Abstract
Introduction:
Osteoporosis is rare in the young having an incidence of 4.1 per 100,000 person-years and can occur secondary to endocrine diseases, inflammatory disorders, malnutrition or malabsorption syndromes, and medications. Pregnancy and lactation-associated osteoporosis (PLO) should be thought of as one of the causes but is often missed as the diagnosis requires a high degree of suspicion along with X-rays and densitometry which are avoided as far as possible during the peripregnancy period. If missed, it can lead to osteoporotic fractures and disability.
Case Report:
We report a case of a 24-year-old primigravida presenting with low back pain 4 months after pregnancy having multiple vertebral compression fractures. On initial workup, a diagnosis of osteoporosis was made and causes of secondary osteoporosis were ruled out after radiological and laboratory investigations. A diagnosis of PLO was made after exclusion of other possible causes. The patient was treated with cessation of lactation and medical management in the form of Calcitonin, Teriparatide, Vitamin D, and calcium supplementation. The patient was symptom-free after 12 months.
Conclusion:
Through this case report, we hope to emphasize that PLO should be considered as a possible etiology in young females with multiple atraumatic fragility fractures presenting in the peri-pregnancy period.
Keywords: Pregnancy, lactation, osteoporosis, spinal fractures, antiosteoporotic agents, teriparatide
Learning Point of the Article:
While osteoporosis is more commonly associated with older age, it can indeed affect younger individuals, especially in the context of specific medical conditions or pregnancy-related factors.
Introduction
Osteoporosis is common in the elderly but is also not uncommon in the younger population. Pregnancy-associated osteoporosis was first described by Nordin and Roper in 1955 as an infrequent condition that results in back pain during pregnancy and the postpartum period, and causes significant disability [1]. The magnitude of the problem is underestimated, and diagnosis is often missed because of the unfamiliarity with the condition, avoidance of radiographs and densitometry during pregnancy, and back pain is often attributed to other factors during pregnancy.
Here, we report a case of pregnancy- and lactation-associated osteoporosis (PLO) involving the dorsolumbar spine presenting with multiple fragility fractures in a lactating primigravida female to highlight the diagnostic challenges, and socio-economic impact and discuss the various treatment modalities mentioned in the literature.
Case Report
A 24-year-old female patient presented to the outpatient clinic with complaints of severe back pain (VAS 8/10). This pain was insidious in onset, dull in nature, and was aggravated by changing sides and was affecting her daily routine activities. She had a full-term vaginal delivery 4 months ago. Her pregnancy was uneventful and was breastfeeding. On clinical examination, she had limitation of flexion and tenderness of the lower dorsal and upper lumbar spinous processes with paraspinal muscle spasm. Neurological evaluation was within normal limits. Anteroposterior (AP) and lateral (Lat) Roentgenogram (X-rays) of the dorsolumbar spine revealed a loss of height from D9-12 vertebral bodies along with decreased cortical thickness and loss of bony trabeculae associated with smooth biconcave deformities, squared-off depressions of the end-plates combined with compressions from the adjacent discs which were representative of cod fish vertebrae suggestive of multiple osteoporotic vertebral fractures (Fig. 1a and b). She was admitted for pain management and further evaluation of her symptoms. She was started on intravenous acetaminophen (paracetamol) three times a day as she was lactating. Magnetic resonance imaging (MRI) showed an anterior wedge collapse of D9-D12 vertebra (Fig. 2a) with hypointense signal on T1 weighted and hyperintense signal in the T2 and short tau inversion recovery (STIR) weighted sagittal and coronal films at D9 and D10 vertebra with normal signal intensity in the intervening disk spaces. The preservation of intraosseous fat reduced the likelihood of a neoplastic lesion (Fig. 2b-d and Fig. 3). On comparing her bone mineral density (BMD) (0.635 g/cm2) to an individual of the same age and sex (Z-score), checked using a dual-energy X-ray absorptiometry (DEXA) scan, revealed a value of -4.2, suggestive of severe osteoporosis and high risk of fragility fractures. Laboratory tests ruled out infective, inflammatory, and metabolic causes and did not reveal any abnormality for secondary causes of osteoporosis such as hyperthyroidism and hyperparathyroidism. All these investigations led to a probable diagnosis of pregnancy and lactation-associated osteoporosis. The patient was advised against breastfeeding and was prescribed a daily intake of calcium carbonate (1000 mg/day), calcitriol (250 ug/day), injectable Teriparatide in a dose of 20 mcg taken subcutaneously and calcitonin nasal spray to be taken as 1 puff in alternate nostril daily for 2 months. She was discharged after 2 days with a VAS of 4/10. On 12-month follow-up, the patient was symptom-free with an increase in BMD to 0.710 g/cm2 (Fig. 4).
