Abstract
Thyroid eye disease is a complex inflammatory disorder of the orbit that has gained tremendous interest over the past years, and numerous scientific efforts have been deployed to elucidate its pathophysiology for novel drug development. Our manuscript will delve into the molecular dysregulations involved in the pathogenesis of thyroid eye disease that led to its clinical manifestations. Abnormalities within the apoptotic pathway, inflammatory cascade, and autoimmune regulatory systems will be covered. We will further discuss the challenges involved in its diagnosis and management and provide a summary of the current diagnostic tools (i.e., molecular biomarkers, diagnostic scores) from the perspective of clinicians. Finally, our comprehensive literature review will provide a thorough summary of most recent preclinical and clinical studies around the topic of thyroid eye disease, with an emphasis on the manuscripts published within the last five years. We believe our manuscript will bring novelty within the field by bridging the fundamental sciences with the clinical aspect of this disease. This review will be a great tool for clinicians in better understanding the pathogenesis of thyroid eye disease while providing an outlook on future perspectives (i.e., liquid biopsies, artificial intelligence).
Keywords: thyroid eye disease, grave’s orbitopathy, grave’s disease, grave’s pathophysiology, deep learning for thyroid eye disease, immunotherapy for grave’s disease
1. Introduction
Thyroid eye disease (TED), also known as thyroid-associated ophthalmopathy or Grave’s orbitopathy (GO), represents the most common extrathyroidal manifestation of Grave’s disease (GD), but may also be seen in other thyroid disorders such as Hashimoto’s thyroiditis (HT) [1]. TED is believed to affect 25–50% of patients with GD and has an annual incidence of 20 cases per 100,000 people [2]. Nearly 5% of patients with TED will further develop sight-threatening complications, such as dysthyroid optic neuropathy (DON) or exposure keratopathy, therefore requiring a surgical intervention in moderate to severe cases [3]. The economic surgical burden of TED was shown to reach approximately 43.5 million dollars in the United States based on the National Ambulatory Surgery Sample (NASS) database [3]. Furthermore, TED was shown to severely impact the quality of life of affected (QOL) individuals; by using the Grave’s Ophthalmopathy QOL instrument, it was shown that individuals who exhibit ocular pain, diplopia, and blurry vision report a lower QOL [4]. The presence of bothersome symptoms in TED patients was shown to be linked to feelings of depression and anxiety and a decline in self-confidence [5]. TED nevertheless comes with a non-negligible economic and psychosocial burden.
TED is characterized by an inflammatory orbital process and remodeling of surrounding connective tissue, with subsequent fat accumulation, myositis, and/or tissue scaring due to autoimmune activation of orbital fibroblasts [6,7]. It is a complex clinical entity with a growing body of literature on its pathogenesis, emerging molecular biomarkers, and novel therapeutics [2,8]. Elucidating the pathophysiology of TED is the cornerstone of evolving diagnostic criteria, drug development, and surgical management. Therefore, this article provides an overview of the pathogenesis of TED and upcoming developments in diagnostic and therapeutic approaches. By bridging the fundamental science to the clinical aspect of TED diagnosis and management, this comprehensive literature review is a crucial tool for many healthcare professionals.
2. Pathogenesis
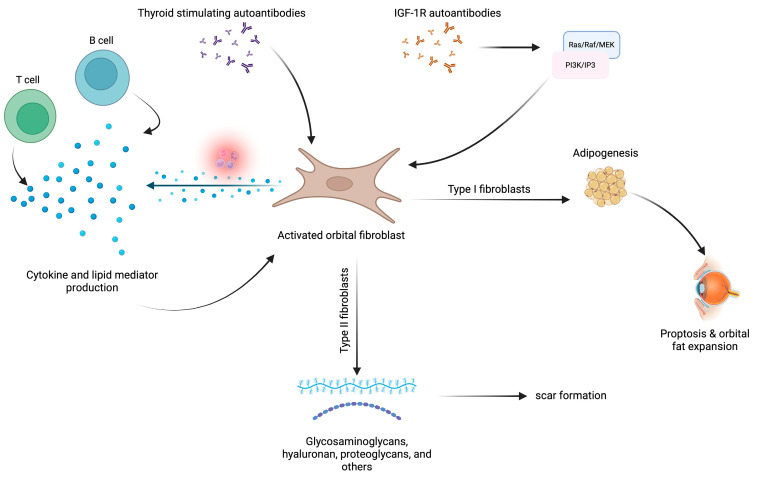
The backbone of TED pathogenesis involves the uncontrolled activation and proliferation of orbital fibroblasts, a multifactorial process (Figure 1). Activated orbital fibroblasts can differentiate into two distinct phenotypes: lipid-rich adipocytes or scar-forming myofibroblasts [6]. This differential is crucial given that clinically, they form two distinct disease entities, which are type I and type II TED, respectively. The ensuing inflammatory process is a result of extracellular matrix production by activated orbital fibroblasts, as well as chemotaxis of B- and T-cells, mast cells, and macrophages to the orbital tissue [9,10,11,12]. Throughout the body of molecular pathways that have been proposed to be involved in orbital fibroblast activation, immune dysregulations are the most important. In this section, we will review the key features involved in TED pathogenesis while discussing the most recent advances.
Figure 1.
Schematic representation of thyroid eye disease pathophysiology. The backbone of TED pathogenesis involves the uncontrolled activation of orbital fibroblasts by thyroid-stimulating autoantibodies, IGF-1R autoantibodies, and through the proinflammatory microenvironment created by T- and B-cells. Activated orbital fibroblasts lead to adipogenesis, which is involved in proptosis and orbital fat expansion, as well as glycosaminoglycan, hyaluronan, and proteoglycan production, markers involved in scar formation. The figure was created with BioRender.com.
2.1. Autoantibodies
The production of autoantibodies directed towards the thyroid stimulating hormone (TSH) receptor (TSHR) and insulin-like growth factor-1 receptor (IGF-1R) constitutes the main key factors involved in orbital fibroblast activation and proliferation [13,14]. A multicenter study amongst pediatric patients demonstrated that the level of TSHR autoantibodies in individuals with GD correlated with disease severity and extrathyroidal manifestation [15]. Conversely, immunoglobulins (Igs) were shown to stimulate IGF-1R in patients with GD; depletion of serum Igs significantly decreased IGF-1R in patients with GD [16]. However, the importance of IGF-1R in TED pathogenesis is yet controversial, with different works confirming or refuting the presence of IGF-1R autoantibodies in GO [17,18,19,20].
TSH-R is a G-protein-coupled transmembrane receptor with major functions in cell proliferation [21]. Using orbital fibroblasts from patients with TED, it was shown that in vitro TSH stimulation significantly increased cell proliferation through the direct induction of the phosphoinositide 3-kinase (PI3K) pathway, and indirect upregulation of the micro-RNAs (miRNA) miR-146a and miR-155 expression was also reported [22]. The activation of the PI3K pathway in orbital fibroblasts leads to cyclic adenosine monophosphate (cAMP) production and subsequent secretion of hyaluronan [23].
The IGF-1R is a member of the tyrosine kinase class of membrane receptors [24]. Its expression was shown to be significantly elevated in orbital fibroblasts and T- and B-cells of individuals with TED [25,26,27,28]. IGF-1R leads to downstream activation of the Ras/Raf/MEK and PI3K signaling pathways, therefore leading to cell proliferation, differentiation, inflammation, adipogenesis, and scar formations—hallmarks in TED [6]. Furthermore, once activated by its ligand, it was shown that IGF-1R localizes in the nucleus of orbital fibroblasts through ADAM17 signaling [25]. Recently, Wang et al. have demonstrated the crucial role of IGF-1R in cellular communication and interaction between orbital fibroblasts and B-cells [26]. Orbital fibroblasts highly expressive in IGF-1R were cocultured with peripheral B-cells, resulting in an observed elevation in the expression of interleukin (IL)-6 and RANTES [26]. The authors also observed significant inhibition of the inflammatory process when pretreating the cells with rituximab [26]. Conversely, the role of IGF-1R in T-cell activation is yet to be fully elucidated in TED. A recent study performed by Kiernan et al. demonstrated that IGF-1R signaling through IGF-1 binding induces Th17 T cell subtype activation, with subsequent IL-17 production and a decrease in reactive oxygen species (ROS) production within Th17 cells [29]. The role of Th17 cells in GO pathogenesis has been underlined in the literature; Th17 cells promote orbital fibroblast and CD34+ fibroblast differentiation, adipogenesis, and orbital fibrosis in patients with GO [30,31].
2.2. Immune Dysregulations Leading to Inflammation, Adipogenesis, Myofibrillogenesis, Hyaluronan Synthesis, and Scarring
The role of lipid mediators (e.g., prostaglandins (PGs)) and cytokines (e.g., IL-1β, IL-6, IL-15, IL-17, tumor necrosis factor (TNF)α, platelet-derived growth factor (PDGF), and transforming growth factor (TGF-β)) in TED pathogenesis have been reported on numerous occasions in various comprehensive literature reviews and studies [6,13,32,33,34,35,36]. This ensuing section reviews the most novel advances in the field while providing the fundamental concepts on TED pathogenesis. We suggest referring to the provided references for further details.
The underlying pathogenesis of TED involves an autoimmune response in which B-cells, T-cells, and CD34+ fibrocytes infiltrate the orbits following TSH-R and IGF1-R stimulation by autoantibodies [37]. The lymphocytes facilitate orbital fibroblast proliferation and differentiation through the release of pro-inflammatory cytokines and chemokines, ultimately leading to additional orbital fibroblast differentiation, adipogenesis, myofibrillogenesis, hyaluronan synthesis, and finally tissue scarring if left untreated (Figure 1) [18].
A fundamental step in the management of TED is to better characterize the pathogenesis of TED and factors inducing the activation of orbital fibroblasts. Recently, it was demonstrated that orbital fibroblast activation was under the regulation of SOX9; SOX9 was shown to activate orbital fibroblasts through the upregulation of mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) 1/2 pathways [38]. Furthermore, orbital fibroblast adipogenesis was observed to be under the regulation of the Piezo1 receptor, a mechanosensitive receptor. It is hypothesized that following increased intraorbital pressure due to extraocular muscle enlargement, Piezo1 receptors, found in orbital fibroblasts, are activated and inhibit adipogenesis of fibroblasts, as characterized by decreased expression of peroxisome proliferator-activated receptor gamma (PPARγ) and CEBPα mRNAs [39]. Conversely, Yes-associated protein (YAP), another mechanosensitive receptor, was recently shown to be involved in chronic fibrosis in GO; pharmacological inhibition of YAP in primary cultured orbital fibroblasts was shown to significantly decrease TGFβ-induced myofibroblast differentiation and subsequent collagen formation [40].
3. Clinical Features
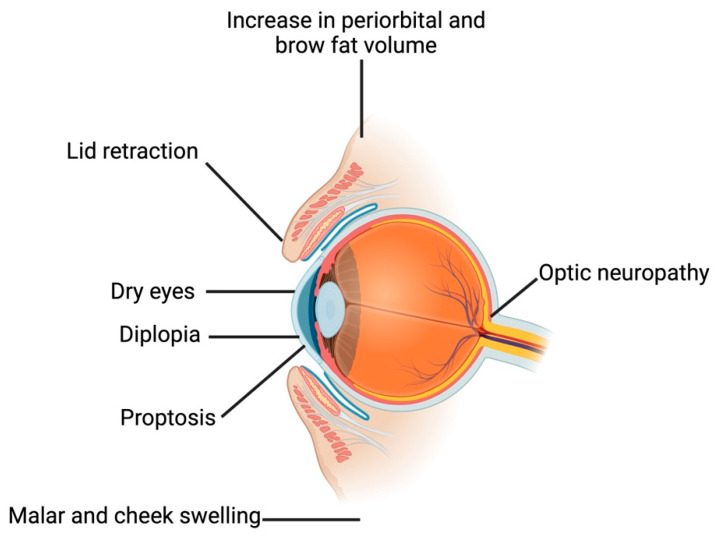
Lid retraction, proptosis, and diplopia are hallmarks of TED (Figure 2), although many patients initially present with more elusive and milder signs and symptoms such as chemosis, dry eyes, or soft-tissue swelling [41]. Inflammation and expansion of the fibroadipose and extraocular muscle (EOM) tissues facilitated by the TSHR-Ab/IGF-1R signaling complex underlies the eyelid retraction, proptosis, diplopia, and the dreaded but rare DON seen in TED [1,42,43]. Changes associated with thyroid disease beyond the orbit include an increase in periorbital and brow fat volume, lateral flare of the eyebrow, and malar and cheek swelling, signifying a diffuse facial soft tissue expansion [44]. TED often presents bilaterally, but unilateral or asymmetric disease occurs as well. It is reported as having a higher incidence in women yet presents with greater severity and at an older age in men [45]. Pediatric patients are rare, with typically milder disease compared to adults [1].
Figure 2.
Schematic representation of the clinical manifestations of thyroid eye disease. The most common clinical symptoms involved in thyroid eye disease are depicted in this illustration. The figure was created with BioRender.com.
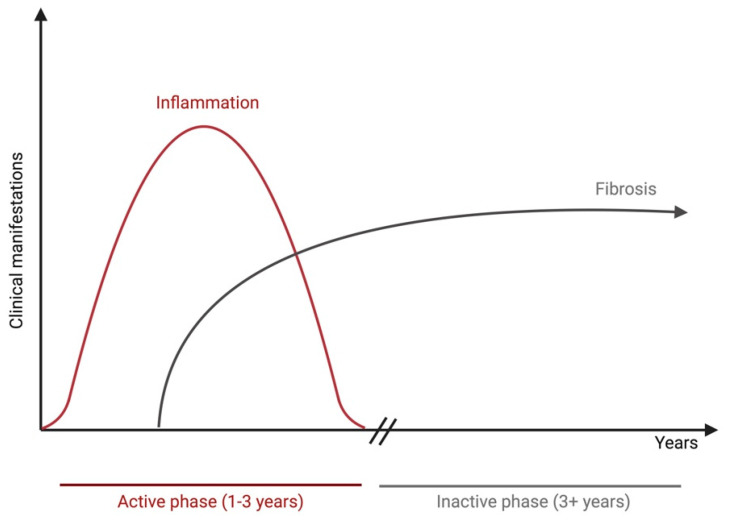
TED is classically described as active or inactive and follows the trajectory of the Rundle’s curve (Figure 3). The active phase constitutes progressive inflammation lasting between 6 and 24 months, ultimately reaching a plateau, at which point the inactive phase commences with fibrosis and regression of inflammation [42]. Reactivation of TED can occur in certain circumstances. Several studies have shown that smoking constitutes one of the major risk factors for TED reactivation [46,47,48]. Smokers with TED were also shown to be at greater risk for TED progression, as well as poor treatment outcomes [46]. Additional risk factors for disease reactivation encompass sustained TSH-R antibodies [14], radioactive iodine treatment (risk of progression of 15–39%) [49,50], and steroid treatment discontinuation in moderate-to-severe TED patients, in which up to 25% of reactivation rates were reported [51,52].
Figure 3.
Schematic representation of the Rundle’s curve. The thyroid eye disease course is composed of an active phase, which lasts from 1 to 3 years and may or may not involve resolution of clinical manifestations during the disease course, and an inactive phase of 3 years duration and more. The figure was created with BioRender.com.
The most common finding is upper eyelid retraction, occurring in 90% of patients. The etiology of upper lid retraction is believed to be multifactorial, with sympathetic overactivity, inflammation, and fibrosis of Muller’s muscle, enlargement and overactivity of the levator palpebrae superioris (LPS) secondary to inferior rectus restriction by way of Hering’s law, and LPS overaction secondary to weakening of the orbicularis oculi muscle. The etiology of lower lid retraction is believed to be in part due to tension on the lid margin from inferior rectus enlargement [42]. Eyelid edema may be attributed to lymphatic dilatation and perivascular lymphocytic infiltrates in the dermis [42].
