Abstract
Background
Evidence on the benefits of fiber-supplemented enteral nutrition (EN) in critically ill patients is inconsistent, and critical care nutrition guidelines lack recommendations based on high-quality evidence. This systematic review and meta-analysis (SRMA) aims to provide a current synthesis of the literature on this topic.
Methods
For this SRMA of randomized controlled trials (RCT), electronic databases (MEDLINE, EMBASE, CENTRAL) were searched systematically from inception to January 2024 and updated in June 2024. Trials investigating clinical effects of fiber-supplemented EN versus placebo or usual care in adult critically ill patients were selected. Two independent reviewers extracted data and assessed the risk of bias of the included studies. Random-effect meta-analysis and trial sequential analysis (TSA) were conducted. The primary outcome was overall mortality, and one of the secondary outcomes was diarrhea incidence. Subgroup analyses were also performed for both outcomes.
Results
Twenty studies with 1405 critically ill patients were included. In conventional meta-analysis, fiber-supplemented EN was associated with a significant reduction of overall mortality (RR 0.66, 95% CI 0.47, 0.92, p = 0.01, I2 = 0%; 12 studies) and diarrhea incidence (RR 0.70, 95% CI 0.51, 0.96, p = 0.03, I2 = 51%; 11 studies). However, both outcomes were assessed to have very serious risk of bias, and, according to TSA, a type-1 error cannot be ruled out. No subgroup differences were found for the primary outcome.
Conclusion
Very low-certainty evidence suggests that fiber-supplemented EN has clinical benefits. High-quality multicenter RCTs with large sample sizes are needed to substantiate any firm recommendation for its routine use in this group of patients.
PROSPERO registration number: CRD42023492829.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-05128-2.
Keywords: Medical nutrition therapy, Fiber, Enteral nutrition, Critical care, Systematic review, Meta-analysis, Trial sequential analysis
Introduction
Critical illness is frequently associated with severe changes in gut function, metabolism and induces a catabolic stress state, often leading to malnutrition and compromised immune function [1–3].
Enteral nutrition (EN) is the preferred route of medical nutrition therapy for critically ill patients [4]. However, a common challenge among critically ill patients is enteral feeding intolerance with a prevalence of up to 75% [5, 6], leading to inadequate nutrient delivery and gastrointestinal (GI) symptoms like constipation or diarrhea [7, 8]. Therefore, inexpensive and safe interventions would be needed to manage this challenge.
Dietary fiber (DF) is a type of carbohydrate that is not/only partially hydrolyzed or absorbed in the human small intestine [9]. DF has been shown to provide various benefits in disease prevention among healthy individuals, including, among other benefits, reduced risk of mortality, type-2 diabetes, and cardiovascular disease [10–13]. A recent narrative review suggested considerable benefits from DF in critically ill patients, attributed to its functions in maintaining gut barrier integrity, modulating immune responses, supporting the gut microbiome, and contributing to systemic anti-inflammatory responses [9]. Formulations containing DF have been introduced attempting to improve GI tolerance of EN in critically ill patients [14]. However, existing trials about DF for critically ill patients yield inconsistent results [13], and there is a lack of up-to-date, high-quality systematic reviews of randomized controlled trials (RCTs) with meta-analysis (SRMA). Consequently, the routine use of DF in intensive care unit (ICU) settings remains unclear. While the American Society for Parenteral and Enteral Nutrition (ASPEN) and the Society of Critical Care Medicine (SCCM) recommend considering the routine use of fermentable soluble DF supplements in stable medical and surgical ICU patients, they advise against the routine use of mixed soluble and insoluble DFs due to concerns about bowel ischemia and dysmotility [15]. Conversely, the European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines do not address the use of DF in the ICU [16].
Prior systematic reviews examining the effects of fiber-supplemented EN in adult critically ill patients [17–19], have included non-RCTs [20–24]. Furthermore, none of the preceding meta-analyses applied trial sequential analysis (TSA), limiting accurate assessment of type-1 and –2 error within the meta-analyses [25]. TSA helps in assessing the robustness of results and minimizes the risk of distortion due to random errors [26].
Therefore, we conducted a SRMA of RCTs and included TSA to generate a higher quality and more precise estimate regarding the efficacy of fiber-supplemented EN in critically ill patients. We also performed GRADE certainty of evidence assessment, thereby enhancing the conclusiveness and reliability of our findings.
Methods
This SRMA was performed in accordance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [27]. The PRISMA 2020 checklist is shown in Additional file 1: Part 1. The protocol was registered in PROSPERO (CRD42023492829).
Eligibility criteria
RCTs of adult (age ≥ 16 years) critically ill patients (defined as admission to the ICU, or if uncertain, a mortality rate of ≥ 5% in the control group or mechanical ventilation at the study inclusion) that compared fiber-supplemented EN with placebo or usual care and reported at least one clinical or GI outcome were included. Pseudorandomized trials and studies that investigated the effects of synbiotics were excluded. Studies among patients with elective or cancer surgery or studies only reporting laboratory, metabolic or nutritional outcomes were also excluded.
Outcomes
The primary outcome was overall mortality. When multiple mortality endpoints were reported in a trial, the data was included in the following order of preference: 28-/30-day mortality > hospital mortality > ICU mortality > other mortality. Secondary outcomes included diarrhea, other GI complications, ICU and hospital length of stay (LOS), duration of mechanical ventilation (MV), infectious complications, metabolic (blood glucose, triglycerides) and nutritional (e.g. tolerated feeding volumes, time to reach energy targets) outcomes.
Information sources and search strategies
MEDLINE, EMBASE, and CENTRAL (Cochrane Database of Systematic Reviews and the Cochrane Central Register of Controlled Trials) were searched through OVID on January 11, 2024, for all relevant RCTs published from database inception to January 09, 2024. No language restrictions were made. The reference lists of previous SRMAs were also reviewed and ClinicalTrials.gov was searched for ongoing studies. The detailed search strategies are presented in Additional file 1: Part 1. The search was repeated on June 10, 2024, to identify potential studies published after the initial search.
Study selection
Search results were exported into Covidence (Veritas Health Innovation, Melbourne, Australia) for screening and removal of duplicates. The article titles and abstracts were screened by two independent reviewers (JK and AH). Full texts of potential eligible trials were retrieved and reviewed independently by the same two reviewers. Disagreements were discussed with a third author (ZYL).
Data collection
Data from eligible trials were extracted independently by two reviewers (JK and AH). Abstracted data including study and patient characteristics, funding sources, feeding information, clinical, metabolic and nutritional outcomes, diarrhea, and adverse events are summarized in Additional file 1: Tables S1–S7. For studies that reported median (Q1–Q3) for continuous outcomes, authors were contacted to obtain the mean and standard deviation (SD). If means and SDs were unavailable, those outcomes were excluded from the meta-analysis. No assumption or data conversion was made if the information could not be obtained.
