Abstract
Background
Immune checkpoint inhibitors (ICI) are associated with a distinct spectrum of toxicities. Data on irAE hospitalization rates and clinical course of patients with thoracic malignancies are lacking.
Methods
Patients with advanced thoracic malignancy treated with ICI (2/2016 to 6/2021) were retrospectively identified. Demographic and clinical data of confirmed irAE hospitalizations were extracted from the medical record and a descriptive analysis was performed.
Results
From February 2016 to June 2021, 1312 patients with thoracic malignancy received ICI (monotherapy, combination with 2nd ICI or other agents) with 102 patients (7.7%) hospitalized for irAEs. Treatment intent was first-line therapy in most patients (N = 50, 49%) with 9% (n = 9) receiving adjuvant ICI (N = 9). Sixty patients (59%) received ICI alone, 32% (N = 33) chemo plus immunotherapy, and 7% (N = 7) dual ICI. The median age on admission was 68 years. The median time between ICI initiation and admission was 64 days (1-935 days). Pneumonitis (32.3%; 33/102) was the most frequent indication for admission followed by gastroenterocolitis (19.6%; 20/102), hepatitis (12.7%; 13/102), myo/pericarditis (9.8%; 10/102), and endocrinopathies (9.8%; 10/102). Multi-organ toxicity occurred in 36% (N = 37) of patients. Overall, 85.2% (87/102) of patients received systemic corticosteroids and 17.6% (18/102) required additional lines of immunosuppression. The median length of hospitalization stay was 7 days (2-28 days) with a 25.5% (n = 26) readmission rate within 60 days and an 11.8% (n = 12) in house mortality rate.
Conclusions
Severe irAE requiring inpatient admission, although infrequent, results in considerable morbidity, mortality, and healthcare utilization. Pneumonitis was the most common irAE requiring inpatient management in our patient population with a significant risk of mortality despite the use of guideline-directed systemic immunosuppression. This study highlights the continued need for collaborative efforts amongst medical specialties for improving the diagnostic and therapeutic management of patients with irAEs.
Keywords: immune-related adverse events, irAEs, immune checkpoint inhibitors, lung cancer, hospitalization
Immune checkpoint inhibitors are associated with a distinct spectrum of toxicities. This brief reports describes the incidence of hospitalization due to immune-related adverse events, describing the toxicities experienced, management, and outcomes.
Introduction
Immune checkpoint inhibition (ICI) has fundamentally altered the treatment paradigm of lung cancer with PD-(L)1 inhibition approved as monotherapy or in various combinations in both early and advanced stage disease. Though ICI is well-tolerated, a portion of patients will require hospitalization to manage immune-related adverse events (irAEs) with the incidence of irAEs necessitating admission ranging from 3.5% to 23%.1,2 To date, there is limited data on the real-world outcomes of patients with thoracic malignancies hospitalized for the management of irAE. In this brief report, we aim to describe the incidence of hospitalization as well as toxicities experienced, management and outcomes.
Methods
In 2017, Massachusetts General Hospital created the Severe Immunotherapy Complications (SIC) Service, a multidisciplinary care team dedicated to improving the management of irAEs.3 Using the SIC database, patients with lung cancer who received ICI from February 2016 to June 2021 and were hospitalized for irAE management were identified. Admissions underwent a 2-stage review process with cases first screened for an irAE based on documentation in the electronic health record, with a second review performed to confirm an irAE using published diagnostic criteria.4 Patient demographics, tumor stage, histologic type, ICI start date, treatment regimen, admission date, immunosuppression regimen, toxicity outcomes (ie, resolution to ≤grade 1), date of last follow-up and death were captured, and a descriptive analysis was performed. The median length of hospitalization, median steroid use (start date to date of irAE resolution to grade ≤1), 60-day mortality rate, and 12-month overall survival were calculated. Overall survival was calculated as the time from admission until the date of death or last follow-up.
