Abstract
Background
This study examined the effects of khat extract on the proinflammatory cytokines, liver, and kidneys of rats. Unlike previous research that focused on broad immune markers and general effects, this study investigated specific proinflammatory cytokines (IL-1β, IL-2, IL-4, IL-6, and TNF-α) and considered gender differences in the immune response. Fresh khat plants and nontoxic doses were used to obtain clear observations relevant to human health.
Material/Methods
Extracts were prepared from young shoots of khat plants and fresh leaves. 150-gram male and female rats were randomly divided into 8 groups (5 rats per group). Six groups received the extract orally for 8 weeks at doses of 100, 300, and 500 mg/kg/day. Blood samples were collected after 56 days and assayed for cytokines using ELISA, while liver enzymes and kidney markers were assayed using kinetics and colorimetric methods.
Results
Khat extract increased the levels of most cytokines, with higher doses causing greater increase. This effect was consistent across sexes for some cytokines (IL-1β, IL-2), but not others (IL-4, IL-6, TNF-α), where sex played a role at specific doses. Various doses of the extract influenced glucose levels in both male and female rats. In addition, liver enzyme markers showed some variations.
Conclusions
This study, with its intriguing findings, illuminates the complex effects of khat on various systems. Variations in AST and ALT levels in the liver support earlier hepatotoxicity research and suggest possible khat-induced damage. This study revealed consistently increased levels of all cytokines as the dose of khat extract increased.
Keywords: Catha, Liver Diseases, Renal Agents, Cytokines, Rats
Introduction
Khat (Catha edulis, Forsk), an evergreen shrub, is commonly used as a natural stimulant. Millions of people chew them in Ethiopia, Kenya, Yemen, Saudi Arabia, Somalia, Djibouti, and other countries. Chewed khat leaves have long been used as a social and recreational stimulant [1,2]. In addition to being a social and entertaining stimulant, khat has long been used to treat various illnesses [3]. The alkaloid cathinone, and to a lesser extent the diastereomers cathine, is primarily responsible for khat-stimulating effects. Cathinone is mostly present in the young, fresh leaves of khat plants and is an intermediary metabolite in the synthesis of cathine. In each session, 100–200 g of fresh khat leaves or shoots is chewed, the juice is drawn out, and the plant residue is left in the mouth. A slightly euphoric state brought on by using khat increased alertness, energy, and chattiness. Farmers, students, and everyday workers utilize khat while working to increase alertness and reduce physical fatigue [1,3–5].
Limited information is available regarding the association between acute and chronic khat use and its impact on liver function, including serum enzyme levels. However, many case studies and brief case series have surfaced recently, mostly from the UK and Europe, as well as among immigrants from areas where khat use is common [6–8]. These findings indicated cases of serious acute and chronic liver damage associated with khat use. Long-term khat usage usually precedes the development of liver damage, which can manifest as acute symptoms, including nausea, exhaustion, pruritus, and jaundice, or as chronic manifestations, including symptoms and consequences associated with portal hypertension. Hepatocellular patterns of elevated liver enzyme levels are often seen in cases of khat-related liver damage; acute presentations show noticeably higher aminotransferase levels [6,7].
On the other hand, because of the plant’s tannin and norpseudoephedrine content, khat is known to increase risk of and vulnerability to cognitive impairment, vascular diseases, stomach ulcers, increased adrenocorticotrophic testosterone levels, urine retention, gastrointestinal tract constipation, and hemorrhage [1,3–5]. Cathinone also briefly increases the heart rate, blood pressure, and respiration rate. It also improves cerebral blood flow, sharpens thinking, and gives people energy [9,10]. It has been shown that the physiological properties of cathinone are comparable to those of the artificial stimulant amphetamine [11]. The structural connections were clear. It is produced in the central nervous system, euphoria, hyperactivity, restlessness, enhanced alertness and happiness, decreased physical tiredness in users, mouth dryness, mydriasis, hunger, hyperthermia, hypertension, and tachycardia [12,13]. Despite these reports of the psychological and physical impacts of khat and cathinone, little research has been conducted on their immunomodulatory properties or biochemical effects.
This study investigated the effects of khat extract on the proinflammatory cytokines, liver, and kidneys of rats. The goal of this study was to overcome the shortcomings of earlier studies and to increase our knowledge of the effects of this plant. Clearer observations that more accurately characterize human body processes were attained by investigation using fresh khat plants [14] and nontoxic dosages. Unlike earlier studies that focused on broad effects and general immune system markers [15–17], this study examined particular proinflammatory cytokines and sex variations in immunological responses. This study provides important new information regarding the complex effects of khat extract on many systems and emphasizes the need for future research to fully understand the potential risks and therapeutic implications of khat use.
Material and Methods
Ethical Considerations
This study underwent a comprehensive review and was approved by the Institutional Research Review and Ethics Committee (MRC-IRB) of the Medical Research Centre at Jazan University, Jazan, Saudi Arabia. The study was approved by approval number 112/SIA/1443. The study strict ly adhered to the ARRIVE Guidelines for reporting in vivo experiments [20] and all experiments were conducted in complete accordance with the guidelines set by the MRC-IRB.
Materials and Reagents
High-quality analytical grade solvents, reagents, and chemicals were used in this study. Reference standards for cathine and cathinone were acquired from Lipomed (Al-Ashban Trading, Kingdom of KSA). Ethanol, chloroform, sodium chloride, potassium chloride, magnesium sulfate, calcium chloride, glucose, ammonium formate buffer, formic acid, and acetonitrile were purchased from reputable suppliers such as Sigma-Aldrich and ThermoFisher (St. Louis, MO, USA).
