Abstract
Viruses are intracellular parasites that utilize organelles, signaling pathways, and the bioenergetics machinery of the cell to replicate the genome and synthesize proteins to build up new viral particles. Mitochondria are key to supporting the virus life cycle by sustaining energy production, metabolism, and synthesis of macromolecules. Mitochondria also contribute to the antiviral innate immune response. Here, we describe the different mechanisms involved in virus–mitochondria interactions. We analyze the effects of viral infections on the metabolism of glucose in the Warburg phenotype, glutamine, and fatty acids. We also describe how viruses directly regulate mitochondrial function through modulation of the activity of the electron transport chain, the generation of reactive oxygen species, the balance between fission and fusion, and the regulation of voltage-dependent anion channels. In addition, we discuss the evasion strategies used to avoid mitochondrial-associated mechanisms that inhibit viral replication. Overall, this review aims to provide a comprehensive view of how viruses modulate mitochondrial function to maintain their replicative capabilities.
Keywords: electron transport chain, glucose, glutamine, fatty acids, innate immunity, metabolic reprogramming, mitochondria, reactive oxygen species, VDACs, virus, Warburg
1. Introduction
As obligatory intracellular parasites, viruses use cellular pathways and organelles to meet their requirements for protein synthesis and replication. The life cycle of a virus is highly dependent on the contribution of mitochondria to the bioenergetics, metabolic, and biosynthetic capabilities of cells. Mitochondria also induce antiviral innate immune mechanisms, like the activation of the mitochondrial antiviral-signaling protein (MAVS) [1] and the type I interferon (IFN) response via the release of mitochondrial DNA (mtDNA) [2]. Furthermore, mitochondria participate in antiviral-signaling pathways, including the activation of toll-like receptors (TLRs) [3] and the NLRP3 inflammasome [4].
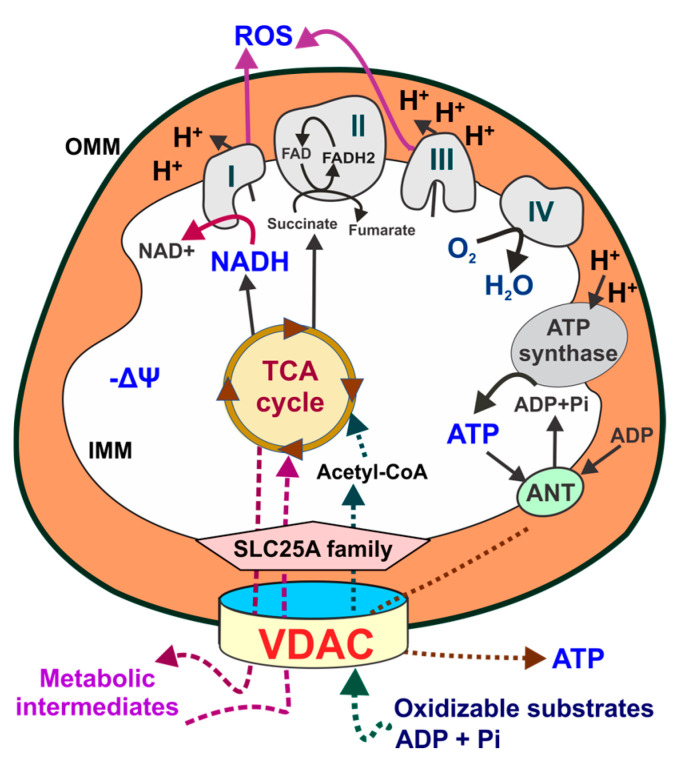
Mitochondrial function and metabolism are supported by fully oxidizable substrates. The tricarboxylic acid (TCA) cycle (also known as Krebs cycle) in the mitochondrial matrix is fueled by the metabolic intermediate acetyl-coenzyme A, which is generated by the oxidation of glucose-derived pyruvate, β-oxidation of fatty acids (FAs), and the amino acids leucine, isoleucine, glycine, serine, and tryptophan. A major byproduct of the TCA cycle is NADH, which is a major donor of the electrons (e−) that flow through the complexes of the electron transport chain (ETC) to reduce the final acceptor molecular O2 to H2O. Oxygen consumption is the parameter used to measure cellular respiration. The flow of e- through the ETC also produces two parallel phenomena: a proton (H+) translocation across the inner mitochondrial membrane and an e- leak. Translocated H+ at complexes I, III, and IV accumulate in the mitochondrial intermembrane space, generating a negative transmembrane potential (mitochondrial membrane potential, ΔΨm) and a positive ΔpH, both components of the proton motive force (Δp). Δp is the force that drives ATP synthesis from ADP and inorganic phosphate (Pi) by Complex V (F1FO-ATP synthase). In non-cancerous and non-proliferating cells, mitochondria produce ~95–98% of total cellular ATP through oxidative phosphorylation (OxPhos). Thus, mitochondrial function is sustained by coupling substrate oxidation with ATP synthesis [5]. Beyond bioenergetics, an e- leak at complexes I, II, and IV of the respiratory chain generates reactive oxygen species (ROS). Quantitatively, mitochondria are the major source of ROS. ROS generation in mitochondria is highly dynamic and modulated by changes in mitochondrial metabolism. Total cellular ROS depends on the balance between production and detoxification by enzymatic and non-enzymatic antioxidants, both in mitochondria and cytosol, that prevent excessive ROS accumulation [6]. Superoxide anion (O2•−), hydroxyl (OH•), peroxyl (ROO•) and alkoxy (RO•) radicals, and hydrogen peroxide (H2O2) originate from molecular O2 [7]. Superoxide dismutases are a family of scavenging enzymes that convert O2•− to H2O2, which is then further reduced to H2O by catalase, glutathione peroxidases, and peroxiredoxins [3]. Only O2•− and H2O2 act as signaling molecules that contribute to maintaining cellular homeostasis and proliferation [8]. The highly toxic HO• and peroxynitrite (ONOO−), for which there are not specific detoxifying systems, do not regulate biochemical pathways. Mitochondrial ROS are either released to the cytosol, mainly H2O2, as signaling molecules or converted to non-reactive species by antioxidant systems. If the antioxidant capability of the cells is exceeded, accumulated ROS cause oxidative stress [9] (Figure 1).
Figure 1.
Schematics of mitochondrial metabolism. Oxidizable substrates, ADP and Pi, cross the outer mitochondrial membrane through VDACs. Acetyl-coenzyme A, generated from respiratory substrates, enters the TCA cycle, generating NADH and FADH2, which fuel the electron transport chain to support oxidative phosphorylation. The TCA cycle also produces metabolic intermediaries released to the cytosol for the synthesis of proteins and lipids. H+ pumping by the respiratory chain across the inner mitochondrial membrane generates a ΔΨ and a proton motive force used by the F1F0-ATP synthase (complex V) to synthesize ATP. Mitochondrial ATP is exported from the matrix by the ANT and released to the cytosol through VDACs. The flow of electrons through complexes I, II, and III also generates ROS. AcCoA: Acetyl CoA; ANT: adenine nucleotide transporter; α-KG: alpha-ketoglutarate; IMM: inner mitochondrial membrane; OMM: outer mitochondrial membrane; Pi: inorganic phosphate; ROS: reactive oxygen species; VDACs: voltage-dependent anion channels; ΔΨ: mitochondrial membrane potential.
Mitochondria are also metabolic hubs that generate metabolites through the catabolism of pyruvate, glutamine, and other respiratory substrates in the TCA cycle. When glucose levels are sufficient for energy generation, α-ketoglutarate and oxalo-acetate, which are glutamine derivatives, are utilized for the synthesis of nonessential amino acids. Citrate, exported to the cytosol, is converted into acetyl-coenzyme A and utilized for the synthesis of FA, cholesterol, and amino acids. Furthermore, glutamine provides nitrogen for the synthesis of purine and pyrimidine and is a precursor for the synthesis of glutathione [6,10,11,12,13,14]. Recently, the transfer of one-carbon units from serine and glycine by a process known as one-carbon metabolism has been shown to be involved in the de novo synthesis of purines and thymidylate synthase in highly proliferative tumors [15].
Mitochondria continuously adapt to metabolic demands by changing the number and morphology through mitochondrial fission, fusion, biogenesis, and mitophagy. Recently, defective mitophagy has been linked to the risk of autoimmune disease due to the accumulation of mtDNA [2].
Because of the multiple roles in bioenergetics and metabolism, mitochondria are essential to maintain cellular homeostasis and a necessary target to favor viral replication. This review summarizes the mechanisms involved in the interactions between viruses and mitochondria. We also describe how viruses benefit from using mitochondrial metabolism and avoid the mechanisms associated with mitochondria that inhibit viral replication. A better understanding of these mechanisms may help to develop novel strategies to control viral infections.
2. Mitochondrial Reactive Oxygen Species during Viral Infections
2.1. Oxidative Stress and Beneficial Effects of Reactive Oxygen Species
ROS generated during viral infections can either favor viral replication and the progression of disease or be deleterious to the infected cell. The outcome depends on the amount of ROS accumulated. Oxidative stress occurs when the production exceeds the cellular antioxidant capacity and the ability to eliminate the reactive intermediates, resulting in damage to DNA, proteins, and lipids [7].
ROS-induced damage to macromolecules has been recognized as a key factor in the pathogenesis of diseases like viral encephalitis, which causes neuronal damage in the central nervous system (CNS) [16]. In the CNS, ROS are mainly produced by microglial cells in response to inflammation or tissue injury [17]. Viruses have evolved strategies to avoid antiviral responses or premature apoptosis caused by excessive accumulation of ROS [18]. Dengue virus (DENV) serotype 4, Japanese encephalitis virus (JEV), rabies virus, and human immunodeficiency virus (HIV) cause oxidative stress in the CNS. The detrimental effects of ROS on neurons after DENV-4 have been demonstrated by Suwanprinya et al. [19]. Although the exact mechanism of ROS production induced by DENV-4 has not been identified, virus attachment to a series of receptors like glycosphingolipids or a component of the viral particle might cause this phenomenon at the early phase of infection. Excessive ROS production, which is more evident after DENV infection progresses, is attributed to a decreased antioxidant response mediated by nuclear factor erythroid 2-related factor 2 (Nrf2) that is targeted by a DENV NS2B3 protease complex. The absence of this antioxidant regulation triggers an increase in ROS levels that favors virus replication, along with upregulation in the expression of inflammatory and apoptotic genes [20]. Consistent with this, an imbalance between ROS and the antioxidant systems has been linked to the development of severe disease [21], mainly mediated by the release of pro-inflammatory cytokines [22], leading to the vascular dysfunction characteristic of severe DENV infection [23]. Furthermore, DENV decreases intracellular GSH levels, which also promotes DENV replication [24]. After JEV infection of neurons, there is a significant upregulation of pyruvate dehydrogenase kinase 1 (PDK-1), stimulating the generation of free radicals. It has been suggested that PDK-1 phosphorylation and the inhibition of the pyruvate dehydrogenase (PDH) complex leads to the accumulation of ROS [25], which contributes to neuronal apoptosis [26]. Oxidative stress during rabies virus infection of dorsal root ganglia cultures contributes to neuronal degeneration. The interaction of the rabies virus P protein with complexes I and IV of the ETC resulted in increased respiration and ROS production [27,28]. Mitochondrial dysfunction was also associated with a high ΔΨm, high NADH/NAD+ ratio, and low levels of ATP [27]. Human immunodeficiency virus (HIV) invades the nervous system through the trafficking of infected immune cells. Infected CNS resident cells, mainly astrocytes, avoid apoptotic death triggered by virus-induced mitochondrial fragmentation. Clearance of injured mitochondria by mitophagy results in astrocytes’ survival. Consequently, infected surviving astrocytes become a long-term virus reservoir in the brain that can also induce bystander cell death caused by mitochondrial dysfunction induced by high levels of mitochondrial ROS [29]. This event relies on the HIV accessory proteins Nef, Vpu, and Vpr and involves mitochondrial membrane depolarization, with cell death triggered by caspase-dependent and independent mechanisms [30].
It has been shown that non-toxic levels of ROS enhance viral replication [18,31,32,33,34]. While several viral proteins involved in ROS formation have been studied, other potential mediators of viral ROS modulation are yet to be identified [31]. The beneficial effects of ROS on the life cycle of viruses, starting as early as a virion binds to its cell receptor, have been demonstrated using antioxidants both in vitro and in animal models [34]. Particularly, RNA viruses are prone to ROS-induced modifications [35]. Viral mutations and the immunosuppressive effect of ROS may contribute to the selection of more virulent strains that escape the immune response [35,36,37]. Viruses that benefit from ROS formation include lymphocytic choriomeningitis virus (LCMV), Kaposi’s sarcoma-associated herpesvirus (KSHV), respiratory syncytial virus (RSV), influenza A virus (IAV), and hepatitis C virus (HCV). Michalek et al. [38] demonstrated increased ROS levels 15 min after the attachment of LCMV to the cell surface. After a weak initial binding, increased ROS levels and receptor modifications induced by ROS, strengthened the virion/cell receptor interaction, favoring the progression of the infection. Furthermore, ROS sensitized the neighboring cells to LCMV binding. The initial phase of ROS generation after infection was followed by a second wave, which was required for efficient viral replication. Similarly, a very early induction of ROS production favored the binding of the KSHV to endothelial cells in the microvasculature [32]. The effects of RSV, which causes severe respiratory disease, on mitochondrial function have been widely studied [39,40,41]. Eighteen hours post-infection (hpi) of adenocarcinoma alveolar cells, increased mitochondrial ROS generation favored RSV replication. This effect was blocked by treatment with MitoQ, a mitochondrial antioxidant, which acted as a potent inhibitor of RSV infection. Furthermore, in a murine model, MitoQ treatment reduced RSV titers and lung inflammation, indicating the importance of mitochondrial ROS production in the pathogenesis of the disease [41]. IAV is another respiratory pathogen that benefits from ROS production in infected cells. IAV infections are responsible for annual, epidemic respiratory diseases, with some strains having pandemic potential [42]. Antioxidant treatment in IAV-infected mice with an intranasally delivered mitochondrial ROS scavenger reduced mortality and lung inflammation [33]. This was consistent with an increase in the type I IFN response and a reduction in IAV titers in the lungs, accompanied by a decrease in pro-inflammatory cytokines at the late stages of infection. Beneficial effects of ROS have also been described for HCV, which causes chronic and persistent infections that are frequently associated with chronic hepatitis, cirrhosis, and hepatocellular carcinoma [43]. Among several HCV proteins that regulate oxidative stress, the core protein—a structural protein that targets the outer mitochondrial membrane—is considered the most potent regulator [44,45]. ROS induction is mediated by the binding of HCV core protein and the inactivation of heat shock protein (Hsp60), a stress response molecular chaperone mainly localized in the mitochondrial matrix. Protein misfolding caused by Hsp60 inactivation leads to mitochondrial dysfunction and ROS production, which in turn sensitizes cells to apoptosis induced by tumor necrosis factor-alpha (TNF-α) [46].
