Abstract
Identifying mechanisms driving the substantial dissolution of biogenic CaCO3 (60 to 80%) in surface and mesopelagic waters of the global ocean is critical for constraining the surface ocean’s alkalinity and inorganic carbon budgets. We examine microzooplankton grazing on coccolithophores, photosynthetic calcifying algae responsible for a majority of open-ocean CaCO3 production, as a mechanism driving shallow dissolution. We show that microzooplankton grazing dissolves 92 ± 7% of ingested coccolith calcite, which may explain 50 to 100% of the observed CaCO3 dissolution in supersaturated surface waters. Microzooplankton grazing on coccolithophores is thus a substantial, previously unrecognized biological mechanism affecting the ballasting of organic carbon to deeper waters, the ecology and fitness of microzooplankton themselves due to buffering of food vacuole pH, and ultimately the continued ability of the surface ocean to take up atmospheric carbon dioxide.
Microzooplankton grazing on coccolithophore calcite provides an important mechanism for shallow dissolution of calcium carbonate.
INTRODUCTION
The marine calcium carbonate (CaCO3) cycle is a crucial component of the global carbon cycle and ocean carbonate chemistry. It involves the production, export, and dissolution of CaCO3, all of which can affect the ocean’s ability to uptake atmospheric CO2 (1). However, uncertainties persist in global production and dissolution rates, preventing an accurate quantification of the magnitude of its contribution to the global carbon cycle (2, 3). One especially paradoxical aspect is the widespread evidence for shallow dissolution of CaCO3 in the upper 1000 m of the ocean water column, which is supersaturated with respect to calcium carbonate and should thermodynamically favor precipitation over dissolution (1, 2, 4, 5). Despite the growing consensus around the necessity of shallow dissolution to explain ocean alkalinity distributions, the processes driving shallow dissolution remain unclear (6). A role for microenvironments, such as those that form in sinking particles or the acidic guts of zooplankton, has been speculated upon for decades. Since the idea was first put forth by Milliman (1), biologically mediated dissolution of biogenic calcite has emerged as a mechanism with promising potential to account for much of the observed CaCO3 dissolution in shallow oceanic regions (1, 7–9). However, the importance of this mechanism globally remains unconstrained.
The biogenic calcite produced by coccolithophores represents a prime candidate as a source for biologically mediated dissolution, as these organisms can be responsible for 50 to 90% of pelagic CaCO3 production (10) and also serve as foundational primary producers in many marine ecosystems (11). In addition, individual coccolithophores sink very slowly out of the surface ocean unless they are aggregated through biological processes, such as grazing and subsequent fecal pellet packaging (12, 13) or transparent exopolymer particle production mediated by stress and/or viral lysis (14, 15). Previous studies have demonstrated that mesozooplankton (size range, 200 to 2000 μm) grazing on coccolithophores can result in up to 73% of ingested calcite dissolving during gut passage (7–9). Microzooplankton (MZP) may also play a role. These single-cell, heterotrophic protists (size range, 20 to 200 μm) are the primary consumers of photosynthetic nanophytoplankton, such as coccolithophores, in nearly all ocean provinces (16, 17). MZP consume five times more primary production than mesozooplankton and are responsible for ingesting up to 60% of coccolithophore calcite production (16, 18). However, because of the small size of waste “minipellets” produced from MZP grazing, their direct contributions to the vertical flux of carbon are thought to be limited (19, 20). Considering MZP’s role as the dominant consumer of marine phytoplankton, we investigated the impact of MZP grazing on the cosmopolitan coccolithophore Emiliania huxleyi (E. hux) on CaCO3 recycling in the surface ocean (3).
Bulk measurements to quantify the ingestion and digestion of coccolith calcite by MZP are complicated by the submicromolar amount of carbon transfer associated with these microscale predators and prey. We cultured E. hux (CCMP374) in growth media containing 13C-labeled dissolved inorganic carbon (DIC) and conducted grazing experiments with two model MZP, Oxyrrhis marina (O. mar) and Gyrodinium dominans (G. dom). We subsequently quantified inorganic carbon transfer at the nanomolar level via measurements of 13C tracer in the particulate and dissolved phases of the experimental system. Grazing experiments were conducted at a set predator-to-prey ratio (data S1), along with seawater-, predator-, uncalcified (naked) prey–, and calcified prey–only controls (see the Supplementary Materials for additional method details and schematic). We confirmed our geochemical carbon tracking results via microscopy using a semiquantitative pH-sensitive fluorescent probe. We then contextualized our lab-derived estimates of dissolution using global calcium carbonate production, export, and dissolution rates to reconcile disparities between observed trends of production and export.
