Abstract
Background
Immune checkpoint inhibitors (ICIs) have revolutionized cancer care with incredible reductions in mortality. One of the most devastating complications of treatment is ICI-related pneumonitis (ICI-p). Despite this, little is known regarding risk factors for severe pneumonitis and treatment effectiveness of various therapeutic options for steroid-refractory disease. To address this, we conducted a retrospective study on patients with cancer who developed ICI-p.
Methods
We examined consecutive patients who received ICIs and developed ICI-p. Risk factors of interest for severe disease and steroid-refractory ICI-p, including pre-treatment pulmonary function tests (PFTs) and chest imaging, were compared between patients with severe (grades 3-5) and mild (grades 1-2) pneumonitis. The clinical and treatment courses for patients with steroid-refractory ICI-p were recorded.
Results
A total of 132 patients developed ICI-p, with 60 patients having mild and 72 with severe disease. We found that lower forced vital capacity percent predicted (66.24 vs 85.05, P = .05), lower total lung capacity percent predicted (85.23 vs 99.71, P = .13), and specific radiographic patterns on pre-treatment chest imaging were predictors of severe disease. Initial corticosteroid dose of less than 1 milligram per kilogram prednisone equivalent (P = .14) was correlated with partially steroid-responsive or steroid-refractory ICI-p. Ten patients had steroid refractory ICI-p, and those who received IVIG alone as the immune suppressant beyond corticosteroids had improved survival (P = 05).
Conclusions
We are the first to identify pre-treatment PFTs and chest imaging abnormalities as risk factors for severe ICI-p. We also found that lower corticosteroid doses were associated with partially steroid-responsive and steroid-refractory ICI-p. Larger, prospective studies are needed to validate our results.
Keywords: checkpoint inhibitor pneumonitis, steroid refractory pneumonitis, immune checkpoint inhibitors, immune-related adverse events
Little is known about risk factors for severe pneumonitis and the treatment effectiveness of various therapeutic options for steroid-refractory disease. This retrospective study focused on cancer patients who developed immune checkpoint inhibitor treatment-related pneumonitis.
Implications for practice.
Our study identified several pre-treatment risk factors for severe ICI-p. These factors can be utilized to identify patients at the highest risk of severe ICI-p before ICI treatment, allowing oncologists to weigh the risks of severe ICI-p with the benefits of cancer treatment. We also found several predictors of partially steroid-responsive and steroid-refractory ICI-p at the time of ICI-p diagnosis, allowing clinicians to target patients who would benefit the most from early and aggressive immune suppression.
Introduction
Immune checkpoint inhibitors (ICIs) have revolutionized treatment in several cancers over the last decade.1 ICIs upregulate the immune system by inhibiting physiologic checkpoints, including programmed cell death ligand 1 (PDL-1), programmed cell death protein 1 (PD-1), cytotoxic T-lymphocyte antigen-4, and their use is rapidly growing.2 Unfortunately, ICI treatment is not without risk, as the increase in immune activity predisposes patients to autoimmune complications, termed immune-related adverse events (IRAE). When immune-mediated inflammation occurs in the lungs following ICI treatment, this is called ICI-pneumonitis (ICI-p).3
ICI-p is one of the most devastating IRAEs and is associated with significant morbidity and mortality. Early studies on ICI-p reported an incidence ranging from 3% to 6%.4 Due to increasing ICI utilization and awareness of IRAEs, recent studies have revealed the incidence to be significantly higher than previously reported,5,6 with severe ICI-p accounting for 30% of all cases.7,8 The reported mortality for ICI-p ranges from 10% to 18%,5,9 with mortality for steroid-refractory ICI-p as high as 57%.6,10 Despite the increasing incidence of ICI-p and the mortality associated with severe ICI-p, little is known regarding the risk factors for severe pneumonitis and the optimal therapeutic choice for steroid-refractory disease.
There are several patient-specific risk factors associated with ICI-p, including prior thoracic radiation, preexisting interstitial lung abnormalities, abnormalities on pulmonary function testing (PFTs), and lab abnormalities such as hypoalbuminemia,8,11-15 but few have been identified specifically for severe ICI-p. Major societies recommend prompt steroid administration and consideration of additional immunosuppression if patients are not responding to corticosteroids within 48-72 hours, but none make a specific recommendation on which steroid refractory medication to utilize.16-19 As ICIs are increasingly used in the neoadjuvant and adjuvant setting and for curative intent,20,21 it is paramount to identify patient-specific risk factors for severe ICI-p to help risk stratify which patients would benefit the most from ICI treatment.
To address this knowledge gap, we conducted a single-center, retrospective study on cancer patients treated with ICIs who developed ICI-p to evaluate whether abnormalities in chest imaging, PFTs, blood work, and previous treatment history would increase the risk of severe ICI-p. Using the same cohort, we compared non-steroid immune suppressants to determine the optimal secondary agent for patients with steroid-refractory ICI-p.
Methods
Data collection
This retrospective cohort study was conducted at a tertiary academic center examining consecutive patients with cancer from 2013 to 2020 who received ICIs and subsequently developed ICI-p. Data collection was completed and managed using REDcap, an electronic data capturing tool that is hosted through The Ohio State University (OSU).22,23 This study protocol was reviewed and approved by the Ohio State Institutional Review Board (2021C0177). Due to the retrospective nature of the study, a waiver of informed consent was granted. The primary outcome being evaluated was the impact of pre-treatment PFTs, chest imaging, lab work, and treatment history on the severity of ICI-p.
Included patients
All patients who received at least one dose of ICI (monotherapy, combination checkpoint inhibitor treatment, combination with chemotherapy, in the setting of a clinical trial) from 2013 to 2020 at Ohio State University were examined. Pneumonitis was determined by documentation of ICI-p by the treating oncologist at the time of toxicity and retrospectively confirmed by a member of the study team (MM, BE, DHO, KH). Pneumonitis was attributed to ICIs based on ICI treatment and reasonable exclusion of alternate etiologies including infection and inflammation from other causes, consistent with other similar studies.24,25
We reviewed and recorded the following potential risk factors: age, gender, race, body mass index, ICI type, cancer type, line of therapy, prior chemotherapy, prior immunotherapy, prior radiation and location of therapy, and diagnostic labs. Pre-treatment PFTs and chest CTs completed within 12 months of ICI initiation were recorded. Treatment courses, including steroid dose and steroid duration, were also recorded.
