Abstract
The cardiovascular exposome encompasses the array of external and internal factors affecting cardiovascular health throughout life, inviting comprehensive monitoring and analysis to enhance prevention, diagnosis, and treatment strategies. Wearable and digital technologies have emerged as promising tools in this domain, offering longitudinal, real-time data on physiological parameters such as heart rate, heart rhythm, physical activity, and sleep patterns. This review explores the advancements in wearable sensor technology, the methodologies for data collection and analysis, and the integration of these technologies into clinical practice and research. Primary findings indicate significant improvements in device accuracy and functionality, facilitated by enhanced sensor technology, artificial intelligence, and data connectivity. These advancements enable precise monitoring, early detection of cardiovascular anomalies, and personalized healthcare interventions. Ultimately, wearables and digital health technologies have the potential to facilitate a deeper understanding of cardiovascular disease and behavior and bridge gaps in traditional healthcare models to help usher in more efficient, personalized, patient-centered care.
Keywords: wearable technologies, cardiovascular exposome, digital health, remote monitoring, personalized health care
Introduction
The cardiovascular exposome represents the comprehensive set of external and internal factors influencing cardiovascular health throughout an individual’s life. These factors include environmental exposures, lifestyle choices, and biological responses, all contributing to the development and progression of cardiovascular diseases (CVDs).1 As CVDs remain a leading cause of death in the United States (US) and globally,2 there is a critical need to better understand the interplay and longitudinal impact of these factors to improve prevention, diagnosis, and treatment strategies.
In recent years, wearable technologies have emerged as powerful tools in the field of cardiovascular health. These devices, which include smartwatches, fitness trackers, and implantable sensors, provide longitudinal, real-time data on various physiological parameters. By monitoring metrics such as heart rate, heart rhythm, physical activity, and sleep, wearable devices may offer valuable insights into an individual’s cardiovascular health and lifestyle behaviors.3
Advancements in sensor technology have significantly enhanced the accuracy and functionality of wearable devices, making them promising tools for both research and clinical practice.4 This review aims to explore the role of wearable technologies in decoding the cardiovascular exposome. We discuss the types of wearable devices available, the key metrics they monitor, and the methods of data collection and analysis they implore. Furthermore, we explore how wearable technology could disrupt traditional healthcare models to help usher in more efficient, patient-centered care while navigating challenges such as device accuracy and socioeconomic disparities, which are crucial for maximizing its impact on healthcare outcomes.
Wearable Technologies in Cardiovascular Health
Overview of Wearable Technologies
Wearables have been defined as “forms of technology that are worn on the body, such as smartwatches or adhesive patches containing sensors…that perform a useful function for the wearer or a caregiver.”5 The popularity of wearable devices and associated health apps has surged, with millions of people globally using these technologies to track their health. It is predicted that wearable devices shipped worldwide will increase from 320 million units in 2022 to nearly 440 million by the end of 2024.6 Common wearables include devices such as smartwatches, smart patches, and smart rings, all designed to monitor various health metrics longitudinally. These devices fall under the broader category of Digital Health Technologies (DHTs), which encompass a wide range of tools and applications aimed at monitoring, diagnosing, and managing health conditions longitudinally.
Importance in Cardiovascular Health
In recent years, certain DHTs have been validated against gold-standard medical-grade devices and have shown high sensitivity and specificity for heart rate and detection of atrial fibrillation (AFib).7 DHT offers a new avenue for the early detection of cardiac events, close monitoring of chronic conditions complicated by frequent hospitalizations, and personalized health interventions. By enabling patients to closely monitor their cardiovascular health outside the traditional clinical settings, wearables have the potential to contribute to proactive health management and timely medical interventions.
Recent Technological Advances
Technological advancements in wearable devices for noninvasive monitoring have significantly improved their accuracy and functionality through enhanced sensor technology, advanced data analytics, and improved connectivity. Innovations such as highly sensitive multi-parameter sensors, artificial intelligence (AI) and machine learning (ML) algorithms for real-time data analysis, and integration with cellular hubs may enable accurate longitudinal monitoring and personalized health insights, transforming the way we manage common cardiovascular problems.8,9 The ongoing integration of wearable technologies with telehealth platforms allows for data sharing between patients and healthcare professionals, which could promote better-informed clinical decisions and remote care.
Impact on Health Care
Wearable health devices have already had a significant impact on US health care. Real-time data collection helps in the early detection and management of chronic conditions, including cardiovascular diseases, enabling timely interventions and potentially reducing the need for frequent hospital visits. Furthermore, these devices empower patients by providing continuous feedback on their health, which can encourage better self-management. This engagement also extends to lifestyle management, as wearables can track physical activity and sleep, helping users make informed decisions about their health.10 Remote patient monitoring also has the potential to contribute to healthcare efficiency and has been shown to reduce acute care use for certain patients with cardiovascular disease.11 According to a KLAS Research report in 2018, 38% of healthcare organizations using remote patient monitoring technologies reported a direct link to reduced hospital admissions, and 17% cited measurable cost savings.12 This review focuses on some of the most commonly used wearable digital health devices (Figure 1).13
Figure 1.