Study quality and risk-of-bias assessment
The quality of the included trials was evaluated independently by two authors using the Canadian Critical Care Nutrition (CCN) Methodological Quality System (JK and ZYL) and the Cochrane Risk of Bias 2 tool (ROB2) (JK and ZYL) [28]. The overall ROB2 assessment was categorized as high risk-of-bias, some concerns, or low risk of bias. The risk of bias traffic light and summary plots were generated by the risk-of-bias visualization (robvis) tool [29]. The CCN Methodological Quality System is used in CCN systematic reviews and allows quality comparisons across topics and time [30]. The methodologic score ranges from 0 to 14 points, where a higher score indicates higher study quality.
Data analysis
All analyses were performed with a random effects model using RevMan 5.4 (Cochrane IMS, Oxford, UK). For dichotomized outcomes, the pooled risk ratio (RR) was estimated by the DerSimonian and Laird random effect meta-analysis. For continuous outcomes, the random effect mean difference (MD) was estimated. Heterogeneity was quantified by the I2 measure. The result of the meta-analysis was presented in the forest plot generated by RevMan. Presence of potential publication bias was evaluated by funnel plots for overall outcomes. Egger’s test for funnel plot asymmetry was performed by using the metafor package in RStudio (version 2023.12.1) if ≥ 10 studies were included in a meta-analysis [31]. All estimates were provided with 95% confidence intervals (CI). A p-value < 0.05 was considered statistically significant.
Subgroup analyses
Subgroup analyses were performed for overall mortality and diarrhea incidence. The following a priori subgroup analyses were conducted: (1) publication date before 2000 versus after 2000, (2a) fermentable versus non-fermentable versus mixed fiber, (2b) viscous versus non-viscous versus mixed fiber, (2c) soluble versus insoluble versus mixed fiber (based on the classification provided by Gill et al [10]), (3) daily fiber dose < 20 g versus ≥ 20 g, (4) average age < 50 years versus ≥ 50 years, (5) average APACHE II score < 17 versus ≥ 17, (6) medical versus surgical versus mixed ICU, (7) intervention start ≤ 24 h versus ≤ 48 h, and (8) minimum duration of intervention < 6 days versus ≥ 6 days. All cut-offs for continuous data were based on the median. The calculation of the daily fiber doses is detailed in Additional file, Table S12. For the age subgroup, an a priori planned cut-off of 65 years was adjusted to the median of 50 years post-hoc to provide a more even distribution of studies while maintaining validity for the comparison of younger versus older study population. Subgroup analyses were not performed for the following pre-planned domains as data were not sufficiently available: patients with abdominal surgery versus others, and patients with shock/vasopressor use versus others. Additionally, no subgroup analysis on study quality was conducted, as none of the included studies had a low risk of bias. The following post-hoc subgroup analyses were added: (9) Co-intervention with immunonutrition versus DF only, (10) funding source of the trial (industry vs. non-industry), and (11) standard formula versus non-standard formula in the control group. All results of the subgroup analyses were not adjusted for multiplicity. Hence, they should be viewed as hypothesis-generating.
Trial sequential analysis
To control for type-1 and type-2 errors [25], TSA was performed for the following outcomes: overall mortality, diarrhea incidence, ICU LOS and hospital LOS. All TSA were performed using the TSA software (0.9.5.10 Beta, The Copenhagen Trial Unit, Denmark) with the pre-specified parameters detailed in Additional file 1: Part 1.
Certainty of evidence
The Grading of Recommendations Assessment, Development, and Evaluation (GRADE) system was used to rate the certainty of evidence for outcomes analyzed with TSA [32]. The certainty of the evidence was rated as high, moderate, low, and very low by considering the risk of bias, inconsistency, indirectness, imprecision, and publication bias. GRADEpro was used to prepare the GRADE evidence profile table [33].
Deviations from the original protocol
While diarrhea was initially considered as secondary outcome alongside other GI complications in the original protocol, we decided to place particular emphasis on it in our analyses for two main reasons. First, diarrhea is a highly prevalent symptom associated with enteral nutrition, with prevalence rates up to 41%, and it significantly impacts patient dignity and morbidity, contributing to issues such as electrolyte imbalances and increased infection risk [34]. Additionally, the incidence of diarrhea was the second most frequently reported outcome in the included studies, after mortality, and provided substantially more data than other secondary outcomes.
Subgroup analyses were performed only for mortality and diarrhea incidence, which was not explicitly specified in the original protocol. This decision was made, as these outcomes were the most clinically relevant and had the most data available, thereby avoiding excessive analyses with limited data.
Results
Study selection
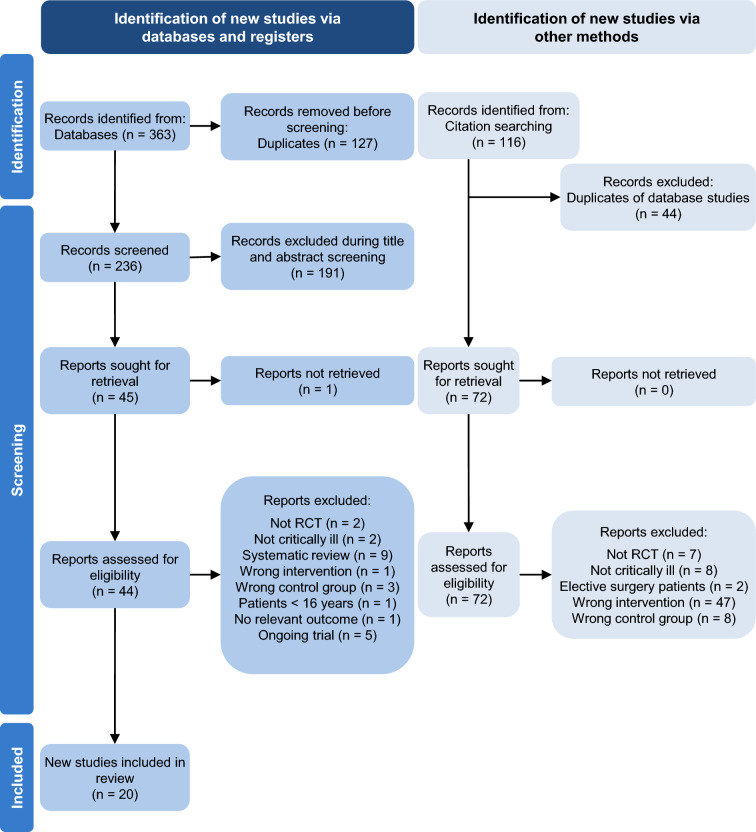
The search identified a total of 363 records from the databases. After removing duplicates, 236 abstracts were screened and of these, 44 full-text articles were assessed for eligibility. Twenty trials with a total of 1405 patients published between 1988 and 2021 were included. The detailed study selection flow is presented in Fig. 1. Five ongoing or unpublished related trials were identified (Additional file 1: Table S8). The excluded trials with the reason for exclusion are listed in Additional file 1: Table S9.
