Simple Summary
Escherichia coli consists of different pathotypes, such as enteropathogenic (EPEC), enterotoxigenic (ETEC), enteroaggregative (EAEC), shiga-toxin (STEC), enterohaemorrhagic (EHEC), and necrotoxigenic (NTEC). They are responsible for severe human clinical forms that often are difficult to treat because of the resistance to one or more antimicrobials. Animals, including birds, may be involved in the epidemiology of E. coli antimicrobial-resistant pathotypes acting as reservoirs. Seagulls are synanthropic wild birds largely present not only along the coastal areas, but also in hinterlands where they can contaminate numerous environments, including urban and farm areas, through their droppings. Monitoring of seagulls is a useful tool to obtain information about the circulation of pathogenic bacteria and to verify the antimicrobial resistance trend.
Keywords: Escherichia coli, seagulls, pathotypes, necrotoxigenic E. coli (NTEC), antimicrobial resistance
Abstract
Seagulls are synanthropic wild birds that can contaminate, through their droppings, beaches, urban and peri-urban environments. This concern is more serious when seagulls eliminate antimicrobial-resistant pathogenic bacteria. This study analyzed the fecal samples from 137 yellow-legged seagulls (Larus michahellis) from Central Italy. A total of 218 Escherichia coli strains were isolated and analyzed for phenotypic and genotypic antimicrobial resistance and to identify the virulence genes characterizing different pathotypes. The disk diffusion method on all isolates found relevant resistance rates to ampicillin (38.99%), tetracycline (23.85%), and enrofloxacin (21.10%). On the basis of all results obtained with this test, 62 (28.44%) isolates were classified as multidrug-resistant (MDR) and 6 (2.75%) as extensive drug-resistant (XDR). Molecular analyses conducted on the strains phenotypically resistant to carbapenems, cephalosporins, and penicillins found 9/37 (24.32%) strains positive for blaOXA-48, 52/103 (50.49%) for blaTEM, 12/103 (11.65%) for blaCMY2, 3/103 (2.91%) for blaCTX, and 1/103 (0.97%,) for blaSHV. PCR to detect virulence genes characterizing different pathotypes found that 40 (18.35%) isolates had the astA gene, indicative of the enteroaggregative (EAEC) pathotype, 2 (0.92%) had cnf1, 2 (0.92%) had cnf2, and 1 (0.46%) had cdt-IV. All five (2.29%) strains were reportable as necrotoxigenic (NTEC), while 4 (1.83%) had both eaeA and escV, reportable as enteropathogenic (EPEC). Measures to limit seagulls’ access where humans and other animals reside are pivotal to reduce the risk of infection with antimicrobial-resistant and pathogenetic E. coli strains.
1. Introduction
Escherichia coli is an opportunistic Gram-negative bacterium, belonging to the family Enterobacteriaceae, commensal of the human and animal intestinal tract. This species encompasses several pathotypes responsible for intestinal and extra-intestinal infections. Urinary and genital tract infections, meningitis, and septicemia, are the most frequent extra-intestinal forms encountered in animals and humans [1]. Different E. coli diarrhoeagenic pathotypes are involved in the enteric forms. They act with different mechanisms in relation to their virulence traits.
Enteropathogenic E. coli (EPEC) strains produce the adherence factor intimin; it is encoded by the eae gene and allows bacteria to adhere to enterocytes causing microvilli loss and consequent diarrhea [2]. Enterotoxigenic E. coli (ETEC) strains are characterized by two groups of virulence factors. The first group includes heat-stable (ST) and heat-labile enterotoxins (LT) [3]. The second group includes colonization factors, such as fimbriae, that help the bacteria to adhere to the ileum [3]. All enteroaggregative E. coli (EAEC) strains are characterized by their aggregative-adherence pattern, designated the stacked-brick configuration, which is mostly mediated by aggregative-adherence fimbriae (AAF) [4]. Most EAEC bacteria harbor additional virulence factors such as the EAEC heat-stable enterotoxin and serine proteases [4]. Shiga toxin-producing E. coli (STEC) are strains producing two of the most potent bacterial toxins: Stx1, homologous to the Stx produced by Shigella dysenteriae type 1, and Stx2, which includes several subtypes. These toxins are toxic to colonic, ileal epithelial, and endothelial cells [5,6,7]. Enterohemorrhagic E. coli (EHEC) are STEC strains having intimin and hemolysin as additional virulence factors. Hemolysin, encoded by the hly gene, contributes to the pathogenesis by different mechanisms such as hemolysis, induction of pro-inflammatory reactions, and epithelial and endothelial cells damage [8]. STEC and EHEC cause different clinical manifestations in humans, including the asymptomatic form, bloody or severe diarrhea and systemic diseases, such as hemorrhagic colitis (HC), and the life-threatening hemolytic–uremic syndrome (HUS), which is the main cause of acute renal failure in children [9]. Necrotoxigenic E. coli (NTEC) have different virulence factors, including fimbrial and afimbrial adhesins, siderophores, and toxins. The cytolethal distending toxin (Cdt) impairs host defense by the holding cell cycle and by apoptosis in epithelial cells and lymphocytes, and subsequent impairing of acquired immunity [10]; it can also alter macrophage function leading to a pro-inflammatory response [10,11]. In addition, NTEC strains have the two cytotoxic necrotizing factors CNF1 and CNF2, which induce multi-nucleation and necrosis of eukaryotic cells [11].
Antimicrobial resistance is a major global challenge affecting animals and humans [12]. Most E. coli strains involved in infections of mammals and birds are resistant to several antimicrobials [13].
Wild birds often harbor pathogens, including antimicrobial-resistant bacteria, which can disseminate in the environment through their feces [14,15,16,17,18]. Seagulls are synanthropic wild birds largely present not only along the coastal areas, but also in hinterlands. They nest on private houses, hotels, large warehouses, and shipyards. They can fly great distances for food, often to landfill sites, especially during the winter, and sewage outlets or agricultural land and farm areas [19]. In addition, they have been proven to transport bacteria from human and animal waste to recreational beaches [19].
Previous studies investigated E. coli populations in wild birds, including seagulls [15,18,20,21,22]. Wild avifauna has been considered a possible bioindicator of antibiotic resistance; therefore, studies have been focused on the determination of the antimicrobial resistance patterns of E. coli. Conversely, to the best of our knowledge, this is the first survey aimed to investigate the role of seagulls present in Italy in the dissemination of antimicrobial-resistant E. coli, as well as of different E. coli pathotypes.
In fact, the aim of the present study was to investigate the occurrence of E. coli strains belonging to different pathotypes in fecal samples collected from yellow-legged seagulls (Larus michahellis) recovered in a rescue center in Central Italy and to study their phenotypic and genotypic characters of antimicrobial resistance.
2. Materials and Methods
2.1. Sampling
During the summer seasons of 2022 and 2023, fecal samples were collected from 137 yellow-legged seagulls (Larus michahellis) recovered at a wildlife rescue center in Central Italy. The gulls were recovering from trauma and kept in single cages; feces were sampled from the bottom of each cage to avoid animals’ stress. Samples were collected as soon as possible after the animal’s arrival at the rescue center, usually within 48 h. Only gulls not yet receiving antibiotic treatments were enrolled in the study. No ethical approval was required because no biological materials were sampled directly from the birds. Each fecal sample was collected in a sterile plastic tube and transferred within 3 h, kept in a cool bag at 4 °C, and sent to the Avian Pathology Laboratories of the Department of Veterinary Sciences, University of Pisa, where it was immediately submitted to bacteriological analyses.
2.2. Escherichia coli Isolation
A swab from each fecal sample was pre-enriched in buffered peptone water (BPW) (Oxoid Ltd., Basingstoke, UK) at 37 °C for 24 h; successively, a loop was streaked onto selective Tryptone Bile X-GLUC (TBX) agar (Biolife, Milan, Italy) and incubated at 42 °C for 24 h. From each sample, 2 distinct colonies were collected, if possible, streaked on Tryptic Soy Agar (TSA) (Biolife).