Depending on the severity of lid retraction, chronic eye exposure may develop, leading to dry eye disease (DES) and, if severe, exposure keratopathy with a risk of corneal scarring, ulceration, perforation, and endophthalmitis [53,54]. This is further exacerbated by the presence of lagophthalmos, decreased blink frequency, and increased tear evaporation [53,54]. In addition to an evaporative dry eye component, there are also aqueous and lipid deficiencies in TED, with decreased tear production and increased meibomian gland dropout [55]. Inflammatory changes in the ocular surface, including squamous cell metaplasia, superior limbic keratoconjunctivitis, and increased pro-inflammatory cytokines such as IL-1β, IL-6, IL-7, L-8, IL-10, IL-17A, TNF-α, lysozyme C, lacritin, and zinc-alpha-2 glycoprotein 1, with subsequently decreased tear breakup time and Schirmer’s test, have been reported [55,56,57,58,59,60,61]. Occasionally, inflammatory DES may be an early symptom of TED; in fact, one single-center study found that nearly 4% of patients with refractory DES had occult TED [62]. Treatment options for DES secondary to TED include lubrication, topical cyclosporine, topical steroids, and tarsorrhaphy [63].
Proptosis affects 60% of patients and is caused by the expansion of EOM and orbital fat, further increasing the risk of eye exposure [53,54] EOM dysfunction, observed in 40% of patients, manifests itself as diplopia and misalignment of the eyes [53]. The inferior rectus, then the medial rectus, and ultimately the superior rectus are most commonly involved, with only rare involvement of the lateral rectus or oblique muscles [64]. The resultant restrictive strabismus, occurring in 15% of patients, manifests as hypotropia and esotropia, which last beyond the active phase of TED due to eventual collagen deposition and fibrosis [65].
TED mainly causes diplopia during upgaze due to inferior rectus muscle hypertrophy, but in some patients, diplopia during downgaze caused by superior rectus hypertrophy is particularly debilitating for daily activities [59]. Diplopia from strabismus may be managed with prisms, monocular occlusion, and botulinum toxin A injection into the affected EOMs, and eventual strabismus surgery once the disease is rendered inactive [53,54,66]. Eye pain, described as dull and pressure-like, is reported in 30% of patients [53].
Dyschromatopsia, visual field loss, and/or visual acuity loss due to DON occurs in 6–15% of patients and is an emergency requiring immediate management, including surgical orbital decompression [53,67]. The mechanism of action for DON includes mechanical compression of the optic nerve by the EOMs, an increase in orbital adipose tissue, crowding of the orbital apex, venous congestion, and resultant ischemia. A component of stretch optic neuropathy from proptosis has also been hypothesized [68]. Older age, male sex, smoking, TSHR-Ab levels, and the presence of the cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) gene are identified as potential risk factors [69]. Additionally, Asian patients, who often present with more diffuse EOM involvement yet less prominent proptosis, may be at a greater risk of DON, possibly due to the shallower and more compact orbital anatomy [67]. Indeed, patients with minimal proptosis were found to be at higher risk of neuropathy due to the restrictive space within the bony walls, leading to a secondary ‘compartment syndrome effect’ [54].
A recent meta-analysis has demonstrated an association between patients with TED and the incidence of glaucoma; approximately 29% of patients with TED had glaucoma in comparison to non-TED patients [70]. Furthermore, the odds ratio (OR) for glaucoma diagnosis in patients with TED was reported to be 6.42 (95% confidence interval 4.76–8.70). However, previous results on this topic are conflicting. Kim et al. previously reported a similar incidence of glaucoma in TED patients when compared to the general population but a greater prevalence (6.8%) of ocular hypertension in TED patients [71].
The increased risk of open-angle glaucoma (OAG) can partially be due to the elevated episcleral venous pressure and changes in ocular perfusion; thus, glaucomatous optic neuropathy must be distinguished from DON [59,69]. The pathophysiology of TED-associated glaucoma remains complex. Alongside changes in episcleral venous pressure, deposition of glycosaminoglycans within the trabecular meshwork was also hypothesized to be involved in its pathophysiology [72]. In addition, the treatment of TED, which is further discussed in the ensuing sections, can raise the intraocular pressure, therefore establishing different pressure gradients within the lamina cribrosa and leading to glaucomatous changes [73]. Nevertheless, additional studies are required to better establish the relationship of TED with the prevalence or incidence of glaucoma in this population.
3.1. Risk Factors
3.1.1. Metabolic and Environmental Risk Factors
Hyperthyroidism—and, to a much lesser extent, hypothyroidism—exacerbates TED; thus, achieving euthyroid status is essential to stabilizing the disease process. In fact, the onset of hyperthyroidism correlates with the onset of TED within 18 months before or after in 85% of cases [59]. Smoking is a significant modifiable risk factor—smokers with GD have nearly a 10-fold higher risk of developing TED compared to non-smokers, tend to develop more severe forms of TED, and respond less favorably to immunosuppressive treatments; these sequelae are diminished in former smokers. Hypoxia and oxidative stress from active smoking are believed to be the culprits [1,31,67]. Recent studies have explored the role of hypercholesterolemia in TED development, with some suggesting a beneficial effect of statins, although the mechanism of both hypercholesterolemia and statins in the role of TED is not well understood [1]. Diabetes, obstructive sleep apnea, and vitamin D deficiency have also been implicated as potential risk factors [67].
3.1.2. Genetic Risk Factors
Although genetic predispositions have been stipulated to be involved in the differences observed for the susceptibility for TED development in different ethnic populations, until today, this aspect remains poorly understood [74]. Single nucleotide polymorphisms (SNPs) in the genes coding for peroxisome proliferator-activated receptor gamma (PPARγ) and TSH-R were proposed as one of the mechanisms [75,76,77].
4. Diagnostic Methods
4.1. History and Ocular Exam
Patient history, including age, sex, ethnicity, comorbidities, systemic associations, pregnancy or pregnancy potential, tobacco smoking, other environmental and psychological stressors, and likelihood of adherence to medical therapies, must be elicited in any new patient with TED. It is important to ensure the absence of overt thyrotoxicosis, which can be life-threatening with complications including ischemia, atrial fibrillation, congestive heart failure, thromboembolism, stroke, psychosis, paralysis, and thyroid storm [43].
A comprehensive ocular examination includes documentation of visual acuity, presence or absence of a relative afferent pupillary defect, intraocular pressure, and color vision (the Hardy–Rand–Rittler plates may be more sensitive than the Ishihara plates for DON, given it would result in an acquired color vision deficiency) [78], Hertel exophthalmometry to document proptosis, and extraocular movements to identify limitations (Table 1). Eyelid changes, including the presence of lateral flare, lid lag, lagophthalmos, Bell’s reflex, and upper and lower eyelid retraction, should be noted. Ocular surface changes, including tear breakup time or Schirmer’s test, presence of superior limbic keratoconjunctivitis, exposure keratopathy, and corneal ulceration, and any related sequelae such as corneal perforation must be noted [59]. Many providers will obtain optical coherence tomography (OCT) of the macula and optic nerve as well as a formal visual field to document baseline structure and function; this is especially pertinent in cases where glaucomatous optic neuropathy—and, of course, DON—may be suspected.
Table 1.
Summary of the required ocular eye exam assessments and their possible findings suggestive of thyroid eye disease.
| Ocular Examination Component | Findings |
|---|---|
| Examination of the orbits and adnexa | |
| Eyelids | Presence of lateral flare, lid lag, lagophthalmos and Bell’s reflex, upper and lower eyelid retraction |
| Extraocular muscle assessment | Extraocular eye movement limitations Presence of proptosis secondary to extraocular muscle enlargement |
| Anterior segment examination | |
| Cornea | Decreased tear breakup time Superior limbic keratoconjunctivitis Exposure keratopathy Corneal ulceration |
| Posterior segment examination | |
| Optic nerve assessment | Increased cup-to-disc ratio Optic nerve head pallor Optic disc swelling Decreased color vision Presence or absence of a relative afferent pupillary defect |
| Neurological investigations | |
| Visual field testing | Presence of any visual field defect secondary to optic nerve damage |
4.2. Assessment of Clinical Activity
Several classification systems to quantify the clinical activity and severity exist and are summarized in Table 2 [59,79]. The NOSPECS and RELIEF classification systems represent now obsolete classification systems historically used to quantify clinical severity without information on clinical activity [43,59,67,79]. The CAS (Clinical Activity Score) grading system assesses clinical activity with a 7-point system for initial evaluation. Successive examinations are scored with a 10-point system. A score greater than 3/7 or 4/10 is considered an active disease. The EUGOGO (European Group of Graves’ Orbitopathy) severity grading system assesses clinical activity and severity with mild, moderate-to-severe, and sight-threatening categories. Mild disease comprises one or more of the following: minor lid retraction < 2 mm, mild soft-tissue involvement, exophthalmos < 3 mm above normal for sex and race, and transient or no diplopia. Moderate-to-severe disease comprises one or more of the following: lid retraction > 2 mm, moderate-to-severe soft-tissue involvement, exophthalmos > 3 mm above normal for sex and race, and inconstant or constant diplopia. Finally, sight-threatening disease includes DON, or corneal breakdown. The VISA (Vision, Inflammation, Strabismus, and Appearance) grading system represents yet another classification system to assess clinical activity and severity; the associated VISA Inflammatory Index is scored out of 10 such that a score < 4/10 represents moderate disease and is managed conservatively, and severe disease with a score > 5/10 is managed more aggressively [43,67,79]. Patients with evidence of disease progression and a score of 5 out of 10 are as well managed more aggressively. The EUGOGO severity grading system is more commonly used in Europe, whereas the VISA grading system is more commonly used in North America [59,79].
Table 2.
Summary of classification systems for clinical activity and severity of thyroid eye disease.
| Grade | Criteria |
|---|---|
| NOSPECS a system | |
| 0 | No physical signs or symptoms |
| I | Only signs, no symptoms |
| II | Soft tissue involvement |
| II-0 | Absent |
| II-1 | Minimal |
| II-2 | Moderate |
| II-3 | Marked |
| III | Proptosis (>2 mm of normal upper limit) |
| III-1 | 3–4 mm |
| III-2 | 5–7 mm |
| III-3 | >8 mm |
| IV | Extraocular muscle involvement |
| IV-0 | Absent |
| IV-1 | Limitation of motion at extremes of gaze |
| IV-2 | Evident restriction of movements |
| IV-3 | Fixed globe(s) |
| V | Corneal involvement due to lagophthalmos |
| V-0 | Absent |
| V-1 | Stippling of cornea |
| V-2 | Ulceration |
| V-3 | Clouding, necrosis, and perforation |
| VI | Sight loss due to optic nerve involvement |
| VI-0 | Absent |
| VI-1 | Disc pallor or visual field defect Visual acuity 20/20 to 20/60 |
| VI-2 | Disc pallor or visual field defect Visual acuity 20/70 to 20/200 |
| VI-3 | Blindness Visual acuity less than 20/200 |
| Mild disease encompasses grades 1 to 3, whereas severe disease encompasses grades 4 to 6. | |
| RELIEF a system | |
| R | Resistance to retropulsion |
| E | Edema of conjunctiva and caruncle |
| L | Lacrimal gland enlargement |
| I | Edema of eyelids |
| F | Fullness of eyelids |
| Clinical activity score | |
| Initial evaluation scored out of 7 | |
| Spontaneous orbital pain | |
| Gaze-evoked orbital pain | |
| Lid edema | |
| Lid erythema | |
| Conjunctival erythema | |
| Chemosis | |
| Caruncle or plica inflammation | |
| Successive evaluations scored out of 10 | |
| Increase of >2 mm proptosis | |
| >8° decrease in ocular motility of one eye in any direction | |
| Decrease in visual acuity of 1 Snellen line | |
| A clinical assessment score (CAS) greater than 3 out of 7 or 4 out of 10 is considered to be an active disease. Each positive element is given one point. | |
| European Group of Graves’ Orbitopathy severity grading system | |
| Soft tissue assessment | |
| Eyelid swelling | |
| 1 | Absent |
| 2 | Mild: none of the features defining moderate or severe categories |
| 3 | Moderate: definite swelling, no lower eyelid festoons or angulation of the upper eyelid skin fold in downgaze |
| 4 | Severe: lower eyelid festoons or upper eyelid fold becomes rounded at 45° in downgaze |
| Eyelid erythema | |
| 1 | Absent |
| 2 | Present |
| Conjunctival erythema | |
| 1 | Absent |
| 2 | Mild: equivocal or minimal |
| 3 | Moderate: <50% |
| 4 | Severe: >50% |
| Conjunctival edema | |
| 1 | Absent |
| 2 | Present |
| Caruncle or plica semilunaris inflammation | |
| 1 | Absent |
| 2 | Present |
| Document | |
| Lid margin assessment at the mid-pupillary line | |
| Palpebral aperture (mm) | |
| Upper and lower lid retraction (mm) | |
| Levator function (mm) | |
| Lagophthalmos | |
| 1 | Absent |
| 2 | Present |
| Bell’s phenomenon | |
| 1 | Absent |
| 2 | Present |
| Proptosis assessment | |
| Hertel exophthalmometry to record intercanthal distance and amount of proptosis | |
| Ocular motility assessment | |
| Prism cover test | |
| Monocular ductions | |
| Head posture | |
| Torsion | |
| Binocular single vision | |
| Corneal integrity assessment | |
| Normal | |
| Punctate keratopathy | |
| Ulcer | |
| Perforation | |
| Optic nerve assessment | |
| Visual acuity | |
| Color vision | |
| Visual field analysis | |
| Optic disc assessment: normal, atrophied, glaucomatous, or edematous | |
| Afferent pupil defect | |
| 1 | Present |
| 2 | Absent |
| Mild disease encompasses one or more of the following: minor lid retraction (<2 mm), mild soft-tissue involvement, exophthalmos < 3 mm above normal for race and gender, no or intermittent diplopia, and corneal exposure responsive to treatments; moderate-to-severe disease encompasses two or more of the following: lid retraction > 2 mm, moderate or severe soft-tissue involvement, exophthalmos > 3 mm above normal for race and gender, inconsistent or consistent diplopia; sight-threatening disease encompasses patients with dysthyroid optic neuropathy and/or corneal breakdown. | |
| VISA a system | |
| 1 point | Vision |
| Visual acuity | |
| Pupillary reflex | |
| Color vision | |
| Visual fields | |
| Optic nerve examination | |
| Visual evoked potentials | |
| 10 points | Inflammation/congestion |
| Caruncular edema | |
| 0 | Absent |
| 1 | Present |
| Chemosis | |
| 0 | Absent |
| 1 | Conjunctiva lies behind the gray line of the lid |
| 2 | Conjunctiva extends anterior to the gray line of the lid |
| Conjunctival erythema | |
| 0 | Absent |
| 1 | Present |
| Lid redness | |
| 0 | Absent |
| 1 | Present |
| Lid edema | |
| 0 | Absent |
| 1 | Present, without redundant tissues |
| 2 | Present, with lower lid festoon and bulging palpebral skin |
| Retrobulbar ache at rest | |
| 0 | Absent |
| 1 | Present |
| Retrobulbar ache with gaze | |
| 0 | Absent |
| 1 | Present |
| Diurnal variation | |
| 0 | Absent |
| 1 | Present |
| 6 points | Strabismus/motility restriction |
| Diplopia | |
| 0 | Absent |
| 1 | Diplopia with horizontal or vertical gaze |
| 2 | Intermittent diplopia in primary gaze |
| 3 | Constant diplopia in primary gaze |
| Ocular restriction | |
| 0 | Duction > 45° |
| 1 | Duction 30–45° |
| 2 | Duction 15–30° |
| 3 | Duction < 15° |
| 3 points | Appearance/exposure |
| Appearance concerns (i.e., proptosis, lid retraction, and fat pockets) | |
| Symptoms from ocular exposure (i.e., foreign body sensation, photophobia, dryness, and secondary tearing) | |
| Measurements including eyelid retraction, scleral show, LPS function, lagophthalmos, and proptosis with Hertel exophthalmometer | |
| Signs of corneal exposure, including punctate epithelial erosions, ulcerations, corneal thinning, and corneal perforation | |
| The VISA classification system tool is useful for assessing disease progression or response to therapy, as well as disease activity or quiescence. | |
a Abbreviations: NOSPECS, No signs or symptoms, Only signs, no symptoms, Soft tissue involvement, Proptosis, Extraocular muscle involvement, Corneal involvement, Sight loss; RELIEF, Resistance to retropulsion, Edema of the conjunctiva and caruncle, Lacrimal gland enlargement, Injection of horizontal rectus muscle insertions, Edema of the eyelids, and Fullness of the eyelids; VISA, Vision, Inflammation, Strabismus, and Appearance.
