Abstract
Signal transduction through multiple distinct pathways regulates and orchestrates the numerous biological processes comprising heart development. This review outlines the roles of the FGFR, EGFR, Wnt, BMP, Notch, Hedgehog, Slit/Robo and other signaling pathways during four sequential phases of Drosophila cardiogenesis—mesoderm migration, cardiac mesoderm establishment, differentiation of the cardiac mesoderm into distinct cardiac cell types, and morphogenesis of the heart and its lumen based on the proper positioning and cell shape changes of these differentiated cardiac cells—and illustrates how these same cardiogenic roles are conserved in vertebrates. Mechanisms bringing about the regulation and combinatorial integration of these diverse signaling pathways in Drosophila are also described. This synopsis of our present state of knowledge of conserved signaling pathways in Drosophila cardiogenesis and the means by which it was acquired should facilitate our understanding of and investigations into related processes in vertebrates.
Keywords: heart development, Drosophila cardiogenesis, cardiac mesoderm specification, cardiac morphogenesis, ostia formation, cardiac valve formation, signaling pathways, FGF signaling, Dpp/BMP signaling, Wnt signaling, Notch signaling, Hedgehog signaling, EGFR signaling, Slit-Robo signaling, Integrin mediated signaling, Pvr/VEGF signaling
INTRODUCTION
Given that the heart first emerged in ancestral bilaterians over 500 million years ago (Romer, 1967; Moorman and Christoffels, 2003; Bishopric, 2005; Simoes-Costa et al., 2005; Ma et al., 2014), it is not surprising that the heart of the fruit fly Drosophila melanogaster exhibits remarkable similarities to that of vertebrates in terms of morphogenetic origins, structure, and regulatory mechanisms. In both Drosophila and vertebrate embryos, the heart originates from two bilaterally symmetrical rows of mesodermal cells that migrate most distally from the point of invagination during gastrulation, become committed to a cardiac fate, and ultimately fuse to form a heart tube at the midline (Bodmer, 1995). Subsequent looping and septa formation leads to the multiple chambered heart in vertebrates while the Drosophila heart remains tubular, albeit divided by an intracardiac valve into a narrow anterior structure termed the aorta and a wider posterior structure referred to as the heart proper (reviewed in Vogler and Bodmer, 2015; Rotstein and Paululat, 2016) (Figure 1).
Fig. 1. The embryonic heart in Drosophila and humans.
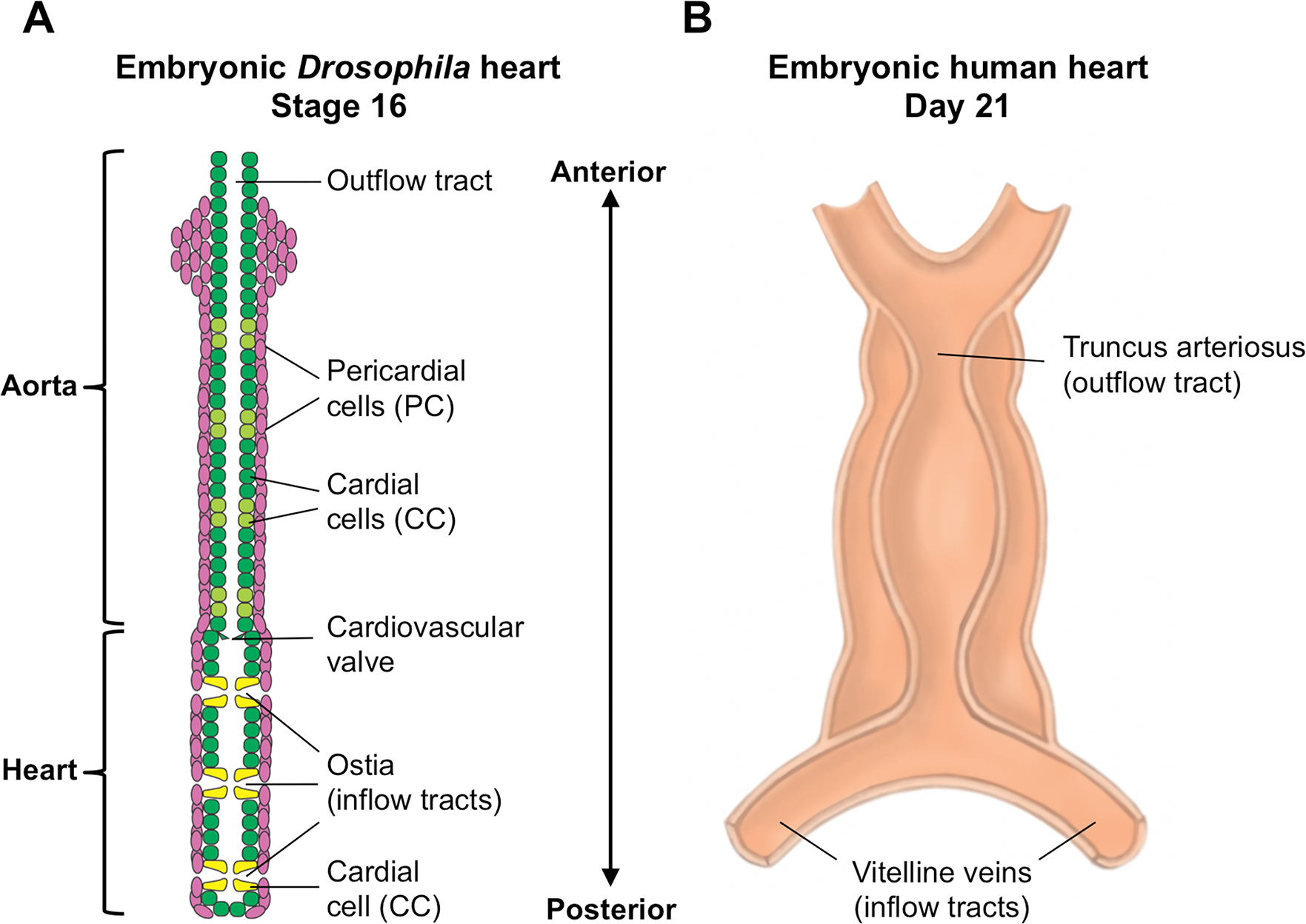
(A) The tubular heart of a stage 16 Drosophila embryo, divided into a narrow anterior structure termed the aorta and a wider posterior structure referred to as the heart proper. Both structures consist of an inner tube of contractile cardial cells (CCs; Tinman-Ladybird-CCs and Tinman-CCs are colored dark green; Seven up-CCs are colored light green and yellow) surrounded by an external sheath of non-muscle nephrocytic pericardial cells (PCs, pink), all arranged metamerically in repeated units. Ostia (inflow tracts) are present only in the posterior compartment, three on each side, with two morphologically distinct Seven up CCs (yellow) acting as a valve to regulate ingress through each ostium. (B) The tubular single-chambered heart of a 21-day human embryo, prior to the looping and septation which will produce four chambers. Note that both hearts are organized as tubes along an anterior-posterior axis, with inflow tracts entering the heart at the posterior part of the organ, and circulatory fluid (hemolymph in Drosophila, blood in humans) being pumped anteriorwards and exiting through the outflow tracts at the anterior.
Proper heart development thus involves four sequential and integrated developmental processes: (i) the internal migration of a subset of mesodermal cells such that they reach locations where they are able to receive appropriate inductive signals, which in turn (ii) enable them to become specified as the cardiac mesoderm (often termed the heart field or cardiogenic mesoderm in vertebrates), the precursor of the heart, (iii) the refinement and differentiation of this cardiac mesoderm into the different cardiac cell types and tissues that comprise the heart, and (iv) the ultimate positioning, arrangement, and changes in shape of these differentiated cardiac cells that complete the morphogenesis of the heart. The genes and proteins that mediate these developmental processes are also conserved, with considerable attention having already been focused on a core set of transcription factors—NK2, GATA, MEF2, T-box, LIM domain and Hand proteins—which govern cardiac progenitor fate specification, cardiac morphogenesis, and the expression of downstream genes which bring about the differentiation of the distinct cell types and tissues of the heart (Bodmer and Venkatesh, 1998; Cripps and Olson, 2002; Olson, 2006; Tao and Schulz, 2007; Tao et al., 2007; Bodmer and Frasch, 2010; Meganathan et al., 2015; Vogler and Bodmer, 2015). This review will highlight the contributions of multiple signaling pathways that function both sequentially and in concert to bring about cardiac development in Drosophila, and show that many of their cardiogenic roles are also conserved in vertebrates.
MESODERM MIGRATION
FGF signaling in mesoderm migration
An essential prerequisite for the development of the heart is the internal migration of mesodermal cells after invagination at gastrulation. This movement ensures that a subset of mesodermal cells reaches stereotyped locations where the cells can receive appropriate position-specific inductive cues which determine their subsequent cardiac fates. In both flies and vertebrates, this migration is mediated by signaling through fibroblast growth factor receptors (FGFRs) expressed in these migrating cells.
In Drosophila, two FGF8-like ligands, Pyramus and Thisbe, are expressed during gastrulation in broad and dynamic patterns in the ectoderm (Gryzik and Muller, 2004; Stathopoulos et al., 2004). Mesodermal cells that have invaginated through the ventral furrow at gastrulation and express an FGFR named Heartless respond to these signals by migrating in a dorsolateral direction under the overlying ectoderm until they have formed a uniform monolayer that reaches as far as the dorsal layer of the epidermis (Figure 2). The observations that (i) the activated form of the FGFR Heartless is detected specifically at the dorsolateral edges of the migrating mesoderm (Gabay et al., 1997), that (ii) mesoderm migration is defective in embryos mutant for pyramus, thisbe, heartless, or genes such as stumps that encode components of the FGF signaling pathway downstream of heartless (Beiman et al., 1996; Gisselbrecht et al., 1996; Shishido et al., 1997; Michelson et al., 1998a; Vincent et al., 1998; Imam et al., 1999; Gryzik and Muller, 2004; Stathopoulos et al., 2004), and that (iii) these mutants also fail to develop dorsal mesodermal structures such as the heart, demonstrate that FGF signaling-mediated mesoderm migration at gastrulation is essential for proper cardiogenesis.
Fig. 2. Mesoderm migration in Drosophila cardiogenesis.
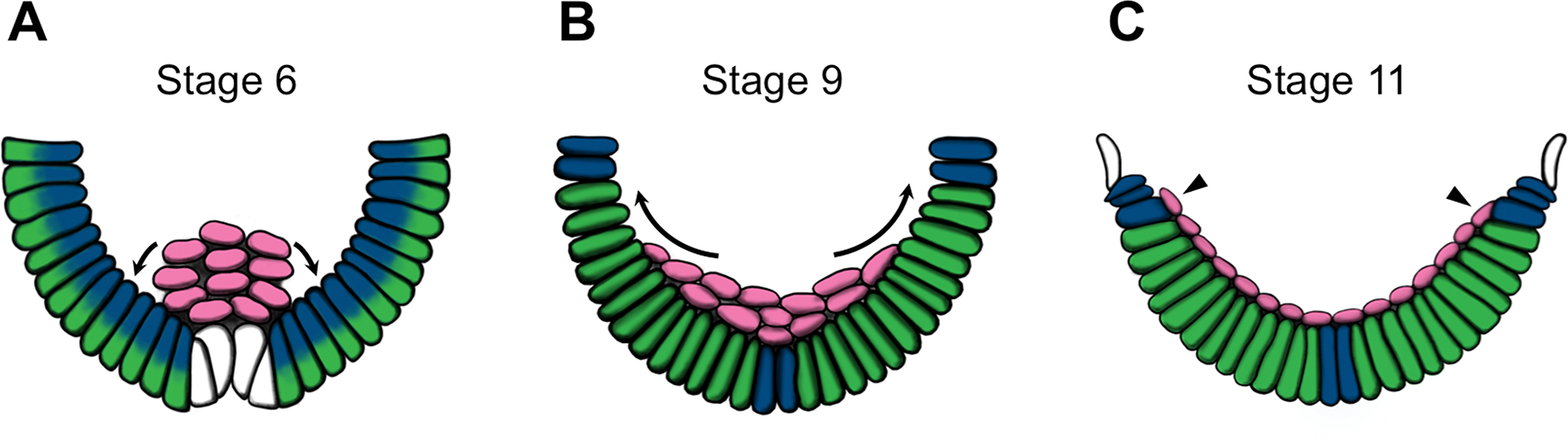
(A-C) Schematic cross-sections of Drosophila embryos at stages 6 (A), 9 (B), and 11 (C) showing the dorsolateral migration (arrows) of the FGFR Heartless-expressing mesodermal cells (pink) after invagination through the ventral furrow at gastrulation. Dynamic and broad expression patterns of the FGFs Pyramus (blue) and Thisbe (green) along the ectoderm direct this migration (cells which are half green and half blue express both FGFs). The dorsalmost cells at stage 11 (arrowheads) receive inductive signals to become specified as the cardiac mesoderm. Adapted from Kadam et al., 2009.
An analogous requirement of FGFR-mediated mesoderm migration for heart development is also observed in mammalian embryos. In mid-streak-staged gastrulating mouse embryos, FGFR1 is concentrated in the posterior mesoderm lateral to the primitive streak and is maintained in the migrating mesodermal wings (Yamaguchi et al., 1992). Analysis of chimeric embryos revealed that cells mutant for Fgfr1 are defective in lateral migration; unlike their wild-type counterparts, they accumulate along the primitive streak and fail to contribute in any significant quantity to the migrating mesodermal wings (Rossant et al., 1997). Furthermore, while Fgfr1 mutant cells have previously been shown to be capable of forming all types of mesoderm (Tam et al., 1997), in the chimeras, they fail to reach the positions that would allow them to contribute to the heart, somites, gut, and cephalic mesenchyme (Rossant et al., 1997).
These results demonstrate that FGFR-mediated mesoderm migration is necessary for heart development in both Drosophila and vertebrates and raise the question of what signals induce these migrated cells to become specified as the cardiac mesoderm.
ESTABLISHING THE CARDIAC MESODERM
In Drosophila, the specification of the dorsal mesoderm as the precursor of dorsal structures such as the heart can be followed by examining the expression pattern of tinman, an NK homeodomain-encoding gene necessary for the development of dorsal mesodermal derivatives. tinman is originally expressed ubiquitously in the uncommitted migrating mesoderm but becomes restricted to the dorsal mesoderm only upon the latter’s specification as a precursor of cardiac and other dorsal mesoderm derivatives in response to position-specific inductive signals (Bodmer et al., 1990; Azpiazu and Frasch, 1993; Bodmer, 1993).
BMP signaling specifies the dorsal mesoderm
At least two secreted factors expressed by the ectoderm act in a combinatorial fashion in Drosophila to induce a cardiogenic fate on the underlying migrated mesoderm. The first of these is Decapentaplegic (Dpp), a TGF-β bone morphogenetic protein (BMP) expressed by the dorsal ectoderm; the role of the second signal, the Wnt protein Wingless, is described in the next section. Three sets of observations demonstrate that signaling through Dpp induces the migrated mesoderm immediately underlying its expression domain in the ectoderm to become specified as the dorsal mesoderm—the precursor of the heart, visceral mesoderm, and dorsal somatic mesoderm. First, the ubiquitous expression of tinman throughout the trunk mesoderm and the T-box-encoding Dorsocross genes become restricted to only the dorsal mesoderm in direct contact with Dpp-expressing ectodermal cells (Frasch, 1995; Reim and Frasch, 2005). Second, loss of function mutations in genes encoding Dpp, its receptor Thickveins, or its downstream SMAD transcription factors Mad and Medea, result in tinman expression fading shortly after gastrulation and a failure to develop dorsal (including cardiac) mesodermal derivatives (Frasch, 1995; Xu et al., 1998; Yin and Frasch, 1998). Finally, ectopic expression of either Dpp throughout the entire ectoderm or a constitutively active form of the Thickveins receptor throughout the entire mesoderm is able to induce tinman in the ventral mesoderm at a stage when its expression is restricted to only the dorsal mesoderm in wild-type embryos (Frasch, 1995; Yin and Frasch, 1998).
