Abstract
Ustilago crameri is a pathogenic basidiomycete fungus that causes foxtail millet kernel smut (FMKS), a devastating grain disease in most foxtail millet growing regions of the world. Carbohydrate-Binding Modules (CBMs) are one of the important families of carbohydrate-active enzymes (CAZymes) in fungi and play a crucial role in fungal growth and development, as well as in pathogen infection. However, there is little information about the CBM family in U. crameri. Here, 11 CBM members were identified based on complete sequence analysis and functional annotation of the genome of U. crameri. According to phylogenetic analysis, they were divided into six groups. Gene structure and sequence composition analysis showed that these 11 UcCBM genes exhibit differences in gene structure and protein motifs. Furthermore, several cis-regulatory elements involved in plant hormones were detected in the promoter regions of these UcCBM genes. Gene ontology (GO) enrichment and protein–protein interaction (PPI) analysis showed that UcCBM proteins were involved in carbohydrate metabolism, and multiple partner protein interactions with UcCBM were also detected. The expression of UcCBM genes during U. crameri infection is further clarified, and the results indicate that several UcCBM genes were induced by U. crameri infection. These results provide valuable information for elucidating the features of U. crameri CBMs’ family proteins and lay a crucial foundation for further research into their roles in interactions between U. crameri and foxtail millet.
Keywords: Ustilago crameri, foxtail millet, CBM, pathogenicity
1. Introduction
Plant pathogenic fungi can degrade host cell walls’ polysaccharide materials by producing a variety of carbohydrate activity enzymes (CAZymes) to facilitate infection and gain nutrition [1]. Carbohydrate-binding modules (CBMs) are widely present in the microorganism, and are important components of the CAZymes of plant pathogenic fungi [2]. CBMs are the most common non-catalytic modules associated with active cell wall hydrolysis enzymes, and they are divided into 87 families in the CAZymes database [3,4]. This protein family plays a key role in the recognition of plant cell wall components and in enhancing the enzyme activity of glycoside hydrolases [5].
More importantly, the roles of CBMs in the pathogenicity of plant pathogens have been confirmed [6,7]. For example, the CBM1 domain protein VdCBM1 from Verticillium dahliae suppressed VdEG1 and VdEG3 protein-induced cell death in Nicotiana benthamiana [8]. The CBM protein PcCBP3, which comes from the oomycete pathogen Phytophthora capsica, was found to be a crucial virulence factor and play a crucial role as an apoplastic effector in interactions between P. capsica and its host [9]. Furthermore, deletion of the swollenin gene that contains an N-terminal CBM1 and C-terminal expansin-like domain results in the significantly reduced virulence of Trichoderma reesei on the host plant [10]. These findings suggest that CBMs are important virulence factors in phytopathogens; the study of CBM proteins would be beneficial to the study of the molecular mechanisms of interactions between pathogens and plants.
Foxtail millet (Setaria italica L.) is one of the world’s oldest cultivated crops, providing the daily dietary intake for millions of people in southern Europe and Asia [11]. Ustilago crameri is an important pathogenic fungus affecting the production of foxtail millet in the world [11]. This disease was first reported in Uttarakhand and then found in India, Karnataka, Andhra Pradesh, Tamil Nadu Maharashtra, and China [11,12,13,14]. A major feature of this pathogen is that it affects grains by producing a dark black powdery mass of spores in grain ears; however, sometimes, a terminal portion of the spike may escape [15]. The ideal soil temperature for infection ranges from 12 to 25 °C; whether the soil is dry or has high-humidity will significantly impact the U. crameri infection [12]. U. crameri can infect foxtail millet at all growth stages and results in a high incidence of infection of 75% in severe years, leading to a significant decrease in foxtail millet yield [11]. This disease is now an increasing threat to the high production of foxtail millet in most foxtail millet production areas. Previous studies have focused on the biological characteristics of U. crameri [11], while little is known about the pathogenic genes of this pathogen.
As of recently, the completion of U. crameri genome sequencing has offered necessary tools for researching pathogenesis at the level of genomes, and has supported the feasibility of performing the large-scale cloning of pathogenicity genes [16,17]. In this study, we identified 11 CBM family genes by searching the genome of U. crameri SCZ-6 using a bioinformatics analysis. Their physicochemical protein properties, conserved motif, gene structure, and promoter cis-acting elements were analyzed. We also detected the expression pattern of these 11 CBM genes during U. crameri infection and constructed their corresponding protein–protein interaction network. The results of this study promote a deeper understanding of CBM genes in U. crameri and provide a theoretical basis for exploring their roles in the pathogenesis of U. crameri.
2. Results
2.1. Identification and Characterization of UcCBM Gene Family
We identified a total of 11 CBM members in U. crameri, which were named UcCBM1-UcCBM11, and these proteins belong to the superfamilies CBM48, CBM4, CBM43, CBM18, CBM21, CBM63, CBM35, CBM50, and CBM32, respectively (Table 1). The protein length, molecular weight, isoelectric points (PIs), grand average of hydropathicity (GRAVH), and subcellular localization are shown in Table 1. The protein length of these UcCBMs ranged from 171 amino acids (aas) (UcCBM11) to 1007 aas (UcCBM5), corresponding to protein molecular weights of 12.14 and 88.78 kDa, respectively. The PI of UcCBM8 was the lowest with 4.57, and the highest was UcCBM4 with 8.83, and the mean isoelectric point of these 11 CBM proteins was 6.18, which indicates acidity. These results indicate that UcCBMs have a wide range of PIs and molecular weights. The GRAVH of these UcCBM proteins was negative, except for UcCBM3, indicating that the majority of UcCBM proteins are associated with hydrophilicity. In addition, the subcellular localization of UcCBM proteins was predicted, and the results showed that the majority of UcCBM proteins were localized extracellularly, indicating that they function primarily in the extracellular space (Table 1).