4.3. Imaging Modalities
4.3.1. Computed Tomography
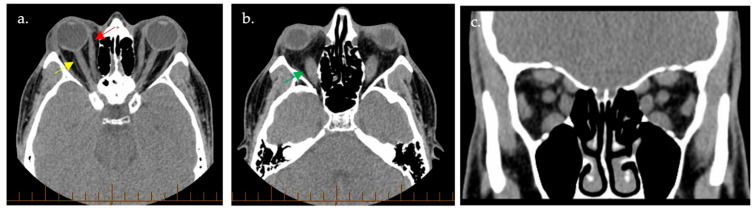
Radiographic imaging is instrumental in guiding the management of TED (Figure 4). Computed tomography (CT) of the orbits classically demonstrates a tendon-sparing enlargement of the EOM bellies, colloquially dubbed the ‘coca-cola sign’, with an increase in the orbital fat volume [53,59,65]. CT imaging may be especially helpful in clarifying the diagnosis of TED by revealing bilateral radiographic disease in cases where the presence of only unilateral disease is suspected clinically [59]. The order of muscle involvement is inferior rectus, medial rectus, and finally, the superior rectus; involvement of the lateral rectus or oblique muscles is not typically seen. Although this is the most frequent finding, it is worth noting that any single or combination of muscle involvement can occur. Other signs include apical crowding and increased angle of the orbital apex (i.e., a deviation from the normal positioning or the orbital apex, which is the point where the optic nerve enters the orbit), increased angle of the medial orbital wall (i.e., the medial orbital wall angles are usually parallel to each other), enlarged superior ophthalmic vein (SOV), orbital fat prolapse, perineural fat effacement, enlarged lacrimal gland, and a taut optic nerve in cases with significant proptosis. Orbitopathy may be radiographically classified as primarily lipogenic (type I), primarily myogenic (type II), or mixed (type III) [59]. CT imaging exophthalmometry should also be measured, as it is more reliable than Hertel exophthalmometry; in cases of lateral orbital decompression where the interzygomatic method of exophthalmometry cannot be utilized, the measurement from the posterior clinoid process to the anterior corneal surface may suffice instead [59,80].
Figure 4.
Computed tomography images demonstrated extraocular muscle enlargement in thyroid eye disease. Computed tomography (CT)-based images in axial (a,b) and coronal (c) planes showcase superior (yellow arrow), medial (red arrow), and inferior (green arrow) rectus muscle enlargement bilaterally.
4.3.2. Magnetic Resonance Imaging
Magnetic resonance imaging (MRI) can provide additional soft tissue resolution, further aiding in the assessment of disease activity [53]. There is a positive correlation between the T1 signal intensity ratio, T1 post-gadolinium signal intensity ratio, and apparent diffusion coefficient with the CAS. T1-weighted images of inactive TED show isointense EOM and facial muscles, with the enhancement of EOMs post-gadolinium. T2-weighted images of inactive TED show hypointense EOMs, whereas active TED has hyperintense EOMs. Barrett’s index, used to assess for the presence of DON, may be derived from CT imaging or MRI—the vertical index is the sum of the vertical muscle diameters divided by the height of the orbit through the optic nerve; the horizontal index is the sum of the horizontal muscle diameters divided by the width of the orbit through the optic nerve. A Barrett’s index greater than 60% is suggestive of DON [59,81].
4.3.3. Orbital Color Doppler Imaging
Orbital color doppler imaging (CDI) is emerging as an important radiographic mean to assess the hemodynamic changes that occur with TED [59]. Orbital and ocular perfusion may be sequentially monitored for prognostication and to assess treatment response [69,82]. Blood flow velocities are measured in the SOV, ophthalmic artery (OA), central retinal artery (CRA), and posterior ciliary artery (PCA). The active phase of TED demonstrates high peak systolic and end-diastolic velocities in the OA and CRA. Reduction or reversal in SOV maximum velocity are noted due to elevation in cone pressure following soft tissue expansion in the orbit; these findings, in addition to the CRA-resistivity index, are improved after orbital decompression [69]. Furthermore, changes in choroidal thickness and the retinochoroidal microvasculature have been noted on OCT with and without angiography [69].
4.4. Serologies and Molecular Biomarkers
TED is associated with hyperthyroidism in 90% of cases, hypothyroidism in 5%, and occurs in euthyroid individuals in the remaining 5% [54]. TED is most commonly linked to GD, which is caused by auto-antibody stimulation of the TSHR with the TSHR-Ab [53]. TSHR-Ab levels correlate with the CAS, representing the only known biomarker for TED and specifically for GO [1,83]. TSHR expression was shown to be elevated in the orbital fat of patients with TED; the activity of TSHR-Ab through TSHR binding stimulates the release of pro-inflammatory cytokines in the orbital tissues, leading to the accumulation of mucopolysaccharides and glycosaminoglycans with resultant soft tissue swelling [84]. TSHR-stimulating immunoglobulins (TSI), a subtype of TSHR-Ab, have also been found to correlate with disease activity and specifically with DON [85,86].
Competitive-binding assays and cell-based bioassays may be used to assess different types of TSHR-Ab throughout the disease course (Table 3). Competitive-binding assays provide information on the presence and concentration of TSHR-Ab through the measurement of TSHR-binding inhibitory immunoglobulins (TBII) and are available globally, whereas cell-based bioassays provide functional information through the measurement of TSI, including whether the antibody is stimulatory or inhibitory, and are generally available in large commercial laboratories or academic centers [83,84]. Both TBII and TSI represent subtypes of the TSHR-Ab. The latest, third-generation competitive-binding assays measure the inhibitory ability of TBII on M22, a labeled F(ab)2 human TSHR-stimulating monoclonal antibody, on the TSHR. TSHR-Ab is detected in 95–98% of patients with GD using third-generation competitive binding assays. Cell-based bioassays utilize stably transfected cell lines or chimeric cell lines expressing TSHR to measure the stimulatory activity in patients with GD, with 99–100% sensitivity and 100% specificity values for antibody function [84]. In contrast, anti-thyroid peroxidase (TPO) and anti-thyroglobulin (anti-Tg) antibodies have not shown a correlation with TED [53].
Table 3.
Sensitivity and specificity of current competitive-binding assays and cell-based bioassays for the diagnosis of thyroid eye disease.
Liquid Biopsy: A Novel Approach
Liquid biopsy is a novel technology that has gained great interest in the past few years for disease diagnosis and monitoring given its non-invasive nature, low cost, highly sensitive nature, and the possibility to provide real-time monitoring of diseases [90]. Although the application of liquid biopsy has been thoroughly discussed in the diagnosis and monitoring of cancers [90,91,92], in this section, we discuss the possibility of translating liquid biopsy technology to TED diagnosis and disease monitoring, a tool not yet applicable given the lack of well-defined, sensitive, and proven biomarkers.
Traditionally, liquid biopsy consists of isolating and analyzing tumor-derived moieties, which encompasses circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), tumor-derived extracellular vesicles (EVs), and circulating cell-free RNA (cfRNA) [90]. The role of miRNAs, which are a subtype of cfRNA, has been discussed in the pathogenesis of TED. It was demonstrated that the serological expression of numerous miRNAs, such as miR-130a [93,94], miR-484, and miR-192-5p [95], is altered in TED. The expression of miR-130a was shown to be elevated in patients with TED, and it modulated adipogenesis through the inhibition of AMPK activity [93,94]. Furthermore, miR-103a-3p was shown to be elevated in TGFβ-activated orbital fibroblasts, where it was shown to promote cell proliferation and differentiation through the ERK-Jun N-terminal kinase (JNK) and TGFβ/Smad signaling pathways [96].
Numerous challenges exist for the implementation of liquid biopsies in the diagnosis, management, and treatment response monitoring of TED. A major drawback is the lack of evidence-based biomarkers. Although several cytokines, miRNAs, and protein expression modifications have been reported in TED, the sensitivity in detecting their serological expression through liquid biopsy has yet to be fully established. Furthermore, technical limitations, such as the lack of resources and well-trained technicians, limit the translation of liquid biopsy into clinical practice for TED management. However, with the growing body of literature on this topic, we believe liquid biopsies could offer a rapid method of screening, diagnosis, and disease monitoring for treatment for TED in the near future.
5. Current Therapeutic Approaches
The management of TED currently involves a dynamic and multidisciplinary approach. In mild cases, pharmacological treatment may not be required. However, in moderate or severe disease, medical intervention, predominantly pharmacological but also surgical, is required in order to prevent sight-threatening complications. This section aims to discuss the current pharmacological landscape in TED to highlight the uses, advantages, and limitations of current drugs used in TED treatment (Table 4). However, before delving into the drugs, it is important to note that there is no current universal gold-standard treatment for TED nor a consensus on an optimal treatment plan. Thus, current management is guided by individual expert opinion. Studies aimed at establishing a gold-standard treatment approach would be highly beneficial to the field. In 2021, the EUGOGO published a clinical guideline for the management of TED to help address this issue; however, it has not been widely adopted in clinical practice globally [97].
Table 4.
A summary of the posology, advantages, and disadvantages of current immunosuppressive and glucocorticoids used to treat thyroid eye disease.
| Treatment | Posology | Advantages | Disadvantages | References |
|---|---|---|---|---|
| First-line treatments | ||||
| Intravenous Methylprednisolone (IVMP) | EUGOGO Regimen
|
|
|
[97,98,99,100,101] |
| Oral Prednisone |
|
|
|
[102] |
| Second-line treatments | ||||
| Azathioprine |
|
|
|
[103,104,105] |
| Cyclosporine |
|
|
|
[106,107,108] |
| Mycophenolate |
|
|
|
[2] |
| Sirolimus |
|
|
|
[109] |
| Methotrexate |
|
|
|
[110,111,112] |
The current first-line treatment is glucocorticoids (GCs). However, biological agents, notably teprotumumab, which was approved in 2020, are increasingly being used as first-line treatment options. One meta-analysis compared these two options, concluding that teprotumumab is more likely to reduce proptosis and diplopia than GCs [113]. However, the lack of direct comparison limits conclusive evidence of clinical superiority, and thus GCs are still widely the preferred first-line treatment in many countries. Furthermore, the greater cost of teprotumumab compared to GCs makes its selection as a first-line treatment less likely in certain countries where the medical financial budget is limited.
Among GCs, there are two clinically relevant therapies: intravenous methylprednisolone (IVMP) and oral prednisone. Both GCs share common mechanisms of action, which include increasing anti-inflammatory processes and suppressing pro-inflammatory aspects of TED [2,114]. Although IVMP is more laborious to administer, it is preferred to oral prednisone, as it has been demonstrated to be more efficacious and with fewer adverse events than oral prednisone in three randomized controlled trials (RCTs) [2,98,101,102,115]. Thus, IVMP is commonly thought of as the gold standard first-line treatment for moderate-to-severe TED.
Teprotumumab has emerged as a promising treatment for moderate-to-severe TED. Teprotumumab is a humanized monoclonal antibody that partially antagonizes IGF-1R by binding its alpha subunit and is demonstrated to be effective in reducing TED clinical activity, as well as reducing the severity of diplopia and proptosis. Teprotumumab was shown to reduce proptosis by 2.31 mm on average (95% confidence interval range 1.17 to 3.45 mm), as well as reduce the CAS to 0 or 1, outlining its great clinical impact on TED [116]. However, its use was shown to induce ototoxicity [117]. Teprotumumab is the first of several promising biologics to be approved for TED and provides an alternative treatment option for patients with moderate-to-severe TED who cannot tolerate or fail to respond to glucocorticoids. Some clinicians now support the use of teprotumumab as first-line treatment, especially in chronic TED, but its clinical use is limited by its cost—which is estimated at USD 360,000 for a 75 kg patient, 5000 times more than intravenous glucocorticoids [118,119]—and a lack of rigorous scientific evidence directly comparing it to IVMP [120].
In refractory cases of TED, there are several second-line treatment options, such as biologics, orbital radiation, and immunosuppressants. Yet, there is a lack of direct evidence supporting one second-line treatment over the other. As a result, the clinical use of these drugs is individualized for the patients through shared decision-making. There are several options a physician can choose from, including immunomodulators, notably tocilizumab targeting IL-6 and rituximab targeting CD-20, both of which will be discussed in more detail. Additionally, there are a multitude of immunosuppressive agents, such as mycophenolate, azathioprine, cyclosporine, methotrexate, and sirolimus, which are used at the discretion of the physician and patient. Altogether, these immunosuppressive agents serve as viable alternatives to IVMP or teprotumumab, but with limited evidence, it is frequently unclear when one specific agent is superior to another.
TED is treated based on severity. Pharmaceutical intervention is considered when TED progresses to moderate, severe, or sight-threatening stages. The absence of medical intervention in mild cases is due to the high frequency of spontaneous resolution. For these patients, it is sufficient to treat local symptoms and monitor the progression carefully [97,121]. However, it is important to note that selenium has been demonstrated to be beneficial in a subset of patients with mild TED [97,122]. Selenium has an important anti-oxidative effect in the thymus, where it exists in high concentrations. RCT evidence supports selenium supplementation for the improvement in quality of life and ophthalmic outcomes in mild cases of TED [2,97,122]. Even though selenium was only studied in mild cases, EUGOGO recommended a 6-month selenium regimen for those with active disease or mild TED due to its positive impact on ophthalmic outcomes [97].
6. Novel Therapeutic Approaches
The cutting-edge advances in TED pathogenesis, as well as in the field of pharmaceutics, have led to the establishment of numerous clinical trials over the past years, with a goal of developing targeted immunotherapy for the treatment of TED (Table 5). In the ensuing section, we will cover the novel therapeutic approaches with strong evidence, the upcoming possibilities, and possible strategies that require further investigation.
Table 5.
An overview of the current and recently completed clinical trials investigating novel treatments for thyroid eye disease.