RESULTS
Substantial calcite dissolution during MZP grazing
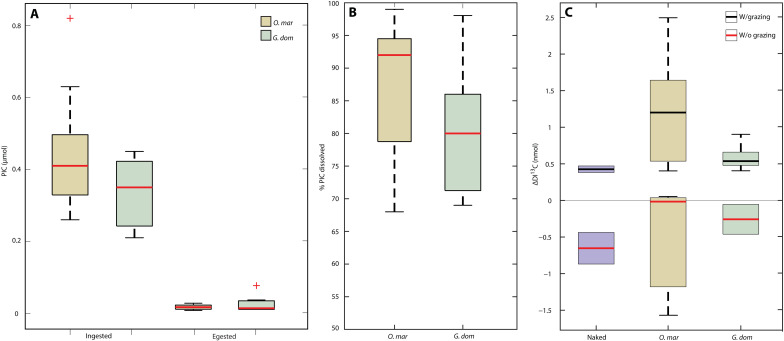
Across all experiments, we observed rapid ingestion of coccolithophores by both MZP, coupled with the dissolution of 92 ± 7% of ingested calcite over a 4-hour digestion period (Fig. 1B and data S1). Over the grazing period, both O. mar and G. dom populations demonstrated similar ranges in particulate inorganic carbon (PIC) ingestion (0.44 ± 0.2; 0.34 ± 0.1 μmol of PIC, respectively; Fig. 1A). O. mar, a coastal dinoflagellate, displayed an average 94% (±2%) dissolution of ingested coccolith calcite, while G. dom, which is representative of open-ocean dinoflagellates, showed an average 87% (±11%) dissolution (Fig. 1B). Bulk seawater undersaturation–driven dissolution rates of coccolithophore calcite in seawater are on the order of fractions of a percent per day and can only support supersaturated dissolution through the production of metabolic acids in confined microenvironments (5). Comparatively, the MZP-mediated dissolution rates reported here (equivalent to 480%/day) are some of the fastest calcite dissolution rates ever measured in seawater and approach the diffusion limit (21, 22).
Fig. 1. MZP digestion promotes dissolution of coccolithophore PIC.
(A) PIC (micromoles) ingested and egested (PIC that remained after the digestion period) for each MZP. (B) % PIC dissolved based on the difference between PIC ingested and egested. (C) Summary plot of the nanomolar change in DI13C for all grazing experiment treatments, including uncalcified naked controls (purple boxes), prey only (“w/o grazing”; red median line), and grazing treatments (“w/ grazing”; black median line). Zero line is drawn on (C) to indicate no change in DI13C, where any points above the zero line signify molar enrichment of 13C and points below the zero line indicate molar depletion of 13C. All calcified and naked prey treatments where grazing occurred fall above the zero line, indicating release of 13C from the digestion of labeled biomaterials. All control treatments without grazing fall at or below the zero line.
To account for the mass transfer of PI13C into the dissolved phase, we analyzed DI13C at the beginning and end of the grazing experiments (Fig. 1C). Because of complications associated with filtration and gas exchange, the DI13C provides a qualitative metric for the release of total labeled carbon from the particulate to the dissolved phase. The addition of the “naked” E. hux grazing treatment allowed us to constrain the DI13C associated with MZP respiration of organic matter alone, as the naked phenotype of the CCMP374 strain does not calcify. Calcified E. hux is characterized by a molar ratio of PIC and particulate organic carbon (POC; PIC:POC, also referred to as the “rain ratio”) of ~1 (23). The average molar enrichment observed in calcified grazing treatments was more than double that of the enrichment observed in naked grazing treatments (1.97 nmol in calcified versus 0.43 nmol in naked), lending additional confidence that our 13C-labeling approach allowed us to disentangle the isotopic enrichment from digestion of organic and inorganic materials.
We used the pH-dependent LysoSensor probe to visualize pH dynamics semiquantitatively during the progression of digestion for both calcified and noncalcified prey. Microscopy demonstrates active LysoSensor fluorescence within the MZP, colocalized with chlorophyll fluorescence from the prey (Fig. 2, A to D). The LysoSensor pKa [DND-167, pKa = 5 (where Ka is the acid dissociation constant)] indicates that these digestive vacuoles are highly acidic (pH < 5), well below both ambient seawater pH and the critical pH for promoting rapid calcite dissolution (24).
Fig. 2. LysoSensor tracks MZP vacuole pH evolution during digestion of calcified and noncalcified prey.
(A to D) Image series for O. mar food vacuoles 3 hours after ingesting calcified E. hux. (A) Bright-field image showing engulfed prey and free prey. Scale bar, 5 μm. (B) False-color blue light image showing chlorophyll fluorescence of ingested and free prey. (C) False-color ultraviolet (UV) light image showing LysoSensor probe fluorescence within MZP vacuoles containing prey. (D) False-color composite of images (A to C) overlaid to aid in the visualization of colocalized prey and LysoSensor fluorescence. (E) Scatterplot of corrected total cell fluorescence (CTCF); purple circles show CTCF for freshly engulfed prey (t = 0 hours), green triangles show ingestion of calcified prey (CCMP374-C) after 3 hours, and tan squares show ingestion of noncalcified prey (CCMP1323) after 3 hours.
Using the microscopy images, we calculate a corrected total cell fluorescence (CTCF; Eq. 5 and data S1C) and compare fluorescence intensity, a proxy for vacuole pH, between uncalcified and calcified prey. We find a much lower CTCF (higher pH) in the calcified prey vacuoles and a higher CTCF (lower pH) in vacuoles filled with noncalcified prey [t(10) = −4.6, P = 0.0005]. The difference in relative pH between these two prey types demonstrates a potential buffering effect that increases pH during calcified prey digestion, through the production of alkalinity from calcite dissolution (Fig. 2E). This buffering effect has been demonstrated within mesozooplankton grazing experiments using Acartia tonsa copepods and the coccolithophore Pleurochrysis carterae (9) and has also been hypothesized to occur during MZP digestion of coccoliths (25).
