Abstract
Epigenetic dysregulation has long been recognized as a significant contributor to tumorigenesis and tumor maintenance, impacting all recognized cancer hallmarks. Although some epigenetic drugs have received regulatory approval for certain hematological malignancies, their efficacy in treating solid tumors has so far been largely disappointing. However, recent advancements in developing new compounds and a deeper understanding of cancer biology have led to success in specific solid tumor subtypes through precision medicine approaches. Moreover, epigenetic drugs may play a crucial role in synergizing with other anticancer treatments, enhancing the sensitivity of cancer cells to various anticancer therapies, including chemotherapy, radiation therapy, hormone therapy, targeted therapy, and immunotherapy. In this review, we critically evaluate the evolution of epigenetic drugs, tracing their development from initial use as monotherapies to their current application in combination therapies. We explore the preclinical rationale, completed clinical studies, and ongoing clinical trials. Finally, we discuss trial design strategies and drug scheduling to optimize the development of possible combination therapies.
Keywords: epigenetic drugs, precision medicine, anticancer treatment, solid tumors
1. Introduction
Epigenetics, defined as the study of changes in gene expression that do not involve alterations to the underlying DNA sequence, has become a key area of research for understanding cancer and developing new treatments. Unlike genetic mutations, which directly modify the DNA code, epigenetic changes involve chemical modifications, such as the addition of methyl groups to DNA or the modification of histone proteins around which DNA is wrapped. These changes can either activate or silence genes, influencing cellular behavior without altering the genetic code itself [1].
In the context of cancer, abnormal epigenetic modifications can lead to the inappropriate activation of oncogenes or the silencing of tumor suppressor genes, driving the uncontrolled growth characteristic of cancer cells. These epigenetic alterations are often reversible, making them attractive targets for therapeutic intervention [2]. Research aimed at reversing these abnormal modifications has led to the development of epigenetic therapies, including DNA methyltransferase (DNMT) inhibitors and histone deacetylase (HDAC) inhibitors. By reprogramming the epigenome, these treatments may restore normal gene expression patterns in cancer cells, potentially slowing disease progression and synergistically enhancing the effectiveness of existing therapies such as chemotherapy, radiation, and immunotherapy [3].
The potential of epigenetics in cancer treatment extends beyond the general approach of targeting common cancer pathways. As our understanding of epigenetics deepens, it is becoming increasingly clear that these mechanisms play a crucial role in cancer development and progression. Nevertheless, epigenetic drugs have been tested both as monotherapies and in combination treatments, with highly variable results so far. Given that the epigenome is highly dynamic and varies between individuals and even among different cells within a tumor, this variability opens the door to personalized medicine, a field in which treatments can be tailored to the specific epigenetic landscape of a patient’s tumor. Consequently, epigenetic therapies represent a promising frontier in the quest for more effective, targeted, and less toxic cancer treatments, offering hope for improved outcomes in a disease that remains one of the leading causes of death worldwide [4].
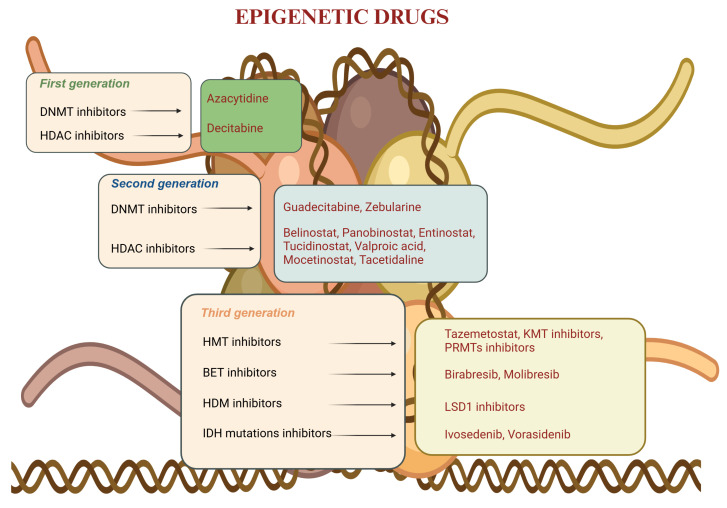
2. Generations of Epigenetic Drugs as Monotherapy
Genome function is regulated at multiple levels by epigenetic enzymes, and in recent years, multiple generations of epigenetic drugs have been developed (Figure 1).
Figure 1.
Generations of epigenetic drugs and key compounds [5]. BET: bromodomain and extra-terminal domain; DNMT: DNA methyltransferase; HDAC: histone deacetylase; HDM: histone demethylase; HMT: Histone methyltransferase; KMT; LSD: lysine-specific histone demethylase; PRMT: protein arginine methyltransferase inhibitor.
First-generation epigenetic drugs include DNMT inhibitors (e.g., azacytidine and decitabine) and HDAC inhibitors (e.g., vorinostat and romidepsin). These compounds are characterized by a very broad spectrum of action against all isoforms of DNMT and HDAC and are not biomarker-driven, following the concept of the “one size fits all”. This lack of specificity represents the major issue of these drugs, as different severe toxicities have been described very frequently. This generation of drugs has shown very limited efficacy and low response rates in solid tumors, whereas, in hematological malignancies, they still represent an important option of treatment in certain settings [6].
In order to guarantee better efficacy with an acceptable safety profile, second-generation epigenetic drugs have been developed. In this family, second-generation DNMT inhibitors (e.g., guadecitabine) and second-generation HDAC inhibitors (e.g., belinostat and panobinostat) are included. These compounds are designed to interfere with only some isoforms of DNMT and HDAC and have demonstrated a much better manageability compared to first-generation inhibitors; nevertheless, efficacy has demonstrated to be largely disappointing, particularly when these agents are used as monotherapy [7]. Thus, combinations with other therapeutic strategies have been tested (chemotherapy, immunotherapy, targeted therapy, etc.), with contrasting results that will be examined in detail later. Subsequently, the concept of developing a therapeutic strategy related to precision medicine led to the development of third-generation epigenetic drugs, including, among others, histone methyltransferases (HMT) inhibitors, bromodomain and extra-terminal domain (BET) inhibitors, lysine-specific histone demethylase 1A (LSD1) inhibitors, isocitrate dehydrogenase (IDH) inhibitors, histone demethylase (HDM) inhibitors, and protein arginine methyltransferase (PRMT) inhibitors. This class of drugs resulted in improved efficacy, leading, in few cases, to the Food and Drug Administration (FDA) approval in solid tumors.
Bromodomain and extra-terminal domain (BET) proteins are structures involved in the regulations of gene expression, and their inhibition represents a promising therapeutic target. Among the BET inhibitors, Birabresib (OTX015/MK-8628) showed efficacy in patients affected by NUT midline carcinoma (NMC) harboring the BRD4–NUT fusion oncoprotein, providing the first proof-of-concept evidence of clinical activity of a BRD inhibitor in targeting BRD4–NUT; three out of ten patients (30%) with NMC had a partial response (PR) with duration of response (DOR) of 1.4 to 8.4 months [8]. The efficacy of Birabresib was then studied in patients affected by NMC with a BRD–NUT gene translocation in a phase 1b trial where Birabresib exhibited clinical activity in NMC and a favorable safety profile; however, the authors suggested that intermittent dosing schedules might help reduce the toxicities associated with chronic administration [9]. The proof of concept for the efficacy of BET inhibitors in NMC was further supported by a phase 1 study, with Molibresib (GSK525762) showing PR in 4 out of 19 patients (21%) and stable disease (SD) in 8 patients (42%) as the best response in patients affected by NMC [10].
Histone methyltransferases (HMTs) are also proteins involved in the regulation of gene expression. Tazemetostat, an inhibitor of the histone-lysine N-methyltransferase enhancer of zeste homolog 2 (EZH2), demonstrated efficacy in a phase II trial including patients with advanced epithelioid sarcoma with INI1/SMARCB1 loss: an objective response rate (ORR) was reached in 15% of patients, with a median progression-free survival (PFS) of 5.5 months (95% CI 3.4–5.9) and median OS (overall survival) of 19.0 months (11.0—not estimable) [11]. Based on these data, in 2020 the FDA granted accelerated approval to tazemetostat as the first treatment option for adults and pediatric patients with metastatic or locally advanced epithelioid sarcoma who are not candidates for complete resection [12].