Plant Material and Extraction
The khat plant was acquired from the Jazan University’s Substance Abuse and Toxicology Research Center, with all necessary government authorizations. The taxonomic identity was confirmed at the Medical Research Center of Jazan University. A voucher specimen (MRC/CE/1442-3) was deposited at the botanical facilities of Jazan University Medical Research Center. Young shoots and fresh leaves were frozen before use. The fresh khat contained the most active ingredients. Young shoots and fresh leaves were cleaned, frozen, and minced using a mortar and pestle. The weight of the fresh plants served as a guide for dosage. The effectiveness of our extraction technique for the detection of cathine and cathinone alkaloids was verified by liquid chromatography-mass spectrometry (LC-MS). Comparable results have been obtained in a previous study [18].
Grouping and Dosing of Experimental Animals
The rats were allowed to acclimatize to the laboratory environment for one–two weeks during the first phase of acclimatization, which was used to optimize experimental conditions. This was the time at which the rats could consume regular laboratory food and water. Animals (150±25 g) were supplied by the Jazan University Medical Research Center in Jazan, Saudi Arabia. To ensure that they were healthy, they were selected based on inclusion criteria. Animals were randomly assigned to experimental groups using a computer-generated randomization sequence or another unbiased method to reduce bias. A pilot experiment to determine the extract doses showed no physical or behavioral harm to rats. We chose doses of 100, 200, and 500 mg/kg for further research [19]. The experiment involved male and female rats 8 weeks old. Forty male and female rats were randomly divided into 8 groups of 5 rats each. Rats in the control group were divided into 2 groups: male and female. Male and female rats comprised the remaining 6 groups, which received an oral dose of the extract for 8 weeks at doses of 100, 300, and 500 mg/kg/day. Fifty-six days after the rats received the extract, their blood and plasma samples were collected for biochemical testing.
Measurement of Serum IL-1β, IL-2, IL-4, IL-6, and TNF-α Levels
Serum IL-1β
Serum levels of IL-1β were quantitatively measured using rat IL-1β ELISA kits (ABCAM, USA). This assay uses a simple sandwich ELISA technique to determine the IL-1β concentration in the serum samples. Both the standards and samples were pipetted into the designated wells and incubated at room temperature for 2.5 hours with the ELISA plate covered with a lid. During this incubation, the IL-1β contained in the samples bound to the wells, which had previously been coated with a specific immobilized antibody. After incubation, the wells were thoroughly washed with a Biotek ELISA washer (Elx50, USA) using 1X wash solution to remove unbound substances. A 1×biotinylated anti-rat IL-1β antibody was then added to each well, and the plate was incubated for 1 hour at room temperature with gentle shaking. The plate was then washed again to remove unbound biotinylated antibody. Horseradish peroxidase (HRP)-conjugated streptavidin was then added to the wells, and after another wash, a substrate solution containing tetramethylbenzidine (TMB) was added. The plate was then incubated for 30 minutes at room temperature in the dark with gentle shaking. Finally, a stop solution was added to stop the reaction. The intensity of the developed color, which is directly proportional to the amount of IL-1β present, was measured at 450 nm using a Biotek ELISA reader (ELX 800, USA). The absorbance value indicated the amount of IL-1β bound to the specific antibody, and the sample concentration was calculated by extrapolation from a standard curve.
Serum IL-2
The serum level of IL-2 was quantitatively determined using an IL-2 ELISA kit for rats (ABCAM, USA). A sandwich ELISA was used to determine the IL-2 concentration in the serum samples. Standard solutions and samples were pipetted simultaneously into the corresponding wells, the plate was covered with a lid and incubated overnight at 4°C with gentle shaking. After incubation, the solution was discarded, and the wells were thoroughly washed with 1×wash solution using a Biotek ELISA washer (ELX 50, USA). Then, 1X biotinylated anti-rat IL-2 antibody was added to each well, and the plate was incubated at room temperature for 1 hour with gentle shaking. After this incubation, the plate was washed again to remove unbound biotinylated antibody. HRP-conjugated streptavidin was then added to the wells, followed by incubation at room temperature for 45 minutes. The wells were washed again as previously described. After the last wash, a tetramethylbenzidine (TMB) solution was added to the wells and the plate was incubated for 30 minutes in the dark with gentle shaking at room temperature. A stop solution was then added to stop the reaction. The IL-2 concentration was determined by measuring the absorbance at 450 nm using a Biotek ELISA reader (ELX 800, USA). The IL-2 concentration in the samples was calculated by extrapolation from a standard curve.
Serum IL-4
Serum interleukin-4 (IL-4) levels were measured quantitatively using an IL-4 ELISA kit for rats (Abcam, USA) in a sandwich ELISA format. A 96-well plate pre-coated with an antibody specific for rat IL-4 was used for the assay and samples and standards were added to the plate. The IL-4 in the samples binds to the immobilized antibody, resulting in a detectable signal. The plate was then sealed and incubated for 2.5 hours. After incubation, the wells were rinsed 4 times with 1X wash buffer in a microplate strip washer (Biotek ELX50, USA). After washing, 100 μL of biotin-labelled IL-4 specific antibody was added to each well and incubated at room temperature for one hour. The plate was washed again 4 times, then 100 μL of HRP-streptavidin solution (enzyme conjugate) was added to each well and incubated for 45 minutes with gentle shaking. Subsequently, 100 μl of TMB substrate was added to all wells and incubated for 30 minutes in the dark. The reaction was then stopped and the absorbance was measured at 450 nm using an ELISA reader (ELx800, USA). The IL-4 concentration in the samples was determined by extrapolation from an IL-4 standard calibration curve.
Serum IL-6
Serum interleukin-6 (IL-6) levels were quantified with a rat IL-6 ELISA kit (MyBioSource, USA) using a double antibody sandwich technique similar to that used for IL-2 and IL-4. Standard solutions and samples (100 μl each) were added to the appropriate wells of the ELISA plate and incubated at 37°C for 1.5 hours. After incubation, the plates were washed twice with wash buffer. Subsequently, 100 μl of the biotinylated IL-6 antibody was added to each well, the plate was sealed and incubated at 37°C for 60 minutes. The wells were then washed thoroughly 3 times using a microplate strip washer (Biotek ELX50, USA). Then, 100 μL of the enzyme complex was added to each well and the plate was incubated at 37°C for 30 minutes. After this incubation, the wells were washed again and 100 μL of the prepared color reagent was added, followed by incubation at 37°C for 30 minutes. After 30 minutes, 100 μl of reagent C was added to each well and the contents were mixed thoroughly on a plate shaker for 1 minute. The absorbance was then measured at 450 nm using an ELISA spectrophotometer (ELx800, USA). The IL-6 concentration in the samples was determined by extrapolation from a standard curve and expressed in pg/ml.
