Abstract
Introduction: Finerenone, a third-generation non-steroidal mineralocorticoid receptor antagonist (MRA), offers a targeted approach to managing cardiovascular outcomes, particularly in patients with chronic kidney disease (CKD) and type 2 diabetes (T2D). Unlike traditional MRAs such as spironolactone and eplerenone, which can cause off-target hormonal side effects and hyperkalemia, Finerenone selectively binds to mineralocorticoid receptors, reducing these risks. Recent randomized controlled trials have demonstrated Finerenone’s potential to improve cardiovascular outcomes, making it a promising alternative in the management of heart failure and other cardiovascular conditions associated with CKD and T2D. Methods: We conducted a scoping review using PRISMA guidelines. A search for “Finerenone” in the PubMed, Embase, and Cochrane Library databases included randomized controlled trials (RCTs), post hoc analyses, and relevant meta-analyses on cardiovascular outcomes. Data were synthesized narratively, assessing study quality through strengths and limitations. Discussion: Finerenone has shown significant benefits and a superior safety profile compared with traditional MRAs like spironolactone and eplerenone in managing CKD, T2D, and heart failure. It effectively reduces cardiovascular and renal events while minimizing risks such as hyperkalemia and hormonal side effects associated with steroidal MRAs. Future studies, including the REDEFINE-HF, FINALITY-HF, and CONFIRMATION-HF trials, will further explore Finerenone’s potential across diverse heart failure phenotypes, including its role in heart failure with mildly reduced and preserved ejection fractions, potentially establishing it as a cornerstone therapy in heart failure management. Conclusions: Finerenone represents a significant advancement in MRA therapy, offering enhanced safety and efficacy in managing cardiovascular outcomes in CKD and T2D patients. The current evidence supports its use as a promising alternative to traditional MRAs, particularly in patients intolerant to steroidal MRAs. Further trials are needed to fully establish its potential across diverse patient populations, including those with varying heart failure phenotypes.
Keywords: finerenone, heart failure, steroidal mineralocorticoid receptor antagonist, spironolactone, eplerenone, systematic review
1. Introduction
Mineralocorticoid receptor antagonists (MRAs) like spironolactone and eplerenone are well-established therapies for managing heart failure with reduced ejection fraction (HFrEF), as recommended by European and US guidelines for symptomatic patients with this condition [1,2]. These medications are effective due to their ability to block the harmful effects of aldosterone on the cardiovascular system. Despite their benefits, the use of these steroidal MRAs is often limited by their associated risks, including hyperkalemia and off-target effects on androgen and progesterone receptors, which can lead to undesirable side effects such as gynecomastia and hormonal imbalances [3,4]. These challenges are particularly pronounced in patients with comorbid conditions like chronic kidney disease (CKD) and type 2 diabetes (T2D), where the risk of adverse events is heightened.
Finerenone, a third-generation non-steroidal MRA, represents a significant advancement in this therapeutic class. By selectively binding to the mineralocorticoid receptor without affecting other steroid receptors, Finerenone offers a more targeted approach, potentially reducing the incidence of hyperkalemia and other side effects seen with traditional MRAs. Recent randomized controlled trials have explored its efficacy in improving cardiovascular outcomes among patients with CKD and T2D, highlighting its role as a promising alternative in the management of heart failure and related conditions. This review aims to synthesize the current evidence on Finerenone’s cardiovascular benefits, providing a comprehensive overview of its potential role in modern clinical practice.
2. Methods
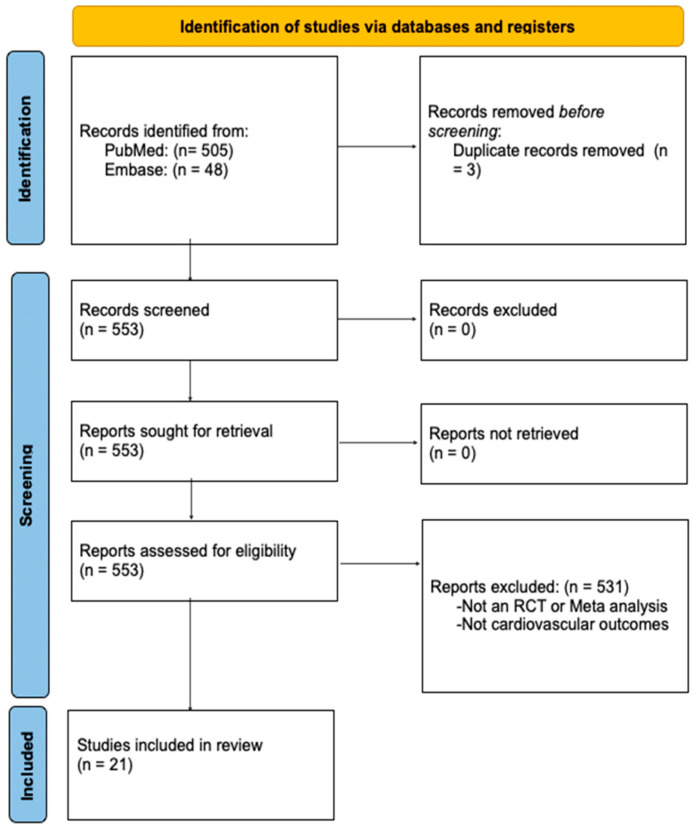
This scoping review aimed to map the available evidence on Finerenone’s impact on cardiovascular health by including randomized controlled trials (RCTs), post hoc analyses, and relevant meta-analyses. We conducted a comprehensive search using the term “Finerenone” without time constraints across the PubMed, Embase, and Cochrane Library databases. We followed PRISMA guidelines and the methodological framework by Arksey and O’Malley [5,6]. RCTs and post hoc analyses were included if they assessed Finerenone’s cardiovascular effects. Meta-analyses were considered if they included eligible RCTs and reported cardiovascular outcomes. No language restrictions were applied. Data were charted to capture the study characteristics, interventions, and key outcomes and synthesized narratively. The quality of the studies was assessed through a discussion of their strengths and limitations rather than through formal quantitative analyses such as risk of bias or heterogeneity assessments. This approach provided an overview of the evidence landscape relevant to Finerenone and cardiovascular health (Figure 1).
Figure 1.
PRISMA flow diagram.