Isocitrate dehydrogenase (IDH) is an enzyme involved in the Krebs cycle that catalyzes the transformation from isocitrate to alpha-ketoglutarate, an irreversible step fundamental for cell metabolism and stability; the inhibition of IDH could lead to alterations in gene expression and be a pro-oncogenic factor [13]. Among IDH mutation inhibitors, ivosidenib demonstrated efficacy in the phase III ClarIDHy trial (NCT02989857), in which 185 patients with IDH1-mutated advanced cholangiocarcinoma previously treated with platinum-based were enrolled. This study showed a statistically significant improvement in PFS with ivosidenib compared to a placebo (HR 0.37, 95% CI, 0.25–0.54, p < 0.001). The median OS was found out to be 10.3 months with ivosidenib versus 7.5 months with the placebo (hazard ratio: 0.79; 95% CI, 0.56–1.12; 1-sided p = 0.09); adjusted for crossover, the mOS with the placebo was 5.1 months (95% CI, 3.8–7.6; HR 0.49 [95% CI, 0.34–0.70]; 1-sided p < 0.001) [14]. Based on these results, in 2021, the FDA approved ivosidenib for adult patients with previously treated advanced IDH1-mutated cholangiocarcinoma [15].
More recently, in 2024, the FDA granted approval for vorasidenib, an oral brain-penetrant inhibitor of mutant IDH1 and IDH2 enzymes, for the treatment of adult and pediatric patients aged 12 and older with grade-2 astrocytoma or oligodendroglioma following surgery (including biopsy, subtotal resection, or gross total resection) and with a susceptible IDH1 or IDH2 mutation [16]. In the randomized phase III INDIGO trial, vorasidenib significantly improved PFS compared to the placebo (mPFS, 27.7 months vs. 11.1 months; HR 0.39; 95% CI, 0.27 to 0.56; p < 0.001); vorasidenib also delayed the time to next intervention (HR 0.26; 95% CI, 0.15 to 0.43; p < 0.001) [17] (Table 1).
Table 1.
Key clinical trials for FDA-approved epigenetic drugs as monotherapy in solid tumors.
| Clinical Trial | Phase | Number of Patients | Type of Solid Tumors | Drug | Results |
|---|---|---|---|---|---|
| NCT02601950 [11] | II | 62 | advanced epithelioid sarcoma with loss of INI1/SMARCB1 | tazemetostat (EZH2 inh) |
ORR: 15% (95% CI 7–26); Median PFS: 5.5 mo (95% CI 3.4–5.9); Median OS: 19.0 mo (11.0–NE). |
| NCT02989857 [14] | III | 185 | IDH1-mutated advanced cholangiocarcinoma | ivosidenib (IDH1 inh) |
Median PFS: 6.9 mo (ivosidenib) vs. 1.4 mo (placebo), HR 0.37, 95% CI, 0.25–0.54, p < 0.001. Median OS: 10.3 mo (ivosidenib) vs. 7.5 mo (placebo), HR 0.79, [95% CI, 0.56–1.12]; 1-sided p = 0.09. |
| NCT04164901 [17] | III | 331 | IDH1 or IDH2 mutated low-grade glioma | vorasidenib (IDH1/IDH2 inh) vs. placebo | Median PFS: 27.7 mo vs. 11.1 mo (HR 0.39; 95% CI, 0.27 to 0.56; p < 0.001) |
CI: confidence interval; EZH2: enhancer of zeste homologue 2; IDH: isocitrate dehydrogenase; inh: inhibitor; HR: hazard ratio; mo: months; NE: not estimable; ORR: objective response rate; OS: overall survival; PFS: progression-free survival.
Another promising target is represented by histone demethylases: in particular, lysine-specific demethylase 1 (LSD1/KDM1A), the first discovered histone lysine demethylase, can disrupt the molecular changes driving the epigenetic plasticity of cancer cells. LSD1 is implicated in various solid tumors, with its overexpression linked to poor prognosis. Recently, LSD1-inhibitors have entered clinical trials for cancer treatment, and results are highly awaited [18].
3. Combining Epigenetic Drugs with Other Anti-Cancer Therapies: Does More Mean Better?
Preclinical evidence has shown that combining epigenetic drugs with other anticancer therapies can enhance therapeutic efficacy and reduce drug resistance, leading to the investigation of many combinations with epigenetic drugs.
3.1. Combination with Chemotherapy
Chemoresistance, often linked to abnormal DNA methylation and alterations in histone acetylation and methylation, can potentially be reversed by epigenetic drugs. On the other hand, preclinical evidence suggests that HDAC inhibitors may enhance the effectiveness of chemotherapeutic agents by promoting chromatin decondensation, potentially leading to a synergistic therapeutic effect [19,20]. However, despite this promise, several clinical trials investigating the combination of epigenetic drugs and chemotherapy across various solid tumors have yielded disappointing results, with many trials being discontinued due to lack of efficacy. Indeed, although a phase II trial investigating carboplatin, paclitaxel, and vorinostat in advanced NSCLC patients showed a trend toward improved ORR with the addition of vorinostat (NCT 00481078) [21], the subsequent phase III trial in the same population failed to meet the primary endpoint of overall survival (OS) in a pre-specified interim analysis, revealing a negative impact of vorinostat on clinical outcomes (NCT00473889) [22]. Similarly, a phase II study of gemcitabine combined with the HDAC inhibitor tacedinaline (CI-994) in advanced pancreatic ductal adenocarcinoma (PDAC) showed no significant improvement in OS, RR, or PFS compared to gemcitabine alone (NCT00004861) [23]. Moreover, despite preclinical evidence suggesting that DNA methylation may contribute to the development of platinum resistance in ovarian cancer (OC), a randomized phase II trial evaluating the combination of the DNA-hypomethylating agent decitabine and carboplatin in recurrent, partially platinum-sensitive cases was terminated due to toxicity and a detrimental effect, irrespective of methylation status (NCT00748527) [24]. Additionally, a phase II trial evaluating the second-generation DNA-hypomethylating agent, guadecitabine, in combination with carboplatin in platinum-resistant ovarian cancer did not result in statistically significant improvements in clinical outcomes. (NCT01696032) [25] (Table 2).
Table 2.
Key clinical trials of epigenetic drugs combined with chemotherapy.
| Trial | Phase | Number of Patients | Type of Solid Tumors | Drugs | Results |
|---|---|---|---|---|---|
| NCT00481078 [21] | II | 94 | advanced NSCLC | CBDCA + TXL + vorinostat vs. CBDCA + TXL + placebo |
ORR (primary endpoint): 34% (CBDCA + TXL + vorinostat) vs. 12%. (CBDCA + TXL + placebo), p = 0.02 |
| NCT00473889 [22] | III | 253 | advanced NSCLC | CBDCA + TXL + vorinostat vs. CBDCA + TXL + placebo |
OS (primary endpoint): 11 mo (0.2 to 17.3) (arm vorinostat + CBDCA + TXL) vs. 14 mo (0.03 to 18.7) (arm placebo+CBDCA + TXL), p = 0.992 |
| NCT00004861 [23] | II | 174 | advanced PDAC | gemcitabine + tacedinaline (CI-994) vs. gemcitabine + placebo |
Median OS (primary endpoint): 194 days (gemcitabine + tacedinaline) vs. 214 days (gemcitabine + placebo), p = 0.908 |
| NCT00748527 [24] | II | 29 | advanced OC with or without methylated hMLH1 | CBDCA (arm A) vs. decitabine + CBDCA (arm B) |
ORR by GCIG criteria in methylated hMLH1 tumor (primary endpoint): Responses (PR/CR) in 9/14 patients (arm A) vs. 3/15 patients (arm B). ORR regardless of methylation status (secondary endpoint): Responses (PR/CR) in 7/13 patients (arm A) vs. 1/12 patients (arm B). |
| NCT01696032 [25] | II | 100 | Platinum-resistance-advanced OC | guadecitabine + carboplatin vs. treatment of choice (topotecan, pegylated liposomal doxorubicin, paclitaxel, or gemcitabine). | Median PFS (primary endpoint): 16.3 w (guadecitabine +carboplatin) vs. 9.1 w (treatment of choice); p = 0.07 |
CBDCA: carboplatin; NSCLC: non-small cell lung cancer; OC: ovarian cancer; ORR: objective response rate; OS: overall survival; PDAC: pancreatic ductal adenocarcinoma; PFS: progression-free survival; TXL: paclitaxel; w: weeks.
