Abstract
Background/Objectives: Socioeconomic status and parental lifestyle influence dietary behaviors, including the administration of oral dietary supplements in children. The aim of this study was to evaluate the effects of selected health, dietary, and sociodemographic factors on the use of dietary supplements by children. Methods: In this cross-sectional observational study, a diagnostic survey based on the computer-assisted web interview method was conducted in November 2022 among 2826 parents whose children attend public educational institutions in Krakow, Poland. The study group included data on 497 nursery children (17.6%), 599 kindergarten children (21.2%), 1594 primary school children (56.4%), and 136 secondary school children (4.8%). Results: Among all respondents, 72.2% were taking oral nutritional supplements, with vitamin D supplementation being particularly popular in all groups. Logistic regression analysis identified predictors of supplement use, including special diet (p < 0.001), use of medication for chronic disease (p = 0.012), regularity of main meals (p = 0.022), and attending a sports school (p = 0.021). A decrease in supplement use was observed with the increasing level of education of children (p < 0.001). Conclusions: These results highlight the importance of various health, dietary, and sociodemographic factors in influencing decisions regarding dietary supplementation in children. Further assessment of dietary supplement usage should be conducted alongside evaluations of nutrient intake from the children’s diet.
Keywords: dietary supplements, consumption, eating behavior, children
1. Introduction
Nutrient consumption among children and adolescents can affect their growth and susceptibility to noncommunicable diseases in adulthood [1]. The use of oral supplements, as in dietary evaluations, should consider the impact of various socioeconomic and lifestyle factors. Greater nutritional quality has been associated with improved socioeconomic status [2,3,4]. Before starting supplementation, it is crucial to consider several important factors, such as nutritional health, dietary quality, current dietary patterns, exercise levels, and pharmaceutical use for chronic illness [5]. In Poland, dietary supplements are very often advertised in various media. However, the legal provisions regulating advertisements are not fully defined, which does not allow consumers to clearly distinguish between medicinal products and dietary supplements [6]. At least approximately 4.4% of the dietary supplement market in Poland is targeted at children [7]. However, there has been limited research on dietary supplement intake among Polish children [8,9]. There are several categories of oral supplements, including vitamins, minerals, herbs or botanicals, amino acids, and components derived from concentrates, metabolites, dietary constituents, or extracts [10].
Minerals and vitamins are the most commonly chosen oral dietary supplements. Vitamin supplementation is recommended for individuals with identified vitamin deficiencies, such as those caused by restrictive availability of certain foods, constrained diets, or impaired uptake. The absence of clear recommendations in these areas may result in improper vitamin intake, with either excessive or insufficient intake. Recently, there has been a rise in the excessive use of nutritional supplements. An informed choice of supplementation guideline takes into account age, body weight, geographic region, and dietary habits, which means that regional or national guidelines may be more applicable in clinical practice [11]. For example, given the high prevalence of vitamin D deficiency in the Polish population, it is recommended that children under 10 years of age receive a vitamin D supplement of 600 to 1000 IU per day, while adolescents and adults should consider a daily intake of 1000 to 2000 IU throughout the year, based on body weight and dietary intake of vitamin D [12]. Recently, the popularity of supplementation with long-chain omega-3 fatty acids, including docosahexaenoic acid (DHA), has also increased, highlighting the clinical importance of ensuring adequate DHA supplementation in childhood [13].
The Committee of Human Nutrition Science of the Polish Academy of Sciences stated that dietary supplements launched in the Polish arena are safe. However, if used inappropriately, they may cause harm. Dietary supplements should be used only after consultation with a nutritional expert, physician, or drug specialist to avoid the risk of overdose or possible interactions with nourishment, drugs, or other health products. The intake of a diverse diet should be regarded as the initial step in enhancing nutritional health and well-being [14]. Since parents usually transfer their own behavioral patterns to their children, it can be assumed that those who use dietary supplements themselves also give them to their children [9]. Therefore, the aim of this study was to assess the effect of selected health, dietary, and sociodemographic factors on the use of dietary supplements by children attending public care and educational institutions in Krakow, Poland.
2. Materials and Methods
2.1. Study Design
This cross-sectional observational study was conducted in November 2022 among parents whose children attended public educational institutions in Krakow. A total of 2789 parents voluntarily participated in the survey. The collected data included 497 children attending nurseries (17.6%), 599 children from kindergartens (21.2%), 1594 children enrolled in primary schools (56.4%), and 136 children in secondary schools (4.8%). A certificate of disability was reported for 151 children. In Poland, children with disabilities can continue compulsory education up to the age of 16 in primary schools and up to the age of 24 in secondary schools.
The research was carried out in line with the ethical principles of medical research outlined in the Declaration of Helsinki. The study protocol had previously received approval from the Bioethics Committee of Jagiellonian University (No. 1072.6120.198.2022; as of 31 August 2022).
2.2. Data Collection
In this study, a diagnostic survey using the computer-assisted web interview method (CAWI) was conducted among parents of children enrolled in public educational institutions under the jurisdiction of the City of Krakow. The link to the questionnaire was initially sent to the directors of the educational institutions and then to the parents of children via an internal mail system or the institution’s newsletter. The responses were collected on the server of Jagiellonian University Medical College as part of its survey system.
The questionnaire for parents included questions related to the assessment of nutrition provided in facilities and the selected eating behaviors of children, including questions regarding the use of dietary supplements. In addition, it contained questions about the sociodemographic and health status of children. The anthropometric data of the children, including their weight and height, were also collected. The body mass index (BMI) was computed and analyzed using national percentile charts [15], and these values were then compared to the BMI thresholds established by the IOTF/WHO (defining the 85th percentile as overweight and the 95th percentile and above as obesity) [16].