Serum TNF-α
Tumour necrosis factor-alpha (TNF-α) levels in serum were quantified using a rat TNF-α ELISA kit (MyBioSource, USA). This kit uses a sandwich enzyme immunoassay for the quantitative in vitro detection of TNF-α in rat serum. In the assay, 100 μL of each of the standards and samples were added to the corresponding wells of the ELISA strips. The plate was sealed and incubated at 37°C for 1.5 hours. After incubation, the medium in each well was replaced with 100 μL of the biotinylated antibody, the plate was resealed and incubated at 37°C for one hour. The wells were then rinsed 3 times with wash buffer using a microplate strip washer (Biotek ELX50, USA), with a two-minute soak time after each wash. Subsequently, 100 μL of the HRP complex was added to each well, the plate was sealed and incubated at 37°C for 30 minutes. The wells were then washed 3 more times using the same soaking procedure. Subsequently, 90 μL of substrate solution was added to each well and the plate was incubated for 15 minutes at 37°C in the dark. Finally, 50 μl of stop reagent was added to each well, and after thoroughly mixing the contents, the optical density was immediately measured at 450 nm using an ELISA reader (ELx800, USA). The TNF-α concentrations in the samples were determined by extrapolation from a standard calibration curve.
Liver and Kidney Markers
Serum concentrations of glucose (mmol/L), blood urea nitrogen (mmol/L), creatinine (μmol/L), sodium (mmol/L), potassium (mmol/L), aspartate aminotransferase (U/L), alanine aminotransferase (U/L), alkaline phosphatase (U/L), and total bilirubin (mg/dL) were measured using an automated clinical chemistry analyzer (Beckman Coulter, Brea, California, USA). Measurements were conducted at the General Directorate of Health Services and University Hospital, Jazan University, Jazan 45142, Saudi Arabia.
Statistical Analyses