3.2. Combination with Radiotherapy
The potential of epigenetic drugs as radiation sensitizers has been demonstrated in preclinical research by disrupting DDR and the cell cycle and increasing oxidative stress [26] and subsequently investigated in many clinical trials, usually concurrently with radiotherapy. Unfortunately, the combination of epigenetic drugs with radiotherapy (RT) in clinical trials has mostly resulted in increased levels of toxicity and minimal patient benefit. A significant challenge lies in identifying the most effective combination and timing for administering epigenetic drugs alongside radiotherapy. While some trials combining RT with epigenetic drugs showed no benefit [27], others have provided encouraging results. A phase I study combining the HDAC inhibitor vorinostat with capecitabine and radiotherapy in patients with localized pancreatic ductal adenocarcinoma (PDAC) was well tolerated, resulting in four R0 resections among 11 patients who underwent surgical exploration and a median overall survival (OS) of 1.1 years (NCT00983268) [28]. Additionally, a phase I trial demonstrated that the combination of vorinostat with vectorized internal radiotherapy using 131I-metaiodobenzylguanidine (MIBG) was tolerable in children with relapsed or refractory high-risk neuroblastoma, achieving an overall response rate (ORR) of 12% across all dose levels and 17% at the recommended phase II dose (NCT01019850) [29]. In the subsequent randomized phase II trial, patients with relapsed or refractory neuroblastoma receiving MIBG plus vorinostat had the highest response rate (32%) compared to 14% in the other treatment arms, meeting the prespecified threshold, with manageable toxicity; in contrast, vincristine and irinotecan did not enhance the response rate to MIBG and were associated with increased toxicity [30]. (Table 3). These findings highlight the need for implementing biomarkers to guide patient selection, since a key challenge lies in identifying the most effective combination and timing for administering epigenetic drugs alongside radiotherapy.
Table 3.
Key clinical trials involving epigenetic drugs with radiotherapy.
| Trial | Phase | Number of Patients | Type of Solid Tumors | Drugs | Results |
|---|---|---|---|---|---|
| NCT00983268 [28] | I | 21 | non-metastatic PDAC | vorinostat + CAPE + RT |
MTD: vorinostat 400 mg daily + CAPE 1000 mg BID (during RT). ORR: 90% SD, 10% PD (at time of surgery). Resection: R0 (4/11 patients) Median OS: 1.1y (95% CI 0.78–1.35). |
| NCT01019850 [29] | I | 27 | relapsed and/or refractory HR neuroblastoma | vorinostat + vectorized internal radiotherapy with 131-I-MIBG. |
Safety: Feasible and tolerable. ORR: 12% at all dose levels and 17% at the RP2D |
| NCT02035137 [30] | II | 114 | relapsed or refractory neuroblastoma | Arm A: MIBG. Arm B: MIBC + vincristine + irinotecan. Arm C: vorinostat + MIBG |
ORR (after 1 course- primary endpoint): 32% (MIBG + vorinostat) vs. 14% (other arms). |
BID: bis in die; CAPE: capecitabine; CI: confidence interval; HR: high-risk; 131-I-MIBG: metaiodobenzylguanidine; MTD: maximum tolerated dose; ORR: objective response rate; OS: overall survival; PD: progressive disease; PDAC: pancreatic ductal adenocarcinoma; RP2D: recommended phase II dose; RT: radiotherapy; SD: stable disease; Y: year.
3.3. Combination with Hormone Therapy
The combination of epigenetic drugs with hormone therapy has been explored in various cancers, particularly breast and prostate cancer. In breast cancer, HDAC inhibitors have long shown the ability to interfere with estrogen-receptor-signaling pathways in estrogen-receptor-positive (ER+) breast cancer (BC) [31]. A phase II trial combining vorinostat with tamoxifen in hormone-therapy-resistant BC reported a 19% ORR and a 40% clinical benefit rate, demonstrating the potential to reverse hormone resistance [32]. The ENCORE 301 phase II trial, which combined entinostat with exemestane in postmenopausal women with advanced hormone-receptor-positive, endocrine-resistant BC showed an improvement in PFS [33]. However, the subsequent phase III E2112 trial failed to demonstrate a significant survival benefit in the same population, with a median OS of 23.4 months for the combination versus 21.7 months for exemestane alone (NCT02115282) [34]. Conversely, the phase III ACE study evaluating tucidinostat with exemestane in postmenopausal patients with advanced hormone-receptor-positive BC showed a significant benefit in PFS (NCT02482753) [35].
In prostate cancer, a phase I/II trial combining the HDAC inhibitor panobinostat with the anti-androgen bicalutamide in castration-resistant prostate cancer (CRPC) demonstrated improved outcomes if compared to historical controls, though high doses of panobinostat were associated with significant toxicity, mostly thrombocytopenia [36]. BET inhibitors, which also target androgen receptor signaling, have shown promise in preclinical CRPC models, including enzalutamide-resistant cases [37]. In a phase Ib/II study, the combination of the BET inhibitor ZEN-3694 with enzalutamide showed acceptable tolerability and potential efficacy in patients with androgen-signaling inhibitors-resistant metastatic CRPC [38]. However, a phase Ib study of the BET inhibitor GS-5829 combined with enzalutamide showed limited efficacy [39]. Another ongoing trial is investigating the novel BET inhibitor NUV-868, both as monotherapy and in combination with olaparib or enzalutamide, across various cancers including prostate cancer (NCT05252390) [40] (Table 4).
Table 4.
Key clinical trials involving epigenetic drugs with hormone therapy.
| Trial | Phase | Number of Patients | Type of Solid Tumors | Drugs | Results |
|---|---|---|---|---|---|
| NCT00365599 [32] | II | 43 | ER-positive, hormone therapy-resistant mBC | vorinostat + tamoxifen |
ORR: 19%. CBR (response or stable disease > 24 w) 40%; Median DOR: 10.3 mo (CI: 8.1–12.4). |
| NCT00676663 [33] | II | 130 | postmenopausal advanced ER +BC | EXE + entinostat vs. EXE + placebo |
Median PFS: 4.28 mo (EXE + entinostat) vs. 2.27 mo (EXE + placebo), HR 0.73, p = 0.06. |
| NCT02115282 [34] | III | 608 | advanced HR + BC | EXE + entinostat vs. EXE + placebo |
Median PFS: 3.3 mo (EXE + entinostat) vs. 3.1 mo (EXE + placebo), HR 0.87; p = 0.30; Median OS: 23.4 mo (EXE + entinostat) vs. 21.7 mo (EXE + placebo), HR 0.99, p = 0.94 |
| NCT02482753 [35] | III | 365 | advanced HR + BC | EXE + tucidinostat vs. EXE + placebo | Median PFS: 7.4 mo (tucidinostat) vs. 3.8 mo (placebo), HR 0.75, p = 0.033. |
| NCT00878436 [36] | I/II | 55 | mCRPC | panobinostat + bicalutamide Arm A: 40 mg panobinostat Arm B: 20 mg panobinostat |
36W-PFS: 47.5% (arm A) vs. 38.5% (arm B). |
| NCT02711956 [38] | Ib/IIa | 75 | mCRPC | ZEN-3694 + ENZA | PFS: 9 mo (95% CI 4.6–12.9) |
CBR: clinical benefit rate; CI: confidence interval; DOR: duration of response; ENZA: enzalutamide; ER: estrogen receptor; EXE: exemestane; HR+: hormone-receptor-positive; mBC: metastatic breast cancer; mCRPC: metastatic castration-resistant prostate cancer; mo: months; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; w: weeks.
3.4. Combination with Targeted Therapy
The use of epigenetics drugs has been explored in combination with targeted therapies in different settings. Preliminary evidence has demonstrated that the use of epigenetic drugs in combination has the potential to overcome or delay the appearance of mechanisms of resistance, which are the most common reason for a target therapy failure. Here, we report significant results in combinations with epidermal growth factor receptor tyrosine kinase family (ErbB) inhibitors, anti-angiogenic agents, mammalian target of rapamycin (mTOR) inhibitors and PARP inhibitors.