Our CTCF data support the hypothesis that coccolith dissolution leads to the buffering of MZP food vacuoles, but the consequences of a less acidic vacuole remain to be fully elucidated, especially its relationship to the fitness and digestive efficiency of natural MZP communities. A food vacuole with lower acidity might lead to decreased efficiency in digesting and assimilating prey, potentially providing a mechanism for the diminished fitness and growth observed in MZP grazers when consuming calcified prey versus noncalcified prey (25).
DISCUSSION
The global importance of MZP grazing on upper ocean carbon cycling
Most global models and data syntheses suggest high rates of euphotic CaCO3 production, accompanied by rapid shallow dissolution within the euphotic and mesopelagic zones (Table 1 and references therein). The very fast dissolution rates measured here suggest a plausible mechanism for this rapid dissolution that can be integrated into current literature estimates of coccolithophore production rates and MZP grazing rates
| (1) |
where P_[PIC] is the globally averaged PIC production rate (Gt PIC year−1), %coccos is the average proportion of global PIC that is produced by coccolithophores, %MZP is the average proportion of calcite production grazed by MZP, and %Diss is the mean proportion of coccolith PIC that dissolves during MZP digestion (Fig. 1B). Calculations were performed for the range of literature values reported in Table 1 using the reported mean production rate, a mean 90% proportion of PIC produced by coccolithophores (10, 26), a mean 60% of calcite production grazed by MZP (18), and a mean of 92% PIC dissolution due to MZP grazing, as determined in this study (Fig. 1B). For the remainder of this discussion, any reference to “shallow” or “surface” dissolution is constrained to the euphotic zone (~200 m), given that the majority of reported production and export values reference the base of the photic zone (Table 1).
Table 1. Summary of global ocean CaCO3 production, export, and extrapolated MZP dissolution rates.
| Data source | Production rate (Gt PIC year−1) | Export rate (Gt PIC year−1) | Export depth (m) | In situ dissolution rate (Gt PIC year−1) | Lab-determined MZP dissolution rate (Gt PIC year−1) |
|---|---|---|---|---|---|
| Milliman (1) | 0.6 | 0.2 | 100 | 0.4 | 0.3 |
| Feely et al. (40) | 0.7 | 0.4 | 200–1100 | 0.3 | 0.4 |
| Berelson et al. (2) | 0.5–1.6 | 0.4–1.8 | ~200 | 1.0 | 0.3–0.8 |
| Sulpis et al. (28) | 1.6 | 0.5–0.9 | 300 | 0.6–1.2 | 0.8 |
| Liang et al. (29) | 1.8–2.0 | 1.0 | 279 | 1.0 | 0.9–1.0 |
| Ziveri et al. (10) | 3.7 | 0.7 | 100–200 | 2.9 | 1.8 |
There now exists a wide range in reported values for global calcium carbonate production, with estimates ranging from 0.5 to 3.7 Gt C year−e (Table 1). The continuous upward trend in global calcium carbonate production estimates can be attributed to several factors, including advances in methodologies and technologies that allow for more accurate measurements (2, 5, 27) and models (6, 28, 29) of calcium carbonate cycling, as well as an evolving understanding of the diversity and distribution of calcium carbonate producing organisms (10, 30). The growing global production estimates are also reflected in the associated export (0.2 to 1.8 Gt C year−1) and dissolution rates (0.3 to 2.9 Gt C year−1), which serve to reinforce the significance of the shallow calcium carbonate dissolution flux. Despite these variations, our extrapolated laboratory dissolution rates (0.3 to 1.8 Gt C year−1) generally align well with the reported values of in situ dissolution rates, accounting for 50 to 100% of observed shallow dissolution rates. Together, these estimates reinforce our findings that MZP-mediated dissolution is a central driver of the rapid cycling of CaCO3 in the euphotic zone.
The important role of MZP in the dissolution of upper ocean CaCO3 is further reinforced when compared with other recognized processes involved in the packaging and processing of biogenic calcite (Fig. 3). Foraminifera and pteropods together likely experience minimal shallow dissolution, as they are expected to quickly sink out of the euphotic and mesopelagic due to their large size (10, 13). Fish-produced carbonates are still highly unconstrained in global CaCO3 production estimates, leaving their contribution to the CaCO3 export and shallow dissolution uncertain (31, 32).
Fig. 3. Fate of biogenic calcium carbonate within the euphotic and mesopelagic ocean.
Values in boxes represent the upper and lower bounds for global CaCO3 fluxes. Arrows represent the flux of CaCO3 in percentage, with letters indicating each distinct process. The top dashed line corresponds to the euphotic zone (~200 m), the middle dashed line represents the aragonite saturation horizon, and the bottom dashed line represents the calcite saturation horizon (where all forms of calcium carbonate are undersaturated). Shallow CaCO3 production values are taken from (2, 10). (A) Portion of global CaCO3 production attributed to large calcifying organisms (10) which sink directly out of the euphotic zone with minimal shallow dissolution. (B) Portion of global CaCO3 production attributed to fish—now unconstrained (32). (C) Portion of global CaCO3 production attributed to coccolithophores (10). (D) Portion of coccolithophore production that is not directly grazed and is available for viral lysis and/or aggregation into sinking particles (16, 17). (E) Portion of coccolithophore production grazed by mesozooplankton [12%; (16, 17)] and subsequently dissolved [38%; (9)]. (F) Portion of coccolithophore production grazed by MZP [60%; (16, 17)] and subsequently dissolved (92%; this study). (G) Estimated extent of dissolution occurring in “mid-saturated” waters [where waters are supersaturated with respect to calcite but undersaturated with respect to aragonite; 29%; (6)].