2.3. Statistical Analysis
The analysis encompassed all parents who finished the questionnaire, regardless of how many responses they provided. Any missing data were omitted from the outcome analyses. The results are reported as means with standard deviations (SDs) or medians with interquartile ranges (IQRs) for continuous variables and as percentages and frequencies for categorical variables. The Pearson χ2 and Kruskal–Wallis tests were used for unadjusted comparisons among children from different educational levels for categorical and continuous variables. A multivariable logistic regression was performed to explore the links between sociodemographic attributes, dietary factors, health-related behaviors, and the use of supplements. Variables were chosen for the final model through a backward elimination approach, with a p-value cutoff of 0.1. Predictive margins were displayed as adjusted means. A p-value of less than 0.05 was deemed statistically significant.
Data were prepared and analyzed using Stata 17SE (StataCorp., College Station, TX, USA) and OriginPro 2023b (OriginLab Corporation, Northampton, MA, USA). The study was carried out according to the Strengthening the Reporting of Observational Studies in Epidemiology Statement [17].
3. Results
3.1. Characteristics of Participants
The study group included 1467 boys (51.9% of the total sample) and 1359 girls (48.1%). Among the respondents, 2628 (93%) lived in urban areas, while 198 (7%) lived in rural areas. The mean age of children was 7.9 years (SD, 4.2; range: 0.6–23.7). Except for age, there were significant differences in the assessment of nutritional status and the characteristics of children according to the type of educational facility they attended. Most respondents had four household members (49.6%; median, 4; IQR, 3–4), with a median of two minors (IQR, 1–2). The majority of the participants’ guardians (79.8% of mothers and 61.9% of fathers) reported having a higher education, and most participants (2240; 79.5%) had both parents employed.
Most children (71.5%) had a normal body weight, while 17% were underweight. Based on BMI, obesity was reported in only 92 participants (3.4%). A total of 382 participants (13.5%) were taking medication for chronic disease; 452 respondents (16.0%) had food intolerances or allergies, the most common being allergies to milk protein (4.2%) and nuts (3%). For this reason, 263 children (9.3%) required a special diet based on a physician’s recommendation, and 173 students (6.1%) followed a special diet based on their parents’ choice (Table 1).
Table 1.
General sociodemographic characteristics and health status of children by educational facility.
| Parameter | Total (N = 2826) |
Nursery (n = 497) |
Kindergarten (n = 599) |
Primary School (n = 1594) |
Secondary School (n = 136) |
p-Value | |
|---|---|---|---|---|---|---|---|
| Age, years | 7.95 (4.4–11.17), 2765 | 1.82 (1.36–2.32), 489 | 4.99 (4.03–5.96), 579 | 10.08 (8.28–12.1), 1565 | 15.57 (14.63–17.22), 132 | (by design) | |
| Sex | Male | 1467 (51.9), 2826 | 262 (52.7), 497 | 318 (53.1), 599 | 807 (50.6), 1594 | 80 (58.8), 136 | 0.249 |
| Female | 1359 (48.1), 2826 | 235 (47.3), 497 | 281 (46.9), 599 | 787 (49.4), 1594 | 56 (41.2), 136 | ||
| BMI category | Normal body weight | 1951 (71.5), 2728 | 370 (77.2), 479 | 416 (72.5), 574 | 1070 (69.3), 1544 | 95 (72.5), 131 | <0.001 |
| Underweight | 463 (17), 2728 | 55 (11.5), 479 | 120 (20.9), 574 | 275 (17.8), 1544 | 13 (9.9), 131 | ||
| Overweight | 222 (8.1), 2728 | 42 (8.8), 479 | 23 (4), 574 | 142 (9.2), 1544 | 15 (11.5), 131 | ||
| Obesity | 92 (3.4), 2728 | 12 (2.5), 479 | 15 (2.6), 574 | 57 (3.7), 1544 | 8 (6.1), 131 | ||
| Place of residence | Rural | 198 (7), 2826 | 22 (4.4), 497 | 13 (2.2), 599 | 115 (7.2), 1594 | 48 (35.3), 136 | <0.001 |
| Urban | 2628 (93), 2826 | 475 (95.6), 497 | 586 (97.8), 599 | 1479 (92.8), 1594 | 88 (64.7), 136 | ||
| Physical activity | Low | 310 (11), 2821 | 4 (0.8), 497 | 13 (2.2), 598 | 244 (15.3), 1591 | 49 (36.3), 135 | <0.001 |
| Moderate | 1188 (42.1), 2821 | 93 (18.7), 497 | 233 (39), 598 | 804 (50.5), 1591 | 58 (43), 135 | ||
| High | 1323 (46.9), 2821 | 400 (80.5), 497 | 352 (58.9), 598 | 543 (34.1), 1591 | 28 (20.7), 135 | ||
| Number of household members | 4 (3–4), 2813 | 4 (3–4), 496 | 4 (3–4), 595 | 4 (3–4), 1587 | 4 (3–5), 135 | <0.001 | |
| Number of minors in the household | 2 (1–2), 2807 | 1 (1–2), 495 | 2 (1–2), 596 | 2 (1–2), 1582 | 1 (1–2), 134 | <0.001 | |
| Mother’s education level | Basic | 19 (0.7), 2819 | 7 (1.4), 496 | 3 (0.5), 598 | 8 (0.5), 1590 | 1 (0.7), 135 | <0.001 |