Pneumonitis definitions
Pneumonitis severity was assigned based on the Common Terminology Criteria for Adverse Events (CTCAE v4), with severe disease defined as grade 3 or higher.26 Pneumonitis resolution was defined as improvement in patient symptoms, resolved inflammatory changes on chest imaging, and a decrease in systemic steroids to less than or equal to the equivalent of 10 milligrams (mg) of prednisone, consistent with society recommendations for resolution of IRAE.17 Steroid refractory pneumonitis was defined as clinical worsening of pneumonitis after a minimum of 48 hours of high-dose corticosteroids (prednisone 1-2 mg/kg/day or more) or no clinical improvement after at least 14 days of high-dose corticosteroids. Steroid-responsive pneumonitis was defined as ICI-p that resulted in complete pneumonitis resolution without an increase or recurrent corticosteroid dose during or following initial treatment, while partially steroid-responsive pneumonitis was defined as ICI-p requiring repeated or increases in corticosteroid courses due to lack of pneumonitis resolution or those that died without documented resolution of ICI-p (includes death for cancer progression, change in code status, or from pneumonitis).
Evaluation of pre-treatment pulmonary function tests
Spirometry (forced expiratory volume in 1 second [FEV1], forced vital capacity [FVC], FEV1/FVC ratio, bronchodilator responsiveness testing), total lung volume (TLC), residual volume (RV), diffusion capacity (DLCO), diffusion capacity corrected for hemoglobin (DLCO corrected for hemoglobin), 6-minute walk test, and oxygen requirement within 12 months of ICI initiation were recorded by the study team and served as the baseline lung function. Testing that did not meet ATS criteria for acceptability and repeatability was not included.27 PFTs closest to the start of ICI diagnosis and treatment were considered. All values were recorded as raw values (liters for FEV1, FVC, TLC, RV, mmol/min/mmHg for DLCO and DLCO corrected for hemoglobin) or as a percent predicted (pp) adjusted for patient’s sex, height, age, and race. When available, post-bronchodilator FEV1/FVC was utilized to determine the presence of obstructive disease.28
Evaluation of pre-treatment chest imaging
As previously described,14,15,29 we defined the group of patients with interstitial abnormalities as those who exhibited at least one of the following in at least 2 lobes of the lung within 12 months of ICI initiation: ground glass appearance, reticular opacity, consolidation, centrilobular nodularity, and honeycombing. Interstitial changes thought to be related to underlying malignancy were not included. As per prior methodology, the extent of interstitial abnormality was measured on a 5-point scale of upper, middle, and lower zones of the lung (0: none, 1: < 5%, 2: 5%-25%, 3: 26%-50%, 4: > 50%). Each zone score was then combined for a total severity score for each patient.14,15 Determination of interstitial abnormality and severity scoring was performed by a board-certified thoracic radiologist on the study team (ED) who was blinded to all other aspects of the patient history.
Statistical analyses
Categorical variables were summarized as frequencies (n) and percentages (%). Continuous variables were summarized as mean and standard deviation (SD) if they were approximately normally distributed; otherwise, median with interquartile range (IQR, 25th and 75th percentile) were used to describe variable distributions. We used univariate logistic regression to identify significant risk factors associated with the development of severe ICI-p, risk factors associated with partially steroid responsive or steroid-refractory ICI-p, and associations between the dose of chest radiation, days of chest radiation from ICI initiation, and severity of pneumonitis. The Kaplan-Meier survival curves and log-rank tests were applied to compare overall survival (using date of last query if alive; death date if deceased) between patients with severe and mild ICI-p. The Spearman correlation was used to evaluate the relationship between PFT parameters and severity of pre-treatment chest imaging findings. We also compared the survival probabilities of patients with steroid-refractory ICI-p receiving different treatments. The statistical analysis was performed using R software (version 4.2.0; R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
Results
Demographics and baseline characteristics
The study demographics and baseline characteristics are summarized in Table 1. A total of 2963 patients with cancer received ICI therapy between 2013 and 2020, and 132 (4.5%) were found to have ICI-p, all of which were treated in the unresectable setting, with 77 requiring hospitalizations. Of patients with ICI-p, the most common tumor types were NSCLC (71/132, 53.8%), renal cell carcinoma (13/132, 9.8%), and melanoma (12/132, 9.1%). Most patients with ICI-p received a PD-1 inhibitor (87/132, 66%) and received ICI as first-line treatment (70/132, 53.0%). Sixty (45.5%) had mild pneumonitis and 72 (54.5%) had severe disease. Patients with mild ICI-p were more likely to have received prior chemotherapy alone (P = .03), chest radiation (P = .03), PDL-1 ICI (P = .05), and have non-small cell lung cancer (NSCLC, P = .04), while patients with severe ICI-p were more likely to have been treated with combination checkpoint inhibitor therapy (P = .01). We found no statistically significant association between the remaining demographic variables and severe ICI-p, including the timing of chest radiation from ICI initiation. Severe ICI-p occurred earlier from ICI initiation compared to mild ICI-p, but this was not statistically significant (80 vs 140 days, P = .07).
Table 1.
Patient demographics and characteristics by severity of ICI-p.
| Variable | Mild ICI-pa | Severe ICI-pa | P-value | |
|---|---|---|---|---|
| Age (mean, SD) | 64.5 (11.4) | 63.2 (10.3) | .52 | |
| Race (%) | White | 53 (88.3) | 68 (94.4) | .21 |
| Other | 7 (11.7) | 4 (5.6) | ||
| Gender (%) | Female | 29 (48.3) | 22 (30.6) | .04 |
| Male | 31 (51.7) | 50 (69.4) | ||
| BMI (mean, SD) | 27.3 (5.8) | 28.3 (6.6) | .34 | |
| ICI treatment (%) | PD-1 | 37 (61.7) | 50 (69.4) | .35 |
| PDL-1 | 22 (36.6) | 11 (15.3) | .005 | |
| CTLA-4 | 0 (0) | 2 (2.8) | .12 | |
| Combination | 1 (1.7) | 9 (12.5) | .01 | |
| ICI doses (mean, IQR) | 8.0 (4.0, 13.2) | 4.0 (2.0, 11.0) | .13 | |
| ICI initiation to ICI-p (days; mean, IQR) | 140 (61.3284.0) | 80 (28.8228.2) | .07 | |
| COPDb (%; n = 72) | Yes | 41 (68.3) | 31 (43.1) | .25 |
| No | 19 (31.7) | 41 (56.9) | ||
| Interstitial lung disease (%; n = 5) | Yes | 2 (3.3%) | 3 (4.2%) | 1.00 |
| No | 58 (96.7%) | 69 (95.8%) | ||
| Cancer type (%) | NSCLC | 38 (63.3) | 33 (45.8) | <.001 |
| Small cell lung cancer | 1 (1.7) | 3 (4.2) | ||
| Melanoma | 4 (6.7) | 8 (11.1) | ||
| Renal cell carcinoma | 0 (.0) | 13 (18.1) | ||
| Other | 17 (28.3) | 15 (20.8) | ||
| NSCLC (%), n = 71 | Yes | 38 (63.3) | 33 (45.8) | .04 |
| No | 22 (36.7) | 39 (54.2) | ||
| Line of therapy (%; n = 70) | 1st line | 33 (55.0) | 37 (51.4) | .68 |
| 2nd or greater | 27 (45.0) | 35 (48.6) | ||
| Prior checkpoint inhibitor (%; n = 25) | Yes | 12 (20.0) | 13 (18.1) | .78 |
| No | 48 (8.0) | 59 (81.9) | ||
| Prior chemotherapy (%; n = 46) | Yes | 15 (25.0) | 31 (43.1) | .03 |
| No | 45 (75.0) | 41 (56.9) | ||
| Prior chest radiation (%; n = 42) | Yes | 27 (48.3) | 15 (25.0) | .003 |
| No | 33 (51.7) | 57 (75.0) | ||
| Date of previous radiation | </= 30 days from ICI initiation | 6 (22.2) | 3 (20.0) | .87 |
| > 30 days from ICI initiation | 21 (77.8) | 12 (80.0) | ||
| </= 90 days from ICI initiation | 17 (63.0) | 7 (46.7) | .31 | |
| > 90 days from ICI initiation | 10 (37.0) | 8 (53.3) | ||
| Radiation dose (Gy; mean, IQR) | 60 .0 (60.0, 60.0) | 55.0 (42.5, 60.0) | .01 |
aGrading of ICI-p by CTCAE criteria version 4; Mild ICI-p (grade 1-2), severe ICI-p (grade 3-5).