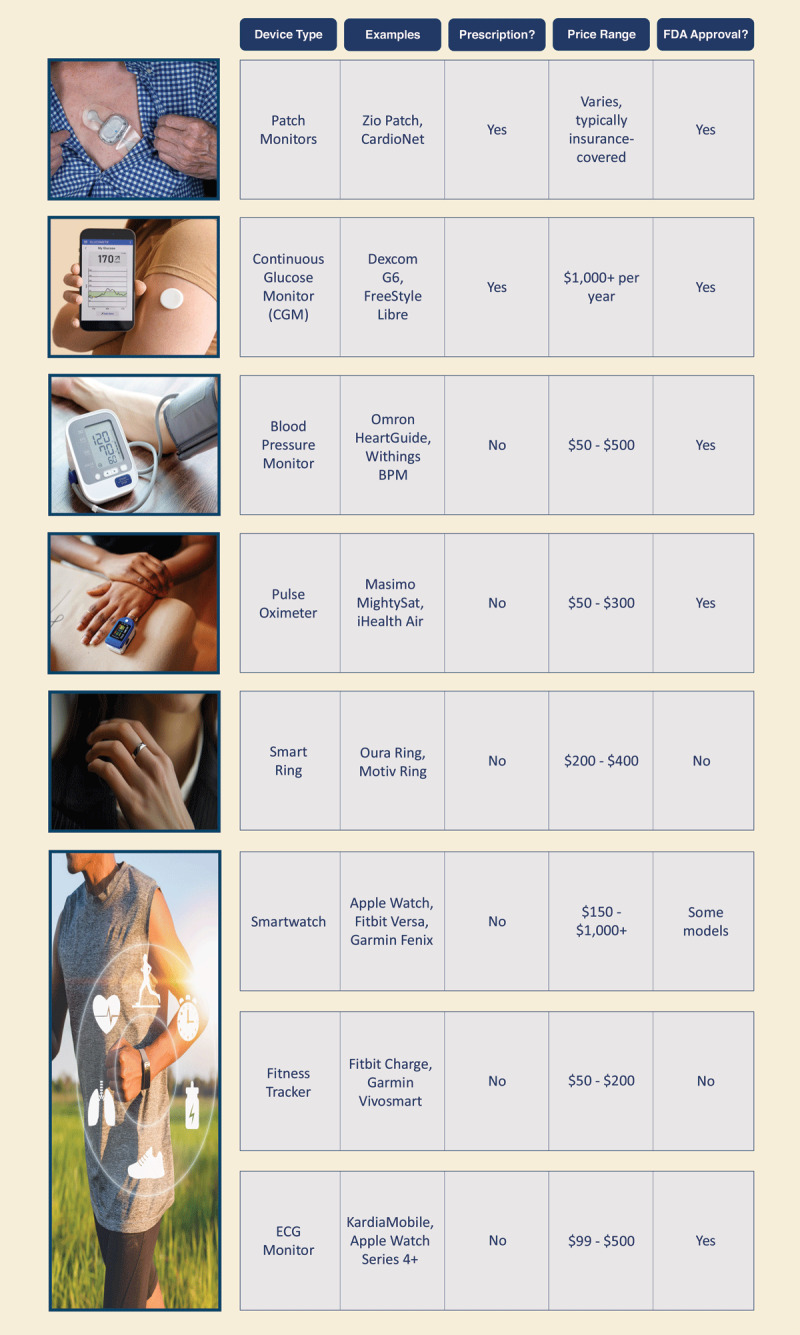
Overview of various wearable devices used in health care, including their types, examples, prescription status, price ranges, and US Food and Drug Administration approval status. The table highlights different categories such as patch monitors, continuous glucose monitors (CGMs), blood pressure monitors, pulse oximeters, smart rings, virtual headsets, smartwatches, fitness trackers, and electrocardiographic monitors. The information underscores the diversity of wearable health technologies and their varying levels of accessibility and regulatory approval.13
Wrist Worn Smartwatches
The smartwatch has become an integral wearable device to monitor personal health data and impact management. A Pew Research Center survey conducted in June 2019 found that 21% of US adults regularly use a smartwatch or fitness tracker.14 These devices provide a wide array of health-related features, combining many functionalities found on separate wearable devices. The convenience and accessibility of smartwatches have driven their popularity and adoption for cardiovascular monitoring. In a survey conducted by the National Heart, Lung, and Blood Institute of wearable device users, 38% of adults with cardiovascular disease used it daily compared with almost half of other adults. It was also found that fewer than one in four adults with or at risk of cardiovascular disease use a wearable device such as a smartwatch to monitor their health.15 This may suggest that while the convenience and accessibility of smartwatches are improving, their adoption for cardiovascular health management remains limited.
Most smartwatches are equipped with photoplethysmography (PPG), accelerometry, and electrodes. This allows the device to continuously or intermittently measure oxygen saturation, heart rate, and cardiac rhythm.7 Recent advancements in smartwatch electrocardiogram (ECG) technology have significantly enhanced their capabilities for monitoring cardiovascular health.16 Using two contact points on the smartwatch, a user can obtain a 30-second rhythm strip by creating a vector from the left arm to the right arm (lead I). More recently, when connected to a smartphone that is equipped with an electrode for the leg region, the user can produce a six-vector ECG tracing. Studies have also demonstrated the high sensitivity and specificity of consumer ECG devices for detecting AFib.17 Indeed, the 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation advised that it is reasonable to use consumer-accessible ECG devices in rhythm monitoring.18
Fitness Trackers
Fitness trackers, while similar in function to smartwatches, are distinct in their design and primary use. These devices, often more streamlined and focused on health metrics, are designed to be worn continuously, offering detailed tracking of physical activity, sleep patterns, and, in some cases, heart rate monitoring. Unlike smartwatches, which often include a range of non-health-related features, fitness trackers are generally more specialized in their approach to health data collection.
In one of the largest studies of its kind, the National Institute of Health’s research program “All of Us” demonstrated that higher daily step counts, as tracked by fitness devices like Fitbits, were associated with a reduced risk of several chronic diseases, including obesity, hypertension, and diabetes.19 Participants who consistently took over 10,000 steps per day saw even greater health benefits, including 44% reduced risk of type 2 diabetes and substantial reductions in obesity (-41%), depression (-33%), gastroesophageal reflux disease (-36%), high blood pressure (-25%), and sleep apnea (-46%).19
Smart Patches
A wearable patch is a device that is typically prescribed for cardiovascular disease management. They are small, adhesive devices that continuously monitor various physiological parameters by sticking to the surface of the skin.20 These patches are designed with comfort and convenience to facilitate extended wear (typically up to about 2 weeks), enabling a continuous feed of data without interrupting the user’s daily activities. They function by using advanced sensors embedded within the adhesive material and can capture the electrical activity of the heart through electrodes in contact with skin, providing continuous ECG monitoring with additional sensors to track vital signs. Some smart patches can deliver near real-time analysis and reporting, whereas others provide analysis only after the entire wear period has concluded.
