Abstract
In recent years, π-conjugated liquid crystalline molecules with optoelectronic functionalities have garnered considerable attention, and integrating these molecules into side-chain liquid crystalline polymers (SCLCPs) holds potential for developing devices that are operational near room temperature. However, it is difficult to design SCLCPs with excellent processability because liquid crystalline mesogens are rigid rods, have low solubility in organic solvents, and have a high isotropization temperature. Recently, we developed near-room-temperature π-conjugated nematic liquid crystals based on “bridged stilbene”. In this work, we synthesized a polyacrylate SCLCP incorporating a bridged stilbene that exhibited a nematic phase near room temperature and could maintain liquid crystallinity for more than three months. We conducted a thorough phase structure analysis and evaluated the optical properties. The birefringence values of the resulting polymers were higher than those of the corresponding monomers because of the enhanced order parameters due to the polymer effect. In addition, the synthesized polymers inherited mesogen-derived AIE properties, with high quantum yields (Φfl = 0.14–0.35) in the solid state. It is noteworthy that the maximum fluorescence wavelength exhibited a redshift of greater than 27 nm as a consequence of film formation. Thus, several unique characteristics of the SCLCPs are unattainable with small molecular systems.
Keywords: side-chain liquid crystalline polymer, nematic liquid crystal, polyacrylate, π-conjugated mesogen, birefringence, aggregation-induced emission
1. Introduction
Liquid crystal polymers are widely used in industrial applications due to their excellent processability and favorable material properties [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. Of particular interest are side-chain liquid crystal polymers (SCLCPs) [15,16,17,18,19,20,21,22,23,24,25], in which mesogenic units are attached to the side chains of linear polymers such as polyacrylate and polymethacrylate. SCLCPs exhibit liquid crystalline phases over a broader temperature range than small molecule liquid crystals and mimic the behavior of small molecule liquid crystals above the glass transition temperature (Tg). This liquid crystalline nature allows for the molecular orientation to be controlled by external fields such as mechanical stress, electric fields, and magnetic fields [1,2,3,4,5,6,7,8,9,10,11,12,13,14]. In addition, the molecular orientation of LC polymers can be controlled using anisotropic surfaces (as in this study) and photoalignment (e.g., [16]). Additionally, SCLCPs can form nanostructures through higher order smectic and columnar phases [26,27,28,29]. Below Tg, the liquid crystal phase that exists above Tg can be vitrified, retaining its anisotropic properties. Among liquid crystal phases, the nematic (N) phase is the most fluid, is highly responsive to external fields, and is suitable for large-area applications [30]. These properties, unique to the N phase, distinguish it from other liquid crystal phases and extend its range of applications beyond traditional uses, including displays [31,32] and polarizing films, into advanced optical materials. The properties exhibited by SCLCPs are highly attractive for the development of materials that leverage the unique optical, luminescent, and electronic properties of π-conjugated systems [33,34,35,36,37,38,39,40,41,42,43,44,45,46]. In particular, SCLCPs with liquid crystalline organic semiconductors in the side chains can be one of the most powerful tools for fabricating film-like devices with bulk arrays of organic semiconductors. In other words, it is effective for the realization of advanced materials based on functional liquid crystalline π-conjugated molecules, such as displays and molecular electronics. However, π-conjugated molecules with functional groups typically have high melting points, and SCLCPs incorporating these as mesogens often struggle to exhibit the nematic (N) phase or only exhibit it at elevated temperatures above 100 °C [45]. In addition, the introduction of a long-chain alkyl group into the mesogen lowers the isotropization temperature but tends to produce a smectic phase with high crystallinity and low operability [47]. As a result, it is challenging to achieve room temperature N-phase behavior in SCLCPs that feature π-conjugated mesogens with photo/electronic functionality.
Recently, we developed a novel aggregation-induced emission luminogen (AIEgen) [48,49,50,51,52,53,54], “seven-membered bridged stilbene”, based on 4-phenyl stilbene (PST) with a seven-membered ring structure, specifically 3,8-diphenyl-6,7-dihydro-5H-benzo[7]annulene (DPB[7]) [55,56,57]. We reported that DPB[7], when modified with appropriate alkyl tails at both ends, exhibits the N phase around room temperature [58]. This bridged stilbene liquid crystal (LC) possesses the unique ability to maintain the N phase over a wide temperature range while exhibiting luminescent properties. Such π-conjugated liquid crystals with low isotropization temperatures have the potential to overcome various limitations of conventional SCLCPs. Using this LC, we have designed a π-conjugated SCLCP that exhibits the N phase at room temperature.
In this study, we synthesized polyacrylate and polymethacrylate SCLCP with the DPB[7] mesogen, which exhibits a nematic phase at room temperature. We analyzed the detailed phase structure and evaluated the birefringence and photophysical properties. Our findings revealed several unique properties of SCLCPs that are not achievable with small molecule systems.
2. Results and Discussion
2.1. Synthesis of Monomers and Polymers
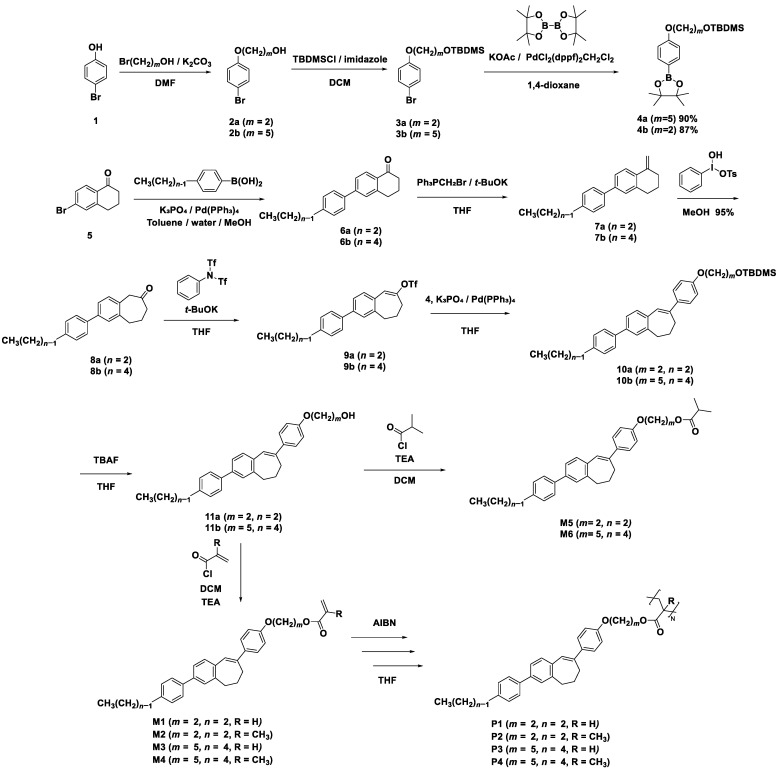
We synthesized side-chain liquid crystalline polyacrylates and polymethacrylates incorporating the 3,8-diphenyl-6,7-dihydro-5H-benzo[7]annulene (DPB[7]) skeleton with alkyl terminals (carbon number: n = 2, 4), alkoxy spacers (carbon number: m = 2, 5), and various terminal ester groups. The chemical structures and synthetic procedures of the monomers (M1–M4), polymers (P1–P4), and model compounds (M5 and M6) are illustrated in Scheme 1. The synthesis of the monomers can be outlined as follows: Suzuki–Miyaura cross-coupling reaction [59,60] between 6-bromo-1-tetralone (5) and 4-alkylphenylboronic acid (with alkyl group carbon numbers: m = 2, 4) provided 6, followed by a Wittig reaction to yield 7; a ring expansion reaction using hypervalent iodine reagent [hydroxy(tosyloxy)iodo]benzene (HTIB) then produced 8; triflation of the carbonyl group yielded 9, and subsequent Suzuki–Miyaura cross-coupling of 9 with 4-alkoxyphenylboronic acid pinacol esters (alkoxy group carbon numbers: n = 2, 5) provided 10; deprotection of the tert-butyldimethylsilyl (TBDMS) group of 10 using tetrabutylammonium fluoride (TBAF) resulted in 11. Finally, the introduction of various acyl chlorides afforded the acrylic monomers (M1 and M3), methacrylic monomers (M2 and M4), and isobutyrate derivatives (M5 and M6). The monomers were purified via column chromatography on silica gel and recrystallization. The chemical structures of the monomers and model compounds were confirmed through 1H-NMR, 13C-NMR, FT-IR, and high-resolution mass spectrometry (HRMS). These spectral data and the synthesis of 1–4 are provided in Supporting Information (SI).
Scheme 1.
Synthesis of acrylic monomers (M1 and M3), methacrylic monomers (M2 and M4), and model compounds of monomer (M5 and M6) with DPB[7] skeleton and flexible chains (m + 1, n), and corresponding polymers (P1–P4).
Radical polymerization of the acrylic monomers (M1 and M3) and methacrylic monomers (M2 and M4) was conducted using 6.5 wt% azobisisobutyronitrile (AIBN) as the initiator, resulting in the corresponding polymers (P1–P4). The crude polymers were purified by reprecipitation in methanol. The polymer structures were confirmed by 1H-NMR and 13C-NMR spectroscopy. The number-average molecular weight (Mn) and weight-average molecular weight (Mw) of the polymers were determined via gel permeation chromatography using polystyrene standards. Furthermore, thermogravimetric analysis (TGA) measurements were performed on P1–P4 at a rate of 20 °C min−1 (Figures S1–S4), determining degradation temperatures at a 10% weight loss (T10). The results are summarized in Table 1. The polymethacrylates exhibited higher molecular weights compared with the polyacrylates.
Table 1.