Results
From February 2016 to June 2021, 1312 patients diagnosed with lung cancer were treated with an ICI-containing regimen. Of those, 102 (7.7%) were admitted to MGH with a confirmed irAE. (Table 1). The median time between ICI initiation and admission was 64 days (1-935 days), with 58.8% (n = 60) receiving anti-PD-(L)1 monotherapy, 32.4% (n = 33) combination chemotherapy and anti-PD-(L)1, and 6.9% (n = 7) combination ICI. The majority (49%) were treated in the front-line metastatic setting, followed by 42.2% in second-line or beyond, with 8.8% receiving adjuvant therapy. The median age was 68.5 years (range 45-89), 58% were male, 92% were current/former smokers, 22.5% had baseline autoimmunity, and the majority (77%) had ECOG PS of 0-1 prior to admission.
Table 1.
Patient demographics, disease, treatment characteristics, and admission characteristics.
| Patient characteristics | No. (%; N = 102) |
|---|---|
| Male | 58 (56.9) |
| Median age (range) years | 68.5 (45-89) |
| Smoking status | |
| Current/former | 94 (92.2) |
| Never | 8 (7.8) |
| Histologic features | |
| Adenocarcinoma | 75 (73.5) |
| Squamous cell carcinoma | 20 (19.6) |
| Small cell lung cancer | 7 (6.9) |
| ECOG | |
| 0-1 | 79 (77.5) |
| ≥2 | 20 (19.6) |
| Unknown | 3 (2.9) |
| Baseline autoimmune disease | 23 (22.5) |
| Endocrinopathy∞ | 13 (12.7) |
| Inflammatory bowel disease | 2 (2.0) |
| Rheumatoid arthritis | 3 (2.9) |
| Psoriasis without arthritis | 4 (3.9) |
| Scleroderma (cutaneous involvement only) | 1 (1%) |
| Baseline immunosuppression | 9 (8.8) |
| Steroids | 8 (7.8) |
| -Prednisone <10 mg (or equivalent) | 2 (2.0) |
| -Prednisone ≥10 mg (or equivalent) | 6 (5.9) |
| Vedolizumab | 1 (1.0) |
| Pulmonary factors | |
| Underlying COPD | 28 (27.5) |
| Supplementary oxygen at baseline | 7 (6.9) |
| Prior thoracic radiation | 46 (45.1) |
| Treatment type at time of admission | |
| PD-1/PD-L1 inhibitor monotherapy | 60 (58.8) |
| Chemotherapy + PD-1/PD-L1 inhibitor | 33 (32.4) |
| PD-1/PD-L1 inhibitor plus CTLA-4 inhibitor | 7 (6.9) |
| Investigational ICI | 1 (1) |
| ICI + investigational agent | 1 (1) |
| Line of therapy | |
| Adjuvant | 9 (8.8) |
| First-line metastatic | 50 (49.0) |
| Second-line metastatic | 23 (22.5) |
| Third-line or beyond metastatic | 20 (19.6) |
| Duration of ICI prior to admission in days | |
| Median (range) | 64 (1-935) |
| Toxicity prompting admission | |
| Pneumonitis | 33 (32.4) |
| Gastrointestinal* | 21 (20.6) |
| Hepatitis | 13 (12.7) |
| Endocrinopathy** | 10 (9.8) |
| Myocarditis/pericarditisΩ | 10 (9.8) |
| Myositis | 5 (4.9) |
| Autoimmune hemolytic anemia | 3 (2.9) |
| Neurotoxicity$ | 3 (2.9) |
| ICI-arthritis | 2 (2.0) |
| Nephritis | 2 (2.0) |
| Treatment of toxicity | |
| Steroids | 87 (85.2) |
| 2L immunosuppression | 18 (17.6) |
| Disposition after discharge | |
| Home | 68 (66.7) |
| Rehab/skilled nursing facility | 10 (9.8) |
| With hospice services | 7 (6.9) |
| Hospitalization length | Days |
| Median (range) | 7 (2-28) |
| Received subsequent cancer therapy | |
| Yes (ICI and non-ICI) | 50 (49) |
| Received subsequent ICI | 21 |
| Delayed after admission only | 7 (6.9%) |
| Discontinued and resumed | 14 (13.7) |
| Alive at time of discharge | |
| Yes | 91 (89.2) |
| No | 11 (10.8) |
∞Endocrinopathy: hypothyroidism (n = 12), adrenal insufficiency (n = 1).