bChronic obstructive pulmonary disease.
Abbreviations: ICI-p, immune checkpoint inhibitor pneumonitis, NSCLC, non-small cell lung cancer; IQR, interquartile range.
Pre-treatment PFTs
Of the 132 patients with ICI-p, 38 patients had spirometry, 30 had lung volume testing, and 34 had diffusion capacity testing performed within 12 months of their ICI initiation. Lower FVCpp (P = .05), FVC < 80% (P = .049), and lower TLCpp (P = .004) was associated with severe ICI-p. There was no noteworthy difference in FEV1, FEV1/FVC ratio, diffusion capacity, or oxygen requirement before treatment between patients with mild and severe ICI-p. The association between pre-treatment PFTs and severity of ICI-p is summarized in Table 2.
Table 2.
Pre-treatment pulmonary function tests by severity of ICI-p.
| Variable | Mild ICI-pa | Severe ICI-pa | P-value | |
|---|---|---|---|---|
| Spirometryb | 20 | 18 | ||
| FEV1 (mean, SD) | 2.17 (0.54) | 2.11 (0.79) | .77 | |
| FEV1pp (mean, SD) | 78.1 (20.4) | 67.1 (22.7) | .11 | |
| FVC (mean, SD) | 3.19 (0.82) | 2.90 (0.95) | 0.29 | |
| FVCpp (mean, SD) | 85.05 (15.54) | 66.24 (24.75) | .005 | |
| FEV1pp | <80% | 10 (50.0) | 12 (66.7) | .30 |
| >/=80% | 10 (50.0) | 6 (33.3) | ||
| FVCpp | <80% | 7 (35.0) | 12 (66.7) | .049 |
| >/=80% | 13 (65.0) | 6 (33.3) | ||
| Lung volumesb | 17 | 13 | ||
| TLC (mean, SD) | 6.15 (1.46) | 5.37 (1.34) | .13 | |
| TLCpp (mean, SD) | 99.71 (17.62) | 83.25 (10.82) | .004 | |
| TLCpp | <80% | 3 (17.6) | 4 (30.8) | .40 |
| >/=80% | 14 (82.4) | 9 (69.2) | ||
| Diffusion capacityc | 18 | 16 | ||
| DLCO (mean, SD) | 17.02 (5.03) | 17.45 (6.09) | .82 | |
| DLCOpp (mean, SD) | 75.50 (20.04) | 73.33 (28.09) | .79 | |
| DLCO corrected for hemoglobin (mean, SD) | 17.28 (4.78) | 18.31 (6.08) | .59 | |
| DLCO corrected for hemoglobin, pp | 79.60 (21.28) | 76.95 (27.82) | .76 | |
| DLCO corrected for hemoglobin, pp | <80% | 8 (53.3) | 10 (62.5) | .61 |
| >/=80% | 7 (46.7) | 6 (37.5) | ||
| Oxygen requirement before ICI (%) | Yes | 6 (10.2) | 13 (18.1) | .31 |
| No | 53 (89.8) | 59 (81.9) |
aGrading of ICI-p by CTCAE criteria version 4; Minor ICI-p (grade 1-2), severe ICI-p (grade 3-5).
bRaw values for FEV1, FVC, and TLC reported in liters.
cRaw values for diffusion capacity reported in mmol/min/mmHg.
Abbreviations: ICI-p, immune checkpoint inhibitor pneumonitis; FEV1, forced expiratory volume in 1 second; FVC, forced viral capacity; TLC, total lung capacity; DLCO, diffusion capacity of the lungs for carbon monoxide; p, percent predicted
Pre-existing interstitial abnormalities
Of the 132 patients with ICI-p, 124 patients had CT imaging completed within 12 months of ICI initiation. Among these patients, the presence of pre-treatment interstitial abnormalities was not associated with severe ICI-p (P = 0.19). Consolidation and bronchiectasis on pre-treatment chest imaging were associated with severe ICI-p (P = .003 and P = .006, respectively), as all patients with either radiographic pattern developed severe disease (7/7 with consolidation and 6/6 with bronchiectasis, Table 3). The severity of pre-treatment interstitial abnormalities was also associated with severe ICI-p (total score 3 [1.25-9.00] vs 2 [1.00-4.00], P = .006, Table 3), and more severe interstitial scores were correlated with lower TLCpp (Supplementary Figure S1). Other radiographic patterns and metastatic pulmonary involvement excluding primary lung malignancies (P = .059) were not associated with the severity of pneumonitis.
Table 3.