3. Results
3.1. Mineralocorticoid Receptor Antagonists
3.1.1. Spironolactone
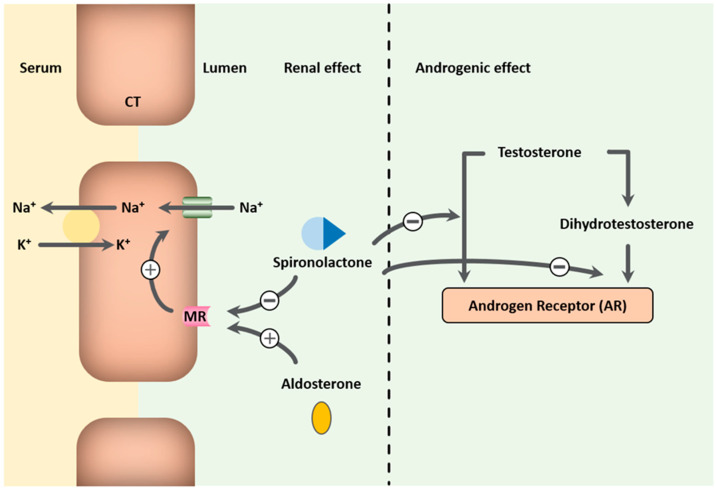
MRAs exhibit diuretic and antihypertensive effects by competitively inhibiting aldosterone at mineralocorticoid receptors in the heart, kidneys, and blood vessels. Spironolactone is a nonselective MRA that not only binds to mineralocorticoid receptors but also to androgen and progesterone receptors (Figure 2). Aldosterone, part of the renin-angiotensin–aldosterone system, acts on the distal tubules and collecting ducts, promoting sodium reabsorption and potassium secretion, and increasing vascular stiffness, cardiac inflammation, fibrosis, and remodeling. Blocking these actions helps reduce the harmful effects of aldosterone that can worsen HFrEF [7,8]. MRAs also help maintain serum potassium levels by decreasing urinary potassium loss, lowering the risk of hypokalemia and related arrhythmias associated with non-potassium-sparing diuretics [9,10,11]. They are primarily used in treating heart failure, particularly HFrEF, and have shown benefits in reducing morbidity and mortality when used alongside standard therapies like angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs) [10,11,12]. The 2017 ACC heart failure guidelines recommend spironolactone for NYHA class II-IV HFrEF patients with a creatinine clearance above 30 mL/min and serum potassium below 5 mEq/L, and they suggest potential benefits for select heart failure with preserved ejection fraction (HFpEF) patients in reducing hospitalizations [1]. However, the use of spironolactone does not come without side effects. Its use can lead to hyperkalemia and impaired renal function, requiring close monitoring of serum potassium levels and kidney function [13]. Despite this, the Effect of Spironolactone on Morbidity and Mortality in Patients with Severe Heart Failure (RALES) trial, the Eplerenone, a Selective Aldosterone Blocker, in Patients with Left Ventricular Dysfunction after Myocardial Infarction (EPHESUS) trial, and the Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms (EMPHASIS-HF) trial have demonstrated the efficacy of MRAs in HFrEF patients being treated with ACE inhibitors. This is likely due to their ability to address aldosterone escape and to reduce aldosterone effects that ACE inhibitors alone cannot manage [9,11,14,15]. Furthermore, spironolactone exhibits anti-androgenic effects due to its activity as a progesterone receptor agonist and androgen receptor antagonist, which can result in gynecomastia, breast tenderness, menstrual irregularities, impotence, and decreased libido, especially at higher doses [3,4]. Hence, it is an off-label, non-Food and Drug Administration (FDA)-approved medication for the treatment of hirsutism and acne vulgaris [16].
Figure 2.
Spironolactone’s action on mineralocorticoid receptors and androgen receptors. Legend: MR = Mineralocorticoid receptor.
3.1.2. Eplerenone
In contrast, eplerenone, a second-generation aldosterone antagonist, is more selective for the mineralocorticoid receptor than spironolactone, with a significantly lower affinity for glucocorticoid, progesterone, and androgen receptors, offering a 100- to 1000-fold reduction in binding to these receptors [17]. It is FDA-approved for improving survival in stable HFrEF patients with congestive heart failure (CHF) post-myocardial infarction, which is supported by findings from the EPHESUS and EMPHASIS-HF trials [10,11]. Eplerenone is also approved for treating hypertension, alone or in combination, particularly when patients experience adverse effects from spironolactone [18]. A multicenter trial showed that eplerenone had fewer endocrine side effects and was better tolerated than spironolactone, although it was less effective in lowering diastolic blood pressure [19]. Thus, starting with spironolactone and switching to eplerenone if side effects arise is a practical approach.
3.1.3. Finerenone
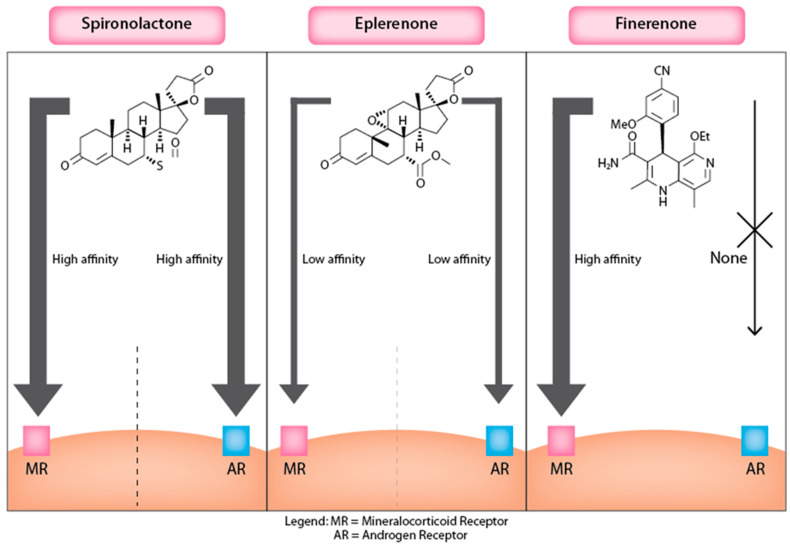
Finerenone is a novel third-generation MRA derived from dihydropyridines, a class distinct from the steroidal backbone of traditional MRAs like spironolactone and eplerenone. This unique structure allows Finerenone to selectively bind to the MR with high affinity while minimizing off-target effects on other steroid receptors, such as androgen and progesterone receptors, which are commonly affected by steroidal MRAs (Figure 3). Quantitatively, the IC50 of Finerenone for the MR is approximately 27.9 nM, compared with 24.2 nM for spironolactone and 990 nM for eplerenone, illustrating its superior binding efficiency [20]. Finerenone’s pharmacokinetic profile is characterized by good oral bioavailability and a predictable, steady drug level that supports once-daily dosing without significant accumulation or prolonged activity from active metabolites, unlike spironolactone [20]. Its binding to the MR is competitive but does not promote the recruitment of transcriptional co-regulators, distinguishing its mechanism of action from traditional steroidal MRAs. This passive antagonism is facilitated by Finerenone’s bulky and rigid molecular structure, which prevents MR activation through specific hydrogen bonding and van der Waals interactions [20]. Its distinct binding characteristics also make Finerenone effective in conditions where MR mutations might render steroidal antagonists less effective, highlighting its potential as a refined third-generation MRA that can better manage risks like hyperkalemia and hormone-related side effects in vulnerable populations [20]. Clinically, the non-steroidal nature of Finerenone offers a targeted and safer option for patients with chronic heart failure and CKD, reducing the likelihood of adverse effects related to steroid receptor cross-reactivity.
Figure 3.
MRA’s action on mineralocorticoid and androgen receptors.
