Simple Summary
The prognostic value of lymphovascular or perineural invasion in prostate cancer specimens regarding oncological outcomes after radical prostatectomy is unclear. Within a contemporary study cohort of 822 prostate cancer patients, 78 (9%) exhibited lymphovascular invasion and 633 (77%) exhibited perineural invasion in RP specimens. In univariable Cox regression models, lymphovascular invasion and perineural invasion were both associated with higher rates of biochemical recurrence (BCR). However, after multivariable adjustment for standard pathologic tumor characteristics, lymphovascular or perineural invasion was not found to be an independent predictor for BCR. These phenomes may be explained by the strong association between the Gleason Grade Group and pathologic tumor stage with lymphovascular as well as perineural invasion.
Keywords: BCR, lymphovascular invasion, perineural invasion, radical prostatectomy, prostate cancer
Abstract
Objective: The aim of this study was to test for the association between lymphovascular invasion or perineural invasion in radical prostatectomy (RP) specimens and biochemical recurrence (BCR). Methods: Relying on a tertiary-care database, we identified prostate cancer patients treated with RP between January 2014 and June 2023. Of these, the majority underwent robotic-assisted RP (81%). Kaplan–Meier survival analyses and Cox regression models addressed BCR according to either lymphovascular invasion or perineural invasion in RP specimens. Additionally, the linear trend test assessed the association between the Gleason Grade Group or pathologic tumor stage and lymphovascular or perineural invasion. Results: Of 822 patients, 78 (9%) exhibited lymphovascular invasion and 633 (77%) exhibited perineural invasion in RP specimens. In survival analyses, the five-year BCR-free survival rates were 62% in patients with lymphovascular invasion vs. 70% in patients without lymphovascular invasion (p = 0.04) and 64% in patients with perineural invasion vs. 82% in patients without perineural invasion (p = 0.01). In univariable Cox regression models, lymphovascular invasion (hazard ratio 1.58, 95% confidence interval 1.01–2.47; p = 0.045) and perineural invasion (hazard ratio 1.77, 95% confidence interval 1.13–2.77; p = 0.013) were both associated with a higher BCR rate. After accounting for age at surgery, PSA value, pathologic tumor stage, Gleason Grade Group, lymph node invasion, positive surgical margin, surgical approach, and adjuvant radiation therapy, lymphovascular (p = 0.740) or perineural invasion (p = 0.341) were not significantly associated with a higher BCR since the Gleason Grade Group and pathologic tumor stage highly correlated with lymphovascular as well as perineural invasion. Conclusions: In univariable models, lymphovascular or perineural invasion is associated with BCR. After adjustment for standard pathologic tumor characteristics, lymphovascular or perineural invasion is not an independent predictor for BCR.
1. Introduction
In patients with localized prostate cancer (PCa), radical prostatectomy (RP) has emerged as a standard of care [1,2,3,4]. Despite curatively intended therapy, some patients may develop biochemical recurrence (BCR) in the postoperative course [5,6,7]. Prognostic factors for BCR of localized PCa after RP include clinical factors such as age [8] and prostate specific antigen (PSA) [9]. In addition, several pathologic features in RP specimens are associated with worse short- and long-term oncological outcomes, such as non-organ confined pathologic tumor stage [10,11], Gleason Grade Group ≥4 [10,12,13], and positive surgical margins [5,7]. However, the clinical impact as well as the prognostic value of lymphovascular invasion in PCa specimens regarding oncological outcomes, such as biochemical recurrence after RP, remain unclear [14,15,16,17,18,19,20,21]. Moreover, while the prognostic role of perineural invasion in prostate cancer samples collected by needle core biopsy is being currently discussed [22,23,24], the prognostic role of perineural invasion in contemporary RP specimens remains controversial [20,25,26,27,28].
We addressed this uncertainty and hypothesized that BCR rates after RP are higher in patients with lymphovascular invasion or perineural invasion compared to those without the respective pathologic feature in RP specimens. To address this hypothesis, we relied on a contemporary cohort of PCa patients treated with RP in a tertiary care referral center.
2. Materials and Methods
2.1. Study Population
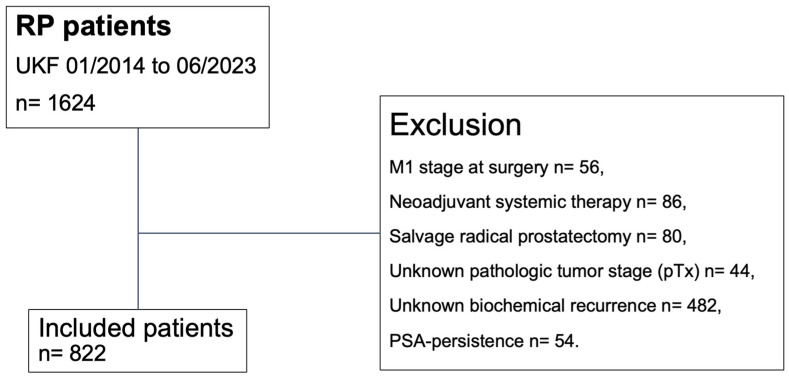
Using our prospectively maintained institutional tertiary-care database, we retrospectively identified patients with histologically confirmed adenocarcinoma of the prostate who underwent RP between January 2014 and June 2023 at the Department of Urology of the Goethe University Hospital Frankfurt, Germany (Figure 1) [29]. In the current study, only patients with known follow-up records of BCR were included. Patients with persistent PSA, defined as post-RP PSA of >0.1 ng/mL within six weeks after surgery, were excluded from the study cohort [30]. Further exclusion criteria consisted of clinical suspicion of metastases at time of surgery (cM1), treatment with neoadjuvant systemic therapy (chemotherapy and/or hormonal therapy), and previous radiation therapy of the prostate (salvage RP). Moreover, patients with an unknown pathologic tumor stage (pTx) were excluded. Informed written consent to participate in this study was given by all patients. Approval by the local ethics committee was obtained prior to data collection. All reporting was reviewed in accordance with the precepts established by the Declaration of Helsinki.
