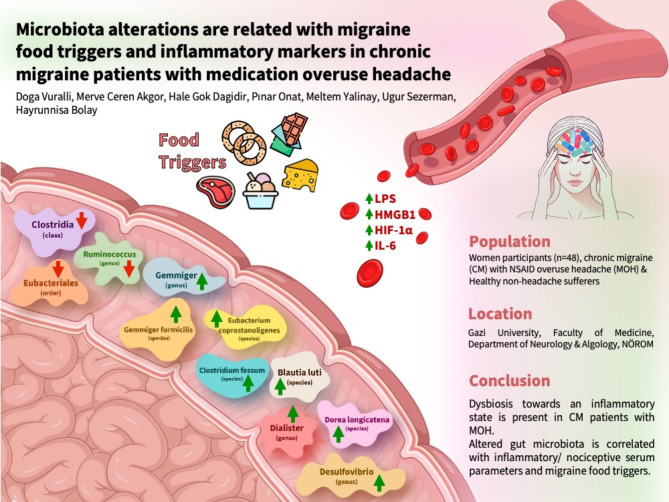
Abstract
Objective
Chronic migraine (CM) patients with medication overuse headache (MOH) were recently shown to be associated with leaky gut and inflammation. We aimed to investigate gut microbiota profiles of CM patients with MOH, and their correlations with inflammatory serum parameters, migraine food triggers, and comorbid anxiety and depression.
Materials and methods
The study included women participants (32 CM patients with NSAID overuse headache, and 16 healthy non-headache sufferers). Migraine duration, monthly migraine headache days, presence of irritable bowel syndrome symptoms, and HADS-D and HADS-A scores were recorded. Serum samples were collected to measure circulating LPS, HMGB1, HIF-1α, and IL-6. The gut microbiota profiles of the patients were evaluated using fecal samples.
Results
Serum LPS, HMGB1, HIF-1α, and IL-6 levels were significantly higher in the CM + MOH group compared to the healthy controls. HADS-A and HADS-D scores were considerably higher in the CM + MOH group compared to the healthy controls. In the microbiota analysis, alpha and beta diversities were similar between the two groups. The class Clostridia, the order Eubacteriales, and the genus Ruminococcus were less abundant in the CM + NSAID overuse headache group compared to the control group. At the genus level Desulfovibrio, Gemmiger, and Dialister and at the species level, Clostridium fessum, Blautia luti, Dorea longicatena, Eubacterium coprostanoligenes, and Gemmiger formicilis were more abundant in the CM + NSAID overuse headache group compared to the control group. Desulfovibrio, Gemmiger, Dialister, Ethanoligenens harbinense, Eubacterium coprostanoligenes, Dorea longicatena, and Thermoclostridium stercorarium showed positive correlations and Clostridia bacteria showed negative correlations with migraine food triggers. Positive correlations were found between LPS and Hapalosiphonaceae, HMGB1 and Melghirimyces, HIF1-α and Rouxeilla and Blautia luti, IL-6 and Melghirimyces and Ruminococcus.
Conclusion
In CM patients with MOH, we have revealed the presence of dysbiosis towards an inflammatory state, and positive correlations were shown between altered gut microbiota and inflammatory serum parameters and migraine food triggers.
Keywords: Medication overuse headache, Chronic migraine, Microbiota, Lipopolysaccharide, Leaky gut, HMGB1, HIF-1α, Inflammation
Graphical abstract
Introduction
Gut microbiota has a pivotal role in the regulation of human physiology and the bidirectional pathways of the gut-brain axis are involved in many neurological diseases and their role in migraine has gained attention in recent years. Factors such as the microbiota composition and inflammatory mediators influence the gut-brain axis. Altered microbiota profiles are suggested to play a crucial role in cardiovascular, neurological, and psychiatric diseases [1]. It has been shown that the microbiota profiles of migraine patients were different compared to the healthy controls [2]. Chronic migraine (CM) and medication overuse headache (MOH) are associated with increased gut permeability [3] and altered gut microbiota composition may underlie the intestinal hyperpermeability and inflammation seen in this headache disorder.
MOH is recognized as a secondary headache frequently seen in CM patients according to the International Classification of Headache Disorders 3rd edition (ICHD-3), even though some clinicians see MOH as a complication of primary headache disorders including CM [4]. Non-steroidal anti-inflammatory drugs (NSAIDs) are the most overused analgesic agents [5] that play a role in the development of MOH in migraine patients. Recently, it was shown that CM patients with MOH were associated with the increased passage of lipopolysaccharide (LPS), bacterial endotoxin, into the bloodstream and consequent release of pro-inflammatory and/or nociceptive molecules such as high mobility group box-1 protein (HMGB1), calcitonin gene-related peptide (CGRP), hypoxia-inducible factor-1α (HIF-1α) and interleukin 6 (IL-6) levels suggesting that leaky gut may be involved in MOH pathophysiology [3].
Microbiota profiles of CM patients with MOH have not been investigated before. We aimed to investigate microbiota profiles of CM patients with MOH and the association between microbiota profiles with migraine food triggers and factors associated with leaky gut and inflammation such as LPS, HMGB1, HIF-1α, and IL-6 levels. This study aims to provide information about the possible link between CM and NSAID overuse headache, altered microbiota profiles, leaky gut, and migraine food triggers.
Methods
Female CM patients with MOH (NSAID overuse headache) were consecutively recruited from Gazi University Faculty of Medicine, Department of Neurology and Algology, Headache outpatient clinic between July 2023 and December 2023. Only women participants were enrolled in the study. CM patients with MOH were included in the study if, (1) the age was between 18 and 65 years of age, (2) they had a definite diagnosis of chronic migraine (1.3) according to ICHD-3, 4) they had a diagnosis of NSAID overuse headache (8.2.3.2) according to ICHD-3, 5) they did not use any migraine prophylactic medications, 6) they agreed to give a blood sample for enzyme-linked immunosorbent assay (ELISA) analysis, 6) they agreed to bring fecal sample and 7) they had normal white blood cell count (WBC), erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels. The inclusion criteria for healthy controls were; (1) age between 18 and 65 years of age, (2) no history of headaches, (3) agreeing to bring fecal samples and (4) having normal WBC, ESR, and CRP levels. Exclusion criteria for the participants were; (1) history of any other chronic or neurological disease or having any laboratory findings suggesting a chronic systemic disease, (2) abnormally hard or loose stool state types [6] or failure to bring the fecal sample, (3) any history of food intolerance or food allergies, 3) chronic daily use of any medications, (4) any infections within 7 days before and after the blood sample or fecal sample collection, (5) history of alcohol or drug abuse (6) smoking and (7) history of any major psychiatric disease. Two headache experts (DV and HB) included the patients in the study after a detailed clinical assessment. The patients were called a month later to question whether they received a diagnosis of any chronic disease within one month after the blood sample and fecal sample collection.
The number of migraine headache days and migraine duration were recorded for CM patients with MOH. Hospital Anxiety and Depression Scale Anxiety (HADS-A) and Depression subscales (HADS-D) scores were recorded for both groups. A detailed migraine food trigger questionnaire was filled out [5] and the irritable bowel syndrome (IBS) symptoms were assessed in both groups according to the Rome IV Criteria [7].
Serum LPS, HMGB1, HIF-1α, and IL-6 levels were assessed using enzyme-linked immunosorbent assay (ELISA). The blood samples collected from both groups were centrifuged (20 min 1000*g at 2–8 °C) to obtain the serum samples and they were stored at − 80 °C until further analysis. The local ethics committee approved the study and it complies with the Declaration of Helsinki.
Enzyme-linked Immuno Sorbent Assay (ELISA)
Serum LPS, HMGB1, HIF-1α, and IL-6 levels were measured with high-sensitivity ELISA kits. ELISA kits were obtained from Elabscience Biotechnology Inc., Houston, TX, USA [IL-6 (E-EL-H6156), HMGB1 (E-EL-H1554), HIF-1 α (E-EL-H6066)] and CUSABIO Inc., Wuhan, China [Human LPS (CSB-E0994h)]. The coefficients of variation were < 10%.
All of the reagents and serum samples were brought to room temperature before the analysis. Dilution of the reference standards provided in each kit was made according to the instructions of the manufacturer. Analytical-grade deionized water was added to the concentrated wash buffers to obtain the wash buffers. The kits were used following the assay procedure. Washing processes were performed using the Combiwash Human ELISA plate washer. A stop solution was added to terminate the enzyme-substrate reaction and a rapid color change to yellow was detected. The optical density of each well at 450 nm was measured using Chromate microplate reader.