“Others: psoriasis without arthritis (n = 4), scleroderma (n = 1).
*Gastrointestinal (gastritis, esophagitis, enteritis, colitis, and pancreatitis).
$Neurotoxicity (transverse myelitis, peripheral neuropathy, and encephalitis).
**Endocrinopathy (diabetes n = 4, hypophysitis n = 1, thyroiditis n = 1, adrenal insufficiency n = 4).
Ω78.6% (11/14) of cases presenting with myocarditis were found to have a concomitant irAE, most frequently myositis and/or myasthenia gravis (35.7%; 5/14).
Investigational agent: mRNA vaccine.
With respect to underlying autoimmune disease (AID), 23 patients had documented preexisting AID, 52% (12/23) of which had hypothyroidism. Of the remaining patients, 4 had psoriasis (without arthritis), 3 had rheumatoid arthritis (RA), 2 inflammatory bowel disease (IBD), 1 had adrenal insufficiency, and 1 displayed quiescent scleroderma (cutaneous involvement only). Of this AID cohort the majority had inactive disease with only 6 patients receiving ≥10 mg of PO prednisone or its equivalent and 2 patients receiving 2L immunosuppression (1 patient with IBD on vedolizumab and 1 patient with RA on methotrexate). Three patients had an AID flare evident on admission, 1 with RA and 2 with IBD. The patient with RA was subsequently resumed on ICI after resolution of symptoms; both patients with IBD had ICI permanently discontinued.
Pneumonitis (32.3%; 33/102) was the most frequent indication for admission followed by gastroenterocolitis (19.6%; 20/102), hepatitis (12.7%; 13/102), myo/pericarditis (9.8%; 10/102), and endocrinopathies (9.8%; 10/102). Of these, 37 patients (36%) had evidence of multi-system toxicity. During admission, 85.2% (87/102) of patients received systemic corticosteroids (I.V and/or PO) and 17.6% (18/102) required additional lines of immunosuppression including intravenous immunoglobulin (IVIG), tocilizumab, infliximab, and mycophenolate mofetil with the most common 2L immunosuppression indications being colitis (n = 6) and pneumonitis (n = 5). Fifteen patients did not receive systemic steroids, this included cases of diabetes mellitus (n = 4), hyper/hypothyroid (n = 2), and gastroenterocolitis (n = 5) of which 4 were started on budesonide for microscopic colitis evident on pathology after discharge, nephrogenic diabetes insipidus (n = 1; treated with DDAVP), grade 1 hepatitis (n = 1), grade 1 pancreatitis (n = 1), and 1 patient with myositis who left against medical advice and was later started on systemic steroids.
During admission, imaging was performed in 78 patients, with radiographic evidence of progressive disease in 22 (28%), stable disease in 29 (37%), and response in 19 (24%); in the adjuvant therapy group all remained without evidence of disease.
The median length of stay for irAE management was 7 days (range 2-28 days) with a 25.5% (n = 26) readmission rate within 60 days and an 11.8% (n = 12) in house mortality rate. Three patients were discharged on hospice. After discharge only 49% (50/102) went on to receives further cancer-directed systemic therapy with 20.5% (21/102) continued on and/or subsequently rechallenged with ICI. Notably, the majority of patients (60%) admitted solely for management of symptomatic endocrinopathies was maintained on ICI although 2 were discontinued due to progressive disease, rather than toxicity concerns. The all cause 60-day, 180-day, and 12-month mortality rate was 37.5% (38/102), 49% (52/102), and 65.6% (67/102), respectively. Myocarditis and hepatitis had a 60-day mortality of 35% and 36%, respectively, with ICI-related pneumonitis (ICIP) associated with the greatest 60-day mortality rate (52.6% [20/38]).