The synergism between HDAC inhibitors and ErbB inhibitors has shown divergent results. The synergism between HDAC inhibitors and ErbB inhibitors was explored in a randomized phase II trial comparing first-generation EGFR inhibitor erlotinib with or without entinostat, an HDAC inhibitor, in 132 patients with advanced and treatment-naive NSCLC. Primary endpoint was 4-month PFS, which was not met (20% with erlotinib and 18% with erlotinib and entinostat, p = 0.7) (NCT00602030) [41]. Similar disappointing results comes from a randomized, placebo-controlled, double-blind phase IIb trial of first-generation EGFR inhibitors (gefitinib or erlotinib) with or without nicotinamide (an HDAC3 inhibitor) in 110 patients with an EGFR-mutant NSCLC; after a median follow-up of 54.3 months, the median PFS and OS were similar between the nicotinamide and the control group (mPFS: 12.7 m vs. 10.9 m, p = 0.2; mOS: 31.0 m vs. 29.4 m, p = 0.2); however, in a subgroup analysis, a significant reduction in mortality risk was observed in the nicotinamide group for females (p = 0.01) and never smokers (p = 0.03) [42].
The combination between HDAC inhibitors and anti-angiogenic agents was also evaluated. A phase I trial explored the combination of vorinostat and bevacizumab in patients affected by clear cell renal cell carcinoma (ccRCC): the combined treatment was tolerable, the ORR was 18%, with 5 partial (PR) and 1 complete response (CR), and the median PFS and OS were 5.7 months and 13.9 months, respectively [43]. Currently, in the same setting, a randomized, double-blind, placebo-controlled phase III trial (RENAVIV) is investigating the combination of pazopanib plus the potent oral pan-HDAC inhibitor abexinostat versus pazopanib alone in patients with advanced ccRCC and without prior exposure to anti-angiogenic TKIs (NCT03592472) [44]. In hepatocellular carcinoma (HCC), resminostat was tested alone or in combination with sorafenib in a phase I/II trial with patients progressing to first-line sorafenib: the combination was safe, and the PFS rate after 12 weeks of treatment (primary endpoint) was 12.5% in the resminostat group and 62.5% in the combination group [45]. Also, the HDAC inhibitor panobinostat was evaluated with bevacizumab in a phase I study with patients affected by high-grade glioma, with promising results (3 PR and 7 SD) and a good tolerability profile [46]. However, a subsequent phase II trial that compared panobinostat plus bevacizumab to bevacizumab alone in patients with recurrent glioblastoma or anaplastic glioma failed to demonstrate a significant benefit in terms of 6 months PFS for the combination arm [47].
The combination between HDAC inhibitors and mTOR inhibitors has been explored in a few early-phase studies. In a phase I trial, the association between the HDAC inhibitor vorinostat and the mTOR inhibitor ridaforolimus showed signals of efficacy in advanced solid tumors, especially ccRCC, despite thrombocytopenia as a significant toxicity [48]. Similarly, another phase I trial showed comparable results with the combination of vorinostat and the mTOR inhibitor sirolimus [49]. Conversely, a single-arm phase II trial exploring the combination of the HDAC inhibitor panobinostat and the mTOR inhibitor everolimus in children and young adults with advanced or recurrent gliomas harboring H3.1 or H3.3 K27M mutation has been withdrawn due to low accrual (NCT03632317) [50] (Table 5).
Table 5.
Key clinical trials involving epigenetic drugs combined with targeted therapy.
| Trial | Phase | Number of Patients | Type of Solid Tumors | Drugs | Results |
|---|---|---|---|---|---|
| NCT02416739 [42] | IIb | 110 | EGFR-mutated NSCLC | erlotinib/gefitinib + nicotinamide vs. erlotinib/gefitinib + placebo |
mPFS (primary endpoint): no statistically significant difference (12.7 m vs. 10.9 m, p = 0.2). Subgroup analysis: significant reduction in mortality risk in females and non-smokers. |
| NCT00324870 [43] | I/II | 36 | ccRCC | BEV + vorinostat |
Feasible and tolerable. ORR: 18% mPFS: 5.7 mo mOS: 13.9 mo |
| NCT00943449 [45] | I/II | 57 | HCC | resminostat +/− sorafenib |
12w-PFS (primary endpoint): 62.5% with the combination, 12.5% with sorafenib. Feasible and tolerable. |
| NCT00859222 [46] | I | 10 | high-grade glioma | BEV + panobinostat | ORR: 30% PR; 70% SD. |
BEV: bevacizumab; ccRCC: clear cell renal cell carcinoma; HCC: hepatocellular carcinoma; NSCLC: non-small cell lung cancer; ORR: objective response rate; OS: overall survival; PFS: progression-free survival; PR: partial response; SD: stable disease.
Due to the uncontrolled growth of cancer cells, DNA damage occurs, making the inhibition of DNA repair proteins a potential therapeutic strategy to effectively disrupt DNA repair mechanisms in cancer cells and thereby enhance cell death. A rationale for testing combination treatments with HDAC and PARP inhibition in cancers not sensitive to PARP inhibitor monotherapy has been defined, given that HDAC inhibition has been shown to be able to induce pharmacologic “BRCAness” in cancer cells with proficient DNA repair activity [51,52]. In BRCA-proficient high-grade serous ovarian and triple-negative breast cancer models, in vivo guadecitabine plus talazoparib treatment decreased xenograft tumor growth and increased overall survival, supporting clinical rationale for PARP inhibitors and epigenetic drugs combinations [53]. A Phase I/Ib clinical trial is currently underway, recruiting to evaluate the safety and preliminary efficacy of the PARP inhibitor olaparib and the HDAC inhibitor vorinostat combination in patients with relapsed, refractory, or metastatic breast cancer (NCT03742245) [54]. Similarly, a phase II trial is exploring the efficacy of ZEN003694, a BET inhibitor, plus talazoparib in patients with recurrent ovarian cancer (NCT05071937) [55].
3.5. Combination with Immunotherapy
Epigenetic drugs can potentially be exploited to modulate antitumor immunity [56,57], and there is increasing evidence that combining epigenetic with immunotherapeutic drugs may be beneficial to overcome acquired resistance to immunotherapy, for example, with DNMT and HDAC inhibitors [58]. Epigenetic drugs can act both on cancer cells and on cells involved in the immune response, exerting a potential role of modulators for immunotherapy [59]. Nevertheless, a randomized phase II study revealed an increased risk of toxicities and no therapeutic benefit from the combination of anti-PD1 pembrolizumab with the second-generation DNMT inhibitor CC-486 (oral azacytidine) [60]. Similarly, only a limited benefit was reported with CC-486 and durvalumab in immunologically cold tumors [61]. Recent results proved that guadecitabine in combination with pembrolizumab can be tolerable and harbors anticancer activity in a phase I dose-escalation study in patients with advanced solid tumors; overall, thirty patients were evaluable for antitumor activity: ORR was 7%, with 37% achieving disease control for 24 weeks or more; of 12 evaluable patients with non-small cell lung cancer (NSCLC), 10 had been previously treated with immune checkpoint inhibitors with 5 patients (42%) who reported a disease control equal or longer than 24 weeks [62]. This may favor the hypothesis of a reversibility of resistance to immunotherapy with the integration of epigenetic drugs [62]. Another study tested the combination of HDAC inhibitor romidepsin with cisplatin and nivolumab in a phase I/II study (NCT02393794) in metastatic TNBC; this association showed a good safety profile: the ORR was 44%, median PFS was 4.4 months, and 1-year PFS rate was 23%; the median OS was 10.3 months [63]. In metastatic melanoma, guadecitabine combined with anti-CTLA4 ipilimumab resulted in an immune-related DCR of 42% and ORR of 26% in the phase Ib NIBIT-M4 study (NCT02608437) [64]. Also, in metastatic melanoma, the addition of entinostat to pembrolizumab (ENCORE-601) demonstrated an ability to restore inflammation in the tumor microenvironment (TME), representing a positive assumption for successful re-treatment with anti-PD-1/PD-L1 [65]. In a phase II trial (NCT02538510), the combination of pembrolizumab and vorinostat in recurrent or metastatic head and neck squamous cell carcinoma (HNSCC) and salivary gland cancer (SGC): partial responses were reported both in HNSCC and in SGC. Among 25 patients with HNSCC, 8 (32%) achieved PR, and 5 (20%) had SD; the median OS was 12.6 months, and the median PFS was 4.5 months; among SGC patients, 4 (16%) out of 25 patients had PR, and 14 (56%) had SD; the median OS was 14 months, and the median PFS was 6.9 months [66]. A pilot study of tazemetostat and pembrolizumab in advanced urothelial carcinoma (ETCTN 10183) showed tolerability; moreover, activity was seen with PR in 3 pts (25%) and SD in 3 (25%). The median PFS was 3.1 months (95%CI: 2.3–NA), and the median overall survival was 8.0 months (95% CI: 4.7–NA) [67]. Hopefully, results from the ongoing study will help define the depth of the degree of reversibility of resistance to immunotherapy. A phase II study of nivolumab plus ipilimumab and ASTX727 versus nivolumab plus ipilimumab in anti-PD-1/PD-L1-resistant melanoma or NSCLC patients is ongoing [68] (Table 6). Finally, future and ongoing trials may elucidate the possible long-term effects of the prolonged use of epigenetic drugs in combination with immunotherapy, including the potential T-cell depletion, which may be induced, for example, by BET inhibitors [69].