Fig. 1.
PRISMA Flowchart. One report was not retrieved because neither the abstract nor the full text was available
Risk of bias and study quality
The CCN score of the studies ranged from 2 to 10, with a median score of 6 (Additional file 1: Table S10). The ROB2 plots are presented in Additional file 1: Fig. S1. None of the included studies had an overall rating of low risk of bias. In 12 studies that reported mortality outcomes, 9/12 (75%) were at high risk of bias and 3/12 (25%) had some concerns. The biases mainly arose from the randomization process, deviations from intended interventions and selection of the reported results.
Study characteristics
Included studies and patient characteristics are summarized in Additional file 1: Table S1. The sample sizes ranged from 20 to 220 (median: 56). Only one study was a multi-center trial [35]. Seven studies enrolled mixed medical and surgical patients [35–41], three included only medical [42–44] and two included only surgical ICU patients [45, 46]. One study included trauma and septic patients with stress diabetes [47], two studies included patients with severe acute pancreatitis [48, 49], two included patients with multi-organ trauma [50, 51], one included patients with traumatic brain injury and hemorrhagic stroke [52], and diseases or ICU admission category were unclear in two studies [53, 54]. In all reviewed studies, EN was administered via feeding tubes. The majority (n = 16) compared fiber-supplemented EN with standard EN [36, 38–45, 48–54]. Two studies added immunomodulating components in the intervention group: one study included arginine and antioxidants (vitamins E and C) [35], and another added glutamine [51], while the control groups lacked these components. Conversely, two studies compared fiber-supplemented EN against control EN formulations that either contained glutamine, arginine, and linolenic acid [46] or were high in protein [46, 47]. The intervention groups in these studies did not receive these additional components. One study administered glutamine in both groups [37], and another provided high-protein formulas in both groups [35]. A detailed summary of the interventions is outlined in Additional file 1: Table S3, and all relevant outcomes are summarized in Additional file 1: Tables S4–S6.
Overall mortality
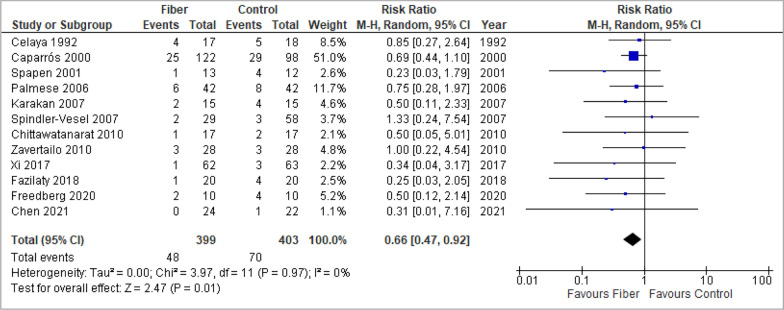
In statistically aggregated data from twelve studies, a significant effect of fiber-supplemented EN on overall mortality was observed (RR 0.66, 95% CI 0.47, 0.92, p = 0.01, I2 = 0%) (Fig. 2).
Fig. 2.
Meta-analysis of overall mortality
No evidence of funnel plot asymmetry was detected in the overall analysis (p = 0.14, Additional file 1: Fig. S9).
There was no evidence for subgroup differences in any of the subgroup analyses. The results of all subgroup analyses are summarized in Additional file 1: Table S11 and visualized in Additional file 1: Fig. S2.
Diarrhea
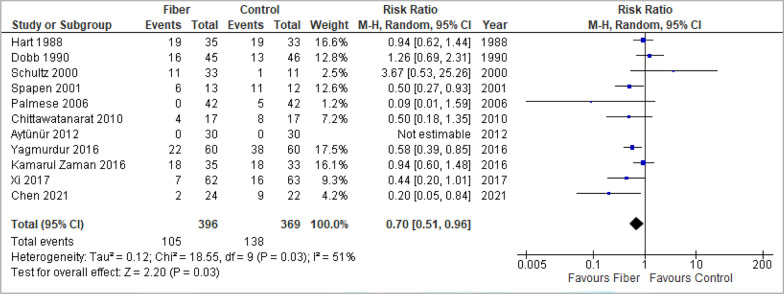
Fiber-supplemented EN was associated with a significant reduction of the diarrhea incidence (RR 0.70, 95% CI 0.51, 0.96, p = 0.03, I2 = 51%; 11 studies) (Fig. 3) and no evidence of funnel plot asymmetry was detected in the overall analysis (p = 0.41, Additional file 1: Fig. S10a).
Fig. 3.
Meta-analysis of diarrhea incidence
Additionally, a significant benefit of fiber-supplemented EN in meta-analysis of diarrhea scores according to the Hart and Dobb diarrhea scale was found (MD -2.77, 95% CI − 4.10, − 1.45, p < 0.0001, I2 = 0%; 3 studies; Additional file 1: Fig. S4). Visual inspection of the funnel plot found no evidence of asymmetry (Additional file 1: Fig. S10b).
In the subgroup analyses, studies published after 2000 indicated a significant reduction of diarrhea events through fiber-supplemented EN (RR 0.59, 95% CI 0.40, 0.85, p = 0.005, I2 = 43%; 9 studies), a result that was not supported by studies published before 2000 (RR 1.04, 95% CI 0.73, 1.46, p = 0.84, I2 = 0%; 2 studies) (test for subgroup differences: p = 0.03, I2 = 79.4%). Providing fiber-supplemented EN in sicker patients (APACHE II ≥ 17 compared to APACHE < 17 and unclear APACHE score) and in medical ICUs (compared to surgical ICUs, mixed ICUs and unclear admission type) seemed to be associated with a significant reduction of diarrhea incidence (tests for subgroup differences: p = 0.01 and p = 0.02, respectively). No evidence for subgroup differences was found in other subgroup analyses, as summarized in Additional file 1: Table S11 and visualized in Additional file 1: Fig. S3.
Other gastrointestinal complications
Four studies reported the overall incidence of GI complications (n = 315). No significant difference was found between groups (RR 0.75, 95% CI 0.49, 1.15, p = 0.19, I2 = 58%) (Additional file 1: Fig. S5a). There was also no significant difference between groups for the incidence of abdominal distension, vomiting, regurgitation and GI bleeding (Additional file 1: Fig. S5b–S5e). However, pooled data from six studies showed a significant benefit of fiber-supplemented EN for the incidence of constipation (RR 0.33, 95% CI 0.19, 0.58, p = 0.0001, I2 = 0%) (Additional file 1: Fig. S5f). Visual inspection of the funnel plots found no evidence of asymmetry (Additional file 1: Fig. S11).
