Abstract
Regulatory T cells (Tregs), an essential component of the human immune system, are a heterogeneous group of T lymphocytes with the ability to suppress immune responses and maintain immune homeostasis. Recent evidence indicates that Tregs may impair antitumor immunity and facilitate cancer progression by weakening functions of effector T cells (Teffs). Consequently, targeting Tregs to eliminate them from tumor microenvironments to improve Teffs’ activity could emerge as an effective strategy for cancer immunotherapy. This review outlines the biology of Tregs, detailing their origins, classification, and crucial markers. Our focus lies on the complex role of Tregs in cancer’s development, progression and treatment, particularly on their suppressive role upon antitumor responses via multiple mechanisms. We delve into Tregs’ involvement in immune checkpoint blockade (ICB) therapy, their dual effect on cancer immunotherapy and their potential biomarkers for ICB therapy effectiveness. We also summarize advances in the therapies that adjust Tregs to optimize ICB therapy, which may be crucial for devising innovative cancer treatment strategies.
Keywords: Regulatory T cells, Tumor microenvironment, Immune checkpoint blockade
Introduction
Regulatory T cells, known as Tregs, are an integral component of the T cell family and play an essential role in sustaining immune equilibrium. In both mice and human peripheral blood, Tregs account for approximately 5–10% of CD4+ T cells [1]. They primarily exert immune-suppressive effect through the secretion of inhibitory cytokines, the inhibition of cytolysis, and by inducing metabolic disruption, further preserving the body’s immunological balance [2]. Nevertheless, recent investigations have revealed that Tregs also contribute to tumor progression. Their capacity for immunosuppression is considered a principal element in enabling tumors to evade immune detection, thereby challenging the effectiveness of cancer immunotherapies [3]. Consequently, an in-depth understanding of Tregs’ immunosuppressive mechanisms is indispensable for the development of more efficacious, tumor-specific therapies. It has been proved that, compared to non-tumor tissues, Tregs within tumors exhibit higher viability and a more active proliferative ability. They promote tumor progression by suppressing anti-tumor immune responses [4]. Therefore, we need to effectively control Tregs to enhance anti-tumor immunotherapies.
Immune checkpoints, encompassing programmed death receptors and their ligands, constitute key elements in maintaining immune system homeostasis. These checkpoint proteins serve as “brakes” on the immune response and are engaged with co-stimulatory molecules to regulate T cell activation and inhibition [5]. Specifically, through the interaction of Tregs with these checkpoints, immune responses may be enhanced or suppressed, reflecting the environmental demands. Tumor cells have demonstrated the ability to evade recognition and clearance by the immune system through bypassing these immune checkpoints [6]. Consequently, blockade strategies targeting immune checkpoint receptors have emerged as a significant approach to cancer immunotherapy, becoming one of the most prominent cancer treatment strategies in recent years [7]. Within the tumor microenvironment, Tregs can effectively suppress anti-tumor immune responses through the expression and amplification of a variety of immune checkpoints. Utilizing immune checkpoint inhibitors (ICIs) can disrupt this interaction and reversely activate the immune components to combat tumors [8]. Recently, ICIs have yielded remarkable results in the treatment of a range of tumors, such as melanoma, hepatocellular carcinoma, lung cancer, gastric cancer, and intestinal cancer, improving the overall survival rate and progression-free survival of patients [9, 10]. A comprehensive review of randomized clinical trials involving ICIs revealed that long-term survivors experienced an approximate 10% increase in survival probability compared to patients not treated with ICIs, highlighting ICIs’ significant clinical benefits [11]. For example, data from clinical trials of non-small cell lung cancer (NSCLC) showed that the one-year survival rate of patients treated with pembrolizumab (anti-PD-1 drug) and chemotherapy was 69.2%, significantly higher than the 49.4% observed in patients who received chemotherapy alone [12]. To date, ICB therapy has achieved considerable success. However, in clinical practice, only a small number of patients (approximately 10–30%) demonstrated a lasting treatment response, and varying degrees of immune-related adverse events (irAEs) [13, 14]. In preclinical models of irAEs, a negative correlation between Treg number and irAEs has been reported [14].
In our review, we first introduced the fundamental aspects of Tregs, discussed the anti-tumor mechanisms of Tregs in the TME, as well as their evolving role in ICB therapy. We analyzed the current application value and unique advantages of combining Tregs-related therapies with ICB therapy based on the expression of various markers in Tregs, and finally explored progressions in targeting tumor-infiltrating Tregs (TI-Tregs).
Basics of tregs biology
Origin and classification of regulatory T cells
Tregs, a subset of CD4+ T lymphocytes, are essential for immune system regulation. They develop primarily in the thymus, with their maturation regulated by three key signals: T cell receptor (TCR) recognition of peptide-MHC ligands, co-stimulatory cluster of differentiation 80 (CD80)/cluster of differentiation 86’s (CD86) interaction with cluster of differentiation 28 (CD28), and activation by interleukin-2 (IL-2) or interleukin-15 [15]. Tregs are categorized into two main types: thymus-derived Tregs (tTregs) and peripherally induced Tregs (pTregs) (Fig. 1). tTregs, which constitute the majority proportion of Tregs, are capable of responding to autoantigens and play a crucial role in maintaining immune self-tolerance [16]. In contrast, pTregs arise from peripheral CD4+ T cells under antigen stimulation and increased FoxP3 expression, often occurring in tissues like the intestine, where it is rich in TGF-β and retinoic acid [17]. Both tTregs and pTregs require IL-2 for their survival and function, with TGF-β being particularly important for iTregs induction in vitro [18]. Tregs are thus characterized by high expression of the transcription factor FoxP3 and the IL-2 receptor alpha chain (CD25), making the CD4+ CD25+ FoxP3+ phenotype a classic identifier for Tregs [19]. These markers facilitate the study of Tregs functions and offer insights into their role in immune tolerance and potential therapeutic applications [20, 21].
Fig. 1.
Generation and classification of tregs. The picture shows two main differentiation routes. The first leads to FoxP3 + nTreg/Tregs, which represent nTregs, also known as thymic Tregs (tTregs). These cells are characterized by the expression of the transcription factor FoxP3 and are crucial for maintaining immune tolerance and preventing autoimmune responses.The second route shows the activation of naïve CD4 + T cells by an immunosuppressive (IS) factor and an antigen, leading to the differentiation into FoxP3 + induced regulatory T cells (iTregs). iTregs are similar to nTregs in their function of immune regulation, but they are induced in the periphery from conventional T cells.There is further differentiation into two other FoxP3- Tregs : Tr1 cells and Th3 cells. Tr1 cells are a type of regulatory T cell that produces high levels of interleukin-10 (IL-10), a cytokine involved in the suppression of inflammatory responses. Th3 cells are another subset of regulatory T cells known for their role in mucosal immunity and their production of transforming growth factor-beta (TGF-β), which also has immunosuppressive properties
There are differences between human and mouse Tregs. Tregs in both humans and mice exhibit conserved expression of the hallmark transcription factor FoxP3; however, in human Tregs, FoxP3 expression is more sensitive to modulation by the inflammatory milieu. While mouse Tregs primarily develop in the thymus, a substantial number of human Tregs are generated in peripheral tissues [22]. Functionally, both human and mouse Tregs suppress immune responses [23]. Human Tregs often rely on external factors (e.g., IL-2) to potentiate their suppressive capacity, whereas mouse Tregs demonstrate a relatively lower dependence on IL-2 [24].
Regulatory T cells in the tumor microenvironment
To gain deeper insight into the role of Tregs in cancer and to explore their potential anti-tumor effects, researchers are increasingly focusing on the tumor microenvironment (TME), which includes immune cells, blood vessels, stromal cells and many other types of cells [25]. In the TME, Tregs inhibit anti-tumor activity in ICB through various pathways, leading to increased drug resistance. The abundant expression of chemokine ligand C-C motif chemokine ligand 22 (CCL22) attracts activated Tregs that express chemokine receptor C-C motif chemokine receptor 4 (CCR4) [26]. These activated Tregs suppress the antigen-presenting function of dendritic cells and induce T cell exhaustion within the tumor by secreting inhibitory cytokines, such as transforming growth factor-β (TGF-β), interleukin-35 (IL-35) and interleukin-10 (IL-10), and by regulating the expression of inhibitory receptors [27]. Moreover, Tregs impair Teffs’ activity through metabolic disruption. In the TME, IL-2 acts as a key growth factor. Tregs compete for IL-2 with Teffs and lead to a scarcity of IL-2 by highly expressing cluster of differentiation 25 (CD25), a high-affinity receptor for IL-2 [28]. Simultaneously, Tregs express the co-inhibitory receptor cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) that binds to cluster of CD80 / b7-1 molecule and CD86 / b7-1 molecule on antigen presenting cells (APCs), disrupting co-stimulatory signals and thereby inhibiting effector T cell activity [29]. In the TME, Tregs create tiny pores in the target cells’ membrane through perforin, facilitating the entry of granzyme B which subsequently activates caspase to induce apoptosis in the target cell, resulting in the suppression of Teffs, NK cells and other anti-tumor immune cells. Through this cytotoxic effect, Tregs maintain an immunosuppressive state in the TME, promoting immune escape and diminishing the efficacy of ICB [30]. The immunosuppressive mechanism of Tregs in TME is also a key factor causing resistance to immunotherapy [31]. Of course, further research is needed to understand the interactions between the different functions of Tregs. Considering the significant role of Tregs in tumor immunity, researchers have proposed two major strategies to enhance tumor immunity: depleting Tregs and reducing their suppressive function.
Engineering tregs
In addition to conventional Tregs, engineered Tregs have garnered the interest of immunology researchers as an emerging area of study, wherein these cells employ the immunosuppressive functions of their natural counterparts for potential clinical therapeutic applications [32]. Using advanced gene editing tools such as CRISPR/Cas9, researchers conducted a loss-of-function screening on approximately 500 nuclear factors to ascertain which genes enhance or inhibit FoxP3 expression. This screening revealed ubiquitin specific peptidase 22 as a positive regulator of FoxP3 expression, while E3 ubiquitin ligase ring finger protein 20 emerged as a negative regulator. These findings not only unveiled previously unknown FoxP3 regulators but also introduced a novel screening method with broad applicability to Treg-based cancer immunotherapy [33]. Subsequent studies have demonstrated that, based on this screening technique, DNA editing can modify multiple genes to regulate Treg functions. By modifying the stability and function of Tregs, this approach establishes the groundwork for engineered Treg-based cancer therapies [34]. Recent advances in this field also include the development of low-immunogenic pluripotent stem cells that can be induced into Tregs through gene editing, a process with important implications for the advancement of engineered Treg cell therapy [35].
Marker genes for tregs
Tregs’ vital role in maintaining immune tolerance has been repeatedly revealed, they suppress autoimmune responses and inhibit tumor-killing immune responses. Researchers have identified a series of marker genes that encode proteins and molecules with specific expression patterns and functions in Tregs [36]. Tregs are notably characterized by the expression of the transcription factor FoxP3, which distinguishes them from other T cell subsets [37]. Although FoxP3 expression is almost exclusive to Tregs in human [38], non-regulatory T cells may also transiently express FoxP3 upon activation in some cases. Therefore, researchers have combined other markers, such as high expression of CD25 and low or no expression of cluster of differentiation 127 (CD127), to more accurately identify Tregs [39]. Helios (IKZF2) and Eos (IKZF4), zinc-finger transcription factors, are also proved to be important marker genes for Tregs and work alongside FoxP3 to regulate key molecules and signaling pathways, maintaining Treg cell development and function [40]. These marker genes can be used as diagnostic tools to identify immune system dysregulation, predict disease progression, and assess therapeutic responses. In addition, a recent study by Michael Delacher et al. employed single-cell gene expression analysis and reported that the transcription factor BATF and chemokine receptor CCR8 expressed by Tregs in mouse and human tissues, are capable of promoting tissue homeostasis and regeneration, indicating that targeting these factors may help balance the immune response and may serve as a potential marker for immunotherapy [41].
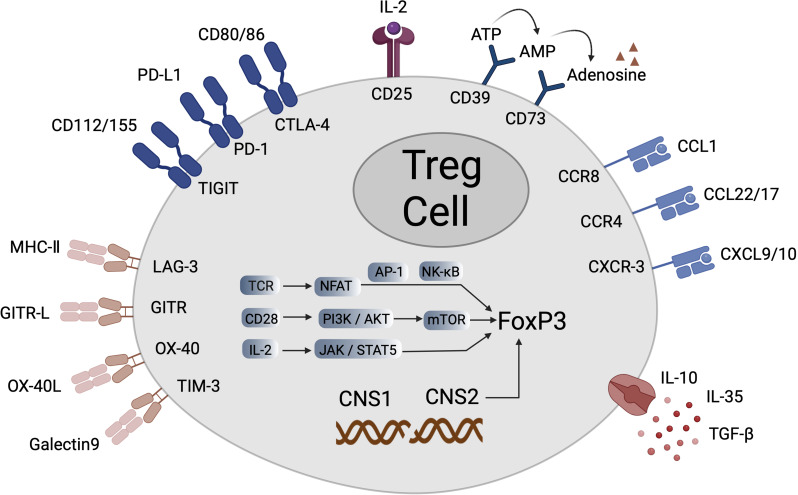
The expression of surface molecules on Tregs is critical for their identification, classification and functional studies, showcasing their distinctiveness (Fig. 2). Key molecules include CD4, cluster of differentiation 39 (CD39), cluster of differentiation 73 (CD73), and CD25, with the latter being vital for Tregs survival and function [42]. Inhibitory co-stimulatory molecules such as CTLA-4, programmed death-1 (PD-1), T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) and T cell immunoreceptors with IG and ITIM domains (TIGIT) play crucial roles in maintaining immune homeostasis and preventing excessive immune responses, with their interactions with co-inhibitory ligands contributing to tumor immune escape [43, 44]. Additionally, TI-Tregs express tumor necrosis factor receptors like glucocorticoid-induced TNFR-related protein (GITR), inducible T-cell costimulatory (ICOS), tumor necrosis factor receptor superfamily member 4 (OX40) and tumor necrosis factor receptor superfamily member 9 (4-1BB) on their surface [45]. In the tumor microenvironment, the presence of chemokine receptors such as CCR4, C-C motif chemokine receptor 6, C-C motif chemokine receptor 8 (CCR8), and tumor necrosis factor receptor superfamily member 4 is essential for Treg migration and expansion, enabling their localization to specific tissues and sites [46]. These marker genes and molecules collectively define the suppressive nature of Tregs. By studying these markers in depth, we gain insights into the biological functions of Tregs and their micro-mechanisms in the tumor microenvironment, providing a theoretical foundation for the treatment of immune- and tumor-related diseases.
Fig. 2.
