4].
In the past 15 years, enormous progress has been made in the prevention and treatment of metastatic melanoma due to the development of BRAF and MEK inhibitors for BRAF V600-mutated melanoma, and even more importantly, immune checkpoint inhibitors (ICIs) [4,5]. Following on the successful results of trials in advanced metastatic melanoma, ICI therapies also showed favorable results in the adjuvant treatment of completely resected locoregional disease [6,7]. Anti-PD1 antibodies were subsequently approved in Europe for the adjuvant treatment of melanoma with completely resected locoregional disease, and in December 2018, the European Medicine Agency (EMA) approved pembrolizumab for the adjuvant treatment of adults with stage III melanoma.
The prospective randomized phase III Keynote 054 (KN054) study evaluated 12 months of adjuvant pembrolizumab therapy in stage III completely resected melanoma patients [7,8,9]. At a median follow-up (FU) of 15 months, recurrence-free survival (RFS) was significantly longer in the pembrolizumab group than in the placebo group (hazard ratio [HR] 0.57; 98.4% confidence interval [CI] 0.43–0.74, p < 0.001) [7] and after an overall median FU of 42.3 months, compared to placebo, pembrolizumab adjuvant therapy significantly improved distant metastasis-free survival (DMFS) (HR 0.60; 95% CI 0.49–0.73, p < 0.0001). Despite these improvements in the treatment of metastatic melanoma, there are substantial differences in survival rates as well as access to innovative treatments for melanoma among European countries, suggesting the existence of significant inequalities in healthcare [10,11].
Although data on patients treated with adjuvant pembrolizumab are available from clinical trials and in the real-world in single countries, there are no multi-country real-world data studies investigating the use of pembrolizumab as adjuvant treatment for stage III melanoma patients. Therefore, this retrospective study was performed to analyze the treatment pattern and clinical outcomes of resected stage III melanoma patients who were treated with adjuvant pembrolizumab on European level using data from the European Melanoma Registry (EUMelaReg). EUMelaReg was founded to address the real-world treatment of melanoma and the outcome of patients across Europe and Israel [12]. It is a disease-entity-based treatment registry specific to collect real-world data on the available diagnoses and treatment patterns of melanoma patients at the European level.
2. Materials and Methods
2.1. Study Design and Patient Selection
This retrospective observational study analyzed adult (age ≥ 18 years) patients from the EUMelaReg database who were diagnosed with resected stage III cutaneous, or melanoma of unknown primary (MUP) and received at least one administration of adjuvant pembrolizumab between 1 January 2019 and 17 April 2021. The country registries that contributed patient-level data to this study were Bulgaria, Croatia Serbia, Bosnia and Herzegovina, Germany, Greece, Italy, Poland, Spain and Switzerland.
Patients were treatment naive for any anti-cancer drugs and must have had at least 12 months of FU and a survival status after the first administration of adjuvant pembrolizumab. Patients were excluded if they had uveal melanoma or received pembrolizumab therapy within a clinical trial or expanded access program.
2.2. Outcomes
The primary objectives were to describe the demographic and clinical characteristics and treatment history, time on adjuvant pembrolizumab treatment (TOT), RFS and DMFS from initiation of pembrolizumab. Secondary objectives included time to next treatment (TTNT) after adjuvant pembrolizumab treatment, next-line therapy and OS from the start of adjuvant pembrolizumab.
2.3. CT/MRI
All patients were staged according to AJCC classification 8th edition. Depending on the country, organs including the brain were screened for metastasis using different techniques and at different frequencies. Computed tomography (CT) or magnetic resonance imaging (MRI) or positron-emission tomography (PET) or PET-CT scans were performed mostly every 3 to 6 months during adjuvant treatment and every 6 months post treatment for up to 3 to 5 years in most of the countries.
2.4. Statistical Analysis
Descriptive statistics were used to summarize baseline study cohort characteristics. For categorical variables, the data are presented as the number of observations and the percentage. For continuous variables, data are presented as the mean ± standard deviation (SD), median, minimum and maximum. For TOT, RFS, DMFS, TTNT and OS, time-to-event analyses were conducted using the Kaplan–Meier method to generate Kaplan–Meier plots and to estimate median time-to-event in months with 95% CI, and events rates with 95% CI at landmark timepoints. TOT is defined as the time from the date of start of treatment to the date of end of treatment. A patient’s date of end of treatment was used as a cutoff date for that patient regardless of whether the treatment was documented as ongoing. RFS is defined as time from the start date of adjuvant pembrolizumab treatment to the date of the first recurrence according to the physician’s assessment or death due to any cause, whichever occurred first. Patients were censored at the start of the next treatment. If neither a subsequent treatment nor death was documented, a patient was censored with the date of last contact. DMFS was defined as time from the start date of adjuvant pembrolizumab treatment to the date of the first documentation of distant metastasis according to the physician’s assessment or death due to any cause, whichever occurred first. Patients were censored at the start of the next treatment. If neither a subsequent treatment nor death was documented, a patient was censored with the date of last contact. TTNT under real-world conditions was calculated for all patients from the start of the adjuvant pembrolizumab therapy to the start date of the next treatment or death, whichever occurred first. All other patients were censored at the last date they were known to be alive. OS is defined as the time from the start date of first pembrolizumab treatment to the date of death due to any cause. The OS for subjects not known to have died was censored at the last date the patient was known to be alive. Follow-up was calculated by Kaplan–Meier analysis with last contact as event and fatal events censored.
For stratified (i.e., sub-group) analyses, Kaplan–Meier plots were created for time to event analyses. Single variable Cox proportional hazards models were analyzed with the stratification factor as the independent variable subject to sample size considerations (a minimum of 10 patients in each level of the stratification factor). Adjacent categories could be combined to meet the minimum number of patients criteria. The HR and 95% CI were summarized for each level of the stratification factor.