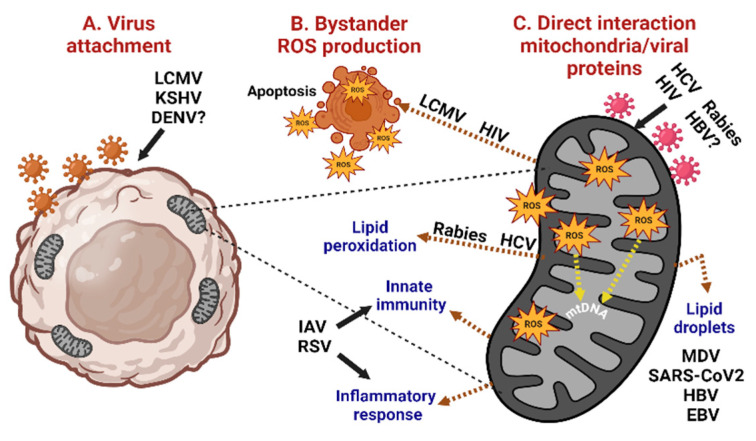
As described above, some viruses trigger mitochondrial ROS production to favor virulence and viral replication, while others regulate ROS generation to induce cell death or foster cell survival in the case of persistent chronic infections (Figure 2).
Figure 2.
Mitochondrial ROS production and the effects on virus infection. (A) Virus attachment triggers ROS generation. (B) ROS favors further virus binding to neighboring cells (bystander effect), which may lead to apoptotic cell death. (C) Direct interaction of viral proteins with mitochondrial components induces ROS production, leading to apoptotic cell death, alterations in lipids metabolism, activation of innate immunity, and the inflammatory response. DENV: dengue virus; LCMV: lymphocytic choriomeningitis virus; HIV: human immunodeficiency virus; KSHV: Kaposi’s sarcoma-associated herpesvirus; HCV: hepatitis C virus; HBV: hepatitis B virus; MDV: Marek´s disease virus; EBV: Epstein–Barr virus; RSV: respiratory syncytial virus; IAV: influenza A virus; mtDNA: mitochondrial DNA.
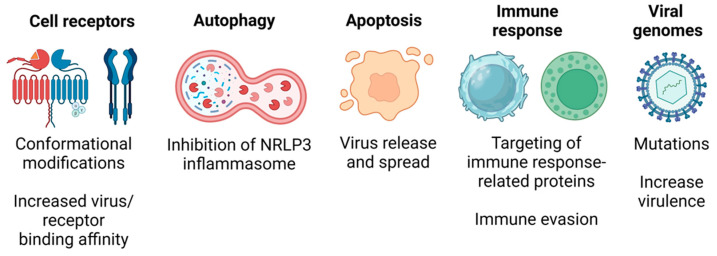
Overall, the beneficial effects of ROS include (a) changes in the conformation of cell receptors that increase virus/cell receptor binding affinity, (b) increased autophagy to repress inflammasome activation, (c) sensitization of cells to the intrinsic apoptotic pathway promoting virus release and spread, (d) targeting of proteins involved in the immune response favoring immune evasion mechanisms, and (e) direct ROS-mediated induction of mutations on the viral genomes increasing virulence (Figure 3).
Figure 3.
ROS-mediated mechanisms favoring viral infections. ROS-induced conformational changes on cell receptors favor virus adsorption, trigger autophagy that leads to inflammasome inactivation, stimulate apoptotic cell death to allow virus release and spread, alter viral proteins that favor evasion of the immune response, and introduce mutations to the virus genome, increasing virulence.
2.2. Cell Death Mediated by Reactive Oxygen Species
Some viruses inhibit cell death to amplify their progeny or to persist in the host, while others promote cell death to spread out to other cells or tissues. Mitochondrial ROS are directly associated with different types of cell death, such as apoptosis, necroptosis, pyroptosis, and ferroptosis, all of which can occur during viral infections (Table 1).
The role and the mechanisms of mitochondrial ROS in virus-induced apoptosis have been well-characterized and reviewed previously [18,47,48]. Sendai virus [49], SARS-CoV-2 [50], porcine epidemic diarrhea virus [51], Rift Valley fever virus [52], HIV-1, HCV, and IAV [47] are among the several viruses associated with ROS-induced apoptosis. The mechanisms of ROS in apoptotic cell death involve the activation of AMP-activated protein kinase (AMPK), which upregulates the transcription factor E2F1 and, consequently, the transcription of pro-apoptotic genes [53]. The intrinsic apoptotic pathway can also be induced by ROS-mediated Bax activation and insertion into the outer mitochondrial membrane, leading to cytochrome C release and activation of downstream caspases, such as the key apoptosis executioner, caspase 3. Additionally, caspases can cleave the p75 subunit of complex I in the ETC, which is followed by ROS generation and further amplification of the apoptotic signals [53,54].
Necroptosis is a type of regulated cell death with morphological characteristics that resemble necrosis. Engagement of cell surface death receptors, such as Fas and tumor necrosis factor-alpha receptor 1, cause loss of plasma membrane integrity, leading to the release of damage-associated molecular patterns (DAMPs) that promote an inflammatory response [55]. The execution of necroptosis relies on the receptor-interacting protein kinases 1 and 3 (RIPK1 and RIPK3) and the pseudokinase mixed-lineage kinase domain-like (MLKL) proteins. Increased mitochondrial ROS induces the mitochondrial translocation of p53 and RIPK1 phosphorylation. RIPK3 is recruited to the necrosome and phosphorylated by RIPK1. Then, RIPK3 phosphorylates MLKL, triggering its oligomerization and destabilizing the plasma membrane [53,55,56]. Furthermore, ROS has been implicated in disulfide bond formation between MLKL subunits, a prerequisite to induce necroptosis [57]. It has been shown that RSV infection of human macrophages induces TLR4 and TLR3 activation and ROS generation, which in turn trigger a RIPK1-independent, TRIF-dependent RIPK3-MLKL necroptotic pathway [58]. Increased ROS production in Theiler’s murine encephalomyelitis virus (TMEV) infection of macrophages has also been linked to necroptotic cell death, particularly when apoptosis is inhibited [59]. Necroptosis has been described for many viruses, including HIV-1 [60], vaccinia virus [61], MCMV [62], and reoviruses [63]. However, a direct link between necroptosis and ROS signaling has not been clearly established or investigated [64].
Pyroptosis is another regulated lytic type of cell death that requires ROS for activation of the NLRP3 inflammasome that triggers caspase-1 cleavage and activation of the pore-forming protein gasdermin, leading to membrane permeabilization and cell death [55]. Therefore, pro-inflammatory mediators (IL-1β and IL-18) are released [65]. RSV infection of macrophages activates TLR2 and ROS production, with ROS having a critical role as a second signal for inflammasome activation [58]. DENV infection or treatment of human endothelial cells with DENV recombinant E protein also induced pyroptosis, necroptosis, and ferroptosis. However, the highest level of cell death has been attributed to ROS-mediated pyroptosis and caspase-1 activity [66]. Similarly, high levels of ROS production and inflammasome activation were evident in the platelets from DENV-infected patients [67].
Ferroptosis is characterized by an iron overload, accumulation of ROS, lipid peroxidation, depletion of glutathione (GSH), and alterations of mitochondrial morphology [55,68]. Morphologically, ferroptotic cells show disruption of the plasma membrane, chromatin condensation, and mitochondrial and nuclear swelling [68]. The exact mechanism of ROS participation in ferroptosis has not been fully elucidated. Viruses may induce ferroptosis to replicate and evade the host immune system [69]. Ferroptotic cell death has been described in the HSV-1 infection of cultured neural cells and in a mouse model of encephalitis. Following HSV-1 infection, Nrf2, which regulates the expression of antioxidative genes, is ubiquitinated and degraded, thus disturbing cellular redox homeostasis and promoting ferroptosis [68]. Furthermore, ferroptosis is considered an effective mechanism for tumor suppression [70]. For example, the Newcastle disease virus (NDV) selectively induces ferroptosis in tumor cells initiated by the activation of p53, followed by nutrient deprivation, suppression of the cystine-glutamate antiporter system Xc-, and induction of ferritin degradation, which stimulates iron release [71]. Iron metabolism dysfunction in COVID-19 patients is associated with ferroptotic cell death in multiple organs. In this case, SARS-CoV-2 infection decreased the GSH pool and downregulated GPX4 gene expression [72]. Ectopic expression of hepatitis A virus (HAV) 3C protease (3Cpro) induced ferroptosis in several human cell lines. However, low cellular levels of 3Cpro did not induce cellular alterations compatible with ferroptosis. Therefore, it has been suggested ferroptosis is a side effect of 3Cpro activity in certain cell types [73]. Latent Epstein–Barr virus (EBV) has also been implicated in the induction of ferroptosis in newly infected human primary B lymphocytes and in transformed B cells. The levels of ferroptotic death vary with the levels of susceptibility according to the distinct states of latency, with Burkitt´s lymphoma cells being highly vulnerable to ROS-induced lipid peroxidation and, consequently, to ferroptosis [74]. Furthermore, these cells have low GSH stores and limited capacity for cystine uptake [75]. Swine influenza virus (SIV) infection increased the iron levels in adenocarcinoma alveolar cells and inhibited the GPx4/system Xc-axis, which is essential for avoiding lipid peroxidation. Thus, high concentrations of intracellular iron, combined with elevated levels of ROS and lipid peroxidation, ultimately caused the death of infected cells by ferroptosis [76].
Table 1.
Types of cell death induced by ROS.
| Cell Death Type | Features | General Mechanisms Involving ROS |
Virus | Model | References |
|---|---|---|---|---|---|
| Necroptosis |
|
|
RSV | Human monocyte cell line (THP-1) | [58] |
| TEMV | Murine macrophage culture | [59] | |||
| DENV | Human endothelial cells (HMEC-1) | [66] | |||
| Pyroptosis |
|
|
RSV | Human monocyte cell line (THP-1) | [58] |
| DENV | Human endothelial cells (HMEC-1) and platelets | [59,67] | |||
| Ferroptosis |
|
|
HSV-1 | Neural cells Encephalitis murine model |
[68] |
| NDV | Glioma cells | [71] | |||
| HAV | Ectopic HAV 3C protease expression in human cell lines | [73] | |||
| EBV | Human primary B cells | [74] | |||
| SARS-CoV2 | Vero (African green monkey) cells | [72] | |||
| SIV | Adenocarcinoma alveolar cells | [76] | |||
| DENV | Human endothelial cells (HMEC-1) | [66] |
mtROS: mitochondrial ROS; RIPK1-3: receptor-interacting protein kinases 1 and 3; MLKL: pseudokinase mixed-lineage kinase domain-like; GSH: glutathione; GPX4: glutathione peroxidase 4; 9RSV: respiratory syncytial virus; TEMV: Theiler’s murine encephalitis virus; DENV: dengue virus; HSV-1: herpes simplex virus 1; NDV: Newcastle disease virus; HAV: hepatitis A virus; EBV: Epstein–Barr virus; SIV: swine influenza virus.
3. Metabolic Effects of Viral Infections
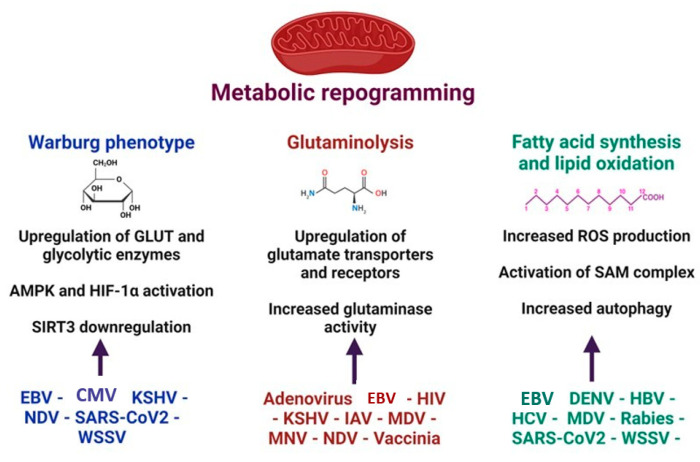
Virus replication is energetically and metabolically very demanding [77]. Viruses cope with these demands, inducing metabolic reprogramming, including the upregulation of enzymes that control the metabolic pathways [78,79]. The metabolic switch enhances glycolysis (Warburg effect), glutaminolysis, and lipid oxidation [78] (Figure 4). An upregulated pentose phosphate pathway and changes in amino acid metabolism after viral infections have also been reported [80]. Some viruses may turn on more than one pathway to support their energetic needs, while different cellular metabolic pathways may be activated at distinct stages of the virus life cycle [81,82].
Figure 4.
Major mitochondrial metabolic pathways and global mechanisms activated during viral infections. Virus replication is an energy demanding process. To cope with this energetic demand, viruses induce cellular metabolic reprogramming, which includes enhanced glycolysis (Warburg phenotype), glutaminolysis, fatty acids synthesis, and lipid oxidation. KSHV: Kaposi´s sarcoma-associated herpesvirus; HCMV: human cytomegalovirus; EBV: Epstein–Barr virus; NDV: Newcastle disease virus; MNV: murine norovirus; MDV: Marek´s disease virus; HBV: hepatitis B virus; HCV: hepatitis C virus; IAV: influenza A virus; WSSV: white spot syndrome virus; HIV: human immunodeficiency virus. GLUT: glucose transporter; AMPK: AMP-activated protein kinase; SAM complex: β-barrel-specific sorting and assembly machinery; mtROS: mitochondrial ROS.
3.1. Warburg Phenotype
The Warburg phenotype, characterized by enhanced glycolysis, even in the presence of physiological concentrations of O2, is displayed by cancer cells and cells with high proliferation rates, like activated T lymphocytes. OxPhos generates 95–98% of the total cellular ATP in quiescent cells, whereas glycolysis and the succinyl-CoA ligase reaction in the TCA cycle provide the remaining 5%. By contrast, cancer cells generate ~10–90% of the total ATP by glycolysis [83,84,85]. Full oxidation of glucose in mitochondria generates ~32 moles of ATP, but only 2 moles of ATP/mole of glucose are produced during glycolysis [83]. Despite this difference in the yield of ATP and arguments in favor or against a higher rate of glycolysis being able to compensate for the relatively lower efficiency, the reality is that the ATP demand for cell division is low compared to the energy requirements for maintaining cellular functions, mainly the activity of the Na+-K+ ATPase. This fact strongly suggests that ATP generation is not limiting for rapid cell proliferation [86,87,88,89]. The current consensus is that enhanced aerobic glycolysis, together with mitochondrial metabolism, is necessary to provide not only ATP but also metabolic intermediates for the synthesis of macromolecules [90,91,92,93]. The shift towards aerobic glycolysis has also been proposed to increase resistance to apoptosis [94,95].
The mechanisms and changes in glucose metabolism induced by viruses are summarized in Table 2.