Table 1.
Nomenclature and characteristics of the predicted carbohydrate binding modules (CBMs) in U. crameri.
| Proposed Gene Name |
Gene ID | Superfamily | CDS Length (bp) | Protein Length (aa) | Mw (KDa) | pI | GRAVH | Predicted Subcellular Localization |
|---|---|---|---|---|---|---|---|---|
| UcCBM1 | Ustilago0G003590 | CBM48 | 2094 | 698 | 79.29 | 5.94 | −0.35 | mitochondrion |
| UcCBM2 | Ustilago0G007370 | CBM4 | 2097 | 699 | 76.23 | 5.82 | −0.24 | extracellular |
| UcCBM3 | Ustilago0G009390 | CBM43 | 1731 | 577 | 60.42 | 4.6 | 0.008 | extracellular |
| UcCBM4 | Ustilago0G030860 | CBM18 | 1137 | 379 | 41.65 | 8.83 | −0.288 | extracellular |
| UcCBM5 | Ustilago0G032100 | CBM21 | 3021 | 1007 | 107.5 | 8.09 | −0.602 | nucleus |
| UcCBM6 | Ustilago0G039330 | CBM18 | 2148 | 716 | 76.63 | 8.71 | −0.512 | extracellular |
| UcCBM7 | Ustilago0G044030 | CBM63 | 1662 | 554 | 57.78 | 5.48 | −0.766 | extracellular |
| UcCBM8 | Ustilago0G046260 | CBM35 | 1410 | 470 | 50.7 | 4.57 | −0.186 | extracellular |
| UcCBM9 | Ustilago0G049680 | CBM35 | 1827 | 609 | 64.41 | 5.09 | −0.204 | extracellular |
| UcCBM10 | Ustilago0G052360 | CBM50 | 771 | 257 | 27.65 | 5.95 | −0.333 | extracellular |
| UcCBM11 | Ustilago0G057940 | CBM32 | 513 | 171 | 18.42 | 4.92 | −0.233 | extracellular |
ID: identity; bp: base pair; aa: amino acid; KDa: kilo Dalton; pI: isoelectric point; Mw: molecular weight; GRAVH: grand average of hydropathicity.
2.2. Phylogenetic Analysis of UcCBM Gene Family
To detect the phylogenetic relationship of CBM family proteins in U. crameri, a phylogenetic tree of these UcCBM proteins and their homologous proteins in other smut fungi, including Sporisorium scitamineum, U. maydis, and U. hordei, was constructed by MEGA7.0 software. The results indicated that these 11 UcCBM proteins could be divided into six groups, among which two CMB members, UcCBM10 and UcCBM3, were in one group. The other group contained UcCBM2 and UcCBM1, and UcCBM7 and UcCBM11 were classified in the same group. Furthermore, UcCBM4 and UcCBM6 had a close relationship, and UcCBM5 was in a separate group (Figure 1). All of the above information shows that these 11 UcCBM proteins have large differences in their evolution; UcCBM5 has especially distant phylogenetic relationships with the other 10 CBM proteins, indicating that this CBM protein may have a specific biological function (Figure 1).
Figure 1.
A phylogenetic tree of the UcCBM proteins and their homologous proteins in other smut fungi, including Sporisorium scitamineum, U. maydis, and U. hordei. The phylogenetic tree was constructed with MEGA7 software using the Neighbor-Joining (NJ) method. Different groups are represented by branches and frames of different colors.
2.3. Conserved Motif, Gene Structure Analysis of UcCBM Gene Family
To understand the characteristics of the U. crameri CBM family, the conserved motifs of the UcCBM proteins were analyzed using the website Multiple Em for Motif Elicitation (MEME). The results showed that 10 different motifs in the UcCBM proteins were found, named Motif 1–10 (Figure 2A,B). Among these 11 UcCBM proteins, UcCBM6 contained the highest number of motifs with five different motifs, while UcCBM11, UcCBM3, and UcCBM8 had no motif. Interestingly, we found that UcCBM6 and UcCBM4 had the same motifs, except for Motif 6, indicating that these two UcCBMs may have the same biological function during infection of a host (Figure 2A). In addition, Motif 7, Motif 4, and Motif 3 were the most common motifs, found in three UcCBM proteins (Figure 2A). Other motifs were detected in two UcCBM proteins (Figure 2A). An analysis of the functional domains found that multiple types of conserved domains related to carbohydrates were found in the 11 CBM family proteins (Figure 2B). However, only UcCBM5, UcCBM9, and UcCBM8 had the conserved domain of typical CBM or GH family proteins (Figure 2B). In addition, the structural features of the 11 UcCBM genes were further detected. As shown in Figure 2C, among 11 UcCBM genes, UcCBM2, UcCBM4, and UcCBM11 had the highest number of exons and introns; they contained two exons and one intron, respectively. However, other UcCBM genes only contained one exon and no introns. The conserved motifs of UcCBMs in the same branch were various, suggesting that these UcCBMs may play different functions in U. cramer virulence (Figure 2A).