Table 6.
Key clinical trials involving epigenetic drugs combined with immunotherapy.
| Trial | Phase | Number of Patients | Type of Solid Tumors | Drugs | Results |
|---|---|---|---|---|---|
| NCT02546986 [60] | II | 100 | NSCLC | azacitidine (CC-486) + PEMBRO | Median PFS (primary endpoint) 2.9 mo (PEMBRO + CC-486) vs. 4.0 mo (PEMBRO + placebo) |
| NCT02393794 [63] | I/II | 51 | locally recurrent or metastatic TNBC | romidepsin + CDDP + NIVO |
ORR: 44%; Median PFS: 4.4 mo; 1-year-PFS: 23%; Median OS: 10.3 mo. |
| NCT02538510 [66] | I/II | 25 HNSCC 25 SGC | recurrent/metastatic HNSCC or salivary gland cancer | vorinostat + PEMBRO | Primary endpoints were safety and ORR: - in HNSCC: CR = 0, PR = 8 (32%), SD = 5 (20%). - in SGCs: CR = 0, PR = 4 (16%), SD = 14 (56%) |
| NCT03854474 [67] | I/II | 12 | advanced UC | tazemetostat + PEMBRO | Primary endpoint: safety: no DLTs. ORR: PR in 3 patients (25%), SD in 3 patients (25%). Median PFS: 3.1 months (95%CI: 2.3–NA); Median OS: 8.0 months (95% CI: 4.7–NA). |
CDDP: cisplatin; CR: complete response; DLTs: dose-limiting toxicities; HNSCC: head and neck squamous cell carcinoma; mo: months; OS: overall survival; PFS: progression-free survival; NA: not available; NIVO: nivolumab; NSCLC: non-small cell lung cancer; ORR: objective response rate; OS: overall survival; PEMBRO: pembrolizumab; PFS: progression-free survival; PR: partial response; SD: stable disease; SGC: salivary gland cancer; TNBC: triple-negative breast cancer; UC: urothelial carcinoma; w: weeks.
4. Current Challenges and Future Directions
The emergence of new precision approaches may represent a transformational force to unlock the potential of the epigenetic therapeutics field, which has been marked by modest success and notable disappointments so far. Historically, one of the main problems of epigenetic drugs has been represented by high toxicities and low specificity [70]. As emphasized by the recent FDA-granted accelerated approval of vorasidenib in low-grade glioma with susceptible IDH1/2 mutations [16], as well as ivosidenib for adult patients with previously treated advanced cholangiocarcinoma with IDH1 mutations [15], epigenetic drugs are becoming increasingly specific for their target enzymes and, thus, their development should follow a precision-medicine approach. Exploration of lower doses and targeted delivery might improve the therapeutic index of epigenetic drugs [71].
To maximize the potentiality of epigenetic drugs, trial design structure, endpoints selection, and dose administration should be carefully determined. Moreover, according to the mechanism of action of the epigenetic treatment, dosage, and schedule of epigenetic drugs should be adequately selected; for example, intermittent dosing might represent a means to reduce severe toxicities [72]. Moreover, long-term follow-up with recording of late responses or clinical benefit in terms of prolonged objective responses or stable disease may be considered to evaluate tumor growth rate [71].
Indeed, to accurately assess the true effectiveness of epigenetic agents, molecular biomarkers, tumor markers, and extended follow-up of PFS and OS may represent alternative outcome measures or epigenetic drug activities, due to the extended time required to modulate epigenetic activity [73], and given that, any clinical trial design should be carefully justified according to the characteristics of drug and population of interest [74].
The traditional dose escalation method has shown itself to be imperfect in earlier trials for determining the optimal dosage and scheduling of epigenetic agents, especially when the goal is to leverage their epigenetic-modifying potential rather than their cytotoxic effects [73]. Combinations with epigenetic drugs have been mostly impaired by the occurrence of acute or chronic dose-limiting toxicities (DLTs); according to a single-center retrospective study, 66% of grade-3 or grade-4 epigenetic drug-related toxicities occurred after the first cycle, advocating for the need to prolong the assessment period [75]. Presumably, cytotoxic effects may not represent the ultimate of these drugs; therefore, the time required for epigenetic drugs to reprogram transcriptional activities and trigger cell differentiation or phenotypic changes in clinical tissues remains a matter of preclinical and clinical investigation [71]. Evaluating biomarker response, methylation, and expression differences between normal and tumor tissues in translational studies should assist the quantitative assessments to determine the therapeutic window of epigenetic drugs [76].
Acknowledgments
MM is supported by the Piano Nazionale di Ripresa e Resilienza (PNRR) INNOVA and HEAL Italia projects. FZ is supported by an AIRC fellowship Italy Post-Doc ID 29854-2023. Figure was generated with biorender.com.
Author Contributions
Conceptualization, A.R. (Alice Rossi) and F.Z.; methodology, M.M.; resources, A.R. (Alice Rossi), F.Z., A.R. (Anna Reni) and M.R.; data curation, M.M. and S.M.; writing—original draft preparation, A.R. (Alice Rossi), F.Z., A.R. (Anna Reni) and M.R.; writing—review and editing, A.R. (Alice Rossi), F.Z., S.P., A.Z. and J.M.; visualization, S.P.; supervision, M.M.; project administration, M.M. and S.M.; funding acquisition, M.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
MM reports honoraria from AstraZeneca (<US $10,000 in a single calendar year), MSD Oncology (<US $10,000 in a single calendar year), Ipsen (<US $10,000 in a single calendar year), Hippocrates Research (<US $10,000 in a single calendar year), Viatris (<US $10,000 in a single calendar year), and Servier (<US $10,000 in a single calendar year); consulting or advisory role for AstraZeneca (<US $10,000 in a single calendar year), MSD Oncology (<US $10,000 in a single calendar year), and Janssen Oncology (<US $10,000 in a single calendar year); research funding from Roche (to institute); travel, accommodations, and expenses managed/covered by AstraZeneca; and other relationship with Novartis and OncoSil. All other authors have declared no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jin N., George T.L., Otterson G.A., Verschraegen C., Wen H., Carbone D., Herman J., Bertino E.M., He K. Advances in epigenetic therapeutics with focus on solid tumors. Clin. Epigenetics. 2021;13:83. doi: 10.1186/s13148-021-01069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu X., Zhao H., Wang R., Chen Y., Ouyang X., Li W., Sun Y., Peng A. Cancer epigenetics: From laboratory studies and clinical trials to precision medicine. Cell Death Discov. 2024;10:28. doi: 10.1038/s41420-024-01803-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang N., Ma T., Yu B. Targeting epigenetic regulators to overcome drug resistance in cancers. Signal Transduct. Target. Ther. 2023;8:69. doi: 10.1038/s41392-023-01341-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sahafnejad Z., Ramazi S., Allahverdi A. An Update of Epigenetic Drugs for the Treatment of Cancers and Brain Diseases: A Comprehensive Review. Genes. 2023;14:873. doi: 10.3390/genes14040873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi A. Created in BioRender. 2024. [(accessed on 18 October 2024)]. Available online: www.BioRender.com/u10j113.
