Abstract
Background/Objectives: Mesothelioma is an aggressive cancer with limited treatment options. Mesothelioma therapy often involves a multimodal approach including surgery, radiotherapy and chemotherapy. However, the prognosis for patients remains poor. Difficult diagnosis, late symptoms when the tumor is in an advanced stage and the onset of chemotherapy resistance make mesothelioma difficult to treat. For this reason, it is essential to discover new pharmacological approaches. Capsaicin (CAPS) is the active compound of chili peppers. Based on CAPS’s anticancer properties on various tumor lines and its chemo-sensitizing action on resistant cells, in this study, we evaluated the effects of CAPS on mesothelioma cells to assess its potential use in mesothelioma therapy. Methods: To evaluate antiproliferative effects of CAPS, we performed MTS assays on various mesothelioma cells, representative of all major mesothelioma subtypes. Transwell migration and wound-healing assays were used to examine the effect of CAPS on mesothelioma cell migration. We also determined the effects of CAPS on oncogenic signaling pathways by assessing the levels of AKT and MAPK activation. Results: In this study, we show that CAPS significantly reduces proliferation of both parental and cisplatin-resistant mesothelioma cells. CAPS promotes S-phase cell cycle arrest and inhibits lateral motility and migration of mesothelioma cells. Accordingly, CAPS suppresses AKT and ERK1/2 activation in MSTO-211H and NCI-H2052 cells. Our results support an antitumor effect of CAPS on cisplatin-resistant mesothelioma cells, suggesting that it may reduce resistance to cisplatin. Conclusions: Our results could pave the way for further studies to evaluate the use of CAPS for mesothelioma treatment.
Keywords: mesothelioma, capsaicin, migration, proliferation, cell cycle, cisplatin resistance, natural drugs, cancer, AKT, ERK1/2
1. Introduction
Malignant mesothelioma (MM) is a rare and very aggressive tumor arising from the mesothelium, the thin membrane that lines several body cavities, such as pleura, peritoneum, pericardium, and tunica vaginalis testis [1]. Malignant pleural mesothelioma (MPM) is the most common in patients (73–85% of mesothelioma cases) [2] and approximately 3500 people are diagnosed with mesothelioma each year in the USA [3]. Mesothelioma predominantly affects men and women in a ratio of 5:1 [2]. Globally, approximately 29,000 mesothelioma deaths occurred in 2019 [4]. MM mostly occurs in patients older than 65 years, and its onset is in general associated with asbestos exposure, after a latency period of 20–50 years [2,5,6,7]. The International Agency for Research on Cancer (IARC) classified asbestos as a carcinogenic substance for humans (Group I) [8]. Several countries have banned the use of asbestos, but, unfortunately, it still represents a global health concern [9].
However, asbestos-unrelated mesothelioma has been diagnosed in young patients carrying germline mutations in BRCA1-associated protein 1 (BAP1) or other tumor suppressor genes [10,11].
MPM is associated with an estimated overall survival of 38% at 1 year and only 7% at 3 years from diagnosis [12]. According to histological features, MM is classified into three main subtypes: epithelioid, sarcomatoid and biphasic, which are associated with a different prognosis [13,14]. In fact, patients diagnosed with sarcomatoid MPM present a lower median survival (4 months) than those affected by epithelioid (19 months) or biphasic (12 months) subtypes [12]. The unfavorable prognosis of MPM patients is related to the late onset of symptoms compared to exposure to asbestos as well as a difficult diagnosis, often carried out when the tumor is in advanced stage [1,11,15,16,17].
The current options for MM therapy are limited. For pleural mesothelioma patients, the standard of care is cisplatin plus pemetrexed, and the combination of two immuno-checkpoint inhibitors, ipilimumab plus nivolumab [18,19]. Although these drug treatments improve patient survival, the prognosis of patients with MM remains poor [20,21]. MPM patients not candidates for surgery exhibited a 41.3% response rate to cisplatin plus pemetrexed in a phase III study [22]. However, the response rate to this combination treatment was significantly lower (26.3%) in other studies [23]. In addition, the development of resistance to chemotherapeutic drugs and the presence of mutations in BAP1 gene result in a reduced response to cisplatin in MM patients [24]. Although the use of ipilimumab plus nivolumab has increased the overall survival of MPM patients compared to chemotherapy [25], the cost–benefit ratio makes ipilimumab plus nivolumab treatment a very limited approach [26,27,28]. Thus, there is the urgent need to develop new therapeutic approaches for treatment and interest in evaluating the beneficial properties of natural compounds in cancer [29,30,31,32].