All descriptive statistical analyses were performed using SAS statistical software (version 9.4 or higher). For survival analyses, the R packages survival and survminer were used. The patients were stratified for the following factors, which included more than 10 patients in each group: sex, age, BRAF status and American Joint Committee on Cancer (AJCC) stage (except stage IIID).
3. Results
3.1. Baseline Characteristics
A total of 200 eligible patients were extracted from the EUMelaReg database for this study. Demographic and patient characteristics at the time of adjuvant pembrolizumab treatment are summarized in Table 1. In total, 117 (58.5%) male patients and 83 (41.5%) female patients were treated with adjuvant pembrolizumab after complete resection. The overall median age was 63 (19.0–88.0) years. Male patients were older (median age: 64.0 [22.0–85.09] years), had a slightly higher percentage of diagnosed MUP of 6.8% and a lower BRAF mutation status (33.3%) compared to female patients with a median age of 59.0 (19.0–88.0) years, 2.4% MUP and 42.2% BRAF mutation (Table 1 and Table S1).
Table 1.
Patient demographics and disease characteristics at initiation of pembrolizumab treatment.
| Female (N = 83) |
Male (N = 117) |
Overall (N = 200) |
|
|---|---|---|---|
| Age (years) | |||
| Mean (SD) | 57.9 (15.3) | 62.0 (14.5) | 60.3 (15.0) |
| Median [Min, Max] | 59.0 [19.0, 88.0] | 64.0 [22.0, 85.0] | 63.0 [19.0, 88.0] |
| Age (years) | |||
| <70 | 61 (73.5%) | 75 (64.1%) | 136 (68.0%) |
| ≥70 | 22 (26.5%) | 42 (35.9%) | 64 (32.0%) |
| Melanoma subtype | |||
| Cutaneous melanoma | 81 (97.6%) | 109 (93.2%) | 190 (95.0%) |
| MUP | 2 (2.4%) | 8 (6.8%) | 10 (5.0%) |
| AJCC stage (8th edition) | |||
| Stage IIIA | 12 (14.5%) | 9 (7.7%) | 21 (10.5%) |
| Stage IIIB | 22 (26.5%) | 30 (25.6%) | 52 (26.0%) |
| Stage IIIC | 47 (56.6%) | 73 (62.4%) | 120 (60.0%) |
| Stage IIID | 2 (2.4%) | 5 (4.3%) | 7 (3.5%) |
| BRAF status | |||
| Negative | 38 (45.8%) | 64 (54.7%) | 102 (51.0%) |
| Positive | 35 (42.2%) | 39 (33.3%) | 74 (37.0%) |
| Unknown | 10 (12.0%) | 14 (12.0%) | 24 (12.0%) |
| ECOG | |||
| 0 | 67 (80.7%) | 89 (76.1%) | 156 (78.0%) |
| 1 | 3 (3.6%) | 3 (2.6%) | 6 (3.0%) |
| Unknown | 13 (15.7%) | 25 (21.4%) | 38 (19.0%) |
| At least one documented comorbidity | |||
| No | 46 (55.4%) | 48 (41.0%) | 94 (47.0%) |
| Yes | 37 (44.6%) | 69 (59.0%) | 106 (53.0%) |
Patient demographics and disease characteristics at start of first pembrolizumab treatment in the adjuvant setting stratified by gender. N: number of patients included in the analysis; SD: standard deviation; Min: minimum; Max: maximum; MUP: melanoma of unknown primary; AJCC: American Joint Committee on Cancer; BRAF: BRAF mutation status; ECOG: Eastern Cooperative Oncology Group.
The most prevalent stage according to the AJCC 8th edition criteria was stage IIIC with 60.0%, followed by stage IIIB with 26.0%, stage IIIA with 10.5% and stage IIID with 3.5% of the total population. The proportion of stage IIIC/D was higher in male patients (66.7%), older patients (≥70 years: 67.2%) and BRAF-negative patients (70.6%) compared to female patients (59.0%), younger patients (>70 years: 61.8%) and BRAF-positive patients (54.1%) (Table 1 and Tables S1–S3). Stratification of patients by AJCC stage showed the highest proportion of stage IIIC/D in male, older and BRAF-negative patients (Table S3).
In total, 78% of the total population had an Eastern Cooperative Oncology Group (ECOG) performance status of 0, which correlated with age, BRAF status and AJCC staging. The higher the age and staging of the patients, the lower the proportion of ECOG status 0 (Table 1 and Tables S1–S3). In total, 47.0% of the patients presented with at least one documented comorbidity.
3.2. Survival Analyses
3.2.1. Time on Adjuvant Treatment
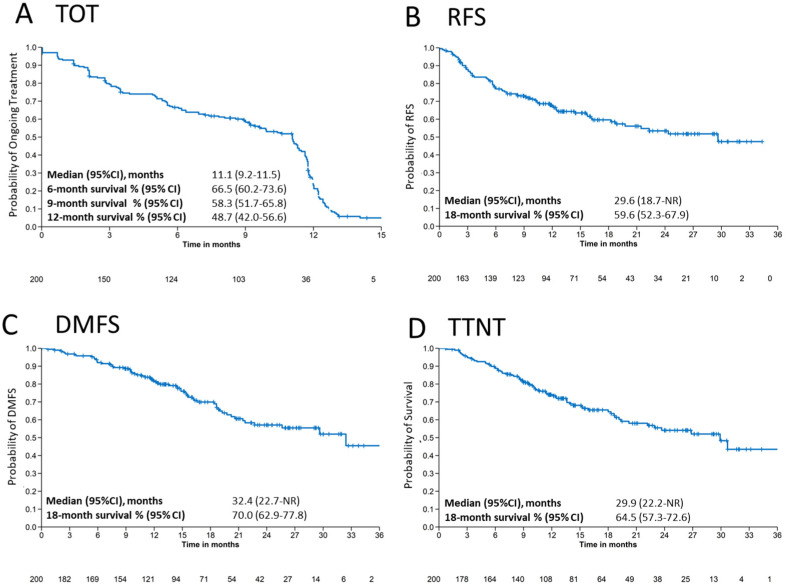
Time on treatment (TOT) with pembrolizumab and reason for discontinuation stratified by gender are shown in Table 2. Median TOT (95% CI) was 11.1 (9.2–11.5) months in the total population (Table 2 and Figure 1A) and longer in female patients (11.1 [9.2–11.5] months) than in male patients (9.9 [6.9–11.6] months). The highest TOT probability (95% CI) was 6 months with 66.5% (60.2–73.6), followed by 9 months with 58.3% (51.7–65.8) and 12 months survival with 48.7% (42.0–56.6).