Length of ICU and hospital stay
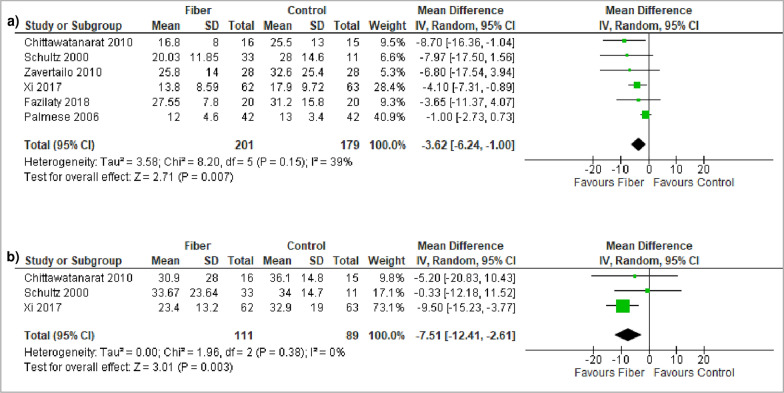
Fiber-supplemented EN was associated with a significantly reduced ICU (MD -3.62, 95% CI − 6.24, − 1.00, p = 0.007, I2 = 39%; 6 studies) and hospital LOS (MD − 7.51, 95% CI − 12.41, − 2.61, p = 0.003, I2 = 0%; 3 studies) (Fig. 4). Visual inspection of the funnel plots found no evidence of asymmetry (Additional file 1: Fig. S12 and S13).
Fig. 4.
Meta-analysis of a ICU LOS and b Hospital LOS
Infectious complications
No association was observed between fiber-supplemented EN and the overall incidence of infectious complications (RR 0.65, 95% CI 0.37, 1.14, p = 0.13, I2 = 0%; 3 studies) (Additional file 1: Fig. S6a). There was no significant evidence for influence on the incidence of pneumonia, urinary tract infection, intra-abdominal infection, sepsis, vascular infection, wound infection and bacteremia (Additional file 1: Fig. S6b–S6h). Visual inspection of the funnel plots found no evidence of asymmetry (Additional file 1: Fig. S14).
Duration of mechanical ventilation
In three studies reporting the duration of MV, there was no significant difference between groups (MD 0.02, 95% CI − 2.30, 2.34, p = 0.98, I2 = 39%) (Additional file 1: Fig. S7) and visual inspection of the funnel plot found no evidence of asymmetry (Additional file 1: Fig. S15).
Metabolic outcomes
One study reported episodes of hypoglycemia, finding a significant benefit of fiber-supplemented EN [54]. Two studies presented blood glucose [47, 48] and one study serum triglyceride levels [47] (Additional file 1: Table S5). Due to heterogeneous timing and units of measurement, the data were unsuitable for a pooled meta-analysis.
Nutritional outcomes
Gastric residual volume, assessed in three studies, showed no significant differences at various timepoints [44, 46, 53]. Five studies [35, 36, 48, 50, 52] measured the administered caloric intake, with one indicating a benefit for fiber supplementation on the mean overall energy intake [48] and one on the intake on specific days [52]. Tolerated feeding volumes were investigated in five studies [38–40, 44, 46], with one revealing significantly greater volumes for the intervention group on specific days [40] and one for the mean daily volume ratio [44] (Additional file 1: Table S5). Due to variability in timing and units of measurement, data was not aggregated for these outcomes.
For meta-analysis, the time to reach energy targets was pooled from two studies, revealing a beneficial effect of fiber-supplemented EN (MD − 2.25, 95% CI − 4.16, − 0.33, p = 0.02, I2 = 53%) (Additional file 1: Fig. S8). Visual inspection of the funnel plot found no evidence of asymmetry (Additional file 1: Fig. S16).
Adverse events
Three studies reported the incidence of adverse events, but they were not pooled due to inconsistency in definitions. One study investigated GI adverse events and observed no significant difference between groups [51]. Another study found no differences between groups for the incidence or severity of adverse health events [42]. One study found no adverse events related to DF but did not provide a specific definition and did not compare these findings to the control group [48] (Additional file 1: Table S7).
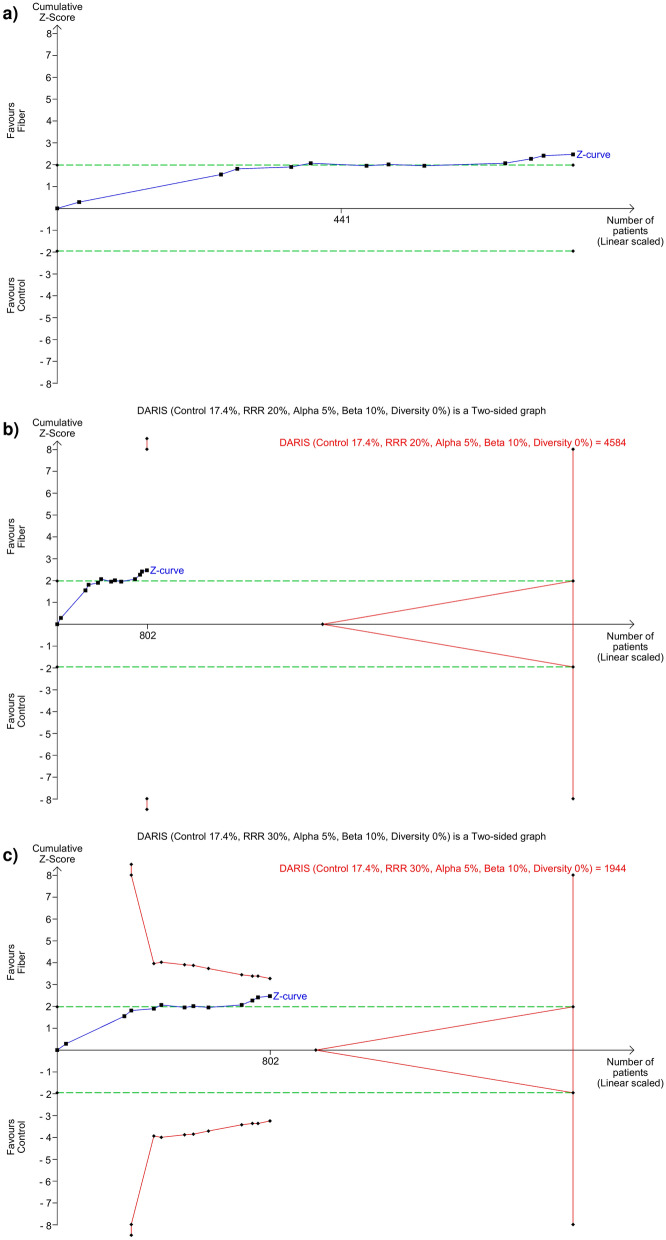
Trial sequential analysis
Results of TSA are summarized in Table 1 and presented in Fig. 5 and Additional file 1: Fig. S17–S19. TSA revealed that the current systematic review did not achieve the required information size (RIS) to detect the pre-specified effect sizes for overall mortality, diarrhea incidence, ICU and hospital LOS. In addition, for all outcomes, the pooled RR crossed the boundaries of conventional meta-analysis (i.e. significant) but did not cross (i.e. not significant) the trial sequential boundaries or the futility boundaries. This suggests the possibility of false positive results, indicating that more adequately designed studies are required to accrue sufficient information to confirm any benefits and justify the routine use of fiber-supplemented EN in critically ill patients. Post-hoc, additional plausible larger effect sizes were tested, and the interpretations were similar (Table 1).