ICIP cohort
Of patients admitted for ICIP, 71.1% (27/38) had a grade 3-4 event, and 15.8% (6/38) had a grade 5 event whereby pneumonitis was deemed the primary contributor to death. Twenty-nine patients (76%; 29/38) required intravenous steroids, with 5 requiring 2L immunosuppression (infliximab [n = 3], IVIG [n = 1], and tocilizumab [n = 1]), and 3 requiring 3L immunosuppression (mycophenolate [n = 2], and IVIG [n = 1]), and 1 requiring 4L immunosuppression (tocilizumab [n = 1]). Nine patients with grade 2 or 3 ICIP were initiated on oral steroids with resolution to grade ≤1. The median duration of steroid treatment for ICIP was 42.5 days (range 1-268) with 36.8% of patients requiring readmission within 60 days. Notably 5 patients were hospitalized for management of other irAEs but found to have grade 1-2 ICIP. Of these five cases, 4 resolved with steroid treatment for alternative irAE management with 1 patient ultimately readmited for management of a grade 2 ICIP flare requiring reescalation of steroids.
In the ICIP cohort, 16 (42%) patients received subsequent systemic cancer-directed therapy with the majority (n = 14) receiving a non-ICI containing regimen. One patient with grade 3 ICIP resumed ICI after completion of steroid taper without flare or development of alternative irAE; 1 patient with grade 3 ICIP was rechallenged resulting in an ICIP flare leading to permanent ICI discontinuation.
Discussion
In a real-world cohort of thoracic cancer patients receiving ICI, 7.7% necessitated hospitalization for irAE management. Although these statistically are in line with the number of patients who experience significant (grades 3-4 toxicity) on PD-(L)1 inhibition with or without chemotherapy,5,6 the hospitalization rate is higher than other published reports.7,8 One potential explanation for increased rates of admission is the high proportion of patients (22.5%) with underlying AID in our cohort and the known association of AID and increased risk of irAE development and hospitalization9 and the focus on a lung cancer population with a known increased risk of hospitalization compared to other tumor types.10
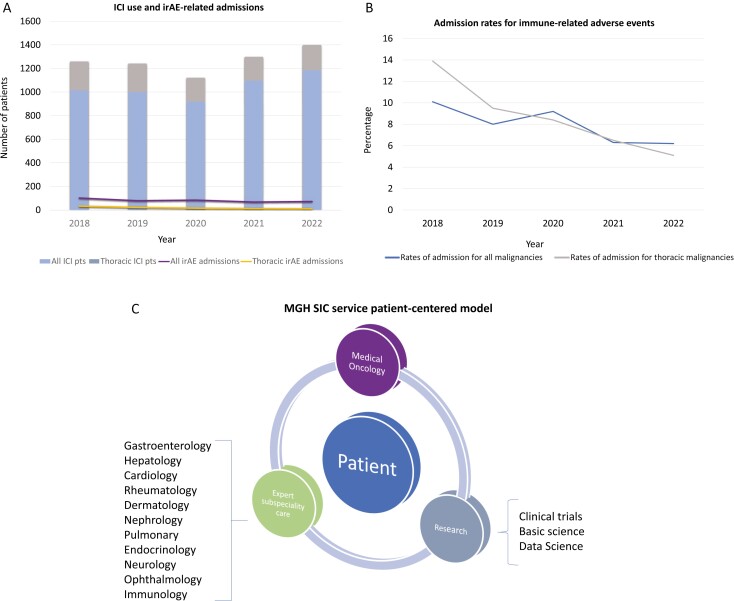
Among our hospitalized cohort, ICIP accounted for the majority admissions (32%) followed by gastroenterocolitis (19%) with 36% found to have multi-system toxicity. Morbidity and mortality were high amongst patients with 11.8% (12/102) dying during admission and only 34.3% (35/102) alive 1 year after admission with the explanation likely multifactorial: (1) a high-risk thoracic patient population, many of whom possess other medical comorbidities, (2) irAE complications and/or steroid-refractory irAEs, (3) receipt of high-dose immunosuppression with subsequent complications, and (4) progressive disease and limitations in delivering additional cancer-directed therapies. For example, in our cohort only 49% of patients received further systemic therapy. These factors likely contributed to increased mortality rates when compared to other publications.8 Furthermore, we hypothesis that differences in the patient populations and the toxicities experienced between studies account for the discrepancy. For example, in the report by Silverstein et al the 1-year OS for their hospitalized cohort was 63% however the majority of patients had melanoma and only 7.2% were admitted for ICIP. Importantly in subgroup analysis, both melanoma and RCC populations had dramatic improvement in 1-year OS when compared to thoracic malignancies, 83% versus 28%, respectively. Additionally, when compared to alternative toxicities, ICIP carried a significant decrement in survival with a median survival of only 82.5 days and 1-year survival of 28.6%. ICIP secondary to PD-(L)1 monotherapy, although uncommon, is associated with significant morbidity and mortality. In fact, it is the most frequently fatal toxicity observed from anti-PD-(L)1 monotherapy.11 Though the incidence of grade ≥3 ICIP reported in clinical