Table 2.
Time on treatment and reason for end of adjuvant treatment.
| Time on Adjuvant Treatment | Female (N = 83) |
Male (N = 117) |
Total (N = 200) |
|---|---|---|---|
| Events, n (%) | 69.0 (83.1%) | 97.0 (82.9%) | 166.0 (83.0%) |
| Median TOT [months] (95% CI) | 11.1 (9.6–11.8) | 9.9 (6.9–11.6) | 11.1 (9.2–11.5) |
| Time on treatment probability (95% CI) * | |||
| 6 months | 73.8 (64.7–84.1) | 61.3 (52.9–71.1) | 66.5 (60.2–73.6) |
| 9 months | 63.3 (53.4–74.9) | 54.8 (46.2–64.9) | 58.3 (51.7–65.8) |
| 12 months | 53.5 (43.3–66.0) | 45.3 (36.7–55.9) | 48.7 (42.0–56.6) |
| Reason for end of adjuvant treatment | |||
| Regularly ended | 32 (38.6%) | 40 (34.2%) | 72 (36.0%) |
| Disease progression | 19 (22.9%) | 34 (29.1%) | 53 (26.5%) |
| Treatment ongoing | 12 (14.5%) | 19 (16.2%) | 31 (15.5%) |
| Toxicity | 9 (10.8%) | 11 (9.4%) | 20 (10.0%) |
| Patient’s wish | 4 (4.8%) | 4 (3.4%) | 8 (4.0%) |
| Lost to follow-up | 1 (1.2%) | 2 (1.7%) | 3 (1.5%) |
| Investigator’s decision | 0 (0%) | 1 (0.9%) | 1 (0.5%) |
| Death | 1 (1.2%) | 0 (0%) | 1 (0.5%) |
| Other | 1 (1.2%) | 5 (4.3%) | 6 (3.0%) |
| Missing | 4 (4.8%) | 1 (0.9%) | 5 (2.5%) |
Time on treatment (TOT) and reason for end of adjuvant treatment stratified by gender. N: number of patients included in the analysis, CI: confidence interval. * Estimates based on Kaplan–Meier survival analysis.
Figure 1.
Survival outcomes. Kaplan–Meier estimates for (A) time on treatment (TOT), (B) recurrence-free survival (RFS), (C) distant metastasis free survival (DMFS) and (D) time to next treatment (TTNT) for patients treated with adjuvant pembrolizumab. CI: confidence interval, NR: not reached.
Stratification of TOT by 6, 9 and 12 months showed that the probability of TOT differ between gender, age, BRAF status and melanoma stage. Patients who were younger (<70 years) and female had a higher chance to stay on treatment for 12 months compared to older (≥70 years) and male patients (Table 3 and Table S4). TOT (95% CI) at 12 months was 52.2% (44.1–61.8) vs. 41.1% (30.0–56.2) for patients >70 years and ≥70 years and 52.2% (44.1–61.8) vs. 41.1% (30.0–56.2) for female and male patients, respectively (Table 3 and Table S4).
Table 3.
Time on treatment rates at 6, 9 and 12 months.
| 6-Month on- Treatment Rates |
9-Month on- Treatment Rates |
12-Month on- Treatment Rates |
|
|---|---|---|---|
| Age | |||
| <70 years | 70.5 (63.0–78.8) | 59.9 (51.9–69.1) | 52.2 (44.1–61.8) |
| ≥70 years | 58.3 (47.2–71.9) | 55.0 (44.0–68.9) | 41.1 (30.0–56.2) |
| Gender | |||
| Female | 73.8 (64.7–84.1) | 63.3 (53.4–74.9) | 53.5 (43.3–66.0) |
| Male | 61.3 (52.9–71.1) | 54.8 (46.2–64.9) | 45.3 (36.7–55.9) |
| BRAF status | |||
| BRAF negative | 64.0 (55.0–74.5) | 59.6 (50.5–70.5) | 49.9 (40.5–61.4) |
| BRAF positive | 64.9 (54.9–76.7) | 49.2 (38.9–62.3) | 41.8 (31.7–55.0) |
| AJCC stage (8th edition) | |||
| Stage IIIA | 85.7 (72.0–100) | 73.8 (56.3–96.8) | 61.5 (42.5–89.2) |
| Stage IIIB | 66.2 (54.3–80.6) | 62.2 (50.1–77.1) | 53.5 (41.2–69.5) |
| Stage IIIC | 63.1 (54.8–72.6) | 53.0 (44.6–63.1) | 43.1 (34.7–53.6) |
| Stage IIID * | 66.7 (37.9–100) | 66.7 (37.9–100) | 66.7 (37.9–100) |
Time on treatment (TOT) at 6, 9 and 12 months. AJCC: American Joint Committee on Cancer; BRAF: BRAF mutation status. * Estimates are uncertain due to the small number of patients (n = 7).
In total, 49.9% (95% CI: 40.5–61.4) of BRAF-negative and 41.8% (95% CI: 31.7–55.0) of BRAF-positive patients stay on treatment for 12 months, but the mutation status had no effect on the time and discontinuation of adjuvant treatment at earlier time points (TOT at 6 months: 64.0%; 95% CI: 55.0–74.5 vs. 64.9%; 95% CI: 54.9–76.7).