Escherichia coli isolated strains were stored, until needed for further analyses, in Brain–Heart Infusion (BHI) broth (Oxoid Ltd.), with the addition of 30% glycerol as a cryoprotectant, at −80 °C.
2.3. Antimicrobial Susceptibility Tests
All E. coli isolates were analyzed for antimicrobial resistance using the Kirby–Bauer disk diffusion test, following CLSI guidelines [23].
A total of 15 different antibiotic molecules, classified into 9 classes, were tested. The following antimicrobial disks (Oxoid, Ltd.) were employed: ampicillin (10 µg), amoxicillin-clavulanate (20/10 µg), cefoxitin (30 µg), cefotaxime (30 µg), ceftiofur (30 µg), imipenem (10 µg), ertapenem (10 µg), aztreonam (30 µg), chloramphenicol (30 µg), tetracycline (30 µg), enrofloxacin (5 µg), ciprofloxacin (5 µg), gentamicin (10 µg), amikacin (30 µg), and trimethoprim-sulfamethoxazole (1.25–23.75 µg).
Escherichia coli ATCC25922 was included as control. The obtained inhibition zones were interpreted according to CLSI [24].
The investigated strains were classified as multidrug-resistant (MDR), extensively drug-resistant (XDR), or pandrug-resistant (PDR) on the basis of the phenotypic resistance results. Briefly, MDR is defined as non-susceptibility to at least one agent in three or more antimicrobial categories. XDR is defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories. PDR is defined as non-susceptibility to all agents in all antimicrobial categories [25].
2.4. Molecular Analyses
DNA was extracted from fresh E. coli strains, cultured on TSA, employing a commercial kit, Quick-DNA Miniprep Plus Kit (Zymo Research, Irvine, CA, USA), following the manufacturer’s instructions.
All PCR assays described below were performed in the automated thermocycler SimpliAmp™ Thermal Cycler, (Applied Biosystems, Waltham, MA, USA). In all PCR assays, sterile water instead of DNA was included as a negative control, whereas DNA from previously isolated and characterized E. coli strains, selected in relation to the searched gene, was added as positive control. All PCR products were analyzed by electrophoresis on 1.5% agarose gel at 100 V for 45 min, using 100 bp DNA Ladder Ready to Load (Solis BioDyne, Tartu, Estonia) as a DNA marker; the gel was stained with ethidium bromide and observed under UV light.
Positive PCR products were submitted to sequencing analyses (BMR Genomics, Padova, Italy). The obtained sequences were analyzed using BioEdit and compared with online gene bank databases: Basic Local Alignment Search Tool (BLAST) and FASTA (https://www.ebi.ac.uk/Tools/sss/fasta/) (Accessed on 10 April 2024).
2.4.1. Identification of E. coli Strains
Pure isolates were confirmed as E. coli by the use of a species-specific PCR, with the primers uspAF (5′-CCGATACGCTGCCAATCAGT-3′) and uspAR (5′-ACGCAGACCGTAGGCCAGAT-3′), which allow the amplification of a 884 bp fragment of the uspA gene; PCR conditions consisted of 30 cycles, each of 94 °C for 2 min, 70 °C for 1 min, and 72 °C for 1 min [26].
2.4.2. Genotypic Resistance
The isolates showing phenotypic resistance to penicillins (ampicillin and/or amoxicillin-clavulanate) and/or cephalosporins (cefoxitin, cefotaxime and/or ceftiofur) were submitted to molecular analyses to investigate the presence of genes blaTEM, blaSHV, and blaCTX-M, coding for extended spectrum β-lactamases (ESBL). The same strains were also tested for the presence of blaCMY1 and blaCMY2 genes coding for AmpC β-lactamases. Furthermore, strains phenotypically resistant to carbapenems (imipenem and/or ertapenem) were tested for the presence of blaNDM, blaVIM, blaIMP, blaKPC, and blaOXA-48 genes, coding for carbapenemases. Primers and PCR conditions were reported in Table 1.
Table 1.
Primers and PCR conditions for the detection of the investigated resistance genes.
| Target Gene | Primers Sequence (5′-3′) | Annealing Temp. (°C) | Amplicons Size (bp) | References |
|---|---|---|---|---|
| bla NDM | GGTTTGGCGATCTGGTTTTC CGGAATGGCTCATCACGATC |
52 | 621 | [27] |
| bla KPC | CGTCTAGTTCTGCTGTCTTG CTTGTCATCCTTGTTAGGCG |
52 | 798 | [27] |
| bla OXA-48 | GCGTGGTTAAGGATGAACAC CATCAAGTTCAACCCAACCG |
52 | 438 | [27] |
| bla IMP | GGAATAGAGTGGCTTAAYTCTC GGTTTAAYAAAACAACCACC |
52 | 232 | [27] |
| blaVIM | GATGGTGTTTGGTCGCATA CGAATGCGCAGCACCAG |
52 | 390 | [27] |
| bla TEM | GCACGAGTGGGTTACATCGA GGTCCTCCGATCGTTGTCAG |
60 | 310 | [28] |
| blaSHV | TTCGCCTGTGTATTATCTCCCTG TTAGCGTTGCCAGTGYTCG |
50 | 854 | [29] |
| blaCTX-M | ATGTGCAGYACCAGTAARGTKATGGC TGGGTRAARTARGTSACCAGAAYCAGCGG |
60 | 593 | [29] |
| blaCMY-1 | GTGGTGGATGCCAGCATCC GGTCGAGCCGGTCTTGTTGAA |
58 | 915 | [29] |
| blaCMY-2 | GCACTTAGCCACCTATACGGCAG GCTTTTCAAGAATGCGCCAGG |
58 | 758 | [29] |
2.4.3. Virulence Factors
The selected E. coli strains were tested for the presence of 20 virulence genes, belonging to 7 pathotypes: STEC, EHEC, EPEC, ETEC, EAEC, EIEC, and NTEC (Table 2). Specifically, these were stx1 and stx2, characteristics of the STEC pathotype, and the hlyA gene specific of EHEC; eaeA gene that is common to both EHEC and EPEC; escV, bfpB, and ent that characterize EPEC; elt, estIa, and estIb for ETEC; astA, aggR, and pic for EAEC; invE for EIEC; and cnf1, cnf2, cdt-I, cdt-II, cdt-III, and cdt-IV for NTEC.
Table 2.
Primers and PCR conditions for the detection of virulence genes.
