Abstract
Background: Pott’s Puffy Tumor (PPT) in young-age patients is a rare clinical entity characterized by osteomyelitis of the frontal bone with a subperiosteal abscess collection. Previous reviews primarily consist of small, retrospective case series and anecdotal reports. This study aims to present the largest, most up-to-date systematic review of essential clinical findings, diagnostic modalities, microbiologic considerations, and treatment approaches for managing PPT in pediatric and adolescent populations. Methods: PubMed, Scopus, and Web of Science databases were systematically screened until 3 January 2024. The protocol of this investigation was registered on PROSPERO in January 2024, and the systematic review was performed according to the PRISMA statement. The study included 184 patients from 109 articles and an additional case from the authors’ institution. Results: PPT commonly stems from untreated rhinosinusitis, respectively, acute pansinusitis, frontal acute rhinosinusitis and chronic rhinosinusitis, and direct head trauma. Infections typically involve a polymicrobial anaerobe-predominant microbiome. Computed tomography and magnetic resonance imaging are routinely used for presurgical assessment and posttreatment surveillance. Intracranial complications were significantly associated with the type of surgical treatment (p value < 0.0001). Conclusions: PPT is a significant and relatively morbid disease often under-recognized and misdiagnosed due to its variable clinical presentation. Management includes both antimicrobial therapy and surgical intervention, emphasizing the importance of an interdisciplinary approach.
Keywords: acute sinusitis, frontal sinusitis, Pott’s puffy tumor, intracranial complication, pediatric sinusitis, acute sinus infection, orbital complications of sinusitis, bacterial sinusitis, frontal bone osteomyelitis, subperiosteal abscess
1. Introduction
Pott’s Puffy Tumor (PPT) is defined as one or more subperiosteal abscesses of the frontal bone associated with underlying osteitis and osteomyelitis [1]. It was first described by Sir Percivall Pott, who related it to earlier forehead trauma in 1768 and to earlier frontal sinusitis in 1775 [2]. It appears as localized swelling of the forehead, with inflammatory signs, tenderness, and swelling of the overlying skin. Associated typical symptoms are headache, periorbital swelling, rhinorrhea, fever, vomiting, and lethargy [1]. PPT is often an indicator of intracranial complications [1]. The infection has the potential to extend into the intracranial cavity through bony erosions, pre-existing pathways, or septic thrombosis via the Haversian canals. This propagation can lead to severe intracranial complications such as meningitis, epidural abscess, subdural empyema, intracerebral abscess, and dural sinus thrombophlebitis [1]. In such cases, computed tomography (CT) is employed for treatment planning, while magnetic resonance imaging (MRI) plays a crucial role in the detection of intracranial complications [2].
PPT was once considered a rare occurrence in the post-antibiotics era, with the majority of reported cases involving adolescents and young adults. This increased susceptibility in the younger age group can be attributed to two main factors: the heightened vascularity of their diploic system and the relative increase in blood supply to the still-developing frontal sinuses [3]. Despite advancements in early detection, targeted antibiotic treatments, and surgical interventions, the morbidity and mortality associated with intracranial complications of sinusitis have significantly decreased. However, intracranial complications still manifest, necessitating swift diagnosis and multidisciplinary treatment to prevent long-term neurological sequelae and fatalities [4]. This study aims to present an up-to-date systematic review of the etiological, clinical, surgical, as well as microbiological findings related to PPT in young patients. Additionally, it aims to foster discussion on the treatment course, which remains a topic of controversy in the literature.
2. Materials and Methods
2.1. Protocol Registration
The protocol of this systematic review was registered on PROSPERO, an international database of prospectively registered systematic reviews in health and social care (Center for Reviews and Dissemination, University of York, York, UK), in January 2024 (registry number CRD42024498741).
2.2. Search Strategy
A systematic literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations [5]. The electronic databases Scopus, Pubmed, and Web of Science were searched from database inception to 3 January 2024. A combination of MeSH terms (“sinusitis” [MeSH Terms], “intracranial complication” [MeSH Terms]) and free-text words (“Pott’s puffy tum*”, “acute sinusitis”, “intracranial complication”) were utilized to search. The reference lists of all the included articles were thoroughly screened to find other relevant articles. References were exported to Zotero bibliography manager (v6.0.10, Center for History and New Media, George Mason University, Fairfax, VA, USA). After duplicates removal, two reviewers (A.D. and T.M.) independently screened all titles and abstracts and then evaluated the full texts of the eligible articles based on the inclusion criteria. Any disagreement between the reviewers involved in the literature search was resolved through discussion with all authors to reach a consensus.
2.3. Selection Criteria
Studies were deemed eligible when the following inclusion criteria were met: (i) confirmed diagnosis of PTT; (ii) patients ≤ 18 years old. Exclusion criteria were as follows: (i) lack of relevant data; (ii) non-original studies (i.e., reviews, recommendations, editorials, conference papers, clinical challenges, and book chapters); (iii) animal model studies; (iv) non-English studies.
2.4. Data Extraction and Quality Assessment
Extracted data were collected in an electronic database including first author, year of publication, sample size, number of patients included, age of the patients, gender, etiology, imaging tests used, types of complication, types of surgery, medical treatment and culture, and outcome. The quality of the studies eligible for inclusion was categorized as Poor, Fair, and Good, in agreement with the National Institute of Health’s quality assessment tool for Observational Cohorts and Cross-Sectional Studies (https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools, accessed on 3 January 2024) [6]. Two reviewers (A.D. and T.M.) independently evaluated the papers, and any disagreement was resolved by discussion.
2.5. Statistical Analysis
Qualitative and quantitative analyses of the data (variance of each variable and descriptive statistics measures of central slope and variability) were performed. Cases were distinguished as intracranial complication or no intracranial complication. Using Chi-square, a statistical association analysis was run between the aforementioned cohorts and the categorical demographic, intraoperative, and postoperative variables. Post hoc analysis for multiple comparisons of categorical data was applied with Bonferroni’s correction. This test assessed each hypothesis at a significance level of α/n, where α is the overall significance level and n is the number of hypotheses being tested. Two-tailed p < 0.05 was considered statistically significant. SPSS version 20 for Windows (IBM Corp, Armonk, NY, USA) was used for all statistical analyses.
3. Results
3.1. Case Report
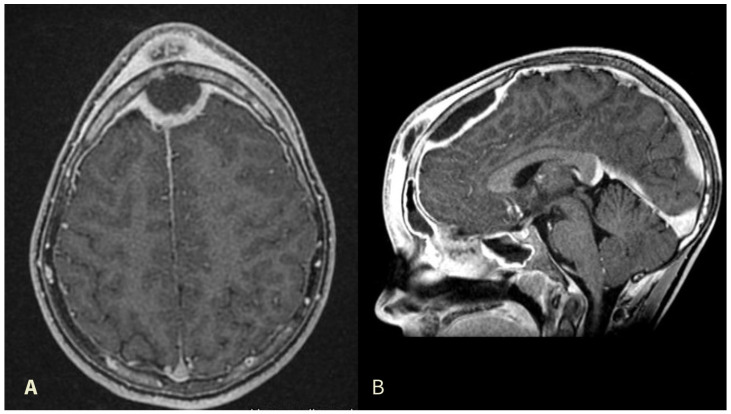
A 12-year-old, Italian male with no comorbidities complained of nasal congestion and headache for one month, who was initially treated by his general practitioner with oral amoxicillin (1 g every 12 h for a week). He presented thereafter to the emergency department clinic with progressively increasing frontal swelling and eyelid edema. He underwent cerebral and head and neck contrast-enhanced CT scan and MRI that revealed a complicated frontal sinusitis with bone erosion of the posterior wall of the frontal sinus and an epidural collection with peripheral rim enhancement at the frontal lobe, suggestive of an epidural abscess with compression of sagittal sinus (Figure 1). He was transferred to our hospital where he received intravenous (IV) vancomycin 40 mg/kg/day, metronidazole 20 mg/kg/day, and ceftazidime 2 g/day. Ophthalmological evaluation excluded orbital complications.
Figure 1.
Preoperative contrast-enhanced MRI image revealing subcutaneous forehead swelling and a frontal epidural abscess in axial (A) and sagittal (B) views.
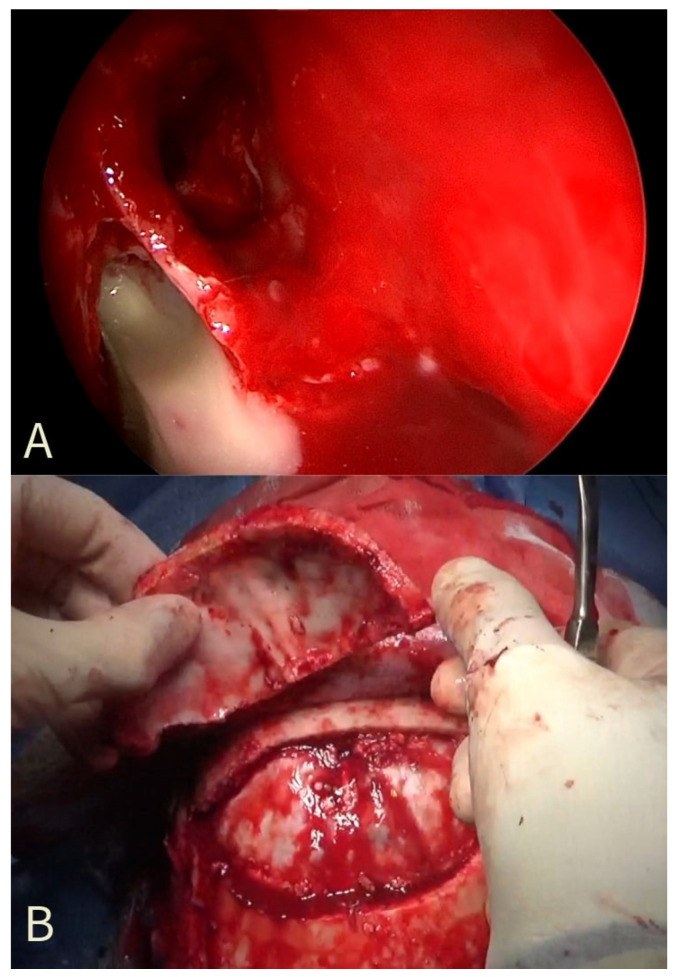
The patient underwent Endoscopic Sinus Surgery (ESS) with a right total ethmoidectomy, right frontal sinusotomy (Draf IIa) (Figure 2A), and maxillary antrostomy. Concomitant craniotomy with trephination and drainage of the brain abscess was performed for the epidural empyema and subperiosteal abscess (Figure 2B). The craniotomy operculum was rebuilt with a custom titanium plate because of the osteomyelitis which involved the frontal bone. Microbiological analysis of the purulent material demonstrated growth of Streptococcus intermedius. The patient remained hospitalized in our department for 6 weeks, and he received IV antibiotics throughout the entire hospital stay. The follow-up MRI performed 6 weeks after surgery revealed the resolution of the subperiosteal and epidural abscesses. He was discharged with saline irrigative 3 times/day with no clinical symptoms and no radiological signs. He continued oral antibiotics for another 4 weeks. Informed consent was obtained for publication purposes.
Figure 2.
Intraoperative photographs. (A): an endoscopic view of the discharge of pus from the frontal sinus; (B): the Pott Puffy Tumor during subperiosteal dissection with inner plate erosions from the epidural granulation tissue.