The results of polymerization: yields, Mn, Mw, polydispersity indexes (Mw/Mn), and degradation temperature at 10% weight loss (T10) of P1–P4.
| Entry | Yield (%) | M n | M w | Mw/Mn | T10 [°C] |
|---|---|---|---|---|---|
| P1 | 40 | 3000 | 5200 | 1.70 | 410 |
| P2 | 34 | 10,400 | 20,000 | 1.93 | 409 |
| P3 | 19 | 5000 | 7000 | 1.40 | 413 |
| P4 | 10 | 17,100 | 26,500 | 1.55 | 412 |
2.2. Phase Transition Behaviors
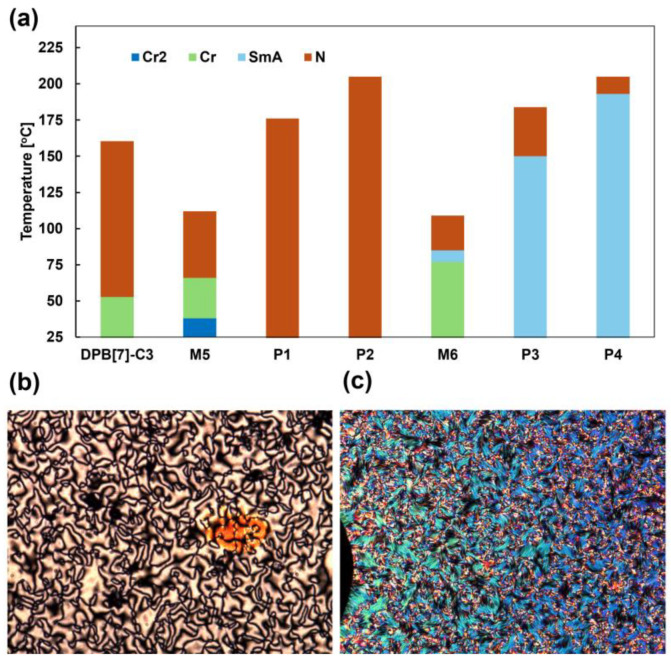
Liquid crystalline phases of the polymers, monomers, and model compounds were identified using polarized optical microscopy (POM). Phase transition temperatures and enthalpies were determined through differential scanning calorimetry (DSC). POM observations suggested that M1 and M2 exhibited only the nematic (N) phase, while M3 and M4 exhibited both the N phase and the smectic A (SmA) phase. It is suspected that the vinyl groups in M1–M4 underwent either conversion to another compound or oligomerization owing to thermal reactions. Therefore, we synthesized model compounds (M5 and M6) in which the (meth)acrylate group was replaced with an isobutyrate group. M5 and M6 were used for comparison with the monomers and polymers in subsequent analyses. The phase transition temperatures and enthalpies (ΔH) of M5 and M6 and P1–P4 are summarized in Table 2, and the typical POM images of P2 and P4 are illustrated in Figure 1. The DSC curves for M5 (second scan), M6 (second scan), and P1–P4 (third scan) are shown in Figures S5–S10, while the POM images for M5, M6, P1, P3, and the rest of P2 and P4 are shown in Figures S11–S16.
Table 2.
Phase transition temperature [°C] (enthalpy ΔH [kJ mol–1]) and the range of LC phase (ΔTLC [°C]) of DPB[7] monomers (M5 and M6) and DPB[7] polymers (P1–P4) upon heating and cooling at a rate of 10 °C min–1 (Cr and Cr2: crystal phase, N: nematic phase, SmA: smectic A phase, Iso: isotropic phase, G: glass state).
| Entry | Phase Transition Behavior | |
|---|---|---|
| Heating [a] | Cooling [b] | |
| DPB[7]-C3 [c] | Cr 52.6 (11.8) N 160.4 (0.85) Iso | Iso 158.3 (0.86) N 4.8 (5.68) Cr |
| M5 | Cr 37.9 (0.15) Cr2 [d] 66.4 (0.24) N 111.6 (0.33) Iso | Iso 109.8 (0.60) N |
| M6 | Cr 77.4 (22.5) SmA 84.8 (0.49) N 109.3 (0.77) Iso | Iso 107.5 (0.41) N 83.0 (0.49) SmA 29.5 (15.6) Cr |
| P1 | G 60.6 N 176.3 (0.22) Iso | Iso 173.4 (0.36) N 57.5 G |
| P2 | N 205.0 (0.32) Iso | Iso 202.5 (0.28) N |
| P3 | G 25.6 SmA 150.3 (0.65) N 184.2 (0.32) Iso | Iso 181.8 (0.46) N 152.8 (1.28) SmA 23.2 G |
| P4 | SmA 193.8 (0.75) N 205.0 (0.19) Iso | Iso 202.0 (0.44) N 181.5 (1.18) SmA |
[a] Third heating for DPB[7]-C3 and P1–P4, and second heating for M5 and M6. [b] Third cooling for DPB[7]-C3 and P1–P4 and second cooling for M5 and M6. [c] Reported by Iwai et al. [54]. [d] A crystal–crystal phase transition was observed only for M5.
Figure 1.
Summary of phase transition behavior. (a) Phase transition temperature and range diagram for DPB[7] monomers (DPB[7]-C3, M5, and M6) and DPB[7] polymers (P1–P4) observed by DSC measurement in second heating (for M5 and M6) and third heating (for DPB[7]-C3 and P1–P4) process. POM images of (b) schlieren texture for P2 at 193 °C and (c) fan-shape texture for P4 at 128 °C.
M5 exhibited the N phase between 66.4 °C and 111.6 °C during the heating process, while M6 exhibited the SmA phase between 77.4 °C and 84.8 °C and the N phase between 84.8 °C and 109.3 °C. POM images of the schlieren textures in the N phase for M5 and M6 are illustrated in Figures S11 and S12, and fan-shaped textures in the SmA phase of M6 are also presented in Figure S12. The flexible chain length, excluding the terminal ester group, (in terms of carbon and oxygen atoms) is three or fewer for M5 and four or more for M6. This trend is similar to our DPB[7] liquid crystals [58], where the SmA phase emerges when the flexible chain length exceeds three atoms.
Next, we describe the phase transition behaviors of P1–P4. P1 and P2 exhibited N phases over the ranges of 25.0–176.3 °C and 25.0–205.0 °C, respectively, during the heating process. In these polymers, the N phase was successfully maintained at room temperature (25 °C), and schlieren textures were observed, as illustrated in Figure 1b. P3 displayed a smectic A (SmA) phase between 25.0 °C and 150.3 °C and an N phase from 150.3 °C to 184.2 °C, while P4 showed a SmA phase from 25.0 °C to 193.8 °C and an N phase from 193.8 °C to 205.0 °C. In the SmA phases of P3 and P4, fan-shaped textures were observed (Figure 1c). The glass transition temperature (Tg) was observed at 60.6 °C for P1, and 25.6 °C for P3, while no Tg was detected for P2 and P4.
The clearing points of P1 and P2 were 64.7 °C and 93.4 °C higher than those of M5, respectively. Similarly, the clearing points of P3 and P4 were higher than those of M6 by 74.9 °C and 95.7 °C, respectively, and the N-SmA phase transition temperatures were higher by 65.5 °C and 109.0 °C. These results, indicating higher phase transition temperatures due to the polymer effect, are consistent with previously reported findings for SCLCPs. Notably, these liquid crystalline phases were maintained even after more than three months, as illustrated in Figures S13 and S14. The room temperature N phase observed in P1 and P2 offers significant advantages for optoelectronic applications operating at room temperature. Finally, upon 25.0 °C, the LC temperature ranges (ΔTLC) for P1–P4 were 151.3 °C, 180.0 °C, 159.2 °C, and 180.0 °C, respectively. These values were considerably larger than those of the model monomers M5 and M6 (ΔTLC = 45.2 °C and 31.9 °C, respectively).
2.3. Structure Analysis of LC Phase
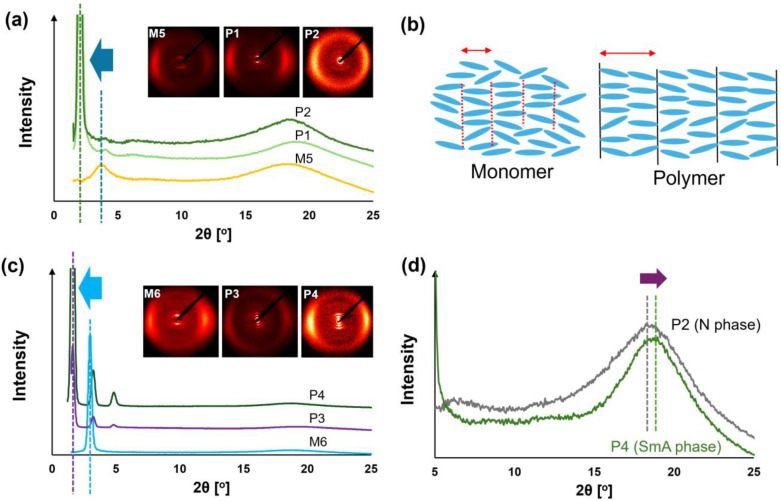
To investigate changes in the molecular arrangement of the LCs due to polymer effect, we performed wide-angle X-ray diffraction (WAXD) measurements for P1–P4 and the corresponding monomers M5 and M6 (Figures S17–S34). The WAXD profiles are summarized in Figure 2, in which the magnetic field is in the vertical direction of the paper. In the N phase, the peak observed in the small-angle region of the WAXD profile (2θsmall) corresponded to the layer spacing (dLC) along the molecular long axis. Also, in the SmA phase, not only the first-order peak (2θsmall) but also second- or third-order peaks were observed for P3 and P4, while the peak in the wide-angle region (2θwide) corresponded to the average distance between mesogens (dmesogen) along the molecular short axis. The values of the small-angle and wide-angle peaks (2θsmall, 2θwide), along with the corresponding d-spacing values (calculated using Bragg’s law) and the molecular length/side-chain length (l) (obtained from DFT calculations (Figures S35 and S36, and Tables S1 and S2)) are presented in Table 3.
Figure 2.
WAXD intensity profiles and expected structure in LC phase. (a) WAXD intensity profile in nematic (N) phase for M5 (80 °C), P1 (40 °C), and P2 (30 °C). The 2θsmall values are shifted to small angle region due to the polymer effect. (b) Schematic illustration of molecular packing in N phase with cybotactic cluster for monomer (monolayer) and polymer (double-layer). (c) WAXD intensity profile in smectic A phase for M6 (80 °C), P3 (30 °C), and P4 (30 °C) The 2θsmall values are shifted to small angle region due to the polymer effect. (d) WAXD intensity profile for P2 and P4 in wide-angle region recorded at 30 °C. The 2θwide value for SmA phase is slightly larger than that for N phase.