| Pathotype | Target Gene |
Primers Sequence (5′-3′) | Annealing Temp. (°C) | Amplicon Size (bp) | Reference |
|---|---|---|---|---|---|
| STEC/ EHEC |
stx1 | ATAAATCGCCATTCGTTGACTAC AGAACGCCCACTGAGATCATC |
60 | 180 | [30] |
| stx2 | GGCACTGTCTGAAACTGCTCC TCGCCAGTTATCTGACATTCTG |
60 | 255 | [30] | |
| hylA | GCATCATCAAGCGTACGTTCC AATGAGCCAAGCTGGTTAAGCT |
60 | 534 | [30] | |
| EHEC/ EPEC |
eaeA | GACCCGGCACAAGCATAAGC CCACCTGCAGCAACAAGAGG |
60 | 384 | [30] |
| EPEC | escV | ATTCTGGCTCTCTTCTTCTTTATGGCTG CGTCCCCTTTTACAAACTTCATCGC |
53 | 544 | [4] |
| bfpB | GACACCTCATTGCTGAAGTCG CCAGAACACCTCCGTTATGC |
53 | 910 | [4] | |
| ent | TGGGCTAAAAGAAGACACACTG CAAGCATCCTGATTATCTCACC |
53 | 629 | [4] | |
| ETEC | LT | GAACAGGAGGTTTCTGCGTTAGGTG CTTTCAATGGCTTTTTTTTGGGAGTC |
53 | 655 | [4] |
| STIa | CCTCTTTTAGYCAGACARCTGAATCASTTG CAGGCAGGATTACAACAAAGTTCACAG |
53 | 157 | [4] | |
| STI | TGTCTTTTTCACCTTTCGCTC CGGTACAAGCAGGATTACAACAC |
53 | 171 | [4] | |
| EIEC | invE | CGATAGATGGCGAGAAATTATATCCCG CGATCAAGAATCCCTAACAGAAGAATCAC |
53 | 766 | [4] |
| EAEC | astA | TGCCATCAACACAGTATATCCG ACGGCTTTGTAGTCCTTCCAT |
53 | 102 | [4] |
| aggR | ACGCAGAGTTGCCTGATAAAG AATACAGAATCGTCAGCATCAGC |
53 | 400 | [4] | |
| pic | AGCCGTTTCCGCAGAAGCC AAATGTCAGTGAACCGACGATTGG |
53 | 1111 | [4] | |
| NTEC | CNF1 | GGGGGAAGTACAGAAGAATTA TTGCCGTCCACTCTCTCACCAGT |
55 | 1111 | [31] |
| CNF2 | TATCATACGGCAGGAGGAAGCACC GTCACAATAGACAATAATTTTCCG |
55 | 1240 | [31] | |
| cdt-I | CAATAGTCGCCCACAGGA ATAATCAAGAACACCACCAC |
56 | 411 | [31] | |
| cdt-II | GAAAATAAATGGAATATAAATGTCCG TTTGTGTTGCCGCCGCTGGTGAAA |
56 | 556 | [31] | |
| cdt-III | GAAAATAAATGGAATATAAATGTCCG TTTGTGTCGGTGCAGCAGGGAAAA |
56 | 555 | [31] | |
| cdt-IV | CCTGATGGTTCAGGAGGCTGGTTC TTGCTCCAGAATCTATACCT |
56 | 350 | [31] |
3. Results
3.1. Escherichia coli Isolation
E. coli strains were isolated from 110 (80.29%, CI: 73.63–86.95%) of the 137 analyzed fecal samples; of these E. coli-positive samples, 2 yielded one isolate for each while the remaining 108 yielded two strains, making a total of 218 isolates.
3.2. Agar Disk Diffusion Method
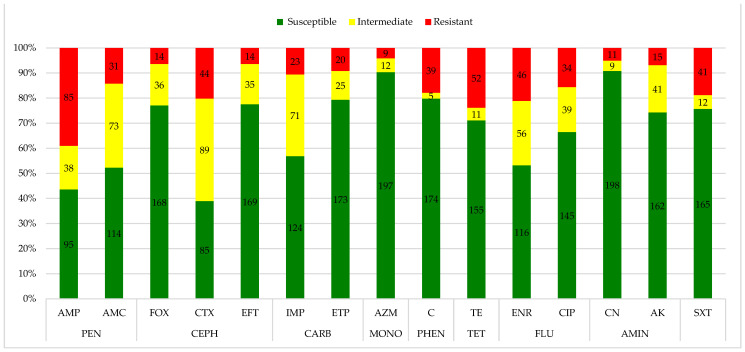
The disk diffusion method on the 218 isolates found relevant resistance rates to ampicillin (38.99%; 95% CI: 33.49–46.49%), tetracycline (23.85%; 95% CI: 18.19–29.51%), and enrofloxacin (21.10%; 95% CI: 15.68–26.52%). The most prevalent susceptibility was observed for gentamicin (90.83%; 95% CI: 87.00–94.66%), aztreonam (90.37%; 95% CI: 86.45–94.29), chloramphenicol (79.82% 95% CI: 74.49–85.15%), ertapenem (79.36%; 95% CI: 73.99–84.73%), ceftiofur (77.52%; 95% CI: 71.98–83.06%), and cefoxitin (77.06%; 95% CI: 71.48–82.64%), while cefotaxime (40.83%; 95% CI: 34.31–47.35%), amoxicillin + clavulanic acid (33.49%; 27.22–39.76%), and imipenem (32.57%; 95% CI: 26.35–38.79%) demonstrated relevant intermediate levels of resistance. Fifty-six (25.68%; 95% CI: 19.88–31.48%) strains were resistant to the penicillins class, 18 (8.25%; 95% CI: 4.60–11.90%) to the cephalosporins class, and 29 (13.30%: 95% CI: 8.79–17.81%) strains were found resistant to both of them, for a total of 103 (47.24%; 95% CI: 40.61–53.87%) strains; while 37 (16.97%; 95% CI: 11.99–21.95%) strains were resistant to the carbapenems class. The results of the agar disk diffusion test are reported in Figure 1 and Table S1.
Figure 1.
Antimicrobial resistance profile of Escherichia coli isolates (n.218) from seagulls. Legend: AMP: ampicillin; AMC: amoxicillin-clavulanate; FOX: cefoxitin; CTX: cefotaxime; EFT: ceftiofur; IMP: imipenem; ETP: ertapenem; AZM: aztreonam; C: chloramphenicol; TE: tetracycline; ENR: enrofloxacin; CIP: ciprofloxacin; CN: gentamicin; AK: amikacin; SXT: trimethoprim-sulfamethoxazole; PEN: penicillins; CEPH: cephalosporins; CARB: carbapenems; MONO: monobactams; PHEN: phenicols; TET: tetracyclines; FLU: fluoroquinolones; AMIN: aminoglycosides.
In relation to the results of the disk diffusion method, it was found that 150 (68.81%; 95% CI: 62.66–74.96%) strains did not fall into any of the resistance classes; among them, 91 (60.66%; 95% CI: 52.84–68.48%) were susceptible to all antimicrobials, 43 (28.66%; 95% CI: 21.42–35.90%) were resistant to at least one antimicrobial of one category, while 16 (10.66%; 95% CI: 5.72–15.60%) were resistant to at least one antimicrobial of two categories (Table 3).
Table 3.
Antimicrobial patterns found with the 150 strains not falling into any of the resistance classes.
| Antimicrobial Pattern | N. of Strains |
|---|---|
| Susceptible to all antimicrobials | 91 |
| CTX | 12 |
| AMP | 8 |
| IMP | 8 |
| ENR | 4 |
| AMP, AMC | 3 |
| AK | 2 |
| ETP | 2 |
| SXT | 2 |
| IMP, ETP | 1 |
| TE | 1 |
| AMP, AMC, FOX, CTX | 2 |
| AMP, ENR | 2 |
| AMP, ENR, CIP | 2 |
| AMP, TE | 2 |
| AMP, AMC, ETP | 1 |
| AMP, CTX | 1 |
| AMP, CTX, EFT | 1 |
| AMP, ETP | 1 |
| AMP, IMP | 1 |
| CTX, ENR, CIP | 1 |
| CTX, IMP, ETP | 1 |
| TE, ENR, CIP | 1 |
Legend. AMP: ampicillin; AMC: amoxicillin-clavulanate; AK: amikacin; CIP: ciprofloxacin; CTX: cefotaxime; EFT: ceftiofur; ENR: enrofloxacin; ETP: ertapenem; FOX: cefoxitin; IMP: imipenem; SXT: trimethoprim-sulfamethoxazole; TE: tetracycline.
Sixty-two (28.44%; 95% CI: 22.45–34.43%) strains were classified as MDR and 6 (2.75%; 95% CI: 0.58–4.92%) as XDR. Table 4 and Table 5 report the antimicrobial patterns for the MDR and XDR strains, respectively.
Table 4.