Figure 1.
Consort diagram. Abbreviations: PSA= prostate-specific antigen; pT = pathologic tumor stage at surgery; RP = radical prostatectomy; UKF = University Hospital Frankfurt.
2.2. Definition of Variables for Analyses
BCR, the primary endpoint of the study, was derived from patients’ self-reports in follow-up and was defined according to American Urological Association (AUA) guidelines as an initial serum PSA value of ≥0.2 ng/mL, with a second confirmatory level of >0.2 ng/mL in follow-up after RP [6,30,31]. Lymphovascular invasion as well as perineural invasion were determined by specialized uropathologists. All pathologic diagnoses were confirmed by a second pathologist.
Covariates consisted of age at surgery (continuously coded), PSA value (continuously coded), pathologic tumour stage (pT2 vs. pT3/pT4), Gleason Grade Group (1 vs. 2 vs. 3 vs. 4 vs. 5), pathologic lymph node stage (pN0 vs. pN1 vs. pNx), positive surgical margin (no vs. yes vs. unknown), surgical approach (open vs. robotic-assisted), and adjuvant radiation therapy (no vs. yes).
2.3. Statistical Analyses
Four analytical steps were performed. First, baseline characteristics were tabulated. Descriptive statistics included medians and interquartile ranges (IQRs) for continuously coded variables and frequencies and proportions for categorical variables. Second, Kaplan–Meier survival analyses addressed BCR-free survival according to either lymphovascular or perineural invasion. Third, univariable, and multivariable Cox regression models addressed BCR. Moreover, we relied on testing for proportional hazards assumption for a Cox regression model fit [32]. Additionally, linear trend tests assessed the association between the Gleason Grade Group or pathologic tumor stage and lymphovascular or perineural invasion.
The R software environment was used for statistical computing and graphics (R version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria) [33]. All tests were two sided, with a level of significance set at p < 0.05.
3. Results
3.1. Descriptive Characteristics
Relying on our institutional tertiary-care database of 1624 PCa patients who underwent RP between January 2014 and June 2023, we identified 822 patients according to the inclusion criteria (Table 1). Overall, the median follow-up of the study cohort was 20 months (IQR: 10–38). Of those, 78 (9%) exhibited lymphovascular invasion and 633 (77%) exhibited perineural invasion in RP specimens.
Table 1.
Descriptive characteristics of 822 prostate cancer patients treated with radical prostatectomy (RP) between January 2014 and June 2023.
| Characteristic | Overall n = 822 1 |
|
|---|---|---|
| Age at surgery (in years) | 66 (61, 71) | |
| PSA (in ng/mL) | 7.1 (5.2, 10.5) | |
| pTstage | pT2 | 473 (58%) |
| pT3/pT4 | 349 (42%) | |
| pNstage | pN0 | 692 (84%) |
| pN1 | 42 (5%) | |
| pNx | 88 (11%) | |
| Gleason Grade Group | 1 | 116 (14%) |
| 2 | 426 (52%) | |
| 3 | 156 (19%) | |
| 4 | 34 (4%) | |
| 5 | 90 (11%) | |
| Lymphovascular invasion | 78 (9%) | |
| Perineural invasion | 633 (77%) | |
| Positive surgical margin | no | 565 (69%) |
| yes | 236 (29%) | |
| unknown | 21 (2%) | |
| Robotic-assisted radical prostatectomy | 664 (81%) | |
| Adjuvant radiation therapy | 77 (10%) |
1 Median (interquartile range); n (%). Abbreviations: pN = pathologic lymph node stage; PSA = prostate-specific antigen; pT = pathologic tumor stage.