| NCT Number (Start-End Date) |
Study Phase | Drug | Mechanism of Action | Study Title |
|---|---|---|---|---|
|
NCT04598815 (2023–2025) |
Phase II | Sirolimus | Inhibits T cell proliferation and fibroblast proliferation | Sirolimus for Graves’ Orbitopathy (GO) (SIRGO) |
|
NCT04936854 (2023–2026) |
Phase II | Sirolimus | Immunosuppression: inhibits T-cell activation, inhibits the IGF-1 pathway, anti-fibroblast effects | Sirolimus vs. Corticosteroids in Treatment of Thyroid Eye Disease |
| NCT05126147 (2022–2024) | Phase IV | Hydroxychloroquine | Inhibits proliferation and adipogenesis in orbital fibroblasts | Hydroxychloroquine in Mild Graves’ Orbitopathy |
| NCT04359979 (2020–2023) | N/A | Tamsulosin | Inhibits catecholamine alpha-1 receptors | Tamsulosin for Thyroid Lid Retraction |
| NCT05394857 (2022–2023) | Phase II | Vunakizumab | Antibody targeting IL-17a, preventing interaction with receptor | Efficacy and Safety of SHR-1314 by Subcutaneous Injection in Active Moderate to Severe Graves’ Orbitopathy Patients |
|
NCT04737330 (2021–2023) |
Phase III | Secukinumab | Antibody targeting IL-17a, preventing interaction with receptor | A Study of the Efficacy and Safety of Secukinumab 300 mg in Patients With Thyroid Eye Disease (TED) (ORBIT) |
|
NCT05331300 (2022–2025) |
Phase I/II | LASN01 | Antibody against IL-11R | A Study to Evaluate the Safety, Preliminary Efficacy, and Pharmacokinetic Properties of LASN01 in Healthy Subjects and in Patients With Pulmonary Fibrosis or Thyroid Eye Disease |
|
NCT05002998 (2021–2025) |
Phase IV | Teprotumumab | Antibody that inhibits IGF-1R | TEPEZZA® (Teprotumumab-trbw) Post-Marketing Requirement Study |
| NCT05276063 (2022–2026) | Phase IIb | Linsitinib | Small molecule that Inhibits intrinsic tyrosine kinase activity of IGF-1R and IR | A Phase 2b, Study of Linsitinib in Subjects With Active, Moderate to Severe Thyroid Eye Disease (LIDS) |
| NCT05176639 (2021–2023) | Phase I, II, III | VRDN-001 | Antibody that Inhibits IGF-1R | A Safety, Tolerability and Efficacy Study of VRDN 001 in Healthy Volunteers and Persons With Thyroid Eye Disease (THRIVE) |
|
NCT06021054 (2023–2025) |
Phase III | VRDN-001 | Antibody that Inhibits IGF-1R | A Randomized, Double-masked, Placebo-controlled Safety, Tolerability, and Efficacy Study of VRDN-001, a Humanized Monoclonal Antibody Directed Against the IGF-1 Receptor, in Participants With Chronic Thyroid Eye Disease (TED) (THRIVE-2) |
|
NCT06384547 (2024–2026) |
Phase III | VRDN-001 | Antibody that Inhibits IGF-1R | A Randomized, Active Controlled, Safety and Tolerability Study of VRDN-001 in Participants With Thyroid Eye Disease (TED) (STRIVE) |
|
NCT05683496 (2023–2024) |
Phase I-II | Lonigutamab | Inhibits IGF-1R | A Phase 1/2, Adaptive, Multiple Dose Ranging Study Evaluating the Safety, Tolerability, Pharmacokinetics, and Clinical Efficacy of Lonigutamab in Subjects With Thyroid Eye Disease (TED) |
|
NCT04876534 (2022–2023) |
Phase II | Tocilizumab | Antibody that inhibits membrane-bound and soluble IL-6R | Tocilizumab in Active Moderate-severe Graves’ Orbitopathy (TOGO) |
| NCT05987423 | Phase III | Satralizumab | Humanized monoclonal antibody targeting IL-6R | Study To Evaluate The Efficacy, Safety, Pharmacokinetics, And Pharmacodynamics Of Satralizumab In Participants With Moderate-To-Severe Thyroid Eye Disease |
|
NCT02378298 (2011–2024) |
Phase IV | Rituximab | Antibody that inhibits CD-20 on B lymphocytes | Rituximab (RTX) Therapy in Patients With Active TAO |
| EudraCT Number: 2015-002127-26 (2015-ongoing) |
Phase II | Belimumab | Antibody targeting BLyS resulting in the inhibition of B cell differentiation and triggering apoptosis | Comparison between treatment with belimumab and methylprednisolone in Graves’hyperthyroidism (GD) and active orbitopathy (GO). |
|
NCT05517421, NCT0552457 (2022–2025) |
Phase III | Batoclimab | Inhibits binding of FcRn to anti-TSHR autoantibodies, leading to degradation of anti-TSHR autoantibodies | Study to Assess Batoclimab in Participants With Active Thyroid Eye Disease |
6.1. Teprotumumab and IGF-1R Inhibitors
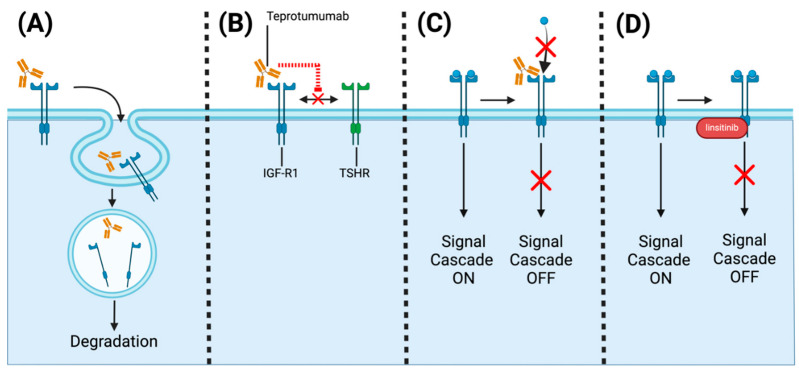
As discussed in the previous section, teprotumumab is a monoclonal antibody that was initially developed as an oncology drug [2]. However, it was repurposed as a treatment for TED. It functions by partially antagonizing IGF-1R, which co-localizes with TSHR on orbital fibroblasts. By binding IGF-1R’s alpha subunit, teprotumumab inhibits IGF-1R through three mechanisms: (i) internalization and degradation of IGF-1R, (ii) IGF-1R activation blockade, and (iii) IGR-1R and TSHR complex formation inhibition (Figure 5) [123,124,125]. As a result, teprotumumab prevents IGF-1R-mediated activation of IL-6 and IL-8 synthesis by TSH, ultimately having an anti-inflammatory effect [123,124,125]. Teprotumumab is currently administered intravenously, with current regimens requiring patients to receive eight infusions, which are 60 to 90 min in length every 3 weeks, a time-consuming and expensive process [120].
Figure 5.
Targeting IGF-R1 for the treatment of thyroid eye disease. (A) Tetrotumumab as well as other antibodies against IGF-1R can trigger the internalization and degradation of IGF-1R. (B) Antibodies against IGF-1R can disrupt the interaction between TSHR and IGF-1R. (C) Antibodies targeting IGF-1R can disrupt ligand binding (red ✕) and downstream signal activation (red ✕). (D) Small molecules disrupt the downstream signaling cascade by targeting tyrosine kinase domains (red ✕). The figure was created with BioRender.com.
In preliminary phase 2 and 3 OPTIAC trials, teprotumumab was compared to placebo in active moderate and severe TED. The primary endpoint was a decrease in CAS of greater than 2, which was achieved in 69% of patients receiving teprotumumab compared to 20% in the placebo arm at 24 weeks. Along with a strong improvement in quality of life, diplopia, and proptosis (decrease of 2 mm or greater) in patients with GO, these data supported its FDA approval in 2020 for use in TED, independently of its severity [126].
Currently, a multi-center phase IV post-marketing study is underway to address the safety of the current regimen and possible alternatives. The study is composed of 300 patients and will test three different cohorts of dosing regimens of teprotumumab (4, 8, or 16 infusions). The study is examining the frequency of treatment-related adverse events and treatment response, which will help clarify the need and clinical use of teprotumumab (NCT05002998).
Teprotumumab, although a promising therapy for TED, is difficult to administer due to its requirement for IV delivery [2,127]. As a result, new IGF-1R antagonists have been sought. VRDN-001 is a full IGF-1R antagonist that was studied in a phase I/II clinical trial at doses of 3 mg/kg, 10 mg/kg, and 20 mg/kg. Key outcomes included an 83% overall responder rate and significant reductions in both proptosis (by 2.4 mm) and CAS (by 4.3 points) [128]. A phase III study is currently being conducted to assess the clinical outcomes of VRDN-001 compared to placebo in patients with moderate to severe TED (NCT05176639). Two other actively recruiting phase III trials are assessing VRDN-001 in specific populations, such as chronic TED patients, and assessing adverse effects over a longer time frame (NCT06021054, NCT06384547). Two new IGF-R1 monoclonal antibodies, VRDN-002 and VRDN-003, are also being studied. They are distinct as they have modified Fc fragments of VRDN-001 to increase the half-life of the drug [129,130,131,132]. In preclinical studies, both VRDN-002 and VRDN-003 were administered to non-human primates intravenously and subcutaneously, with the latter presenting a clinical advantage over teprotumumab. In healthy volunteers, IV administration of VRDN-002 was well tolerated with an increased half-life [130]. The results of these preclinical studies are promising and may fuel future VRDN-002 and VRDN-003 clinical investigations [126]. A notable advantage of these drugs is subcutaneous administration, which is less laborious than IV administration. Lonigutamab is another subcutaneous IGF-1R inhibitor currently in phase I/II clinical study evaluating its safety and efficacy in patients with TED (NCT05683496).
Linsitinib is a small-molecule dual inhibitor of both IGF-1R and insulin receptors. Linsitinib inhibits the tyrosine kinase domain of IGF-1R, preventing autophosphorylation and downstream signaling [133,134]. In preclinical studies in murine models of GO, researchers successfully prevented the development and progression of TED, serving as foundational evidence for clinical studies of linsitinib [133]. A key advantage of linsitinib is its oral route of administration. Currently, a phase IIb RCT (LIDS) is underway to assess the safety and efficacy of linsitinib compared to placebo in patients with active moderate-to-severe TED (NCT05276063).
6.2. Tocilizumab and IL-6 Receptor Antagonists
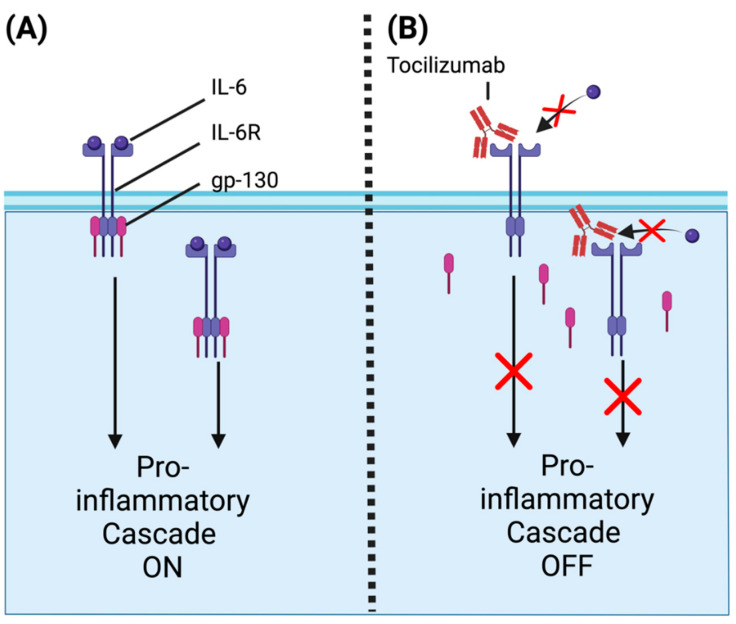
Tocilizumab is a monoclonal antibody widely used in the treatment of autoimmune conditions such as rheumatoid arthritis. It targets interleukin-6 receptors (IL-6R), which recruit gp-130 that then dimerizes to activate a pro-inflammatory cascade involved in B and T cell activation [2]. IL-6R exists both in the soluble and membrane form in a multitude of immune cells, both of which are inhibited by tocilizumab, resulting in the prevention of downstream homodimer formation with gp-130 (Figure 6). The end result is the inhibition of IL-6R-mediated pro-inflammatory pathways such as antibody and acute phase protein synthesis [135].
Figure 6.
Mechanism of action of antibodies targeting IL-6R. (A) Uninhibited IL-6R is activated by its ligand, IL-6, leading to the dimerization of gp-130 and activation of a signaling cascade that activates the pro-inflammatory response. (B) Tocilizumab disrupts the interaction between IL-6R and its ligand (red ✕), preventing gp-130 dimerization and inhibiting the signaling cascade that triggers pro-inflammatory responses (red ✕). The figure was created with BioRender.com.
Prior to its study in TED, tocilizumab was mainly used to treat rheumatoid arthritis. The foundation for its use in TED came from a small RCT (n = 32) with GO patients who had failed IV corticosteroid therapy. In patients receiving IV tocilizumab, there were greater reductions in CAS (86% achieving CAS lower than 3 vs. 39% in the placebo) at 0, 4, 8, and 12 weeks [136]. Other small observational studies, as well as a systematic review, have also supported tocilizumab as a second-line therapy [137,138,139]. However, a larger, long-term RCT would be needed to favor widespread clinical implementation of tocilizumab [137,138,139]. Currently, a phase 2 trial of unknown status is comparing tocilizumab to IVMP in patients with moderate-to-severe TED (NCT04876534), and additional studies would greatly clarify the optimal use of tocilizumab for TED.
6.3. Rituximab and Targeting B Cells
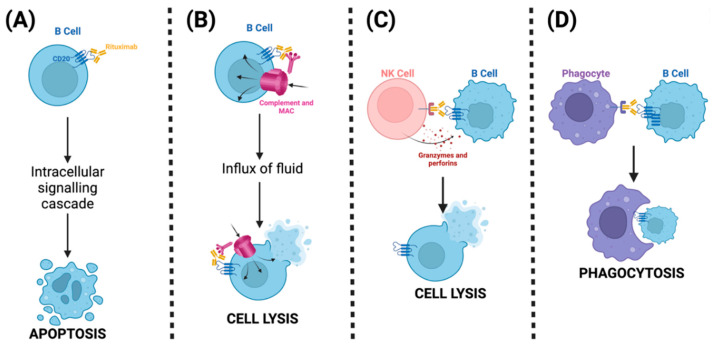
Initially developed as the first molecule to treat non-Hodgkin’s lymphoma, rituximab (RTX) is now increasingly used to treat various autoimmune diseases. RTX is a monoclonal antibody directed against CD20 surface antigen, which is involved in B-cell differentiation and activation [140,141]. RTX inhibits B cells through several pathways. Firstly, RTX activates intracellular signaling pathways, resulting in cell apoptosis (Figure 7) [140,141]. Its Fc domain activates the classical pathway of the complement system by activating C1, leading to the formation of membrane attack complexes (MAC), causing cell lysis [140,141]. Another pathway mediated by RTX’s Fc domain is the activation of NK cells through their fragment crystallizable receptor (FCR), which results in the release of granzymes and perforins, causing cytotoxic death of B cells [140,141]. Finally, through FCRs of phagocytes, such as macrophages, RTX induces phagocytosis of B cells, resulting in their intracellular degradation [140,141].
Figure 7.
Mechanism of action of Rituximab, an antibody specific to CD20 on the surface of B cells. (A) Rituximab binds its ligand CD20, which can trigger an intracellular signaling cascade, leading to apoptosis of B cells. (B) Complement activation by rituximab leads to deposition of MACs that cause an influx of fluid and cell lysis of B cells. (C) Fc fragments of rituximab trigger Fc receptors on NK cells, activating them and leading to the release of granzymes and perforins and cell lysis of B cells. (D) Phagocytes are activated through their Fc receptor, recognizing the Fc fragment of rituximab, leading to the degradation of the B cell through phagocytosis. The figure was created with BioRender.com.
The use of rituximab to treat TED is based on two RCTs, one conducted in Italy and the other in the United States. However, these two small single-center studies provided conflicting results. In the American study, patients with moderate-to-severe TED were given either RTX or placebo, and no advantage or reduction in CAS was seen in the experimental arm [142]. However, the Italian study compared RTX (1000 mg 2 weeks apart) to IVMP and found that RTX outperformed IVMP, with all patients showing inactivation of GO at 24 weeks in the RTX arm, compared to 69% in the IVMP arm [143]. Despite the conflicting results, RTX is used as a second-line treatment; however, future studies could aim to elucidate which subset of TED patients would benefit most from RTX.
Another strategy to target B-cells has also been investigated. Belimumab, an immunomodulator approved for lupus treatment, is a monoclonal antibody that targets B-lymphocyte stimulator protein (BLyS, aka. BAFF), a cytokine involved in B-cell differentiation [144]. By binding its target BLyS, belimumab inhibits BLyS interaction with BCR as well as other proteins, ultimately inhibiting B-cell differentiation into plasma cells and triggering apoptosis [144]. Belimumab is being studied as a treatment for TED on the basis that BLyS levels are elevated in TED patients. An ongoing RCT comparing belimumab to IVMP is being conducted in Europe in patients with moderate-severe GO (EudraCT Number: 2015-002127-26) [145]. The interim analysis of this study provides evidence that belimumab is as effective as IVMP in treating GO, albeit with a slower effect but notably with improved tolerability [145].
6.4. Future Therapeutic Possibilities
TSHR Inhibitors
TSHR inhibitors are an emerging, promising therapeutic strategy for TED. This is largely due to past evidence demonstrating that TSHR has a synergistic relationship with IGF-R1 and has been seen to be overexpressed in TED, leading to adipogenesis [2,146]. Even though TSHR’s involvement in TED is not fully understood, drugs targeting it are under study (Table 6).
Table 6.