An increased expression of glucose transporters (GLUT) and enzymes involved in the glycolytic pathway has been shown in several viral infections [78]. Human cytomegalovirus (HCMV) replication increased glucose uptake [96] by downregulating GLUT1 and upregulating the more efficient GLUT4 [97]. HCMV infection also upregulated the glycolytic enzymes phosphofructose kinase-1, hexokinase, PDH, and also increased glycolytic intermediates and lactate [98,99,100,101]. In addition, these changes in glucose metabolism increased FA and lipid synthesis [101]. GLUT3 and hexokinase II have been found to be upregulated in KSHV-latently infected endothelial cells [94].
Several viral infections cause enhanced glycolysis and metabolic reprogramming through the activation of hypoxia-inducible factor 1-alpha (HIF-1α). An initial infection with SARS-CoV2 is characterized by enhanced aerobic glycolysis and high virus titers [82]. Elevated glucose levels promote viral replication and cytokine expression, leading to rapid tissue dissemination and exacerbated inflammation [82,102]. In monocytes, enhanced glycolysis and the pro-inflammatory state responsible for the lung injury observed in COVID-19 patients were mediated by HIF-1α [102]. Epstein–Barr virus (EBV) is a human oncogenic gamma-herpesvirus that is associated with several types of lymphocytic disorders and epithelial tumors [103]. The EBV latent membrane protein (LMP)-1 oncoprotein regulates tumorigenesis and shifts the metabolic program towards aerobic glycolysis by activating HIF-1α [104,105,106]. KSHV also activates HIF-1α, which is required for activating KSHV oncogenes [79].
In other cases, the Warburg effect in virus-infected cells is accompanied by decreased activity of the mitochondrial respiratory chain complexes and mitochondrial dysfunction without a complete shutdown of OxPhos. Newcastle disease virus (NDV) is an avian virus oncolytic to mammalian cells [107]. Recently, it was shown that NDV induces mitochondrial damage, leading to the degradation of SIRT3 via mitophagy [108]. SIRT3 is a mitochondrial member of the sirtuin family of the NAD-dependent ADP-ribosyl transferases and/or protein deacetylases involved in metabolism and stress response [109]. Reduced or lack of SIRT3 activity shifts mitochondrial bioenergetic metabolism toward glycolysis, which contributes to viral replication [108].
Finally, a mitochondrial metabolic switch has also been well-characterized in viral infections of crustaceans caused by the white spot syndrome virus (WSSV). The stage of viral genome replication in the shrimp immune cells is characterized by increased expression and activity of glycolytic enzymes, such as hexokinase and phosphofructokinase [110]. Furthermore, AMPK expression and phosphorylation are also significantly upregulated in WSSV-infected shrimp. Via the mTORC2-AKT pathway, AMPK phosphorylates glycolytic enzymes, promoting the expression of HIF-1α [111,112]. Thus, enhanced glycolysis provided energy and biomolecules for virus replication [110]. Additionally, Chen et al. [113] reported that an induced metabolic shift into the Warburg phenotype can counteract the high levels of ROS produced in response to WSSV infection.
Table 2.
Mechanisms involved in the virus-induced shift towards aerobic glycolysis.
| Virus | Mechanism | References |
|---|---|---|
| HCMV | Downregulation of GLUT1 Upregulation of GLUT4 and glycolytic intermediates. Increase in lactate production Involvement of AMPK pathway |
[97,98,99,100,101] |
| SARS-CoV2 | Induction and activation of HIF-1α Enhanced aerobic glycolysis |
[82] |
| EBV | Upregulation of GLUT1, lactate dehydrogenase A, and PDK-1 LMP-1-induced expression of HIF-1α |
[104,105,106] |
| KSHV | Induction and activation of HIF-1α Upregulation of GLUT3 and hexokinase II |
[94] |
| NDV | Degradation of SIRT3 | [108,109] |
| WSSV | Increased expression of hexokinase, phosphofructokinase, and AMPK Induction and activation of HIF-1α |
[110,111,112] |
GLUT: glucose transporter; AMPK: AMP-activated protein kinase; PDK-1: pyruvate dehydrogenase kinase 1; HIF-1α: hypoxia-inducible factor 1-alpha; LMP-1: latent membrane protein 1; SIRT3: sirtuin 3; HCMV: human cytomegalovirus; EBV: Epstein–Barr virus; KSHV: Kaposi´s sarcoma-associated herpesvirus; NDV: Newcastle disease virus; WSSV: white spot syndrome virus.
3.2. Reverse Warburg Effect
Oncoviruses can induce the reverse Warburg effect, which is characterized by enhanced aerobic glycolysis in cancer-associated fibroblasts. In this two-compartment model of metabolic symbiosis, cancer cells secrete H2O2 and cause oxidative stress in neighboring fibroblasts, which produce metabolic intermediates, such as pyruvate, ketone bodies, FA, and lactic acid that feed the tumor cells [114].
The life cycle of the oncovirus KSHV comprises a quiescent, latent state and a lytic, replicative phase. In contrast to the abundant expression of viral genes observed during lytic infection, latency is characterized by the episomic persistence of the viral genome with restricted viral gene expression [115]. The latency-associated nuclear antigen (LANA) protein is the main latency-regulatory viral protein, whereas the switch to the lytic phase is controlled by the replication and transcription activator (RTA) protein [116]. Different latency programs with distinct patterns of gene expression have been described. EBV nuclear antigen (EBNA) 1 is expressed in Burkitt’s lymphoma, LMPs in nasopharyngeal carcinoma, Hodgkin’s lymphoma, and NK/T cell lymphoproliferative diseases [117,118], and all EBNAs and LMPs are expressed in EBV-associated post-transplantation lymphoproliferative disorders, acute infectious mononucleosis, and X-linked lymphoproliferative syndrome [119]. It is well-established that in a latent KSHV infection, gene expression is restricted by several microRNAs (miRNAs) [120]. miRNAs are highly conserved noncoding RNAs that intervene in a wide array of biological processes [121]. Comparable to cellular miRNAs, virus-encoded miRNAs also regulate energetic metabolism and angiogenesis [122]. It has been demonstrated that KSHV-encoded miRNAs induce aerobic glycolysis in infected cells [123] while others are transferred to uninfected neighboring cells, inducing the reverse Warburg effect [79,124]. Induction of the glycolytic pathway is also observed in EBV-induced nasopharyngeal carcinoma. In this case, BART1-5P miRNAs increased glucose consumption and lactate production via regulation of the AMP-activated protein kinase (AMPK)/mTOR/HIF-1α pathway. The discovery of virus-encoded miRNAs unveiled a mechanism used by many viruses, particularly those that maintain a latent state, to induce a metabolic shift in the surrounding cells that favor their own persistence [125].
In general, viruses utilize a broad spectrum of strategies to directly or indirectly utilize the Warburg phenotype and mitochondrial metabolism to support the energetic and synthetic requirements of viral replication.
3.3. Fatty Acids Synthesis and Lipid Peroxidation
Disturbances in the synthesis, accumulation, and oxidation of FAs are common in viral infections. Lipid droplets (LDs), formed by a core of triacylglycerols, cholesteryl esters, and retinyl esters surrounded by a phospholipid monolayer, play an important role in viral infections [126]. Catabolism of stored triglycerides yields FAs that undergo mitochondrial β-oxidation, resulting in ATP formation. Aside from being used to store energy, FAs released from phospholipids and triglycerides act as signaling molecules [127,128,129]. During viral infections, changes in the lipid composition of LDs influence the pathogenicity and replication of the viruses [130]. For example, virus-dependent increased LD levels activate the NLRP3 inflammasome and modulate the innate immune response [131]. Below, we describe how viruses alter lipid metabolism.
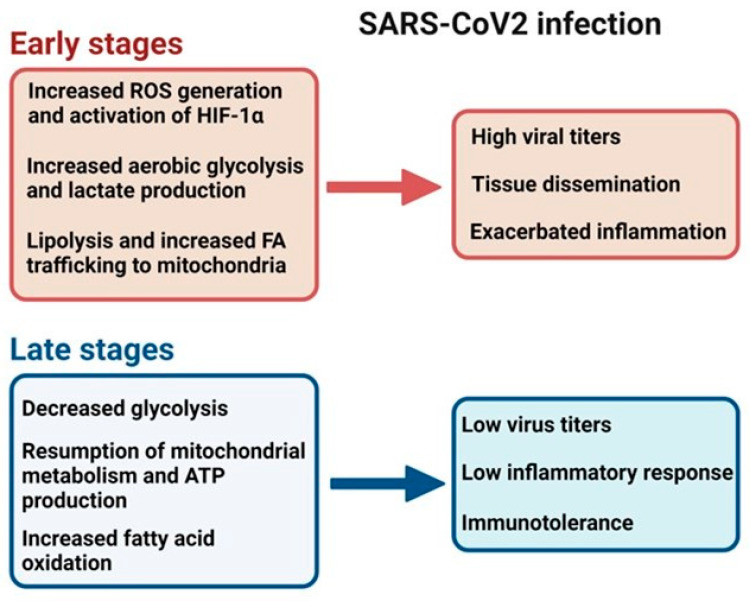
Inhibition of FA synthesis decreases the production of infectious virus particles [132]. FA inhibition rendered KSHV intracellular virions non-infectious, suggesting a FA-dependent blockage of virion assembly and/or maturation [81]. By contrast, gallid herpesvirus 2 or Marek´s disease virus (MDV), an alpha herpesvirus of chickens, increased FA synthesis in infected cells and induced LD formation, which facilitated virus replication [133]. Lipolysis has been described as a mechanism that provides energy for WSSV during virus replication. However, virus-induced lipogenesis is also required at later stages when long-chain FAs must be supplied for virus morphogenesis [134]. Recently, it was shown that the ORF6 protein of SARS-CoV2 that inserts into LDs and interacts with mitochondria binding to the SAM (β-barrel-specific sorting and assembly machinery) complex in the outer mitochondrial membrane induces lipolysis and stimulates FA trafficking into mitochondria [135,136]. Opposite to the initial stages of SARS-CoV2 infection, the second phase is characterized by low virus titers, reduced glycolysis, and oxidative metabolism, with increased FA oxidation [82]. The accessory SARS-CoV2 ORF3c protein, localized to the outer mitochondrial membrane, causes this metabolic shift, probably by an indirect mechanism that involves the transport of pyruvate from the cytoplasm to the mitochondrial matrix [136]. SARS-CoV2 infection demonstrates how a virus manipulates mitochondria according to the specific needs at each step of the infectious process (Table 3 and Figure 5).
Table 3.
SARS-CoV2 accessory proteins and mitochondrial dysfunction.
| Protein | Mitochondrial Target | Mitochondrial Dysfunction | References |
|---|---|---|---|
| ORF3a | mPTP | Increase mitochondrial Ca++ Increase ROS Promotes HIF-1α expression Release of mtDNA |
[137] |
| ORF3b | MAVS-Drp1 | Suppression of IFN response | [138] |
| ORF3c | TOM20 and TOM70 (OMM) MAVS |
Increase of FA synthesis Suppression of IFN response |
[136,139] |
| ORF7a | MAVS | Suppression of IFN response | [140] |
| ORF8a | MAVS | Suppression of IFN response | [141] |
| ORF9b | MAVS TOM70 |
Suppression of IFN response | [140,141,142,143,144] |
| ORF10 | MAVS Mitophagy receptor NIX |
Suppression of IFN response | [145] |
mPTP: mitochondrial permeability transition pore; MAVS: mitochondrial antiviral-signaling protein; Drp1: Dynamin-related protein 1; LD: lipid droplet; SAM complex: Sorting and Assembly Machinery complex; OMM: outer mitochondrial membrane; TOM70: translocase of OMM.
Figure 5.
Metabolic switch in SARS-CoV2 infection. SARS-CoV2 infection follows a bimodal metabolic reprogramming. Initial SARS-CoV2 infection is characterized by mitochondrial ROS production that promotes hypoxia-inducible factor 1-alpha (HIF-1α) expression, lipolysis, and an increase in FA synthesis. Together with the induction of the Warburg effect, virus replication is enhanced, accompanied by a severe pro-inflammatory response (cytokine storm). During the second stage, glycolysis and oxygen consumption decrease, FA oxidation increases, and the mitochondria return to regular respiration and ATP production. It is a hypo-inflammatory stage, with decreased virus titers and immunotolerance [92,133].
Enveloped viruses need lipids for the viral envelope, while positive-sense RNA viruses require lipids to constitute the replication and assembly compartments [146]. These compartments are partially closed double-membrane structures formed by the rearrangement of membranes of different organelles. Infections with RNA viruses cause redistribution of LDs that become more accessible to viral non-structural proteins to initiate replication [147,148]. ROS generation also modulates LD formation. High mitochondrial ROS production increased the number of LDs and stimulated HBV gene expression in HepG2 human hepatocellular carcinoma cells expressing hepatitis B virus HBx protein [149]. Treatment with the antioxidant N-acetylcysteine decreased LD accumulation in a time-dependent manner [150]. In addition to the effect on LD formation, ROS induce the peroxidation of lipids rich in polyunsaturated FAs, both in cell membranes and viral envelopes. HCV infections cause liver steatosis and induce the peroxidation of lipids and viral enzymes of the replicase complex, particularly NS3/4A and NS5B [147]. Attenuated replication may facilitate long-term viral persistence [151]. It has been consistently demonstrated that peroxidation of the viral envelope causes disintegration of the viral particle [152]. The pathological role of lipid peroxidation has been demonstrated for neurotropic viruses, such as rabies virus. In a dorsal root ganglia model, Jackson et al. [153] demonstrated the presence of multiple axonal swellings, concomitantly with positive viral antigen and immunostaining for 4-hydroxynonenal (4-HNE), a marker of lipid peroxidation associated with oxidative stress. It was suggested that lipid peroxidation induced modifications in mitochondrial and cytoskeletal proteins, which were finally responsible for axonal swelling. Therefore, mitochondrial dysfunction and altered lipid metabolism, induced by virus replication, are key mediators of neuronal degeneration in rabies virus infection.
The cellular lipid metabolism in DENV-infected cells is characterized by activation of FA synthesis, accumulation of LDs, and mobilization of FAs [153], being FAs the main energetic substrate for efficient replication [154,155]. By an autophagy-dependent process, DENV increases β-oxidation, which generates ATP while depleting LDs and triglycerides [155].
Another alteration of lipid metabolism induced by viruses is caused by the immediate-early EBV protein BRLF1 that induces the expression of the FA synthase and several proteins involved in FA metabolism and cholesterol biosynthesis in epithelial and B cells [150].
Overall, alterations in lipid metabolism caused by viral infections range from increased FA synthesis and lipid droplet accumulation to increased FA oxidation and lipid peroxidation. Ultimately, the mechanisms seem to be virus-specific.
3.4. Glutamine Metabolism
Glutamine is the most abundant amino acid in the body and is used for the synthesis of proteins, nucleotides, and lipids [156]. Glutaminolysis is the catabolic conversion of glutamine into nitrogen-containing metabolites of the TCA cycle that both support OxPhos and are used for the synthesis of amino acids and nucleotides [157,158]. During viral infections, enhanced glutamine catabolism sustains virus replication [124].