3.2. Chronic Kidney Disease and Type 2 Diabetes Mellitus
In patients with CKD and T2D, Finerenone has shown promising efficacy and safety in reducing both cardiovascular and kidney risks. Early evidence from the Effect of Finerenone on Albuminuria in Patients With Diabetic Nephropathy (ARTS-DN) trial demonstrated Finerenone’s ability to significantly reduce proteinuria in patients with diabetic nephropathy who were already on ACEi’s or ARBs, with reductions in the urinary albumin–creatinine ratio (UACR) at higher doses [21]. Building on this, the Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes (FIDELIO-DKD) trial enrolled patients with more advanced CKD stages and showed that Finerenone not only reduced the risk of cardiovascular events by 14% but also slowed kidney disease progression by 18% [22]. These benefits did come with higher rates of hyperkalemia. The Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes (FIGARO-DKD) trial focused more on cardiovascular protection and earlier CKD stages and showed a significant reduction in heart failure hospitalizations [23]. While the renal benefits were less pronounced in FIGARO-DKD compared with FIDELIO-DKD, there was still a significant reduction in the UACR and lower incidences of end-stage kidney disease. A major criticism was the possibly that some of the patients in these studies were actually benefiting from the sodium–glucose transport protein 2 inhibitor (SGLT2i) and not actually from Finerenone. The pooled FIDELITY analysis, which combined the FIDELIO-DKD and FIGARO-DKD trials, confirmed that Finerenone’s benefits on cardiovascular and kidney outcomes were consistent, whether in the presence or not of an SGLT2i as an adjunct, suggesting an additive protective effect on patient health [24]. A summary of these RCT’s can be seen in Table 1.
Table 1.
Summary of RCT’s on the efficacy and safety of Finerenone in patients with CKD and T2DM.
| Study (Year) | Design | Intervention | Control | Main Outcomes | Effect Size |
|---|---|---|---|---|---|
| ARTS-DN (2015) [21] | RCT | Finerenone 1.25–20 mg once daily | Placebo | UACR over 90 days | There were reductions in UACR across all doses of Finerenone |
| FIDELIO-DKD (2020) [22] | RCT | Finerenone 10 or 20 mg once daily | Placebo | Kidney composite | Finerenone reduced the risk of CKD progression by 18% |
| FIGARO-DKD (2021) [23] | RCT | Finerenone 10 or 20 mg once daily | Placebo | Cardiovascular composite | Finerenone reduced the risk of composite CV events by 13% |
| The FIDELITY analysis (2022) [24] | Pooled analysis of FIDELIO-DKD and FIGARO-DKD | Finerenone 10 or 20 mg once daily | Finerenone + SGLT2i | Cardiovascular composite and kidney composite | Finerenone without an SGLT2i was equally as effective in reducing cardiorenal outcomes as Finerenone with SGLT2i |
RCT = randomized controlled trial; SGLT2i = sodium–glucose transport protein 2 inhibitor; UACR = urine albumin–creatinine ratio; CKD = chronic kidney disease; CV = cardiovascular.
3.3. Blood Pressure
In 2023, the Effect of Finerenone on Ambulatory Blood Pressure in Chronic Kidney Disease in Type 2 Diabetes (ARTS-DN ABPM) subanalysis explored the effects of Finerenone on ambulatory blood pressure in the ARTS-DN trial. By day 90, Finerenone had led to reductions in the 24 h systolic blood pressure of −8.3 mmHg, −11.2 mmHg, and −9.9 mmHg at doses of 10 mg, 15 mg, and 20 mg, respectively. The study demonstrated consistent blood pressure improvements across all doses, despite Finerenone’s once-daily dosing and short half-life, with no incidents of hypotension reported [25].
3.4. Heart Failure
Finerenone has emerged as a promising alternative to traditional MRAs for managing heart failure, particularly because it lacks the unwanted side effects of non-steroidal MRAs. The ARTS study (2013) was the first to evaluate Finerenone in HFrEF patients. It showed that Finerenone at doses of 5 and 10 mg daily reduced biomarkers like BNP, NT-proBNP, and albuminuria as effectively, or better than, spironolactone, with fewer incidents of hyperkalemia and less renal function decline, suggesting a superior safety profile [26]. Compared with eplerenone, considered the safer alternative to spironolactone, Finerenone was similarly effective in reducing NT-proBNP but demonstrated a safer clinical profile, placing it at the forefront of MRAs for safety, as demonstrated in the AA randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease (ARTS-HF) trial [27].
The FIGARO-DKD trial further supported Finerenone’s benefits in patients with chronic kidney disease and type 2 diabetes, showing an 18% reduction in the risk of cardiovascular death or first heart failure hospitalization and a 29% reduction in first hospitalization for heart failure [28]. The FIDELITY-DKD analysis, which pooled the FIGARO-DKD and FIDELIO-DKD studies, confirmed Finerenone’s efficacy in reducing cardiovascular events, particularly heart failure hospitalizations, and showed beneficial kidney outcomes, reinforcing the importance of early treatment in this patient population [29]. Another pooled analysis of these studies further demonstrated Finerenone’s effectiveness in reducing cardiovascular risk, especially for heart failure, across varying severities of chronic kidney disease and levels of albuminuria [30]. Finerenone has thus proven to be a safer and more efficacious alternative to traditional MRAs for HFrEF, but its role in patients with mildly reduced or preserved systolic function remains to be fully explored.
The Finerenone in Heart Failure with Mildly Reduced or Preserved Ejection Fraction (FINEARTS-HF) trial, completed in August 2024, enrolled patients with heart failure with mildly reduced ejection fraction (HFmrEF) and HFpEF. The trial found that Finerenone significantly reduced heart failure events, primarily by decreasing recurrent hospitalizations or urgent visits, although it did not achieve a statistically significant reduction in cardiovascular mortality [31]. A subgroup analysis divided patients between an LVEF < 60% and >60% without distinguishing between preserved and mildly reduced ejection fraction at the 50% cutoff. While 36.5% of patients in the Finerenone group and 36% in the control group had an LVEF < 50%, fitting the mildly reduced group, there was no separate subgroup analysis for this category. The inconsistency in reporting LVEF baselines but not consistently applying these cutoffs to outcomes could lead to misinterpretations. Nonetheless, significant benefits were seen within the <60% group, reinforcing possible positive effects on HFmrEF patients. For those with an LVEF > 60%, no significant difference from placebo was observed in reducing cardiovascular outcomes, suggesting a need for further analyses specifically for HFpEF. Importantly, Finerenone’s benefits were consistent regardless of whether patients were on SGLT2 inhibitors or not, which are strongly recommended in this population.
A large pooled analysis of the FIDELIO-DKD, FIGARO-DKD, and FINEARTS-HF trials demonstrated reductions in heart failure hospitalization and composite kidney outcomes, with a notable reduction in all-cause mortality, although the reduction in cardiovascular death did not reach statistical significance [32]. Overall, Finerenone has shown consistent efficacy and safety across various heart failure phenotypes and comorbid conditions, particularly in reducing heart failure-related events and hospitalizations, establishing its role in the comprehensive management of cardiovascular and renal outcomes in patients with heart failure. A summary of the studies that examined Finerenone in patients with heart failure can be seen in Table 2.