3.2. Search Results and Quality Assessment
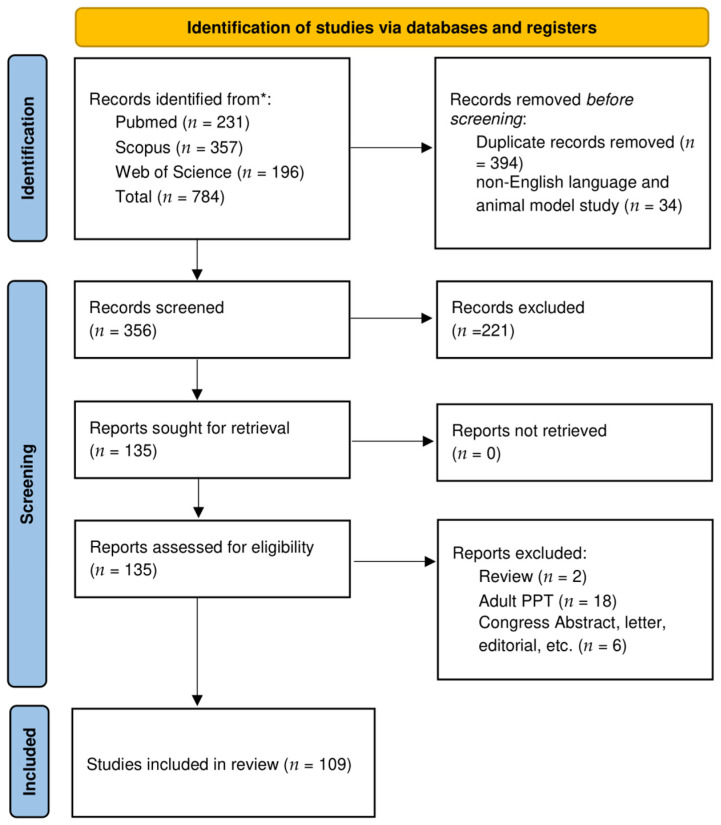
After duplicates removal and exclusion of 221 records due to coherence with the inclusion/exclusion criteria, 135 articles relevant to the topic were examined. No records were unavailable for retrieving. Finally, 109 were included in the review [2,3,4,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112]. A detailed flowchart of the search process is shown in Figure 3.
Figure 3.
PRISMA diagram representing the Electronic Database Search and inclusion/exclusion process of the review. Legend * date of last search: 3 January 2024.
In accordance with the National Institute of Health’s quality assessment tool for Observational Cohorts and Cross-Sectional Studies [6], 20 studies (18.3%) were deemed of Good quality, 69 (63.4%) Fair, and 20 studies (18.3%) as Poor, due to the lack of reporting clinical data (Table S1).
3.3. Included Studies’ Characteristics
Among the 110 studies included in the qualitative analysis, 99 were case papers [2,4,7,8,9,10,11,12,13,14,16,17,18,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,41,42,43,44,45,46,47,48,49,50,51,52,53,54,56,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,77,79,80,81,82,83,84,85,86,87,88,89,90,92,93,94,95,96,97,98,99,100,101,103,104,105,107,108,109,110,111,112], while only 11 studies were case series [3,15,19,40,55,57,76,78,91,102,106]. These studies were published between 1949 and 2023 (see Table 1).
Table 1.
Studies included in the systematic review.
| Author | Year | Age (Years) | Sex | Etiology | Imaging | Complication | Surgery | Treatment | Culture |
|---|---|---|---|---|---|---|---|---|---|
| Adnani et al. [7] | 2023 | 8 | F | ARS | ce-CT | PSC | NA | NA | NA |
| Allfather et al. [8] | 2017 | 7 | F | FARS | ce-CT | EDA | ESS, CRA | MER, VAN | Staphylococcus, Fusobacterium |
| AlMoosa et al. [9] | 2016 | 9 | F | T | ce-CT, ce-MRI | None | ESS, CRA | empirical: CLI, CEF; AC: VOR | Aspergillus Fumigatus |
| Amstrup et al. [10] | 2023 | 9 | M | ARS | ce-CT, ce-MRI | SSST, EDA | None | CEF, MET | S. anginosus |
| Arnold et al. [11] | 2009 | 10 | M | T | CT | EDA | EXD, CRE | CEF, VAN, MET | S. intermedius |
| Arora et al. [12] | 2014 | 14 | M | FARS | ce-CT | None | EXD | CEF, CLI | NA |
| Avcu et al. [13] | 2015 | 12 | M | ARS | ce-CT, ce-MRI | PSC | EXD, ESS | CEF, VAN, MET | NA |
| Bağdatoğlu et al. [14] | 2001 | 18 | M | ARS | ce-CT | EDA, SDA | CRA | empirical: IM gentamycin AC: VAN, RIF, MET |
STE |
| Bambakidis et al. [15] | 2001 | 11 | M | ARS | CT, only 2 pts MRI | SDA, OA | CRA | BSATB |
Fusobacterium, S. pneumoniae |
| 11 | M | ARS | EDA, SDA | CRA | BSATB | S. milleri | |||
| 16 | M | ARS | SDA | EXD, CRA | BSATB | Klebsiella sp., Peptostreptococcus | |||
| 18 | M | ARS | EDA | EXD, CRA | BSATB | S. microaerophilic | |||
| 15 | M | ARS | EDA | CRA | BSATB | Peptostreptococcus | |||
| 14 | M | ARS | EDA | CRA | BSATB | S. viridans | |||
| 11 | F | ARS | EDA, SDA, BA | CRA | BSATB | S. pyogenes | |||
| Behbahani et al. [3] | 2020 | 7 | M | ARS | ce-CT or ce-MRI | EDA | ESS, CRA | CEF | S. pyogenes, Corynebacterium, Pseudodiphtheriticum |
| 13 | M | ARS | SDA | ESS, CRA | CEF, MET | S. anginosus | |||
| 14 | M | ARS | EDA | ESS, CRA | CEF, MET | S. intermedius | |||
| 6 | M | ARS | EDA | ESS, CRA | CEF | S. pyogenes | |||
| 12 | M | ARS | EDA, SDA | ESS, CRA | CEF, CLI | S. anginosus | |||
| 14 | M | ARS | CER | ESS, CRA | CEF, MET | S. anginosus, S. intermedius, Staph. epidermidis | |||
| 13 | M | ARS | EDA | ESS, CRA | CEF, MET | S. intermedius | |||
| 10 | F | ARS | EDA, CER | ESS, CRA | CEF, MET | S. intermedius | |||
| 11 | M | ARS | EDA | ESS | CEF, MET |
Staph epidermidis, Propionibacterium, Avidum Diphtheroids |
|||
| 5 | M | ARS | CER | ESS | CEF, MET | S. pyogenes | |||
| 5 | F | ARS | EDA, MEN | ESS | CEF, MET | S. intermedius | |||
| 12 | M | ARS | SDA | ESS, CRA | CEF, MET | S, intermedius, Staph. aureus, Staph. epidermidis | |||
| Belharti et al. [16] | 2023 | 15 | M | FARS | ce-CT | EDA | NA | NA | NA |
| Bhalla et al. [17] | 2016 | 5 | M | ARS | ce-CT | subperiosteal OA, EDA | ESS | CEF, CLI, MET | NA |
| Blackman et al. [18] | 2005 | 9 | M | ARS | ce-CT | OC | EXD | CEF, VAN, MET | Staph. saccharolyticus |
| Blumfield et al. [19] | 2011 | 9–16 | 8M 1F |
ARS | 8 ce-CT, 1 ce-MRI | 2 SSST 6 EDA 1 SDA |
3: EXD, ESS, CRA 2: ESS, 2: EXD, CRE 1: none, 1: transferred to another hospital |
BSATB |
S. milleri, S. pneumonia, S. group F, Staph. aureus |
| Butskiy et al. [20] | 2017 | 10 | M | ARS | ce-CT | None | EXD | CEF, CLI | S. intermedius |
| Cannon et al. [21] | 2017 | 5 | M | ARS | ce-MRI | None | EXD | NA | S. anginosus |
| Cheng et al. [22] | 2009 | 32 months | M | SEP | ce-CT | SSST | EXD | CLI | Staph. aureus |
| Costa et al. [23] | 2020 | 13 | M | ARS | ce-CT | BA, OA | EXD, ESS, CRA | CEF, VAN, MET | NA |
| Davidson et al. [24] | 2006 | 14 | F | ARS | ce-CT | EDA, fistula | CRE | NA | NA |
| Dayan et al. [25] | 2020 | 4 | F | ARS | ce-CT | None | EXD | BSATB | NA |
| Durur-Subasi et al. [26] | 2008 | 14 | F | ARS | ce-CT | EDA, BA | EXD | NA | NA |
| Faridi et al. [27] | 2022 | 4 | F | ARS | ce-CT, ce-MRI | CER | EXD | CEF, VAN | NA |
| Feder et al. [28] | 1987 | 6 | F | ARS | X-ray, ce-CT | None | ESS | empirical: cefuroxime and gentamicin; AC: CLI |
Peptostreptococcus, Bacteroides melaninogenicus |
| 12 | F | ARS | ce-CT | EDA | ESS, CRA | empirical: CLI AC: penicillin |
Alpha Hemolytic S. | ||
| Forgie et al. [29] | 2008 | 16 | M | ARS | NA | None | EXD | BSATB | NA |
| Fu B. [30] | 2010 | 16 | M | FARS | ce-CT | None | EXD | BSATB | NA |
| Fullerton et al. [31] | 2016 | 11 | M | FARS | ce-MRI | EDA | EXD, ESS, CRA | CEF | S. pyogenes |
| Gildener-Leapman et al. [32] | 2012 | 5 | M | FARS | ce-CT | EDA, SDA, CER | EXD, ESS, CRA | CEF, VAN, MET | S. intermedius |
| Gozgec et al. [33] | 2022 | 15 | M | ARS | ce-CT | OC | EXD | BSATB | NA |
| Guillén et al. [34] | 2001 | 12 | F | ARS | ce-CT | OC, EDA, SDA | CRA | CEF, VAN, MET | NA |
| Gupta et al. [35] | 2004 | 3 | M | FARS | ce-CT, ce-MRI | EDA | ESS, CRA | CEF, MET, cloxacillin | STE |
| Haider et al. [36] | 2012 | 14 | M | ARS | ce-CT, ce-MRI | MEN, SDA | EXD, ESS, CRA | MER, MET | F. necrophorum |
| Hassan et al. [37] | 2020 | 15 | M | T | ce-CT, ce-MRI | EDA | EXD, ESS | empirical: VAN, AMPS AC: CEF, MET |
F. nucleatum |
| Hayek et al. [38] | 2007 | 9 | M | ARS | ce-CT | PSC | EXD, ESS | AMPS, then CEF | NA |
| Heale et al. [39] | 2015 | 5 | F | NA | ce-CT | PSC, EDA | EXD, CRA | CEF, ET | S. anginosus |
| Hicks et al. [40] | 2011 | NA | NA | ARS | ce-CT, MRI | EDA, OC | ESS | BSATB | S. milleri |
| NA | NA | ARS | ce-CT, MRI | EDA. SDA, SSST | EXD, ESS | BSATB | S. milleri | ||
| NA | NA | ARS | ce-CT | SDA | ESS, CRA | BSATB | S. milleri | ||
| NA | NA | ARS | ce-CT, MRI | EDA, SDA | ESS, CRA | BSATB | Cutibacterium acnes | ||
| NA | NA | ARS | ce-CT, MRI | SDA | EXD, ESS | BSATB | NA | ||
| NA | NA | ARS | ce-CT | BA | CRA | BSATB | S. milleri | ||
| Hitti et al. [41] | 2010 | 6 | F | ARS | ce-CT | EDA | ESS, CRE | empirical: CEF, VAN, MET AC: AMPS |
S. pyogenes |
| Holder et al. [42] | 1991 | 17 | M | ARS | X-ray, ce-CT | BA | ESS | empirical: ceftazidime and MET AC: ceftazidime, MET, penicillin, chloramphenicol |
Non-hemolytic S. |
| Hore et al. [43] | 2000 | 12 | F | ARS | CT | None | EXD, ESS | CEF, MET | S. milleri |
| Huijssoon et al. [44] | 2003 | 8 | M | ARS | ce-CT | OC | EXD | BSATB | S. milleri |
| Ikoma et al. [45] | 2020 | 12 | M | FARS | ce-CT, ce-MRI | EDA, PNC | EXD, CRA | MER, VAN then CEF, MET | S. constellatus |
| Is et al. [46] | 2007 | 11 | F | NA | ce-CT | EDA | EXD | AMP, CEF, MET | Peptostreptococcus, Veillonella, E. Coli |
| Jafri et al. [47] | 2015 | 11 | F | ARS | ce-CT | EDA | EXD, CRA | AMO | S. intermedius |
| Joo et al. [48] | 2019 | 7 | F | FARS | ce-CT | OC | EXD | AMPS | NA |
| Kalkan et al. [49] | 2017 | 14 | M | ARS | ce-CT, ce-MRI | EDA | ESS, CRA | BSATB | STE |
| Karadaghy et al. [50] | 2022 | 23 months | M | ARS | ce-CT | PSC | EXD, ESS | AMPS, then LEV | S. intermedius, Granulicatella adiacens |
| Karaman et al. [51] | 2008 | 7 | F | ARS | ce-CT | None | EXD, ESS | AMPS | S. milleri |
| Ketenci et al. [52] | 2011 | 12 | F | ARS | ce-CT | SDA, BA | EXD, ESS, CRA | AMPS, VAN, MET | Peptostreptococcus |
| 13 | M | ARS | ce-CT | SDA, BA | EXD, ESS, CRA | CEF, AMPS, MET | S. pyogenes | ||
| Khan et al. [53] | 2006 | 10 | F | MAS | ce-CT | EDA | EXD, CRA, Cortical Mastoidectomy | CEF, CLI | STE |