Pre-treatment chest imaging and severity of ICI-p.
| Mild ICI-pa | Severe ICI-pa | P-value | ||
|---|---|---|---|---|
| Pre-treatment interstitial abnormalities | Yes | 35 (62.5) | 50 (73.5) | .19 |
| No | 21 (37.5) | 18 (26.5) | ||
| Consolidation | Yes | 0 (0.0) | 7 (10.3) | .003 |
| No | 56 (100.0) | 61 (89.7) | ||
| Ground glass | Yes | 23 (41.1) | 27 (39.7) | .88 |
| No | 33 (58.9) | 41 (60.3) | ||
| Reticular | Yes | 17 (30.4) | 30 (44.1) | .11 |
| No | 39 (69.7) | 38 (56.0) | ||
| Centrilobular nodules or bronchiolitis | Yes | 7 (12.5) | 12 (17.6) | .43 |
| No | 49 (87.5) | 56 (82.4) | ||
| Bronchiectasis | Yes | 0 (0.0) | 6 (8.8) | .006 |
| No | 56 (100.0) | 62 (91.2) | ||
| Honeycombing | Yes | 1 (1.8) | 2 (2.9) | .67 |
| No | 55 (98.2) | 66 (97.1) | ||
| Total interstitial severity score | 2.00 (1.00, 4.00) | 3.00 (1.25, 9.00) | .006 | |
| Pulmonary metastasesb | Yes | 8 (38.1) | 23 (63.9) | .059 |
| No | 13 (61.9) | 13 (36.1) |
aGrading of ICI-p by CTCAE criteria version 4; Minor ICI-p (grade 1-2), severe ICI-p (grade 3-5).
bOnly includes patients with metastatic pulmonary disease. Primary lung malignancies were not included.
Abbreviations: ICI-p, immune checkpoint inhibitor pneumonitis.
Diagnostic labs
Blood work was documented at the time of ICI-p diagnosis. We found that lower absolute eosinophil count, lower absolute lymphocyte count, and lower albumin were associated with severe ICI-p, (P = .004, P = .001, P < .001, respectively, Table 4). Absolute neutrophil counts and absolute monocyte counts were not associated with the severity of pneumonitis.
Table 4.
Labs at pneumonitis diagnosis by severity of ICI-p.
| Variable | Mild ICI-pa | Severe ICI-pa | P-value |
|---|---|---|---|
| Absolute neutrophil countb (median, IQR) | 5230 (3700, 7820) | 5310 (978, 9103) | .56 |
| Absolute lymphocyte countb (median, IQR) | 896 (546, 1365) | 645 (223 953) | .01 |
| Absolute monocyte countb (median, IQR) | 735 (500 980) | 620 (85, 1000) | .11 |
| Absolute eosinophil countb (median, IQR) | 175 (21 430) | 50 (0, 200) | .004 |
| Albuminc (mean, SD) | 3.65 (0.34) | 3.26 (0.49) | <.001 |
aGrading of ICI-p by CTCAE criteria version 4; Minor ICI-p (grade 1-2), severe ICI-p (grade 3-5).
bMeasured in K/µL.
cMeasure in g/dL.
Abbreviations: ICI-p, immune checkpoint inhibitor pneumonitis.
Risk factors for partially steroid-responsive or steroid-refractory pneumonitis
Of the 132 ICI-p patients, 74 (56%) had partially steroid-responsive or steroid-refractory pneumonitis (22 patients required recurrent steroids after initial pneumonitis treatment, 27 patients required an increase in steroids during initial pneumonitis treatment, 25 died while being treated for active pneumonitis) and 58 (44%) did not. Patients who received less than 1 mg/kg prednisone equivalent of corticosteroid at ICI-p diagnosis had more partially steroid-responsive and steroid-refractory disease (P = .014, Table 5); patients in the < 1 mg/kg prednisone equivalent group received a median dose of 60 mg (0.67 mg/kg) of prednisone, while the group treated with at least 1 mg/kg prednisone equivalent steroids received a median dose of 82.5 mg (1.06 mg/kg) of prednisone (Supplementary Table S1). There was no difference in days to steroid initiation from pneumonitis imaging and days from initial steroid administration to first steroid taper between the two groups (Supplementary Table S2).
Table 5.
Risk factors for partially steroid-responsive or steroid-refractory ICI-p.
| Variable | Steroid-responsive ICI-p (n = 58) | Partially steroid-responsive or steroid-refractory ICI-p (n = 74) | P-value | |
|---|---|---|---|---|
| Steroids < 1 mg/kg (prednisone equivalent) at ICI-p diagnosis | Yes | 19 (32.8) | 40 (54.1) | .014 |
| No | 39 (67.2) | 34 (45.9) | ||
| Steroid administrationa | Intravenous | 13 (23.2) | 27 (37.0) | .091 |
| Oral | 43 (76.8) | 46 (63.0) | ||
| Pre-treatment chest imagingb | ||||
| Interstitial abnormalities | Yes | 33 (61.1) | 52 (74.3) | .12 |
| No | 21 (38.9) | 18 (25.7) | ||
| Consolidation | Yes | 1 (1.9) | 6 (8.6) | .09 |
| No | 53 (98.1) | 64 (91.4) | ||
| Ground glass | Yes | 14 (25.9) | 36 (51.4) | .004 |
| No | 40 (74.1) | 34 (48.6) | ||
| Reticular | Yes | 19 (35.2) | 28 (40.0) | .58 |
| No | 35 (64.8) | 42 (60.0) | ||
| Centrilobular nodules or Bronchiolitis | Yes | 7 (13.0) | 12 (17.1) | .52 |
| No | 47 (87.0) | 58 (82.9) | ||
| Bronchiectasis | Yes | 1 (1.9) | 5 (7.1) | .15 |
| No | 53 (98.1) | 65 (92.9) | ||
| Honeycombing | Yes | 1 (1.9) | 2 (2.9) | .72 |
| No | 53 (98.1) | 68 (97.1) | ||
| Severity score | 2.00 (1.00, 3.00) | 3.00 (1.00, 8.25) | .001 | |
| Pneumonitis chest imagingc | ||||
| Consolidation | Yes | 15 (27.3) | 30 (46.9) | .03 |
| No | 40 (72.7) | 34 (53.1) | ||
| Ground glass | Yes | 52 (94.5) | 58 (90.6) | .42 |
| No | 3 (5.5) | 6 (9.4) | ||
| Reticular | Yes | 33 (60.0) | 35 (54.7) | .56 |
| No | 22 (40.0) | 29 (45.3) | ||
| Centrilobular nodules or Bronchiolitis | Yes | 8 (14.5) | 10 (15.6) | .87 |
| No | 47 (85.5) | 54 (84.4) | ||
| Bronchiectasis | Yes | 9 (16.4) | 13 (20.3) | .58 |
| No | 46 (83.6) | 51 (79.7) | ||
| Honeycombing | Yes | 3 (5.5) | 7 (10.9) | .28 |
| No | 52 (94.5) | 57 (89.1) |
aThree patients did not receive steroid treatment.
bOne-hundred twenty-four patients had chest CT available for review before ICI initiation.
cOne-hundred nineteen patients had chest CT available for review at the time of ICI-p diagnosis.
Of the 124 patients with pre-treatment chest imaging, the presence of ground glass (P = .004) and severe interstitial abnormalities (P = .001) were associated with partially steroid-responsive and steroid-refractory ICIa-p. Of the 132 ICI-p patients, 64 of the 74 patients with partially steroid-responsive or steroid-refractory ICI-p and 55 of the 58 patients with steroid-responsive ICI-p had a chest CT following diagnosis of ICI-p. The presence of consolidative changes at the time of ICI-p diagnosis was associated with severe and partially steroid-responsive ICI-p (P = .03, Table 5). Other radiographic patterns at ICI-p diagnosis and the route of steroid administration were not associated with steroid-refractory or partially steroid-responsive disease.