Various genes expressed by Tregs and their surface molecules. In Tregs, FoxP3 is the key transcription factor that maintains their regulatory function. The expression of FoxP3 is closely related to the function of Tregs. The recognition of antigens by the T-cell receptor (TCR) triggers signal transduction, activating NFAT, which works in conjunction with AP-1 or NF-κB to promote the expression of FoxP3. Concurrently, co-stimulatory molecules like CD28 activate the downstream PI3K/Akt signaling pathway, thereby affecting the mTOR pathway, which is crucial for the stable expression of FoxP3 and the function of Tregs. Additionally, the cytokine signal IL-2 promotes the expression of FoxP3 through the JAK/STAT pathway, particularly STAT5.Other surface molecules also play an important role in the development and function of Tregs
Tregs in antitumor immunity regulation
Cancer remains a significant threat to global health and is a major concern for humanity. According to the American Association for Cancer Research, by 2040, the worldwide cancer patient population is predicted to reach 28 million, with approximately 16.2 million expected to succumb to the disease (AACR Cancer Progress Report 2022). The evolution of cancer treatment has transitioned from traditional modalities such as surgery, radiation, and chemotherapy to contemporary approaches including targeted therapies and immunotherapy [47]. Immunotherapy, which harnesses the body’s immune system’s ability to identify and attack cancer cells, represents a groundbreaking advancement in anti-cancer research. In particular, ICB therapy has exhibited remarkable clinical efficacy by blocking immunosuppressive signaling pathways and enhancing the anti-tumor response of T cells [48]. Within this context, Tregs play a pivotal role in tumor therapy. In tumor tissues, the proliferation of Tregs is often markedly increased, which is believed to be a strategy by which tumors evade immune system attacks [49]. Meanwhile, TI-Treg is a key mediator of resistance to cancer immunotherapy. Tregs impair the anti-tumor immune response by inhibiting the activity of other immune cells, particularly CD8+ T cells. As a result, targeted immunotherapy reduce the ratio of Tregs to Teffs in the TME has emerged as a highly promising avenue in cancer therapy via leveraging the immunosuppressive role of Tregs, especially in ICB therapy [50, 51]. To thoroughly understand the role of Tregs in tumor immunity, scientists are actively exploring their subpopulations and functional regulatory mechanisms. This endeavor includes modulating the number and function of Tregs through drug intervention, gene editing, or immunotherapy to bolster the body’s immune response to tumors (Fig. 3).
Fig. 3.
Tregs in antitumor immunity regulation. A. Tregs degrade ATP to produce adenosine via CD39 and CD73, and adenosine inhibits the function of NK and effector T cells through A2aR.At the same time, tumor cells cause lactate accumulation due to Warburg effect, which promotes the generation of Tregs. B. Tregs compete with effector T cells for IL-2 through CD25 and secrete inhibitory cytokines such as IL-10, IL-35, TGF-β, and VEGF, Suppressing the immune response and promoting tumorigenesis. C. Tregs exhibit CTLA-4, LAG-3, and PD-1 on their surface, which interact with molecules on tumor cells and antigen-presenting cells (APC), suppressing effector T cells. D. Tregs respond to chemokines such as CCL17, CCL22, and CCL1 by expressing receptors like CCR4, CCR8, and PD-1, leading to their migration to the tumor microenvironment (TME)
FoxP3+ tregs
The human FoxP3 gene, located on the p-arm of the X chromosome, encodes a transcription factor that leads to the differentiation of T cells into Tregs [52]. FoxP3 plays a crucial role in the development of Tregs, serving as a distinctive marker for their identification and is essential for the establishment and maintenance of gene expression [53]. Benjamin Bilgüvar and Alexander Varki et al. demonstrated the importance of FoxP3 in maintaining immune homeostasis by studying mutations in the FoxP3 gene in mice and human. Mutations of this gene give rise to severe autoimmune diseases in mice and are associated with human immune polymorphic inflammatory syndrome [54]. The transcription of FoxP3 is mainly regulated by five elements, including conserved non-coding sequences located in the FoxP3 locus in Tregs, on which transcription initiation and maintenance of FoxP3 are highly dependent [55]. In particular, the CpG region in CNS2, known as the Treg-specific demethylated region, maintains a highly demethylated state to preserve FoxP3 expression [15, 56].
In TME, under lactate-rich and hypoxic conditions, FoxP3 expression alters the metabolic modalities of Tregs, enabling them to function normally in a low-glycemic and high-lactic acid environment, thus adapting better to the TME [57]. In contrast, glycolysis-dependent Teffs are suppressed. Additionally, studies have shown that tumor cells and associated cells can secrete factors like TGF-β and IL-10, inducing Tregs’ expression of FoxP3 and increase in number [58]. Activation of inflammatory factors and immune cells may also regulate FoxP3 expression [59]. Therefore, targeted therapy against various sources of FoxP3 in the TME holds significant potential value. AstraZeneca has developed antisense oligonucleotides targeting FoxP3 (AZD8701), a therapy specifically targeting Tregs and has been evaluated in patients with advanced solid tumors (NCT04504669), revealing potential to enhance CD8+ T cell activation. Simultaneous studies in the A20 tumor model that combined FoxP3 ASO with anti-PD-1 treatment demonstrated that FoxP3 ASO significantly inhibited tumor growth and increased the number of complete responses (CR) or near-complete responses (near-CR) in mice [60]. In addition, a novel chemically modified self-delivered antisense oligonucleotide (FANA ASO) reduced the mouse tumor volume by targeting FoxP3 and reduced the mRNA level of FoxP3 and the number of Tregs. Several immune checkpoints, including CTLA-4, Tim-3, PD-1, LAG-3, and TIGIT, were down-regulated and are currently being investigated in combination with ICIs [61].
CD25+ tregs
CD25, the α-chain of the IL-2 receptor, is predominantly expressed on Tregs and forms the IL-2 receptor along with the β-chain (CD122) and the γ-chain (CD132) [62]. However, not all CD25-expressing cells are Tregs, necessitating their identification in combination with other markers [63]. IL-2 serves multiple roles as a cell growth factor, including promoting antibody-secretion of B cells, activating Teffs and NK cells and promoting Treg cell growth and differentiation [64]. CD25 binds to receptors on Tregs, activating Janus kinase [65], which leads to the phosphorylation of signal transducer and activator of transcription 5 (STAT5). Activated STAT5 then binds to the promoter of FoxP3 and CNS2, enhancing transcriptional activation and expression in Tregs [66]. In the TME, Tregs’ high CD25 expression allows IL-2 a strong affinity for its receptor IL-2Rαβγ, competing for IL-2 and inhibiting Teffs that require IL-2 signaling for survival and function [67]. This mechanism enables Tregs to protect the tumor from the immune system by suppressing the immune response.
Despite the crucial role of the molecule, Treg-targeted therapies that focus on CD25 exhibit limited clinical efficacy. The primary issue is that systemic Treg depletion induces severe responses of inflammation and autoimmune, while also disrupting IL-2 signaling in Teffs, instead of selectively acting on Tregs. This unintended action undermines therapeutic efficacy [68]. This challenge is prevalent across all Treg-targeted therapies. Hence, designing optimized anti-CD25 antibodies that specifically target Tregs presents a promising strategy. 7D4, an IgM antibody, binds to CD25 without inhibiting IL-2/IL-2R signaling. It represents a novel type of optimized antibody. RG6292, derived from 7D4, selectively depletes Tregs via antibody-dependent cell-mediated cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) while sparing CD4+ and CD8+ Teffs [69]. Moreover, studies in mouse models demonstrated that combining optimized anti-CD25 antibodies with PD-1 inhibitors significantly amplifies anti-tumor effects and improves ICB efficacy [70, 71]. h7B7-15 S, a humanized anti-CD25 monoclonal antibody that does not block IL-2, further enhances Treg depletion when paired with anti-CTLA-4, leading to substantial improvements in the remodeling of the tumor immune microenvironment [72].
GITR+ tregs
GITR is a constituent of the tumor necrosis factor superfamily which exhibits elevated expression levels under the influence of FoxP3 in mature Tregs [73]. Within the TME, the interaction of GITR on Tregs with its natural ligand initiates the activation of several pivotal cellular signaling cascades, including the NF-κB signaling pathway, the MAPK pathway, and the PI3k/pkB pathway [74]. These signaling cascades are crucial for the activation, survival and functionality of Tregs, in addition to facilitating the proliferation and effector capacities of Teffs [75].
Studies have indicated variability in GITR expression on tumor-infiltrating Tregs and lymphocytes across different tumor types, highlighting that patients with NSCLC, renal cell carcinoma and melanoma might benefit from anti-GITR therapy [76]. Helios plays a key role in ensuring that Tregs maintain their stable suppressive phenotype by enhancing FoxP3 expression, while the downregulation of Helios expression following GITR activation induces a Th1 effector-like phenotype in Tregs, thereby destabilizing FoxP3 expression and compromising their suppressive function within the tumor microenvironment [3, 77].
GITR’s dual functions influencing both Tregs and Teffs make it a compelling and important target in the field of tumor immunotherapy [78]. Research concerning GITR agonist monoclonal antibodies showed that although efficacy has been seen with combination therapy with ICIs in phase I/II trials, they do not appear to be as effective as monotherapy, but rather respond to combination therapy, particularly with the addition of PD-1 blockade. The addition of PD-1 blockade may produce synergistic and complementary antitumor effects by reversing CD8+ T cell exhaustion [74, 79]. DTA-1, an antibody to rat immunoglobulin g2a of GITR, significantly prolonged survival and induced durable tumor-specific immunity in a mouse ID8 ovarian cancer model with combined anti-PD-1 and GITR therapy. Combination therapy reduced tumor burden [80]. In phase I trials, TRX518 (anti-GITR) was administered alone or in combination with PD-1 inhibitors such as pembrolizumab or nivolumab. Considerable clinical responses were demonstrated when TI-Tregs were significantly depleted while CD8+ T cell infiltration was increased, suggesting that combination therapy may be particularly effective when targeting tumors with high Treg content [81].
CCR4+ tregs
CCR4 is found on Tregs and other Th cells and interacts with the ligand CCL17 and CCL22 [82]. This receptor manifests elevated expression in T-cell malignancies such as adult T-cell leukemia/lymphoma and cutaneous T-cell lymphomas [83]. Within the TME, tumor cells and tumor-associated immune cells secrete CCL17 and CCL22. Tregs exhibiting high expression of CCR4 are recruited into the TME through recognizing and binding to these chemokines, thereby facilitating immune evasion from cancer [84]. The team led by Christine Ménétrier-Caux has demonstrated the CCL22-mediated recruitment of Tregs in breast cancer across two different studies [85]. At the same time, the study found that when cancer patients receive ICB treatment, it will lead to the activation of inflammatory responses in the TME, the expression of pro-inflammatory and immunomodulatory signaling pathways, including CCL17 and CCL12. This promotes the migration of CCR4+ Tregs into the tumor will promote tumor progression and resistance to ICB treatment [86, 87].
Therefore, anti-CCR4 monoclonal antibodies play a crucial role in evoking and enhancing anti-tumor immunity in cancer patients by selectively depleting Tregs. For instance, mogamulizumab is a fully humanized and deglycosylated monoclonal anti-CCR4 antibody. The researchers conducted a phase I clinical study to evaluate the safety and efficacy of nivolumab and mogamulizumab monoclonal antibodies in patients with advanced solid tumors. Analysis of paired biopsy samples from 12 patients showed that in most cases, TI-Tregs decreased and CD8+ T cells increased. Solid objective responses were observed in tumors such as hepatocellular carcinoma and NSCLC [88]. However, David S. et al.‘s study on patients with locally advanced or metastatic solid tumors found that the synergistic effect of mogamulizumab combined with nivolumab was not observed. This may be since CD8 and NK cells also express CCR4, resulting in additional consumption [89, 90], which requires further research and verification. However, it is essential to recognize that, due to the high expression of CCR4 in skin tissue, mogamulizumab treatment may result in skin-related adverse reactions and necessitates careful monitoring [91].
CCR8+ tregs
CCR8 serves as a chemokine receptor that binds to its ligand CCL1, thus mediating cell chemotaxis [92]. CCR8 is recognized as a potential specific marker for TI-Tregs and is selectively enhanced by Tregs within tumors across a broad spectrum of human cancer types, including breast, hepatocellular, colorectal, NSCLC and metastatic melanoma [93]. CCR8 is predominantly expressed in Tregs expanded in tumors, with negligible expression in tumor-infiltrating effector T cells (TI-Teffs) or peripheral Tregs in both human and mice [94]. Meanwhile, CCR8+ Tregs are deemed as a stable subtype possessing enhanced immunosuppressive capacity, and their frequency increases with disease progression [95]. Although evidence suggests that the CCR8 signaling pathway is not essential for Tregs to exert tumor suppressive immunity and may not be involved in the recruitment of tumor Tregs, targeting CCR8 remains a critical component in tumor therapy [94], thereby serving as an effective strategy for TI-Treg cell targeting. Anti-cancer therapeutic antibodies targeting CCR8 can eliminate tumor-associated Tregs via Fc region-mediated ADCC and ADCP [96]. Therefore, anti-CCR8 antibodies may mitigate the adverse effects of extensive Treg depletion induced by anti-CTLA-4 or anti-PD-1 therapy and prevent the onset of fatal autoimmune reactions. S-531,011 is identified as a monoclonal antibody, which uniquely interacts with CCR8 among all known chemokines and possesses potent ADCC activity that neutralizes the CCL1-CCR8 signaling pathway, resulting in the depletion of Tregs within the tumor and producing a significant anti-tumor effect [97]. Van Damme et al. demonstrated in a study using NSCLC mouse models that the combination of anti-CCR8 antibodies and PD-1 monoclonal antibodies yielded enhanced therapeutic benefits [93]. Similarly, a phase 1/2 clinical trial report indicated that LM-108, an Fc-optimized anti-CCR8 monoclonal antibody exhibited encouraging anti-tumor efficacy in gastric cancer patients resistant to PD-1 therapy when administered in combination with anti-PD-1 antibodies [98].
CD39 / CD73+ tregs
CD39 and CD73 are nucleic acid ectonucleotidases that play pivotal roles in immune regulation and the TME. These enzymes are predominantly expressed in Tregs and regulate the immune response by synergizing within the TME [99]. Initially, CD39, a nucleotide triphosphatase, progressively degrades extracellular ATP into ADP and AMP, marking a crucial initiating step. Subsequently, CD73 efficiently transforms AMP into adenosine [100] which is a potent immunosuppressive molecule that can diminish the anti-tumor immune response by binding to adenosine receptors 2a (A2aR) or 2b (A2bR) on the surface of CD8+ T cells, NK cells, and APCs, thereby inhibiting their activation and cytotoxic functions [101]. This mechanism further compromises the immune system’s capacity to attack the tumor, offering a pathway for the tumor to circumvent immune surveillance [102].