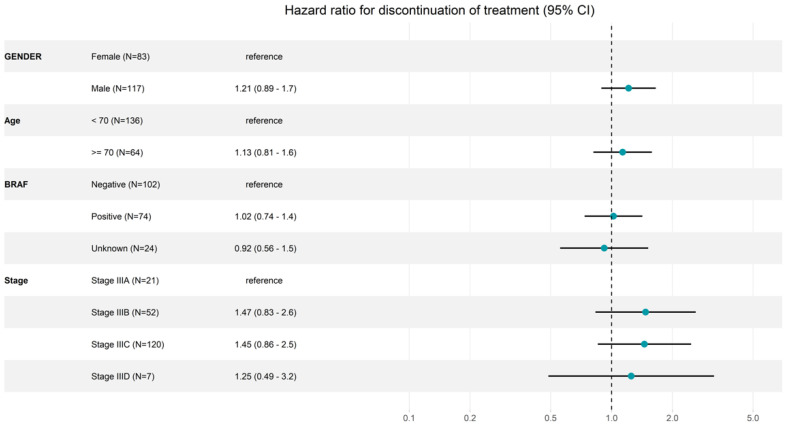
In addition to age, gender and BRAF status, the melanoma stage has an impact on the discontinuation of adjuvant treatment. Patients with stage IIIA had a 6-month TOT (95% CI) of 85.7% (72–100) and 12-month TOT of 61.5% (42.5–89.2) compared to patients with stage IIIC with 6-month TOT of 63.1% (37.9–72.6) and 12-month TOT of 43.1% (34.7–53.6) (Table 2 and Figure 2). However, male patients, older patients (≥70 years) and patients with stage IIIC/D discontinued treatment more frequently at earlier time points (Table 3 and Figure 2).
Figure 2.
Forest plot for discontinuation of adjuvant pembrolizumab treatment. N: number of patients included in the analysis; Age: age in categories at therapy start; stage: AJCC (8th edition) stage at therapy start; CI: confidence interval.
The most common reasons for stopping adjuvant treatment were regular completion of treatment (n = 72, 36.0%) and disease recurrence (n = 53, 26.5%). In total, 10% (n = 20) of patients discontinued adjuvant treatment due to tolerability. No differences were observed between female and male patients (Table 2).
3.2.2. Recurrence-Free Survival and Distant Metastasis-Free Survival
Kaplan–Meier estimates show a median RFS of 29.6 (95% CI: 18.7–not reached [NR]) months and an 18-month RFS median rate of 59.6% (95% CI: 52.3–67.9) for the total population (Figure 1B). Age and gender had no effect on 18-month RFS. However, recurrence was correlated with melanoma stage, showing a higher RFS rate (95% CI) at 18 months of 76.6% (55.8–100) for stage IIIA, 66.7% (53.9–82.6) for stage IIIB and 53.0% (43.8–64.2) for stage IIIC (Table 4). The same trend was observed at 24 months, with a decrease in RFS with progression of AJCC stage (Figure S2D). BRAF-negative patients had a slightly longer RFS rate (95% CI) at 18 months (59.5% (48.8–73.6) and 24 months compared to BRAF-mutated patients (18 months: 53.4 [42.4–67.2]) (Table 4 and Figure S2C).
Table 4.
Recurrence-free survival and distant metastasis-free survival rates at 18 months.
| 18-Month RFS Rates (N = 200) |
18-Month DMFS Rates (N = 200) |
|
|---|---|---|
| Age | ||
| <70 years | 59.0 (50.3–69.3) | 72.2 (63.8–81.7) |
| ≥70 years | 60.6 (48.3–76.0) | 64.7 (52.5–79.9) |
| Gender | ||
| Female | 59.9 (48.8–73.6) | 70.7 (60.1–83.1) |
| Male | 59.8 (50.7–70.5) | 69.6 (60.5–80.1) |
| BRAF status | ||
| BRAF negative | 59.5 (49.4–71.7) | 74.0 (64.5–84.9) |
| BRAF positive | 53.4 (42.4–67.2) | 61.1 (50.0–74.6) |
| AJCC stage (8th edition) | ||
| Stage IIIA | 76.6 (55.8–100) | 82.0 (65.2–100) |
| Stage IIIB | 66.7 (53.9–82.6) | 77.2 (64.8–91.9) |
| Stage IIIC | 53.0 (43.8–64.2) | 64.4 (55.1–75.2) |
| Stage IIID * | 66.7 (37.9–100) | 83.3 (58.3–100) |
Recurrence-free survival (RFS) and distant metastasis-free survival (DMSF) at 18 months. N: number of patients included in the analysis; AJCC: American Joint Committee on Cancer; BRAF: BRAF mutation status. * Estimates are uncertain due to the small number of patients (n = 7).
Median DMFS was 32.4 (95% CI: 22.7–NR) months and the median rate at 18 months was 70.0% (95% CI: 62.9–77.8) in the total population (Figure 1C). Subgroup analysis of DMFS showed a longer DMFS rate (95% CI) at 18 months for younger (72.2% [63.8–81.7]) and BRAF-negative (74.0% [64.5–84.9]) patients than for older (64.7% [52.5–79.9]) and BRAF-positive (61.1% [50.0–74.6]) patients. The same was observed at 24 months (Table 4; Figure S3A,C). Stage IIIC patients exhibited distant metastases faster than stage IIIB and stage IIIC patients (stage IIIC: 64.4% [95% CI: 55.1–75.2]; stage IIIB: 77.2% [95% CI: 64.8–91.9]; stage IIIA: 82.0% [95% CI: 65.2–100]) at 18 months (Table 4 and Figure S3D). However, median values were not significant (p = 0.32). DMFS appeared similar between female and male patients (Figure S3B).