The role of Dpp in Drosophila cardiogenesis described above led researchers to examine whether BMPs also induced cardiac mesoderm in vertebrates. At the stage when the mesodermal cells that will ultimately give rise to the heart are located in two bilateral regions in the anterolateral part of the chicken embryo, BMP4 and BMP7 are expressed by the adjacent ectoderm while BMP2 and BMP5 are expressed by the adjacent endoderm (Schultheiss et al., 1997; Somi et al., 2004). Overlapping expression of these BMPs along the borders of these cardiac precursors persists until and beyond both the onset of Nkx2.5 (the vertebrate ortholog of tinman) expression and the fusion of the cardiac precursors into the heart tube (Somi et al., 2004). While their overlapping expression patterns allow the BMPs to function redundantly during cardiogenesis, over 16 percent of mouse embryos homozygous for a Bmp2 null mutation formed no heart cells at all (that could be detected by Nkx2.5 expression), while the remainder all exhibited defective heart development (Zhang and Bradley, 1996). Double mutant mice homozygous for null mutations in both the Bmp5 and Bmp7 genes exhibited significantly delayed heart development compared to wild-type, with the double mutant hearts also displaying disorganized heart morphology (Solloway and Robertson, 1999). Finally, BMP7, BMP2, and BMP4 were all able to induce Nkx2.5 expression in chicken embryo anterior medial mesoendoderm explant cultures, with the latter two also inducing complete cardiogenesis (Schultheiss et al., 1997). Collectively, these data indicate that BMPs induce vertebrate mesodermal cells to commit to a cardiac mesodermal fate in a manner analogous to that in flies.
Intersections of Dpp and Wnt signaling domains specify the cardiac mesoderm
The dorsal mesodermal identity induced by Dpp in Drosophila gives rise to the visceral mesoderm and dorsal somatic muscles in addition to the heart, implying that an additional second signal must act in concert with Dpp to specify the more restricted cardiac mesoderm. Wingless, the Drosophila ortholog of the secreted glycoprotein Wnt1 (Baker, 1987), and which is expressed in 15 transverse stripes along the trunk ectoderm overlying the mesoderm (van den Heuvel et al., 1989), functions as this second signal. Eliminating Wingless function using a temperature-sensitive wingless mutation at the time when tinman expression becomes restricted to the dorsal mesoderm results in a reduction of heart cells without any effect on the visceral mesoderm or dorsal somatic muscles (Wu et al., 1995). This decrease in heart cell number is accompanied by the loss of tinman expression in cardiac precursor cells. Cardiac precursor cells are also missing in embryos lacking both Frizzled and Frizzled2, the receptors of Wingless (Bhanot et al., 1999; Chen and Struhl, 1999); Dishevelled, a downstream component of the Wingless signal transduction pathway; or Armadillo (β-catenin), a transcription factor in the canonical Wnt pathway (Park et al., 1996). Finally, overexpression of Dishevelled is able to rescue defective heart development in wingless mutants (Park et al., 1996), indicating that signaling through the Wnt pathway is indeed essential for Drosophila cardiogenesis. Taken together, these data show that segmentally arranged mesodermal cells underlying the intersections of Dpp and Wingless expression in the ectoderm become specified as the cardiac mesoderm (Lockwood and Bodmer, 2002). These cardiac progenitor cells then merge to ultimately form the continuous heart along the anterior-posterior axis (Borkowski et al., 1995).
The role of Wnt signaling in specifying cardiac progenitors in vertebrates is considerably more complex, with canonical Wnt signaling both inducing and suppressing cardiac differentiation depending on the time of action. First, canonical Wnt signaling utilizing Wnt3a and β-catenin is necessary for mesoderm specification before gastrulation—Wnt3a- or β-catenin-deficient mouse embryos failed to generate mesodermal tissue (Liu et al., 1999; Huelsken et al., 2000), while early inhibition of canonical Wnt signaling in murine embryonic stem (ES) cells blocked the expression of mesodermal marker genes (Lindsley et al., 2006).
However, the next step in vertebrate cardiogenesis, the specification of multipotent cardiac progenitors from these mesodermal cells, is suppressed by canonical Wnt/β-catenin signaling. In both chicken and Xenopus embryos, activation of the canonical Wnt pathway by ectopic expression of Wnt3a or Wnt8 suppressed cardiac differentiation as indicated by the downregulation of cardiac markers such Nkx2.5, while inhibition of the pathway through Wnt antagonists such as Crescent and Dkk-1 favored cardiac-specific gene expression (Marvin et al., 2001; Schneider and Mercola, 2001). Consistent with these results, β-catenin knockouts in mouse embryos also produced multiple ectopic hearts (Lickert et al., 2002). Collectively, these data indicate that the diffusion of Wnt antagonists from the Spemann organizer coupled with the inhibitory effect of canonical Wnt/β-catenin signaling on cardiogenesis at this stage of development serve to delimit the domains of the cardiogenic mesoderm.
This biphasic role, both positive and negative, of canonical Wnt/β-catenin signaling on cardiac specification depends on its time of action. This was elegantly illustrated in transgenic zebrafish where heat shock-induced expression of Wnt8 before gastrulation resulted in more cardiac progenitors than in wild-type embryos, whereas induced Wnt8 expression after gastrulation produced fewer cardiac progenitors (Ueno et al., 2007). Conversely, induced expression of the Wnt antagonist Dkk-1 before gastrulation inhibited cardiogenesis, while expression after gastrulation enhanced it (Ueno et al., 2007). A similar temporal biphasic effect was also seen in mouse ES cells, where treatment with Wnt ligands and inhibitors showed that Wnt/β-catenin signaling at an earlier time point during differentiation led to increased cardiac gene expression and larger contractile areas within embryoid bodies, while canonical Wnt/β-catenin signaling at a later time point had the reverse effect (Naito et al., 2006; Ueno et al., 2007).
Not all Wnts play an inhibitory role during this stage of cardiogenesis. In particular, noncanonical signaling through Wnt11 is essential for vertebrate cardiac specification of the mesodermal cells. Loss of function experiments showed that Wnt11 was required for cardiac gene expression and normal heart development in Xenopus embryos while its overexpression was sufficient to induce contractile phenotypes in Xenopus explants (Pandur et al., 2002; Afouda et al., 2008). Further analysis showed that this cardiogenic activity of Wnt11 in Xenopus was mediated by a PKC and JNK-mediated noncanonical Wnt pathway (Pandur et al., 2002). Similar positive roles for Wnt11 in cardiac specification were also seen with both quail mesoderm and mouse ES cells (Eisenberg et al., 1997; Eisenberg and Eisenberg, 1999; Terami et al., 2004; Ueno et al., 2007).
Of note, this alternating biphasic role of canonical Wnt signaling and the incorporation of noncanonical Wnt signaling pathways is utilized in vertebrate cardiogenesis even after the specification of the cardiac mesoderm: canonical Wnt/β-catenin signaling brings about the proliferation of cardiac progenitor cells in the second heart field (Ai et al., 2007; Klaus et al., 2007; Kwon et al., 2007; Lin et al., 2007; Tian et al., 2010) but inhibits the subsequent terminal differentiation of cardiomyocytes (Brott and Sokol, 2005; Lavery et al., 2008; Zhu et al., 2008). The latter process is mediated instead by noncanonical Wnt signaling through Wnt11 (Garriock et al., 2005; Gessert et al., 2008). This elaborate step by step variation in Wnt signaling pathways in vertebrate cardiogenesis likely reflects the overall complexity of spatiotemporally integrating multiple processes such as the specification, proliferation, and differentiation of distinct cardiac progenitors from diverse ancestral and intermediate cell populations in different heart fields.
An additional role for FGF signaling in specifying the dorsal mesoderm
In addition to its initial role in bringing about mesoderm migration in Drosophila embryos, the FGFR Heartless has a second, later function in specifying the dorsal mesoderm. When this later function is disrupted by expressing a dominant negative version of the FGFR throughout the mesoderm at a stage when mesoderm migration is not affected, a reduction in the number of heart cells and dorsal somatic muscle cells, as detected by the expression of the Even skipped marker, is observed (Michelson et al., 1998b). Furthermore, ectopic overexpression of either of the FGF ligands Pyramus or Thisbe results in an increase in the number of these Even skipped-expressing cells (Kadam et al., 2009; Klingseisen et al., 2009). Thus, the induction of the dorsal (and hence, cardiac) mesoderm in Drosophila requires both Dpp and FGF signaling.
A similar requirement of both FGFs and BMPs is observed for cardiogenic induction in vertebrates. In both the chick and mouse embryos, cells fated to become cardiac cells are positioned close to FGF- and BMP-expressing cells (Garcia-Martinez and Schoenwolf, 1993; Crossley and Martin, 1995; Schultheiss et al., 1997; Shamim and Mason, 1999). Combined treatment with both FGFs and BMPs, but neither signal alone, promotes cardiogenesis in non-precardiac mesoderm explants from chick embryos (Lough et al., 1996; Barron et al., 2000). Ectopic application of FGF8 in regions of the chick embryo where BMP2 and/or BMP4 are already present results in expanded expression of cardiac markers such as Nkx2.5 (Alsan and Schultheiss, 2002). Similarly, in the zebrafish embryo, FGF8 is able to induce Nkx2.5 expression in the presence of BMP2 (Reifers et al., 2000).
Signaling through EGFR is required for the survival of the cardiac mesoderm
Once specified, signaling through the epidermal growth factor receptor (EGFR) is required for maintaining the cardiac mesoderm in Drosophila. Targeted overexpression of EGFR in the mesoderm results in an increase in the number of heart cells, suggesting a role in cardiac cell proliferation and/or maintenance (Grigorian et al., 2011). Loss of function mutations in EGFR result in an almost complete absence of most cardiac cell types accompanied by significant amounts of cell death (Grigorian et al., 2011). These observations support the model that EGFR activity is required for the survival of the cardiac mesoderm during and after specification.
A similar role in cell survival for EGFR is observed in mice, albeit in vascular smooth muscle cells (VSMCs). Targeted inactivation of EGFR in VSMCs resulted in significantly higher lactate dehydrogenase (LDH) release, indicating reduced cell viability, as well as elevated caspase-3 activity, indicating an enhanced basal apoptosis rate (Schreier et al., 2011).
Notch signaling restricts cardiac mesodermal fate
Signaling through Wingless, as described previously, positively regulates the cardiac mesoderm in Drosophila: cells in the Dpp-induced dorsal mesoderm that also receive the Wingless signal become specified as cardiac progenitors. In contrast, signaling through the Notch receptor restricts the number of cardiac progenitors that are specified in the dorsal mesoderm (Carmena et al., 1998). Eliminating Notch using a temperature-sensitive allele during this cardiac mesoderm specification stage results in embryos with significantly larger numbers of every cardiac cell type (Hartenstein et al., 1992; Mandal et al., 2004).
Research performed on embryonic stem (ES) cells suggests that Notch signaling also has a similar restrictive effect on cardiogenic mesoderm specification in vertebrates. Utilizing Notch1 knockouts as well as activated and dominant negative versions of the mammalian homolog of Suppressor of Hairless [Su(H)], a critical transcription factor in the Notch pathway, investigators demonstrated that Notch signaling led to ES cells adopting neuroectodermal and not cardiogenic fates (Schroeder et al., 2003; Lowell et al., 2006; Nemir et al., 2006).
In summary, and as outlined in Figure 3, the convergence of positive regulatory inputs through the FGFR (Heartless), EGFR, Wnt (Wingless) and BMP (Dpp) signal transduction pathways, and negative regulation by the Notch signaling pathway specify and maintain the cardiac mesoderm in Drosophila. And, as described above, orthologous counterparts of each of these signaling pathways, playing similar cardiogenic roles, are found in vertebrates.
Fig. 3. Regulatory network of signaling pathways responsible for establishing the Drosophila cardiac mesoderm.
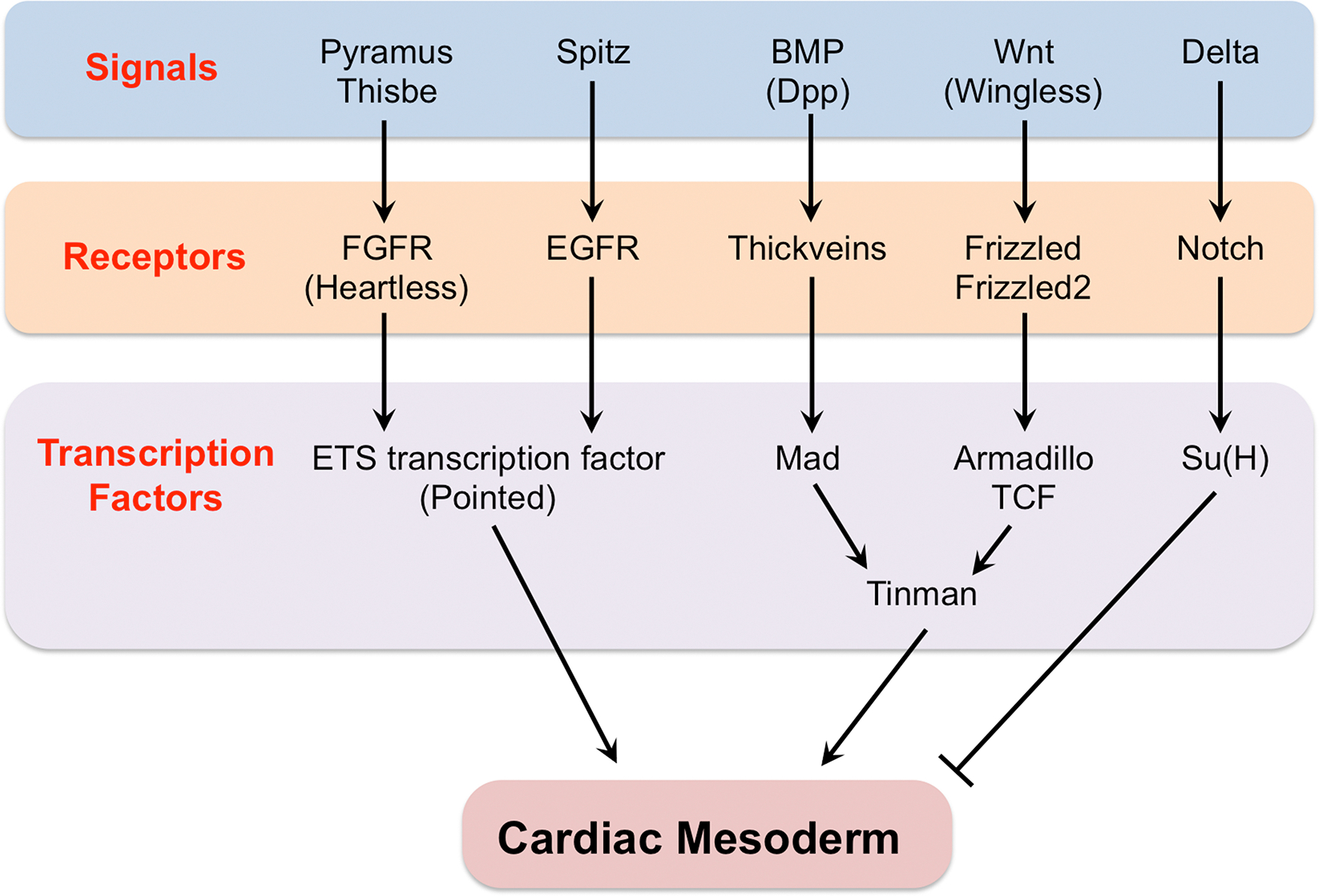
Only the signaling ligands, their receptors, and the terminal transcription factors of these signal transduction pathways are shown.
