Abstract
Background
Surgical site infections (SSI) result in increased morbidity and mortality, prolonged recovery, longer hospital length of stay for medication or possible additional surgeries, and escalated health care costs. The purpose of this randomized controlled trial was to compare SSI rates and overall skin flora burden between those using chlorhexidine (CHG) cloths versus soap and water preoperatively in the adult spine surgery population.
Methods
Subjects were randomized preoperatively to use 2% CHG cloths versus soap and water the night before and morning of surgery prior to the operation. A skin culture was obtained at enrollment prior to any cleansing, again at post-operation day 4 or hospital discharge (whichever came first), and finally at the surgeons’ postoperative visits. A blinded advanced practice nurse served as the assessor for SSI.
Results
Those enrolled in the research arm had more growth on their screening skin culture than the control arm (P = .02). While there was no difference in rates of SSI between groups, the CHG group had lower skin flora burden at hospital discharge (P = .004), indicating residual protection.
Conclusion
Surgical incisions are most vulnerable to bacterial entry prior to 72 hours post-operation before completion of epithelialization, which establishes a barrier from microbes. The use of CHG, which has a residual impact for up to 4 days, could offer additional risk reduction for SSI development.
Keywords: chlorhexidine gluconate (CHG), neuro-spine surgery, skin bacterial load, surgical site infections (SSI)
Introduction
Hospital-acquired infections (HAI) are nosocomial illnesses that surface within 48 hours of hospitalization, inflicting a toll on human suffering and increasing health care costs.1 Out of all 5 of the HAIs, surgical site infections (SSIs) are tied with pneumonia as the most frequently occurring infections at 21.8% of the overall rates of all hospital-acquired infections.1 For many patients, SSIs can result in discomfort or pain, reduced functional health, or in extreme cases may even result in death as well as prolonging hospitalizations, often requiring readmissions for additional medication or surgeries.2 Published studies indicate that the risk of death increases as high as 11 times in individuals experiencing SSI, with 77% of those deaths directly related to the SSI.1,3,4 Additionally, SSIs are listed as one of the most common and costly HAIs, with estimates as high as $10 billion spent annually on health care treatment and therapies related to these infections in the United States (US).2,5
SSI rates vary according to the type of infection, duration of the surgical procedure, and if the patients’ core temperature and blood glucose were well managed.6 According to the Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network, SSIs are a frequent complication of surgery, occurring in an estimated 21.8% of adult patients in the US.1,7 In addition, SSIs following spinal surgery are a frequent complication occurring in as high as 12.0% of cases.8 This study was completed at an academic medical center in the southeastern US. At the time, the SSI rate was 7% in the adult spine surgical patients at the site where this study was completed.
The CDC identifies 3 classes of SSIs, (1) SSIs related to superficial incisions, (2) SSIs related to deep incisions, and (3) organ/space SSIs. Recently, the Society of Healthcare Epidemiology of America (SHEA) issued a list of patient-related risk factors for SSIs, inclusive of advanced age, obesity, smoking, diabetes or poor glucose control, immunosuppressant therapies, an American Society of Anesthesiology (ASA) score of greater than 2, hospitalized preoperatively, and Staphylococcus aureus nasal colonization. 2,9 Microbial contamination of the surgical site may occur mainly during the surgical intervention or during hospitalization.
There are multiple practices focused on mitigating the risk of developing SSIs. In addition to aseptic surgical technique, infection-mitigating practices include pre-incision scrubbing and avoidance of clipping or shaving hair prior to incision. Preoperative testing of high-risk patients for nasal colonization of Methicillin-resistant Staphylococcus aureus (MRSA) followed by decolonizing prior to admission for a procedure further reduces the risk of incision contamination. Other mitigating strategies to reduce SSI include intraoperative antiseptic wound lavage, antimicrobial suture utilization, administration of IV antimicrobial prophylaxis 30 to 60 minutes prior to incision, reducing operating room noise, and optimizing or maintaining good blood glucose control.10,11
Accurate identification of modifiable risk factors for SSIs as well as the development of therapies for mitigating SSIs remain important in the development of strategies to prevent these potentially devastating infections.8 While the elimination of contamination from the surgical suite may be one strategy, many are caused by microorganisms present in patients’ skin flora preoperatively such as S aureus.9,12 Due in part to this, recommendations include modifying patient risk factors for developing SSIs whenever possible through the use of adequate protocols for antimicrobial prophylaxis with antibiotics as recommended by SHEA and Association of periOperative Registered Nurses.9,12,13 Preventative actions to reduce the total number of microorganisms on patients’ skin using topical agents such as chlorhexidine gluconate (CHG) in addition to cleansing nasal mucosa (using mupirocin ointment or provodine-iodine) preoperatively, have been successful in decreasing rates of incision infections in subjects preoperatively colonized with S. aureus.12
Over 2 decades ago, the CDC reported that cleansing with 2% CHG reduced skin bacterial load by 9-fold and subsequently released a recommendation to cleanse with CHG prior to surgical incision to reduce SSIs.5 This recommendation was followed by the CDC definitions for SSIs that included the infection must occur within 30 days of the incision to be considered surgery-related. Additionally, Graling and Vasaly18 found a statistically significant reduction in SSI rates with CHG bathing in 335 subjects compared to 284 control subjects in which there was no active intervention. This highlights CHG bathing as an interesting strategy to explore.