Figure 1.
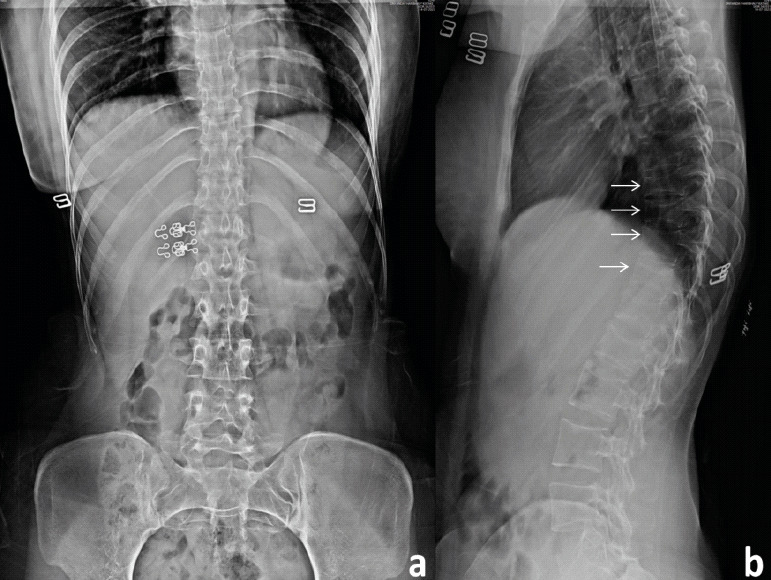
X-ray: Anteroposterior (a) and lateral view (b) of the dorsolumbar spine: showing a loss of height from D9-12 vertebral bodies along with decreased cortical thickness and loss of bony trabeculae associated with smooth biconcave deformities, squared-off depressions of the end-plates combined with compressions from the adjacent discs which were representative of cod fish vertebrae suggestive of multiple osteoporotic vertebral fractures.
Figure 2.
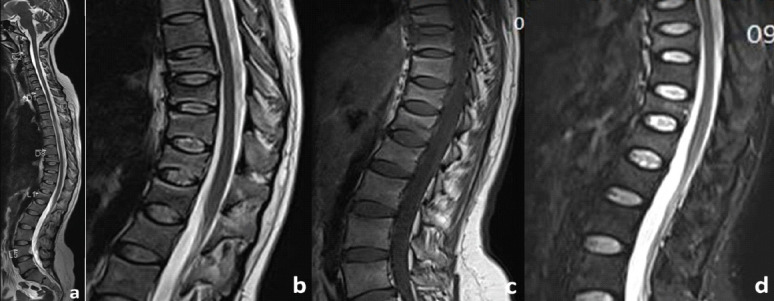
MRI: Sagittal images of whole spine (a) T2 showing anterior wedge collapse at D9-D12 vertebra. Sagittal images of dorsolumbar spine T2 (b) hyperintense, T1 (c) hypointense, and STIR (d) hyperintense signal in the D9 and D10 vertebra with normal signal intensity in the intervening disk spaces with preservation of intraosseous fat.
Figure 3.