Capsaicin (trans-8-methyl-N-vanillyl-6-nonenamide) (CAPS) is the main phytochemical compound responsible for the spicy flavor of red chili peppers. CAPS exhibits several beneficial properties on human health, including antibacterial, anti-inflammatory, analgesic, and anticancer activities [33]. In particular, CAPS exerts antiproliferative and antimigratory effects against various cancer cell lines [34,35,36,37,38]. It inhibits the activation of AKT and ERK1/2, serine-threonine kinases which are commonly involved in several cellular processes, including proliferation, cell growth, and migration [39,40,41,42,43]. In addition, CAPS promotes cancer cell apoptosis, and cell cycle arrest at different phases depending on cell lines and tumor models [39,44,45,46,47]. Interestingly, oral CAPS administration in xenograft mouse models of prostate cancer sensitized tumor cells to radiotherapy, resulting in a more marked reduction in tumor mass than radiotherapy alone [48,49]. Moreover, several studies have demonstrated a strong antitumor action of CAPS on cancer cells resistant to conventional chemotherapy. CAPS impairs cell cycle in various cisplatin-resistant cancer cell lines and, when combined with cisplatin, induces apoptosis in cisplatin-resistant gastric cancer cells [50,51]. However, the effects of CAPS on various MM cells have not been investigated. Given the properties of CAPS on other cancers, in this study, we evaluated the antitumor properties of CAPS in different MM lines, representative of major MM subtypes. Specifically, we show that CAPS exhibits strong antiproliferative action on both parental and cisplatin-resistant MM cells. Moreover, a further novel element of our study was to evaluate the effect of CAPS on migration and invasion, which are among the main hallmarks of tumor aggressiveness, in various mesothelioma lines. We provide the first evidence of the anti-migratory and anti-invasive action of CAPS and its inhibitory effect on AKT and ERK1/2 activation in MM cells. Our in vitro results are very encouraging and could pave the way for future clinical research on the use of CAPS as a possible therapeutic approach for patients with malignant pleural mesothelioma, even with the occurrence of cisplatin resistance.
2. Materials and Methods
2.1. Cell Lines and Reagents
MSTO-211H, NCI-H2052, NCI-H2452 and NCI-H28 human MM cell lines were purchased from ATCC (Manassas, VA, San Diego, CA, USA). These MM lines are representative of all MM subtypes: sarcomatoid (NCI-H2052), epithelioid (NCI-H2452, NCI-H28) and biphasic (MSTO-211H). All cell lines were cultured in RPMI-1640 medium containing GlutaMAX (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 1% penicillin–streptomycin (Pen/Strep) and 10% Fetal Bovine Serum (FBS) (R&D Systems, Minneapolis, MN, USA). The cells were kept at 37 °C in a humidified atmosphere containing 5% CO2.
2.2. Generation of Cisplatin-Resistant MM Cell Lines
Cisplatin-resistant MSTO-211H and NCI-H2052 cells were generated in our laboratory by long-term exposure to increasing concentrations of cisplatin (Calbiochem, San Diego, CA, USA) (5 µM, 10 µM, 15 µM, 20 µM, 25 µM), as described previously [52]. Following 72 h treatment with each concentration, the cells were grown in cisplatin-free medium for 10 days and then treated with the next higher concentration of cisplatin up to 25 µM. The cells were treated with cisplatin during their exponential growth phase. Selected resistant cells were maintained in RPMI-1640 medium containing 20 µM cisplatin (IC50) [53], 10% FBS and 1% Pen/Strep.
2.3. Capsaicin and Treatment Medium Preparation
Capsaicin (≥95%, from Capsicum sp.) (M2028) was purchased from Sigma Aldrich (St. Louis, MO, USA) and dissolved in Dimethyl sulfoxide (DMSO) to prepare a 200 mM stock solution. The stock solution was diluted in media to obtain the desired CAPS concentration used in the experiments.