| Basic vocational | 74 (2.6), 2819 | 4 (0.8), 496 | 16 (2.7), 598 | 43 (2.7), 1590 | 11 (8.2), 135 | ||
| Secondary | 477 (16.9), 2819 | 62 (12.5), 496 | 79 (13.2), 598 | 291 (18.3), 1590 | 45 (33.3), 135 | ||
| Higher | 2249 (79.8), 2819 | 423 (85.3), 496 | 500 (83.6), 598 | 1248 (78.5), 1590 | 78 (57.8), 135 | ||
| Father’s education level | Basic | 60 (2.1), 2817 | 12 (2.4), 496 | 12 (2), 597 | 32 (2), 1589 | 4 (3), 135 | <0.001 |
| Basic vocational | 261 (9.3), 2817 | 24 (4.8), 496 | 43 (7.2), 597 | 166 (10.5), 1589 | 28 (20.7), 135 | ||
| Secondary | 753 (26.7), 2817 | 148 (29.8), 496 | 155 (26), 597 | 397 (25), 1589 | 53 (39.3), 135 | ||
| Higher | 1743 (61.9), 2817 | 312 (62.9), 496 | 387 (64.8), 597 | 994 (62.6), 1589 | 50 (37), 135 | ||
| Parent with occupational activity | Father | 405 (14.4), 2817 | 62 (12.5), 497 | 96 (16), 599 | 229 (14.4), 1587 | 18 (13.4), 134 | 0.001 |
| Mother | 172 (6.1), 2817 | 14 (2.8), 497 | 30 (5), 599 | 113 (7.1), 1587 | 15 (11.2), 134 | ||
| Both parents | 2240 (79.5), 2817 | 421 (84.7), 497 | 473 (79), 599 | 1245 (78.5), 1587 | 101 (75.4), 134 | ||
| Self-assessed financial situation | Average | 1925 (68.2), 2822 | 367 (73.8), 497 | 407 (68), 599 | 1056 (66.4), 1591 | 95 (70.4), 135 | 0.002 |
| Above average | 709 (25.1), 2822 | 104 (20.9), 497 | 163 (27.2), 599 | 417 (26.2), 1591 | 25 (18.5), 135 | ||
| Below average | 188 (6.7), 2822 | 26 (5.2), 497 | 29 (4.8), 599 | 118 (7.4), 1591 | 15 (11.1%), 135 | ||
| Medication for chronic disease | 382 (13.5%), 2823 | 36 (7.2), 497 | 76 (12.7), 599 | 247 (15.5), 1592 | 23 (17), 135 | <0.001 | |
| Certificate of disability | 151 (5.4%), 2819 | 20 (4), 496 | 28 (4.7), 597 | 87 (5.5), 1591 | 16 (11.9), 135 | 0.004 | |
| Special diet | No | 2390 (84.6), 2826 | 416 (83.7), 497 | 521 (87), 599 | 1352 (84.8), 1594 | 101 (74.3), 136 | 0.001 |
| Based on parents’ choice | 173 (6.1), 2826 | 23 (4.6), 497 | 27 (4.5), 599 | 106 (6.7), 1594 | 17 (12.5), 136 | ||
| Based on a physician’s recommendation | 263 (9.3), 2826 | 58 (11.7), 497 | 51 (8.5), 599 | 136 (8.5), 1594 | 18 (13.2), 136 | ||
| Child in a special educational facility | 172 (6.1), 2826 | 28 (5.6), 497 | 25 (4.2), 599 | 100 (6.3), 1594 | 19 (14), 136 | <0.001 | |
| Child in a sports school or club | 265 (9.4), 2826 | 8 (1.6), 497 | 9 (1.5), 599 | 232 (14.6), 1594 | 16 (11.8), 136 | <0.001 | |
| Child in a facility with integration groups/classes | 574 (20.3), 2826 | 90 (18.1), 497 | 96 (16), 599 | 371 (23.3), 1594 | 17 (12.5), 136 | <0.001 | |
Data presented as median (IQR), total number of responses for continuous variables, or frequency (percentage), total number of responses for categorical variables.
3.2. Selected Eating Habits of Children and Adolescents
This research also evaluated the specific dietary habits of children and teenagers (Table 2). Most participants (75.8%) used collective nutrition offered at the educational facility. This included almost all children attending nurseries and kindergartens. A total of 2423 children (85.8%) were reported to eat main meals regularly. Snacking between meals every day or more often was reported in 1344 children. The most popular snacks were fruit (82.8%), sweet snacks (52.7%), and unsweetened milk drinks and desserts (43.1%).
Table 2.
Characteristics of eating habits among children and teenagers by educational facility.
| Total (N = 2826) |
Nursery (n = 497) |
Kindergarten (n = 599) |
Primary School (n = 1594) |
Secondary School (n = 136) |
p-Value | ||
|---|---|---|---|---|---|---|---|
| Use of collective nutrition in the facility | 2141 (75.8), 2826 | 479 (96.4), 497 | 578 (96.5), 599 | 1034 (64.9), 1594 | 50 (36.8), 136 | <0.001 | |
| Number of meals eaten in the facility | 2 (1–3), 2826 | 4 (4–4), 497 | 3 (3–4), 599 | 1 (1–2), 1594 | 1 (1–2), 136 | <0.001 | |
| Eating between meals (snack) | Never | 70 (2.5), 2826 | 13 (2.6), 497 | 9 (1.5), 599 | 36 (2.3), 1594 | 12 (8.8), 136 | <0.001 |
| 1–3 times a month | 165 (5.8), 2826 | 32 (6.4), 497 | 46 (7.7), 599 | 82 (5.1), 1594 | 5 (3.7), 136 | ||
| Once a week | 282 (10), 2826 | 53 (10.7), 497 | 63 (10.5), 599 | 159 (10), 1594 | 7 (5.2), 136 | ||
| A few times a week | 965 (34.2), 2826 | 137 (27.6), 497 | 209 (34.9), 599 | 567 (35.6), 1594 | 52 (38.2), 136 | ||
| Once a day | 828 (29.3), 2826 | 154 (31), 497 | 180 (30.1), 599 | 461 (28.9), 1594 | 33 (24.3), 136 | ||
| Several times a day | 516 (18.3), 2826 | 108 (21.7), 497 | 92 (15.4), 599 | 289 (18.1), 1594 | 27 (19.9), 136 | ||
| Type of snacks | Fruit | 2332 (82.5), 2826 | 452 (91), 497 | 541 (90.3), 599 | 1253 (78.6), 1594 | 86 (63.2), 136 | <0.001 |