Data were analyzed using SPSS version 21.00. Descriptive statistics are reported as the mean±standard error of the mean. Statistical comparisons between groups were performed using one-way analysis of variance (ANOVA) and Student’s t-test. Post hoc analysis was conducted using the least significant difference (LSD) test. The significance level for all tests was set at α=0.05. Figures were generated using GraphPad Prism version 9 (GraphPad Software, San Diego, CA, USA).
Results
Cytokine network
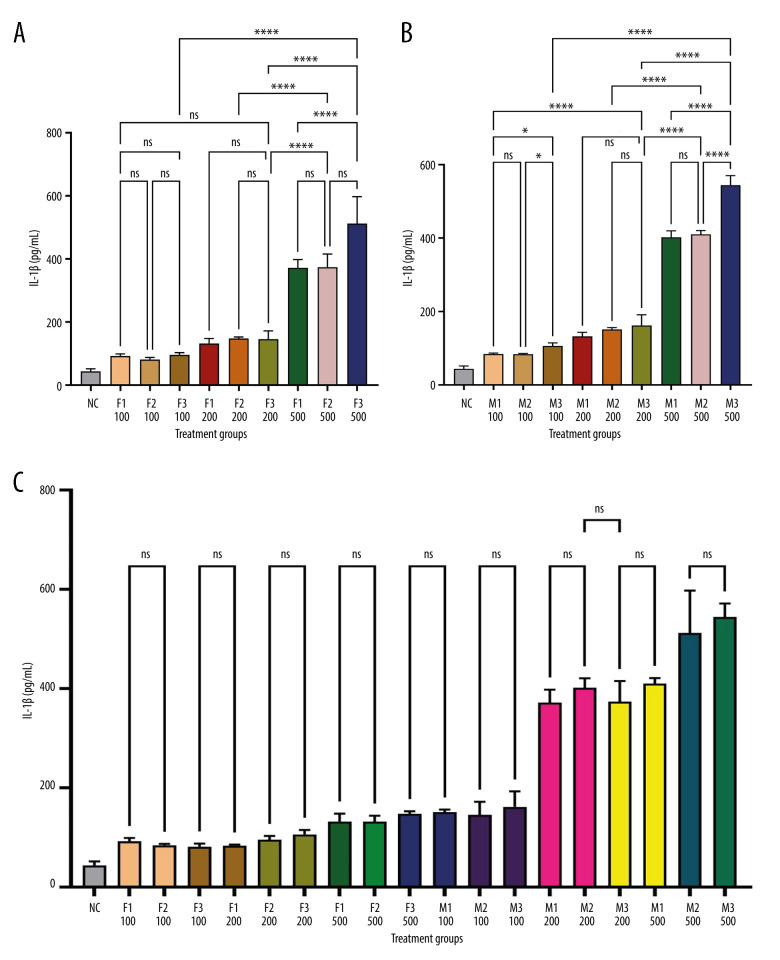
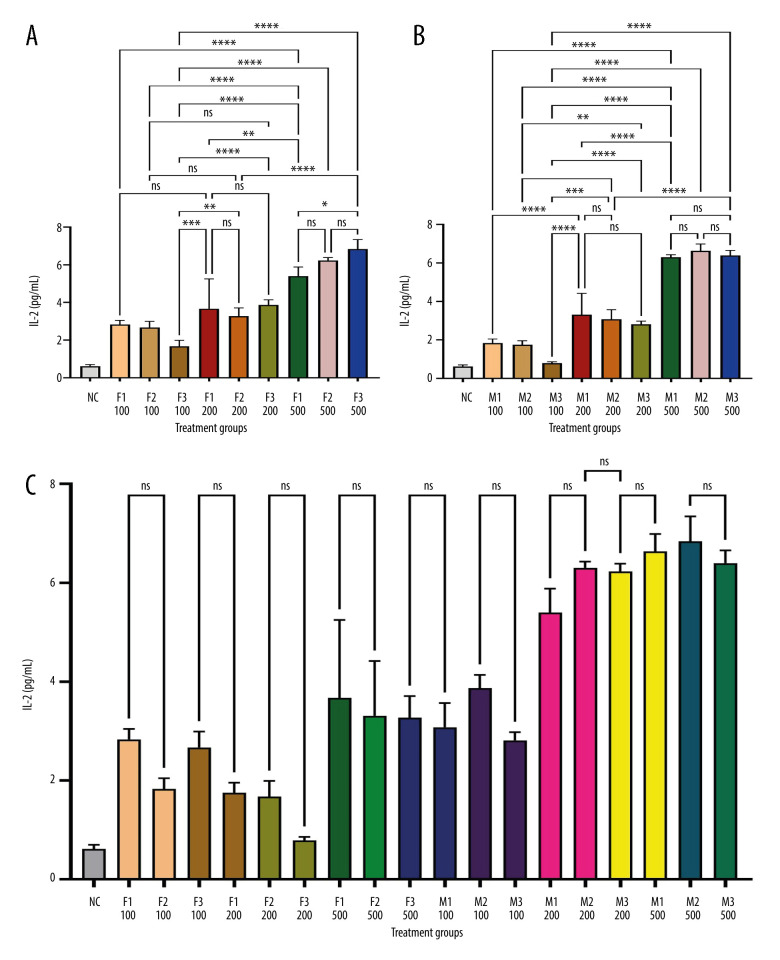
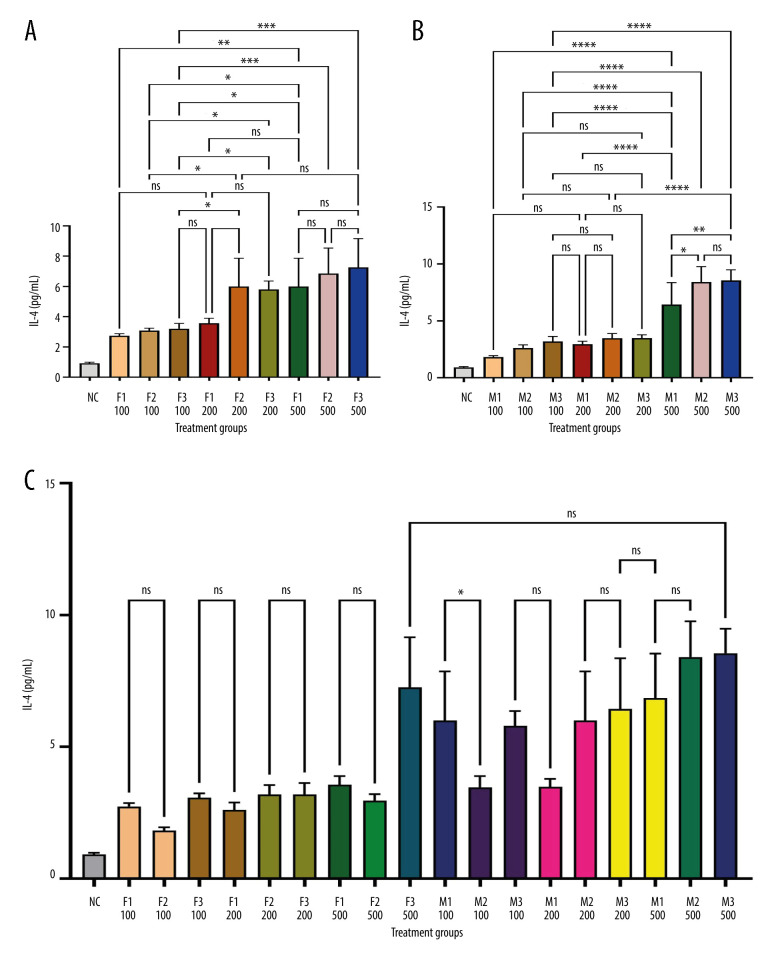
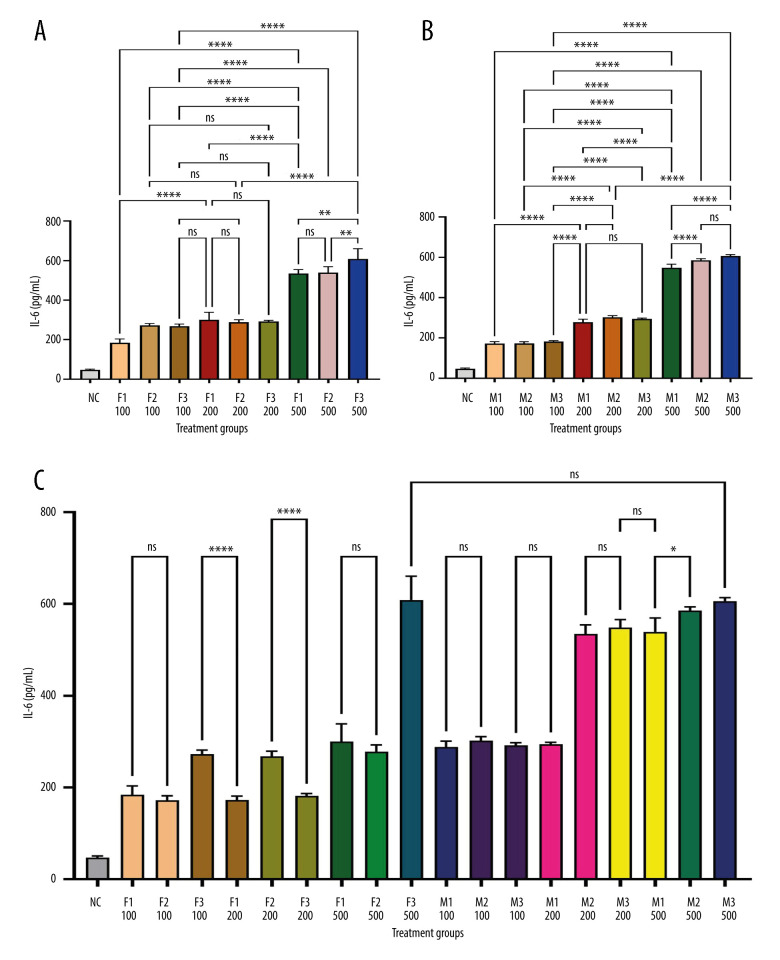
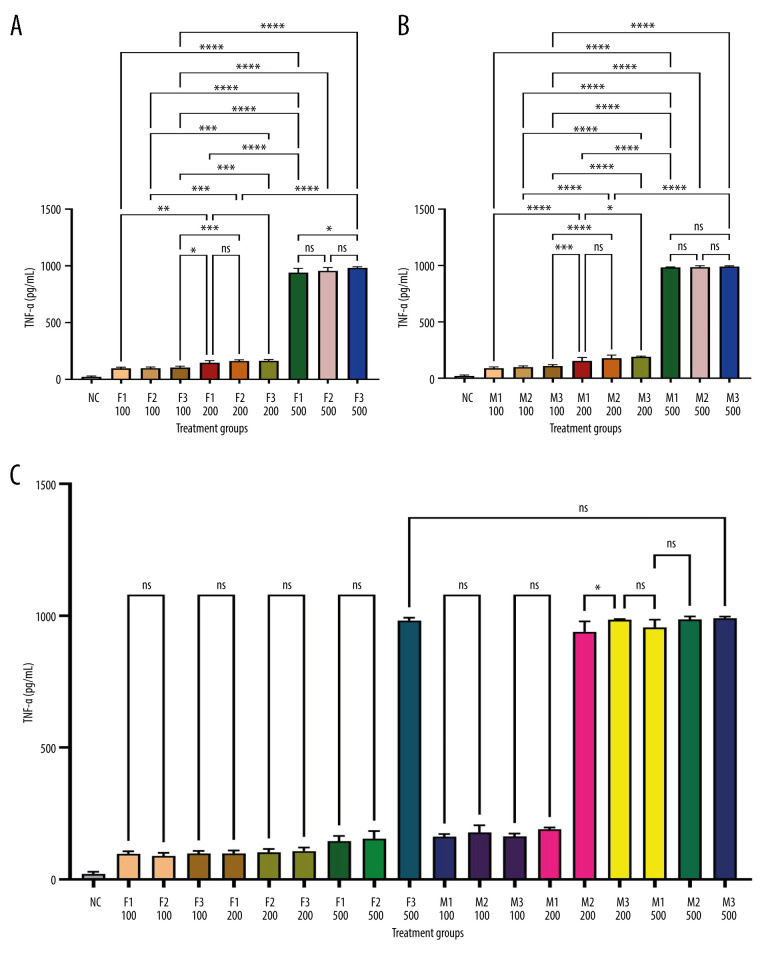
Figure 1 depicts IL-1β levels in response to varying doses of khat extract. The findings revealed that at higher extract dosages, IL-1β levels significantly increased. However, there were no appreciable differences in IL-1β levels between the male and female groups. In contrast, Figure 2 shows IL-2 levels in response to various khat extract dosages. These results demonstrated that the extract affected IL-2 levels in a dose-dependent manner (P<0.05). Moreover, no appreciable differences in IL-2 levels were observed between the male and female groups, indicating that the dose-dependent effect of khat extract on IL-2 levels was unaffected by sex. Figure 3 displays the dose-dependent effects on IL-4 levels and potential sex differences in rats treated with various khat extract doses. Increasing the extract dosage led to increased IL-4 levels. Significantly, a sex difference was noted at a dosage of 200, suggesting a unique reaction to this particular khat extract dosage in terms of IL-4 synthesis. These results emphasize how the complex khat extract dose, IL-4 levels, and sex affect the immunological responses of rats. Figures 4 and 5 show that, at higher dosages of khat extract, IL-6 and TNF-α levels increased in a dose-dependent manner. Significant sex differences were noted in this instance, too, at dosages of 100 mg/kg and 500 mg/kg, respectively, indicating sex-specific changes in IL-6 and TNF-α synthesis.
