Abstract
A convenient and effective synthesis of imidazo[1,2-a]pyrimidine derivatives has been developed under microwave irradiations using Al2O3 as a catalyst in solvent-free conditions. The functionalized imidazo[1,2-a]pyrimidine derivatives are useful in biochemistry and medical science. In our investigation, the antimicrobial activity of the synthesized compounds was evaluated against 13 microorganisms, including 6 Gram-positive bacteria, 4 Gram-negative bacteria, and 3 pathogenic fungi. Bioactivity tests revealed that the majority of the compounds exhibited good antimicrobial activity. Finally, molecular docking simulations and ADME-T predictions were performed, showing that the most active compounds have good binding modes with microbial targets and promising pharmacokinetic safety profiles.
Keywords: imidazo[1,2-a]pyrimidine; microwave; Al2O3 catalyst; solvent free; antimicrobial activity; in silico study
1. Introduction
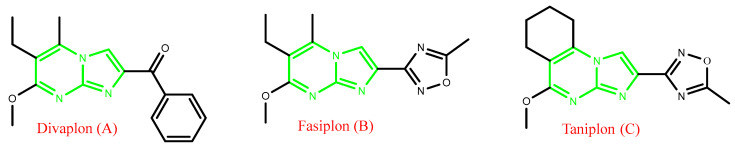
Aza-heterocycles have found their outstanding place among bioactive products due to their important functions in the molecular forms of life. They are considered as an essential type of bio-organic molecules playing a particular role among a variety of significant natural and medicinal products [1,2]. As one of the aromatic heterocyclic rings and imidazopyrimidines, they show pharmaceutical properties of interest because of their important therapeutic activities [3]. Imidazo[1,2-a]pyrimidines are a heterocyclic scaffold of great interest because they exhibit a variety of physiological properties, such as antioxidant [4], anti-inflammatory [5], anxiolytic [6], anticonvulsant [7], benzodiazepine receptor agonist [8], calcium channel blocker [9], antitubercular [10], anticancer [11], antimalarial [12], antiviral [13], antimicrobial [14], and antifungal effects [15]. Moreover, imidazo[1,2-a]pyrimidines are found in various drugs as a basic unit of commercially accessible compound libraries, for example, divaplon, fasiplon, and taniplon (Figure 1) [16]. This is possibly a result of their exclusive physicochemical characteristics and their similarity to natural substrates like purines [17]. Imidazo[1,2-a]pyrimidine heterocycles exhibit significant biological activities. Their structural analogy to purine heterocycles and the guanidine functionality suggest multiple possible biological activities. Nevertheless, a deeper investigation is important to fully evaluate the biological effectiveness and range of this bicyclic framework, particularly in the area of antimicrobial research [14].
Figure 1.
Pharmacologically active drugs holding imidazo[1,2-a]pyrimidine scaffolds.
Antimicrobial resistance occurs when viruses, bacteria, and fungi do not respond effectively to drugs anymore. It complicates the treatment of infection, increases the risk of disease transmission, and may even cause death [18]. It is not surprising, therefore, that considerable efforts have been directed toward developing synthetic approaches for constructing various antimicrobial compounds. As a part of our continuous works [19,20,21,22,23,24,25], we herein aim to report a new synthesis of imidazo[1,2-a]pyrimidine derivatives from 2-aminopyrimidine and α-bromoketones. The reaction was activated with an aluminum-based catalyst, irradiated by a domestic microwave under solvent-free conditions, marking the first synthesis of 2-arylimidazo[1,2-a]pyrimidines utilizing Al2O3 as a catalyst. To the best of our knowledge, compounds 3e and 3k are novel derivatives of the imidazo[1,2-a]pyrimidine family, characterized by the presence of a methoxy group in the ortho position and two methyl groups at the meta and para positions of the benzene ring, respectively. The antimicrobial activity of synthesized heterocyclic compounds was examined by in vitro and in silico studies.
The design, manufacturing, and synthesis of compounds valuable as human therapeutic molecules are one of the most important goals of medicinal chemistry and organic synthesis [26,27]. For this purpose, and to study their antimicrobial inhibitory activities, eleven imidazo[1,2-a]pyrimidines were synthesized under microwave irradiation catalyzed by an aluminum-based catalyst. The in vitro antimicrobial inhibitory activities of all prepared heterocyclic compounds were evaluated. Moreover, the inhibitory mechanism of some imidazo[1,2-a]pyrimidine derivatives was investigated through molecular docking and enzyme kinetic studies. Our study can provide lead molecules for the search and expansion of natural compounds based on antimicrobial candidates.
2. Results and Discussion
2.1. Chemistry
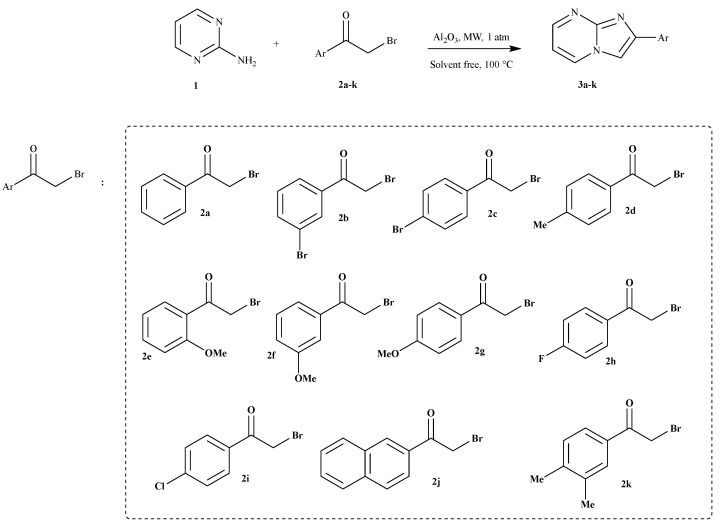
As shown in Scheme 1, imidazo[1,2-a]pyrimidines 3a–k were synthesized by a condensation reaction of 2-aminopyrimidine and several 2-bromoarylketones without solvent. They were catalyzed by basic alumina (Al2O3) and irradiated using a domestic microwave.
Scheme 1.
Synthesis of imidazo[1,2-a]pyrimidine derivatives 3a–k.
To optimize the synthesis of 2-(4-chlorophenyl)imidazo[1,2-a]pyrimidine, the amount of catalyst was varied to assess its impact on reaction efficiency. Table 1 summarizes the results of this optimization, highlighting the relationship between the amount of Al2O3 and the corresponding yields.
Table 1.
Influence of catalyst load on the imidazo[1,2-a]pyrimidine synthesis.

| |||
| Entry | Catalyst (% w/w) | Yield (%) | Reaction Time (s) |
|---|---|---|---|
| 1 | 10 | 26 | 150 |
| 2 | 20 | 45 | 120 |
| 3 | 30 | 65 | 90 |
| 4 | 40 | 63 | 90 |
| 5 | 50 | 34 | 120 |
With these results in hand, the scope of the reaction was then studied (Table 2). This protocol was found suitable for a range of 2-bromoketones bearing electron-rich (Me or MeO) or electron-withdrawing groups (F or Br) on the ortho, para, or meta position of the benzene ring and proceeded smoothly, affording the corresponding products in 52–68% yields. It should be noted that unsubstituted 2-phenylimidazo[1,2-a]pyrimidine and the 2-naphtylimidazo[1,2-a]pyrimidine were also suitable for this transformation, and satisfactory yields (70 and 67%) were, respectively, obtained. The 3,4-dimethyl-substituted substrate worked well and provided a product with a 64% yield. In this study, we found that basic Al2O3 is an environmentally friendly catalytic system for the synthesis of imidazo[1,2-a]pyrimidine derivatives with good catalytic efficiency.
Table 2.
Structure and physical parameters of the synthesized imidazo[1,2-a]pyrimidine derivatives.
| Entry | Structure | Yield (%) | Yield (Ref %) | Melting Point (°C) | Melting Point (Ref °C) | Reaction Time (s) |
|---|---|---|---|---|---|---|
| 3a |
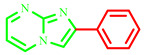
|
70 | 80 [28] | 195–197 | 190–192 [28] | 240 |
| 3b |
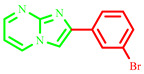
|
56 | 80 [18] | 191–193 | 220 [18] | 300 |
| 3c |

|
63 | 94 [9] | 209–211 | 212–214 [9] | 90 |
| 3d |
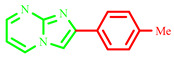
|
68 | 75 [28] | 229–231 | 236–238 [28] | 150 |
| 3e |

|
57 | ----- | 264–266 | Not described | 200 |
| 3f |
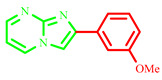
|
53 | 90 [9] | 219–221 | 222–224 [9] | 200 |
| 3g |
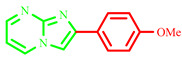
|
52 | 70 [28] | 189–191 | 188–190 [28] | 200 |
| 3h |
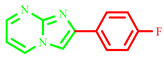
|
63 | 85 [28] | 230–232 | 236–238 [28] | 110 |
| 3i |
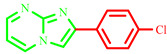
|
65 | 83 [10] | 273–275 | 267–269 [10] | 90 |
| 3j |
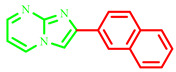
|
67 | 91 [10] | 249–251 | 276–278 [10] | 220 |
| 3k |
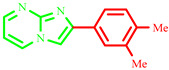
|
64 | ----- | 231–233 | Not described | 180 |
Despite the advantages of the use of Al2O3 as a catalyst like low toxicity, high surface area, and thermal stability, it led to lower-than-expected yields in this synthesis. Nonetheless, it is essential to evaluate this result in the context of green chemistry. Al2O3 serves as a non-toxic, abundant, and sustainable catalyst that reduces environmental harm. While the yields were lower than our expectations, our results highlight the prospective avenues for further optimization of reaction conditions and the investigation of hybrid catalytic systems that could enhance yields while adhering to green practices.
2.2. Biology
The results of the antimicrobial potency of 3c–k are listed in Table 3. They show that compounds 3a, 3c, 3j, 3g, and 3k exhibited strong activity against C. albicans species with an inhibitory zone ranging from 12.3 ± 0.5 mm to 19.3 ± 0.3 mm (see Supplementary Materials). Even in the case where compounds 3c, 3g, and 3j displayed moderate activity against S. aureus and M. luteus, compound 3g exhibited excellent activity against B. subtilis (19.3 ± 0.3 mm). However, none of the compounds revealed any activity against Gram-negative bacteria, L. monocytogenes and E. faecalis. In general, Gram-positive bacteria are more sensitive than Gram-negative ones. This sensitivity might be related to the cell envelope’s composition. The Gram-positive wall is formed by a thick layer of peptidoglycan, which facilitates the penetration of active agents in the action site [29]. From a structural point of view, it can be concluded that Br, OMe, Cl, and F in the para direction exhibited excellent antimicrobial potency; however, other substituents revealed quenching activity.
Table 3.