Coccolithophores appear to be the dominant producer of open-ocean calcium carbonate (10, 30). When considering the different processes that affect the attenuation of coccolithophore calcite, only 5% of the calcite ingested by MZP is exported from the euphotic zone (Eq. 1, Fig. 1, and Table 1). Similarly, mesozooplankton processing results in the export of 7% of coccolithophore calcite, but their ultimate contribution to shallow dissolution is likely smaller than MZP due to their different trophic role within food webs (9, 16, 17). All the remaining coccolithophore production (28%) we assume has the potential to aggregate into marine snow particles, which are large enough to sink rapidly out of the euphotic zone. It is worth noting that this remaining 28% includes the viral loss rate, which can also contribute to aggregation and particle formation (33) but may also cause coccolith shedding that may or may not become entrained in particles, thus hindering a full accounting of the fate of CaCO3 associated with these processes.
Alongside MZP grazing, particle-driven dissolution of CaCO3 is emerging as another major biological mechanism that controls attenuation of shallow CaCO3. Recent global estimates of dissolution occurring in waters, supersaturated with respect to calcite but undersaturated with respect to aragonite, imply a dissolution of 30% of the euphotic zone flux within mesopelagic waters (6). Respiration within sinking particle aggregates may drive the formation of acidic microenvironments that could facilitate further dissolution as they sink from the euphotic zone (5). It is also possible for protists, fungi, fish larvae, and micro- and mesozooplankton to feed on and degrade particle aggregates, leading to a now unconstrained dissolution flux within particles that sink below the euphotic zone and deeper in the mesopelagic (34, 35). While particle-driven dissolution represents an unconstrained aspect of the CaCO3 cycle, our study highlights the demonstrative role of MZP ingestion and subsequent dissolution as a fundamental biogeochemical mechanism working to rapidly recycle a substantial portion of CaCO3 within the supersaturated surface ocean.
In the broader context of ocean ecosystems and carbon transport, MZP grazing–mediated dissolution of coccolithophores may reduce the calcite pool available for packaging into heavy, sinking particles. The ballasting of sinking particles by calcium carbonate is proposed to be an important control on the efficiency of the biological carbon pump (BCP), which is how photosynthetically fixed carbon moves from the surface to deep ocean (11, 36). Our results suggest that MZP grazing may reduce the efficiency of the BCP indirectly by reducing the amount of coccolith calcite available for ballasting sinking particles. The modulation of MZP grazing on the ballast effect of coccolith calcite within the context of the BCP remains to be tested and should be explored via direct in situ observations that explicitly measure coccolithophore production, MZP grazing, and particulate export fluxes. Field studies of coccolithophore bloom dynamics with a focus on calcite production, grazing and viral infection mortality rates, associated particle aggregation, and export at various depths would provide a more comprehensive understanding of the dynamic mechanisms that control the calcium carbonate counter pump and, by extension, the ocean’s ability to absorb CO2.
This study identifies MZP grazing as a previously ignored biological mechanism that contributes to the shallow regeneration of alkalinity and, by extension, maintains the buffering capacity of the surface ocean (6). The application of 13C labeling in cultures can accurately and reliably quantify micro- and nanoscale processes involving the transformation and movement of carbon. In addition, we have demonstrated a potential time-dependent buffering effect within the food vacuoles of MZP due to the dissolution of ingested calcite materials, which likely affects grazer fitness (25) and dynamics of CaCO3 export within natural communities. The extrapolation of our lab results to global production and dissolution rates lends additional confidence in our identification of MZP grazing as an essential mechanism that recycles up to 50% of biogenic calcite production within the surface and mesopelagic ocean. This study highlights the importance of considering biological activity and ecological interactions in the recycling of shallow alkalinity, especially with regard to improving models and predictions of the ocean’s ability to absorb atmospheric CO2.
MATERIALS AND METHODS
General culturing procedures
13C-labeled seawater was prepared using sterile filtered seawater (FSW; 0.2-μm pore size). DIC and total alkalinity (TA) were completely removed via HCl addition and aeration and were then restored to typical surface values (2000 μmol kg−1 and 2200 μequiv kg−1, respectively) using 13C-labeled sodium bicarbonate (NaH13CO3) and sodium hydroxide (NaOH). f/2-Si culture medium was prepared according to (37) using the 13C-labeled seawater and used to culture E. hux (strain CCMP374 from K. Bidle at Rutgers University). Cultures were maintained in exponential growth at 16°C with a light intensity of ~75 μmol photon m−2 s−1 on a 12/12-hour light/dark cycle. Cultures were allowed to grow under these conditions until their PIC reached a f13C of ~50% before any experiments were performed, with the f13C being the ratio of 13C to total inorganic C (13C + 12C). A minimum value of 50% for f13C was chosen because it allows for substantial enrichment in the PI13C pool, thus providing a useful tracer for the fate of coccolith calcite during grazing.