Microbiota analysis
Sample processing and sequencing
After obtaining the samples, bacterial DNA isolation was performed with the ZymoBIOMICS DNA Miniprep kit. The obtained DNAs were checked by measuring both the precise DNA amount with Qubit 2.0 and the purity with NanodropONEc. In the cases where the purity of the DNA was not found sufficient, cleaning was performed with the magnetic bead method. Since amplicon creation for 16 S rRNA-targeted amplicon sequencing was carried out by the polymerase chain reaction (PCR) method, DNA purity was very important. DNAs additionally purified with magnetic beads were amplified with 27 F-1453R universal 16 S primers to create an amplicon library. After measurements of the amplified PCR products were made with Qubit 2.0, they were purified again with the magnetic bead method to remove impurities caused by PCR. The DNA library of the purified samples was prepared with the 16S024 kit provided by Oxford Nanopore Technologies. Barcoded samples for adapter and multiplexing were purified again with magnetic beads, then combined with sequencing buffer and sequenced with MinION FLO-MIN106D flowcells. Sequencing was performed to obtain at least 30,000 sequence readings from each sample. Unlike the 16 S (V3-V4 regions) sequencing performed with Illumina MiSeq technology, since all regions for 16 S were sequenced, the average quality score (phred score) according to the Lambda phage loaded with the barcoding kit for long multiple runs rather than the high number of sequence reads is Q17. After sequencing, the read values were optimized and debugged according to the reference of the 3.6 kb Lambda phage included in the kit, and ONT FAST5 signal Hiles were converted into fastq Hiles. Consensus sequences were created with the obtained Fastq Hiles and annotated according to the NCBI 16 S database. Taxonomy was created at the species or genus level based on 95% accuracy in taxonomy creation.
Statistical analysis
Data analysis was performed using IBM SPSS statistical software version 22.0 (USA) and python 3.11.4. The normal distribution of data was investigated by the Shapiro-Wilk normality test. Continuous variables were indicated as mean ± standard deviation and categorical variables were given as frequency and percentage values. The analysis of normally distributed continuous variables was performed with a student t-test. The analysis of non-normally distributed continuous variables was performed with the Mann-Whitney’s U test. p < 0.05 was considered statistically significant.
Explorative analyses
In order to explore the dataset using visualizations and in terms of bacterial diversity, firstly relative abundance plots were created using the plot functionality of the pandas package.
Each group’s mean/median diversity was then compared using either Mann Whitney U test or t-test (based on the results of the Shapiro-Wilk normality test, if both groups were normally distributed, t-test was used, if not, Mann Whitney U was used) using python package scipy, and its related functions. For alpha diversities, Shannon index, Simpson index, and Observed OTU metrics were used. Differences in alpha diversities were visualized using boxplots made using the seaborn package. To explore the beta diversities, PCoA was applied using the skbio package. As the distance metric, Bray-Curtis was chosen. To show the features (bacteria) most involved in the first two components, a loadings plot was created along with the scatter plot of the original PCoA, both done with the seaborn package.
Comparative analyses
Comparative analyses were applied with comparative statistical testing, applying either Mann Whitney U or t-test to group comparisons depending on the normality of the groups. The results were then visualized with seaborn boxplots. Bacteria that were seen in significantly different levels in compared groups were then selected (p value < 0.05) to be visualized in a plot showing both the log fold changes (logFCs) and the p-values. For this, a violin plot was created with the seaborn package.
Correlation analyses
Correlation analyses were applied on either continuous and categorical data (when correlating bacterial percentage values and migraine food trigger categories), or continuous and continuous data (when correlating bacterial percentage values and inflammatory serum marker levels). For correlating continuous and categorical data, Point Biserial Correlation (scipy package) was used, and for correlating continuous and continuous groups, Spearman correlation (scipy package) was used, as most groups contain many zeros and not normally distributed. Each correlation analysis was visualized using seaborn heatmaps.
In correlation analyses including bacterial value data, only the bacteria that were seen significantly different in the related group comparisons were used, to limit the size of the graphs and to filter the output information.
Results
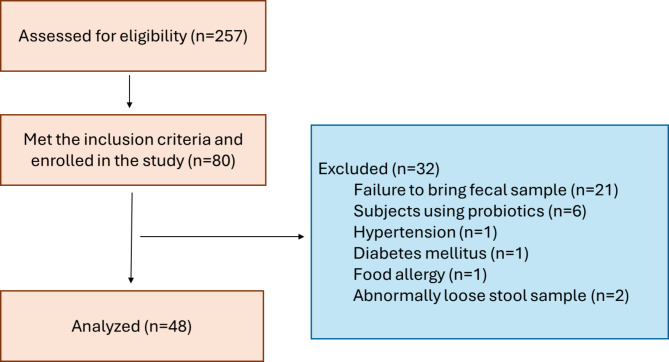
Flow-chart of the study is given in Fig. 1. Thirty-two CM + MOH patients and 16 healthy controls were included in the study. The mean age was 41.4 ± 8.5 years in the chronic migraine and MOH group and 41.0 ± 12.1 years in the healthy controls (p = 0.89). In the chronic migraine and MOH group, migraine duration was 244.1 ± 78.8 months, and mean monthly migraine headache days was 22.2 ± 7.4 days.
Fig. 1.
The flowchart of the study
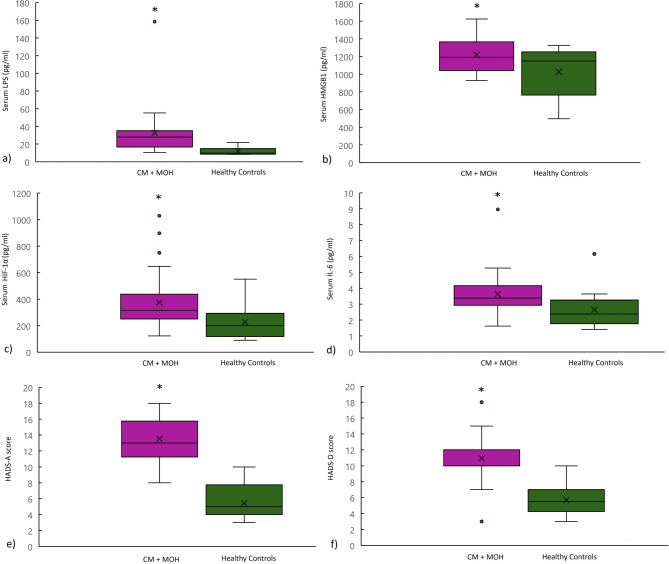
Serum LPS, HMGB1, HIF-1α and IL-6 levels were significantly higher in the CM + MOH patients compared to the healthy controls (p = 0.002, p = 0.023, p = 0.009 and p = 0.042 respectively) (Fig. 2). HADS-A scores and HADS-D scores were significantly higher in the CM + MOH patients compared to the healthy controls (p < 0.0001 and p < 0.0001 respectively) (Fig. 2). IBS symptoms were defined by 20 CM + MOH patients (62.5%) and 5 healthy controls (31.3%). IBS symptoms were higher in CM + MOH patients compared to the healthy controls (p < 0.0001). Twenty-five CM patients with MOH (78.1%) were fast eaters with a chewing time of each bite less than 20 s while 14 healthy controls (87.5%) were fast eaters. The percentage of fast eaters was similar between the two groups (p = 0.43).
Fig. 2.
Serum (a) LPS, (b) HMGB1, (c) HIF-1α and (d) IL-6 levels were significantly higher in CM + MOH patients compared to healthy controls (p = 0.002, p = 0.023, p = 0.009 and p = 0.042 respectively). CM + MOH patients had higher (e) HADS-A and (f) HADS-D scores compared to healthy controls (p < 0.0001 and p < 0.0001 respectively). Data are shown as whisker-box plots (whisker: full range; line: median; cross: mean)
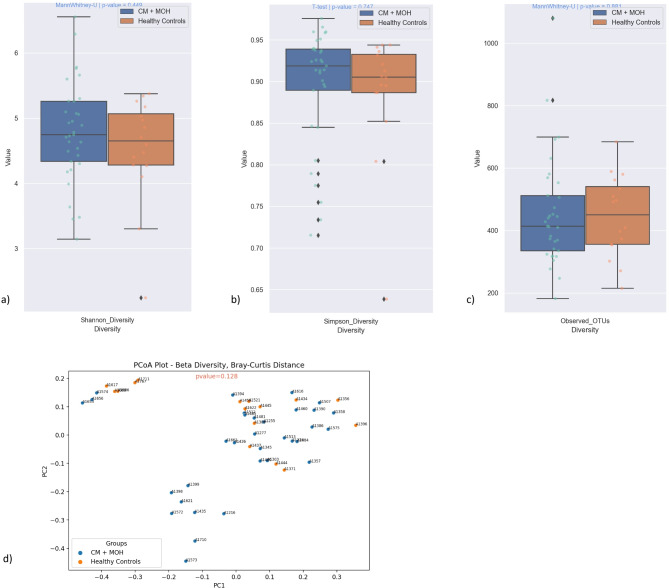
Alpha diversity represents the richness and evenness of microbial community. Alpha diversities evaluated by Shannon and Simpson indices and Observed OTU richness were similar between CM and control groups (p = 0.45, p = 0.75 and p = 0.88 respectively) (Fig. 3). Beta diversity shows the dissimilarity of community composition between the groups. PCoA analysis with Bray-Curtis dissimilarity index was performed to evaluate overall diversity of gut microbiota between CM patients with MOH and healthy non-headache sufferers. PCoA with Bray-Curtis dissimilarity index for beta diversity at the genus level revealed no significant difference between the groups (p = 0.128) (Fig. 3).
Fig. 3.