| Kim et al. [54] | 2012 | 18 | M | ARS | ce-MRI | EDA, SDA | EXD, CRA | AMO, then VAN, ceftazidime, MET | STE |
| Klivitsky et al. [55] | 2023 | 11 | M | ARS | ce-CT, ce-MRI | PSC, SSST, CST | EXT, CRA | CEF and MET | S. pneumoniae |
| 9 | F | ARS | CT, MRI | None | EXD | CEF and CLI | Negative | ||
| 12 | F | ARS | ce-CT, MRI | EDA, SSST | EXD, ESS, CRA | CEF and MET | S. constellatus | ||
| 14 | M | ARS | CT, ce-CT, MRI | EDA, BA | ESS | CEF and MET | S. intermedius | ||
| 15 | M | ARS | CT, ce-CT, MRI | EDA | EXD, ESS, CRA | CEF and MET | STE | ||
| 13 | M | ARS | CT, ce-CT, MRI | EDA | ESS | CEF and MET | Prevotella | ||
| 17 | F | ARS | CT | None | ESS | CEF and CLI | S. pneumoniae | ||
| 14 | M | ARS | CT, MRI | EDA | EXD, ESS, CRA | CEF and MET | STE | ||
| 10 | F | ARS | CT | EDA | EXD | CEF and MET | STE | ||
| 9 | M | ARS | CT | PSC | EXXD | cefuroxime and MET | Staph. aureus | ||
| Kombogiorgas et al. [2] | 2006 | 11 | M | ARS | ce-MRI | EDA | EXD, ESS, CRA | CEF, MET | STE |
| Kuhar et al. [56] | 2023 | 3 | F | ARS | ce-CT, ce-MRI | OC, EDA, SSST | EXD, ESS, CRA | CEF | S. intermedius |
| Kühn et al. [57] | 2022 | 6 | M | FARS | ce-CT, ce-MRI | SDA, MEN, CER | ESS, CRA | cefotaxime, CLI | S. intermedius |
| 17 | M | ARS | ce-CT, ce-MRI | EDA | ESS | CEF | NA | ||
| 9 | F | ARS | ce-CT, ce-MRI | EDA | ESS, CRA | cefotaxime, CLI | S. intermedius | ||
| Lang et al. [58] | 2001 | 14 | F | FARS | ce-CT | SDA, SSST | EXD, CRA | cephalosporin, MET | STE |
| 15 | F | ARS | ce-CT | SDA, SSST | EXD, CRA | cephalosporin, MET | S. pyogenes | ||
| 12 | F | ARS | ce-CT | EDA, SDA | EXD, ESS, CRA | cephalosporin, MET | H. influenzae | ||
| Lauria et al. [59] | 2014 | 14 | M | FARS | ce-CT | None | EXD, ESS | VAN, MET, ceftazidime, then AMPS | S. constellatus |
| Ling et al. [60] | 2021 | 9 | M | ARS | ce-RM | EDA | NA | BSATB | NA |
| Linton et al. [61] | 2019 | 16 | M | T | ce-CT | OA | EXD, ESS | AMOC MET | STE |
| Liu et al. [62] | 2015 | 10 | F | ARS | ce-CT | EDA | ESS, CRA | MER, then cephalexin | Alpha Hemolytic S. |
| Maheshwar et al. [63] | 2001 | 14 | M | T | ce-CT | None | ESS | AMOC, then flucloxacillin, fusidic acid, MET, RIF | S. intermedius |
| Marzuillo et al. [64] | 2017 | 9 | F | ARS | ce-CT | Fistula | NA | AMOC | NA |
| McGee et al. [65] | 2022 | NA | M | ARS | ce-CT | None | EXD, ESS | CEF, MET | Staph. aureus |
| Morley et al. [66] | 2009 | 7 | M | ARS | ce-CT, ce-MRI | EDA | CRA | benzylpenicillin, flucloxacillin then CLI | NA |
| Moser et al. [67] | 2009 | 14 | F | ARS | ce-CT | EDA | EXD, ESS | CEF | STE |
| Moses et al. [68] | 2018 | 15 | M | FB | ce-CT | SDA | ESS, CRA | empirical: TAZO AC: MER, MET |
F. necrophorum |
| Nastovska et al. [69] | 2017 | 15 | M | FARS | ce-MRI | None | ESS, CRA | benzylpenicillin | S. anginosus |
| Nicoli et al. [70] | 2014 | 13 | M | FARS | ce-CT, ce-MRI | EDA | ESS, CRA | BSATB | S. intermedius |
| Nourkami-Tutdibi et al. [71] | 2020 | 6 | M | FARS | ce-CT, ce-MRI | EDA | CRA | sultamicillin, then cefotaxime, CLI | S. intermedius |
| Olmaz et al. [72] | 2019 | 12 | M | ARS | CT- ce-MRI | EDA | CRA | CEF, VAN, MET | NA |
| Onesimo et al. [73] | 2011 | 8 | F | ARS | ce-MRI | EDA | NA | NA | S. intermedius |
| Özkaya Parlakay et al. [74] | 2012 | 13 | M | ARS | ce-CT | None | None | cefotaxime and VAN | NA |
| Öztürk et al. [75] | 2020 | 15 | M | FARS | ce-CT | None | NA | CEF and teicoplanin | NA |
| Palabiyik et al. [76] | 2016 | 18 | M | ARS | ce-CT, ce-MRI | PSC | ESS | AMPS, MET, or CEF | 1 E. Coli, 1 S. Epidermidis, 6 STE |
| 17 | M | ARS | ce-CT, ce-MRI | None | ESS | ||||
| 9 | M | ARS | ce-CT, ce-MRI | EDA | EXD, CRA | ||||
| 11 | F | ARS | ce-CT, ce-MRI | EDA | ESS, CRA | ||||
| 7 | M | T | ce-CT, ce-MRI | None | ESS | ||||
| 17 | M | ARS | ce-CT, ce-MRI | None | EXD | ||||
| Palacios-García et al. [77] | 2019 | 15 | M | ARS | ce-CT | None | EXD, ESS | empirical: CLI AC: LEV |
S. intermedius |
| Parida et al. [78] | 2012 | 10 | M | ARS | ce-CT | PSC | EXD, ESS | BSATB | Staph. aureus |
| 15 | F | CRS | ce-CT | None | EXD, ESS | BSATB | STE | ||
| 9 | F | ARS | ce-CT | None | ESS | BSATB | STE | ||
| 11 | M | T | ce-CT | None | EXD | BSATB | Pseudomonas aeruginosa | ||
| 13 | M | ARS | ce-CT | None | ESS | BSATB | STE | ||
| Patel et al. [79] | 2021 | 13 | M | SD | ce-CT | MEN | ESS | CEF, VAN, MET | NA |
| Patel et al. [80] | 2011 | 11 | M | ARS | ce-CT, ce-MRI | fistula, MEN | EXD, CRE | empirical: AMOC AC: MER, amphotericin B, then fluconazole |
Candida parapsilosis |
| Pender [81] | 1990 | 13 | M | ARS | CT | None | ESS | oxacillin and ampicillin | Staph. aureus, S-viridans |
| 17 | M | I | CT | EDA and OA | ESS | cefuroxime and MET | Coagulase neg Staph. | ||
| Podolsky-Gondim et al. [82] | 2018 | 14 | M | ARS | ce-CT, ce-MRI | EDA | EXD, CRA | CEF, oxacillin, MET | Peptostreptococcus |
| Przybysz et al. [83] | 2018 | 8 | F | ARS | CT | None | NA | NA | NA |
| Queen et al. [84] | 2001 | 14 | M | ARS | CT, MRI | SDA | EXD, ESS, CRE | BSATB | NA |
| Reddan et al. [85] | 2018 | 6 | M | ARS | CT | None | ESS | NA | NA |
| Rogers [86] | 1949 | 11 | M | NA | NA | NA | EXD, CRE | NA | B. Alkaligenes faecalis |
| 16 | M | NA | NA | NA | EXD, CRE | NA | STE | ||
| Rogo et al. [87] | 2013 | 5 | F | ARS | CT | EDA | None | MER and VAN | NA |
| Russ et al. [88] | 2022 | 11 | M | I | ce-CT | EDA | ESS, CRA | CEF, VAN, MET | S. anginosus |
| Sabatiello et al. [89] | 2010 | 15 | M | ARS | ce-CT | SDA, BA | NA | cefotaxime, CLI | Peptostreptococcus, Fusobacterium |
| Sade et al. [90] | 2016 | 12 | M | FARS | ce-MRI | BA | NA | NA | NA |
| Salomão et al. [91] | 2014 | 11 | F | CRS | ce-CT | EDA | EXD, CRA | BSATB | STE |
| 9 | M | CRS | ce-CT | EDA | None | BSATB | S. aureus | ||
| 14 | M | FARS | ce-CT | EDA, SDA | EXD, CRA | BSATB | S. pyogenes | ||
| 12 | M | T | ce-CT | EDA | EXD, CRA | BSATB | S. pyogenes | ||
| 12 | M | CRS | ce-CT | EDA | EXD, CRA | BSATB | STE | ||
| 13 | M | T | ce-CT | EDA, fistula | EXD, CRA | BSATB | STE | ||
| Sharma et al. [4] | 2017 | 8 | F | FARS | CT, ce-MRI | EDA | ESS, CRA | BSATB | S. intermedius |
| Shehu et al. [92] | 2008 | 10 | F | ARS | CT | SDA | EXD, CRA | BSATB | NA |
| Shemesh et al. [93] | 2015 | 11 | M | NA | ce-CT | None | NA | NA | NA |
| Sheth et al. [94] | 2018 | 15 | M | FB | ce-CT | SDA | ESS, CRA | empirical: CEF, VAN, TAZO AC: MER, MET |
F. necrophorum |
| 17 | M | FARS | ce-CT, ce-MRI | SDA, SSST | CRA | CEF, CLI, VAN | F. necrophorum | ||
| 14 | M | ARS | ce-CT | EDA, PNC, CER | CRA | CEF, VAN, MET | F. necrophorum, S. constellatum | ||
| Silva et al. [95] | 2022 | 16 | F | ARS | CT, ce-MRI | SDA, BA | CRA | CEF, MET | None |
| Stark et al. [96] | 2016 | 14 | M | FARS | ce-CT | None | EXD, ESS | empirical: flucloxacillin AC: CLI, RIF |
Staph. aureus |
| Stoddard et al. [97] | 2019 | 13 | M | FARS | ce-MRI | EDA | CRA | CEF, VAN, MET | Staph. aureus |
| Strony et al. [98] | 2007 | 4 | M | ARS | ce-CT | EDA | EXD, CRA | CEF, VAN, MET | S. viridans |
| Sugiyama et al. [99] | 2016 | 17 | M | ARS | ce-CT, ce-MRI | EDA, PNC | EXD, ESS | CEF, MET | Peptostreptococcus, Collinsella aerofaciens, Staph. lugdunensis |
| Suwan et al. [100] | 2012 | 8 | F | ARS | CT | EDA | EXD, ESS, CRE | VAN, cefotaxime, MET, then AMPS | S. constellatus, F. necrophorum |
| Tibesar et al. [101] | 2021 | 15 | M | ARS | ce-CT | PSC | EXD, ESS | CEF, VAN, MET | S. intermedius |
| Tsai et al. [102] | 2010 | 14 | M | ARS | ce-CT | SSST | EXD, ESS, CRA | BSATB | F. nucleatum |
| 13 | M | T | ce-CT | SDA, BA | CRA | BSATB | Veillonella sp., Peptostreptococcus micros, F. nucleatum, S. viridans, Eikenella corrodens | ||
| 15 | M | CRS | ce-CT | SDA | EXD, ESS, CRA | BSATB | Peptostreptococcus micros; Coagulase neg Staph. | ||
| 12 | M | CRS | ce-CT | SDA | ESS, CRA | BSATB | Coagulase neg Staph., Prevotella sp. | ||
| 9 | F | AP | ce-CT | SDA | CRE, fistula repair | BSATB | P. aeruginosa | ||
| 13 | M | ARS | ce-CT | EDA, PNC, SDA | EXD, ESS, CRA | CEF, VAN, then penicillin G | S. constellatus, Beta-hemolytic non-group A streptococci | ||
| Tudor et al. [103] | 1981 | 16 | M | T | CT | EDA | EXD, CRA | nafcillin, gentamicin, then AMO | NA |
| Urík et al. [104] | 2015 | 6 | M | ARS | ce-CT | EDA, MEN | EXD | CEF, CLI, oral ketoconazole | STE |
| Vadiee et al. [105] | 2023 | 12 | F | IB | CT, ce-MRI | EDA | CRA | ceftazidime, VAN, MET | Polybacterial |
| van der Poel et al. [106] | 2016 | 7 | F | T | ce-CT, ce-MRI | SDA, SSST | ESS, CRE | penicillin | S. intermedius |