O. mar and G. dom were obtained from the Phyto Lab in the College of Life Sciences and Agriculture at the University of New Hampshire and maintained in the Subhas Lab at the Woods Hole Oceanographic Institution. Cultures were transferred and fed weekly with Isochrysis galbana (CCMP1323) to a final concentration of 10,000 cells ml−1. MZP were maintained in an incubator with the same conditions as coccolithophore cultures. Before experiments, MZP were starved in sterile FSW for 5 days to ensure that their vacuoles were empty.
Organism abundance
During experiments, E. hux cell density was assessed via triplicate 300-μl aliquots, run on a flow cytometer (Guava, Millipore), with optimized settings determined based on chlorophyll a fluorescence, side scatter (SSC), and forward scatter. To determine MZP abundance, 2-ml aliquots were preserved using 1% Lugol’s solution and counted using a Sedgewick-Rafter chamber and a light microscope.
MZP ingestion tests
Before conducting grazing dissolution experiments, ingestion tests were conducted with both MZP to determine the optimal grazing density on non–13C-labeled E. hux. Tests were performed using a range of prey-to-predator ratios (50 to 1000) to identify which ratio allowed for the greatest prey ingestion within a 4-hour ingestion period. Prey ingestion was quantified by calculating the change in cell density every hour for 6 hours total. It was determined that a prey-to-predator ratio of ~500 for O. mar and ~200 for G. dom allowed for maximum ingestion of E. hux within a 4-hour period, and all subsequent grazing experiments were performed at that ratio.
Coccolith dissolution experimental protocol
Experimental bottles were prepared at a set prey:predator ratio, along with seawater-, predator-, naked prey–, and calcified prey–only controls. All bottles were sampled for initial measurements (t = 0) of organism abundance, PIC, and DIC and placed in an incubator for the 4-hour ingestion period (fig. S1). Total prey ingestion was quantified via flow cytometry by calculating the change in prey abundance over the ingestion period (t = 4). MZP were then separated and recovered using a series of FSW rinses through a 5-μm polycarbonate track-etch membrane filter that allowed for uneaten E. hux cells to pass through. Recovered MZP were placed in a clean bottle with new FSW and were allowed to digest for another 4-hour period. Following MZP recovery, organism abundances were sampled to verify that the majority of uneaten E. hux was removed and that >50% of the initial MZP were recovered. At the end of the 4-hour digestion period (t = 8), final samples were collected for organism abundance, PIC, and DIC. Controls were carried through the entire experimental setup.
Corrections for MZP regurgitation of ingested coccolithophores
During the digestion periods, it was observed that some coccolithophore cells were “regurgitated” by the MZP. Regurgitation of cells was first observed on the microscope when confirming that live MZP were recovered following the filtration of uneaten coccolithophores. Within a matter of seconds, an individual MZP was observed to spin rapidly and eject a coccolithophore cell that it had ingested. Haunost et al. (38) also observed this phenomenon and showed that flow cytometry could be used to quantify the number of partially digested cells that had been regurgitated (they defined this phenomenon as egestion, but here, we use the term “regurgitation”). We inspected the flow cytometry plots and correlated the regurgitated cells to a small population that had a similar SSC value but reduced fluorescence compared to the uneaten E. hux (fig. S2). We then corrected our “cells ingested” and subsequent “PIC ingested” calculations by gating and subtracting the regurgitated cells.
PIC ingestion and dissolution calculations
We related the measured cellular PIC quota (picomol PIC per cell) to the number of cells ingested to quantify the amount of PIC ingested during MZP grazing. PIC ingestion was then calculated using the following equation
| (2) |
where the cell density at the end of the ingestion period is subtracted from the cell density at the start of the grazing experiment and multiplied by the total experimental volume (mlexp) and the cellular PIC quota.
The following equations were used to convert the raw signals from the Picarro into moles dissolved of 13C during MZP grazing. The f13C, which is the ratio of 13C/totC, was determined using the following equation
| (3) |
where DI13C is the amount of 13C in the dissolved phase, and DI12C + DI13C is the total DIC. Assuming negligible additions of 12C relative to 13C due to the enrichment of 13C within the coccoliths (f13C > 50%), the change in the isotopic abundance from the start and end of grazing was converted to a change in moles of 13C added to the seawater using the following equation [modified from (39)]
| (4) |
where mi is the mass of seawater at the start of the grazing experiments, and [DIC]f/2,i is the initial DIC of the f/2-Si media control in micromoles per kilogram. is the fractional abundance measured at the final time point for the treatment, and is the fractional abundance measured at the final time point for the grazing-only control. Therefore, ΔDI13Cf−i represents the change in moles of 13C between the start and end of the grazing experiments. Because of unpredictable changes in [DIC] from filtration steps in the experimental method, we chose to use [DIC]f/2,i because it represents the true initial [DIC] without any impacts from biology or other experimental artifacts.
While the PIC data constrain the high end of coccolith dissolution due to confounding effects of regurgitation, or “spitting out” partially digested materials (fig. S2), the DI13C data represent the lower limit of coccolith dissolution due to the potential loss of 13CO2 due to outgassing during filtration steps. One-way, single-factor analysis of variance (ANOVA) confirms that the DI13C enrichment in the calcified grazing bottles was significantly different from the enrichment associated with the nongrazing controls (P < 0.05; Fig. 1C and data S1). The naked E. hux grazing treatment (374-N) allowed us to constrain the DI13C associated with MZP respiration of labeled organic matter and was not statistically different from the enrichment associated with calcified grazing treatments via one-way ANOVA (P = 0.409).