The transcription factor c-myc, which regulates the expression of genes associated with cancer cell metabolism, favors viral infections [142]. The adenovirus E4ORF1 protein enhances adenovirus replication in human lung cells by activating c-myc, which, in turn, upregulates glutamine transporters and increases the activity of the rate-limiting enzyme glutaminase [159]. It has also been shown that during latency, the LANA protein of KSHV, as well as EBV infection, upregulates glutaminase expression by activating c-myc [160]. IAV infection of primary human bronchial epithelial cells and bone marrow-dendritic cells from pediatric patients showed a switch to glutamine utilization that was also dependent on c-myc [161].
Other viruses enhance glutaminolysis independently of c-myc. The highly virulent avian pathogen NDV induces upregulation of the glutamate transporter SLC1A3 and increases glutaminase activity [128]. In infected chicken embryonic fibroblasts with MDV, glutamine was converted to α-ketoglutarate as an intermediate for the TCA cycle to support virus replication [133]. Since glutamine is necessary for the efficient function of lymphocytes and macrophages [162], it was suggested that glutamine catabolism during MDV may contribute to avoiding the antiviral immune response [133]. A murine norovirus (MNV) model has been used to study metabolic reprogramming in MNV-infected macrophages. Noroviruses strains causing acute or persistent infection require glutaminolysis as a carbon source for genome replication. The viral non-structural protein NS1/2 has been identified as responsible for the increase in glutaminase levels. Although the effect of glutaminolysis on norovirus replication is clear, there is no current information on the effect of this catabolic process on infected macrophages [163]. Serum glutamine and glutamate depletion concomitantly with increased phosphatidylcholine biosynthesis suggest that both pathways contribute to HBV replication and progression to the hepatocellular carcinoma associated with HBV infection [164,165]. Glutaminolysis is also the major pathway supplying intermediates for the TCA cycle and OxPhos in HIV-1-infected naïve and memory CD4+ T cells [166,167]. Elevated levels of intracellular glutamine are observed during HIV-1 infection [167,168]. Although the entry of glutamine-derived carbon into the citric acid cycle is not affected, the secretion of glutamine-derived glutamic acid and protein levels of enzymes that metabolize glutamine to glutamic acid are significantly increased [168]. Glutaminolysis regulates the early steps of infection, favors virus replication, increases susceptibility to HIV-1 infection, and drives CD4+ T-cell proliferation [167,168].
Although many viruses require both increased glycolysis and glutaminolysis for efficient replication, the vaccinia virus, the causal agent of smallpox, only requires the anaplerotic reactions derived from glutamine catabolism without enhancing glycolysis. Glutamine is specifically used for vaccinia virus protein synthesis without a significant effect on transcription [169]. A similar finding has been described for the KSHV infection, in which glutaminolysis is required for early protein translation with no impact on virus gene transcription [79].
4. Mitochondria in the Immune Response to Viral Infections
The effects of ROS on the innate immune response are broad, altering protein function and introducing post-translational modifications that affect the immune signaling pathways. ROS participate in the antiviral immune response by inducing MAVS and by activating TLRs and DNA-sensing pathways, like STING (stimulator of interferon genes) [170,171]. A signaling cascade, initiated after nucleic acid-sensing receptors associated with the adaptor MAVS, leads to the expression of antiviral genes, mainly type I IFN. The robustness of the response depends on the activation/degradation of molecules like IRF-3 and IFN-stimulated genes (ISG) [172]. Other viruses have a direct effect on MAVS. The HCV protease NS3/4a binds to mitochondria and cleaves MAVS [173], whereas the PB1-F2 protein of IAV, as well as SARS-CoV2 ORF10 protein, induce mitophagy and MAVS degradation, suppressing the type I IFN response [174]. The Murine herpesvirus-68 (MHV-68), closely related to KSHV and EBV, is also recognized by the cytosolic DNA-sensing pathway cGAS/STING. Tao et al. [170] demonstrated that increased ROS levels antagonized the IFN-β response to MHV-68 by oxidizing a STING-cysteine residue. Thus, by manipulating the ROS levels, MHV-68 inhibits the innate immune response, favoring its own replication.
Participation of ROS in the inflammatory response is also linked to their role as a second signal for activation of the NLRP3 inflammasome [172,175], which leads to cleavage and maturation of the pro-inflammatory cytokines, IL-1β and IL-18. Furthermore, Gasdermin-D, a key molecule during pyroptosis, is also cleaved upon ROS signaling [175]. Mitochondrial ATP, which is released after virus-induced cell death, has also been implicated in the activation of the NLRP3 inflammasome [175,176].
Mitochondrial DNA (mtDNA) is a double-stranded circular DNA that encodes 13 subunits of the mitochondrial respiratory chain. Its proximity to the inner mitochondrial membrane—where ROS are produced in the ETC—and the lack of protection by histones make mtDNA highly vulnerable. mtDNA plays a critical role in the mitochondrial-derived immune response [177]. Following mitochondrial damage or cell death, mtDNA is released and sensed by innate immune receptors, triggering the inflammatory response [3]. Several members of the Picornaviridae family, including enterovirus 71 (EV-A71), Seneca Valley virus (SVV), and foot-and-mouth disease virus (FMDV), trigger mtDNA release into the cytosol after induction of mitochondrial damage. An opening of the mitochondrial permeability transition pore (mPTP) and VDAC1/Bak/BaX-dependent mtDNA leakage into the cytoplasm has been shown during SVV infection, whereas EV-A71 and FMDV also induce mPTP opening and VDAC1-dependent mtDNA release without involving Bak/BaX-activity. Released mtDNA also binds to cGAS (cyclic GMP-AMP synthase), activating the antiviral immune response. These picornaviruses are able to surpass the antiviral immunity by encoding the 2C protein, a highly conserved non-structural protein, which degrades cGAS or blocks the activation of the signaling cascade [178].
Independently of which is the mitochondrial target, viruses display an array of evasion strategies to avoid the mitochondria-derived immune response.
5. Virus Interaction with Voltage-Dependent Anion Channels
The voltage-dependent anion channels (VDACs) 1, 2, and 3 are β-barrel structures in the outer mitochondrial membrane of all eukaryotic cells [179,180,181,182]. The influx of oxidizable substrates, ADP, inorganic phosphate, and glycolytic ATP into mitochondria and the efflux of ATP through the outer mitochondrial membrane occurs only through VDACs [180,183,184]. VDACs also contribute to the regulation of calcium import to mitochondria [185]. Once inside the matrix, oxidizable substrates enter the TCA cycle, generating NADH that fuels the ETC. Overall, VDACs operate as a biological switch that, in the open state, maximizes the flux of metabolites for optimal mitochondrial function, whereas during the closed state, it lowers mitochondrial metabolism [183,186]. Thus, regulation of only this channel has an amplifying effect on several intra- and extra-mitochondrial pathways that modulate cancer metabolism and bioenergetics. Since mitochondrial ROS production directly depends on the activity of the ETC, VDAC opening or closing is a major driver for ROS formation [183]. Moreover, VDAC regulation may serve as an adjustable rheostat, with a range of operational levels that depend on the magnitude and duration of VDAC opening [183,186].
Information about the interactions between VDACs and viral proteins is still very scarce. The DENV E protein is a viral receptor binding protein that interacts with the cellular chaperone GRP78, which in turn interacts with VDAC1. As a result of these interactions, VDAC1 is re-localized during DENV infection, with mitochondria moving toward the endoplasmic reticulum. It was suggested that the re-localization of VDAC1 is required to traffic metabolites near the sites of DENV replication in proximity to the endoplasmic reticulum. The relevance of the DENV E/GRP78/VDACs was confirmed by VDAC1 silencing, which significantly reduced DENV protein expression, percentage of infection, and extracellular virus titers [187]. Infectious bursal disease virus (IBDV) is a double-stranded RNA (dsRNA) virus that causes severe immunosuppression in chickens. VDAC1 has been found to be upregulated during IBDV infection, being key in mediating IBDV polymerase activity [188]. VDAC1 interacts with IBDV VP1 and VP3 proteins, which are components of the polymerase complex. This interaction is crucial for stabilizing the complex and promoting polymerase activity that enhances viral replication and transcription. Cytomegalovirus (CMV) is a herpesvirus with high seropositivity in humans [189]. Although still controversial, it was suggested that CMV increases the risk of developing Alzheimer’s disease and cognitive alterations [190]. Recently, in a murine CMV model, an association between cognitive disorders and increased permeability of the blood–brain barrier was demonstrated. Among several altered mitochondrial parameters, it was shown that VDAC1 expression and mitochondrial ROS increased in brain microvascular endothelial cells after repeated MCMV infections during a 12-month period, which was suggestive of a cell dependence on OxPhos. Likely, cell metabolic reprogramming alters the integrity of the blood–brain barrier, favoring T-cell infiltration and facilitating cognitive decline [191].
The enterovirus 71 (EV 71) 2B protein, which is a viroporin, directly interacts with VDAC3 to increase ROS production and enhance viral replication, as evidenced by the inhibition of EV 71 replication after VDAC3 knockdown. This interaction also suppresses the synthesis of taurine/hypotaurine, which has antioxidant activity. Although the exact mechanism is unknown, it was suggested that the 2B/VDAC3 interaction would suppress the expression of the enzymes involved in hypotaurine metabolism. By contrast, siRNA silencing of VDAC3 increased the antioxidant capacity of infected cells, implying that VDAC3 might be involved in a negative regulation of this antioxidant mechanism [192]. Ca2+ storage organelles, such as the ER and mitochondria, are important in calcium homeostasis [193]. Tight contact between mitochondria and ER triggers a rapid mitochondrial Ca2+ influx and Ca2+ overload that can disrupt Δψm and alter OxPhos [185]. Additionally, elevated mitochondrial Ca2+ levels increase ROS production [194,195] by stimulating the flow of electrons in the ETC or by altering the structure of the respiratory complexes [173]. VDACs participate in efficient Ca2+ transfer to the mitochondria by forming multi-protein complexes with Ca2+ channels in other organelles. The mitochondrial permeability transition pore (mPTP) is a non-specific pore that is permeable to solutes of <1.5 kDa. mPTP opens in the inner mitochondrial membrane under elevated levels of Ca2+ in the matrix, especially under conditions of oxidative stress and low levels of adenine nucleotides. The opening of the mPTP causes mitochondrial swelling, disruption of the outer membrane, and release of mitochondrial components that induce apoptosis. HCV core and NS5a proteins are known to cause ER stress with the release of Ca2+ and direct transfer from the ER to the mitochondria. This results in alterations of the ETC, increasing ROS production and sensitivity to mitochondrial permeability transition and cell death [196,197]. Particularly, the induction of mPTP has been attributed to HCV core protein [197]. HBV is another virus that modifies mitochondrial function by interacting with VDAC3. The HBV HBx regulatory protein has a binding affinity for VDAC3. The HBx/VDAC3 association decreased ΔΨm and altered mitochondrial physiology [198]. It is well-established that HBV can alter Ca2+ signaling to create a cellular environment favorable for virus replication [199]. Interaction of the viral protein with VDAC3 enhances Ca2+ trafficking into the mitochondria. Furthermore, HBx regulates the mPTP, promoting mitochondrial Ca2+ outflow [200]. Mitochondrial dysfunction during HBV infection has been linked to chronic hepatitis, cirrhosis, and oncogenesis. It is likely that the HBx/VDAC3 interaction and mPTP regulation mediated by HBx plays a pivotal role in the outcome of the disease. Pharmaceutical targeting of Ca2+ signaling may provide a potent strategy for controlling HBV.
The porcine respiratory and reproductive syndrome virus (PRRSV) causes one of the most important diseases that affect the swine industry. PRRSV GP5 proteins are key for viral infectivity. Interaction of GP5 with VDAC1 promoted VDAC oligomerization and enhanced mitochondrial Ca2+ uptake from the ER by promoting ER-mitochondria contact. This resulted in the induction of mROS release and triggered autophagy, which repressed NLRP3 inflammasome activation and increased viral replication [201].
Although few, these studies demonstrate that the virus’s influence on VDAC function may be key to altering the physiological status of the mitochondria to sustain virus infection.
6. Viral Effects on the Electron Transport Chain
Several viruses have been shown to regulate the activity of ETC. RSV manipulation of the mitochondrial metabolism and signaling pathways has been widely recognized. Among several RSV-mitochondrial targets, the inhibition of Complex I is central to RSV pathogenesis [34]. High viral loads in infected cells are associated with decreased mitochondrial respiration and increased ROS production caused by the inhibition of complex I. It has been shown that HCV limits OxPhos by downregulating the core subunits of Complexes I and IV at early time points after infection, while Complex V activity decreases at the later stages [106]. Derakhshan et al. [202] demonstrated that the HSV-1 Us3 protein kinase mediated the inhibition of cellular respiration by blocking electron transport between complexes II and III. HIV-1 causes neurological disorders without infecting neurons. The neurotoxic HIV-1 transactivator of transcription (Tat) protein is secreted by infected T cells and macrophages/microglia affecting bystander cells, such as neurons [203]. Tat inserts into mitochondria through a basic domain, induces mitochondrial hyperpolarization, and decreases the activity of complexes III and IV in isolated neuronal mitochondria [204,205]. Thus, the effect of Tat on respiratory chain complexes in neurons and the global effects on mitochondrial function might be partially responsible for the neurological signs observed in HIV-1-infected patients. In the case of human herpes simplex virus 1 (HSV-1) infection, a decline in ATP levels has been attributed to mitochondrial dysfunction [206]. The highly oncogenic MDV infects immune cells, causing a deadly lymphoproliferative disease in chickens [207]. The phosphorylated p38 protein (pp38) of MDV is required to lyse lymphocytes B, to induce latency, and to prevent apoptosis in T cells. pp38 increased the activity of mitochondrial succinate dehydrogenase, which is part of Complex II and feeds electrons directly into the ubiquinone/ubiquinol pool. The mechanism by which pp38 upregulates Complex II activity is currently unknown. However, co-localization with mitochondria could not be demonstrated, suggesting that the effect is indirect [208].
7. Virus-Triggered Mitochondrial Fission
Mitochondria are dynamic organelles that undergo morphological adaptations through cycles of fusion and fission to fuse or divide individual mitochondria, respectively [3]. Mitochondrial dynamics is involved in the regulation of the cell cycle, the immune response, and programmed cell death [209]. Beyond the changes in morphology, the number of mitochondria is determined by the rate of mitochondrial biogenesis and the removal of damaged mitochondria by mitophagy [210]. Disruption of mitochondrial fission leads to altered metabolism, proliferation, and apoptosis [211]. Some viruses exert a tight control on mitochondria dynamics. Checking when mitochondria fragmentation and mitophagy are required is clued to promptly escape from the mitochondria-induced immune response. AMPK is an energy sensor that detects low ATP levels [212,213] and affects mitochondrial dynamics. During NDV infection, under energetic stress, AMPK induces mitochondrial fission and controls mitophagy [108]. EBV-encoded BHRF1 protein is a BCL2 homolog that stimulates DNM1L (dynamin 1-like proteins)/Drp1-mediated mitochondrial fission and drives the reorganization of mitochondria into perinuclear aggregates. BHRF1 inhibits the IFN type I response by interacting with autophagosomes and stimulating mitophagy [214].