DIFFERENTIATION OF THE CARDIAC MESODERM INTO DISTINCT CARDIAC CELL TYPES
The cardiac progenitor cells comprising the Drosophila cardiac mesoderm undergo a stereotyped series of asymmetric and symmetric cell divisions to differentiate into two classes of heart cells: an inner row of contractile cardial cells (CCs), and an external sheath of non-muscle nephrocytic pericardial cells (PCs) which surrounds the CCs. However, neither the CCs nor the PCs constitute a uniform population, being comprised of eight different cell types distinguishable by their distinct cell lineages and individual gene expression programs: Seven up-CCs, Tinman-Ladybird-CCs, Tinman-CCs, Seven up-PCs, Odd skipped-PCs, Even skipped-PCs, Tinman-Ladybird-PCs, and Tinman-PCs, all named for the transcription factors they express (Figure 4) (Azpiazu and Frasch, 1993; Bodmer, 1993; Jagla et al., 1997; Ward and Skeath, 2000; Alvarez et al., 2003; Han and Bodmer, 2003). Some CCs become differentiated yet further to give rise to the valves that ensure that the circulatory fluid is only pumped anteriorwards (Lehmacher et al., 2012) or to the valves that regulate the opening and closing of the ostia, channels which allow the Drosophila circulatory fluid to enter the heart (Rizki, 1978). These differentiation processes are also mediated by conserved signaling mechanisms.
Fig. 4. Schematic diagram showing the stereotyped positions of the eight different cell types comprising the Drosophila heart.
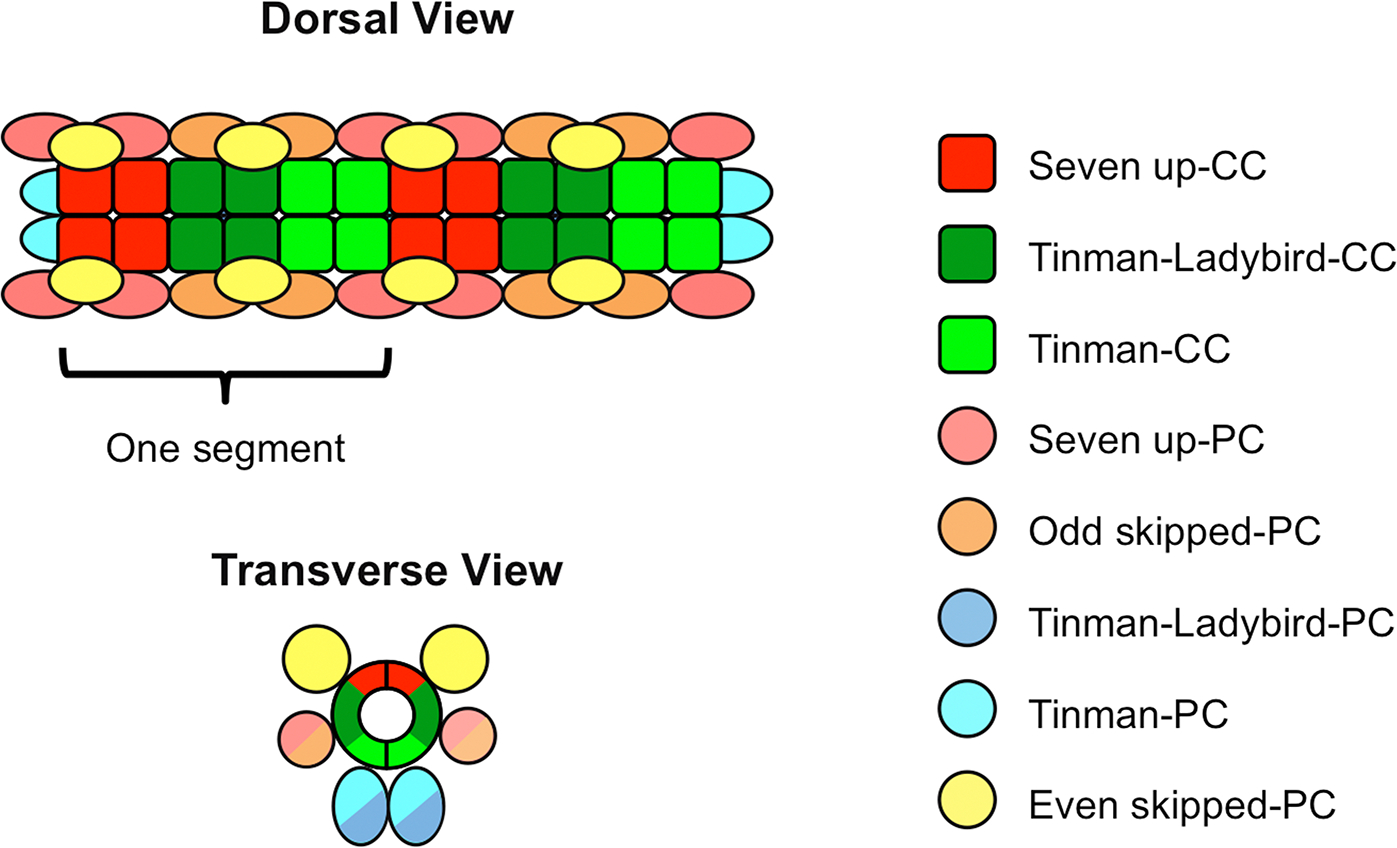
An individual heart segment, composed of two contralateral hemisegments mirroring each other across the dorsal midline, is indicated.
Notch signaling brings about the differentiation of the pericardial cells (PCs)
While signaling through the Notch pathway initially plays a suppressive role during the specification of the cardiac mesoderm, once the cardiac mesoderm has been established, Notch signaling is utilized again, this time for the differentiation of a subset of heart cells into pericardial cells. When this later function of Notch signaling is inactivated after cardiac mesoderm specification using the temperature-sensitive Notch mutation or with mutations of its ligand Delta, supernumerary CCs are formed at the expense of PCs (Hartenstein et al., 1992; Mandal et al., 2004; Grigorian et al., 2011). Conversely, overexpression of an activated form of Notch at this later stage converts CCs into PCs (Han and Bodmer, 2003; Ahmad et al., 2014).
In particular, this later function of Notch plays a critical role in determining the distinct fates of cardiac cell progeny produced by asymmetric cell divisions, thereby leading to cellular diversification. An antagonist of Notch activity, the membrane-associated protein Numb, becomes localized to one side of asymmetrically dividing progenitor cells and thus segregates primarily to only one of the two daughter cells (Rhyu et al., 1994; Spana and Doe, 1996; Ahmad et al., 2012). The daughter cell that inherits the bulk of the Numb protein is unable to respond to the Notch signal and therefore, in contrast to its pericardial sibling, adopts a myogenic or cardial cell fate. In the cardiac mesoderm of embryos mutant for numb, however, Notch signaling is not antagonized in any of the progeny of asymmetric cell division, resulting in all of them becoming pericardial (Park et al., 1998; Ward and Skeath, 2000; Han and Bodmer, 2003).
Previous studies had shown that in the absence of Notch signaling, the transcription factor Su(H) forms a repressor complex with co-repressors such as Groucho or C-terminal binding protein that binds to the enhancers of target genes to prevent their transcription (Bray and Furriols, 2001; Barolo and Posakony, 2002; Bray and Bernard, 2010). The activation of Notch receptors by ligand binding produces a proteolytic cleavage that releases the Notch intracellular domain (Nicd) from the plasma membrane, allowing it to enter the nucleus where it associates with Su(H), displaces the co-repressor and converts the Su(H) complex from a transcriptional repressor into an activator. That prior knowledge, along with the observations that the expression of Delta, a ligand for Notch, is specifically restricted to CCs (Grigorian et al., 2011), and that Su(H) binding sites are enriched along the enhancers of genes specifically expressed in PCs compared to those in CCs (Ahmad et al., 2014) ultimately led to our understanding of the mechanism by which Notch signaling induces pericardial, as opposed to myocardial CC fate (Figure 5).
Fig. 5. Pericardial fate specification by the Notch signaling pathway.
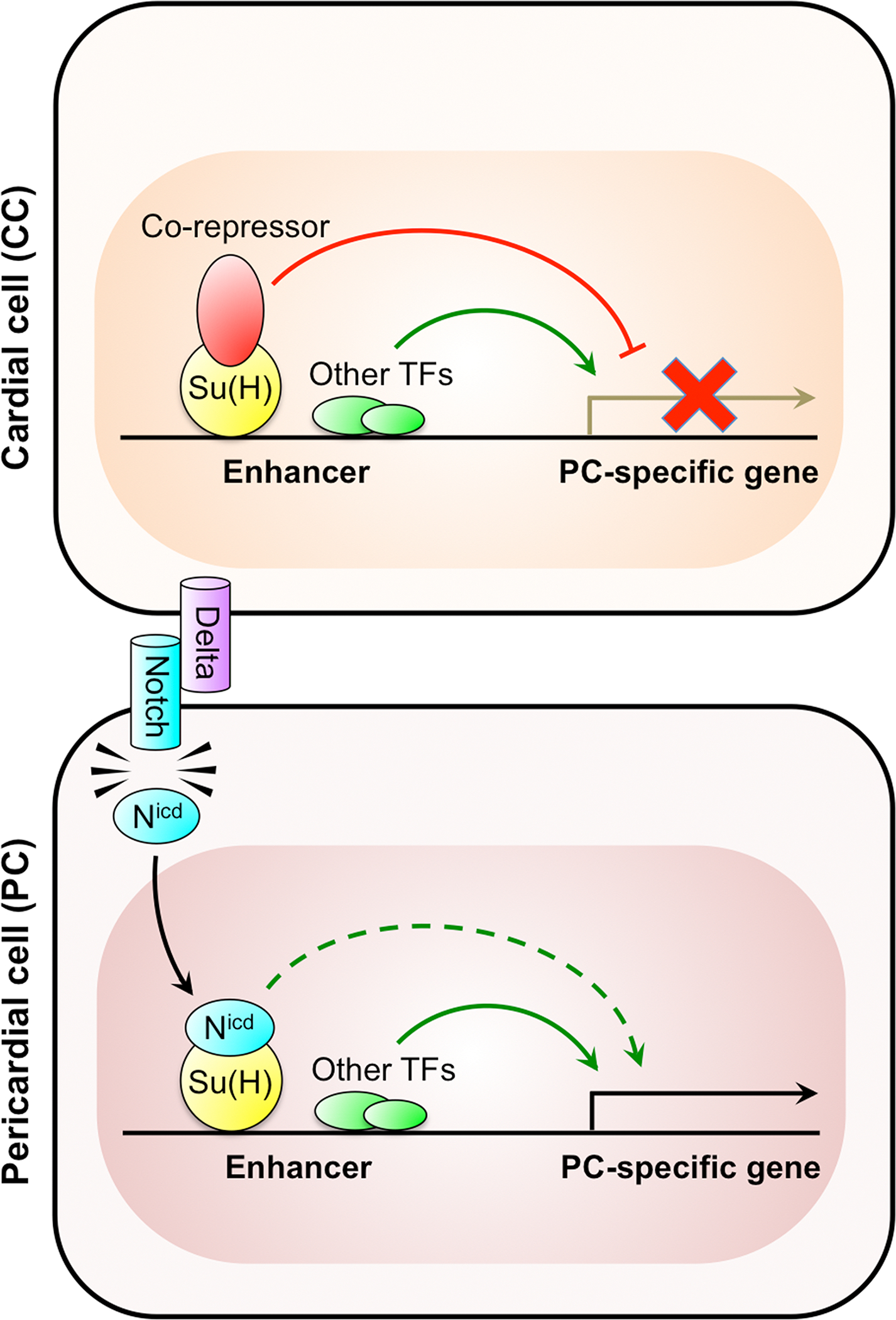
Schematic showing the regulation of pericardial cell (PC)-specific genes. Binding sites for the Su(H) transcription factor are enriched along the enhancers of PC-specific genes. In cardial cells (CCs), the enhancers of PC-specific genes are repressed by the binding of the Su(H)-co-repressor complex. However, the Delta ligand expressed specifically by the CCs activates the Notch receptor in the neighboring cells, with the resulting cleaved Nicd fragment associating with Su(H) and displacing the co-repressor. The resulting elimination of the repressor complex is sufficient to initiate transcription of the PC-specific genes due to the presence of other local activators, leading these neighboring cells to adopt a pericardial fate. Transcription of the PC-specific genes is further enhanced by the Nicd-Su(H) complex acting as an activator. Adapted from Ahmad et al., 2014.
In cardial cells, PC-specific genes are repressed by the Su(H)-co-repressor complex. Delta, expressed by CCs, activates Notch receptor in the neighboring cells fated to become PCs, with the resulting Nicd associating with Su(H) and displacing the co-repressor. The resulting elimination of the repressor complex is sufficient to initiate transcription of PC-specific genes, leading these neighboring cells to adopt a pericardial fate. This mechanism was confirmed by examining the effects of eliminating either the Su(H) protein by RNAi, or Su(H) binding by mutating its binding sites on the enhancers of PC genes, both of which led to presumptive CCs expressing PC-specific genes (Ahmad et al., 2014).
Intriguingly, Notch signaling in vertebrates also leads to cells in the established heart field adopting non-myocardial fates, suggesting that this later function of Notch in discriminating between distinct cardiac cell fates is also conserved. Conditional activation of Notch signaling in Xenopus embryos by injecting mRNA encoding an activated form of Su(H) leads to cells adopting a mesocardial or pericardial roof fate instead of a myocardial one (Rones et al., 2000). Similarly, constitutive activation of Notch by expressing Nicd in the developing chick heart promoted conduction cell differentiation and inhibited cardiomyocyte differentiation, while inactivating the Notch pathway by expression of a dominant negative version of Su(H) increased myocardial lineage markers at the expense of conduction cell markers (Chau et al., 2006).
Hedgehog signaling differentiates cardiac cells into distinct subtypes
The signaling molecule Hedgehog, expressed and secreted by the ectoderm in stripes adjacent to the Wingless-expressing cells, has three distinct functions in Drosophila cardiogenesis. First, it plays an indirect role in cardiac mesoderm specification by maintaining and reinforcing the Wingless expression from ectodermal cells necessary for the induction of cardiac progenitors (Park et al., 1996). These cardiac progenitor cells comprise non-overlapping subpopulations expressing distinct transcription factor-encoding genes such as even skipped and ladybird along each segment. The second function of Hedgehog signaling, in combination with the RAS pathway, is to specify and distinguish the even skipped-expressing subpopulation of cardiac progenitors from the ladybird-expressing subpopulation (Liu et al., 2006). Third, Hedgehog signaling initiates the differentiation of the cardial cells arising from the cardiac progenitors into distinct cell subtypes by bringing about the expression of the seven up gene in the anterior two pairs of cardial cells in a segment. Seven up inhibits the expression of tinman in these four cells, thus dividing each heart segment into two anterior pairs of Seven up-CCs and four posterior pairs of Tinman-Ladybird-CCs and Tinman-CCs (Figure 4) (Gajewski et al., 2000; Lo and Frasch, 2001; Ponzielli et al., 2002). The Seven up-CCs subsequently differentiate into the valves regulating the opening and closing of the inflow tracts in the embryonic or adult heart (Molina and Cripps, 2001). Thus, Hedgehog, by specifying the Seven up-CCs, establishes heart segment polarity and the location and differentiation of the inflow tracts in Drosophila.