Figure 2.
Motif distribution, conserved domain, and gene structure analysis of UcCBMs. (A) Phylogenetic trees and conserved motifs of 11 UcCBMs. (B) nserved motif sequence logo of UcCBM proteins. (C) Domain analysis of UcCBMs. (D) Exon–intron structures of UcCBMs.
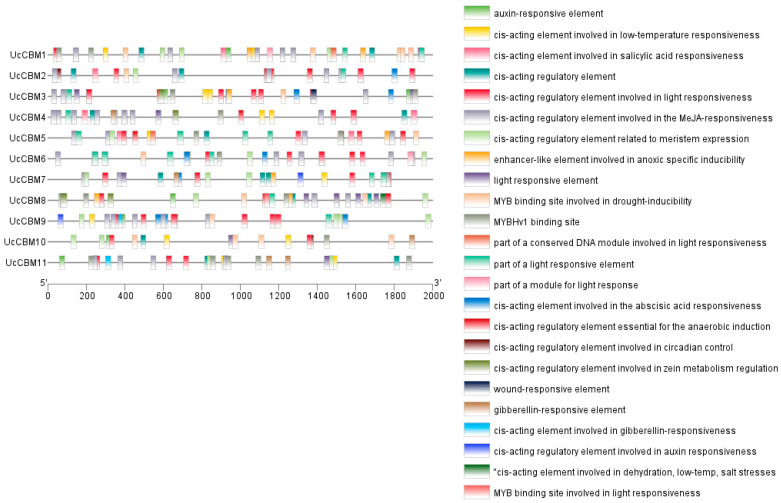
2.4. Analysis of Cis-Acting Promoter Elements of UcCBM Gene Family
To explore the UcCBM-involved signal regulation pathways, the cis-elements in the 2000 bp sequence upstream of the 11 UcCBM gene families were analyzed. According to the results, multiple cis-elements were identified in the UcCBMs (Figure 3 and Table S1). Among them, the hormone response elements included an auxin-responsive element, salicylic acid-responsive element, MeJA-responsive elements, abscisic acid-responsive element, and gibberellin-responsive element. The latter was the most frequent in the UcCBM genes, and all 11 genes contained hormone response elements, which is the most in the UcCBM gene family, suggesting that UcCBMs may be involved in regulating the hormones of U. crameri (Figure 3 and Table S1). There were 7 and 8 UcCBM genes containing low-temperature and drought response elements, respectively, indicating that the UcCBMs may also play a crucial role in the mitigation of stress (Figure 3 and Table S1). Furthermore, we found that an MYBHv1 binding site was contained in 9 UcCBM genes, suggesting that the transcriptional expression of these 9 UcCBMs can be regulated by the MYB transcription factor (Figure 3 and Table S1). Therefore, these results confirm that UcCBMs may be involved in multiple biological processes, and provide important information for studying the biological function of the UcCBM gene family in U. crameri growth and development.
Figure 3.
Cis-acting elements of UcCBM family members in U. crameri. The 2000 bp promoter sequences of U. crameri UcCBM genes contain a variety of cis-acting elements, including hormone responsive elements, drought, low-temperature, and other response elements, as well as an MYBHv1 binding site.
2.5. Protein Structure Analysis of UcCBM Gene Family Members
To better understand the biological function of CBM genes in U. crameri, the secondary structure of the 11 UcCBMs in U. crameri was detected, and the main component of their secondary structure was random coil. Specifically, alpha helices ranged from 5.42% to 32.41%, beta turns from 0.6% to 5.84%, random coil from 50.57% to 87.39%, and extended stand from 6.06% to 29.15% (Table S2). Furthermore, the three-dimensional (3D) protein structures of 11 UcCBM genes were further predicted. As shown in Figure 4, the 3D structure of these 11 UcCBM proteins exhibited substantial differences, highlighting the structural diversity prevalent within the UcCBM family.
Figure 4.
Three-dimensional (3D) modeling of the UcCBM proteins that were predicted, displayed at a confidence level > 0.7.
2.6. Gene Ontology Enrichment Analysis of UcCBM Genes
Gene Ontology (GO) enrichment analysis can help us understand the functions of genes in biological processes [18]. To further investigate the functions and metabolic pathways of the identified CBM proteins in U. crameri, GO enrichment analysis of the 11 UcCBMs was performed. The results indicated that 11 genes were enriched in 43 GO terms (Table S3). We analyzed the top 20 terms, which yielded the majority of UcCBM proteins, including UcCBM1, UcCBM2, UcCBM3, UcCBM4, UcCBM8, and UcCBM9, as having been enriched in the carbohydrate metabolic process (GO:0005975) (Figure 5). UcCBM1 was also enriched in the glycogen biosynthetic (GO:0005978), glycogen metabolic process (GO:0005977), and energy reserve metabolic process (GO:0006112) (Figure 5). UcCBM2 was enriched in the arabinose metabolic process (GO:0019566), L-arabinose metabolic process (GO:0046373), and pentose metabolic process (GO:0019321) (Figure 5). These findings show that the UcCBM genes play an important role in the carbohydrate metabolic process, especially in glycogen metabolic, arabinose metabolic, L-arabinose metabolic, and pentose metabolic processes, which are a component of plant cell walls [19].