- 6.Bohl S.R., Bullinger L., Rücker F.G. Epigenetic therapy: Azacytidine and decitabine in acute myeloid leukemia. Expert Rev. Hematol. 2018;11:361–371. doi: 10.1080/17474086.2018.1453802. [DOI] [PubMed] [Google Scholar]
- 7.Rius M., Lyko F. Epigenetic cancer therapy: Rationales, targets and drugs. Oncogene. 2012;31:4257–4265. doi: 10.1038/onc.2011.601. [DOI] [PubMed] [Google Scholar]
- 8.Stathis A., Zucca E., Bekradda M., Gomez-Roca C., Delord J.-P., de La Motte Rouge T., Uro-Coste E., De Braud F., Pelosi G., French C.A. Clinical response of carcinomas harboring the BRD4–NUT oncoprotein to the targeted bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 2016;6:492–500. doi: 10.1158/2159-8290.CD-15-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewin J., Soria J.-C., Stathis A., Delord J.-P., Peters S., Awada A., Aftimos P.G., Bekradda M., Rezai K., Zeng Z., et al. Phase Ib Trial With Birabresib, a Small-Molecule Inhibitor of Bromodomain and Extraterminal Proteins, in Patients With Selected Advanced Solid Tumors. J. Clin. Oncol. 2018;36:3007–3014. doi: 10.1200/JCO.2018.78.2292. [DOI] [PubMed] [Google Scholar]
- 10.Piha-Paul S.A., Hann C.L., French C.A., Cousin S., Braña I., Cassier P.A., Moreno V., De Bono J.S., Harward S.D., Ferron-Brady G., et al. Phase 1 study of molibresib (GSK525762), a bromodomain and extra-terminal domain protein inhibitor, in NUT carcinoma and other solid tumors. JNCI Cancer Spectr. 2019;4:pkz093. doi: 10.1093/jncics/pkz093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gounder M., Schöffski P., Jones R.L., Agulnik M., Cote G.M., Villalobos V.M., Attia S., Chugh R., Chen T.W.-W., Jahan T., et al. Tazemetostat in advanced epithelioid sarcoma with loss of INI1/SMARCB1: An international, open-label, phase 2 basket study. Lancet Oncol. 2020;21:1423–1432. doi: 10.1016/S1470-2045(20)30451-4. [DOI] [PubMed] [Google Scholar]
- 12.Tazemetostat FDA Approval. [(accessed on 7 August 2024)]; Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-option-specifically-patients-epithelioid-sarcoma-rare-soft-tissue.
- 13.Pirozzi C.J., Yan H. The implications of IDH mutations for cancer development and therapy. Nat. Rev. Clin. Oncol. 2021;18:645–661. doi: 10.1038/s41571-021-00521-0. [DOI] [PubMed] [Google Scholar]
- 14.Zhu A.X., Macarulla T., Javle M.M., Kelley R.K., Lubner S.J., Adeva J., Cleary J.M., Catenacci D.V.T., Borad M.J., Bridgewater J.A., et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma with IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021;7:1669–1677. doi: 10.1001/jamaoncol.2021.3836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivosidenib FDA Approval. [(accessed on 7 August 2024)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-advanced-or-metastatic-cholangiocarcinoma.
- 16.Vorasidenib FDA Approval. [(accessed on 7 August 2024)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-vorasidenib-grade-2-astrocytoma-or-oligodendroglioma-susceptible-idh1-or-idh2-mutation.
- 17.Mellinghoff I.K., Bent M.J.v.D., Blumenthal D.T., Touat M., Peters K.B., Clarke J., Mendez J., Yust-Katz S., Welsh L., Mason W.P., et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. New Engl. J. Med. 2023;389:589–601. doi: 10.1056/NEJMoa2304194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hosseini A., Minucci S. A comprehensive review of lysine-specific demethylase 1 and its roles in cancer. Epigenomics. 2017;9:1123–1142. doi: 10.2217/epi-2017-0022. [DOI] [PubMed] [Google Scholar]
- 19.Yan B., Chen Q., Shimada K., Tang M., Li H., Gurumurthy A., Khoury J.D., Xu B., Huang S., Qiu Y. Histone deacetylase inhibitor targets CD123/CD47-positive cells and reverse chemoresistance phenotype in acute myeloid leukemia. Leukemia. 2019;33:931–944. doi: 10.1038/s41375-018-0279-6. [DOI] [PubMed] [Google Scholar]
- 20.Li J., Hao D., Wang L., Wang H., Wang Y., Zhao Z., Li P., Deng C., Di L.-J. Epigenetic targeting drugs potentiate chemotherapeutic effects in solid tumor therapy. Sci. Rep. 2017;7:4035. doi: 10.1038/s41598-017-04406-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramalingam S.S., Maitland M.L., Frankel P., Argiris A.E., Koczywas M., Gitlitz B., Thomas S., Espinoza-Delgado I., Vokes E.E., Gandara D.R., et al. Carboplatin and paclitaxel in combination with either vorinostat or placebo for first-line therapy of advanced non-small-cell lung cancer. J. Clin. Oncol. 2010;28:56–62. doi: 10.1200/JCO.2009.24.9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. NCT00473889. ClinicalTrials.gov. [(accessed on 30 August 2024)]; Available online: https://clinicaltrials.gov/study/NCT00473889?tab=results.
- 23.Richards D.A., Boehm K.A., Waterhouse D.M., Wagener D.J., Krishnamurthi S.S., Rosemurgy A., Grove W., Macdonald K., Gulyas S., Clark M., et al. Gemcitabine plus CI-994 offers no advantage over gemcitabine alone in the treatment of patients with advanced pancreatic cancer: Results of a phase II randomized, double-blind, placebo-controlled, multicenter study. Ann. Oncol. 2006;17:1096–1102. doi: 10.1093/annonc/mdl081. [DOI] [PubMed] [Google Scholar]
- 24.Glasspool R.M., Brown R., E Gore M., Rustin G.J.S., A McNeish I., Wilson R.H., Pledge S., Paul J., Mackean M., Hall G.D., et al. A randomised, phase II trial of the DNA-hypomethylating agent 5-aza-2′-deoxycytidine (decitabine) in combination with carboplatin vs carboplatin alone in patients with recurrent, partially platinum-sensitive ovarian cancer. Br. J. Cancer. 2014;110:1923–1929. doi: 10.1038/bjc.2014.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oza A.M., Matulonis U.A., Secord A.A., Nemunaitis J., Roman L.D., Blagden S.P., Banerjee S., McGuire W.P., Ghamande S., Birrer M.J., et al. A Randomized Phase II Trial of Epigenetic Priming with Guadecitabine and Carboplatin in Platinum-resistant, Recurrent Ovarian Cancer. Clin. Cancer Res. 2020;26:1009–1016. doi: 10.1158/1078-0432.CCR-19-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camphausen K., Tofilon P.J. Inhibition of histone deacetylation: A strategy for tumor radiosensitization. Clin. Oncol. 2007;25:4051–4056. doi: 10.1200/JCO.2007.11.6202. [DOI] [PubMed] [Google Scholar]
- 27.Galanis E., Anderson S.K., Miller C.R., Sarkaria J.N., Jaeckle K., Buckner J.C., Ligon K.L., Ballman K.V., Moore D.F., Nebozhyn M., et al. Phase I/II trial of vorinostat combined with temozolomide and radiation therapy for newly diagnosed glioblastoma: Results of Alliance N0874/ABTC 02. Neuro Oncol. 2018;20:546–556. doi: 10.1093/neuonc/nox161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chan E., Arlinghaus L.R., Cardin D.B., Goff L., Berlin J.D., Parikh A., Abramson R.G., Yankeelov T.E., Hiebert S., Merchant N., et al. Phase i trial of vorinostat added to chemoradiation with capecitabine in pancreatic cancer. Radiother. Oncol. 2016;119:312–318. doi: 10.1016/j.radonc.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DuBois S.G., Groshen S., Park J.R., Haas-Kogan D.A., Yang X., Geier E., Chen E., Giacomini K., Weiss B., Cohn S.L., et al. Phase I study of vorinostat as a radiation sensitizer with 131I-metaiodobenzylguanidine (131I-MIBG) for patients with relapsed or refractory neuroblastoma. Clin. Cancer Res. 2015;21:2715–2721. doi: 10.1158/1078-0432.CCR-14-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DuBois S.G., Granger M.M., Groshen S., Tsao-Wei D., Ji L., Shamirian A., Czarnecki S., Goodarzian F., Berkovich R., Shimada H., et al. Randomized Phase II Trial of MIBG Versus MIBG, Vincristine, and Irinotecan Versus MIBG and Vorinostat for Patients With Relapsed or Refractory Neuroblastoma: A Report From NANT Consortium. J. Clin. Oncol. 2021;39:3506–3514. doi: 10.1200/JCO.21.00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Margueron R., Duong V., Bonnet S., Escande A., Vignon F., Balaguer P., Cavailles V. Histone Deacetylase Inhibition and Estrogen Receptor Levels Modulate the Transcriptional Activity of Partial Antiestrogens. J. Mol. Endocrinol. 2004;32:583–594. doi: 10.1677/jme.0.0320583. [DOI] [PubMed] [Google Scholar]