| 10 | F | ARS | ce-CT, ce-MRI | None | ESS | AMOC | Commensale flora | ||
| 12 | F | ARS | ce-CT, ce-MRI | EDA | ESS, CRE | AMOC, then penicillin, MET | S. constellatus | ||
| 13 | M | ARS | ce-CT, ce-MRI | None | None | AMOC | Streptococcus | ||
| 17 | M | ARS | ce-CT, ce-MRI | EDA | ESS, CRE | penicillin, MET | S. intermedius | ||
| Vanderveken et al. [107] | 2012 | 5 | M | ARS | ce-CT | EDA | EXD, CRA | AMOC | STE |
| Vaphiades et al. [108] | 2023 | 10 | M | ARS | ce-MRI | EDA, SSST, MEN | EXD, CRA | BSATB | NA |
| Verma et al. [109] | 2021 | 14 | M | ARS | ce-CT | EDA | EXD, ESS, CRA | CEF | Streptococcus |
| Verma et al. [110] | 2018 | 15 | M | ARS | ce-CT | None | ESS | CEF, VAN, MET | NA |
| 12 | M | AFRS | CT | EDA | ESS | itraconazole | NA | ||
| 12 | F | ARS | CT | None | ESS | BSATB | STE | ||
| Weinberg et al. [111] | 2005 | 11 | F | ARS | ce-CT | None | ESS | cefotaxime, VAN, MET | Group C Beta Streptococcus |
| Wu et al. [112] | 2009 | 12 | F | AP | ce-CT | None | EXD | ceftazidime and gentamicin | P. aeruginosa |
| Our case | 2024 | 12 | M | ARS | ce-CT, ce-MRI | EDA | ESS, CRA | ceftazidime, VAN, MET | S. intermedius |
Abbreviations: AC = after culture; AFRS = allergic fungal rhinosinusitis; AMO = amoxicillin; AMOC = amoxicillin/clavulanate; AMP = ampicillin; AMPS = ampicillin/sulbactam; AP = acupuncture; ARS = acute pansinusitis; BA = brain abscess; BSATB = IV broad-spectrum antibiotics; ce-CT = contrast-enhanced computed tomography; ce-MRI = contrast-enhanced magnetic resonance imaging; CEF = ceftriaxon; CER = cerebritis; CLI = clindamycin; CRA = craniotomy; CRE = craniectomy; CRS = chronic rhinosinusitis; CST = cavernous sinus thrombosis; EDA = epidural abscess; ESS = Endoscopic Sinus Surgery; EXD = external drainage; F = female; FARS = frontal acute rhinosinusitis; FB = foreign body; FR = full recovery; I = influenza; IB = insect bite; LEV = levofloxacin; M = male; MAS = mastoiditis; MEN = meningitis; MER = meropenem; MET = metronidazole; NA = not available; OA = orbital abscess; OC = orbital cellulitis; PNC = pneumocephalus; PSC = preseptal cellulitis; RIF = rifampicin; S = streptococcus; SEP = septicemia; SD = scuba diving; SDA = subdural abscess; SSST = superior sagittal sinus thrombosis; Staph = staphylococcus; STE = sterile; T = trauma; TAZO = piperacillin/tazobactam; VAN = vancomycin; VOR = voriconazole.
3.4. Included Patients’ Characteristics
The total number of patients was 184, where 124 (67.4%) were male and 54 female (29.3%). The mean age of the patients was 12.06 ± 3.29 years (range 1.92–18 years) (refer to Table 2).
Table 2.
Demographic data and etiologies of studies included in the systematic review.
| Demographic Data | N (Range) | % |
|---|---|---|
| Patients | 184 | |
| Sex | 124M/54F/6NA | |
| Age | 12.06 ± 3.29 (1.92–18) |
|
| Year of publication | 1949–2023 | |
| Etiology | ||
| Acute rhinosinusitis | 128 | 69.56 |
| Frontal acute rhinosinusitis | 22 | 11.95 |
| Chronic rhinosinusitis | 6 | 3.27 |
| Trauma | 12 | 6.52 |
| Allergic fungal rhinosinusitis | 1 | 0.55 |
| Acupuncture | 2 | 1.08 |
| Insect bite | 1 | 0.55 |
| Influenza | 2 | 1.08 |
| Mastoiditis | 1 | 0.55 |
| Foreign body | 2 | 1.08 |
| Scuba diving | 1 | 0.55 |
| Septicemia | 1 | 0.55 |
| Not available | 5 | 2.71 |
Abbreviations: M = male; F = female; NA = not available.
The most common etiology was acute rhinosinusitis (69.35%) described in terms of pansinusitis, while frontal acute rhinosinusitis was counted in 22 patients (11.89%). Chronic rhinosinusitis as etiology was clearly stated for six patients (3.26%). Head trauma was found to be the most common cause excluding sinusitis (12; 6.52%). Other etiologies are reported in Table 2.
3.5. Imaging Assessment
In the current analysis, the majority of authors relied on ce-CT as the primary imaging modality for diagnosing PTT and its complications, accounting for 86 cases (46.73%). Additionally, a combination of ce-CT and ce-MRI was utilized in 17.93% of cases (refer to Table 1).
3.6. Pathogens
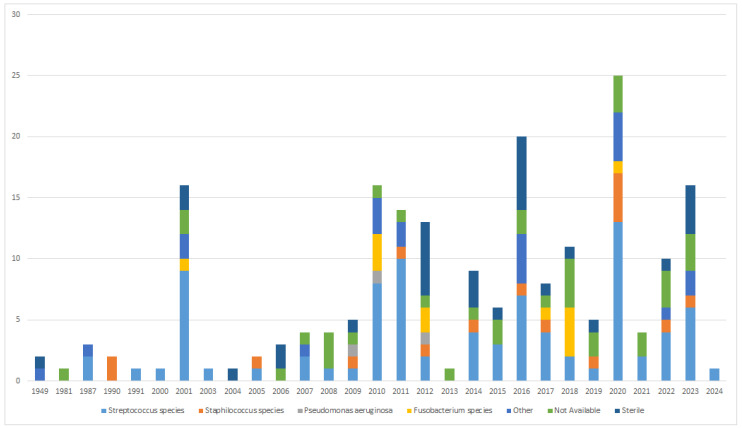
Microbiological analysis frequently resulted in multiple growth, with streptococci being the most prevalent individual pathogens (85, 40.66%). Among streptococci, Streptococcus intermedius was the most frequently cultured (11.96%), while staphylococci accounted for 9.38% of cases. Sterile cultures were prevalent (14.35%) (refer to Table 3). As illustrated in Figure 4, there has been no substantial variation in pathogens over time, with the most frequent being those from the Streptococcus species.
Table 3.
Pathogens cultured from Pott’s Puffy Tumor.
| Pathogens Cultured | N | % |
|---|---|---|
| Streptococcus intermedius | 25 | 11.96 |
| Streptococcus pyogenes | 10 | 4.78 |
| Peptostreptococcus | 10 | 4.78 |
| Streptococcus milleri | 9 | 4.30 |
| Streptococcus anginosus | 8 | 3.82 |
| Streptococcus constellatus | 7 | 3.35 |
| Streptococcus viridans | 4 | 1.91 |
| Streptococcus pneumoniae | 4 | 1.91 |
| Other Streptococcus species | 8 | 3.82 |
| Staphylococcus aureus | 10 | 4.78 |
| Staphylococcus Epidermidis | 4 | 1.91 |
| Coagulase neg Staph. | 3 | 1.43 |
| Other Staphylococcus species | 3 | 1.43 |
| Pseudomonas aeruginosa | 3 | 1.43 |
| Escherichia Coli | 2 | 0.95 |
| Fusobacterium necrophorum | 6 | 2.87 |
| Other Fusobacterium species | 6 | 2.87 |
| Other | 21 | 10.05 |
| Not available | 36 | 17.22 |
| Sterile | 30 | 14.35 |
Figure 4.
Changes in the various pathogens identified over time.
3.7. Medical Treatment
All patients received antibiotic therapy, with the duration of treatment ranging from 10 days to 6 months, averaging 6.8 weeks. The predominant antibiotics utilized were ceftriaxone (20.73%) and metronidazole (20.73%), either individually or in combination (see Table 4).
Table 4.
Medical treatment of Pott’s Puffy Tumor.
| Medical Treatment | N | % |
|---|---|---|
| Ceftriaxone | 68 | 20.73 |
| Metronidazole | 68 | 20.73 |
| Vancomycin | 32 | 9.75 |
| Clindamycin | 20 | 6.09 |
| Ampicillin-sulbactam | 12 | 3.65 |
| Amoxicillin-clavulanate | 9 | 2.74 |
| Meropenem | 7 | 2.13 |
| Cefotaxime | 7 | 2.13 |
| Ceftazidime | 6 | 1.82 |
| Gentamycin | 4 | 1.22 |
| Rifampicin | 3 | 0.91 |
| Flucloxacillin | 3 | 0.91 |
| Cefuroxime | 3 | 0.91 |
| Piperacillin-tazobactam | 2 | 0.61 |
| Levofloxacin | 2 | 0.61 |
| Benzylpenicillin | 2 | 0.61 |
| Oxacillin | 2 | 0.61 |
| Amoxicillin | 2 | 0.61 |
| Voriconazole | 1 | 0.30 |
| Ampicillin | 1 | 0.30 |
| Beta-lattamic ndd | 7 | 2.13 |
| Cephalosporin ndd | 4 | 1.22 |
| Other | 8 | 2.44 |
| IV broad-spectrum antibiotics | 43 | 13.11 |
| Not available | 12 | 3.65 |
3.8. Intracranial Extension
Based on our examination, 131 (71.19%) out of the patients considered in this analysis experienced intracranial complications. Among them, 38 patients were ≤10 years of age (29.00%), 81 patients (61.83%) were between 11 and 18 years old, and 6 patients (4.58%) did not have their age reported.
The predominant intracranial complication observed was epidural abscess (42.59%), succeeded by subdural empyema (17.12%), thrombosis of the superior sagittal sinus (6.48%), and brain abscess (5.55%). Multiple intracranial complications were identified in 38 patients (29.00%). Age, sex, type of imaging assessment, pathogen type, and culture species were not statistically correlated with the development of intracranial complications (p value > 0.05). Intracranial complications were significantly associated with the type of surgical treatment (p value < 0.0001). Bonferroni correction for multiple comparisons showed the preference for a combined surgical approach in patients with intracranial complications than in those without intracranial involvement, compared to external (p = 0.022) and endoscopic interventions (p = 0.0002). The details of the intracranial complications from the studies included are provided in Table 5. No other relevant associations were found between intracranial complications and the clinical variables considered (Table 6).
Table 5.
Complications associated with Pott’s Puffy Tumor.
| Intracranial Complications | N | % |
|---|---|---|
| Epidural abscess | 92 | 42.59 |
| Subdural abscess | 37 | 17.12 |
| Brain abscess | 12 | 5.55 |
| Superior sagittal sinus thrombosis | 14 | 6.48 |
| Pneumocephalus | 3 | 1.39 |
| Fistula | 4 | 1.85 |
| Cerebritis | 7 | 3.24 |
| Meningitis | 7 | 3.24 |
| Cavernous sinus thrombosis | 1 | 0.46 |
| Not available | 2 | 0.92 |
| None | 37 | 17.13 |
Table 6.