Several key studies underscore the effectiveness of these devices in improving diagnostic accuracy and patient outcomes. For instance, the mHealth Screening To Prevent Strokes (mSToPS) trial demonstrated that Zio patches (iRhythm Technologies, Inc.) are particularly effective in detecting asymptomatic AFib in high-risk populations, with significant implications for early intervention and stroke prevention.21,22 More recently, the Cardiac Ambulatory Monitor Evaluation of Outcomes and Time to Events (CAMELOT) study, which analyzed data from more than 287,000 Medicare patients, highlighted that the Zio patch not only achieved the highest diagnostic yield for arrhythmias but also significantly reduced the need for retesting and subsequent healthcare utilization.23
Smart Rings
The smart ring has since emerged as a compact and convenient alternative to other wearable health devices. Most smart rings are designed to be worn continuously, typically on the middle or index finger. Commercially available smart rings use PPG to monitor heart rate, oxygen saturation, and rhythm. Some devices also include accelerometers that provide the data necessary to track physical activity and sleep patterns.
One study validated the accuracy of smart rings, specifically the Oura ring (Oura Health), in monitoring heart rate in real-time.24 The study demonstrated that the Oura ring’s heart rate measurements were highly comparable to those obtained through standard ECG monitoring. This finding underscores the potential of smart rings to provide reliable, continuous heart rate data, which is useful for managing patients with CVD. Continuous heart rate monitoring can help detect arrhythmias, monitor stress levels, and track overall cardiovascular health, enabling timely interventions and personalized care strategies.24
Data Collection & Analysis
Wearable devices such as smartwatches, fitness trackers, and patch monitors are equipped with sensors such as accelerometers, ECG, and PPG that gather real-time physiological data (Figure 2).25 These sensors measure various health metrics including heart rate/rhythm, physical activity, and sleep patterns. The integration of these sensors with sophisticated data analytics algorithms may enable the detection of abnormalities, prediction of health issues, and generation of personalized health insights. By synchronizing with smartphones and health applications, these devices facilitate long-term health tracking and enable users to share their data with clinicians, thus enhancing the potential for proactive health management and improved clinical outcomes (Figure 3). The following section delves into the various methods of data collection utilized by wearable technologies and the advanced analytical techniques employed to process and interpret these data, emphasizing their role in modern health care.
Figure 2.
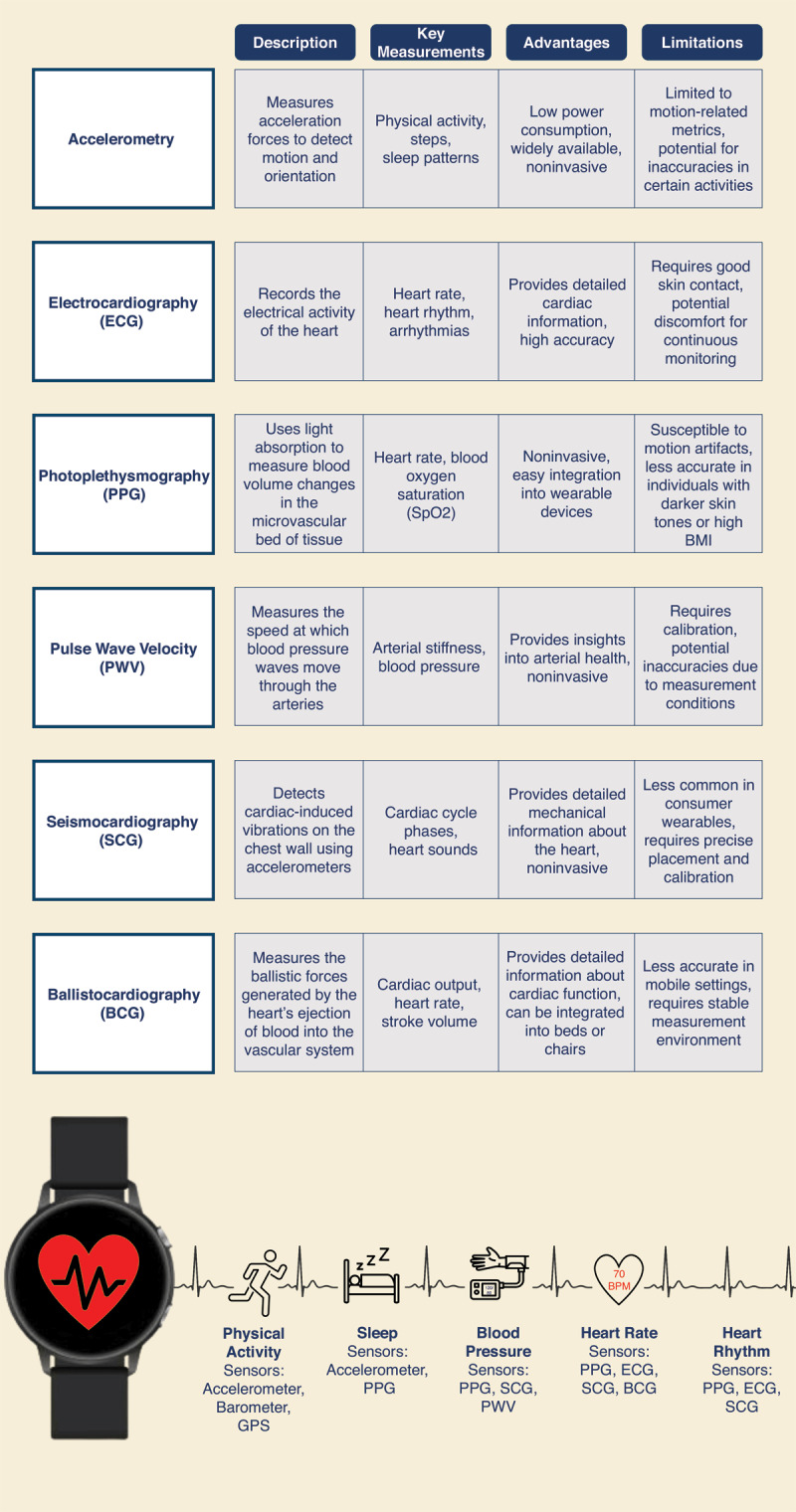
Summary of key measurements and technologies used in wearable health devices, including accelerometry, electrocardiography (ECG), photoplethysmography (PPG), pulse wave velocity (PWV), seismocardiography (SCG), and ballistocardiography (BCG). Each technology is described along with its primary applications, advantages, and limitations. The figure provides an overview of how these sensors contribute to monitoring various physiological parameters such as heart rate, blood pressure, physical activity, and sleep patterns, highlighting the potential and challenges of each measurement method.25
Figure 3.