Table 2.
Summary of randomized controlled trials of Finerenone on heart failure.
| Study (Year) | Design | Population | Intervention vs. Control | Primary Outcome | Result |
|---|---|---|---|---|---|
| ARTS part A and B (2013) [26] | RCT | HFrEF and CKD | Finerenone 2.5–10 mg once daily vs. placebo (part A) and spironolactone (part B) | Change in serum potassium | Finerenone 10 mg once daily and 5 mg twice daily led to higher mean increases in serum potassium than the placebo but lower levels than spironolactone. |
| ARTS-HF (2016) [27] | RCT | HFrEF and T2D and/or CKD | Finerenone 2.5–15 mg once daily vs. eplerenone 25–50 mg once daily | >30% decline in NT-proBNP | There was no difference in NT-proBNP decline in Finerenone versus eplerenone. |
| Filippatos et al., 2022 [28] | Post hoc | Finerenone 10 or 20 mg once daily vs. placebo | First HHF, the combined risk of cardiovascular death or first HHF, and the risk of new-onset heart failure. | Finerenone had a 29% reduction in first HHF, 18% in cardiovascular death or first HHF, and 32% in new-onset HF. | |
| Agarwal et al. (2022) [29] | Post hoc | CKD and T2D | Finerenone 10 or 20 mg once daily vs. placebo | Cardiovascular and renal outcomes | Finerenone reduced the risk of cardiovascular and kidney outcomes vs. placebo across the spectrum of CKD in patients with type 2 diabetes. |
| Agarwal et al. (2023) [30] | Post hoc | CKD and T2D | Finerenone 10 or 20 mg once daily vs. placebo | Incidence rates of cardiovascular events | Finerenone was associated with a reduction in composite cardiovascular risk irrespective of eGFR and UACR. |
| FINEARTS-HF (2024) [31] | RCT | HFmrEF and HFpEF | Finerenone 20 or 40 mg once daily vs. placebo | Composite of total worsening heart failure events and death from cardiovascular causes | Finerenone resulted in a lower rate of total worsening heart failure events and death from cardiovascular causes than the placebo. |
| FINE-HEART (2024) [32] | Pooled analysis of FIGARO-DKD, FIDELIO-DKD, and FINEARTS-HF trials | - | Finerenone 20 or 40 mg once daily vs. placebo | Cardiorenal outcomes | Failed to demonstrate significant reductions in cardiovascular death; however, Finerenone was associated with significantly lower deaths of any cause, cardiovascular events, and kidney outcomes |
RCT = randomized controlled trial; HFrEF = heart failure with reduced ejection fraction; CKD = chronic kidney disease; T2D = type 2 diabetes; NT-proBNP = N-terminal pro B-type natriuretic peptide; HHF = hospitalization for heart failure; HF = heart failure; HFmrEF = heart failure with mildly reduced ejection fraction; HFpEF = heart failure with preserved ejection fraction; eGFR = estimated glomerular filtration rate; UACR = urine albumin-to-creatinine ratio.
3.5. Atrial Fibrillation
Filippatos et al. conducted a secondary analysis of the FIDELIO-DKD trial. Among these, 461 patients had a history of atrial fibrillation (AFib) or flutter. During the study, new-onset AFib occurred in 82 patients treated with Finerenone and 117 patients in the placebo group. The same composite kidney and cardiovascular outcomes were assessed. The analysis revealed that Finerenone’s effect on primary and key secondary kidney and cardiovascular outcomes was not significantly influenced by baseline AFib or flutter. Importantly, Finerenone was associated with a reduced risk of new-onset AFib or flutter, and it demonstrated a consistent reduction in the risk of kidney or cardiovascular disease, regardless of the AFib status at baseline [33].
3.6. Ethnicity and Sex Differences
Recent studies have examined the effects of Finerenone on cardiovascular and kidney outcomes across ethnicity and gender. Koya et al. (2023) found that Finerenone lowered cardiovascular event rates in Asians, with 8.6% in the Finerenone group versus 10.0% in the placebo group, and the results were consistent across Asian and non-Asian populations [34]. Bansal et al. (2024) noted that Finerenone’s effect on heart failure hospitalizations was stronger in males, and it reduced kidney events in patients under 75. The drug consistently reduced albuminuria and eGFR decline regardless of sex or age, and gynecomastia was uncommon in males, underscoring its broad efficacy and safety [35].
3.7. Head-to-Head Studies with SGLT-2s, GLP-1 RAs, and MRAs
Finerenone’s role in cardiovascular care is emerging through comparisons with traditional MRAs like spironolactone and eplerenone, but comparisons with SGLT2i and glucagon-like peptide-1 receptor agonists (GLP-1 RAs) remain indirect. In HFrEF patients, the ARTS study demonstrated that Finerenone at 5 and 10 mg doses matched or exceeded spironolactone in reducing BNP, NT-proBNP, and albuminuria levels, with fewer incidents of increases in serum potassium and lower rates of hyperkalemia, suggesting a favorable safety profile [26]. When compared with eplerenone in HFrEF patients, the ARTS-HF study found similar reductions in proBNP levels between the two drugs, but also hinted at potential benefits of Finerenone in reducing a composite outcome of death, cardiovascular hospitalizations, or emergency presentations, although this finding was exploratory [27]. Direct head-to-head studies comparing Finerenone with SGLT2i and GLP-1 RAs are lacking. However, a network meta-analysis in patients with T2DM and CKD indicated that SGLT2 inhibitors were superior in reducing renal outcomes and heart failure hospitalizations. While all three drug classes similarly reduced the risk of major adverse cardiovascular events, only SGLT2 inhibitors significantly lowered the cardiovascular death risk, underscoring their superior benefits for renal and heart failure outcomes over Finerenone and GLP-1 RAs [36]. A summary of these studies can be seen in Table 3.
Table 3.
Summary of Finerenone in head-to-head randomized controlled trials.
| Study (Year) | Design | Population | Intervention vs. Control | Primary Outcome | Effect Size |
|---|---|---|---|---|---|
| Zhang et al. (2022) [36] | Network meta-analysis | - | Finerenone vs. SGLT2i and GLP-1 agonist | Cardiorenal outcomes | SGLT2i significantly decreased the risk of renal events and HHF in comparison. All three were comparable in MACE, ACD, and CVD. |
| ARTS part A and B (2013) [26] | RCT | HFrEF and CKD | Finerenone 2.5–10 mg once daily vs. placebo (part A) and spironolactone (part B) | Change in potassium | Finerenone 10 mg once daily and 5 mg twice daily led to higher mean increases in serum potassium than the placebo but lower levels than spironolactone |
| ARTS-HF (2016) [27] | RCT | HFrEF and T2D and/or CKD | Finerenone 2.5–15 mg once daily vs. eplerenone 25–50 mg once daily | >30% decline in NT-proBNP | There was no difference in the NT-proBNP decline in Finerenone versus eplerenone |
RCT = randomized controlled trial; HFrEF = heart failure with reduced ejection fraction; CKD = chronic kidney disease; T2D = type 2 diabetes; SGLT2i = sodium–glucose cotransporter-2 inhibitor; GLP-1 agonist = glucagon-like peptide-1 receptor agonist; NT-proBNP = N-terminal prohormone of brain natriuretic peptide; HHF = hospitalization for heart failure; MACE = major adverse cardiovascular events; ACD = all-cause death; CVD = cardiovascular death.