trials ranges between 3% and 5%12-15 real-world data suggests higher rates (3.3%-26%).16-19 In our study, although the overall rate of pneumonitis among patients receiving ICI was low (2.9%, 38/1312) it accounted for the majority of admissions with dismal outcomes including a 15% in-house mortality rate and a 60-day mortality rate of 52.6%. Despite the typical responsiveness of ICIP to systemic corticosteroids, leading to symptom resolution within a few weeks, this study sheds light on a subgroup of patients who experience a more virulent, steroid-refractory course, defined as a failure of clinical improvement after a minimum of 48 hours of high-dose corticosteroids (prednisone 1-2 g/kg/day or more).20 As observed in both our cohort and other studies, limited effective second-line immunosuppressive regimens can often necessitate extended courses of steroids. Moreover, it is noteworthy that other data series have also demonstrated the substantial mortality rate of ICIP, up to 75%.20,21 This study raises awareness about the severe and potentially life-threatening nature of this toxicity, particularly in patient population vulnerable to pulmonary complications, and emphasizes the necessity for trials, such as NCT04438382, to optimize the management of these complex cases.20 Furthermore, it advocates for the development of clinical trials investigating more robust and effective upfront immunosuppressive interventions. Lastly, it emphasizes the importance of prompt identification and management of serious irAEs. MGH established the SIC Service in 2017 with the aim of cultivating proficiency in identifying and managing irAEs. Similarly, other institutions have since established irAE tumor boards22 and specialized treatment teams23,24 dedicated to the management of toxicities. Since its inception, the MGH SIC service—comprised of medical oncologists, expert subspecialists and translational researchers—provides clinical care and performs novel translational research in this space. Initially focused on inpatient management, the scope of the SIC team has extended to outpatient clinical referrals to subspecialists (ie, outpatient GI referral with consideration of endoscopy), resulting in decreasing rates of irAE admissions despite increased ICI use (Figure 1). This observation is in line with other studies that have demonstrated that multidisciplinary management aids in mitigating rates of hospitalization.25 Future directions in irAE management focus on both refining multidisciplinary management of these novel side effects, particularly those refractory to 1L immunosuppression as well as performing transformative research to enable prediction and ideally prevention of these life-altering side effects.
Figure 1.
Immune checkpoint inhibitors (ICIs) administration and irAE-related admissions. (A) Total number of patients across malignancies, including those with thoracic malignancies, receiving an ICI-containing regimens and those admitted for irAE evaluation. (B) Rates of irAE-related admissions across tumor types with (C) illustrating the multidisciplinary Severe Immunotherapy Complications (SIC) model for patients with irAEs.
This report adds to an important body of irAE literature. However, there are several limitations in this single-center retrospective study including underestimation of irAEs requiring admission as other patients may have been admitted outside our academic network. Conversely as this study was performed at a tertiary care center, with a large referral base, this cohort may be enriched in patients with higher acuity and overall worse outcomes than patients managed in the community. Furthermore, with the subspeciality expertise—including the SIC service—at the performing institution these findings may not be generalizable. Additionally, there remain challenges to ensure proper irAE adjudication; however, we attempted to mitigate this with the use of 2-step validation process, including the use of published criteria.