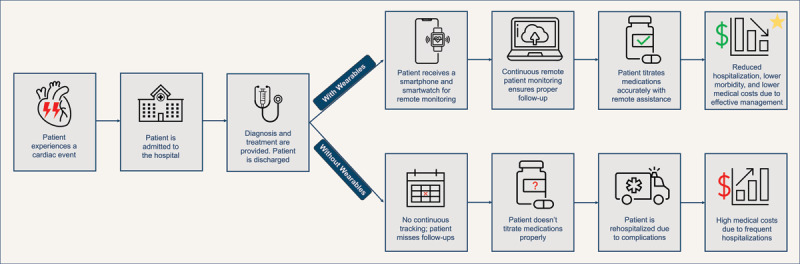
Comparison of patient outcomes with and without the use of wearable devices for remote monitoring. The figure illustrates a scenario where a patient receives a smartphone and smartwatch for continuous remote monitoring, leading to accurate medication titration, reduced hospitalizations, and better health outcomes. In contrast, the absence of wearable technology results in missed follow-ups, improper medication management, and higher medical costs due to frequent hospitalizations. The figure underscores the potential benefits of wearables in improving patient management and reducing healthcare costs.
Photoplethysmography
Photoplethysmography is a noninvasive optical technique used in wearable devices to measure heart rate, oxygen saturation, and cardiac rhythm. It works by shining light into the skin and detecting changes in light absorption caused by blood flow.26 For example, during systole, more blood is present in the arteries, leading to greater light absorption and reduced reflection. These changes are processed to produce a waveform, which can be analyzed to extract various physiologic metrics.26 PPG is used widely in smartwatches and fitness trackers due to its ease of use and continuous monitoring capabilities.
Pulse Wave Velocity
Pulse wave velocity measures the speed at which blood pressure pulses travel through the arterial tree. It is an indicator of artery stiffness, with high values indicating stiffer arteries, which are associated with an increased risk of cardiovascular events.27
Accelerometry
Accelerometers in wearable devices measure movement and activity levels by detecting changes in velocity and direction. They operate using three main principles: capacitive, piezoresistive, and piezoelectric effects.28 Capacitive accelerometers measure changes in capacitance caused by movement, piezoresistive accelerometers detect changes in electrical resistance due to stress or strain on a material, and piezoelectric accelerometers generate an electric charge in response to mechanical stress. These sensors provide data on how fast and in which direction the device is moving, allowing the device to track physical activities such as steps taken, distance traveled, and overall activity levels.
Seismocardiography
This noninvasive technology uses an accelerometer to detect signals from the local vibration of the chest wall that is emitted from the movements of the heart and its valves.29 In addition, this technology has been of interest for assessing the clinical status of patients with heart failure.30
Ballistocardiography
Ballistocardiography is a noninvasive technique that measures the mechanical activity of the heart by detecting the body’s subtle movements caused by blood ejected with each heartbeat.31 It works by using sensors placed on a modified weighing scale, bed, or table system to record time recoil and movements in the cranial to caudal direction during systole.31 Similar to PPG, wearable devices incorporating ballistocardiography technology can monitor heart rate and heart rate variability29 and are of interest in managing patients with heart failure.32
Applications of Wearable Data in Cardiovascular Diseases
Wearables hold promise in addressing barriers in the episodic traditional healthcare model. In addition to its episodic nature, the traditional healthcare model can be financially and logistically burdensome for patients who live far from healthcare facilities or must take time off work for appointments, exacerbating disparities in healthcare access.33 The application of wearables in conditions such as AFib and coronary artery disease holds promise for enhancing early detection, monitoring, and personalized treatment strategies.
Applications in Measuring Environmental Exposures
Data captured by wearables reaches beyond traditional health measures to encompass environmental exposures, providing a more comprehensive understanding of the cardiovascular exposome. The National Academies of Sciences, Engineering, and Medicine 2022 report highlights several practical applications of wearable technologies in environmental health monitoring.34 For instance, wearables can measure variations in air pollution levels and correlate these data with cardiovascular diseases and/or events. Furthermore, these devices can track other environmental stressors, such as noise and heat, providing a multifaceted view of the urban exposome. Multiparameter assessment of environmental factors via wearables may help address the complex and interrelated nature of environmental exposures. Overall, the integration of wearable data into larger epidemiological studies could enhance our understanding of how environmental factors are associated with cardiovascular health (Figure 4).
Figure 4.