3.2. Biochemical Recurrence Rates According to Lymphovascular Invasion
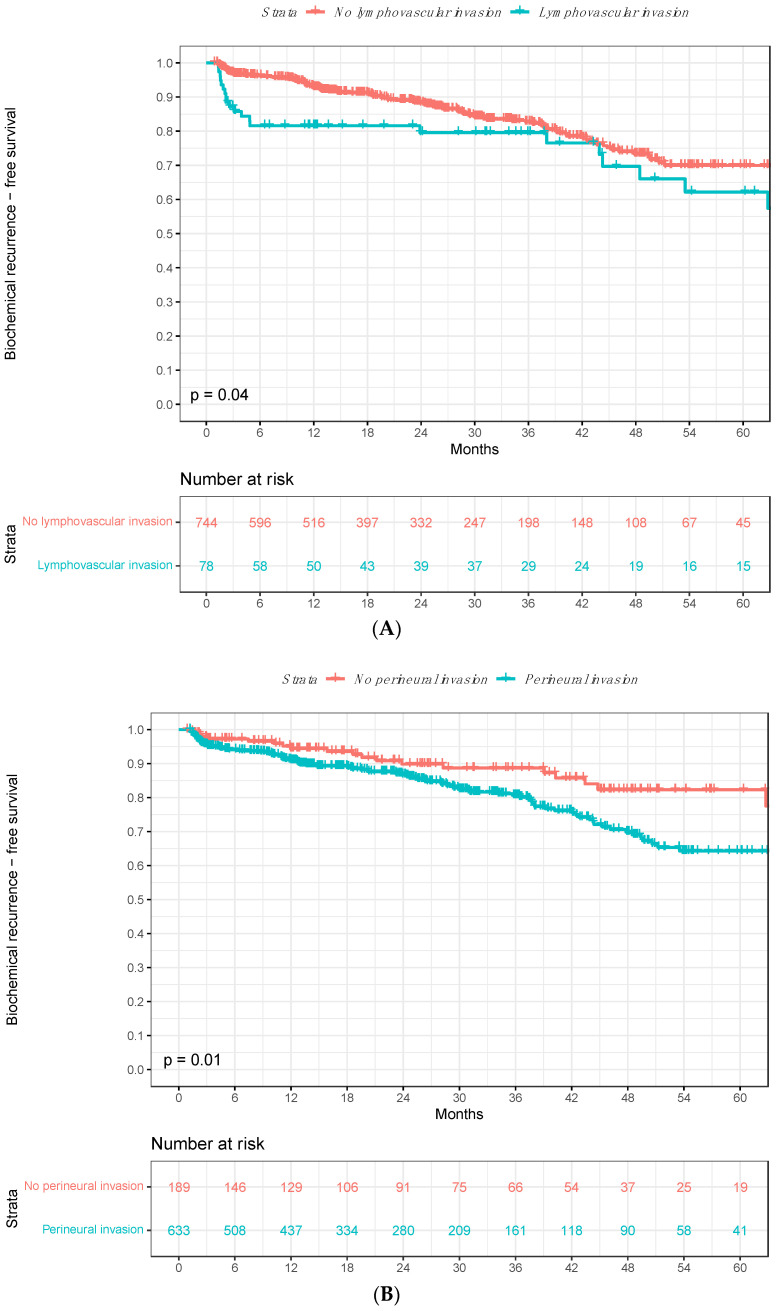
The five-year BCR-free survival rates were 62% in patients with lymphovascular invasion vs. 70% in patients without lymphovascular invasion in Kaplan–Meier survival analyses (Δ8%; p = 0.04; Figure 2A). In univariable Cox regression models, lymphovascular invasion predicted higher BCR rates after RP (hazard ratio [HR]: 1.58, 95% confidence interval [CI]: 1.01–2.47; p = 0.045; Table 2). After adjustment for age at surgery, PSA value, pathologic tumor stage, Gleason Grade Group, lymph node invasion, positive surgical margin, surgical approach, and adjuvant radiation therapy in multivariable models, lymphovascular invasion was not significantly associated with higher BCR rates (HR 0.91, 95% CI 0.50–1.63; p = 0.740) since the Gleason Grade Group and pathologic tumor stage highly correlated with higher rates of lymphovascular invasion in the linear trend test (p < 0.001; Figure 3A,B).
Figure 2.
Kaplan–Meier survival analyses addressing biochemical recurrence (BCR)-free survival after radical prostatectomy (RP) according to presence vs. absence of (A) lymphovascular invasion and (B) perineural invasion in RP specimens. Abbreviations: BCR = biochemical recurrence; BCRFS = biochemical recurrence-free survival; RP = radical prostatectomy.
Table 2.
Univariable and multivariable Cox regression models addressing rates of biochemical recurrence (BCR) after radical prostatectomy (RP), according to presence vs. absence of lymphovascular invasion or perineural invasion in RP specimens.
| Univariable | Multivariable * | |||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Lymphovascular invasion (Ref. no) | 1.58 | 1.01, 2.47 | 0.045 | 0.91 | 0.50, 1.63 | 0.740 |
| Perineural invasion (Ref. no) | 1.77 | 1.13, 2.77 | 0.013 | 1.26 | 0.78, 2.04 | 0.341 |
* adjusted for age at surgery, PSA value, pTstage, Gleason Grade Group, pNstage, positive surgical margin, surgical approach, and adjuvant radiation therapy. Abbreviations: CI = confidence interval; HR = hazard ratio; pN = pathologic lymph node stage; PSA = prostate-specific antigen; pT = pathologic tumor stage. Bold highlights statistically significant hazard ratio and corresponding p-value.