Figure 5.
Gene Ontology enrichment analysis of UcCBM genes.
2.7. Response of UcCBM Family Genes During U. crameri Infection in Foxtail Millet
To identify the potential candidate UcCBM genes involved in the pathogenesis of U. crameri, the expression of 11 UcCBM genes in U. crameri after inoculation was detected based on transcriptome sequencing data reported earlier [16]. The expression pattern of the 11 UcCBM family genes based on fragments per kilobase of exon model per million mapped fragments (FPKMs) values at four inoculation time points (0, 12, 24, and 72 h) is showed in Figure 6. The result indicates that the expression of six genes, UcCBM7, UcCBM3, UcCBM5, UcCBM9, UcCBM6, and UcCBM2, were downregulated after U. crameri inoculation, while the expression levels of UcCBM8, UcCBM4, and UcCBM1 were significantly upregulated at 48 and 72 h post-inoculation, respectively. These results confirm that UcCBM genes are involved in the pathogenicity of U. crameri, and play a crucial role in interactions between U. crameri and foxtail millet.
Figure 6.
Expression of UcCBM gene in U. crameri at different inoculation time points.
2.8. Protein−Protein Interaction Network
To further clarify the functional characteristics of UcCBM family proteins, the protein–protein interaction (PPI) network was analyzed using STRING v12.0. The results showed that there are 31 nodes and 53 edges, with an average node degree of 3.42 and an average local clustering coefficient of 0.645 (p−value< 1.0 × 10−16). We set the confidence level greater than 0.7 to predict the functional partner proteins of the UcCBMs, and found that multiple partner proteins interact with the UcCBMs. For instance, UcCBM1 (UHOR_06976) interacted with glycogen [starch] synthase (UHOR_01531), PGM2-phosphoglucomutase (UHOR_00757), glycogenin-2 beta (UHOR_01677), 4-alpha-glucanotransferase (UHOR_05985), and SPT14-N-acetylglucosaminyltransferase (UHOR_03240); UcCBM5 (UHOR_00973) interacted with serine/threonine protein phosphatase (UHOR_07708), ALG1-beta-mannosyltransferase (UHOR_06695), and glycogen [starch] synthase (UHOR_01531); and UcCBM8 (UHOR_04077) interacted with the Fe-S protein (UHOR_06641), cytochrome b5 family protein (UHOR_02592), and Fe-S cluster assembly protein (DRE2) (Figure 7). In this interaction network, UcCBM1 (UHOR_06976) and UcCBM5 (UHOR_00973) form the core of the interactions (Figure 7). These functional partner proteins may work together with UcCBMs to regulate the growth and development of pathogens, and are involved in the interactions between pathogens and their hosts.
Figure 7.
Protein–protein interaction network of UcCBM family proteins, constructed based on smut fungi U. hordei genome. UHOR_06976: UcCBM1; UHOR_02432: UcCBM3; UHOR_02718: UcCBM2; UHOR_00509: UcCBM4 or UcCBM6; UHOR_00973: UcCBM5; UHOR_02067: UcCBM7; UHOR_04077: UcCBM8; UHOR_06273: UcCBM9; UHOR_03469: UcCBM10; UHOR_03282: UcCBM11.
3. Discussion
Foxtail millet kernel smut (FMKS) has become an important factor in restricting the production of foxtail millet in the world [20]. Recently, although the genome sequencing of U. crameri has been completed [16], the study of pathogenicity function genes has been limited. Many studies have confirmed that CBM proteins of plant pathogens can act as effectors and play crucial roles in the interactions between pathogens and their hosts [21,22]. In this study, 11 CBM genes were identified via a bioinformatics analysis based on the genome data of U. crameri. The number of UcCBM genes was less than those of the necrotrophic fungus Rhizoctonia solani [23] and the hemibiotroph Magnaporthe grisea [24], which may be due to the fact that the biotrophic U. crameri did not destroy the host cell wall during the infection.
Only five UcCBM proteins have the conserved domain of typical CBM or GH family proteins; however, the CDS of UcCBM4 and UcCBM6 contains a carbohydrate-binding site. Furthermore, several LysM domains have a chitin-binding ability, and are known as CBM protein family 50 domains [25]. The proteins within the LysM domain directly play a role either in the immunity regulation of pattern recognition receptors (PRRs) or as effectors in pathogenic fungi that suppress plant immunity [26,27,28]. Our result indicates that UcCBM10 contains two LysMs conserved domains; we speculate that this gene may act as an effector and play crucial roles in the U. crameri–host interaction. VdEG3, an effector of V. dahlia, which has both the CBM1 and GH12 conserved domains, could trigger cell death in N. benthamiana via its GH12 (VdEG3GH12) domain [8]. Interestingly, the CMB1 domain of VdEG3 could suppress the cell death-inducing activity of VdEG3GH12. We also found that UcCBM10 containing both CBM and GH conserved domains and their domain features was consistent with VdEG3. Thus, UcCBM10 and VdEG3 may have similarities in function when it comes to pathogen–host interactions.