Figure 1.
Quantitative assessment of IL-1β levels in serum using sandwich ELISA. This figure demonstrates the measurement of IL-1β concentration in rat serum using a sandwich ELISA. Serum samples were collected and analyzed using IL-1β rat ELISA kits from MyBioSource (USA). The optical density was measured at 450 nm with an ELISA reader (ELx800, USA). IL-1β levels were calculated based on a standard curve and expressed in pg/mL. * Indicates statistically significant differences; ns – no significant changes. (A) Female groups; (B) male groups; (C) comparison between male and female groups. (This figure was generate using GraphPad Prism version 9, GraphPad Software, San Diego, California, USA).
Figure 2.
Sandwich ELISA quantitative serum IL-2 assessment. IL-2 rat ELISA kits from MyBioSource (USA) were used to examine serum samples. We assessed optical density at 450 nm with an ELISA reader (ELx800, USA). IL-2 levels were determined in pg/mL using a standard curve. The picture depicts the experimental technique and quantifies serum IL-2 levels. * Indicates statistical significance; ns – no significant differences. (A) Female groups; (B) male groups; (C) contrast between male and female groupings. (This figure was generate using GraphPad Prism version 9, GraphPad Software, San Diego, California, USA).
Figure 3.
Sandwich ELISA quantitative serum IL-4 assessment. IL-4 rat ELISA kits from MyBioSource (USA) were used to examine serum samples. We assessed optical density at 450 nm with an ELISA reader (ELx800, USA). IL-4 levels were determined in pg/mL using a standard curve. The picture depicts the experimental technique and quantifies serum IL-4 levels. * Indicates statistical significance; ns indicates no significant differences. (A) Female groups; (B) male groups; (C) comparison between male and female groups. (This figure was generate using GraphPad Prism version 9, GraphPad Software, San Diego, California, USA).