Table 3.
The 2θ peak angle in small-angle region (2θsmall [°]) and wide-angle region (2θwide [°]) obtained from WAXD profile, their 2θ d-spacing values (dLC and dmesogen [Å]), calculated molecular length (l [Å]), and the value of dLC/l.
| Entry | 2θsmall [°] | dLC[a] [Å] | l[b] [Å] | dLC/l [-] | 2θwide [°] | dmesogen [Å] |
|---|---|---|---|---|---|---|
| M5 | 3.60 [c] | 24.6 | 24.2 | 1.0 | 18.5 [c] | 4.78 |
| P1 | 2.09 | 42.4 | 22.4 | 1.9 | 18.9 [d] | 4.71 |
| P2 | 2.01 [e] | 44.0 | 22.4 | 2.0 | 18.3 [e] | 4.85 |
| M6 | 2.97 [c] | 29.8 | 30.2 | 1.0 | 18.5 [c] | 4.78 |
| P3 | 1.61 [e] | 55.1 | 28.9 | 1.9 | 19.3 [e] | 4.60 |
| P4 | 1.61 [e] | 55.1 | 28.9 | 1.9 | 18.9 [e] | 4.69 |
[a] Layer spacing of LC phase (nematic phase for M5, P1, P2, and smectic A phase for M6, P3, P4) calculated from 2θsmall [°] value. [b] Whole molecular length (or length of side-chain for polymers) when alkyl chains are assumed to be fully extended in all-trans (calculated using DFT calculation at the B3LYP/6-31G(d) level of theory). [c] Measured at 80 °C. [d] Measured at 40 °C. [e] Measured at 30 °C.
In the N phase of M5 (at 80 °C), P1 (at 40 °C), and P2 (at 30 °C), broad scattering peaks in the wide-angle region and sharp peaks in the small-angle region were observed (Figure 2a). In the N phase, there was no long-range positional order, and typically, only broad scattering in the wide-angle region was detected in WAXD measurements. Therefore, the observed small-angle peaks suggest the formation of smectic-like micro aggregates, specifically cybotactic clusters. The azimuthal profiles of diffraction in the small-angle region for M5, P1, and P2 (Figures S19, S25 and S28) demonstrated that the scattering in the small-angle region was orthogonal to that in the wide-angle region. These results indicate that the cybotactic clusters [61,62,63] are SmA-type, with no uniform tilt in the domain. Notably, the ratio of the small-angle peak intensity to the wide-angle peak intensity was more than 10 times greater in P1 and P2 than in M5, suggesting that cybotactic clusters form more readily in P1 and P2 than in M5.
The 2θsmall values for M5, P1, and P2 were 3.60°, 2.09°, and 2.01°, respectively, corresponding to dLC values of 24.6 Å, 42.4 Å, and 44.0 Å, respectively. DFT calculations revealed that the molecular lengths (l) for M5, P1, and P3 were 24.2 Å, 22.4 Å, and 22.4 Å, respectively, when the flexible chains were in the all-trans configuration and fully extended. Consequently, the dLC/l ratios for M5, P1, and P3 were calculated to be 1.0, 1.9, and 2.0, respectively. This indicates that the cybotactic clusters in the N phase of M5 form a monolayer structure, while those in P1 and P3 form a double-layer structure (Figure 2b).
In the SmA phase of M6 (80 °C), P3 (30 °C), and P4 (30 °C), broad scattering peaks were observed in the wide-angle region, while sharp peaks appeared in the small-angle region. The azimuthal profiles of diffraction in the small-angle region for M6, P3, and P4 (Figures S22, S31, and S34) showed that the scattering in the small-angle region was orthogonal to that in the wide-angle region, a characteristic of the SmA phase. The 2θsmall values for M6, P3, and P4 were 2.97°, 1.61°, and 1.61°, respectively, corresponding to dLC values of 29.8 Å, 55.1 Å, and 55.1 Å. DFT calculations revealed l = 30.2 Å, 28.9 Å, and 28.9 Å for M6, P3, and P4, respectively, from which the dLC/l ratios were determined to be 1.0, 1.9, and 1.9. This suggests that M6 has a monolayer structure in the SmA phase, while P3 and P4 exhibit double-layer structures (Figure 2b).
In summary, M5 and M6 exhibit monolayer structures in the LC phase, while P1–P4 form double-layer structures. The formation of a double-layer structure is commonly reported in SCLCPs [64,65]. The 2θwide values observed in the N phase of M5, P1, and P2 were 18.5°, 18.9°, and 19.3°, respectively, while those observed in the SmA phase of M6, P3, and P4 were 18.5°, 18.9°, and 19.3°, respectively. Consequently, dmesogen values for M5, P1, P2, M6, P3, and P4 were calculated to be 4.78 Å, 4.71 Å, 4.85 Å, 4.78 Å, 4.60 Å, and 4.69 Å, respectively. Although there was no difference between the dmesogen values of M5 and M6, the values of P3 and P4 were 0.02–0.25 Å smaller than those of P1 and P2.
2.4. Birefringence Properties
To evaluate the optical properties of P1–P4, temperature-variable birefringence (Δn) measurements were performed following our previously reported method [66,67,68,69,70]. For comparison with the corresponding polymers, ∆n measurements were also conducted for M5 and M6 because of the lack of heat stability of M1–M4. First, the nematic liquid crystals (NLCs) were filled into homogeneously aligned polyimide cells. When preparing LC cells for P1–P4, the acrylic polymers P1 and P3 could be filled into the polyimide cells via capillary force; however, the methacrylic polymers P2 and P4 could not be filled due to their high viscosity, likely caused by differences in molecular weight. To confirm uniaxial orientation, POM observations were carried out. The prepared LC cells appeared dark when the polarizer was aligned with the rubbing direction and brightest when at 45° to the rubbing direction (Figure S37). These results confirm that the nematic director was aligned with the rubbing direction.
Next, we employed a micro-spectroscopic method, observing the transmitted light through the LC cell as a function of wavelength (λ) under cross-polarized conditions. The nematic director was set at 45° to the polarizer. A typical transmission light plot is shown in Figure S38 (dot). The transmitted light intensity (I) was fitted using the following Equation (1) and Cauchy’s Equation (2) to determine the coefficients a, b, and c, where A is a constant.
| (1) |
| (2) |
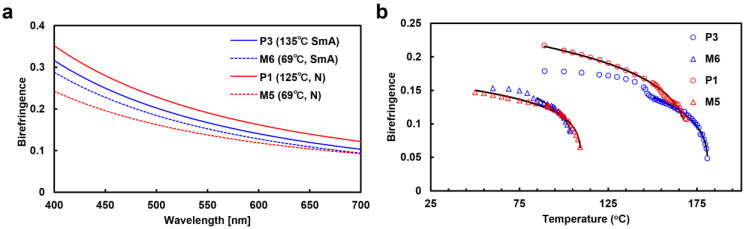
The theoretical curve obtained from Equation (2) fitted well to the transmitted light plot (Figure S38 (solid line)). From this fitting, we obtained the Δn data presented in Figure 3. Figure 3a illustrates the wavelength dependence of Δn at T/Ti = 0.9 (Ti; isotropic temperature), while Figure 3b shows Δn plotted against temperature for M5, M6, P1, and P3 at 550 nm. As shown in Figure 3a, the Δn values of P1 and P3 were higher than those of M5 and M6, indicating that Δn increased as a result of polymerization. Additionally, P1 and P3 exhibited Δn values of 0.24 and 0.20 at room temperature (25 °C), respectively.
Figure 3.
Birefringence is dependent on (a) wavelength and (b) temperature for M5, M6, P1, and P3. (a) Measured at the same reduced temperature (T/Ti = 0.9) in each LC phase. (b) Plotted against temperature at 550 nm and fitted by Equations (3) and (4). For M6 and P3, the fitting was performed within the nematic phase.
As illustrated in Figure 3b, Δn decreased with increasing temperature. The relationship between Δn and temperature can be explained by the temperature dependence of the order parameter (S) of the nematic director, which is described by Haller’s approximation:
| (3) |
| (4) |
where Δn0 is the extrapolated value for the perfectly oriented birefringence (S = 1) of the N LC, Ti is the clearing point, and β is a material constant characteristic of the NLC. The theoretical curve obtained from Equation (4) fitted well to the plot of Δn for each compound (Figure 3), and the values of β and Δn0 were determined. Additionally, the order parameter S was calculated using Equation (3) from the measured Δn values and the corresponding Δn0 values.
The values of S at 90 °C were calculated, as this was the temperature at which M5, M6, P1, and P3 could be measured, and are shown in Table 4 along with the corresponding values of β and Δn0. The Δn0 values for M5, M6, P1, and P3 were 0.21, 0.26, 0.31, and 0.26, respectively, and the β values were 0.18, 0.23, 0.22, and 0.23, respectively. The S values at 90 °C were 0.60, 0.50, 0.69, and 0.69, respectively. Comparison of these values indicates an improvement in S due to the polymer effect [21].
Table 4.
Determined values of ∆n0 and β by Haller’s approximation and estimated birefringence (∆n) and order parameter (S) at 90 °C.
| Entry | ∆n0 | β | ∆n (90 °C) | S (90 °C) |
|---|---|---|---|---|
| M5 | 0.21 | 0.18 | 0.12 | 0.60 |
| M6 | 0.26 | 0.23 | 0.13 [a] | 0.50 [b] |
| P1 | 0.31 | 0.22 | 0.22 | 0.69 |
| P3 | 0.26 | 0.23 | 0.18 [a] | 0.69 [b] |
[a] Value in smectic A phase. [b] calculated using Δn (90 °C) value in smectic A phase.
Furthermore, a comprehensive evaluation of the S values, combined with the results from DSC and WAXD measurements, led to the conclusion that the significant increase in the higher phase transition temperatures (SmA–N and N–isotropic transitions) of P1–P4 might be attributed to the enhancement of S, which resulted from the formation of a double-layer structure in the liquid crystalline phase.