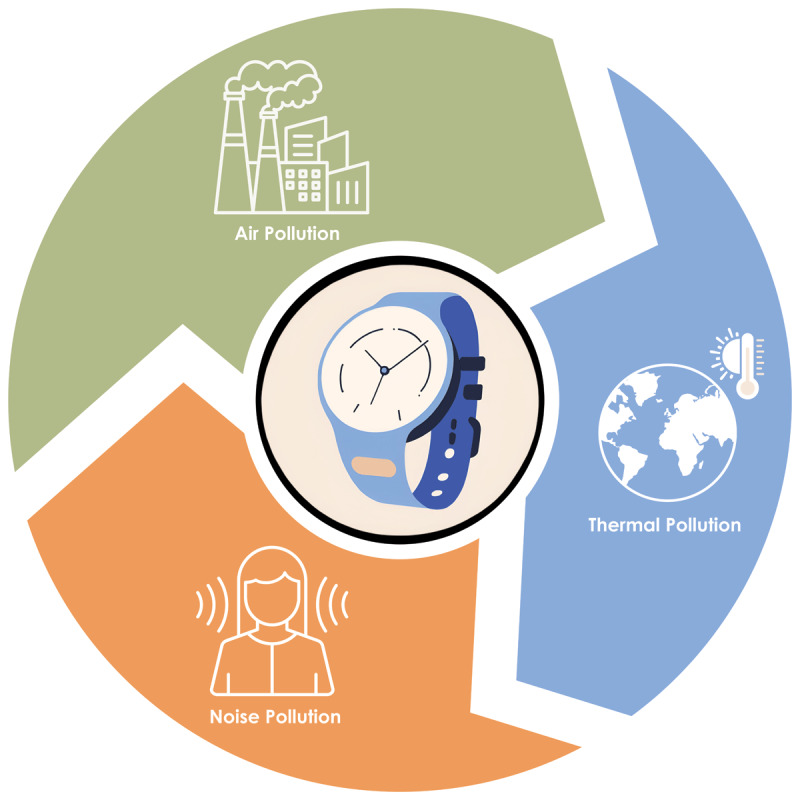
Visualization of different environmental exposures monitored by wearable devices, including air pollution, sound pollution, and thermal pollution. The figure highlights how wearables can track various environmental factors that contribute to the cardiovascular exposome, offering a comprehensive view of the external factors impacting cardiovascular health.
Advancing AFib Care: Wearables in Detection and Therapy
AFib impacts about 4% of adults over 60 years old and 10% of those over age 80.35 It may go undetected until complications occur, such as heart failure or stroke. Evidence is increasing for consumer wearable devices as a means for early detection of AFib. The Apple Heart Study,36 involving over 419,000 participants, demonstrated that the Apple Watch could detect AFib with a positive predictive value of 84% using PPG technology. Similarly, the Fitbit-based Study enrolled over 455,000 participants and confirmed the ability of Fitbit devices to detect AFib, reporting a positive predictive value of 98% for identifying irregular heart rhythms.37 Meanwhile, the Huawei-based study38 involved more than 187,000 participants and showed that the Huawei smartwatch could effectively detect AFib, with results comparable to those of traditional ECG methods. In an additional study of Apple Watch-based PPG technology, a deep neural network (DNN) algorithm trained with heuristic pretraining demonstrated very high accuracy in predicting AFib, with a C-statistic of 0.97 compared with the widely accepted 12-lead electrocardiogram.39 Furthermore, after AFib is diagnosed, wearable monitoring can enable assessment of AFib burden, effectiveness of antiarrhythmic treatments, and heart rate control.40
Coronary Artery Disease: Supporting Cardiac Rehabilitation and Guideline-Driven Self-Management
Coronary artery disease is one of the major clinical indications for cardiac rehabilitation (CR), which is a class I guideline-recommended program to improve patient outcomes. One significant challenge of center-based CR is the limited number of accredited facilities available across the US: 74% of adults live in areas where there is less than one CR center per 100,000 people, and 14% of the population resides in regions entirely devoid of CR centers.41 This shortage is especially severe in rural and economically disadvantaged areas, posing significant barriers to access for these communities.
To address this challenge, digital CR programs offer a promising solution to expand access to home-based CR. The evolving science of digital technologies in CR was recently detailed in a science advisory from the American Heart Association.38 Digital health technologies can automate recording home exercise while providing immediate feedback, allowing for flexible goal adjustments and supporting comprehensive risk factor modification beyond exercise.42,43
The MiCORE (Myocardial infarction, COmbined-device, Recovery Enhancement) study illustrates the value of comprehensive risk factor modification in the secondary prevention setting. MiCORE enrolled 200 patients who had experienced type I myocardial infarction across four hospitals.44 These participants were provided with a self-management program guided by established protocols, including a mobile app connected to an Apple Watch and a Bluetooth blood pressure monitor. Results showed a 52% decrease in all-cause hospital readmissions within 30 days among those enrolled in the self-management program compared with a propensity-matched historical control group. Moreover, a cost-effectiveness analysis projected potential savings of $6,000 USD per patient with the adoption of this intervention.45
Building on the MiCORE results, the ongoing mTECH-Rehab (Impact of a Mobile Technology Enabled Corrie CR Program) trial aims to assess a hybrid CR program in 200 patients.46 This hybrid approach may be the future of CR by combining in-person with home-based digitally enabled CR in a manner tailored to the needs of the individual patient. As the field of digital CR evolves and presents opportunities for improvement, this trial compares the tech-driven approach, which emphasizes health equity, with conventional care methods to determine its effectiveness for patients recovering from heart-related events or procedures.
Challenges and Limitations
Advancements in wearable technologies offer a promising avenue for patient-centered health care, enabling new methods to monitor and enhance patient outcomes. Yet, challenges such as accuracy persist with technologies like single-lead ECGs and PPG. Addressing these is crucial in future studies.