| Vegetables | 767 (27.1), 2826 | 157 (31.6), 497 | 169 (28.2), 599 | 414 (26), 1594 | 27 (19.9), 136 | 0.018 | |
| Unsweetened milk drinks and desserts | 1217 (43.1), 2826 | 256 (51.5), 497 | 284 (47.4), 599 | 621 (39), 1594 | 56 (41.2), 136 | <0.001 | |
| Sweetened milk drinks and desserts | 860 (30.4), 2826 | 109 (21.9), 497 | 197 (32.9), 599 | 503 (31.6), 1594 | 51 (37.5), 136 | <0.001 | |
| Sweet snacks | 1490 (52.7), 2826 | 139 (28), 497 | 340 (56.8), 599 | 944 (59.2), 1594 | 67 (49.3), 136 | <0.001 | |
| Salty snacks | 904 (32), 2826 | 113 (22.7), 497 | 181 (30.2), 599 | 558 (35), 1594 | 52 (38.2), 136 | <0.001 | |
| Nuts, almonds, seeds, kernels | 1040 (36.8), 2826 | 126 (25.4), 497 | 260 (43.4), 599 | 613 (38.5), 1594 | 41 (30.2), 136 | <0.001 | |
| Regular consumption of main meals (breakfast, dinner, supper) | Definitely yes | 418 (14.8), 2826 | 103 (20.7), 497 | 135 (22.5), 599 | 164 (10.3), 1594 | 16 (11.8), 136 | <0.001 |
| Rather yes | 2005 (71), 2826 | 373 (75.1), 497 | 417 (69.6), 599 | 1143 (71.7), 1594 | 72 (52.9), 136 | ||
| Rather no | 350 (12.4), 2826 | 19 (3.8), 497 | 44 (7.4), 599 | 251 (15.8), 1594 | 36 (26.5), 136 | ||
| Definitely no | 53 (1.9), 2826 | 2 (0.4), 497 | 3 (0.5), 599 | 36 (2.3), 1594 | 12 (8.8), 136 | ||
| Most common products bought from a school or nearby store | Sweets | 539 (19.1), 2826 | 7 (1.4), 497 | 23 (3.8), 599 | 480 (30.1), 1594 | 29 (21.3), 136 | <0.001 |
| Confectionery bread | 318 (11.3), 2826 | 5 (1), 497 | 22 (3.7), 599 | 248 (15.6), 1594 | 43 (31.6), 136 | <0.001 | |
| Fruit | 42 (1.5), 2826 | 7 (1.4), 497 | 10 (1.7), 599 | 20 (1.3), 1594 | 5 (3.7), 136 | 0.158 | |
| Sweetened carbonated drinks | 170 (6), 2826 | 1 (0.2), 497 | 5 (0.8), 599 | 143 (9), 1594 | 21 (15.4), 136 | <0.001 | |
| Chips | 160 (5.7), 2826 | 2 (0.4), 497 | 5 (0.8), 599 | 142 (8.9), 1594 | 11 (8.1), 136 | <0.001 | |
| Yogurts, cheeses, milk | 42 (1.5), 2826 | 3 (0.6), 497 | 6 (1), 599 | 26 (1.6), 1594 | 7 (5.2), 136 | 0.001 | |
| Mineral water | 469 (16.6), 2826 | 11 (2.2), 497 | 28 (4.7), 599 | 368 (23.1), 1594 | 62 (45.6), 136 | <0.001 | |
| Sandwiches | 95 (3.4), 2826 | 0 (0), 497 | 3 (0.5), 599 | 59 (3.7), 1594 | 33 (24.3), 136 | <0.001 | |
Data presented as median (IQR), total number of responses for continuous variables, or frequency (percentage), total number of responses for categorical variables.
3.3. Dietary Supplement Consumption Among Children and Adolescents
Among all respondents, 72.2% reported administering dietary supplements to their children. The types of supplementation are presented in Table 3. Vitamin D intake was the most popular in every group. The highest percentage of multivitamin supplementation was observed in children attending kindergartens (13.7%). A decrease in the intake of most supplements was observed with the increasing level of education.
Table 3.
Number and percentage of children using specific types of supplementation by educational facility.
| Supplementation | Total (N = 2821) |
Nursery (n = 497) | Kindergarten (n = 598) |
Primary School(n = 1592) | Secondary School (n = 134) |
p-Value |
|---|---|---|---|---|---|---|
| Any | 2038 (72.2) | 424 (85.3) | 465 (77.8) | 1070 (67.2) | 79 (59) | <0.001 |
| Iron | 102 (3.6) | 24 (4.8) | 25 (4.2) | 46 (2.9) | 7 (5.2) | 0.112 |
| Calcium | 83 (2.9) | 8 (1.6) | 13 (2.2) | 54 (3.4) | 8 (5.9) | 0.022 |
| Magnesium | 173 (6.1) | 5 (1) | 15 (2.5) | 132 (8.3) | 21 (15.4) | <0.001 |
| Multivitamin | 302 (10.7) | 34 (6.8) | 82 (13.7) | 171 (10.7) | 15 (11) | 0.004 |
| Mineral sets | 97 (3.4) | 8 (1.6) | 18 (3) | 61 (3.8) | 10 (7.4) | 0.006 |
| Vitamin C | 698 (24.7) | 140 (28.2) | 162 (27.1) | 362 (22.7) | 34 (25) | 0.039 |
| Vitamin D | 1777 (62.9) | 404 (81.3) | 416 (69.5) | 902 (56.6) | 55 (40.4) | <0.001 |
| Omega-3 | 538 (19) | 104 (20.9) | 147 (24.5) | 268 (16.8) | 19 (14) | <0.001 |
| Others | 162 (5.7) | 31 (6.2) | 33 (5.5) | 91 (5.7) | 7 (5.2) | 0.951 |
Data presented as frequency (percentage); 5 responses indicating “don’t know” were excluded.
Logistic regression identified the following predictors of dietary supplement use in the study group (Table 4): education level (p < 0.001), special diet (p < 0.001), consumption of nuts, almonds, seeds, or kernels between meals (p < 0.001), use of medication for chronic disease (p = 0.012), consumption of sweetened milk drinks between meals (p = 0.015), regularity of main meals (p = 0.022), attending a sports school (p = 0.021), number of meals eaten at the facility (p = 0.041), and buying chips in a school or nearby store (p = 0.037).