Prior studies have linked the use of 2% CHG cloths with decreased rates of SSIs and reported a 50% reduction in SSIs in adult patients following total knee replacements.14 Similarly, results of a study conducted with patients in medical intensive care unit patients bathed daily with CHG discovered bacterial skin concentration was inversely associated with the density of skin microorganisms over a 24-hour period, potentially representing a residual antimicrobial activity on the skin by the product.15 Incisional wounds epithelialize within 72 hours, suggesting that the highest risk for exposure to infectious agents occurs intraoperatively and in the first 3 days post-operation. Despite this fact, in many hospitals the use of CHG cloths preoperatively is not a standard practice (with some only washing with soap and water).6,7,14 This represents a gap in current nursing practice and best evidence-based care that could improve patient outcomes and reduce health care costs, making this worthy of additional exploration.
Methods
The purpose of this single-site randomized controlled trial (RCT) of adult neuro-spine patients was to compare SSI rates and overall changes in skin flora burden between cleansing with 2% CHG skin preparation cloths to cleansing with soap and water. Those subjects randomized to CHG, cleansed with the CHG cloths the night before at home and the morning of the surgery prior to the operation. The CHG cloths the researchers used were impregnated with aloe vera instead of alcohol as the investigators felt the subjects may better tolerate the non-drying impact of aloe vera. The research arm subjects were matched to patients using standard-of-care skin prep with soap and water only. Bacterial skin burden was evaluated through skin cultures collected at screening pre-cleansing, on post-operation day 4 or the day of hospital discharge (whichever came first), and again at the surgeons’ postoperative visit (approximately 30 days after the procedure). These repeated measurements allowed for the exploration of residual reduction in skin bacterial load for up to 4 and 30 days.
Approval, Recruitment, Consent, and Enrollment
Review and approval for this single-site RCT were provided by the Institutional Review Board overseeing the research at the participating institution (IRB# 150310). Adult patients scheduled for neurosurgical spine surgery were evaluated preoperatively by the researchers and, if they met inclusion and exclusion criteria, the individuals were approached for interest in participation by the research team. Patients were then provided written informed consent followed by discussions where the participants’ questions were answered. If the individual expressed interest in participating in the study, signed consent was obtained, participants were given a copy, and then they were enrolled and randomized. In alignment with good clinical practice, the design and reporting for the study followed the recommendations included in the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines. Study data were captured in the Research Electronic Data Capture System (REDCap®), a HIPAA-compliant, web-based, password-protected data repository.
Eligibility
For this trial, participants were eligible to participate if they were (1) 18 years or older, (2) scheduled for a neuro-spine procedure, and (3) had at least 2 of the following risk factors for SSI: diabetes, a BMI greater than 30, an ASA score greater than 2, preoperatively hospitalized, over 60 years old, taking chronic steroids or immunosuppressive medications, or having any prior history of SSIs.
During screening, potential participants were excluded if they were unable to provide consent, non-English speaking, or had a known allergy to any of the ingredients contained in the 2% CHG cloths required for the intervention. In addition, potential participants who were experiencing an infection at the time or had a history of spine infections were excluded because the required treatment of the infection with antibiotics immediately pre-operation would affect the microbes present. Potential participants were also excluded if they had intradural spinal tumors because prior investigations suggested CHG self-cleansing was contraindicated for operations addressing this type of pathology.