Zones of inhibition (in mm) of compounds 3a–k (concentration used: 50 mg/mL of DMSO).
| Compounds | Gram-Positive Bacteria | ||||||
|---|---|---|---|---|---|---|---|
| S. aureus | M. luteus | L. monocytogenes | E. faecalis | B. cereus | B. subtilis | ||
| 3a | 8.6 ± 0.3 | 8.3 ± 0.3 | / | / | / | 8.3 ± 0.3 | |
| 3b | 8.3 ± 0.3 | / | / | / | / | / | |
| 3c | 12.3 ± 0.3 | 15.6 ± 0.3 | / | / | 11 ± 0.6 | 9.3 ± 0.3 | |
| 3d | / | / | / | / | / | ||
| 3e | 8.6 ± 0,3 | 9.6 ± 0.3 | 7 ± 0 | 7 ± 0 | / | ||
| 3f | 8.3 ± 0.3 | 8 ± 0.0 | / | 10.6 ± 0.3 | 9.3 ± 0.3 | ||
| 3g | 12.6 ± 0.3 | / | / | / | / | 19.3 ± 0.3 | |
| 3h | 7.3 ± 0.3 | / | / | 7 ±0.0 | 7 ± 0.0 | ||
| 3i | 8.6 ± 0.3 | / | / | / | / | 7.6 ± 0.3 | |
| 3j | 12.3 ± 0.3 | 13.3 ± 0.3 | / | 11.3 ± 0.3 | 8.6 ± 0.3 | ||
| 3k | 10.6 ± 0.3 | 9.3 ± 0.3 | / | 12.3 ± 0.3 | 9.3 ± 0.3 | ||
| Gram-Negative Bacteria | Yeasts | ||||||
| Compounds | E. coli | K. pneumoniae | P. areuginosa | S. typhymerium | C. albicans ATCC 26790 | C. albicans ATCC 10231 | C. albicans IPP 444 |
| 3a | 8.6 ± 0.3 | / | / | / | 9.6 ± 0.3 | 15 ± 1 | / |
| 3b | / | / | / | / | / | / | / |
| 3c | 6 ± 0.0 | / | / | / | 13 ± 0.57 | 18.3 ± 0.6 | / |
| 3d | / | / | / | / | 8.6 ± 0.3 | / | / |
| 3e | / | / | / | / | / | / | / |
| 3f | / | / | / | / | 10 ± 0.5 | 11.6 ± 0.3 | / |
| 3g | / | / | / | / | 13 ± 0.5 | 19.3 ± 0.3 | / |
| 3h | / | / | / | / | 14 ± 0.57 | / | / |
| 3i | / | / | / | / | / | / | / |
| 3j | / | / | / | / | 15 ± 0.3 | 12.3 ± 0.5 | 14 ± 0.5 |
| 3k | / | / | / | / | 15.6 ± 0.3 | 12.6 ± 0.3 | 14.6 ± 0.3 |
The antimicrobial potency of the active compounds that have an inhibitory zone was equal to or greater than 12 mm; these compounds were assessed against the sensitive microorganisms to determine their minimum inhibitory concentration (MIC).
Ten serial dilutions were prepared from an initial concentration of 80 mg/mL by reducing the stock solutions twofold. The results of the active compounds are summarized in Table 4. The results revealed varied activities among the active compounds, with MIC values ranging from 20 mg/mL to 2.5 mg/mL (Table 4). Compounds 3j and 3k exhibited excellent activities against C. albicans, while 3g demonstrated activity against B. subtilis and C. albicans.
Table 4.
In vitro MIC and MBC (mg/mL) of synthesized active compounds.
| Product | S. aureus | M. luteus | B. cereus | B. subtilis | C. albicans 26790 | C. albicans 10231 | C. albicans IPP 444 | |
|---|---|---|---|---|---|---|---|---|
| 3a | MIC | / | / | / | / | / | 5 | / |
| MBC | / | / | / | / | / | 10 | / | |
| 3c | MIC | / | / | / | / | 5 | 5 | / |
| MBC | / | / | / | / | 10 | 10 | / | |
| 3g | MIC | 5 | / | / | 2.5 | 5 | 2.5 | / |
| MBC | 10 | / | / | 2.5 | 10 | 5 | / | |
| 3h | MIC | / | / | / | / | 5 | / | / |
| MBC | / | / | / | / | 5 | / | / | |
| 3j | MIC | 10 | 5 | / | / | 2.5 | 5 | 5 |
| MBC | 10 | 10 | / | / | 5 | 5 | 10 | |
| 3k | MIC | / | / | 20 | / | 2.5 | 5 | 2.5 |
| MBC | / | / | 20 | / | 2.5 | 10 | 5 |
The MIC values were equal to MBC values in the most active imidazo[1,2-a]pyrimidine compounds. The MBC/MIC ratio indicated that the bactericidal effect was based on the record of Payveld [30].
Interestingly, the presence of a halogen particularly Cl or a methyl substituent in the para position on the imidazo[1,2-a]pyrimidine rings augments the activity threefold. Clearly, imidazo[1,2-a]pyrimidines having Br or F in the para position have moderate activity compared with compounds having a Cl substituent in the same position. It is worth noting that all imidazo[1,2-a]pyrimidines with halogenate substituents are more active against Gram-positive bacteria and C. albicans species. Further investigations are currently focused on anti-candidosis activity in our laboratory.
2.3. Molecular Docking Simulation
2.3.1. Protein-Ligand Interaction
The docking analysis of the three compounds 3g, 3k, and 3j with six X-ray crystal structures of the studied targets was performed, and the detailed results of the docking simulation (semi-flexible) are outlined in Table 5.
Table 5.
Docking score energy, RMSD values, and interactions between the most active compounds and native ligands with active site residues of antibacterial and antifungal targets.
| S. aureus (PDB ID: 4URM) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Compounds | S-Score (kcal/mol) | RMSD (Å) |
Bonds Between Atoms of Compounds and Active Site Residues | |||||
| Atom of Compound |
Involved Receptor Atoms |
Involved Receptor Residues |
Category | Type of Interaction |
Distance (Å) |
|||
| 3g | −5.241 | 2.016 | N | HH21 | ARG144(A) | Hydrogen Bond | Conventional H-Bond | 2.70 |
| N | OE2 | GLU58(A) | Hydrogen Bond | Conventional H-Bond | 3.23 | |||
| H | OD2 | ASP81(A) | Hydrogen Bond | Carbon–H-Bond | 2.93 | |||
| H | OD2 | ASP81(A) | Hydrogen Bond | Carbon–H-Bond | 2.61 | |||
| / | NH2 | ARG84(A) | Electrostatic | Pi–Cation | 3.84 | |||
| / | NH2 | ARG84(A) | Electrostatic | Pi–Cation | 4.46 | |||
| / | OE1 | GLU58(A) | Electrostatic | Pi–Anion | 4.48 | |||
| / | OE2 | GLU58(A) | Electrostatic | Pi–Anion | 3.64 | |||
| / | C | ASN54(A) | Hydrophobic | Amide–Pi Stacked | 4.94 | |||
| / | C | GLY85(A) | Hydrophobic | Amide–Pi Stacked | 5.31 | |||
| / | / | ARG84(A) | Hydrophobic | Pi–Alkyl | 5.11 | |||
| / | / | PRO87(A) | Hydrophobic | Pi–Alkyl | 4.75 | |||
| / | / | ARG84(A) | Hydrophobic | Pi–Alkyl | 5.42 | |||
| / | / | PRO87(A) | Hydrophobic | Pi–Alkyl | 5.13 | |||
| / | / | ILE86(A) | Hydrophobic | Pi–Alkyl | 4.31 | |||
| 3k | −4.871 | 1.898 | / | NH2 | ARG84(A) | Electrostatic | Pi–Cation | 4.78 |
| / | NH2 | ARG84(A) | Electrostatic | Pi–Cation | 4.49 | |||
| / | OE1 | GLU58(A) | Electrostatic | Pi-Anion | 4.57 | |||
| C | / | ILE86(A) | Hydrophobic | Alkyl | 4.99 | |||
| / | / | PRO87(A) | Hydrophobic | Pi–Alkyl | 4.44 | |||
| / | / | PRO87(A) | Hydrophobic | Pi–Alkyl | 4.42 | |||
| / | / | ILE86(A) | Hydrophobic | Pi–Alkyl | 4.53 | |||
|
Native
ligand (XAM) |
−5.813 | 1.408 | OBB | HZ3 | LYS93(A) | Hydrogen Bond | Conventional H-Bond | 2.19 |
| OBY | HZ2 | LYS11(A) | Hydrogen Bond | Conventional H-Bond | 1.99 | |||
| CLX | HD3 | ARG84(A) | Hydrogen Bond | Carbon–H-Bond | 3.02 | |||
| / | / | ALA108(A) | Hydrophobic | Alkyl | 4.86 | |||
| CLW | / | PRO87(A) | Hydrophobic | Alkyl | 5.09 | |||
| CLX | / | ARG84(A) | Hydrophobic | Alkyl | 3.98 | |||
| CLX | / | PRO87(A) | Hydrophobic | Alkyl | 4.70 | |||
| CAK | / | MET94(A) | Hydrophobic | Alkyl | 5.09 | |||
| CAK | / | VAL105(A) | Hydrophobic | Alkyl | 4.36 | |||
| / | / | PRO87(A) | Hydrophobic | Pi–Alkyl | 4.22 | |||
| E. coli (PDB ID: 3FV5 ) | ||||||||
| 3k | −3.662 | 0.884 | H | OD2 | ASP69(A) | Hydrogen Bond | Carbon–H-Bond | 2.13 |
| H | OD2 | ASP69(A) | Hydrogen Bond | Carbon–H-Bond | 2.41 | |||
| / | OE2 | GLU46(A) | Electrostatic | Pi–Anion | 3.96 | |||
| / | C | ASN42(A) | Hydrophobic | Amide–Pi Stacked | 4.27 | |||
| / | C | ASN42(A) | Hydrophobic | Amide–Pi Stacked | 4.21 | |||
| C | / | PRO75(A) | Hydrophobic | Alkyl | 4.58 | |||
| / | / | MET74(A) | Hydrophobic | Pi–Alkyl | 4.78 | |||
| / | / | VAL165(A) | Hydrophobic | Pi–Alkyl | 5.19 | |||
| / | / | MET74(A) | Hydrophobic | Pi–Alkyl | 4.03 | |||
| / | / | MET74(A) | Hydrophobic | Pi–Alkyl | 5.06 | |||
|
Native
Ligand (1EU) |
−3.552 | 1.362 | N24 | HH11 | ARG132(A) | Hydrogen Bond | Conventional H-Bond | 2.50 |
| H6 | OD2 | ASP69(A) | Hydrogen Bond | Conventional H-Bond | 1.76 | |||
| H7 | OD2 | ASP69(A) | Hydrogen Bond | Conventional H-Bond | 2.35 | |||
| H5 | OG | SER43(A) | Hydrogen Bond | Carbon–H-Bond | 2.89 | |||
| H17 | O | GLY73(A) | Hydrogen Bond | Carbon–H-Bond | 2.63 | |||