Alpha diversities measured by Shannon and Simpson indices and Observed OTU richness were comparable between CM + MOH and control groups (p = 0.45, p = 0.75 and p = 0.88 respectively). PCoA with Bray-Curtis dissimilarity index for beta diversity at the genus level showed no significant difference between CM + MOH and healthy control groups (p = 0.128)
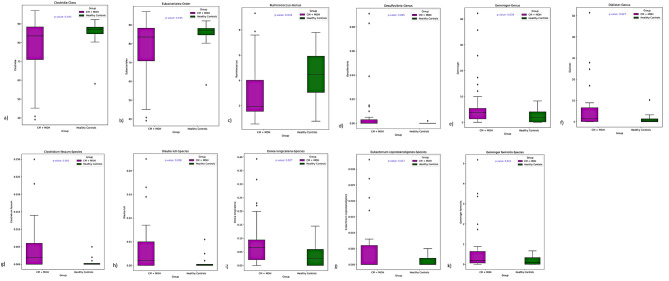
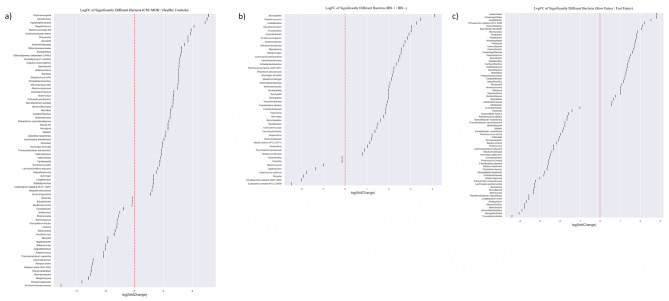
The microbiota analysis was performed at the class, order, family, genus, and species levels (Fig. 4). At the class level, Clostridia was less abundant in the CM + NSAID overuse headache group compared to the control group (p = 0.035). At the order level, Eubacteriales was less abundant in the CM + NSAID overuse headache group compared to the control group (p = 0.035). At the genus level, Ruminococcus was less abundant and Desulfovibrio, Gemmiger and Dialister were more abundant in the CM + NSAID overuse headache group compared to the healthy controls (p = 0.034, p = 0.045, p = 0.034 and p = 0.027 respectively). At the species level, Clostridium fessum, Blautia luti, Dorea longicatena, Eubacterium coprostanoligenes, and Gemmiger formicilis showed relative positive predominancy in the CM + NSAID overuse headache group compared to the control group (p = 0.002, p = 0.016, p = 0.027 and p = 0.024 respectively). Clostridium fessum was also significantly abundant in fast eater CM + MOH patients compared to fast eater healthy controls (p = 0.002). LogFC of significantly different bacteria between chronic migraine patients and healthy controls is given in Fig. 5a. LogFC of significantly different bacteria between subjects with and without irritable bowel syndrome is given in Fig. 5b. LogFC of significantly different bacteria between subjects with a chewing time of less or more than 20 s for each bite is given in Fig. 5c.
Fig. 4.
(a) The class Clostridia (p = 0.035), (b) the order Eubacteriales (p = 0.035) and (c) the genus Ruminococcus (p = 0.034) were less abundant in the CM + MOH group compared to the control group. The genera (d) Desulfovibrio, (e) Gemmiger and (f) Dialister were predominant in the CM + MOH group compared to the healthy controls (p = 0.045, p = 0.034 and p = 0.027 respectively). At the species level, (g) Clostridium fessum, (h) Blautia luti, (i) Dorea longicatena, (j) Eubacterium coprostanoligenes, and (k) Gemmiger formicilis were more abundant in the CM + MOH group compared to the control group (p = 0.002, p = 0.016, p = 0.027 and p = 0.024 respectively)
Fig. 5.
(a) Log Fold Change (LogFC) calculated as Chronic migraine / Healthy Controls. Positive logFC indicates bacteria that were significantly more abundant in chronic migraine group and negative logFC indicates bacteria that were significantly more abundant in healthy controls. (b) LogFC of significantly different bacteria between subjects with and without IBS. Positive logFC indicates bacteria that were significantly more abundant in subjects with IBS and negative logFC indicates bacteria that were significantly more abundant in subjects without IBS. (c) LogFC of significantly different bacteria between subjects with a chewing time of less or more than 20 s for each bite. Positive logFC indicates bacteria that were more abundant in subjects with a chewing time of more than 20 s and negative logFC indicates bacteria that were more abundant in subjects with a chewing time of less than 20 s. The fold change of all bacteria that showed significant differences between the two groups in statistical tests with a p-value less than 0.05 is shown in this figure
Correlation analysis
The correlations between migraine food triggers and altered gut bacteria in the chronic migraine group were analyzed (Fig. 6). The relative abundance of Desulfovibrio was positively correlated with peanut butter (r = 0.88, p = 0.015), grapefruit (r = 0.59, p < 0.001), soya sauce (r = 0.45, p = 0.01), coffee cream/milk powder (r = 0.38, p = 0.03) and ice cream (r = 0.38, p = 0.03). Increase in Gemmiger was positively associated with ice cream (r = 0.37, p = 0.035). The genus Dialister was positively correlated with frozen meat and chicken products (r = 0.58, p < 0.001), hamburger/pizza (r = 0.53, p = 0.002), red meat/minced meat (r = 0.52, p = 0.002 ), aged cheese (r = 0.51, p = 0.003), ketchup (r = 0.49, p = 0.005), coffee cream/milk powder (r = 0.47, p = 0.006), cream filled pastries (r = 0.47, p = 0.006), pickles (r = 0.47, p = 0.007), sweet sauces (r = 0.43, p = 0.01), soya sauce (r = 0.41, p = 0.02), vinegar (r = 0.40, p = 0.02) and chocolate/cacao (r = 0.38, p = 0.03). Ethanoligenens harbinense was positively correlated with chocolate/cacao (r = 0.38, p = 0.03), canned tuna fish (r = 0.56, p < 0.001), ready-to-eat canned foods (r = 0.56, p < 0.001), cream filled pastries (r = 0.36, p = 0.04) and excessive carbohydrate consumption in a single meal (r = 0.43, p = 0.01). Eubacterium coprostanoligenes was positively associated with peanut (r = 0.74, p < 0.001 ), onion/garlic (r = 0.51, p = 0.003), pastries/simit (Turkish bagel) (r = 0.37, p = 0.035), orange juice (r = 0.47, p = 0.006), cream filled pastries (r = 0.36, p = 0.04), cream cheese (r = 0.75, p < 0.001 ), ready-to-use sauces (such as pesto sauce) (r = 0.38, p = 0.03) and ready-to-eat canned foods (r = 0.63, p < 0.001). Dorea longicatena was positively correlated with yoghurt (r = 0.45, p = 0.009), soudjouk/sausage (r = 0.64, p < 0.001), salami/pastrami (r = 0.48, p < 0.001), green pepper/green beans (r = 0.45, p = 0.009). Thermoclostridium stercorarium was positively correlated with peanut (r = 0.41, p < 0.001) and cream cheese (r = 0.43, p < 0.001). Clostridia bacteria was negatively correlated with chocolate/cacao (r = -0.4, p = 0.02), pickles (r = -0.49, p = 0.004), butter (r = -0.46, p = 0.008), chicken (r = -0.46, p = 0.008), milk (r = -0.6, p < 0.001), pastrami/salami (r = -0.44, p = 0.01), mushroom (r = -0.46, p = 0.008 ), red meat/minced meat (r = -0.45, p = 0.01), ketchup (r = -0.44, p = 0.01), frozen meat and chicken products (r =-0.53, p = 0.002) and chickpea and beans (r = -0.46, p = 0.008).
Fig. 6.
Heatmaps showing correlations between migraine food triggers and altered gut microbiota
LPS was positively correlated with Hapalosiphonaceae (r = 0.37, p = 0.01 ), HMGB1 was positively associated with Melghirimyces (r = 0.39, p = 0.002), HIF1-α was positively correlated with Rouxeilla (r = 0.40, p = 0.005) in both groups and Blautia luti (r = 0.30, p = 0.048) in CM + MOH group (Fig. 7), IL-6 was positively correlated with Melghirimyces (r = 0.35, p = 0.048 ) and Ruminococcus (r = 0.60, p < 0.001) in CM + MOH patients (Fig. 7) while negatively correlated with Subdoligranulum variabile (r = -0.48, p = 0.006) and the genus Subdoligranulum (r = -0.45, p = 0.009) in CM + MOH patients. HAD anxiety scores were positively correlated with an increase in Subdoligranulum variabile (r = 0.41, p = 0.001) and, Desulfovibrio (r = 0.45, p = 0.002), Anoxybacillus (r = 0.48, p = 0.002) and Aphanothecaceae (r = 0.36, p = 0.01) in both groups and HAD depression scores were positively associated with Moryella (r = 0.38, p = 0.03) in CM + MOH patients and negatively associated with Neglectibacter (r = -0.44, p = 0.002) in both groups (Fig. 7).
Fig. 7.