Promoter cis-acting element analysis is key to studying gene function [29]. Previous studies showed that CBM proteins could provide energy for plant growth and development and stress response by becoming involved in starch biosynthesis and degradation [30,31,32]. For instance, the rice CBM protein FLO6 can bind to starch by its CBM48 domain and plays a critical role in starch synthesis [33]. Our results showed that the promoter region of the UcCBM gene family possessed more hormone response and stress response elements, indicating that the UcCBMs were involved in fungi growth and development and the hormonal regulation of fungi biological processes. Interestingly, we also found that UcCBM1 and UcCBM5 interacted with glycogen [starch] synthase (UHOR_01531), which involved starch synthesis through PPI analysis. However, the functions of these UcCBM genes and how UcCBM regulates the growth and development of U. crameri need to be further studied.
The CBM protein family plays an essential role in hydrolyzing the wall polysaccharides of a plant [34]. The GO enrichment analysis results of this study showed that the function of UcCBM genes is mainly related to carbohydrate metabolic processes, and in particular the glycogen metabolic process, arabinose metabolic process, L-arabinose metabolic process, and pentose metabolic process. We found that most of the UcCBM proteins were downregulated during the early stages of U. crameri infection; this may have been due to the fact that U. crameri, a biotrophic fungal pathogen, did not break the cell walls of the plant during infection. Furthermore, the expression of effector genes in phytopathogens is typically induced during their infection of a host plant [35,36,37,38]. In our study, UcCBM8, UcCBM4, and UcCBM1 were upregulated during U. crameri infection, similarly to most virulence factors of phytopathogenic fungi, such as SCRE1 and UvGHF1 of Ustilaginoidea virens, which causes rice false smut [39,40]. Thus, we speculate that these proteins may act as an effector and play an important role in U. crameri infection.
These findings establish a foundation for developing a deeper understanding of the potential roles of CBM genes in fungi. Whole-genome analysis enables us to preliminarily characterize the UcCBMs in U. crameri. Our results are mainly based on bioinformatics analysis, and the true role of UcCBMs in U. crameri growth and pathogenicity still needs more experiments to be verified. The molecular mechanism of UcCBM genes involved in interactions between U. crameri and its host also need further exploration. For example, determining the heterologous expression of these UcCBM proteins in plants is a good method for validating their virulence function [41,42]. Furthermore, developing the small interfering RNAs (siRNAs) of these UcCBM genes will also help us in understanding the influence of UcCBMs on plant immunity and may lay a foundation for the potential practical application of UcCBMs in foxtail millet anti-disease methods and breeding [43,44,45].
4. Materials and Methods
4.1. Identification and Physicochemical Property Analysis of UcCBM Family Genes
The U. crameri SCZ-6 genomic data and relevant annotation files were obtained from our previously reported study [16]. We used the CAZy database (http://www.cazy.org/, accessed on 5 September 2024) with an e-value of less than 1 × e−5 to search the CMB proteins in the U. crameri genome. The online ExPASy software (https://web.expasy.org/protparam/, accessed on 5 September 2024) was used to predict the physicochemical properties of the UcCBM proteins [46]. The website https://wolfpsort.hgc.jp/, accessed on 5 September 2024, was used to predict the subcellular localization of the UcCBM proteins.
4.2. Multiple Sequence Alignment and Phylogenetic Analysis
The homologous proteins of UcCBMs in Sporisorium scitamineum, U. maydis, and U. hordei were identified by using BLAST with the NCBI website (https://www.ncbi.nlm.nih.gov/, accessed on 5 September 2024). All 11 UcCBM family proteins and their homologous proteins were aligned using Clustal W and default parameters. The phylogenetic tree was constructed using the MEGA 7.0 software with a Neighbor-Joining (NJ) method.
4.3. Gene Structure, Protein Motif, and 3D Structure Analysis
GSDS (https://gsds.gao-lab.org/index.php, accessed on 5 September 2024) was used to predict the exons or introns of the UcCBM genes [47]. The MEME website (http://meme-suite.org, accessed on 5 September 2024) was used to analyze the conservative motifs of the UcCBM proteins, with a pattern count of 10 and other parameters set to default values [48]. The 3D structures of the UcCBMs were created using the website https://www.swissmodel.expasy.org/, accessed on 5 September 2024, [49,50].
4.4. Promoter Cis-Acting Regulatory Elements Analysis
The promoter sequences 2000 bp upstream of the transcription start site of the UcCBM genes were generated from the U. crameri SCZ-6 genome sequences [16]. Cis-acting regulatory elements of 11 UcCBM genes were identified through the PlantCARE online network server (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 5 September 2024).
4.5. GO Enrichment and Protein–Protein Interaction Analysis
We used the website (https://www.omicshare.com/tools, accessed on 10 September 2024) to perform the GO function enrichment of the UcCBM proteins; and the website https://cn.string-db.org/, accessed on 10 September 2024, was used to identify the partner proteins interacting with the UcCBM family proteins. The smut fungi U. hordei was selected as a reference genome.
4.6. Analysis of Expression Patterns of UcCBM Family Genes
According to the previous study [16], the transcriptome expression levels of 11 UcCBM genes at different time points (0 h, 12 h, 24 h, and 72 h) after U. crameri inoculation were obtained. The expression heat map of 11 UcCBM genes was drawn using the following website (https://www.omicshare.com/tools, accessed on 10 September 2024).