| / | NH1 | ARG72(A) | Electrostatic | Pi–Cation | 3.63 | |||
| / | OE2 | GLU46(A) | Electrostatic | Pi–Anion | 4.09 | |||
| / | / | MET74(A) | Hydrophobic | Pi–Alkyl | 4.51 | |||
| / | / | PRO75(A) | Hydrophobic | Pi–Alkyl | 4.91 | |||
| / | / | ARG72(A) | Hydrophobic | Pi–Alkyl | 5.09 | |||
| / | / | PRO75(A) | Hydrophobic | Pi–Alkyl | 4.70 | |||
| B. Cereus (PDB ID: 3DUW ) | ||||||||
| 3j | −5.855 | 1.164 | N | HA | GLU90(A) | Hydrogen Bond | Carbon–H-Bond | 2.76 |
| / | OE2 | GLU90(A) | Electrostatic | Pi–Anion | 4.16 | |||
| / | OD1 | ASP140(A) | Electrostatic | Pi–Anion | 4.36 | |||
| / | / | HIS40(A) | Hydrophobic | Pi–Pi Stacked | 5.95 | |||
| / | C | ALA141(A) | Hydrophobic | Amide–Pi Stacked | 4.79 | |||
| / | C | ALA141(A) | Hydrophobic | Amide–Pi Stacked | 4.17 | |||
| / | / | ALA91(A) | Hydrophobic | Pi–Alkyl | 3.52 | |||
| / | / | ALA119(A) | Hydrophobic | Pi–Alkyl | 5.01 | |||
| / | / | ALA141(A) | Hydrophobic | Pi–Alkyl | 4.08 | |||
| / | / | ALA91(A) | Hydrophobic | Pi–Alkyl | 4.36 | |||
| / | / | ALA141(A) | Hydrophobic | Pi–Alkyl | 3.69 | |||
| / | / | ALA141(A) | Hydrophobic | Pi–Alkyl | 4.62 | |||
| / | / | LEU68(A) | Hydrophobic | Pi–Alkyl | 5.08 | |||
|
Native
Ligand (SAH) |
−5.616 | 1.623 | N7 | H | ALA119(A) | Hydrogen Bond | Conventional H-Bond | 2.56 |
| O | HZ1 | LYS143(A) | Hydrogen Bond | Conventional H-Bond | 2.19 | |||
| HN1 | O | ASP140(A) | Hydrogen Bond | Conventional H-Bond | 1.88 | |||
| HN61 | OH | TYR149(A) | Hydrogen Bond | Conventional H-Bond | 2.78 | |||
| O | HA | ALA141(A) | Hydrogen Bond | Carbon–H-Bond | 2.93 | |||
| / | OD1 | ASP142(A) | Electrostatic | Pi–Anion | 3.36 | |||
| / | OD2 | ASP142(A) | Electrostatic | Pi–Anion | 3.14 | |||
| HN2 | OD2 | ASP140(A) | Electrostatic | Attractive charge | 2.00 | |||
| OXT | NZ | LYS143(A) | Electrostatic | Attractive charge | 4.31 | |||
| SD | / | HIS40(A) | Other | Pi–Sulfur | 5.65 | |||
| / | / | ALA91(A) | Hydrophobic | Pi–Alkyl | 3.80 | |||
| / | / | LEU118(A) | Hydrophobic | Pi–Alkyl | 5.30 | |||
| / | / | ALA119(A) | Hydrophobic | Pi–Alkyl | 5.09 | |||
| / | / | ALA141(A) | Hydrophobic | Pi–Alkyl | 4.75 | |||
| / | / | ALA91(A) | Hydrophobic | Pi–Alkyl | 4.69 | |||
| / | / | LEU118(A) | Hydrophobic | Pi–Alkyl | 4.66 | |||
| B. Subtilis (PDB ID: 2RHL) | ||||||||
| 3g | −5.205 | 1. 634 | O | HG1 | THR109(A) | Hydrogen Bond | Conventional H-Bond | 2.95 |
| H | O | MET105(A) | Hydrogen Bond | Carbon–H-Bond | 2.70 | |||
| / | NH1 | ARG143(A) | Electrostatic | Pi–Cation | 4.97 | |||
| / | OE1 | GLU139(A) | Electrostatic | Pi–Anion | 4.72 | |||
| / | C | GLY104(A) | Hydrophobic | Amide–Pi Stacked | 4.68 | |||
| / | C | GLY104(A) | Hydrophobic | Amide–Pi Stacked | 4.52 | |||
|
Native
Ligand (GDP) |
−6.077 | 1.336 | O1A | H | GLY21(A) | Hydrogen Bond | Conventional H-Bond | 2.01 |
| O2 | H | GLY22(A) | Hydrogen Bond | Conventional H-Bond | 1.82 | |||
| O2 | H | GLY23(A) | Hydrogen Bond | Conventional H-Bond | 2.76 | |||
| O3B | H | ALA71(A) | Hydrogen Bond | Conventional H-Bond | 2.19 | |||
| O3B | H | ALA72(A) | Hydrogen Bond | Conventional H-Bond | 2.65 | |||
| O3B | H | ALA73(A) | Hydrogen Bond | Conventional H-Bond | 2.13 | |||
| O2B | H | GLY108(A) | Hydrogen Bond | Conventional H-Bond | 2.30 | |||
| O2B | H | THR109(A) | Hydrogen Bond | Conventional H-Bond | 1.86 | |||
| O2B | HG1 | THR109(A) | Hydrogen Bond | Conventional H-Bond | 2.30 | |||
| O6 | HD21 | ASN166(A) | Hydrogen Bond | Conventional H-Bond | 2.19 | |||
| HO3 | O | GLY104(A) | Hydrogen Bond | Conventional H-Bond | 2.90 | |||
| O2 | HA2 | GLY22(A) | Hydrogen Bond | Carbon–H-Bond | 3.03 | |||
| N3 | HA2 | GLY22(A) | Hydrogen Bond | Carbon–H-Bond | 2.57 | |||
| O1B | HA | ALA73(A) | Hydrogen Bond | Carbon–H-Bond | 2.65 | |||
| O2B | HA2 | GLY108(A) | Hydrogen Bond | Carbon–H-Bond | 2.98 | |||
| O6 | HA | PRO135(A) | Hydrogen Bond | Carbon–H-Bond | 2.59 | |||
| H2 | O | GLY104(A) | Hydrogen Bond | Carbon–H-Bond | 2.16 | |||
| O1B | NH2 | ARG143(A) | Electrostatic | Attractive charge | 4.10 | |||
| / | / | PHE183(A) | Hydrophobic | Pi–Pi Shaped | 4.82 | |||
| M. luteus (PDB ID: 3AQC) | ||||||||
| 3j | −4.958 | 1.424 | H | O | LYS170(B) | Hydrogen Bond | Carbon–H-Bond | 2.61 |
| / | OE2 | GLU146(B) | Electrostatic | Pi–Anion | 3.69 | |||
| / | OE2 | GLU146(B) | Electrostatic | Pi–Anion | 4.02 | |||
| / | HB2 | SER80(B) | Hydrophobic | Pi–Sigma | 2.66 | |||
| / | / | HIS83(B) | Hydrophobic | Pi-Pi Stacked | 5.61 | |||
| / | / | HIS83(B) | Hydrophobic | Pi-Pi Stacked | 5.72 | |||
| / | / | VAL142(B) | Hydrophobic | Pi–Alkyl | 5.49 | |||
| / | / | VAL142(B) | Hydrophobic | Pi–Alkyl | 5.31 | |||
| / | / | CYS143(B) | Hydrophobic | Pi–Alkyl | 5.11 | |||
|
Native
Ligand (2DE) |
−7.534 | 1.144 | O1A | HZ2 | LYS170(B) | Hydrogen Bond | Conventional H-Bond | 1.71 |
| O1A | HE3 | LYS170(B) | Hydrogen Bond | Carbon–H-Bond | 2.87 | |||
| H2 | OE2 | GLU146(B) | Hydrogen Bond | Carbon–H-Bond | 2.47 | |||
| O3B | HZ1 | LYS225(B) | Electrostatic | Attractive charge | 2.13 | |||
| O3B | NH1 | ARG93(B) | Electrostatic | Attractive charge | 4.10 | |||
| O2B | NZ | LYS170(B) | Electrostatic | Attractive charge | 4.96 | |||
| O2B | NZ | LYS170(B) | Electrostatic | Attractive charge | 4.58 | |||
| O2B | NZ | LYS225(B) | Electrostatic | Attractive charge | 3.84 | |||
| PA | OD2 | ASP84(B) | Electrostatic | Attractive charge | 3.69 | |||
| PA | OD2 | ASP88(B) | Electrostatic | Attractive charge | 3.87 | |||
| PA | OE2 | GLU146(B) | Electrostatic | Attractive charge | 5.36 | |||
| PB | OD2 | ASP84(B) | Electrostatic | Attractive charge | 4.52 | |||
| PB | OD2 | ASP88(B) | Electrostatic | Attractive charge | 4.40 | |||
| PB | OD2 | ASP211(B) | Electrostatic | Attractive charge | 5.34 | |||
| PB | OD1 | ASP230(B) | Electrostatic | Attractive charge | 5.06 | |||
| C10 | / | VAL142(B) | Hydrophobic | Alkyl | 5.13 | |||
| C14 | / | ILE87(B) | Hydrophobic | Alkyl | 5.25 | |||
| C15 | / | HIS83(B) | Hydrophobic | Pi–Alkyl | 5.30 | |||
| C. albicans (PDB ID: 3Q70) | ||||||||
| 3k | −5.040 | 1.501 | H | OG1 | THR221(A) | Hydrogen Bond | Carbon–H-Bond | 2.65 |
| / | / | TYR84(A) | Hydrophobic | Pi–Pi Stacked | 5.27 | |||
| / | / | TYR225(A) | Hydrophobic | Pi–Pi Stacked | 5.02 | |||
| C | / | ILE119(A) | Hydrophobic | Alkyl | 4.00 | |||
| C | / | ILE123(A) | Hydrophobic | Alkyl | 5.22 | |||
| C | / | ILE123(A) | Hydrophobic | Alkyl | 4.97 | |||
| C | / | TYR84(A) | Hydrophobic | Pi–Alkyl | 4.43 | |||
| C | / | TYR84(A) | Hydrophobic | Pi–Alkyl | 4.56 | |||
|
Native
ligand (RIT) |
−5.107 | 1.464 | O24 | HN | GLY85(A) | Hydrogen Bond | Conventional H-Bond | 2.42 |
| H5 | O | GLY220(A) | Hydrogen Bond | Conventional H-Bond | 2.87 | |||
| H5 | OG1 | THR221(A) | Hydrogen Bond | Conventional H-Bond | 2.54 | |||
| H18 | O | GLY220(A) | Hydrogen Bond | Conventional H-Bond | 2.38 | |||
| H4 | OD2 | ASP218(A) | Hydrogen Bond | Carbon H-Bond | 3.09 | |||
| / | / | TYR225(A) | Hydrophobic | Pi–Pi Stacked | 4.90 | |||
| / | / | TYR51(A) | Hydrophobic | Pi–Pi Stacked | 4.40 | |||
| C64 | / | ILE30(A) | Hydrophobic | Alkyl | 5.20 | |||
| C68 | / | VAL12(A) | Hydrophobic | Alkyl | 5.49 | |||
| C68 | / | ILE30(A) | Hydrophobic | Alkyl | 5.29 | |||
| C86 | / | TYR51(A) | Hydrophobic | Pi–Alkyl | 4.03 | |||
In order to estimate all possible interactions, the docking outputs generated by MOE software (https://www.chemcomp.com/en/Products.htm, accessed on 18 October 2024) were converted into (.pdb) files and visualized with the default parameters of the BIOVIA DS visualizer package (Dassault Systèmes BIOVIA, Discovery Studio Modeling Environment, 2020).