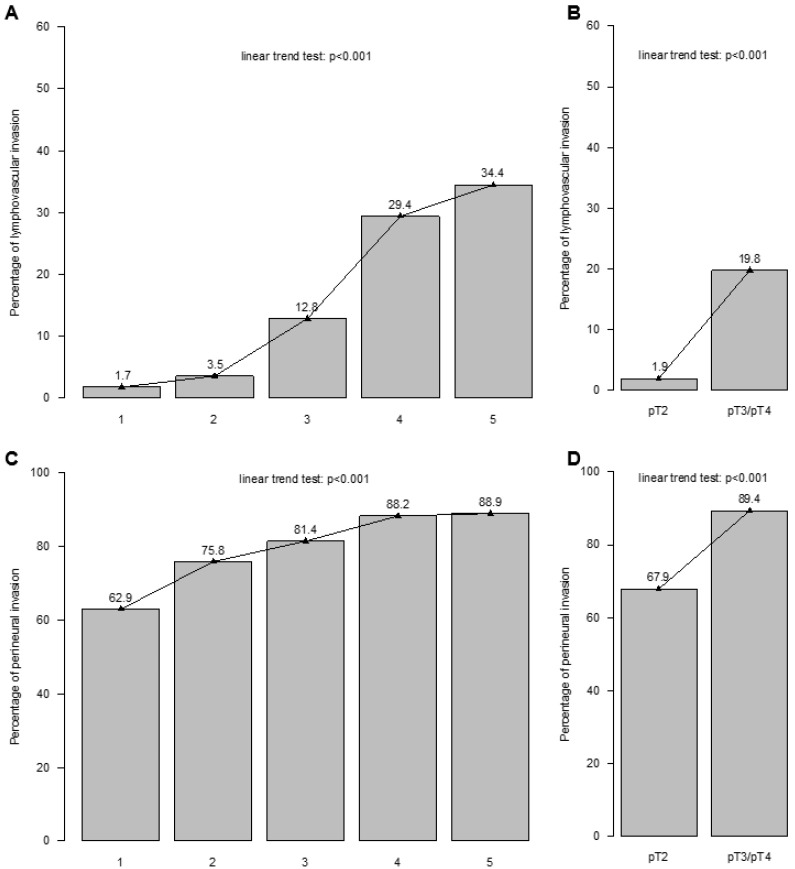
Figure 3.
The association between (A) Gleason Grade Group and lymphovascular invasion, (B) pathologic tumor stage (pTstage) and lymphovascular invasion, (C) Gleason Grade Group and perineural invasion, and (D) pTstage and perineural invasion in radical prostatectomy specimen. Abbreviation: pT = pathologic tumor stage.
3.3. Biochemical Recurrence Rates According to Perineural Invasion
In Kaplan–Meier survival analyses, the five-year BCR-free survival rates were 64% in patients with perineural invasion vs. 82% in patients without perineural invasion (Δ18%; p = 0.01; Figure 2B). In univariable Cox regression models, perineural invasion was statistically significantly associated with BCR after RP (HR 1.77, 95% CI 1.13–2.77; p = 0.013; Table 2). After adjustment for age at surgery, PSA value, pathologic tumor stage, Gleason Grade Group, lymph node invasion, positive surgical margin, surgical approach, and adjuvant radiation therapy in multivariable models, perineural invasion was not significantly associated with higher BCR rates (HR 1.26, 95% CI 0.78–2.04; p = 0.341) since the Gleason Grade Group and pathologic tumor stage highly correlated with higher rates of perineural invasion in the linear trend test (p < 0.001; Figure 3C,D).
4. Discussion
We hypothesized that BCR rates after RP are higher in patients with lymphovascular invasion or perineural invasion compared to those without the respective pathologic feature in RP specimens. Addressing this hypothesis in a contemporary cohort of PCa patients treated with RP at a tertiary care referral center between January 2014 and June 2023, we made several important observations.
First, we tabulated the proportion of patients diagnosed with lymphovascular invasion in our contemporary cohort of localized PCa patients treated with RP. Specifically, 78 of 822 patients (9%) exhibited lymphovascular invasion in RP specimens. Lymphovascular invasion rates in RP specimens in the literature range from 10 to 30% [14,15,16,20]. Thus, the lymphovascular invasion rate of 9% reported in the present study is slightly lower. However, these small differences in lymphovascular invasion rates may be explained by differences in pathologic tumor characteristics (e.g., pathologic tumor stage, Gleason Grade Group) in the study cohort [16,20,34]. As a consequence, to reduce uncontrolled bias or confounding due to differences in pathologic tumor characteristics, it is essential to adjust for these covariates in multivariable models, as in the present study.
Second, we recorded the proportion of patients diagnosed with perineural invasion in our contemporary cohort of localized PCa patients treated with RP. Specifically, 633 of 822 patients (77%) exhibited perineural invasion in RP specimens. The currently reported perineural invasion rate in RP specimens demonstrates that perineural invasion represents a common pathologic feature in RP specimens. Moreover, it is consistent with those reported in previous historical series, ranging from 44 to 78% [20,25,26,27,28,35]. This broad variability observed across different studies may be explained by differences in patient populations as well as interobserver variability. Therefore, it is essential to rely on a central pathology review of RP specimens, as in our tertiary referral center.