8. Concluding Remarks
Viruses modulate mitochondrial function and cellular metabolism to favor their own replication, persist in the host, and increase their virulence. Many viruses target more than one mitochondrial component and signaling pathway. Inhibition of the ETC, increased or decreased ROS production, differential utilization of glucose, glutamine, and FAs, and changes in mitochondrial dynamics are the mechanisms triggered by viral infections. Viruses use numerous strategies to bypass or avoid cellular mechanisms, which can prevent the infection from spreading or decrease the virulence. Several viruses are considered the main etiological agents of emerging diseases with pandemic potential. Therefore, besides understanding viral pathogenesis, gaining knowledge of virus/mitochondria interactions may provide novel opportunities for therapeutic interventions that can eventually be applied to the treatment of emergent viral diseases or to overcome the antiviral resistance of certain strains.
Author Contributions
Conceptualization, E.N.M., M.G. and S.E.P.; investigation, E.N.M., M.G. and S.E.P.; resources, E.N.M., M.G. and S.E.P.; writing—original draft preparation, E.N.M., M.G. and S.E.P.; writing—review and editing, E.N.M. and S.E.P.; supervision, E.N.M.; project administration, E.N.M.; funding acquisition, E.N.M., M.G. and S.E.P. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was supported partially by the following funding from High Innovation-High Reward South Carolina Translational Research Grant UL1 TR001450, granted to E.N.M., from the Chan Zuckerberg Initiative and the Silicon Valley Community Foundation to M.G. (IS1R-0000000014), and ANPCyT PICT2019-2019-00436 to S.P.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Chen S., Liao Z., Xu P. Mitochondrial control of innate immune responses. Front. Immunol. 2023;14:1166214. doi: 10.3389/fimmu.2023.1166214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rai P., Janardhan K., Meacham J., Madenspacher J., Lin W., Karmaus P., Martinez J., Li Q., Yan M., Zeng J., et al. IRGM1 links mitochondrial quality control to autoimmunity. Nat. Immunol. 2021;22:312–321. doi: 10.1038/s41590-020-00859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banoth B., Cassel S. Mitochondria in innate immune signaling. Transl. Res. 2018;202:52–68. doi: 10.1016/j.trsl.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blevins H., Xu Y., Biby S., Zhang S. The NLRP3 Inflammasome Pathway: A Review of Mechanisms and Inhibitors for the Treatment of Inflammatory Diseases. Front. Aging Neurosci. 2022;14:879021. doi: 10.3389/fnagi.2022.879021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholls D.G., Ferguson S.J. Bioenergetics. Academic Press; Cambridge, MA, USA: 2013. 419p [Google Scholar]
- 6.Adam-Vizi V., Chinopoulos C. Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol. Sci. 2006;27:639–645. doi: 10.1016/j.tips.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Freinbichler W., Colivicchi M.A., Stefanini C., Bianchi L., Ballini C., Misini B., Weinberger P., Linert W., Varešlija D., Tipton K.F., et al. Highly reactive oxygen species: Detection, formation, and possible functions. Cell. Mol. Life Sci. 2011;68:2067–2079. doi: 10.1007/s00018-011-0682-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banerjee S., Ghosh S., Mandal A., Ghosh N., Sil P. ROS-associated immune response and metabolism: A mechanistic approach with implication of various diseases. Arch. Toxicol. 2020;94:2293–2317. doi: 10.1007/s00204-020-02801-7. [DOI] [PubMed] [Google Scholar]
- 9.Figueira T.R., Barros M., Camargo A., Castilho R.F., Ferreira J.C., Kowaltowski A.J., Sluse F., Souza-Pinto N.C., Vercesi A.E. Mitochondria as a source of reactive oxygen and nitrogen species: From molecular mechanisms to human health. Antioxid. Redox Signal. 2013;18:2029–2074. doi: 10.1089/ars.2012.4729. [DOI] [PubMed] [Google Scholar]
- 10.Stincone A., Prigione A., Cramer T., Wamelink M.M., Campbell K., Cheung E., Olin-Sandoval V., Grüning N.M., Krüger A., Tauqeer Alam M., et al. The return of metabolism: Biochemistry and physiology of the pentose phosphate pathway. Biol. Rev. Camb. Philos. Soc. 2015;90:927–963. doi: 10.1111/brv.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBerardinis R.J., Cheng T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li T., Copeland C., Le A. Glutamine Metabolism in Cancer. Adv. Exp. Med. Biol. 2021;1311:17–38. doi: 10.1007/978-3-030-65768-0_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Owen O.E., Kalhan S.C., Hanson R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002;277:30409–30412. doi: 10.1074/jbc.R200006200. [DOI] [PubMed] [Google Scholar]
- 14.Still E.R., Yuneva M.O. Hopefully devoted to Q: Targeting glutamine addiction in cancer. Br. J. Cancer. 2017;116:1375–1381. doi: 10.1038/bjc.2017.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meiser J., Tumanov S., Maddocks O., Labuschagne C.F., Athineos D., Van Den Broek N., Mackay G.M., Gottlieb E., Blyth K., Vousden K., et al. Serine one-carbon catabolism with formate overflow. Sci. Adv. 2016;2:e1601273. doi: 10.1126/sciadv.1601273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salim S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017;360:201–205. doi: 10.1124/jpet.116.237503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Colton C., Wilt S., Gilbert D., Chernyshev O., Snell J., Dubois-Dalcq M. Species differences in the generation of reactive oxygen species by microglia. Mol. Chem. Neuropathol. 1996;28:15–20. doi: 10.1007/BF02815200. [DOI] [PubMed] [Google Scholar]
- 18.Foo J., Bellot G., Pervaiz S., Alonso S. Mitochondria-mediated oxidative stress during viral infection. Trends Microbiol. 2022;30:679–692. doi: 10.1016/j.tim.2021.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Suwanprinya L., Morales N., Sanvarinda P., Dieng H., Okabayash T., Morales Vargas R. Dengue Virus-Induced Reactive Oxygen Species Production in Rat Microglial Cells. Jpn. J. Infect. Dis. 2017;70:383–387. doi: 10.7883/yoken.JJID.2016.236. [DOI] [PubMed] [Google Scholar]
- 20.Ferrari M., Zevini A., Palermo E., Muscolini M., Alexandridi M., Etna M., Coccia E.M., Fernandez-Sesma A., Coyne C., Zhang D.D., et al. Dengue Virus Targets Nrf2 for NS2B3-Mediated Degradation Leading to Enhanced Oxidative Stress and Viral Replication. J. Virol. 2020;94:e01551-20. doi: 10.1128/JVI.01551-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pillai A., Muthuraman K.R., Mariappan V., Belur S.S., Lokesh S., Rajendiran S. Oxidative stress response in the pathogenesis of dengue virus virulence, disease prognosis and therapeutics: An update. Arch. Virol. 2019;164:2895–2908. doi: 10.1007/s00705-019-04406-7. [DOI] [PubMed] [Google Scholar]
- 22.Soundravally R., Hoti S.L., Patil S.A., Cleetus C.C., Zachariah B., Kadhiravan T., Narayanan P., Kumar B.A. Association between proinflammatory cytokines and lipid peroxidation in patients with severe dengue disease around defervescence. Int. J. Infect. Dis. 2014;18:68–72. doi: 10.1016/j.ijid.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 23.Parikh S.M., Mammoto T., Schultz A., Yuan H.T., Christiani D., Karumanchi S.A., Sukhatme V.P. Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans. PLoS Med. 2006;3:e46. doi: 10.1371/journal.pmed.0030046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian Y., Jiang W., Gao N., Zhang J., Chen W., Fan D., Zhou D., An J. Inhibitory effects of glutathione on dengue virus production. Biochem. Biophys. Res. Commun. 2010;3:420–424. doi: 10.1016/j.bbrc.2010.05.108. [DOI] [PubMed] [Google Scholar]
- 25.Glushakova L.G., Judge S., Cruz A., Pourang D., Mathews C.E., Stacpoole P.W. Increased superoxide accumulation in pyruvate dehydrogenase complex deficient fibroblasts. Mol. Genet. Metab. 2011;104:255–260. doi: 10.1016/j.ymgme.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chakraborty S., Sen E., Basu A. Pyruvate dehydrogenase kinase 1 promotes neuronal apoptosis upon Japanese encephalitis virus infection. IBRO Neurosci. Rep. 2022;13:410–419. doi: 10.1016/j.ibneur.2022.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alandijany T., Kammouni W., Roy Chowdhury S., Fernyhough P., Jackson A.C. Mitochondrial dysfunction in rabies virus infection of neurons. J. Neurovirol. 2013;19:537–549. doi: 10.1007/s13365-013-0214-6. [DOI] [PubMed] [Google Scholar]
- 28.Kammouni W., Wood H., Jackson A. Lyssavirus phosphoproteins increase mitochondrial complex I activity and levels of reactive oxygen species. J. Neurovirol. 2017;23:756–762. doi: 10.1007/s13365-017-0550-z. [DOI] [PubMed] [Google Scholar]
- 29.Ojeda D., Grasso D., Urquiza J., Till A., Vaccaro M., Quarleri J. Cell Death Is Counteracted by Mitophagy in HIV-Productively Infected Astrocytes but Is Promoted by Inflammasome Activation Among Non-productively Infected Cells. Front. Immunol. 2018;9:2633. doi: 10.3389/fimmu.2018.02633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cevallos C., Ojeda D., Sánchez L., Urquiza J., Delpino M., Quarleri J. HIV-induced bystander cell death in astrocytes requires cell-to-cell viral transmission. J. Neurochem. 2022;163:338–356. doi: 10.1111/jnc.15703. [DOI] [PubMed] [Google Scholar]
- 31.Sander W.J., Fourie C., Sabiu S., O’Neill F., Pohl C.H., O’Neill H.G. Reactive oxygen species as potential antiviral targets. Rev. Med. Virol. 2022;32:e2240. doi: 10.1002/rmv.2240. [DOI] [PubMed] [Google Scholar]
- 32.Bottero V., Chakraborty S., Chandran B. Reactive oxygen species are induced by Kaposi’s sarcoma-associated herpesvirus early during primary infection of endothelial cells to promote virus entry. J. Virol. 2013;87:1733–1749. doi: 10.1128/JVI.02958-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.To E., Erlich J., Liong F., Luong R., Liong S., Esaq F., Oseghale O., Anthony D., McQualter J., Bozinovski S., et al. Mitochondrial Reactive Oxygen Species Contribute to Pathological Inflammation During Influenza A Virus Infection in Mice. Antioxid. Redox Signal. 2020;32:929–942. doi: 10.1089/ars.2019.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu M., Bogoyevitch M.A., Jans D.A. Subversion of Host Cell Mitochondria by RSV to Favor Virus Production is Dependent on Inhibition of Mitochondrial Complex I and ROS Generation. Cells. 2019;8:1417. doi: 10.3390/cells8111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domingo E., Menéndez-Arias L., Holland J.J. RNA virus fitness. Rev. Med. Virol. 1997;7:87–96. doi: 10.1002/(SICI)1099-1654(199707)7:2<87::AID-RMV188>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Beck M., Shi Q., Morris V.G., Levander O.A. Rapid genomic evolution of a non-virulent coxsackievirus B3 in seleniumdeficient mice results in selection of identical virulent isolates. Nat. Med. 1995;1:433–436. doi: 10.1038/nm0595-433. [DOI] [PubMed] [Google Scholar]
- 37.Beck M., Kolbeck P.C., Rohr L., Shi Q., Morris V., Levander O.A. Vitamin E deficiency intensifies the myocardial injury of coxsackievirus B3 infection of mice. J. Nutr. 1994;124:345–358. doi: 10.1093/jn/124.3.345. [DOI] [PubMed] [Google Scholar]
- 38.Michalek R., Pellom S., Holbrook B., Grayson J.M. The requirement of reactive oxygen intermediates for lymphocytic choriomeningitis virus binding and growth. Virology. 2008;379:205–212. doi: 10.1016/j.virol.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hosakote Y., Liu T., Castro S.M., Garofalo R., Casola A. Respiratory syncytial virus induces oxidative stress by modulating antioxidant enzymes. Am. J. Respir. Cell Mol. Biol. 2009;41:348–357. doi: 10.1165/rcmb.2008-0330OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kipper S., Hamad S., Caly L., Avrahami D., Bacharach E., Jans D.A., Gerber D., Bajorek M. New host factors important for respiratory syncytial virus (RSV) replication revealed by a novel microfluidics screen for interactors of matrix (M) protein. Mol. Cell Proteom. 2015;14:532–543. doi: 10.1074/mcp.M114.044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu M., Li H.M., Bogoyevitch M., Jans D.A. Mitochondrial protein p32/HAPB1/gC1qR/C1qbp is required for efficient respiratory syncytial virus production. Biochem. Biophys. Res. Commun. 2017;489:460–465. doi: 10.1016/j.bbrc.2017.05.171. [DOI] [PubMed] [Google Scholar]
- 42.Hussain M., Galvin H., Haw T., Nutsford A., Husain M. Drug resistance in influenza A virus: The epidemiology and management. Infect. Drug Resist. 2017;10:121–134. doi: 10.2147/IDR.S105473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farinati F., Cardin R., Bortolami M., Burra P., Russo F., Rugge M., Guido M., Sergio A., Naccarato R. Hepatitis C virus: From oxygen free radicals to hepatocellular carcinoma. J. Viral Hepat. 2007;14:821–829. doi: 10.1111/j.1365-2893.2007.00878.x. [DOI] [PubMed] [Google Scholar]
- 44.Wang T., Weinman S. Causes and consequences of mitochondrial reactive oxygen species generation in hepatitis C. J. Gastroenterol. Hepatol. 2006;3:S34–S37. doi: 10.1111/j.1440-1746.2006.04591.x. [DOI] [PubMed] [Google Scholar]