Limitations of Single-Lead ECG Devices
A study examined three commercially available smartwatch single-lead ECG devices and found that their sensitivity for diagnosing AFib ranged from 78% to 88% while their specificity ranged from 80% to 86%.47 However, these devices face challenges such as noise and artifacts, which can complicate interpretation and render 2% to 15% of ECGs uninterpretable. Furthermore, single-lead ECG detection can fall short in diagnosing intricate arrhythmias or critical conditions such as myocardial infarction.48
Challenges with PPG Technology
Wearables using PPG technology face challenges in maintaining accuracy. PPG works optimally when directly in contact with the skin, which may not always be the case with devices secured by straps.48 Factors such as skin color, moisture levels, and even tattoos can affect the accuracy of PPG readings, potentially undermining their reliability. Moreover, the widespread availability of PPG technology in personal devices can lead to excessive self-monitoring, which has the potential to cause anxiety among users and may contribute to unnecessary healthcare costs due to frequent consultations and tests triggered by false positives or minor, nonthreatening irregularities.
Reimbursement, Socioeconomic Disparities, and Digital Access
Medicare reimbursement for remote patient monitoring mandates regular physiological data collection and transmission for over half of the days in a month.13 This requirement may be excessive for managing conditions such as hypertension and inadequate for complex diseases such as diabetes. In addition, socioeconomic disparities significantly impact wearable adoption. A survey among 4,272 US adults revealed a notable discrepancy in wearable use between income brackets.14 Individuals earning $75,000 or more were three times more likely to use wearables compared with those earning less than $30,000. Such disparities could exacerbate existing healthcare inequalities, potentially subjecting economically disadvantaged individuals to substandard care.
Integration Challenges
Incorporating data gathered from wearable digital health trackers into electronic health records (EHRs) involves transforming the information into standardized formats that allow for smooth sharing across different clinical systems.49 Currently, clinicians face challenges in managing and interpreting disparate and extensive datasets. Healthcare systems will need to establish a robust framework and strategy for integrating validated wearable digital health trackers into EHRs and clinical workflow.
Future Directions
The future of wearable technologies in cardiovascular health is promising, with several emerging trends and innovations poised to further transform the field. One such advancement is the development of AI-driven chatbots, such as the Smart AI Resource Assistant for Health (S.A.R.A.H.) developed by the World Health Organization. These chatbots could assist in managing chronic conditions by offering personalized recommendations and reminders, thereby improving patient adherence to treatment plans and reducing clinician burden.50 Additionally, noninvasive continuous glucose monitors are already transforming the care of patients with diabetes. Their expanded use and integration with wearables are expected to enhance the longitudinal assessment of cardiometabolic health and could enable precision care.51
The potential for personalized medicine through wearables is becoming increasingly viable. Advanced AI and ML algorithms can analyze vast amounts of data collected from wearables to identify patterns and predict health outcomes, allowing for tailored interventions based on individual risk profiles. Additionally, AI analysis of single-lead ECG has the potential to detect heart failure, and wearable devices collecting single-lead ECG data may provide robust information on many aspects of cardiovascular health.52 Collaborative efforts in research and development are crucial to overcoming current limitations and ensuring the widespread adoption of these technologies. For instance, systematic reviews have highlighted the need for standardized protocols and robust validation studies to ensure the accuracy and reliability of wearable data.53 By addressing these challenges, the integration of wearable technologies with traditional healthcare practices can be optimized to support a more personalized approach to cardiovascular care and drive better health outcomes.
Conclusion
Wearable technologies have revolutionized the landscape of cardiovascular health by providing real-time data longitudinally that empowers both patients and healthcare professionals. These devices, ranging from smartwatches and fitness trackers to advanced ECG monitors, are beginning to demonstrate their potential to improve early detection, monitoring, and management of cardiovascular diseases. The integration of advanced sensor technologies and AI-driven data analytics has significantly enhanced the accuracy and functionality of these wearables, offering valuable insights into the cardiovascular exposome. By enabling proactive health management and personalized interventions, wearables and digital health technologies have the potential to enhance patient outcomes.
Despite these advancements, challenges remain in fully harnessing the potential of wearable technologies. Issues such as device accuracy, data integration with healthcare systems, and socioeconomic disparities around accessing these technologies need to be addressed to maximize their impact. Additionally, long-term studies are necessary to understand the sustained benefits and potential drawbacks of wearables in cardiovascular health management. As technology continues to evolve, collaborative efforts in research and development, alongside policy initiatives to ensure equitable access, will be important in realizing the full potential of wearable technologies in decoding the cardiovascular exposome and improving global health outcomes.
Key Points
Comprehensive Monitoring: Wearable technologies can provide longitudinal, real-time data on key cardiovascular metrics such as heart rate, heart rhythm, physical activity, and sleep patterns.
Technological Advancements: Significant advancements in sensor technology, data analytics, and connectivity have enhanced the accuracy and functionality of wearables, making them attractive tools in both research and clinical practice.
Environmental Exposures: Wearable devices equipped with advanced sensors can monitor environmental factors such as air quality, temperature, and ultraviolet radiation, which could unlock new insights into how these exposures impact cardiovascular health.
Personalized Health Insights: The integration of wearable data with artificial intelligence and machine learning algorithms could enable personalized health analysis to inform health-related decisions and behaviors.
Challenges and Opportunities: While wearable technologies are exciting tools in cardiovascular health, challenges such as device accuracy, data integration, and socioeconomic disparities in access must be addressed to maximize their impact on healthcare outcomes.
Contributor Information
Geyner A. Gaona, Email: ggaona1@jh.edu.
Seth S. Martin, Email: smart100@jhmi.edu.
Competing Interests
The authors have no competing interests to declare.