2.3.2. Orientation and Bonding Interaction of the Compounds at the Active Site of Receptors
As shown in Table 5, we found that compounds 3g and 3k were predicted to be the strongest binders to the S. aureus target (PDB ID: 4URM) binder that forms the complexes, with the stability confirmed by the negative score energy of −5.241, and −4.871 Kcal/mol, respectively. Notably, the score value of compound 3g is very close to that of the native ligand (XAM): −5.813 kcal/mol (Table 5).
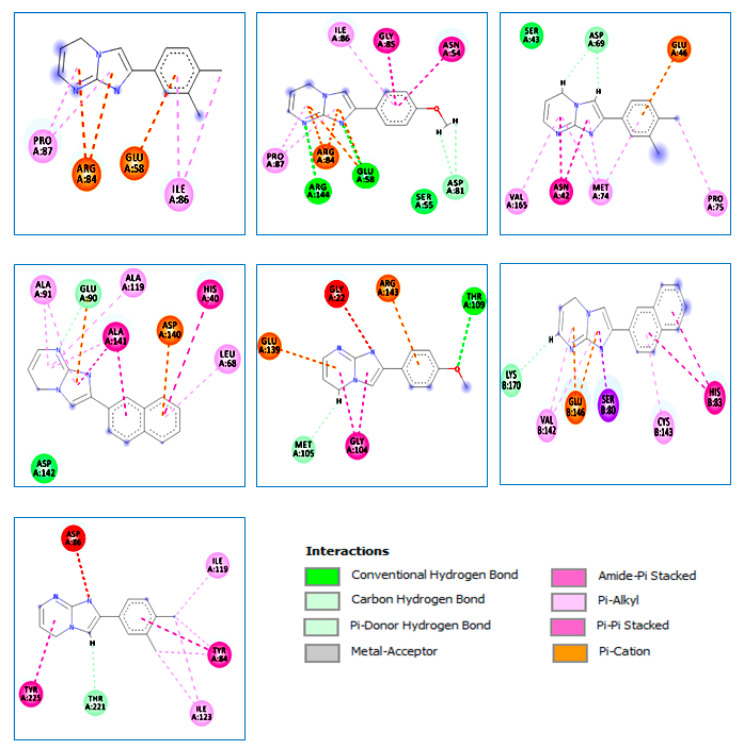
From the obtained results, it is apparent that compound 3g formed four strong hydrogen bonds [29] with the active site residue of S. aureus target (PDB ID: 4URM); two conventional H-bonds, namely N/ARG144(A)-HH21/bond distance = 2.70Å and N/GLU58(A)-OE2/bond distance = 3.23Å; and two carbon–H-bonds, H/ASP81(A)-OD2/bond distance = 2.93Å and H/ASP81(A)-OD2/bond distance = 2.61Å. Four electrostatic interactions were observed with ARG84(A) and GLU58(A), while this compound formed seven hydrophobic interactions with the active site of the enzyme (Table 5; Figure 2).
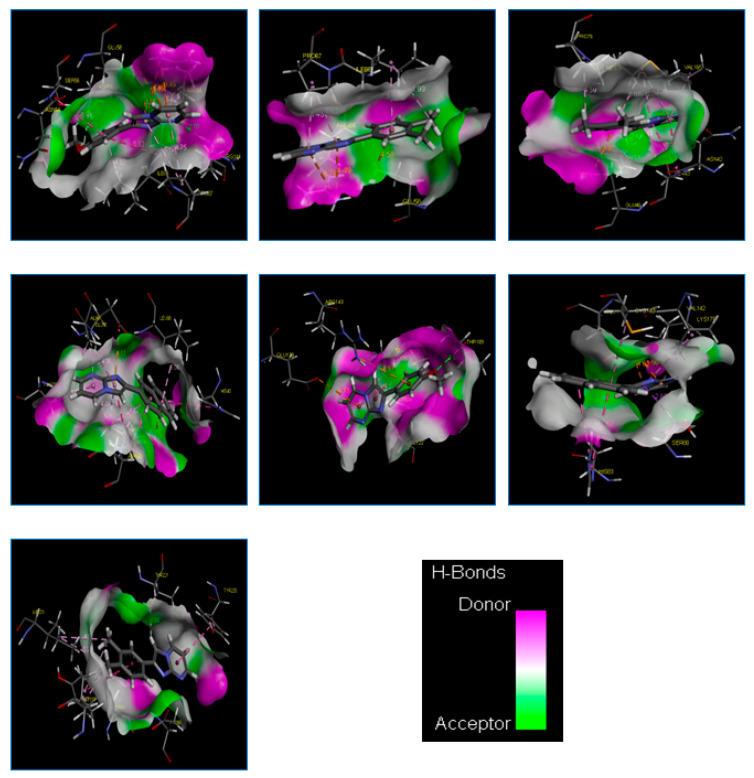
Figure 2.
The 2D diagrams of the interaction between 3g and S. aureus (PDB ID:4URM); 3k and S. aureus (PDB ID:4URM); 3k and E. coli (PDB ID:3FV5); 3j and B. cereus (PDB ID:3DUW); 3g and B. subtilis (PDB ID:2RHL); 3j and M. luteus (PDB ID:3AQC); and 3k and C. albicans (PDB ID:3Q70).
Similarly, compound 3k revealed three electrostatic interactions with the active site residue of the S. aureus target (PDB ID: 4URM) and four hydrophobic interactions with the pocket of the target (Table 5; Figure 2). In this regard, several studies [30,31,32] revealed that ARG84(A) plays an important role in the inhibition of the S. aureus (PDB ID: 4URM) target (Figure 3 and Figure 4).
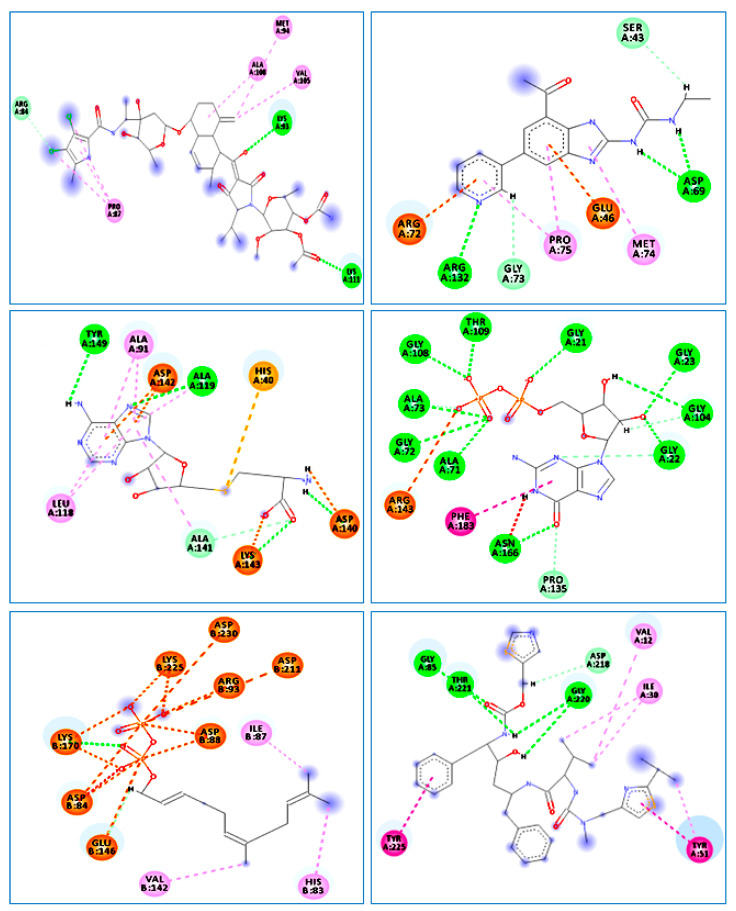
Figure 3.
The 2D diagrams of the interaction between XAM and S. aureus (PDB ID:4URM); 1EU and E. coli (PDB ID:3FV5); SAH and B. cereus (PDB ID:3DUW); GDP and B. subtilis (PDB ID:2RHL); 2DE and M. luteus (PDB ID:3AQC); and RIT and C. albicans (PDB ID:3Q70).
Figure 4.
The 3D diagrams of the interaction between 3g and S. aureus (PDB ID:4URM); 3k and S. aureus (PDB ID:4URM); 3k and E. coli (PDB ID:3FV5); 3j and B. cereus (PDB ID:3DUW); 3g and B. subtilis (PDB ID:2RHL); 3j and M. luteus (PDB ID:3AQC); and 3k and C. albicans (PDB ID: 3Q70).
Notably, the complex formed by compound 3g exhibited the best negative energy value of −3.662 kcal/mol compared to the native ligand (1EU) (−3.552 kcal/mol) (Table 5). The docked conformation of compound 3g with the E. coli target (PDB ID: 3FV5) is shown in Figure 2. We note that this compound formed two strong carbon–H-bonds: H/ ASP69(A)-OD2/bond distance = 2.13Å and H/ASP69(A)-OD2/bond distance = 2.41Å (Table 5; Figure 2). At the same time, compound 3g formed one electrostatic interaction and seven hydrophobic interactions with the target active site residue of the target. Notably, several studies [33,34] have reported that ASN42(A), GLU46(A), and PRO75(A) play a central role in E. coli receptor (PDB ID: 3FV5) inhibition.
Compound 3j formed the most stable complex with the B. cereus (PDB ID: 3DUW) target compared with the native ligand (SAH), as confirmed by the negative score energy values of −5.855 kcal/mol and −5.616 kcal/mol, respectively. This compound exhibited one strong carbon–hydrogen bond with the residue GLU90(A) (bond distance = 2.76Å Å) in addition to two electrostatic interactions with GLU90(A) and ASP140(A). Moreover, it exhibited ten hydrophobic interactions with the active site residue of the B. cereus target (PDB ID: 3DUW) (Table 5; Figure 2). Sokolova N. et al. [35] confirmed that these residues are critical in the formation of different interactions in the active site of this target.