Analytical techniques
DIC samples were collected via gravity filtration by passing ~20 ml of seawater through a combusted 0.45-μm glass fiber filter, and PIC samples were taken from the same filters used for DIC collection. PIC samples were dried in an oven at 65°C for a minimum of 4 hours and stored in combusted tin foil inside the fridge until analyzed. All samples were analyzed using a Picarro Cavity Ring-Down Spectroscopy system, allowing simultaneous δ13 C and total PIC or DIC determination.
To account for the mass transfer of PIC into the dissolved phase, we also analyzed DI13C at the beginning and end of the grazing experiments (Fig. 1C and fig. S3). Where the PIC data constrain the high end of coccolith dissolution, the DI13C data represent the lower limit of coccolith dissolution due to the potential loss of 13CO2 due to outgassing during filtration steps. Other controls were incorporated into the experimental design to correct for various processes that might affect the PI13C and DI13C data. The media-only control served as a constraint on any isotope effects due to filtration and methodological artifacts. The grazer-only control allowed us to account for any effects arising from ambient grazer respiration, which affects [DIC] and appeared to enrich the experiment media in DI12C (e.g., negative values in Fig. 1C). The prey-only control served as a constraint on any background dissolution that occurred on the calcified strains (i.e., from coccolith shedding/disintegration, microbially mediated dissolution, etc).
Vacuole pH determination using LysoSensor probe
Using LysoSensor Blue (DND-167), an acidotropic fluorescent probe, and an epifluorescence microscope equipped with a camera, we evaluated the acidity of food vacuoles during a 3-hour digestion period for both I. galbana (noncalcified prey) and E. hux (calcified prey). To understand the dynamics of pH evolution as the food vacuole forms and prey is digested, MZP were “pulse-fed” with prey, wherein they were initially starved, fed with prey (104 cells/ml), and imaged every ~30 min for 3 hours. Two microliters of the LysoSensor probe was added to 1 ml of the MZP cultures (final probe concentration, 2 μM) and then incubated in darkness for 10 min at room temperature. After incubation, the cultures were anesthetized using 3 μl of NiSO4 per 1 ml of culture to slow MZP movement to obtain clear images. Images were then collected using (i) transmitted light for normal color images, (ii) blue light for chlorophyll fluorescence of prey, and (iii) ultraviolet light for LysoSensor probe fluorescence (Fig. 2B). We then calculated the CTCF associated with the lysosome as follows
| (5) |
See data S1C for raw data and CTCF calculations. See data S2 for all photos collected and used for the CTCF analysis.
Acknowledgments
We thank K. Thamatrokoln, K. Bidle, and L. Haramaty (Rutgers) for providing the calcified and naked phenotypes of E. hux (CCMP374), as well as culturing expertise. We thank M. Hayden (WHOI) for helping with preliminary method development and experimental design. We acknowledge and thank the editors and reviewers for helpful comments and feedback.
Funding: This work was supported by the National Sciences Foundation (GCR 2020878 to A.V.S. and E.L.H.).
Author contributions: Conceptualization: A.V.S., E.L.H., and C.L.D. Methodology: C.L.D., E.L.H., A.V.S., and M.D.J. Resources: A.V.S., M.D.J., and C.L.D. Investigation: C.L.D. and M.D.J. Data curation: C.L.D. and A.V.S. Visualization: C.L.D. Formal analysis: C.L.D. and A.V.S. Validation: C.L.D. and A.V.S. Funding acquisition: A.V.S. and E.L.H. Project administration: A.V.S. and C.L.D. Supervision: A.V.S., E.L.H., M.D.J., and C.L.D. Writing—original draft: C.L.D. Writing—review and editing: C.L.D., A.V.S., E.L.H., and M.D.J.
Diversity, equity, ethics, and inclusion: We acknowledge that Woods Hole Oceanographic Institution (WHOI) is located on the unceded ancestral and contemporary land of the Wôpanâak people. We recognize the perpetuated detrimental effects that systemic government oppressions have had on Indigenous communities as a result of colonization. We are committed to taking action to interrupt the legacies of colonialism and build authentic and mutual relationships with Indigenous communities toward justice.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: The dataset and statistics generated for the current study are available as the Supplementary Materials alongside this manuscript. Experimental data and statistics are included in the Excel workbook “data S1,” and all LysoSensor microscopy photos are included in the zip file “data S2.” Datasets are also available at www.bco-dmo.org/project/865363.