At the same time, it affects the body’s immunotherapy. The activation of adenosine/A2AR signaling can increase the expression of immune checkpoints on the surface of immune cells, including PD-1, CTLA-4 and LAG3, while promoting Treg proliferation and secretion of immunosuppressive factors (including TGFβ and IL-10) [103]. The high expression of CD39 and CD73 is usually induced by hypoxic conditions within tumors and plays an important role in creating an immunosuppressive microenvironment, thereby reducing the efficacy of ICB [104]. When the adenosine level in the TME is high (such as breast cancer and lung cancer), or when tumors grow rapidly and promote TME hypoxia, the use of anti-CD39 and CD73 antibodies can help enhance the efficacy of ICB [105]. For example, high levels of CD73 are expressed in EGFR-mutated NSCLC, thereby inhibiting T cell activity. The combination of anti-PD-L1 and anti-CD73 therapy significantly improved T cell responses and reduced tumor growth in EGFR-mutated NSCLC compared with either therapy alone [106].
CTLA-4+ treg
CTLA-4 is an inhibitory receptor expressed on the surface of T cells and plays an important role in activating Tregs and maintaining the stability of the immune system [107]. In the TME, Tregs express significantly more CTLA-4 than Teffs [108].CTLA-4 on the surface of Tregs competes with the co-stimulatory molecule CD28 for binding to the B7 molecule (CD80/CD86) on the surface of APCs. When CTLA-4 binds to the B7 molecule, it deprives CD28 of the required co-stimulatory signals, effectively inhibiting T cell activation [26, 109]. Meanwhile, CTLA-4 also carries CD80 and CD86 on the surface of antigen-presenting cells into Tregs through endocytosis [110], which reduces the number of co-stimulatory molecules on the surface of APCs and indirectly decreases T cell activation and expansion. CTLA-4 is essential for Tregs function because T cells require essential amino acids, such as tryptophan, for protein synthesis and metabolic activities. CTLA-4 induces the production of indoleamine-2,3-dioxygenase (IDO) through interaction with APCs, which promotes catabolism of tryptophan and generates pro-apoptotic metabolites that inhibit the activation of effector T cells [17, 111]. In addition, CTLA-4 signaling can interfere with the proximal signaling of T cell receptor and CD28 [112]. All these findings suggest that CTLA-4 serves as an important target for clinical therapy and provides new avenues for the treatment of various types of cancer.
TIGIT+ tregs
TIGIT, a member of the immunoglobulin superfamily featuring immunoglobulin and immunoreceptor tyrosine kinase structural domains, is extensively expressed across the immune system, encompassing Teffs, NK cells and Tregs [113]. Elevated TIGIT expression levels on Tregs in peripheral blood mononuclear cells of both healthy individuals and cancer patients correlate with hypomethylation of the TIGIT locus and the binding of FoxP3 to Tregs. Furthermore, TIGIT expression is notably upregulated within the TME [114]. TIGIT manifests immunosuppressive effects by binding to two ligands, CD155 (PVR) and CD112 (PVRL2), thereby inhibiting T and NK cell activation through competition for ligands with other molecules, such as CD226 (DNAM-1) or CD96 [115]. Within the TME, TIGIT binding to its ligands activates signaling pathways resulting in Treg attachment and stimulates the expression of the effector molecule fibrinogen-like protein 2, thus promoting Treg cell-mediated suppression of Teff proliferation [116]. Additionally, researchers have observed that TIGIT+ Tregs in peripheral and tumor sites demonstrate increased expression of various characteristic marker genes, such as FoxP3, Helios, CTLA-4, PD-1, and lymphocyte activation gene-3 (LAG-3) [117]. This shows that blockade of TIGIT increasesthe immune-suppressing ability of Tregs, thereby affecting the efficacy of ICB therapy.
Therefore, blockade of TIGIT has become a key area of research in recent years following anti-PD-1 therapy. Dual blockers of PD-1 and TIGIT are a promising approach for tumor immunotherapy. Dual PD-1/TIGIT blockers boost the growth and activity of tumor-specific CD8+ T cells and tumor-infiltrating lymphocytes (TILs) compared with a single blocker [118]. Tiragolumab, a popular inhibitor of the TIGIT target, has shown encouraging clinical results in combination with PD-1 or PD-L1, particularly in NSCLC [119]. The latest Phase II study found that Tiragolumab, which activates tumor and circulating myeloid cells via the Fc receptor, greatly improved the objective remission rate and progression-free survival [120].
Other types of treg
Indeed, there are other types of Tregs that play a crucial role in regulating tumor immunity. LAG-3 is a cell surface protein which is prominently expressed on Tregs. Typically, LAG-3 attaches to MHC II molecules on the surface of antigen-presenting cells and delivers inhibitory signals that interfere with CD4-MHC II interactions, thus inhibiting T cell activation and facilitating tumor cell evasion of immune attacks [121, 122]. Within the TME, TGF-β + Tregs suppress the function of effector T cells through the release of TGF-β, which additionally induces the differentiation of undifferentiated T cells into Tregs, thus promoting tumor invasiveness and metastatic ability [123]. The dysfunction of TIM-3+ Tregs and CD8+ tumor-infiltrating T lymphocytes, as well as the expansion of Tregs, are positively correlated [124]. Cluster of differentiation 103 is recognized as a hallmark of TI-Tregs and facilitates their specific migration and localization within tissues, notably in sites such as the intestinal mucosa, thereby enhancing the inhibitory effect upon Teff proliferation [125, 126]. CXCR3+ Treg specifically binds to IFN-γ-related ligands such as CXCL9, CXCL10 and CXCL11, and continuously migrates to the TME, which can not only guide immune activation in an inflammatory environment, but also enhance the immunosuppressive function of Tregs in tumors. It has a dual role and is closely related to tumor immune escape. Focusing on the expression of different genes and molecules may provide a deeper understanding of the immunoregulatory mechanisms within the TME, potentially leading to the development of new therapeutic strategies designed to modulate the function of these cells to enhance the anti-tumor immune response.
Tregs in ICB
Molecules such as CTLA-4, PD-1, and PD-L1 operate through co-inhibitory signaling pathways and is also a key target for ICB therapy at the same time. ICB therapy blocks the interaction of these molecules to enhance Teffs’ activity and improve their ability to attack tumors (Fig. 4). After treatment, changes in the number and function of Tregs within the TME are influenced by various factors, including Treg molecular expression, tumor type, metabolic regulation, and individual immune responses [3]. Current study aims to investigate how these factors contribute to variations in therapeutic efficacy and design new therapies with improved anti-tumor efficacy without overexposing patients to irAEs.
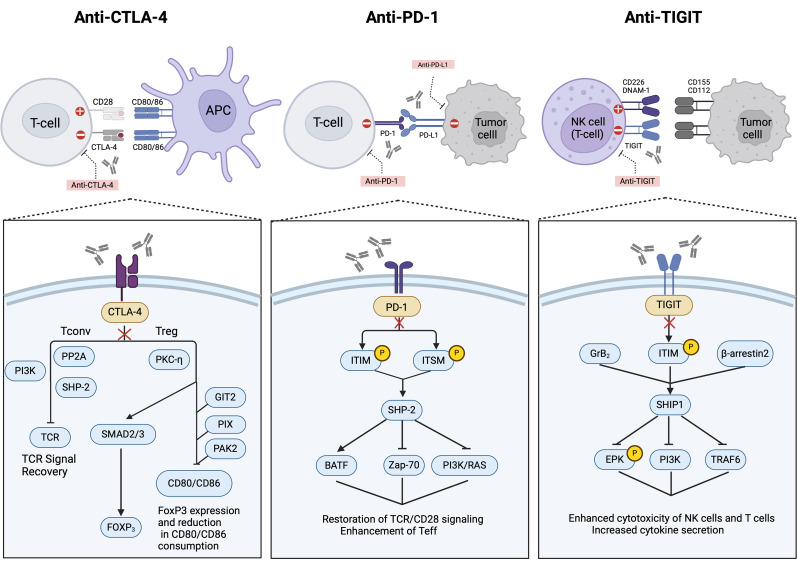
Fig. 4.
The mechanisms of Tregs in immune checkpoint blockade therapy. Anti-CTLA-4 enhances the recovery of T-cell receptor (TCR) signaling by blocking CTLA-4 on T cells, thereby boosting the activity of Teffs. It also reduces the competition for CD80/CD86 by Tregs, indirectly decreasing the expression of FoxP3 and the immunosuppressive function of Tregs; Anti-PD-1 disrupts the interaction between PD-1 on T cells and its ligand PD-L1 on tumor cells, restoring TCR/CD28 signaling and enhancing the function of effector T cells (Teff). This enhancement also involves affecting downstream pathways such as PI3K/RAS, thereby improving cell survival; Anti-TIGIT blocks TIGIT, thereby enhancing the cytotoxicity of natural killer (NK) cells and T cells, and increasing cytokine secretion. It also restores the competitiveness of CD226, DNAM-1. This is achieved by inhibiting molecules that interact with TIGIT, such as SH2 domain-containing proteins (SHIP1), and intracellular T-cell signaling pathways like EPK and PI3K
Surface biomarkers and antibody molecular mechanisms
Tregs and CTLA-4 blockade immunotherapy
During ICB treatment, the expression level of specific targets in Tregs and their molecular mechanisms are crucial to the therapeutic effect. Blocking different targets leads to varying effects. Initially, CTLA-4 inhibitors were believed to primarily function by reactivating dysfunctional Teffs. However, later studies revealed that CTLA-4 is predominantly expressed by Tregs within the TME [127]. Anti-CTLA-4 therapy promotes Treg depletion by inhibiting CD80/86-CTLA-4 interactions or through ADCC and ADCP, thus enhancing T cell activation [128, 129]. In anti-tumor immunity, the differential expression of CTLA-4 in Tregs and Teffs allows anti-CTLA-4 monoclonal antibodies to selectively deplete Tregs while preserving activity of Teffs, thereby enhancing anti-tumor immune responses [130] .
However, the precise contribution of anti-CTLA-4 to overall antitumor efficacy remains a subject of debate. Studies have indicated that with employment of anti-CTLA-4 treatment, the number of Tregs in the TME may not decrease significantly [26]. For instance, quantitative immunohistochemical analysis of patients with melanoma, prostate cancer, and bladder cancer after ipilimumab treatment revealed increased infiltration of CD4+ and CD8+ T cells in the tumor, whereas the number of FoxP3+ Tregs remained mostly unchanged [131]. Consequently, some studies suggest that anti-CTLA-4 antibodies may alter the migration patterns and activation status of Tregs. This effect may be attributed to the CTLA-4 blockade removing constraints on Treg expansion, leading to an increase in peripheral blood Tregs that subsequently replenish Tregs within the tumor [132, 133]. This also explains the failure to detect substantial TI-Treg depletion following treatment with depleting anti-CTLA-4 antibodies. Furthermore, Francesco et al. demonstrated in a mouse subcutaneous tumor transplantation model that Tregs regulate their population size by relying on CD28 costimulatory signals to deplete CD80 and CD86 expression. CTLA-4 inhibitors disrupt this balance, potentially resulting in excessive Treg proliferation within tumors [134]. Moreover, non-selective CTLA-4 targeting weakens the anti-tumor response because other immune cells in the TME, such as activated Teffs and DCs also express CTLA-4 [135].
Anti-CTLA-4 antibodies exert anti-tumor effects by binding to Fc receptors (FcγRs) via their Fc regions and this mechanism has become a significant hotspot in recent years. Research has shown that anti-CTLA-4 antibodies initiate ADCC or ADCP by binding to FcγRs, leading to the clearance and depletion of Tregs [136]. The rate of Treg cell depletion is influenced by the IgG isotype of anti-CTLA-4 antibodies and the polymorphisms of FcγRs [137]. For instance, tuvirumab, an IgG2 antibody targeting CTLA-4, has low affinity for activating FcγRs and primarily functions by blocking CTLA-4 signaling, with weak ADCC-mediated Treg clearance capabilities. In contrast, ipilimumab (IgG1) exhibits higher affinity for FcγRs, effectively inducing ADCC and promoting Treg cell depletion [136, 138]. Thus, selecting the appropriate antibody structure for specific tumor types is essential. However, if anti-CTLA-4 antibodies preferentially bind to inhibitory FcγRIIB on the surface of Tregs, it may reduce the effectiveness of antibody-mediated Treg clearance, ultimately diminishing the efficacy of ICB therapy [139]. Optimizing the Fc region structure of antibodies to balance Treg clearance efficiency and minimize side effects remains a critical focus in antibody drug development. For example, XTX101 is designed with an Fc enhancement region and is covalently linked to a masking peptide that blocks the complementarity determining region. This design enables it to be specifically activated in the TME, thereby minimizing systemic side effects [140].
Tregs and PD-1 blockade immunotherapy
PD-1 is an inhibitory costimulatory molecule that is broadly expressed on the surface of Teffs [141]. PD-L1 is a transmembrane protein typically expressed on tumor cells and specific immune cells, including DCs and macrophages [142]. PD-L1 upregulates FoxP3 expression in Tregs and influences anti-tumor therapies. Numerous studies suggest that elevated PD-L1 expression correlates with poor patient prognosis [143]. When PD-1 interacts with PD-L1 on tumor cell surfaces, it results in the inactivation of Teff function, thereby promoting tumor immune evasion. Blocking the PD-1/PD-L1 interaction reactivates Teffs, thereby strengthening the immune system’s response to tumor cells [144]. PD-1/PD-L1 blockers represent the most extensively researched ICB therapies to date. Studies indicate a significant improvement in median overall survival in metastatic NSCLC to 21.9 months [145]. Toor et al. demonstrated that pembrolizumab, an anti-PD-1 antibody, inhibited FoxP3 expression on Tregs when applied to peripheral blood mononuclear cells from melanoma patients. This inhibition reduced the immunosuppressive function of Tregs [146]. Additionally, blocking PD-1 with pembrolizumab monoclonal antibody in melanoma patients showed similar results [147]. Unlike CTLA-4, PD-1 expression on Teffs within the TME is typically higher than on Tregs. Consequently, the primary aim of anti-PD-1 therapy is to relieve the inhibition of Teffs and restore their anti-tumor activity [148]. However, unchecked Tregs may impair treatment efficacy. This may be because that anti-PD-1 antibodies not only enhance CD8+ T cell activity but also activate PD-1+ Tregs through TCR and CD28 signaling pathways, thus maintaining their immunosuppressive effects [149, 150]. However, in patients unresponsive to PD-1/PD-L1 ICIs, elevated numbers of PD-1+ Tregs are linked to reduced therapeutic efficacy [148, 151]. Geels’ team therefore showed that Treg cell accumulation after PD-1 blockade may be indirectly related to activated CD8+ T cells. IL-2 production by CD8+ T cells lead to upregulation of ICOS by TI-Treg, thereby promoting their accumulation. Administration of ICOSL inhibitors prior to anti-PD-1 therapy reduces Treg cell accumulation while significantly enhancing the efficacy of anti-PD-1 therapy in immunogenic melanoma [152]. Gulijk et al. found that in mice models, anti-PD-L1 treatment preferentially activated Tregs in resistant tumors. In contrast, Teffs were not activated in the TME, which is a key factor contributing to treatment resistance [153].