2.4. MTS Assay
MSTO-211H, NCI-H2052, NCI-H2452 and NCI-H28 cells were seeded onto 96-well plates at a density of 1000 cells/well (MSTO-211H) or 2000 cells/well (NCI-H2052, NCI-H2452 and NCI-H28). The cells were treated for 24, 48 and 72 h with 5% FBS-RPMI 1640 medium containing CAPS at concentrations ranging from 50 µM to 200 µM for all MM cell lines, except for NCI-H28 cells, for which we used up to 300 µM.
Cell viability was assessed by the MTS assay (cat. no. G3582; CellTiter 96®AQueous One Solution Cell Proliferation Assay, Promega, Madison, WI, USA), following the manufacturer’s instructions. The absorbance at 490 nm was measured with a plate reader (Victor XS, Perkin Elmer, Waltham, MA, USA). The percentage of increase in cell growth from time zero (% increase over time 0) was calculated as follows:
| % increase over time 0 = ((ABS Time T − ABS Time 0)/ABS Time 0) × 100, |
where ABS Time T represented absorbance measured after 24, 48, or 72 h of treatment, while ABS Time 0 was absorbance at time zero.
2.5. Half-Maximal Inhibitory Concentration (IC50) Evaluation
Following 24, 48 and 72 h of CAPS treatment (50, 100, 200, 250 and 300 µM), IC50 values were calculated for MSTO-211H, NCI-H2052, NCI-H2452 and NCI-H28 cells by GraphPad Prism 8 software through a dose–response curve, selecting log(inhibitor) vs. normalized response-variable slope.
2.6. Cytofluorimetric Analysis of MM Cell Cycle
MSTO-211H (2.5 × 105 cells), NCI-H2052 (3 × 105 cells), NCI-H2452 (3 × 105 cells) and NCI-H28 (3 × 105 cells) were seeded onto 100 mm plates the day before treatment was started. After treatment for 48 or 72 h with 5% serum-RPMI 1640 medium containing DMSO or CAPS, the cells were fixed in cold 70% ethanol and stained with propidium iodide following instructions of the propidium iodide flow cytometry kit (Abcam, Cambridge, UK), as described previously [54]. Cell cycle analyses were performed by FACS (BD LSR II 14-Color Flow Cytometer, Biosciences) at the Wistar Institute Cytofluorimetry Core Facility (Philadelphia, PA, USA).
2.7. Wound-Healing Assay
A confluent monolayer of NCI-H2052, NCI-H2452 and NCI-H28 cells, seeded onto 6-well plates, was wounded using a 200 μL micropipette tip. After washing with Dulbecco’s phosphate-buffered saline (DPBS) (Gibco, Thermo Fisher Scientific), the cells were treated with medium containing DMSO or CAPS at concentrations that did not affect cell proliferation. Images of the wounded area were acquired by a DMi1 (Leica, Wetzlar, Germany) microscope (4× objective) both at time 0 (immediately after scratching) and at 24, 48 and 72 h of treatment until wound closure. The images were examined by using Wound_healing_size_tool update, an ImageJ/Fiji® plugin [55,56]. The wound closure rate was measured as follows:
| % wound closure = ((Wound area time 0 − Wound area time T)/Wound area time 0) × 100), |
where wound area time 0 was the wound area after scratching, and wound area time T represented the wound area at the end of each treatment time.
2.8. Transwell Migration Assay
After starvation in serum-free medium (SFM) for 24 h, MSTO-211H, NCI-H2052, and NCI-H28 cells were seeded at a density of 2 × 104 cells in SFM in the upper chamber of a 6.5 mm Transwell with an 8.0µm pore size polyester membrane insert (Corning REF 3464, Corning, NY, USA). SFM (for NCI-H2052) or 5% serum-RPMI 1640 medium (for MSTO-211H and NCI-H28) was introduced in the lower chamber. DMSO or CAPS were added to both chambers of the Transwell. Migration was measured at 16 h for NCI-H2052, 24 h for NCI-H28 and 27.5 h for MSTO-211H. After removing cells on the upper surface of the membrane using a cotton swab, the cells on the lower surface of the filter were fixed in ice-cold methanol and stained with Coomassie Brilliant Blue, as described previously [57]. Images were acquired by an inverted microscope (DMi1, Leica, Wetzlar, Germany).