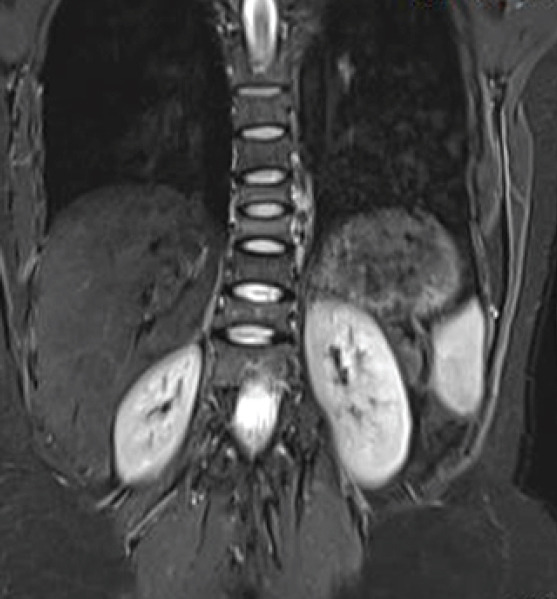
MRI: Coronal images of dorsolumbar spine T2 hyperintense signal in the D9 and D10 vertebra with normal signal intensity in the intervening disk spaces with preservation of intraosseous fat.
Figure 4.
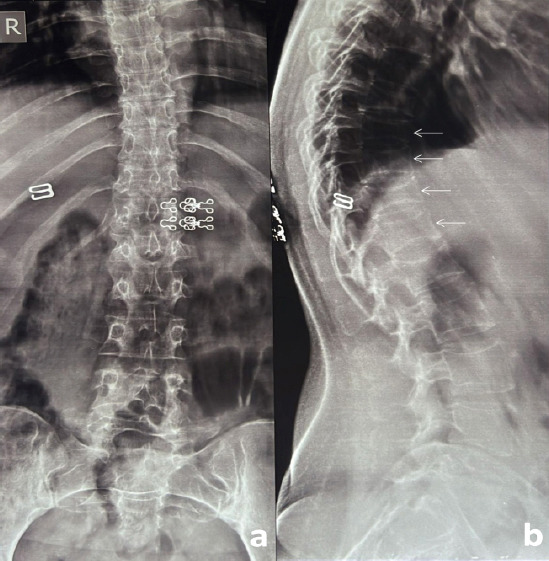
X-ray: Anteroposterior (a) and lateral view (b) of the dorsolumbar spine at 1-year follow-up: showing no further progression of kyphosis.
Discussion
Osteoporosis in young
Osteoporosis, though rare, can occur in the young secondary to hyperparathyroidism, hyperthyroidism, diabetes, thalassemia, multiple myeloma, intestinal malabsorption, leukemia, liver disease, metastatic bone disease, Cushing’s syndrome, acromegaly, scurvy, Marfan’s syndrome and medications such as antacids containing aluminum, heparin, anticonvulsants, thyroxine, and steroid use.
PLO epidemiology and pathophysiology
PLO is a rare type of premenopausal osteoporosis that occurs mainly in the third trimester or immediately after the delivery [2-4]. Due to its infrequent occurrence, the exact prevalence is not known. Although the etiology of PLO is unclear, family history, genetic factors as well as dietary habits seem to be predisposing factors [5-8]. Excessive resorption of calcium from the skeleton, increased rate of bone turnover induced by pregnancy, and additional changes in mineral metabolism that occur during lactation further increase the risk of PLO in susceptible women [9]. Another cause may be a sort of calcitonin deficiency which exacerbates the normal losses of calcium during lactation [9].
Presentation and recurrence
The main complaint is severe pain in the lower back and lower extremity joints, accompanied by reduced general mobility. More often than not, pain appearing in the final trimester of pregnancy or immediately postpartum is diagnosed as another problem associated with pregnancy and lactation. Although vertebral fractures, regardless of osteoporosis or other causes, are rare complications in new and expecting mothers, they should always be considered in women presenting with acute pain in the peripregnancy period [10,11]. Kyvernitakis et al. [12] and Sullivan et al. [13] have reported recurrence rates of 20% in subsequent pregnancies. Kyvernitakis et al. found a significant correlation between the number of fractures at the time of diagnosis and subsequent fractures, irrespective of a second pregnancy. The risk increases from 10% with a single fracture to 27% with more than one fracture [12].