5. Conclusions
In summary, the members of the UcCBM proteins were identified and analyzed at the genome-wide level to explore their biological function in interactions between U. crameri and plants. A total of 11 UcCBM proteins were identified from the U. crameri genome, and their physicochemical properties, evolutionary relationships, and potential biological functions were further predicted and clarified. This study provides a reference for exploring the gene functions of UcCBMs, and lays a foundation for future efforts to detect the molecular mechanisms of interactions between U. crameri and foxtail millet.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms252111790/s1.
Author Contributions
A.W. designed and directed the research. A.W., D.Z. and Y.Z. performed the bioinformatics analysis and wrote the original draft. T.X., N.W. and F.N. helped perform the bioinformatics analysis. A.W., D.Y. and D.X. revised and polished the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the science and technology project of Hubei province, China—breeding technology of genome design (No. HBNYHXGG2023-7).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Zhao Z.T., Liu H.Q., Wang C.F., Xu J.R. Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genom. 2013;14:274. doi: 10.1186/1471-2164-14-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cantarel B.L., Coutinho P.M., Rancurel C., Bernard T., Lombard V., Henrissat B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009;37:D233–D238. doi: 10.1093/nar/gkn663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lombard V., Golaconda Ramulu H., Drula E., Coutinho P.M., Henrissat B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:D490–D495. doi: 10.1093/nar/gkt1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert H., Knox J.P., Boraston A.B. Advances in understanding the molecular basis of plant cell wall polysaccharide recognition by carbohydrate-binding modules. Curr. Opin. Struct. Biol. 2013;23:669–677. doi: 10.1016/j.sbi.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 5.Freelove A.C.J., Bolam D.N., White P., Hazlewood G.P., Gilbert H.J. A Novel Carbohydrate-binding Protein Is a Component of the Plant Cell Wall-degrading Complex of Piromyces equi. J. Biol. Chem. 2001;276:43010–43017. doi: 10.1074/jbc.M107143200. [DOI] [PubMed] [Google Scholar]
- 6.Lamour K.H., Mudge J., Gobena D., Hurtado-Gonzales O.P., Schmutz J., Kuo A., Miller N.A., Rice B.J., Raffaele S., Cano L.M., et al. Genome sequencing and mapping reveal loss of heterozygosity as a mechanism for rapid adaptation in the vegetable pathogen Phytophthora capsici. Mol. Plant-Microbe Interact. 2012;25:1350–1360. doi: 10.1094/MPMI-02-12-0028-R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gui Y.J., Zhang W.Q., Zhang D.D., Zhou L., Short D.P., Wang J., Dai X.F. A Verticillium dahliae extracellular cutinase modulates plant immune responses. Mol. Plant-Microbe Interact. 2018;31:260–273. doi: 10.1094/MPMI-06-17-0136-R. [DOI] [PubMed] [Google Scholar]
- 8.Gui Y.J., Chen J.Y., Zhang D.D., Li N.Y., Li T.G., Zhang W.Q., Dai X.F. Verticillium dahliae manipulates plant immunity by glycoside hydrolase 12 proteins in conjunction with carbohydrate-binding module 1. Environ. Microbiol. 2017;19:1914–1932. doi: 10.1111/1462-2920.13695. [DOI] [PubMed] [Google Scholar]
- 9.Yin Z.Y., Wang N., Duan W.W., Pi L., Shen D.Y., Dou D.L. Phytophthora capsici CBM1-containing protein CBP3 is an apoplastic effector with plant immunity-inducing activity. Mol. Plant Pathol. 2021;22:1358–1369. doi: 10.1111/mpp.13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brotman Y., Briff E., Viterbo A., Chet I. Role of swollenin, an expansin-like protein from Trichoderma, in plant root colonization. Plant Physiol. 2008;147:779–789. doi: 10.1104/pp.108.116293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar B. First record of smut disease of foxtail millet caused by Ustilago crameri Korn. J. Mycol. Plant Pathol. 2011;41:459–461. [Google Scholar]
- 12.Wang C.S. Physiologic specialization and the control of millet smut. Phytopathology. 1944;34:1050–1055. [Google Scholar]
- 13.Lata C., Gupta S., Prasad M. Foxtail millet: A model crop for genetic and genomic studies in bioenergy grasses. Crit. Rev. Biotechnol. 2013;33:328–343. doi: 10.3109/07388551.2012.716809. [DOI] [PubMed] [Google Scholar]
- 14.Muthamilarasan M., Prasad M. Advances in Setaria genomics for genetic improvement of cereals and bioenergy grasses. Theoretical and applied genetics. Theor. Und Angew. Genet. 2015;128:1–14. doi: 10.1007/s00122-014-2399-3. [DOI] [PubMed] [Google Scholar]
- 15.Hao L., Liu J., Zhang A., Han Y., Yi H. Differential gene expression in foxtail millet during interaction with the smut fungus ustilago crameri. Physiol. Mol. Plant Pathol. 2020;110:101459. doi: 10.1016/j.pmpp.2020.101459. [DOI] [Google Scholar]