- 45.Pal S., Polyak S., Bano N., Qiu W., Carithers R., Shuhart M., Gretch D.R., Das A. Hepatitis C virus induces oxidative stress, DNA damage and modulates the DNA repair enzyme NEIL1. J. Gastroenterol. Hepatol. 2010;25:627–634. doi: 10.1111/j.1440-1746.2009.06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang S., Kim S., Kim J.H., Lee W., Kim G., Lee K.H., Choi K., Oh J. Interaction of hepatitis C virus core protein with Hsp60 triggers the production of reactive oxygen species and enhances TNF-alpha-mediated apoptosis. Cancer Lett. 2009;279:230–237. doi: 10.1016/j.canlet.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Reshi M.L., Su Y.C., Hong J.R. RNA Viruses: ROS-Mediated Cell Death. Int. J. Cell Biol. 2014;2014:467452. doi: 10.1155/2014/467452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Redza-Dutordoir M., Averill-Bates D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta. 2016;1863:2977–2992. doi: 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 49.Gao P., Tchernyshyov I., Chang T.C., Lee Y.S., Kita K., Ochi T., Zeller K.I., De Marzo A.M., Van Eyk J.E., Mendell J.T., et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kundura L., Gimenez S., Cezar R., André S., Younas M., Lin Y.L., Portalès P., Lozano C., Boulle C., Reynes J., et al. Angiotensin II induces reactive oxygen species, DNA damage, and T-cell apoptosis in severe COVID-19. J. Allergy Clin. Immunol. 2022;150:594–603.e2. doi: 10.1016/j.jaci.2022.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X., Xu Y., Zhang Q., Yang F., Yin Z., Wang L., Li Q. Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/p53, but not p38 MAPK and SAPK/JNK signalling pathways. Vet. Microbiol. 2019;232:1–12. doi: 10.1016/j.vetmic.2019.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Narayanan A., Amaya M., Voss K., Chung M., Benedict A., Sampey G., Kehn-Hall K., Luchini A., Liotta L., Bailey C., et al. Reactive oxygen species activate NFκB (p65) and p53 and induce apoptosis in RVFV infected liver cells. Virology. 2014;449:270–286. doi: 10.1016/j.virol.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 53.Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nat. Chem. Biol. 2014;10:9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 54.Burdon R.H. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radic. Biol. Med. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-S. [DOI] [PubMed] [Google Scholar]
- 55.Rius-Pérez S., Pérez S., Toledano M.B., Sastre J. Mitochondrial Reactive Oxygen Species and Lytic Programmed Cell Death in Acute Inflammation. Antioxid. Redox Signal. 2023;39:708–727. doi: 10.1089/ars.2022.0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y., Han S.J., Park I., Kim I., Chay K.O., Kim S.M., Jang D.I., Lee T., Lee S.R. Redox Regulation of the Tumor Suppressor PTEN by Hydrogen Peroxide and Tert-Butyl Hydroperoxide. Int. J. Mol. Sci. 2017;18:982. doi: 10.3390/ijms18050982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu S., Liu H., Johnston A., Hanna-Addams S., Reynoso E., Xiang Y., Wang Z. MLKL forms disulfide bond-dependent amyloid-like polymers to induce necroptosis. Proc. Natl. Acad. Sci. USA. 2017;114:E7450–E7459. doi: 10.1073/pnas.1707531114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bedient L., Pokharel S.M., Chiok K.R., Mohanty I., Beach S.S., Miura T.A., Bose S. Lytic Cell Death Mechanisms in Human Respiratory Syncytial Virus-Infected Macrophages: Roles of Pyroptosis and Necroptosis. Viruses. 2020;12:932. doi: 10.3390/v12090932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Son K.N., Lipton H.L. Inhibition of Theiler’s virus-induced apoptosis in infected murine macrophages results in necroptosis. Virus Res. 2015;195:177–182. doi: 10.1016/j.virusres.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ting P., Wu S.Q., He X., Luo H., Zhang Y., Fan M., Geng G., Ruiz V.C., Zjang J., Mills L., et al. Necroptosis takes place in human immunodeficiency virus type-1 (HIV-1)-infected CD4+ T lymphocytes. PLoS ONE. 2014;9:e93944. doi: 10.1371/journal.pone.0093944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cho Y., Chalia S., Moquin D., George R., Ray T.D., Guildford M., Chan F.K.M. Phosphorylation-driven assembly of the RIP1–RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Upton J.W., Kaiser W.J., Mocarski E.S. Virus inhibition of RIP3-dependent necrosis. Cell Host Microbe. 2010;7:302–313. doi: 10.1016/j.chom.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berger A.K., Danthi P. Reovirus activates a caspase-independent cell death pathway. mBio. 2013;4:e00178. doi: 10.1128/mBio.00178-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang S., Yu X., Meng X., Huo W., Su Y., Liu J., Liu Y., Zhang J., Wang S., Yu J. Coxsackievirus A6 Induces Necroptosis for Viral Production. Front. Microbiol. 2020;11:42. doi: 10.3389/fmicb.2020.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meissner F., Molawi K., Zychlinsky A. Superoxide dismutase 1 regulates caspase-1 and endotoxic shock. Nat. Immunol. 2008;9:866–872. doi: 10.1038/ni.1633. [DOI] [PubMed] [Google Scholar]
- 66.Lien T.S., Sun D.S., Wu C.Y., Chang H.H. Exposure to Dengue Envelope Protein Domain III Induces Nlrp3 Inflammasome-Dependent Endothelial Dysfunction and Hemorrhage in Mice. Front. Immunol. 2021;12:617251. doi: 10.3389/fimmu.2021.617251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hottz E.D., Lopes J.F., Freitas C., Valls-de-Souza R., Oliveira M.F., Bozza M.T., Bozza P.T. Platelets mediate increased endothelium permeability in dengue through NLRP3-inflammasome activation. Blood. 2013;122:3405–3414. doi: 10.1182/blood-2013-05-504449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu X.Q., Xu T., Ji W., Wang C., Ren Y., Xiong X., Zhou X., Lin S.H., Xu Y., Qiu Y. Herpes Simplex Virus 1-Induced Ferroptosis Contributes to Viral Encephalitis. mBio. 2023;14:e0237022. doi: 10.1128/mbio.02370-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen J., Fu J., Zhao S., Zhang X., Chao Y., Pan Q., Sun H., Zhang J., Li B., Xue T., et al. Free Radical and Viral Infection: A Review from the Perspective of Ferroptosis. Vet. Sci. 2023;10:456. doi: 10.3390/vetsci10070456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stockwell B.R., Friedmann Angeli J.P., Bayir H., Bush A.I., Conrad M., Dixon S.J., Fulda S., Gascon S., Hatzios S.K., Kagan V.E. Ferroptosis: A regulated cell death Nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kan X., Yin Y., Song C., Tan L., Qiu X., Liao Y., Liu W., Meng S., Sun Y., Ding C. Newcastle-disease-virus-induced ferroptosis through nutrient deprivation and ferritinophagy in tumor cells. iScience. 2021;24:102837. doi: 10.1016/j.isci.2021.102837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y., Huang J., Sun Y., Stubbs D., He J., Li W., Wang F., Liu Z., Ruzicka J.A., Taylor E.W., et al. SARS-CoV-2 suppresses mRNA expression of selenoproteins associated with ferroptosis, endoplasmic reticulum stress and DNA synthesis. Food Chem. Toxicol. 2021;153:12286. doi: 10.1016/j.fct.2021.112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Komissarov A.A., Karaseva M.A., Roschina M.P., Shubin A.V., Lunina N.A., Kostrov S.V., Demidyuk I.V. Individual Expression of Hepatitis A Virus 3C Protease Induces Ferroptosis in Human Cells In Vitro. Int. J. Mol. Sci. 2021;22:7906. doi: 10.3390/ijms22157906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burton E.M., Voyer J., Gewurz B.E. Epstein-Barr virus latency programs dynamically sensitize B cells to ferroptosis. Proc. Natl. Acad. Sci. USA. 2022;119:e2118300119. doi: 10.1073/pnas.2118300119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Banjac A., Perisic T., Sato H., Seiler A., Bannai S., Weiss N., Kölle P., Tschoep K., Issels R.D., Daniel P.T., et al. The cystine/cysteine cycle: A redox cycle regulating susceptibility versus resistance to cell death. Oncogene. 2008;27:1618–1628. doi: 10.1038/sj.onc.1210796. [DOI] [PubMed] [Google Scholar]
- 76.Cheng J., Tao J., Li B., Shi Y., Liu H. Swine influenza virus triggers ferroptosis in A549 cells to enhance virus replication. Virol. J. 2022;19:104. doi: 10.1186/s12985-022-01825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahmoudabadi G., Milo R., Phillips R. Energetic cost of building a virus. Proc. Natl. Acad. Sci. USA. 2017;114:E4324–E4333. doi: 10.1073/pnas.1701670114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sanchez E.L., Lagunoff M. Viral activation of cellular metabolism. Virology. 2015;480:609–618. doi: 10.1016/j.virol.2015.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu X., Zhu C., Wang Y., Wei F., Cai Q. KSHV Reprogramming of Host Energy Metabolism for Pathogenesis. Front. Cell. Infect. Microbiol. 2021;11:621156. doi: 10.3389/fcimb.2021.621156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allen C., Arjona S.P., Santerre M., Sawaya B.E. Hallmarks of Metabolic Reprogramming and Their Role in Viral Pathogenesis. Viruses. 2022;14:602. doi: 10.3390/v14030602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sanchez E., Pulliam T.H., Dimaio T., Thalhofer A., Clark T., Lagunoff M. Glycolysis, Glutaminolysis, and Fatty Acid Synthesis Are Required for Distinct Stages of Kaposi’s Sarcoma-Associated Herpesvirus Lytic Replication. J. Virol. 2017;91:e02237-16. doi: 10.1128/JVI.02237-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang X., Buechler N.L., Woodruff A.G., Long D.L., Zabalawi M., Yoza B.K., McCall C.E., Vachharajani V. Sirtuins and Immuno-Metabolism of Sepsis. Int. J. Mol. Sci. 2018;19:2738. doi: 10.3390/ijms19092738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nakashima R., Paggi M., Pedersen P.L. Contributions of glycolysis and oxidative phosphorylation to adenosine 5′-triphosphate production in AS-30D hepatoma cells. Cancer Res. 1984;44:5702–5706. [PubMed] [Google Scholar]
- 84.Griguer C.E., Oliva C.R., Gillespie G.Y. Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J. Neuro-Oncol. 2005;74:123–133. doi: 10.1007/s11060-004-6404-6. [DOI] [PubMed] [Google Scholar]
- 85.Brand M.D. The efficiency and plasticity of mitochondrial energy transduction. Biochem. Soc. Trans. 2005;33:897–904. doi: 10.1042/BST0330897. [DOI] [PubMed] [Google Scholar]
- 86.Kilburn D.G., Lilly M.D., Webb F.C. The energetics of mammalian cell growth. J. Cell Sci. 1969;4:645–654. doi: 10.1242/jcs.4.3.645. [DOI] [PubMed] [Google Scholar]
- 87.Schwenke W., Soboll S., Seitz H.J., Sies H. Mitochondrial and cytosolic ATP/ADP ratios in rat liver in vivo. Biochem. J. 1981;200:405–408. doi: 10.1042/bj2000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Keenan M.M., Chi J.T. Alternative fuels for cancer cells. Cancer J. 2015;21:49–55. doi: 10.1097/PPO.0000000000000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Seyfried T.N., Arismendi-Morillo G., Mukherjee P., Chinopoulos C. On the Origin of ATP Synthesis in Cancer. iScience. 2020;23:101761. doi: 10.1016/j.isci.2020.101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fernandez-de-Cossio-Diaz J., Vazquez A. Limits of aerobic metabolism in cancer cells. Sci. Rep. 2017;7:13488. doi: 10.1038/s41598-017-14071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vaupel P., Schmidberger H., Mayer A. The Warburg effect: Essential part of metabolic reprogramming and central contributor to cancer progression. Int. J. Radiat. Biol. 2019;95:912–919. doi: 10.1080/09553002.2019.1589653. [DOI] [PubMed] [Google Scholar]
- 93.Vander Heiden M., Cantley L., Thompson C. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Clark S., Vazquez A., Furiya K., Splattstoesser M.K., Bashmail A., Schwartz H., Russell M., Bhark S., Moreno O., McGovern M., et al. Rewiring of the Host Cell Metabolome and Lipidome during Lytic Gammaherpesvirus Infection Is Essential for Infectious-Virus Production. J. Virol. 2023;97:e0050623. doi: 10.1128/jvi.00506-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Delisle L., Fuhrmann M., Quéré C., Pauletto M., Pichereau V., Pernet F., Corporeau C. The Voltage-Dependent Anion Channel (VDAC) of Pacific Oysters Crassostrea gigas Is Upaccumulated During Infection by the Ostreid Herpesvirus-1 (OsHV-1): An Indicator of the Warburg Effect. Mar. Biotechnol. 2018;20:87–97. doi: 10.1007/s10126-017-9789-x. [DOI] [PubMed] [Google Scholar]
- 96.Landini M.P. Early enhanced glucose uptake in human cytomegalovirus-infected cells. J. Gen. Virol. 1984;65:1229–1232. doi: 10.1099/0022-1317-65-7-1229. [DOI] [PubMed] [Google Scholar]
- 97.Yu Y., Clippinger A.J., Alwine J.C. Viral effects on metabolism: Changes in glucose and glutamine utilization during human cytomegalovirus infection. Trends Microbiol. 2011;19:360–367. doi: 10.1016/j.tim.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Munger J., Bennett B.D., Parikh A., Feng X., McArdle J., Rabitz H.A., Shenk T., Rabinowitz J.D. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008;26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Terry L.J., Vastag L., Rabinowitz J.D., Shenk T. Human kinome profiling identifies a requirement for AMP-activated protein kinase during human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA. 2012;109:3071–3076. doi: 10.1073/pnas.1200494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McArdle J., Moorman N.J., Munger J. HCMV targets the metabolic stress response through activation of AMPK whose activity is important for viral replication. PLoS Pathog. 2012;8:e1002502. doi: 10.1371/journal.ppat.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alwine J.C. The human cytomegalovirus assembly compartment: A masterpiece of viral manipulation of cellular processes that facilitates assembly and egress. PLoS Pathog. 2012;8:e1002878. doi: 10.1371/journal.ppat.1002878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Codo A.C., Davanzo G.G., Monteiro L.B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., Prodonoff J.S., Carregari V.C., de Biagi Junior C., Crunfli F., et al. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent Axis. Cell Metab. 2020;32:437–446.e5. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Heawchaiyaphum C., Yoshiyama H., Iizasa H., Burassakarn A., Tumurgan Z., Ekalaksananan T., Pientong C. Epstein-Barr Virus Promotes Oral Squamous Cell Carcinoma Stemness through the Warburg Effect. Int. J. Mol. Sci. 2023;24:14072. doi: 10.3390/ijms241814072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sung W., Chen P., Liao M., Lee J. Enhanced aerobic glycolysis of nasopharyngeal carcinoma cells by Epstein-Barr virus latent membrane protein 1. Exp. Cell Res. 2017;359:94–100. doi: 10.1016/j.yexcr.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 105.Hulse M., Caruso L., Madzo J., Tan Y., Johnson S., Tempera I. Poly(ADP-ribose) polymerase 1 is necessary for coactivating hypoxia-inducible factor-1-dependent gene expression by Epstein-Barr virus latent membrane protein 1. PLoS Pathog. 2018;14:e1007394. doi: 10.1371/journal.ppat.1007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gerresheim G., Roeb E., Michel A., Niepmann M. Hepatitis C Virus Downregulates Core Subunits of Oxidative Phosphorylation, Reminiscent of the Warburg Effect in Cancer Cells. Cells. 2019;8:1410. doi: 10.3390/cells8111410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zamarin D., Palese P. Oncolytic Newcastle disease virus for cancer therapy: Old challenges and new directions. Future Microbiol. 2012;7:347–367. doi: 10.2217/fmb.12.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gong Y., Tang N., Liu P., Sun Y., Lu S., Liu W., Tan L., Song C., Qiu X., Liao Y., et al. Newcastle disease virus degrades SIRT3 via PINK1-PRKN-dependent mitophagy to reprogram energy metabolism in infected cells. Autophagy. 2022;18:1503–1521. doi: 10.1080/15548627.2021.1990515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hirschey M., Shimazu T., Huang J., Schwer B., Verdin E. SIRT3 regulates mitochondrial protein acetylation and intermediary metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011;76:267–277. doi: 10.1101/sqb.2011.76.010850. [DOI] [PubMed] [Google Scholar]