2.9. Transwell Invasion Assay
The invasive capacity of MM cells was assessed using Transwell inserts with an 8 µm pore size membrane coated with homogeneous layer of Matrigel (Corning, NY, USA), as previously reported [57]. Briefly, 2 × 104 NCI-H2052 cells were seeded, and invasion was stopped after 24 h of treatment in SFM containing DMSO alone or CAPS at a concentration of 100 µM. Cells on the upper surface of the membrane were removed, while cells on the lower surface of the membrane were stained with Coomassie Brilliant Blue after fixation in ice-cold methanol. Then, images were acquired by an inverted microscope (DMi1, Leica, Wetzlar, Germany).
2.10. Western Blotting
Cells were lysed using RIPA buffer (Thermo Fisher Scientific) supplemented with halt protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Protein concentration was measured by the BCA assay (Thermo Fisher Scientific). The following primary antibodies pAKT S473 (#4060), pan-AKT (#4691), pERK1/2 (#4370), ERK1/2 (#9102) from Cell Signaling Technology (Danvers, MA, USA) and GAPDH (sc-365062) (Santa Cruz Biotechnology, Dallas, TX, USA) were used at a 1:1000 dilution, while the secondary antibodies anti-rabbit IgG-HRP-linked (sc-2357) and m-IgGk BP-HRP antibody (sc-516102) from Santa Cruz Biotechnology were used at a dilution of 1:5000. Chemiluminescence images of immunoblots were obtained using Odyssey Fc (LI-COR, Lincoln, NE, USA).
3. Results
3.1. Capsaicin (CAPS) Reduces Cell Proliferation Both in Parental and Cisplatin-Resistant Mesothelioma Cells
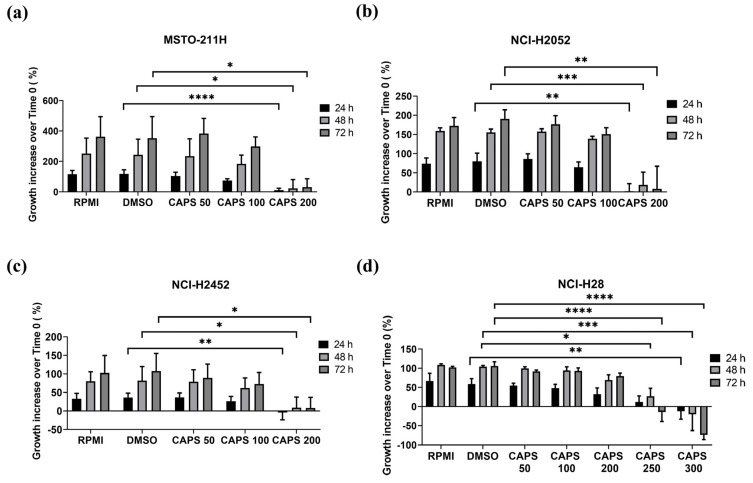
To evaluate the anti-proliferative effect of CAPS on MM cells, we performed an MTS assay on several mesothelioma cell lines, representative of all MM subtypes, such as sarcomatoid (NCI-H2052), epithelioid (NCI-H2452, NCI-H28) and biphasic (MSTO-211H) (Figure 1). We assessed cell growth at 24, 48 and 72 h of treatment with various CAPS concentrations, and the results are expressed as % cell growth increase compared to time zero. To confirm that the effect of CAPS on cell proliferation was not related to cytotoxicity of DMSO used to dissolve CAPS, we also treated cells for 24, 48 and 72 h with 5% serum-RPMI 1640 medium containing DMSO at the same %v/v of cells treated with the highest concentration of CAPS. Cells treated in this way constituted the vehicle control (DMSO). As shown in Figure 1, no difference in cell growth was observed in vehicle control cells (DMSO) compared to cells exposed to RPMI 1640 medium (RPMI). Thus, no vehicle toxicity was detected since DMSO treatment did not affect cell growth of all MM lines tested.
Figure 1.