Similar requirements for Hedgehog signaling in both cardiac progenitor specification and subsequent differentiation are seen in vertebrates. In zebrafish, Hedgehog specifies both the myocardial and endocardial progenitors and brings about the differentiation of the latter (Thomas et al., 2008; Wong et al., 2012). Furthermore, loss of function in mouse embryos of Sonic hedgehog, one of the three mammalian orthologs of the hedgehog gene in Drosophila, leads to defects in second heart field-derived cardiac structures, such as enlarged inflow tracts (Tsukui et al., 1999) and shortened and incorrectly positioned outflow tracts (Washington Smoak et al., 2005).
MIGRATION, POSITIONING, POLARITY, ADHESION AND CELL SHAPE CHANGES IN HEART TUBE MORPHOGENESIS
The differentiating cardial and pericardial cells from each side of the Drosophila embryo continue to migrate as attached rows of cells to the dorsal midline. In the process (Figure 6), each CC first becomes constricted along its dorsal domain and makes initial contact with its contralateral counterpart across the midline along this constricted dorsal domain. Each cell in these dorsally conjoined CC pairs then adopts a crescent shape, thereby allowing the ventral domains of the CCs to also join, and form a tube which encloses an internal central lumen (Medioni et al., 2008). Pericardial cells that migrated along with the cardial cells flank and remain intimately connected with the resulting myocardial tube, and thus occupy positions somewhat further from the dorsal midline. This final phase of heart tube morphogenesis thus entails the proper migration and positioning of CCs and PCs with respect to one another at the dorsal midline, adhesion between relevant cells, appropriate polarization of the CCs to determine leading edges during migration and contact between the relevant membrane domains, and cell shape changes that ensure tube and lumen formation. All these processes are also coordinated by conserved signaling pathways.
Fig. 6. Schematic representation of two cardial cells illustrating the temporal sequence of events during heart tube and lumen formation.
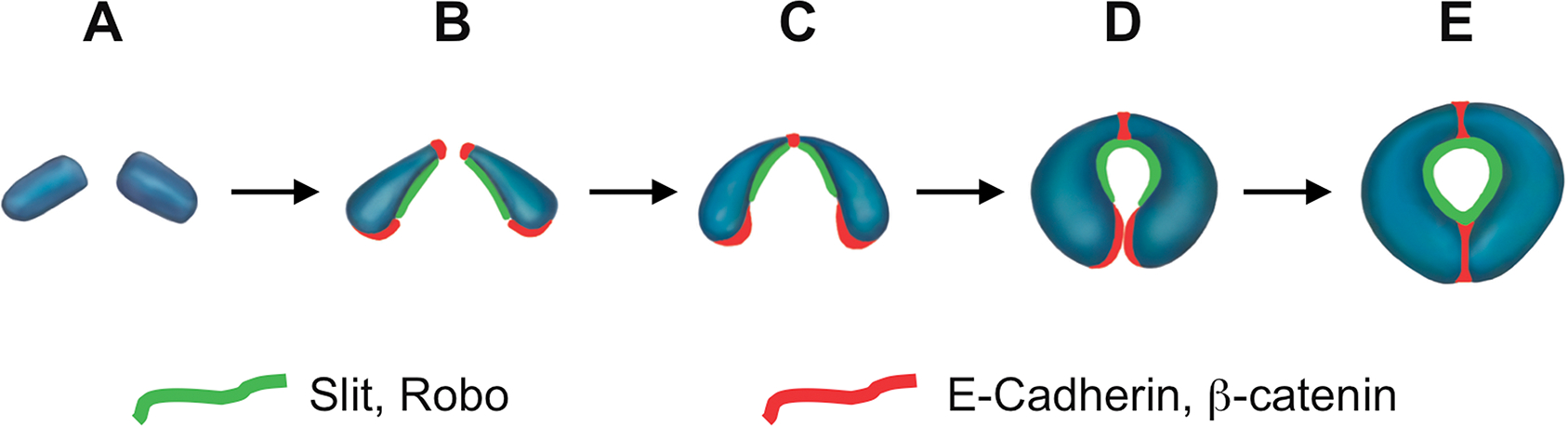
(A) At an early stage in migration both cardial cells (CCs) are cuboidal. (B) As they approach one another, they become constricted along their dorsal domain. (C) Each CC makes initial contact with its contralateral counterpart across the midline along this constricted dorsal domain. (D) Each cell in these dorsally conjoined CC pairs then begins adopting a crescent shape. (E) Ultimately, this shape change also allows the ventral domains of the CCs to join and form a tube which encloses an internal central lumen. Localization of Slit and Robo is shown in green while that of E-cadherin and β-catenin is shown in red. Adapted from Medioni et al., 2008 and Santiago-Martinez et al., 2008.
Signaling through the Slit/Robo pathway mediates migration, positioning, polarity, adhesion and shape change of heart cells
The highly conserved Slit protein and its receptors, Roundabout (Robo) and Roundabout 2 (Robo2) (Kramer et al., 2001; Wong et al., 2002), play critical roles in all of the processes described above. Embryos lacking either slit or robo2 function exhibit delayed migration of CCs and PCs towards the midline (MacMullin and Jacobs, 2006). The respective positions of both cardial and pericardial cells are also defective in slit, robo, and robo2 loss of function mutants. In embryos lacking slit function, the CCs fail to form two continuous rows of cells attached to each other at the midline with PCs flanking them: frequent gaps in both CC and PC rows are observed with the cells often forming inappropriate and ectopic clusters, suggesting defects in cell adhesion. Furthermore, many CCs fail to reach the dorsal midline, becoming inappropriately interspersed within the PCs. Similar phenotypes are seen in double mutants which disrupt both robo and robo2 functions, with relatively milder cardiac positioning and alignment defects being detected when only one receptor is eliminated (Qian et al., 2005; MacMullin and Jacobs, 2006; Santiago-Martinez et al., 2006).
Slit is expressed by the cardial cells and ultimately accumulates at the dorsal midline; Robo is expressed by both cardial and pericardial cells; and Robo2 is expressed solely by the pericardial cells (Qian et al., 2005; MacMullin and Jacobs, 2006; Santiago-Martinez et al., 2006). Misexpression studies showed that the correct migration, alignment, and positioning of both CCs and PCs were both critically dependent on the specific localization of Slit and its receptors, and, further, that the correct localization of Slit required wild-type robo and robo2 expression and functions, while that of Robo necessitated normal slit function (Qian et al., 2005; Santiago-Martinez et al., 2006).
In addition, Slit/Robo signaling mediates heart lumen formation by regulating membrane polarity, adhesion, and the shape of the cardial cells. In wild-type CCs, the cell adhesion proteins E-cadherin and β-catenin are specifically localized at the membranes along the constricted dorsal domain where the contralateral CCs make initial contact and at the ventral domain where they subsequently join after adopting a crescent shape. Cell-cell adhesion at these two junctional domains but not in the intervening luminal domain (where Slit and Robo are concentrated) ensures that a proper lumen is enclosed. However, in embryos lacking slit or robo function, both E-cadherin and β-catenin localization is expanded into the presumptive luminal domain thus creating an extended region of cell-cell adhesion that prevents lumen encapsulation (Qian et al., 2005; Medioni et al., 2008; Santiago-Martinez et al., 2008). Furthermore, Slit and its receptors are also required for the initial constriction of CCs along their dorsal junctional domain where the cells make initial contact with their contralateral counterparts. In mutants lacking either Slit or both Robo and Robo2 receptors, the CCs remain rounded and come into contact along most of their apposing surfaces, including the presumptive luminal domain, thereby also blocking lumen formation (Medioni et al., 2008).
Similarly, expression of Slit2, Slit3, Robo1, and Robo2 is detected in the developing mouse heart (Medioni et al., 2010), and mutational analysis has revealed functional roles for these genes in the correct migration and positioning of cells of the vertebrate cardiovascular system. In zebrafish, both slit2 and robo1 are required during heart tube formation for the proper migration of endocardial cells (Fish et al., 2011); in mice, Slit3, Robo1 and Robo2 are required for the proper development of the pericardium and the sinus horn myocardium, the alignment of caval veins, and the adhesion and migration of cardiac neural crest cells (Mommersteeg et al., 2013).
Integrins operate in concert with Slit and its receptors to bring about the migration, alignment, and polarization of differentiated heart cells in addition to lumen formation
The transmembrane Integrin receptors Myospheroid (Mys) and Scab (Scb), their ligands, and their downstream intracellular messengers are also involved in the aspects of cardiac morphogenesis mediated by Slit/Robo signaling. Delays in CC and PC migration, errors in the alignment and positioning of these cells, and defective lumen encapsulation similar to those seen in slit loss of function mutants or robo/robo2 double mutants are observed in embryos lacking these Integrins and their Laminin ligands (Yarnitzky and Volk, 1995; Stark et al., 1997; Martin et al., 1999; MacMullin and Jacobs, 2006; Vanderploeg et al., 2012). Furthermore, double heterozygote assays in which the cardiac phenotypes of heterozygous slit mutations were found to be enhanced by reducing the functions of these Integrins, their Laminin and Collagen ligands, or their downstream signaling components such as Talin or Integrin Linked Kinase indicated synergistic interactions and therefore likely cooperative signaling between the Slit/Robo- and Integrin-mediated pathways (MacMullin and Jacobs, 2006).
Consistent with their roles during CC migration and lumen encapsulation, these Integrins are predominantly localized at the presumptive luminal domain of the leading edges of the cells. They are required for the leading edge motility necessary for the joining of contralateral CCs—extensions of lamelipodial and filopodial processes from the leading edge were significantly reduced in embryos lacking zygotic mys or scb function (Vanderploeg et al., 2012). These Integrins also mediate the localization of Slit and Robo proteins necessary for lumen formation at the presumptive luminal domain—in embryos mutant for the Integrin-coding genes, Slit and Robo are mislocalized and present at significantly reduced levels, and the lumen is consequently missing or defective (Vanderploeg et al., 2012).
The lumen encapsulation by the two juxtaposed cardial cells in the Drosophila heart shares considerable similarities with lumen development in the mammalian dorsal aorta, where two apposed endothelial cells undergo cell shape changes to give rise to an extracellular lumen (Strilic et al., 2009). Thus, it is worth noting that disrupting B1-Integrin function in vertebrates results in absent or defective vascular lumens reminiscent of the cardiac phenotypes of Integrin mutants in Drosophila (Drake et al., 1992; Zovein et al., 2010). Similar contributions of Integrins to migration and adhesion during vertebrate vascular development are also seen (Davis and Senger, 2005; Hynes, 2007; Silva et al., 2008).
The small GTPase Cdc42, the nonmuscle myosin II Zipper, and the formins dDAAM and Diaphanous mediate lumen formation
The small GTPase Cdc42 plays a critical role in cardiac lumen formation in Drosophila. Contralateral CCs in embryos that lack Cdc42 function exhibit defective cell shape changes during lumen formation, failing to make ventral contacts after the initial dorsal contacts. In particular, Cdc42 plays an integral role in the actomyosin network: functioning in concert with dDAAM and Diaphanous, formins involved in actin polymerization, Cdc42 ensures the proper dynamic localization of the Drosophila nonmuscle myosin II Zipper at the leading edge of the CCs that brings about the requisite cell shape changes for lumen formation (Vogler et al., 2014).
Cdc42 also plays critical roles in mammalian heart morphogenesis. The deletion of Cdc42 in the developing mouse heart results in early embryonic lethality, and functional Cdc42 is required for leukocyte-inhibitory factor (LIF)-induced cell shape changes in cultured rat cardiomyocytes (Nagai et al., 2003; Maillet et al., 2009). Given the parallels described earlier between lumen formation in the Drosophila heart and the mammalian dorsal aorta, it is worth noting that Cdc42 is also needed for lumen development in human endothelial cell cultures (Bayless and Davis, 2002; Koh et al., 2008).
METAMORPHOSIS OF THE EMBRYONIC/LARVAL DROSOPHILA HEART INTO THE ADULT HEART
During the pupal stage, the once embryonic and subsequently larval heart tube undergoes extensive morphological changes to metamorphose into the adult heart (Curtis et al., 1999; Zikova et al., 2003; Monier et al., 2005). Most of the posterior wider region of the embryonic heart, referred to as the heart proper, is histolyzed, while the narrower, anterior part of the heart referred to as the aorta becomes widened. New ostia (inflow tracts) develop from the Seven up-CCs of what used to be the embryonic aorta, and three new intracardiac valves are formed (Molina and Cripps, 2001; Monier et al., 2005; Lehmacher et al., 2012). Finally, a subset of the syncytial alary muscles that connect the heart to the lateral exoskeleton dedifferentiate into mononucleate myoblasts and subsequently redifferentiate to form a new addition to the adult heart: a layer of longitudinal muscles associated with the heart tube along its ventral surface (Molina and Cripps, 2001; Lehmacher et al., 2012; Schaub et al., 2015).
Signaling through both the FGFR Heartless and Notch pathways was found to be necessary for the differentiation of the alary muscle-derived myoblasts, i.e. cells not originally part of the heart tube, into the ventral longitudinal muscles of the adult heart (Zeitouni et al., 2007; Schaub et al., 2015). Similarly, in vertebrates, FGF and Notch signaling mediate the proliferation and differentiation of cells from the second heart field—mesodermal cells that were not originally part of the initial heart tube but which contribute to tube extension and most of the cardiac structures (Reifers et al., 2000; Abu-Issa et al., 2002; Alsan and Schultheiss, 2002; Frank et al., 2002; Ilagan et al., 2006; Park et al., 2006; Chen et al., 2008; Park et al., 2008; Zhang et al., 2008; Kwon et al., 2009).
Furthermore, inhibition of the canonical Wnt/β-catenin signaling pathway in Drosophila was found to be necessary for the transdifferentation of the cardial cells of a segment of the wider embryonic heart proper into those of the narrower, posterior terminal chamber of the adult heart (Zeitouni et al., 2007). Of note, a similar reduction of canonical Wnt/β-catenin signaling is required to bring about the terminal differentiation of cardiomyocytes in the vertebrate heart (Brott and Sokol, 2005; Lavery et al., 2008; Zhu et al., 2008)
Additional roles of Wnt and PDGF-VEGF signaling in the formation of ostial and intracardiac valves are discussed in the following section.
VALVE FORMATION
Wnt signaling is required for ostia valve development
In both Drosophila and vertebrates, valves regulate the unidirectional flow of the circulatory fluid into the heart through the inflow tracts, and from the heart into the outflow tracts. Channels termed ostia serve as the inflow tracts in Drosophila (Rizki, 1978; Molina and Cripps, 2001). By undergoing a process of cellular constriction, extension out of the cardial cell layer into the heart lumen, and partial delamination, the two Seven up-cardial cells that comprise each ostium achieve a distinct morphology that enables them to function as valves to regulate inflow into the heart. Signaling by Wnt4, which is expressed in these ostia cells at much higher levels than in other CCs, and Wingless, which is expressed specifically in the ostia-lining Seven up-CCs, is essential for proper development of the ostia: embryos in which canonical Wnt signaling is disrupted with Wnt4 mutations, Wnt4 RNAi, wingless mutations, wingless RNAi, frizzled RNAi, frizzled2 RNAi, or dominant negative versions of the downstream TCF transcription factor exhibit absence of Seven up-CCs or severe defects in ostia cell morphology, and thus in ostia formation (Tauc et al., 2012; Chen et al., 2016; Trujillo et al., 2016).