Survival analysis of mild versus severe pneumonitis
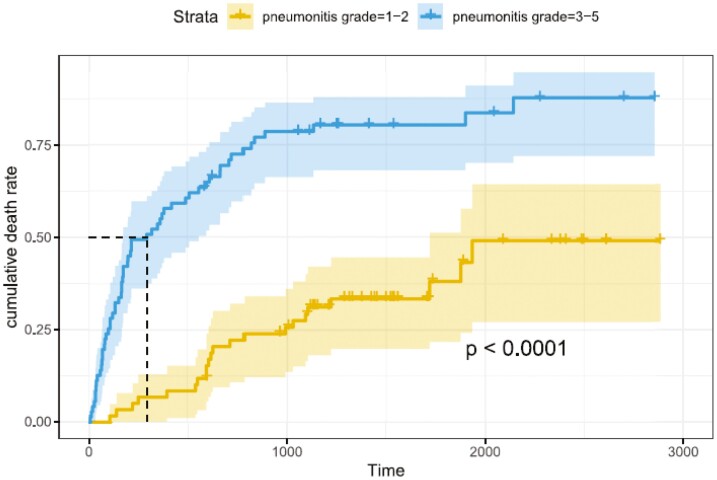
Severity of ICI-p and its impact on survival was evaluated as a secondary outcome. The severity of ICI-p was divided into mild (grade 1-2) and severe (grade 3-5). Of our 132 patients, 60 patients developed mild pneumonitis and 72 patients developed severe pneumonitis. Patients with severe pneumonitis had worse overall survival when compared to mild pneumonitis (P < .0001, Figure 1).
Figure 1.
Survival by severity of ICI-p
Survival analysis of IVIG alone versus other steroid-alternative agents
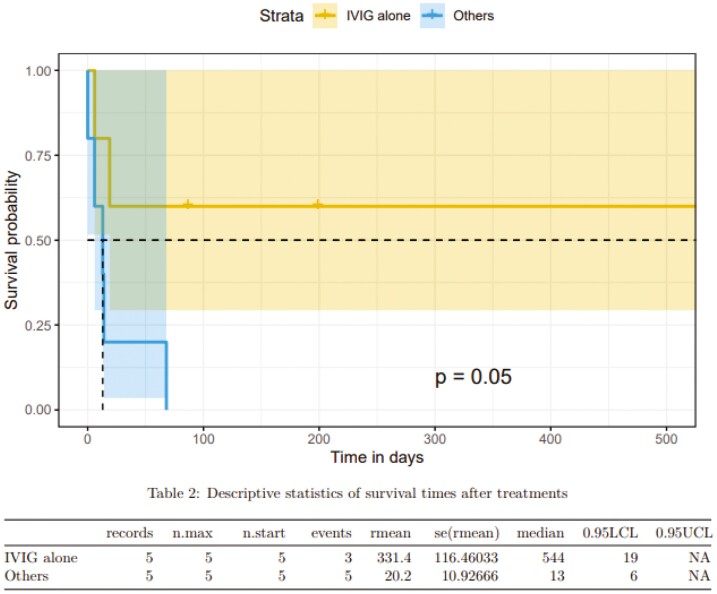
A total of 10 patients were treated with immunosuppressive medications for steroid-refractory ICI-p. Four of the 10 patients initially received <1 mg/kg prednisone equivalent as the initial steroid dose, and all patients received at least 1 mg/kg prednisone equivalent steroids during their clinical course. Nine of the 10 patients received steroids within 1 day of diagnosis, with 1 patient not receiving steroids until 21 days after initial imaging findings of ICI-p. Of the 10 patients, 5 received intravenous immunoglobulin (IVIG) alone, 2 received infliximab, 1 patient received IVIG and abatacept, 1 patient received IVIG and cyclophosphamide, and 1 patient received rituximab. Three of the 10 patients had pneumonitis resolution after treatment with IVIG alone, and patients who received IVIG alone had improved survival (P = 0.05, Figure 2).
Figure 2.
Survival in steroid-refractory ICI-p patients treated with IVIG alone.
Discussion
In this single-center retrospective analysis of consecutive patients with cancer treated with ICI, we found that abnormal FVCpp and TLCpp, specific and severe radiographic pre-treatment chest imaging abnormalities, and lower absolute lymphocyte and eosinophil counts at the time of diagnosis were risk factors for severe ICI-p. We also found that less than 1 mg/kg prednisone equivalent of corticosteroid as initial treatment was associated with partially steroid-responsive and steroid-refractory ICI-p. This study is the first to concurrently evaluate pre-treatment risk factors for severe ICI-p and the impact of initial corticosteroid dosing on the clinical course of ICI-p. Our study highlights several pre-treatment risk factors that may help stratify which patients are at the highest risk for severe complications from ICI and the importance of aggressive and appropriate immune suppression for preventing partially steroid-responsive ICI-p.
Several groups have shown that pre-treatment interstitial abnormalities may be a risk factor for developing ICI-p, but few have focused on whether imaging abnormalities are a risk factor for severe disease. Shimoji et al reported that pre-existing interstitial lung abnormalities,30 particularly ground-glass attenuation, were a risk factor for ICI-p, while several groups showed that pulmonary fibrosis and the extent of honeycombing on pre-treatment imaging increases the risk of ICI-p.11,31 None found a link between pre-treatment chest imaging abnormalities and severe ICI-p. In our previous study,14 we also found that chest imaging abnormalities were a risk factor for ICI-p but not for severe disease. However, our study only included patients up to 2017, with most patients receiving ICI for palliative intent, limiting the amount of time for ICI-p to develop. In our current study, the number of ICI-p patients is higher, and a majority received ICI as first-line treatment. We found that consolidation and bronchiectasis on pre-treatment chest imaging, along with the extent of interstitial abnormality, were risk factors for developing severe ICI-p. As most cancer patients have chest imaging completed before ICI initiation, pre-treatment chest imaging is a reliable and non-invasive tool to help determine which patients may be at the highest risk for severe pulmonary complications (hospitalization, intensive unit admission, death).