Hyperprogressive disease (HPD) refers to the phenomenon where tumors in cancer patients experience rapid acceleration following ICB treatment [154]. Kamada’s team found that in patients with advanced gastric cancer, approximately 10% of anti-PD-1 treatments lead to HPD [150]. PD-1 blockade may promote the proliferation of highly suppressive PD-1+ eTregs in HPD, which in turn diminishes the efficacy of ICB therapy. Therefore, the presence of actively proliferating PD-1+ eTregs in tumors serves as a reliable indicator of HPD [155]. Research indicated that the ratio of the frequency of PD-1+CD8+ T cells to the frequency of PD-1+ Tregs in the TME served as a more reliable predictor of the clinical effectiveness of PD-1 blockade therapies compared to the predictors PD-L1 and tumor mutational load [148]. In a mouse model, single-cell analysis revealed that PD-1 signaling promotes lipid metabolism, proliferation, and inhibitory pathways in TI-Tregs. Conditional deletion or blockade of PD-1 diminishes TI-Treg function while enhancing anti-tumor immunity [156]. Thus, combined approaches targeting PD-1 and other Treg markers that promote Teff activity while suppressing Treg functionality could be crucial for enhancing the efficacy of anti-PD-1 therapy.
TGF-β promotes PD-L1 expression through the MRTF-A/NF-κB pathway, leading to immune escape in NSCLC [157]. In the TME, TGF-β induces naive T cells to differentiate into iTregs, while Tregs enhance immunosuppression by expressing TGF-β [158]. Blocking TGF-β enhances the effect of anti-PD-1/PD-L1 therapy. The fusion protein M7824, which blocks both PD-L1 and TGF-β, prolonged survival and induced long-term effective anti-tumor immunity in a mouse model [159]. Y332D inhibited both TGF-β and VEGF signaling, exhibiting enhanced anti-cancer activity when combined with PD-1 inhibitors [160]. Anti-PD-1 treatment up-regulates other suppressor molecules on T cells (e.g., TIM-3, LAG-3, TIGIT), which increases the suppressive capacity of Tregs [115, 161]. Clinical trials targeting these suppressor molecules in combination with anti-PD-1 have shown promising efficacy [162]. The effects of anti-PD-1/PD-L1 antibody therapy on Tregs are multifaceted and complex, requiring further research to clarify their relationship.
Interactions between CTLA-4 and PD-1 blockade immunotherapy
Anti-CTLA-4 primarily acts during the initial activation phase of T cells by blocking the interaction between CTLA-4 and B7 molecules. This process occurs primarily in the lymph nodes, where it promotes anti-tumor effects by enhancing naïve T cell activation and reducing the suppressive function of Tregs [163]. In contrast to anti-CTLA-4, PD-1 expression on effector T cells in the TME is typically higher than on Tregs, and anti-PD-1 therapy mainly restores Teff function [164] Clinically, anti-PD-1 therapy has demonstrated superior efficacy compared to anti-CTLA-4 therapy [165]. Anti-CTLA-4 therapy affects not only Tregs in the TME but also systemic Tregs, leading to their depletion and frequently causing irAEs such as colitis, rash, and hepatitis [166]. Consequently, anti-PD-1 therapy has gained wider clinical application. Subsequent clinical trials have confirmed that combining anti-CTLA-4 and anti-PD-1 therapies significantly enhances efficacy. This combined strategy enhances CD4+/CD8+ T cell infiltration into tumor tissues, amplifies co-stimulatory signals and promotes the infiltration and activity of T cells and other immune cells within the TME [163, 167].
However, when the traditional anti-CTLA-4 antibody, ipilimumab, is administered alongside anti-PD- (L)1 monoclonal antibody therapy, the likelihood of irAEs rises. Generally, more than 50% of patients encounter irAEs, which signifies a markedly higher incidence than that observed with either treatment alone [168]. Therefore, this adverse effect can be mitigated by altering the structure of the previously mentioned anti-CTLA-4 antibody. AGEN1181 is a novel anti-CTLA-4 antibody that exhibits increased affinity for FcγRIIIa due to the S239D/I332E mutations and is capable of selectively depleting TI-Tregs and diminishing systemic immune activation. It has confirmed clinical efficacy in patients who have undergone multiple treatments, either as a monotherapy or in combination with the anti-PD-1 antibody Balstilimab [169]. Botensilimab, another engineered anti-CTLA-4 antibody, has shown sustained clinical responses across nine distinct immune-resistant or poorly immunogenic tumor types when combined with Balstilimab in patients with advanced solid tumors, establishing a foundation for future trials [170].
Metabolic regulation and TME environmental factors
Amino acid metabolism
In the TME, besides cell surface biomarkers, the unique metabolic mechanisms of Tregs and environmental factors also play a crucial role. In amino acid metabolism, the tryptophan-kynurenine (Trp-Kyn) pathway is closely associated with local immunosuppression within the TME. Tryptophan degradation inhibits the mTORC1 signaling pathway, activating GCN2 and resulting in T cell cycle arrest. Meanwhile, kynurenine and its metabolites act as potent agonists of the aryl hydrocarbon receptor (AhR), further activating Tregs and myeloid-derived suppressor cells (MDSCs), thereby inducing immune regulation [171, 172]. Targeting this metabolic pathway may enhance the efficacy of ICB. A phase II/III study assessed the efficacy of Indoximod (an IDO inhibitor) in combination with pembrolizumab for advanced melanoma treatment. The clinical trial (NCT02073123) reported an objective response rate of 56% and a complete response rate of 19%, demonstrating promising therapeutic efficacy [173].
Glycolysis and lactate metabolism
Glycolysis plays a crucial role in the efficacy of ICB therapy. Cancer cells rely on aerobic glycolysis to consume glucose for survival, leading to a substantial reduction in glucose levels within the TME [174]. Reduced glucose in the TME impairs mTOR activity, glycolysis, and IFN-γ production in TILs, which subsequently results in diminished TIL efficacy [175]. Tregs predominantly rely on oxidative phosphorylation to maintain their suppressive function, whereas Teffs depend on glycolysis and are more susceptible to damage [176]. The lactic acid produced during metabolism influences Treg activity in ICB therapy through several mechanisms. Roberta et al. found that blocking CTLA-4 promotes metabolic adaptability in T cells within tumors exhibiting low glycolytic activity, destabilizing Tregs and shifting them towards an inflammatory phenotype that produces cytokines like IFN-γ and TNF. This transformation enhances immune cell infiltration and improves therapeutic outcomes, particularly in tumors with glycolytic defects [177]. Additionally, Ding et al. demonstrated that lactate promotes RNA splicing and CTLA-4 expression in TI-Tregs via the lactate-FoxP3-USP39-CTLA-4 signaling axis, maintaining the immunosuppressive function of Tregs and impacting ICB efficacy. Thus, in tumors with low glycolytic activity, the efficacy of anti-CTLA-4 therapy is enhanced, and combining CTLA-4 blockade with glycolysis inhibitors may offer additional therapeutic benefits [178]. During anti-PD-1 treatment, in a low-glucose and high-lactate environment, eTregs take up lactate via MCT1 and upregulate PD-1 expression, whereas CD8+ T cells exhibit the opposite PD-1 expression pattern. This divergence contributes to the failure of high-glycolytic tumors to respond to PD-1 inhibitors, ultimately driving disease progression [179]. In an NSCLC model, combining the glycolysis inhibitor IACS-010759 with a PD-1 inhibitor demonstrated superior efficacy compared to monotherapy [180]. For different tumor types, a detailed analysis of their TME is essential to formulate personalized treatment strategies.
Hypoxic environment
Hypoxia is one of the main markers that distinguish solid tumors from normal tissues [181]. It promotes the polarization of tumor-associated macrophages (TAMs) via hypoxia-inducible factor, facilitating their shift to the immunosuppressive M2 phenotype. M2-like macrophages secrete immunosuppressive cytokines, including IL-10 and TGF-β, which promote Treg cell activity and diminish the efficacy of ICB [182, 183]. Additionally, hypoxia can activate the transcription factor HIF-1α, inducing FoxP3 expression and promoting the differentiation of CD4+ T lymphocytes into Tregs [184]. HIF-1α also upregulates PD-L1 expression, enabling Tregs to more effectively suppress T cell function, thereby limiting the efficacy of ICB therapy in this immunosuppressive environment. Studies have shown that directly eliminating TME hypoxia can improve cancer immunotherapy in mice [185, 186]. Li et al. demonstrated in a mouse model that the combination of hyperbaric oxygen therapy and chemotherapy agents, such as teniposide, against hepatocellular carcinoma can activate the cGAS-STING signaling pathway, thereby enhancing anti-tumor immune responses and increasing sensitivity to PD-1 antibody immunotherapy [187]. Bailey’s team found that targeting HIF-1α reduced PD-L1 expression on tumor cells and tumor-infiltrating myeloid cells. However, through an IFNγ-dependent mechanism, it elevated PD-L1 expression in normal tissues, abolished the PD-1/PD-L1 checkpoint in the TME, diminished Treg-mediated immunosuppression and enhanced immune tolerance checkpoint activity in normal tissues [188]. Therefore, HIF-1α inhibitors, such as PX-478, may be ideal partners for CTLA-4 targeted immunotherapy, offering potential therapeutic synergy [189].
Improve ICB efficacy by treg changes
Presently, researchers are exploring therapies capable of selectively depleting Tregs within tumors while augmenting ICB efficacy without triggering autoimmune responses. An optimal target would be one highly expressed on intra-tumoral Tregs, making the combination of Treg cell depletion with ICIs a potentially superior therapeutic approach [190]. Simultaneous targeting of two inhibitory receptors to enhance anti-tumor immune responses. Clinical agents under development, targeting molecules like CD25, CCR4, OX40, ICOS, or GITR, are being considered for use in conjunction with ICIs (Table 1). In the previous sections, we described a variety of existing popular targets and analyzed their relationship with regulatory Tregs in ICB therapy, highlighting the positive effects of combination therapy. Nevertheless, researchers are concurrently investigating novel Treg cell targets to attain highly specific targeting of intra-tumoral Tregs, thereby enhancing the efficacy of ICB.
Table 1.
Recent experiments on ICB tumor therapy were associated with the corresponding Treg cell changes
| Treatment Plan | Trial phase Trial registration |
Tumor types | Effects | Tregs |
|---|---|---|---|---|
| Ipilimumab + Cetuximab + Radiotherapy |
phase 1 |
Locally advanced head and neck cancer |
3-year PFS: 61%; 3-year DFS: 72%; 3-year OS: 72% |
Elimination of the less inhibitory Treg population may alter the balance of immune surveillance and immune escape |
| Camrelizumab + Apatinib + Oxaliplatin |
Phase II |
Locally advanced gastric cancer |
ORR: 28% CPR: 15.8% MPR: 26.3% |
Treg became more enriched in the tumor microenvironment post-treatment in some non-major pathological response (non-MPR) cases |
| Nivolumab + Ipilimumab |
Phase II/III |
Malignant pleural mesothelioma |
PFS: 6.25% OS: 23% |
Treatment caused changes in the Treg cell ratio and proliferation rate, reflecting the adjustment of the immune response |
| Ipilimumab + Rituximab |
Phase I |
Relapsed / Refractory B-cell lymphoma |
mPFS: 2.6 months ORR: 24% |
The CD45RA-Treg cell ratio increases after treatment and may help to predict treatment response |
| Mogamulizumab + Durvalumab |
Phase I |
Advanced solid tumors |
ORR: 5.3% mPFS: 1.9months mOS: 8.9months |
Most patients are almost completely depleted of peripheral eTreg with reduced intratumoral Treg |
| Ipilimumab + Nivolumab + Anthracycline-based |
Phase II |
Metastatic hormone receptor-positive breast cancer |
mPSF: 5.1months CBR: 55% |
Addition of the drug to chemotherapy did not improve efficacy but caused a decrease in Treg levels, indicating a degree of immunomodulatory activity |
| Nivolumab + Abemaciclib + Endocrine therapy |
Phase II JapicCTI-194,782 jRCT2080224706 UMIN000036970 |
HR-positive HER2- negative metastatic breast cancer |
ORR(FUL): 54.5% ORR(LET): 40.0% DCR(FUL): 90.9% DCR(LET): 80.0% |
Lower number of treg cells. Combination therapies have potential immunomodulatory effects, and monitoring Treg levels may help to understand therapeutic efficacy |
| Mogamulizumab + Nivolumab |
Phase I/II |
Locally advanced or metastatic solid tumors |
ORR: 10.5% mPFS: 2.6months mOS: 9.5months |
Treatment causes depletion of CCR 4 + effector Treg in the peripheral blood of most patients, benefiting Nivolumab play a role |
| Mogamulizumab + Nivolumab |
Phase I |
Advanced or metastatic solid tumors | Differ from one another | The observed depletion of effector Tregs in the peripheral blood and in the tumor microenvironment, may enhance the patient immune response |
| Pembrolizumab + Low-dose Cyclophosphamide + G100 (TLR4 agonist) |
Phase II |
Advanced pretreated soft tissue sarcoma |
mPFS: 1.8months mOS: 10.6months |
The reduced CD8 / FoxP 3 + CD4 ratio indicates an increase in Treg cells and may affect the limited efficacy of the treatment |
| Pembrolizumab + Pelareorep |
Phase II |
Advanced pancreatic adenocarcinoma |
mPFS: 1.9months mOS: 6.3months, |
The abundance of Treg cells decreased in the peripheral blood of the CBR group and not in Treg cells in the peripheral blood of the NR |
| Anti-GITR-TRX518 + Nivolumab |
Phase I |
Advanced solid tumors |
DCR: 50% ORR: 12.5% |
The Treg cells were initially reduced, but increased after the TRX518 dose |
| Durvalumab + Tremelimumab |
phase II |
Malignant Pleural Mesothelioma |
mOS<14.0months DFS<8.4months |
Both ICB regimens induced CD8 T-cell infiltration into MPM tumors but did not alter CD8/Treg ratios |
| Tiragolumab + Atezolizumab |
Phase IB |
Advanced HER2-negative breast cancer |
mPFS: 3.5 months mOS: 11 months |
Decreased circulating Treg cells in non-progressors, suggesting enhanced immune response against the tumor. |
Itahashi et al. found that the Basic Leucine Zipper ATF-like Transcription Factor (BATF) plays a vital role in activating Tregs in mice and the TME by analyzing TAC-seq and ChIP-seq data. Additionally, BATF+ Tregs were linked to poor clinical responses to PD-1 blockade [191]. Tregs activated by BATF showed higher expression of genes related to TCR and NF-κB signaling. Moreover, BATF might affect how Tregs move into the tumor area by downregulating specific chemokine receptor signaling genes [191]. Therefore, focusing on Tregs with high BATF expression alongside PD-1 blockade therapy is an important direction for future research. Additionally, endoglin acts as a co-receptor for TGFβ through immune mechanisms. Research has shown that Tregs expressing endoglin are present in both mouse and human rectal cancer tissues. A study by Schoonderwoerd and colleagues found that combining anti-endoglin and anti-PD-1 antibodies significantly improved treatment outcomes in various colorectal cancer models [192]. Li and others demonstrated that targeting Bcl6 in Tregs effectively slowed tumor growth and enhanced the effectiveness of ICB treatment when used with anti-CTLA-4 or anti-PD-1 therapies. These new targets show strong anti-tumor effects, expanding options for ICB therapy and offering new possibilities for future clinical use [193].