Randomization Schema
Following screening for eligibility and documentation of written informed consent, subjects were randomized 1:1 to either the intervention or control arms of the trial using a 4-block randomization table that the statisticians loaded into REDCap. The research team was unable to see the assignment in queue for the current or next subject, and REDCap maintained the blinding of each assignment until enrollment was complete. The blinded surgical site assessors were advanced practice nurses and experts on the subject matter of SSIs. These evaluators did not have access to the randomization allocation in the database, manage consent form retrievals, enroll subjects, or educate patients in using the CHG cloths for cleansings. They remained blinded to the randomization of the subjects for the entire 2-year period of the study.
Intervention Description
Neurosurgical patients randomized to the intervention arm received skin cleansing cloths impregnated with 2%-CHG to use at home the night before the surgery, and printed instructions were provided with education on preoperative cleansing from the neckline to the toes. Nurses educated the patients on the preoperative cleansing with CHG cloths, stressing not to shower or rinse skin after application and allowing the product to air dry on the skin. The cleansing was repeated preoperatively a second time on the day of surgery. The printed instructions had space for the research patients to place the peel-off label from the package of the CHG cloths, which they signed and included the date and time when the home cleansing was completed. The subjects brought the completed instruction sheet with them on the day of the surgery and returned it to research staff for documentation. The research staff also verbally confirmed adherence to the cleansing. One subject shared that they showered after cleaning with the CHG cloth because they felt tickling on their skin, and the research personnel removed this subject from the study. Patients randomized to the control arm were educated to cleanse with soap and water only the night before surgery.
All consented subjects had one skin culture swab obtained close to the intended incision line on the day of the screening prior to cleansing with 2% CHG cloths or soap/water. Additional skin culture swabs were collected at the site of incision after the operation on day 4 or the day of discharge (whichever occurred first) as well as at the post-operative follow-up visit with the surgeon (approximately 4 to 6 weeks after the procedure).
Both groups were evaluated daily for the development of SSIs by study personnel until post-operation day 4 or hospital discharge, whichever one came first. The final evaluation for SSI was completed during the post-operation follow-up with the surgeon. Two nurse practitioners served as blinded SSI evaluators utilizing the CDC outpatient procedure component of the SSI surveillance guideline to evaluate and grade the incision line for the presence of SSI.7 Measurements for a change in skin flora were completed by comparing skin swab cultures results of the intervention group versus the standard of care group and individual changes preoperatively and postoperatively.
Outcomes and Measures
All outcome measures were selected a priori. The primary outcome of this trial was development of SSIs. The secondary outcome for this trial was a change in skin flora burden (measured via skin cultures from pre-intervention compared to the culture collected at hospital discharge and at the 30-day follow-up visit with the surgeon). All patient data in the database were de-identified by research personnel with assigned codes instead of names.
Statistical Analyses
The statistical plan included a study of independent cases and controls with 1 control per case. Sample size calculation for the study, which included evaluation of prior research, indicated if the rate of infection in the control group (those with risk factors, including superficial SSI) was 0.5, then just 150 were needed to detect a 50% or greater reduction (80% power) and resulted in a calculation of a sample of 150, with 75 in each arm.14 We described patient demographic and baseline characteristics using the median and interquartile range (IQR: 25th, 75th percentiles) for continuous variables and a frequency and percentage for categorical variables. We compared patient demographics and baseline characteristics between study groups using the Wilcoxon rank sum test for continuous variables and the