3.2.3. Time to Next Treatment
Median TTNT was 29.9 (95% CI: 22.2-NR) months and the median 18-month survival rate (95% CI) was 64.5% (57.3–72.6) (Figure 1D and Table 5). At 18 months, TTNT (95% CI) was higher in BRAF-negative (66.2% [56.2–78.0]) than in BRAF-positive (54.9% [44.0–68.6]) patients, but similar between male and female patients and between elderly and younger patients (Table 5). At 24 months, there was no difference in TTNT between elderly and younger patients (Table S7), male and female patients (Table S8), or BRAF-positive and BRAF-negative patients (Table S9). However, at all timepoints, patients with more advanced stages predictably had shorter TTNT, and at 24 months, TTNT (95% CI) was substantially better in stage IIIA (80.7% [62.5–100]) than in stage IIIB (59.4% [44.4–79.4]) and stage IIIC (46.3% [36.0–59.6]) patients (Table S10).
Table 5.
Time to next treatment and overall survival rates at 18 months.
| 18-Month TTNT Rates (N = 200) | 18-Month OS Rates (N = 200) |
|
|---|---|---|
| Total [%] (95% CI) | 64.5 (57.3–72.6) | 88.1 (82.7–93.8) |
| Age | ||
| <70 years | 65.6 (56.9–75.7) | 90.6 (84.8–96.9) |
| ≥70 years | 62.0 (50.1–76.7) | 82.3 (71.5–94.8) |
| Gender | ||
| Female | 63.5 (52.4–77.0) | 90.4 (83.2–98.3) |
| Male | 65.1 (56.1–75.6) | 86.5 (79.1–94.5) |
| BRAF status | ||
| BRAF negative | 54.9 (44.0–68.6) | 81.0 (70.7–92.9) |
| BRAF positive | 66.2 (56.2–78.0) | 91.4 (85.3–97.8) |
| AJCC stage (8th edition) | ||
| Stage IIIA | 80.7 (62.5–100) | 100 (100–100) |
| Stage IIIB | 72.1 (59.7–87.1) | 91.2 (82.1–100) |
| Stage IIIC | 57.7 (48.3–68.8) | 85.5 (78.2–93.4) |
| Stage IIID * | 83.3 (58.3–100) * | 83.3 (58.3–100) * |
Time on next treatment (TTNT) and overall survival (OS) at 18 months. N: number of patients included in the analysis, AJCC: American Joint Committee on Cancer; BRAF: BRAF mutation status. * Estimates are uncertain due to the small number of patients (n = 7).
3.2.4. Overall Survival
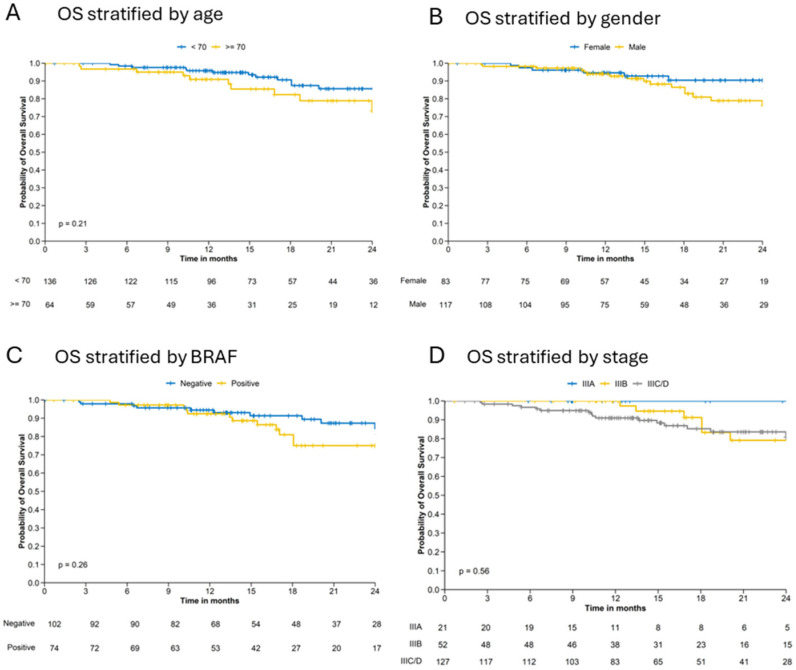
Median 18-month OS rate (95% CI) was 88.1% (82.7–93.8) (Table 5) and lower in elderly (82.3% [71.5–94.8]) than in younger (90.6% [84.8–96.9]) patients, in BRAF-positive (81.0% [70.7–92.9]) than in BRAF-negative (91.4% [85.3–97.8]) patients and in stage IIIC patients (85.5% [78.2–93.4]) than in stage IIIB (91.2% [82.1–100]) and IIIA (100% [100–100]) patients (Table 5).
At 24 months, both male patients and elderly patients tended to have worse outcomes than female and younger patients (Tables S8 and S9). OS at 24 months was 100% for stage IIIA but was approximately 80% for stages IIIB and stage IIIC (Table S10).
Previously observed differences can also be observed for OS survival. Differences in OS Kaplan–Meier curves show a better trend for younger (<70 years) patients, female patients and BRAF negative patient (Figure 3A–C). However, differences between female and male patients and between BRAF positive and negative patients are observed after 15 months. Stratification of OS by melanoma stage show the highest OS probability for stage IIIA patients. Patients with stage IIIB have a slightly better OS survival up to 18 months than patients in stage IIIC/D (Figure 3D).
Figure 3.
Kaplan–Meier estimates for overall survival (OS) stratified by (A) age, (B) gender, (C) BRAF mutation status and (D) stage.
4. Discussion
This retrospective study analyzed 200 fully resected stage III melanoma patients who were treated with pembrolizumab as an adjuvant therapy with a minimum of 1 year follow-up (median 16.5 months). This study provides real-world data obtained in a European setting, and therefore supplements other data on adjuvant anti-PD1 treatment of melanoma including the results of KN054 clinical trial, but without the usual restrictions of a clinical trial.
While randomized clinical trials remain the gold standard for evaluating the efficacy and safety of new cancer therapies, previous systematic reviews have demonstrated that clinical trials results may differ in important ways from those achieved in a real-world setting. Due to enrollment procedures and inclusion or exclusion criteria, a clinical trial may, for example, underrepresent certain demographic populations or miss differences in outcomes among disease status groups or subpopulations [13].