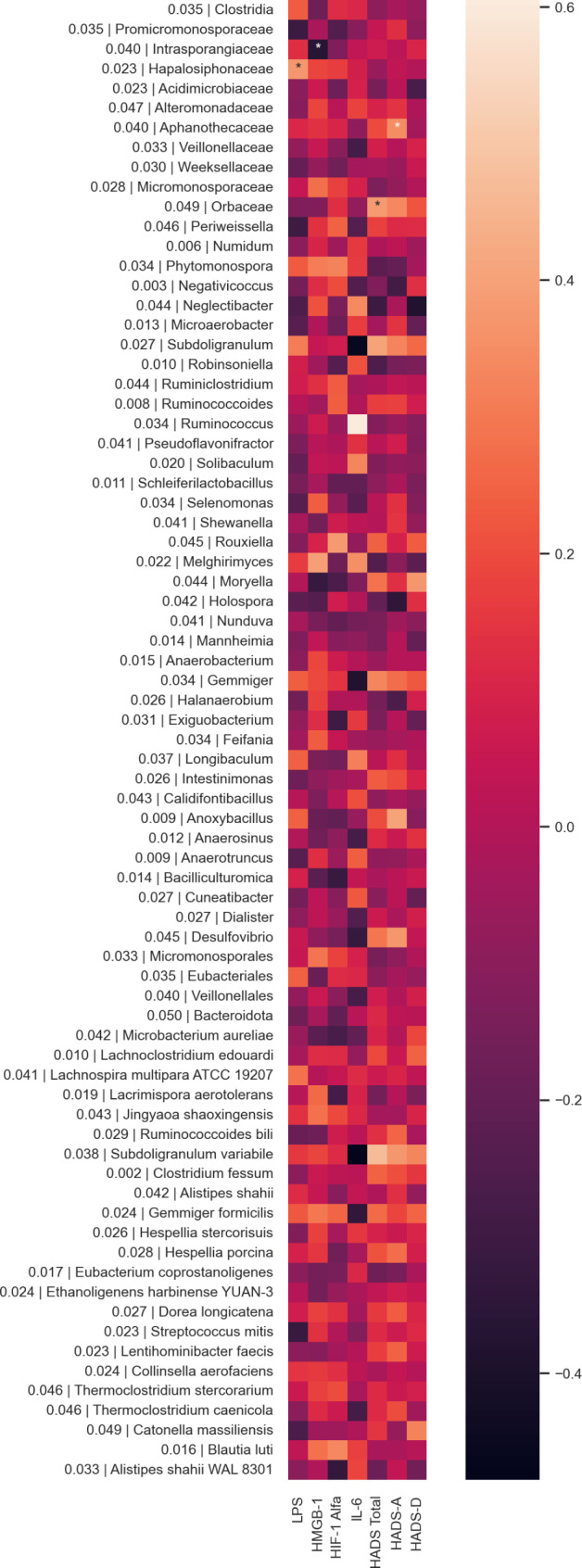
Heatmaps showing correlations between LPS, HMGB1, HIF-1α, IL-6, HADS-A, HADS-D scores and altered gut microbiota
Discussion
We showed the presence of dysbiosis in the gut microbiota of CM patients with MOH towards an inflammatory state as evidenced by the relative abundance of Desulfovibrio, Dialister, Dorea longicatena, and Blautia luti and relative depletion of Ruminoccoccus. Inflammatory serum parameters such as LPS, HMGB1, IL-6, and HIF1-α levels were positively correlated with these certain bacteria in the altered gut microbiota of CM patients. Additionally, alterations in microbiota composition were correlated with migraine food triggers. The microbiota changes were also linked to anxiety and depression scores, and the presence of irritable bowel syndrome symptoms.
Studies have addressed strong correlations between gut microbiota alterations and commonly used medications such as NSAIDs [8]. Intestinal injury induced by NSAIDs is also shown to be associated with alterations in gut microbiota and dysbiosis. Several animal studies have assessed the role of NSAIDs on gut microbiota, however, there are only few human studies that have investigated the effect of NSAIDs on gut bacteria. The use of NSAIDs, both in preclinical and clinical studies had no clear effect on alpha diversity [9–14]. In our study, there was no difference regarding the alpha diversity between CM patients with NSAID overuse headache and healthy controls. Previously Edogawa et al. [9] showed that indomethacin decreased the abundance of Ruminococcus in women and similarly, in this study, CM patients with NSAID overuse headache had less abundance of Ruminococcus compared to healthy non-headache sufferers. In a study conducted by Rogers and Aronoff in their community residents, the bacteria family Desulfovibrionaceae was shown to be more common in NSAID users than in the controls [11]. In the present study, the genus Desulfovibrio was more abundant in the CM + NSAID overuse group than in the healthy controls. In a recent study that investigated the composition of gut microbiota in pediatric migraine patients, similar to our results, Gemmiger was more abundant in migraine patients [15]. Previously, Gemmiger was also suggested to have role in depression [16]. The genus Ruminococcus was less abundant in CM patients with MOH. Migraine is known to be associated with certain comorbidities such as depression, irritable bowel syndrome and inflammatory bowel disease. Ruminococcus depletion has been observed in major depressive disorder, irritable bowel syndrome and inflammatory bowel disease patients [17–19].
Blautia luti was another bacteria species that was more abundant in the CM patients with NSAID overuse headache and it was also positively correlated with HIF1-α levels. In previous studies, Blautia luti was significantly increased in inflammatory bowel disease patients compared to the control group [20]. Blautia luti was also reported to be higher in abundance in depression patients compared to healthy controls [21]. However, there are controversial results regarding Blautia luti species. Decreased incidence of Blautia luti was shown in gut microbiota of children with obesity and the decrease was more pronounced in the gut microbiota of obese children who also had insulin resistance suggesting that depletion of Blautia luti could be associated with metabolic inflammation [22].
Dorea longicatena was more abundant in CM patients with MOH compared to healthy non-headache sufferers in our study. Dorea longicatena abundance was reported to be associated with being overweight and obesity [23] and insulin resistance after gastric bypass [24]. Dorea longicatena also metabolizes sialic acid found at the terminal ends of mucins and release of sialic acid is associated with mucin degradation that increases gut permeability [25]. IL-6 levels were negatively correlated with Subdoligranulum in this study. Similarly, in a previous study, subdoligranulum was also shown to be negatively correlated with IL-6 levels in overweight and obese individuals [26].
Desulfovibrio (DSV) bacteria are sulfate-reducing and anaerobic bacteria that are associated with many diseases such as irritable bowel syndrome, inflammatory bowel diseases, Parkinson’s disease, bacteremia, autism, liver and chronic kidney diseases, autoimmune diseases and metabolic syndrome [27]. Desulfovibrio desulfuricans has been reported to have inflammatory effects through LPS [28–30]. Desulfovibrio fairfieldensis have outer membrane vesicles that are associated with increased inflammation and tight junction dysfunction in the intestinal barrier [31]. DSV abundance also has a role in impaired cognition and memory deficits [32]. DSV abundance was also shown to be correlated with anxiety and depression in ulcerative colitis patients [33]. Similarly in our study, in CM patients with MOH, the abundance of DSV bacteria was positively correlated with HAD anxiety scores.
In a previous study that investigated alterations in gut microbiota in pediatric migraine patients, Dialister was relatively abundant in female migraine patients compared to female healthy controls whereas, Desulfovibrio was more abundant in the migraine patients compared to healthy controls in the male group [34]. A positive correlation between the abundance of the genus Dialister and Ankylosing Spondylitis Disease Activity Score was shown in patients with spondyloarthritis and it was suggested as a marker of inflammation [35]. Desulfovibrio and Dialister were also more abundant in the CM patients with NSAID overuse headache compared to the healthy controls in our study.
Alterations in microbiota composition may contribute to the role of certain foods as migraine triggers. Probable mechanisms underlying the correlation between certain bacteria alterations and significantly related migraine food triggers are given in Table 1. Clostridia, Dialister, DSV, Gemmiger, Ethanoligenens harbinense, Dorea longicatena, Thermoclostridium stercorarium, and Eubacterium coprostanoligenes that are involved in tryptophan metabolism, sulfate reduction, histaminergic mechanisms, ethanol-type fermentation, polysaccharide degradation were shown to be associated with migraine food triggers. Clostridia bacteria was negatively correlated with chocolate/cacao, pickles, butter, chicken, pastrami/salami, red meat/minced meat, frozen meat, and chicken products. Chocolate/cacao, red meat and red meat products, milk and milk products, and poultry are rich in tryptophan [36–38]. Clostridia has been shown to produce indole acetic acid through tryptophan metabolism [34]. Tryptophan metabolism could be one of the mechanisms underlying the association between food triggers and microbiota. Tryptophan is a precursor for serotonin, a neurotransmitter involved in migraine pathophysiology and high brain serotonin levels were shown in migraine patients between the attacks [39]. The genus Dialister was positively correlated with frozen meat and chicken products, red meat/minced meat, aged cheese, and chocolate/cacao in our study. These migraine food triggers are rich in tryptophan. Dialister in the caecum microbiota of weaned piglets was shown to be significantly increased by dietary tryptophan [40]. The genus Dialister was also shown to be increased with 4-week interventions with a vegan diet and whole grain barley [41, 42]. DSV is a sulfate-reducing bacteria and hydrogen sulfide is a by-product of DSV which may be toxic to the host in high concentrations. Sulfate and sulfite are derived from dietary sources especially food preservatives [27]. Sulfites are suggested to provoke headache via the release of histamine [43, 44]. Gemmiger is a butyrate-producing bacteria and butyrate influences serotonin release from intestinal cells [45]. The abundance of Gemmiger was positively associated with ice cream. Milk products contain tryptophan [36–38]. Ultra-processed food consumption (> 5 servings per day) was also shown to be associated with an increase in the genus Gemmiger [46]. Ethanoligenens harbinense is capable of ethanol-type fermentation and converts sugars into cellular energy and produces ethanol and carbon dioxide (CO2) [47]. The abundance of thermoclostridium stercorarium was correlated with peanut and cream cheese. Thermoclostridium stercorarium is a thermophilic bacteria that degrades polysaccharides and produces acetate, ethanol, CO2, and hydrogen [48]. Ethanol-producing bacteria is associated with ethanol-mediated activation of the nuclear factor- κB signaling pathway which changes the gut barrier integrity and increases systemic endotoxemia [49]. Dorea longicatena is involved in glucose fermentation and produces hydrogen and CO2 [50]. Dorea longicatena also metabolizes sialic acid found in mucins and the release of sialic acid is associated with mucin degradation which increases gut permeability and thus results in mild systemic inflammation. Eubacterium coprostanoligenes is a cholesterol-reducing bacteria that converts cholesterol to coprostanol and ferments carbohydrates into acetic, formic, and succinic acids [51, 52]. Succinate was shown to be correlated with colonic inflammation [53]. Monosodium glutamate (MSG) is also accused of triggering migraine attacks and can be found in canned and frozen foods, ketchup, and sauces [54]. MSG consumption was shown to induce changes in microbiota and metabolic dysbiosis in a systematic review [55]. Onions contain sulfites and sulfites trigger headaches via histamine release [43, 44]. Migraine patients are more susceptible to histamine-induced headache compared to controls [56]. Histamine infusion was shown to provoke a delayed migraine attack in migraine patients [57]. Elevated plasma histamine levels were reported both during and between the attacks in migraine patients [57]. Peanuts, citrus fruits, pickled and fermented foods contain tyramine and excess dietary tyramine may interfere with gastrointestinal homeostasis and is suggested to be involved in intestinal inflammation. Dietary tyramine and histamine were shown to have synergistic cytotoxic effects on human intestinal cell culture [58]. Tyramine was also suggested to play a role in dietary migraine [59, 60].