Antimicrobial patterns found among the 62 MDR strains.
| Antimicrobial Pattern | N. of Strains |
|---|---|
| AMP, AMC, CTX, EFT, ETP, ATM, ENR, CIP, AK | 1 |
| AMP, AMC, CTX, IMP, ETP, ATM, ENR, AK | 1 |
| AMP, AMC, CTX, IMP, C, TE, ENR, CIP | 1 |
| AMP, CTX, EFT, IMP, ETP, ATM, ENR, CIP, AK | 1 |
| AMP, CTX, IMP, C, TE, SXT | 1 |
| AMC, FOX, CTX, EFT, TE, ENR, SXT | 1 |
| AMP, AMC, C, TE, ENR, CIP, SXT | 4 |
| AMP, AMC, CTX, EFT, ATM, C, TE | 1 |
| AMP, AMC, ETP, C, ENR, CIP, SXT | 2 |
| AMP, AMC, FOX, CTX, TE, AK, SXT | 1 |
| AMP, C, TE, ENR, CIP, SXT | 2 |
| AMP, C, TE, ENR, SXT | 1 |
| C, TE, ENR, CIP, CN, SXT | 3 |
| AMP, AMC, C, ENR, CIP, SXT | 1 |
| AMP, AMC, C, TE, ENR, CIP | 1 |
| AMP, AMC, C, TE, SXT | 1 |
| AMP, AMC, CTX, EFT, IMP, SXT | 1 |
| AMP, AMC, CTX, EFT, TE, SXT | 1 |
| AMP, AMC, CTX, IMP, ENR | 1 |
| AMP, AMC, FOX, CTX, EFT, ATM, TE | 1 |
| AMP, AMC, TE, ENR, CIP, SXT | 2 |
| AMP, C, TE, ENR | 1 |
| AMP, C, TE, ENR, CIP | 4 * |
| AMP, C, TE, SXT | 6 * |
| AMP, CTX, EFT, IMP, AK | 2 ** |
| AMP, CTX, IMP, AK | 1 |
| FOX, CTX, EFT, ETP, ATM, CN, AK | 1 |
| FOX, CTX, ETP, ATM, CN, AK | 1 |
| FOX, ETP, ATM, CN, AK | 1 |
| AMP, AMC, CN, SXT | 1 |
| AMP, AMC, FOX, SXT | 1 ** |
| AMP, AMC, TE, ENR, CIP | 1 |
| AMP, C, TE | 4 |
| AMP, CTX, ETP | 2 |
| AMP, CTX, TE | 1 |
| AMP, ENR, CIP, SXT | 1 |
| AMP, TE, ENR | 1 |
| AMP, TE, SXT | 3 |
| FOX, CTX, IMP, ETP, AK | 1 |
Legend. AMP: ampicillin; AMC: amoxicillin-clavulanate; AK: amikacin; ATM: aztreonam; C: chloramphenicol; CIP: ciprofloxacin; CN: gentamicin; CTX: cefotaxime; EFT: ceftiofur; ENR: enrofloxacin; ETP: ertapenem; FOX: cefoxitin; IMP: imipenem; SXT: trimethoprim-sulfamethoxazole; TE: tetracycline; *: 2 EAEC; **: 1 EAEC.
Table 5.
Antimicrobial patterns found among the 6 XDR strains.
| Antimicrobial Pattern | N. of Strains |
|---|---|
| AMP, FOX, CTX, EFT, ETP, ATM, C, TE, ENR, CIP, CN, AK, SXT | 1 |
| AMP, FOX, CTX, IMP, ETP, C, TE, ENR, CIP, AK, SXT | 1 |
| AMP, AMC, FOX, C, TE, ENR, CIP, CN, SXT | 1 |
| AMP, AMC, FOX, CTX, ETP, C, TE, ENR, CIP, SXT | 1 |
| AMP, CTX, EFT, C, TE, ENR, CIP, CN, SXT | 2 |
Legend. AMP: ampicillin; AMC: amoxicillin-clavulanate; AK: amikacin; ATM: aztreonam; C: chloramphenicol; CIP: ciprofloxacin; CN: gentamicin; CTX: cefotaxime; EFT: ceftiofur; ENR: enrofloxacin; ETP: ertapenem; FOX: cefoxitin; IMP: imipenem; SXT: trimethoprim-sulfamethoxazole; TE: tetracycline.
3.3. Genotypic Resistance
Molecular analyses conducted on strains phenotypically resistant to carbapenems, cephalosporins, and penicillins found 9/37 (24.32%; 95% CI: 10.50–38.14%) strains positive for blaOXA-48, 52/103 (50.49%; 95% CI: 40.83–60.15%) for blaTEM, 12/103 (11.65%; 95% CI: 5.45–17.85%) for blaCMY2, 3/103 (2.91%; 95% CI: 0.00–6.16%) for blaCTX, and 1/103 (0.97%; 95% CI: 0.00–2.86%) for blaSHV (Table 6). On the basis of these results, 9 isolates having only the blaCMY2 gene were identified as AmpC β-lactamases producers; 9 with only the blaOXA-48 gene were classified as carbapenemases producers; and 54 isolates having blaTEM and/or blaCTX and or blaSHV alone or in combination were identified as ESBLs.
Table 6.
Antimicrobial resistance genes in Escherichia coli isolates from seagulls.
| Genes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| bla NDM | bla KPC | bla OXA-48 | bla VIM | bla IMP | bla CMY1 | bla CMY2 | bla SHV | bla CTX | bla TEM | |
| No. positive isolates | 0 | 0 | 9 (24.32%) | 0 | 0 | 0 | 12 (11.65%) |
1 (0.97%) |
3 (2.91%) |
52 (50.49%) |
| No. tested isolates | 37 | 37 | 37 | 37 | 37 | 103 | 103 | 103 | 103 | 103 |
Sequencing analyses of the amplicons showed 100% homology with the correspondent sequence reported in GenBank and therefore confirmed the positive results.
3.4. Virulence Factors
Molecular analyses to detect genes coding for the virulence factors characterizing different pathotypes found the astA gene in 40/218 (18.35%; 95% CI:13.21–23.49%) isolates, potentially indicative of the EAEC pathotype. Additionally, 4/218 (1.83%; 95% CI: 0.05–3.61%) isolates had both eaeA and escV, potentially placing the strains within the EPEC pathotype. Moreover, 2/218 (0.92%; 95% CI: 0.00–2.19%) isolates tested positive for cnf1, 2/218 (0.92%; 95% CI: 0.00–2.19%) for cnf2, and 1/218 (0.46%; 95% CI: 0.00–1.36%) for cdt-IV, for a total of 5 (2.29%; 95% CI: 0.30–4.28%) potential NTEC strains. Among the strains classified as belonging to the investigated pathotypes, only 6 EAEC isolates were MDR.
Sequencing analyses of the amplicons showed 100% homology with the correspondent sequence reported in GenBank and therefore confirmed the positive results.
4. Discussion
The results obtained in the present survey show that seagulls may disperse through their droppings E. coli strains reportable as belonging to different pathotypes able to determine disease both in animals and humans. Seagulls fly long distances, reaching not only coastal zones, but also urban, peri-urban, and farm areas where they find human and animal waste from which they may acquire different pathogens. On the other hand, they can transport these microorganisms to additional areas, including beaches, acting as sources of infection.
E. coli is known to be ubiquitous and it has been frequently found in seabirds. However, few studies have investigated the occurrence of E. coli pathotypes in avifauna including gulls. Recently, Cardoso et al. [32], in Brazil, detected virulence genes of the EAEC, ETEC, or EPEC pathotypes in 30% of the identified strains, the first two described in seabirds for the first time. EPEC were previously reported in seagulls and other seabirds in the USA [33], and in wild birds, but not gulls, in Japan [34]. A survey carried out in wild birds of different species sampled in Italy evaluated the presence of virulence genes directly in birds’ feces; one or more virulence genes belonging to EPEC, EHEC, and STEC were found in 21/121 birds; 3 and 5 yellow-legged seagulls (L. michaehellis) had stx1 and eaeA genes, respectively [35]. Sanches et al. [2] in Brazil isolated 401 E. coli strains from 516 wild birds and molecular analyses detected EPEC (2.99%) and STEC (0.74%). Similarly, Borges et al. [36] identified STEC (0.8%) and EPEC (2.0%) in feces of 123 free living wild birds from Brazil. No strains isolated in our survey were positive for stx1 and/or stx2 genes, suggesting that the examined gulls were not shedders of STEC strains. This result is in agreement with previous studies that did not detect STEC in feces sampled from gulls and other wild birds [34,37]. Conversely, other surveys found STEC in feces of wild birds belonging to different species, although with low rates [2,35,36,38,39,40,41,42]. Among the isolates of our study, 18.35% had the astA gene reportable as an EAEC strain, and 1.83% had the eaeA and escV genes typical of EPEC. These findings confirm wild avifauna, in particular seagulls, as possible spreaders of EAEC and EPEC.