Real-world populations may therefore provide a less selective view with respect to demographic characteristics, e.g., in terms of age structure, performance status, comorbidities and other factors [13,14,15]. Hence, the current study was performed to evaluate the patient characteristics and survival outcomes of adjuvant pembrolizumab in a patient population in a real-world setting in Europe.
In KN054 only 8% of the patients were in IIIA as compared to 10.5% in EUMelaReg; IIIB 34.5 vs. 26.0, IIIC 49.7% vs. 60% and IIID 3.7% vs. 3.5%. From the AJCC 8th edition it is evidenced that stage IIIC is associated with a 5-year survival rate of 69% and 10-year survival rate of 60%, which correlates with EUMelaReg results and reflects the real-world situation.
Results may be also compared to a separate real-world study conducted using the US-based USON registry [16]. Treatment adherence was similar between the two real-world analyses, with a median duration of treatment at 11.1 (9.2–11.5) months in EUMelaReg patients and 11.8 (11.6–11.8) months in USON patients showing good therapy adherence in both cohorts. All three studies were performed in adult patients aged 18 years or older with stage III melanoma treated with complete resection and subsequent adjuvant pembrolizumab therapy.
We found that 12-month rates for RFS and DMFS in our study were substantially lower compared to KN054. These differences likely reflect differences in patient characteristics, as patients in our study were older (median age, 63 years) than those in the KN054 trial (median age, 54 years) and tended to have more advanced-stage melanoma (patients with grade IIIA-D accounted for 10.5%, 26%, 60% and 3.5% in EUMelaReg and 8.2%, 31.7%, 51.9% and 3.9% in KN054 with 4.3% unevaluable).
Nevertheless, comparing 12-month RFS rates for EUMelaReg and KN054 stratified by AJCC 8 stage still shows differences, which were especially pronounced in stage IIIC (60.1% in EUMelaReg vs. 73.6% in KN054). This might partially still be related to different substage distribution, since, e.g., patients with in-transit metastases were excluded in KN054, as were stage IIIA patients with a sentinel node tumor burden of less than 1.0 mm.
Conversely, in the USON real-world study RFS was higher (81.0%) than in both the KN054 or a real-world European setting. This could well be due to a shorter median follow-up time (9.3 months in the USON registry vs. 16.5 months in the EUMelaReg analysis and 15 months in KN054) and the substantially lower proportion of US registry patients who had more advanced-stage melanoma (only 40.4% of patients had stage IIIC and stage IIID melanoma). OS was not reached in any of the three studies.
Overall, we found that patient characteristics and risk profiles varied in important ways, including age and cancer stage, across the two registry-based real-world studies and the KN054 clinical trial. These differences are likely to have resulted in the numerically lower survival outcomes observed in our European study and the numerically higher survival outcomes observed in the US study when compared to the pivotal KN054 clinical trial.
This study has certain limitation related to the observational nature of the data collection leading to several sources of bias, including most importantly selection bias, missing data and underreporting of informative variables, such as comorbidities and other co-variates related to treatment selection or outcomes. Conversely, our study covered a more representative population than would normally be found in a clinical trial as our patients were not excluded based on ECOG score, age, co-morbidities or lymph node status. Additionally, the proportion of 37% of BRAF V600 mutated patients treated with pembrolizumab underscores the relevance of adjuvant immunotherapy in melanoma despite a given alternative of using BRAF/MEK-inhibitors in the adjuvant setting [17].
5. Conclusions
The patients in the EUMelaReg study had a lower recurrence-free survival outcomes than the clinical trial patients both in the overall population and by substage. This was likely due to a different spectrum of patients, i.e., the real-world patients seem to bear a worse prognosis, e.g., due to age and tumor substage. The study suggests that patient populations in clinical trials may not be fully representative for real-world populations, and therefore outcomes in clinical practice are important to study.
Acknowledgments
We like to thank the following EUMelaReg Contributors: Nethanel Asher, Sheba, Israel; Dimitrios Bafaloukos, Athens, Greece; Maja Banjin, Sarajevo, Bosnia and Herzegovina; Lars Bastholt, Odense, Denmark; Marija Buljan, Zagreb, Croatia; Daška Štulhofer Buzina, Zagreb, Croatia; Eva Couselo, Barcelona, Spain; Razvan Curca, Alba Lulia, Romania; Alexander Gerasimov, Sofia, Bulgaria; John Haanen, Amsterdam, Netherlands; Davorin Herceg, Zagreb, Croatia; Amina Jalovčić, Sarajevo, Bosnia and Herzegovina; Ahmed Kontilev, Sofia, Bulgaria; Ulrike Leiter-Stöppke, Tübingen, Germany; Mario Mandala, Perugia, Italy; Mihai Marinca, Lasi, Romania; Željko Mijušković, Belgrade, Serbia; Alessandro Minisini, Udine, Italy; Serban Negru, Timisoara, Romania; Guiseppe Palmieri, Rome, Italy; Jan Poleszczuk, Warsaw, Poland; Teresa Puértolas, Zaragoza, Spain; Michael Schenker, Craiova, Romania; Henrik Schmidt, Aarhus, Denmark; Mirna Šitum, Zagreb, Croatia; Eva Ellebæk Steensgaard, Herlev, Denmark; Bozena Cybulska Stopa, Kraków, Poland; Inge Marie Svane, Herlev, Denmark; Michel Wouters, Amsterdam, The Netherlands; Daniela Zob, Bucharest, Romania.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers16213558/s1, Table S1: Baseline demographics and clinical characteristics stratified by age; Table S2: Baseline demographics and clinical characteristics stratified by BRAF mutation status; Table S3: Baseline demographics and clinical characteristics stratified by AJCC stage (8th edition); Table S4: Time on treatment by demographic and disease characteristics; Table S5: Recurrence-free survival by demographic and disease characteristics; Table S6: Distant metastasis-free survival by demographic and disease characteristics; Figure S1. Time on adjuvant pembrolizumab treatment stratified by (A) gender, (B) age, (C) BRAF mutation status, and (D) stage; Figure S2. Recurrence-free survival stratified by (A) gender, (B) age, (C) BRAF mutation status, and (D) stage; Figure S3: Kaplan-Meier curves of DMFS stratified by (A) age, (B) gender, (C) BRAF mutation status, and (D) stage; Figure S4: Kaplan-Meier curves of TTNT stratified by (A) age, (B) gender, (C) BRAF mutation status, and (D) stage; Figure S5: Kaplan-Meier curves of OS stratified by (A) age, (B) gender, (C) BRAF mutation status, and (D) stage; Table S7: Survival endpoints stratified by age; Table S8: Survival endpoints stratified by gender; Table S9: Survival endpoints stratified by BRAF mutation status; Table S10: Survival endpoints stratified by AJCC stage (8th edition).