Table 1.
Summary of results of TSA
| Effect size | Incidence, or variance | I2 (%) | D2 (%) | RIS | % of RIS attained | Pooled effect (TSA adjusted 95% CI) | Z-curve passed the conventional boundaries? | Z-curve passed the TSA boundaries? | Z-curve passed the futility boundaries? |
|---|---|---|---|---|---|---|---|---|---|
| Overall mortality (12 studies, n = 802) | |||||||||
| RRR: 10.0% | 17.4% | 0 | 0 | 19,155 | 4.19 | NA | Yes | No | No |
| RRR: 20.0%* | 17.4% | 0 | 0 | 4584 | 17.5 | 0.66 (0.17, 2.55) | Yes | No | No |
| RRR: 30.0%* | 17.4% | 0 | 0 | 1944 | 41.3 | 0.66 (0.38, 1.14) | Yes | No | No |
| Intensive care unit length of stay (6 studies, n = 380) | |||||||||
| MIREDIF 1 day | 51.1 | 39 | 69.9 | 7119 | 18.7 | − 3.62 (− 14.30, 7.06) | Yes | No | No |
| MIREDIF 2 days* | 51.1 | 39 | 69.9 | 1781 | 21.3 | − 3.62 (− 9.80, 2.56) | Yes | No | No |
| MIREDIF 3 days* | 51.1 | 39 | 69.9 | 792 | 48.0 | − 3.62 (− 7.73, 0.49) | Yes | No | No |
| Hospital length of stay (3 studies, n = 200) | |||||||||
| MIREDIF 1 day | 312.2 | 0 | 0 | 13,122 | 1.5 | NA | Yes | No | No |
| MIREDIF 2 days* | 312.2 | 0 | 0 | 3281 | 3.1 | − 7.51 (− 27.50, 12.48) | Yes | No | No |
| MIREDIF 3 days* | 312.2 | 0 | 0 | 1459 | 13.7 | − 7.51 (− 27.50, 12.48) | Yes | No | No |
| Diarrhea incidence (11 studies, n = 765) | |||||||||
|
RRR: 15.0%* |
37.4 | 51 | 60.5 | 7644 | 10.0 | 0.70 (0.20, 2.49) | Yes | No | No |
| RRR: 25.0% | 37.4 | 51 | 60.5 | 2678 | 28.6 | 0.70 (0.38, 1.29) | Yes | No | No |
|
RRR: 35.0%* |
37.4 | 51 | 60.5 | 1325 | 57.7 | 0.70 (0.45, 1.09) | Yes | No | No |
TSA trial sequential analysis, I2 Between-trial heterogeneity, D2 diversity-estimate, RRR relative risk reduction, MIREDIF minimally relevant difference, RIS required information size, NA not applicable, CI confidence interval
*Post-hoc sensitivity analyses
Fig. 5.
Trial Sequential Analysis (TSA) for overall mortality. a RRR = 10%, b RRR = 20%, c RRR = 30%. DARIS diversity-adjusted required information size; RRR relative risk reduction. The Z curve in blue measures the treatment effect (pooled relative risk). The parallel lines in green are the boundaries of conventional meta-analysis (alpha 5%). The red lines, located outside the parallel lines, are the boundaries of benefit and harm. These are boundaries of conventional meta-analysis adjusted for between-trial heterogeneity and multiple statistical testing (TSA boundaries). A treatment effect outside the TSA boundaries of benefit/harm indicates reliable evidence for a pre-defined magnitude of treatment effect, and a treatment effect within the futility zone (the triangle between the parallel lines) indicates that there is reliable evidence of an absence of a pre-defined magnitude of treatment effect
GRADE certainty of the evidence
The overall certainty of evidence using GRADE was rated as very low for all examined outcomes, implicating that the true effect is likely to be substantially different from the estimated effect (Table 2). The level of evidence was mainly downgraded due to very serious risk of bias and serious imprecision.
Table 2.
GRADE certainty assessment and summary of findings table
| Certainty assessment | Summary of findings | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Participants (studies) follow-up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall certainty of evidence | Study event rates (%) | Relative effect (95% CI) | Anticipated absolute effects | ||
| With control | With fiber | Risk with control | Risk difference with fiber | ||||||||
| Overall mortality | |||||||||||
| 802 (12 RCTs) | Very seriousa | Not serious | Not serious | Seriousb | None | ⨁◯◯◯ Very low | 70/403 (17.4%) | 48/399 (12.0%) | RR 0.66 (0.47, 0.92) | 174 per 1000 | 59 fewer per 1000 (from 92 to 14 fewer) |
| Diarrhea incidence | |||||||||||
| 765 (11 RCTs) | Very seriousc | Seriousd | Not serious | Seriousb | None | ⨁◯◯◯ Very low | 138/369 (37.4%) | 105/396 (26.5%) | RR 0.70 (0.51, 0.96) | 374 per 1000 | 112 fewer per 1000 (from 183 to 15 fewer) |
| ICU LOS | |||||||||||
| 380 (6 RCTs) | Very seriouse | Not serious | Not serious | Seriousb | None | ⨁◯◯◯ Very low | 179 | 201 | – | The mean ICU LOS was 0 | MD 3.62 lower (6.24 lower to 1 lower) |
| Hospital LOS | |||||||||||
| 200 (3 RCTs) | Very seriousf | Not serious | Not serious | Seriousb | None | ⨁◯◯◯ Very low | 89 | 111 | – | The mean hospital LOS was 0 | MD 7.51 lower (12.41 lower to 2.61 lower) |
CI confidence interval, MD mean difference, RR risk ratio
a3/12 studies had some concerns and 9/12 studies had high risk of bias
bWide trial sequential analysis adjusted confidence interval
c1/11 studies had some concerns and 10/11 studies had high risk of bias
dThe overall heterogeneity is I2 = 51%
e1/6 studies had some concerns and 5/6 studies had high risk of bias
fAll included trials had high risk of bias
Discussion
Summary of main findings
Overall, this SRMA of 20 RCTs found very low-certainty evidence suggesting the benefits of fiber-supplemented EN in critically ill patients. Although the latter indicated a potential improvement in clinical and diarrheal outcomes, TSA suggested that the accrued information size is insufficient, and more trials are needed to confirm these benefits. Furthermore, the overall certainty of evidence was compromised by a serious risk of bias in the trials.
Interpretation of the results in the context of other evidence
One SRMA by Cara et al. in 2021 assessed the safety of EN with DF based on 19 studies, including RCTs, retrospective cohort studies, case reports and case series [17]. They found no significant effects on diarrheal events, other GI complications, mortality, or ICU and hospital LOS.