Table 1.
The correlation between certain bacteria alterations and significantly related migraine food triggers in this study and the probable underlying mechanisms
| Altered bacteria | Migraine food triggers | Explanation | The probable mechanism |
|---|---|---|---|
| Clostridia (class) ↓ | Chocolate/cacao, pickles, butter, chicken, milk, pastrami, sausage, mushroom, red meat / minced meat, ketchup and frozen meat and chicken products |
Clostridia has been shown to produce indole acetic acid through tryptophan metabolism [38]. Chocolate/cacao, red meat and red meat products, milk and milk products and poultry are rich in tryptophan [36, 37]. A decrease in the class clostridia could result in excess tryptophan. |
Tryptophan metabolism and serotonergic mechanisms |
| Desulfovibrio (genus) ↑ | Peanut butter, grapefruit, soya sauce, clotted cream, custard, ice cream and excessive carbohydrate consumption in a single meal |
Desulfovibrio is a sulfate-reducing bacteria and sulfate and sulfite are derived from dietary sources especially food preservatives [27]. Sulfites trigger headache via histamine release. |
Histaminergic mechanisms |
| Gemmiger (genus) ↑ | Ice cream |
Gemmiger is a butyrate producing bacteria and butyrate has an influence on serotonin release from intestinal cells [45]. |
Tryptophan metabolism and serotonergic mechanisms |
| Dialister (genus) ↑ | Frozen meat and chicken products, hamburger, pizza, coffee cream and milk powder, ketchup, cream filled pastries, red meat/minced meat, pickles, aged cheese, chocolate/cacao, sweet sauces, soya sauce and vinegar |
Chocolate/cacao, red meat and red meat products, milk and milk products and poultry are rich in tryptophan [36–38]. Dietary tryptophan significantly increased the relative abundance of Dialister in the caecum microbiota of weaned piglets [40]. |
Tryptophan metabolism and serotonergic mechanisms |
| Ethanoligenens harbinense (species) ↑ | Chocolate/cacao, canned tuna fish, cream filled pastries, ready-to-eat canned foods and excessive eating during one meal | Ethanoligenens harbinense is capable of ethanol-type fermentation and converts sugars into cellular energy and produces ethanol and carbon dioxide [47]. | Ethanol-type fermentation |
| Eubacterium coprostanoligenes (species) ↑ | Peanut, pastries, simit (Turkish bagel), cream filled pastries, cream cheese, ready-to-use sauces (such as pesto sauce), ready-to-eat canned foods, onion/garlic, orange juice | Eubacterium coprostanoligenes ferments carbohydrates into acetic, formic and succinic acids [51, 52]. Succinate was shown to be correlated with colonic inflammation [53]. | Colonic inflammation and decreased gut barrier function and increased intestinal permeability |
| Dorea longicatena (species) ↑ | Yoghurt, soudjouk, salami, pastrami, bell pepper and beans |
Dorea longicatena is involved in glucose fermentation and produces hydrogen and carbon dioxide [50]. Dorea longicatena metabolizes sialic acid found in mucins and release of sialic acid is associated with mucin degradation which increases gut permeability [25]. |
Decreased gut barrier function and increased intestinal permeability |
| Thermoclostridium stercorarium (species) ↑ | Peanut and cream cheese | Thermoclostridium stercorarium is a thermophilic bacteria that degrades polysaccharides and produces acetate, ethanol, carbon dioxide and hydrogen [48]. | Ethanol mediated activation of nuclear factor- κB signaling pathway, decreased barrier integrity and increased intestinal permeability |
The tryptophan-kynurenine (Trp-Kyn) pathway was suggested to be one of the main pathways in the microbiota-gut-brain axis [61]. The gut bacterial strains have synthetic enzymes that may produce tryptophan metabolites and tryptophan is the precursor of serotonin [62, 63]. The germ-free mice were reported to have higher TRP plasma levels [64] and higher serotonin turnover [65]. The probiotic supplementations were shown to reduce 5-hydroxyindoleacetic acid and kynurenine in the frontal cortex [66]. Inflammation is associated with tryptophan metabolism [67]. Tryptophan is metabolized mainly by the indole pathway by the intestinal microbiota. Indican, a metabolite of indole, was found to be high in the urine of pediatric migraine patients [15]. The production of indican by intestinal bacteria increases when there is bacterial overgrowth and dysbiosis [15].
Mainly dietary patterns influence gut microbiota, however, other factors such as age, sex, lifestyle, exercise, genetics, geographic location, cultural traditions, antibacterial drugs, psychological situation, and stress also play a role in this dynamic ecosystem [68, 69]. The same factors may also influence migraine headache frequency in patients with migraine. The association between microbiota and migraine food triggers and serum inflammatory parameters could be influenced by these confounding factors.
The disruption in intestinal barrier integrity results in the leakage of LPS into the circulation and triggers a low-grade systemic inflammatory response. LPS triggers the release of HMGB1, a potent proinflammatory molecule of innate immunity that mediates trigeminal nociception [70, 71, 72]. LPS and HMGB1 act through toll-like receptor-4 (TLR-4) and amplify the immune response including IL-6 [70, 73, 74]. Proinflammatory cytokines such as IL-6 and IL-1β released upon LPS leak, are also nociceptive in the trigeminovascular system and play a role in the development of headache [3, 70, 75].
Increased systemic pro-inflammatory cytokine levels such as TNF-α and IL-6 have been shown in migraine patients [76, 77]. Increased production of interferon-gamma and granulocyte/ macrophage colony-stimulating factor was shown in dietary migraine patients after the induction of a migraine headache attack with the dietary trigger [78]. Although elevated systemic inflammatory markers were demonstrated in migraine patients, the underlying cause of the systemic inflammation is unknown. We recently showed increased serum LPS, LPS binding protein, HIF-1α, VE-cadherin, and IL-6 levels in chronic migraine patients with NSAID overuse headache [3] suggesting leaky gut and LPS escape into the circulation as a source of inflammation. In this study, we have shown correlations between altered microbiota and systemic inflammatory parameters further supporting the role of dysbiosis and consequent leaky gut in CM patients with NSAID headache. Moreover, a link between microbiota changes and migraine food triggers is revealed. Alterations in microbiota composition were also correlated with migraine food triggers shedding light on the mechanisms that may contribute to certain foods’ role as migraine triggers. The dysbiosis in gut microbiota was shown previously in migraine patients [79]. The intestinal dysbiosis was suggested to play a role in migraine by increased intestinal permeability and consequent inflammatory processes [80]. Therapeutic strategies targeting LPS and HMGB1 should be investigated in migraine patients.
Gut dysbiosis reduces the barrier integrity and increases gut permeability and dysregulates immune responses resulting in inflammation and oxidative stress [81]. LPS in the systemic circulation is evidence of intestinal hyperpermeability. Short-chain fatty acids are produced by gut bacteria and play an important role in the gut barrier integrity [82]. SCFAs have neuroprotective and anti-inflammatory effects on the central nervous system [83]. SCFAs exhibit anti-inflammatory effects by regulating the expression of proinflammatory cytokines such as tumor necrosis factor-α and IL-6 [84]. Dysbiosis was shown to impair the production of SCFAs [85]. Colon dysbiosis was shown to be associated with migraine-like pain via TNF-α up-regulation in the trigeminal nociceptive system in mice [86].