- 32.Munster P.N., Thurn K.T., Thomas S., Raha P., Lacevic M., Miller A., Melisko M., Ismail-Khan R., Rugo H., Moasser M., et al. A phase II study of the histone deacetylase inhibitor vorinostat combined with tamoxifen for the treatment of patients with hormone therapy-resistant breast cancer. Br. J. Cancer. 2011;104:1828–1835. doi: 10.1038/bjc.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yardley D.A., Ismail-Khan R.R., Melichar B., Lichinitser M., Munster P.N., Klein P.M., Cruickshank S., Miller K.D., Lee M.J., Trepel J.B. Randomized phase II, double-blind, placebo-controlled study of exemestane with or without entinostat in postmenopausal women with locally recurrent or metastatic estrogen receptor-positive breast cancer progressing on treatment with a nonsteroidal aromatase inhibitor. J. Clin. Oncol. 2013;31:2128–2135. doi: 10.1200/JCO.2012.43.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeruva S.L.H., Zhao F., Miller K.D., Tevaarwerk A.J., Wagner L.I., Gray R.J., Sparano J.A., Connolly R.M. E2112: Randomized Phase III Trial of Endocrine Therapy Plus Entinostat or Placebo in Hormone Receptor-Positive Advanced Breast Cancer. A Trial of the ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 2021;39:3171–3181. doi: 10.1200/JCO.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Z., Li W., Hu X., Zhang Q., Sun T., Cui S., Wang S., Ouyang Q., Yin Y., Geng C., et al. Tucidinostat plus exemestane for postmenopausal patients with advanced, hormone receptor-positive breast cancer (ACE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:806–815. doi: 10.1016/S1470-2045(19)30164-0. [DOI] [PubMed] [Google Scholar]
- 36.Ferrari A.C., Alumkal J.J., Stein M.N., Taplin M.-E., Babb J.S., Barnett E.S., Gomez-Pinillos A., Liu X., Moore D.F., DiPaola R.S., et al. Epigenetic therapy with panobinostat combined with bicalutamide rechallenge in castration-resistant prostate cancer. Clin. Cancer Res. 2019;25:52–63. doi: 10.1158/1078-0432.CCR-18-1589. [DOI] [PubMed] [Google Scholar]
- 37.Welti J., Sharp A., Yuan W., Dolling D., Rodrigues D.N., Figueiredo I., Gil V., Neeb A., Clarke M., Seed G., et al. Targeting Bromodomain and Extra-Terminal (BET) family proteins in Castration-Resistant Prostate Cancer (CRPC) Clin. Cancer Res. 2018;24:3149–3162. doi: 10.1158/1078-0432.CCR-17-3571. [DOI] [PubMed] [Google Scholar]
- 38.Aggarwal R.R., Schweizer M.T., Nanus D.M., Pantuck A.J., Heath E.I., Campeau E., Attwell S., Norek K., Snyder M., Bauman L., et al. A phase Ib/IIa study of the Pan-BET inhibitor ZEN-3694 in combination with enzalutamide in patients with metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2020;26:5338–5347. doi: 10.1158/1078-0432.CCR-20-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aggarwal R., Starodub A.N., Koh B.D., Xing G., Armstrong A.J., Carducci M.A. Phase Ib Study of the BET Inhibitor GS-5829 as Monotherapy and Combined with Enzalutamide in Patients with Metastatic Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2022;28:3979–3989. doi: 10.1158/1078-0432.CCR-22-0175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. NCT05252390. ClinicalTrials.Gov. [(accessed on 30 August 2024)]; Available online: https://clinicaltrials.gov/study/NCT05252390.
- 41.Neal J.W., Sequist L.V. Complex role of histone deacetylase inhibitors in the treatment of non-small-cell lung cancer. J. Clin. Oncol. 2012;30:2280–2282. doi: 10.1200/JCO.2011.41.0860. [DOI] [PubMed] [Google Scholar]
- 42.Oh H.-J., Bae S.-C., Oh I.-J., Park C.-K., Jung K.-M., Kim D.-M., Lee J.-W., Kang C.K., Park I.Y., Kim Y.-C. Nicotinamide in Combination with EGFR-TKIs for the Treatment of Stage IV Lung Adenocarcinoma with EGFR Mutations: A Randomized Double-Blind (Phase IIb) Trial. Clin. Cancer Res. 2024;30:1478–1487. doi: 10.1158/1078-0432.CCR-23-3059. [DOI] [PubMed] [Google Scholar]
- 43.Pili R., Liu G., Chintala S., Verheul H., Rehman S., Attwood K., Lodge M.A., Wahl R., Martin J.I., Miles K.M., et al. Combination of the histone deacetylase inhibitor vorinostat with bevacizumab in patients with clear-cell renal cell carcinoma: A multicentre, single-arm phase I/II clinical trial. Br. J. Cancer. 2017;116:874–883. doi: 10.1038/bjc.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. NCT03592472. ClinicalTrials.gov. [(accessed on 30 August 2024)]; Available online: https://clinicaltrials.gov/study/NCT03592472.
- 45.Bitzer M., Horger M., Giannini E.G., Ganten T.M., Wörns M.A., Siveke J.T., Dollinger M.M., Gerken G., Scheulen M.E., Wege H., et al. Resminostat plus sorafenib as second-line therapy of advanced hepatocellular carcinoma—The SHELTER study. J. Hepatol. 2016;65:280–288. doi: 10.1016/j.jhep.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 46.Drappatz J., Lee E.Q., Hammond S., Grimm S.A., Norden A.D., Beroukhim R., Gerard M., Schiff D., Chi A.S., Batchelor T.T., et al. Phase i study of panobinostat in combination with bevacizumab for recurrent high-grade glioma. J. Neuro-Oncol. 2011;107:133–138. doi: 10.1007/s11060-011-0717-z. [DOI] [PubMed] [Google Scholar]
- 47.Lee E.Q., Reardon D.A., Schiff D., Drappatz J., Muzikansky A., Grimm S.A., Norden A.D., Nayak L., Beroukhim R., Rinne M.L., et al. Phase II study of panobinostat in combination with bevacizumab for recurrent glioblastoma and anaplastic glioma. Neuro Oncol. 2015;17:862–867. doi: 10.1093/neuonc/nou350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zibelman M., Wong Y.-N., Devarajan K., Malizzia L., Corrigan A., Olszanski A.J., Denlinger C.S., Roethke S.K., Tetzlaff C.H., Plimack E.R. Phase i study of the mTOR inhibitor ridaforolimus and the HDAC inhibitor vorinostat in advanced renal cell carcinoma and other solid tumors. Investig. New Drugs. 2015;33:1040–1047. doi: 10.1007/s10637-015-0261-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Park H., Garrido-Laguna I., Naing A., Fu S., Falchook G.S., Piha-Paul A.S., Wheler J.J., Hong D.S., Tsimberidou A.M., Subbiah V., et al. Phase I Dose-Escalation Study of the mTOR Inhibitor Sirolimus and the HDAC Inhibitor Vorinostat in Patients with Advanced Malignancy. Oncotarget. 2016;7:67521–67531. doi: 10.18632/oncotarget.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. NCT03632317. ClinicalTrials.gov. [(accessed on 30 August 2024)]; Available online: https://clinicaltrials.gov/study/NCT03632317.
- 51.Lord C.J., Ashworth A. BRCAness revisited. Nat. Rev. Cancer. 2016;16:110–120. doi: 10.1038/nrc.2015.21. [DOI] [PubMed] [Google Scholar]
- 52.Ramos L., Truong S., Zhai B., Joshi J., Ghaidi F., Lizardo M.M., Shyp T., Kung S.H., Rezakhanlou A.M., Oo H.Z., et al. A Bifunctional PARP-HDAC Inhibitor with Activity in Ewing Sarcoma. Clin. Cancer Res. 2023;29:3541–3553. doi: 10.1158/1078-0432.CCR-22-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pulliam N., Fang F., Ozes A.R., Tang J., Adewuyi A., Keer H., Lyons J., Baylin S.B., Matei D., Nakshatri H., et al. An effective epigenetic-PARP inhibitor combination therapy for breast and ovarian cancers independent of BRCA mutations. Clin. Cancer Res. 2018;24:3163–3175. doi: 10.1158/1078-0432.CCR-18-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. NCT03742245. ClinicalTrials.gov. [(accessed on 30 August 2024)]; Available online: https://clinicaltrials.gov/study/NCT03742245?cond=NCT03742245&rank=1.