A similar role for Wnt signaling in valve formation is also seen in vertebrates. A subset of vertebrate endothelial/endocardial cells also undergo constriction, extension and delamination in an epithelial-mesenchymal transition (EMT) process as they leave the endothelial layer and migrate into the cardiac jelly to form the cardiac cushions that give rise to the cardiac valves (Armstrong and Bischoff, 2004). This early EMT process is associated with high levels of both Wnt gene expression and Wnt signaling in mouse, chicken, and zebrafish embryos (Armstrong and Bischoff, 2004; Liebner et al., 2004; Alfieri et al., 2010; Moro et al., 2012). Furthermore, disruption of the Wnt signaling pathway with β-catenin mutations, β-catenin antagonists, Wnt antagonists, or Wnt inhibitors also blocks cushion formation and thus, valve differentiation (Hurlstone et al., 2003; Liebner et al., 2004; Alfieri et al., 2010).
PDGF-VEGF signaling is required for adult heart valve formation
During metamorphosis in Drosophila, extensive remodeling of the CCs of the larval heart brings about the formation of the adult heart (Molina and Cripps, 2001; Monier et al., 2005). Programmed cell death eliminates most of what was the posterior larval heart chamber, new ostia differentiate in what used to be the larval aorta (anterior compartment), and three new pairs of valves form which ensure that the circulatory fluid is pumped only anteriorwards. PDGF- and VEGF-receptor related (Pvr), a receptor tyrosine kinase related to mammalian PDGF and VEGF receptors, is specifically expressed in the precursors of these new adult valves. Additionally, expression of a dominant negative version of Pvr in the heart partially repressed adult valve formation, while ectopic expression of a constitutionally activated form of Pvr occasionally induced additional ectopic valves, indicating a role for the PDGF-VEGF signaling pathway in adult cardiac valve formation (Zeitouni et al., 2007).
In vertebrates, signaling through the VEGF receptor is used to induce EMT-mediated cardiac cushions from the endocardium and ultimately gives rise to the cardiac valves (Armstrong and Bischoff, 2004). Ligands of the VEGF receptor become restricted to the endocardial cells in the cushion forming regions (Miquerol et al., 1999; Dor et al., 2001). Furthermore, blocking VEGF signaling with inhibitors of the VEGF receptor causes loss of valve differentiation markers along with structural and functional defects in cardiac valve development (Lee et al., 2006).
Pygopus is required for adult heart valve differentiation
Another protein involved in Drosophila adult heart valve formation is Pygopus, which had previously been identified as a component of the canonical Wnt signaling pathway mediated by Wingless, Armadillo, and TCF (Belenkaya et al., 2002; Kramps et al., 2002; Parker et al., 2002). At the cardial cells comprising the intracardiac adult valves, the heart tube lumen is narrower and the myofibrillar network is denser than at the adjacent CCs. RNAi knockdown of pygopus results in both a lack of high-density myofibrils and an increase in the heart tube diameter at the valves. Furthermore, loss of function mutants of pygopus did not exhibit any defects in cardial cell number, indicating that Pygopus played a role in cardiac valve differentiation, but not in specification. RNAi knockdown of other components of the Wingless signaling pathway also exhibited significant valve dilations, albeit not as severe as that in the pygopus knockdown, and no genetic interaction was detected between pygopus and the genes encoding these components in double heterozygote assays (Tang et al., 2014). While this does not completely rule out the possibility that Pygopus might be mediating the differentiation of adult intracardiac valves through the canonical Wnt signaling pathway also utilized for valve formation in vertebrates (Armstrong and Bischoff, 2004; Liebner et al., 2004; Alfieri et al., 2010; Moro et al., 2012), it does raise the question of whether Pygopus could be using a different signaling pathway instead.
SIGNALING PATHWAYS UTILIZED IN CONSERVED CARDIOGENIC PROCESSES ALSO REGULATE PROCESSES UNIQUE TO VERTEBRATE CARDIOGENESIS
While this review has focused primarily on signaling pathways mediating aspects of heart development that are common to both Drosophila and vertebrates in terms of origins, structure, morphogenesis, and regulatory mechanisms, there are certain features of vertebrate cardiogenesis that have no counterparts in the fly. Many of the signaling pathways that regulate the common conserved aspects of heart development, however, are also used to mediate these cardiogenic processes unique to vertebrates.
For example, both the Drosophila and the vertebrate embryonic hearts originate as linear tubes running along the anterior-posterior axis, with circulatory fluid entering the lower/posterior part of the heart through inflow tracts and being pumped out from the upper/anterior region through the outflow tract (Figure 1). However, in vertebrates, but not in Drosophila, looping of this originally linear tube leads to its anterior-posterior polarity being converted into right-left polarity, with the atria, the previously posterior region where blood enters the heart, becoming ultimately anterior to the presumptive ventricles, the part of the heart which pumps blood out (Manner, 2000). The direction of cardiac looping is dependent on the left-right asymmetry set up in vertebrate embryos by asymmetrical expression of the genes Nodal, Lefty and Pitx2 on the left side of the body, which in turn is mediated by the Notch, Hedgehog, Wnt and BMP signaling pathways (Levin et al., 1995; Sampath et al., 1997; Boettger et al., 1999; Meyers and Martin, 1999; Schilling et al., 1999; Zhang et al., 2001; Krebs et al., 2003; Raya et al., 2003; Tsiairis and McMahon, 2009). The actual looping process itself is mediated by modulators of cytoskeletal changes such as the protein Xin, which in turn is induced by BMP2 signals (Wang et al., 1999).
Another unique aspect of vertebrate heart development is the separation of the undivided lumen of the looped heart tube into four distinct chambers. This entails the formation and fusion of endocardial cushions that divide the heart tube into right and left atrioventricular channels, the ventral growth of two atrial septa from the roof of the atrium into these endocardial cushions, and the development of the interventricular septum from the myocytes at the floor of the developing ventricles and extensions of the endocardial cushions (Schleich et al., 2013). Signaling through BMP and canonical Wnt pathways is necessary for forming the endocardial cushions by epithelial-mesenchymal transitions (Hurlstone et al., 2003; Liebner et al., 2004; Ma et al., 2005; Person et al., 2005; Rivera-Feliciano and Tabin, 2006), while the development and growth of the septa also require Hedgehog in addition to Wnt/β-catenin and BMP signaling (Goddeeris et al., 2008; Tian et al., 2010; Briggs et al., 2013).
Yet another feature that distinguishes vertebrate cardiogenesis from that in Drosophila is the colonization of and contribution to the vertebrate heart by an extracardiac nonmesodermal progenitor population—the cardiac neural crest cells. Arising originally from the dorsal neural tube, the cardiac neural crest cells migrate through the caudal pharyngeal arches into the outflow tract, bringing about its septation, and contributing to endocardial cushions, valves, smooth muscle tissue, and in the case of zebrafish, the myocardium (Kirby and Waldo, 1995; Hutson and Kirby, 2003; Li et al., 2003; Sato and Yost, 2003). The migration of the cardiac neural crest cells is mediated by signaling through the Hedgehog, BMP, Slit-Robo, and noncanonical Wnt pathways (Kaartinen et al., 2004; De Calisto et al., 2005; Jia et al., 2005; Washington Smoak et al., 2005; Calmont et al., 2009), while their proliferation and survival involves Hedgehog, FGF, and primarily canonical but also noncanonical Wnt signaling (Gage et al., 1999; Brault et al., 2001; Abu-Issa et al., 2002; Hamblet et al., 2002; Kioussi et al., 2002; Goddeeris et al., 2007).
SPATIOTEMPORAL EXPRESSION PATTERNS OF SIGNALING PATHWAY COMPONENTS REGULATE CARDIOGENIC PROCESSES
The diverse cardiogenic processes mediated by the distinct signaling mechanisms described in this review must be correctly orchestrated in time and space to bring about proper heart development. Not surprisingly, in many instances, it is the regulation of the spatiotemporal expression patterns of the ligands of these signaling pathways which determines where and when the latter are activated. For example, it is the localized expression patterns of the FGF ligands Pyramus and Thisbe that ensure the dorsal migration of the mesodermal monolayer (Kadam et al., 2009; Klingseisen et al., 2009); of Dpp and Wingless that bring about the specification of the cells immediately underlying their intersection as the cardiac mesoderm (van den Heuvel et al., 1989; Frasch, 1995; Wu et al., 1995; Yin and Frasch, 1998; Lockwood and Bodmer, 2002); and of Delta, expressed by the cardial cells, that induces a pericardial fate on neighboring cells (Grigorian et al., 2011; Ahmad et al., 2014).
However, an observation often not fully appreciated is that the regulation of expression patterns of other components of the signaling pathways may also be used to determine the time and location of a particular cardiogenic process. One illustration of this involves the cells which will become specified as the cardiac mesoderm by the later FGFR-mediated signaling process once mesoderm migration is completed (Michelson et al., 1998b). These localized cells must necessarily continue to produce the FGFR Heartless, a feat which is mediated by a heartless enhancer driving expression specifically in the presumptive cardiac mesodermal cells (Ahmad et al., 2016).
INTEGRATING MULTIPLE SIGNALS DURING CARDIOGENESIS
Given the number of distinct signaling pathways discussed so far, especially those involved in establishing the cardiac mesoderm, a particularly germane question is how the inputs from these diverse pathways are effectively combined to bring about proper heart development. An investigation into the regulation of even skipped, a gene expressed by a subset of cardiac progenitor cells, indicated that this integration is achieved at the transcriptional level in cardiac genes.
As illustrated in Figure 3, the signaling pathways involved in cardiac mesoderm specification act through downstream transcription factors: ETS domain transcription factors in the case of FGFR and EGFR, TCF in the case of Wingless, and Mad in the case of Dpp. A 312 bp minimal Muscle and Heart Enhancer (MHE) of the even skipped gene that was sufficient to drive its cardiac mesodermal expression contained functional binding sites for ETS domain proteins, TCF and Mad in addition to those for Twist and Tinman, transcription factors ubiquitously expressed throughout the presumptive cardiac mesoderm (Halfon et al., 2000). These binding sites were critical for MHE function, since mutating individual sites significantly reduced reporter expression driven by the MHE. Furthermore, the transcription factors binding at these sites act in a synergistic manner, since mutating two distinct sites resulted in expression defects significantly more severe than the additive effects of mutating the sites independently. Collectively, these data demonstrate that cardiac genes can integrate the inputs from many distinct signaling pathways by combinatorial binding of multiple pathway-specific transcription factors to their enhancers. Many other examples of cardiac genes utilizing their enhancers to combine inputs from diverse signaling pathways have since been observed, indicating that this is a favored method for integration (Halfon et al., 2002; Han et al., 2002; Philippakis et al., 2006; Zhu et al., 2012; Ahmad et al., 2014; Busser et al., 2015a).
Of note, the integration of signaling pathway inputs in establishing the cardiac mesoderm also provides an effective approach for identifying novel heart genes (Ahmad et al., 2012). As described previously, activation of the FGFR, EGFR, Dpp and Wingless signaling pathways would produce more cardiac mesodermal cells, and thus elevate the expression of cardiac genes within the embryo, while signaling through Notch would result in fewer cardiac mesodermal cells and reduced levels of cardiac gene expression. Consequently, mutations or transgenes that inactivated or constitutively activated these signaling pathways would be expected to alter cardiac gene expression in a predictable manner. Genome-wide transcriptional expression profiles from wild-type embryos and multiple genetic backgrounds that perturbed individual signaling pathways in both these ways were thus obtained, and a statistical meta-analysis method fitted to a training set of forty known cardiac mesodermal genes was used to rank all Drosophila genes by their likelihood of being expressed in the heart based on their collective behavior in these transcriptional expression profiles. In situ hybridization assays performed on the highly ranked genes in this meta-analysis identified seventy novel cardiac genes, with the cardiogenic roles of several being subsequently elucidated (Ahmad et al., 2012; Ahmad et al., 2014).
PERSPECTIVES
The leading causes of deaths worldwide are cardiovascular diseases (Go et al., 2014; Nichols et al., 2014). In particular, congenital heart disease (CHD) remains the most prevalently diagnosed birth defect with a postnatal incidence of 0.75% and an incident rate of 10% in stillbirths (Hoffman, 1995; Hoffman and Kaplan, 2002; Reller et al., 2008; Marelli et al., 2014). Understanding the molecular and genetic defects underlying CHDs and cardiomyopathies is thus a pressing reason to aim for a complete and comprehensive analysis of heart development.
Multiple approaches have been used to study cardiogenesis. They include the use of cytogenetic analysis, linkage and association studies, copy number variation and DNA microarray analysis, and whole exome sequencing with CHD-affected individuals in humans (Fahed et al., 2013; Andersen et al., 2014; Digilio and Marino, 2016); of cell-based assays using either embryonic stem cells or induced pluripotent stem cells induced to differentiate along the cardiac lineage (Alexander and Bruneau, 2010); of vertebrate models such as the mouse, chicken, zebrafish and Xenopus (Bruneau, 2008; Staudt and Stainier, 2012; Kain et al., 2014; Duncan and Khokha, 2016; Grant et al., 2017); and, of course, of the invertebrate model, Drosophila (Tao and Schulz, 2007; Vogler and Bodmer, 2015; Frasch, 2016; Lovato and Cripps, 2016; Rotstein and Paululat, 2016).
Each approach has its advantages and shortcomings, and a thorough understanding of heart development will require using all of them. As described previously, there are a few late vertebrate cardiogenic processes which are not recapitulated in Drosophila. But the relatively reduced level of complexity of the Drosophila heart compared to its vertebrate counterpart; the metamerically repeated and stereotyped arrangements of its cells that allow even subtle phenotypes to be detected and quantitated easily; its amenability to genetic analyses, such as the ability to disrupt any of its genes in any domain via RNAi and the ease of performing genetic interaction, epistasis, and rescue assays; the ability to examine heart development at single cell resolution; the capacity of the fly to survive to the larval level of development even with a disrupted heart; and the fact that the relatively low level of gene duplication in Drosophila reduces redundancy issues associated with paralogs all serve to make it a particularly tractable system for studying cardiogenesis. The observation that not merely cardiogenic transcription factors, but entire signaling pathways and the cardiogenic functions they mediate are conserved between Drosophila and vertebrate heart development further highlight the importance of utilizing investigations in Drosophila to enhance and facilitate our understanding of vertebrate cardiogenesis. Part of the power of Drosophila lies in being able to use its genetic toolkit to unravel these signaling pathways. This is particularly relevant in the context of the small number of genes identified as involved in CHD from human subjects: a significant fraction of these genes encode components of signaling pathways (Fahed et al., 2013; Andersen et al., 2014). They would be of little value if we were unable to elucidate the relevant cardiogenic signaling pathways that they contribute to based on research performed in Drosophila and other vertebrate models.
Similarly, attempts at understanding cardiogenesis using Drosophila should consider using integrative strategies which both leverage the distinct advantages of different systems and focus on genes and pathways likely to be playing critical roles in mammalian cardiogenesis, and thus be of particular relevance to human heart development. One elegant example of such an integrated approach utilized histone marks and gene expression profiles from human and mouse embryonic stem cells differentiating along the cardiac lineage to create an epigenetic signature to predict putative mammalian cardiogenic genes. The effects of knocking down their orthologs in the developing Drosophila cardiac mesoderm and heart were then examined to rapidly and efficiently assess whether these predicted genes actually possessed cardiogenic roles and determine which to analyze further (Busser et al., 2015b). Conversely, with more and more new genome-wide approaches for studying heart development being utilized in Drosophila (reviewed in Frasch, 2016), it has become even more important to explore and assess what the resulting findings can tell us about vertebrate, and ultimately human, cardiogenesis.