Most notable preclinical studies exploring possible novel treatments for thyroid eye disease.
| Drug | Mechanism of Action | Results | References |
|---|---|---|---|
| VRDN-002 | Antibody targeting IGF-R1 | Safely administered to primates intravenously and subcutaneously with increased half-life compared to VRDN-001 | [129,130] |
| VRDN-003 | Antibody Targeting IGF-R1 | Safely administered to primates intravenously and subcutaneously with increased half-life compared to VRDN-001 | [131,132] |
| S37A | Small molecule that interferes with signal transduction of TSHR | Can inhibit TSHR signaling in the presence of its ligand TSH | [147] |
| ANTAG3 and Lisitinib | Antibody targeting both TSHR and IGF-R1 | A synergistic effect of the drug combination was seen in GO fibroblasts | [148] |
In preclinical studies, one such drug, K1-70, a recombinant human antibody, has been shown to interfere with TSHR signaling by interfering with ligand binding [149]. These findings led to a phase 1 clinical trial that demonstrated tolerable IV and intramuscular (IM) administration in patients with Grave’s disease [150]. It is not yet used for TED treatment, as no further clinical trials have yet been conducted. Other TSHR antagonists are being investigated as potential therapies. S37A is a small molecule that interferes with signal transduction by inhibiting TSHR signaling in the presence of its ligand in cell lines and has shown promising results in studies [2,147]. These studies have not yet been translated into practice, but nevertheless, they show that TSHR is a potential target for TED treatments. As TSHR and IGF-R1 cross-communicate, there is an idea to inhibit both targets concurrently. This has been investigated in cultures of GO fibroblasts [148,151]. These studies revealed a synergistic effect, one that could be beneficial to reduce required doses or compensate for the inefficiency of targeting solely one receptor [148]. Further studies are required to conclude if there is any clinical significance of this combination strategy.
6.5. Future Therapeutic Strategies
6.5.1. Targeting Cytokines
The promising results of recently FDA-approved immunomodulating agents have further driven the exploration of novel immune markers, cytokines, and cells as targets. Most of the drug candidates have been validated in preclinical models and are currently being investigated in clinical trials. The first notable target being studied is IL-17. IL-17 has been associated with TED pathophysiology, and thus a group is examining vunakizumab (SHR-1314), a subcutaneously administered monoclonal antibody targeting IL-17a, preventing its interaction with its receptor. A phase I study in psoriasis patients demonstrated that vunakizumab is tolerable in human subjects, and this study is being translated into a phase II clinical trial for patients with moderate-to-severe TED (NCT05394857). Another group conducted a phase III clinical trial for secukimumab, another monoclonal antibody targeting IL-17a, in patients with TED (NCT04737330). However, this study was terminated early due to the low probability of meeting the primary endpoint. Therefore, targeting IL-17a as a therapy for TED still remains unconfirmed [152].
Another cytokine being targeted in phase I and II clinical trials in patients with TED and pulmonary fibrosis is IL-11 (NCT05331300, NCT0622545). Although the role of IL-11 in TED is not well understood, it is postulated that IL-11 may play a role in the altered phenotype of orbital fibroblasts [2,153]. Thus, researchers are currently striving to inhibit the IL-11 receptor (IL-11R) with LASN01, a fully human antibody. The goal of this strategy is to prevent signaling and pro-inflammatory activity mediated by IL-11R, which would act as a potential therapy for TED.
6.5.2. Neonatal Fragment Crystallizable Receptor
The neonatal fragment crystallizable receptor (FcRn) plays a crucial role in transporting immunoglobulin G (IgG) across barriers and protecting it from degradation. FcRn inhibitors have recently emerged as a potential treatment for various autoimmune diseases, including Myasthenia Gravis [154]. Batoclimab is a monoclonal antibody that targets FcRn, preventing it from recycling IgG [126]. In TED, this may enhance the breakdown of pathogenic autoantibodies targeting TSHR and IGF-R1 [126]. In a phase IIa trial (ASCEND-GO 1), seven patients with active moderate-to-severe TED received batoclimab as weekly subcutaneous injections. The results showed a 64.8% decrease in serum IgG levels and a 56.7% reduction in anti-TSHR antibodies [155]. However, the subsequent phase IIb trial (ASCEND-GO 2) was terminated due to increased serum cholesterol levels in participants [2,126]. It is unclear if batoclimab will be used in TED clinically. To address this, a phase III, multi-center, randomized, placebo-controlled study is currently ongoing to evaluate the efficacy of batoclimab for the treatment of TED (NCT05517421, NCT0552457).
6.5.3. Sirolimus
Sirolimus is an immunosuppressive agent that has recently been studied in clinical trials to treat TED. It acts by suppressing the sensitivity of lymphocytes to cytokines, predominantly to IL-2, thus reducing lymphocyte activation [2]. In a preliminary observational study comparing sirolimus to IVMP, the sirolimus cohort outperformed IVMP at 24 weeks (86.6% to 26.6%) [109]. This study subsequently fueled a phase II trial, comparing first-line use of sirolimus to IVMP in patients with GO, striving to provide additional evidence to clarify the clinical significance of sirolimus (NCT04598815).
6.5.4. Statins
Statins are used to reduce cholesterol levels by inhibiting 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA). In recent studies, statins have been shown to be effective in treating TED. This is due to their anti-inflammatory and anti-immunomodulatory properties. Their anti-inflammatory effects include inhibiting the induction of pro-inflammatory cytokines released from T lymphocytes. One recent phase II clinical trial evaluated the effects of adding atorvastatin to intravenous glucocorticoids in patients with GO and hypercholesterolemia. Results showed that adding a statin to IVMP improved outcomes in patients with GO, although a bigger population is needed to confirm the results found in this study [156].
7. Novel Advances in Artificial Intelligence for the Management of Thyroid Eye Disease
Artificial intelligence (AI) is rapidly becoming immersed in medical practices. Its application within the field of ophthalmology has grown quickly as it presents the possibility to optimize diagnostics, prognostics, and disease management. TED is a highly complex and variable disease, reliant on a thorough diagnosis from imaging, clinical assessment, and thyroid function assessment. AI can be a valuable tool in managing TED, aiding every step of the way, from diagnosis and tracking disease progression to personalizing treatments.
7.1. Diagnostic Applications
Traditionally, TED diagnosis relies on a physician’s expertise, which can lead to inconsistencies in diagnosis and can limit the early detection of disease [157]. To address current limitations, AI modalities are being developed to facilitate the diagnosis and screening of TED through the accurate identification of early-stage clinical markers of TED. For example, current methods of measuring proptosis are limited by their lack of reproducibility and unreliability, largely due to the high degree of subjectivity involved [158]. To objectify this measurement, one study developed an AI model that can quickly and reliably identify clinically relevant proptosis based on orbital CT scans. Their model measured proptosis at a concordance correlation coefficient (CCC) of 0.9895 on axial CT images and a CCC of 0.9902 on sagittal CT images, which were both comparable to the results of clinician-based measurements [158]. With more advancements, an improved model would be a promising alternative to current standards that would optimize the reliability and accuracy of proptosis measurements. Another group applied their machine learning model to facial scans to assess the facial symptoms of TED. They trained their model to recognize specific symptoms, including but not limited to eyelid retraction, ocular dyskinesia, and conjunctival congestion. They concluded that their model could reliably identify patients with facial symptoms of TED with an average area under the curve (AUC) of 0.85 [159].
Beyond identifying and quantifying symptoms of TED, AI-based modalities have been explored for the purpose of screening for TED. One study developed an AI-powered tool aimed at diagnosing TED without clinician input based on orbital CT scans [160]. The model was externally verified (AUC of 0.919), and through non-inferiority experiments, the researchers concluded that their model could diagnose TED (85.67%) as accurately as residents (84.33%), whose clinical interpretation is the current benchmark in TED diagnosis [160]. If such a model could be perfected to outperform clinicians, they could be used to screen patients at risk, providing a valid, reliable, and automated diagnostic tool [157,160].
7.2. Disease Monitoring
AI could also aid in the management of TED by quantifying the severity of the disease to track progression and assist in the selection of an optimal treatment plan. To explore this application, one study developed an AI-powered program that could quantify inflammation of extraocular muscles based on contrast-enhanced MRI in order to evaluate the activity stage of TED [161]. The study examined three machine learning models, and the light gradient boosting machine (LightGBM) had the best diagnostic classification performance with an AUC of 0.9260 [161]. Another study examined a machine learning model’s ability to assess CAS. They found that their model could predict the CAS based on patients’ facial scans with an accuracy of 84.6% when training the model with an unfiltered data set, and the accuracy increased to 89.0% when trained with filtered data sets with consistent results [162]. Additionally, an AI-based model was trained off orbital CT scans to differentiate between active and inactive TED, achieving an AUC of 0.871 [163]. Altogether, these studies all developed AI-based modalities with the potential to be used clinically to analyze images to quickly provide clinicians with clinically relevant information that could assist in tracking disease progression and activity.
7.3. Decision Making Tool
The information acquired from AI-driven systems can also help clinicians guide their treatment selection. This was demonstrated by a group of researchers whose convolutional neural network (CNN), specifically tailored for image-centric tasks, could analyze orbital CT scans to quantify proptosis and identify patients that required surgical drainage [164]. Although this model’s measurements of proptosis differed significantly from those made by physicians, it still showed promise, as the linear and volumetric measurements of proptosis made by the CNN were more predictive of the requirement of surgery (AUC of 0.78 and 0.79, respectively) than physician measurements (AUC of 0.70) [164]. The volumetric AI-based proptosis measurements from CNN could be a valuable clinical tool to predict the likelihood of surgery. Another group examined the use of AI models in predicting TED patients’ responses to treatment. Their model, XGBoost, analyzed clinical characteristics and laboratory results, predicting the response of TED patients to steroid treatment with an accuracy of 0.861 [52]. When investigating the factors most impactful on XGBoost performance, the researchers determined that specific patterns of both thyroid-stimulating immunoglobulin and low-density lipoprotein cholesterol were the most significant in predicting the XGBoost’s ability to predict response [52]. These results further demonstrate the use of AI-based modalities in identifying specific clinical patterns that can impact a patient’s response to treatment or guide a physician in choosing the optimal treatment.
Altogether, the data that the AI tool can acquire from facial scans, imaging modalities (e.g., CT scans and MRIs), and laboratory diagnostics can open the possibility of quicker and improved clinical decision-making. Recent efforts to integrate AI in the clinical management of TED have demonstrated its potential to improve diagnosis, progression assessment, and clinical decision-making. As these systems continue to advance, their clinical integration will grow, offering clinicians valuable tools to deliver optimal and comprehensive care. However, major challenges constitute drawbacks to the translation of AI-based deep learning models for the management of TED to clinical practice [157]. The complexity of TED and the variability in its clinical manifestations are significant barriers. Greater training data sets are required to achieve excellent performance of deep learning models and enable their generalization to different populations. Furthermore, such models come with a great cost—high costs in their development and future application to clinical medicine. Additional technical limitations are also faced, such as the ethical aspects surrounding data privacy and the necessity for validation steps to filter and manage potential AI-derived medical errors.
8. Current Challenges and Future Perspectives
Autoimmune diseases, such as TED, have strikingly varying phenotypes and encompass complex pathogenesis. A current limitation in the management of TED is the lack of reliable markers to diagnose, assess the severity of disease, and establish treatment response [108]. Currently, ocular examination, repetitive laboratory investigations, and imaging modalities form the backbone of TED diagnosis. However, numerous efforts have been deployed in recent years to develop less invasive, cost-friendly, and rapid diagnostic tools such as liquid biopsy and AI-derived deep learning models. However, their translation to clinical practice is yet to be optimized. The limited understanding of disease pathology currently limits the exploitation of biological agents for TED treatment. For example, although TED patients do have IGF-1R autoantibodies, the presence of such markers has not been associated with the severity of the disease nor with susceptibility to specific treatments [108]. Further studies are thus required to close the fundamental science knowledge gap regarding TED pathogenesis.
Management of patients with TED involves a multidisciplinary approach that may require input from several specialists. Currently, the lack of consensus on the optimal treatment plan results in varying treatment approaches. IVMP and GCs have been used as the gold standard, followed by immunosuppressive agents. However, new biologics have emerged that may offer enhanced therapeutic benefits with fewer adverse events [108]. Teprotumumab is now viewed as a first-line treatment in TED patients in the United States [165], but its use is limited worldwide in the same setting, given its greater cost compared to GCs. We believe that through rigorous studies and the development of alternative options by pharmaceutical companies, these medications will offer specialists more diverse options to combat TED. Overall, biological agents will be most effective in an era of personalized medicine, whereby specific diagnostic markers can guide clinicians to optimal treatment regimens. Thus, linking specific markers to susceptibility to biologics may be required to fully reach the potential of biologics in treating TED.