2.5. Fluorescence Properties
To investigate the fluorescence properties of M1–M4 and P1–P4, absorption spectra, fluorescence spectra, and fluorescence quantum yields (Φfl) were measured in a dilute THF solution and in the solid state. Additionally, the fluorescence spectra of cast films of P1–P4 were measured. The obtained values for maximum absorption wavelength (λabs), maximum fluorescence wavelength (λfl), and Φfl for M1–M4 and P1–P4 are summarized in Table 5.
Table 5.
Spectroscopic properties for M1–M4 and P1–P4 in THF solution, solid state, and film.
| Entry | λabs [nm] | Φfl [-] | λfl [nm] | |||
|---|---|---|---|---|---|---|
| THF | THF | Solid [a] | THF | Solid [a] | Film | |
| M1 | 316 | 0.02 | 0.43 | 399 | 417 | - |
| M2 | 316 | 0.02 | 0.58 | 399 | 407 | - |
| M3 | 316 | 0.02 | 0.61 | 401 | 413 | - |
| M4 | 316 | 0.02 | 0.48 | 400 | 408 | - |
| P1 | 316 | 0.03 | 0.14 | 407 | 415 | 442 |
| P2 | 316 | 0.02 | 0.16 | 399 | 413 | 456 |
| P3 | 316 | 0.03 | 0.35 | 400 | 413 | 469 |
| P4 | 316 | 0.03 | 0.18 | 407 | 413 | 473 |
[a] Polycrystalline solid.
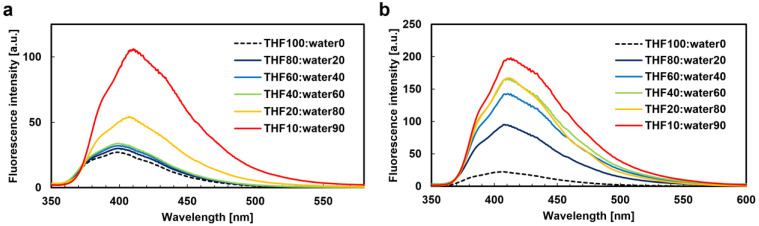
The λabs values for all the monomers (M1–M4) in THF were 316 nm. The Φfl values for M1–M4 in THF solution were 0.02, while in the solid state, they were 0.43, 0.58, 0.61, and 0.48, respectively. This indicates that M1–M4 exhibited aggregation-induced emission (AIE) properties. The AIE properties were further confirmed by aggregation experiments on M2 and P2 (Figure 4). The λfl values for M1–M4 in the THF solution were approximately 400 nm, while in the solid state (polycrystalline state), they were 417, 407, 413, and 408 nm, respectively. These results suggest that the fluorescence in the solid state may be derived from aggregates rather than isolated monomers. This behavior was similar to that of our previously reported AIEgen, bridged stilbene [55].
Figure 4.
Aggregation experiments. Fluorescence spectra of (a) M2 and (b) P2 in THF/water mixtures with different water contents (vol %) excited at each λabs; the concentration is 10−5 M.
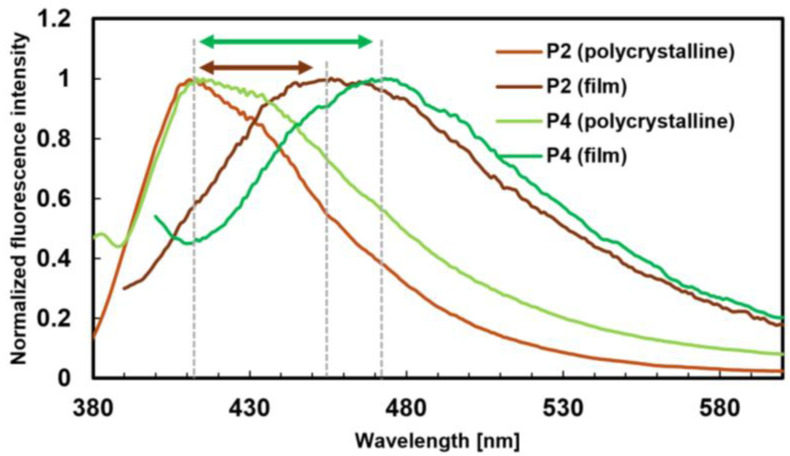
The λabs value for P1–P4 in THF was 316 nm. The Φfl value for P1–P4 in the THF solution was approximately 0.03, while in the solid state, the values were 0.14, 0.15, 0.35, and 0.18, respectively. P1–P4 exhibited the same AIE properties as the monomers, and aggregation experiments with P2 and P4 yielded similar results to those of the monomers (Table S3). Similar redshifts in the λfl values for P1–P4 in both the THF solution and the solid state were observed, mirroring the behavior of the monomers. These findings revealed that the luminescence properties of the bulk polymers and monomers were nearly identical. Interestingly, the λfl values for P1–P4 in the polymer films were 442, 456, 469, and 473 nm, respectively, redshifted by more than 27 nm compared with those in the solid state (polycrystalline state) (Figure 5). The relationship between the λfl of the films and the liquid crystalline phase at room temperature was also investigated. The λfl values for P1 and P2 (which exhibited the N phase) were around 450 nm, while those for P3 and P4 (which exhibited the SmA phase) were around 470 nm. These results suggest that the λfl in the films may vary depending on the liquid crystalline phase, with a relatively shorter wavelength shift in the N phase and a longer wavelength shift in the SmA phase. This indicates that the molecular orientation and interactions in different LC phases may affect the fluorescence properties.
Figure 5.
Fluorescence spectra of P2 and P4 excited at 370 nm (polycrystalline) and at 380 nm (film).
3. Experimental
3.1. Materials
Unless otherwise noted, all solvents and chemicals were commercially available and used without further purification. Column chromatography was performed on silica gel (Silica Gel 60N, 63–210 μm, Kanto chemical Co., Inc., Tokyo, Japan). [Hydroxy(tosyloxyl)iodo]benzene, tetrakis(triphenylphoshine)palladium (0) (Pd(PPh3)4), [1,1-bis(diphenylphosphino)ferrocene]palladium (0) (Pd(dppf)Cl2·CH2Cl2), and 6-bromo-1-tetralone were purchased from TCI (Tokyo, Japan).
3.2. Instruments
1H-NMR and 13C-NMR spectra were recorded on BRUKER 500 (Yokohama, Japan) (500 MHz) and JEOL 400 (Tokyo, Japan) (100 MHz) spectrometers, respectively, for CDCl3 solution using tetramethylsilane (TMS) as an internal standard. 1H-NMR spectra were reported as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet), integration, and coupling constants in units of Hz. 13C-NMR spectra were reported as chemical shifts in ppm. The FT-IR spectra were recorded on a JASCO FT-IR 4600 spectrometer (Tokyo, Japan). High-resolution EI mass spectra (HRMS) were recorded on a double-focusing mass spectrometer JEOL JMS-700, measured at the Tokyo Institute of Technology Open Facility Center. This center is independent of our laboratory to ensure fairness. Size exclusion chromatography (SEC) was performed using a JASCO system (PU-2080, CL-NETII/ADC, CO-2060, UV-2075, RI-2031, Tokyo, Japan) equipped with TSK gel columns (TOSOH G3000H xl, Tokyo, Japan) with THF as the eluent at the following rate of 0.85 mL min−1 at 40 °C after calibration with polystyrene standards. Polarized optical microscopy (POM) was performed using a Leica DM2500P microscopy (Wetzlar, Germany) with a Mettler FP90 hot stage (Greifensee, Switzerland). Differential scanning calorimetry (DSC) was performed using PerkinElmer DSC 8500 equipment (Waltham, MA, USA) at a scanning rate of 10 °C min−1 under a flow of dry nitrogen. Thermo-gravimetric analysis (TGA) was performed using a Rigaku Thermo Plus EVO2 series TG-DTA 8122 (Tokyo, Japan) at a heating rate of 20 °C min–1 under a flow of dry nitrogen. The initial mass of the samples was 3–6 mg. X-ray investigations were carried out with samples kept in glass capillary tubes (1.0 mm diameter) for oriented patterns under a magnetic field. Wide-angle X-ray diffraction (WAXD) patterns were obtained using a Bruker D8 DISCOVER (Billerica, MA, USA) equipped with a Vantec-500 detector and Cu Kα radiation. UV-Vis spectra were recorded on a JASCO V-670 UV-vis spectrophotometer (Tokyo, Japan) and fluorescence spectra were recorded on a JASCO FP-6500 spectrofluorometer (Tokyo, Japan). Absolute quantum yields were measured by Hamamatsu Photonics Quantaurus QY apparatus (Hamamatsu City, Japan). All sample solutions were de-aerated by bubbling with argon for 15 min prior to the quantum yield measurement.
3.3. Birefringence
Measurement of birefringence was performed in uniaxially aligned LC cells containing indium tin oxide (ITO) purchased from EHC. The cell gap (d) of 3–5 μm was determined by the interferometric method [66,67,68,69,70].
3.4. Theoretical Calculations
Theoretical calculations were carried out on the Gaussian 16 program [71]. Geometry optimizations were carried out using DFT methods at the B3LYP with the 6-31G(d) basis. Whether the optimized geometry was at the stationary point without any imaginary frequency was checked by the frequency calculation performed at the optimized geometries using their level of theory.
3.5. Synthesis
6-(4-ethylphenyl)-3,4-dihydronaphthalen-1(2H)-one (6a)
To a solution of 6-bromo-1-tetralone (5) (3.4 g, 15 mmol), 4-alkylphenylboronic acid (3.4 g, 22 mmol), potassium phosphate (9.5 g, 45 mmol) in solvent (30 mL/12 mL/6 mL; toluene/water/methanol), Pd(PPh3)4 (0.55 g, 0.48 mmol) was added under argon atmosphere, and the mixture was refluxed (100 °C) for 3 hours. After the reaction, the mixture was cooled to room temperature, then extracted with dichloromethane. The organic layer was washed with water three times, dried over MgSO4, filtrated, and evaporated in vacuo. The residue was purified by column chromatography on silica gel (6/1 (v/v) hexane/ethyl acetate) to afford 6a as a brown solid; yield 96%; 1H-NMR (500 MHz, CDCl3) δ 8.09 (d, J = 8.2 Hz, Ar-H, 1H), 7.56–7.52 (m, Ar-H, 3H), 7.47–7.45 (m, Ar-H, 1H), 7.31–7.29 (m, Ar-H, 2H), 3.02 (t, J = 6.1 Hz, -CH2-, 2H), 2.73–2.67 (m, -CH2-, 4H), 2.20–2.15 (m, -CH2-, 2H), 1.28 (t, J = 7.6 Hz, -CH3, 3H) ppm (Figure S47).