Tregs not only promote tumor escape but also help control immune-related toxicity in ICB therapy [194]. The number of Tregs is often not reduced under ICB therapy, and the modulation of either the number or function of these cells to enhance the effects of ICB therapy is a hot topic. In different tumor microenvironments, significant variations exist in the phenotype and function of Tregs. Recently, single-cell RNA sequencing technology has unveiled the diversity of Tregs in different tumor types [195]. For example, Tregs in breast cancer demonstrate high PD-1 and PD-L1 expression [196], while those in ovarian cancer display high PD-1 and 4-1BB expression [197]. This diversity can result in varying behaviors across different tumor microenvironments, potentially impacting ICB efficacy. In common tumors such as those of the lung and liver, Tregs play an immunosuppressive role. However, in colorectal cancer and head and neck tumors, Tregs are associated with a better prognosis, partly by suppressing microbial-induced inflammation and reducing susceptibility to tumors [198, 199]. Tregs situated in various parts of the tumor exhibit distinct functions. In colorectal cancer, Tregs in the tumor mesenchyme contribute to a better prognosis compared to those in the tumor nests and swelling margins [200]. For example, CCR8, targeting tumor-expanding Tregs specifically while preserving peripheral Tregs, represents an important candidate therapeutic target for future therapies. Surface Oncology has developed SRF114, a highly selective humanized anti-CCR8 desialylated antibody, currently undergoing a phase I clinical trial (NCT05635643). Preliminary results indicate that in patients with head and neck squamous cell carcinoma, SRF114 effectively reduces TI-Tregs and modifies the TME to facilitate immune attack [201].
Additionally, the number or function of Tregs can be modulated using low-dose adjuvant therapy or immunomodulators, enhancing ICB efficacy. Using low-dose chemotherapeutic agents or radiotherapy can selectively reduce the number of Tregs and, when combined with ICB therapy, decrease the immunosuppressive effect of Tregs [202, 203]. For example, the combination of Ipilimumab and Nivolumab with anthracycline chemotherapeutic agents (e.g., adriamycin) and Cyclophosphamide for metastatic hormone receptor-positive breast cancer has demonstrated clinical benefits following the discontinuation of chemotherapy in some patients, despite a higher risk of high-grade adverse events [204].
Other immunomodulators, including variants of IL-2, are primarily intended to enhance Teffs over Tregs, or to indirectly influence Treg cell function by modulating other immunosuppressive factors, such as PD-1/PD-L1 [205]. A low-affinity IL-2, developed by Ren et al. and combined with an anti-PD-1 antibody, targeted intratumorally infiltrating CD8+ T cells, without significantly affecting Tregs. This combination therapy not only significantly boosted the antitumor effect but also synergized with anti-PD-L1 therapy to overcome tumor resistance to immune checkpoint inhibition without significant toxicity [144]. Another study investigated the use of a PD-1-linked IL-2 agonist to enhance effector T-cell antitumor capacity and effectively overcome immunosuppression in chronic infection and cancer [206]. Other recent studies such as the use of MALT 1 inhibitors to reprogram Tregs to lose their immunosuppressive function and secrete INF-γ, thereby enhancing the immune response to tumors. This approach has shown potential benefits in combination with anti-PD-1 in animal models and is being evaluated in clinical trials [207].
Challenges and future directions
While ICB stands as a groundbreaking advancement in antitumor therapy, enhancing therapeutic outcomes, it also presents several limitations, particularly in terms of the role of Tregs. Initially, certain patients might develop resistance to ICB over time, a process underpinned by numerous intricate mechanisms [208]. Firstly, tumor-intrinsic factors, including insufficient tumor antigenicity, defective INF-γ signaling, and the absence of endogenous MHC, can contribute to ICB resistance [209]. Additionally, external factors in the TME, such as immunosuppressive Tregs, myeloid-derived suppressor cells (MDSCs), and TGF-β, also contribute to ICB resistance through their inhibitory effects [210]. Metabolic alterations within the TME further impair immune cell function, exacerbating drug resistance [211]. This article had explored the impact of Treg cell-mediated immunosuppression on treatment response and discusses potential strategies to overcome this challenge. Furthermore, ICB therapy can lead to chronic immune-related adverse events impacting various organs, such as the endocrine and rheumatologic systems, potentially affecting as many as 40% of patients [212, 213]. Moreover, the absence of reliable biomarkers for predicting patient responses to ICB therapy hinders the prediction of outcomes with ICB combination therapies and the discovery of novel targets [214]. Ultimately, the efficacy of ICB therapy exhibits considerable variation among patients and across different types of cancer, with some experiencing significant benefits while others see no response [209]. These challenges underscore the necessity for additional research and clinical trials to address these issues, enhance both the efficacy and safety of ICB therapy, and broaden its applicability across a more diverse patient demographic.
To achieve this goal, it is imperative to explore the intricate mechanisms of action of Tregs and the signaling pathways that connect their isoforms with distinct effector features. Understanding how Tregs influence the efficacy of ICB is essential to fully leveraging Tregs as an immunotherapeutic target [3, 215]. Future research endeavors might concentrate on the development of drugs designed specifically to target Treg in the TME. Such drugs, including antibody-drug conjugates, immunotoxins, and small interfering RNA conjugates, aim to either eliminate Tregs or inhibit their functions without compromising other immune cells [216].
Furthermore, targeted drug delivery systems represent a promising approach to specifically eliminate Tregs from the tumor environment, thereby bolstering the body’s anticancer response and augmenting the efficacy of ICB [217]. Particularly noteworthy is the advancement of nanodrug delivery systems, which encapsulate drug carriers within nanoparticles to accurately target tumor sites, enhancing drug stability and biocompatibility, extending the drug action duration, and significantly lowering toxicities. Nanoparticles in NDDS are specifically designed to target Tregs [218]. For example, in tumor models such as breast cancer 4T1 and colon cancer CT-26, the tumor-activated biomimetic lipoprotein carrier system is used to break through the intratumoral delivery barrier, efficiently deliver immunogenic cell death (ICD) inducers to intratumoral tumor cells and activate anti-tumor resistance. The tumor immune response weakens the dominance of Tregs and significantly enhances the therapeutic effect of immune checkpoint blockade (ICB) such as anti-PD-1 [219]. By combining targeted delivery systems with ICB therapy, it is possible to overcome the immunosuppression caused by Tregs, thereby improving the patient’s response rate to immunotherapy. This strategy is expected to become an important tool in future cancer immunotherapy [220].
Considering the variability among individuals, developing personalized medical strategies that adapt treatment regimens to the unique immune microenvironment and Treg characteristics of each patient is crucial. This development requires evaluating the efficacy and safety of diverse therapies via large-scale, multicenter clinical trials [221]. Research into Treg utilization in ICB therapy represents a rapidly advancing field, confronted with myriad challenges. By integrating the latest advancements in biotechnology, clinical trials, and data analysis, there is an expectation of significantly improving personalized oncology treatment strategies, enhancing therapeutic efficacy, and reducing side effects in the future.
Conclusion
In summary, Tregs embody a paradoxical nature, serving as a double-edged sword by both mitigating undue activation of the immune system to avert autoimmune diseases and facilitating tumor progression. As research into Tregs advances, a growing repertoire of Treg markers has been delineated within tumor tissues, offering insights into their roles and mechanisms of action in tumorigenesis. These markers hold potential not only as diagnostic biomarkers for cancer but also as therapeutic targets. Nevertheless, the translation of therapeutic interventions from disease samples, cellular, and animal models to clinical application remains limited. This gap underscores the need for extensive research to unearth the clinical utility of Tregs in cancer diagnostics and treatment. The challenge of indiscriminately depleting Tregs, which may trigger immune dysregulation, accentuates the importance of devising strategies that precisely target tumor-associated Tregs without compromising systemic immune homeostasis. ICB therapy has marked a significant leap in reinvigorating the immune system’s capacity to detect and eradicate cancer cells, addressing a pivotal tumor immune evasion tactic. Despite this progress, ICB therapy encounters obstacles, including therapy resistance and adverse effects. The trajectory of ICB therapy hinges on the adoption of personalized medical strategies, bolstered by genomic analysis, biomarker identification, and a nuanced understanding of the tumor microenvironment. In particular, the role of Tregs in modulating immune responses and their potential to diminish ICB therapy’s effectiveness is a pivotal research avenue, heralding new discoveries and enhancements in oncology. Furthermore, advancements in gene editing and cell engineering technologies portend the development of highly specialized Tregs, paving the way for more targeted and efficacious cancer therapies in the foreseeable future.
Acknowledgements
Not applicable.
Abbreviations
- Treg
Regulatory T
- ICB
immune checkpoint blockade
- CCL22
C-C motif chemokine ligand 22
- CCR4
C-C motif chemokine receptor 4
- TGF-β
transforming growth factor-β
- IL-35
interleukin-35
- IL-10
interleukin-10
- IL-2
interleukin-2
- CD25
cluster of differentiation 25
- CTLA-4
cytotoxic T-Lymphocyte-Associated protein 4
- CD80
cluster of differentiation 80
- APCs
antigen presenting cells
- TCR
T cell receptor
- CD28
cluster of differentiation 28
- tTreg
thymus-derived Tregs
- pTreg
peripherally induced Tregs
- nTregs
natural Tregs
- FoxP3+ Treg
forkhead box P3+ Treg
- iTreg
induced regulatory T cells
- CD39
cluster of differentiation 39
- CD73
cluster of differentiation 73
- PD-1
programmed death-1
- TIM-3
T cell immunoglobulin and mucin domain-containing protein 3
- TIGIT
T cell immunoreceptor with IG and ITIM domains
- GITR
glucocorticoid-induced TNFR-related protein
- OX40
tumor necrosis factor receptor superfamily member 4
- 4-1BB
tumor necrosis factor receptor superfamily member 9
- CCR8
C-C motif chemokine receptor 8
- TME
tumor microenvironment
- STAT5
signal transducer and activator of transcription 5
- ADCC
antibody-dependent cell-mediated cytotoxicity
- ADCP
antibody-dependent cell-mediated phagocytosis
- CTL
cytotoxic T lymphocyte
- NSCLC
non-small cell lung cancer
- IDO
indoleamine-2,3-dioxygenase
- LAG-3
lymphocyte activation gene-3
- ICIs
immune checkpoint inhibitors
- PD-L1
programmed cell death-ligand 1
- ICIs
immune checkpoint inhibitors
- irAEs
related adverse events
- TI-Tregs
tumor-infiltrating Tregs
Author contributions
AZ, TF, YL and GY conceptualized and designed the study and reviewed and revised the manuscript. AZ and TF were involved in data acquisition and analysis.GY, CL and ZJ supervised the study. All of the authors have read and approved the final manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2020AAA0109501), the CAMS Innovation Fund for Medical Sciences (CIFMS) (No. 2021-I2M-1-015), the National Natural Science Foundation of China (82473443) and the Beijing Natural Science Foundation (L248050, 7242119).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An Zhang, Tao Fan, Yixiao Liu and Guanhua Yu contributed equally to this work and share first authorship.