Existing literature suggests there may be an association between pre-treatment PFT abnormalities and the development of ICI-p, but none shows whether these physiologic changes are associated with severe disease. FEV1pp, FVCpp, and TLCpp have all been implicated in previous studies as risk factors for ICI-p.32,33 All of these studies, including our previous work, have not found a link between these abnormalities and severe ICI-p as most are limited by the available pre-treatment PFTs and the small number of patients with severe ICI-p.14,32 In our current study, we had a larger number of patients with severe disease but were still limited by the small percentage of patients that had PFTs completed within 12 months of ICI initiation. We found that reduced pre-treatment FVCpp and TLCpp were associated with severe ICI-p, and lower TLCpp was associated with more severe pre-treatment interstitial changes. FVCpp and TLCpp are closely linked with the development and progression of interstitial lung disease,34,35 therefore lower pre-treatment values may be predictive of patients at higher risk for severe ICI-p. As ICIs are increasingly utilized in the neoadjuvant and curative setting, preventing severe ICI-p, which can worsen survival in patients with potentially curable early-stage cancer, is of paramount importance. Integrating pre-treatment PFTs into the treatment planning for these patients, especially for patients with more severe pre-treatment interstitial abnormalities, may identify patients at highest risk for severe ICI-p, those in which the risk of toxicity outweighs the benefit of treatment, and those who may need more frequent monitoring (office visits, shorter interval chest CTs) early in the ICI treatment course as severe ICI-p tends to present early compared to mild disease.10
While pre-treatment chest and PFT abnormalities are relatively well established as potential risk factors for ICI-p, the association with serologic biomarkers is not clear. Zhou et al found that lower albumin levels were associated with the development of ICI-p,11 and Chu et al showed that higher pre-treatment peripheral eosinophilia was associated with ICI-p and better overall survival.36 Neither examined whether these or other serologic markers were predictive for severe ICI-p. We found that lower albumin level, lower absolute lymphocyte count, and lower absolute eosinophil count at the time of ICI-p diagnosis were associated with severe ICI-p. Serum albumin may be difficult to utilize prognostically for severe ICI-p as low levels have been correlated with lower survival in cancer patients treated with ICI,37 but higher absolute eosinophil counts have been linked to steroid-responsive pulmonary diseases,38,39 which typically have a better prognosis compared to non-eosinophilic interstitial lung diseases. We found that patients with higher eosinophil count at ICI-p diagnosis had more mild disease compared to those with lower eosinophil counts, which may reflect a better response to initial corticosteroid treatment preventing the development of severe ICI-p. Further studies will be needed to validate our findings by using albumin, lymphocyte count, and eosinophil count as potential biomarkers for severe ICI-p.
Corticosteroids are a mainstay in ICI-p treatment, but the dosing of steroids and its impact on the disease course for ICI-p is unknown. Major societies all recommend treatment with steroids at a dose of at least 1 mg/kg (prednisone equivalent) as first-line therapy.16,19,40 However, this recommendation is based on expert opinion, extrapolated from other ICI toxicities, and not based on evidence showing superiority of corticosteroid dosing being at least 1 mg/kg. Furthermore, previous literature on drug-related pneumonitis suggested initial corticosteroid dosing at 1 mg/kg but only up to a maximum of 60 mg daily.41 Although utilized frequently, corticosteroids at high doses are not harmless and can increase the risk of opportunistic infections, exacerbate other chronic illnesses, and decrease quality of life. In our study, we are the first to show that patients who received less than 1 mg/kg prednisone equivalent of steroids, despite a median dose of 60 mg of prednisone, were more likely to need an increase or repeated steroid course, additional immune suppression, or have incompletely resolved ICI-p compared to those who received at least 1 mg/kg corticosteroid as initial therapy, suggesting this dose is necessary to prevent partially steroid responsive or refractory ICI-p.
We found that patients with severe ICI-p have decreased survival compared to those with mild ICI-p, consistent with prior studies.8,15 Severe ICI-p, particularly in patients with steroid-refractory ICI-p, is associated with significant morbidity and mortality.10,42 Small studies and case series have shown varying success with cyclophosphamide,42 IVIG,43 infliximab,44 mycophenolate,10 and tocilizumab.45 Society guidelines recommend additional, non-steroid immune suppression, but the optimal secondary agent is unknown due to a lack of direct comparison between treatment options.16,19,40 In our study, we found that patients with steroid-refractory ICI-p treated with IVIG may live longer compared to other treatment options, but this was limited by small sample size, suboptimal steroid dosing, and delayed treatment initiation at pneumonitis diagnosis. Larger, prospective studies are needed to fully evaluate the role of IVIG against other therapeutic options for steroid-refractory ICI-p.
Prior thoracic radiation is a known risk factor for ICI-p in patients with lung cancer,15,46,47 but whether this leads to more severe disease is unknown. In a study of lung cancer patients treated with ICI, Deng et al found that radiation treatment (not limited to thoracic radiation) during ICI was associated with severe ICI-p,46 but this same association was not seen in radiation treatment preceding ICI initiation. Barron et al found that patients with severe ICI-p all received previous radiotherapy,47 but the severe ICI-p cohort was small (4 patients) and prior radiation treatment was not limited to thoracic radiation. Surprisingly, in our study, we found that NSCLC and previous chest radiation were associated with mild ICI-p. This may be driven by the subset of stage III NSCLC patients that received concurrent chemoradiation followed by ICI who developed mild ICI-p (required corticosteroids but never required oxygen supplementation or hospitalization). Further studies will be needed to validate our findings as this has important prognostic implications for integrating ICI into the multimodality treatment plan for patients with lung cancer.
There are several limitations to our study. First, this study is retrospective in nature, and there may be unidentifiable confounders that affect the associations among pre-treatment imaging abnormalities, PFT abnormalities, lab abnormalities, and severe ICI-p. Additionally, P-values for demographic variables are unadjusted for multiple comparisons, making significant findings prone to false positives. Second, our sample size for steroid-refractory ICI-p is small and several patients received delayed or suboptimal corticosteroid dosing, making it difficult to make definitive conclusions on the optimal secondary treatment agent for refractory disease. Third, patients included in the partially steroid-responsive cohort were heterogeneous, and identified risk factors may not apply to each subgroup. However, there are currently no predictors for steroid-responsive and partially steroid-responsive ICI-p, and we believe our study gives valuable insight into a previously understudied area of ICI-p. Lastly, the diagnosis of ICI-p is made clinically, requiring reasonable exclusion of alternate etiologies, leading to variability in diagnosis and practice patterns. To address this, all pneumonitis cases documented by the treating oncologist were retrospectively reviewed by a member of the study team for agreement. Our pneumonitis incidence is within the range of recent studies of ICI-p, suggesting generalizability of our results.48
Conclusion
We found that pre-treatment chest imaging abnormalities, PFT abnormalities, and lower lymphocyte and eosinophil count at the time of ICI-p diagnosis may be associated with severe ICI-p. We also found that an initial corticosteroid dose of less than 1 mg/kg was associated with partially steroid-responsive ICI-p. Larger, prospective studies will be needed to validate our results.