6-(4-ethylphenyl)-1-methylene-1,2,3,4-tetrahydronaphthalene (7a)
Methyltriphenylphosphonium bromide (7.1 g, 19 mmol) was dissolved in THF (40 mL) under argon atmosphere. The solution was cooled to 0 °C, and then potassium tert-butoxide (2.4 g, 21 mmol) was added. Following stirring for 10 minutes, 6a (14.5 mmol) was added, warming to room temperature and stirring overnight. The reaction mixture was quenched by adding NH4Cl aq to the solution and extracted with ethyl acetate. The organic layer was washed with water three times, dried over MgSO4, filtrated, and evaporated in vacuo. The residue was purified by column chromatography on silica gel (4/1 (v/v) hexane/dichloromethane) to afford 7a as a colorless solid; yield 90%; 1H-NMR (500 MHz, CDCl3) δ 7.71 (d, J = 8.2 Hz, Ar-H, 1H), 7.51 (d, J = 8.2 Hz, Ar-H, 2H), 7.40–7.37 (m, Ar-H, 1H), 7.33–7.32 (m, Ar-H, 1H), 7.26 (d, J = 7.6 Hz, Ar-H, 2H), 5.51 (s, C=CH, 1H), 4.96 (s, C=CH, 1H), 2.90 (t, J = 6.3 Hz, -CH2-, 2H), 2.69 (q, J = 7.6 Hz, -CH2-, 2H), 2.57 (t, J = 6.1 Hz, -CH2-, 2H), 1.94–1.89 (m, -CH2-, 2H), 1.27 (t, J = 7.6 Hz, -CH3, 3H) ppm (Figure S49).
2-(4-ethylphenyl)-5,7,8,9-tetrahydro-6H-benzo[7]annulen-6-one (8a)
To a solution of 7a (13 mmol) in solvent (38 mL/2 mL; methanol/water), HTIB ([hydroxy(tosyloxy)iodo]benzene; 5.6 g, 14 mmol) was added under air, and stirred at room temperature for 20 min. After the reaction, the mixture was extracted with dichloromethane. The organic layer was washed with water three times, dried over MgSO4, filtrated, and evaporated in vacuo. The residue was purified by column chromatography on silica gel (6/1 (v/v) hexane/ethyl acetate) to afford 8a as a colorless solid; yield 92%; 1H-NMR (500 MHz, CDCl3) δ 7.51 (d, J = 8.2 Hz, Ar-H, 2H), 7.41–7.38 (m, Ar-H, 2H), 7.27 (d, J = 8.2 Hz, Ar-H, 2H), 7.21 (d, J = 7.6 Hz, Ar-H, 1H), 3.76 (s, -CH2-, 2H), 3.01 (t, J = 6.4 Hz, -CH2-, 2H), 2.69 (q, J = 7.6 Hz, -CH2-, 2H), 2.60 (t, J = 7.0 Hz, -CH2-, 2H), 2.06–2.01 (m, -CH2-, 2H), 1.28 (t, J = 7.6 Hz, -CH3, 3H) ppm (Figure S51).
3-(4-ethylphenyl)-6,7-dihydro-5H-benzo[7]annulen-8-yl trifluoromethanesulfonate (9a)
The mixture of 8a (12 mmol) and THF (30 mL) was cooled to −20 °C, and then tert-butoxide (2.4 g, 21 mmol) was added. The mixture was stirred at 0 °C for 1 h, and then cooled to −20 °C, then N-phenylbis(trifluolomethanesulfonimide) (5.3 g, 15 mmol) was added, and stirred at −20 °C for a further 1 h, and then warmed to 0 °C, and stirred for 4 h. After that, the reaction was quenched by the dropwise addition of water (20 mL), and organic products were extracted with ethyl acetate. The organic layer was washed with water three times, dried over MgSO4, filtrated, and evaporated in vacuo. The residue was purified by column chromatography on silica gel (6/1 (v/v) hexane/ethyl acetate) to afford 9a as a slight yellow solid; yield 98%; 1H-NMR (500 MHz, CDCl3) δ 7.51 (d, J = 7.9 Hz, Ar-H, 2H), 7.43–7.40 (m, Ar-H, 1H), 7.34–7.33 (m, Ar-H, 1H), 7.27 (d, J = 8.2 Hz, Ar-H, 2H), 7.22 (d, J = 7.9 Hz, Ar-H, 1H), 6.62 (s, Ar-CH-, 1H), 2.95 (t, J = 5.2 Hz, -CH2-, 2H), 2.81 (t, J = 6.4 Hz, -CH2-, 2H), 2.70 (q, J = 7.6 Hz, -CH2-, 2H), 2.06–2.01 (m, -CH2-, 2H), 1.28 (t, J = 7.6 Hz, -CH3, 3H) ppm (Figure S53).
tert-butyl(2-(4-(3-(4-ethylphenyl)-6,7-dihydro-5H-benzo[7]annulen-8-yl)phenoxy)ethoxy)dimethylsilane (10a)
To a solution of 9 (8 mmol), 4a (16 mmol), K3PO4·nH2O (2.4 g, 16 mmol) in THF (40 mL), Pd(PPh3)4 (0.28 g, 0.24 mmol) was added under argon atmosphere, and stirred at 50 °C overnight. After the reaction, the mixture was cooled to room temperature, then extracted with dichloromethane. The organic layer was washed with water three times, dried over MgSO4, filtrated, and evaporated in vacuo. The residue was purified by column chromatography on silica gel (4/1 (v/v) hexane/dichloromethane) to afford 10a as a colorless solid; yield 54%; 1H-NMR (500 MHz, CDCl3) δ 7.55 (d, J = 7.9 Hz, Ar-H, 2H), 7.45–7.39 (m, Ar-H, 4H), 7.27–7.26 (m, 3H), 6.91 (d, J = 8.5 Hz, Ar-H, 2H), 6.77 (s, Ar-H, 1H), 4.07 (t, J = 5.3 Hz, -CH2-, 2H), 3.99 (t, J = 5.0 Hz, -CH2-, 2H), 2.87 (t, J = 6.0 Hz, -CH2-, 2H), 2.72–2.66 (m, -CH2-, 4H), 2.23 (t, J = 6.3 Hz, -CH2-, 2H), 1.28 (t, J = 7.6 Hz, -CH3, 3H), 0.92 (s, CH3, 9H), 0.12 (s, -CH3, 6H) ppm (Figure S55).
2-(4-(3-(4-ethylphenyl)-6,7-dihydro-5H-benzo[7]annulen-8-yl)phenoxy)ethan-1-ol (11a)
To a solution of 10a (2.6 mmol) in THF (5.0 mL), 12M HCl aq (4.0 mL) was added, and stirred at room temperature for 10 min. After that, the reaction was quenched by the dropwise addition of NaHCO3 aq (20 mL), and organic products were extracted with dichloromethane. The organic layer was washed with water three times, dried over MgSO4, filtrated, and evaporated in vacuo. Purification by recrystallization (CHCl3/Hex) gave 11a as a colorless solid; yield 87%; 1H-NMR (500 MHz, CDCl3) δ 7.55 (d, J = 8.2 Hz, Ar-H, 2H), 7.46 (d, J = 8.7 Hz, Ar-H, 2H), 7.42 (dd, J = 7.8, 2.0 Hz, Ar-H, 1H), 7.39 (s, Ar-H, 1H), 7.28–7.26 (m, 3H), 6.93 (d, J = 8.9 Hz, Ar-H, 2H), 6.78 (s, Ar-H, 1H), 4.12 (t, J = 4.6 Hz, -CH2-, 2H), 4.00–3.97 (m, -CH2-, 2H), 2.88 (t, J = 6.1 Hz, -CH2-, 2H), 2.72–2.66 (m, -CH2-, 4H), 2.26–2.21 (m, -CH2-, 2H), 1.28 (t, J = 7.5 Hz, -CH2-, 3H) ppm (Figure S57).
2-(4-(3-(4-ethylphenyl)-6,7-dihydro-5H-benzo[7]annulen-8-yl)phenoxy)ethyl acrylate (M1)
To a solution of compound 11a (1.3 mmol) and triethylamine (0.27 mL, 2.0 mmol) in dichloromethane (5.0 mL) was added acyl chloride (1.7 mmol) and the mixture was stirred at room temperature for 1 h. After the reaction, the solvent was removed by evaporation, and the residue was filtered with hexane. The organic layer was washed with water three times, dried over MgSO4, filtrated, and evaporated in vacuo. The residue was purified by column chromatography on silica gel (1/1 (v/v) hexane/ethyl acetate) to afford crude M1-M6. Purification by recrystallization (hexane/dichloromethane) gave pure M1 as a colorless solid; yield: 53%; 1H-NMR (500 MHz, CDCl3) δ 7.55 (d, J = 8.2 Hz, Ar-H, 2H), 7.47–7.39 (m, Ar-H, 4H), 7.27–7.25 (m, 3H), 6.93 (d, J = 8.5 Hz, Ar-H, 2H), 6.77 (s, Ar-H, 1H), 6.48–6.44 (m, =CH, 1H), 6.18 (dd, J = 17.4, 10.4 Hz, -CH=, 1H), 5.87 (dd, J = 10.5, 1.4 Hz, =CH, 1H), 4.53 (t, J = 4.7 Hz, -CH2-, 2H), 4.25 (t, J = 4.9 Hz, -CH2-, 2H), 2.88 (t, J = 6.1 Hz, -CH2-, 2H), 2.72–2.66 (m, -CH2-, 4H), 2.23 (t, J = 6.1 Hz, -CH2-, 2H), 1.28 (t, J = 7.6 Hz, -CH3, 3H) ppm (Figure S59); 13C-NMR (100 MHz, CDCl3) δ 166.2, 157.9, 143.4, 142.3, 141.6, 139.2, 138.4, 137.4, 136.4, 131.5, 131.1, 128.4, 128.2, 127.7, 127.5, 127.4, 127.0, 124.5, 114.5, 66.1, 63.0, 35.0, 33.1, 30.3, 28.6, 15.7 ppm (Figure S60). HRMS (EI) Calcd for C30H30O3: 438.5664, Found 438.2195 (Figure S57).