Another SRMA by Liu et al. from 2022 included 20 RCTs and one cohort study, investigating interventions with fiber, probiotics or synbiotics. Liu et al. revealed no significant impact of DF on all clinical outcomes in fiber-only studies [18].
The most recent SRMA from the same group from 2023 included 13 RCTs [19], although one of the included studies was a pseudorandomized trial [55]. They concluded that DF might (or might not) reduce mortality, diarrhea, other GI complications, ICU and hospital LOS and the time to reach full EN.
Given our meticulous search strategy, which was significantly more thorough in both scope and detail, we were able to include a larger number of RCTs than previous SRMAs [36, 37, 47, 50, 52, 53]. Nevertheless, all studies were of low quality and the information size is insufficient to draw definitive conclusions regarding the benefits (or harms) of fiber-supplemented EN.
Impact of the results on clinical practice and future research
The complexity of clinical decision-making regarding the routine administration of DF in critically ill patients is reflected by the absence of clear guidance in major nutritional guidelines. ESPEN guidelines do not address the use of DF in the ICU at all [16]. In contrast, ASPEN recommends caution with the use of insoluble fiber [15] due to historical concerns about bowel obstruction, a potential adverse event documented in two case reports from 1990 that investigated five surgical and trauma patients receiving insoluble fiber [23, 24]. The studies in our meta-analyses are small and of low quality, and adverse effects of DF, especially bowel obstruction, were rarely reported outcomes. This is making it difficult to confirm or dismiss ASPEN’s cautious approach.
Generally, classifying fiber only by its solubility is increasingly recognized as outdated, and recent expert opinion papers highlight the importance of considering additional physicochemical characteristics such as viscosity and fermentability [9, 10]. A nuanced understanding of fiber's properties and biological mechanisms could further inform the design of future clinical trials, increasing the possibility of detecting a true clinically significant benefit and potential adverse effects.
Most importantly, our GRADE assessments implicate that the true effect of fiber-supplemented EN is likely to be substantially different from the estimated effect. The TSA results suggest that the findings of our meta-analyses may be at risk of type-1 errors, and more robust studies are needed to validate whether a real difference exists. The high risk of bias among the included studies underscores the potential for overestimation of benefits. Overall, given the very low-certainty of evidence, no strong recommendations can be made regarding the routine use of fiber-supplemented EN in critically ill patients. Although diarrhea is a common and relevant symptom in critically ill patients and fiber-supplemented EN is relatively inexpensive, its potential benefits should be approached with caution. Given the potential type-1 errors and overestimations of effect, the possibility that DF could even be harmful cannot be excluded. Therefore, high-quality RCTs are needed to accumulate sufficient evidence and substantiate the efficacy and safety of fiber-supplemented EN. Until such evidence is available, clinicians should consider individual patient circumstances when deciding on the use of DF supplementation.
The results of our TSA further suggest that future trials should not be powered for mortality unless the expected effect size is large (e.g. a RRR of 30%), a magnitude more commonly observed in pharmaceutical trials. Instead, future studies should be powered for diarrhea incidence due to its high prevalence among critically ill patients and because the required sample size is relatively more achievable compared to the other outcomes.
Strengths and limitations
Our SRMA has numerous strengths. We conducted a meticulous systematic search and performed robust quality and GRADE assessments. In addition, the meta-analysis of accurately selected RCTs enhances the overall quality of evidence of our SRMA compared to previous SRMAs. Including non-RCTs in a meta-analysis can lead to reduced reliability due to higher susceptibility to biases, increased methodological variability, and higher heterogeneity [26]. Finally, our SRMA is the first to explore the effects of fiber-supplemented EN through TSA, allowing us to estimate the required sample size for future trials. Overall, our SRMA provides the highest quality of evidence available on fiber-supplemented EN in critically ill patients.
Our SRMA also faces important limitations. Firstly, the studies included were predominantly single-centered and all had small sample sizes. None of the studies had a low risk of bias and most had a high risk of bias, which can lead to overestimations of benefits and underestimations of harm. Secondly, despite our efforts to obtain missing data from the authors, not all studies could be aggregated in the statistical analyses due to the diverse ways outcomes were measured and reported. For most outcomes, fewer than ten studies provided data for the meta-analysis, resulting in low patient numbers. Thirdly, as we excluded trials that only reported on metabolic or nutritional outcomes, our analysis provides an incomplete view of the evidence regarding these outcomes. Finally, in most subgroup analyses, not all studies from the overall analyses could be categorized into a defined subgroup due to missing data on subgroup characteristics. We also observed an uneven distribution of studies and population sizes across most of the subgroup analyses. Generally, the validity of our subgroup analyses is very low due to the very low certainty of evidence characterizing the overall results, which limits the interpretability and significance of these findings. Also, the multiplicity of subgroup analyses was not corrected for, and the findings from these analyses should be considered hypothesis-generating rather than confirmatory. Nonetheless, our subgroup analyses suggest several areas for future trials on tailored enteral nutrition, including the optimal type, dosage, start timing, and treatment duration of DF, as well as the patient populations that benefit most from DF supplementation.
Conclusion
This SRMA with TSA shows very low-certainty evidence suggesting that fiber-supplemented EN has clinical benefits, and future trials should explore diarrheal incidence as a primary outcome. Overall, given the very low-certainty of evidence, current findings should not be considered definitive to guide clinical practice. Large-scale, high-quality RCTs are required to accumulate sufficient evidence to justify recommendations for the routine use of fiber-supplemented EN in critically ill patients. Additionally, the optimal DF type, dosage, start timing, and treatment duration, as well as the critically ill patient population that benefits the most from fiber-supplemented EN should be explored.
Supplementary Information
Abbreviations
- ASPEN
American Society for Parenteral and Enteral Nutrition
- CCN
Critical care nutrition
- D2
Diversity estimate
- DARIS
Diversity adjusted required information size
- DF
Dietary fiber
- EN
Enteral nutrition
- ESPEN
European Society for Clinical Nutrition and Metabolism
- ICU
Intensive care unit
- LOS
Length of stay
- MD
Mean difference
- MIREDIF
Minimally relevant difference
- MV
Mechanical ventilation
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
Randomized controlled trial
- RIS
Required information size
- ROB2
Cochrane Risk of Bias 2
- RR
Risk ratio
- RRR
Relative risk reduction
- SCCM
Society of Critical Care Medicine
- SD
Standard deviation
- TSA
Trial sequential analysis
Author contributions
All authors contributed to the conception and design of this review. JLK, AH and ZYL performed the literature search, data extraction, and quality assessment. JLK, AH, ZYL and CCHL contributed to the data analysis. JLK generated the summary tables and figures. JLK, AH, ZYL, CCHL, CS and DKH interpreted the data. The manuscript was drafted by JLK. All authors provided critical revisions and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. None.