All these pathotypes are relevant in human medicine. EPEC causes the loss of intestinal microvillus and induces a high child mortality rate, mainly in developing countries [42]. The EAEC pathotype is known as a major cause of acute and persistent diarrhea, and death among children in developing countries, but it is a cause of sporadic diarrhea and a common cause of traveler’s diarrhea, as well [43].
A relevant result obtained in the present study is the 2.29% (5/218) of strains with cnf1, cnf2, or cdt-IV genes coding for the typical virulence factors of the NTEC pathotype. To the best of our knowledge, data about the occurrence of NTEC in wild avifauna are not available in literature; therefore, this is the first report suggesting that gulls can harbor this pathotype, as well. NTEC strains were reported for the first time in neonatal enteritis [44]; in humans, these are associated with dysenteric syndrome, but it is also frequently involved in extra-intestinal infections, such as urinary tract infections [45]. Some investigations reported the presence of NTEC in mammals: NTEC1 strains have been isolated from ruminants, pigs, horses, dogs, and rabbits with enteritis and from pigs, dogs [46], and cats [47] with extra-intestinal infections; NTEC2 strains have been mainly cultured from ruminants with septicemia or intestinal infections [48,49,50,51,52].
Although the antimicrobial resistance rates were not very high, our study provides evidence that seagulls may contribute to the dissemination of E. coli strains characterized by resistance to different antimicrobials. The highest percentages of strains were resistant to ampicillin (38.99%), tetracycline (23.85%) and enrofloxacin (21.10%). These values are quite in accordance with the results of other surveys that evaluated the antimicrobial resistance of E. coli isolated from seagulls’ feces. Ahmed et al. [21] found 28%, 32%, and 24% of isolates were resistant to ampicillin, tetracyclines, and enrofloxacin, respectively, in Turkey. In Alaska, 27% of E. coli isolates were resistant to ampicillin and 44% to tetracyclines [53]. Moreover, our results are in line with research conducted on seagulls in other European areas. In a study conducted in the Czech Republic, 19.1% (49/257) of a total of 257 E. coli strains were found to be resistant to tetracycline and 11.7% (30/257) to ampicillin [54]. Resistance rates of 35% for tetracycline and 34% for amoxicillin were found in E. coli isolates from 179 seagull fecal samples in Portugal [55].
High percentages of resistance were found for ampicillin (72.22%), tetracycline (44.44%), and enrofloxacin (38.88%) in E. coli isolated from storks and seagulls in Central Spain [18]. In addition, Zendri et al. [56] found 100% resistance to ampicillin and 56.8% to tetracycline in E. coli from seagull feces in the UK. Furthermore, a survey conducted on E. coli isolated from seagull feces collected from nine different European countries (Denmark, England, Ireland, Latvia, Netherland, Poland, Portugal, Spain, Sweden) identified overall prevalence rates of 19.0% and 18.01% resistance to tetracycline and ampicillin, respectively [57]. Ampicillin, tetracycline, and enrofloxacin are among the most widely used antibiotics in both human and veterinary medicine in Europe, including Italy [58,59]. Ampicillin is commonly prescribed in human medicine for common bacterial infections, while tetracycline and enrofloxacin are widely used in veterinary medicine to treat bacterial infections in companion and farm animals [60,61].
Molecular analyses of our study revealed a high prevalence of the blaTEM gene (50.49%) in agreement with previous surveys. Dolejska et al. [54] found this gene in 29 of 30 E. coli isolates resistant to beta-lactam antibiotics in the Czech Republic. Poeta et al. [62] found 8/11 strains positive for blaTEM-52 gene in Berlengas Island (Portugal) and, in the same area, Alves et al. [55] found that blaTEM was the most prevalent gene in isolates from seagull feces (38% of 68 isolates resistant to penicillins). This gene encodes the TEM β-lactamase. This enzyme was originally linked to penicillin resistance, but over time, its variants have evolved into extended spectrum β-lactamases (ESBLs), enabling them to evade a broader range of β-lactam antibiotics, including cephalosporins and monobactams [63,64].
The blaCTX gene was found at a lower percentage (2.91%) than in E. coli from seagull feces from Porto (Portugal) beaches [20], where 98% of the 45 ESBL producers carried the blaCTX-M gene, and lower than the 41.6% (30/72) of E. coli isolated from gulls in Barcelona (Spain) [65]. Zendri et al. [56] found the blaCTX-M gene in 21/60 (35%) of the extended-spectrum cephalosporin-resistant E. coli strains isolated from seagulls in the UK. The blaCTX gene is responsible for the production of CTX-M β-lactamase, an ESBL particularly effective against cephalosporins, such as cefotaxime, contributing to the widespread antibiotic resistance in clinical bacteria [63].
Regarding the blaCMY-2 gene, this is the most common plasmid-mediated AmpC β-lactamase in Enterobacterales and confers resistance to cephalosporins, penicillins, and combinations of antibiotics with β-lactamase inhibitors [66,67]. In our study, it was detected in 11.65% (12/103) of the analyzed strains, while Vergara et al. [65] found it in 2.8% (2/72) of E. coli strains isolated from gulls in Spain. Poirel et al. [68] reported a high percentage (29%) of blaCMY-2 positive strains among resistant E. coli isolated from wild seagull feces in Miami Beach (USA).
Only one (0.97%, 1/103) of our strains was found positive for blaSHV, less than the 52.8% (38/72) detected in Spain [66]. The blaSHV gene encodes SHV β-lactamase, an ESBL that destroys penicillins and broad-spectrum cephalosporins, commonly found in nosocomial infections [63]. SHV β-lactamases currently encompass a large number of allelic variants including extended-spectrum β-lactamases (ESBL), which are the majority, non-ESBL strains and several not classified variants [69].
The blaOXA-48 gene was found in 24.32% (9/37) of the carbapenems-resistant strains. Poeta et al. [62] found 1/11 resistant strain having the blaOXA-1 gene from seagulls of Berlengas Island. In contrast, Alves et al. [55] and Dolejska et al. [54] found no strain having the blaOXA gene among 157 and 30 resistant E. coli isolates studied in Portugal and the Czech Republic, respectively. The blaOXA-48 gene encodes the enzyme OXA-48, a carbapenemase that makes bacteria resistant to carbapenems, which are used as antibiotics of last resort. Carbapenems are used as last-resort antibiotics to treat severe infections caused by MDR bacteria when other treatments have failed, owing to their limited vulnerability to most beta-lactam resistance determinants [68,70].
Finally, analyses of the distribution of resistance classes identified 28.44% of isolates as MDR and 2.75% as XDR. These results are similar to those found in other seagull populations, where the prevalence of MDR strains was 29% [21]. Similar values were also found by Martín-Maldonado et al. [18], who detected 63.2% (12/19) of the tested seagulls had antimicrobial-resistant E. coli strains, and four (30%) of them were considered MDR.
Our study could have some limitations. The first one concerns the analyzed samples. In fact, the number of fecal specimens was not high; however, only seagulls that had not received any antimicrobial treatment and had been recovered for no more than 48 h were selected. A further limit could be related to the health status of the gulls involved in the study; birds brought to rehabilitation centers usually are not in good health and therefore they are more susceptible to acquiring pathogens; however, the short time between the birds arriving and the sampling time should reduce this risk. A concern could also regard the antimicrobial susceptibility results, which might not reflect the real scenario, because the Kirby–Bauer method might be less sensitive than the broth microdilution test. In addition, molecular analyses to detect other antimicrobial resistance genes may be useful to better understand the role of E. coli strains as potential donors of these genes. These analyses should include the genes related to the detected resistance, as well as genes that may not been expressed.