Supplementary material
Supplementary material is available at The Oncologist online.
Contributor Information
Meghana Moodabagil, Department of Internal Medicine, The Ohio State Wexner Medical Center, Columbus, OH 43210, United States.
Robert Easterling, Department of Pulmonary, Critical Care, and Sleep Medicine, Medical University of South Carolina, Charlestown, SC 29425, United States.
Jing Peng, Center for Biostatistics, The Ohio State University, James Comprehensive Cancer Center, Columbus, OH 43210, United States.
Hamzah Abu-Sbeih, Division of Medical Oncology, Department of Internal Medicine, The Ohio State University, James Comprehensive Cancer Center, Columbus, OH 43210, United States.
Alexa Meara, Division of Medical Oncology, Department of Internal Medicine, The Ohio State University, James Comprehensive Cancer Center, Columbus, OH 43210, United States.
Edwin Donnelly, Division of Radiology, The Ohio State Wexner Medical Center, Columbus, OH 43210, United States.
Dwight H Owen, Division of Medical Oncology, Department of Internal Medicine, The Ohio State University, James Comprehensive Cancer Center, Columbus, OH 43210, United States.
Kevin Ho, Division of Pulmonary, Critical Care, and Sleep Medicine, The Ohio State Wexner Medical Center, Columbus, OH 43210, United States.
Author contributions
Meghana Moodabagil: collection and assembly of data, data analysis and interpretation, manuscript preparation. Robert Easterling: collection and assembly of data, data analysis, and interpretation. Jing Peng: data analysis and interpretation, manuscript preparation. Hamzah Abu-Sbeih: collection and assembly of data. Alexa Meara: conception and design, manuscript preparation. Edwin Donnelly: conception and design, collection and assembly of data, data analysis and interpretation. Dwight H. Owen: conception and design, collection and assembly of data, data analysis and interpretation, manuscript preparation. Kevin Ho: conception and design, collection and assembly of data, data analysis and interpretation, manuscript preparation. All authors reviewed the manuscript and approved the final submission. Dwight H. Owen, and Kevin Ho: contributed equally to this work.
Funding
The authors declare no funding.
Conflicts of interest
None declared.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Robert CA. decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kalisz KR, Ramaiya NH, Laukamp KR, Gupta A. Immune checkpoint inhibitor therapy-related pneumonitis: patterns and management. Radiographics. 2019;39(7):1923-1937. 10.1148/rg.2019190036 [DOI] [PubMed] [Google Scholar]
- 3. Marrone KA, Naidoo J, Brahmer JR. Immunotherapy for lung cancer: no longer an abstract concept. Semin Respir Crit Care Med. 2016;37(5):771-782. 10.1055/s-0036-1592298 [DOI] [PubMed] [Google Scholar]
- 4. Naidoo J, Wang X, Woo KM, et al. Pneumonitis in patients treated with anti-programmed death-1/programmed death ligand 1 therapy. J Clin Oncol. 2017;35(7):709-717. 10.1200/JCO.2016.68.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho JY, Kim J, Lee JS, et al. Characteristics, incidence, and risk factors of immune checkpoint inhibitor-related pneumonitis in patients with non-small cell lung cancer. Lung Cancer. 2018;125:150-156. 10.1016/j.lungcan.2018.09.015 [DOI] [PubMed] [Google Scholar]
- 6. Suresh K, Voong KR, Shankar B, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: incidence and risk factors. J Thorac Oncol. 2018;13(12):1930-1939. 10.1016/j.jtho.2018.08.2035 [DOI] [PubMed] [Google Scholar]
- 7. Petri CR, Patell R, Batalini F, Rangachari D, Hallowell RW. Severe pulmonary toxicity from immune checkpoint inhibitor treated successfully with intravenous immunoglobulin: case report and review of the literature. Respir Med Case Rep. 2019;27:100834. 10.1016/j.rmcr.2019.100834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atchley WT, Alvarez C, Saxena-Beem S, et al. Immune checkpoint inhibitor-related pneumonitis in lung cancer: real-world incidence, risk factors, and management practices across six health care centers in North Carolina. Chest. 2021;160(2):731-742. 10.1016/j.chest.2021.02.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tiu BC, Zubiri L, Iheke J, et al. Real-world incidence and impact of pneumonitis in patients with lung cancer treated with immune checkpoint inhibitors: a multi-institutional cohort study. J ImmunoTher Cancer. 2022;10(6):e004670. 10.1136/jitc-2022-004670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beattie J, Rizvi H, Fuentes P, et al. Success and failure of additional immune modulators in steroid-refractory/resistant pneumonitis related to immune checkpoint blockade. J ImmunoTher Cancer. 2021;9(2):e001884. 10.1136/jitc-2020-001884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou P, Zhao X, Wang G. Risk factors for immune checkpoint inhibitor-related pneumonitis in cancer patients: a systemic review and meta-analysis. Respiration. 2022;101(11):1035-1050. 10.1159/000526141 [DOI] [PubMed] [Google Scholar]
- 12. Khunger M, Rakshit S, Pasupuleti V, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: a systematic review and meta-analysis of trials. Chest. 2017;152(2):271-281. 10.1016/j.chest.2017.04.177 [DOI] [PubMed] [Google Scholar]
- 13. Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of programmed cell death 1 inhibitor-related pneumonitis in patients with advanced cancer: a systematic review and meta-analysis. JAMA Oncol. 2016;2(12):1607-1616. 10.1001/jamaoncol.2016.2453 [DOI] [PubMed] [Google Scholar]
- 14. Wong A, Riley M, Zhao S, et al. Association between pre-treatment chest imaging and pulmonary function abnormalities and immune checkpoint inhibitor pneumonitis. Cancer Immunol Immunother. 2023;72(6):1727-1735. 10.1007/s00262-023-03373-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wong A, Riley M, Zhao S, et al. Association between pretreatment chest imaging and immune checkpoint inhibitor pneumonitis among patients with lung cancer. J Natl Compr Canc Netw. 2023;21(11):1164-1171.e5. 10.6004/jnccn.2023.7059 [DOI] [PubMed] [Google Scholar]
- 16. Schneider BJ, Naidoo J, Santomasso BD, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: ASCO guideline update. J Clin Oncol. 2021;39(36):4073-4126. 10.1200/JCO.21.01440 [DOI] [PubMed] [Google Scholar]
- 17. Brahmer JR, Lacchetti C, Thompson JA. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline Summary. J Oncol Pract. 2018;14(4):247-249. 10.1200/JOP.18.00005 [DOI] [PubMed] [Google Scholar]
- 18. Haanen J, Obeid M, Spain L, et al. ; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Management of toxicities from immunotherapy: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(12):1217-1238. 10.1016/j.annonc.2022.10.001 [DOI] [PubMed] [Google Scholar]