Correlation of demographics and clinical details findings between PPT patients with and without intracranial complications.
| Intracranial Complications (131 Cases) |
No Intracranial Complications (53 Cases) |
p Value | ||
|---|---|---|---|---|
| Age | Median (IQR) | 12.00 (9–14) | 12.00 (10–14) | 0.865 |
| Sex | Male | 34 (66.7%) | 88 (70.4%) | 0.626 |
| Female | 17 (33.3%) | 37 (29.6%) | ||
| Imaging | TC | 39 (78.0%) | 64 (57.1%) | 0.039 |
| MRI | 2 (4.0%) | 9 (8.0%) | ||
| TC + MRI | 9 (18.0%) | 39 (34.8%) | ||
| Surgery type | External | 15 (34.1%) | 25 (20.7%) | <0.0001 |
| Endoscopic | 14 (31.8%) | 12 (9.9%) | ||
| Combined | 15 (34.1%) | 84 (69.4%) | ||
| Pathogen | Gram+ | 20 (76.9%) | 69 (82.1%) | 0.569 |
| Gram- | 2 (7.7%) | 8 (9.5%) | ||
| Other | 4 (15.4%) | 7 (8.3%) | ||
| Culture | Single pathogen | 21 (72.4%) | 66 (70.2%) | 0.820 |
| Multiple pathogens | 8 (27.6%) | 28 (29.8%) |
Abbreviation: IQR = interquartile range.
3.9. Surgical Treatment
Only seven patients (3.80%) did not undergo any surgery, and out of these, four (2.17%) had intracranial involvement. The type of surgery was not available for 10 cases (5.43%). In all other instances, surgery using various approaches was carried out.
The majority of the authors opted for an external surgical approach for draining subperiosteal abscesses (11.41%). Some authors employed endoscopic endonasal treatment either independently (15.21%) or in conjunction with external drainage (9.78%). Regarding intracranial complications, craniotomy was the primary surgical method in most articles, either on its own (9.23%) or in combination with external drainage (11.41%), endonasal surgery (15.21%), or as a combination of all three modalities (10.32%). Additional combinations of surgeries are detailed in Table 7.
Table 7.
Surgical treatment of Pott’s Puffy Tumor.
| Type of Surgery | N | % |
|---|---|---|
| External drainage | 21 | 11.41 |
| Endoscopic Sinus Surgery | 28 | 15.21 |
| Craniotomy | 17 | 9.23 |
| External drainage + Endoscopic Sinus Surgery | 18 | 9.78 |
| External drainage + craniotomy | 21 | 11.41 |
| Endoscopic Sinus Surgery + craniotomy | 28 | 15.21 |
| External drainage + Endoscopic Sinus Surgery + craniotomy | 19 | 10.32 |
| Craniectomy | 2 | 1.08 |
| External drainage, craniectomy | 6 | 3.26 |
| Endoscopic Sinus Surgery, craniectomy | 4 | 2.17 |
| External drainage + Endoscopic Sinus Surgery + craniectomy | 2 | 1.08 |
| External drainage, craniotomy, Cortical Mastoidectomy | 1 | 0.54 |
| Transferred to other hospital | 1 | 0.54 |
| None | 6 | 3.26 |
| Not available | 10 | 5.43 |
4. Discussion
4.1. Epidemiology
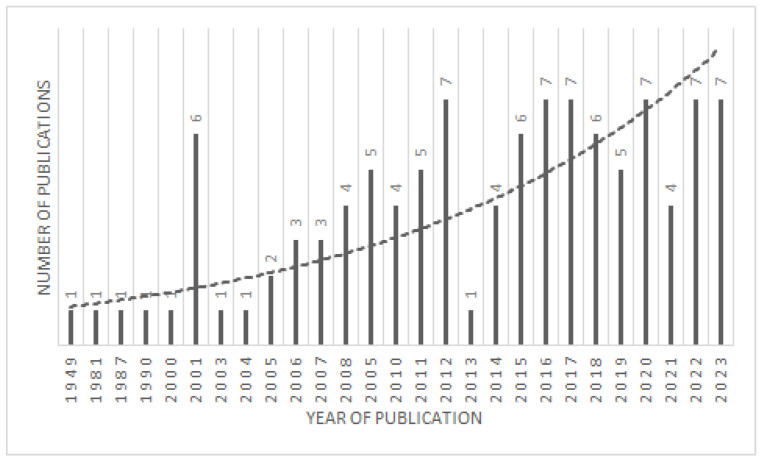
The determination of frequency measures for PPT is challenging due to its rare occurrence. Although it is experiencing minor annual variations, there seems to be an upward trend in the reported cases of PPT in recent years, as illustrated in Figure 5. While these fluctuations may be influenced by publishing pattern, they underscore the significance of promptly recognizing factors that could contribute to PPT predisposition. Frontal sinuses become pneumatized at 6 years of age, and they reach their adult configuration at the age of 15 [28]; that is why teenagers are especially affected by this entity. To our knowledge, only two cases have been reported in children younger than 3 years of age in the literature in the post-antibiotic era [22,50].
Figure 5.
Graph showing the number of published papers trending.
4.2. Pathophysiology
PPT typically manifests following sinusitis, particularly in cases of pansinusitis. Originating from the frontal sinus, the infection progresses through the frontal bone marrow cavity, inducing osteomyelitis that erodes the external table, leading to the formation of a subperiosteal abscess. Additionally, the infection may extend to the posterior table, giving rise to an epidural abscess. Despite the relative impermeability of the dura mater and arachnoid membranes, the infection can breach these barriers, spreading to the subdural space and causing subdural collections or cerebritis [28].
Consideration should also be given to the hematogenous route, as valveless diploic veins can become infected, resulting in septic thrombophlebitis of the sagittal sinus, subdural empyema, and brain abscess [113]. This phenomenon is more common in children than adults [113]. Persistent bacterial overgrowth in the frontal sinus cavity and adjacent soft tissues allows for small vessel thrombosis and venous congestion [48]. The disruption of the frontal periosteal blood supply initiates an inflammatory response characterized by increased intraosseous pressure, leading to extensive necrosis of the trabecular bone matrix. The resulting avascular and ischemic conditions favor the transition from an aerobic to an anaerobic environment, promoting the growth of opportunistic microorganisms that give rise to abscesses and cortical sinus tracts.
In certain instances, the infection can involve the floor of the frontal sinus, extending to the orbits and causing either orbital cellulitis or an orbital abscess [113].
These theories may partly explain the difference in the incidence of intracranial complications between adult and juvenile populations. In fact, a recent systematic review on intra-orbital complications of Pott’s Puffy Tumor in adults reported an incidence of around 30%, compared to approximately 70% in our review of younger patients [114]. In our study, the proportion of cases in individuals under 18 years of age was 59.5% (184/309), while cases in adults accounted for 40.5% (125/309) [114].
4.3. Clinical Presentation
A gradually tendered tumefaction of the scalp at the forehead is a distinctive indicator of PPT. The initial symptoms and signs of PPT often present subtly, resembling frontal sinusitis. The onset of pronounced symptoms, especially heightened headache and fever, or the manifestation of signs indicating increased intracranial pressure (such as nausea, vomiting, lethargy), periorbital complaints, or a lack of symptom resolution despite antibiotic treatment, necessitate imaging evaluation to detect potential silent intracranial involvement, even in the absence of overt neurological symptoms [113].
4.4. Imaging Modalities
Early diagnosis of PPT is crucial for minimizing morbidity and mortality, necessitating a heightened level of suspicion. Diagnosis primarily relies on a comprehensive evaluation of the patient’s history, clinical examination, and imaging studies. When a subperiosteal abscess is suspected, appropriate imaging is essential to confirm the diagnosis and assess potential complications.
The diagnostic workup should involve a ce-CT scan with brain and bony sequences, as it excels in visualizing bone structures and effectively delineates air–bone and air–soft tissue interfaces crucial for sinus surgeons [109]. CT scans can reveal sinusitis, bone erosion, subperiosteal collections, and intracranial extensions, with osteomyelitis indicated by low-attenuated areas of lytic bone destruction. CT is both quick and widely accessible, with pediatric protocols recommended to minimize radiation exposure in children [4].
MRI offers superior soft tissue resolution, making it the gold standard for detecting intracranial complications, dural sinus thrombosis, and bone edema [52]. However, its drawbacks include increased time consumption, the need for anesthesia in younger children, limitations in evaluation bony destruction, and limited availability, even though it can be used to reduce radiation exposure. MRI venography should be added when clinical suspicion of dural or cavernous sinus thrombosis arises [19]. While ultrasound has been proposed for PPT detection in children [85], its diagnostic value remains inadequately investigated in the literature. Additionally, bone Tc-mMP scintigraphy may offer heightened sensitivity compared to CT in early-stage osteomyelitis, though it proves less sensitive in an acute sinusitis setting [115].
Based on the current review, CT emerges as the most effective and commonly used imaging modality for PPT diagnosis. However, in cases with suspected intracranial involvement, MRI is recommended.
4.5. Intracranial Manifestations
The risk of intracranial complications associated with PPT is noteworthy, with reported incidences ranging from 43% to 85% in various studies [1,52,113,116]. The anterior pericranium is particularly susceptible to infection spread due to its rich venous plexus, directly communicating with the diploic veins of the frontal sinus cavity. This anatomical feature enables retrograde flow of septic emboli into the cranial vault, seeding the intracranial space, with or without concurrent erosion of the posterior table of the frontal sinus. PPT can lead to brain abscess, epidural and subdural abscess, superior sagittal sinus thrombosis, pneumocephalus, and meningitis. Less commonly, it can lead to cerebritis and fistula formation [52].
While the frequency of each type of collection varies in published reports, epidural collections are suggested to be the most common focal intracranial manifestation of PPT [1,52,113,116]. Our review found that 92 patients had epidural abscesses, accounting for over 40% of the total cases and more than half of the patients with intracranial complications.
Intracranial complications are often associated with leukocytosis, elevated ESR, and raised CRP levels [34], indicative of the bacterial origin of the complication.
Given the high incidence of seizures in intracranial abscesses (ranging from 19% to 80% of affected patients), immediate initiation of anticonvulsant therapy as prophylaxis against seizures is recommended for all patients with intracranial complications of sinusitis [40,117]. Extracranial complications, such as orbital infections, often co-occur with intracranial disease and shape the clinical presentation, particularly in pediatric patients lacking neurological symptoms. Visible craniofacial manifestations of PPT may serve as early indicators for patients, prompting them to seek medical evaluation sooner.
4.6. Microbiology
Due to the comparatively lower oxygen concentration in the frontal sinus, microaerophilic streptococci, including alpha-hemolytic Streptococcus, Peptostreptococcus, Bacteroides spp., and various anaerobes (such as Prevotella, Porphyromonas, Fusobacterium, and Peptostreptococcus spp.), were predominantly cultured from sinogenic sources. Additionally, less-frequently encountered organisms included Hemophilus influenza, Staphylococcus aureus, and Enterococcus spp. [2,15,118].
In our review, the most prevalent bacteria were identified as Streptococcus species, notably Streptococcus intermedius, accounting for 11.96% of cases. The prompt initiation of empirical intravenous antibiotic treatment during the initial surgery likely played a significant role in the absence of identifiable pathogens in the subsequent cultures.
4.7. Treatment
The initial treatment of a patient with a PPT is high-dose intravenous antibiotics. The selected antibiotics should possess the capability to cross the blood–brain barrier and provide coverage against both aerobic and anaerobic bacteria [3]. A commonly chosen combination includes a third-generation cephalosporin, metronidazole, and penicillin or vancomycin [see Table 1]. The antibiotic course is typically extended for a minimum of 6–8 weeks postoperatively [2,118]. The successful management of PPT involves a combination of broad-spectrum antibiotic therapy and surgical intervention. Adequate treatment has significantly reduced the mortality rate of sinogenic intracranial complications from 60% to 3.7% [118].
Historically, the osteoplastic flap was a conventional surgical access method to the anterior frontal sinus table in PPT. However, with the introduction of endoscopes and powered instrumentation, these methods have been largely replaced by ESS [106]. ESS is superior to classic techniques, providing effective management of the ostio–meatal complex and in opening the frontal recess, which cannot be externally approached [119]. While ESS is often highly effective, select cases with significant pericranial extension may necessitate an external approach with osteoplasties.