The complex formed by compound 3g exhibited the lowest binding energy score (−5.205 kcal/mol), which is very close to native ligand GDP 5 (−6.077 Kcal/mol) (Table 5). Furthermore, compound 3g exhibited one strong hydrogen bond (conventional and carbon types) with the residues THR109(A) in MET105(A), respectively (bond distance = 2.95 Å and 2.70 Å). Two electrostatic interactions (Pi–cation and Pi–anion) were detected with GLU90(A) and ASP140(A), respectively. Consequently, two hydrophobic interactions were formed between this compound and the same residue, i.e., GLY104(A) (Table 5; Figure 2). These results have been confirmed by recent studies [34,35,36,37,38,39].
As presented in Table 5, we found that compound 3j has a very close affinity with the M. Luteus (PDB ID: 3AQC) target compared to native ligand 2DE, with a value of −7.534 kcal/mol. This is through the formation of a stable complex with a negative energy score of −4.958 kcal/mol and a low RMSD value.
The docked conformation of compound 3 in the active site pocket of M. luteus (PDB ID: 3AQC) exhibited one strong H-bond formed between compound 3j and the residue LYS170(B) (bond distance = 2.61Å). Also, two electrostatic interactions (Pi–anion) were observed, which were formed between this compound and the same residue GLU146(B). Additionally, compound 3j exhibited six hydrophobic interactions with the different residues in the active site of the M. luteus (PDB ID: 3AQC) target (Table 5; Figure 2). It should be noted that these results were supported by previous studies [40,41,42].
Finally, the docking results of compound 3k with the C. albicans (PDB ID: 3Q70) active pocket show that the complex binding score (S-score) was −5.040 kcal/mol, which is very close to the native ligand (3Q70-RIT), at −5.107 kcal/mol (Table 5). In addition, it should be noted that compound 3k revealed one strong H-bond with the pocket of the C. albicans (PDB ID: 3Q70) receptor, formed between the compound and the residue THR221(A) (bond distance = 2.65Å). Moreover, seven hydrophobic interactions were detected between this compound and the different residues in the active site of the studied target (Table 5; Figure 2). These results were supported by Barakat et al. [43].
2.4. Drug-Likeness Evaluation
Different parameters of physicochemical properties were calculated in order to verify the drug-likeness rules by using Swiss ADME [44] online servers. All the results are listed in Table 6.
Table 6.
Physicochemical properties and drug-likeness predictions of compounds 3g, 3k, and 3j.
| Compounds | Physicochemical Properties | Medicinal Chemistry | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Drug-Likeness Rules | |||||||||
| TPSA (Å2) |
n-ROT | MW (g/mol) |
MLog P | n-HA | n-HD | Lipinski | Veber | Egan | |
| WLogP | |||||||||
| (0~140) | (0~11) | (100~500) | (0~5) | (0~12) | (0~7) | ||||
| 3g | 39.42 | 2 | 225.25 | 1.49 | 3 | 0 | Accepted | Accepted | Accepted |
| 2.40 | |||||||||
| 3k | 30.19 | 1 | 223.27 | 2.33 | 2 | 0 | Accepted | Accepted | Accepted |
| 3.01 | |||||||||
| 3j | 30.19 | 1 | 245.28 | 2.65 | 2 | 0 | Accepted | Accepted | Accepted |
| 3.55 | |||||||||
TPSA: topological polar surface area, n-ROT: number of rotatable bonds, MW: molecular weight, Log P: logarithm of partition coefficient of compound between n-octanol and water, n-HA: number of hydrogen bond acceptors, n-HD: Number of hydrogen bonds donors.
As shown in Table 6, it is apparent that in all compounds, namely 3g, 3k, and 3j, the number of hydrogen bond donors was < 5 (n-HD: (0~7)), and the number of hydrogen bond acceptors was < 10 (n-HA: (0~10)). In addition, the molecular weight values of these compounds ranged from 100 to 500 g/mol, and the MLogP and WLogP values were <5. Also, nROTB values were <11, which denotes the flexibility of these compounds. On the other hand, all TPSA values obtained were less than 140 Å. According to these results, it can be concluded that all compounds satisfy all the criteria of drug-likeness without any violation of Lipinski, Veber, and Egan rules. Clearly, not all compounds pose problems with oral bioavailability and pharmacokinetic parameters.
2.5. ADME-T Properties
The absorption, distribution, metabolism, excretion, and toxicity parameters were calculated by using the online server pkCSM [45]. All the results are presented in Table 7.
Table 7.
ADMET/pharmacokinetic properties of compounds 3g, 3k, and 3j.
| ADME | Parameters | 3g | 3k | 3j |
|---|---|---|---|---|
| Absorption | Caco2 (10−6 cm/s) | 1.178 | 1.417 | 1.526 |
| HIA (%) | 99.207 | 97.682 | 97.629 | |
| Distribution | CNS (log PS) | −1.800 | −1.692 | −1.462 |
| BBB (log BB) | 0.311 | 0.030 | 0.123 | |
| Metabolism | CYP1A2 inhibitor | Yes | Yes | Yes |
| CYP2C19 Inhibitor | No | No | No | |
| CYP2D6 substrate | No | No | No | |
| CYP3A4 substrate | No | No | No | |
| Excretion | Renal OCT2 substrate | No | No | No |
| Total Clearance (log mL/min/kg) | 0.744 | 0.780 | 0.737 | |
| Toxicity | hERG I and II inhibitors | No | No | No |
| Hepatotoxicity | No | No | No |
Caco-2: colon adenocarcinoma, HIA: human intestinal absorption, CNS: central nervous system permeability, BBB: blood–brain barrier permeability, Renal OCT2 substrate: organic cation transporter 2, hERG: human ether-à-go-go-related gene.
From the analysis in Table 7, we can draw the following conclusions:
All compounds had Caco-2 values greater than −5.15 (>−5.15 cm/s). This means that these compounds exhibit good permeability. In addition, it is evident that all compounds had HIA values greater than 30%, indicating that the orally administered drug candidates are absorbed from the gastrointestinal system into the bloodstream of the human body.
All three compounds could penetrate the CNS, as confirmed by logPS values, which were in the range of −3 < logPS < −2. Additionally, the logBB values of compounds 3g, 3k, and 3j were 0.311, 0.030, and 0.123, respectively. This indicates that compound 3g readily crosses the blood–brain barrier and that compounds 3k and 3j are poorly distributed in the brain (Table 7).
A P450 CYP inhibitor test was performed to determine whether the drug blocked or decreased the activity of one or more isoforms of the CYP450 enzyme. Data from this table indicate that compounds 3g, 3k, and 3j are inhibitors of the CYP1A2 isoform but not CYP2C19 inhibitors. In addition, these compounds are not CYP2D6 and CYP3A4 substrates.
Further analysis of the table showed that none of the compounds were likely to be an OCT2 substrate. Moreover, it can be clearly seen that these compounds have a low excretion clearance (<5 mL/min/kg) (Table 7).
Additionally, the selected compounds were neither hERG I nor hERG II inhibitors. However, none of these compounds posed a hepatotoxicity risk.
3. Experimental Section
3.1. Chemistry
3.1.1. General Information
All solvents and reagents were purchased from Aldrich (St. Louis, MO, USA)/Merck (Rahway, NJ, USA) and used as received without any further purification. Routine monitoring of reactions was performed by thin-layer chromatography (TLC). Melting points were taken using a Bank Kofer Hrizbank apparatus standard WME 50–266 °C and were uncorrected. The 1H and 13C NMR spectra were recorded on Magritek SPINSOLVE 91 spectrometers (Magritek (Aachen, Germany)) at 91 and 23 MHz, respectively. Samples were recorded in DMSO-d6 solutions using TMS as an internal standard. The chemical shifts are expressed in δ units (ppm) and quoted downfield from TMS. Signals are abbreviated as follows: s, singlet; d, doublet; dd, doublet of doublet; m, multiplet. The electron impact mass spectra were obtained using a Bruker 320-MS instrument (Bruker, Billerica, MA, USA). Microwave irradiation was performed using a household microwave oven.
3.1.2. General Procedure for the Synthesis of 2-arylimidazo[1,2-a]Pyrimidines (3a–k)
As described in Scheme 1, imidazo[1,2-a]pyrimidine derivatives (3a–k) were synthesized by a condensation reaction of 2-aminopyrimidine 1 (10 mmol) and several 2-bromoarylketones 2 (a–k) (10 mmol) without solvent, catalyzed by Al2O3, and irradiated using a domestic microwave for 90–300 s. The amount of the catalyst was 30% (w/w). The TLC method was used to track the reaction progress. Then, the crude was cooled at room temperature, filtered, and washed several times with a mixture of ethyl ether and ethanol to afford a pure solid with a good yield without the need for any purification.
2-phenylimidazo[1,2-a]pyrimidines (3a)
Yield: 70%; light brown solid; mp 195–197 °C; 1H NMR (91 MHz, DMSO d6): 9.30 (dd, J = 6.7, 1.9 Hz, 1H), 8.98 −7.44 (m, 8H); 13C NMR (23 MHz, DMSO-d6): 157.29, 138.22, 131.07, 130.02, 126.81, 114.31; MS (ESI) calcd for C12H9N3 [M+H]+ 195.23; found 196. The characterization data are consistent with the results reported in the literature [28].
2-(3-bromophenyl)imidazo[1,2-a]pyrimidine (3b)
Yield: 56%; light brown solid; mp 191–193 °C; 1H NMR (91 MHz, DMSO d6): 9.02 (dd, J = 6.7, 1.9 Hz, 1H), 8.59 (d, J = 9.6 Hz, 2H), 8.31 –7.17 (m, 7H); 13C NMR (23 MHz, DMSO-d6): 152.43, 142.05, 136.07, 134.74, 131.57, 131.40, 128.58, 125.02, 122.62, 110.37, 109.10; MS (ESI) calcd for C12H8BrN3 [M+H]+ 274.12; found 276. The obtained characterization results match those reported in the literature [18].
2-(4-bromophenyl)imidazo[1,2-a]pyrimidine (3c)
Yield: 63%; white solid; mp 209–211 °C; 1H NMR (91 MHz, DMSO-d6): 9.21 (dd, J = 6.6, 1.9 Hz, 1H), 8.86–7.45 (m, 7H); MS (ESI) calcd for C12H8BrN3 [M+H]+ 274.12; found 276. The characterization results for this compound align with those available in the literature [9].
2-(p-tolyl)imidazo[1,2-a]pyrimidine (3d)
Yield: 68%; pale solid; mp 229–231 °C; 1H NMR (91 MHz, DMSO d6): δ 9.48 (dd, J = 6.9, 1.7 Hz, 1H), 9.13–7.37 (m, 7H), 2.74–2.55 (m, 3H); 13C NMR (23 MHz, DMSO-d6): 156.90, 144.71, 140.87, 137.96, 136.74, 130.35, 126.51, 124.12, 114.14, 109.40, 21.16; MS (ESI) calcd for C13H11N3 [M+H]+ 209.25; found 210. The characterization data are consistent with the results reported in the literature [28].