- 55. NCT05071937. ClinicalTrials.gov. [(accessed on 18 October 2024)]; Available online: https://clinicaltrials.gov/study/NCT05071937?term=Epigenetics&intr=PARP%20inhibitor&rank=1.
- 56.Lau C.M., Adams N.M., Geary C.D., Weizman O.-E., Rapp M., Pritykin Y., Leslie C.S., Sun J.C. Epigenetic control of innate and adaptive immune memory. Nat. Immunol. 2018;19:963–972. doi: 10.1038/s41590-018-0176-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aspeslagh S., Morel D., Soria J.C., Postel-Vinay S. Epigenetic modifiers as new immunomodulatory therapies in solid tumours. Ann. Oncol. 2018;29:812–824. doi: 10.1093/annonc/mdy050. [DOI] [PubMed] [Google Scholar]
- 58.Sun W., Lv S., Li H., Cui W., Wang L. Enhancing the anticancer efficacy of immunotherapy through combination with histone modification inhibitors. Genes. 2018;9:633. doi: 10.3390/genes9120633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Majchrzak-Celinska A., Warych A., Szoszkiewicz M. Novel approaches to epigenetic therapies: From drug combinations to epigenetic editing. Genes. 2021;12:208. doi: 10.3390/genes12020208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levy B.P., Giaccone G., Besse B., Felip E., Garassino M.C., Gomez M.D., Garrido P., Piperdi B., Ponce-Aix S., Menezes D., et al. Randomised phase 2 study of pembrolizumab plus CC-486 versus pembrolizumab plus placebo in patients with previously treated advanced non-small cell lung cancer. Eur. J. Cancer. 2019;108:120–128. doi: 10.1016/j.ejca.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 61.Taylor K., Yau H.L., Chakravarthy A., Wang B., Shen S.Y., Ettayebi I., A Ishak C., Bedard P.L., Razak A.A., Hansen A.R., et al. An open-label, phase II multicohort study of an oral hypomethylating agent CC-486 and durvalumab in advanced solid tumors. J. Immunother. Cancer. 2020;8:e000883. doi: 10.1136/jitc-2020-000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papadatos-Pastos D., Yuan W., Pal A., Crespo M., Ferreira A., Gurel B., Prout T., Ameratunga M., Chénard-Poirier M., Curcean A., et al. Phase 1, dose-escalation study of guadecitabine (SGI-110) in combination with pembrolizumab in patients with solid tumors. J. Immunother. Cancer. 2022;10:e004495. doi: 10.1136/jitc-2022-004495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma P., Abramson V.G., O’Dea A., Nye L.E., Mayer I.A., Crane G.J., Elia M., Yoder R., Staley J.M., Schwensen K., et al. Romidepsin (HDACi) plus cisplatin and nivolumab triplet combination in patients with metastatic triple negative breast cancer (mTNBC) J. Clin. Oncol. 2021;39((Suppl. S15)):1076. doi: 10.1200/JCO.2021.39.15_suppl.1076. [DOI] [Google Scholar]
- 64.Di Giacomo A.M., Covre A., Finotello F., Rieder D., Danielli R., Sigalotti L., Giannarelli D., Petitprez F., Lacroix L., Valente M., et al. Guadecitabine plus ipilimumab in unresectable melanoma: The NIBIT-M4 clinical trial. Clin. Cancer Res. 2019;25:7351–7362. doi: 10.1158/1078-0432.CCR-19-1335. [DOI] [PubMed] [Google Scholar]
- 65.Agarwala S.S., Moschos S.J., Johnson M.L., Opyrchal M., Gabrilovich D., Danaher P., Wang F., Brouwer S., Ordentlich P., Sankoh S., et al. Efficacy and safety of entinostat (ENT) and pembrolizumab (PEMBRO) in patients with melanoma progressing on or after a PD-1/L1 blocking antibody. J. Clin. Oncol. 2018;36:9530. doi: 10.1200/JCO.2018.36.15_suppl.9530. [DOI] [Google Scholar]
- 66.Rodriguez C.P., Wu Q., Voutsinas J., Fromm J.R., Jiang X., Pillarisetty V.G., Lee S.M., Santana-Davila R., Goulart B., Baik C.S., et al. A phase II trial of pembrolizumab and vorinostat in recurrent metastatic head and neck squamous cell carcinomas and salivary gland cancer. Clin. Cancer Res. 2020;26:837–845. doi: 10.1158/1078-0432.CCR-19-2214. [DOI] [PubMed] [Google Scholar]
- 67.Hussain M.H.A., Kocherginsky M., Singh P., Myint Z., Jiang D.M., Wulff-Burchfield E.M., Sharon E., Piekarz R., Meeks J.J., VanderWeele D.J. A pilot study of tazemetostat and pembrolizumab in advanced urothelial carcinoma (ETCTN 10183) J. Clin. Oncol. 2023;41:506. doi: 10.1200/JCO.2023.41.6_suppl.506. [DOI] [Google Scholar]
- 68.Di Giacomo A., Rossi G., Calabro L., Pascucci A., Vegni V., Simonetti E., Colucci M., Valente M., Gibilisco G., Frongia F., et al. 123P A phase II study of nivolumab (N) plus ipilimumab (I) and ASTX727 or N plus I in PD-1/PD-L1 resistant melanoma or NSCLC patients: The run-in phase of the NIBIT Foundation ML1 study. Immuno-Oncol. Technol. 2023;20:100595. doi: 10.1016/j.iotech.2023.100595. [DOI] [Google Scholar]
- 69.Bolden J.E., Tasdemir N., Dow L.E., van Es J.H., Wilkinson J.E., Zhao Z., Clevers H., Lowe S.W. Inducible in vivo silencing of Brd4 identifies potential toxicities of sustained BET protein inhibition. Cell Rep. 2014;8:1919–1929. doi: 10.1016/j.celrep.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feehley T., O’Donnell C.W., Mendlein J., Karande M., McCauley T. Drugging the epigenome in the age of precision medicine. Clin. Epigenetics. 2023;15:6. doi: 10.1186/s13148-022-01419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Morel D., Jeffery D., Aspeslagh S., Almouzni G., Postel-Vinay S. Combining epigenetic drugs with other therapies for solid tumours—Past lessons and future promise. Nat. Rev. Clin. Oncol. 2019;17:91–107. doi: 10.1038/s41571-019-0267-4. [DOI] [PubMed] [Google Scholar]
- 72.Kitajima S., Asahina H., Chen T., Guo S., Quiceno L.G., Cavanaugh J.D., Merlino A.A., Tange S., Terai H., Kim J.W., et al. Overcoming Resistance to Dual Innate Immune and MEK Inhibition Downstream of KRAS. Cancer Cell. 2018;34:439–452.e6. doi: 10.1016/j.ccell.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Juo Y.-Y., Gong X.-J., Mishra A., Cui X., Baylin S.B., Azad N.S., Ahuja N. Epigenetic therapy for solid tumors: From bench science to clinical trials. Epigenomics. 2015;7:215–235. doi: 10.2217/epi.14.73. [DOI] [PubMed] [Google Scholar]
- 74.Seymour L., Ivy S.P., Sargent D., Spriggs D., Baker L., Rubinstein L., Ratain M.J., Le Blanc M., Stewart D., Crowley J., et al. The design of phase II clinical trials testing cancer therapeutics: Consensus recommendations from the Clinical Trial Design Task Force of the National Cancer Institute Investigational Drug Steering Committee. Clin. Cancer Res. 2010;16:1764–1769. doi: 10.1158/1078-0432.CCR-09-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Leroy L., Satar T., Baldini C., Martin-Romano P., Hollebecque A., Michot J.-M., Ribrag V., Massard C., Paoletti X., Vinay S.P. Safety profile of epigenetic therapies in early phase trials: Do epidrugs deserve specific drug development processes? Ann. Oncol. 2019;30:i5. doi: 10.1093/annonc/mdz029.003. [DOI] [Google Scholar]
- 76.Azad N., Zahnow C.A., Rudin C.M., Baylin S.B. The future of epigenetic therapy in solid tumours—Lessons from the past. Nat. Rev. Clin. Oncol. 2013;10:256–266. doi: 10.1038/nrclinonc.2013.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.