Investigations
Diagnosis can be made by exclusion where preliminary investigations in the form of X-ray, MRI scan, DEXA, and laboratory parameters are required to rule out any secondary causes of osteoporosis. Serum tartrate-resistant acid phosphatase-5b and urinary N-terminal telopeptide of type I collagen (NTX) are markers of bone resorption which can be used to confirm the diagnosis whereas serum bone alkaline phosphatase (BAP) and N-terminal propeptide of type I procollagen are bone formation markers measured using a chemiluminescent enzyme immunoassay and antibody radioimmunoassay which can be used to assess response to treatment.
Management
There is no mutually agreed opinion or guideline in the treatment of this condition. The treatment options are limited to those practiced in the reported cases. A common first step in the treatment of a patient with PLO is delactation as is recommended in most cases [10, 11, 14] with supplementation of calcium with or without Vitamin D [2]. Other options include bed rest, corset application, and medical treatment which include calcium and Vitamin D [10], bisphosphonates [13], teriparatide [15, 16], and strontium ranelate [17]. Kyphoplasty, to treat postpartum vertebral fractures, has also been tried in cases of intractable pain and spinal deformity [18].
The World Health Organization recommends exclusively breastfeeding for 4–6 months followed by weaning and introduction a supplemental diet. However, in developing countries, breastfeeding is commonly continued beyond 6 months and early weaning has a significant social impact [19]. The majority of developing countries disagree with early weaning and hence while treating PLO, doctors need to keep in mind the safety profile of drugs during lactation. Lactation results in the loss of 300–400 mg of calcium daily, which is compensated for by 5–10% skeletal calcium loss. However, this loss is gained back within a few months of weaning [20]. Therefore, weaning is advised to treat PLO. Calcium and Vitamin D supplementation increases the levels of calcium in the body and calcium absorption, respectively.
Vitamin K2 is one option for osteoporosis treatment. The effects of Vitamin K2 on bone are improving bone quality by promoting gamma-carboxylation of osteocalcin, and advancing bone formation and calcification through steroid and xenobiotic receptors (SXR) [21, 22]. One report mentions that Vitamin K2 effectively prevents fractures and sustains BMD in osteoporosis [23]. Vitamin K2 is administered to newborns or pregnant women for the treatment of melena neonatorum [24], so this medicine is safer than the other treatments. It may be difficult to conclude that Vitamin K2 treatment improved symptoms of post-pregnancy osteoporosis earlier than these treatments. However, we can easily use it for women who may be pregnant due to its safety.
Strontium Ranolate (SrRan) was used for patients with PLO in limited cases [17, 25]. It has a dual effect on bone, that is, stimulation of new bone formation and inhibition of bone resorption [26]. Treatment with SrRan resulted in significant reductions in the risks of new vertebral and non-vertebral fractures and increased both the lumbar spine and femoral neck bone mineral density in postmenopausal patients [27]. SrRan provides a rapid increase in BMD. Patients with PLO treated with weaning, and supplementation of SrRan, calcium, and cholecalciferol achieved a 31–33% increase in their lumbar BMD and an almost 20% increase in their total hip BMD at 12–21 months [17, 25]. However, the long-term safety and potential adverse events regarding prenatal impairment that are associated with the use of SrRan are unclear [25].
Teriparatide is one of the most effective drugs in preventing spinal fractures in osteoporotic treatment. Choe et al. reported that daily Teriparatide should be considered for young patients with PLO, especially those with multiple vertebral fractures, to avoid long-term morbidity [26-28]. In another study, Teriparatide treatment resulted in an increase in BMD and no additional fractures during observation over several years [14]. Teriparatide is more effective than BPs concerning BMD increase [29] but has a limited period of use. It is a strong candidate for patients with pregnancy and lactation-associated OP and fractures [15,28], but no drugs have been established to inhibit fracture within half a year of OP onset.