ACKNOWLEDGMENTS
I am grateful to Linda K. Castor for providing many of the illustrations. This work was supported by the American Heart Association Grant 16SDG31390005 (Shaad M. Ahmad).
Footnotes
DEDICATION
This review is dedicated to the memory of Dr. Alan M. Michelson, scientist, mentor, friend, and fellow explorer of the Drosophila heart.
REFERENCES
- Abu-Issa R, Smyth G, Smoak I, Yamamura K, Meyers EN. 2002. Fgf8 is required for pharyngeal arch and cardiovascular development in the mouse. Development 129:4613–4625. [DOI] [PubMed] [Google Scholar]
- Afouda BA, Martin J, Liu F, Ciau-Uitz A, Patient R, Hoppler S. 2008. GATA transcription factors integrate Wnt signalling during heart development. Development 135:3185–3190. [DOI] [PubMed] [Google Scholar]
- Ahmad SM, Bhattacharyya P, Jeffries N, Gisselbrecht SS, Michelson AM. 2016. Two Forkhead transcription factors regulate cardiac progenitor specification by controlling the expression of receptors of the fibroblast growth factor and Wnt signaling pathways. Development 143:306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad SM, Busser BW, Huang D, Cozart EJ, Michaud S, Zhu X, Jeffries N, Aboukhalil A, Bulyk ML, Ovcharenko I, Michelson AM. 2014. Machine learning classification of cell-specific cardiac enhancers uncovers developmental subnetworks regulating progenitor cell division and cell fate specification. Development 141:878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad SM, Tansey TR, Busser BW, Nolte MT, Jeffries N, Gisselbrecht SS, Rusan NM, Michelson AM. 2012. Two forkhead transcription factors regulate the division of cardiac progenitor cells by a Polo-dependent pathway. Dev Cell 23:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. 2007. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci U S A 104:9319–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JM, Bruneau BG. 2010. Lessons for cardiac regeneration and repair through development. Trends Mol Med 16:426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfieri CM, Cheek J, Chakraborty S, Yutzey KE. 2010. Wnt signaling in heart valve development and osteogenic gene induction. Dev Biol 338:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsan BH, Schultheiss TM. 2002. Regulation of avian cardiogenesis by Fgf8 signaling. Development 129:1935–1943. [DOI] [PubMed] [Google Scholar]
- Alvarez AD, Shi W, Wilson BA, Skeath JB. 2003. pannier and pointedP2 act sequentially to regulate Drosophila heart development. Development 130:3015–3026. [DOI] [PubMed] [Google Scholar]
- Andersen TA, Troelsen Kde L, Larsen LA. 2014. Of mice and men: molecular genetics of congenital heart disease. Cell Mol Life Sci 71:1327–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. 2004. Heart valve development: endothelial cell signaling and differentiation. Circ Res 95:459–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azpiazu N, Frasch M. 1993. tinman and bagpipe: two homeo box genes that determine cell fates in the dorsal mesoderm of Drosophila. Genes Dev 7:1325–1340. [DOI] [PubMed] [Google Scholar]
- Baker NE. 1987. Molecular cloning of sequences from wingless, a segment polarity gene in Drosophila: the spatial distribution of a transcript in embryos. EMBO J 6:1765–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barolo S, Posakony JW. 2002. Three habits of highly effective signaling pathways: principles of transcriptional control by developmental cell signaling. Genes Dev 16:1167–1181. [DOI] [PubMed] [Google Scholar]
- Barron M, Gao M, Lough J. 2000. Requirement for BMP and FGF signaling during cardiogenic induction in non-precardiac mesoderm is specific, transient, and cooperative. Developmental dynamics : an official publication of the American Association of Anatomists 218:383–393. [DOI] [PubMed] [Google Scholar]
- Bayless KJ, Davis GE. 2002. The Cdc42 and Rac1 GTPases are required for capillary lumen formation in three-dimensional extracellular matrices. J Cell Sci 115:1123–1136. [DOI] [PubMed] [Google Scholar]
- Beiman M, Shilo BZ, Volk T. 1996. Heartless, a Drosophila FGF receptor homolog, is essential for cell migration and establishment of several mesodermal lineages. Genes Dev 10:2993–3002. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Standley HJ, Lin X, Houston DW, Heasman J, Lin X. 2002. pygopus Encodes a nuclear protein essential for wingless/Wnt signaling. Development 129:4089–4101. [DOI] [PubMed] [Google Scholar]
- Bhanot P, Fish M, Jemison JA, Nusse R, Nathans J, Cadigan KM. 1999. Frizzled and Dfrizzled-2 function as redundant receptors for Wingless during Drosophila embryonic development. Development 126:4175–4186. [DOI] [PubMed] [Google Scholar]
- Bishopric NH. 2005. Evolution of the heart from bacteria to man. Ann N Y Acad Sci 1047:13–29. [DOI] [PubMed] [Google Scholar]
- Bodmer R 1993. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development 118:719–729. [DOI] [PubMed] [Google Scholar]
- Bodmer R 1995. Heart development in Drosophila and its relationship to vertebrates. Trends Cardiovasc Med 5:21–28. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Frasch M. 2010. Development and Aging of the Drosophila Heart. Heart Development and Regeneration:47–86. [Google Scholar]
- Bodmer R, Jan LY, Jan YN. 1990. A new homeobox-containing gene, msh-2, is transiently expressed early during mesoderm formation of Drosophila. Development 110:661–669. [DOI] [PubMed] [Google Scholar]
- Bodmer R, Venkatesh TV. 1998. Heart development in Drosophila and vertebrates: conservation of molecular mechanisms. Dev Genet 22:181–186. [DOI] [PubMed] [Google Scholar]
- Boettger T, Wittler L, Kessel M. 1999. FGF8 functions in the specification of the right body side of the chick. Curr Biol 9:277–280. [DOI] [PubMed] [Google Scholar]
- Borkowski OM, Brown NH, Bate M. 1995. Anterior-posterior subdivision and the diversification of the mesoderm in Drosophila. Development 121:4183–4193. [DOI] [PubMed] [Google Scholar]
- Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, Sommer L, Boussadia O, Kemler R. 2001. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development 128:1253–1264. [DOI] [PubMed] [Google Scholar]
- Bray S, Bernard F. 2010. Notch targets and their regulation. Curr Top Dev Biol 92:253–275. [DOI] [PubMed] [Google Scholar]
- Bray S, Furriols M. 2001. Notch pathway: making sense of suppressor of hairless. Curr Biol 11:R217–221. [DOI] [PubMed] [Google Scholar]
- Briggs LE, Phelps AL, Brown E, Kakarla J, Anderson RH, van den Hoff MJ, Wessels A. 2013. Expression of the BMP receptor Alk3 in the second heart field is essential for development of the dorsal mesenchymal protrusion and atrioventricular septation. Circ Res 112:1420–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brott BK, Sokol SY. 2005. A vertebrate homolog of the cell cycle regulator Dbf4 is an inhibitor of Wnt signaling required for heart development. Dev Cell 8:703–715. [DOI] [PubMed] [Google Scholar]
- Bruneau BG. 2008. The developmental genetics of congenital heart disease. Nature 451:943–948. [DOI] [PubMed] [Google Scholar]
- Busser BW, Haimovich J, Huang D, Ovcharenko I, Michelson AM. 2015a. Enhancer modeling uncovers transcriptional signatures of individual cardiac cell states in Drosophila. Nucleic Acids Res 43:1726–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busser BW, Lin Y, Yang Y, Zhu J, Chen G, Michelson AM. 2015b. An Orthologous Epigenetic Gene Expression Signature Derived from Differentiating Embryonic Stem Cells Identifies Regulators of Cardiogenesis. PLoS One 10:e0141066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmont A, Ivins S, Van Bueren KL, Papangeli I, Kyriakopoulou V, Andrews WD, Martin JF, Moon AM, Illingworth EA, Basson MA, Scambler PJ. 2009. Tbx1 controls cardiac neural crest cell migration during arch artery development by regulating Gbx2 expression in the pharyngeal ectoderm. Development 136:3173–3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmena A, Gisselbrecht S, Harrison J, Jimenez F, Michelson AM. 1998. Combinatorial signaling codes for the progressive determination of cell fates in the Drosophila embryonic mesoderm. Genes Dev 12:3910–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau MD, Tuft R, Fogarty K, Bao ZZ. 2006. Notch signaling plays a key role in cardiac cell differentiation. Mech Dev 123:626–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Struhl G. 1999. Wingless transduction by the Frizzled and Frizzled2 proteins of Drosophila. Development 126:5441–5452. [DOI] [PubMed] [Google Scholar]
- Chen VC, Stull R, Joo D, Cheng X, Keller G. 2008. Notch signaling respecifies the hemangioblast to a cardiac fate. Nat Biotechnol 26:1169–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Zhu JY, Fu Y, Richman A, Han Z. 2016. Wnt4 is required for ostia development in the Drosophila heart. Dev Biol 413:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripps RM, Olson EN. 2002. Control of cardiac development by an evolutionarily conserved transcriptional network. Developmental biology 246:14–28. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martin GR. 1995. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121:439–451. [DOI] [PubMed] [Google Scholar]
- Curtis NJ, Ringo JM, Dowse HB. 1999. Morphology of the pupal heart, adult heart, and associated tissues in the fruit fly, Drosophila melanogaster. J Morphol 240:225–235. [DOI] [PubMed] [Google Scholar]
- Davis GE, Senger DR. 2005. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization. Circ Res 97:1093–1107. [DOI] [PubMed] [Google Scholar]
- De Calisto J, Araya C, Marchant L, Riaz CF, Mayor R. 2005. Essential role of non-canonical Wnt signalling in neural crest migration. Development 132:2587–2597. [DOI] [PubMed] [Google Scholar]
- Digilio MC, Marino B. 2016. What Is New in Genetics of Congenital Heart Defects? Front Pediatr 4:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dor Y, Camenisch TD, Itin A, Fishman GI, McDonald JA, Carmeliet P, Keshet E. 2001. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development 128:1531–1538. [DOI] [PubMed] [Google Scholar]
- Drake CJ, Davis LA, Little CD. 1992. Antibodies to beta 1-integrins cause alterations of aortic vasculogenesis, in vivo. Dev Dyn 193:83–91. [DOI] [PubMed] [Google Scholar]
- Duncan AR, Khokha MK. 2016. Xenopus as a model organism for birth defects-Congenital heart disease and heterotaxy. Semin Cell Dev Biol 51:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg CA, Eisenberg LM. 1999. WNT11 promotes cardiac tissue formation of early mesoderm. Dev Dyn 216:45–58. [DOI] [PubMed] [Google Scholar]
- Eisenberg CA, Gourdie RG, Eisenberg LM. 1997. Wnt-11 is expressed in early avian mesoderm and required for the differentiation of the quail mesoderm cell line QCE-6. Development 124:525–536. [DOI] [PubMed] [Google Scholar]
- Fahed AC, Gelb BD, Seidman JG, Seidman CE. 2013. Genetics of congenital heart disease: the glass half empty. Circ Res 112:707–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish JE, Wythe JD, Xiao T, Bruneau BG, Stainier DY, Srivastava D, Woo S. 2011. A Slit/miR-218/Robo regulatory loop is required during heart tube formation in zebrafish. Development 138:1409–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DU, Fotheringham LK, Brewer JA, Muglia LJ, Tristani-Firouzi M, Capecchi MR, Moon AM. 2002. An Fgf8 mouse mutant phenocopies human 22q11 deletion syndrome. Development 129:4591–4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch M 1995. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature 374:464–467. [DOI] [PubMed] [Google Scholar]
- Frasch M 2016. Genome-wide approaches to Drosophila heart development. J Cardiovasc Dev Dis 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay L, Seger R, Shilo BZ. 1997. MAP kinase in situ activation atlas during Drosophila embryogenesis. Development 124:3535–3541. [DOI] [PubMed] [Google Scholar]
- Gage PJ, Suh H, Camper SA. 1999. Dosage requirement of Pitx2 for development of multiple organs. Development 126:4643–4651. [DOI] [PubMed] [Google Scholar]
- Gajewski K, Choi CY, Kim Y, Schulz RA. 2000. Genetically distinct cardial cells within the Drosophila heart. Genesis 28:36–43. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez V, Schoenwolf GC. 1993. Primitive-streak origin of the cardiovascular system in avian embryos. Dev Biol 159:706–719. [DOI] [PubMed] [Google Scholar]
- Garriock RJ, D’Agostino SL, Pilcher KC, Krieg PA. 2005. Wnt11-R, a protein closely related to mammalian Wnt11, is required for heart morphogenesis in Xenopus. Dev Biol 279:179–192. [DOI] [PubMed] [Google Scholar]
- Gessert S, Maurus D, Brade T, Walther P, Pandur P, Kuhl M. 2008. DM-GRASP/ALCAM/CD166 is required for cardiac morphogenesis and maintenance of cardiac identity in first heart field derived cells. Dev Biol 321:150–161. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht S, Skeath JB, Doe CQ, Michelson AM. 1996. heartless encodes a fibroblast growth factor receptor (DFR1/DFGF-R2) involved in the directional migration of early mesodermal cells in the Drosophila embryo. Genes Dev 10:3003–3017. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB, American Heart Association Statistics C, Stroke Statistics S. 2014. Executive summary: heart disease and stroke statistics--2014 update: a report from the American Heart Association. Circulation 129:399–410. [DOI] [PubMed] [Google Scholar]
- Goddeeris MM, Rho S, Petiet A, Davenport CL, Johnson GA, Meyers EN, Klingensmith J. 2008. Intracardiac septation requires hedgehog-dependent cellular contributions from outside the heart. Development 135:1887–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddeeris MM, Schwartz R, Klingensmith J, Meyers EN. 2007. Independent requirements for Hedgehog signaling by both the anterior heart field and neural crest cells for outflow tract development. Development 134:1593–1604. [DOI] [PubMed] [Google Scholar]
- Grant MG, Patterson VL, Grimes DT, Burdine RD. 2017. Modeling Syndromic Congenital Heart Defects in Zebrafish. Curr Top Dev Biol 124:1–40. [DOI] [PubMed] [Google Scholar]
- Grigorian M, Mandal L, Hakimi M, Ortiz I, Hartenstein V. 2011. The convergence of Notch and MAPK signaling specifies the blood progenitor fate in the Drosophila mesoderm. Dev Biol 353:105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gryzik T, Muller HA. 2004. FGF8-like1 and FGF8-like2 encode putative ligands of the FGF receptor Htl and are required for mesoderm migration in the Drosophila gastrula. Curr Biol 14:659–667. [DOI] [PubMed] [Google Scholar]
- Halfon MS, Carmena A, Gisselbrecht S, Sackerson CM, Jimenez F, Baylies MK, Michelson AM. 2000. Ras pathway specificity is determined by the integration of multiple signal-activated and tissue-restricted transcription factors. Cell 103:63–74. [DOI] [PubMed] [Google Scholar]
- Halfon MS, Grad Y, Church GM, Michelson AM. 2002. Computation-based discovery of related transcriptional regulatory modules and motifs using an experimentally validated combinatorial model. Genome Res 12:1019–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblet NS, Lijam N, Ruiz-Lozano P, Wang J, Yang Y, Luo Z, Mei L, Chien KR, Sussman DJ, Wynshaw-Boris A. 2002. Dishevelled 2 is essential for cardiac outflow tract development, somite segmentation and neural tube closure. Development 129:5827–5838. [DOI] [PubMed] [Google Scholar]