In conclusion, as ICI indications expand the incidence of patients experiencing significant irAEs undoubtedly increases. Collaborative efforts amongst medical specialties are essential for improving the diagnostic and therapeutic management of patients with irAEs, particularly ICIP where effective treatments for steroid-refractory ICIP is urgently needed.
Contributor Information
Ayo Falade, Department of Medicine, Salem Hospital, Salem, MA, United States.
Leyre Zubiri, Mass General Cancer Center, Division of Hematology-Oncology, Department of Medicine, Massachusetts General Hospital, Boston, MA, United States.
Chia-Yun Wu, Mass General Cancer Center, Division of Hematology-Oncology, Department of Medicine, Massachusetts General Hospital, Boston, MA, United States.
Katherine Perlman, Mass General Cancer Center, Division of Hematology-Oncology, Department of Medicine, Massachusetts General Hospital, Boston, MA, United States.
Joie Sun, Mass General Cancer Center, Division of Hematology-Oncology, Department of Medicine, Massachusetts General Hospital, Boston, MA, United States.
Nora Hathaway, Mass General Cancer Center, Division of Hematology-Oncology, Department of Medicine, Massachusetts General Hospital, Boston, MA, United States.
Kelley Grealish, Mass General Cancer Center, Division of Hematology-Oncology, Department of Medicine, Massachusetts General Hospital, Boston, MA, United States.
Jackie Lopiccolo, Mass General Cancer Center, Division of Hematology-Oncology, Department of Medicine, Massachusetts General Hospital, Boston, MA, United States.
Kerry Reynolds, Mass General Cancer Center, Division of Hematology-Oncology, Department of Medicine, Massachusetts General Hospital, Boston, MA, United States.
Meghan J Mooradian, Mass General Cancer Center, Division of Hematology-Oncology, Department of Medicine, Massachusetts General Hospital, Boston, MA, United States.
Author contributions
Ayo Falade (Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Writing—original draft, Writing—review & editing), Leyre Zubiri (Data curation, Formal Analysis, Project administration, Resources, Writing—original draft, Writing—review & editing), Chia-Yun Wu (Data curation, Resources), Katherine Perlman (Data curation), Joie Sun (Data curation), Nora Hathaway (Data curation), Kelley Grealish (Data curation), Jackie Lopiccolo (Data curation), Kerry Reynolds (Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing), Meghan J. Mooradian (Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing)
Conflicts of interest
L.Z. reported scientific advisory board for GSK and Merck. K.R. reported advisory board for SAGA Diagnostics, educational speaker for MedScape, and CME Outfitters, institutional research funding from Bristol Myers Squibb.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
References
- 1. Nice L, Bycroft R, Wu X, et al. Assessment of hospitalization rates for immune-related adverse events with immune checkpoint inhibitors. J Oncol Pharm Pract. 2021;27(7):1736-1742. 10.1177/1078155220968909 [DOI] [PubMed] [Google Scholar]
- 2. Balaji A, Zhang J, Wills B, et al. Immune-related adverse events requiring hospitalization: spectrum of toxicity, treatment, and outcomes. J Oncol Pract. 2019;15(9):e825-e834. 10.1200/JOP.18.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zubiri L, Molina GE, Mooradian MJ, et al. Effect of a multidisciplinary Severe Immunotherapy Complications Service on outcomes for patients receiving immune checkpoint inhibitor therapy for cancer. J ImmunoTher Cancer. 2021;9(9):e002886. 10.1136/jitc-2021-002886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Molina GE, Zubiri L, Cohen JV, et al. Temporal trends and outcomes among patients admitted for immune-related adverse events: A Single-Center Retrospective Cohort Study from 2011 to 2018. Oncologist. 2021;26(6):514-522. 10.1002/onco.13740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Martin R, Delvys R-A, Robinson Andrew G, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. [DOI] [PubMed] [Google Scholar]
- 6. Leena G, Delvys R-A, Shirish G, et al. Pembrolizumab plus chemotherapy in metastatic non–small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092. [DOI] [PubMed] [Google Scholar]