3.8. Future Studies
Finerenone’s success will be further tested in several upcoming studies, particularly in managing cardiovascular outcomes and heart failure. The FINALITY-HF, CONFIRMATION-HF, and REDEFINE-HF trials aim to position Finerenone as a potential cornerstone in heart failure treatment, potentially replacing traditional MRAs like spironolactone and eplerenone. REDEFINE-HF builds on the findings of the FINEARTS-HF trial by evaluating cardiovascular outcomes in hospitalized patients with HFpEF and HFmrEF [37]. FINALITY-HF will assess the efficacy and safety of Finerenone in patients with heart failure and reduced ejection fraction who are intolerant of or ineligible for steroidal MRAs, potentially establishing it as not just an alternative but a first-line MRA in heart failure treatment [38].
The CONFIDENCE study serves as a strong precursor to CONFIRMATION-HF by exploring the combined effects of Finerenone and empagliflozin on albuminuria reduction in CKD patients with type 2 diabetes, underscoring the potential of combination therapies to enhance both renal and cardiovascular outcomes [39]. CONFIRMATION-HF will further this research by investigating Finerenone combined with SGLT2 inhibitors in a broader HF population, regardless of the LVEF, with the goal of reducing heart failure events and cardiovascular mortality [40]. This could help cement Finerenone’s role as an adjunct to current guideline-directed medical therapies in heart failure.
The FIVE-STAR study contributes to the cardiovascular narrative by focusing on vascular stiffness and cardiorenal biomarkers in patients with type 2 diabetes and CKD, highlighting Finerenone’s potential impact on vascular health [41]. Meanwhile, the FIONA and FINE-ONE studies, though more niche, demonstrate Finerenone’s expanding utility beyond traditional adult populations. FIONA explores its efficacy in pediatric patients with CKD and proteinuria, marking Finerenone’s entry into younger demographics [42]. FIONA-OLE is an extension study of FIONA that will assess the long-term safety (18 months) of Finerenone in the same cohort [42]. Similarly, FINE-ONE investigates its role in type 1 diabetes, indicating Finerenone’s growing application in managing conditions beyond CKD and heart failure [43]. FIND-CKD will look to assess Finerenone’s effect on cardiorenal outcomes in patients with CKD but without diabetes [44].
Collectively, these studies intend to propel Finerenone’s expanding role in cardiovascular care, not only in CKD and diabetes but also in heart failure (Table 4).
Table 4.
Anticipated randomized controlled trials.
| Study | Design | Anticipated Completion Date | Population | Intervention vs. Control | Primary Outcome |
|---|---|---|---|---|---|
| REDEFINE-HF [37] | RCT | April 2026 | Adults with heart failure with an EF > 40% | Finerenone vs. placebo | Composite total of HF events and CV death |
| FINALITY-HF [38] | RCT | August 2026 | HFrEF and intolerant of non steroidal MRAs | Finerenone vs. placebo | Composite total of HF events and CV death |
| CONFIDENCE [39] | RCT | January 2025 | Patients with CKD and T2DM on ACEis or ARBs | Finerenone + empagliflozin vs. placebo + Finerenone or empagliflozin | Urinary albumin to-creatinine ratio (UACR) |
| CONFIRMATION-HF [40] | RCT | November 2025 | Adults with heart failure | Finerenone + empagliflozin vs. standard of care | Clinical benefit |
| FIVE-STAR [41] | RCT | July 2026 | CKD and T2DM | Finerenone vs. placebo | Change in CAVI (Cardio–ankle vascular index) at 24 weeks after initiation of the protocol treatment compared with baseline |
| FIONA [42] | RCT | March 2027 | Children (6 months to 18 years old) | Finerenone + ACEi/ARB vs. placebo | Urinary protein-to-creatinine ratio (UPCR) reduction of at least 30% from baseline to day 180 |
| FIONA-OLE [42] | RCT | 18-month extension of FIONA | Children (6 months to 18 years old) | Finerenone + ACEi/ARB vs. placebo | Long-term safety |
| FINE-ONE [43] | RCT | October 2025 | Adults with T1DM | Finerenone vs. placebo | Change in UACR |
| FIND-CKD [44] | RCT | February 2026 | CKD without diabetes | Finerenone vs. placebo | Change in eGFR from baseline to 32 months |
RCT = randomized controlled trial; EF = ejection fraction; HF = heart failure; CV = cardiovascular; HFrEF = heart failure with reduced ejection fraction; MRA = mineralocorticoid receptor antagonist; CKD = chronic kidney disease; T2DM = type 2 diabetes mellitus; ACEi = angiotensin-converting enzyme inhibitor; ARB = angiotensin II receptor blocker; UACR = urinary albumin-to-creatinine ratio; CAVI = cardio–ankle vascular index; UPCR = urinary protein-to-creatinine ratio; T1DM = type 1 diabetes mellitus; eGFR = estimated glomerular filtration rate.
4. Discussion
Finerenone is a non-steroidal, selective antagonist of the mineralocorticoid receptor specifically developed to counteract the harmful effects of MR overactivation. Its targeted action on the MR distinguishes it from traditional MRAs like spironolactone and eplerenone, which tend to have off-target effects in varying degrees. It reduces cardiovascular damage linked to excessive MR activation while minimizing side effects associated with androgen and progesterone receptor interactions. Currently approved in over 90 countries for the treatment of CKD associated with T2DM, Finerenone’s broader therapeutic potential is being explored through the FINEOVATE research program, which includes several Phase III trials discussed in this review.
Finerenone has shown significant cardiovascular and renal benefits in patients with CKD and T2D, making it a promising alternative to existing MRAs. Current guidelines from the American College of Cardiology (ACC) and the European Society of Cardiology (ESC) recommend MRAs like spironolactone and eplerenone for patients with HFrEF. However, as evidence supporting Finerenone’s broader efficacy and safety in CKD, diabetes, and heart failure continues to grow, these guidelines may evolve to include Finerenone, especially for patients who are intolerant of or less responsive to traditional steroidal MRAs. As HF management continues to evolve, especially for patients with CKD and T2DM, the current guidelines may not fully address the challenges these patients face. Finerenone’s effectiveness and better safety profile in these groups highlight the need for updated guidelines that include newer treatment options, which could improve outcomes for these high-risk patients. Finerenone may also find a unique role in managing HFmrEF and HFpEF, areas where traditional MRAs have limited impact.