- 110.Ng Y.S., Lee D.Y., Liu C.H., Tung C.Y., He S.T., Wang H.C. White Spot Syndrome Virus Triggers a Glycolytic Pathway in Shrimp Immune Cells (Hemocytes) to Benefit Its Replication. Front. Immunol. 2022;13:901111. doi: 10.3389/fimmu.2022.901111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang P., Fu H.J., Lv L.X., Liu C.F., Han C., Zhao X.F., Wang J.X. WSSV exploits AMPK to activate mTORC2 signaling for proliferation by enhancing aerobic glycolysis. Commun. Biol. 2023;6:361. doi: 10.1038/s42003-023-04735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Godoy-Lugo J.A., Miranda-Cruz M.M., Rosas-Rodríguez J.A., Adan-Bante N.P., Icedo-García R., Soñanez-Organis J.G. Hypoxia inducible factor -1 regulates WSSV-induced glycolytic genes in the white shrimp Litopenaeus vannamei. Fish Shellfish Immunol. 2019;92:165–171. doi: 10.1016/j.fsi.2019.05.040. [DOI] [PubMed] [Google Scholar]
- 113.Chen I.T., Lee D.Y., Huang Y.T., Kou G.H., Wang H.C., Chang G.D., Lo C.F. Six Hours after Infection, the Metabolic Changes Induced by WSSV Neutralize the Host’s Oxidative Stress Defenses. Sci. Rep. 2016;9:27732. doi: 10.1038/srep27732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liang L., Li W., Li X., Jin X., Liao Q., Li Y., Zhou Y. ‘Reverse Warburg effect’ of cancer-associated fibroblasts (Review) Int. J. Oncol. 2022;60:67. doi: 10.3892/ijo.2022.5357. [DOI] [PubMed] [Google Scholar]
- 115.Manners O., Murphy J., Coleman A., Hughes D., Whitehouse A. Contribution of the KSHV and EBV lytic cycles to tumourigenesis. Curr. Opin. Virol. 2018;32:60–70. doi: 10.1016/j.coviro.2018.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Broussard G., Damania B. Regulation of KSHV Latency and Lytic Reactivation. Viruses. 2020;12:1034. doi: 10.3390/v12091034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kuppers R. B cells under influence: Transformation of B cells by Epstein-Barr virus. Nat. Rev. Immunol. 2003;3:801–812. doi: 10.1038/nri1201. [DOI] [PubMed] [Google Scholar]
- 118.Gulley M.L., Tang W. Laboratory assays for Epstein-Barr virus-related disease. J. Mol. Diagn. 2008;10:279–292. doi: 10.2353/jmoldx.2008.080023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsuchiya S. Diagnosis of Epstein-Barr virus-associated diseases. Crit. Rev. Oncol. Hematol. 2002;44:227–238. doi: 10.1016/S1040-8428(02)00114-2. [DOI] [PubMed] [Google Scholar]
- 120.Yogev O., Henderson S., Hayes M.J., Marelli S.S., Ofir-Birin Y., Regev-Rudzki N., Herrero J., Enver T. Herpesviruses shape tumour microenvironment through exosomal transfer of viral microRNAs. PLoS Pathog. 2017;13:e1006524. doi: 10.1371/journal.ppat.1006524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Miska E.A. How microRNAs control cell division, differentiation and death. Curr. Opin. Genet. Dev. 2005;15:563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 122.Li W., Yan Q., Ding X., Shen C., Hu M., Zhu Y., Qin D., Lu H., Krueger B.J., Renne R., et al. The SH3BGR/STAT3 Pathway Regulates Cell Migration and Angiogenesis Induced by a Gammaherpesvirus MicroRNA. PLoS Pathog. 2016;12:e1005605. doi: 10.1371/journal.ppat.1005605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu P., Tang N., Meng C., Yin Y., Qiu X., Tan L., Sun Y., Song C., Liu W., Liao Y., et al. SLC1A3 facilitates Newcastle disease virus replication by regulating glutamine catabolism. Virulence. 2022;13:1407–1422. doi: 10.1080/21505594.2022.2112821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Martinez-Outschoorn U., Lin Z., Trimmer C., Flomenberg N., Wang C., Pavlides S., Pestell R.G., Howell A., Sotgia F., Lisanti M. Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: Implications for PET imaging of human tumors. Cell Cycle. 2011;10:2504–2520. doi: 10.4161/cc.10.15.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lyu X., Wang J., Guo X., Wu G., Jiao Y., Faleti O.D., Liu P., Liu T., Long Y., Chong T., et al. EBV-miR-BART1-5P activates AMPK/mTOR/HIF1 pathway via a PTEN independent manner to promote glycolysis and angiogenesis in nasopharyngeal carcinoma. PLoS Pathog. 2018;14:e1007484. doi: 10.1371/journal.ppat.1007484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ducharme N.A., Bickel P.E. Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 127.Voet D., Voet J.G. Biochemistry. 3rd ed. John Wiley & Sons; Danvers, MA, USA: 2004. [Google Scholar]
- 128.Liu S., Alexander R.K., Lee C.H. Lipid metabolites as metabolic messengers in inter-organ communication. Trends Endocrinol. Metab. 2014;25:356–363. doi: 10.1016/j.tem.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kotlyarov S., Kotlyarova A. Involvement of Fatty Acids and Their Metabolites in the Development of Inflammation in Atherosclerosis. Int. J. Mol. Sci. 2022;23:1308. doi: 10.3390/ijms23031308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cloherty A., Olmstead A., Ribeiro C., Jean F. Hijacking of Lipid Droplets by Hepatitis C, Dengue and Zika Viruses-From Viral Protein Moonlighting to Extracellular Release. Int. J. Mol. Sci. 2020;21:7901. doi: 10.3390/ijms21217901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Negash A., Ramos H.J., Crochet N., Lau D., Doehle B., Papic N., Delker D.A., Jo J., Bertoletti A., Hagedorn C.H., et al. IL-1β production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog. 2013;9:e1003330. doi: 10.1371/journal.ppat.1003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li Y., Webster-Cyriaque J., Tomlinson C.C., Yohe M., Kenney S. Fatty Acid Synthase Expression is Induced by the Epstein-Barr Virus Immediate-Early Protein BRLF1 and is Required for Lytic Viral Gene Expression. J. Virol. 2004;78:4197–4206. doi: 10.1128/JVI.78.8.4197-4206.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Boodhoo N., Kamble N., Sharif S., Behboudi S. Glutaminolysis and Glycolysis Are Essential for Optimal Replication of Marek’s Disease Virus. J. Virol. 2020;94:e01680-19. doi: 10.1128/JVI.01680-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ng Y.S., Cheng C.S., Ando M., Tseng Y.T., He S.T., Li C.Y., Cheng S.W., Chen Y.M., Kumar R., Liu C., et al. White spot syndrome virus (WSSV) modulates lipid metabolism in white shrimp. Commun. Biol. 2023;6:546. doi: 10.1038/s42003-023-04924-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yue M., Hu B., Li J., Chen R., Yuan Z., Xiao H., Chang H., Jiu Y., Cai K., Ding B. Coronaviral ORF6 protein mediates inter-organelle contacts and modulates host cell lipid flux for virus production. EMBO J. 2023;42:e112542. doi: 10.15252/embj.2022112542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mozzi A., Oldani M., Forcella M.E., Vantaggiato C., Cappelletti G., Pontremoli C., Valenti F., Forni D., Saresella M., Biasin M., et al. SARS-CoV-2 ORF3c impairs mitochondrial respiratory metabolism, oxidative stress, and autophagic flux. iScience. 2023;26:107118. doi: 10.1016/j.isci.2023.107118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ramachandran K., Maity S., Muthukumar A., Kandala S., Tomar D., Abd El-Aziz T., Allen C., Sun Y., Venkatesan M., Madaris T., et al. SARS-CoV-2 infection enhances mitochondrial PTP complex activity to perturb cardiac energetics. iScience. 2022;25:103722. doi: 10.1016/j.isci.2021.103722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu J., Shi Y., Pan X., Wu S., Hou R., Zhang Y., Zhong T., Tang H., Du W., Wang L., et al. SARS-CoV-2 ORF9b inhibits RIG-I-MAVS antiviral signaling by interrupting K63-linked ubiquitination of NEMO. Cell Rep. 2021;34:108761. doi: 10.1016/j.celrep.2021.108761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stewart H., Lu Y., O’Keefe S., Valpadashi A., Cruz-Zaragoza L.D., Michel H., Nguyen S.K., Carnell G., Lukhovitskaya N., Milligan R., et al. The SARS-CoV-2 protein ORF3c is a mitochondrial modulator of innate immunity. iScience. 2023;26:108080. doi: 10.1016/j.isci.2023.108080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jia H., Liu C., Li D., Huang Q., Liu D., Zhang Y., Ye C., Zhou D., Wang Y., Tan Y., et al. Metabolomic analyses reveal new stage-specific features of COVID-19. Eur. Respir. J. 2022;59:2100284. doi: 10.1183/13993003.00284-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Jiang H., Zhang H., Meng Q., Xie J., Li Y., Chen H., Zheng Y., Wang X., Qi H., Zhang J., et al. SARS-CoV-2 Orf9b suppresses type I interferon responses by targeting TOM70. Cell. Mol. Immunol. 2020;17:998–1000. doi: 10.1038/s41423-020-0514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dang C., Le A., Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin. Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gao X., Zhu K., Qin B., Olieric V., Wang M., Cui S. Crystal structure of SARS-CoV-2 Orf9b in complex with human TOM70 suggests unusual virus-host interactions. Nat. Commun. 2021;12:2843. doi: 10.1038/s41467-021-23118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brandherm L., Kobaš A., Klöhn M., Brüggemann Y., Pfaender S., Rassow J., Kreimendahl S. Phosphorylation of SARS-CoV-2 Orf9b Regulates Its Targeting to Two Binding Sites in TOM70 and Recruitment of Hsp90. Int. J. Mol. Sci. 2021;22:9233. doi: 10.3390/ijms22179233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li X., Hou P., Ma W., Wang X., Wang H., Yu Z., Chang H., Wang T., Jin S., Wang X., et al. SARS-CoV-2 ORF10 suppresses the antiviral innate immune response by degrading MAVS through mitophagy. Cell Mol. Immunol. 2023;20:686. doi: 10.1038/s41423-023-01023-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Herker E. Lipid Droplets in Virus Replication. FEBS Lett. 2024;598:1299–1300. doi: 10.1002/1873-3468.14819. [DOI] [PubMed] [Google Scholar]
- 147.Awadh A.A. The Role of Cytosolic Lipid Droplets in Hepatitis C Virus Replication, Assembly, and Release. Biomed. Res. Int. 2023;2023:5156601. doi: 10.1155/2023/5156601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Shulla A., Randall G. (+) RNA virus replication compartments: A safe home for (most) viral replication. Curr. Opin. Microbiol. 2016;32:82–88. doi: 10.1016/j.mib.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cho H., Kim S.Y., Yoo S.K., Choi Y.H., Cheong J. Fatty acids increase hepatitis B virus X protein stabilization and HBx-induced inflammatory gene expression. FEBS J. 2014;281:2228–2239. doi: 10.1111/febs.12776. [DOI] [PubMed] [Google Scholar]
- 150.Wang L., Wang Z., Ersing I., Nobre L., Guo R., Jiang S., Trudeau S., Zhao B., Weekes M.P., Gewurz B.E. Epstein-Barr virus subverts mevalonate and fatty acid pathways to promote infected B-cell proliferation and survival. PLoS Pathog. 2019;15:e1008030. doi: 10.1371/journal.ppat.1008030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yamane D., McGivern D., Wauthier E., Yi M., Madden V.J., Welsch C., Antes I., Wen Y., Chugh P.E., McGee C., et al. Regulation of the hepatitis C virus RNA replicase by endogenous lipid peroxidation. Nat. Med. 2014;20:927–935. doi: 10.1038/nm.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Baugh M.A. HIV: Reactive oxygen species, enveloped viruses and hyperbaric oxygen. Med. Hypotheses. 2000;55:232–238. doi: 10.1054/mehy.2000.1048. [DOI] [PubMed] [Google Scholar]
- 153.Jackson A., Kammouni W., Zherebitskaya E., Fernyhough P. Role of oxidative stress in rabies virus infection of adult mouse dorsal root ganglion neurons. J. Virol. 2010;84:4697–4705. doi: 10.1128/JVI.02654-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fernandes-Siqueira L.O., Zeidler J.D., Sousa B.G., Ferreira T., Da Poian A.T. Anaplerotic Role of Glucose in the Oxidation of Endogenous Fatty Acids during Dengue Virus Infection. mSphere. 2018;313:e00458-17. doi: 10.1128/mSphere.00458-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Heaton N.S., Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe. 2010;8:422–432. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Mayers J.R., Vander Heiden M.G. Famine versus feast: Understanding the metabolism of tumors in vivo. Trends Biochem. Sci. 2015;40:130–140. doi: 10.1016/j.tibs.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yang L., Venneti S., Nagrath D. Glutaminolysis: A Hallmark of Cancer Metabolism. Annu. Rev. Biomed. Eng. 2017;19:163–194. doi: 10.1146/annurev-bioeng-071516-044546. [DOI] [PubMed] [Google Scholar]
- 158.Walker M.C., van der Donk W.A. The many roles of glutamate in metabolism. J. Ind. Microbiol. Biotechnol. 2016;43:419–430. doi: 10.1007/s10295-015-1665-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Thai M., Thaker S., Feng J., Du Y., Hu H., Ting T., Wu T., Graeber G., Braas H., Christofk R. MYC-induced reprogramming of glutamine catabolism supports optimal virus replication. Nat. Commun. 2015;6:8873. doi: 10.1038/ncomms9873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Krishna G., Soman Pillai V., Valiya Veettil M. Upregulation of GLS1 Isoforms KGA and GAC Facilitates Mitochondrial Metabolism and Cell Proliferation in Epstein-Barr Virus Infected Cells. Viruses. 2020;12:811. doi: 10.3390/v12080811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Smallwood H.S., Duan S., Morfouace M., Rezinciuc S., Shulkin B.L., Shelat A., Zink E.E., Milasta S., Bajracharya R., Oluwaseum A.J., et al. Targeting Metabolic Reprogramming by Influenza Infection for Therapeutic Intervention. Cell Rep. 2017;19:1640–1653. doi: 10.1016/j.celrep.2017.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Calder P.C., Yaqoob P. Glutamine and the immune system. Amino Acids. 1999;17:227–241. doi: 10.1007/BF01366922. [DOI] [PubMed] [Google Scholar]