Author Contributions
Conceptualization, M.W., P.M., S.J. and T.B.; data curation, M.W., J.M., G.K.S., L.K., V.C.-S., P.M., T.S.K., P.T., E.E., M.B., P.A.A., H.G. and I.M.R.; formal analysis, M.W., P.S. and M.B.; investigation, M.W., J.M., I.G., I.L., G.K.S., L.K., V.C.-S., P.M., T.S.K., E.E., P.C., P.A.A., H.G., I.M.R., P.R., D.S. and R.D.; methodology, M.W., P.S. and M.B.; validation, M.W. and M.B.; writing—original draft, M.W.; writing—review and editing, J.M., I.G., I.L., L.K., P.M., S.J., T.B., P.R. and D.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study is a retrospective database analysis. Submission of this study to an Institutional Review Board/Independent Ethics Committee was not required.
Informed Consent Statement
Patient informed consent was obtained by patients in writing before documentation or transfer of any data occurred into a national country or multi-country registry. Since the national informed consent covered data transfers from a country or multi-country registry to other international scientific research organizations such as EUMelaReg, no further consent was necessary. For the anonymous analysis of EUMelaReg patient data within this study, no separate patient information was needed.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
Author Philipp Schnecko was employed by the company Alcedis GmbH. The following co-authors have participated on Advisory Boards for one or more of the following companies: V.C. and G.K.S. (MSD and Pierre Fabre); E.E. and I.L. (MSD); H.G. (Sanofi, Bristol Myers Squibb, MSD); D.S. (4SC, Amgen, Array Biopharma, Astra Zeneca, Bristol Myers Squibb, Daiichi Sankyo, Immunocore, InFlarX, MSD, Nektar, Neracare, Novartis, OncoSec, Pfizer, Philogen, Pierre Fabre, Roche, Replimune, Sandoz, Sanofi/Regeneron and SUN-Pharma); L.K. (MSD, Bristol Myers Squibb, Roche, Novartis, Abbvie and Janssen); P.A.A. (Bristol Myers Squibb, Roche-Genentech, Merck Sharp & Dohme, Novartis, AstraZeneca, Immunocore, Boehringer-Ingelheim, Eisai, Regeneron, Daiichi Sankyo, Oncosec, Nouscom, Seagen, iTeos) and P.M. (MSD, Pierre Fabre, GSK, Roche, Bristol Myers Squibb, Novartis, Sanofi, Beiersdorf, Allmiral, Hermal, AMGEN, Sun-Pharma); D.S. (from 4SC, Amgen, Array Biopharma, Astra Zeneca, BMS, Daiichi Sankyo, Haystack, Immunocore, InFlarX, Innovent, Labcorp, Merck Serono, MSD, Nektar, Neracare, Novartis, OncoSec, Pfizer, Philogen, Pierre Fabre, Replimune, Roche, Sandoz, Sanofi/Regeneron, SUN-Pharma); L.K. (from MSD, BMS, Roche, Novartis, Abbvie and Janssen). Research Grants: P.A.A., P.R., D.S. and P.S. have received research grants and/or contracts outside this project from various pharmaceutical companies. P.S., M.B. and M.W. received funding to EUMelaReg and Alecdis GmbH (employer) from Merck & Co., Inc. for this study. Consulting Fees and or Payment for Honoraria: The following co-authors have received consulting fees and/or payment honoraria for lectures, presentations, expert testimony and/or speaking events outside the submitted work: G.K.S. (from Roche, MSD and Merck, Novartis, BMS, Pier-Fabre); P.A.A. (from Bristol Myers Squibb, Roche-Genentech, Merck & Co., Inc., Novartis, Merck Serono, Pierre-Fabre, Sun Pharma, Sanofi, Idera, Sandoz, 4SC, Italfarmaco, Nektar, Pfizer/Array, Lunaphore, Medicenna. Bio-Al Health, ValoTx, Replimmune); R.D. (from Novartis, Merck & Co., Inc., Bristol-Myers Squibb, Roche, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator, T3 Pharma, MaxiVAX SA, Pfizer and touchIME); I.G. (from MSD, Bristol Myers Squibb, Merck & Co., Inc., Roche, Sanofi, Novartis); I.L. (from Roche, Astra, BMS and Novartis); I.M. (from Bristol Myers Squibb, Merck, Novartis, Pierre Fabre, Roche, Sanofi, Astra Zeneca); P.R. (from MSD: BMS, Novartis, Merck & Co Inc, Sanofi, Pierre Fabre, Blueprint Medicines, Philogen). I.G. is as a leadership or fiduciary role at the Bulgarian Association of Dermatology Oncology and D.S. has a leadership or fiduciary role in other board, society, committee or advocacy groups (paid or unpaid) including: EuMelaReg, Dermatologic Cooperative Oncology Group (DeCOG), German Cancer Society, Hiege Stiftung, Deutsche Hautkrebsstiftung, NVKH E.V. J.S. and T.B. are employees of Merck & Co., Inc. and have stock in the company. M.B. has financial stock in Biotech and Affimed. T.K. and P.T. have no disclosures to report. The authors declare that this study received funding from Merck & Co., Inc. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Funding Statement
The authors declare that this study received funding from Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. The funder was involved in the study design.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Olsen C.M., Whiteman D.C. Clinical Epidemiology of Melanoma. In: Balch C.M., Atkins M.B., Garbe C., Gershenwald J.E., Halpern A.C., Kirkwood J.M., McArthur G.A., Thompson J.F., Sober A.J., editors. Cutaneous Melanoma. Springer International Publishing; Cham, Switzerland: 2020. pp. 425–449. [Google Scholar]