In clinical trials, Finerenone has demonstrated similar or superior efficacy in reducing biomarkers like NT-proBNP compared with spironolactone and eplerenone, and it shows fewer adverse effects. Beyond its impact on cardiorenal outcomes, Finerenone has also been shown to effectively lower blood pressure in patients with CKD and T2D, with significant reductions in systolic blood pressure reported in the ARTS-DN trial. Additionally, secondary analyses from the FIGARO-DKD trial suggest that Finerenone may reduce the incidence of new-onset atrial fibrillation, setting it apart from other MRAs. Finerenone’s ability to reduce proteinuria and slow the progression of CKD further establishes its role as an essential therapeutic option in the management of diabetes, complementing its cardiovascular benefits in heart failure patients.
Finerenone’s safety profile is another key advantage, as it is associated with a lower incidence of hyperkalemia compared with spironolactone and demonstrates a more favorable renal safety profile, with fewer instances of acute kidney injury (AKI). Unlike steroidal MRAs, Finerenone does not cause anti-androgenic side effects such as gynecomastia, impotence, and menstrual irregularities. The ARTS-HF study further supports Finerenone’s safety, showing fewer increases in serum potassium and less deterioration of renal function compared with eplerenone, making it an even safer alternative for patients who cannot tolerate spironolactone.
In the broader context of heart failure treatment, Finerenone’s role among established therapies remains under investigation. While SGLT2 inhibitors and GLP-1 RAs have demonstrated significant cardiovascular and renal benefits in CKD and T2D populations, network meta-analyses indicate that SGLT2 inhibitors outperform both Finerenone and GLP-1 RAs in reducing renal outcomes and heart failure hospitalizations [34]. Although all three classes reduce major adverse cardiovascular events, only SGLT2 inhibitors have shown a significant impact in reducing cardiovascular death. Finerenone may find its niche in combination therapy, as ongoing trials like CONFIDENCE and CONFIRMATION explore the potential synergistic effects of combining Finerenone with SGLT2 inhibitors, potentially broadening its therapeutic applications and enhancing patient outcomes.
This positioning of Finerenone reflects its evolving role in comprehensive cardiovascular and renal management, with future studies poised to further clarify its standing alongside, or in conjunction with, other established therapies. We provided a table that summarizes the comparisons of Finerenone to steroidal MRAs with respect to various characteristics and side effects (Table 5).
Table 5.
Comparison of the characteristics and effects of MRAs.
| Steroidal MRA | Non-Steroidal MRA | ||
|---|---|---|---|
| Characteristics | Spironolactone | Eplerenone | Finerenone |
| Antagonism over MR | High | Low | High |
| Antagonism over AR | High | Low | Low |
| Effect on proteinuria and kidney damage | Moderate | Low | High |
| BP reduction | High | Low | High |
| Effect on HFpEF | Moderate | Moderate | High |
| Effect on HFmrEF | Moderate | Moderate | High |
| Effect on HFrEF | High | High | High |
| Hyperkalemia | High | Moderate | Low |
| Gynecomastia | High | Moderate | Low |
MRA = mineralocorticoid receptor antagonist; MR = mineralocorticoid receptor; AR = androgen receptor; BP = blood pressure; HFpEF = heart failure with preserved ejection fraction; HFmrEF = heart failure with moderately reduced ejection fraction; HFrEF = heart failure with reduced ejection fraction.
5. Conclusions
Finerenone represents a significant advancement in the management of heart failure, CKD, and T2D, offering a clearly better side-effect profile compared with traditional MRAs, especially in HFrEF, where it demonstrates superior safety and potential efficacy. While the early data suggest possible benefits in patients with HFmrEF, its role in HFpEF remains less certain and requires further investigation. More randomized controlled trials and subgroup analyses, particularly from the FINEART-HF trial, are necessary to better assess outcomes in specific groups. Additionally, new RCTs that focus specifically on these populations, ideally in a head-to-head format, are essential to solidify Finerenone’s position in cardiovascular and renal care and to determine its full therapeutic potential across different heart failure phenotypes.
Author Contributions
Conceptualization, M.S.; methodology, M.S.; software, M.S.; validation, M.S., J.T. and S.S.; formal analysis, M.S.; investigation, M.S.; resources, M.S.; data curation, M.S.; writing—original draft preparation, M.S.; writing—review and editing, M.S., J.T., S.S., A.L., M.G. and A.B.; visualization, M.S.; supervision, M.S.; project administration, M.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. As this is a scoping review involving publicly available data, no Institutional Review Board (IRB) approval was required.
Informed Consent Statement
Not applicable, as this study did not involve human participants.
Data Availability Statement
In this scoping review, all data used were sourced from publicly accessible online databases, and no new data were generated. Consequently, no additional materials, data collection forms, or analytic code are available beyond what is already publicly accessible. All referenced data can be accessed directly through the original sources as cited in the review.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Jr., Colvin M.M., Drazner M.H., Filippatos G.S., Fonarow G.C., Givertz M.M., et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2017;70:776–803. doi: 10.1016/j.jacc.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 2.McMurray J.J., Adamopoulos S., Anker S.D., Auricchio A., Böhm M., Dickstein K., Falk V., Filippatos G., Fonseca C., Gomez-Sanchez M.A., et al. ESC Committee for Practice Guidelines. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 3.Young D.B., Smith M.J., Jr., Jackson T.E., Scott R.E. Multiplicative interaction between angiotensin II and K concentration in stim-ulation of aldosterone. Am. J. Physiol. 1984;247:E328. doi: 10.1152/ajpendo.1984.247.3.E328. [DOI] [PubMed] [Google Scholar]
- 4.Ghose R.P., Hall P.M., Bravo E.L. Medical management of aldosterone-producing adenomas. Ann. Intern. Med. 1999;131:105–108. doi: 10.7326/0003-4819-131-2-199907200-00005. [DOI] [PubMed] [Google Scholar]