- 163.Hafner A., Meurs N., Garner A., Azar E., Passalacqua K.D., Nagrath D., Wobus C.E. Norovirus NS1/2 protein increases glutaminolysis for efficient viral replication. PLoS Pathog. 2023;19:2023. doi: 10.1371/journal.ppat.1011909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yu L., Zeng Z., Tan H., Feng Q., Zhou Q., Hu J., Li Y., Wang J., Yang W., Feng J., et al. Significant metabolic alterations in patients with hepatitis B virus replication observed via serum untargeted metabolomics shed new light on hepatitis B virus infection. J. Drug Target. 2022;30:442–449. doi: 10.1080/1061186X.2021.2009841. [DOI] [PubMed] [Google Scholar]
- 165.Li H., Zhu W., Zhang L., Lei H., Wu X., Guo L., Chen X., Wang Y., Tang H. The metabolic responses to hepatitis B virus infection shed new light on pathogenesis and targets for treatment. Sci. Rep. 2015;5:8421. doi: 10.1038/srep08421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Clerc I., Moussa D.A., Vahlas Z., Tardito S., Oburoglu L., Hope T.J., Sitbon M., Dardalhon V., Mongellaz C., Taylor N. Entry of glucose- and glutamine-derived carbons into the citric acid cycle supports early steps of HIV-1 infection in CD4 T cells. Nat. Metab. 2019;1:717–730. doi: 10.1038/s42255-019-0084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hegedus A., Kavanagh Williamson M., Khan M.B., Dias Zeidler J., Da Poian A.T., El-Bacha T., Struys E.A., Huthoff H. Evidence for Altered Glutamine Metabolism in Human Immunodeficiency Virus Type 1 Infected Primary Human CD4+ T Cells. AIDS Res. Hum. Retroviruses. 2017;33:1236–1247. doi: 10.1089/aid.2017.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Nakaya M., Xiao Y., Zhou X., Chang J.H., Chang M., Cheng X., Blonska M., Lin X., Sun S.C. Inflammatory T cell responses rely on amino acid transporter ASCT2 facilitation of glutamine uptake and mTORC1 kinase activation. Immunity. 2014;40:692–705. doi: 10.1016/j.immuni.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fontaine K.A., Camarda R., Lagunoff M. Vaccinia virus requires glutamine but not glucose for efficient replication. J. Virol. 2014;88:4366–4374. doi: 10.1128/JVI.03134-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tao L., Lemoff A., Wang G., Zarek C., Lowe A., Yan N., Reese T. Reactive oxygen species oxidize STING and suppress interferon production. eLife. 2020;9:e57837. doi: 10.7554/eLife.57837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gonzalez-Dosal R., Horan K.A., Paludan S.R. Mitochondria-derived reactive oxygen species negatively regulates immune innate signaling pathways triggered by a DNA virus, but not by an RNA virus. Biochem. Biophys. Res. Commun. 2012;418:806–810. doi: 10.1016/j.bbrc.2012.01.108. [DOI] [PubMed] [Google Scholar]
- 172.Soucy-Faulkner A., Mukawera E., Fink K., Martel A., Jouan L., Nzengue Y., Lamarre D., Vande Velde C., Grandvaux N. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog. 2010;6:e1000930. doi: 10.1371/journal.ppat.1000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang T., Weinman S.A. Interactions Between Hepatitis C Virus and Mitochondria: Impact on Pathogenesis and Innate Immunity. Curr. Pathobiol. Rep. 2013;1:179–187. doi: 10.1007/s40139-013-0024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang R., Zhu Y., Ren C., Yang S., Tian S., Chen H., Jin M., Zhou H. Influenza A virus protein PB1-F2 impairs innate immunity by inducing mitophagy. Autophagy. 2021;17:496–511. doi: 10.1080/15548627.2020.1725375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Rosa C.P., Belo T., Santos N., Silva E.N., Gasparotto J., Corsetti P.P., de Almeida L.A. Reactive oxygen species trigger inflammasome activation after intracellular microbial interaction. Life Sci. 2023;15:122076. doi: 10.1016/j.lfs.2023.122076. [DOI] [PubMed] [Google Scholar]
- 176.Iyer S., Pulskens W., Sadler J., Butter L., Teske G., Ulland T., Eisenbarth S., Florquin S., Flavell R., Leemans J., et al. Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cortopassi G., Wang E. Modelling the effects of age-related mtDNA mutation accumulation; complex I deficiency, superoxide and cell death. Biochim. Biophys. Acta. 1995;1271:171–176. doi: 10.1016/0925-4439(95)00025-Y. [DOI] [PubMed] [Google Scholar]
- 178.Liu H., Zhu Z., Xue Q., Yang F., Li Z., Xue Z., Cao W., He J., Guo J., Liu X., et al. Innate sensing of picornavirus infection involves cGAS-STING-mediated antiviral responses triggered by mitochondrial DNA release. PLoS Pathog. 2023;19:e1011132. doi: 10.1371/journal.ppat.1011132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Colombini M. Structure and mode of action of a voltage dependent anion-selective channel (VDAC) located in the outer mitochondrial membrane. Ann. N. Y. Acad. Sci. 1980;341:52–63. doi: 10.1111/j.1749-6632.1980.tb47198.x. [DOI] [PubMed] [Google Scholar]
- 180.Colombini M. VDAC structure, selectivity, and dynamics. Biochim. Biophys. Acta. 2012;1818:1457–1465. doi: 10.1016/j.bbamem.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.De Pinto V., Guarino F., Guarnera A., Messina A., Reina S., Tomasello F.M., Palermo V., Mazzoni C. Characterization of human VDAC isoforms: A peculiar function for VDAC3? Biochim. Biophys. Acta. 2012;1797:1268–1275. doi: 10.1016/j.bbabio.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 182.Sampson M.J., Lovell R., Craigen W.J. The murine voltage-dependent anion channel gene family. Conserved structure and function. J. Biol. Chem. 1997;272:18966–18973. doi: 10.1074/jbc.272.30.18966. [DOI] [PubMed] [Google Scholar]
- 183.Fang D., Maldonado E.N. VDAC Regulation: A Mitochondrial Target to Stop Cell Proliferation. Adv. Cancer Res. 2018;138:41–69. doi: 10.1016/bs.acr.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Heslop K.A., Milesi V., Maldonado E.N. VDAC Modulation of Cancer Metabolism: Advances and Therapeutic Challenges. Front. Physiol. 2021;29:742839. doi: 10.3389/fphys.2021.742839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Rosencrans W.M., Rajendran M., Bezrukov S.M., Rostovtseva T.K. VDAC regulation of mitochondrial calcium flux: From channel biophysics to disease. Cell Calcium. 2021;94:102356. doi: 10.1016/j.ceca.2021.102356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Maldonado E.N. VDAC-Tubulin, an Anti-Warburg Pro-Oxidant Switch. Front. Oncol. 2017;7:4. doi: 10.3389/fonc.2017.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Jitobaom K., Tongluan N., Smith D.R. Involvement of voltage-dependent anion channel (VDAC) in dengue infection. Sci. Rep. 2016;6:35753. doi: 10.1038/srep35753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Han C., Zeng X., Yao S., Gao L., Zhang L., Qi X., Duan Y., Yang B., Gao Y., Liu C., et al. Voltage-Dependent Anion Channel 1 Interacts with Ribonucleoprotein Complexes To Enhance Infectious Bursal Disease Virus Polymerase Activity. J. Virol. 2017;91:e00584-17. doi: 10.1128/JVI.00584-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hoehl S., Berger A., Ciesek S., Rabenau H. Thirty years of CMV seroprevalence-a longitudinal analysis in a German university hospital. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1095–1102. doi: 10.1007/s10096-020-03814-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Lee K.H., Kwon D.E., Do H., La Y., Han S.H. Association between cytomegalovirus end-organ diseases and moderate-to-severe dementia: A population-based cohort study. BMC Neurol. 2020;20:216. doi: 10.1186/s12883-020-01776-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Harrison M., Morris S., Rudman G., Rittenhouse D.J., Monk C., Sakamuri S., Mehedi Hasan M., Shamima Khatun M., Wang H., Garfinkel L.P., et al. Intermittent cytomegalovirus infection alters neurobiological metabolism and induces cognitive deficits in mice. Brain. Behav. Immun. 2024;117:36–50. doi: 10.1016/j.bbi.2023.12.033. [DOI] [PubMed] [Google Scholar]
- 192.Cheng M.L., Wu C., Chien K., Lai C., Li G., Liu Y., Lin G., Ho H. Enteroviral 2B Interacts with VDAC3 to Regulate Reactive Oxygen Species Generation That Is Essential to Viral Replication. Viruses. 2022;14:1717. doi: 10.3390/v14081717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Marchi S., Patergnani S., Missiroli S., Morciano G., Rimessi A., Wieckowski M.R., Giorgi C., Pinton P. Mitochondrial and endoplasmic reticulum calcium homeostasis and cell death. Cell Calcium. 2018;69:62–72. doi: 10.1016/j.ceca.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 194.Brookes P.S., Yoon Y., Robotham L., Anders M., Sheu S.-S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol.-Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 195.Arruda A.P., Pers B.M., Parlakgul G., Guney E., Inouye K., Hotamisligil G.S. Chronic enrichment of hepatic endoplasmic reticulum–mitochondria contact leads to mitochondrial dysfunction in obesity. Nat. Med. 2014;20:1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Korenaga M., Wang T., Li Y., Showalter L.A., Chan T., Sun J., Weinman S.A. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J. Biol. Chem. 2005;280:37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 197.Wang T., Campbell R.V., Yi M.K., Lemon S.M., Weinman S.A. Role of Hepatitis C virus core protein in viral-induced mitochondrial dysfunction. J. Viral Hepat. 2010;17:784–793. doi: 10.1111/j.1365-2893.2009.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rahmani Z., Huh K.W., Lasher R., Siddiqui A. Hepatitis B virus X protein colocalizes to mitochondria with a human voltage-dependent anion channel, HVDAC3, and alters its transmembrane potential. J. Virol. 2000;74:2840–2846. doi: 10.1128/JVI.74.6.2840-2846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Xia W., Shen Y., Xie H., Zheng S. Involvement of endoplasmic reticulum in hepatitis B virus replication. Virus Res. 2006;121:116–121. doi: 10.1016/j.virusres.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 200.Kong F., Zhang F., Liu X., Qin S., Yang X., Kong D., Pan X., You H., Zheng K., Tang R. Calcium signaling in hepatitis B virus infection and its potential as a therapeutic target. Cell Commun. Signal. 2021;19:82. doi: 10.1186/s12964-021-00762-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang S., Zeng L., Su B.Q., Yang G., Wang J., Ming S.L., Chu B.B. The glycoprotein 5 of porcine reproductive and respiratory syndrome virus stimulates mitochondrial ROS to facilitate viral replication. mBio. 2023;14:e0265123. doi: 10.1128/mbio.02651-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Derakhshan M., Willcocks M.M., Salako M., Kass G., Carter M.J. Human herpesvirus 1 protein US3 induces an inhibition of mitochondrial electron transport. J. Gen. Virol. 2006;87:2155–2159. doi: 10.1099/vir.0.81949-0. [DOI] [PubMed] [Google Scholar]
- 203.Van de Bovenkamp M., Nottet C., Pereira F. Interactions of human immunodeficiency virus-1 proteins with neurons: Possible role in the development of human immunodeficiency virus-1-associated dementia. Eur. J. Clin. Investig. 2002;32:619–627. doi: 10.1046/j.1365-2362.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- 204.Scheffler I.E. Mitochondria make a comeback. Adv. Drug Deliv. Rev. 2001;49:3–26. doi: 10.1016/S0169-409X(01)00123-5. [DOI] [PubMed] [Google Scholar]
- 205.Norman J.P., Perry S., Kasischke K., Volsky D., Gelbard H.A. HIV-1 trans activator of transcription protein elicits mitochondrial hyperpolarization and respiratory deficit, with dysregulation of complex IV and nicotinamide adenine dinucleotide homeostasis in cortical neurons. J. Immunol. 2007;178:869–876. doi: 10.4049/jimmunol.178.2.869. [DOI] [PubMed] [Google Scholar]
- 206.Murata T., Goshima F., Daikoku T., Inagaki-Ohara K., Takakuwa H., Kato K., Nishiyama Y. Mitochondrial distribution and function in herpes simplex virus-infected cells. J. Gen. Virol. 2000;81:401–406. doi: 10.1099/0022-1317-81-2-401. [DOI] [PubMed] [Google Scholar]
- 207.Bertzbach L., Kohn M., You Y., Kossak L., Sabsabi M.A., Kheimar A., Härtle S., Kaufer B.B. In vitro infection of primary chicken lymphocytes with Marek’s disease virus. STAR Protoc. 2023;4:102343. doi: 10.1016/j.xpro.2023.102343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Piepenbrink M., Li X., O’Connell P., Schat K.A. Marek’s disease virus phosphorylated polypeptide pp38 alters transcription rates of mitochondrial electron transport and oxidative phosphorylation genes. Virus Genes. 2009;39:102–112. doi: 10.1007/s11262-009-0372-z. [DOI] [PubMed] [Google Scholar]
- 209.Tilokani L., Nagashima S., Paupe V., Prudent J. Mitochondrial dynamics: Overview of molecular mechanisms. Essays Biochem. 2018;62:341–360. doi: 10.1042/EBC20170104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Twig G., Hyde B., Shirihai O.S. Mitochondrial fusion, fission and autophagy as a quality control axis: The bioenergetic view. Biochim. Biophys. Acta. 2008;1777:1092–1097. doi: 10.1016/j.bbabio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kleele T., Rey T., Winter J., Zaganelli S., Mahecic D., Perreten Lambert H., Ruberto F., Nemir M., Wai T., Pedrazzini T., et al. Distinct fission signatures predict mitochondrial degradation or biogenesis. Nature. 2021;593:435–439. doi: 10.1038/s41586-021-03510-6. [DOI] [PubMed] [Google Scholar]
- 212.Lin S., Hardie D. AMPK: Sensing glucose as well as cellular energy status. Cell Metab. 2018;27:299–313. doi: 10.1016/j.cmet.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 213.Herzig S., Shaw R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Vilmen G., Glon D., Siracusano G., Lussignol M., Shao Z., Hernandez E., Perdiz D., Quignon F., Mouna L., Poüs C., et al. BHRF1, a BCL2 viral homolog, disturbs mitochondrial dynamics and stimulates mitophagy to dampen type I IFN induction. Autophagy. 2021;17:1296–1315. doi: 10.1080/15548627.2020.1758416. [DOI] [PMC free article] [PubMed] [Google Scholar]