Pearson’s chi-square test for categorical variables. We compared the rate of SSI between groups pre-cleansing, post-operation day 4 or discharge, and at the 30-day follow-up visit, and we used the chi-square test or Fisher’s exact test on skin flora burden data from the post-operation day 4/hospital discharge and the 30-day follow-up visit groups as appropriate. We fit the logistic model to further examine the strength of the relationship the intervention had on SSI and skin flora burden on post-op day 4/discharge and at the 30-day follow-up visit. The randomized controlled trial method greatly reduces the potential of the data being confounding, so we examined the balance of variables between the 2 arms, and these variables were distributed evenly. Additionally, we had a relatively small effective sample size of 38 events, including all baseline variables in the model for adjustment would cause model overfitting. Therefore, we did not adjust for baseline variables in the logistic model. We observed a higher skin culture growth rate in the research arm at screening, so we fit the same logistic model but adjusted for the results differences in the baseline skin culture in a sensitivity analysis. We showed the effect estimates in terms of the odds ratio of any growth of skin bacteria (odds ratio [OR], 95% confidence interval [CI], P value). We used a 2-sided P of less than .05 significance level for statistical inference. All analyses were conducted using R, version 4.3.0,16 specifically the regression modeling strategies package.17
Results
Study Population
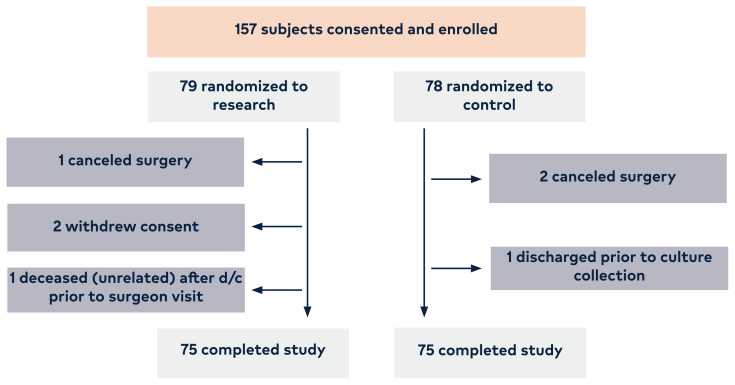
A total of 157 adult patients undergoing any type of neuro-surgical spine surgery at the participating site were consented. Subjects were enrolled over a 2-year period, with 150 consented subjects (96%) completing study participation, resulting in a total of 75 subjects per arm (Figure 1).
Figure 1.
The enrollment and allocation of subjects into the 2 arms of the study.
Demographics collected allowed for comparisons between subjects in the research (2% CHG cloths) arm and the control (soap and water) arm in regards to age, gender, race, risk factors, total operating room time in minutes, and surgical procedure (Table 1).
Table 1.
Table of Demographics and Baseline Characteristics (Screening Timepoint)
| Characteristic | Overall (N = 150) | Study group A-research (n = 75) | Study group B-control (n = 75) | P value |
|---|---|---|---|---|
| Age (years) | 65.0 (59.3, 72.0) | 66.0 (61.0, 72.0) | 63.0 (57.0, 71.5) | .09 |
| Patient > 60 years old | 113 (75%) | 61 (81%) | 52 (69%) | .09 |
| Gender | .33 | |||
| Male | 78 (52%) | 42 (56%) | 36 (48%) | |
| Female | 72 (48%) | 33 (44%) | 39 (52%) | .53 |
| Race | ||||
| White | 133 (89%) | 66 (88%) | 67 (89%) | |
| Black | 11 (7.3%) | 6 (8.0%) | 5 (6.7%) | |
| Hispanic | 2 (1.3%) | 0 (0%) | 2 (2.7%) | |
| Asian | 4 (2.7%) | 3 (4.0%) | 1 (1.3%) | |
| Body Mass Index (kg/m2) | 31.3 (27.0, 36.0) | 31.3 (27.5, 34.3) | 30.9 (26.8, 37.4) | .67 |
| BMI > 30 | 79 (53%) | 40 (53%) | 39 (52%) | .87 |
| Is the patient currently a smoker or have they smoked within the last year? | 21 (14%) | 10 (14%) | 11 (15%) | .84 |
| (Missing) | 1 | 1 | 0 | |
| Is the patient a diabetic? | 53 (35%) | 23 (31%) | 30 (40%) | .23 |
| ASA > 2 | 146 (98%) | 74 (99%) | 72 (97%) | .62 |
| (Missing) | 1 | 0 | 1 | |
| Has the patient been on chronic steroids or immunosuppressive medications? | 47 (31%) | 25 (33%) | 22 (29%) | .60 |
| Has the patient ever had a SSI in the past? | 22 (15%) | 11 (15%) | 11 (15%) | >.99 |
| Pre-operatively hospitalized | 2 (1.3%) | 0 (0%) | 2 (2.7%) | .50 |
| Length of procedure in minutes | 194.0 (155.0, 248.0) | 182.5 (152.0, 229.0) | 217.0 (162.5, 276.5) | .08 |
| (Missing) | 1 | 1 | 0 | |
| Total OR time in minutes | 281.0 (237.0, 352.0) | 265.0 (234.3, 323.3) | 287.0 (241.5, 367.5) | .07 |
| (Missing) | 1 | 1 | 0 | |
| Screening/BL skin culture result (binary) | .02 | |||
| No growth | 68 (45%) | 27 (36%) | 41 (55%) | |
| Growth | 82 (55%) | 48 (64%) | 34 (45%) | |
| Screening/BL skin culture category | .01 | |||
| No growth | 68 (45%) | 27 (36%) | 41 (55%) | |
| Staph aureus | 3 (2.0%) | 0 (0%) | 3 (4.0%) | |
| VRE | 1 (0.7%) | 1 (1.3%) | 0 (0%) | |
| Other | 78 (52%) | 47 (63%) | 31 (41%) |
Note: We displayed the median (25th, 75th percentile) for continuous variables and frequency (percentage) for categorical variables. P values were calculated using Pearson’s chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables.