5. Conclusions
The results obtained in this study showed that seagulls often harbor E. coli strains belonging to pathotypes responsible for diseases in humans and animals. Yellow-legged seagulls seem to be also involved in the epidemiology of the NTEC strains, which had never been detected in wild birds. Moreover, many E. coli strains isolated in this study were MDR and XDR and other isolates, even if not classified in these groups, were characterized by multiple antimicrobial resistances. The finding of resistance genes highlighted the additional issue related to the possibility that E. coli strains act as donors of these genes to other bacteria, contributing to the amplification of the antimicrobial resistance. Considering that seagulls are free-living wild birds not submitted to antimicrobial treatments, the detection of antimicrobial-resistant bacteria, including important pathogens, shows that they easily acquire bacteria from contaminated environments. Similarly, they can significantly contribute to the dissemination of pathogenic antimicrobial-resistant bacteria.
Therefore, targeted interventions, including improved waste management practices and public awareness campaigns, are essential to reduce seagulls’ access to areas where humans and other animals reside.
Seagulls could be used as effective indicators for monitoring the dissemination of different E. coli pathotypes and for studying old and new antimicrobial-resistances.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ani14213048/s1, Table S1: Antimicrobial resistance profile of Escherichia coli isolates (n.218) from seagulls.
Author Contributions
Conceptualization, V.V.E.; methodology, G.C., F.B., R.C. and V.V.E.; formal analysis, G.C., F.B. and V.V.E.; writing—original draft preparation, G.C., F.B. and V.V.E.; writing—review and editing, V.V.E. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study because no samples were collected directly from the animals involved in the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are reported in the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by University of Pisa, Fondi Ateneo 2022.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wasiński B. Extra-intestinal pathogenic Escherichia coli-threat connected with food-borne infections. Ann. Agric. Environ. Med. 2019;26:532–537. doi: 10.26444/aaem/111724. [DOI] [PubMed] [Google Scholar]
- 2.Sanches L.A., Gomes M.D.S., Teixeira R.H.F., Cunha M.P.V., Oliveira M.G.X., Vieira M.A.M., Gomes T.A.T., Knobl T. Captive wild birds as reservoirs of enteropathogenic E. coli (EPEC) and Shiga-toxin producing E. coli (STEC) Braz. J. Microbiol. 2017;48:760–763. doi: 10.1016/j.bjm.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arenas-Hernandez M.M., Martınez-Laguna Y., Torres A.G. Clinical implications of enteroadherent Escherichia coli. Curr. Gastroenterol. Rep. 2012;14:386–394. doi: 10.1007/s11894-012-0277-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Müller D., Greune L., Heusipp G., Karch H., Fruth A., Tschäpe H., Schmidt M.A. Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR. Appl. Environ. Microbiol. 2007;73:3380–3390. doi: 10.1128/AEM.02855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tozzoli R., Grande L., Michelacci V., Ranieri P., Maugliani A., Caprioli A., Morabito S. Shiga toxin-converting phages and the emergence of new pathogenic Escherichia coli: A world in motion. Front. Cell. Infect. Microbiol. 2014;4:80. doi: 10.3389/fcimb.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahamtan Y., Hayati M., Namavari M. Prevalence and distribution of the stx1, stx2 genes in Shiga toxin producing E. coli (STEC) isolates from cattle. Iran. J. Microbiol. 2010;2:8–13. [PMC free article] [PubMed] [Google Scholar]
- 7.Melton-Celsa A.R. Shiga Toxin (Stx) Classification, Structure, and Function. Microbiol. Spectr. 2014;2:4. doi: 10.1128/microbiolspec.EHEC-0024-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bielaszewska M., Rüter C., Kunsmann L., Greune L., Bauwens A., Zhang W., Kuczius T., Sik Kim K., Mellmann A., Schmidt M.A., et al. Enterohemorrhagic Escherichia coli Hemolysin Employs Outer Membrane Vesicles to Target Mitochondria and Cause Endothelial and Epithelial Apoptosis. PLoS Pathog. 2013;9:e1003797. doi: 10.1371/journal.ppat.1003797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruyand M., Mariani-Kurkdjian P., Gouali M., de Valk H., King L.A., Le Hello S., Bonacorsi S., Loirat C. Hemolytic uremic syndrome due to Shiga toxin-producing Escherichia coli infection. Med. Mal. Infect. 2018;48:167–174. doi: 10.1016/j.medmal.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Scuron M.D., Boesze-Battaglia K., Dlakic M., Shenker B.J. The cytolethal distending toxin contributes to microbial virulence and disease pathogenesis by acting as a tri-perditious toxin. Front. Cell. Infect. Microbiol. 2016;6:168. doi: 10.3389/fcimb.2016.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sobhy N.M., Yousef S.G.A., Aboubakr H.A., Nisar M., Nagaraja K.V., Mor S.K., Valeris-Chacin R.J., Goyal S.M. Virulence factors and antibiograms of Escherichia coli isolated from diarrheic calves of Egyptian cattle and water buffaloes. PLoS ONE. 2020;15:e0232890. doi: 10.1371/journal.pone.0232890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antimicrobial Resistance Collaborators Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet. 2022;399:629–655. doi: 10.1016/S0140-6736(21)02724-0. Erratum in Lancet 2022, 400, 1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuentes-Castillo D., Castro-Tardón D., Esposito F., Neves I., Rodrigues L., Fontana H., Fuga B., Catão-Dias J.L., Lincopan N. Genomic evidences of gulls as reservoirs of critical priority CTX-M-producing Escherichia coli in Corcovado Gulf, Patagonia. Sci. Total Environ. 2023;874:162564. doi: 10.1016/j.scitotenv.2023.162564. [DOI] [PubMed] [Google Scholar]
- 14.Bonnedahl J., Drobni M., Gauthier-Clerc M., Hernandez J., Granholm S., Kayser Y., Melhus A., Kahlmeter G., Waldenström J., Johansson A., et al. Dissemination of Escherichia coli with CTX-M type ESBL between humans and yellow-legged gulls in the south of France. PLoS ONE. 2009;4:e5958. doi: 10.1371/journal.pone.0005958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolejská M., Bierosová B., Kohoutová L., Literák I., Cízek A. Antibiotic-resistant Salmonella and Escherichia coli isolates with integrons and extended-spectrum beta-lactamases in surface water and sympatric black-headed gulls. J. Appl. Microbiol. 2009;106:1941–1950. doi: 10.1111/j.1365-2672.2009.04155.x. [DOI] [PubMed] [Google Scholar]