2-(2-methoxyphenyl)imidazo[1,2-a]pyrimidine (3e)
Yield: 57%; yellow solid; mp 264–266 °C; 1H NMR (91 MHz, DMSO-d6): 9.09–8.73 (m, 1H), 8.12–7.00 (m, 7H), 4.02 (s, 3H); 13C NMR (23 MHz, DMSO-d6): 157.00, 139.61, 133.97, 132.23, 131.69, 129.37, 128.17, 121.40, 117.66, 114.77, 114.18, 112.72, 56.53; MS (ESI) calcd for C13H11N3O [M+H]+ 225.25; found 226.
2-(3-methoxyphenyl)imidazo[1,2-a]pyrimidine (3f)
Yield: 53%; brown solid; mp 219–221 °C; 1H NMR (91 MHz, DMSO-d6): 9.31 (dd, J = 6.7, 1.8 Hz, 1H), 9.13–6.86 (m, 7H), 3.85 (d, J = 1.7 Hz, 3H); 13C NMR (23 MHz, DMSO-d6): 160.25, 157.27, 144.80, 138.09, 136.54, 131.11, 128.26, 118.91, 116.77, 114.26, 111.90, 110.38, 56.03; MS (ESI) calcd for C13H11N3O [M+H]+ 225.25; found 226. The characterization results for this compound align with those available in the literature [9].
2-(4-methoxyphenyl)imidazo[1,2-a]pyrimidine (3g)
Yield: 52%; yellow solid; mp 189–191 °C; 1H NMR (91 MHz, DMSO-d6): 9.52 (s, 1H), 8.15 –7.04 (m, 7H), 3.87 (d, J = 2.2 Hz, 3H); 13C NMR (23 MHz, DMSO-d6): 169.47, 164.43, 131.29, 127.30, 114.66, 107.81, 56.03; MS (ESI) calcd for C13H11N3O [M+H]+ 225.25; found 226. The characterization data are consistent with the results reported in the literature [28].
2-(4-fluorophenyl)imidazo[1,2-a]pyrimidine (3h)
Yield: 63%; light brown solid; mp 230–232 °C; 1H NMR (91 MHz, DMSO-d6): 9.29 (dd, J = 6.7, 1.9 Hz, 1H), 8.96–7.26 (m, 7H); 13C NMR (23 MHz, DMSO-d6): 157.01, 144.81, 137.99, 135.90, 129.26, 128.89, 123.79, 117.39, 116.44, 114.06, 109.88; MS (ESI) calcd for C12H8FN3 [M+H]+ 213.22; found 214. The characterization data are consistent with the results reported in the literature [28].
2-(4-chlorophenyl)imidazo[1,2-a]pyrimidine (3i)
Yield: 65%; light brown solid; mp 273–175 °C; 1H NMR (91 MHz, DMSO-d6): 9.25 (d, J = 6.9 Hz, 1H), 8.92–7.26 (m, 7H); 13C NMR (23 MHz, DMSO-d6): 156.39, 144.72, 137.56, 136.05, 128.91, 128.53, 123.83, 117.05, 116.09, 113.47, 109.32; MS (ESI) calcd for C12H8ClN3 [M+H]+ 229.67; found 214. The experimental data correspond well with those previously reported in the literature [10].
2-(naphthalen-2-yl)imidazo[1,2-a]pyrimidine (3j)
Yield: 67%; pale solid; mp 249–251 °C; 1H NMR (91 MHz, DMSO-d6): 9.25 (dd, J = 6.8, 1.9 Hz, 1H), 8.96–7.46 (m, 10H); 13C NMR (23 MHz, DMSO-d6): 156.03, 145.74, 138.58, 137.64, 133.77, 133.21, 129.49, 128.84, 128.29, 127.85, 127.63, 126.06, 125.87, 123.79, 113.27, 110.08; MS (ESI) calcd for C16H11N3 [M+H]+ 245.29; found 246. The experimental data correspond well with those previously reported in the literature [10].
2-(3,4-dimethylphenyl)imidazo[1,2-a]pyrimidine (3k)
Yield: 64%; light brown solid; mp 231–233 °C; 1H NMR (91 MHz, DMSO-d6): 9.27 (dd, J = 6.8, 1.9 Hz, 1H), 8.95–7.35 (m, 6H), 2.64–2.20 (m, 6H); 13C NMR (23 MHz, DMSO-d6): 156.47, 144.27, 139.32, 137.52, 136.42, 130.39, 127.05, 123.98, 123.63, 113.69, 108.88, 19.41, 19.32; MS (ESI) calcd for C14H13N3 [M+H]+ 223.28; found 224.
It should be noted that, even with the use of NMR spectrometers operating at 91 MHz and 23 MHz for 1H and 13C NMR, respectively, the products obtained correspond to those documented in the literature. The MS analysis revealed dominant peaks that precisely match the expected molar masses of the synthesized compounds, confirming their identification. Moreover, the 1H NMR spectra we acquired are identical to those stated in previous research, further confirming the chemical structure of the compounds. Although the 13C NMR spectra showed fewer peaks for some products—likely due to the performance and lower sensitivity of the instruments used—most of the signals align well with the literature data. The noted chemical shifts may indicate overlapping carbons, clarifying the absence of peaks that correspond to theoretical carbon positions. This supports compelling evidence for the effective synthesis of the desired compounds.
3.2. Biology
The antimicrobial potency of all imidazo[1,-2a]pyrimidine compounds was screened in vitro against 13 microorganisms, comprising 6 Gram-positive bacteria, 4 Gram-negative bacteria, and 3 yeasts (Table 8), using the disk diffusion method as described by Nariya et al. [46].
Table 8.
Microorganism strains and their ATCC numbers.
| Gram-Positive Bacteria | |||||
|---|---|---|---|---|---|
|
Staphylococcus aureus ATCC 25923 |
Micrococcus luteus ATCC 9341 |
Listeria monocytogenes ATCC 15313 |
Bacillus cereus ATCC 10876 |
Bacillus subtilis ATCC6633 |
Enterococcus faecalis ATCC29212 |
| Gram-Negative Bacteria | |||||
| Escherichia coli ATCC 25912 | Pseudomonas aeruginosa ATCC 27853 |
Klebsiella pneumoniae ATCC 700603 |
Salmonella typhimurium ATCC 13311 |
||
| Yeasts | |||||
| Candida albicans ATCC 26790 | Candida albicans ATCC 10231 | Candida albicans IPP 444 | |||
Briefly, 50 mg/mL of the synthesized compounds 3a–k was dissolved into dimethylsulphoxide. Then, ten microliters of each solution was added to a disk. The inhibitory zones were calculated (mm) after 24 h of incubation at 37 °C. All the samples were taken in triplicates. The means and the SD were calculated using PAST 4.3 software.
The active compounds were determined by their minimum inhibitory concentration (MIC) on an ELISA plate using the method described by Premsai Rai et al. [47], with some modifications. Briefly, bacteria and yeast were inoculated into brain–heart infusion broth (BHIB) and Sabouraud broth, respectively, at 37 °C for 24 h. The optical density was adjusted to 5 × 106 colony-forming units/mL for bacteria and to 5 × 105 colony-forming units/mL for yeast, measured at 620 nm.
The graded concentrations of the active imidazo[1,2-a]pyrimidine compounds (0.039–20 mg/mL) and suspension microorganisms were added into the first ten wells of the ELISA plate to obtain 200 µL as the final concentration, and the samples were then incubated for 24 h at 37 °C. The MIC is expressed as the lowest concentration of compounds that showed no visible growth. The 11th well was considered a positive control, which contained the medium and inoculum. The 12th well was considered a negative control, which contained just the medium. The minimum bactericidal concentrations (MBCs) were assessed from the subcultured MIC wells. Ten microliters of each solution was added to Müller–Hinton agar or Sabouraud agar and incubated aerobically for 24 h at 37 °C. The lowest concentration with no growth, shown on the MHA plate, was recorded as the MBC.
3.3. In Vitro Assay for PDE5 Inhibitors
3.3.1. Protein Expression and Purification
Ligands and Target Preparations
The 3D structures of the most active compounds (3g, 3k, and, 3j) were optimized using the semi-empirical method AM1 [48], which was implemented in Hyperchem 8.0.8 software (Version 8.0.8, Hypercube, Gainesville, FL, USA). In addition, all these structures were converted into the .*mdb format in order to be used as input MOE docking.
Six crystal structures of targets were downloaded from Protein Data Bank (http://www.rcsb.org) and selected as antibacterial targets, namely gyrase B (PDB ID: 4URM) [49] from Staphylococcus aureus; the DNA topoisomerase complex (PDB ID: 3FV5) [48] of E. coli; BcOMT2 (PDB ID: 3DUW) [50] from Bacillus cereus; BsFtsZ (PDB ID: 2RHL) [51] from Bacillus subtili; and hexaprenyl diphosphate synthase (PDB ID: 3AQC) [39] from Micrococcus luteus. Secreted aspartic protease (PDB ID: 3Q70) [50] from Candida albicans was chosen as an antifungal target in order to understand the antibacterial activities of these compounds. Some information related to the target structures is provided in Table 9.
Table 9.
Data related to the six targets studied.
| Targets PDB |
Methods | Organism | Chain | Sequence Length | Resolution (Å) | Native Ligands |
|---|---|---|---|---|---|---|
| 4URM | X-ray diffraction | Staphylococcus aureus | A, B | 123 | 1.85 | 9JH |
| 3FV5 | X-ray diffraction | E. coli K-12 | A, B | 201 | 1.80 | 1EU |
| 3DUW | X-ray diffraction | B. cereus | A, B | 223 | 1.20 | SAH |
| 2RHL | X-ray diffraction | Bacillus subtilis | A, B | 325 | 2.45 | GDP |
| 3AQC | X-ray diffraction | M. luteus | A, B, C, D | 325 | 2.61 | 2DE |
| 3Q70 | X-ray diffraction | C. albicans | A | 342 | 1.40 | RIT |
Protocol and validation of the Docking method
Molecular docking simulations were performed using the MOE software [51] to discover and identify the binding interactions of compounds within the active site residues of six targets. The molecular docking protocol steps were followed and detailed in our previous research [52,53] using the following default parameters: Placement: Triangle Matcher, Rescoring 1: London dG. The scoring function used was London dG.
Furthermore, the method was validated by re-docking all the native ligands to their targets. The RSMD values of the obtained complexes (target-crystallized ligands) were less than 2.0 Å [54], implying that the docking method is accurate and successful.
ADME-Tox evaluation
In order to validate the drug-likeness rules, namely Lipinski, Veber ,and Ghose, different parameters of physicochemical properties (TPSA, nROT, MW, LogP, number of hydrogen bond acceptors (nHAs), and number of hydrogen bond donors (nHDs)) were calculated using the SwissADME server (http://www.swissadme.ch/) [44].