Overall, both groups were similar in age, race, and risk factors for SSIs. Our total patient population was approximately 50% male:female (n = 72 female, 48%), with 52% (n = 39) females in the CHG arm and 44% (n = 33) in the control arm. White patients represented the largest percentage of the population enrolled at 89% (n = 133), followed by Black patients at 7.3% (n = 11), Asians at 2.7% (n = 4), and Hispanics at 1.3% (n = 2), showing some diversity of individuals participating. This diversity was similarly represented in both arms of the study. Length of surgical procedure in minutes revealed an overall median of 194 minutes (IQR 155.0, 248.0), and the control group had slightly longer median surgical procedure times at 217 minutes (IQR 162.5, 276.5) compared to the research arm of 182.5 minutes (IQR 152.0, 229.0) without reaching statistical significance (Table 1).
Surgical Site Infections
Overall, only 21 SSIs occurred in both arms of the study by post-operation day 30. Superficial incisional site infection occurred in 4.7% (n = 7) of cases and was evenly distributed, with 4% (n = 3) of SSIs occurring within the control arm and 5.4% (n = 4) in the research arm (P = .72). A total of 14 subjects (9.5%) sought medical attention due to any other concern over their incision after hospital discharge with 6 (8.1%) in the control arm and 8 (11%) in the research arm (P = .57) (Table 2).
Table 2.
Primary and Secondary Outcomes at Timepoints POD1, POD2, POD3, POD4/Discharge, and 30-Day Follow-up
| Clinical Outcomes | Overall (N= 150) | Study group A– research (N = 75) | Study group B–control (N = 75) | P value |
|---|---|---|---|---|
| Skin culture result (POD 4/Hospital Discharge) | .004 | |||
| Growth | 34 (30%) | 9 (17%) | 25 (42%) | |
| No growth | 78 (70%) | 44 (83%) | 34 (58%) | |
| Lost to follow-up or discharged early | 38 | 22 | 16 | |
| Skin culture result (30-day FU) | .45 | |||
| Growth | 56 (61%) | 25 (57%) | 31 (65%) | |
| No growth | 36 (39%) | 19 (43%) | 17 (35%) | |
| (Unknown) | 58 | 31 | 27 | |
| Diagnosis of SSI (POD 1) | 1 (0.9%) | 1 (1.9%) | 0 (0%) | .47 |
| (Unknown) | 34 | 21 | 13 | |
| Diagnosis of SSI (POD 2) | 0 (0%) | 0 (0%) | 0 (0%) | > .99 |
| (Unknown) | 64 | 34 | 30 | |
| Diagnosis of SSI (POD 3) | 0 (0%) | 0 (0%) | 0 (0%) | > .99 |
| (Unknown) | 104 | 56 | 48 | |
| Diagnosis of SSI (POD 4/Discharge) | 0 (0%) | 0 (0%) | 0 (0%) | > .99 |
| (Unknown) | 112 | 58 | 54 | |
| Diagnosis of SSI (30-day FU) | 7 (4.7%) | 4 (5.4%) | 3 (4.0%) | .72 |
| (Unknown) | 1 | 1 | 0 | |
| Sought medical attention regarding the incision since discharge | 14 (9.5%) | 8 (11%) | 6 (8.1%) | .57 |
| (Unknown) | 2 | 1 | 1 |
Note: We displayed frequency (percentage) for categorical outcome variables and compared them between two groups using Pearson’s chi-square test or Fisher’s exact test as needed.