A ketogenic diet regulates intestinal microbiota and has anti-inflammatory effects [87] that may have positive effects on metabolic dysbiosis seen in chronic migraine with NSAID overuse headache. Moreover, ketosis was also shown to prevent neurogenic inflammation in mouse models [88]. Inulin supplementation was shown to increase total antioxidant capacity and decrease high sensitivity-CRP levels and oxidative stress index levels in migraine patients [89]. Zonulin, a tight junction protein, was also shown to be decreased after inulin supplementation but it was not significant compared to the control group [89]. The decrease in inflammatory parameters was suggested to be due to improved gut integrity and reduced LPS leakage into the bloodstream [89]. A low glycemic diet al.so was shown to modify inflammatory responses [90]. TNF-α receptors II and CRP levels were significantly decreased with a low glycemic diet [91]. High omega-3 with low omega-6 diet resulted in a higher headache improvement compared to low omega-6 diet in chronic migraine patients [92]. In a randomized controlled trial (RCT) in episodic migraine patients, omega-3 and nano-curcumin supplementation reduced the expression of TNF-α mRNA and serum TNF-α level [93]. The effects of elimination diets on migraine frequency were studied in two RCTs [94, 95]. In these studies, personalized migraine food triggers were eliminated and a beneficial effect on migraine headache frequency was shown in one of them [94]. Histaminergic mechanisms are also involved in migraine food triggers. Histamine-free diet was also shown to decrease headache attacks and analgesic consumption in chronic headache patients [96]. More studies are required to evaluate the effects of dietary interventions on microbiota, migraine food triggers, and inflammatory parameters in migraine.
There are some limitations of the study. Our sample size was small due to strict inclusion criteria for both CM patients with MOH and healthy controls. We only included female chronic migraine patients with NSAID overuse headache. We conducted the study in this specific group of patients since we have provided recent evidence of leaky gut and enhanced inflammation in female CM patients with NSAID overuse headache [3]. However, the inclusion of such a specific group of patients was also a strength of our study to define the correlations between microbiota and inflammatory markers and migraine food triggers. Another limitation of the study was that we had not studied an extensive inflammatory marker panel. We only evaluated inflammatory markers that were significantly different in CM patients with NSAID overuse headache in our previous study [3]. A more detailed inflammatory marker panel may reveal more specific mechanisms of gut dysbiosis. We have not assessed the microbiota profile of episodic migraine patients and compared the altered microbiota in episodic migraine and chronic migraine patients. This comparison remains to be established. The changes in microbiota and inflammatory markers after the preventive treatment of migraine remain to be evaluated in longitudinal studies. Both CM and MOH are more prevalent in women and NSAIDs are the most overused analgesics worldwide. Therefore, we only included female subjects and patients overusing NSAIDs in our study. However, the latter limits the generalizability of our results to all migraine patients. The microbiota changes should be investigated in patients using other analgesic medications such as triptans. The correlations revealed in our study do not imply a causal relationship, experimental studies are required to show causality. The strength of our study is that we evaluated the correlations between microbiota, inflammatory markers, and migraine food triggers for the first time in a specific group of female CM patients with NSAID overuse headache without using any migraine prophylactic treatments. We involved subjects without any other chronic disease and chronic use of other medications to exclude the influence of other drugs and diseases on gut microbiota.
Conclusion
In the gut microbiota of CM patients with MOH, we have demonstrated the presence of dysbiosis towards an inflammatory state, and inflammatory serum parameters such as LPS, HMGB1, IL-6, and HIF1-α levels were shown to be positively correlated with certain bacteria in the gut microbiota. The associations between migraine food triggers and altered gut bacteria in CM patients with MOH were significant for some bacteria, even though the exact mechanisms underlying these associations are not known. Few studies investigated the complex link between migraine and microbiota and this is the first study that evaluated the correlation between migraine food triggers, microbiota changes, and inflammatory parameters in chronic migraine with NSAID overuse headache. Further studies are required to enlighten the role of microbiota in migraine and migraine food triggers and to identify promising biomarkers and their correlations with clinical features that will enlighten new pathways for therapeutic targets. Metabolites of probable pathways underlying the link between microbiota and migraine food triggers, especially the tryptophan pathway, should be investigated in both serum and urine in further studies to verify our findings. The role of dietary interventions, prebiotics, and probiotics on these pathways remains to be investigated.
Acknowledgements
The study was supported by Gazi University ADEP project grant (TGA-2022-7857) and partially supported by TÜBA (Turkish Academy of Science).
Abbreviations
- CM
Chronic migraine
- MOH
Medication overuse headache
- NSAIDs
Non-steroidal anti-inflammatory drugs
- LPS
Lipopolysaccharide
- HMGB1
High mobility group box-1 protein
- CGRP
Calcitonin gene-related peptide
- HIF-1α
Hypoxia-inducible factor-1α
- IL-6
Interleukin 6
- ICHD-3
International Classification of Headache Disorders 3rd edition
- ELISA
Enzyme-linked immunosorbent assay
- WBC
White blood cell count
- ESR
Erythrocyte sedimentation rate
- CRP
C-reactive protein
- HADS-A
Hospital Anxiety and Depression Scale Anxiety subscale
- HADS-D
Hospital Anxiety and Depression Scale Depression subscale
- IBS
Irritable bowel syndrome
- PCR
Polymerase chain reaction
- logFC
Log fold change
- DSV
Desulfovibrio
- CO2
Carbon dioxide
- Trp
Tryptophan
- Kyn
Kynurenine
- SCFAs
Short-chain fatty acids
- RCT
Randomized controlled trial
Author contributions
DV and HB designed the study. DV, MCA, HGD, and HB collected the data. DV, MCA, PO, MY, US, and HB analyzed the data. DV and HB wrote the manuscript. All authors reviewed, contributed, and edited the final draft. All authors approved the final version.
Funding
The study was supported by Gazi University ADEP project grant (TGA-2022-7857) and partially supported by TÜBA (Turkish Academy of Science).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study was approved by the Clinical Research Ethics Committee of Gazi University Faculty of Medicine. Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
HB is an Associate Editor of the Journal of Headache and Pain. The rest of the authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lynch SV, Pedersen O (2016) The human intestinal microbiome in Health and Disease. N Engl J Med 375(24):2369–2379 [DOI] [PubMed] [Google Scholar]
- 2.Crawford J, Liu S, Tao F (2022) Gut microbiota and migraine. Neurobiol Pain 11:100090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vuralli D, Ceren Akgor M, Gok Dagidir H, Gulbahar O, Yalinay M, Bolay H, Lipopolysaccharide VE-cadherin (2024) HMGB1, and HIF-1α levels are elevated in the systemic circulation in chronic migraine patients with medication overuse headache: evidence of leaky gut and inflammation. J Headache Pain 25(1):23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandenbussche N, Laterza D, Lisicki M, Lloyd J, Lupi C, Tischler H et al (2018) Medication-overuse headache: a widely recognized entity amidst ongoing debate. J Headache Pain 19(1):50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ceren Akgor M, Vuralli D, Sucu DH, Gokce S, Tasdelen B, Gultekin F et al (2023) Distinct Food Triggers for Migraine, Medication Overuse Headache and irritable bowel syndrome. J Clin Med. ;12(20) [DOI] [PMC free article] [PubMed]
- 6.Blake MR, Raker JM, Whelan K (2016) Validity and reliability of the Bristol Stool Form Scale in healthy adults and patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 44(7):693–703 [DOI] [PubMed] [Google Scholar]
- 7.Schmulson MJ, Drossman DA (2017) What is New in Rome IV. J Neurogastroenterol Motil 23(2):151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vich Vila A, Collij V, Sanna S, Sinha T, Imhann F, Bourgonje AR et al (2020) Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat Commun 11(1):362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edogawa S, Peters SA, Jenkins GD, Gurunathan SV, Sundt WJ, Johnson S et al (2018) Sex differences in NSAID-induced perturbation of human intestinal barrier function and microbiota. Faseb j 32(12):fj201800560R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prizment AE, Staley C, Onyeaghala GC, Vivek S, Thyagarajan B, Straka RJ et al (2020) Randomised clinical study: oral aspirin 325 mg daily vs placebo alters the gut microbial composition and bacterial taxa associated with colorectal cancer risk. Aliment Pharmacol Ther 52(6):976–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogers MAM, Aronoff DM (2016) The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect 22(2):178.e1-.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lázár B, László SB, Hutka B, Tóth AS, Mohammadzadeh A, Berekméri E et al (2021) A comprehensive time course and correlation analysis of indomethacin-induced inflammation, bile acid alterations and dysbiosis in the rat small intestine. Biochem Pharmacol 190:114590 [DOI] [PubMed] [Google Scholar]