- 16.Liang J., Yin D.S., Shu X.Y., Xiang T., Zhang C., Li H.L., Wang A.J. Integrated Genome Sequencing and Transcriptome Analysis Identifies Candidate Pathogenicity Genes from Ustilago crameri. J. Fungi. 2024;10:82. doi: 10.3390/jof10010082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y., Hao Z., Sun H., Liu J., Shen S., Zhou C., Li Z. Genome Sequence Resource of Ustilago crameri, a Fungal Pathogen Causing Millet Smut Disease of Foxtail Millet. Plant Dis. 2023;107:546–548. doi: 10.1094/PDIS-06-22-1439-A. [DOI] [PubMed] [Google Scholar]
- 18.Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vincken J.P., Schols H.A., Oomen R.J.F.J., McCann M.C., Ulvskov P., Voragen A.G.J., Visser R.G.F. If homogalacturonan were a side chain of rhamnogalacturonan I. Implications for cell wall architecture. Plant Physiol. 2003;132:1781–1789. doi: 10.1104/pp.103.022350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang B., Wang Z., Jin K. Investigation on occurrence and control measures of millet smut in 2003 and 2004 in southeastern Shanxi Province. Shaanxi Agric. Sci. 2007;1:94–95. [Google Scholar]
- 21.Gaulin E., Dramé N., Lafitte C., Torto-Alalibo T., Martinez Y., Ameline-Torregrosa C., Khatib M., Mazarguil H., Villalba-Mateos F., Kamoun S., et al. Cellulose binding domains of a Phytophthora cell wall protein are novel pathogen-associated molecular patterns. Plant Cell. 2006;18:1766–1777. doi: 10.1105/tpc.105.038687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larroque M., Barriot R., Bottin A., Barre A., Rougé P., Dumas B., Gaulin E. The unique architecture and function of cellulose-interacting proteins in oomycetes revealed by genomic and structural analyses. BMC Genom. 2012;13:605. doi: 10.1186/1471-2164-13-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng A.P., Lin R.M., Zhang D.H., Qin P.G., Xu L.Z., Ai P., Ding L., Wang Y.R., Chen Y., Liu Y., et al. The evolution and pathogenic mechanisms of the rice sheath blight pathogen. Nat. Commun. 2013;4:1424. doi: 10.1038/ncomms2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dean R.A., Talbot N.J., Ebbole D.J., Farman M.L., Mitchell T.K., Orbach M.J., Thon M., Kulkarni R., Xu J.R., Pan H., et al. The genome sequence of the rice blast fungus Magnaporthe grisea. Nature. 2005;434:980. doi: 10.1038/nature03449. [DOI] [PubMed] [Google Scholar]
- 25.Akcapinar G.B., Kappel L., Sezerman O.U., Seidl-Seiboth V. Molecular diversity of LysM carbohydrate-binding motifs in fungi. Curr. Genet. 2015;61:103–113. doi: 10.1007/s00294-014-0471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Jonge R., Thomma B.P.H.J. Fungal LysM effectors: Extinguishers of host immunity? Trends Microbiol. 2009;17:151–157. doi: 10.1016/j.tim.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Bolton M.D., Van Esse H.P., Vossen J.H., De Jonge R., Stergiopoulos I., Stulemeijer I.J.E., Berg G.C.M.V.D., Borrás-Hidalgo O., Dekker H.L., De Koster C.G., et al. The novel Cladosporium fulvum lysin motif effector Ecp6 is a virulence factor with orthologues in other fungal species. Mol. Microbiol. 2008;69:119–136. doi: 10.1111/j.1365-2958.2008.06270.x. [DOI] [PubMed] [Google Scholar]
- 28.Marshall R., Kombrink A., Motteram J., Loza-Reyes E., Lucas J., Hammond-Kosack K.E., Thomma B.P., Rudd J.J. Analysis of two in planta expressed LysM effector homologs from the fungus Mycosphaerella graminicola reveals novel functional properties and varying contributions to virulence on wheat. Plant Physiol. 2011;156:756–769. doi: 10.1104/pp.111.176347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su Y., Zeeshan U.l., Haq M., Liu X., Li Y., Yu J., Yang D., Wu Y., Liu Y. A Genome-Wide Identification and Expression Analysis of the Casparian Strip Membrane Domain Protein-like Gene Family in Pogostemon cablin in Response to p-HBA-Induced Continuous Cropping Obstacles. Plants. 2023;12:3901. doi: 10.3390/plants12223901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz-Gayosso A., Rodríguez-Sotres R., Martínez-Barajas E., Coello P. A role for the carbohydrate-binding module (CBM) in regulatory SnRK1 subunits: The effect of maltose on SnRK1 activity. Plant J. 2018;96:163–175. doi: 10.1111/tpj.14026. [DOI] [PubMed] [Google Scholar]
- 31.Christiansen C., Hachem M.A., Janecek S., Vikso-Nielsen A., Blennow A., Svensson B. The carbohydrate-binding module family 20–diversity, structure, and function. FEBS J. 2009;276:5006–5029. doi: 10.1111/j.1742-4658.2009.07221.x. [DOI] [PubMed] [Google Scholar]