Author Contributions
Conceptualization, M.K.; writing—original draft preparation, M.K., S.M.T., N.T., J.D. and A.D.; writing—review and editing, M.K., F.A., B.A. and C.E.-H.; visualization, M.K. and N.T.; supervision, B.A. and C.E.-H. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bartalena L., Gallo D., Tanda M.L., Kahaly G.J. Thyroid Eye Disease: Epidemiology, Natural History, and Risk Factors. Ophthal. Plast. Reconstr. Surg. 2023;39:S2–S8. doi: 10.1097/IOP.0000000000002467. [DOI] [PubMed] [Google Scholar]
- 2.Moledina M., Damato E.M., Lee V. The changing landscape of thyroid eye disease: Current clinical advances and future outlook. Eye. 2024;38:1425–1437. doi: 10.1038/s41433-024-02967-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh G., Sehgal M., Mithal A., Alabiad C., Kossler A. Severe thyroid eye disease in the US: A national perspective. J. Endocr. Soc. 2021;5((Suppl. S1)):A843. doi: 10.1210/jendso/bvab048.1720. [DOI] [Google Scholar]
- 4.Cockerham K.P., Padnick-Silver L., Stuertz N., Francis-Sedlak M., Holt R.J. Quality of Life in Patients with Chronic Thyroid Eye Disease in the United States. Ophthalmol. Ther. 2021;10:975–987. doi: 10.1007/s40123-021-00385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith T.J., Hegedüs L., Lesser I., Perros P., Dorris K., Kinrade M., Troy-Ott P., Wuerth L., Nori M. How patients experience thyroid eye disease. Front. Endocrinol. 2023;14:1283374. doi: 10.3389/fendo.2023.1283374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta V., Hammond C.L., Roztocil E., Gonzalez M.O., Feldon S.E., Woeller C.F. Thinking inside the box: Current insights into targeting orbital tissue remodeling and inflammation in thyroid eye disease. Surv. Ophthalmol. 2022;67:858–874. doi: 10.1016/j.survophthal.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith T.J. New advances in understanding thyroid-associated ophthalmopathy and the potential role for insulin-like growth factor-I receptor. F1000Research. 2018;7:134. doi: 10.12688/f1000research.12787.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khong J.J., McNab A.A., Ebeling P.R., Craig J.E., Selva D. Pathogenesis of thyroid eye disease: Review and update on molecular mechanisms. Br. J. Ophthalmol. 2016;100:142. doi: 10.1136/bjophthalmol-2015-307399. [DOI] [PubMed] [Google Scholar]
- 9.Bahn R.S. Graves’ ophthalmopathy. N. Engl. J. Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo N., Woeller C.F., Feldon S.E., Phipps R.P. Peroxisome proliferator-activated receptor γ ligands inhibit transforming growth factor-β-induced, hyaluronan-dependent, T cell adhesion to orbital fibroblasts. J. Biol. Chem. 2011;286:18856–18867. doi: 10.1074/jbc.M110.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawlowski P., Wawrusiewicz-Kurylonek N., Eckstein A., Reszec J., Luczynski W., Johnson K., Kretowski A., Bakunowicz-Lazarczyk A., Gorska M., Szamatowicz J., et al. Disturbances of Modulating Molecules (FOXP3, CTLA-4/CD28/B7, and CD40/CD40L) mRNA Expressions in the Orbital Tissue from Patients with Severe Graves’ Ophthalmopathy. Mediat. Inflamm. 2015;2015:340934. doi: 10.1155/2015/340934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang I.-H., Rose G.E., Ezra D.G., Bailly M. Macrophages promote a profibrotic phenotype in orbital fibroblasts through increased hyaluronic acid production and cell contractility. Sci. Rep. 2019;9:9622. doi: 10.1038/s41598-019-46075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Łacheta D., Miśkiewicz P., Głuszko A., Nowicka G., Struga M., Kantor I., Poślednik K.B., Mirza S., Szczepański M.J. Immunological Aspects of Graves’ Ophthalmopathy. BioMed Res. Int. 2019;2019:7453260. doi: 10.1155/2019/7453260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roos J.C.P., Paulpandian V., Murthy R. Serial TSH-receptor antibody levels to guide the management of thyroid eye disease: The impact of smoking, immunosuppression, radio-iodine, and thyroidectomy. Eye. 2019;33:212–217. doi: 10.1038/s41433-018-0242-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diana T., Brown R.S., Bossowski A., Segni M., Niedziela M., König J., Bossowska A., Ziora K., Hale A., Smith J., et al. Clinical Relevance of Thyroid-Stimulating Autoantibodies in Pediatric Graves’ Disease—A Multicenter Study. J. Clin. Endocrinol. Metab. 2014;99:1648–1655. doi: 10.1210/jc.2013-4026. [DOI] [PubMed] [Google Scholar]
- 16.Varewijck A.J., Boelen A., Lamberts S.W.J., Fliers E., Hofland L.J., Wiersinga W.M., Janssen J.A.M.J.L. Circulating IgGs May Modulate IGF-I Receptor Stimulating Activity in a Subset of Patients with Graves’ Ophthalmopathy. J. Clin. Endocrinol. Metab. 2013;98:769–776. doi: 10.1210/jc.2012-2270. [DOI] [PubMed] [Google Scholar]
- 17.Minich W.B., Dehina N., Welsink T., Schwiebert C., Morgenthaler N.G., Köhrle J., Eckstein A., Schomburg L. Autoantibodies to the IGF1 receptor in Graves’ orbitopathy. J. Clin. Endocrinol. Metab. 2013;98:752–760. doi: 10.1210/jc.2012-1771. [DOI] [PubMed] [Google Scholar]
- 18.Gontarz-Nowak K., Szychlińska M., Matuszewski W., Stefanowicz-Rutkowska M., Bandurska-Stankiewicz E. Current knowledge on Graves’ orbitopathy. J. Clin. Med. 2020;10:16. doi: 10.3390/jcm10010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weightman D.R., Perros P., Sherif I.H., Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity. 1993;16:251–257. doi: 10.3109/08916939309014643. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y., Zhang J., Deng W., Mo C., Liang Y., Huang K., Xu F., Tang F. Comparison of orbital fibroblasts from Graves’ ophthalmopathy and healthy control. Heliyon. 2024;10:e28397. doi: 10.1016/j.heliyon.2024.e28397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tuncel M. Thyroid Stimulating Hormone Receptor. Mol. Imaging Radionucl. Ther. 2017;26((Suppl. S1)):87–91. doi: 10.4274/2017.26.suppl.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woeller C.F., Roztocil E., Hammond C., Feldon S.E. TSHR Signaling Stimulates Proliferation Through PI3K/Akt and Induction of miR-146a and miR-155 in Thyroid Eye Disease Orbital Fibroblasts. Investig. Ophthalmol. Vis. Sci. 2019;60:4336–4345. doi: 10.1167/iovs.19-27865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieger C.C., Kahaly G.J., Azam A., Klubo-Gwiezdzinska J., Neumann S., Gershengorn M.C. Graves’ Autoantibodies Exhibit Different Stimulating Activities in Cultures of Thyrocytes and Orbital Fibroblasts Not Reflected by Clinical Assays. Thyroid®. 2022;32:90–96. doi: 10.1089/thy.2021.0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.LeRoith D., Holly J.M.P., Forbes B.E. Insulin-like growth factors: Ligands, binding proteins, and receptors. Mol. Metab. 2021;52:101245. doi: 10.1016/j.molmet.2021.101245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoa N., Tsui S., Afifiyan N.F., Sinha Hikim A., Li B., Douglas R.S., Smith T.J. Nuclear targeting of IGF-1 receptor in orbital fibroblasts from Graves’ disease: Apparent role of ADAM17. PLoS ONE. 2012;7:e34173. doi: 10.1371/journal.pone.0034173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang R., Song D., Zhong Y., Li H. Potential role of IGF-1R in the interaction between orbital fibroblasts and B lymphocytes: An implication for B lymphocyte depletion in the active inflammatory phase of thyroid-associated ophthalmopathy. BMC Immunol. 2024;25:31. doi: 10.1186/s12865-024-00613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohyi M., Smith T.J. IGF1 receptor and thyroid-associated ophthalmopathy. J. Mol. Endocrinol. 2018;61:T29–T43. doi: 10.1530/JME-17-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paik J.S., Kim S.-E., Kim J.H., Lee J.-Y., Yang S.-W., Lee S.-B. Insulin-like growth factor-1 enhances the expression of functional TSH receptor in orbital fibroblasts from thyroid-associated ophthalmopathy. Immunobiology. 2020;225:151902. doi: 10.1016/j.imbio.2019.151902. [DOI] [PubMed] [Google Scholar]
- 29.Kiernan K., Alwarawrah Y., Nichols A.G., Danzaki K., MacIver N.J. Insulin and IGF-1 have both overlapping and distinct effects on CD4+ T cell mitochondria, metabolism, and function. Sci. Rep. 2024;14:4331. doi: 10.1038/s41598-024-54836-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fang S., Huang Y., Zhong S., Li Y., Zhang Y., Li Y., Sun J., Liu X., Wang Y., Zhang S., et al. Regulation of Orbital Fibrosis and Adipogenesis by Pathogenic Th17 Cells in Graves Orbitopathy. J. Clin. Endocrinol. Metab. 2017;102:4273–4283. doi: 10.1210/jc.2017-01349. [DOI] [PubMed] [Google Scholar]
- 31.Buonfiglio F., Ponto K.A., Pfeiffer N., Kahaly G.J., Gericke A. Redox mechanisms in autoimmune thyroid eye disease. Autoimmun. Rev. 2024;23:103534. doi: 10.1016/j.autrev.2024.103534. [DOI] [PubMed] [Google Scholar]
- 32.Dik W.A., Virakul S., van Steensel L. Current perspectives on the role of orbital fibroblasts in the pathogenesis of Graves’ ophthalmopathy. Role Ocul. Fibros. Ophthalmic Dis. 2016;142:83–91. doi: 10.1016/j.exer.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Rapoport B., Pillarisetty R.J., Herman E.A., Congco E.G. Evidence for prostaglandin production by human lymphocytes during culture with human thyroid cells in monolayer: A possible role for prostaglandins in the pathogenesis of graves’ disease. Biochem. Biophys. Res. Commun. 1977;77:1245–1250. doi: 10.1016/S0006-291X(77)80113-7. [DOI] [PubMed] [Google Scholar]
- 34.Aoun T., Danielova Gueorguieva D., Wu K.Y. Orbital Inflammation in Thyroid Eye Disease: Stress Responses and Their Implications. Stresses. 2024;4:54–78. doi: 10.3390/stresses4010004. [DOI] [Google Scholar]
- 35.Ahsanuddin S., Wu A.Y. Single-cell transcriptomics in thyroid eye disease. Taiwan J. Ophthalmol. 2023 doi: 10.4103/tjo.TJO-D-23-00096. [DOI] [Google Scholar]
- 36.Patrick C., Roztocil E., Husain F., Feldon S.E., Woeller C. Activating the aryl hydrocarbon receptor with naturally occurring compounds to mitigate thyroid eye disease orbital fibroblast activation. Investig. Ophthalmol. Vis. Sci. 2024;65:3052. [Google Scholar]
- 37.Girnita L., Smith T.J., Janssen J.A.M.J.L. It Takes Two to Tango: IGF-I and TSH Receptors in Thyroid Eye Disease. J. Clin. Endocrinol. Metab. 2022;107((Suppl. S1)):S1–S12. doi: 10.1210/clinem/dgac045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou M., Lin B., Wu P., Ke Y., Huang S., Zhang F., Hei X., Mao Z., Li X., Wan P., et al. SOX9 Induces Orbital Fibroblast Activation in Thyroid Eye Disease Via MAPK/ERK1/2 Pathway. Investig. Ophthalmol. Vis. Sci. 2024;65:25. doi: 10.1167/iovs.65.2.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Galgoczi E., Orsos I., Molnar Z., Ujhelyi B., Steiber Z., Szabo L., Dienes B., Csernoch L., Nagy E.V., Katko M. Endocrine Abstracts. Volume 101 Bioscientifica; Bristol, UK: 2024. Modelling of mechanical stimuli through piezo1 receptor activation and its effect on adipogenic differentiation of orbital fibroblasts. [Google Scholar]
- 40.Ko J., Kim Y.J., Choi S.H., Lee C.S., Yoon J.S. Yes-Associated Protein Mediates the Transition from Inflammation to Fibrosis in Graves’ Orbitopathy. Thyroid®. 2023;33:1465–1475. doi: 10.1089/thy.2023.0309. [DOI] [PubMed] [Google Scholar]
- 41.Chin Y.H., Ng C.H., Lee M.H., Koh J.W.H., Kiew J., Yang S.P., Sundar G., Khoo C.M. Prevalence of thyroid eye disease in Graves’ disease: A meta-analysis and systematic review. Clin. Endocrinol. 2020;93:363–374. doi: 10.1111/cen.14296. [DOI] [PubMed] [Google Scholar]
- 42.Osaki T.H., Monteiro L.G., Osaki M.H. Management of eyelid retraction related to thyroid eye disease. Taiwan J. Ophthalmol. 2022;12:12–21. doi: 10.4103/tjo.tjo_57_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoang T.D., Stocker D.J., Chou E.L., Burch H.B. 2022 Update on Clinical Management of Graves’ Disease and Thyroid Eye Disease. Endocrinol. Metab. Clin. N. Am. 2022;51:287–304. doi: 10.1016/j.ecl.2021.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ugradar S., Goldberg R.A., Douglas R.S. Changing the face of thyroid eye disease. Eye. 2023;37:197–199. doi: 10.1038/s41433-022-02186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Subramaniam D.L., Yadalla D., Rajagopalan J. Are Severe Forms of Thyroid Eye Disease Common in the Indian Population? Clinical Characteristics of 136 Patients from a Tertiary Eye Care Centre. Indian J. Endocrinol. Metab. 2023;27:56–61. doi: 10.4103/ijem.ijem_280_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornton J., Kelly S.P., Harrison R.A., Edwards R. Cigarette smoking and thyroid eye disease: A systematic review. Eye. 2007;21:1135–1145. doi: 10.1038/sj.eye.6702603. [DOI] [PubMed] [Google Scholar]
- 47.Patel P., Khandji J., Kazim M. Recurrent Thyroid Eye Disease. Ophthal. Plast. Reconstr. Surg. 2015;31:445–448. doi: 10.1097/IOP.0000000000000371. [DOI] [PubMed] [Google Scholar]
- 48.Kimball L.E., Kulinskaya E., Brown B., Johnston C., Farid N.R. Does smoking increase relapse rates in Graves’ disease? J. Endocrinol. Investig. 2002;25:152–157. doi: 10.1007/BF03343979. [DOI] [PubMed] [Google Scholar]
- 49.Rashad R., Pinto R., Li E., Sohrab M., Distefano A.G. Thyroid Eye Disease. Life. 2022;12:2084. doi: 10.3390/life12122084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stan M.N., Bahn R.S. Risk factors for development or deterioration of Graves’ ophthalmopathy. Thyroid. 2010;20:777–783. doi: 10.1089/thy.2010.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zang S., Ponto K., Kahaly G. Intravenous glucocorticoids for Graves’ orbitopathy: Efficacy and morbidity. J. Clin. Endocrinol. Metab. 2011;96:320–332. doi: 10.1210/jc.2010-1962. [DOI] [PubMed] [Google Scholar]
- 52.Park J., Kim J., Ryu D., Choi H. Factors related to steroid treatment responsiveness in thyroid eye disease patients and application of SHAP for feature analysis with XGBoost. Front. Endocrinol. 2023;14:1079628. doi: 10.3389/fendo.2023.1079628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szelog J., Swanson H., Sniegowski M.C., Lyon D.B. Thyroid Eye Disease. Mo. Med. 2022;119:343–350. [PMC free article] [PubMed] [Google Scholar]
- 54.Li Z., Cestari D.M., Fortin E. Thyroid eye disease: What is new to know? Curr. Opin. Ophthalmol. 2018;29:528. doi: 10.1097/ICU.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 55.Rana H.S., Akella S.S., Clabeaux C.E., Skurski Z.P., Aakalu V.K. Ocular Surface Disease in Thyroid Eye Disease: A Narrative Review. Ocul. Surf. 2022;24:67–73. doi: 10.1016/j.jtos.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim Y.S., Kwak A.Y., Lee S.Y., Yoon J.S., Jang S.Y. Meibomian gland dysfunction in Graves’ orbitopathy. Can. J. Ophthalmol. J. Can. Ophtalmol. 2015;50:278–282. doi: 10.1016/j.jcjo.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 57.Gürdal C., Saraç O., Genç I., Kırımlıoğlu H., Takmaz T., Can I. Ocular surface and dry eye in Graves’ disease. Curr. Eye Res. 2011;36:8–13. doi: 10.3109/02713683.2010.526285. [DOI] [PubMed] [Google Scholar]
- 58.Aass C., Norheim I., Eriksen E.F., Børnick E.C., Thorsby P.M., Pepaj M. Comparative proteomic analysis of tear fluid in Graves’ disease with and without orbitopathy. Clin. Endocrinol. 2016;85:805–812. doi: 10.1111/cen.13122. [DOI] [PubMed] [Google Scholar]
- 59.Shah S.S., Patel B.C. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. [(accessed on 2 August 2024)]. Thyroid Eye Disease. Available online: http://www.ncbi.nlm.nih.gov/books/NBK582134/ [PubMed] [Google Scholar]