Figure 4.
Quantitative assessment of IL-6 levels in serum using sandwich ELISA using IL-6 rat ELISA kits from MyBioSource (USA). * Indicates statistically significant differences; ns – no significant changes. (A) Female groups; (B) male groups; (C) comparison between male and female groups. (This figure was generate using GraphPad Prism version 9, GraphPad Software, San Diego, California, USA).
Figure 5.
Quantitative assessment of TNF-α levels in serum using sandwich ELISA. Serum samples were collected and analyzed using TNF-α rat ELISA kits from MyBioSource (USA). The optical density was measured at 450 nm with an ELISA reader (ELx800, USA). TNF-α levels were calculated based on a standard curve and expressed in pg/mL. The figure provides an overview of the experimental procedure and represents the quantitative assessment of TNF-α levels in serum. * Indicates statistically significant differences; ns – no significant changes. (A) Female groups; (B) male groups; (C) comparison between male and female groups. (This figure was generate using GraphPad Prism version 9, GraphPad Software, San Diego, California, USA).
Effect of Khat on the Blood Glucose Levels
Table 1 displays the sex-based glucose values (mmol/L). The glucose level variations between the groups were statistically significant as the threshold of significance was less than 0.05. The letters (a, b) in the table indicate one-way ANOVA-analyzed statistical significance between groups. The control, 100 mg/kg, 300 mg/kg, and 500 mg/kg treated groups were included. In male rats, the control group had a mean glucose level of 6.12±0.14 mmol/L, followed by the 100 mg/kg group at 6.04±0.71, the 300 mg/kg group at 5.67±0.09, and the 500 mg/kg group at 6.73±0.30. Among female rats, the control group had a mean glucose level of 6.55±0.10 mmol/L, followed by the 100 mg/kg group at 5.98±0.25, the 300 mg/kg group at 6.17±0.79, and the 500 mg/kg group at 6.47±0.39 mmol/L.
Table 1.
Effect of khat on the blood glucose levels.
| Groups | (mmol/L) | |
|---|---|---|
| Male | Female | |
| Control | 6.12±0.14a | 6.55±0.10a |
| 100 mg/kg | 6.04±0.71a | 5.98±0.25b |
| 300 mg/kg | 5.67±0.09a | 6.17±0.79a,b |
| 500 mg/kg | 6.73±0.30a | 6.47±0.39a,b |
The level of significance was set at <0.05, indicating that the differences between the groups were statistically significant. The letters (a, b) in the table represent the specific statistical significance between groups, as analyzed by one-way ANOVA.
Renal and Hepatic Markers
For males, the aspartate aminotransferase (AST) levels ranged from 267.33 U/L to 336.33 U/L, with the Control group having the highest level (Table 2). For females, the AST levels ranged from 216.67 U/L to 322.00 U/L, with the Control group having the lowest level. In males, the alanine aminotransferase (ALT) levels ranged from 92.67 U/L to 110.33 U/L. The Control group had the lowest ALT level, whereas the 100 mg/kg group had the highest ALT level. However, the differences in ALT levels between groups were not statistically significant. In females, the ALT levels ranged from 85.67 U/L to 92.33 U/L. The Control group had the highest ALT levels, whereas the 300 and 500 mg/kg groups had relatively lower ALT levels. The differences in ALP levels between groups were not statistically significant. No significant difference was observed in total bilirubin levels between the male and female groups.
Table 2.
Glucose, hepatic markers and total bilirubin of female and male rats fed with the extract.
| Groups | AST (U/L) | ALT (U/L) | ALP (U/L) | TBI (mg/Dl) | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Control | 336.33± 18.64a | 216.67± 29.84a | 109.67± 13.87a | 92.33± 10.79a | 227.00± 84.48a | 144.00± 30.0a | 0.17± 0.06a | 0.10± 0.01a |
| 100 mg/kg | 270.33± 90.15b | 277.33± 36.96a | 110.33± 16.26a | 85.67± 21.94a | 265.33± 75.48a | 125.33± 22.01a | 0.13± 0.06b | 0.17± 0.06a |
| 300 mg/kg | 270.67± 62.32b | 251.67± 33.65a | 92.67± 11.72b | 86.00± 3.46a | 171.33± 16.80a | 200.00± 60.36a | 0.17± 0.06a | 0.13± 0.06a |
| 500 mg/kg | 267.33± 21.78b | 322.00± 26.89b | 98.00± 11.53b | 91.00± 7.21a | 213.00± 59.00a | 178.67± 23.63a | 0.17± 0.06a | 0.20± 0.01a |
The level of significance was set at <0.05, indicating that the differences between the groups were statistically significant. The letters (a, b) in the table represent the specific statistical significance between groups, as analyzed by one-way ANOVA.
Table 3 summarizes various biochemical parameters, including blood urea nitrogen (BUN) in mmol/L, creatinine in μmol/L, sodium (Na) in mmol/L, and potassium (K) in mmol/L, for the different groups categorized by sex. Notable findings included significantly higher BUN levels in females in the 100 mg/kg and 300 mg/kg groups than in the control group. No significant differences were observed in the other parameters between the groups. Specific statistical comparisons and significance levels are not provided in the given information.
Table 3.