On the other hand, the pkCSM server (http://biosig.unimelb.edu.au/pkcsm/prediction, accessed on 18 October 2024) [45] was used for the analysis of ADMET profiles by calculating the following parameters: absorption (Caco-2: colon adenocarcinoma and HIA: human intestinal absorption), distribution (CNS: central nervous system permeability and BBB: blood–brain barrier permeability), metabolism (CYP1A2 inhibitor, CYP2C19 inhibitor, and CYP2D6 inhibitor), excretion (Renal OCT2 substrate: organic cation transporter 2 and total clearance), and toxicity (hERG: human ether-à-go-go-related gene and hepatotoxicity).
4. Conclusions
The reported method for the synthesis of imidazo[1,2-a]pyrimidine compounds assisted by microwave irradiation offers several advantages with respect to the mechano-synthesis and thermal treatment methods, such as higher yields, very short reaction times, and easy work-up procedure. In addition, the condensation reaction to produce imidazo[1,2-a]pyrimidine derivatives assisted by microwave irradiation and catalyzed by Al2O3 without any solvent was performed under more environmentally friendly conditions compared to other evaluation methods. In this study, all the compounds were tested, and they showed good activity against Gram-positive bacteria and yeasts but not against Gram-negative bacteria. Some of the compounds exhibited better antimicrobial activity compared to others.
Furthermore, the molecular docking study showed that compounds 3g, 3k, and 3j have high affinity for the binding site of the antimicrobial target, which was confirmed by the lowest energy score with good binding modes, leading to the formation of different interaction types. Additionally, in silico ADME and toxicity predictions were performed on these compounds, and most of them were found to comply with the Lipinski, Veber, and Egan rules with good drug-likeness, some oral bioavailability properties, and good pharmacokinetic profiles. This study confirms that these derivatives are not hepatotoxic and are not carcinogenic.
Finally, this research identifies new imidazo[1,2-a]pyrimidines derivatives that may aid in the design and development of novel antibacterial agents.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29215058/s1, compounds 3a–3k.
Author Contributions
Conceptualization, Z.K. and N.C.-B.; methodology, D.B. and N.A.; software, I.D.; validation, N.C.-B., Z.K. and D.B.; writing—original draft preparation, Z.K., N.A. and I.D.; writing—review and editing, N.C.-B., Z.K., J.A.S., and visualization, J.A.S. and M.P.V.-T.; C.Z.-C. and L.B.; supervision, N.C.-B., Z.K. and I.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The authors are grateful to the Directorate General for Scientific Research and Technological Development (DGRSDT) and the University of Tlemcen for their financial support. JAS and MPVT thank the Ministerio de Ciencia y Tecnología (Project MAT2017-86109P) for financial support.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Nasirmahale L.N., Shirini F., Bayat Y., Mazloumi M. Solvent-Free Synthesis of Imidazo[1,2-a]Pyrimidine-3-Carbonitriles and 1,2,4-Triazolo[4,3-a]Pyrimidines under the Catalytic Performance of TiO2-[Bip]-NH2+ C(NO2)3− as a Novel Nanocatalyst. J. Mol. Struct. 2023;1272:134210. doi: 10.1016/j.molstruc.2022.134210. [DOI] [Google Scholar]
- 2.Nezhadramezan-Ghasemabadi H., Mazloumi M., Azimi S.C., Shirini F. One-Pot Three Component Synthesis of Pyrido[2,3-d]Pyrimidines and Benzo[4,5]Imidazo[1,2-a]-Pyrimidine-3-Carbonitrile Catalyzed by Acidic Ionic Liquid Immobilized on Nanoporous TiO2. J. Mol. Struct. 2023;1274:134435. doi: 10.1016/j.molstruc.2022.134435. [DOI] [Google Scholar]
- 3.Rawat M., Rawat D.S. Copper Oxide Nanoparticle Catalysed Synthesis of Imidazo[1,2-a]Pyrimidine Derivatives, Their Optical Properties and Selective Fluorescent Sensor towards Zinc Ion. Tetrahedron Lett. 2018;59:2341–2346. doi: 10.1016/j.tetlet.2018.05.005. [DOI] [Google Scholar]
- 4.Rehan T.A., Al-Lami N., Alanee R.S. Anti-Cancer and Antioxidant Activities of Some New Synthesized 3-Secondary Amine Derivatives Bearing Imidazo [1,2-A] Pyrimidine. Eurasian Chem. Commun. 2021;3:339–351. doi: 10.22034/ecc.2021.277531.1151. [DOI] [Google Scholar]
- 5.Shang L.L., Feng Y., Gao X.L., Chen Z.R., Xia Y., Jin W.W., Liu C.J. DMAP-Catalyzed C—N Bond Formation for Diverse Synthesis of Imidazo[1,2-a]Pyrimidine and Pyrimido[1,2-a]Benzimidazole Derivatives. Chin. J. Chem. 2020;38:1595–1599. doi: 10.1002/cjoc.202000214. [DOI] [Google Scholar]
- 6.Güngör T. Microwave Assisted, Sequential Two-Step, One-Pot Synthesis of Novel Imidazo[1,2-a] Pyrimidine Containing Tri/Tetrasubstituted Imidazole Derivatives. Turk. J. Chem. 2021;45:219–230. doi: 10.3906/kim-2009-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goel R., Luxami V., Paul K. Synthetic Approaches and Functionalizations of Imidazo[1,2-a]Pyrimidines: An Overview of the Decade. RSC Adv. 2015;5:81608–81637. doi: 10.1039/C5RA14795F. [DOI] [Google Scholar]
- 8.Kobak R.Z.U., Akkurt B. Formation and Uses of Imidazo[1,2-a]Pyrimidines and Related Compounds: A Review Comprising Years 2000–2021. J. Turk. Chem. Soc. Sect. A Chem. 2022;9:1335–1386. doi: 10.18596/jotcsa.1110922. [DOI] [Google Scholar]
- 9.Atif H.Y.S., Wagare D.S., Ahmed A.Z., Durrani A.N. Ultrasound Promoted One-Pot Synthesis of 2-Arylimidazo[1,2-a]Pyrimidines in Glycerol. Rasayan J. Chem. 2021;14:2645–2651. doi: 10.31788/RJC.2021.1446502. [DOI] [Google Scholar]
- 10.Aeluri R., Alla M., Polepalli S., Jain N. Synthesis and Antiproliferative Activity of Imidazo[1,2-a]Pyrimidine Mannich Bases. Eur. J. Med. Chem. 2015;100:18–23. doi: 10.1016/j.ejmech.2015.05.037. [DOI] [PubMed] [Google Scholar]
- 11.Annareddygari S., Kasireddy V., Reddy J. Synthesis of Novel Amide-Functionalized Imidazo[1,2-a]Pyrimidin-5(1H)-Ones and Their Biological Evaluation as Anticancer Agents. Russ. J. Org. Chem. 2022;58:412–418. doi: 10.1134/S1070428022030216. [DOI] [Google Scholar]
- 12.Ravindar L., Hasbullah S.A., Rakesh K.P., Hassan N.I. Pyrazole and Pyrazoline Derivatives as Antimalarial Agents: A Key Review. Eur. J. Pharm. Sci. 2023;183:106365. doi: 10.1016/j.ejps.2022.106365. [DOI] [PubMed] [Google Scholar]
- 13.Azzouzi M., El Ouafi Z., Azougagh O., Daoudi W., Ghazal H., Barkany S.E., Abderrazak R., Mazières S., El Aatiaoui A., Oussaid A. Design, Synthesis, and Computational Studies of Novel Imidazo[1,2-a]Pyrimidine Derivatives as Potential Dual Inhibitors of HACE2 and Spike Protein for Blocking SARS-CoV-2 Cell Entry. J. Mol. Struct. 2023;1285:135525. doi: 10.1016/j.molstruc.2023.135525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ramírez-Trinidad Á., Carrillo-Jaimes K., Rivera-Chávez J.A., Hernández-Vázquez E. Synthesis and Cytotoxic/Antimicrobial Screening of 2-Alkenylimidazo[1,2-a]Pyrimidines. Med. Chem. Res. 2023;32:144–157. doi: 10.1007/s00044-022-02997-6. [DOI] [Google Scholar]
- 15.Güngör T. One Pot, Multicomponent Protocol for the Synthesis of Novel Imidazo[1,2-a]Pyrimidine-Based Pyran Analogs: A Potential Biological Scaffold. Monatshefte Fur Chem. 2020;151:781–789. doi: 10.1007/s00706-020-02601-w. [DOI] [Google Scholar]
- 16.Mantipally M., Gangireddy M.R., Gundla R., Badavath V.N., Mandha S.R., Maddipati V.C. Rational Design, Molecular Docking and Synthesis of Novel Homopiperazine Linked Imidazo[1,2-a]Pyrimidine Derivatives as Potent Cytotoxic and Antimicrobial Agents. Bioorganic Med. Chem. Lett. 2019;29:2248–2253. doi: 10.1016/j.bmcl.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 17.Gómez-García O., Andrade-Pavón D., Campos-Aldrete E., Ballinas-Indilí R., Méndez-Tenorio A., Villa-Tanaca L., Álvarez-Toledano C. Synthesis, Molecular Docking, and Antimycotic Evaluation of Some 3-Acyl Imidazo[1,2-a]Pyrimidines. Molecules. 2018;23:599. doi: 10.3390/molecules23030599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rival Y., Grassy G., Taudou A., Ecalle R. Antifungal Activity in Vitro of Some Imidazo[1,2-a]Pyrimidine Derivatives. Eur. J. Med. Chem. 1991;26:13–18. doi: 10.1016/0223-5234(91)90208-5. [DOI] [Google Scholar]
- 19.Chatzopoulou M., Martínez R.F., Willis N.J., Claridge T.D.W., Wilson F.X., Wynne G.M., Davies S.G., Russell A.J. The Dimroth Rearrangement as a Probable Cause for Structural Misassignments in Imidazo[1,2-a]Pyrimidines: A 15N-Labelling Study and an Easy Method for the Determination of Regiochemistry. Tetrahedron. 2018;74:5280–5288. doi: 10.1016/j.tet.2018.06.033. [DOI] [Google Scholar]
- 20.Kumar G., Kiran Tudu A. Tackling Multidrug-Resistant Staphylococcus Aureus by Natural Products and Their Analogues Acting as NorA Efflux Pump Inhibitors. Bioorg. Med. Chem. 2023;80:117187. doi: 10.1016/j.bmc.2023.117187. [DOI] [PubMed] [Google Scholar]
- 21.Kibou Z., Aissaoui N., Daoud I., Seijas J.A., Vázquez-Tato M.P., Klouche-Kheli N., Choukchou-Braham N. Efficient Synthesis of 2-Aminopyridine Derivatives: Antibacterial Activity Assessment and Molecular Docking Studies. Molecules. 2022;27:3439. doi: 10.3390/molecules27113439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baba-Ahmed I., Kibou Z., Choukchou-Braham N. Recent Advances in the Synthesis of Tacrine Derivatives as Multifunctional Agents for Alzheimer’s Disease. Curr. Org. Chem. 2021;25:2579–2624. doi: 10.2174/1385272825666210716154531. [DOI] [Google Scholar]