- 60.Bajkowska D., Szelachowska M., Buczyńska A., Krętowski A.J., Siewko K. Tears as a Source of Biomarkers in the Diagnosis of Graves’ Orbitopathy. Biomolecules. 2022;12:1620. doi: 10.3390/biom12111620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Khazaei H., Khazaei D., Verma R., Ng J., Wilmarth P.A., David L.L., Rosenbaum J.T. The Potential of Tear Proteomics for Diagnosis and Management of Orbital Inflammatory Disorders including Graves’ ophthalmopathy. Exp. Eye Res. 2021;213:108813. doi: 10.1016/j.exer.2021.108813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gupta A., Sadeghi P.B., Akpek E.K. Occult Thyroid Eye Disease in Patients Presenting with Dry Eye Symptoms. Am. J. Ophthalmol. 2009;147:919–923. doi: 10.1016/j.ajo.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 63.Gürdal C., Genç İ., Saraç Ö., Gönül İ., Takmaz T., Can İ. Topical Cyclosporine in Thyroid Orbitopathy-Related Dry Eye: Clinical Findings, Conjunctival Epithelial Apoptosis, and MMP-9 Expression. Curr. Eye Res. 2010;35:771–777. doi: 10.3109/02713683.2010.490320. [DOI] [PubMed] [Google Scholar]
- 64.Rana K., Juniat V., Patel S., Selva D. Extraocular muscle enlargement. Graefes Arch. Clin. Exp. Ophthalmol. Albrecht Graefes Arch. Klin. Exp. Ophthalmol. 2022;260:3419–3435. doi: 10.1007/s00417-022-05727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Glatt H.J. Optic nerve dysfunction in thyroid eye disease: A clinician’s perspective. Radiology. 1996;200:26–27. doi: 10.1148/radiology.200.1.8657923. [DOI] [PubMed] [Google Scholar]
- 66.Akbari M.R., Mirmohammadsadeghi A., Mahmoudzadeh R., Veisi A. Management of Thyroid Eye Disease-Related Strabismus. J. Curr. Ophthalmol. 2020;32:1–13. doi: 10.1016/j.joco.2019.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu C.Y., Ford R.L., Wester S.T., Shriver E.M. Update on thyroid eye disease: Regional variations in prevalence, diagnosis, and management. Indian J. Ophthalmol. 2022;70:2335–2345. doi: 10.4103/ijo.IJO_3217_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Agarwal A., Khanam S. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. [(accessed on 2 August 2024)]. Dysthyroid Optic Neuropathy. Available online: http://www.ncbi.nlm.nih.gov/books/NBK564299/ [PubMed] [Google Scholar]
- 69.Goel R., Shah S., Sundar G., Arora R., Gupta S., Khullar T. Orbital and ocular perfusion in thyroid eye disease. Surv. Ophthalmol. 2023;68:481–506. doi: 10.1016/j.survophthal.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 70.Delavar A., Radha Saseendrakumar B., Lee T.C., Topilow N.J., Ting M.A., Liu C.Y., Korn B.S., Weinreb R.N., Kikkawa D.O., Baxter S.L. Associations Between Thyroid Eye Disease and Glaucoma Among Those Enrolled in the National Institutes of Health All of Us Research Program. Ophthal. Plast. Reconstr. Surg. 2023;39:336–340. doi: 10.1097/IOP.0000000000002310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J.W., Ko J., Woo Y.J., Bae H.W., Yoon J.S. Prevalence of Ocular Hypertension and Glaucoma as Well as Associated Factors in Graves’ Orbitopathy. J. Glaucoma. 2018;27:464–469. doi: 10.1097/IJG.0000000000000942. [DOI] [PubMed] [Google Scholar]
- 72.Knepper P.A., Goossens W., Hvizd M., Palmberg P.F. Glycosaminoglycans of the human trabecular meshwork in primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 1996;37:1360–1367. [PubMed] [Google Scholar]
- 73.Wu J., Du Y., Li J., Fan X., Lin C., Wang N. The influence of different intraocular pressure on lamina cribrosa parameters in glaucoma and the relation clinical implication. Sci. Rep. 2021;11:9755. doi: 10.1038/s41598-021-87844-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee M.H., Chin Y.H., Ng C.H., Nistala K.R.Y., Ow Z.G.W., Sundar G., Yang S.P., Khoo C.M. Risk Factors of Thyroid Eye Disease. Endocr. Pract. 2021;27:245–253. doi: 10.1016/j.eprac.2020.11.011. [DOI] [PubMed] [Google Scholar]
- 75.Yin X., Latif R., Bahn R., Tomer Y., Davies T.F. Influence of the TSH receptor gene on susceptibility to Graves’ disease and Graves’ ophthalmopathy. Thyroid. 2008;18:1201–1206. doi: 10.1089/thy.2008.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pawlak-Adamska E., Daroszewski J., Bolanowski M., Oficjalska J., Janusz P., Szalinski M., Frydecka I. PPARg2 Ala 12 variant protects against Graves’ orbitopathy and modulates the course of the disease. Immunogenetics. 2013;65:493–500. doi: 10.1007/s00251-013-0702-0. [DOI] [PubMed] [Google Scholar]
- 77.Alevizaki M., Mantzou E., Cimponeriu A., Saltiki K., Philippou G., Wiersinga W. The Pro12Ala PPARγ gene polymorphism: Possible modifier of the activity and severity of thyroid-associated orbitopathy (TAO) Clin. Endocrinol. 2009;70:464–468. doi: 10.1111/j.1365-2265.2008.03343.x. [DOI] [PubMed] [Google Scholar]
- 78.Huna-Baron R., Glovinsky Y., Habot-Wilner Z. Comparison between Hardy–Rand–Rittler 4th edition and Ishihara color plate tests for detection of dyschromatopsia in optic neuropathy. Graefes Arch. Clin. Exp. Ophthalmol. 2013;251:585–589. doi: 10.1007/s00417-012-2073-x. [DOI] [PubMed] [Google Scholar]
- 79.Barrio-Barrio J., Sabater A.L., Bonet-Farriol E., Velázquez-Villoria Á., Galofré J.C. Graves’ Ophthalmopathy: VISA versus EUGOGO Classification, Assessment, and Management. J. Ophthalmol. 2015;2015:249125. doi: 10.1155/2015/249125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tiong T.Y.V., Sundar G., Young S.M., Makmur A., Yong H.R.C., Wong Y.L.J., Lang S.S., Tan A.P. A Novel Method of CT Exophthalmometry in Patients with Thyroid Eye Disease. Asia-Pac. J. Ophthalmol. 2020;9:39–43. doi: 10.1097/01.APO.0000617908.29733.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Monteiro M.L.R., Gonçalves A.C.P., Silva C.T.M., Moura J.P., Ribeiro C.S., Gebrim E.M.M.S. Diagnostic Ability Of Barrett’s Index to Detect Dysthyroid Optic Neuropathy Using Multidetector Computed Tomography. Clinics. 2008;63:301–306. doi: 10.1590/S1807-59322008000300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shah S.S., Khanam S. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. [(accessed on 2 August 2024)]. Orbital Color Doppler Imaging. Available online: http://www.ncbi.nlm.nih.gov/books/NBK572120/ [PubMed] [Google Scholar]
- 83.Kahaly G.J., Diana T., Olivo P.D. TSH Receptor Antibodies: Relevance & Utility. Endocr. Pract. 2020;26:97–106. doi: 10.4158/EP-2019-0363. [DOI] [PubMed] [Google Scholar]
- 84.Diana T., Kahaly G.J. Thyroid Stimulating Hormone Receptor Antibodies in Thyroid Eye Disease—Methodology and Clinical Applications. Ophthal. Plast. Reconstr. Surg. 2018;34:S13. doi: 10.1097/IOP.0000000000001053. [DOI] [PubMed] [Google Scholar]
- 85.Diana T., Ponto K.A., Kahaly G.J. Thyrotropin receptor antibodies and Graves’ orbitopathy. J. Endocrinol. Investig. 2021;44:703–712. doi: 10.1007/s40618-020-01380-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ponto K.A., Diana T., Binder H., Matheis N., Pitz S., Pfeiffer N., Kahaly G.J. Thyroid-stimulating immunoglobulins indicate the onset of dysthyroid optic neuropathy. J. Endocrinol. Investig. 2015;38:769–777. doi: 10.1007/s40618-015-0254-2. [DOI] [PubMed] [Google Scholar]
- 87.Bitcon V., Donnelly J., Kiaei D. Sensitivity of assays for TSH-receptor antibodies. J. Endocrinol. Investig. 2016;39:1195–1196. doi: 10.1007/s40618-016-0520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.López Ortega J.M., Martínez P.S., Acevedo-León D., Capell N.E. Anti-TSH receptor antibodies (TRAb): Comparison of two third generation automated immunoassays broadly used in clinical laboratories and results interpretation. PLoS ONE. 2022;17:e0270890. doi: 10.1371/journal.pone.0270890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee J.I., Jang H.W., Kim S.K., Choi J.Y., Kim J.Y., Hur K.Y., Kim J.H., Min Y.-K., Chung J.H., Kim S.W. Diagnostic value of a chimeric TSH receptor (Mc4)-based bioassay for Graves’ disease. Korean J. Intern. Med. 2011;26:179–186. doi: 10.3904/kjim.2011.26.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lone S.N., Nisar S., Masoodi T., Singh M., Rizwan A., Hashem S., El-Rifai W., Bedognetti D., Batra S.K., Haris M., et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer. 2022;21:79. doi: 10.1186/s12943-022-01543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Halawa T., Baeesa S., Fadul M.M., Badahdah A.A., Enani M., Fathaddin A.A., Kawass D., Alkhotani A., Bahakeem B., Kurdi M. The Role of Liquid Biopsy in the Diagnosis and Prognosis of WHO Grade 4 Astrocytoma. Cureus. 2023;15:e41221. doi: 10.7759/cureus.41221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kulbay M., Marcotte E., Remtulla R., Lau T.H., Paez-Escamilla M., Wu K.Y., Burnier M.N. Uveal Melanoma: Comprehensive Review of Its Pathophysiology, Diagnosis, Treatment, and Future Perspectives. Biomedicines. 2024;12:1758. doi: 10.3390/biomedicines12081758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Douglas C.E., Roztocil E., Hammond C., Gonzalez M., Feldon S.E., Woeller C. Investigating the role of circulating microRNAs as potential biomarkers for Thyroid Eye Disease. Investig. Ophthalmol. Vis. Sci. 2021;62:3349. [Google Scholar]
- 94.Hammond C.L., Roztocil E., Gonzalez M.O., Feldon S.E., Woeller C.F. MicroRNA-130a Is Elevated in Thyroid Eye Disease and Increases Lipid Accumulation in Fibroblasts Through the Suppression of AMPK. Investig. Ophthalmol. Vis. Sci. 2021;62:29. doi: 10.1167/iovs.62.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kim N., Choung H., Kim Y.J., Woo S.E., Yang M.K., Khwarg S.I., Lee M.J. Serum microRNA as a potential biomarker for the activity of thyroid eye disease. Sci. Rep. 2023;13:234. doi: 10.1038/s41598-023-27483-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xie B., Xiong W., Zhang F., Wang N., Luo Y., Chen Y., Cao J., Chen Z., Ma C., Chen H. The miR-103a-3p/TGFBR3 axis regulates TGF-β-induced orbital fibroblast activation and fibrosis in thyroid-eye disease. Mol. Cell. Endocrinol. 2023;559:111780. doi: 10.1016/j.mce.2022.111780. [DOI] [PubMed] [Google Scholar]
- 97.Bartalena L., Kahaly G.J., Baldeschi L., Dayan C.M., Eckstein A., Marcocci C., Marinò M., Vaidya B., Wiersinga W.M., EUGOGO The 2021 European Group on Graves’ orbitopathy (EUGOGO) clinical practice guidelines for the medical management of Graves’ orbitopathy. Eur. J. Endocrinol. 2021;185:G43–G67. doi: 10.1530/EJE-21-0479. [DOI] [PubMed] [Google Scholar]
- 98.Marcocci C., Bartalena L., Tanda M.L., Manetti L., Dell’Unto E., Rocchi R., Barbesino G., Mazzi B., Bartolomei M.P., Lepri P., et al. Comparison of the effectiveness and tolerability of intravenous or oral glucocorticoids associated with orbital radiotherapy in the management of severe Graves’ ophthalmopathy: Results of a prospective, single-blind, randomized study. J. Clin. Endocrinol. Metab. 2001;86:3562–3567. doi: 10.1210/jcem.86.8.7737. [DOI] [PubMed] [Google Scholar]
- 99.van Geest R.J., Sasim I.V., Koppeschaar H.P.F., Kalmann R., Stravers S.N., Bijlsma W.R., Mourits M.P. Methylprednisolone pulse therapy for patients with moderately severe Graves’ orbitopathy: A prospective, randomized, placebo-controlled study. Eur. J. Endocrinol. 2008;158:229–237. doi: 10.1530/EJE-07-0558. [DOI] [PubMed] [Google Scholar]
- 100.Bartalena L. Prevention of Graves’ ophthalmopathy. Best Pract. Res. Clin. Endocrinol. Metab. 2012;26:371–379. doi: 10.1016/j.beem.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 101.Kahaly G.J., Pitz S., Hommel G., Dittmar M. Randomized, single blind trial of intravenous versus oral steroid monotherapy in Graves’ orbitopathy. J. Clin. Endocrinol. Metab. 2005;90:5234–5240. doi: 10.1210/jc.2005-0148. [DOI] [PubMed] [Google Scholar]
- 102.Stiebel-Kalish H., Robenshtok E., Hasanreisoglu M., Ezrachi D., Shimon I., Leibovici L. Treatment modalities for Graves’ ophthalmopathy: Systematic review and metaanalysis. J. Clin. Endocrinol. Metab. 2009;94:2708–2716. doi: 10.1210/jc.2009-0376. [DOI] [PubMed] [Google Scholar]
- 103.Strianese D. Efficacy and Safety of Immunosuppressive Agents for Thyroid Eye Disease. Ophthal. Plast. Reconstr. Surg. 2018;34((Suppl. S1)):S56–S59. doi: 10.1097/IOP.0000000000001131. [DOI] [PubMed] [Google Scholar]
- 104.Perros P., Weightman D.R., Crombie A.L., Kendall-Taylor P. Azathioprine in the treatment of thyroid-associated ophthalmopathy. Acta Endocrinol. 1990;122:8–12. doi: 10.1530/acta.0.1220008. [DOI] [PubMed] [Google Scholar]
- 105.Rajendram R., Taylor P.N., Wilson V.J., Harris N., Morris O.C., Tomlinson M., Yarrow S., Garrott H., Herbert H.M., Dick A.D., et al. Combined immunosuppression and radiotherapy in thyroid eye disease (CIRTED): A multicentre, 2 × 2 factorial, double-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2018;6:299–309. doi: 10.1016/S2213-8587(18)30021-4. [DOI] [PubMed] [Google Scholar]
- 106.Matsuda S., Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology. 2000;47:119–125. doi: 10.1016/s0162-3109(00)00192-2. [DOI] [PubMed] [Google Scholar]
- 107.Prummel M.F., Mourits M.P., Berghout A., Krenning E.P., van der Gaag R., Koornneef L., Wiersinga W.M. Prednisone and cyclosporine in the treatment of severe Graves’ ophthalmopathy. N. Engl. J. Med. 1989;321:1353–1359. doi: 10.1056/NEJM198911163212002. [DOI] [PubMed] [Google Scholar]
- 108.Barbesino G., Salvi M., Freitag S.K. Future Projections in Thyroid Eye Disease. J. Clin. Endocrinol. Metab. 2022;107((Suppl. S1)):S47–S56. doi: 10.1210/clinem/dgac252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lanzolla G., Maglionico M.N., Comi S., Menconi F., Piaggi P., Posarelli C., Figus M., Marcocci C., Marinò M. Sirolimus as a second-line treatment for Graves’ orbitopathy. J. Endocrinol. Investig. 2022;45:2171–2180. doi: 10.1007/s40618-022-01862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yong K.-L., Chng C.-L., Ming Sie N., Lang S., Yang M., Looi A., Choo C.-T., Shen S., Seah L.L. Methotrexate as an Adjuvant in Severe Thyroid Eye Disease: Does It Really Work as a Steroid-Sparing Agent? Ophthal. Plast. Reconstr. Surg. 2019;35:369–373. doi: 10.1097/IOP.0000000000001279. [DOI] [PubMed] [Google Scholar]