Availability of data and materials
All generated data are presented within the manuscript or the additional file.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
AH holds a research stipend from the Medical Faculty RWTH Aachen (“Habilitationsstipendium”), grants for IITs from the German Research Foundation, the Lotte and John Hecht Memorial Foundation, Fresenius Kabi, Pascoe Pharma and Baxter. She receives lecture fees and travel honoraria from Fresenius Kabi and Baxter. CS has served as consultant and received honorarium as speaker for Fresenius, BRAUN and Baxter. ED has received speaker honoraria from Baxter. None of the above mentioned are related to this specific systematic review. All other authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zheng-Yii Lee and Aileen Hill Joint last author.
References
- 1.Sharma K, Mogensen KM, Robinson MK. Pathophysiology of critical illness and role of nutrition. Nutr Clin Pract. 2019;34(1):12–22. 10.1002/ncp.10232. [DOI] [PubMed] [Google Scholar]
- 2.Hoffer LJ, Bistrian BR. Nutrition in critical illness: a current conundrum. F1000Res. 2016;5:2531. 10.12688/f1000research.9278.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moron R, Galvez J, Colmenero M, Anderson P, Cabeza J, Rodriguez-Cabezas ME. The importance of the microbiome in critically ill patients: role of nutrition. Nutrients. 2019. 10.3390/nu11123002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reintam Blaser A, et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017;43(3):380–98. 10.1007/s00134-016-4665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaser AR, Starkopf J, Kirsimagi U, Deane AM. Definition, prevalence, and outcome of feeding intolerance in intensive care: a systematic review and meta-analysis. Acta Anaesthesiol Scand. 2014;58(8):914–22. 10.1111/aas.12302. [DOI] [PubMed] [Google Scholar]
- 6.Wang K, McIlroy K, Plank LD, Petrov MS, Windsor JA. Prevalence, outcomes, and management of enteral tube feeding intolerance: a retrospective cohort study in a tertiary center. JPEN J Parenter Enteral Nutr. 2017;41(6):959–67. 10.1177/0148607115627142. [DOI] [PubMed] [Google Scholar]
- 7.McClave SA, Gualdoni J, Nagengast A, Marsano LS, Bandy K, Martindale RG. Gastrointestinal dysfunction and feeding intolerance in critical illness: Do we need an objective scoring system? Curr Gastroenterol Rep. 2020;22(1):1. 10.1007/s11894-019-0736-z. [DOI] [PubMed] [Google Scholar]
- 8.Heyland DK, et al. Incidence, risk factors, and clinical consequence of enteral feeding intolerance in the mechanically ventilated critically ill: an analysis of a multicenter, multiyear database. Crit Care Med. 2021;49(1):49–59. 10.1097/CCM.0000000000004712. [DOI] [PubMed] [Google Scholar]
- 9.McClave SA, Omer E, Eisa M, Klosterbauer A, Lowen CC, Martindale RG. The importance of providing dietary fiber in medical and surgical critical care. Nutr Clin Pract. 2023. 10.1002/ncp.11092. [DOI] [PubMed] [Google Scholar]
- 10.Gill SK, Rossi M, Bajka B, Whelan K. Dietary fibre in gastrointestinal health and disease. Nat Rev Gastroenterol Hepatol. 2021;18(2):101–16. 10.1038/s41575-020-00375-4. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393(10170):434–45. 10.1016/S0140-6736(18)31809-9. [DOI] [PubMed] [Google Scholar]
- 12.Slavin J. Fiber and prebiotics: mechanisms and health benefits. Nutrients. 2013;5(4):1417–35. 10.3390/nu5041417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Green CH, Busch RA, Patel JJ. Fiber in the ICU: Should it be a regular part of feeding? Curr Gastroenterol Rep. 2021;23(9):14. 10.1007/s11894-021-00814-5. [DOI] [PubMed] [Google Scholar]
- 14.Atkinson M, Worthley LI. Nutrition in the critically ill patient: part III. Enteral nutrition. Crit Care Resusc. 2003;5(3):207–15. [PubMed] [Google Scholar]
- 15.McClave SA, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: society of critical care medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159–211. 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 16.Singer P, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48–79. 10.1016/j.clnu.2018.08.037. [DOI] [PubMed] [Google Scholar]
- 17.Cara KC, Beauchesne AR, Wallace TC, Chung M. Safety of using enteral nutrition formulations containing dietary fiber in hospitalized critical care patients: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. 2021;45(5):882–906. 10.1002/jpen.2210. [DOI] [PubMed] [Google Scholar]
- 18.Liu T, et al. Effect of dietary fiber on gut barrier function, gut microbiota, short-chain fatty acids, inflammation, and clinical outcomes in critically ill patients: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr. 2022;46(5):997–1010. 10.1002/jpen.2319. [DOI] [PubMed] [Google Scholar]
- 19.Liu T, Feng P, Wang C, Ojo O, Wang YY, Wang XH. Effects of dietary fibre on enteral feeding intolerance and clinical outcomes in critically ill patients: a meta-analysis. Intensive Crit Care Nurs. 2023;74:103326. 10.1016/j.iccn.2022.103326. [DOI] [PubMed] [Google Scholar]
- 20.Fu Y, et al. Relationship between dietary fiber intake and short-chain fatty acid-producing bacteria during critical illness: a prospective cohort study. JPEN J Parenter Enteral Nutr. 2020;44(3):463–71. 10.1002/jpen.1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura K, et al. Pectin-containing liquid enteral nutrition for critical care: a historical control and propensity score matched study. Asia Pac J Clin Nutr. 2019;28(1):57–63. 10.6133/apjcn.201903_28(1).0009. [DOI] [PubMed] [Google Scholar]
- 22.O’Keefe SJ, et al. Effect of fiber supplementation on the microbiota in critically ill patients. World J Gastrointest Pathophysiol. 2011;2(6):138–45. 10.4291/wjgp.v2.i6.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scaife CL, Saffle JR, Morris SE. Intestinal obstruction secondary to enteral feedings in burn trauma patients. J Trauma. 1999;47(5):859–63. 10.1097/00005373-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 24.McIvor AC, Meguid MM, Curtas S, Warren J, Kaplan DS. Intestinal obstruction from cecal bezoar; a complication of fiber-containing tube feedings. Nutrition. 1990;6(1):115–7. [PubMed] [Google Scholar]
- 25.Shah A, Smith AF. Trial sequential analysis: adding a new dimension to meta-analysis. Anaesthesia. 2020;75(1):15–20. 10.1111/anae.14705. [DOI] [PubMed] [Google Scholar]
- 26.Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol. 2017;17(1):39. 10.1186/s12874-017-0315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Page MJ, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne JAC, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 29.McGuinness LA, Higgins JPT. Risk-of-bias visualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021;12(1):55–61. 10.1002/jrsm.1411. [DOI] [PubMed] [Google Scholar]
- 30.Lee ZY, et al. Benefits and harm of probiotics and synbiotics in adult critically ill patients. A systematic review and meta-analysis of randomized controlled trials with trial sequential analysis. Clin Nutr. 2023;42(4):519–31. 10.1016/j.clnu.2023.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Sterne JA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 32.Guyatt G, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–94. 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 33.GRADEpro GDT: GRADEpro Guideline Development Tool [Software]. McMaster University and Evidence Prime, 2024. Available from gradepro.org.