Third, we identified a strong association between the Gleason Grade Group and pathologic tumor stage with lymphovascular and perineural invasion in RP specimens in the linear trend tests. The lymphovascular invasion rate increased from 1.7% in Gleason Grade Group 1 to 34.4% in Gleason Grade Group 5 and from 1.9% in pT2 to 19.8% in pT3/pT4. This observation is not unexpected since lymphovascular invasion, defined as the invasion of vessel walls by tumor cells and/or the presence of tumor emboli surrounded by endothelial cells, represents a pathologic tumor feature that occurs in an advanced stage of PCa [34]. Similarly, the perineural invasion rate increased from 62.9% in Gleason Grade Group 1 to 88.9% in Gleason Grade Group 5 and from 67.9% in pT2 to 89.4% in pT3/pT4. To the best of our knowledge, we are the first to graphically depict these associations.
Fourth, in survival analyses, we identified important differences in BCR after RP according to the presence vs. absence of lymphovascular invasion in RP specimens. The five-year BCR-free survival rates were 62% in patients with lymphovascular invasion vs. 70% in patients without lymphovascular invasion (Δ8%; p = 0.04). Similarly, lymphovascular invasion predicted a 1.6-fold higher BCR rate after RP in univariable Cox regression models (p = 0.045). However, in multivariable models adjusting for age at surgery, PSA value and other pathologic tumor characteristics, including pathologic tumor stage, Gleason Grade Group, lymph node stage, positive surgical margin, surgical approach and adjuvant radiation therapy, the BCR rate did not statistically significantly differ according to the presence vs. absence of lymphovascular invasion in RP specimens (p = 0.740). The lack of consistent association of lymphovascular invasion in RP specimens with BCR may be explained in several ways. First, it may be postulated that the lack of association between lymphovascular invasion and BCR after multivariable adjustment for pathologic tumor characteristics and adjuvant radiation therapy relates to a stronger association between the Gleason Grade Group as well as pathologic tumor stage and lymphovascular invasion in RP specimens, as described above. Second, lymphovascular invasion is associated with a lower prognostic strength in the current study cohort compared to historical reports [14,20]. Third, recently published analyses by Kawase et al. indicate that lymphovascular invasion may be of prognostic value only in select RP patients [15]. Moreover, other unmeasured variables may underly this lack of association. For example, the magnitude of differences in BCR rates between patients with vs. without lymphovascular invasion may be too small to detect independent predictor status. The proposed explanations are preliminary at best. Nevertheless, contemporary prostate cancer guidelines of the European Association of Urology (EAU) recommend reporting lymphovascular invasion as a mandatory element in pathology reports [30]. Moreover, the presence of lymphovascular invasion appears to be associated with pathogenic germline DNA-repair gene mutations in men with PCa [36].
Finally, we also addressed BCR after RP according to the presence vs. absence of perineural invasion in RP specimens. In Kaplan–Meier survival analyses, five-year BCR-free survival rates were 64% in patients with perineural invasion vs. 82% in patients without perineural invasion (Δ18%; p = 0.01). In univariable Cox regression models, perineural invasion predicted a 1.8-fold higher BCR rate after RP (p = 0.013). However, perineural invasion was not statistically significantly associated with BCR after RP in multivariable Cox regression models (p = 0.341). These observations validate historical studies, where perineural invasion did not provide additional information for BCR risk prediction after accounting for standard pathologic tumor characteristics, such as pathologic tumor stage, Gleason score, and positive surgical margin [25,27,37]. However, these findings are in contrast to observations recorded by Kang et al. and Stankovic et al., who identified perineural invasion as an independent predictor of BCR after RP [20,26]. Nonetheless, analyses by Wu et al. suggest that a minimum of three foci of perineural invasion is required to predict BCR [27]. Moreover, Gertsen et al. recently reported that apex-localized perineural invasion independently predicted higher BCR compared to mid- or base-localized perineural invasion [38]. In consequence, analyses categorizing perineural invasion as presence vs. absence may underestimate the prognostic value of perineural invasion in RP specimens.
Taken together, in our contemporary study cohort, only 9% of patients exhibited lymphovascular invasion and 77% exhibited perineural invasion in RP specimens. In univariable models, lymphovascular or perineural invasion are both associated with BCR. However, after adjustment for age at surgery, PSA value, standard pathologic tumor characteristics, surgical approach, and adjuvant radiation therapy, lymphovascular or perineural invasion were not statistically significantly associated with BCR. Nevertheless, our observations may indicate that reporting lymphovascular or perineural invasion in RP specimens may not provide additional prognostic value beyond standard pathologic tumor characteristics.
Despite our important observations, the present study has limitations. First, due to its retrospective nature, a potential for residual selection biases, despite systematic adjustment for biases and confounders in multivariable models, remained. This limitation is applicable to all studies relying on a retrospective study design [14,20,25,34]. Second, our study relies on a limited sample size. Specifically, only 24 patients with lymphovascular invasion and 115 patients with perineural invasion experienced BCR. In consequence, further sub-stratification such as stage-specific subgroup analyses was not possible. Third, we relied on a study cohort of PCa patients treated with RP between 2014 and 2023. The study period of around 10 years may introduce biases, as medical treatment protocols as well as surgical techniques have slightly changed over time. However, this limitation applies to both patients with vs. without lymphovascular or perineural invasion in RP specimens. Fourth, in our cohort, lymphovascular invasion as well as perineural invasion were categorized as presence vs. absence. The determination of the vascular status in RP specimens must be evaluated with great caution in the event of negative findings. Unlike in other organs, a more intensive immunohistochemical examination would be required for a more reliable assessment. Nonetheless, previous analyses indicate that ambiguous RP specimens exhibit similar prognostic outcomes compared to those classified as positive [39]. Moreover, the extent of perineural and vascular invasion as well as their localization (intra- vs. extratumoral) should be determined in future studies [27,28]. Finally, postoperative follow-up within our study cohort was also limited. Therefore, other study endpoints that could be equally as interesting as BCR, such as metastasis-free, cancer-specific or overall survival, could not be addressed.