- 19. Puzanov I, Diab A, Abdallah K, et al. ; Society for Immunotherapy of Cancer Toxicity Management Working Group. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J ImmunoTher Cancer. 2017;5(1):95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ge S, Huang C. Immune checkpoint inhibitors in neoadjuvant therapy of non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis. 2022;14(2):333-342. 10.21037/jtd-21-1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Antonia SJ, Villegas A, Daniel D, et al. ; PACIFIC Investigators. PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377(20):1919-1929. 10.1056/NEJMoa1709937 [DOI] [PubMed] [Google Scholar]
- 22. Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a Harris metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li M, Spakowicz D, Zhao S, et al. Brief report: inhaled corticosteroid use and the risk of checkpoint inhibitor pneumonitis in patients with advanced cancer. Cancer Immunol Immunother. 2020;69(11):2403-2408. 10.1007/s00262-020-02674-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shankar B, Zhang J, Naqash AR, et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol. 2020;6(12):1952-1956. 10.1001/jamaoncol.2020.5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. CommonTerminologyCriteriaforAdverseEvents(CTCAE).Version4.03.2010.Availableat:https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. Accessed August 2, 2023. [Google Scholar]
- 27. Graham BL, Steenbruggen I, Miller MR, et al. Standardization of Spirometry 2019 update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am J Respir Crit Care Med. 2019;200(8):e70-e88. 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sterk PJ. Let's not forget: the GOLD criteria for COPD are based on post-bronchodilator FEV1. Eur Respir J. 2004;23(4):497-498. 10.1183/09031936.04.00017104 [DOI] [PubMed] [Google Scholar]
- 29. Kanai O, Kim YH, Demura Y, et al. Efficacy and safety of nivolumab in non-small cell lung cancer with preexisting interstitial lung disease. Thorac Cancer. 2018;9(7):847-855. 10.1111/1759-7714.12759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shimoji K, Masuda T, Yamaguchi K, et al. Association of preexisting interstitial lung abnormalities with immune checkpoint inhibitor-induced interstitial lung disease among patients with nonlung cancers. JAMA Netw Open. 2020;3(11):e2022906. 10.1001/jamanetworkopen.2020.22906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yamaguchi T, Shimizu J, Oya Y, et al. Risk factors for pneumonitis in patients with non-small cell lung cancer treated with immune checkpoint inhibitors plus chemotherapy: a retrospective analysis. Thorac Cancer. 2022;13(5):724-731. 10.1111/1759-7714.14308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reuss JE, Brigham E, Psoter KJ, et al. Pretreatment lung function and checkpoint inhibitor pneumonitis in NSCLC. JTO Clin Res Rep. 2021;2(10):100220. 10.1016/j.jtocrr.2021.100220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suzuki Y, Karayama M, Uto T, et al. Assessment of immune-related interstitial lung disease in patients with NSCLC treated with immune checkpoint inhibitors: a multicenter prospective study. J Thorac Oncol. 2020;15(8):1317-1327. 10.1016/j.jtho.2020.04.002 [DOI] [PubMed] [Google Scholar]
- 34. Veit T, Barnikel M, Crispin A, et al. Variability of forced vital capacity in progressive interstitial lung disease: a prospective observational study. Respir Res. 2020;21(1):270. 10.1186/s12931-020-01524-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med. 2000;161(2 Pt 1):646-664. [DOI] [PubMed] [Google Scholar]
- 36. Chu X, Zhao J, Zhou J, et al. Association of baseline peripheral-blood eosinophil count with immune checkpoint inhibitor-related pneumonitis and clinical outcomes in patients with non-small cell lung cancer receiving immune checkpoint inhibitors. Lung Cancer. 2020;150:76-82. 10.1016/j.lungcan.2020.08.015 [DOI] [PubMed] [Google Scholar]
- 37. Guven DC, Sahin TK, Erul E, et al. The association between albumin levels and survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front Mol Biosci. 2022;9:1039121. 10.3389/fmolb.2022.1039121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allen J, Wert M. Eosinophilic pneumonias. J Allergy Clin Immunol Pract. 2018;6(5):1455-1461. 10.1016/j.jaip.2018.03.011 [DOI] [PubMed] [Google Scholar]
- 39. Bafadhel M, McKenna S, Terry S, Mistry V, Pancholi M, Venge P, Lomas DA, Barer MR, Johnston SL, Pavord ID, Brightling CE. Blood eosinophils to direct corticosteroid treatment of exacerbations of chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Am J Respir Crit Care Med. 2012;186(1):48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Brahmer JR, Lacchetti C, Schneider BJ, et al. ; National Comprehensive Cancer Network. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36(17):1714-1768. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Müller NL, White DA, Jiang H, Gemma A. Diagnosis and management of drug-associated interstitial lung disease. Br J Cancer 2004;91(Suppl 2):S24-S30. 10.1038/sj.bjc.6602064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Camard M, Besse B, Cariou PL, et al. Prevalence and outcome of steroid-resistant/refractory pneumonitis induced by immune checkpoint inhibitors. Respir Med Res. 2022;82:100969. 10.1016/j.resmer.2022.100969 [DOI] [PubMed] [Google Scholar]
- 43. Balaji A, Hsu M, Lin CT, et al. Steroid-refractory PD-(L)1 pneumonitis: incidence, clinical features, treatment, and outcomes. J ImmunoTher Cancer. 2021;9(1):e001731. 10.1136/jitc-2020-001731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lai KA, Sheshadri A, Adrianza AM, et al. Role of infliximab in immune checkpoint inhibitor-induced pneumonitis. J Immunother Precis Oncol. 2020;3(4):172-174. 10.36401/JIPO-20-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Stroud CR, Hegde A, Cherry C, et al. Tocilizumab for the management of immune mediated adverse events secondary to PD-1 blockade. J Oncol Pharm Pract. 2019;25(3):551-557. 10.1177/1078155217745144 [DOI] [PubMed] [Google Scholar]
- 46. Deng H, Deng J, Lin X, et al. A Risk-scoring model for severe checkpoint inhibitor-related pneumonitis: a case-control study. Clin Drug Investig. 2023;43(5):347-357. 10.1007/s40261-023-01267-6 [DOI] [PubMed] [Google Scholar]
- 47. Barrón F, Sánchez R, Arroyo-Hernández M, et al. Risk of developing checkpoint immune pneumonitis and its effect on overall survival in non-small cell lung cancer patients previously treated with radiotherapy. Front Oncol. 2020;10:570233. 10.3389/fonc.2020.570233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang H, Guo X, Zhou J, et al. Clinical diagnosis and treatment of immune checkpoint inhibitor-associated pneumonitis. Thorac Cancer. 2020;11(1):191-197. 10.1111/1759-7714.13240 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.