- 13.Liang X, Bittinger K, Li X, Abernethy DR, Bushman FD, FitzGerald GA (2015) Bidirectional interactions between indomethacin and the murine intestinal microbiota. Elife 4:e08973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maseda D, Zackular JP, Trindade B, Kirk L, Roxas JL, Rogers LM et al (2019) Nonsteroidal anti-inflammatory drugs alter the Microbiota and exacerbate Clostridium difficile Colitis while dysregulating the inflammatory response. mBio. ;10(1) [DOI] [PMC free article] [PubMed]
- 15.Papetti L, Del Chierico F, Frattale I, Toto F, Scanu M, Mortera SL et al (2024) Pediatric migraine is characterized by traits of ecological and metabolic dysbiosis and inflammation. J Headache Pain 25(1):171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Wang H, Zhang H, Chen X, Zhang Y, Wu J et al (2022) Toward a deeper understanding of gut microbiome in Depression: the Promise of Clinical Applicability. Adv Sci (Weinh) 9(35):e2203707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maes M, Vasupanrajit A, Jirakran K, Klomkliew P, Chanchaem P, Tunvirachaisakul C et al (2022) Exploration of the gut microbiome in Thai patients with major depressive disorder uncovered a specific bacterial profile with depletion of the < em > Ruminococcus genus as a putative biomarker. medRxiv. :2022.11.06.22282014 [DOI] [PMC free article] [PubMed]
- 18.Chen H, Ou R, Tang N, Su W, Yang R, Yu X et al (2023) Alternation of the gut microbiota in irritable bowel syndrome: an integrated analysis based on multicenter amplicon sequencing data. J Translational Med 21(1):117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Reau AJ, Suen G (2018) The Ruminococci: key symbionts of the gut ecosystem. J Microbiol 56(3):199–208 [DOI] [PubMed] [Google Scholar]
- 20.Abdelbary MMH, Hatting M, Bott A, Dahlhausen A, Keller D, Trautwein C et al (2022) The oral-gut axis: salivary and fecal microbiome dysbiosis in patients with inflammatory bowel disease. Front Cell Infect Microbiol 12:1010853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park S, Li C, Wu X, Zhang T (2023) Gut microbiota alterations and their functional differences in Depression according to Enterotypes in Asian individuals. Int J Mol Sci. ;24(17) [DOI] [PMC free article] [PubMed]
- 22.Benítez-Páez A, Del Gómez EM, López-Almela I, Moya-Pérez Á, Codoñer-Franch P, Sanz Y (2020) Depletion of Blautia Species in the microbiota of obese children relates to intestinal inflammation and metabolic phenotype worsening. mSystems. ;5(2) [DOI] [PMC free article] [PubMed]
- 23.Companys J, Gosalbes MJ, Pla-Pagà L, Calderón-Pérez L, Llauradó E, Pedret A et al (2021) Gut microbiota Profile and its association with clinical variables and dietary intake in Overweight/Obese and lean subjects: a cross-sectional study. Nutrients 13(6):2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prudêncio APA, Fonseca DC, Machado NM, Alves JTM, Sala P, Fernandes GR et al (2023) Red Meat Intake, Indole-3-Acetate, and Dorea longicatena together affect insulin resistance after gastric bypass. Nutrients. ;15(5) [DOI] [PMC free article] [PubMed]
- 25.Crost EH, Tailford LE, Le Gall G, Fons M, Henrissat B, Juge N (2013) Utilisation of Mucin glycans by the human gut Symbiont Ruminococcus gnavus is strain-dependent. PLoS ONE 8(10):e76341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Hul M, Le Roy T, Prifti E, Dao MC, Paquot A, Zucker JD et al (2020) From correlation to causality: the case of Subdoligranulum. Gut Microbes 12(1):1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh SB, Carroll-Portillo A, Lin HC (2023) Desulfovibrio Gut: Enemy within? Microorganisms. ;11(7) [DOI] [PMC free article] [PubMed]
- 28.Weglarz L, Dzierzewicz Z, Skop B, Orchel A, Parfiniewicz B, Wiśniowska B et al (2003) Desulfovibrio desulfuricans lipopolysaccharides induce endothelial cell IL-6 and IL-8 secretion and E-selectin and VCAM-1 expression. Cell Mol Biol Lett 8(4):991–1003 [PubMed] [Google Scholar]
- 29.Kapral M, Węglarz L, Parfiniewicz B, Lodowska J, Jaworska-Kik M (2010) Quantitative evaluation of transcriptional activation of NF-κB p65 and p50 subunits and IκBα encoding genes in colon cancer cells by Desulfovibrio desulfuricans endotoxin. Folia Microbiol (Praha) 55(6):657–661 [DOI] [PubMed] [Google Scholar]
- 30.Dzierżewicz Z, Szczerba J, Lodowska J, Wolny D, Gruchlik A, Orchel A et al (2010) The role of Desulfovibrio desulfuricans lipopolysaccharides in modulation of periodontal inflammation through stimulation of human gingival fibroblasts. Arch Oral Biol 55(7):515–522 [DOI] [PubMed] [Google Scholar]
- 31.Nie Y, Xie X-Q, Zhou L, Guan Q, Ren Y, Mao Y et al (2022) Desulfovibrio fairfieldensis-derived outer membrane vesicles damage epithelial barrier and induce inflammation and Pyroptosis in macrophages. Cells 12:89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritz NL, Burnett BJ, Setty P, Reinhart KM, Wilson MR, Alcock J et al (2016) Sulfate-reducing bacteria impairs working memory in mice. Physiol Behav 157:281–287 [DOI] [PubMed] [Google Scholar]
- 33.Humbel F, Rieder JH, Franc Y, Juillerat P, Scharl M, Misselwitz B et al (2020) Association of alterations in intestinal microbiota with impaired psychological function in patients with inflammatory bowel diseases in Remission. Clin Gastroenterol Hepatol 18(9):2019–29e11 [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Xi K, Zhang L, Han M, Wang Q, Liu X (2024) Tryptophan metabolites and gut microbiota play an important role in pediatric migraine diagnosis. J Headache Pain 25(1):2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tito RY, Cypers H, Joossens M, Varkas G, Van Praet L, Glorieus E et al (2017) Brief report: Dialister as a microbial marker of Disease Activity in Spondyloarthritis. Arthritis Rheumatol 69(1):114–121 [DOI] [PubMed] [Google Scholar]
- 36.Górska-Warsewicz H, Laskowski W, Kulykovets O, Kudlińska-Chylak A, Czeczotko M, Rejman K (2018) Food Products as sources of protein and amino acids-the case of Poland. Nutrients. ;10(12) [DOI] [PMC free article] [PubMed]
- 37.Soh N, Walter G (2011) Tryptophan and depression: can diet alone be the answer? Acta Neuropsychiatrica 23:3–11 [Google Scholar]
- 38.Gao J, Xu K, Liu H, Liu G, Bai M, Peng C et al (2018) Impact of the gut microbiota on intestinal immunity mediated by Tryptophan Metabolism. Front Cell Infect Microbiol 8:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deen M, Hansen HD, Hougaard A, Nørgaard M, Eiberg H, Lehel S et al (2018) High brain serotonin levels in migraine between attacks: a 5-HT4 receptor binding PET study. NeuroImage: Clin 18:97–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rao Z, Li J, Shi B, Zeng Y, Liu Y, Sun Z et al (2021) Dietary tryptophan levels Impact Growth Performance and Intestinal Microbial Ecology in Weaned piglets via Tryptophan metabolites and intestinal antimicrobial peptides. Animals 11(3):817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohnert E, Kreutz C, Binder N, Hannibal L, Gorkiewicz G, Müller A et al (2021) Changes in gut microbiota after a four-week intervention with Vegan vs. Meat-Rich diets in healthy participants: a Randomized Controlled Trial. Microorganisms. ;9(4) [DOI] [PMC free article] [PubMed]
- 42.Martínez I, Lattimer JM, Hubach KL, Case JA, Yang J, Weber CG et al (2013) Gut microbiome composition is linked to whole grain-induced immunological improvements. Isme j 7(2):269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva M, Gama J, Pinto N, Pivi G, Brancal H, Carvalho L et al (2019) Sulfite concentration and the occurrence of headache in young adults: a prospective study. Eur J Clin Nutr 73(9):1316–1322 [DOI] [PubMed] [Google Scholar]
- 44.Dahl R, Henriksen JM, Harving H (1986) Red wine asthma: a controlled challenge study. J Allergy Clin Immunol 78(6):1126–1129 [DOI] [PubMed] [Google Scholar]
- 45.Reigstad CS, Salmonson CE, Rainey JF III, Szurszewski JH, Linden DR, Sonnenburg JL et al (2015) Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J 29(4):1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cuevas-Sierra A, Milagro FI, Aranaz P, Martínez JA, Riezu-Boj JI (2021) Gut microbiota differences according to Ultra-processed Food Consumption in a Spanish Population. Nutrients 13:8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruane J, Sonnino A, Agostini A (2010) Bioenergy and the potential contribution of agricultural biotechnologies in developing countries. Biomass Bioenergy 34(10):1427–1439 [Google Scholar]
- 48.Poehlein A, Zverlov VV, Daniel R, Schwarz WH, Liebl W (2013) Complete genome sequence of Clostridium stercorarium subsp. stercorarium strain DSM 8532, a thermophilic degrader of Plant Cell Wall fibers. Genome Announc 1(2):e0007313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F (2024) Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Intern Emerg Med 19(2):275–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taras D, Simmering R, Collins MD, Lawson PA, Blaut M (2002) Reclassification of Eubacterium formicigenerans Holdeman and Moore 1974 as Dorea formicigenerans gen. nov., comb. nov., and description of Dorea longicatena sp. nov., isolated from human faeces. Int J Syst Evol Microbiol 52(Pt 2):423–428 [DOI] [PubMed] [Google Scholar]