Capsaicin (CAPS) reduces proliferation of malignant mesothelioma (MM) cells. MSTO-211H (a), NCI-H2052 (b), NCI-H2452 (c), NCI-H28 (d) cells were treated with RPMI 1640 medium alone (RPMI); medium containing DMSO (DMSO) at the same %v/v of CAPS maximum concentration; and various CAPS concentrations (in µM) for 24, 48 and 72 h. Data are expressed as percentage of growth increase over time 0 and results are shown as mean ± standard deviation of three independent experiments. Statistical differences were evaluated by one-way analysis of variance (ANOVA) (* p-value < 0.05, ** p-value < 0.01; *** p-value < 0.001; **** p-value < 0.0001 versus DMSO).
Regarding CAPS treatments, concentrations of 50 µM and 100 µM did not impair growth of all MM cell lines when compared to DMSO alone. However, treatment with CAPS at 200 µM significantly reduced growth of MSTO-211H, NCI-H2052 and NCI-H2452 cells (Figure 1a, Figure 1b, Figure 1c, respectively), while it did not affect proliferation of NCI-H28 cells, which were resistant to these CAPS concentrations. Notably, CAPS concentrations at 250 µM and 300 µM significantly reduced growth of NCI-H28 cells (Figure 1d). Thus, CAPS shows strong anti-proliferative effect on MM cells when compared to DMSO-control-treated cells.
Then, we evaluated the IC50 following CAPS treatment for 24, 48 and 72 h for each MM line. The IC50 values are reported in Table 1.
Table 1.
Capsaicin (CAPS) half-maximal inhibitory concentration (IC50) values on MM cells. IC50 values refer to CAPS concentrations in µM. IC50 was evaluated following 24, 48 and 72 h of CAPS treatment.
| MM Cell Line | IC50 (24 h Treatment) | IC50 (48 h Treatment) | IC50 (72 h Treatment) |
|---|---|---|---|
| MSTO-211H | 193.6 | 192.8 | 189.5 |
| NCI-H2052 | 216.1 | 198.1 | 173.0 |
| NCI-H2452 | 231.4 | 215.0 | 191.6 |
| NCI-H28 | 325.2 | 275.9 | 242.4 |
As shown in Table 1, IC50 values were even lower for cells representing sarcomatoid (NCI-H2052) and biphasic (MSTO-211H) subtypes than for epithelioid (NCI-H2452 and NCI-H28) subtypes, suggesting the possible use of CAPS in particularly aggressive MM subtypes.
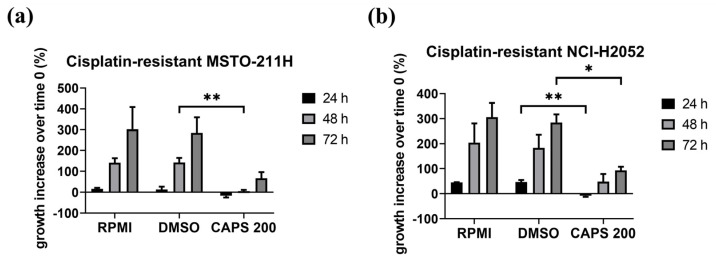
Since occurrence of resistance to conventional chemotherapeutic drugs, such as cisplatin, results in treatment failure in patients [24,58,59], we evaluated the effect of CAPS on cell growth of cisplatin-resistant MSTO-211H and NCI-H2052 cells, generated in our laboratory following prolonged exposure to increasing concentrations of cisplatin [52], as described in Section 2. Significantly, treatment with CAPS 200 µM reduced cell proliferation of both cisplatin-resistant MSTO-211H and NCI-H2052 cells as compared to DMSO-treated cells (Figure 2a,b). Since no cytotoxicity of DMSO was observed in cisplatin-resistant cells, the antiproliferative effects were indeed related to CAPS action.
Figure 2.
Capsaicin (CAPS) reduces proliferation of cisplatin-resistant malignant mesothelioma (MM) cell lines. Cisplatin-resistant MSTO-211H (a) and NCI-H2052 (b) cells were treated with RPMI 1640 medium alone (RPMI); medium containing DMSO (DMSO) at the same %v/v as CAPS concentration; medium containing CAPS at a concentration of 200 µM (CAPS 200) for 24, 48 and 72 h. Data are expressed as percentage of growth increase over time 0 and represent results obtained by two independent experiments performed in triplicate. Statistical differences were evaluated by one-way analysis of variance (ANOVA) (* p-value < 0.05, ** p-value < 0.01 versus DMSO).