- 2.Board R., Smittenaar R., Lawton S., Liu H., Juwa B., Chao D., Corrie P. Metastatic melanoma patient outcomes since introduction of immune checkpoint inhibitors in England between 2014 and 2018. Int. J. Cancer. 2020;148:868–875. doi: 10.1002/ijc.33266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swetter S.M., Thompson J.A., Albertini M.R., Barker C.A., Baumgartner J., Boland G., Chmielowski B., DiMaio D., Durham A., Fields R.C., et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2024. J. Natl. Compr. Cancer Netw. 2024;22:290–298. doi: 10.6004/jnccn.2024.0036. [DOI] [PubMed] [Google Scholar]
- 4.Boutros A., Croce E., Ferrari M., Gili R., Massaro G., Marconcini R., Arecco L., Tanda E.T., Spagnolo F. The treatment of advanced melanoma: Current approaches and new challenges. Crit. Rev. Oncol./Hematol. 2024;196:104276. doi: 10.1016/j.critrevonc.2024.104276. [DOI] [PubMed] [Google Scholar]
- 5.Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020;11:3801. doi: 10.1038/s41467-020-17670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber J., Mandalà M., Del Vecchio M., Gogas H.J., Arance A.M., Cowey C.L., Dalle S., Schenker M., Chiarion-Sileni V., Marquez-Rodas I., et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N. Engl. J. Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 7.Eggermont A.M.M., Blank C.U., Mandalà M., Long G.V., Atkinson V., Dalle S., Haydon A., Lichinitser M., Khattak A., Carlino M.S., et al. Adjuvant Pembrolizumab versus Placebo in Resected Stage III Melanoma. N. Engl. J. Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 8.Eggermont A.M., Blank C.U., Mandala M., Long G.V., Atkinson V.G., Dalle S., Haydon A., Lichinitser M., Khattak A., Carlino M.S., et al. Prognostic and predictive value of AJCC-8 staging in the phase III EORTC1325/KEYNOTE-054 trial of pembrolizumab vs lacebo in resected high-risk stage III melanoma. Eur. J. Cancer. 2019;116:148–157. doi: 10.1016/j.ejca.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Eggermont A.M., Kicinski M., Blank C.U., Mandala M., Long G.V., Atkinson V., Dalle S., Haydon A., Meshcheryakov A., Khattak A., et al. Five-Year Analysis of Adjuvant Pembrolizumab or Placebo in Stage III Melanoma. NEJM Évid. 2022;1:EVIDoa2200214. doi: 10.1056/EVIDoa2200214. [DOI] [PubMed] [Google Scholar]
- 10.Minicozzi P., Walsh P.M., Sanchez M.J., Trama A., Innos K., Marcos-Gragera R., Dimitrova N., Botta L., Johannesen T.B., Rossi S., et al. Is low survival for cancer in Eastern Europe due principally to late stage at diagnosis? Eur. J. Cancer. 2018;93:127–137. doi: 10.1016/j.ejca.2018.01.084. [DOI] [PubMed] [Google Scholar]
- 11.Sekulovic L.K., Guo J., Agarwala S., Hauschild A., McArthur G., Cinat G., Wainstein A., Caglevic C., Lorigan P., Gogas H., et al. Access to innovative medicines for metastatic melanoma worldwide: Melanoma World Society and European Association of Dermato-oncology survey in 34 countries. Eur. J. Cancer. 2018;104:201–209. doi: 10.1016/j.ejca.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Weichenthal M., van Akkooi A., Mohr P., Ellebaek E., Svane I.-M., Ugurel-Becker S., Leiter U., Hoejberg L., Haanen J., Schmidt H., et al. EUMelaReg: A European platform for outcome research on real world treatment data of patients with advanced melanoma. Ann. Oncol. 2018;29:viii459. doi: 10.1093/annonc/mdy289.042. [DOI] [Google Scholar]
- 13.Donia M., Kimper-Karl M.L., Høyer K.L., Bastholt L., Schmidt H., Svane I.M. The majority of patients with metastatic melanoma are not represented in pivotal phase III immunotherapy trials. Eur. J. Cancer. 2017;74:89–95. doi: 10.1016/j.ejca.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Liu F., Ou W., Diede S., Whitman E. Real-world experience with pembrolizumab in patients with advanced melanoma: A large retrospective observational study. Medicine. 2019;98:e16542. doi: 10.1097/MD.0000000000016542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowey C.L., Liu F.X., Black-Shinn J., Stevinson K., Boyd M., Frytak J.R., Ebbinghaus S.W. Pembrolizumab Utilization and Outcomes for Advanced Melanoma in US Community Oncology Practices. J. Immunother. 2018;41:86–95. doi: 10.1097/CJI.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowey C.L., Sura S., Beeks A., Scherrer E., Krepler C., Jiang R. Real-world outcomes of adjuvant pembrolizumab for completely resected stage III cutaneous melanoma; Proceedings of the 18th International Congress of the Society for Melanoma Research (SMR); Virtual. 28–31 October 2021. [Google Scholar]
- 17.Dummer R., Brase J.C., Garrett J., Campbell C.D., Gasal E., Squires M., Gusenleitner D., Santinami M., Atkinson V., Mandalà M., et al. Adjuvant dabrafenib plus trametinib versus placebo in patients with resected, BRAF(V600)-mutant, stage III melanoma (COMBI-AD): Exploratory biomarker analyses from a randomised, phase 3 trial. Lancet Oncol. 2020;21:358–372. doi: 10.1016/S1470-2045(20)30062-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.