- 51.Juste C, Gérard P (2021) Cholesterol-to-Coprostanol Conversion by the gut microbiota: what we know, suspect, and ignore. Microorganisms 9(9):1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freier TA, Beitz D, Li L, Hartman PA (1994) Characterization of Eubacterium coprostanoligenes sp. nov., a cholesterol-reducing anaerobe. Int J Syst Bacteriol 44:137–142 [DOI] [PubMed] [Google Scholar]
- 53.Connors J, Dawe N, Van Limbergen J (2018) The role of Succinate in the regulation of intestinal inflammation. Nutrients. ;11(1) [DOI] [PMC free article] [PubMed]
- 54.Spekker E, Nagy-Grócz G (2023) All roads lead to the gut: the importance of the Microbiota and Diet in Migraine. Neurol Int 15(3):1174–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahangari H, Bahramian B, Khezerlou A, Tavassoli M, Kiani-Salmi N, Tarhriz V et al (2024) Association between Monosodium glutamate consumption with changes in gut microbiota and related metabolic dysbiosis—A systematic review. Food Sci Nutr 12(8):5285–5295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.VON STORCH TJC. RELATION OF EXPERIMENTAL HISTAMINE HEADACHE TO MIGRAINE AND NONMIGRAINE HEADACHE (1940) Archives Neurol Psychiatry 44(2):316–322 [Google Scholar]
- 57.Lassen LH, Thomsen LL, Olesen J (1995) Histamine induces migraine via the H1-receptor. Support for the NO hypothesis of migraine. NeuroReport 6(11):1475–1479 [DOI] [PubMed] [Google Scholar]
- 58.Del Rio B, Redruello B, Linares DM, Ladero V, Fernandez M, Martin MC et al (2017) The dietary biogenic amines tyramine and histamine show synergistic toxicity towards intestinal cells in culture. Food Chem 218:249–255 [DOI] [PubMed] [Google Scholar]
- 59.Smith I, Kellow AH, Mullen PE, Hanington E (1971) Dietary migraine and tyramine metabolism. Nature 230(5291):246–248 [DOI] [PubMed] [Google Scholar]
- 60.Kohlenberg RJ (1982) Tyramine sensitivity in dietary migraine: a critical review. Headache 22(1):30–34 [DOI] [PubMed] [Google Scholar]
- 61.Arzani M, Jahromi SR, Ghorbani Z, Vahabizad F, Martelletti P, Ghaemi A et al (2020) Gut-brain Axis and migraine headache: a comprehensive review. J Headache Pain 21(1):15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maqsood R, Stone TW (2016) The gut-brain Axis, BDNF, NMDA and CNS disorders. Neurochem Res 41(11):2819–2835 [DOI] [PubMed] [Google Scholar]
- 63.Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F et al (2013) The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol Psychiatry 18(6):666–673 [DOI] [PubMed] [Google Scholar]
- 64.Yano Jessica M, Yu K, Donaldson Gregory P, Shastri Gauri G, Ann P, Ma L et al (2015) Indigenous Bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell 161(2):264–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lukić I, Getselter D, Koren O, Elliott E (2019) Role of Tryptophan in Microbiota-Induced Depressive-Like Behavior: evidence from Tryptophan Depletion Study. Front Behav Neurosci. ;13 [DOI] [PMC free article] [PubMed]
- 66.Saulnier DM, Ringel Y, Heyman MB, Foster JA, Bercik P, Shulman RJ et al (2013) The intestinal microbiome, probiotics and prebiotics in neurogastroenterology. Gut Microbes 4(1):17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanaka M, Tóth F, Polyák H, Szabó Á, Mándi Y, Vécsei L (2021) Immune influencers in Action: metabolites and enzymes of the Tryptophan-Kynurenine metabolic pathway. Biomedicines. ;9(7) [DOI] [PMC free article] [PubMed]
- 68.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M et al (2012) Human gut microbiome viewed across age and geography. Nature 486(7402):222–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang J, Wu Je, Li Y, Zhang Ye, Cho WC, Ju X et al (2021) Gut bacteria formation and influencing factors. FEMS Microbiol Ecol. ;97(4) [DOI] [PubMed]
- 70.Dağıdır HG, Topa E, Vuralli D, Bolay H (2023) Medication overuse headache is associated with elevated lipopolysaccharide binding protein and pro-inflammatory molecules in the bloodstream. J Headache Pain 24(1):150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bolay H, Karadas Ö, Oztürk B, Sonkaya R, Tasdelen B, Bulut TDS et al (2021) HMGB1, NLRP3, IL-6 and ACE2 levels are elevated in COVID-19 with headache: a window to the infection-related headache mechanism. J Headache Pain 22(1):94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vuralli D, Dağidir HG, Topa EA, Belen HB. (2023) Leaky gut and inflammatory biomarkers in a medication overuse headache model in male rats. Turk J Med Sci 54(1):33–41. [DOI] [PMC free article] [PubMed]
- 73.Yang H, Wang H, Andersson U (2020) Targeting inflammation driven by HMGB1. Front Immunol 11:484 [DOI] [PMC free article] [PubMed]
- 74.Rathinam VAK, Zhao Y, Shao F (2019) Innate immunity to intracellular LPS. Nat Immunol 20(5):527–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Socała K, Doboszewska U, Szopa A, Serefko A, Włodarczyk M, Zielińska A et al (2021) The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol Res 172:105840 [DOI] [PubMed] [Google Scholar]
- 76.Thuraiaiyah J, Erritzøe-Jervild M, Al-Khazali HM, Schytz HW, Younis S (2022) The role of cytokines in migraine: a systematic review. Cephalalgia 42(14):1565–1588 [DOI] [PubMed] [Google Scholar]
- 77.Vuralli D, Bolay H (2023) Migraine chronification is associated with higher body mass index and elevated serum interleukin-6 levels. Annals Med Res 30(9):1149–1152 [Google Scholar]
- 78.Martelletti P, Stirparo G, Rinaldi C, Frati L, Giacovazzo M (1993) Disruption of the Immunopeptidergic Network in Dietary Migraine. Headache: J Head Face Pain 33(10):524–527 [DOI] [PubMed] [Google Scholar]
- 79.Yong D, Lee H, Min HG, Kim K, Oh HS, Chu MK (2023) Altered gut microbiota in individuals with episodic and chronic migraine. Sci Rep 13(1):626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gazerani P, Papetti L, Dalkara T, Cook CL, Webster C, Bai J (2024) The brain, the eating plate, and the gut microbiome: partners in Migraine Pathogenesis. Nutrients 16(14):2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yoo JY, Groer M, Dutra SVO, Sarkar A, McSkimming DI (2020) Gut microbiota and Immune System interactions. Microorganisms. ;8(10) [DOI] [PMC free article] [PubMed]
- 82.Wang L, Christophersen CT, Sorich MJ, Gerber JP, Angley MT, Conlon MA (2012) Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig Dis Sci 57(8):2096–2102 [DOI] [PubMed] [Google Scholar]
- 83.Noble EE, Hsu TM, Kanoski SE (2017) Gut to Brain Dysbiosis: mechanisms linking western Diet Consumption, the Microbiome, and cognitive impairment. Front Behav Neurosci 11:9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vinolo MAR, Rodrigues HG, Nachbar RT, Curi R (2011) Regulation of inflammation by short chain fatty acids. Nutrients 3(10):858–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ikeda T, Nishida A, Yamano M, Kimura I (2022) Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases. Pharmacol Ther 239:108273 [DOI] [PubMed] [Google Scholar]
- 86.Tang Y, Liu S, Shu H, Yanagisawa L, Tao F (2020) Gut microbiota dysbiosis enhances Migraine-Like Pain Via TNFα Upregulation. Mol Neurobiol 57(1):461–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Lorenzo C, Ballerini G, Barbanti P, Bernardini A, D’Arrigo G, Egeo G et al (2021) Applications of ketogenic diets in patients with headache: clinical recommendations. Nutrients 13(7):2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramachandran R (2018) Neurogenic inflammation and its role in migraine. Semin Immunopathol 40(3):301–314 [DOI] [PubMed] [Google Scholar]
- 89.Vajdi M, Khorvash F, Askari G (2024) The effects of inulin supplementation on clinical indices, oxidative stress and inflammatory biomarkers in women with migraine: a double-blind, placebo-controlled, randomized trial. J Funct Foods 121:106422 [Google Scholar]
- 90.Razeghi Jahromi S, Ghorbani Z, Martelletti P, Lampl C, Togha M (2019) On behalf of the School of Advanced Studies of the European Headache F. Association of diet and headache. J Headache Pain 20(1):106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hartman TJ, Albert PS, Zhang Z, Bagshaw D, Kris-Etherton PM, Ulbrecht J et al (2010) Consumption of a legume-enriched, low-glycemic index diet is associated with biomarkers of insulin resistance and inflammation among men at risk for colorectal cancer. J Nutr 140(1):60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramsden CE, Zamora D, Makriyannis A, Wood JT, Mann JD, Faurot KR et al (2015) Diet-induced changes in n-3- and n-6-derived endocannabinoids and reductions in headache pain and psychological distress. J Pain 16(8):707–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Abdolahi M, Tafakhori A, Togha M, Okhovat AA, Siassi F, Eshraghian MR et al (2017) The synergistic effects of ω-3 fatty acids and nano-curcumin supplementation on tumor necrosis factor (TNF)-α gene expression and serum level in migraine patients. Immunogenetics 69(6):371–378 [DOI] [PubMed] [Google Scholar]
- 94.Aydinlar EI, Dikmen PY, Tiftikci A, Saruc M, Aksu M, Gunsoy HG et al (2013) IgG-based elimination diet in migraine plus irritable bowel syndrome. Headache 53(3):514–525 [DOI] [PubMed] [Google Scholar]
- 95.Mitchell N, Hewitt CE, Jayakody S, Islam M, Adamson J, Watt I et al (2011) Randomised controlled trial of food elimination diet based on IgG antibodies for the prevention of migraine like headaches. Nutr J 10:85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wantke F, Götz M, Jarisch R (1993) Histamine-free diet: treatment of choice for histamine-induced food intolerance and supporting treatment for chronic headaches. Clin Exp Allergy 23(12):982–985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.