- 16.Ebani V.V., Guardone L., Bertelloni F., Perrucci S., Poli A., Mancianti F. Survey on the Presence of Bacterial and Parasitic Zoonotic Agents in the Feces of Wild Birds. Vet. Sci. 2021;8:171. doi: 10.3390/vetsci8090171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cagnoli G., Bertelloni F., Interrante P., Ceccherelli R., Marzoni M., Ebani V.V. Antimicrobial-Resistant Enterococcus spp. in Wild Avifauna from Central Italy. Antibiotics. 2022;11:852. doi: 10.3390/antibiotics11070852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martín-Maldonado B., Rodríguez-Alcázar P., Fernández-Novo A., González F., Pastor N., López I., Suárez L., Moraleda V., Aranaz A. Urban Birds as Antimicrobial Resistance Sentinels: White Storks Showed Higher Multidrug-Resistant Escherichia coli Levels Than Seagulls in Central Spain. Animals. 2022;12:2714. doi: 10.3390/ani12192714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alm E.W., Daniels-Witt Q.R., Learman D.R., Ryu H., Jordan D.W., Gehring T.M., Santo Domingo J. Potential for gulls to transport bacteria from human waste sites to beaches. Sci. Total Environ. 2018;615:123–130. doi: 10.1016/j.scitotenv.2017.09.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simões R.R., Poirel L., Da Costa P.M., Nordmann P. Seagulls and beaches as reservoirs for multidrug-resistant Escherichia coli. Emerg. Infect. Dis. 2010;16:110–112. doi: 10.3201/eid1601.090896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ahmed N.A., Gulhan T. Determination of antibiotic resistance patterns and genotypes of Escherichia coli isolated from wild birds. Microbiome. 2024;12:8. doi: 10.1186/s40168-023-01729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erika E., Scarpellini R., Celli G., Marliani G., Zaghini A., Mondo E., Rossi G., Piva S. Wild birds as potential bioindicators of environmental antimicrobial resistance: A preliminary investigation. Res. Vet. Sci. 2024;180:105424. doi: 10.1016/j.rvsc.2024.105424. [DOI] [PubMed] [Google Scholar]
- 23.CLSI (Clinical and Laboratory Standards Institute) M02-A12—Performance Standards for Antimicrobial Disk Susceptibility Tests. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2015. pp. 1–96. Approved Standard—Twelfth Edition. [Google Scholar]
- 24.CLSI (Clinical and Laboratory Standards Institute) 33rd ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2023. Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100. [Google Scholar]
- 25.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen J., Griffiths M.W. PCR differentiation of Escherichia coli from other gram-negative bacteria using primers derived from the nucleotide sequences flanking the gene encoding the universal stress protein. Lett. Appl. Microbiol. 1998;27:369–371. doi: 10.1046/j.1472-765X.1998.00445.x. [DOI] [PubMed] [Google Scholar]
- 27.Poirel L., Walsh T.R., Cuvillier V., Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011;70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 28.Dahshan H., Shahada F., Chuma T., Moriki H., Okamoto K. Genetic analysis of multidrug-resistant Salmonella enterica serovars Stanley and Typhimurium from cattle. Vet. Microbiol. 2010;145:76–83. doi: 10.1016/j.vetmic.2010.02.035. [DOI] [PubMed] [Google Scholar]
- 29.Hasman H., Mevius D., Veldman K., Olesen I., Aarestrup F.M. β-Lactamases among extended-spectrum β-lactamase (ESBL)-resistant Salmonella from poultry, poultry products and human patients in The Netherlands. J. Antimicrob. Chemother. 2005;56:115–121. doi: 10.1093/jac/dki190. [DOI] [PubMed] [Google Scholar]
- 30.Paton A.W., Paton J.C. Direct detection and characterization of Shiga toxigenic Escherichia coli by multiplex PCR for stx1, stx2, eae, ehxA, and saa. J. Clin. Microbiol. 2002;40:271–274. doi: 10.1128/JCM.40.1.271-274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Borriello G., Lucibelli M.G., De Carlo E., Auriemma C., Cozza D., Ascione G., Scognamiglio F., Iovane G., Galiero G. Characterization of enterotoxigenic E. coli (ETEC), Shiga-toxin producing E. coli (STEC) and necrotoxigenic E. coli (NTEC) isolated from diarrhoeic Mediterranean water buffalo calves (Bubalus bubalis) Res. Vet. Sci. 2012;93:18–22. doi: 10.1016/j.rvsc.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cardoso M.D., Gonçalves V.D., Grael A.S., Pedroso V.M., Pires J.R., Travassos C.E.P.F., Domit C., Vieira-Da-Motta O., Dos Prazeres Rodrigues D., Siciliano S. Detection of Escherichia coli and other Enterobacteriales members in seabirds sampled along the Brazilian coast. Prev. Vet. Med. 2023;218:105978. doi: 10.1016/j.prevetmed.2023.105978. [DOI] [PubMed] [Google Scholar]
- 33.Steele C.M., Brown R.N., Botzler R.G. Prevalences of zoonotic bacteria among seabirds in rehabilitation centers along the Pacific Coast of California and Washington, USA. J. Wildl. Dis. 2005;41:735–744. doi: 10.7589/0090-3558-41.4.735. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi H., Kanazaki M., Hata E., Kubo M. Prevalence and characteristics of eae- and stx-positive strains of Escherichia coli from wild birds in the immediate environment of Tokyo Bay. Appl. Environ. Microbiol. 2009;75:292–295. doi: 10.1128/AEM.01534-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertelloni F., Lunardo E., Rocchigiani G., Ceccherelli R., Ebani V.V. Occurrence of Escherichia coli virulence genes in feces of wild birds from Central Italy. Asian Pac. J. Trop. Med. 2019;12:142–146. doi: 10.4103/1995-7645.254941. [DOI] [Google Scholar]
- 36.Borges C.A., Cardozo M.V., Beraldo L.G., Oliveira E.S., Maluta R.P., Barboza K.B., Werther K., Ávila F.A. Wild birds and urban pigeons as reservoirs for diarrheagenic Escherichia coli with zoonotic potential. J. Microbiol. 2017;5:344–348. doi: 10.1007/s12275-017-6523-3. [DOI] [PubMed] [Google Scholar]
- 37.Wani S.A., Samanta I., Bhat M.A., Nishikawa Y. Investigation of shiga toxin-producing Escherichia coli in avian species in India. Lett. Appl. Microbiol. 2004;39:389–394. doi: 10.1111/j.1472-765X.2004.01586.x. [DOI] [PubMed] [Google Scholar]
- 38.Silva V.L., Nicoli J.R., Nascimento T.C., Diniz C.G. Diarrheagenic Escherichia coli strains recovered from urban pigeons (Columba livia) in Brazil and their antimicrobial susceptibility patterns. Curr. Microbiol. 2009;59:302–308. doi: 10.1007/s00284-009-9434-7. [DOI] [PubMed] [Google Scholar]
- 39.Hughes L.A., Bennett M., Coffey P., Elliott J., Jones T.R., Jones R.C., Lahuerta-Marin A., McNiffe K., Norman D., Williams N.J., et al. Risk factors for the occurrence of Escherichia coli virulence genes eae, stx1 and stx2 in wild bird populations. Epidemiol. Infect. 2009;137:1574–1582. doi: 10.1017/S0950268809002507. [DOI] [PubMed] [Google Scholar]
- 40.Caballero M., Rivera I., Jara L.M., Ulloa-Stanojlovic F.M., Shiva C. Isolation and molecular identification of potentially pathogenic Escherichia coli and Campylobacter jejuni in feral pigeons from an urban area in the city of lima, Peru. Rev. Inst. Med. Trop. Sao Paulo. 2015;57:393–396. doi: 10.1590/S0036-46652015000500004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koochakzadeh A., Askari Badouei M., Zahraei Salehi T., Aghasharif S., Soltani M., Ehsan M. Prevalence of shiga toxin-producing and enteropathogenic Escherichia coli in wild and pet birds in Iran. Rev. Bras. Cienc. Avic. 2015;17:445–450. doi: 10.1590/1516-635X1704445-450. [DOI] [Google Scholar]
- 42.Kobuszewska A., Wysok B. Pathogenic Bacteria in Free-Living Birds, and Its Public Health Significance. Animals. 2024;14:968. doi: 10.3390/ani14060968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajan A., Robertson M.J., Carter H.E., Poole N.M., Clark J.R., Green S.I., Criss Z.K., Zhao B., Karandikar U., Xing Y., et al. Enteroaggregative E. coli Adherence to Human Heparan Sulfate Proteoglycans Drives Segment and Host Specific Responses to Infection. PLoS Pathog. 2020;16:e1008851. doi: 10.1371/journal.ppat.1008851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caprioli A., Falbo V., Roda L.G., Ruggeri F.M., Zona C. Partial purification and characterization of an Escherichia coli toxic factor that induces morphological cell alterations. Infect. Immun. 1983;39:1300–1306. doi: 10.1128/iai.39.3.1300-1306.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rahman H., Deka M. Detection & characterization of necrotoxin producing Escherichia coli (NTEC) from patients with urinary tract infection (UTI) Indian J. Med. Res. 2014;139:632–637. [PMC free article] [PubMed] [Google Scholar]