- 111.Rivera-Grana E., Lin P., Suhler E.B., Rosenbaum J.T. Methotrexate as a Corticosteroid-Sparing Agent for Thyroid Eye Disease. J. Clin. Exp. Ophthalmol. 2015;6:422. doi: 10.4172/2155-9570.1000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Strianese D., Iuliano A., Ferrara M., Comune C., Baronissi I., Napolitano P., D’Alessandro A., Grassi P., Bonavolontà G., Bonavolontà P., et al. Methotrexate for the treatment of thyroid eye disease. J. Ophthalmol. 2014;2014:128903. doi: 10.1155/2014/128903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Douglas R.S., Dailey R., Subramanian P.S., Barbesino G., Ugradar S., Batten R., Qadeer R.A., Cameron C. Proptosis and Diplopia Response with Teprotumumab and Placebo vs the Recommended Treatment Regimen with Intravenous Methylprednisolone in Moderate to Severe Thyroid Eye Disease: A Meta-analysis and Matching-Adjusted Indirect Comparison. JAMA Ophthalmol. 2022;140:328. doi: 10.1001/jamaophthalmol.2021.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Längericht J., Krämer I., Kahaly G.J. Glucocorticoids in Graves’ orbitopathy: Mechanisms of action and clinical application. Ther. Adv. Endocrinol. Metab. 2020;11:204201882095833. doi: 10.1177/2042018820958335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aktaran Ş., Akarsu E., Erbağci İ., Araz M., Okumuş S., Kartal M. Comparison of intravenous methylprednisolone therapy vs. oral methylprednisolone therapy in patients with Graves’ ophthalmopathy: IVGC vs. OGC in Graves’ ophthalmopathy. Int. J. Clin. Pract. 2006;61:45–51. doi: 10.1111/j.1742-1241.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 116.Lin F., Yao Q., Yu B., Deng Z., Qiu J., He R. The Efficacy and Safety of Teprotumumab in Thyroid Eye Disease: Evidence from Randomized Controlled Trials. Int. J. Clin. Pract. 2023;2023:6638089. doi: 10.1155/2023/6638089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Najjar W., Yu J. Audiologic Demonstration of Ototoxicity from Teprotumumab Treatment in a Patient with Thyroid Eye Disease. OTO Open. 2022;6:2473974X221097097. doi: 10.1177/2473974X221097097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Perros P., Hegedüs L. Teprotumumab in thyroid eye disease: Wonder drug or great divider? Eur. Thyroid J. 2023;12:e230043. doi: 10.1530/ETJ-23-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Burch H.B., Perros P., Bednarczuk T., Cooper D.S., Dolman P.J., Leung A.M., Mombaerts I., Salvi M., Stan M.N. Management of thyroid eye disease: A consensus statement by the American Thyroid Association and the European Thyroid Association. Eur. Thyroid J. 2022;11:e220189. doi: 10.1530/ETJ-22-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Douglas R.S., Wang Y., Dailey R.A., Harris G.J., Wester S.T., Schiffman J.S., Tang R.A., Fowler B., Fleming J., Smith T.J. Teprotumumab in Clinical Practice: Recommendations and Considerations from the OPTIC Trial Investigators. J. Neuroophthalmol. 2021;41:461–468. doi: 10.1097/WNO.0000000000001134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bartalena L., Baldeschi L., Boboridis K., Eckstein A., Kahaly G.J., Marcocci C., Perros P., Salvi M., Wiersinga W.M., on behalf of the European Group on Graves’ Orbitopathy (EUGOGO) The 2016 European Thyroid Association/European Group on Graves’ Orbitopathy Guidelines for the Management of Graves’ Orbitopathy. Eur. Thyroid J. 2016;5:9–26. doi: 10.1159/000443828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Marcocci C., Kahaly G.J., Krassas G.E., Bartalena L., Prummel M., Stahl M., Altea M.A., Nardi M., Pitz S., Boboridis K., et al. Selenium and the Course of Mild Graves’ Orbitopathy. N. Engl. J. Med. 2011;364:1920–1931. doi: 10.1056/NEJMoa1012985. [DOI] [PubMed] [Google Scholar]
- 123.Yvon C., Khong J.J., Malhotra R., Patel B.C. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2024. [(accessed on 2 August 2024)]. Teprotumumab. Available online: http://www.ncbi.nlm.nih.gov/books/NBK585036/ [Google Scholar]
- 124.Ugradar S., Kang J., Kossler A.L., Zimmerman E., Braun J., Harrison A.R., Bose S., Cockerham K., Douglas R.S. Teprotumumab for the treatment of chronic thyroid eye disease. Eye. 2022;36:1553–1559. doi: 10.1038/s41433-021-01593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chen H., Mester T., Raychaudhuri N., Kauh C.Y., Gupta S., Smith T.J., Douglas R.S. Teprotumumab, an IGF-1R blocking monoclonal antibody inhibits TSH and IGF-1 action in fibrocytes. J. Clin. Endocrinol. Metab. 2014;99:E1635–E1640. doi: 10.1210/jc.2014-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kamboj A., Harrison A.R., Mokhtarzadeh A. Emerging therapies in the medical management of thyroid eye disease. Front. Ophthalmol. 2023;3:1295902. doi: 10.3389/fopht.2023.1295902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Douglas R.S., Kahaly G.J., Ugradar S., Elflein H., Ponto K.A., Fowler B.T., Dailey R., Harris G.J., Schiffman J., Tang R., et al. Teprotumumab Efficacy, Safety, and Durability in Longer-Duration Thyroid Eye Disease and Re-treatment. Ophthalmology. 2022;129:438–449. doi: 10.1016/j.ophtha.2021.10.017. [DOI] [PubMed] [Google Scholar]
- 128.Moster M., Douglas R., Cockerham K., Turbin R., Nijhawan N., Schiffman J., Ugradar S., O’Shaughnessy D., Summerfelt R., She A., et al. Phase 1/2 Clinical Study of VRDN-001, A Full Antagonist Antibody to the Insulin-Like Growth Factor-1 Receptor (IGF-1R), in Patients with Thyroid Eye Disease (TED) (P3-9.001) Neurology. 2023;100((Suppl. S2)):2098. [Google Scholar]
- 129.Dickinson B. VRDN-002, A Second-Generation Insulin Like Growth Factor-1 Receptor (IGF-1R) Inhibitory Antibody for Thyroid Eye Disease: Preclinical Pharmacokinetics and Clinical Promise. Investig. Ophthalmol. Vis. Sci. 2022;63:3995–A0337. [Google Scholar]
- 130.Foster K., She A., Summerfelt R.M., Dickinson B., Katz B., Wiczling P., Bedian V. VRDN-002, a Next-Generation Half-life Extended Antagonist Antibody to IGF-1 Receptor for Thyroid Eye Disease (TED): Safety and Pharmacokinetic/Pharmacodynamic (PK/PD) Results in Healthy Volunteers. Investig. Ophthalmol. Vis. Sci. 2023;64:4038. [Google Scholar]
- 131.Dickinson B., Foster K., Bedian V. Preclinical Pharmacokinetics of VRDN-003, A Next-Generation Half-life Extended Antibody to the IGF-1 Receptor for Thyroid Eye Disease. Investig. Ophthalmol. Vis. Sci. 2023;64:4043. [Google Scholar]
- 132.Newell R., Zhao Y., Bedian V. Design and Preclinical Characterization of VRDN-003, a Next-Generation, Half-life Extended Antibody to IGF-1 Receptor in Development for Thyroid Eye Disease (TED) Investig. Ophthalmol. Vis. Sci. 2023;64:4044. [Google Scholar]
- 133.Gulbins A., Horstmann M., Daser A., Flögel U., Oeverhaus M., Bechrakis N.E., Banga J.P., Keitsch S., Wilker B., Krause G., et al. Linsitinib, an IGF-1R inhibitor, attenuates disease development and progression in a model of thyroid eye disease. Front. Endocrinol. 2023;14:1211473. doi: 10.3389/fendo.2023.1211473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Puzanov I., Lindsay C.R., Goff L., Sosman J., Gilbert J., Berlin J., Poondru S., Simantov R., Gedrich R., Stephens A., et al. A Phase I Study of Continuous Oral Dosing of OSI-906, a Dual Inhibitor of Insulin-Like Growth Factor-1 and Insulin Receptors, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015;21:701–711. doi: 10.1158/1078-0432.CCR-14-0303. [DOI] [PubMed] [Google Scholar]
- 135.Okuda Y. Review of tocilizumab in the treatment of rheumatoid arthritis. Biol. Targets Ther. 2008;2:75–82. doi: 10.2147/btt.s1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perez-Moreiras J.V., Gomez-Reino J.J., Maneiro J.R., Perez-Pampin E., Romo Lopez A., Rodríguez Alvarez F.M., Castillo Laguarta J.M., Del Estad Cabello A., Gessa Sorroche M., España Gregori E., et al. Efficacy of Tocilizumab in Patients with Moderate-to-Severe Corticosteroid-Resistant Graves Orbitopathy: A Randomized Clinical Trial. Am. J. Ophthalmol. 2018;195:181–190. doi: 10.1016/j.ajo.2018.07.038. [DOI] [PubMed] [Google Scholar]
- 137.Ceballos-Macías José J., Rivera-Moscoso R., Flores-Real Jorge A., Vargas-Sánchez J., Ortega-Gutiérrez G., Madriz-Prado R., Velasco-Ramos P.D.L.C., Muñoz-Monroy Omar E., Meneses-Pérez Anna C., Fernández-Morales Irma N., et al. Tocilizumab in glucocorticoid-resistant graves orbitopathy. A case series report of a mexican population. Ann. Endocrinol. 2020;81:78–82. doi: 10.1016/j.ando.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 138.Sánchez-Bilbao L., Martínez-López D., Revenga M., López-Vázquez Á., Valls-Pascual E., Atienza-Mateo B., Valls-Espinosa B., Maiz-Alonso O., Blanco A., Torre-Salaberri I., et al. Anti-IL-6 Receptor Tocilizumab in Refractory Graves’ Orbitopathy: National Multicenter Observational Study of 48 Patients. J. Clin. Med. 2020;9:2816. doi: 10.3390/jcm9092816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Duarte A.F., Xavier N.F., Sales Sanz M., Cruz A.A.V. Efficiency and Safety of Tocilizumab for the Treatment of Thyroid Eye Disease: A Systematic Review. Ophthal. Plast. Reconstr. Surg. 2024;40:367–373. doi: 10.1097/IOP.0000000000002573. [DOI] [PubMed] [Google Scholar]
- 140.Randall K.L. Rituximab in autoimmune diseases. Aust. Prescr. 2016;39:131–134. doi: 10.18773/austprescr.2016.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ravani P., Bonanni A., Rossi R., Caridi G., Ghiggeri G.M. Anti-CD20 Antibodies for Idiopathic Nephrotic Syndrome in Children. Clin. J. Am. Soc. Nephrol. CJASN. 2016;11:710–720. doi: 10.2215/CJN.08500815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stan M.N., Garrity J.A., Carranza Leon B.G., Prabin T., Bradley E.A., Bahn R.S. Randomized Controlled Trial of Rituximab in Patients with Graves’ Orbitopathy. J. Clin. Endocrinol. Metab. 2015;100:432–441. doi: 10.1210/jc.2014-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Salvi M., Vannucchi G., Currò N., Campi I., Covelli D., Dazzi D., Simonetta S., Guastella C., Pignataro L., Avignone S., et al. Efficacy of B-Cell Targeted Therapy with Rituximab in Patients with Active Moderate to Severe Graves’ Orbitopathy: A Randomized Controlled Study. J. Clin. Endocrinol. Metab. 2015;100:422–431. doi: 10.1210/jc.2014-3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blair H.A., Duggan S.T. Belimumab: A Review in Systemic Lupus Erythematosus. Drugs. 2018;78:355–366. doi: 10.1007/s40265-018-0872-z. [DOI] [PubMed] [Google Scholar]
- 145.Lazzaroni E., Covelli D., Currò N., Campi I., Vannucchi G., Dazzi D., Muller I., Guastella C., Dolci A., Arosio M., et al. Endocrine Abstracts. Bioscientifica; Bristol, UK: 2020. [(accessed on 2 August 2024)]. Comparison between the efficacy of the Anti-BAFF monoclonal antibody belimumab vs methylprednisolone in active moderate-severe graves’ orbitopathy: An interim analysis. Available online: http://www.endocrine-abstracts.org/ea/0070/ea0070yi9.htm. [Google Scholar]
- 146.Zhang L., Baker G., Janus D., Paddon C.A., Fuhrer D., Ludgate M. Biological Effects of Thyrotropin Receptor Activation on Human Orbital Preadipocytes. Investig. Opthalmology Vis. Sci. 2006;47:5197. doi: 10.1167/iovs.06-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Marcinkowski P., Hoyer I., Specker E., Furkert J., Rutz C., Neuenschwander M., Sobottka S., Sun H., Nazare M., Berchner-Pfannschmidt U., et al. A New Highly Thyrotropin Receptor-Selective Small-Molecule Antagonist with Potential for the Treatment of Graves’ Orbitopathy. Thyroid. 2019;29:111–123. doi: 10.1089/thy.2018.0349. [DOI] [PubMed] [Google Scholar]
- 148.Place R.F., Krieger C.C., Neumann S., Gershengorn M.C. Inhibiting thyrotropin/insulin-like growth factor 1 receptor crosstalk to treat Graves’ ophthalmopathy: Studies in orbital fibroblasts in vitro: Inhibiting TSHR/IGF-1R crosstalk in Graves’ ophthalmopathy. Br. J. Pharmacol. 2017;174:328–340. doi: 10.1111/bph.13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Núñez Miguel R., Sanders P., Allen L., Evans M., Holly M., Johnson W., Sullivan A., Sanders J., Furmaniak J., Rees Smith B. Structure of full-length TSH receptor in complex with antibody K1-70TM. J. Mol. Endocrinol. 2023;70:e220120. doi: 10.1530/JME-22-0120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Furmaniak J., Sanders J., Sanders P., Li Y., Rees Smith B. TSH receptor specific monoclonal autoantibody K1-70TM targeting of the TSH receptor in subjects with Graves’ disease and Graves’ orbitopathy—Results from a phase I clinical trial. Clin. Endocrinol. 2022;96:878–887. doi: 10.1111/cen.14681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Neumann S., Krieger C.C., Gershengorn M.C. Targeting TSH and IGF-1 Receptors to Treat Thyroid Eye Disease. Eur. Thyroid J. 2020;9((Suppl. S1)):59–65. doi: 10.1159/000511538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cieplińska K., Niedziela E., Kowalska A. Immunological Processes in the Orbit and Indications for Current and Potential Drug Targets. J. Clin. Med. 2023;13:72. doi: 10.3390/jcm13010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wu P., Lin B., Huang S., Meng J., Zhang F., Zhou M., Hei X., Ke Y., Yang H., Huang D. IL-11 Is Elevated and Drives the Profibrotic Phenotype Transition of Orbital Fibroblasts in Thyroid-Associated Ophthalmopathy. Front. Endocrinol. 2022;13:846106. doi: 10.3389/fendo.2022.846106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yan C., Duan R.-S., Yang H., Li H.-F., Zou Z., Zhang H., Zhou H., Li X.-L., Zhou H., Jiao L., et al. Therapeutic Effects of Batoclimab in Chinese Patients with Generalized Myasthenia Gravis: A Double-Blinded, Randomized, Placebo-Controlled Phase II Study. Neurol. Ther. 2022;11:815–834. doi: 10.1007/s40120-022-00345-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Janes J., Kahaly G., Liang S., Tedeschi P. ODP509 Proof-of-concept, Multicenter, Open-Label Phase 2a Study of Batoclimab in Active and Moderate-to-Severe Thyroid Eye Disease. J. Endocr. Soc. 2022;6((Suppl. S1)):A778–A779. doi: 10.1210/jendso/bvac150.1609. [DOI] [Google Scholar]
- 156.Lanzolla G., Sabini E., Leo M., Menconi F., Rocchi R., Sframeli A., Piaggi P., Nardi M., Marcocci C., Marinò M. Statins for Graves’ orbitopathy (STAGO): A phase 2, open-label, adaptive, single centre, randomised clinical trial. Lancet Diabetes Endocrinol. 2021;9:733–742. doi: 10.1016/S2213-8587(21)00238-2. [DOI] [PubMed] [Google Scholar]
- 157.Yi C., Niu G., Zhang Y., Rao J., Liu G., Yang W., Fei X. Advances in artificial intelligence in thyroid-associated ophthalmopathy. Front. Endocrinol. 2024;15:1356055. doi: 10.3389/fendo.2024.1356055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Zhang Y., Rao J., Wu X., Zhou Y., Liu G., Zhang H. Automatic measurement of exophthalmos based orbital CT images using deep learning. Front. Cell Dev. Biol. 2023;11:1135959. doi: 10.3389/fcell.2023.1135959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Huang X., Ju L., Li J., He L., Tong F., Liu S., Li P., Zhang Y., Wang X., Yang Z., et al. An Intelligent Diagnostic System for Thyroid-Associated Ophthalmopathy Based on Facial Images. Front. Med. 2022;9:920716. doi: 10.3389/fmed.2022.920716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Song X., Liu Z., Li L., Gao Z., Fan X., Zhai G., Zhou H. Artificial intelligence CT screening model for thyroid-associated ophthalmopathy and tests under clinical conditions. Int. J. Comput. Assist. Radiol. Surg. 2021;16:323–330. doi: 10.1007/s11548-020-02281-1. [DOI] [PubMed] [Google Scholar]
- 161.Li Y., Ma J., Xiao J., Wang Y., He W. Use of extreme gradient boosting, light gradient boosting machine, and deep neural networks to evaluate the activity stage of extraocular muscles in thyroid-associated ophthalmopathy. Graefes Arch. Clin. Exp. Ophthalmol. 2024;262:203–210. doi: 10.1007/s00417-023-06256-1. [DOI] [PubMed] [Google Scholar]
- 162.Moon J.H., Shin K., Lee G.M., Park J., Lee M.J., Choung H., Kim N. Machine learning-assisted system using digital facial images to predict the clinical activity score in thyroid-associated orbitopathy. Sci. Rep. 2022;12:22085. doi: 10.1038/s41598-022-25887-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee J., Lee S., Lee W.J., Moon N.J., Lee J.K. Neural network application for assessing thyroid-associated orbitopathy activity using orbital computed tomography. Sci. Rep. 2023;13:13018. doi: 10.1038/s41598-023-40331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Fu R., Bandos A., Leader J.K., Melachuri S., Pradeep T., Bhatia A., Narayanan S., Campbell A.A., Zhang M., Sahel J.-A., et al. Artificial Intelligence Automation of Proptosis Measurement: An Indicator for Pediatric Orbital Abscess Surgery. Ophthalmol. Ther. 2023;12:2479–2491. doi: 10.1007/s40123-023-00754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Douglas R.S., Kossler A.L., Abrams J., Briceño C.A., Gay D., Harrison A., Lee M., Nguyen J., Joseph S.S., Schlachter D., et al. Expert Consensus on the Use of Teprotumumab for the Management of Thyroid Eye Disease Using a Modified-Delphi Approach. J. Neuroophthalmol. 2022;42:334–339. doi: 10.1097/wno.0000000000001560. [DOI] [PMC free article] [PubMed] [Google Scholar]