Abbreviations: SSI = surgical site infections; FU = follow-up; POD = post-operative day
Skin Flora Burden
A total of 55% (n = 82) of baseline pre-intervention skin cultures grew out microbes with a larger percentage in the research arm 64% (n = 48) compared to the control arm 45% (n = 34) (P = .02). Subjects’ skin cultures from the day of hospital discharge revealed 30% (n = 34) of all participants grew bacteria with 17% (n = 9) in the research arm and 42% (n = 25) in the control arm, and this difference was statistically significant at P = .004. To further examine the relationship between these variables (study arm and growth on skin culture at pre-cleansing and hospital discharge), a logistic regression analysis was completed with and without adjusting for the skin cultures at screening. Results were consistent and indicated the research arm was significantly less likely to have dermal microbe growth at hospital discharge (Not adjusted: OR = 0.28, 95% CI 0.12–0.67, P = .01; Adjusted: OR = 0.24, 95% CI 0.10–0.61, P = .003). This medium effect size resulted in the predicted probability of no growth in the research arm of 83% (95% CI 70–91%). In the adjusted logistic model, the screening skin culture was not significantly associated with dermal microbe growth (P = .26). By the time of the surgeons’ follow-up visit, there was no difference in bacterial dermal burden between the study and control arms (P = .45)
Discussion
The purpose of this RCT was to compare subjects’ SSI rates and overall changes in skin flora burden between adult neuro-spine patients randomized to receive either antiseptic skin cleanings with 2% CHG skin preparation cloths twice prior to surgery or standard soap and water. Skin cultures were collected pre-cleansing, at hospital discharge, and again at the postoperative visit with the surgeon (approximately 30 days after the procedure) for exploration of residual reduction in skin bacterial load for up to 4 and 30 days. To our knowledge, this is the first study to evaluate changes in skin flora burden or dermal microbe growth over time (preoperative to hospital discharge) by comparing CHG cleansing to soap and water.
Findings included no difference in SSI grade or incidence between the 2 groups, which could be due to the study being underpowered. The initial power analysis for the study’s sample size was based upon the findings from the study completed by Eiselt revealing a 50% reduction in SSI in their study of orthopedic lower extremity surgeries, a different population than our adult neuro-spine patients.14 The research arm entered the study with a statistically-significant higher bacterial skin burden pre-cleansing (P = .02) yet experienced the same number of SSIs as the control group. The research arm containing more patients with higher bacterial skin burden may be a reason that there was not a lower rate of SSI post-procedure. Perhaps SSIs were not a good choice for an outcome variable as only 21 total SSIs were observed in this study. Even if there was an enrollment of 1000 subjects per arm, assuming similar SSI results, the study could have revealed no difference. There are also multiple publications of smaller effect sizes. The literature lacks clear guidance and conflicting reports, and additional research may be needed to better describe the effect size of utilizing 2% CHG for the reduction of SSIs in clean surgeries.19,20
However, those who used the CHG cloths in our study had statically significantly reduced skin flora burden at discharge (P = .004) even though the last cleansing with the cloths occurred on the day of the procedure prior to the operation, indicating that residual protection can last up to 4 days. By the time of the surgeons’ follow-up visit approximately 30 days later, there was no difference in skin bacterial load between the 2 groups, indicating that the difference seen at discharge was due to the CHG cloths and that the protection offered by the CHG cloths declined over time.
Historically, hospitals are where the journey to healing and overcoming illnesses occurs. Unfortunately, sometimes it is also where patients contract illnesses in the form of HAI. As these HAIs result in patients’ reduced quality of life, discomfort, pain, and, rarely, lasting impaired function and prolonged hospitalization, SSIs can be devastating and costly to patients. The surgical incision site is most vulnerable prior to epithelization, which typically occurs by 72 hours post-incision.21 Therefore, maintaining skin that is free from bacteria and potentially infectious agents is desired and beneficial during this critical time period. Our findings of reduced bacterial skin burden at the time of discharge for the research arm support this concept of protecting patients from SSIs during the most vulnerable time of healing. The baseline skin cultures (collected prior to any cleansing) in the research arm were statically significant for higher levels of bacterial skin burden (P = .007) compared to the control arm. This finding resulted in a logistic regression model to correct for higher bacterial skin burden pre-cleansing in the study arm. In light of this, our findings of reduced dermal microbes at the time of discharge in the research arm suggest that the 2% CHG cleansings may have an even stronger effect.