The neurosurgical approach to focal intracranial suppuration, particularly subdural empyema, is a subject of debate. Craniotomy is favored in former series examining subdural empyema secondary to sinusitis [117,120]. Small intracranial involvement without focal neurological deficits may initially be managed conservatively but may require craniotomies for abscesses refractory to medical management. Joint neurosurgical drainage and endoscopic or external sinus drainage have been shown to be more effective, leading to faster recovery and shorter hospital length of stay [121].
Frontal lobe abscesses can be treated based on the patient’s condition and the maturity of the abscess wall, either through aspiration with radiological localization or total excision via craniotomy. In our study, we observed that in the presence of intracranial complications, there is a higher prevalence of using a combined approach. This suggests that employing multiple techniques may be more effective for draining intracranial abscesses, whether epidural, subdural, or cerebral. Regardless of the principal treatment, the primary goal remains to drain the abscess and re-establish adequate frontoethmoidal drainage through surgical opening of the sinuses and prolonged antibiotic treatment. Successful treatment of PPT necessitates close collaboration between otorhinolaryngologists, neurosurgeons, pediatricians, bacteriologists, and other related departments.
4.8. Limitations of the Study
The present study has several limitations that are worthy of mention:
-
-
Case reports: the review primarily included case reports and short case series, which can limit the generalizability of findings due to variability and heterogeneity.
-
-
Missing data: some older articles lacked details relevant to the study, leading to the exclusion of some information from the analyses.
-
-
Evolution of treatment: the evolution of surgical technique and overall management of PPT that has taken place over the last decades may have improved outcomes, potentially affecting the comparability of older data with more recent data.
5. Conclusions
The rarity of PPT poses challenges in defining its frequency accurately, with reported cases displaying an upward trend in recent years. Early diagnosis is imperative for mitigating morbidity and mortality, requiring a heightened level of suspicion. Imaging, particularly contrast-enhanced CT scans, plays a crucial role in confirming the diagnosis and evaluating potential complications.
The risk of intracranial complications in PPT is significant, emphasizing the necessity for a combined approach involving high-dose intravenous antibiotics and surgery. This strategy is crucial in preventing long-term neurological complications and sequelae. Collaborative efforts among multidisciplinary teams, including otorhinolaryngologists, neurosurgeons, pediatricians, and bacteriologists, are indispensable for effective patient management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm13216428/s1, Table S1: Quality Assessment.
Author Contributions
Conceptualization, A.D. and T.M.; methodology, A.D. and T.M.; validation, A.D., T.M. and E.E.; investigation, A.D.; data curation, A.D. and T.M.; writing—original draft preparation, A.D.; writing—review and editing, A.D., T.M., F.B., E.S., G.S., D.C. and E.E.; visualization, F.B., E.S., G.S. and D.C.; supervision, E.E.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the principles of the Helsinki Declaration, the Italian privacy and data laws, and the in-house rules for retrospective studies of the Otolaryngology Section at Padova University (Italy). Patients signed a detailed informed consent form regarding the processing and publication of their data. They consented to “the use of the clinical data for scientific research purposes in the medical, biomedical and epidemiological fields, also in order to be recalled in the future for follow-up needs”. The data were examined in agreement with the Italian privacy and sensitive data laws and the internal regulations of the University of Padova.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available at request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Koltsidopoulos P., Papageorgiou E., Skoulakis C. Pott’s puffy tumor in children: A review of the literature. Laryngoscope. 2020;130:225–231. doi: 10.1002/lary.27757. [DOI] [PubMed] [Google Scholar]
- 2.Kombogiorgas D., Solanki G.A. The Pott puffy tumor revisited: Neurosurgical implications of this unforgotten entity: Case report and review of the literature. J. Neurosurg. 2006;105:143–149. doi: 10.3171/ped.2006.105.2.143. [DOI] [PubMed] [Google Scholar]
- 3.Behbahani M., Burokas L., Rosinski C.L., Rosenberg D.M., Chaudhry N.S., Sherman J.M. Treatment of pediatric extra-axial sinogenic infection: Case series and literature review. Child’s Nerv. Syst. 2020;36:755–766. doi: 10.1007/s00381-019-04378-8. [DOI] [PubMed] [Google Scholar]
- 4.Sharma P., Sharma S., Gupta N., Kochar P., Kumar Y. Pott Puffy Tumor. Bayl. Univ. Med. Cent. Proc. 2017;30:179–181. doi: 10.1080/08998280.2017.11929575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.NHLBI NIH Study Quality Assessment Tools. [(accessed on 3 January 2024)]; Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools.
- 7.Adnani A., Wawrzyniak M., Eilbert W. Girl with a frontal headache and fever. J. Am. Coll. Emerg. Physicians Open. 2023;4:e12994. doi: 10.1002/emp2.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allfather P., Nagler J. Seven-Year-Old Girl With Forehead Swelling. Ann. Emerg. Med. 2017;70:771–796. doi: 10.1016/j.annemergmed.2017.05.027. [DOI] [PubMed] [Google Scholar]
- 9.AlMoosa Z.A., AlFawaz T., AlFawaz F. Pott’s puffy tumor due to Aspergillus fumigatus: A case report and review. Int. J. Pediatr. Adolesc. Med. 2016;3:128–131. doi: 10.1016/j.ijpam.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amstrup J., Penning L.W., Hansen J.K., Brix N., Andersen G. A 9-year-old boy with a nonmalignant forehead tumor—A rare case of pediatric Pott’s puffy tumor. BMC Pediatr. 2023;23:300. doi: 10.1186/s12887-023-04117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arnold P.M., Govindan S., Anderson K.K. Spontaneous cranial osteomyelitis in an otherwise healthy ten-year-old male. Pediatr. Neurosurg. 2009;45:407–409. doi: 10.1159/000270154. [DOI] [PubMed] [Google Scholar]
- 12.Arora H.S., Abdel-Haq N. A 14-year-old male with swelling of the forehead. Pott’s puffy tumor. Pediatr. Ann. 2014;43:479–481. doi: 10.3928/00904481-20141124-05. [DOI] [PubMed] [Google Scholar]
- 13.Avcu G., Belet N., Kurnaz S.C., Karli A., Sensoy G. Pott’s puffy tumor in a 12-year-old boy. Pediatr. Int. 2015;57:163–165. doi: 10.1111/ped.12440. [DOI] [PubMed] [Google Scholar]
- 14.Bağdatoğlu C., Güleryüz A., Ersöz G., Talas D.U., Kandemir O., Köksel T. A rare clinical entity: Pott’s puffy tumor. A case report. Pediatr. Neurosurg. 2001;34:156–158. doi: 10.1159/000056011. [DOI] [PubMed] [Google Scholar]
- 15.Bambakidis N.C., Cohen A.R. Intracranial complications of frontal sinusitis in children: Pott’s puffy tumor revisited. Pediatr. Neurosurg. 2001;35:82–89. doi: 10.1159/000050395. [DOI] [PubMed] [Google Scholar]
- 16.Belharti A., Nasri S., Kennoudi N., Ziani H., Mojahid A., Aichouni N., Kamaoui I., Skiker I. What is hidden behind a forehead swelling: Pott’s puffy tumor? Pediatr. Neonatol. 2023;64:611–612. doi: 10.1016/j.pedneo.2023.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Bhalla V., Khan N., Isles M. Pott’s puffy tumour: The usefulness of MRI in complicated sinusitis. J. Surg. Case Rep. 2016;2016:rjw038. doi: 10.1093/jscr/rjw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blackman S.C., Schleiss M.R. Forehead swelling caused by Pott’s puffy tumor in a 9-year-old boy with sinusitis. Pediatr. Int. 2005;47:704–707. doi: 10.1111/j.1442-200x.2005.02129.x. [DOI] [PubMed] [Google Scholar]
- 19.Blumfield E., Misra M. Pott’s puffy tumor, intracranial, and orbital complications as the initial presentation of sinusitis in healthy adolescents, a case series. Emerg. Radiol. 2011;18:203–210. doi: 10.1007/s10140-010-0934-3. [DOI] [PubMed] [Google Scholar]
- 20.Butskiy O., Remillard A., Kozak F. Forehead swelling in a 10-year-old male: A case report. Br. Columbia Med. J. 2017;59:407–411. [Google Scholar]
- 21.Cannon L., Zwemer E., Stephens J.R. Puff laddy: A 5-year-old-boy with forehead swelling. BMJ Case Rep. 2017;2017:bcr-2017-223340. doi: 10.1136/bcr-2017-223340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng S., Vu P. Pott’s Puffy Tumor in a Premature Neonate: The New Youngest Case Reported in the Post-Antibiotic Era. Orbit. 2009;28:412–414. doi: 10.3109/01676830903104736. [DOI] [PubMed] [Google Scholar]
- 23.Costa L., Mendes Leal L., Vales F., Santos M. Pott’s puffy tumor: Rare complication of sinusitis. Braz. J. Otorhinolaryngol. 2020;86:812–814. doi: 10.1016/j.bjorl.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davidson L., McComb J.G. Epidural-cutaneous fistula in association with the Pott puffy tumor in an adolescent: Case report. J. Neurosurg. 2006;105:235–237. doi: 10.3171/ped.2006.105.3.235. [DOI] [PubMed] [Google Scholar]
- 25.Dayan E.R., Sanders J.E. Girl With Fever and Forehead Swelling. Ann. Emerg. Med. 2020;76:679–694. doi: 10.1016/j.annemergmed.2020.05.013. [DOI] [PubMed] [Google Scholar]
- 26.Durur-Subasi I., Kantarci M., Karakaya A., Orbak Z., Ogul H., Alp H. Pott’s puffy tumor: Multidetector computed tomography findings. J. Craniofac. Surg. 2008;19:1697–1699. doi: 10.1097/SCS.0b013e31818eed33. [DOI] [PubMed] [Google Scholar]
- 27.Faridi M.M.A., Pandey S., Shamsi S. Pott’s Puffy Tumor Presenting as Pyogenic Meningitis in an Infant. Case Rep. Pediatr. 2022;2022:4732287. doi: 10.1155/2022/4732287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feder H.M., Cates K.L., Cementina A.M. Pott puffy tumor: A serious occult infection. Pediatrics. 1987;79:625–629. [PubMed] [Google Scholar]
- 29.Forgie S.E., Marrie T.J. Pott’s puffy tumor. Am. J. Med. 2008;121:1041–1042. doi: 10.1016/j.amjmed.2008.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Fu B. Pott’s puffy tumour: A rare complication from frontal sinusitis. Emerg. Med. J. 2010;27:521. doi: 10.1136/emj.2009.081042. [DOI] [PubMed] [Google Scholar]