- 5.Levac D., Colquhoun H., O’Brien K.K. Scoping studies: Advancing the methodology. Implement. Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mizuno Y., Yoshimura M., Yasue H., Sakamoto T., Ogawa H., Kugiyama K., Harada E., Nakayama M., Nakamura S., Ito T., et al. Aldosterone production is activated in failing ventricle in humans. Circulation. 2001;103:72–77. doi: 10.1161/01.CIR.103.1.72. [DOI] [PubMed] [Google Scholar]
- 8.Lijnen P., Petrov V. Induction of cardiac fibrosis by aldosterone. J. Mol. Cell. Cardiol. 2000;32:865–879. doi: 10.1006/jmcc.2000.1129. [DOI] [PubMed] [Google Scholar]
- 9.Cooper H.A., Dries D.L., Davis C.E., Shen Y.L., Domanski M.J. Diuretics and risk of arrhythmic death in patients with left ventricular dysfunction. Circulation. 1999;100:1311–1315. doi: 10.1161/01.CIR.100.12.1311. [DOI] [PubMed] [Google Scholar]
- 10.Pitt B., Remme W., Zannad F., Neaton J., Martinez F., Roniker B., Bittman R., Hurley S., Kleiman J., Gatlin M. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N. Engl. J. Med. 2003;348:1309–1321. doi: 10.1056/NEJMoa030207. [DOI] [PubMed] [Google Scholar]
- 11.Zannad F., McMurray J.J., Krum H., van Veldhuisen D.J., Swedberg K., Shi H., Vincent J., Pocock S.J., Pitt B. Eplerenone in patients with systolic heart failure and mild symptoms. N. Engl. J. Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 12.Pitt B., Zannad F., Remme W.J., Cody R., Castaigne A., Perez A., Palensky J., Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N. Engl. J. Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 13.Milliez P., Girerd X., Plouin P.F., Blacher J., Safar M.E., Mourad J.-J. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J. Am. Coll. Cardiol. 2005;45:1243. doi: 10.1016/j.jacc.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 14.Jorde U.P., Vittorio T., Katz S.D., Colombo P.C., Latif F., Le Jemtel T.H. Elevated plasma aldosterone levels despite complete inhibition of the vascular angioten-sin-converting enzyme in chronic heart failure. Circulation. 2002;106:1055. doi: 10.1161/01.CIR.0000030935.89559.04. [DOI] [PubMed] [Google Scholar]
- 15.Lim P.O., Young W.F., MacDonald T.M. A review of the medical treatment of primary aldosteronism. J. Hypertens. 2001;19:353–361. doi: 10.1097/00004872-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Layton A.M., Eady E.A., Whitehouse H., Del Rosso J.Q., Fedorowicz Z., van Zuuren E.J. Oral Spironolactone for Acne Vulgaris in Adult Females: A Hybrid Systematic Review. Am. J. Clin. Dermatol. 2017;18:169–191. doi: 10.1007/s40257-016-0245-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Struthers A., Krum H., Williams G.H. A Comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin. Cardiol. 2008;31:153–158. doi: 10.1002/clc.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Collins K.J., Dennison Himmelfarb C., DePalma S.M., Gidding S., Jamerson K.A., Jones D.W., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Pre-vention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2018;71:e127–e248. doi: 10.1016/j.jacc.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 19.Parthasarathy H.K., Ménard J., White W.B., Young W.F., Williams G.H., Williams B., Ruilope L.M., McInnes G.T., Connell J.M., MacDonald T.M. A double-blind, randomized study comparing the antihypertensive effect of eplerenone and spironolactone in patients with hypertension and evidence of primary aldosteronism. J. Hypertens. 2011;29:980–990. doi: 10.1097/HJH.0b013e3283455ca5. [DOI] [PubMed] [Google Scholar]
- 20.Fagart J., Hillisch A., Huyet J., Bärfacker L., Fay M., Pleiss U., Pook E., Schäfer S., Rafestin-Oblin M.-E., Kolkhof P. A new mode of mineralocorticoid receptor antagonism by a potent and selective nonsteroidal molecule. J. Biol. Chem. 2010;285:29932–29940. doi: 10.1074/jbc.M110.131342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bakris G.L., Agarwal R., Chan J.C., Cooper M.E., Gansevoort R.T., Haller H., Remuzzi G., Rossing P., Schmieder R.E., Nowack C., et al. Effect of Finerenone on Albuminuria in Patients with Diabetic Nephropathy: A Randomized Clinical Trial. JAMA. 2015;314:884–894. doi: 10.1001/jama.2015.10081. [DOI] [PubMed] [Google Scholar]
- 22.Bakris G.L., Agarwal R., Anker S.D., Pitt B., Ruilope L.M., Rossing P., Kolkhof P., Nowack C., Schloemer P., Joseph A., et al. Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2020;383:2219–2229. doi: 10.1056/NEJMoa2025845. [DOI] [PubMed] [Google Scholar]
- 23.Pitt B., Filippatos G., Agarwal R., Anker S.D., Bakris G.L., Rossing P., Joseph A., Kolkhof P., Nowack C., Schloemer P., et al. Cardiovascular Events with Finerenone in Kidney Disease and Type 2 Diabetes. N. Engl. J. Med. 2021;385:2252–2263. doi: 10.1056/NEJMoa2110956. [DOI] [PubMed] [Google Scholar]
- 24.Rossing P., Anker S.D., Filippatos G., Pitt B., Ruilope L.M., Birkenfeld A.L., McGill J.B., Rosas S.E., Joseph A., Gebel M., et al. Finerenone in Patients with Chronic Kidney Disease and Type 2 Diabetes by Sodium–Glucose Cotransporter 2 Inhibitor Treatment: The FIDELITY Analysis. Diabetes Care. 2022;45:2991–2998. doi: 10.2337/dc22-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agarwal R., Ruilope L.M., Ruiz-Hurtado G., Haller H., Schmieder R.E., Anker S.D., Filippatos G., Pitt B., Rossing P., Lambelet M., et al. Effect of finerenone on ambulatory blood pressure in chronic kidney disease in type 2 diabetes. J. Hypertens. 2022;41:295–302. doi: 10.1097/HJH.0000000000003330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pitt B., Kober L., Ponikowski P., Gheorghiade M., Filippatos G., Krum H., Nowack C., Kolkhof P., Kim S.-Y., Zannad F. Safety and tolerability of the novel non-steroidal mineralocorticoid receptor antagonist BAY 94-8862 in patients with chronic heart failure and mild or moderate chronic kidney disease: A randomized, double-blind trial. Eur. Heart J. 2013;34:2453–2463. doi: 10.1093/eurheartj/eht187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filippatos G., Anker S.D., Böhm M., Gheorghiade M., Køber L., Krum H., Maggioni A.P., Ponikowski P., Voors A.A., Zannad F., et al. A randomized controlled study of finerenone vs. eplerenone in patients with worsening chronic heart failure and diabetes mellitus and/or chronic kidney disease. Eur. Heart J. 2016;37:2105–2114. doi: 10.1093/eurheartj/ehw132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filippatos G., Anker S.D., Agarwal R., Ruilope L.M., Rossing P., Bakris G.L., Tasto C., Joseph A., Kolkhof P., Lage A., et al. Finerenone Reduces Risk of Incident Heart Failure in Patients with Chronic Kidney Disease and Type 2 Diabetes: Analyses From the FIGARO-DKD Trial. Circulation. 2022;145:437–447. doi: 10.1161/CIRCULATIONAHA.121.057983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal R., Filippatos G., Pitt B., Anker S.D., Rossing P., Joseph A., Kolkhof P., Nowack C., Gebel M., Ruilope L.M., et al. Cardiovascular and kidney outcomes with Finerenone in patients with type 2 diabetes and chronic kidney disease: The FIDELITY pooled analysis. Eur. Heart J. 2022;43:474–484. doi: 10.1093/eurheartj/ehab777. Correction in Eur. Heart J. 2022, 43, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agarwal R., Pitt B., Rossing P., Anker S.D., Filippatos G., Ruilope L.M., Kovesdy C.P., Tuttle K., Vaduganathan M., Wanner C., et al. Modifiability of Composite Cardiovascular Risk Associated with Chronic Kidney Disease in Type 2 Diabetes with Finerenone. JAMA Cardiol. 2023;8:732–741. doi: 10.1001/jamacardio.2023.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Solomon S.D., McMurray J.J., Vaduganathan M., Claggett B., Jhund P.S., Desai A.S., Henderson A.D., Lam C.S., Pitt B., Senni M., et al. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N. Engl. J. Med. 2024;391:789–799. doi: 10.1056/NEJMoa2407107. [DOI] [PubMed] [Google Scholar]