References
- 1.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017. Sep 16;390(10100):1151-1210. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan SU, Javed Z, Lone AN, et al. Social Vulnerability and Premature Cardiovascular Mortality Among US Counties, 2014 to 2018. Circulation. 2021. Oct 19;144(16):1272-1279. doi: [DOI] [PubMed] [Google Scholar]
- 3.Bhatnagar A. Environmental Determinants of Cardiovascular Disease. Circ Res. 2017. Jul 7;121(2):162-180. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Powell-Wiley TM. Disentangling Ancestry From Social Determinants of Health in Hypertension Disparities-An Important Step Forward. JAMA Cardiol. 2021. Apr 1;6(4):398-399. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baum A, Wisnivesky J, Basu S, Siu AL, Schwartz MD. Association of Geographic Differences in Prevalence of Uncontrolled Chronic Conditions With Changes in Individuals’ Likelihood of Uncontrolled Chronic Conditions. JAMA. 2020. Oct 13;324(14):1429-1438. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson AE, Zhu J, Garrard W, et al. Area Deprivation Index and Cardiac Readmissions: Evaluating Risk-Prediction in an Electronic Health Record. J Am Heart Assoc. 2021. Jul 6;10(13):e020466. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamad R, Öztürk B, Foverskov E, et al. Association of Neighborhood Disadvantage With Cardiovascular Risk Factors and Events Among Refugees in Denmark. JAMA Netw Open. 2020. Aug 3;3(8):e2014196. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodward M, Peters SA, Batty GD, et al. Socioeconomic status in relation to cardiovascular disease and cause-specific mortality: a comparison of Asian and Australasian populations in a pooled analysis. BMJ Open. 2015. Mar 17;5(3):e006408. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic Status and Cardiovascular Outcomes: Challenges and Interventions. Circulation. 2018. May 15;137(20):2166-2178. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bevan GH, Nasir K, Rajagopalan S, Al-Kindi S. Socioeconomic Deprivation and Premature Cardiovascular Mortality in the United States. Mayo Clin Proc. 2022. Jun;97(6):1108-1113. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kucharska-Newton AM, Harald K, Rosamond WD, Rose KM, Rea TD, Salomaa V. Socioeconomic indicators and the risk of acute coronary heart disease events: comparison of population-based data from the United States and Finland. Ann Epidemiol. 2011. Aug;21(8):572-9. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerber Y, Koton S, Goldbourt U, et al. Poor neighborhood socioeconomic status and risk of ischemic stroke after myocardial infarction. Epidemiology. 2011. Mar;22(2):162-9. doi: [DOI] [PubMed] [Google Scholar]
- 13.The Lancet Public Health. Education: a neglected social determinant of health. Lancet Public Health. 2020. Jul;5(7):e361. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silhol R, Zins M, Chauvin P, Chaix B. Investigating the spatial variability in incidence of coronary heart disease in the Gazel cohort: the impact of area socioeconomic position and mediating role of risk factors. J Epidemiol Community Health. 2011. Feb;65(2):137-43. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan SU, Kumar P, Arshad A, et al. Social Vulnerability and Potentially Preventable Cardiovascular Deaths Among Younger Adults in the U.S. Counties, 2014-2018. JACC Adv. 2023. Feb 8;2(2):100196. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy NM, Mayne SL, Pool LR, et al. Exposure to Neighborhood-Level Racial Residential Segregation in Young Adulthood to Midlife and Incident Subclinical Atherosclerosis in Black Adults: The Coronary Artery Risk Development in Young Adults Study. Circ Cardiovasc Qual Outcomes. 2022. Feb;15(2):e007986. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones MR, Diez-Roux AV, Hajat A, et al. Race/ethnicity, residential segregation, and exposure to ambient air pollution: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Public Health. 2014. Nov;104(11):2130-7. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenstock S, Whitman S, West JF, Balkin M. Racial disparities in diabetes mortality in the 50 most populous US cities. J Urban Health. 2014. Oct;91(5):873-85. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abrahamowicz AA, Ebinger J, Whelton SP, Commodore-Mensah Y, Yang E. Racial and Ethnic Disparities in Hypertension: Barriers and Opportunities to Improve Blood Pressure Control. Curr Cardiol Rep. 2023. Jan;25(1):17-27. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Al Rifai M, Mahtta D, Kherallah R, et al. Prevalence and Determinants of Difficulty in Accessing Medical Care in U.S. Adults. Am J Prev Med. 2021. Oct;61(4):492-500. doi: [DOI] [PubMed] [Google Scholar]
- 21.Gupta T, Kalra A, Kolte D, et al. Regional Variation in Utilization, In-hospital Mortality, and Health-Care Resource Use of Transcatheter Aortic Valve Implantation in the United States. Am J Cardiol. 2017. Nov 15;120(10):1869-1876. doi: [DOI] [PubMed] [Google Scholar]
- 22.Damluji AA, Epstein R, Moscucci M, et al. Healthcare access to TAVR procedures by population density: a focus on healthcare disparity in Florida. Circulation. 2019;140:A14981-A. [Google Scholar]
- 23.Cross SH, Mehra MR, Bhatt DL, et al. Rural-Urban Differences in Cardiovascular Mortality in the US, 1999-2017. JAMA. 2020. May 12;323(18):1852-1854. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Danielli S, Ashrafian H, Darzi A. Healthy city: global systematic scoping review of city initiatives to improve health with policy recommendations. BMC Public Health. 2023. Jul 1;23(1):1277. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al Rifai M, Mahtta D, Kherallah R, et al. Prevalence and Determinants of Difficulty in Accessing Medical Care in U.S. Adults. Am J Prev Med. 2021. Oct;61(4):492-500. doi: [DOI] [PubMed] [Google Scholar]