References
- 1.Zou W, Regulatory T. Cells, tumour immunity and immunotherapy. Nat Rev Immunol. 2006;6(4):295–307. [DOI] [PubMed] [Google Scholar]
- 2.Dikiy S, Rudensky AY. Principles of regulatory T cell function. Immunity. 2023;56(2):240–55. [DOI] [PubMed] [Google Scholar]
- 3.Li C, Jiang P, Wei S, Xu X, Wang J. Regulatory T cells in tumor microenvironment: new mechanisms, potential therapeutic strategies and future prospects. Mol Cancer. 2020;19(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun L, Su Y, Jiao A, Wang X, Zhang B. T cells in health and disease. Signal Transduct Target Therapy. 2023;8(1):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morad G, Helmink BA, Sharma P, Wargo JA. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell. 2021;184(21):5309–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng X, Hou Z, Qian Y, Zhang Y, Cui Q, Wang X, et al. Tumors evade immune cytotoxicity by altering the surface topology of NK cells. Nat Immunol. 2023;24(5):802–13. [DOI] [PubMed] [Google Scholar]
- 7.Huo JL, Wang YT, Fu WJ, Lu N, Liu ZS. The promising immune checkpoint LAG-3 in cancer immunotherapy: from basic research to clinical application. Front Immunol. 2022;13:956090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Miguel M, Calvo E. Clinical challenges of Immune Checkpoint inhibitors. Cancer Cell. 2020;38(3):326–33. [DOI] [PubMed] [Google Scholar]
- 9.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in Cancer: mechanisms of Action, Efficacy, and limitations. Front Oncol. 2018;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carlino MS, Larkin J, Long GV. Immune checkpoint inhibitors in melanoma. Lancet (London England). 2021;398(10304):1002–14. [DOI] [PubMed] [Google Scholar]
- 11.Lin EP, Hsu CY, Berry L, Bunn P, Shyr Y. Analysis of Cancer Survival Associated with Immune checkpoint inhibitors after Statistical Adjustment: a systematic review and Meta-analyses. JAMA Netw open. 2022;5(8):e2227211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garassino MC, Gadgeel S, Speranza G, Felip E, Esteban E, Dómine M, et al. Pembrolizumab Plus Pemetrexed and Platinum in Nonsquamous Non-small-cell Lung Cancer: 5-Year outcomes from the phase 3 KEYNOTE-189 study. J Clin Oncol. 2023;41(11):1992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma P, Hu-Lieskovan S, Wargo JA, Ribas A. Primary, adaptive, and Acquired Resistance to Cancer Immunotherapy. Cell. 2017;168(4):707–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar P, Saini S, Prabhakar BS. Cancer immunotherapy with check point inhibitor can cause autoimmune adverse events due to loss of Treg homeostasis. Semin Cancer Biol. 2020;64:29–35. [DOI] [PubMed] [Google Scholar]
- 15.Savage PA, Klawon DEJ, Miller CH. Regulatory T Cell Development. Annu Rev Immunol. 2020;38:421–53. [DOI] [PubMed] [Google Scholar]
- 16.Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T cells and human disease. Annu Rev Immunol. 2020;38:541–66. [DOI] [PubMed] [Google Scholar]
- 17.Raffin C, Vo LT, Bluestone JA. T(reg) cell-based therapies: challenges and perspectives. Nat Rev Immunol. 2020;20(3):158–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hippen KL, Hefazi M, Larson JH, Blazar BR. Emerging translational strategies and challenges for enhancing regulatory T cell therapy for graft-versus-host disease. Front Immunol. 2022;13:926550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohkura N, Sakaguchi S. Transcriptional and epigenetic basis of Treg cell development and function: its genetic anomalies or variations in autoimmune diseases. Cell Res. 2020;30(6):465–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Yang M, Tang L, Wang F, Huang S, Liu S, et al. TLR4 regulates RORγt(+) regulatory T-cell responses and susceptibility to colon inflammation through interaction with Akkermansia muciniphila. Microbiome. 2022;10(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dong Y, Yang C, Pan F. Post-translational regulations of Foxp3 in Treg Cells and their therapeutic applications. Front Immunol. 2021;12:626172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheru N, Hafler DA, Sumida TS. Regulatory T cells in peripheral tissue tolerance and diseases. Front Immunol. 2023;14:1154575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shao Q, Gu J, Zhou J, Wang Q, Li X, Deng Z, et al. Tissue tregs and maintenance of tissue homeostasis. Front Cell Dev Biol. 2021;9:717903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shakhar G, Lindquist RL, Skokos D, Dudziak D, Huang JH, Nussenzweig MC, et al. Stable T cell-dendritic cell interactions precede the development of both tolerance and immunity in vivo. Nat Immunol. 2005;6(7):707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Y, Yu D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol Ther. 2021;221:107753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanaka A, Sakaguchi S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017;27(1):109–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12(4):265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Śledzińska A, Vila de Mucha M, Bergerhoff K, Hotblack A, Demane DF, Ghorani E, et al. Regulatory T cells restrain Interleukin-2- and Blimp-1-Dependent Acquisition of cytotoxic function by CD4(+) T cells. Immunity. 2020;52(1):151–e666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Togashi Y, Shitara K, Nishikawa H. Regulatory T cells in cancer immunosuppression - implications for anticancer therapy. Nat Reviews Clin Oncol. 2019;16(6):356–71. [DOI] [PubMed] [Google Scholar]
- 30.Yan Y, Huang L, Liu Y, Yi M, Chu Q, Jiao D, et al. Metabolic profiles of regulatory T cells and their adaptations to the tumor microenvironment: implications for antitumor immunity. J Hematol Oncol. 2022;15(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Cui M, Sun Y, Liu S, Jiang W. Mechanisms, combination therapy, and biomarkers in cancer immunotherapy resistance. Cell Commun Signal. 2024;22(1):338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eggenhuizen PJ, Ng BH, Ooi JD. Treg Enhancing Therapies to treat Autoimmune diseases. Int J Mol Sci. 2020;21(19). [DOI] [PMC free article] [PubMed]
- 33.Cortez JT, Montauti E, Shifrut E, Gatchalian J, Zhang Y, Shaked O, et al. CRISPR screen in regulatory T cells reveals modulators of Foxp3. Nature. 2020;582(7812):416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schumann K, Raju SS, Lauber M, Kolb S, Shifrut E, Cortez JT, et al. Functional CRISPR dissection of gene networks controlling human regulatory T cell identity. Nat Immunol. 2020;21(11):1456–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferreira LMR, Muller YD, Bluestone JA, Tang Q. Next-generation regulatory T cell therapy. Nat Rev Drug Discovery. 2019;18(10):749–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikami N, Kawakami R, Sakaguchi S. New Treg cell-based therapies of autoimmune diseases: towards antigen-specific immune suppression. Curr Opin Immunol. 2020;67:36–41. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Lee DS, Liang Y, Zheng Y, Dixon JR. Foxp3 orchestrates reorganization of chromatin architecture to establish regulatory T cell identity. Nat Commun. 2023;14(1):6943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hori S. FOXP3 as a master regulator of T(reg) cells. Nat Rev Immunol. 2021;21(10):618–9. [DOI] [PubMed] [Google Scholar]
- 39.Aly MG, Ibrahim EH, Karakizlis H, Weimer R, Opelz G, Morath C, et al. CD4 + CD25 + CD127-Foxp3 + and CD8 + CD28- tregs in renal transplant recipients: phenotypic patterns, Association with immunosuppressive drugs, and Interaction with Effector CD8 + T cells and CD19 + IL-10 + bregs. Front Immunol. 2021;12:716559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powell MD, Read KA, Sreekumar BK, Oestreich KJ. Ikaros Zinc Finger transcription factors: regulators of Cytokine Signaling pathways and CD4(+) T helper cell differentiation. Front Immunol. 2019;10:1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delacher M, Simon M, Sanderink L, Hotz-Wagenblatt A, Wuttke M, Schambeck K, et al. Single-cell chromatin accessibility landscape identifies tissue repair program in human regulatory T cells. Immunity. 2021;54(4):702–e2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wegrzyn AS, Kedzierska AE, Obojski A. Identification and classification of distinct surface markers of T regulatory cells. Front Immunol. 2022;13:1055805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang R, Sun L, Li CF, Wang YH, Yao J, Li H, et al. Galectin-9 interacts with PD-1 and TIM-3 to regulate T cell death and is a target for cancer immunotherapy. Nat Commun. 2021;12(1):832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schnell A, Bod L, Madi A, Kuchroo VK. The Yin and Yang of co-inhibitory receptors: toward anti-tumor immunity without autoimmunity. Cell Res. 2020;30(4):285–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JH, Kim BS, Lee SK. Regulatory T Cells in Tumor Microenvironment and Approach for Anticancer Immunotherapy. Immune Netw. 2020;20(1):e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korbecki J, Grochans S, Gutowska I, Barczak K, Baranowska-Bosiacka I. CC chemokines in a Tumor: a review of Pro-cancer and Anti-cancer properties of receptors CCR5, CCR6, CCR7, CCR8, CCR9, and CCR10 ligands. Int J Mol Sci. 2020;21(20). [DOI] [PMC free article] [PubMed]
- 47.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yap TA, Parkes EE, Peng W, Moyers JT, Curran MA, Tawbi HA. Development of Immunotherapy combination strategies in Cancer. Cancer Discov. 2021;11(6):1368–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kang JH, Zappasodi R. Modulating Treg stability to improve cancer immunotherapy. Trends cancer. 2023;9(11):911–27. [DOI] [PubMed] [Google Scholar]
- 50.Scott EN, Gocher AM, Workman CJ, Vignali DAA, Regulatory T, Cells. Barriers of Immune Infiltration into the Tumor Microenvironment. Front Immunol. 2021;12:702726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McRitchie BR, Akkaya B. Exhaust the exhausters: targeting regulatory T cells in the tumor microenvironment. Front Immunol. 2022;13:940052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wing JB, Tanaka A, Sakaguchi S. Human FOXP3(+) Regulatory T cell heterogeneity and function in autoimmunity and Cancer. Immunity. 2019;50(2):302–16. [DOI] [PubMed] [Google Scholar]
- 53.Xu C, Li HB, Flavell RA. A special collection of reviews on frontiers in immunology. Cell Res. 2020;30(10):827–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zemmour D, Charbonnier LM, Leon J, Six E, Keles S, Delville M, et al. Single-cell analysis of FOXP3 deficiencies in humans and mice unmasks intrinsic and extrinsic CD4(+) T cell perturbations. Nat Immunol. 2021;22(5):607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dominguez-Villar M, Hafler DA. Regulatory T cells in autoimmune disease. Nat Immunol. 2018;19(7):665–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhuo C, Li Z, Xu Y, Wang Y, Li Q, Peng J, et al. Higher FOXP3-TSDR demethylation rates in adjacent normal tissues in patients with colon cancer were associated with worse survival. Mol Cancer. 2014;13:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Gong R, Zhao C, Lei K, Sun X, Ren H. Human FOXP3 and tumour microenvironment. Immunology. 2023;168(2):248–55. [DOI] [PubMed] [Google Scholar]
- 58.Grainger JR, Smith KA, Hewitson JP, McSorley HJ, Harcus Y, Filbey KJ, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-β pathway. J Exp Med. 2010;207(11):2331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Colamatteo A, Carbone F, Bruzzaniti S, Galgani M, Fusco C, Maniscalco GT, et al. Molecular mechanisms Controlling Foxp3 expression in Health and Autoimmunity: from epigenetic to post-translational regulation. Front Immunol. 2019;10:3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Revenko A, Carnevalli LS, Sinclair C, Johnson B, Peter A, Taylor M et al. Direct targeting of FOXP3 in Tregs with AZD8701, a novel antisense oligonucleotide to relieve immunosuppression in cancer. J Immunother Cancer. 2022;10(4). [DOI] [PMC free article] [PubMed]
- 61.Akimova T, Wang L, Bartosh Z, Eruslanov E, Albelda S, Singhal S, et al. Abstract 3255: Targeting CD4 + FOXP3 + Tregs to enhance anti-tumor immunity. Cancer Res. 2024;84(6Supplement):3255. [Google Scholar]
- 62.van der Veen EL, Suurs FV, Cleeren F, Bormans G, Elsinga PH, Hospers GAP, et al. Development and evaluation of Interleukin-2-Derived Radiotracers for PET Imaging of T Cells in mice. Journal of nuclear medicine: official publication. Soc Nuclear Med. 2020;61(9):1355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic kupffer cells. Hepatology (Baltimore MD). 2008;48(3):978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou P. Emerging mechanisms and applications of low-dose IL-2 therapy in autoimmunity. Cytokine Growth Factor Rev. 2022;67:80–8. [DOI] [PubMed] [Google Scholar]
- 65.Hernandez R, Põder J, LaPorte KM, Malek TR. Engineering IL-2 for immunotherapy of autoimmunity and cancer. Nat Rev Immunol. 2022;22(10):614–28. [DOI] [PubMed] [Google Scholar]
- 66.Ross SH, Cantrell DA. Signaling and function of Interleukin-2 in T lymphocytes. Annu Rev Immunol. 2018;36:411–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chinen T, Kannan AK, Levine AG, Fan X, Klein U, Zheng Y, et al. An essential role for the IL-2 receptor in T(reg) cell function. Nat Immunol. 2016;17(11):1322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oh DS, Kim H, Oh JE, Jung HE, Lee YS, Park JH, et al. Intratumoral depletion of regulatory T cells using CD25-targeted photodynamic therapy in a mouse melanoma model induces antitumoral immune responses. Oncotarget. 2017;8(29):47440–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Solomon I, Amann M, Goubier A, Arce Vargas F, Zervas D, Qing C, et al. CD25-T(reg)-depleting antibodies preserving IL-2 signaling on effector T cells enhance effector activation and antitumor immunity. Nat Cancer. 2020;1(12):1153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Arce Vargas F, Furness AJS, Solomon I, Joshi K, Mekkaoui L, Lesko MH, et al. Fc-Optimized Anti-CD25 depletes Tumor-Infiltrating Regulatory T Cells and synergizes with PD-1 blockade to Eradicate established tumors. Immunity. 2017;46(4):577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zammarchi F, Havenith K, Bertelli F, Vijayakrishnan B, Chivers S, van Berkel PH. CD25-targeted antibody-drug conjugate depletes regulatory T cells and eliminates established syngeneic tumors via antitumor immunity. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed]
- 72.Peng Y, Fu Y, Liu H, Zhao S, Deng H, Jiang X, et al. Non-IL-2-blocking anti-CD25 antibody inhibits tumor growth by depleting Tregs and has synergistic effects with anti-CTLA-4 therapy. Int J Cancer. 2024;154(7):1285–97. [DOI] [PubMed] [Google Scholar]
- 73.Wang F, Chau B, West SM, Kimberlin CR, Cao F, Schwarz F, et al. Structures of mouse and human GITR-GITRL complexes reveal unique TNF superfamily interactions. Nat Commun. 2021;12(1):1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buzzatti G, Dellepiane C, Del Mastro L. New emerging targets in cancer immunotherapy: the role of GITR. ESMO Open. 2020;4(Suppl 3):e000738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tian J, Zhang B, Rui K, Wang S. The role of GITR/GITRL Interaction in Autoimmune diseases. Front Immunol. 2020;11:588682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li Q, Lu J, Li J, Zhang B, Wu Y, Ying T. Antibody-based cancer immunotherapy by targeting regulatory T cells. Front Oncol. 2023;13:1157345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu MF, Jin C, Wu T, Chen EH, Lu M, Qin HL. Helios serves as a suppression marker to reduce regulatory T cell function in pancreatic cancer patients. Immunol Res. 2021;69(3):275–84. [DOI] [PubMed] [Google Scholar]