- Han Z, Bodmer R. 2003. Myogenic cells fates are antagonized by Notch only in asymmetric lineages of the Drosophila heart, with or without cell division. Development 130:3039–3051. [DOI] [PubMed] [Google Scholar]
- Han Z, Fujioka M, Su M, Liu M, Jaynes JB, Bodmer R. 2002. Transcriptional integration of competence modulated by mutual repression generates cell-type specificity within the cardiogenic mesoderm. Dev Biol 252:225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartenstein AY, Rugendorff A, Tepass U, Hartenstein V. 1992. The function of the neurogenic genes during epithelial development in the Drosophila embryo. Development 116:1203–1220. [DOI] [PubMed] [Google Scholar]
- Hoffman JI. 1995. Incidence of congenital heart disease: II. Prenatal incidence. Pediatr Cardiol 16:155–165. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S. 2002. The incidence of congenital heart disease. J Am Coll Cardiol 39:1890–1900. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Vogel R, Brinkmann V, Erdmann B, Birchmeier C, Birchmeier W. 2000. Requirement for beta-catenin in anterior-posterior axis formation in mice. J Cell Biol 148:567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. 2003. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature 425:633–637. [DOI] [PubMed] [Google Scholar]
- Hutson MR, Kirby ML. 2003. Neural crest and cardiovascular development: a 20-year perspective. Birth Defects Res C Embryo Today 69:2–13. [DOI] [PubMed] [Google Scholar]
- Hynes RO. 2007. Cell-matrix adhesion in vascular development. J Thromb Haemost 5 Suppl 1:32–40. [DOI] [PubMed] [Google Scholar]
- Ilagan R, Abu-Issa R, Brown D, Yang YP, Jiao K, Schwartz RJ, Klingensmith J, Meyers EN. 2006. Fgf8 is required for anterior heart field development. Development 133:2435–2445. [DOI] [PubMed] [Google Scholar]
- Imam F, Sutherland D, Huang W, Krasnow MA. 1999. stumps, a Drosophila gene required for fibroblast growth factor (FGF)-directed migrations of tracheal and mesodermal cells. Genetics 152:307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagla K, Frasch M, Jagla T, Dretzen G, Bellard F, Bellard M. 1997. ladybird, a new component of the cardiogenic pathway in Drosophila required for diversification of heart precursors. Development 124:3471–3479. [DOI] [PubMed] [Google Scholar]
- Jia L, Cheng L, Raper J. 2005. Slit/Robo signaling is necessary to confine early neural crest cells to the ventral migratory pathway in the trunk. Dev Biol 282:411–421. [DOI] [PubMed] [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. 2004. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development 131:3481–3490. [DOI] [PubMed] [Google Scholar]
- Kadam S, McMahon A, Tzou P, Stathopoulos A. 2009. FGF ligands in Drosophila have distinct activities required to support cell migration and differentiation. Development 136:739–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kain KH, Miller JW, Jones-Paris CR, Thomason RT, Lewis JD, Bader DM, Barnett JV, Zijlstra A. 2014. The chick embryo as an expanding experimental model for cancer and cardiovascular research. Dev Dyn 243:216–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussi C, Briata P, Baek SH, Rose DW, Hamblet NS, Herman T, Ohgi KA, Lin C, Gleiberman A, Wang J, Brault V, Ruiz-Lozano P, Nguyen HD, Kemler R, Glass CK, Wynshaw-Boris A, Rosenfeld MG. 2002. Identification of a Wnt/Dvl/beta-Catenin --> Pitx2 pathway mediating cell-type-specific proliferation during development. Cell 111:673–685. [DOI] [PubMed] [Google Scholar]
- Kirby ML, Waldo KL. 1995. Neural crest and cardiovascular patterning. Circ Res 77:211–215. [DOI] [PubMed] [Google Scholar]
- Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. 2007. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci U S A 104:18531–18536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingseisen A, Clark IB, Gryzik T, Muller HA. 2009. Differential and overlapping functions of two closely related Drosophila FGF8-like growth factors in mesoderm development. Development 136:2393–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh W, Mahan RD, Davis GE. 2008. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci 121:989–1001. [DOI] [PubMed] [Google Scholar]
- Kramer SG, Kidd T, Simpson JH, Goodman CS. 2001. Switching repulsion to attraction: changing responses to slit during transition in mesoderm migration. Science 292:737–740. [DOI] [PubMed] [Google Scholar]
- Kramps T, Peter O, Brunner E, Nellen D, Froesch B, Chatterjee S, Murone M, Zullig S, Basler K. 2002. Wnt/wingless signaling requires BCL9/legless-mediated recruitment of pygopus to the nuclear beta-catenin-TCF complex. Cell 109:47–60. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R, Saijoh Y, O’Brien TP, Hamada H, Gridley T. 2003. Notch signaling regulates left-right asymmetry determination by inducing Nodal expression. Genes Dev 17:1207–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. 2007. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A 104:10894–10899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C, Qian L, Cheng P, Nigam V, Arnold J, Srivastava D. 2009. A regulatory pathway involving Notch1/beta-catenin/Isl1 determines cardiac progenitor cell fate. Nat Cell Biol 11:951–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavery DL, Martin J, Turnbull YD, Hoppler S. 2008. Wnt6 signaling regulates heart muscle development during organogenesis. Dev Biol 323:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Cope JJ, Ackermann GE, Goishi K, Armstrong EJ, Paw BH, Bischoff J. 2006. Vascular endothelial growth factor receptor signaling is required for cardiac valve formation in zebrafish. Dev Dyn 235:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmacher C, Abeln B, Paululat A. 2012. The ultrastructure of Drosophila heart cells. Arthropod Struct Dev 41:459–474. [DOI] [PubMed] [Google Scholar]
- Levin M, Johnson RL, Stern CD, Kuehn M, Tabin C. 1995. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell 82:803–814. [DOI] [PubMed] [Google Scholar]
- Li YX, Zdanowicz M, Young L, Kumiski D, Leatherbury L, Kirby ML. 2003. Cardiac neural crest in zebrafish embryos contributes to myocardial cell lineage and early heart function. Dev Dyn 226:540–550. [DOI] [PubMed] [Google Scholar]
- Lickert H, Kutsch S, Kanzler B, Tamai Y, Taketo MM, Kemler R. 2002. Formation of multiple hearts in mice following deletion of beta-catenin in the embryonic endoderm. Dev Cell 3:171–181. [DOI] [PubMed] [Google Scholar]
- Liebner S, Cattelino A, Gallini R, Rudini N, Iurlaro M, Piccolo S, Dejana E. 2004. Beta-catenin is required for endothelial-mesenchymal transformation during heart cushion development in the mouse. J Cell Biol 166:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Cui L, Zhou W, Dufort D, Zhang X, Cai CL, Bu L, Yang L, Martin J, Kemler R, Rosenfeld MG, Chen J, Evans SM. 2007. Beta-catenin directly regulates Islet1 expression in cardiovascular progenitors and is required for multiple aspects of cardiogenesis. Proc Natl Acad Sci U S A 104:9313–9318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley RC, Gill JG, Kyba M, Murphy TL, Murphy KM. 2006. Canonical Wnt signaling is required for development of embryonic stem cell-derived mesoderm. Development 133:3787–3796. [DOI] [PubMed] [Google Scholar]
- Liu J, Qian L, Wessells RJ, Bidet Y, Jagla K, Bodmer R. 2006. Hedgehog and RAS pathways cooperate in the anterior-posterior specification and positioning of cardiac progenitor cells. Dev Biol 290:373–385. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. 1999. Requirement for Wnt3 in vertebrate axis formation. Nat Genet 22:361–365. [DOI] [PubMed] [Google Scholar]
- Lo PC, Frasch M. 2001. A role for the COUP-TF-related gene seven-up in the diversification of cardioblast identities in the dorsal vessel of Drosophila. Mech Dev 104:49–60. [DOI] [PubMed] [Google Scholar]
- Lockwood WK, Bodmer R. 2002. The patterns of wingless, decapentaplegic, and tinman position the Drosophila heart. Mech Dev 114:13–26. [DOI] [PubMed] [Google Scholar]
- Lough J, Barron M, Brogley M, Sugi Y, Bolender DL, Zhu X. 1996. Combined BMP-2 and FGF-4, but neither factor alone, induces cardiogenesis in non-precardiac embryonic mesoderm. Dev Biol 178:198–202. [DOI] [PubMed] [Google Scholar]
- Lovato TL, Cripps RM. 2016. Regulatory networks that direct the development of specialized cell Types in the Drosophila heart. J Cardiovasc Dev Dis 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell S, Benchoua A, Heavey B, Smith AG. 2006. Notch promotes neural lineage entry by pluripotent embryonic stem cells. PLoS Biol 4:e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. 2005. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development 132:5601–5611. [DOI] [PubMed] [Google Scholar]
- Ma X, Cong P, Hou X, Edgecombe GD, Strausfeld NJ. 2014. An exceptionally preserved arthropod cardiovascular system from the early Cambrian. Nat Commun 5:3560. [DOI] [PubMed] [Google Scholar]
- MacMullin A, Jacobs JR. 2006. Slit coordinates cardiac morphogenesis in Drosophila. Dev Biol 293:154–164. [DOI] [PubMed] [Google Scholar]
- Maillet M, Lynch JM, Sanna B, York AJ, Zheng Y, Molkentin JD. 2009. Cdc42 is an antihypertrophic molecular switch in the mouse heart. J Clin Invest 119:3079–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L, Banerjee U, Hartenstein V. 2004. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet 36:1019–1023. [DOI] [PubMed] [Google Scholar]
- Manner J 2000. Cardiac looping in the chick embryo: a morphological review with special reference to terminological and biomechanical aspects of the looping process. Anat Rec 259:248–262. [DOI] [PubMed] [Google Scholar]
- Marelli AJ, Ionescu-Ittu R, Mackie AS, Guo L, Dendukuri N, Kaouache M. 2014. Lifetime prevalence of congenital heart disease in the general population from 2000 to 2010. Circulation 130:749–756. [DOI] [PubMed] [Google Scholar]
- Martin D, Zusman S, Li X, Williams EL, Khare N, DaRocha S, Chiquet-Ehrismann R, Baumgartner S. 1999. wing blister, a new Drosophila laminin alpha chain required for cell adhesion and migration during embryonic and imaginal development. J Cell Biol 145:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. 2001. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev 15:316–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medioni C, Astier M, Zmojdzian M, Jagla K, Semeriva M. 2008. Genetic control of cell morphogenesis during Drosophila melanogaster cardiac tube formation. J Cell Biol 182:249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medioni C, Bertrand N, Mesbah K, Hudry B, Dupays L, Wolstein O, Washkowitz AJ, Papaioannou VE, Mohun TJ, Harvey RP, Zaffran S. 2010. Expression of Slit and Robo genes in the developing mouse heart. Dev Dyn 239:3303–3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meganathan K, Sotiriadou I, Natarajan K, Hescheler J, Sachinidis A. 2015. Signaling molecules, transcription growth factors and other regulators revealed from in-vivo and in-vitro models for the regulation of cardiac development. Int J Cardiol 183:117–128. [DOI] [PubMed] [Google Scholar]
- Meyers EN, Martin GR. 1999. Differences in left-right axis pathways in mouse and chick: functions of FGF8 and SHH. Science 285:403–406. [DOI] [PubMed] [Google Scholar]
- Michelson AM, Gisselbrecht S, Buff E, Skeath JB. 1998a. Heartbroken is a specific downstream mediator of FGF receptor signalling in Drosophila. Development 125:4379–4389. [DOI] [PubMed] [Google Scholar]
- Michelson AM, Gisselbrecht S, Zhou Y, Baek KH, Buff EM. 1998b. Dual functions of the heartless fibroblast growth factor receptor in development of the Drosophila embryonic mesoderm. Dev Genet 22:212–229. [DOI] [PubMed] [Google Scholar]
- Miquerol L, Gertsenstein M, Harpal K, Rossant J, Nagy A. 1999. Multiple developmental roles of VEGF suggested by a LacZ-tagged allele. Dev Biol 212:307–322. [DOI] [PubMed] [Google Scholar]
- Molina MR, Cripps RM. 2001. Ostia, the inflow tracts of the Drosophila heart, develop from a genetically distinct subset of cardial cells. Mech Dev 109:51–59. [DOI] [PubMed] [Google Scholar]
- Mommersteeg MT, Andrews WD, Ypsilanti AR, Zelina P, Yeh ML, Norden J, Kispert A, Chedotal A, Christoffels VM, Parnavelas JG. 2013. Slit-roundabout signaling regulates the development of the cardiac systemic venous return and pericardium. Circ Res 112:465–475. [DOI] [PubMed] [Google Scholar]
- Monier B, Astier M, Semeriva M, Perrin L. 2005. Steroid-dependent modification of Hox function drives myocyte reprogramming in the Drosophila heart. Development 132:5283–5293. [DOI] [PubMed] [Google Scholar]
- Moorman AF, Christoffels VM. 2003. Cardiac chamber formation: development, genes, and evolution. Physiol Rev 83:1223–1267. [DOI] [PubMed] [Google Scholar]
- Moro E, Ozhan-Kizil G, Mongera A, Beis D, Wierzbicki C, Young RM, Bournele D, Domenichini A, Valdivia LE, Lum L, Chen C, Amatruda JF, Tiso N, Weidinger G, Argenton F. 2012. In vivo Wnt signaling tracing through a transgenic biosensor fish reveals novel activity domains. Dev Biol 366:327–340. [DOI] [PubMed] [Google Scholar]
- Nagai T, Tanaka-Ishikawa M, Aikawa R, Ishihara H, Zhu W, Yazaki Y, Nagai R, Komuro I. 2003. Cdc42 plays a critical role in assembly of sarcomere units in series of cardiac myocytes. Biochem Biophys Res Commun 305:806–810. [DOI] [PubMed] [Google Scholar]
- Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. 2006. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc Natl Acad Sci U S A 103:19812–19817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemir M, Croquelois A, Pedrazzini T, Radtke F. 2006. Induction of cardiogenesis in embryonic stem cells via downregulation of Notch1 signaling. Circ Res 98:1471–1478. [DOI] [PubMed] [Google Scholar]
- Nichols M, Townsend N, Scarborough P, Rayner M. 2014. Cardiovascular disease in Europe 2014: epidemiological update. Eur Heart J 35:2929. [DOI] [PubMed] [Google Scholar]
- Olson EN. 2006. Gene regulatory networks in the evolution and development of the heart. Science 313:1922–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandur P, Lasche M, Eisenberg LM, Kuhl M. 2002. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature 418:636–641. [DOI] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. 2006. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development 133:2419–2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Watanabe Y, Smyth G, Miyagawa-Tomita S, Meyers E, Klingensmith J, Camenisch T, Buckingham M, Moon AM. 2008. An FGF autocrine loop initiated in second heart field mesoderm regulates morphogenesis at the arterial pole of the heart. Development 135:3599–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Wu X, Golden K, Axelrod JD, Bodmer R. 1996. The wingless signaling pathway is directly involved in Drosophila heart development. Dev Biol 177:104–116. [DOI] [PubMed] [Google Scholar]
- Park M, Yaich LE, Bodmer R. 1998. Mesodermal cell fate decisions in Drosophila are under the control of the lineage genes numb, Notch, and sanpodo. Mech Dev 75:117–126. [DOI] [PubMed] [Google Scholar]