- 26.Chen Z, Dazard J-E, Khalifa Y, Motairek I, Al-Kindi S, Rajagopalan S. Artificial intelligence-based assessment of built environment from Google Street View and coronary artery disease prevalence. Eur Heart J. 2024. May 7;45(17):1540-1549. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu M, Patel VR, Salas RN, et al. Neighborhood Environmental Burden and Cardiovascular Health in the US. JAMA Cardiol. 2024. Feb 1;9(2):153-163. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khan SU, Al-Mallah MH. Air pollution and acute coronary syndrome: The air we breathe. Atherosclerosis. 2024. Mar:390:117453. doi: [DOI] [PubMed] [Google Scholar]
- 29.Crowley R, Mathew S, Hilden D. Health and Public Committee of the American College of Physicians. Modernizing the United States’ Public Health Infrastructure: A Position Paper From the American College of Physicians. Ann Intern Med. 2023. Aug;176(8):1089-1091. doi: [DOI] [PubMed] [Google Scholar]
- 30.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004. Jun 1;109(21):2655-71. doi: [DOI] [PubMed] [Google Scholar]
- 31.Brook RD, Rajagopalan S, Pope CA 3rd., et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010. Jun 1;121(21):2331-78. doi: [DOI] [PubMed] [Google Scholar]
- 32.Pope CA 3rd., Muhlestein JB, May HT, Renlund DG, Anderson JL, Horne BD. Ischemic heart disease events triggered by short-term exposure to fine particulate air pollution. Circulation. 2006. Dec 5;114(23):2443-8. doi: [DOI] [PubMed] [Google Scholar]
- 33.Beelen R, Hoek G, van den Brandt PA, et al. Long-term effects of traffic-related air pollution on mortality in a Dutch cohort (NLCS-AIR study). Environ Health Perspect. 2008. Feb;116(2):196-202. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan SU. Cardiovascular Disease Prevention With mHealth Innovations: Transforming Wellness Through Wireless. Circ Cardiovasc Qual Outcomes. 2024. Jul;17(7):e011005. doi: [DOI] [PubMed] [Google Scholar]
- 35.Gerber Y, Weston SA, Killian JM, Therneau TM, Jacobsen SJ, Roger VL. Neighborhood income and individual education: effect on survival after myocardial infarction. Mayo Clin Proc. 2008. Jun;83(6):663-9. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Méjean C, Droomers M, van der Schouw YT, et al. The contribution of diet and lifestyle to socioeconomic inequalities in cardiovascular morbidity and mortality. Int J Cardiol. 2013. Oct 15;168(6):5190-5. doi: [DOI] [PubMed] [Google Scholar]
- 37.Dupre ME, George LK, Liu G, Peterson ED. The cumulative effect of unemployment on risks for acute myocardial infarction. Arch Intern Med. 2012. Dec 10;172(22):1731-7. doi: [DOI] [PubMed] [Google Scholar]
- 38.Deo SV, Motairek I, Nasir K, et al. Association Between Historical Neighborhood Redlining and Cardiovascular Outcomes Among US Veterans With Atherosclerotic Cardiovascular Diseases. JAMA Netw Open. 2023. Jul 3;6(7):e2322727. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swope CB, Hernández D. Housing as a determinant of health equity: A conceptual model. Soc Sci Med. 2019. Dec:243:112571. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Lancet Public Health. Education: a neglected social determinant of health. Lancet Public Health. 2020. Jul;5(7):e361. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Erqou S, Clougherty JE, Olafiranye O, et al. Particulate Matter Air Pollution and Racial Differences in Cardiovascular Disease Risk. Arterioscler Thromb Vasc Biol. 2018. Apr;38(4):935-942. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joynt KE, Whellan DJ, O’Connor CM. Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry. 2003. Aug 1;54(3):248-61. doi: [DOI] [PubMed] [Google Scholar]
- 43.Sumner JA, Khodneva Y, Muntner P, et al. Effects of Concurrent Depressive Symptoms and Perceived Stress on Cardiovascular Risk in Low- and High-Income Participants: Findings From the Reasons for Geographical and Racial Differences in Stroke (REGARDS) Study. J Am Heart Assoc. 2016. Oct 10;5(10):e003930. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaw LJ, Merz CN, Bittner V, et al. Importance of socioeconomic status as a predictor of cardiovascular outcome and costs of care in women with suspected myocardial ischemia. Results from the National Institutes of Health, National Heart, Lung and Blood Institute-sponsored Women‘s Ischemia Syndrome Evaluation (WISE). J Womens Health (Larchmt). 2008. Sep;17(7):1081-92. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fabreau GE, Leung AA, Southern DA, et al. Sex, socioeconomic status, access to cardiac catheterization, and outcomes for acute coronary syndromes in the context of universal healthcare coverage. Circ Cardiovasc Qual Outcomes. 2014. Jul;7(4):540-9. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campanera M, Gasull M, Gracia-Arnaiz M. Food Security as a Social Determinant of Health: Tackling Inequalities in Primary Health Care in Spain. Health Hum Rights. 2023. Jun;25(1):9-21 [PMC free article] [PubMed] [Google Scholar]
- 47.Takahashi EA, Schwamm LH, Adeoye OM, et al. An Overview of Telehealth in the Management of Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation. 2022. Dec 20;146(25):e558-e568. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haleem A, Javaid M, Singh RP, Suman R. Telemedicine for healthcare: Capabilities, features, barriers, and applications. Sens Int. 2021:2:100117. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ladeiras-Lopes R, Baciu L, Grapsa J, et al. Social media in cardiovascular medicine: a contemporary review. Eur Heart J Digit Health. 2020. Nov 30;1(1):10-19. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Syme SL. Social determinants of health: the community as an empowered partner. Prev Chronic Dis. 2004. Jan;1(1):A02. [PMC free article] [PubMed] [Google Scholar]
- 51.Thompson B, Molina Y, Viswanath K, Warnecke R, Prelip ML. Strategies To Empower Communities To Reduce Health Disparities. Health Aff (Millwood). 2016. Aug 1;35(8):1424-8. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nasir K, Javed Z, Khan SU, Jones SL, Andrieni J. Big Data and Digital Solutions: Laying the Foundation for Cardiovascular Population Management CME. Methodist Debakey Cardiovasc J. 2020. Oct-Dec;16(4):272-282. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gondi S, Chokshi DA. Public Health and Payers-Bridging the Gap to Boost Public Health Investment. JAMA Health Forum. 2022. Jul 1;3(7):e222750. doi: [DOI] [PubMed] [Google Scholar]