- 31.Fullerton L., Huckerby L., Gandhi M., Jabeen F., Maity S. Trauma-related Pott’s puffy tumour. Arch. Dis. Child. 2016;101:184. doi: 10.1136/archdischild-2015-309172. [DOI] [PubMed] [Google Scholar]
- 32.Gildener-Leapman N., Lin A. Pott’s puffy tumor in a 5-year-old male and a review of the literature. Int. J. Pediatr. Otorhinolaryngol. Extra. 2012;7:48–51. doi: 10.1016/j.pedex.2011.05.003. [DOI] [Google Scholar]
- 33.Gozgec E., Ogul H., Kılıc K. Pott’s Puffy Tumor with Intraorbital Abscess. Rev. Soc. Bras. Med. Trop. 2022;55:e01262022. doi: 10.1590/0037-8682-0126-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guillén A., Brell M., Cardona E., Claramunt E., Costa J.M. Pott’s puffy tumour: Still not an eradicated entity. Child’s Nerv. Syst. 2001;17:359–362. doi: 10.1007/s003810000420. [DOI] [PubMed] [Google Scholar]
- 35.Gupta M., El-Hakim H., Burgava R., Mehta V. Pott’s puffy tumour in a pre-adolescent child: The youngest reported in the post-antibiotic era. Int. J. Pediatr. Otorhinolaryngol. 2004;68:373–378. doi: 10.1016/j.ijporl.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Haider H.R., Mayatepek E., Schaper J., Vogel M. Pott’s puffy tumor: A forgotten differential diagnosis of frontal swelling of the forehead. J. Pediatr. Surg. 2012;47:1919–1921. doi: 10.1016/j.jpedsurg.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 37.Hassan S., Rahmani B., Rastatter J.C., Jaju A.I., Kurup S.P. Trauma-associated Pott’s puffy tumor: An ophthalmologic perspective. Orbit. 2020;39:38–40. doi: 10.1080/01676830.2019.1573909. [DOI] [PubMed] [Google Scholar]
- 38.Hayek B.R., Sitole S., Andreoli M., Banich A., Ahmad A.Z. Bilateral eyelid edema and orbital cellulitis associated with Pott’s puffy tumor. Ophthalmic Plast. Reconstr. Surg. 2007;23:163–165. doi: 10.1097/IOP.0b013e31803316b5. [DOI] [PubMed] [Google Scholar]
- 39.Heale L., Zahanova S., Bismilla Z. Cases: Pott puffy tumour in a five-year-old girl. CMAJ. 2015;187:433–435. doi: 10.1503/cmaj.140202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hicks C.W., Weber J.G., Reid J.R., Moodley M. Identifying and Managing Intracranial Complications of Sinusitis in Children: A Retrospective Series. Pediatr. Infect. Dis. J. 2011;30:222–226. doi: 10.1097/INF.0b013e3181f86398. [DOI] [PubMed] [Google Scholar]
- 41.Hitti E., Love E. Pott’s puffy tumor in a six-year-old female. J Hosp Med. 2010;5:E4–E5. doi: 10.1002/jhm.814. [DOI] [PubMed] [Google Scholar]
- 42.Holder J., Corbin D., Marquez S., Clarke H., Walcott J., Thomas R. Pott’s puffy tumour and subdural empyema following frontal sinusitis. West Indian Med. J. 1991;40:33–36. [PubMed] [Google Scholar]
- 43.Hore I., Mitchell R.B., Radcliffe G., De Casso Moxo C. Pott’s puffy tumour: A rare cause of forehead swelling in a child. Int. J. Clin. Pract. 2000;54:267–268. doi: 10.1111/j.1742-1241.2000.tb11900.x. [DOI] [PubMed] [Google Scholar]
- 44.Huijssoon E., Woerdeman P.A., van Diemen-Steenvoorde R.A.A.M., Hanlo P.W., Plötz F.B. An 8-year-old boy with a Pott’s puffy tumor. Int. J. Pediatr. Otorhinolaryngol. 2003;67:1023–1026. doi: 10.1016/S0165-5876(03)00183-6. [DOI] [PubMed] [Google Scholar]
- 45.Ikoma N., Aizawa Y., Sasaki T., Oishi M., Saito N., Yoshida H., Saitoh A. Pott’s puffy tumour: A rare and life-threatening disease. Lancet Infect. Dis. 2020;20:1482. doi: 10.1016/S1473-3099(20)30684-8. [DOI] [PubMed] [Google Scholar]
- 46.Is M., Karatas A., Aytekin H., Dosoglu M., Gezen F. An 11-year-old girl with Pott’s puffy tumour. Int. J. Pediatr. Otorhinolaryngol. Extra. 2007;2:215–217. doi: 10.1016/j.pedex.2007.06.002. [DOI] [Google Scholar]
- 47.Jafri L., Farooq O. Pott’s puffy tumor. Pediatr. Neurol. 2015;52:250–251. doi: 10.1016/j.pediatrneurol.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 48.Joo M.J., Schapira K.E. Pott’s Puffy Tumor: A Potentially Deadly Complication of Sinusitis. Cureus. 2019;11:e6351. doi: 10.7759/cureus.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalkan H., Eryılmaz M.A., Kıresi D., Arbag H., Yesildag A. Pott’s puffy tumor: The role of ultrasound, computed tomography and magnetic resonance imaging in diagnosis. Eur. J. Gen. Med. 2017;14:51–53. doi: 10.29333/ejgm/81883. [DOI] [Google Scholar]
- 50.Karadaghy O.A., Lucas J.C., Paroya S., Jensen D. Pott’s puffy tumor in a 23-month-old: Youngest known case of a rare disease. Auris Nasus Larynx. 2022;49:713–716. doi: 10.1016/j.anl.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Karaman E., Hacizade Y., Isildak H., Kaytaz A. Pott’s puffy tumor. J. Craniofac. Surg. 2008;19:1694–1697. doi: 10.1097/SCS.0b013e31818b432e. [DOI] [PubMed] [Google Scholar]
- 52.Ketenci I., Unlü Y., Tucer B., Vural A. The Pott’s puffy tumor: A dangerous sign for intracranial complications. Eur. Arch. Otorhinolaryngol. 2011;268:1755–1763. doi: 10.1007/s00405-011-1660-5. [DOI] [PubMed] [Google Scholar]
- 53.Khan M.A. Pott’s puffy tumor: A rare complication of mastoiditis. Pediatr. Neurosurg. 2006;42:125–128. doi: 10.1159/000090469. [DOI] [PubMed] [Google Scholar]
- 54.Kim H.Y., Hwang E.H., Han Y.M., Kim S.H., Kim Y.M. Pott’s puffy tumor in an adolescent boy. Pediatr. Int. 2012;54:158–160. doi: 10.1111/j.1442-200X.2011.03419.x. [DOI] [PubMed] [Google Scholar]
- 55.Klivitsky A., Erps A., Regev A., Ashkenazi-Hoffnung L., Pratt L.T., Grisaru-Soen G. Pott’s Puffy Tumor in Pediatric Patients: Case Series and Literature Review. Pediatr. Infect. Dis. J. 2023;42:851–856. doi: 10.1097/INF.0000000000004026. [DOI] [PubMed] [Google Scholar]
- 56.Kuhar B.G., Dunn T.M., Liming B.J., Yakopson V.S. Pott’s Puffy Tumor: A Rare, Life-Threatening Presentation of Periorbital Edema. Mil. Med. 2023;188:3696–3698. doi: 10.1093/milmed/usad291. [DOI] [PubMed] [Google Scholar]
- 57.Kühn J.P., Linsler S., Nourkami-Tutdibi N., Meyer S., Becker S.L., Yilmaz U., Schick B., Bozzato A., Kulas P. Pott’s puffy tumor: A need for interdisciplinary diagnosis and treatment. HNO. 2022;70:8–13. doi: 10.1007/s00106-021-01134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lang E.E., Curran A.J., Patil N., Walsh R.M., Rawluk D., Walsh M.A. Intracranial complications of acute frontal sinusitis. Clin. Otolaryngol. Allied Sci. 2001;26:452–457. doi: 10.1046/j.1365-2273.2001.00499.x. [DOI] [PubMed] [Google Scholar]
- 59.Lauria R.A., Laffitte Fernandes F., Brito T.P., Pereira P.S.G., Chone C.T. Extensive Frontoparietal Abscess: Complication of Frontal Sinusitis (Pott’s Puffy Tumor) Case Rep. Otolaryngol. 2014;2014:632464. doi: 10.1155/2014/632464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ling C., Ng D.C.E., Kumar T.A.P.B.A. More than a Bump on the Head. J. Pediatr. 2021;238:331. doi: 10.1016/j.jpeds.2021.06.052. [DOI] [PubMed] [Google Scholar]
- 61.Linton S., Pearman A., Joganathan V., Karagama Y. Orbital abscess as a complication of Pott’s puffy tumour in an adolescent male. BMJ Case Rep. 2019;12:e229664. doi: 10.1136/bcr-2019-229664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu A., Powers A.K., Whigham A.S., Whitlow C.T., Shetty A.K. A Child With Fever and Swelling of the Forehead. Pott’s puffy tumor and epidural abscess complicating frontal sinusitis. Clin. Pediatr. 2015;54:803–805. doi: 10.1177/0009922815584945. [DOI] [PubMed] [Google Scholar]
- 63.Maheshwar A.A., Harris D.A., Al-Mokhthar N., Evans R.A. Pott’s puffy tumour: An unusual presentation and management. J. Laryngol. Otol. 2001;115:497–499. doi: 10.1258/0022215011908036. [DOI] [PubMed] [Google Scholar]
- 64.Marzuillo P., Aliberti F., Tipo V. A revealing forehead cutaneuos lesion: Pott’s “puffy” tumor. Türk Pediatri Arşivi. 2017;52:57. doi: 10.5152/TurkPediatriArs.2016.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McGee A.E., Cooper F., Maini S.K., Vallamkondu V. Unusual Presentation of Pott’s puffy tumour in a child: Our recent experience and review of the literature. BMJ Case Rep. 2022;15:e247325. doi: 10.1136/bcr-2021-247325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Morley A.M.S. Pott’s puffy tumour: A rare but sinister cause of periorbital oedema in a child. Eye. 2009;23:990–991. doi: 10.1038/eye.2008.129. [DOI] [PubMed] [Google Scholar]
- 67.Moser R., Schweintzger G., Uggowitzer M., Urban C., Stammberger H., Eder H., Kerbl R. Recurrent Pott’s puffy tumor—Atypical presentation of a rare disorder. Wien. Klin. Wochenschr. 2009;121:719–722. doi: 10.1007/s00508-009-1268-6. [DOI] [PubMed] [Google Scholar]
- 68.Moses S.F., Roben E.C. Deep Impact. Clin. Pediatr. Emerg. Med. 2018;19:187–192. doi: 10.1016/j.cpem.2018.06.002. [DOI] [Google Scholar]
- 69.Nastovska R., Lim L.L. Sinusitis complicated by frontal bone osteomyelitis in a young patient. Med. J. Aust. 2017;207:376. doi: 10.5694/mja16.01434. [DOI] [PubMed] [Google Scholar]
- 70.Nicoli T.K., Mäkitie A. Frontal sinusitis causing epidural abscess and puffy tumor. N. Engl. J. Med. 2014;370:e18. doi: 10.1056/NEJMicm1307740. [DOI] [PubMed] [Google Scholar]
- 71.Nourkami-Tutdibi N., Linsler S., Yilmaz U., Pfeifer J., Derouet C., Becker S.L., Zemlin M., Meyer S., Kulas P. A 6-Year-Old Boy with a Frontal Mass: Pott Puffy Tumor. J. Pediatr. 2020;217:211. doi: 10.1016/j.jpeds.2019.09.047. [DOI] [PubMed] [Google Scholar]
- 72.Olmaz B., Cingoz M., Akdogan E., Kandemirli S.G. Correlation of imaging and intraoperative findings in Pott’s puffy tumour. Scott. Med. J. 2019;64:25–29. doi: 10.1177/0036933018803787. [DOI] [PubMed] [Google Scholar]
- 73.Onesimo R., Scalzone M., Valetini P., Caldarelli M. Pott’s puffy tumour by Streptoccocus intermedius a rare complication of sinusitis. Case Rep. 2011;2011:bcr0820114660. doi: 10.1136/bcr.08.2011.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Özkaya Parlakay A., Kara A., Cengiz A., Ceyhan M. Puffy Frontal Edema: A Serious Life-Threatening Finding of Pott’s Puffy Tumor: Case Report. Turk. Klin. Tip Bilim. Derg. 2012;32:850–853. doi: 10.5336/medsci.2010-18408. [DOI] [Google Scholar]
- 75.Öztürk N., Atay K., Çekin İ.E., Erkul B.E., Karademir F. A rare case in childhood: Pott’s puffy tumor developing secondary to frontal sinus osteoma. Türk Pediatri Arşivi. 2020;55:445–448. doi: 10.14744/TurkPediatriArs.2020.28863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Palabiyik F.B., Yazici Z., Cetin B., Celebi S., Hacimustafaoglu M. Pott puffy tumor in children: A rare emergency clinical entity. J. Craniofacial Surg. 2016;27:e313–e316. doi: 10.1097/SCS.0000000000002573. [DOI] [PubMed] [Google Scholar]