- Parker DS, Jemison J, Cadigan KM. 2002. Pygopus, a nuclear PHD-finger protein required for Wingless signaling in Drosophila. Development 129:2565–2576. [DOI] [PubMed] [Google Scholar]
- Person AD, Garriock RJ, Krieg PA, Runyan RB, Klewer SE. 2005. Frzb modulates Wnt-9a-mediated beta-catenin signaling during avian atrioventricular cardiac cushion development. Dev Biol 278:35–48. [DOI] [PubMed] [Google Scholar]
- Philippakis AA, Busser BW, Gisselbrecht SS, He FS, Estrada B, Michelson AM, Bulyk ML. 2006. Expression-guided in silico evaluation of candidate cis regulatory codes for Drosophila muscle founder cells. PLoS Comput Biol 2:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzielli R, Astier M, Chartier A, Gallet A, Therond P, Semeriva M. 2002. Heart tube patterning in Drosophila requires integration of axial and segmental information provided by the Bithorax Complex genes and hedgehog signaling. Development 129:4509–4521. [DOI] [PubMed] [Google Scholar]
- Qian L, Liu J, Bodmer R. 2005. Slit and Robo control cardiac cell polarity and morphogenesis. Curr Biol 15:2271–2278. [DOI] [PubMed] [Google Scholar]
- Raya A, Kawakami Y, Rodriguez-Esteban C, Buscher D, Koth CM, Itoh T, Morita M, Raya RM, Dubova I, Bessa JG, de la Pompa JL, Izpisua Belmonte JC. 2003. Notch activity induces Nodal expression and mediates the establishment of left-right asymmetry in vertebrate embryos. Genes Dev 17:1213–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reifers F, Walsh EC, Leger S, Stainier DY, Brand M. 2000. Induction and differentiation of the zebrafish heart requires fibroblast growth factor 8 (fgf8/acerebellar). Development 127:225–235. [DOI] [PubMed] [Google Scholar]
- Reim I, Frasch M. 2005. The Dorsocross T-box genes are key components of the regulatory network controlling early cardiogenesis in Drosophila. Development 132:4911–4925. [DOI] [PubMed] [Google Scholar]
- Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. 2008. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr 153:807–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhyu MS, Jan LY, Jan YN. 1994. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell 76:477–491. [DOI] [PubMed] [Google Scholar]
- Rivera-Feliciano J, Tabin CJ. 2006. Bmp2 instructs cardiac progenitors to form the heart-valve-inducing field. Dev Biol 295:580–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizki TM. 1978. The circulatory system and associated cells and tissues. In: Ashburner M, Wright TRF, editors. The Genetics and Biology of Drosophila. London and New York: Academic Press. pp 397–452. [Google Scholar]
- Romer AS. 1967. Major steps in vertebrate evolution. Science 158:1629–1637. [DOI] [PubMed] [Google Scholar]
- Rones MS, McLaughlin KA, Raffin M, Mercola M. 2000. Serrate and Notch specify cell fates in the heart field by suppressing cardiomyogenesis. Development 127:3865–3876. [DOI] [PubMed] [Google Scholar]
- Rossant J, Ciruna B, Partanen J. 1997. FGF signaling in mouse gastrulation and anteroposterior patterning. Cold Spring Harb Symp Quant Biol 62:127–133. [PubMed] [Google Scholar]
- Rotstein B, Paululat A. 2016. On the morphology of the Drosophila heart. J Cardiovasc Dev Dis 3:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath K, Cheng AM, Frisch A, Wright CV. 1997. Functional differences among Xenopus nodal-related genes in left-right axis determination. Development 124:3293–3302. [DOI] [PubMed] [Google Scholar]
- Santiago-Martinez E, Soplop NH, Kramer SG. 2006. Lateral positioning at the dorsal midline: Slit and Roundabout receptors guide Drosophila heart cell migration. Proc Natl Acad Sci U S A 103:12441–12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago-Martinez E, Soplop NH, Patel R, Kramer SG. 2008. Repulsion by Slit and Roundabout prevents Shotgun/E-cadherin-mediated cell adhesion during Drosophila heart tube lumen formation. J Cell Biol 182:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Yost HJ. 2003. Cardiac neural crest contributes to cardiomyogenesis in zebrafish. Dev Biol 257:127–139. [DOI] [PubMed] [Google Scholar]
- Schaub C, Marz J, Reim I, Frasch M. 2015. Org-1-dependent lineage reprogramming generates the ventral longitudinal musculature of the Drosophila heart. Curr Biol 25:488–494. [DOI] [PubMed] [Google Scholar]
- Schilling TF, Concordet JP, Ingham PW. 1999. Regulation of left-right asymmetries in the zebrafish by Shh and BMP4. Dev Biol 210:277–287. [DOI] [PubMed] [Google Scholar]
- Schleich JM, Abdulla T, Summers R, Houyel L. 2013. An overview of cardiac morphogenesis. Arch Cardiovasc Dis 106:612–623. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Mercola M. 2001. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev 15:304–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreier B, Dohler M, Rabe S, Schneider B, Schwerdt G, Ruhs S, Sibilia M, Gotthardt M, Gekle M, Grossmann C. 2011. Consequences of epidermal growth factor receptor (ErbB1) loss for vascular smooth muscle cells from mice with targeted deletion of ErbB1. Arterioscler Thromb Vasc Biol 31:1643–1652. [DOI] [PubMed] [Google Scholar]
- Schroeder T, Fraser ST, Ogawa M, Nishikawa S, Oka C, Bornkamm GW, Nishikawa S, Honjo T, Just U. 2003. Recombination signal sequence-binding protein Jkappa alters mesodermal cell fate decisions by suppressing cardiomyogenesis. Proc Natl Acad Sci U S A 100:4018–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultheiss TM, Burch JB, Lassar AB. 1997. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev 11:451–462. [DOI] [PubMed] [Google Scholar]
- Shamim H, Mason I. 1999. Expression of Fgf4 during early development of the chick embryo. Mech Dev 85:189–192. [DOI] [PubMed] [Google Scholar]
- Shishido E, Ono N, Kojima T, Saigo K. 1997. Requirements of DFR1/Heartless, a mesoderm-specific Drosophila FGF-receptor, for the formation of heart, visceral and somatic muscles, and ensheathing of longitudinal axon tracts in CNS. Development 124:2119–2128. [DOI] [PubMed] [Google Scholar]
- Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE. 2008. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol 28:1703–1713. [DOI] [PubMed] [Google Scholar]
- Simoes-Costa MS, Vasconcelos M, Sampaio AC, Cravo RM, Linhares VL, Hochgreb T, Yan CY, Davidson B, Xavier-Neto J. 2005. The evolutionary origin of cardiac chambers. Dev Biol 277:1–15. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Robertson EJ. 1999. Early embryonic lethality in Bmp5;Bmp7 double mutant mice suggests functional redundancy within the 60A subgroup. Development 126:1753–1768. [DOI] [PubMed] [Google Scholar]
- Somi S, Buffing AA, Moorman AF, Van Den Hoff MJ. 2004. Dynamic patterns of expression of BMP isoforms 2, 4, 5, 6, and 7 during chicken heart development. Anat Rec A Discov Mol Cell Evol Biol 279:636–651. [DOI] [PubMed] [Google Scholar]
- Spana EP, Doe CQ. 1996. Numb antagonizes Notch signaling to specify sibling neuron cell fates. Neuron 17:21–26. [DOI] [PubMed] [Google Scholar]
- Stark KA, Yee GH, Roote CE, Williams EL, Zusman S, Hynes RO. 1997. A novel alpha integrin subunit associates with betaPS and functions in tissue morphogenesis and movement during Drosophila development. Development 124:4583–4594. [DOI] [PubMed] [Google Scholar]
- Stathopoulos A, Tam B, Ronshaugen M, Frasch M, Levine M. 2004. pyramus and thisbe: FGF genes that pattern the mesoderm of Drosophila embryos. Genes Dev 18:687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudt D, Stainier D. 2012. Uncovering the molecular and cellular mechanisms of heart development using the zebrafish. Annu Rev Genet 46:397–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strilic B, Kucera T, Eglinger J, Hughes MR, McNagny KM, Tsukita S, Dejana E, Ferrara N, Lammert E. 2009. The molecular basis of vascular lumen formation in the developing mouse aorta. Dev Cell 17:505–515. [DOI] [PubMed] [Google Scholar]
- Tam PP, Parameswaran M, Kinder SJ, Weinberger RP. 1997. The allocation of epiblast cells to the embryonic heart and other mesodermal lineages: the role of ingression and tissue movement during gastrulation. Development 124:1631–1642. [DOI] [PubMed] [Google Scholar]
- Tang M, Yuan W, Bodmer R, Wu X, Ocorr K. 2014. The role of pygopus in the differentiation of intracardiac valves in Drosophila. Genesis 52:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Schulz RA. 2007. Heart development in Drosophila. Semin Cell Dev Biol 18:3–15. [DOI] [PubMed] [Google Scholar]
- Tao Y, Wang J, Tokusumi T, Gajewski K, Schulz RA. 2007. Requirement of the LIM homeodomain transcription factor tailup for normal heart and hematopoietic organ formation in Drosophila melanogaster. Mol Cell Biol 27:3962–3969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauc HM, Mann T, Werner K, Pandur P. 2012. A role for Drosophila Wnt-4 in heart development. Genesis 50:466–481. [DOI] [PubMed] [Google Scholar]
- Terami H, Hidaka K, Katsumata T, Iio A, Morisaki T. 2004. Wnt11 facilitates embryonic stem cell differentiation to Nkx2.5-positive cardiomyocytes. Biochem Biophys Res Commun 325:968–975. [DOI] [PubMed] [Google Scholar]
- Thomas NA, Koudijs M, van Eeden FJ, Joyner AL, Yelon D. 2008. Hedgehog signaling plays a cell-autonomous role in maximizing cardiac developmental potential. Development 135:3789–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Yuan L, Goss AM, Wang T, Yang J, Lepore JJ, Zhou D, Schwartz RJ, Patel V, Cohen ED, Morrisey EE. 2010. Characterization and in vivo pharmacological rescue of a Wnt2-Gata6 pathway required for cardiac inflow tract development. Dev Cell 18:275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo GV, Nodal DH, Lovato CV, Hendren JD, Helander LA, Lovato TL, Bodmer R, Cripps RM. 2016. The canonical Wingless signaling pathway is required but not sufficient for inflow tract formation in the Drosophila melanogaster heart. Dev Biol 413:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsiairis CD, McMahon AP. 2009. An Hh-dependent pathway in lateral plate mesoderm enables the generation of left/right asymmetry. Curr Biol 19:1912–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukui T, Capdevila J, Tamura K, Ruiz-Lozano P, Rodriguez-Esteban C, Yonei-Tamura S, Magallon J, Chandraratna RA, Chien K, Blumberg B, Evans RM, Belmonte JC. 1999. Multiple left-right asymmetry defects in Shh(−/−) mutant mice unveil a convergence of the shh and retinoic acid pathways in the control of Lefty-1. Proc Natl Acad Sci U S A 96:11376–11381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. 2007. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A 104:9685–9690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel M, Nusse R, Johnston P, Lawrence PA. 1989. Distribution of the wingless gene product in Drosophila embryos: a protein involved in cell-cell communication. Cell 59:739–749. [DOI] [PubMed] [Google Scholar]
- Vanderploeg J, Vazquez Paz LL, MacMullin A, Jacobs JR. 2012. Integrins are required for cardioblast polarisation in Drosophila. BMC Dev Biol 12:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S, Wilson R, Coelho C, Affolter M, Leptin M. 1998. The Drosophila protein Dof is specifically required for FGF signaling. Mol Cell 2:515–525. [DOI] [PubMed] [Google Scholar]
- Vogler G, Bodmer R. 2015. Cellular Mechanisms of Drosophila Heart Morphogenesis. J Cardiovasc Dev Dis 2:2–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler G, Liu J, Iafe TW, Migh E, Mihaly J, Bodmer R. 2014. Cdc42 and formin activity control non-muscle myosin dynamics during Drosophila heart morphogenesis. J Cell Biol 206:909–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DZ, Reiter RS, Lin JL, Wang Q, Williams HS, Krob SL, Schultheiss TM, Evans S, Lin JJ. 1999. Requirement of a novel gene, Xin, in cardiac morphogenesis. Development 126:1281–1294. [DOI] [PubMed] [Google Scholar]
- Ward EJ, Skeath JB. 2000. Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development 127:4959–4969. [DOI] [PubMed] [Google Scholar]
- Washington Smoak I, Byrd NA, Abu-Issa R, Goddeeris MM, Anderson R, Morris J, Yamamura K, Klingensmith J, Meyers EN. 2005. Sonic hedgehog is required for cardiac outflow tract and neural crest cell development. Dev Biol 283:357–372. [DOI] [PubMed] [Google Scholar]
- Wong K, Park HT, Wu JY, Rao Y. 2002. Slit proteins: molecular guidance cues for cells ranging from neurons to leukocytes. Curr Opin Genet Dev 12:583–591. [DOI] [PubMed] [Google Scholar]
- Wong KS, Rehn K, Palencia-Desai S, Kohli V, Hunter W, Uhl JD, Rost MS, Sumanas S. 2012. Hedgehog signaling is required for differentiation of endocardial progenitors in zebrafish. Dev Biol 361:377–391. [DOI] [PubMed] [Google Scholar]
- Wu X, Golden K, Bodmer R. 1995. Heart development in Drosophila requires the segment polarity gene wingless. Dev Biol 169:619–628. [DOI] [PubMed] [Google Scholar]
- Xu X, Yin Z, Hudson JB, Ferguson EL, Frasch M. 1998. Smad proteins act in combination with synergistic and antagonistic regulators to target Dpp responses to the Drosophila mesoderm. Genes Dev 12:2354–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Conlon RA, Rossant J. 1992. Expression of the fibroblast growth factor receptor FGFR-1/flg during gastrulation and segmentation in the mouse embryo. Dev Biol 152:75–88. [DOI] [PubMed] [Google Scholar]
- Yarnitzky T, Volk T. 1995. Laminin is required for heart, somatic muscles, and gut development in the Drosophila embryo. Dev Biol 169:609–618. [DOI] [PubMed] [Google Scholar]
- Yin Z, Frasch M. 1998. Regulation and function of tinman during dorsal mesoderm induction and heart specification in Drosophila. Dev Genet 22:187–200. [DOI] [PubMed] [Google Scholar]
- Zeitouni B, Senatore S, Severac D, Aknin C, Semeriva M, Perrin L. 2007. Signalling pathways involved in adult heart formation revealed by gene expression profiling in Drosophila. PLoS Genet 3:1907–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bradley A. 1996. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development 122:2977–2986. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lin Y, Zhang Y, Lan Y, Lin C, Moon AM, Schwartz RJ, Martin JF, Wang F. 2008. Frs2alpha-deficiency in cardiac progenitors disrupts a subset of FGF signals required for outflow tract morphogenesis. Development 135:3611–3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP. 2001. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell 106:781–792. [PubMed] [Google Scholar]
- Zhu W, Shiojima I, Ito Y, Li Z, Ikeda H, Yoshida M, Naito AT, Nishi J, Ueno H, Umezawa A, Minamino T, Nagai T, Kikuchi A, Asashima M, Komuro I. 2008. IGFBP-4 is an inhibitor of canonical Wnt signalling required for cardiogenesis. Nature 454:345–349. [DOI] [PubMed] [Google Scholar]
- Zhu X, Ahmad SM, Aboukhalil A, Busser BW, Kim Y, Tansey TR, Haimovich A, Jeffries N, Bulyk ML, Michelson AM. 2012. Differential regulation of mesodermal gene expression by Drosophila cell type-specific Forkhead transcription factors. Development 139:1457–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zikova M, Da Ponte JP, Dastugue B, Jagla K. 2003. Patterning of the cardiac outflow region in Drosophila. Proc Natl Acad Sci U S A 100:12189–12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, Fassler R, Mellman I, Lane TF, Iruela-Arispe ML. 2010. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell 18:39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]