5. Conclusions
In univariable models, lymphovascular or perineural invasion is associated with BCR. However, after adjustment for standard pathologic tumor characteristics, lymphovascular or perineural invasion is not an independent predictor for BCR.
Acknowledgments
Carolin Siech was awarded a scholarship by the STIFTUNG GIERSCH.
Author Contributions
C.S.: concept and design, draft of the manuscript, statistical analysis, and analysis and interpretation of the data. N.G.: acquisition of the data. M.W., C.C.G., C.H. and F.J.K.: acquisition of the data, critical revision of the manuscript and important intellectual content. Z.T.: statistical analysis, analysis, and interpretation of the data. P.I.K.: critical revision of the manuscript and important intellectual content. L.A.K.: acquisition of the data, supervision, critical revision of the manuscript, and important intellectual content. F.K.H.C.: acquisition of the data and supervision. B.H.: acquisition of the data, supervision, concept and design, critical revision of the manuscript, and important intellectual content, P.M.: acquisition of the data, supervision, concept and design, critical revision of the manuscript, and important intellectual content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the institutional review boards of the University Cancer Centre Frankfurt and the Ethical Committee at the University Hospital Frankfurt (SUG-1-2018_A2023, 18 April 2023).
Informed Consent Statement
Informed written consent to participate in this study was given by all patients.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Oberlin D.T., Flum A.S., Lai J.D., Meeks J.J. The effect of minimally invasive prostatectomy on practice patterns of American urologists. Semin. Preserv. Strateg. Bladder Cancer. 2016;34:255.e1–255.e5. doi: 10.1016/j.urolonc.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preisser F., Cooperberg M.R., Crook J., Feng F., Graefen M., Karakiewicz P.I., Klotz L., Montironi R., Nguyen P.L., D’Amico A.V. Intermediate-risk Prostate Cancer: Stratification and Management. Eur. Urol. Oncol. 2020;3:270–280. doi: 10.1016/j.euo.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Williams I.S., McVey A., Perera S., O’Brien J.S., Kostos L., Chen K., Siva S., Azad A.A., Murphy D.G., Kasivisvanathan V., et al. Modern paradigms for prostate cancer detection and management. Med. J. Aust. 2022;217:424–433. doi: 10.5694/mja2.51722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chierigo F., Wenzel M., Amling C., Flammia R.S., Horlemann B., Zhe T., Saad F., Chun F.K.H., Graefen M., Gallucci M., et al. Survival after Radical Prostatectomy versus Radiation Therapy in High-Risk and Very High-Risk Prostate Cancer. J. Urol. 2022;207:375–384. doi: 10.1097/JU.0000000000002250. [DOI] [PubMed] [Google Scholar]
- 5.Van den Broeck T., van den Bergh R.C.N., Briers E., Cornford P., Cumberbatch M., Tilki D., De Santis M., Fanti S., Fossati N., Gillessen S., et al. Biochemical Recurrence in Prostate Cancer: The European Association of Urology Prostate Cancer Guidelines Panel Recommendations. Eur. Urol. Focus. 2020;6:231–234. doi: 10.1016/j.euf.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Pisansky Thomas M., Thompson Ian M., Valicenti Richard K., D’Amico Anthony V., Selvarajah S. Adjuvant and Salvage Radiotherapy after Prostatectomy: ASTRO/AUA Guideline Amendment 2018–2019. J. Urol. 2019;202:533–538. doi: 10.1097/JU.0000000000000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tilki D., Chen M.-H., Wu J., Huland H., Graefen M., Wiegel T., Böhmer D., Mohamad O., Cowan J.E., Feng F.Y., et al. Adjuvant Versus Early Salvage Radiation Therapy for Men at High Risk for Recurrence Following Radical Prostatectomy for Prostate Cancer and the Risk of Death. J. Clin. Oncol. 2021;39:2284–2293. doi: 10.1200/JCO.20.03714. [DOI] [PubMed] [Google Scholar]
- 8.Öbek C., Lai S., Sadek S., Civantos F., Soloway M.S. Age as a prognostic factor for disease recurrence after radical prostatectomy. Urology. 1999;54:533–538. doi: 10.1016/S0090-4295(99)00168-5. [DOI] [PubMed] [Google Scholar]
- 9.Tilki D., Mandel P., Karakiewicz P.I., Heinze A., Huland H., Graefen M., Knipper S. The impact of very high initial PSA on oncological outcomes after radical prostatectomy for clinically localized prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2020;38:379–385. doi: 10.1016/j.urolonc.2019.12.027. [DOI] [PubMed] [Google Scholar]
- 10.Würnschimmel C., Wenzel M., Wang N., Tian Z., Karakiewicz P.I., Graefen M., Huland H., Tilki D. Radical prostatectomy for localized prostate cancer: 20-year oncological outcomes from a German high-volume center. Urol. Oncol. Semin. Orig. Investig. 2021;39:830.e17–830.e26. doi: 10.1016/j.urolonc.2021.04.031. [DOI] [PubMed] [Google Scholar]