Renal markers female and male rats fed with the extract.
| Groups | BUN (mmol/L) | Creatinine (μmol/L) | Na (mmol/) | K (mmol/L) | ||||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | Female | |
| Control | 8.23± 0.35a | 7.53± 0.32a | 35.69± 4.51a | 41.87± 2.86a | 143.00± 1.00a | 139.67± 1.53a | 7.40± 0.36a | 7.77± 0.32a |
| 100 mg/kg | 8.13± 1.17a | 9.60± 0.61b | 32.06± 9.43a | 47.20± 3.17a | 141.33± 3.51a | 142.67± 0.58a | 6.93± 0.59a | 7.10± 1.21a |
| 300 mg/kg | 7.10± 1.18a | 9.70± 1.83b | 33.87± 7.00a | 43.35± 4.29a | 144.00± 1.00a | 142.33± 0.58a | 6.97± 0.38a | 7.10± 0.30a |
| 500 mg/kg | 7.23± 0.75a | 7.47± 0.12a | 29.15± 9.01a | 42.44± 10.68a | 140.00± 4.58a | 141.33± 2.52a | 7.33± 0.21a | 7.53± 0.49a |
The level of significance was set at <0.05, indicating that the differences between the groups were statistically significant. The letters (a, b) in the table represent the specific statistical significance between groups, as analyzed by one-way ANOVA.
Discussion
Variations in AST and ALT levels in the liver support earlier hepatotoxicity research and suggest possible khat-induced damage. However, further investigation is required to verify the degree of damage and identify countermeasures. Higher BUN levels in females at higher khat dosages suggested possible malfunction. The main objective of this study was to assess the effects of khat extract on the immune system as well as hepatic and renal markers, using rats as a model animal. As it tackles the constraints that impede the detection of autoimmune effects, this study deviates from earlier studies [2,17]. Clearer observations made possible by the use of fresh plants and nontoxic doses in this study increased the precision of the results in describing the functions occurring in the human body.
Khat is associated with hepatotoxicity. Studies in rats have demonstrated that khat increases inflammation and oxidative stress in the liver [1,4,21]. Changes in the liver serum are indicators of this injury [1,21]. Studies of normal hepatocytes have also indicated in vitro cytotoxicity prompted by khat [22]. Beyond the potential harm to the liver, khat consumption may also lower blood cell production [4] and interfere with liver-metabolized drugs [21]. Although these studies highlight the potential risk of liver damage associated with chewing khat, Coenzyme Q10 supplementation may offer some protection against the negative effects of khat in mice [4].
The current results demonstrate the relevance of the differences in AST and ALT levels between various male and female groups in the context of possible hepatotoxicity. High AST and ALT values in the control group, especially in the females, were suggestive of potential liver damage. Studies linking khat usage to liver damage are consistent with the rise in these liver enzymes because khat increases oxidative stress and inflammation in the liver. The abnormalities identified in this study were consistent with the symptoms of liver damage associated with khat use [1,2]. Although there was no statistically significant difference in the ALT levels between the groups, khat could still be bad for the liver.
According to previous research, chewing khat can lead to nephrotoxicity [1,5]. Markers of impaired kidney function, creatinine, and blood urea nitrogen increased following khat use [1,5] lend credence. Al-Hashem et al. [1] also reported that khat increases kidney markers of oxidative stress and interferes with antioxidant enzyme activity [1]. Remarkably, khat may aggravate renal impairment caused by other drugs [5]. Although earlier studies indicate that khat use can damage the kidneys in a dose-dependent manner, with higher dosages causing more harm [1,5], Table 2 provides some evidence. In females from the 100 mg/kg and 300 mg/kg groups, the table indicates noticeably higher blood BUN values than in the control group, which is consistent with the anticipated increase in renal dysfunction markers at higher khat dosages. A more definitive conclusion is hampered by the paucity of data on other indicators, such as antioxidant enzyme activity or interactions with other drugs and the absence of appreciable increases in creatinine levels. Future research imitating this design with thorough statistical analysis and the addition of other kidney function markers is required to provide a more accurate picture.
The investigation of khat and its immunological effects revealed a complex dose-dependent relationship. Reduced khat extract dosages boost the immune system, as seen by increased IgG and IgM antibodies and CD4+ T helper cells in mouse studies [17]. Conversely, a decrease in CD8+, CD3+, and white blood cell counts suggested that increased khat extract dosages inhibited the cellular immunological response in mice [17]. Although khat addiction may not have a major impact on resistin levels in tuberculosis (TB) patients as opposed to TB patients without addiction, one study suggested that khat addicts may have a higher bacterial burden in their lungs [15]. The exact mechanism by which khat alters the immune system remains unclear. More research is needed to validate these findings in humans and to clarify their implications for human health [16,17].
Drawing on other studies [16,17], this study presents a unique perspective on the immunological effects of khat extract. Research on the impact of certain proinflammatory cytokines (IL-1β, IL-2, IL-4, IL-6, and TNF-α) extends beyond that of general immune system markers such antibodies and cell counts. Remarkably, our results show that in contrast to earlier theories of possible dose-dependent immunological stimulation or inhibition, all assessed cytokines increased consistently with increasing khat extract doses [17]. Moreover, our study revealed differences in IL-4, IL-6, and TNF-α synthesis between male and female rats, thereby shedding light on the possible effect of sex on the immune system. However, previous studies have not addressed this issue [15–17]. Considering sex differences and concentrating on particular cytokine responses, the present study adds a great deal to the body of knowledge based on earlier studies.
This study has a limitation in the manner of extracting the khat juice in humans in contrast to extracting the plant material manually to the animal model and the inability to measure the degree of damage in this study. Estimation of hepatocellular damage and kidney damage require more markers measurement to highlight the effect of khat in these 2 organs.
Conclusions
This study examined the effects of khat extract on the liver, kidneys, and cytokines in rats. This provides new insights for future studies. Variations in AST and ALT levels in the liver support earlier hepatotoxicity research and suggest possible khat-induced damage. However, further investigation is required to verify the degree of damage and identify countermeasures. Higher BUN levels in females at higher khat dosages suggested possible malfunction, which was partially in line with previous research that related khat use to dose-dependent kidney injury. In contrast to earlier studies, in this study consistently increased all cytokines as the dose of the khat extract increased. It also brings to light possible variations in the immune response by sex, which calls for further research. This study clarifies the intricate impact of khat use on different systems and highlights the need for more research to completely comprehend the possible hazards and therapeutic implications of khat use.
Acknowledgment
The authors gratefully acknowledge the funding from the Deanship of Graduate Studies and Scientific Research, Jazan University, Saudi Arabia, through Project Number RG24-M014.