- 46.Starcic M., Johnson J.R., Stell A.L., van der Goot J., Hendriks H.G., van Vorstenbosch C., van Dijk L., Gaastra W. Haemolytic Escherichia coli isolated from dogs with diarrhea have characteristics of both uropathogenic and necrotoxigenic strains. Vet. Microbiol. 2002;85:361–377. doi: 10.1016/S0378-1135(02)00003-2. [DOI] [PubMed] [Google Scholar]
- 47.Sura R., Van Kruiningen H.J., DebRoy C., Hinckley L.S., Greenberg K.J., Gordon Z., French R.A. Extraintestinal pathogenic Escherichia coli-induced acute necrotizing pneumonia in cats. Zoonoses Public Health. 2007;54:307–313. doi: 10.1111/j.1863-2378.2007.01067.x. [DOI] [PubMed] [Google Scholar]
- 48.Cid D., Blanco M., Blanco J.E., Ruiz Santa Quiteria J.A., De La Fuente R., Blanco J. Serogroups, toxins and antibiotic resistance of Escherichia coli strains isolated from diarrheic goat kids in Spain. Vet. Microbiol. 1996;53:349–353. doi: 10.1016/S0378-1135(96)01222-9. [DOI] [PubMed] [Google Scholar]
- 49.Blanco J.E., Blanco M., Blanco J., Mora A., Balaguer L., Mourino M., Juarez A., Jansen W.H. O serogroups, biotypes and eae genes in Escherichia coli strains isolated from diarrheic and healthy rabbits. J. Clin. Microbiol. 1996;34:3101–3107. doi: 10.1128/jcm.34.12.3101-3107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pohl P., Imberechts H., Marin M., Schlicker C., Stockmans F. Prévalence des gènes codant pour les cytotoxines nécrosantes (CNF1 et CNF2) chez des Escherichia coli isolées de bovins malades ou asymptomatiques. Ann. Med. Vet. 1997;141:161–164. [Google Scholar]
- 51.Van Bost S., Roles S., Mainil J. Necrotoxigenic Escherichia coli type-2 invade and cause diarrhoea during experimental infection in colostrum-restricted newborn calves. Vet. Microbiol. 2001;81:315–329. doi: 10.1016/S0378-1135(01)00360-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khawaskar D.P., Sinha D.K., Lalrinzuala M.V., Athira V., Kumar M., Chhakchhuak L., Mohanapriya K., Sophia I., Abhishek, Kumar O.R.V. Pathotyping and antimicrobial susceptibility testing of Escherichia coli isolates from neonatal calves. Vet. Res. Commun. 2022;46:353–362. doi: 10.1007/s11259-021-09857-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atterby C., Ramey A.M., Hall G.G., Järhult J., Börjesson S., Bonnedahl J. Increased prevalence of antibiotic-resistant E. coli in gulls sampled in Southcentral Alaska is associated with urban environments. Infect. Ecol. Epidemiol. 2016;6:32334. doi: 10.3402/iee.v6.32334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dolejska M., Cizek A., Literak I. High prevalence of antimicrobial-resistant genes and integrons in Escherichia coli isolates from Black-headed Gulls in the Czech Republic. J. Appl. Microbiol. 2007;103:11–19. doi: 10.1111/j.1365-2672.2006.03241.x. [DOI] [PubMed] [Google Scholar]
- 55.Alves M.S., Pereira A., Araújo S.M., Castro B.B., Correia A.C., Henriques I. Seawater is a reservoir of multi-resistant Escherichia coli, including strains hosting plasmid-mediated quinolones resistance and extended-spectrum beta-lactamases genes. Front. Microbiol. 2014;5:426. doi: 10.3389/fmicb.2014.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zendri F., Maciuca I.E., Moon S., Jones P.H., Wattret A., Jenkins R., Baxter A., Timofte D. Occurrence of ESBL-Producing Escherichia coli ST131, Including the H30-Rx and C1-M27 Subclones, Among Urban Seagulls from the United Kingdom. Microb. Drug Resist. 2020;26:697–708. doi: 10.1089/mdr.2019.0351. [DOI] [PubMed] [Google Scholar]
- 57.Stedt J., Bonnedahl J., Hernandez J., McMahon B.J., Hasan B., Olsen B., Drobni M., Waldenström J. Antibiotic resistance patterns in Escherichia coli from gulls in nine European countries. Infect. Ecol. Epidemiol. 2014;4:21565. doi: 10.3402/iee.v4.21565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.European Centre for Disease Prevention and Control (ECDC) Surveillance of Antimicrobial Resistance in Europe 2020. 2021. [(accessed on 25 March 2024)]. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2020.
- 59.European Medicines Agency (EMA) Sales of Veterinary Antimicrobial Agents in 31 European Countries 2022 Trends (2010–2022) 2022. [(accessed on 25 March 2024)]. Available online: https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2022-trends-2010-2022-thirteenth-esvac-report_en.pdf.
- 60.Grabowski Ł., Gaffke L., Pierzynowska K., Cyske Z., Choszcz M., Węgrzyn G., Węgrzyn A. Enrofloxacin-The Ruthless Killer of Eukaryotic Cells or the Last Hope in the Fight against Bacterial Infections? Int. J. Mol. Sci. 2022;23:3648. doi: 10.3390/ijms23073648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caneschi A., Bardhi A., Barbarossa A., Zaghini A. The Use of Antibiotics and Antimicrobial Resistance in Veterinary Medicine, a Complex Phenomenon: A Narrative Review. Antibiotics. 2023;12:487. doi: 10.3390/antibiotics12030487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Poeta P., Radhouani H., Igrejas G., Gonçalves A., Carvalho C., Rodrigues J., Vinué L., Somalo S., Torres C. Seagulls of the Berlengas natural reserve of Portugal as carriers of fecal Escherichia coli harboring CTX-M and TEM extended-spectrum beta-lactamases. Appl. Environ. Microbiol. 2008;74:7439–7441. doi: 10.1128/AEM.00949-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Paterson D.L., Bonomo R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pfeifer Y., Cullik A., Witte W. Resistance to cephalosporins and carbapenems in Gram-negative bacterial pathogens. Int. J. Med. Microbiol. 2010;300:371–379. doi: 10.1016/j.ijmm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 65.Vergara A., Pitart C., Montalvo T., Roca I., Sabaté S., Hurtado J.C., Planell R., Marco F., Ramírez B., Peracho V., et al. Prevalence of Extended-Spectrum-β-Lactamase- and/or Carbapenemase-Producing Escherichia coli Isolated from Yellow-Legged Gulls from Barcelona, Spain. Antimicrob. Agents Chemother. 2017;61:e02071-16. doi: 10.1128/AAC.02071-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Denisuik A.J., Lagacé-Wiens P.R., Pitout J.D., Mulvey M.R., Simner P.J., Tailor F., Karlowsky J.A., Hoban D.J., Adam H.J., Zhanel G.G., et al. Molecular epidemiology of extended-spectrum β-lactamase-, AmpC β-lactamase- and carbapenemase-producing Escherichia coli and Klebsiella pneumoniae isolated from Canadian hospitals over a 5 year period: CANWARD 2007-11. J. Antimicrob. Chemother. 2013;68((Suppl. 1)):i57–i65. doi: 10.1093/jac/dkt027. [DOI] [PubMed] [Google Scholar]
- 67.Meini S., Tascini C., Cei M., Sozio E., Rossolini G.M. AmpC β-lactamase-producing Enterobacterales: What a clinician should know. Infection. 2019;47:363–375. doi: 10.1007/s15010-019-01291-9. [DOI] [PubMed] [Google Scholar]
- 68.Poirel L., Potron A., De La Cuesta C., Cleary T., Nordmann P., Munoz-Price L.S. Wild coastline birds as reservoirs of broad-spectrum-β-lactamase-producing Enterobacteriaceae in Miami Beach, Florida. Antimicrob. Agents Chemother. 2012;56:2756–2758. doi: 10.1128/AAC.05982-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liakopoulos A., Mevius D., Ceccarelli D. A Review of SHV Extended-Spectrum β-Lactamases: Neglected Yet Ubiquitous. Front. Microbiol. 2016;7:1374. doi: 10.3389/fmicb.2016.01374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Meletis G. Carbapenem resistance: Overview of the problem and future perspectives. Ther. Adv. Infect. Dis. 2016;3:15–21. doi: 10.1177/2049936115621709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are reported in the manuscript.