- 11.Preisser F., Chun F.K.H., Pompe R.S., Heinze A., Salomon G., Graefen M., Huland H., Tilki D. Persistent Prostate-Specific Antigen After Radical Prostatectomy and Its Impact on Oncologic Outcomes. Eur. Urol. 2019;76:106–114. doi: 10.1016/j.eururo.2019.01.048. [DOI] [PubMed] [Google Scholar]
- 12.Epstein J.I., Egevad L., Amin M.B., Delahunt B., Srigley J.R., Humphrey P.A., The Grading Committee The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: Definition of Grading Patterns and Proposal for a New Grading System. Am. J. Surg. Pathol. 2016;40:244–252. doi: 10.1097/PAS.0000000000000530. [DOI] [PubMed] [Google Scholar]
- 13.Egevad L., Delahunt B., Srigley J.R., Samaratunga H. International Society of Urological Pathology (ISUP) grading of prostate cancer—An ISUP consensus on contemporary grading. APMIS. 2016;124:433–435. doi: 10.1111/apm.12533. [DOI] [PubMed] [Google Scholar]
- 14.May M., Kaufmann O., Hammermann F., Loy V., Siegsmund M. Prognostic impact of lymphovascular invasion in radical prostatectomy specimens. BJU Int. 2007;99:539–544. doi: 10.1111/j.1464-410X.2006.06650.x. [DOI] [PubMed] [Google Scholar]
- 15.Kawase M., Ebara S., Tatenuma T., Sasaki T., Ikehata Y., Nakayama A., Toide M., Yoneda T., Sakaguchi K., Teishima J., et al. Prognostic Importance of Lymphovascular Invasion for Specific Subgroup of Patients with Prostate Cancer After Robot-Assisted Radical Prostatectomy (The MSUG94 Group) Ann. Surg. Oncol. 2024;31:2154–2162. doi: 10.1245/s10434-023-14691-x. [DOI] [PubMed] [Google Scholar]
- 16.Sathianathen N.J., Furrer M.A., Mulholland C.J., Katsios A., Soliman C., Lawrentschuk N., Peters J.S., Zargar H., Costello A.J., Hovens C.M., et al. Lymphovascular Invasion at the Time of Radical Prostatectomy Adversely Impacts Oncological Outcomes. Cancers. 2024;16:123. doi: 10.3390/cancers16010123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semba R., Uchida K., Hirokawa Y., Shiraishi T., Onishi T., Sasaki T., Inoue T., Watanabe M. Short-term prognosis of low-risk prostate cancer patients is favorable despite the presence of pathological prognostic factors: A retrospective study. BMC Urol. 2023;23:174. doi: 10.1186/s12894-023-01345-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang W., Zhang L., Wu B., Zha Z., Zhao H., Jun Y., Jiang Y. The impact of lymphovascular invasion in patients with prostate cancer following radical prostatectomy and its association with their clinicopathological features: An updated PRISMA-compliant systematic review and meta-analysis. Medicine. 2018;97:e13537. doi: 10.1097/MD.0000000000013537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fajkovic H., Mathieu R., Lucca I., Hiess M., Hübner N., Al Awamlh B.A.H., Lee R., Briganti A., Karakiewicz P., Lotan Y., et al. Validation of lymphovascular invasion is an independent prognostic factor for biochemical recurrence after radical prostatectomy. Urol. Oncol. Semin. Orig. Investig. 2016;34:233.e1–233.e6. doi: 10.1016/j.urolonc.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Kang M., Oh J.J., Lee S., Hong S.K., Lee S.E., Byun S.-S. Perineural Invasion and Lymphovascular Invasion are Associated with Increased Risk of Biochemical Recurrence in Patients Undergoing Radical Prostatectomy. Ann. Surg. Oncol. 2016;23:2699–2706. doi: 10.1245/s10434-016-5153-z. [DOI] [PubMed] [Google Scholar]
- 21.Yee D.S., Shariat S.F., Lowrance W.T., Maschino A.C., Savage C.J., Cronin A.M., Scardino P.T., Eastham J.A. Prognostic significance of lymphovascular invasion in radical prostatectomy specimens. BJU Int. 2011;108:502–507. doi: 10.1111/j.1464-410X.2010.09848.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teramoto Y., Numbere N., Wang Y., Miyamoto H. Clinical Significance of Perineural Invasion by Prostate Cancer Detected on Needle Core Biopsy: Unilateral vs Bilateral Involvement. Am. J. Clin. Pathol. 2023;159:116–119. doi: 10.1093/ajcp/aqac142. [DOI] [PubMed] [Google Scholar]
- 23.Teramoto Y., Wang Y., Miyamoto H. Risk Stratification by Quantification of Perineural Cancer Invasion on Prostate Needle Core Biopsy: Should It Be Counted? J. Urol. 2023;210:639–648. doi: 10.1097/JU.0000000000003618. [DOI] [PubMed] [Google Scholar]
- 24.Bell P.D., Teramoto Y., Gurung P.M.S., Numbere N., Yang Z., Miyamoto H. The Clinical Significance of Perineural Invasion by Prostate Cancer on Needle Core Biopsy: Involvement of Single Versus Multiple Sextant Sites. Arch. Pathol. Lab. Med. 2022;146:1252–1257. doi: 10.5858/arpa.2021-0248-OA. [DOI] [PubMed] [Google Scholar]