Poly[2-(4-(3-(4-ethylphenyl)-6,7-dihydro-5H-benzo[7]annulen-8-yl)phenoxy)ethyl] acrylate (P1)
To a pressure-resident tube, which contained M1 (0.37 g, 0.83 mmol) and a portion of THF (3.0 mL), AIBN (azobis(isobutyronitrile), 9.0 mg, 6.5 wt%, was added, and the mixture was stirred at 60 °C for 24 h. The mixture was poured dropwise with an excess amount of methanol, and it was filtered. The solids were purified by column chromatography on SephadexTM G100 (eluted with THF), and reprecipitated with methanol; colorless solid; yield 40.3%; 1H-NMR (500 MHz, CDCl3) δ 7.50–7.05 (brm, Ar-H, 9H), 6.88–6.71 (brm, Ar-H, 3H), 4.26–4.10 (brm, 4H), 2.88–2.36 (brm, 6H), 2.17–2.13 (brm, 2H), 1.84 (br, 1H), 1.26 (br, 3H) ppm (Figure S71); 13C-NMR (100 MHz, CDCl3) δ 174.7, 157.8, 143.3, 142.1, 141.6, 139.0, 138.3, 138.2, 137.2, 136.3, 135.9, 131.2, 128.3, 127.4, 127.3, 126.9, 125.6, 124.5, 114.5, 65.8, 62.9, 41.3, 41.2, 35.0, 34.3, 33.1, 31.6, 30.4, 30.1, 29.6, 28.6, 21.3, 15.7 ppm (Figure S72).
4. Conclusions
We synthesized P1–P4 as SCLCPs with varying flexible chain lengths by radical polymerization of acrylate monomers containing π-extended bridged stilbene mesogens. The resulting polymers exhibited liquid crystalline phases over a temperature range exceeding 150 °C. P1 and P2 exhibited a nematic phase at room temperature, which was maintained for more than three months. These stable nematic phases are highly advantageous for device applications operating at room temperature. WAXD measurements revealed that the polymers possessed a double-layer structure in the liquid crystalline phase. Additionally, the polymers demonstrated larger order parameter (S) values compared with small molecules and exhibited higher birefringence. The polymers were also found to retain the AIE characteristics of the DPB[7] skeleton, with high quantum yields (Φfl = 0.14–0.35) in the solid state (polycrystalline). Furthermore, film formation resulted in a redshift of the maximum fluorescence wavelength (λfl) by more than 27 nm, yielding λfl values in the range of 442–473 nm. The magnitude of the redshift may be related to the liquid crystalline phase exhibited at room temperature, though further investigation is required to clarify the details. These optically and luminescent-functionalized SCLCPs hold promise for applications in luminescent semiconductors and optoelectronic materials, including holograms [72,73,74] and polarized light-emitting devices [75,76,77,78,79,80,81,82,83,84,85].
Acknowledgments
We thank Masato Koizumi (Materials Analysis Division, Tokyo Institute of Technology) for the HRMS measurements. This division is independent of our laboratory to ensure fairness. Riki Iwai and Y. Shimomura thank JSPS Research Fellowships for Young Scientists.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules29215220/s1: Figures S1–S4: TGA curves; Figures S5–S10: DSC thermogram; Figures S11–S16: POM images; Figures S17–S34: 1D- or 2D-WAXD profiles; Figures S35 and S36: Optimized structure (DFT calculation); Figure S37: POM images of nematic phase in polyimide cell; Figure S38: Wavelength dependence of light transmittance; Figure S39: Absorption spectra; Figure S40: Fluorescence spectra; Figures S41–S78: NMR spectra; Figures S79–S84: FT-IR spectra; Figures S85–S88: HRMS spectra; Tables S1 and S2: Atomic coordination and absolute energy (DFT calculation); Table S3: Aggregation experiments.
Author Contributions
Conceptualization, G.-i.K. and R.I.; methodology, M.T.; Formal analysis, Y.S. (Yoshimichi Shimomura), Y.S. (Yuki Sawatari) and M.T.; investigation, Y.S. (Yuki Sawatari), Y.S. (Yoshimichi Shimomura) and T.T.; writing—original draft, G.-i.K., Y.S. (Yuki Sawatari) and T.T.; writing—review and editing, G.-i.K.; project administration, G.-i.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in this article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This project was supported in part by MEXT/JSPS KAKENHI grants 23H02036 (G.-i.K.), JST PRESTO (G.-i.K.), Toshiaki Ogasawara Memorial Foundation (G.-i.K.), Iketani Science and Technology Foundation, Murata Science and Education Foundation (G.-i.K.), and Science Tokyo SPRING Foundation (Y.S.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jackson W.J., Jr. Liquid crystal polymers. XI. Liquid crystal aromatic polyesters: Early history and future trends. Mol. Cryst. Liq. Cryst. 1989;169:23. doi: 10.1080/00268948908062732. [DOI] [Google Scholar]
- 2.White T., Broer D. Programmable and adaptive mechanics with liquid crystal polymer networks and elastomers. Nat. Mater. 2015;14:1087. doi: 10.1038/nmat4433. [DOI] [PubMed] [Google Scholar]
- 3.Imrie C.T., Henderson P.A. Liquid crystal dimers and higher oligomers: Between monomers and polymers. Chem. Soc. Rev. 2007;36:2096. doi: 10.1039/b714102e. [DOI] [PubMed] [Google Scholar]
- 4.Watanabe J., Hayashi M., Nakata Y., Niori T., Tokita M. Smectic liquid crystals in main-chain polymers. Prog. Polym. Sci. 1997;22:1053. doi: 10.1016/S0079-6700(97)00016-6. [DOI] [Google Scholar]
- 5.Watanabe Y., Kato R., Fukushima K., Kato T. Degradable and nanosegregated elastomers with multiblock sequences of biobased aromatic mesogens and biofunctional aliphatic oligocarbonates. Macromolecules. 2022;55:10285. doi: 10.1021/acs.macromol.2c01747. [DOI] [Google Scholar]
- 6.Watanabe J., Hayashi M. Thermotropic liquid crystals of polyesters having a mesogenic p,p′-bibenzoate unit. 1. Smectic A mesophase properties of polyesters composed of p,p′-bibenzoic acid and alkylene glycols. Macromolecules. 1988;21:278. doi: 10.1021/ma00179a059. [DOI] [Google Scholar]
- 7.Watanabe J., Hayashi M. Thermotropic liquid crystals of polyesters having a mesogenic p,p′-bibenzoate unit. 2. X-ray study on smectic mesophase structures of BB-5 and BB-6. Macromolecules. 1989;22:4083. doi: 10.1021/ma00200a046. [DOI] [Google Scholar]
- 8.Zhang Y., Wang X., Yang W., Yan H., Zhang X., Han D., He Y., Li C., Sun L. Programmable Complex Shape Changing of Polysiloxane Main-Chain Liquid Crystalline Elastomers. Molecules. 2023;28:4858. doi: 10.3390/molecules28124858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaya J., Ribierre J.-C., Correia G., Dappe Y.J., Mathevet F., Mager L., Heinrich B., Méry S. Control of the Organization of 4,4′-bis(carbazole)-1,1′-biphenyl (CBP) Molecular Materials through Siloxane Functionalization. Molecules. 2023;28:2038. doi: 10.3390/molecules28052038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J.G., Lee J.G., Wie J.J. Confinement-Induced Fabrication of Liquid Crystalline Polymeric Fibers. Molecules. 2022;27:5639. doi: 10.3390/molecules27175639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou Z., Li W., Qian J., Liu W., Wang Y., Zhang X., Guo Q., Yashchyshyn Y., Wang Q., Shi Y., et al. Flexible Liquid Crystal Polymer Technologies from Microwave to Terahertz Frequencies. Molecules. 2022;27:1336. doi: 10.3390/molecules27041336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie C., Yang S., He R., Liu J., Chen Y., Guo Y., Guo Z., Qiu T., Tuo X. Recent Advances in Self-Assembly and Application of Para-Aramids. Molecules. 2022;27:4413. doi: 10.3390/molecules27144413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimomura Y., Kawamura A., Tokita M., Watanabe J., Konishi G. Fluorinated Poly(pentylene 4,4′-bibenzoate)s with Low Isotropization Temperatures and Unique Phase Transition Behavior. Macromolecules. 2023;56:5152. doi: 10.1021/acs.macromol.3c00773. [DOI] [Google Scholar]
- 14.Kasai H., Hayakawa Y., Akiyama H., Ozaki M., Kato T., Kakimoto M. New Fabrication Approach to Develop a High Birefringence Photo-Crosslinked Film Based on a Sulfur-Containing Liquid Crystalline Molecule with Large Temperature Dependence of Birefringence. Mol. Cryst. Liq. Cryst. 2018;671:28–35. doi: 10.1080/15421406.2018.1467619. [DOI] [Google Scholar]
- 15.McArdle C.B. Side Chain Liquid Crystal Polymers. Springer; Dordrecht, The Netherlands: Loctite Ltd.; Dublin, Ireland: 1989. [Google Scholar]
- 16.Ikeda T., Tsutsumi O. Optical Switching and Image Storage by Means of Azobenzene Liquid-Crystal Films. Science. 1995;268:1873. doi: 10.1126/science.268.5219.1873. [DOI] [PubMed] [Google Scholar]