Denosumab is a human monoclonal antibody that binds to the receptor activator of nuclear factor κB-ligand (RANKL) and inhibits the activation of osteoclasts and their precursors, leading to a suppression of bone turnover and an increase in BMD. Stumpf et al. (2021) reported a case of PLO, treated with Denosumab along with calcium and Vitamin D supplementation, which led to an increase in BMD at the lumbar spine, femoral neck, and total hip by 21.2%, 5.6%, and 8.0% at 12 months and by 32.0%, 13.0%, and 11.5% at 18 months, respectively [30]. Another patient with PLO was treated with weekly Teriparatide (56.5 µg/ week) for 6 weeks, followed by Denosumab (60 mg) for 6 months, and at 12 months and exhibited an increase in BMD from baseline at the lumbar spine (L2–L4) and femoral neck by 16.5% and 3.9%, respectively [31]. These outcomes suggested that Denosumab can be considered one of the effective treatments for PLO.
Bisphosphonates (BP) have not been preferred for multiple reasons. Stathopoulos et al. [32] identified 78 cases involving fetuses whose mothers had been exposed to bisphosphonates before conception or during pregnancy, along with seven cases of bisphosphonates exposure before or during lactation. Most of the mothers and infants did not demonstrate serious adverse effects. However, there were cases of shortened gestational age, low neonatal birth weight, and transient hypocalcemia of the newborns, while the very few reported cases of spontaneous abortions and congenital anomalies probably resulted from maternal underlying diseases and concomitant medication [32]. The half-life of a bisphosphonate is as long as 10 years and can pass through the placenta and may have teratogenic effects on a fetus [33-35]. The use of bisphosphonates may, therefore, be risky for the subsequent gestations of PLO patients. Regarding the prevention of subsequent fractures, Winarno et al. recently described a case of severe pregnancy- and lactation-associated OP with 11 vertebral fractures. According to the authors, unsatisfactory results were obtained with oral and intravenous BPs in combination with calcium and Vitamin D supplementation [15]. These findings indicate that BPs may not be able to halt the development of subsequent fractures.
Regarding treatment and follow-up, both Sullivan et al. and Ott et al. recommended a treatment duration of 5 years, followed by clinical and bone mineral density (BMD) review [7,13]. This suggests that ongoing monitoring is crucial to assess treatment efficacy and prevent recurrence of fractures (Fig. 4).
Most of the young women who are diagnosed with PLO do not have a prior BMD measurement, like our patient and the possibility of a prior low bone mass cannot be ruled out. However, there was nothing in the patients’ histories to suggest inadequate intake of calcium or Vitamin D and the absence of decalcification and pseudofractures in the limb bones, despite the codfish vertebrae, pointed to a diagnosis of osteoporosis rather than osteomalacia.
Conclusion
PLO although rare should be considered as one of the differentials in all peripartum women complaining of acute lower back pain and diagnosed with osteoporosis on a BMD scan.
Clinical Message.
PLO should be considered as a differential in young peripartum patient diagnosed with osteoporosis.
Biography



Footnotes
Conflict of Interest: Nil
Source of Support: Nil
Consent: The authors confirm that informed consent was obtained from the patient for publication of this case report
References
- 1.Nordin BE, Roper A. Post-pregnancy osteoporosis;A syndrome? Lancet. 1955;268:431–4. doi: 10.1016/s0140-6736(55)90214-2. [DOI] [PubMed] [Google Scholar]
- 2.Kovacs CS, Ralston SH. Presentation and management of osteoporosis presenting in association with pregnancy or lactation. Osteoporos Int. 2015;26:2223–41. doi: 10.1007/s00198-015-3149-3. [DOI] [PubMed] [Google Scholar]