- 25.Reeves F., Hovens C.M., Harewood L., Battye S., Peters J.S., Costello A.J., Corcoran N.M. Does perineural invasion in a radical prostatectomy specimen predict biochemical recurrence in men with prostate cancer? Can. Urol. Assoc. J. 2015;9:E252–E255. doi: 10.5489/cuaj.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stankovic M., Wolff L., Wieder T., Mendes J., Schumacher B. Perineural invasion as predictor of biochemical recurrence in prostate cancer following open radical prostatectomy: A single-center experience. World J. Urol. 2022;40:2695–2700. doi: 10.1007/s00345-022-04158-1. [DOI] [PubMed] [Google Scholar]
- 27.Wu S., Xie L., Lin S.X., Wirth G.J., Lu M., Zhang Y., Blute M.L., Dahl D.M., Wu C.-L. Quantification of perineural invasion focus after radical prostatectomy could improve predictive power of recurrence. Hum. Pathol. 2020;104:96–104. doi: 10.1016/j.humpath.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Lubig S., Thiesler T., Müller S., Vorreuther R., Leipner N., Kristiansen G. Quantitative perineural invasion is a prognostic marker in prostate cancer. Pathology. 2018;50:298–304. doi: 10.1016/j.pathol.2017.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Siech C., Wenzel M., Lange C., Cano Garcia C., Humke C., Tian Z., Karakiewicz P.I., Traumann M., Kluth L.A., Chun F.K.H., et al. The Association between Patient Characteristics and Biochemical Recurrence after Radical Prostatectomy. Medicina. 2024;60:1119. doi: 10.3390/medicina60071119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.EAU Guidelines Office EAU Guidelines on Prostate Cancer. Edn. Presented at the EAU Annual Congress Paris 2024. 2024. [(accessed on 23 October 2024)]. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/
- 31.Cookson M.S., Aus G., Burnett A.L., Canby-Hagino E.D., D’Amico A.V., Dmochowski R.R., Eton D.T., Forman J.D., Goldenberg S.L., Hernandez J., et al. Variation in the Definition of Biochemical Recurrence in Patients Treated for Localized Prostate Cancer: The American Urological Association Prostate Guidelines for Localized Prostate Cancer Update Panel Report and Recommendations for a Standard in the Reporting of Surgical Outcomes. J. Urol. 2007;177:540–545. doi: 10.1016/j.juro.2006.10.097. [DOI] [PubMed] [Google Scholar]
- 32.Grambsch P.M., Therneau T.M. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515–526. doi: 10.1093/biomet/81.3.515. [DOI] [Google Scholar]
- 33.R Core Team R: A Language and Environment for Statistical Computing. [(accessed on 27 August 2023)]. Available online: https://www.R-project.org/
- 34.Kang Y.J., Kim H.-S., Jang W.S., Kwon J.K., Yoon C.Y., Lee J.Y., Cho K.S., Ham W.S., Choi Y.D. Impact of lymphovascular invasion on lymph node metastasis for patients undergoing radical prostatectomy with negative resection margin. BMC Cancer. 2017;17:321. doi: 10.1186/s12885-017-3307-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Mauro E., Di Bello F., Califano G., Morra S., Creta M., Celentano G., Abate M., Fraia A., Pezone G., Marino C., et al. Incidence and Predicting Factors of Histopathological Features at Robot-Assisted Radical Prostatectomy in the mpMRI Era: Results of a Single Tertiary Referral Center. Medicina. 2023;59:625. doi: 10.3390/medicina59030625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isaacsson Velho P., Silberstein J.L., Markowski M.C., Luo J., Lotan T.L., Isaacs W.B., Antonarakis E.S. Intraductal/ductal histology and lymphovascular invasion are associated with germline DNA-repair gene mutations in prostate cancer. The Prostate. 2018;78:401–407. doi: 10.1002/pros.23484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lian Z., Zhang H., He Z., Ma S., Wang X., Liu R. Impact of positive surgical margin location and perineural invasion on biochemical recurrence in patients undergoing radical prostatectomy. World J. Surg. Oncol. 2020;18:201. doi: 10.1186/s12957-020-01977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gertsen B.G., Teramoto Y., Wang Y., Tsuzuki T., Miyamoto H. Clinical significance of location of perineural cancer invasion detected on prostate needle core biopsy. Virchows Arch. 2024 doi: 10.1007/s00428-024-03779-8. [DOI] [PubMed] [Google Scholar]
- 39.Galiabovitch E., Hovens C.M., Peters J.S., Costello A.J., Battye S., Norden S., Ryan A., Corcoran N.M. Routinely reported ‘equivocal’ lymphovascular invasion in prostatectomy specimens is associated with adverse outcomes. BJU Int. 2017;119:567–572. doi: 10.1111/bju.13594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.