Table 4.
Association between the probability of taking a dietary supplement and the study variables.
| Variable | OR (95% CI) | p-Value | |
|---|---|---|---|
| Use of medication for chronic disease | No | Reference | |
| Yes | 1.41 (1.08–1.84) | 0.012 | |
| Educational facility | Nursery | Reference | |
| Kindergarten | 0.56 (0.40–0.78) | 0.001 | |
| Primary school | 0.44 (0.29–0.67) | <0.001 | |
| Secondary school | 0.32 (0.18–0.55) | <0.001 | |
| Overall effect | - | <0.001 | |
| Regular consumption of main meals (breakfast, dinner, supper) | Definitely yes | Reference | |
| Rather yes | 0.74 (0.57–0.98) | 0.032 | |
| Rather no | 0.62 (0.44–0.87) | 0.006 | |
| Definitely no | 0.48 (0.25–0.90) | 0.023 | |
| Overall effect | - | 0.022 | |
| Number of meals eaten at the facility | Per additional meal | 1.14 (1.01–1.29) | 0.041 |
| Special diet | No | Reference | |
| Based on parents’ choice | 2.01 (1.32–3.04) | 0.001 | |
| Based on a physician’s recommendation | 1.65 (1.18–2.29) | 0.003 | |
| Overall effect | - | <0.001 | |
| Buying chips in a school or nearby store | No | Reference | |
| Yes | 0.69 (0.49-0.98) | 0.037 | |
| Snack between meals: sweetened milk drinks and desserts | No | Reference | |
| Yes | 1.27 (1.05–1.53) | 0.015 | |
| Snack between meals: nuts, almonds, seeds, kernels | No | Reference | |
| Yes | 1.77 (1.47–2.13) | <0.001 | |
| Attending a sports school | No | Reference | |
| Yes | 1.43 (1.06–1.94) | 0.021 |
OR, odds ratio from the logistic regression (N = 2818, intercept = 3.47, 95% CI: 1.93–6.26, log-pseudolikelihood = −1569).
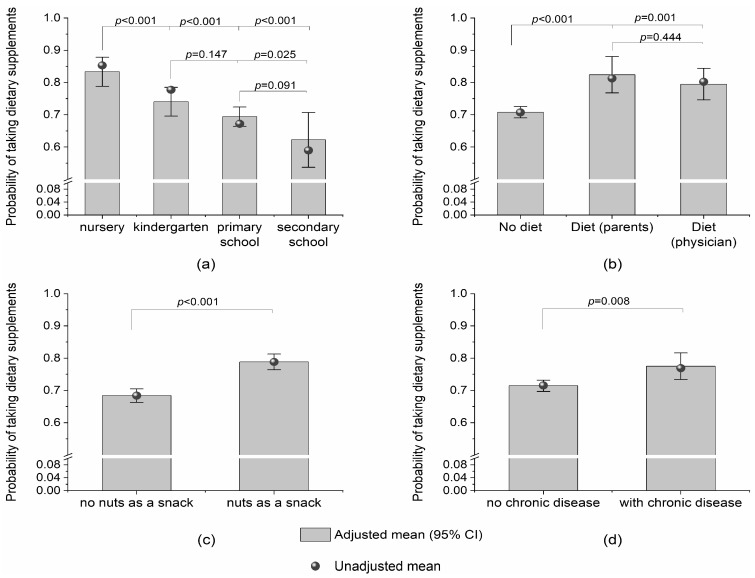
The probability of taking dietary supplements was lower with the increasing level of education (which was strongly correlated with age). Participants on a special diet and those taking medication for chronic disease were more likely to take dietary supplements compared with those who were not on a special diet or did not take medication for chronic disease (Figure 1).
Figure 1.
Adjusted (bars with 95% CI) and unadjusted (bullets) probabilities of taking dietary supplements by (a) level of education; (b) special diet; (c) consumption of nuts, almonds, seeds, or kernels between meals; and (d) use of medication for chronic disease.
4. Discussion
Our study, conducted in 2022, revealed that 72.2% of children aged 0.6 to 23.7 years who attended public care and educational institutions in Krakow used dietary supplements. The most popular type of supplement in each group was vitamin D, which is recommended for the Polish population, followed by vitamin C and omega-3 fatty acids. However, the study was conducted in the fall and winter among well-educated parents of children, mostly from a large city.
Similarly, a high consumption of dietary supplements was also observed in 79% of all children aged 0–3 years recruited from five pediatric outpatient clinics in Poland during 2019–2022 [8].
A 2009 study across nine European nations found that voluntary supplement intake varied by country, significantly affecting nutrient consumption disparities. Supplement use was highest in Finland and Denmark, while Poland’s intake was moderate compared to other European countries [11]. According to Stierman et al., an increasing number of parents are choosing to introduce dietary supplements into their children’s nutrition, especially vitamin and mineral supplements. Among adolescents, the consumption of any nutritional supplements rose significantly in a linear trend from 2009–2010 (22.1%) to 2017–2018 (29.7%). The most common supplements were multivitamins and minerals (23.8%), and their use increased with higher family income and education level [18].
Prior to the COVID-19 pandemic (2018), a study examined the use of dietary supplements among healthy, non-medicated children from the town and municipality of Niepołomice and Kraków. The results indicated that only one-third of these children used nutritional supplements, with higher usage observed in rural areas compared to urban ones and a higher prevalence among boys than girls [5].
During the COVID-19 health crisis, the market for dietary supplements in Poland and around the world experienced rapid and continuous growth [19]. The consumption of dietary supplements by parents may be associated with their administration to children [9]. The consumption of dietary supplements containing zinc and vitamin D increased especially among Polish people with higher or medical and paramedical education [20].