- 23.Belhadj F., Kibou Z., Benabdallah M., Aissaoui M., Rahmoun M.N., Villemin D., Choukchou-Braham N. Synthesis and Biological Evaluation of New Chromenes and Chromeno[2,3-d] Pyrimidines. S. Afr. J. Chem. 2021;75:150–155. doi: 10.17159/0379-4350/2021/v75a18. [DOI] [Google Scholar]
- 24.Medjdoub A., Belhadj F., Saidi Merzouk A., Baba Hamed Y., Kibou Z., Choukchou-Braham N., Merzouk H. In Vitro Peripheral Blood Mononuclear Cell Proliferation, Cytokine Secretion and Oxidative Stress Modulation by Pyrido[2,3-d] Pyrimidines. Chem. Pap. 2020;74:903–913. doi: 10.1007/s11696-019-00924-5. [DOI] [Google Scholar]
- 25.Belhadj F., Kibou Z., Cheikh N., Choukchou-Braham N., Villemin D. Convenient Access to New 4-Substituted Aminopyrido[2,3-d]Pyrimidine Derivatives. Tetrahedron Lett. 2015;56:5999–6002. doi: 10.1016/j.tetlet.2015.09.042. [DOI] [Google Scholar]
- 26.Nouali F., Sousa J.L.C., Albuquerque H.M.T., Mendes R.F., Paz F.A.A., Saher L., Kibou Z., Choukchou-Braham N., Talhi O., Silva A.M.S. Microwave-Assisted Synthesis of 4,6-Disubstituted Isoindoline-1,3-Diones by Diels-Alder Reactions. J. Mol. Struct. 2023;1275:134608. doi: 10.1016/j.molstruc.2022.134608. [DOI] [Google Scholar]
- 27.Kibou Z., Villemin D., Lohier J.F., Cheikh N., Bar N., Choukchou-Braham N. Easy Solventless Synthesis of New Mono and Bis Amino-5H-Chromeno [3,4-c] Pyridin-5-One Derivatives. Tetrahedron. 2016;72:1653–1661. doi: 10.1016/j.tet.2016.01.063. [DOI] [Google Scholar]
- 28.Xie Y.Y. Organic Reactions in Ionic Liquids: Ionic Liquid-Accelerated One-Pot Synthesis of 2-Arylimidazo[1,2-a]Pyrimidines. Synth. Commun. 2005;35:1741–1746. doi: 10.1081/SCC-200063911. [DOI] [Google Scholar]
- 29.Sindi A., Chawn M.V.B., Hernandez M.E., Green K., Islam M.K., Locher C., Hammer K. Anti-Biofilm Effects and Characterisation of the Hydrogen Peroxide Activity of a Range of Western Australian Honeys Compared to Manuka and Multifloral Honeys. Sci. Rep. 2019;9:17666. doi: 10.1038/s41598-019-54217-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wasihun A.G., Kasa B.G. Evaluation of Antibacterial Activity of Honey against Multidrug Resistant Bacteria in Ayder Referral and Teaching Hospital, Northern Ethiopia. Springerplus. 2016;5:1–8. doi: 10.1186/s40064-016-2493-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imberty A., Hardman K.D., Carver J.P., Perez S. Molecular Modelling of Protein-Carbohydrate Interactions. Docking of Monosaccharides in the Binding Site of Concanavalin A. Glycobiology. 1991;1:631–642. doi: 10.1093/glycob/1.6.631. [DOI] [PubMed] [Google Scholar]
- 32.Wade R.C., Goodford P.J. The Role of Hydrogen-Bonds in Drug Binding. Prog. Clin. Biol. Res. 1989;289:433–444. [PubMed] [Google Scholar]
- 33.Skok Ž., Barančoková M., Benek O., Cruz C.D., Tammela P., Tomašič T., Zidar N., Mašič L.P., Zega A., Stevenson C.E.M., et al. Exploring the Chemical Space of Benzothiazole-Based DNA Gyrase B Inhibitors. ACS Med. Chem. Lett. 2020;11:2433–2440. doi: 10.1021/acsmedchemlett.0c00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kowalczyk A., Paneth A., Trojanowski D., Paneth P., Zakrzewska-Czerwińska J., Stączek P. Thiosemicarbazide Derivatives Decrease the ATPase Activity of Staphylococcus Aureus Topoisomerase IV, Inhibit Mycobacterial Growth, and Affect Replication in Mycobacterium Smegmatis. Int. J. Mol. Sci. 2021;22:3881. doi: 10.3390/ijms22083881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sokolova N., Zhang L., Deravi S., Oerlemans R., Groves M.R., Haslinger K. Structural Characterization and Extended Substrate Scope Analysis of Two Mg2+-Dependent O-Methyltransferases from Bacteria**. ChemBioChem. 2023;24:e202300076. doi: 10.1002/cbic.202300076. [DOI] [PubMed] [Google Scholar]
- 36.Aljohny B.O., Rauf A., Anwar Y., Naz S., Wadood A. Antibacterial, Antifungal, Antioxidant, and Docking Studies of Potential Dinaphthodiospyrols from Diospyros Lotus Linn Roots. ACS Omega. 2021;6:5878–5885. doi: 10.1021/acsomega.0c06297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aarjane M., Slassi S., Tazi B., Amine A. Synthesis and Biological Evaluation of Novel Isoxazole Derivatives from Acridone. Arch. Pharm. 2021;354:e2000261. doi: 10.1002/ardp.202000261. [DOI] [PubMed] [Google Scholar]
- 38.Hosseini Z.S., Housaindokht M.R., Razzaghi-Asl N., Miri R. Virtual Screening of Some Heterocyclic Structures toward Novel Antibacterial Agents. J. Iran. Chem. Soc. 2018;15:621–628. doi: 10.1007/s13738-017-1262-2. [DOI] [Google Scholar]
- 39.Miguel A., Hsin J., Liu T., Tang G., Altman R.B., Huang K.C. Variations in the Binding Pocket of an Inhibitor of the Bacterial Division Protein FtsZ across Genotypes and Species. PLoS Comput. Biol. 2015;11:e1004117. doi: 10.1371/journal.pcbi.1004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Then A., Zhang H., Ibrahim B., Schuster S. Bioinformatics Analysis of the Periodicity in Proteins with Coiled-Coil Structure—Enumerating All Decompositions of Sequence Periods. Int. J. Mol. Sci. 2022;23:8692. doi: 10.3390/ijms23158692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Desai J., Liu Y., Wei H., Liu W., Ko T., Guo R., Oldfield E. Structure, Function, and Inhibition of Staphylococcus Aureus Heptaprenyl Diphosphate Synthase. ChemMedChem. 2016;11:1915–1923. doi: 10.1002/cmdc.201600311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sasaki D., Fujihashi M., Okuyama N., Kobayashi Y., Noike M., Koyama T., Miki K. Crystal Structure of Heterodimeric Hexaprenyl Diphosphate Synthase from Micrococcus Luteus B-P 26 Reveals That the Small Subunit Is Directly Involved in the Product Chain Length Regulation. J. Biol. Chem. 2011;286:3729–3740. doi: 10.1074/jbc.M110.147991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barakat A., Al-Majid A.M., Al-Qahtany B.M., Ali M., Teleb M., Al-Agamy M.H., Naz S., Ul-Haq Z. Synthesis, Antimicrobial Activity, Pharmacophore Modeling and Molecular Docking Studies of New Pyrazole-Dimedone Hybrid Architectures. Chem. Cent. J. 2018;12:29. doi: 10.1186/s13065-018-0399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daina A., Michielin O., Zoete V. SwissADME: A Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pires D.E.V., Blundell T.L., Ascher D.B. PkCSM: Predicting Small-Molecule Pharmacokinetic and Toxicity Properties Using Graph-Based Signatures. J. Med. Chem. 2015;58:4066–4072. doi: 10.1021/acs.jmedchem.5b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nariya P.B., Bhalodia N.R., Shukla V.J., Nariya M.B. In Vitro Evaluation of Antimicrobial and Antifungal Activity of Cordia Macleodii Bark. (HOOK.F. & THOMSON) Int. J. PharmTech Res. 2010;2:2522–2526. [Google Scholar]
- 47.Rai N.P., Narayanaswamy V.K., Govender T., Manuprasad B.K., Shashikanth S., Arunachalam P.N. Design, Synthesis, Characterization, and Antibacterial Activity of {5-Chloro-2-[(3-Substitutedphenyl-1,2,4-Oxadiazol-5-Yl)-Methoxy]-Phenyl} -(Phenyl)-Methanones. Eur. J. Med. Chem. 2010;45:2677–2682. doi: 10.1016/j.ejmech.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 48.Stewart J.J.P. Optimization of Parameters for Semiempirical Methods V: Modification of NDDO Approximations and Application to 70 Elements. J. Mol. Model. 2007;13:1173–1213. doi: 10.1007/s00894-007-0233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu J., Patel S., Sharma N., Soisson S.M., Kishii R., Takei M., Fukuda Y., Lumb K.J., Singh S.B. Structures of Kibdelomycin Bound to Staphylococcus Aureus GyrB and ParE Showed a Novel U-Shaped Binding Mode. ACS Chem. Biol. 2014;9:2023–2031. doi: 10.1021/cb5001197. [DOI] [PubMed] [Google Scholar]
- 50.Cho J.H., Park Y., Ahn J.H., Lim Y., Rhee S. Structural and Functional Insights into O-Methyltransferase from Bacillus Cereus. J. Mol. Biol. 2008;382:987–997. doi: 10.1016/j.jmb.2008.07.080. [DOI] [PubMed] [Google Scholar]
- 51.Raymond A., Lovell S., Lorimer D., Walchli J., Mixon M., Wallace E., Thompkins K., Archer K., Burgin A., Stewart L. Combined Protein Construct and Synthetic Gene Engineering for Heterologous Protein Expression and Crystallization Using Gene Composer. BMC Biotechnol. 2009;9:37. doi: 10.1186/1472-6750-9-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Daoud I., Melkemi N., Salah T., Ghalem S. Combined QSAR, Molecular Docking and Molecular Dynamics Study on New Acetylcholinesterase and Butyrylcholinesterase Inhibitors. Volume 74. Elsevier Ltd.; Amsterdam, The Netherlands: 2018. [DOI] [PubMed] [Google Scholar]
- 53.Daoud I., Mesli F., Melkemi N., Ghalem S., Salah T. Discovery of Potential SARS-CoV 3CL Protease Inhibitors from Approved Antiviral Drugs Using: Virtual Screening, Molecular Docking, Pharmacophore Mapping Evaluation and Dynamics Simulation. J. Biomol. Struct. Dyn. 2022;40:12574–12591. doi: 10.1080/07391102.2021.1973563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bajda M., Wiȩckowska A., Hebda M., Guzior N., Sotriffer C.A., Malawska B. Structure-Based Search for New Inhibitors of Cholinesterases. Int. J. Mol. Sci. 2013;14:5608–5632. doi: 10.3390/ijms14035608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is contained within the article or Supplementary Materials.