Supplementary Materials
The PDF file includes:
Figs. S1 to S3
Legends for data S1 and S2
Other Supplementary Material for this manuscript includes the following:
Data S1 and S2
REFERENCES AND NOTES
- 1.Milliman J. D., Production and accumulation of calcium carbonate in the ocean: Budget of a nonsteady state. Global Biogeochem. Cy. 7, 927–957 (1993). [Google Scholar]
- 2.Berelson W. M., Balch W. M., Najjar R., Feely R. A., Sabine C., Lee K., Relating estimates of CaCO3 production, export, and dissolution in the water column to measurements of CaCO3 rain into sediment traps and dissolution on the sea floor: A revised global carbonate budget. Global Biogeochem. Cy. 21, doi.org/10.1029/2006GB002803 (2007). [Google Scholar]
- 3.Iglesias-Rodríguez M. D., Brown C. W., Doney S. C., Kleypas J., Kolber D., Kolber Z., Hayes P. K., Falkowski P. G., Representing key phytoplankton functional groups in ocean carbon cycle models: Coccolithophorids. Global Biogeochem. Cy. 16, 47-1–47-20 (2002). [Google Scholar]
- 4.Gangstø R., Gehlen M., Schneider B., Bopp L., Aumont O., Joos F., Modeling the marine aragonite cycle: Changes under rising carbon dioxide and its role in shallow water CaCO3 dissolution. Biogeosciences 5, 1057–1072 (2008). [Google Scholar]
- 5.Subhas A. V., Dong S., Naviaux J. D., Rollins N. E., Ziveri P., Gray W., Rae J. W. B., Liu X., Byrne R. H., Chen S., Moore C., Martell-Bonet L., Steiner Z., Antler G., Hu H., Lunstrum A., Hou Y., Kemnitz N., Stutsman J., Pallacks S., Dugenne M., Quay P., Berelson W. M., Adkins J. F., Shallow calcium carbonate cycling in the North Pacific Ocean. Global Biogeochem. Cy. 36, e2022GB007388 (2022). [Google Scholar]
- 6.Kwon E. Y., Dunne J. P., Lee K., Biological export production controls upper ocean calcium carbonate dissolution and CO2 buffer capacity. Sci. Adv. 10, eadl0779 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harris R. R., Zooplankton grazing on the coccolithophore Emiliania huxleyi and its role in inorganic carbon flux. Mar. Biol. 119, 431–439 (1994). [Google Scholar]
- 8.Langer G., Nehrke G., Jansen S., Dissolution of Calcidiscus leptoporus coccoliths in copepod guts? A morphological study. Mar. Ecol. Prog. Ser. 331, 139–146 (2007). [Google Scholar]
- 9.White M. M., Waller J. D., Lubelczyk L. C., Drapeau D. T., Bowler B. C., Balch W. M., Fields D. M., Coccolith dissolution within copepod guts affects fecal pellet density and sinking rate. Sci. Rep. 8, 9758 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziveri P., Gray W. R., Anglada-Ortiz G., Manno C., Grelaud M., Incarbona A., Rae J. W. B., Subhas A. V., Pallacks S., White A., Adkins J. F., Berelson W., Pelagic calcium carbonate production and shallow dissolution in the North Pacific Ocean. Nat. Commun. 14, 805 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.B. Rost, U. Riebesell, “Coccolithophores and the biological pump: Responses to environmental changes” in Coccolithophores: From Molecular Processes to Global Impact, H. R. Thierstein, J. R. Young, Eds. (Springer, 2004), pp. 99–125.
- 12.Bach L. T., Riebesell U., Sett S., Febiri S., Rzepka P., Schulz K. G., An approach for particle sinking velocity measurements in the 3–400 μm size range and considerations on the effect of temperature on sinking rates. Mar. Biol. 159, 1853–1864 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Subhas A. V., Pavia F. J., Dong S., Lam P. J., Global trends in the distribution of biogenic minerals in the ocean. J. Geophys. Res. Oceans 128, e2022JC019470 (2023). [Google Scholar]
- 14.Nissimov J. I., Pagarete A., Ma F., Cody S., Dunigan D. D., Kimmance S. A., Allen M. J., Coccolithoviruses: A review of cross-kingdom genomic thievery and metabolic thuggery. Viruses 9, 52 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sheyn U., Rosenwasser S., Lehahn Y., Barak-Gavish N., Rotkopf R., Bidle K. D., Koren I., Schatz D., Vardi A., Expression profiling of host and virus during a coccolithophore bloom provides insights into the role of viral infection in promoting carbon export. ISME J. 12, 704–713 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmoker C., Hernández-León S., Calbet A., Microzooplankton grazing in the oceans: Impacts, data variability, knowledge gaps and future directions. J. Plankton Res. 35, 691–706 (2013). [Google Scholar]
- 17.Steinberg D. K., Landry M. R., Zooplankton and the ocean carbon cycle. Annu. Rev. Mar. Sci. 9, 413–444 (2017). [DOI] [PubMed] [Google Scholar]
- 18.Mayers K. M. J., Poulton A. J., Daniels C. J., Wells S. R., Woodward E. M. S., Tarran G. A., Widdicombe C. E., Mayor D. J., Atkinson A., Giering S. L. C., Growth and mortality of coccolithophores during spring in a temperate Shelf Sea (Celtic Sea, April 2015). Prog. Oceanogr. 177, 101928 (2019). [Google Scholar]
- 19.Gowing M. M., Silver M. W., Minipellets: A new and abundant size class of marine fecal pellets. J. Mar. Res. 43, 395–418 (1985). [Google Scholar]