In Polish recommendations, the risk groups for vitamin D deficiency are veganism and other types of vegetarianism, an allergy to cow’s milk, and a low-fat diet [12]. Similarly, a French expert consensus recommended vitamin D supplementation in children in cases of decreased availability of this vitamin (obesity, black ethnicity, lack of skin exposure to sun) or decreased intake (vegan diet) [21]. Vitamin supplementation is recommended for nutritional deficiencies, including malabsorption syndromes, atypical diets, or insufficient vitamin consumption. Vitamin C deficiency is more common in Western countries, particularly among individuals who follow restrictive diets or experience reduced absorption due to gastrointestinal issues. Vitamin C supplementation appears to be beneficial for those with recurrent respiratory infections [22]. Although an international overview observed an increase in the use of specific dietary supplements due to the COVID-19 pandemic, their efficacy and appropriateness remain unconfirmed. Considering the relative safety of these substances, their use might still be recommended, especially for children [23].
Vorilhon et al. suggested that one of the main reasons for using dietary supplements in children is to improve overall health and strengthen the immune system, which is particularly important in times of increased susceptibility to infection [24].
In our study, we observed a significant increase in the consumption of dietary supplements in children attending care and educational institutions in Krakow. It was found to be significantly associated with special diets and the use of drugs for chronic illness. Children were twice as likely to be given supplements if they were on a diet based on parental choice rather than a physician’s recommendation. Supplements were also more likely to be given to children who were on a physician-recommended diet than to those who were not on any diet.
In the study of nursery and kindergarten children in Krakow, food allergies were the primary reason for special diets. Among the participants, only 11 parents reported that their child was vegetarian, and 3 reported vegan diets [25]. In a cross-sectional study involving Polish children aged 5–10 years (63 vegetarians, 52 vegans, and 72 matched omnivores), nearly one-third of children adhering to either vegetarian or vegan diets received no supplementation with vitamin B12 or B12-fortified foods, and a similar proportion used vitamin D supplements [26]. According to the recommendations for children on vegan diets, regular monitoring and supplementation with vitamins B12 and D are essential, with iron, calcium, DHA, and zinc supplemented as needed [27].
The use of dietary supplements by children with chronic diseases had a beneficial effect on their health. Szymelfejnik et al. studied 2258 individuals attending primary schools in the Kujawsko-Pomorskie Voivodeship, Poland. They found that the main reason for using dietary supplements was the health condition of a child. The use of nutritional supplements was also strongly linked to the location of residence. The greatest consumption of dietary supplements was observed in children from medium-sized towns, whereas the lowest consumption of dietary supplements was noted among those living in small cities [28].
As previously mentioned, adults and children with obesity are at risk of vitamin D deficiency [12,21]. In our research, a small proportion of children were identified as overweight (8.1%) or obese (3.4%) according to their BMI interpretation, while as many as 17% of children were underweight. The use of dietary supplements in the form of oral nutritional supplements can significantly improve the health of children with low body weight. A review and meta-analysis by Zhang et al. demonstrated that nutritional supplements are beneficial for improving growth outcomes in undernourished children, especially those with mild to moderate undernutrition [29]. On the other hand, a systematic review conducted in 2023 assessed the effectiveness of vitamin D supplementation in overweight children and adolescents. The results were ambiguous, with the authors claiming that vitamin D supplementation slightly increases 25(OH)D levels in children and adolescents with overweight and obesity [30]. In 2024, another meta-analysis assessed the use of vitamin D in children with obesity. The results indicated that the use of an extremely high dose (>4000 IU/day) may be the most optimal strategy in terms of reducing inflammatory responses and improving insulin resistance in children and adolescents with overweight and obesity [31].
In the present study, most children consumed meals prepared in educational institutions. Children who did not regularly eat main meals and ate snacks between meals were significantly more likely to receive dietary supplements. Moreover, the intake of dietary supplements decreased with the age of the children. The use of dietary supplements was also found to be significantly associated with attending a sports school.
This finding is in line with a study by Ishitsuka et al., who showed that the higher frequency of sports participation among Japanese elementary school children was significantly associated with higher odds of using amino acids or proteins and multivitamins [32]. Bolesławska et al. studied two age groups of students from sports championship schools in Poznań, Poland. The study showed that the diets of adolescents with increased physical activity were mostly properly balanced in terms of nutritional value, except for calcium, sodium, phosphorus, and vitamin D content [33]. In a review assessing the nutrition knowledge of adolescent athletes conducted from 2010 to April 2022, it was revealed that knowledge about supplements was consistently low [34].
The increased use of dietary supplements in children results from the growing health awareness among parents. However, because dietary supplements are not regulated in the same way as medications and because they are readily available outside pharmacies without pediatrician consultation, there are risks associated with their uncontrolled use, which may lead to inadequate doses, lack of efficacy, or overdoses. The risk of incorrect dosing increases for formulations with multiple nutrients. On the other hand, pediatricians often view vitamin supplementation as a supportive treatment rather than a crucial therapeutic intervention [22].
Strengths and Limitations of the Study
The strength of the present study lies in its assessment of dietary supplement intake among a large group of children and adolescents of various ages who attend care and educational institutions. The results may be influenced by the timing of the study, namely, the fall–winter season, a period when dietary supplements are more commonly used. A limitation of this study is the lack of information on the dosages of dietary supplements administered to the children and the small number of responses received from the parents of secondary school students. Another limitation of the study is its online nature. Since individuals with higher education are more likely to use the Internet, the representativeness of our sample in terms of educational levels is diminished. Additionally, people with higher education are generally more interested in health-related topics [35]. In our study, over 80% of the participants held a higher education degree, which is not representative of the education level distribution in the general population.
5. Conclusions
This study revealed that approximately two-thirds of children were given dietary supplements during the study period, indicating that these products are popular among the studied group. There was a noticeable decrease in the use of dietary supplements with increasing education levels (correlated with older age). Children on special diets or taking medication for chronic diseases were more predisposed to take dietary supplements than their counterparts. Lifestyle factors, such as the regularity of main meals and participation in sports activities, were also significant predictors of dietary supplement use. These findings highlight the importance of health, dietary, and sociodemographic factors in parental decisions regarding the use of dietary supplements by their children. They also highlight potential areas for health education and dietary interventions targeting supplement use. Further assessment of dietary supplement usage should be conducted alongside evaluations of nutrient intake from the children’s diet.