- 3.Laroche M, Talibart M, Cormier C, Roux C, Guggenbuhl P, Degboe Y. Pregnancy-related fractures:A retrospective study of a French cohort of 52 patients and review of the literature. Osteoporos Int. 2017;28:3135–42. doi: 10.1007/s00198-017-4165-2. [DOI] [PubMed] [Google Scholar]
- 4.Mäkitie O, Zillikens MC. Early-onset osteoporosis. Calcif Tissue Int. 2022;110:546–61. doi: 10.1007/s00223-021-00885-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peris P, Monegal A, Martínez MA, Moll C, Pons F, Guañabens N. Bone mineral density evolution in young premenopausal women with idiopathic osteoporosis. Clin Rheumatol. 2007;26:958–61. doi: 10.1007/s10067-006-0405-0. [DOI] [PubMed] [Google Scholar]
- 6.Rubin LA, Hawker GA, Peltekova VD, Fielding LJ, Ridout R, Cole DE. Determinants of peak bone mass:Clinical and genetic analyses in a young female Canadian cohort. J Bone Miner Res. 1999;14:633–43. doi: 10.1359/jbmr.1999.14.4.633. [DOI] [PubMed] [Google Scholar]
- 7.Ott SM. Bone density in adolescents. N Engl J Med. 1991;325:1646–7. doi: 10.1056/NEJM199112053252310. [DOI] [PubMed] [Google Scholar]
- 8.Bonjour JP, Theintz G, Buchs B, Slosman D, Rizzoli R. Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab. 1991;73:555–63. doi: 10.1210/jcem-73-3-555. [DOI] [PubMed] [Google Scholar]
- 9.Kovacs CS. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 2005;10:105–18. doi: 10.1007/s10911-005-5394-0. [DOI] [PubMed] [Google Scholar]
- 10.Terzi R, Terzi H, Özer T, Kale A. A rare cause of postpartum low back pain:Pregnancy- and lactation-associated osteoporosis. BioMed Res Int. 2014;2014:287832. doi: 10.1155/2014/287832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva L, Sampaio L, Pinto J, Brito JS, Ventura FS. Osteoporotic fractures in pregnancy - conjunction of factors? Acta Reumatol Port. 2009;34:641–5. [PubMed] [Google Scholar]
- 12.Kyvernitakis I, Reuter TC, Hellmeyer L, Hars O, Hadji P. Subsequent fracture risk of women with pregnancy and lactation-associated osteoporosis after a median of 6 years of follow-up. Osteoporos Int. 2018;29:135–42. doi: 10.1007/s00198-017-4239-1. [DOI] [PubMed] [Google Scholar]
- 13.O'Sullivan SM, Grey AB, Singh R, Reid IR. Bisphosphonates in pregnancy and lactation-associated osteoporosis. Osteoporos Int. 2006;17:1008–12. doi: 10.1007/s00198-006-0112-3. [DOI] [PubMed] [Google Scholar]
- 14.Ozturk C, Atamaz FC, Akkurt H, Akkoc Y. Pregnancy-associated osteoporosis presenting severe vertebral fractures. J Obstet Gynaecol Res. 2014;40:288–92. doi: 10.1111/jog.12157. [DOI] [PubMed] [Google Scholar]
- 15.Winarno AS, Kyvernitakis I, Hadji P. Successful treatment of 1-34 Parathyroid Hormone (PTH) after failure of bisphosphonate therapy in a complex case of pregnancy associated osteoporosis and multiple fractures. Z Geburtshilfe Neonatol. 2014;218:171–3. doi: 10.1055/s-0034-1382069. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Hong MK, Park SW, Park HM, Kim J, Ahn J. A case of teriparatide on pregnancy-induced osteoporosis. J Bone Metab. 2013;20:111–4. doi: 10.11005/jbm.2013.20.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanriover MD, Oz SG, Sozen T, Kilicarslan A, Guven GS. Pregnancy- and lactation-associated osteoporosis with severe vertebral deformities:Can strontium ranelate be a new alternative for the treatment? Spine J. 2009;9:e20–4. doi: 10.1016/j.spinee.2008.06.451. [DOI] [PubMed] [Google Scholar]