- 20.Beaumont K., Nash G., Davidson A., Ultrastructure, morphology and flux of microzooplankton faecal pellets in an east Antarctic fjord. Mar. Ecol. Prog. Ser. 245, 133–148 (2002). [Google Scholar]
- 21.Subhas A. V., Adkins J. F., Rollins N. E., Naviaux J., Erez J., Berelson W. M., Catalysis and chemical mechanisms of calcite dissolution in seawater. Proc. Natl. Acad. Sci. U.S.A. 114, 8175–8180 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjöberg E. L., Rickard D. T., Calcite dissolution kinetics: Surface speciation and the origin of the variable pH dependence. Chem. Geol. 42, 119–136 (1984). [Google Scholar]
- 23.Krumhardt K. M., Lovenduski N. S., Iglesias-Rodriguez M. D., Kleypas J. A., Coccolithophore growth and calcification in a changing ocean. Prog. Oceanogr. 159, 276–295 (2017). [Google Scholar]
- 24.Naviaux J. D., Subhas A. V., Rollins N. E., Dong S., Berelson W. M., Adkins J. F., Temperature dependence of calcite dissolution kinetics in seawater. Geochim. Cosmochim. Acta 246, 363–384 (2019). [Google Scholar]
- 25.Harvey E. L., Bidle K. D., Johnson M. D., Consequences of strain variability and calcification in Emiliania huxleyi on microzooplankton grazing. J. Plankton Res. 37, 1137–1148 (2015). [Google Scholar]
- 26.Schiebel R., Planktic foraminiferal sedimentation and the marine calcite budget. Global Biogeochem. Cy. 16, 3-1–3-21 (2002). [Google Scholar]
- 27.Neukermans G., Bach L. T., Butterley A., Sun Q., Claustre H., Fournier G. R., Quantitative and mechanistic understanding of the open ocean carbonate pump - perspectives for remote sensing and autonomous in situ observation. Earth-Sci. Rev. 239, 104359 (2023). [Google Scholar]
- 28.Sulpis O., Jeansson E., Dinauer A., Lauvset S. K., Middelburg J. J., Calcium carbonate dissolution patterns in the ocean. Nat. Geosci. 14, 423–428 (2021). [Google Scholar]
- 29.Liang H., Lunstrum A. M., Dong S., Berelson W. M., John S. G., Constraining CaCO3 export and dissolution with an ocean alkalinity inverse model. Global Biogeochem. Cy. 37, e2022GB007535 (2023). [Google Scholar]
- 30.Knecht N. S., Benedetti F., Hofmann Elizondo E. U., Bednaršek N., Chaabane S., De Weerd C., Peijnenburg K. T. C. A., Schiebel R., Vogt M., The impact of zooplankton calcifiers on the marine carbon cycle. Global Biogeochem. Cy. 37, e2022GB007685 (2023). [Google Scholar]
- 31.Wilson R. W., Millero F. J., Taylor J. R., Walsh P. J., Christensen V., Jennings S., Grosell M., Contribution of fish to the marine inorganic carbon cycle. Science 323, 359–362 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Oehlert A. M., Garza J., Nixon S., Frank L., Folkerts E. J., Stieglitz J. D., Lu C., Heuer R. M., Benetti D. D., del Campo J., Gomez F. A., Grosell M., Implications of dietary carbon incorporation in fish carbonates for the global carbon cycle. Sci. Total Environ. 916, 169895 (2024). [DOI] [PubMed] [Google Scholar]
- 33.Laber C. P., Hunter J. E., Carvalho F., Collins J. R., Hunter E. J., Schieler B. M., Boss E., More K., Frada M., Thamatrakoln K., Brown C. M., Haramaty L., Ossolinksi J., Fredericks H., Nissimov J. I., Vandzura R., Sheyn U., Lehahn Y., Chant R. J., Martins A. M., Coolen M. J. L., Vardi A., DiTullio G. R., Van Mooy B. A. S., Bidle K. D., Coccolithovirus facilitation of carbon export in the North Atlantic. Nat. Microbiol. 3, 537–547 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Bishop J. K. B., Collier R. W., Kettens D. R., Edmond J. M., The chemistry, biology, and vertical flux of particulate matter from the upper 1500 m of the Panama Basin. Deep Sea Res. 27, 615–640 (1980). [Google Scholar]
- 35.Tsukamoto K., Miller M. J., The mysterious feeding ecology of leptocephali: A unique strategy of consuming marine snow materials. Fish. Sci. 87, 11–29 (2021). [Google Scholar]
- 36.De La Rocha C. L., Passow U., Factors influencing the sinking of POC and the efficiency of the biological carbon pump. Deep Sea Res. Pt. II: Trop. Stud. Oceanogr. 54, 639–658 (2007). [Google Scholar]
- 37.R. R. L. Guillard, “Culture of phytoplankton for feeding marine invertebrates” in Culture of Marine Invertebrate Animals, W. L. Smith, M. H. Chanley, Eds. (Plenum Press, 1975), pp 26–60. [Google Scholar]
- 38.Haunost M., Riebesell U., D’Amore F., Kelting O., Bach L. T., Influence of the calcium carbonate shell of coccolithophores on ingestion and growth of a dinoflagellate predator. Front. Marine Science. 8, 664269 (2021). [Google Scholar]
- 39.Subhas A. V., Rollins N. E., Berelson W. M., Dong S., Erez J., Adkins J. F., A novel determination of calcite dissolution kinetics in seawater. Geochim. Cosmochim. Acta 170, 51–68 (2015). [Google Scholar]
- 40.Feely R. A., Sabine C. L., Lee K., Millero F. J., Lamb M. F., Greeley D., Bullister J. L., Key R. M., Peng T.-H., Kozyr A., Ono T., Wong C. S., In situ calcium carbonate dissolution in the Pacific Ocean. Global Biogeochem. Cyc. 16, 91-1–91-12 (2002). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S3
Legends for data S1 and S2
Data S1 and S2