Acknowledgments
We would like to thank the parents of the children and the directors of care and educational institutions under the Municipality of Krakow for their participation in the study.
Author Contributions
Conceptualization, B.P.; methodology, B.P.; software, P.H.; validation, B.P., P.H. and P.K.; formal analysis, P.H. and B.P.; investigation, B.P.; data curation, W.O., K.S., E.C.-M., D.S. and K.K.; writing—original draft preparation, W.O., K.S., E.C.-M., D.S., K.K. and B.P.; writing—review and editing, B.P. and P.K.; visualization, P.H.; supervision, P.K.; project administration, B.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Bioethics Committee of Jagiellonian University (No. 1072.6120.198.2022; as of 31 August 2022).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was financed by the Municipality of Krakow.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kaikkonen J.E., Mikkilä V., Raitakari O.T. Role of childhood food patterns on adult cardiovascular disease risk. Curr. Atheroscler. Rep. 2014;16:443. doi: 10.1007/s11883-014-0443-z. [DOI] [PubMed] [Google Scholar]
- 2.Rydén P., Hagfors L. Diet cost, diet quality, and socio-economic position: How are they related and what contributes to differences in diet costs? Public Health Nutr. 2011;14:1680–1692. doi: 10.1017/S1368980010003642. [DOI] [PubMed] [Google Scholar]
- 3.Vilar-Compte M., Burrola-Méndez S., Lozano-Marrufo A., Ferré-Eguiluz I., Flores D., Gaitán-Rossi P., Teruel G., Pérez-Escamilla R. Urban poverty and nutrition challenges associated with accessibility to a healthy diet: A global systematic literature review. Int. J. Equity Health. 2021;20:40. doi: 10.1186/s12939-020-01330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brettschneider A.K., Barbosa C.L., Haftenberger M., Lehmann F., Mensink G.B.M. Adherence to food-based dietary guidelines among adolescents in Germany according to socio-economic status and region: Results from Eating Study as a KiGGS Module (EsKiMo) II. Public Health Nutr. 2021;24:1216–1228. doi: 10.1017/S136898002100001X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piórecka B., Koczur K., Cichocki R., Jagielski P., Kawalec P. Socio-Economic Factors Influencing the Use of Dietary Supplements by Schoolchildren from Małopolska Voivodship (Southern Poland) Int. J. Environ. Res. Public Health. 2022;19:7826. doi: 10.3390/ijerph19137826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Książczak K., Nieścior B. Advertisement of Diet Supplement—Legal Analysis. Zesz. Nauk. Szkoły Głównej Gospod. Wiej. W Warszawie. 2018;18:130–142. doi: 10.22630/PRS.2018.18.1.12. [DOI] [Google Scholar]
- 7.Euromonitor International . Vitamins and Dietary Supplements in Poland (Report) Euromonitor International; London, UK: 2016. [Google Scholar]
- 8.Woźniak D., Przysławski J., Banaszak M., Drzymała-Czyż S. Dietary Supplements among Children Ages 0-3 Years in Poland-Are They Necessary? Foods. 2022;12:16. doi: 10.3390/foods12010016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piekara A., Krzywonos M., Kaczmarczyk M. What Do Polish Parents and Caregivers Think of Dietary Supplements for Children Aged 3–12? Nutrients. 2020;12:3076. doi: 10.3390/nu12103076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Parliament and The Council of the European Union Directive 2002/46/EC of the European Parliament and of the Council of 10 June 2002. Off. J. Eur. Communities. 2002;12:51–57. [Google Scholar]
- 11.Flynn A., Hirvonen T., Mensink G.B., Ocke M.C., Serra-Majem L., Stos K., Szponar L., Tetens I., Turrini A., Fletcher R., et al. Intake of selected nutrients from foods, from fortification and from supplements in various European countries. Food Nutr. Res. 2009;53:2038. doi: 10.3402/fnr.v53i0.2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Płudowski P., Kos-Kudła B., Walczak M., Fal A., Zozulińska-Ziółkiewicz D., Sieroszewski P., Peregud-Pogorzelski J., Lauterbach R., Targowski T., Lewiński A., et al. Guidelines for Preventing and Treating Vitamin D Deficiency: A 2023 Update in Poland. Nutrients. 2023;15:695. doi: 10.3390/nu15030695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mazurek M., Mól N., Zasada M., Zasada W., Pyznar O., Kwinta P. Dietary supplements use among children from south-eastern Poland. Pediatr. Pol. 2022;97:13–19. doi: 10.5114/polp.2022.114951. [DOI] [Google Scholar]
- 14.Wawrzyniak A., Przybyłowicz K., Wądołowska L., Charzewska J., Górecka D., Lange E., Members of the Human Nutrition Science Committee of the Polish Academy of Sciences Statement of the Committee of Human Nutrition Science of the Polish Academy of Sciences on the use of dietary supplements containing vitamins and minerals by adults. Rocz. Panstw. Zakł. Hig. 2021;77:1–6. doi: 10.32394/rpzh.2021.0168. [DOI] [PubMed] [Google Scholar]
- 15.Kulaga Z., Rózdzynska-Swiatkowska A., Grajda A., Gurzkowska B., Wojtylo M., Gózdz M. Percentile charts for growth and nutritional status assessment in Polish children and adolescents from birth to 18 years of age. Stand. Med. Pediatr. 2015;12:119–135. [Google Scholar]
- 16.Cole T.J., Lobstein T. Extended international (IOTF) body mass index cut-offs for thinness, overweight and obesity. Pediatr. Obes. 2012;7:284–294. doi: 10.1111/j.2047-6310.2012.00064.x. [DOI] [PubMed] [Google Scholar]