- 78.Zappasodi R, Sirard C, Li Y, Budhu S, Abu-Akeel M, Liu C, et al. Rational design of anti-GITR-based combination immunotherapy. Nat Med. 2019;25(5):759–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ono S, Suzuki S, Kondo Y, Okubo I, Goto M, Ogawa T, et al. Trametinib improves Treg selectivity of anti-CCR4 antibody by regulating CCR4 expression in CTLs in oral squamous cell carcinoma. Sci Rep. 2022;12(1):21678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu L, Xu X, Zhang B, Zhang R, Ji H, Wang X. Combined PD-1 blockade and GITR triggering induce a potent antitumor immunity in murine cancer models and synergizes with chemotherapeutic drugs. J Transl Med. 2014;12:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Davar D, Zappasodi R, Wang H, Naik GS, Sato T, Bauer T, et al. Phase IB Study of GITR agonist antibody TRX518 singly and in combination with Gemcitabine, Pembrolizumab, or Nivolumab in patients with Advanced Solid tumors. Clin Cancer Res. 2022;28(18):3990–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: can Treg cells be a new therapeutic target? Cancer Sci. 2019;110(7):2080–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Watanabe K, Gomez AM, Kuramitsu S, Siurala M, Da T, Agarwal S, et al. Identifying highly active anti-CCR4 CAR T cells for the treatment of T-cell lymphoma. Blood Adv. 2023;7(14):3416–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Korbecki J, Kojder K, Simińska D, Bohatyrewicz R, Gutowska I, Chlubek D et al. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4. International journal of molecular sciences. 2020;21(21). [DOI] [PMC free article] [PubMed]
- 85.Kohli K, Pillarisetty VG, Kim TS. Key chemokines direct migration of immune cells in solid tumors. Cancer Gene Ther. 2022;29(1):10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marshall LA, Marubayashi S, Jorapur A, Jacobson S, Zibinsky M, Robles O et al. Tumors establish resistance to immunotherapy by regulating T(reg) recruitment via CCR4. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed]
- 87.Li Y, Cao H, Jiang Z, Yan K, Shi J, Wang S, et al. CCL17 acts as an antitumor chemokine in micromilieu-driven immune skewing. Int Immunopharmacol. 2023;118:110078. [DOI] [PubMed] [Google Scholar]
- 88.Yoshie O. CCR4 as a therapeutic target for Cancer Immunotherapy. Cancers (Basel). 2021;13:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hong DS, Rixe O, Chiu VK, Forde PM, Dragovich T, Lou Y, et al. Mogamulizumab in Combination with Nivolumab in a phase I/II study of patients with locally advanced or metastatic solid tumors. Clin Cancer Res. 2022;28(3):479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kurose K, Ohue Y, Wada H, Iida S, Ishida T, Kojima T, et al. Phase Ia study of FoxP3 + CD4 Treg Depletion by infusion of a humanized Anti-CCR4 antibody, KW-0761, in Cancer patients. Clin Cancer Res. 2015;21(19):4327–36. [DOI] [PubMed] [Google Scholar]
- 91.Hirotsu KE, Neal TM, Khodadoust MS, Wang JY, Rieger KE, Strelo J, et al. Clinical characterization of Mogamulizumab-Associated Rash during treatment of Mycosis Fungoides or Sézary Syndrome. JAMA Dermatol. 2021;157(6):700–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gombert M, Dieu-Nosjean M-C, Winterberg F, Bünemann E, Kubitza RC, Da Cunha L, et al. CCL1-CCR8 interactions: an Axis mediating the Recruitment of T Cells and Langerhans-Type dendritic cells to sites of atopic skin Inflammation1. J Immunol. 2005;174(8):5082–91. [DOI] [PubMed] [Google Scholar]
- 93.Van Damme H, Dombrecht B, Kiss M, Roose H, Allen E, Van Overmeire E et al. Therapeutic depletion of CCR8(+) tumor-infiltrating regulatory T cells elicits antitumor immunity and synergizes with anti-PD-1 therapy. J Immunother Cancer. 2021;9(2). [DOI] [PMC free article] [PubMed]
- 94.Ueyama A, Nogami W, Nashiki K, Haruna M, Miwa H, Hagiwara M et al. Immunotherapy Targeting CCR8 + Regulatory T Cells Induces Antitumor Effects via Dramatic Changes to the Intratumor CD8 + T Cell Profile. Journal of immunology (Baltimore, Md: 1950). 2023;211(4):673 – 82. [DOI] [PubMed]
- 95.Wang T, Zhou Q, Zeng H, Zhang H, Liu Z, Shao J, et al. CCR8 blockade primes anti-tumor immunity through intratumoral regulatory T cells destabilization in muscle-invasive bladder cancer. Cancer Immunol Immunotherapy: CII. 2020;69(9):1855–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moser B. Chemokine receptor-targeted therapies: special case for CCR8. Cancers. 2022;14(3). [DOI] [PMC free article] [PubMed]
- 97.Nagira Y, Nagira M, Nagai R, Nogami W, Hirata M, Ueyama A, et al. S-531011, a Novel Anti-human CCR8 antibody, induces potent antitumor responses through Depletion of Tumor-Infiltrating CCR8-Expressing Regulatory T Cells. Mol Cancer Ther. 2023;22(9):1063–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Luo J, Huang W, Yang J, Li J, Li Y, Fei D, et al. Abstract 6008: effective depletion of tumor-infiltrating Tregs by a novel anti-CCR8 antibody (LM-108): addressing resistance associated with immune checkpoint inhibitors. Cancer Res. 2022;82(12Supplement):6008. [Google Scholar]
- 99.de Leve S, Wirsdörfer F, Jendrossek V. Targeting the Immunomodulatory CD73/Adenosine system to improve the therapeutic gain of Radiotherapy. Front Immunol. 2019;10:698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kepp O, Bezu L, Yamazaki T, Di Virgilio F, Smyth MJ, Kroemer G, et al. ATP and cancer immunosurveillance. EMBO J. 2021;40(13):e108130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Allard B, Allard D, Buisseret L, Stagg J. The adenosine pathway in immuno-oncology. Nat Reviews Clin Oncol. 2020;17(10):611–29. [DOI] [PubMed] [Google Scholar]
- 102.Yegutkin GG, Boison D. ATP and Adenosine Metabolism in Cancer: Exploitation for Therapeutic Gain. Pharmacol Rev. 2022;74(3):797–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xia C, Yin S, To KKW, Fu L. CD39/CD73/A2AR pathway and cancer immunotherapy. Mol Cancer. 2023;22(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Z, Liu X, Shen H, Xu X, Zhao X, Fu R. Adenosinergic axis and immune checkpoint combination therapy in tumor: a new perspective for immunotherapy strategy. Front Immunol. 2022;13:978377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vignali PDA, DePeaux K, Watson MJ, Ye C, Ford BR, Lontos K, et al. Hypoxia drives CD39-dependent suppressor function in exhausted T cells to limit antitumor immunity. Nat Immunol. 2023;24(2):267–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tu E, McGlinchey K, Wang J, Martin P, Ching SL, Floc’h N et al. Anti-PD-L1 and anti-CD73 combination therapy promotes T cell response to EGFR-mutated NSCLC. JCI Insight. 2022;7(3). [DOI] [PMC free article] [PubMed]
- 107.Hosseini A, Gharibi T, Marofi F, Babaloo Z, Baradaran B. CTLA-4: from mechanism to autoimmune therapy. Int Immunopharmacol. 2020;80:106221. [DOI] [PubMed] [Google Scholar]
- 108.Zhang A, Ren Z, Tseng KF, Liu X, Li H, Lu C, et al. Dual targeting of CTLA-4 and CD47 on T(reg) cells promotes immunity against solid tumors. Sci Transl Med. 2021;13:605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Watanabe T, Ishino T, Ueda Y, Nagasaki J, Sadahira T, Dansako H, et al. Activated CTLA-4-independent immunosuppression of Treg cells disturbs CTLA-4 blockade-mediated antitumor immunity. Cancer Sci. 2023;114(5):1859–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kennedy A, Waters E, Rowshanravan B, Hinze C, Williams C, Janman D, et al. Differences in CD80 and CD86 transendocytosis reveal CD86 as a key target for CTLA-4 immune regulation. Nat Immunol. 2022;23(9):1365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Erkers T, Stikvoort A, Uhlin M. Lymphocytes in placental tissues: Immune Regulation and translational possibilities for Immunotherapy. Stem Cells Int. 2017;2017:5738371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tay C, Tanaka A, Sakaguchi S. Tumor-infiltrating regulatory T cells as targets of cancer immunotherapy. Cancer Cell. 2023;41(3):450–65. [DOI] [PubMed] [Google Scholar]
- 113.Godfrey J, Chen X, Sunseri N, Cooper A, Yu J, Varlamova A et al. TIGIT is a key inhibitory checkpoint receptor in lymphoma. J Immunother Cancer. 2023;11(6). [DOI] [PMC free article] [PubMed]
- 114.Harjunpää H, Guillerey C. TIGIT as an emerging immune checkpoint. Clin Exp Immunol. 2020;200(2):108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, et al. Treg cells expressing the coinhibitory molecule TIGIT selectively inhibit proinflammatory Th1 and Th17 cell responses. Immunity. 2014;40(4):569–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chauvin JM, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8(2). [DOI] [PMC free article] [PubMed]
- 118.Chu X, Tian W, Wang Z, Zhang J, Zhou R. Co-inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: mechanisms and clinical trials. Mol Cancer. 2023;22(1):93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rousseau A, Parisi C, Barlesi F. Anti-TIGIT therapies for solid tumors: a systematic review. ESMO Open. 2023;8(2):101184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guan X, Hu R, Choi Y, Srivats S, Nabet BY, Silva J, et al. Anti-TIGIT antibody improves PD-L1 blockade through myeloid and T(reg) cells. Nature. 2024;627(8004):646–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chocarro L, Blanco E, Zuazo M, Arasanz H, Bocanegra A, Fernández-Rubio L et al. Understanding LAG-3 signaling. Int J Mol Sci. 2021;22(10). [DOI] [PMC free article] [PubMed]
- 122.Cai L, Li Y, Tan J, Xu L, Li Y. Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy. J Hematol Oncol. 2023;16(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Larson C, Oronsky B, Carter CA, Oronsky A, Knox SJ, Sher D, et al. TGF-beta: a master immune regulator. Expert Opin Ther Targets. 2020;24(5):427–38. [DOI] [PubMed] [Google Scholar]
- 124.Rueda CM, Jackson CM, Chougnet CA. Regulatory T-Cell-mediated suppression of conventional T-Cells and dendritic cells by different cAMP intracellular pathways. Front Immunol. 2016;7:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anz D, Mueller W, Golic M, Kunz WG, Rapp M, Koelzer VH, et al. CD103 is a hallmark of tumor-infiltrating regulatory T cells. Int J Cancer. 2011;129(10):2417–26. [DOI] [PubMed] [Google Scholar]
- 126.Qiu Z, Khairallah C, Chu TH, Imperato JN, Lei X, Romanov G et al. Retinoic acid signaling during priming licenses intestinal CD103 + CD8 TRM cell differentiation. J Exp Med. 2023;220(5). [DOI] [PMC free article] [PubMed]
- 127.Mousa AM, Enk AH, Hassel JC, Reschke R. Immune checkpoints and Cellular Landscape of the Tumor Microenvironment in Non-melanoma skin Cancer (NMSC). Cells. 2024;13(19):1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hong MMY, Maleki Vareki S. Addressing the Elephant in the immunotherapy room: Effector T-Cell Priming versus Depletion of Regulatory T-Cells by Anti-CTLA-4 therapy. Cancers (Basel). 2022;14(6). [DOI] [PMC free article] [PubMed]
- 129.Tekguc M, Wing JB, Osaki M, Long J, Sakaguchi S. Treg-expressed CTLA-4 depletes CD80/CD86 by trogocytosis, releasing free PD-L1 on antigen-presenting cells. Proc Natl Acad Sci U S A. 2021;118:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tanaka A, Sakaguchi S. Targeting Treg cells in cancer immunotherapy. Eur J Immunol. 2019;49(8):1140–6. [DOI] [PubMed] [Google Scholar]
- 131.Sharma A, Subudhi SK, Blando J, Scutti J, Vence L, Wargo J, et al. Anti-CTLA-4 Immunotherapy does not deplete FOXP3(+) Regulatory T cells (Tregs) in human cancers. Clin Cancer Res. 2019;25(4):1233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Huang AC, Zappasodi R. A decade of checkpoint blockade immunotherapy in melanoma: understanding the molecular basis for immune sensitivity and resistance. Nat Immunol. 2022;23(5):660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang Y, Du X, Liu M, Tang F, Zhang P, Ai C, et al. Hijacking antibody-induced CTLA-4 lysosomal degradation for safer and more effective cancer immunotherapy. Cell Res. 2019;29(8):609–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Marangoni F, Zhakyp A, Corsini M, Geels SN, Carrizosa E, Thelen M, et al. Expansion of tumor-associated Treg cells upon disruption of a CTLA-4-dependent feedback loop. Cell. 2021;184(15):3998–e401519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bolton HA, Zhu E, Terry AM, Guy TV, Koh WP, Tan SY, et al. Selective Treg reconstitution during lymphopenia normalizes DC costimulation and prevents graft-versus-host disease. J Clin Invest. 2015;125(9):3627–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Arce Vargas F, Furness AJS, Litchfield K, Joshi K, Rosenthal R, Ghorani E, et al. Fc effector function contributes to the activity of human Anti-CTLA-4 antibodies. Cancer Cell. 2018;33(4):649–e634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sanseviero E, O’Brien EM, Karras JR, Shabaneh TB, Aksoy BA, Xu W, et al. Anti-CTLA-4 activates Intratumoral NK Cells and combined with IL15/IL15Rα complexes enhances Tumor Control. Cancer Immunol Res. 2019;7(8):1371–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Korman AJ, Garrett-Thomson SC, Lonberg N. Author correction: the foundations of immune checkpoint blockade and the ipilimumab approval decennial. Nat Rev Drug Discov. 2022;21(2):163. [DOI] [PubMed] [Google Scholar]
- 139.Knorr DA, Blanchard L, Leidner RS, Jensen SM, Meng R, Jones A, et al. FcγRIIB is an Immune Checkpoint limiting the activity of Treg-Targeting antibodies in the Tumor Microenvironment. Cancer Immunol Res. 2024;12(3):322–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jenkins KA, Park M, Pederzoli-Ribeil M, Eskiocak U, Johnson P, Guzman W et al. XTX101, a tumor-activated, Fc-enhanced anti-CTLA-4 monoclonal antibody, demonstrates tumor-growth inhibition and tumor-selective pharmacodynamics in mouse models of cancer. J Immunother Cancer. 2023;11(12). [DOI] [PMC free article] [PubMed]
- 141.Somasundaram R, Connelly T, Choi R, Choi H, Samarkina A, Li L, et al. Tumor-infiltrating mast cells are associated with resistance to anti-PD-1 therapy. Nat Commun. 2021;12(1):346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms Controlling PD-L1 expression in Cancer. Mol Cell. 2019;76(3):359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Overacre-Delgoffe AE, Vignali DAA. Treg Fragility: a Prerequisite for Effective Antitumor Immunity? Cancer Immunol Res. 2018;6(8):882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boyer M, Şendur MAN, Rodríguez-Abreu D, Park K, Lee DH, Çiçin I, et al. Pembrolizumab Plus Ipilimumab or Placebo for metastatic non-small-cell lung Cancer with PD-L1 tumor proportion score ≥ 50%: Randomized, double-blind phase III KEYNOTE-598 study. J Clin Oncol. 2021;39(21):2327–38. [DOI] [PubMed] [Google Scholar]
- 146.Toor SM, Syed Khaja AS, Alkurd I, Elkord E. In-vitro effect of pembrolizumab on different T regulatory cell subsets. Clin Exp Immunol. 2018;191(2):189–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4 + CD25(hi) regulatory T cells. Int Immunol. 2009;21(9):1065–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. 2020;21(11):1346–58. [DOI] [PubMed] [Google Scholar]