- 17.Hisano K., Aizawa M., Ishizu M., Kurata Y., Nakano W., Akamatsu N., Christopher J., Shishido A. Scanning wave photopolymerization enables dye-free alignment patterning of liquid crystals. Sci. Adv. 2017;3:e1701610. doi: 10.1126/sciadv.1701610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi K., Taguchi D., Kajitani T., Fukushima T., Kubo S., Shishido A. Synthesis and Characterization of Side-Chain Liquid-Crystalline Block Copolymers Containing Cyano-Terminated Phenyl Benzoate Moieties. Molecules. 2023;28:7849. doi: 10.3390/molecules28237849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shigeyama T., Matsumoto K., Hisano K., Tsutsumi O. Tunable Reflection through Size Polydispersity of Chiral-Nematic Liquid Crystal Polymer Particles. Molecules. 2023;28:7779. doi: 10.3390/molecules28237779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arakawa Y., Nakajima S., Kang S., Konishi G., Watanabe J. Synthesis and evaluation of high-birefringence polymethacrylate having a diphenyl-diacetylene LC moiety in the side chain. J. Mater. Chem. 2012;22:14346. doi: 10.1039/c2jm32489j. [DOI] [Google Scholar]
- 21.Kang S., Nakajima S., Arakawa Y., Tokita M., Watanabe J., Konishi G. Highly birefringent side-chain LC polymethacrylate with a dinaphthyl-acetylene mesogenic unit. Polym. Chem. 2014;5:2253. doi: 10.1039/C3PY01528A. [DOI] [Google Scholar]
- 22.Ma X., Li Q., Song C., Zhang H., Zhi Y., He X. The effect of different photoluminescent side-chain length on the phase behaviour of chiral liquid crystal polymer. Liq. Cryst. 2022;49:1498–1510. doi: 10.1080/02678292.2022.2044527. [DOI] [Google Scholar]
- 23.Castro L.D.C., Lub J., Oliveira O.N., Jr., Schenning A.P.H.J. Mechanochromic Displays Based on Photoswitchable Cholesteric Liquid Crystal Elastomers. Angew. Chem. Int. Ed. 2024:e202413559. doi: 10.1002/anie.202413559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiong Y., He P. A review on electrocatalysis for alkaline oxygen evolution reaction (OER) by Fe-based catalysts. J. Mater. Sci. 2023;58:2041. doi: 10.1007/s10853-023-08176-1. [DOI] [Google Scholar]
- 25.Arakawa Y., Kuwahara H., Sakajiri K., Kang S., Tokita M., Konishi G. Highly birefringent polymer films from the photo-crosslinking polymerisation of bistolane-based methacrylate monomers. Liq. Cryst. 2015;42:1419. doi: 10.1080/02678292.2015.1053542. [DOI] [Google Scholar]
- 26.O’Neill M., Kelly S.M. Ordered Materials for Organic Electronics and Photonics. Adv. Mater. 2010;23:566. doi: 10.1002/adma.201002884. [DOI] [PubMed] [Google Scholar]
- 27.Swager T.M. Molecular Shape and Polar Order in Columnar Liquid Crystals. Acc. Chem. Res. 2022;55:3010. doi: 10.1021/acs.accounts.2c00452. [DOI] [PubMed] [Google Scholar]
- 28.Hoeben F.J.M., Jonkheijm P., Meijer E.W., Schenning A.P.H.J. About Supramolecular Assemblies of π-Conjugated Systems. Chem. Rev. 2005;105:1491. doi: 10.1021/cr030070z. [DOI] [PubMed] [Google Scholar]
- 29.Sakurai T., Kato K., Shimizu M. Side-Chain Labeling Strategy for Forming Self-Sorted Columnar Liquid Crystals from Binary Discotic Systems. Crystals. 2023;13:1473. doi: 10.3390/cryst13101473. [DOI] [Google Scholar]
- 30.Demus D., Goodby J., Gray G.W., Spiess H.W., Vill V. Physical Properties of Liquid Crystals. Wiley-VCH; Weinheim, Germany: New York, NY, USA: Chichester, UK: Brisbane, Australia: Singapore: Toronto, ON, Canada: 1999. [Google Scholar]
- 31.Heilmeier G.H., Zanoni L.A., Barton L.A. Guest-Host Interactions in Nematic Liquid Crystals. A New Electro-Optic Effect. Appl. Phys. Lett. 1968;13:91. doi: 10.1063/1.1652529. [DOI] [Google Scholar]
- 32.Heilmeier G.H. Liquid crystal displays: An experiment in interdisciplinary research that worked. IEEE Trans. Electron. Devices. 1976;23:780. doi: 10.1109/T-ED.1976.18482. [DOI] [Google Scholar]
- 33.O’Neill M., Kelly S.M. Liquid crystals for charge transport, luminescence, and photonics. Adv. Mater. 2003;15:1135. doi: 10.1002/adma.200300009. [DOI] [Google Scholar]
- 34.Giménez R., Piñol M., Serrano J.L. Luminescent liquid crystals derived from 9,10-bis(phenylethynyl)anthracene. Chem. Mater. 2004;16:1377. doi: 10.1021/cm030582u. [DOI] [Google Scholar]
- 35.Fleischmann E.K., Zentel R. Liquid-crystalline ordering as a concept in materials science: From semiconductors to stimuli-responsive devices. Angew. Chem. Int. Ed. 2013;52:8810. doi: 10.1002/anie.201300371. [DOI] [PubMed] [Google Scholar]
- 36.Iino H., Usui T., Hanna J. Liquid crystals for organic thin-film transistors. Nat. Commun. 2015;6:6828. doi: 10.1038/ncomms7828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padalkar V.S., Tsutsui Y., Sakurai T., Sakamaki D., Tohnai N., Kato K., Takata M., Akutagawa T., Sakai K., Seki S. Optical and structural properties of ESIPT inspired HBT–fluorene molecular aggregates and liquid crystals. J. Phys. Chem. B. 2017;121:10407. doi: 10.1021/acs.jpcb.7b08073. [DOI] [PubMed] [Google Scholar]
- 38.Zhang W., Sakurai T., Aotani M., Watanabe G., Yoshida H., Padalkar V.S., Tsutsui Y., Sakamaki D., Ozaki M., Seki S. Highly fluorescent liquid crystals from excited-state intramolecular proton transfer molecules. Adv. Opt. Mater. 2019;7:1801349. doi: 10.1002/adom.201801349. [DOI] [PubMed] [Google Scholar]
- 39.Arakawa Y., Sasaki S., Igawa K., Tokita M., Konishi G., Tsuji H. Birefringence and photoluminescence properties of diphenylacetylene-based liquid crystal dimers. New J. Chem. 2020;44:17531. doi: 10.1039/D0NJ04426A. [DOI] [Google Scholar]
- 40.Voskuhl J., Giese M. Mesogens with aggregation-induced emission properties: Materials with a bright future. Aggregate. 2022;3:e124. doi: 10.1002/agt2.124. [DOI] [Google Scholar]
- 41.Yamada S., Yoshida K., Uto E., Yoshida K., Sakurai T., Konno T. Development of photoluminescent liquid-crystalline dimers bearing two fluorinated tolane-based luminous mesogens. J. Mol. Liq. 2023;363:119884. doi: 10.1016/j.molliq.2022.119884. [DOI] [Google Scholar]
- 42.Yamada S., Konno T. Development of donor-π-acceptor-type fluorinated tolanes as compact condensed phase luminophores and applications in photoluminescent liquid-crystalline molecules. Chem. Rec. 2023;23:e202300094. doi: 10.1002/tcr.202300094. [DOI] [PubMed] [Google Scholar]
- 43.Wu X., Niu X., Zhu S., Tian M., Liu W. A novel multi-stimuli responsive fluorescence liquid crystal material with aggregationinduced emission effect. Liq. Cryst. 2023;50:1035. doi: 10.1080/02678292.2023.2200263. [DOI] [Google Scholar]
- 44.Sawatari Y., Shimomura Y., Takeuchi M., Iwai R., Tanaka T., Tsurumaki E., Tokita M., Watanabe J., Konishi G. Supramolecular liquid crystals from the dimer of L-shaped molecules with tertiary amide end groups. Aggregate. 2024;5:e507. doi: 10.1002/agt2.507. [DOI] [Google Scholar]
- 45.Iida Y., Shimomura Y., Tokita M., Konishi G. Push-pull biphenyl and tolane derivatives as novel luminescent liquid crystals: Synthesis and properties. Liq. Cryst. Early View. 2024:1–14. doi: 10.1080/02678292.2024.2381547. [DOI] [Google Scholar]
- 46.Pathak S.K., Pradhan B., Gupta M., Pal S.K., Sudhakar A.A. Liquid-Crystalline Star-Shaped Supergelator Exhibiting Aggregation-Induced Blue Light Emission. Langmuir. 2016;32:9301. doi: 10.1021/acs.langmuir.6b02509. [DOI] [PubMed] [Google Scholar]
- 47.Demus D. One Century Liquid Crystal Chemistry: From Vorländer’s Rods to Disks, Stars and Dendrites. Mol. Cryst. Liq. Cryst. 2006;364:25. doi: 10.1080/10587250108024978. [DOI] [Google Scholar]
- 48.Mei J., Leung N.L., Kwok R.T., Lam J.W., Tang B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015;115:11718. doi: 10.1021/acs.chemrev.5b00263. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki S., Suzuki S., Sameera W.M.C., Igawa K., Morokuma K., Konishi G. Highly Twisted N,N-Dialkylamines as a Design Strategy to Tune Simple Aromatic Hydrocarbons as Steric Environment-Sensitive Fluorophores. J. Am. Chem. Soc. 2016;138:8194. doi: 10.1021/jacs.6b03749. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki S., Sasaki S., Sairi A.S., Iwai R., Tang B.Z., Konishi G.I. Principles of Aggregation-Induced Emission: Design of Deactivation Pathways for Advanced AIEgens and Applications. Angew. Chem. Int. Ed. 2020;59:9856. doi: 10.1002/anie.202000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen Z., Pan Y., Yan D., Wang D., Tang B.Z. AIEgen-Based Nanomaterials for Bacterial Imaging and Antimicrobial Applications: Recent Advances and Perspectives. Molecules. 2023;28:2863. doi: 10.3390/molecules28062863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo W., Tan Y., Gui Y., Yan D., Wang D., Tang B.Z. Near-Infrared-Emissive AIE Bioconjugates: Recent Advances and Perspectives. Molecules. 2022;27:3914. doi: 10.3390/molecules27123914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhou L., Zheng L.L., Gao M.Y., Xu C.J., Ge Y.F., Bai T.X., Wen J., Cheng Y.H., Zhu M.F. Confinement fluorescence effect of an aggregation-induced emission luminogen in crystalline polymer. Aggregate. 2023;4:e338. doi: 10.1002/agt2.338. [DOI] [Google Scholar]