- 32.Janecek S., Svensson B., MacGregor E.A. Structural and evolutionary aspects of two families of non-catalytic domains present in starch and glycogen binding proteins from microbes, plants and animals. Enzym. Microb. Technol. 2011;49:429–440. doi: 10.1016/j.enzmictec.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L., Li N., Zhang J., Zhao L.L., Qiu J.J., Wei C.X. The CBM48 domain-containing protein FLO6 regulates starch synthesis by interacting with SSIVb and GBSS in rice. Plant Mol. Biol. 2022;108:343–361. doi: 10.1007/s11103-021-01178-0. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y., Wang P., Tian J., Seidi F., Guo J., Zhu W., Xiao H., Song J. Carbohydrate-Binding Modules of Potential Resources: Occurrence in Nature, Function, and Application in Fiber Recognition and Treatment. Polymers. 2022;14:1806. doi: 10.3390/polym14091806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Z., Song T., Zhu L., Ye W., Wang Y., Shao Y., Wang Y. A Phytophthora sojae glycoside hydrolase 12 protein is a major virulence factor during soybean infection and is recognized as a PAMP. Plant Cell. 2015;27:2057–2072. doi: 10.1105/tpc.15.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Niu X., Yamamoto N., Yang G., Lin H., Jiang L., Liu Y., Zheng A. A small secreted protein, RsMf8HN, in Rhizoctonia solani triggers plant immune response, which interacts with rice OsHIPP28. Microbiol. Res. 2023;266:127219. doi: 10.1016/j.micres.2022.127219. [DOI] [PubMed] [Google Scholar]
- 37.Tan X., Hu Y., Jia Y., Hou X., Xu Q., Han C., Wang Q. A conserved glycoside hydrolase family 7 cellobiohydrolase PsGH7a of Phytophthora sojae is required for full virulence on soybean. Front. Microbiol. 2020;11:1285. doi: 10.3389/fmicb.2020.01285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiang J., Li X., Yin L., Liu Y., Zhang Y., Qu J., Lu J. A candidate RxLR effector from Plasmopara viticola can elicit immune responses in Nicotiana benthamiana. BMC Plant Biol. 2017;17:75. doi: 10.1186/s12870-017-1016-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang N., Yang J., Fang A., Wang J., Li D., Li Y., Sun W. The essential effector SCRE1 in Ustilaginoidea virens suppresses rice immunity via a small peptide region. Mol. Plant Pathol. 2020;21:445–459. doi: 10.1111/mpp.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou J., Jiang C., Qiu S., Duan G., Wang G., Li D., Sun W. An Ustilaginoidea virens glycoside hydrolase 42 protein is an essential virulence factor and elicits plant immunity as a PAMP. Mol. Plant Pathol. 2023;24:1414–1429. doi: 10.1111/mpp.13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stergiopoulos I., de Wit P.J.G.M. Fungal effector proteins. Annu. Rev. Phytopathol. 2009;47:233–263. doi: 10.1146/annurev.phyto.112408.132637. [DOI] [PubMed] [Google Scholar]
- 42.Wang L., Liu H., Zhang M., Ye Y., Wang L., Zhu J., Wang Y. Microbe-derived non-necrotic glycoside hydrolase family 12 proteins act as immunogenic signatures triggering plant defenses. J. Integr. Plant Biol. 2022;64:1966–1978. doi: 10.1111/jipb.13337. [DOI] [PubMed] [Google Scholar]
- 43.Chen X.Y., Pei Z.X., Liu H., Huang J.B., Chen X.L., Luo C.X., Hsiang T., Zheng L. Host-induced gene silencing of fungal-specific genes of Ustilaginoidea virens confers effective resistance to rice false smut. Plant Biotechnol. J. 2021;20:253–255. doi: 10.1111/pbi.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li D.Y., Li S., Wei S.H., Sun W.X. Strategies to manage rice sheath blight: Lessons from interactions between rice and Rhizoctonia solani. Rice. 2021;14:21. doi: 10.1186/s12284-021-00466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Q., Wang J.F., Zhao J.R., Xu J.H., Sun S.T., Zhang H.F., Wu J.J., Tang C.L., Kang Z.S., Wang X.J. A polysaccharide deacetylase from Puccinia striiformis f. sp. tritici is an important pathogenicity gene that suppresses plant immunity. Plant Biotechnol. J. 2020;18:1830–1842. doi: 10.1111/pbi.13345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfand J.M., LeFevre G.H., Luthy R.G. Metabolization and degradation kinetics of the urban-use pesticide fipronil by white rot fungus Trametes versicolor. Environ. Sci. Process. Impacts. 2016;18:1256–1265. doi: 10.1039/C6EM00344C. [DOI] [PubMed] [Google Scholar]
- 47.Cajthaml T. Biodegradation of endocrine-disrupting compounds by ligninolytic fungi: Mechanisms involved in the degradation. Environ. Microbiol. 2015;17:4822–4834. doi: 10.1111/1462-2920.12460. [DOI] [PubMed] [Google Scholar]
- 48.Gasteiger E., Hoogland C., Gattiker A., Duvaud S., Wilkins M.R., Appel R.D., Bairoch A. The Proteomics Protocols Handbook. Humana Press; Totowa, NJ, USA: 2005. Protein identification and analysis tools on the expasy server; pp. 571–607. [Google Scholar]
- 49.Bairoch A., Apweiler R. The SWISS-PROT protein sequence database and its supplement TrEMBL in 2000. Nucleic Acids Res. 2000;28:45–48. doi: 10.1093/nar/28.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kriventseva E.V., Fleischmann W., Zdobnov E.M., Apweiler R. CluSTr: A database of clusters of SWISS-PROT+TrEMBL proteins. Nucleic Acids Res. 2001;29:33–36. doi: 10.1093/nar/29.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.