- 149.Aksoylar HI, Boussiotis VA. PD-1(+) T(reg) cells: a foe in cancer immunotherapy? Nat Immunol. 2020;21(11):1311–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, et al. PD-1(+) regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci U S A. 2019;116(20):9999–10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Genova C, Dellepiane C, Carrega P, Sommariva S, Ferlazzo G, Pronzato P, et al. Therapeutic implications of Tumor Microenvironment in Lung Cancer: Focus on Immune Checkpoint Blockade. Front Immunol. 2021;12:799455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Geels SN, Moshensky A, Sousa RS, Murat C, Bustos MA, Walker BL, et al. Interruption of the intratumor CD8(+) T cell:Treg crosstalk improves the efficacy of PD-1 immunotherapy. Cancer Cell. 2024;42(6):1051–e667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.van Gulijk M, van Krimpen A, Schetters S, Eterman M, van Elsas M, Mankor J, et al. PD-L1 checkpoint blockade promotes regulatory T cell activity that underlies therapy resistance. Sci Immunol. 2023;8(83):eabn6173. [DOI] [PubMed] [Google Scholar]
- 154.Ferrara R, Mezquita L, Texier M, Lahmar J, Audigier-Valette C, Tessonnier L, et al. Hyperprogressive Disease in patients with Advanced Non-small Cell Lung Cancer treated with PD-1/PD-L1 inhibitors or with single-Agent Chemotherapy. JAMA Oncol. 2018;4(11):1543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tay C, Qian Y, Sakaguchi S. Hyper-progressive disease: the potential role and consequences of T-Regulatory cells foiling Anti-PD-1 Cancer Immunotherapy. Cancers (Basel). 2020;13(1). [DOI] [PMC free article] [PubMed]
- 156.Kim MJ, Kim K, Park HJ, Kim GR, Hong KH, Oh JH, et al. Deletion of PD-1 destabilizes the lineage identity and metabolic fitness of tumor-infiltrating regulatory T cells. Nat Immunol. 2023;24(1):148–61. [DOI] [PubMed] [Google Scholar]
- 157.Du F, Qi X, Zhang A, Sui F, Wang X, Proud CG, et al. MRTF-A-NF-κB/p65 axis-mediated PDL1 transcription and expression contributes to immune evasion of non-small-cell lung cancer via TGF-β. Exp Mol Med. 2021;53(9):1366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ansa-Addo EA, Zhang Y, Yang Y, Hussey GS, Howley BV, Salem M, et al. Membrane-organizing protein moesin controls Treg differentiation and antitumor immunity via TGF-β signaling. J Clin Invest. 2017;127(4):1321–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med. 2018;10(424). [DOI] [PubMed]
- 160.Niu M, Yi M, Wu Y, Lyu L, He Q, Yang R, et al. Synergistic efficacy of simultaneous anti-TGF-β/VEGF bispecific antibody and PD-1 blockade in cancer therapy. J Hematol Oncol. 2023;16(1):94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Shayan G, Ferris RL. PD-1 blockade upregulate TIM-3 expression as a compensatory regulation of immune check point receptors in HNSCC TIL. J Immunother Cancer. 2015;3(Suppl 2):P196. [Google Scholar]
- 162.Gu L, Khadaroo PA, Su H, Kong L, Chen L, Wang X, et al. The safety and tolerability of combined immune checkpoint inhibitors (anti-PD-1/PD-L1 plus anti-CTLA-4): a systematic review and meta-analysis. BMC Cancer. 2019;19(1):559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rotte A. Combination of CTLA-4 and PD-1 blockers for treatment of cancer. J Exp Clin Cancer Res. 2019;38(1):255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Naimi A, Mohammed RN, Raji A, Chupradit S, Yumashev AV, Suksatan W, et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun Signal. 2022;20(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Weber JS, Kudchadkar RR, Yu B, Gallenstein D, Horak CE, Inzunza HD, et al. Safety, efficacy, and biomarkers of nivolumab with vaccine in ipilimumab-refractory or -naive melanoma. J Clin Oncol. 2013;31(34):4311–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Du X, Liu M, Su J, Zhang P, Tang F, Ye P, et al. Uncoupling therapeutic from immunotherapy-related adverse effects for safer and effective anti-CTLA-4 antibodies in CTLA4 humanized mice. Cell Res. 2018;28(4):433–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Willsmore ZN, Coumbe BGT, Crescioli S, Reci S, Gupta A, Harris RJ, et al. Combined anti-PD-1 and anti-CTLA-4 checkpoint blockade: treatment of melanoma and immune mechanisms of action. Eur J Immunol. 2021;51(3):544–56. [DOI] [PubMed] [Google Scholar]
- 168.Yang F, Shay C, Abousaud M, Tang C, Li Y, Qin Z, et al. Patterns of toxicity burden for FDA-approved immune checkpoint inhibitors in the United States. J Exp Clin Cancer Res. 2023;42(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.O’Day S, khoueiry AE, Ramamurthy C, Bullock A, Shapiro I, Han H, et al. 398 AGEN1181, an fc engineered anti-CTLA-4 antibody, demonstrates clinical activity, alone or in combination with balstilimab (anti-PD-1), and broadens the therapeutic potential of CTLA-4 therapy. J Immunother Cancer. 2020;8(Suppl 3):A242–A. [Google Scholar]
- 170.Delepine C, Levey D, Krishnan S, Kim K-S, Sonabend A, Wilkens M, et al. 470 Botensilimab, an Fc-enhanced CTLA-4 antibody, enhances innate and adaptive immune activation to promote superior anti-tumor immunity in cold and I-O refractory tumors. J Immunother Cancer. 2022;10(Suppl 2):A490–A. [Google Scholar]
- 171.Feng X, Liao D, Liu D, Ping A, Li Z, Bian J. Development of indoleamine 2,3-Dioxygenase 1 inhibitors for Cancer Therapy and Beyond: a recent perspective. J Med Chem. 2020;63(24):15115–39. [DOI] [PubMed] [Google Scholar]
- 172.Röhrig UF, Reynaud A, Majjigapu SR, Vogel P, Pojer F, Zoete V. Inhibition mechanisms of indoleamine 2,3-Dioxygenase 1 (IDO1). J Med Chem. 2019;62(19):8784–95. [DOI] [PubMed] [Google Scholar]
- 173.Yentz S, Smith D. Indoleamine 2,3-Dioxygenase (IDO) inhibition as a strategy to Augment Cancer Immunotherapy. BioDrugs. 2018;32(4):311–7. [DOI] [PubMed] [Google Scholar]
- 174.Liberti MV, Locasale JW. The Warburg Effect: how does it Benefit Cancer cells? Trends Biochem Sci. 2016;41(3):211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Qin D, Zhang Y, Shu P, Lei Y, Li X, Wang Y. Targeting tumor-infiltrating tregs for improved antitumor responses. Front Immunol. 2024;15:1325946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ho PC, Kaech SM. Reenergizing T cell anti-tumor immunity by harnessing immunometabolic checkpoints and machineries. Curr Opin Immunol. 2017;46:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zappasodi R, Serganova I, Cohen IJ, Maeda M, Shindo M, Senbabaoglu Y, et al. CTLA-4 blockade drives loss of T(reg) stability in glycolysis-low tumours. Nature. 2021;591(7851):652–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Ding R, Yu X, Hu Z, Dong Y, Huang H, Zhang Y, et al. Lactate modulates RNA splicing to promote CTLA-4 expression in tumor-infiltrating regulatory T cells. Immunity. 2024;57(3):528–e406. [DOI] [PubMed] [Google Scholar]
- 179.Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. 2022;40(2):201–. – 18.e9. [DOI] [PubMed] [Google Scholar]
- 180.Lemberg KM, Gori SS, Tsukamoto T, Rais R, Slusher BS. Clinical development of metabolic inhibitors for oncology. J Clin Invest. 2022;132(1). [DOI] [PMC free article] [PubMed]
- 181.Noman MZ, Desantis G, Janji B, Hasmim M, Karray S, Dessen P, et al. PD-L1 is a novel direct target of HIF-1α, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med. 2014;211(5):781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Henze AT, Mazzone M. The impact of hypoxia on tumor-associated macrophages. J Clin Invest. 2016;126(10):3672–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Park JE, Dutta B, Tse SW, Gupta N, Tan CF, Low JK, et al. Hypoxia-induced tumor exosomes promote M2-like macrophage polarization of infiltrating myeloid cells and microRNA-mediated metabolic shift. Oncogene. 2019;38(26):5158–73. [DOI] [PubMed] [Google Scholar]
- 184.Arias C, Sepúlveda P, Castillo RL, Salazar LA. Relationship between hypoxic and Immune pathways activation in the progression of Neuroinflammation: role of HIF-1α and Th17 cells. Int J Mol Sci. 2023;24(4). [DOI] [PMC free article] [PubMed]
- 185.Wu Q, You L, Nepovimova E, Heger Z, Wu W, Kuca K, et al. Hypoxia-inducible factors: master regulators of hypoxic tumor immune escape. J Hematol Oncol. 2022;15(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sasidharan Nair V, Saleh R, Toor SM, Cyprian FS, Elkord E. Metabolic reprogramming of T regulatory cells in the hypoxic tumor microenvironment. Cancer Immunol Immunother. 2021;70(8):2103–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Li K, Gong Y, Qiu D, Tang H, Zhang J, Yuan Z et al. Hyperbaric oxygen facilitates teniposide-induced cGAS-STING activation to enhance the antitumor efficacy of PD-1 antibody in HCC. J Immunother Cancer. 2022;10(8). [DOI] [PMC free article] [PubMed]
- 188.Bailey CM, Liu Y, Liu M, Du X, Devenport M, Zheng P et al. Targeting HIF-1α abrogates PD-L1-mediated immune evasion in tumor microenvironment but promotes tolerance in normal tissues. J Clin Invest. 2022;132(9). [DOI] [PMC free article] [PubMed]
- 189.Shurin MR, Umansky V. Cross-talk between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy. J Clin Invest. 2022;132(9). [DOI] [PMC free article] [PubMed]
- 190.Ellis GI, Riley JL. How to kill T(reg) cells for immunotherapy. Nat cancer. 2020;1(12):1134–5. [DOI] [PubMed] [Google Scholar]
- 191.Itahashi K, Irie T, Yuda J, Kumagai S, Tanegashima T, Lin YT, et al. BATF epigenetically and transcriptionally controls the activation program of regulatory T cells in human tumors. Sci Immunol. 2022;7(76):eabk0957. [DOI] [PubMed] [Google Scholar]
- 192.Schoonderwoerd MJA, Koops MFM, Angela RA, Koolmoes B, Toitou M, Paauwe M, et al. Targeting Endoglin-Expressing Regulatory T Cells in the Tumor Microenvironment enhances the effect of PD1 checkpoint inhibitor immunotherapy. Clin Cancer Res. 2020;26(14):3831–42. [DOI] [PubMed] [Google Scholar]
- 193.Li Y, Wang Z, Lin H, Wang L, Chen X, Liu Q, et al. Bcl6 preserves the Suppressive Function of Regulatory T Cells during Tumorigenesis. Front Immunol. 2020;11:806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Iglesias-Escudero M, Arias-González N, Martínez-Cáceres E. Regulatory cells and the effect of cancer immunotherapy. Mol Cancer. 2023;22(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Sjaastad LE, Owen DL, Tracy SI, Farrar MA. Phenotypic and Functional Diversity in Regulatory T Cells. Front Cell Dev Biol. 2021;9:715901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Liu J, Wang X, Deng Y, Yu X, Wang H, Li Z. Research Progress on the Role of Regulatory T Cell in Tumor Microenvironment in the treatment of breast Cancer. Front Oncol. 2021;11:766248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Toker A, Nguyen LT, Stone SC, Yang SYC, Katz SR, Shaw PA, et al. Regulatory T cells in Ovarian Cancer are characterized by a highly activated phenotype distinct from that in Melanoma. Clin Cancer Res. 2018;24(22):5685–96. [DOI] [PubMed] [Google Scholar]
- 198.Okuyama K, Naruse T, Yanamoto S. Tumor microenvironmental modification by the current target therapy for head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2023;42(1):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Qu X, Tang Y, Hua S. Immunological approaches towards Cancer and inflammation: a Cross talk. Front Immunol. 2018;9:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li Y, Zhang C, Jiang A, Lin A, Liu Z, Cheng X, et al. Potential anti-tumor effects of regulatory T cells in the tumor microenvironment: a review. J Translational Med. 2024;22(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Panduro M, Ren Y, Masia R, Yang Y, Lake AC, Palombella VJ, et al. Abstract 5125: depletion of CCR8 + tumor Treg cells with SRF114 or anti-CCR8 therapy promotes robust antitumor activity and reshapes the tumor microenvironment toward a more pro-inflammatory milieu. Cancer Res. 2023;83(7Supplement):5125. [Google Scholar]
- 202.Vafaei S, Zekiy AO, Khanamir RA, Zaman BA, Ghayourvahdat A, Azimizonuzi H, et al. Combination therapy with immune checkpoint inhibitors (ICIs); a new frontier. Cancer Cell Int. 2022;22(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Li JY, Chen YP, Li YQ, Liu N, Ma J. Chemotherapeutic and targeted agents can modulate the tumor microenvironment and increase the efficacy of immune checkpoint blockades. Mol Cancer. 2021;20(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Andresen NK, Røssevold AH, Quaghebeur C, Gilje B, Boge B, Gombos A et al. Ipilimumab and Nivolumab combined with anthracycline-based chemotherapy in metastatic hormone receptor-positive breast cancer: a randomized phase 2b trial. J Immunother Cancer. 2024;12(1). [DOI] [PMC free article] [PubMed]
- 205.Holcomb EA, Zou W. A forced marriage of IL-2 and PD-1 antibody nurtures tumor-infiltrating T cells. J Clin Investig. 2022;132(3). [DOI] [PMC free article] [PubMed]
- 206.Hashimoto M, Araki K, Cardenas MA, Li P, Jadhav RR, Kissick HT, et al. PD-1 combination therapy with IL-2 modifies CD8(+) T cell exhaustion program. Nature. 2022;610(7930):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Keller P, Mazo I, Gao Y, Reddy V, Caballero F, Kazer S, et al. Abstract P106: reprogramming regulatory T cells (Treg) using a MALT1 inhibitor for cancer therapy. Mol Cancer Ther. 2021;20(12Supplement):P106–P. [Google Scholar]
- 208.Gu SS, Wang X, Hu X, Jiang P, Li Z, Traugh N, et al. Clonal tracing reveals diverse patterns of response to immune checkpoint blockade. Genome Biol. 2020;21(1):263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Kalbasi A, Ribas A. Tumour-intrinsic resistance to immune checkpoint blockade. Nat Rev Immunol. 2020;20(1):25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Lee J, Kim EH. Mechanisms underlying response and resistance to immune checkpoint blockade in cancer immunotherapy. Front Oncol. 2023;13:1233376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Li H, Zhou L, Zhou J, Li Q, Ji Q. Underlying mechanisms and drug intervention strategies for the tumour microenvironment. J Exp Clin Cancer Res. 2021;40(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19(4):254–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Patrinely JR Jr., Johnson R, Lawless AR, Bhave P, Sawyers A, Dimitrova M, et al. Chronic Immune-related adverse events following adjuvant Anti-PD-1 therapy for high-risk Resected Melanoma. JAMA Oncol. 2021;7(5):744–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Ciccolini J, Milano G. Immune check points in cancer treatment: current challenges and perspectives. Br J Cancer. 2023;129(9):1365–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Grover P, Goel PN, Greene MI, Regulatory T, Cells. Regulation of identity and function. Front Immunol. 2021;12:750542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Pandey PR, Young KH, Kumar D, Jain N. RNA-mediated immunotherapy regulating tumor immune microenvironment: next wave of cancer therapeutics. Mol Cancer. 2022;21(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Yang J, Bae H. Drug conjugates for targeting regulatory T cells in the tumor microenvironment: guided missiles for cancer treatment. Exp Mol Med. 2023;55(9):1996–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhang J, Wang S, Zhang D, He X, Wang X, Han H, et al. Nanoparticle-based drug delivery systems to enhance cancer immunotherapy in solid tumors. Front Immunol. 2023;14:1230893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Li J, Wang H, Wang Y, Gong X, Xu X, Sha X, et al. Tumor-activated size-enlargeable Bioinspired Lipoproteins Access Cancer cells in Tumor to Elicit Anti-tumor Immune responses. Adv Mater. 2020;32(38):e2002380. [DOI] [PubMed] [Google Scholar]
- 220.Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P. Nanoparticle-based immunotherapy for cancer. ACS Nano. 2015;9(1):16–30. [DOI] [PubMed] [Google Scholar]
- 221.Serr I, Kral M, Scherm MG, Daniel C. Advances in human Immune System Mouse models for Personalized Treg-based immunotherapies. Front Immunol. 2021;12:643544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.