Implications
The outcome of this study has implications for nursing practice and empowering patients’ participation in their own care. The study participants were involved in preoperative care that could reduce SSIs by using the 2% CHG cloths the night before surgery. This might have created a sense of being part of decision-making related to their surgery outcomes. Additionally, this study revealed a prolonged reduction in the quantity of microbes on the skin and therefore offers lasting protection from the double preoperative antiseptic cleansing using 2% CHG cloths during the critical time of the incision’s epithelization. The particular cloths used for this project used aloe vera as the carrier instead of the alcohol utilized in similar clothes. The use of aloe vera may offer improved hydration of epithelial layers possibly allowing CHG to penetrate deeper into the dermal layers and more research is needed to compare these cloths to those utilizing alcohol. Nurses can educate patients on the double preoperative cleansing with CHG cloths, stressing not to shower, bathe, or rinse after application and allowing the product to air dry on the skin (Figure 2).
Figure 2.
The Patient Education on the use of CHG cloths that were given to participants enrolled in the research arm along with the packages of 2% CHG cloths.
Limitations
Our study was a single-site department study confined to the use of CHG cloths for the prevention of SSIs in adults undergoing neuro-spine surgeries. In this study, adult surgical cases other than ortho-neuro spine were not included. There is conflicting evidence of the effect size of the intervention, and more research is needed to accurately determine this. The sample size calculation for this project was based upon the 50% effect size reported by Eiselt, resulting in an underpowered study and inability to assess the impact of 2% CHG cloths on SSI.14 Certain factors such as the length of surgeries and/or other medical conditions were not considered in this study. Lastly, the researchers did not hold blood glucose constant during the entire hospitalization between groups, which also could have impacted the results.
Conclusion
While unable to ascertain if the use of 2% CHG cloths twice preoperatively could reduce the rates of SSI, possibly due to our sample size, findings do support reduced microbial density on the skin from CHG cleansing that lasted until hospital discharge. This is an important finding that could offer protection during the critical first phase of healing of an incision. Additional research is needed to understand effect size and if these findings can be replicated in other patient populations.
Funding Statement
Dr Shotwell thanks and acknowledges the National Institutes of Health for the funding given to Vanderbilt University Medical Center in order to make this work possible (Grant: TUL1 TR000445 from NCATS/NIH).
Footnotes
Conflicts of Interest: Dr Card thanks and acknowledges Sage Therapeutics for the grant that made this work possible. The opinions expressed reflect those of the authors and not necessarily those of Sage Therapeutics.
Dr Card serves on the Board of Directors of the Council on Surgical and Perioperative Safety.
Dr Wells reports consulting fees from Vanderbilt-Ingram Cancer Center.
Dr Cheng is a member of the AO Spine North America Board of Directors and the American Association of Neurological Surgeons Board of Directors.
Authors Shi and Adesinasi, and Drs Hall and Sherwood declare they have no conflicts of interest.
Dr Card reports personal honoraria from the American Society of PeriAnesthesia Nurses and financial support from the Emergency Nurses Association.
Dr Shotwell thanks and acknowledges the National Institutes of Health for the funding given to Vanderbilt University Medical Center in order to make this work possible (Grant: TUL1 TR000445 from NCATS/NIH). The opinions expressed reflect those of the authors and not necessarily those of the National Institutes of Health.
References
- 1.Monegro AF, Muppidi V, Regunath H. StatPearls. StatPearls Publishing; 2024. Hospital-acquired infections. Updated February 12, 2023 https://www.ncbi.nlm.nih.gov/books/NBK441857. [PubMed] [Google Scholar]
- 2.Tan SA, Sanchez JA. Surgical site and other acquired perioperative infections. In: Sanchez JA, Higgins RSD, Kent PS, editors. Handbook of Perioperative and Procedural Patient Safety. Elsevier; 2024. pp. 191–204. [Google Scholar]
- 3.Wan YI, Patel A, Abbott TEF, Achary C, Mac-Donald N, Duceppe E, et al. Prospective observational study of postoperative infection and outcomes after noncardiac surgery: analysis of prospective date from the VISION cohort. Br J Anaesth. 2020;125(1):87–89. doi: 10.1016/j.bja.2020.03.027. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien WJ, Gupta K, Itani KMF. Association of postoperative infection with risk of long-term infection and mortality. JAMA Surg. 2020;155(1):61–68. doi: 10.1001/jamasurg.2019.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR Hospital Infection Control Practices Advisory Committee. Guideline for prevention of surgical site infection, 1999. Infect Control Hosp Epidemiol. 1999;20(4):250–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 6.Schweizer ML, Chiang HY, Septimus E, Moody J, Braun B, Hafner J, et al. Association of a bundled intervention with surgical site infections among patients undergoing cardiac, hip, or knee surgery. JAMA. 2015;313(21):2162–2171. doi: 10.1001/jama.2015.5387. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Outpatient procedure component surgical site infection (OPC-SSI) surveillance. [Accessed February 12, 2023]. https://www.cdc.gov/nhsn/pdfs/opc/opc-ssi-protocol-current-508.pdf .