- 17.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008;61:344–349. doi: 10.1016/j.jclinepi.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Stieman B., Mishra S., Gahche J.J., Potischman N., Hales C.M. Dietary supplement use in children and adolescents aged ≤19 years—United States, 2017–2018. Morb. Mortal. Wkly. Rep. 2020;69:1557–1562. doi: 10.15585/mmwr.mm6943a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Djaoudene O., Romano A., Bradai Y.D., Zebiri F., Ouchene A., Yousfi Y., Amrane-Abider M., Sahraoui-Remini Y., Madani K. A Global Overview of Dietary Supplements: Regulation, Market Trends, Usage during the COVID-19 Pandemic, and Health Effects. Nutrients. 2023;15:3320. doi: 10.3390/nu15153320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puścion-Jakubik A., Bielecka J., Grabia M., Mielech A., Markiewicz-Żukowska R., Mielcarek K., Moskwa J., Naliwajko S.K., Soroczyńska J., Gromkowska-Kępka K.J., et al. Consumption of Food Supplements during the Three COVID-19 Waves in Poland-Focus on Zinc and Vitamin D. Nutrients. 2021;13:3361. doi: 10.3390/nu13103361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacchetta J., Bacchetta J., Edouard T., Edouard T., Laverny G., Laverny G., Bernardor J., Bernardor J., Bertholet-Thomas A., Bertholet-Thomas A., et al. Vitamin D and calcium intakes in general pediatric populations: A French expert consensus paper. Arch. Pediatr. 2022;29:312–325. doi: 10.1016/j.arcped.2022.02.008. [DOI] [PubMed] [Google Scholar]
- 22.Martini L., Pecoraro L., Salvottini C., Piacentini G., Atkinson R., Pietrobelli A. Appropriate and inappropriate vitamin supplementation in children. J. Nutr. Sci. 2020;9:e20. doi: 10.1017/jns.2020.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scapaticci S., Neri C.R., Marseglia G.L., Staiano A., Chiarelli F., Verduci E. The impact of the COVID-19 pandemic on lifestyle behaviors in children and adolescents: An international overview. Ital. J. Pediatr. 2022;48:22. doi: 10.1186/s13052-022-01211-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vorilhon P., Arpajou B., Roussel H.V., Merlin E., Pereira B., Cabaillot A. Efficacy of vitamin C for the prevention and treatment of upper respiratory tract infection. A meta-analysis in children. Eur. J. Clin. Pharmacol. 2019;75:303–311. doi: 10.1007/s00228-018-2601-7. [DOI] [PubMed] [Google Scholar]
- 25.Piórecka B., Kozioł-Kozakowska A., Holko P., Kowalska-Bobko I., Kawalec P. Provision of special diets to children in public nurseries and kindergartens in Krakow (Poland) Front. Nutr. 2024;11:1341062. doi: 10.3389/fnut.2024.1341062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Desmond M.A., Sobiecki J.G., Jaworski M., Płudowski P., Antoniewicz J., Shirley M.K., Eaton S., Książyk J., Cortina-Borja M., De Stavola B., et al. Growth, body composition, and cardiovascular and nutritional risk of 5- to 10-y-old children consuming vegetarian, vegan, or omnivore diets. Am. J. Clin. Nutr. 2021;113:1565–1577. doi: 10.1093/ajcn/nqaa445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemale J., Mas E., Jung C., Bellaiche M., Tounian P. French-speaking Pediatric Hepatology, Gastroenterology and Nutrition Group (GFHGNP). Vegan diet in children and adolescents. Recommendations from the French-speaking Pediatric Hepatology, Gastroenterology and Nutrition Group (GFHGNP) Arch.Pediatr. 2019;26:442–450. doi: 10.1016/j.arcped.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Szymelfejnik E., Jaroch A., Ameryk M., Balcerzak W., Świątkowki M., Popławski C. Evaluation of the use of dietary supplements among first-grade primary school children from Poland in the context of the socioeconomic status of the family and children’s health status. J. Food Nutr. Res. 2019;58:255–265. [Google Scholar]
- 29.Zhang Z., Li F., Hannon B.A., Hustead D.S., Aw M.M., Liu Z., Chuah K.A., Low Y.L., Huynh D.T.T. Effect of Oral Nutritional Supplementation on Growth in Children with Undernutrition: A Systematic Review and Meta-Analysis. Nutrients. 2021;13:3036. doi: 10.3390/nu13093036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corsello A., Macchi M., D’oria V., Pigazzi C., Alberti I., Treglia G., De Cosmi V., Mazzocchi A., Agostoni C., Milani G.P. Effects of vitamin D supplementation in obese and overweight children and adolescents: A systematic review and meta-analysis. Pharmacol. Res. 2023;192:106793. doi: 10.1016/j.phrs.2023.106793. [DOI] [PubMed] [Google Scholar]
- 31.Zhu L., Li S., Zhong L., Xu S., Zhu H. Optimal vitamin D supplement dosage for improving insulin resistance in children and adolescents with overweight/obesity: A systematic review and network meta-analysis. Eur. J. Nutr. 2024;63:763–775. doi: 10.1007/s00394-023-03301-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishitsuka K., Sasaki S., Mezawa H., Konishi M., Igarashi M., Yamamoto-Hanada K., Nakayama S.F., Ohya Y. Dietary supplement use in elementary school children: A Japanese web-based survey. Environ. Health Prev. Med. 2021;26:63. doi: 10.1186/s12199-021-00985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bolesławska I., Żwirska J., Błaszczyk-Bębenek E., Przysławski J. Assessment of the nutrients consumption and selected nutrition status indicators in youth with increased physical activity. Bromatol. Chem. Toksykol. 2020;53:100–108. (In Polish) [Google Scholar]
- 34.Hulland S.C., Trakman G.L., Alcock R.D. Adolescent athletes have better general than sports nutrition knowledge and lack awareness of supplement recommendations: A systematic literature review. Br. J. Nutr. 2024;131:1362–1376. doi: 10.1017/S0007114523002799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bach R.L., Wenz A. Studying health related Internet and mobile device use using weblogs and smartphone records. PLoS ONE. 2020;15:e0234663. doi: 10.1371/journal.pone.0234663. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.