Footnotes
Financial support: Deanship of Graduate Studies and Scientific Research, Jazan University, Saudi Arabia, through Project Number RG24-M014
Conflict of interest: None declared
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Declaration of Figures’ Authenticity: All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References
- 1.Al-Hashem FH, Bin-Jaliah I, Dallak MA, et al. Khat (Catha edulis) extract increases oxidative stress parameters and impairs renal and hepatic functions in rats. Bahrain Medical Bulletin. 2011;33(1):1–9. [Google Scholar]
- 2.Alsalahi A, Abdulla MA, Al-Mamary M, et al. Toxicological features of Catha edulis(Khat) on livers and kidneys of male and female Sprague-Dawley rats: A subchronic study. Evid Based Complement Alternat Med. 2012;2012:829401. doi: 10.1155/2012/829401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Githua KK, Maitho TE, Nguta JM, Okumu MO. Studies on the ethnopharmacology, antimicrobial activity, and toxicity of Catha edulis (Vahl.) Endl., in Sprague Dawley rats. F1000Res. 2022;11:286. doi: 10.12688/f1000research.109243.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy C, Okanya P, Nyariki JN, et al. Coenzyme Q10 nullified khat-induced hepatotoxicity, nephrotoxicity and inflammation in a mouse model. Heliyon. 2020;6(9):e04917. doi: 10.1016/j.heliyon.2020.e04917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shewamene Z, Engidawork E. Subacute administration of crude khat (Catha edulis F.) extract induces mild to moderate nephrotoxicity in rats. BMC Complement Altern Med. 2014;14:66. doi: 10.1186/1472-6882-14-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins M, Handslip R, Kumar M, et al. Reversible khat-induced hepatitis: Two case reports and review of the literature. Frontline Gastroenterol. 2013;4(4):278–81. doi: 10.1136/flgastro-2013-100318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaikhain G, Gaballah M, Alhazmi A, et al. Fatalities involving khat in Jazan, Saudi Arabia, 2018 to 2021. Toxics. 2023;11(6):506. doi: 10.3390/toxics11060506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patel H, Kumar K, Essrani RK, et al. Acute hepatitis in a Yemeni immigrant associated with khat: A “biological amphetamine” carried in cultures. Clin Pract. 2021;11(1):167–73. doi: 10.3390/clinpract11010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alsanosy R, Alhazmi HA, Sultana S, et al. Phytochemical screening and cytotoxic properties of ethanolic extract of young and mature khat leaves. Journal of Chemistry. 2020;2020(1):7897435. [Google Scholar]
- 10.Atnafie SA, Muluneh NY, Getahun KA, et al. Pesticide residue analysis of Khat leaves and health risks among Khat Chewers in the Amhara region, Northwestern Ethiopia. J Environ Public Health. 2021;2021(1):4680573. doi: 10.1155/2021/4680573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Magnavacca A, Giuliani C, Roda G, et al. Catha edulis Leaves: Morphological characterization and anti-inflammatory properties in an in vitro model of gastritis. Plants. 2024;13(11):1538. doi: 10.3390/plants13111538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bereda G. Catha Edulis Forsk and its adverse effects on health: Current and ongoing factuality. Ann Clin Med Case Rep. 2021;7(13):1–10. [Google Scholar]
- 13.Malasevskaia I, Al-Awadhi AA, Mohammed L. Tea in the morning and khat afternoon: health threats due to khat chewing. Cureus. 2020;12(12):e12363. doi: 10.7759/cureus.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atlabachew M, Chandravanshi BS, Redi M. Selected secondary metabolites and antioxidant activity of khat (Catha edulis Forsk) chewing leaves extract. International Journal of Food Properties. 2014;17(1):45–64. [Google Scholar]
- 15.Alvi A, Fatima N, Jerah AA, et al. Correlation between resistin, tuberculosis and khat addiction: A study from south western province of Saudi Arabia. PLoS One. 2015;10(10):e0140245. doi: 10.1371/journal.pone.0140245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alvi A, Rizwan M, Sunosi RAL, Jerah ABA. Does khat chewing increases the risk of Mycobacterium tuberculosis infection by macrophage immune modulation? Med Hypotheses. 2014;82(6):667–69. doi: 10.1016/j.mehy.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 17.Ketema T, Yohannes M, Alemayehu E, Ambelu A. Evaluation of immunomodulatory activities of methanolic extract of khat (Forsk) and cathinone in Swiss albino mice. BMC Immunol. 2015;16(1):9. doi: 10.1186/s12865-015-0072-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohan S, Abdelwahab SI, Hobani YH, et al. Catha edulis Extract Induces H9c2 cell apoptosis by increasing reactive oxygen species generation and activation of mitochondrial proteins. Pharmacogn Mag. 2016;12(Suppl 3):S321–26. doi: 10.4103/0973-1296.185732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jerah A, Taha M, Farasani A. Insights into the anorexic mechanism of khat: An integrated in vivo, ex vivo, and in silico investigations. Tradit Med Res. 2024;9(10):58. [Google Scholar]
- 20.Madkhali OA, Moni SS, Sultan MH, et al. Design and characterization of Lactotransferrin peptide-loaded dextran-docosahexaenoic acid nanoparticles: An immune modulator for hepatic damage. Sci Rep. 2023;13(1):13537. doi: 10.1038/s41598-023-40674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alqahtani AS, Parvez MK, Alqahtani AM, et al. Effects of Catha edulis (khat) on the pharmacokinetics of metformin in diabetic rats using UPLC/MS/MS analysis and its impact on hepatic CYP450 enzymes. Separations. 2023;10(8):442. [Google Scholar]
- 22.Taha MME, Abdelwahab SI, Al-Sanousi R. In vitro hepatotoxcity of Catha edulis Forsk. (khat) phenolic-rich extract on human hepatocytes. Journal of Applied Pharmaceutical Science. 2014;4(11):42–46. [Google Scholar]