- 7. Kalinich M, Murphy W, Wongvibulsin S, et al. Prediction of severe immune-related adverse events requiring hospital admission in patients on immune checkpoint inhibitors: study of a population level insurance claims database from the USA. J ImmunoTher Cancer. 2021;9(3):e001935. 10.1136/jitc-2020-001935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Silverstein J, Wright F, Wang M, et al. Evaluating survival after hospitalization due to immune-related adverse events from checkpoint inhibitors. Oncologist. 2023;28(10):e950-e959. 10.1093/oncolo/oyad135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kehl KL, Yang S, Awad MM, et al. Pre-existing autoimmune disease and the risk of immune-related adverse events among patients receiving checkpoint inhibitors for cancer. Cancer Immunol Immunother. 2019;68(6):917-926. 10.1007/s00262-019-02321-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kress JP, Christenson J, Pohlman AS, Linkin DR, Hall JB. Outcomes of critically ill cancer patients in a University Hospital setting. Am J Respir Crit Care Med. 1999;160(6):1957-1961. 10.1164/ajrccm.160.6.9812055 [DOI] [PubMed] [Google Scholar]
- 11. Wang DY, Salem JE, Salem JE, et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721-1728. 10.1001/jamaoncol.2018.3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Velasco G, Je Y, Bossé D, et al. Comprehensive meta-analysis of key immune-related adverse events from CTLA-4 and PD-1/PD-L1 inhibitors in cancer patients. Cancer Immunol Res. 2017;5(4):312-318. 10.1158/2326-6066.CIR-16-0237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152(2):271-281. 10.1016/j.chest.2017.04.177 [DOI] [PubMed] [Google Scholar]
- 14. Wang Y, Zhou S, Yang F, et al. Treatment-related adverse events of PD-1 and PD-L1 inhibitors in clinical trials: a systematic review and meta-analysis. JAMA Oncol. 2019;5(7):1008-1019. 10.1001/jamaoncol.2019.0393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor–related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607-1616. 10.1001/jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- 16. Liu Q, Chenan Z, Chenan Z, et al. Abstract CT086: pneumonitis incidence in patients with non-small cell lung cancer treated with immunotherapy or chemotherapy in clinical trials and real-world data. Cancer Res. 2020;80. [Google Scholar]
- 17. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with Anti–Programmed Death-1/Programmed Death Ligand 1 Therapy. J Clin Oncol. 2017;35(7):709-717. 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930-1939. [DOI] [PubMed] [Google Scholar]
- 19. Aiad M, Fresco K, Prenatt Z, et al. Comparison of pneumonitis rates and severity in patients with lung cancer treated by immunotherapy, radiotherapy, and immunoradiotherapy. Cureus. 2022;14(6):e25665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balaji A, Hsu M, Lin CT, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J ImmunoTher Cancer. 2021;9(1):e001731. 10.1136/jitc-2020-001731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. 2019;25(3):551-557. 10.1177/1078155217745144 [DOI] [PubMed] [Google Scholar]
- 22. Kennedy LC, Wong KM, Kamat NV, et al. Untangling the multidisciplinary care web: streamlining care through an Immune-Related Adverse Events (IRAE) Tumor Board. Target Oncol. 2020;15(4):541-548. 10.1007/s11523-020-00739-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cole S, Zibelman M, Bertino E, Yucebay F, Reynolds K. Managing immuno-oncology toxicity: top 10 innovative institutional solutions. Am Soc Clin Oncol Educ Book. 2019;39(39):96-104. 10.1200/EDBK_100018 [DOI] [PubMed] [Google Scholar]
- 24. Naidoo J, Zhang J, Lipson EJ, et al. A multidisciplinary toxicity team for cancer immunotherapy–related adverse events. J Natl Compr Canc Netw. 2019;17(6):712-720. 10.6004/jnccn.2018.7268 [DOI] [PubMed] [Google Scholar]
- 25. Bonanno L, Lorenzi M, Massa D, et al. Immune-related diarrhea and colitis in non-small cell lung cancers: impact of multidisciplinary management in a real-world setting. Oncologist. 2024;29(1):e118-e130. 10.1093/oncolo/oyad238 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.