- 77.Palacios-García J.M., Moreno-Luna R., Molina-Fernandez E.M., Sanchez-Gomez S. Bilateral involvement of frontal sinuses in a pott’s puffy tumor: A case report. Otorhinolaryngol. Clin. 2019;11:67–69. doi: 10.5005/jp-journals-10003-1340. [DOI] [Google Scholar]
- 78.Parida P.K., Surianarayanan G., Ganeshan S., Saxena S.K. Pott’s puffy tumor in pediatric age group: A retrospective study. Int. J. Pediatr. Otorhinolaryngol. 2012;76:1274–1277. doi: 10.1016/j.ijporl.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 79.Patel A., Vuppula S., Hayward H., Lakhani A., Lighter J. A Case of Pott’s Puffy Tumor Associated With Barosinusitis from Scuba Diving. Pediatr. Emerg. Care. 2021;37:e51–e54. doi: 10.1097/PEC.0000000000001657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Patel S.A., Chatterjee A., Simonsen K. Recurrent midline frontal mass in a patient with sinusitis. Clin. Pediatr. 2011;50:266–268. doi: 10.1177/0009922809352680. [DOI] [PubMed] [Google Scholar]
- 81.Pender E.S. Pott’s puffy tumor: A complication of frontal sinusitis. Pediatr. Emerg. Care. 1990;6:280–284. doi: 10.1097/00006565-199012000-00008. [DOI] [PubMed] [Google Scholar]
- 82.Podolsky-Gondim G.G., Santos M.V., Carneiro V.M., Pires Augusto L., Da Costa Pacheco Neto R., Santos de Oliveira R.M. Neurosurgical Management of Pott’s Puffy Tumor in an Obese Adolescent with Asthma: Case Report with a Brief Review of the Literature. Cureus. 2018;10:e2836. doi: 10.7759/cureus.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Przybysz P., Hartmann P., Jackowska T. Paranasal sinusitis complicated by Pott’s puffy tumour. Pediatr. Med. Rodz.-Paediatr. Fam. Med. 2018;14:331–336. doi: 10.15557/PiMR.2018.0041. [DOI] [Google Scholar]
- 84.Queen J.R., Anderson E. A fourteen-year old with Pott’s Puffy Tumor. J. Emerg. Med. 2001;21:185–188. doi: 10.1016/S0736-4679(01)00365-1. [DOI] [PubMed] [Google Scholar]
- 85.Reddan T., Connor P. Not just a bump on the head: Ultrasound as first-line imaging in a boy with Pott’s puffy tumour. J. Med. Radiat. Sci. 2018;65:71–73. doi: 10.1002/jmrs.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rogers L. Pott’s puffy tumour. Br. J. Surg. 1949;36:315. doi: 10.1002/bjs.18003614315. [DOI] [PubMed] [Google Scholar]
- 87.Rogo T., Schwartz R.H. Pott puffy tumor in a 5-year-old girl with frontal sinusitis. Ear Nose Throat J. 2013;92:E24. [PubMed] [Google Scholar]
- 88.Russ A.E., Morse A.M., Spiro D.M. When a Headache Is More than the Flu: A Case Report. Clin. Pract. Cases Emerg. Med. 2022;6:240–243. doi: 10.5811/cpcem2022.6.56491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sabatiello M., Vanhooteghem O., Mostinckx S., De La Brassinne M. The Pott’s puffy tumor: An unusual complication of frontal sinusitis, methods for its detection. Pediatr. Dermatol. 2010;27:406–408. doi: 10.1111/j.1525-1470.2010.01182.x. [DOI] [PubMed] [Google Scholar]
- 90.Sade R., Polat G., Kantarci M. Unusual cause of seizure: Pott puffy tumor. J. Craniofacial Surg. 2016;27:e548–e549. doi: 10.1097/SCS.0000000000002860. [DOI] [PubMed] [Google Scholar]
- 91.Salomão J.F., Cervante T.P., Bellas A.R., Boechat M.C., Pone S.M., Pone M.V., de APereira B. Neurosurgical implications of Pott’s puffy tumor in children and adolescents. Child’s Nerv. Syst. 2014;30:1527–1534. doi: 10.1007/s00381-014-2480-x. [DOI] [PubMed] [Google Scholar]
- 92.Shehu B.B., Mahmud M.R. Pott’s puffy tumour: A case report. Ann. Afr. Med. 2008;7:138–140. doi: 10.4103/1596-3519.55663. [DOI] [PubMed] [Google Scholar]
- 93.Shemesh A.J., Panebianco N.L., Chen A.E. An Uncommon Complication of Sinusitis in a Young Adolescent. Pediatr. Emerg. Care. 2015;31:531–532. doi: 10.1097/PEC.0000000000000491. [DOI] [PubMed] [Google Scholar]
- 94.Sheth S.P., Ilkanich P., Congeni B. Complicated fusobacterium sinusitis: A case report. Pediatr. Infect. Dis. J. 2018;37:E246–E248. doi: 10.1097/INF.0000000000001927. [DOI] [PubMed] [Google Scholar]
- 95.Silva A.C.V., Lins C.M., Mendes R.F.d.A., Silva M.H.S., de Alencar Neto J.F., Lopes C.C.M., Ferraz G.L.D.S., de Sousa D.F.R., Bem Junior L.S., Valença M.M., et al. Case Report: Pott’s Edematous Tumor: Complicated Frontal Sinusitis—An Unremembered Diagnosis. Front. Surg. 2022;9:889463. doi: 10.3389/fsurg.2022.889463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stark P., Ghumman R., Thomas A., Sawyer S.M. Forehead swelling in a teenage boy. J. Paediatr. Child Health. 2015;51:731–733. doi: 10.1111/jpc.12790. [DOI] [PubMed] [Google Scholar]
- 97.Stoddard T.J., Tung P., Kelly M.N. Pott’s Puffy Tumor: A Case Report. J. Pediatr. Health Care. 2019;33:585–588. doi: 10.1016/j.pedhc.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 98.Strony R.J., Dula D. Pott puffy tumor in a 4-year-old boy presenting in status epilepticus. Pediatr. Emerg. Care. 2007;23:820–822. doi: 10.1097/PEC.0b013e31815a05f5. [DOI] [PubMed] [Google Scholar]
- 99.Sugiyama A., Kobayashi M., Moriishi H., Tanaka H., Mitsuyoshi R., Matsunaga T., Kuwabara S. Pneumocephalus associated with Pott’s puffy tumor. J. Neurol. Sci. 2016;362:196–197. doi: 10.1016/j.jns.2016.01.056. [DOI] [PubMed] [Google Scholar]
- 100.Suwan P.T., Mogal S., Chaudhary S. Pott’s Puffy Tumor: An Uncommon Clinical Entity. Case Rep. Pediatr. 2012;2012:386104. doi: 10.1155/2012/386104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tibesar R.J., Azhdam A.M., Borrelli M. Pott’s Puffy Tumor. Ear Nose Throat J. 2021;100:870S–872S. doi: 10.1177/01455613211039031. [DOI] [PubMed] [Google Scholar]
- 102.Tsai B.Y., Lin K.L., Lin T.Y., Chiu C.H., Lee W.J., Hsia S.H., Wu C.T., Wang H.S. Pott’s puffy tumor in children. Child’s Nerv. Syst. 2010;26:53–60. doi: 10.1007/s00381-009-0954-z. [DOI] [PubMed] [Google Scholar]
- 103.Tudor R.B., Carson J.P., Pulliam M.W., Hill A. Pott’s puffy tumor, frontal sinusitis, frontal bone osteomyelitis, and epidural abscess secondary to a wrestling injury. Am. J. Sports Med. 1981;9:390–391. doi: 10.1177/036354658100900609. [DOI] [PubMed] [Google Scholar]
- 104.Urík M., Machač J., Šlapák I., Hošnová D. Pott’s puffy tumor: A rare complication of acute otitis media in child: A case report. Int. J. Pediatr. Otorhinolaryngol. 2015;79:1589–1591. doi: 10.1016/j.ijporl.2015.06.029. [DOI] [PubMed] [Google Scholar]
- 105.Vadiee G., Beshali M., Jahangiri S., Eghlidos Z., Rahimian Z., Mirzaei F. Pott’s puffy tumor: A case report. Clin. Case Rep. 2023;11:e7815. doi: 10.1002/ccr3.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Van Der Poel N.A., Hansen F.S., Georgalas C., Fokkens W.J. Minimally invasive treatment of patients with Pott’s puffy tumour with or without endocranial extension—A case series of six patients: Our Experience. Clin. Otolaryngol. 2016;41:596–601. doi: 10.1111/coa.12538. [DOI] [PubMed] [Google Scholar]
- 107.Vanderveken O.M., De Smet K., Dogan-Duyar S., Desimpelaere J., Duval E.L., De Praeter M., Van Rompaey D. Pott’s puffy tumour in a 5-year old boy: The role of ultrasound and contrast-enhanced CT imaging; surgical case report. B-ENT. 2012;8:127–129. [PubMed] [Google Scholar]
- 108.Vaphiades M.S., Isen D., Tavakoli M., Bilyk J.R. Pott luck. Surv. Ophthalmol. 2023;68:830–833. doi: 10.1016/j.survophthal.2022.11.005. [DOI] [PubMed] [Google Scholar]
- 109.Verma R.K., Abraham S. Potts Puffy Tumor: A Rare Cause of Seizure in Children. Eur. J. Rhinol. Allergy. 2021;4:99–102. doi: 10.5152/ejra.2021.21035. [DOI] [Google Scholar]
- 110.Verma R., Behera S. Endoscopic management of Pott’s puffy tumour—Still a common entity in-developing country a case series of three patients: Our experience. Int. J. Pediatr. Otorhinolaryngol. Extra. 2018;19:10–13. doi: 10.1016/j.pedex.2017.07.001. [DOI] [Google Scholar]
- 111.Weinberg B., Gupta S., Thomas M.J., Stern H. Pott’s puffy tumor: Sonographic diagnosis. J. Clin. Ultrasound. 2005;33:305–307. doi: 10.1002/jcu.20130. [DOI] [PubMed] [Google Scholar]
- 112.Wu C.T., Huang J.L., Hsia S.H., Lee H.Y., Lin J.J. Pott’s puffy tumor after acupuncture therapy. Eur. J. Pediatr. 2009;168:1147–1149. doi: 10.1007/s00431-008-0892-x. [DOI] [PubMed] [Google Scholar]
- 113.Rohde R.L., North L.M., Murray M., Khalili S., Poetker D.M. Pott’s puffy tumor: A comprehensive review of the literature. Am. J. Otolaryngol. 2022;43:103529. doi: 10.1016/j.amjoto.2022.103529. [DOI] [PubMed] [Google Scholar]
- 114.Sideris G., Davoutis E., Panagoulis E., Maragkoudakis P., Nikolopoulos T., Delides A. A Systematic Review of Intracranial Complications in Adults with Pott Puffy Tumor over Four Decades. Brain Sci. 2023;13:587. doi: 10.3390/brainsci13040587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Uren R.F., Howman-Giles R. Pott’s Puffy Tumor: Scintigraphic Findings. Clin. Nucl. Med. 1992;17:724–727. doi: 10.1097/00003072-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 116.Akiyama K., Karaki M., Mori N. Evaluation of adult pott’s puffy tumor: Our five cases and 27 literature cases. Laryngoscope. 2012;122:2382–2388. doi: 10.1002/lary.23490. [DOI] [PubMed] [Google Scholar]
- 117.Clayman G.L., Adams G.L., Paugh D.R., Koopmann C.F. Intracranial complications of paranasal sinusitis: A combined institutional review. Laryngoscope. 1991;101:234–239. doi: 10.1288/00005537-199103000-00003. [DOI] [PubMed] [Google Scholar]
- 118.Brook I. Microbiology and antimicrobial treatment of orbital and intracranial complications of sinusitis in children and their management. Int. J. Pediatr. Otorhinolaryngol. 2009;73:1183–1186. doi: 10.1016/j.ijporl.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 119.Pendolino A.L., Koumpa F.S., Zhang H., Leong S.C., Andrews P.J. Draf III frontal sinus surgery for the treatment of Pott’s puffy tumour in adults: Our case series and a review of frontal sinus anatomy risk factors. Eur. Arch. Otorhinolaryngol. 2020;277:2271–2278. doi: 10.1007/s00405-020-05980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kaufman D.I., Litman N., Miller M.H. Sinusitis: Induced subdural empyerna. Neurology. 1983;33:123. doi: 10.1212/WNL.33.2.123. [DOI] [PubMed] [Google Scholar]
- 121.Garin A., Thierry B., Leboulanger N., Blauwblomme T., Grevent D., Blanot S., Garabedian N., Couloigner V. Pediatric sinogenic epidural and subdural empyema: The role of endoscopic sinus surgery. Int. J. Pediatr. Otorhinolaryngol. 2015;79:1752–1760. doi: 10.1016/j.ijporl.2015.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available at request.