- 18.Bayram S, Ozturk C, Sivrioglu K, Aydinli U, Kucukoglu S. Kyphoplasty for pregnancy-associated osteoporotic vertebral fractures. Joint Bone Spine. 2006;73:564–6. doi: 10.1016/j.jbspin.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Caleyachetty A, Krishnaveni GV, Veena SR, Hill J, Karat SC, Fall CH, et al. Breastfeeding duration, age of starting solids and high BMI risk and adiposity in Indian children. Matern Child Nutr. 2011;9:199–216. doi: 10.1111/j.1740-8709.2011.00341.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pearson D, Kaur M, San P, Lawson N, Baker P, Hosking D. Recovery of pregnancy mediated bone loss during lactation. Bone. 2004;34:570–8. doi: 10.1016/j.bone.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 21.Shearer MJ. Vitamin K. Lancet. 1995;345:229–34. doi: 10.1016/s0140-6736(95)90227-9. [DOI] [PubMed] [Google Scholar]
- 22.Tabb MM, Sun A, Zhou C, Grün F, Errandi J, Romero K, et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem. 2003;278:43919–27. doi: 10.1074/jbc.M303136200. [DOI] [PubMed] [Google Scholar]
- 23.Shiraki M, Shiraki Y, Aoki C, Miura M. Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res. 2000;15:515–21. doi: 10.1359/jbmr.2000.15.3.515. [DOI] [PubMed] [Google Scholar]
- 24.Shimada H, Himeno K, Michimoto T, Tanada S, Ikeuchi M, Suwa M, et al. Prevention of Vitamin K deficiency in the early neonatal period--prophylactic oral administration of VK to the mother. Nihon Sanka Fujinka Gakkai Zasshi. 1990;42:705–10. [PubMed] [Google Scholar]
- 25.Zarattini G, Buffoli P, Isabelli G, Marchese M. Pregnancy-associated osteoporosis with seven vertebral compression fractures, a case treated with strontium ranelate. Clin Cases Miner Bone Metab. 2014;11:139–41. [PMC free article] [PubMed] [Google Scholar]
- 26.Bonnelye E, Chabadel A, Saltel F, Jurdic P. Dual effect of strontium ranelate:Stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42:129–38. doi: 10.1016/j.bone.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 27.Silverman S, Christiansen C. Individualizing osteoporosis therapy. Osteoporos Int. 2012;23:797–809. doi: 10.1007/s00198-011-1775-y. [DOI] [PubMed] [Google Scholar]
- 28.Choe EY, Song JE, Park KH, Seok H, Lee EJ, Lim SK, et al. Effect of teriparatide on pregnancy and lactation-associated osteoporosis with multiple vertebral fractures. J Bone Miner Metab. 2012;30:596–601. doi: 10.1007/s00774-011-0334-0. [DOI] [PubMed] [Google Scholar]
- 29.Diab DL, Watts NB. Postmenopausal osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2013;20:501–9. doi: 10.1097/01.med.0000436194.10599.94. [DOI] [PubMed] [Google Scholar]
- 30.Stumpf U, Kraus M, Hadji P. Influence of denosumab on bone mineral density in a severe case of pregnancy-associated osteoporosis. Osteoporos Int. 2021;32:2383–7. doi: 10.1007/s00198-021-06008-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ijuin A, Yoshikata H, Asano R, Tsuburai T, Kikuchi R, Sakakibara H. Teriparatide and denosumab treatment for pregnancy and lactation-associated osteoporosis with multiple vertebral fractures:A case study. Taiwan J Obstet Gynecol. 2017;56:863–6. doi: 10.1016/j.tjog.2017.10.028. [DOI] [PubMed] [Google Scholar]
- 32.Stathopoulos IP, Liakou CG, Katsalira A, Trovas G, Lyritis GG, Papaioannou NA, et al. The use of bisphosphonates in women prior to or during pregnancy and lactation. Hormones (Athens) 2011;10:280–91. doi: 10.14310/horm.2002.1319. [DOI] [PubMed] [Google Scholar]
- 33.Hassen-Zrour S, Korbâa W, Béjia I, Saidani Z, Bergaoui N. Maternal and fetal outcome after long-term bisphosphonate exposure before conception. Osteoporos Int. 2010;21:709–10. doi: 10.1007/s00198-009-0983-1. [DOI] [PubMed] [Google Scholar]
- 34.Ornoy A, Wajnberg R, Diav-Citrin O. The outcome of pregnancy following pre-pregnancy or early pregnancy alendronate treatment. Reprod Toxicol. 2006;22:578–9. doi: 10.1016/j.reprotox.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Losada I, Sartori L, Di Gianantonio E, Zen M, Clementi M, Doria A. Bisphosphonates in patients with autoimmune rheumatic diseases:Can they be used in women of childbearing age? Autoimmun Rev. 2010;9:547–52. doi: 10.1016/j.autrev.2010.03.002. [DOI] [PubMed] [Google Scholar]