- 54.Kumari B., Dahiwadkar R., Kanvah S. White light emission from AIE-active luminescent organic materials. Aggregate. 2022;3:e191. doi: 10.1002/agt2.191. [DOI] [Google Scholar]
- 55.Iwai R., Suzuki S., Sasaki S., Sairi A.S., Igawa K., Suenobu T., Morokuma K., Konishi G. Bridged Stilbenes: AIEgens Designed via a Simple Strategy to Control the Non-radiative Decay Pathway. Angew. Chem. Int. Ed. 2020;59:10566. doi: 10.1002/anie.202000943. [DOI] [PubMed] [Google Scholar]
- 56.Shimomura Y., Konishi G. Flexible Alkylene Bridges as a Tool to Engineer Crystal Distyrylbenzene Structures Enabling Highly Fluorescent Monomeric Emission. Chem. Eur. J. 2022;28:e202201884. doi: 10.1002/chem.202201884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shimomura Y., Konishi G. Push-Pull Bridged Distyrylbenzene with Highly Bright Solid-State Red-Orange Aggregation-Induced Emission. Chem. Eur. J. 2023;29:e20231191. doi: 10.1002/chem.202301191. [DOI] [PubMed] [Google Scholar]
- 58.Iwai R., Yoshida H., Arakawa Y., Sasaki S., Iida Y., Igawa K., Sakurai T., Suzuki S., Tokita M., Watanabe J., et al. Near-room-temperature π-conjugated nematic liquid crystals in molecules with a flexible seven-membered ring structure. Aggregate. 2024;5:e660. doi: 10.1002/agt2.660. [DOI] [Google Scholar]
- 59.Miyaura N., Suzuki A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995;95:245. doi: 10.1021/cr00039a007. [DOI] [Google Scholar]
- 60.Sen A., Yamada Y.M. Latest Developments on Palladium- and Nickel-Catalyzed Cross-Couplings Using Aryl Chlorides: Suzuki–Miyaura and Buchwald–Hartwig Reactions. Synthesis. 2024;56 doi: 10.1055/a-2344-5677. [DOI] [Google Scholar]
- 61.Arakawa Y., Sasaki Y., Haraguchi N., Itsuno S., Tsuji H. Synthesis, phase transitions and birefringence of novel liquid crystalline 1,4-phenylene bis(4-alkylthio benzoates) and insights into the cybotactic nematic behaviour. Liq. Cryst. 2017;45:821. doi: 10.1080/02678292.2017.1385103. [DOI] [Google Scholar]
- 62.Shanker G., Prehm M., Nagaraj M., Vij J.K., Tschierske C. Development of polar order in a bent-core liquid crystal with a new sequence of two orthogonal smectic and an adjacent nematic phase. J. Mater. Chem. 2011;21:18711. doi: 10.1039/c1jm14653j. [DOI] [Google Scholar]
- 63.Mohamed A., Silvio P., Christoph K., Christoph K., Alexey E., Carsten T. Cluster phases of 4-cyanoresorcinol derived hockey-stick liquid crystals. J. Mater. Chem. C. 2017;5:8454. doi: 10.1039/C7TC01816A. [DOI] [Google Scholar]
- 64.Yuan Y., Li J., He L., Liu Y., Zhang H. Preparation and properties of side chain liquid crystalline polymers with aggregation-induced emission enhancement characteristics. J. Mater. Chem. C. 2018;6:7119. doi: 10.1039/C8TC01641K. [DOI] [Google Scholar]
- 65.Ni B., Liao J., Chen S., Zhang H. Influence of alkoxy tail length on the phase behaviors of side-chain liquid crystalline polymers without the spacer. RSC Adv. 2015;5:9035. doi: 10.1039/C4RA15361H. [DOI] [Google Scholar]
- 66.Arakawa Y., Nakajima S., Ishige R., Uchimura M., Kang S., Konishi G., Watanabe J. Synthesis of diphenyl-diacetylene-based nematic liquid crystals and their high birefringence properties. J. Mater. Chem. 2012;22:8394. doi: 10.1039/c2jm16002a. [DOI] [Google Scholar]
- 67.Kang S., Nakajima S., Arakawa Y., Konishi G., Watanabe J. Large extraordinary refractive index in highly birefringent nematic liquid crystals of dinaphthyldiacetylene-based materials. J. Mater. Chem. C. 2013;1:4222. doi: 10.1039/c3tc30640b. [DOI] [Google Scholar]
- 68.Arakawa Y., Kang S., Nakajima S., Sakajiri K., Cho Y., Kawauchi S., Watanabe J., Konishi G. Diphenyltriacetylenes: Novel nematic liquid crystal materials and analysis of their nematic phase-transition and birefringence behaviours. J. Mater. Chem. C. 2013;1:8094. doi: 10.1039/c3tc31658k. [DOI] [Google Scholar]
- 69.Arakawa Y., Kang S., Tsuji H., Watanabe J., Konishi G. The design of liquid crystalline bistolane-based materials with extremely high birefringence. RSC Adv. 2016;6:92845. doi: 10.1039/C6RA14093A. [DOI] [Google Scholar]
- 70.Arakawa Y., Kang S., Tsuji H., Watanabe J., Konishi G. Development of novel bistolane-based liquid crystalline molecules with an alkylsulfanyl group for highly birefringent materials. RSC Adv. 2016;6:16568. doi: 10.1039/C5RA25122B. [DOI] [Google Scholar]
- 71.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Petersson G.A., Nakatsuji H., et al. Gaussian 16, Revision C.01. Gaussian, Inc.; Wallingford, CT, USA: 2016. [Google Scholar]
- 72.Kim J., Li Y., Miskiewicz M.N., Oh C., Kudenov M.W., Escuti M.J. Fabrication of ideal geometric-phase holograms with arbitrary wavefronts. Optica. 2015;2:958. doi: 10.1364/OPTICA.2.000958. [DOI] [Google Scholar]
- 73.Pi D., Liu J., Wang Y. Review of computer-generated hologram algorithms for color dynamic holographic three-dimensional display. Light Sci. Appl. 2022;11:231. doi: 10.1038/s41377-022-00916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shishido A. Rewritable holograms based on azobenzene-containing liquid-crystalline polymers. Polym. J. 2010;42:525. doi: 10.1038/pj.2010.45. [DOI] [Google Scholar]
- 75.Kobashi J., Yoshida H., Ozaki M. Polychromatic Optical Vortex Generation from Patterned Cholesteric Liquid Crystals. Phys. Rev. Lett. 2016;116:253903. doi: 10.1103/PhysRevLett.116.253903. [DOI] [PubMed] [Google Scholar]
- 76.Morita F., Kishida Y., Sato Y., Sugiyama H., Abekura M., Nogami J., Toriumi N., Nagashima Y., Kinoshita T., Fukuhara G., et al. Design and enantioselective synthesis of 3D π-extended carbohelicenes for circularly polarized luminescence. Nat. Synth. 2024;3:774. doi: 10.1038/s44160-024-00527-3. [DOI] [Google Scholar]
- 77.Hsieh Y.-Y., Shyue J.-J., Chao Y.-C., Wong K.-T., Bassani D.M. Chiral Binaphthalene Building Blocks for Self-Assembled Nanoscale CPL Emitters. Molecules. 2023;28:3382. doi: 10.3390/molecules28083382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Furoida A., Daitani M., Hisano K., Tsutsumi O. Aggregation-Enhanced Room-Temperature Phosphorescence from Au(I) Complexes Bearing Mesogenic Biphenylethynyl Ligands. Molecules. 2021;26:7255. doi: 10.3390/molecules26237255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kato K., Iwano R., Tokuda S., Yasuzawa K., Gon M., Ohtani S., Furukawa S., Tanaka K., Ogoshi T. Circularly polarized luminescence from a common alkoxy pillar [5]arene and its co-aggregates with π-conjugated rods. Aggregate. 2024;5:e482. doi: 10.1002/agt2.482. [DOI] [Google Scholar]
- 80.Gong Z.L., Li Z.Q., Zhong Y.W. Circularly polarized luminescence of coordination aggregates. Aggregate. 2022;3:e177. doi: 10.1002/agt2.177. [DOI] [Google Scholar]
- 81.Yan H., He Y., Wang D., Han T., Tang B.Z. Aggregation-induced emission polymer systems with circularly polarized luminescence. Aggregate. 2023;4:e331. doi: 10.1002/agt2.331. [DOI] [Google Scholar]
- 82.Uchimura M., Watanabe Y., Araoka F., Watanabe J., Takezoe H., Konishi G. Development of Laser Dyes to Realize Low Threshold in Dye-Doped Cholesteric Liquid Crystal Lasers. Adv. Mater. 2010;22:4473. doi: 10.1002/adma.201001046. [DOI] [PubMed] [Google Scholar]
- 83.Wan S.-P., Lu H.-Y., Li M., Chen C.-F. Advances in circularly polarized luminescent materials based on axially chiral compounds. J. Photochem. Photobiol. C. 2022;50:100500. doi: 10.1016/j.jphotochemrev.2022.100500. [DOI] [Google Scholar]
- 84.Hasegawa Y., Kitagawa Y. Luminescent lanthanide coordination polymers with transformative energy transfer processes for physical and chemical sensing applications. J. Photochem. Photobiol. C. 2022;51:100485. doi: 10.1016/j.jphotochemrev.2022.100485. [DOI] [Google Scholar]
- 85.Cho S., Takahashi M., Fukuda J., Yoshida H., Ozaki M. Directed self-assembly of soft 3D photonic crystals for holograms with omnidirectional circular-polarization selectivity. Commun. Mater. 2021;2:39. doi: 10.1038/s43246-021-00146-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in this study are included in this article/Supplementary Materials, further inquiries can be directed to the corresponding author.