- 8.Zhang X, Liu P, You J. Risk factors for surgical site infection following spinal surgery: a meta-analysis. Medicine (Baltimore) 2022;101(8):e28836. doi: 10.1097/MD.0000000000028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calderwood MS, Anderson DJ, Bratzler DW, Dellinger EP, Garcia-Houchins S, Maragakis LL, et al. Strategies to prevent surgical site infections in acute-care hospitals: 2022 update. Infect Control Hosp Epidemiol. 2022;44(5):695–720. doi: 10.1017/ice.2023.67. doi:0.1017/ice.2023.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mcleod RWJ, Myint-Wilks L, Davies SE, Elhassan HA. The impact of noise in the operating theatre: a review of the evidence. Ann R Coll Surg Eng. 2021;103(2):83–87. doi: 10.1308/rcsann.2020.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.2021 National and state healthcare-associated infections progress report 2022. Centers for Disease Control and Prevention (CDC); [Accessed February 12, 2023]. Current HAI progress report. Updated 2022. https://www.cdc.gov/hai/data/portal/progress-report.html . [Google Scholar]
- 12.Phillips M, Rosenberg A, Shopsin B, Cuff G, Skeete F, Foti A, et al. Preventing surgical site infections: a randomized, open-label trial of nasal mupirocin ointment and nasal povidone-iodine solution. Infect Control Hospital Epidemiol. 2014;35(7):826–832. doi: 10.1086/676872.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Association of Perioperative Registered Nurses. Guidelines for Perioperative Practice. AORN; 2022. Guideline for preoperative patient skin antisepsis; pp. 623–680. [Google Scholar]
- 14.Eiselt D. Presurgical skin preparation with a novel 2% chlorhexidine gluconate cloth reduces rates of surgical site infection in orthopaedic surgical patients. Orthop Nurs. 2009;28(3):141–145. doi: 10.1097/NOR.0b013e3181a469db. [DOI] [PubMed] [Google Scholar]
- 15.Popovich KJ, Lyles R, Hayes R, Hota B, Trick W, Weinstein RA, et al. Relationship between chlorhexidine gluconate skin concentration and microbial density on the skin of critically ill patients bathed daily with chlorhexidine gluconate. Infection Control Hosp Epidemiol. 2012;33(9):889–896. doi: 10.1086/667371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Core Team. R: a language and environment for statistical computing. R Foundation for Statistical Computing Updated. 2023. https://www.R-project.org/
- 17.Harrell FE., Jr Package ‘rms’. Vanderbilt Univ. 2017;229:Q8. [Google Scholar]
- 18.Graling PR, Vasaly FW. Effectiveness of 2% CHG cloth bathing for reducing surgical site infections. AORN J. 2013;97(5):547–551. doi: 10.1016/j.aorn.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Bak J, Le J, Takayama T, Gibson A, Zerbel S, Safdar N, et al. Effect of 2% chlorhexidine gluconate-impregnated cloth on surgical site infections in vascular surgery. Ann Vasc Surg. 2017;43:197–202. doi: 10.1016/j.avsg.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 20.Chlebicki MP, Safdar N, O’Horo JC, Maki DG. Preoperative chlorhexidine shower or bath for prevention of surgical site infection: a meta-analysis. Am J Infect Control. 2013;41(2):167–73. doi: 10.1016/j.ajic.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Kordestani SS. Atlas of Wound Healing: A Tissue Engineering Approach. Elsevier; 2019. [Google Scholar]