- 34.Tirlapur N, et al. Diarrhoea in the critically ill is common, associated with poor outcome, and rarely due to Clostridium difficile. Sci Rep. 2016;6:24691. 10.1038/srep24691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caparrós T, Lopez J, Grau T. Early enteral nutrition in critically ill patients with a high-protein diet enriched with arginine, fiber, and antioxidants compared with a standard high-protein diet. The effect on nosocomial infections and outcome. JPEN J Parenter Enteral Nutr. 2001;25(6):299–308. 10.1177/0148607101025006299. (discussion 308-9). [DOI] [PubMed] [Google Scholar]
- 36.Kamarul Zaman M. The effects of prebiotics on fecal microbiota in critical care patients receiving enteral nutrition, Master of Medical Science, Faculty of Medicine, University of Malaya, Kuala Lumpur, 2016.
- 37.Palmese S, Odierna I, Scarano D, Scibilia A, Natale A, Pezza M. Early enteral nutrition enriched with FOS and intravenous glutamine supplementation in intensive care unit patients. Nutr Therapy Metab. 2006;24(3):140–6. [Google Scholar]
- 38.Hart GK, Dobb GJ. Effect of a fecal bulking agent on diarrhea during enteral feeding in the critically ill. JPEN J Parent Enteral Nutr. 1988;12(5):465–8. 10.1177/0148607188012005465. [DOI] [PubMed] [Google Scholar]
- 39.Dobb GJ, Towler SC. Diarrhoea during enteral feeding in the critically ill: a comparison of feeds with and without fibre. Intensive Care Med. 1990;16(4):252–5. 10.1007/bf01705161. [DOI] [PubMed] [Google Scholar]
- 40.Rushdi TA, Pichard C, Khater YH. Control of diarrhea by fiber-enriched diet in ICU patients on enteral nutrition: a prospective randomized controlled trial. Clin Nutr. 2004;23(6):1344–52. 10.1016/j.clnu.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 41.Schultz AA, Ashby-Hughes B, Taylor R, Gillis DE, Wilkins M. Effects of pectin on diarrhea in critically ill tube-fed patients receiving antibiotics. Am J Crit Care. 2000;9(6):403–11. [PubMed] [Google Scholar]
- 42.Freedberg DE, et al. Impact of fiber-based enteral nutrition on the gut microbiome of ICU patients receiving broad-spectrum antibiotics: a randomized pilot trial. Crit Care Explor. 2020;2(6):e0135. 10.1097/CCE.0000000000000135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spapen H, Diltoer M, Van Malderen C, Opdenacker G, Suys E, Huyghens L. Soluble fiber reduces the incidence of diarrhea in septic patients receiving total enteral nutrition: a prospective, double-blind, randomized, and controlled trial. Clin Nutr. 2001;20(4):301–5. 10.1054/clnu.2001.0399. [DOI] [PubMed] [Google Scholar]
- 44.Yagmurdur H, Leblebici F. Enteral nutrition preference in critical care: fibre-enriched or fibre-free? Asia Pac J Clin Nutr. 2016;25(4):740–6. 10.6133/apjcn.122015.12. [DOI] [PubMed] [Google Scholar]
- 45.Chittawatanarat K, Pokawinpudisnun P, Polbhakdee Y. Mixed fibers diet in surgical ICU septic patients. Asia Pac J Clin Nutr. 2010;19(4):458–64. [PubMed] [Google Scholar]
- 46.Spindler-Vesel A, Bengmark S, Vovk I, Cerovic O, Kompan L. Synbiotics, prebiotics, glutamine, or peptide in early enteral nutrition: a randomized study in trauma patients. JPEN J Parent Enteral Nutr. 2007;31(2):119–26. 10.1177/0148607107031002119. [DOI] [PubMed] [Google Scholar]
- 47.Celaya S, et al. Experiencia con una dieta enteral con fibra y alto contenido en grasas en pacientes de UCI con intolerancia a la glucosa. Nutritción Hospitalaria. 1992;7(4):260–9. [PubMed] [Google Scholar]
- 48.Chen T, Ma Y, Xu L, Sun C, Xu H, Zhu J. Soluble dietary fiber reduces feeding intolerance in severe acute pancreatitis: a randomized study. JPEN J Parent Enteral Nutr. 2021;45(1):125–35. 10.1002/jpen.1816. [DOI] [PubMed] [Google Scholar]
- 49.Karakan T, Ergun M, Dogan I, Cindoruk M, Unal S. Comparison of early enteral nutrition in severe acute pancreatitis with prebiotic fiber supplementation versus standard enteral solution: a prospective randomized double-blind study. World J Gastroenterol. 2007;13(19):2733–7. 10.3748/wjg.v13.i19.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fazilaty Z, Chenari H, Shariatpanahi ZV. Effect of ss-glucan on serum levels of IL-12, hs-CRP, and clinical outcomes in multiple-trauma patients: a prospective randomized study. Ulus Travma Acil Cerrahi Derg. 2018;24(4):287–93. 10.5505/tjtes.2017.34514. [DOI] [PubMed] [Google Scholar]
- 51.Wang S-Y. Clinical effects of glutamine and dietary fiber enhanced enteral nutrition in critically ill trauma patients. World Chin J Digestol. 2014. 10.11569/wcjd.v22.i18.2626. [Google Scholar]
- 52.Zavertailo LL, Semenkova GV, Leiderman IN. Effect of an original enteral feeding protocol on clinical outcome indicators in patients with acute cerebral damage of vascular and traumatic genesis. Anesteziol Reanimatol. 2010;4:35–8. [PubMed] [Google Scholar]
- 53.Aytünür CS, Özcan N, Özcan A, Kaymak Ç, Başar H, Köse B. Lif İçeren ve İçermeyen Enteral Ürünlerle Beslenen Hastalarda Gastrik Rezidüel Volüm ve Gastrointestinal Komplikasyonların Karşılaştırılması. Türk Yoğun Bakım Derneği Dergisi. 2012;10(2):46–51. 10.4274/Tybdd.10.08. [Google Scholar]
- 54.Xi F, et al. Efficacy and safety of pectin-supplemented enteral nutrition in intensive care: a randomized controlled trial. Asia Pac J Clin Nutr. 2017;26(5):798–803. 10.6133/apjcn.082016.07. [DOI] [PubMed] [Google Scholar]
- 55.Tuncay P, et al. Use of standard enteral formula versus enteric formula with prebiotic content in nutrition therapy: a randomized controlled study among neuro-critical care patients. Clin Nutr ESPEN. 2018;25:26–36. 10.1016/j.clnesp.2018.03.123. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All generated data are presented within the manuscript or the additional file.