- 32.Vaduganathan M., Filippatos G., Claggett B.L., Desai A.S., Jhund P.S., Henderson A., Brinker M., Kolkhof P., Schloemer P., Lay-Flurrie J., et al. Finerenone in Heart Failure and Chronic Kidney Disease with Type 2 Diabetes: The FINE-HEART pooled analysis of cardi-ovascular, kidney, and mortality outcomes. Nat. Med. 2024 doi: 10.1038/s41591-024-03264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filippatos G., Bakris G.L., Pitt B., Agarwal R., Rossing P., Ruilope L.M., Butler J., Lam C.S., Kolkhof P., Roberts L., et al. Finerenone Reduces New-Onset Atrial Fibrillation in Patients with Chronic Kidney Disease and Type 2 Diabetes. J. Am. Coll. Cardiol. 2021;78:142–152. doi: 10.1016/j.jacc.2021.04.079. [DOI] [PubMed] [Google Scholar]
- 34.Koya D., Anker S.D., Ruilope L.M., Rossing P., Liu Z., Lee B.W., Lee C.-T., Scott C., Kolkhof P., Lawatscheck R., et al. Cardiorenal Outcomes with Finerenone in Asian Patients with Chronic Kidney Disease and Type 2 Diabetes: A FIDELIO-DKD post hoc Analysis. Am. J. Nephrol. 2023;54:370–378. doi: 10.1159/000532102. [DOI] [PubMed] [Google Scholar]
- 35.Bansal S., Canziani M.E.F., Birne R., Anker S.D., Bakris G.L., Filippatos G., Rossing P., Ruilope L.M., Farjat A.E., Kolkhof P., et al. Finerenone cardiovascular and kidney outcomes by age and sex: FIDELITY post hoc analysis of two phase 3, multicentre, double-blind trials. BMJ Open. 2024;14:e076444. doi: 10.1136/bmjopen-2023-076444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y., Jiang L., Wang J., Wang T., Chien C., Huang W., Fu X., Xiao Y., Fu Q., Wang S., et al. Network meta-analysis on the effects of finerenone versus SGLT2 inhibitors and GLP-1 receptor agonists on cardiovascular and renal outcomes in patients with type 2 diabetes mellitus and chronic kidney disease. Cardiovasc. Diabetol. 2022;21:232. doi: 10.1186/s12933-022-01676-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Randomized Trial to Determine the Efficacy and Safety of Finerenone on Morbidity and Mortality Among Heart Failure Patients with Left Ventricular Ejection Fraction Greater than or Equal to 40% Hospitalized Due to an Episode of Acute Decompensated Heart Failure (REDEFINE-HF) [(accessed on 1 August 2024)]; ClinicalTrials.gov Identifier: NCT06008197. EudraCT: 2023-508581-15-00. Available online: https://clinicaltrials.gov/ct2/show/NCT06008197.
- 38.Colorado Prevention Center A Study to Evaluate Finerenone on Clinical Efficacy and Safety in Patients with Heart Failure Who Are Intolerant or Not Eligible for Treatment with Steroidal Mineralocorticoid Receptor Antagonists (FINALITY-HF) [(accessed on 1 August 2024)]; ClinicalTri-als.gov Identifier: NCT06033950. Updated 17 April 2024. Available online: https://clinicaltrials.gov/ct2/show/NCT06033950.
- 39.Green J.B., Green J.B., Mottl A.K., Mottl A.K., Bakris G., Bakris G., Heerspink H.J.L., Heerspink H.J.L., Mann J.F.E., Mann J.F.E., et al. Design of the Combination effect of Finerenone and Empagliflozin in participants with chronic kidney disease and type 2 diabetes using a UACR Endpoint study (CONFIDENCE) Nephrol. Dial. Transplant. 2023;38:894–903. doi: 10.1093/ndt/gfac198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colorado Prevention Center A Study to Determine the Efficacy and Safety of Finerenone and SGLT2i in Combination in Hos-pitalized Patients with Heart Failure (CONFIRMATION-HF) (CONFIRMATION) [(accessed on 1 August 2024)]; ClinicalTrials.gov Identifier: NCT06024746. Updated 22 July 2024. Available online: https://clinicaltrials.gov/ct2/show/NCT06024746.
- 41.Tanaka A., Shibata H., Imai T., Yoshida H., Miyazono M., Takahashi N., Fukuda D., Okada Y., Teragawa H., Suwa S., et al. Rationale and design of an investigator-initiated, multicenter, prospective, placebo-controlled, double-blind, randomized trial to evaluate the effects of finerenone on vascular stiffness and cardiorenal biomarkers in type 2 diabetes and chronic kidney disease (FIVE-STAR) Cardiovasc. Diabetol. 2023;22:194. doi: 10.1186/s12933-023-01928-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schaefer F., Montini G., Kang H.G., Walle J.V., Zaritsky J., Schreuder M.F., Litwin M., Scalise A., Scott H., Potts J., et al. Investigating the use of finerenone in children with chronic kidney disease and proteinuria: Design of the FIONA and open-label extension studies. Trials. 2024;25:203. doi: 10.1186/s13063-024-08021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heerspink H.J., Birkenfeld A.L., Cherney D.Z., Colhoun H.M., Ji L., Mathieu C., Groop P.-H., Pratley R.E., Rosas S.E., Rossing P., et al. Rationale and design of a randomised phase III registration trial investigating finerenone in participants with type 1 diabetes and chronic kidney disease: The FINE-ONE trial. Diabetes Res. Clin. Pract. 2023;204:110908. doi: 10.1016/j.diabres.2023.110908. [DOI] [PubMed] [Google Scholar]
- 44.Heerspink H.J.L., Agarwal R., Bakris G.L., Cherney D.Z.I., Lam C.S.P., Neuen B.L., Sarafidis P.A., Tuttle K.R., Wanner C., Brinker M.D., et al. Design and baseline characteristics of the Finerenone, in addition to standard of care, on the progression of kidney disease in patients with Non-Diabetic Chronic Kidney Disease (FIND-CKD) randomized trial. Nephrol. Dial. Transplant. 2024:gfae132. doi: 10.1093/ndt/gfae132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
In this scoping review, all data used were sourced from publicly accessible online databases, and no new data were generated. Consequently, no additional materials, data collection forms, or analytic code are available beyond what is already publicly accessible. All referenced data can be accessed directly through the original sources as cited in the review.