Collectively, we demonstrated the antiproliferative action of CAPS on all major MM subtypes. Furthermore, our results indicate that CAPS effectively reduces proliferation in cisplatin-resistant mesothelioma cells, suggesting that CAPS treatment could overcome cisplatin resistance of MM cells.
3.2. CAPS Impairs Cell Cycle in Mesothelioma Cells
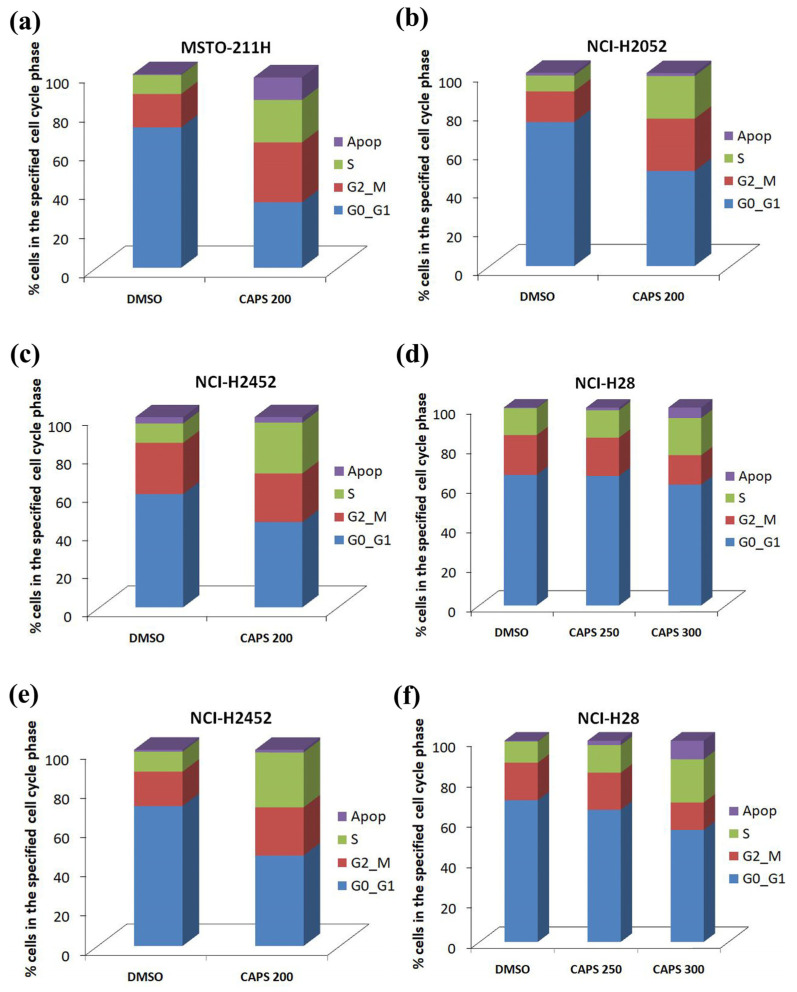
We then analyzed the effects of CAPS on the cell cycle and performed cytofluorometric analysis on MSTO-211H, NCI-H2052, NCI-H2452 and NCI-H28 cells following 48 h of CAPS treatment. As shown in Figure 3a, in MSTO-211H cells, CAPS reduced the fraction of cells in the G0/G1 phase (33.85% ± 8.27 in CAPS 200 versus 72.5% ± 3.54 in DMSO, * p-value < 0.05),while significantly increasing the S-phase cell population (21.9% ± 0.566 in CAPS 200 versus 9.8% ± 3.39 in DMSO, * p-value < 0.05). Furthermore, in MSTO-211H cells treated with CAPS 200 µM for 48 h, we observed an increase in the number of apoptotic cells compared to DMSO alone.
Figure 3.
Capsaicin (CAPS) impairs cell cycle in malignant mesothelioma (MM) cells. Cell cycle analysis was performed in duplicate on MM cells after 48 h (a–d) or 72 h (e,f) treatment with medium containing Dimethyl sulfoxide (DMSO) used as vehicle at the same %v/v as the highest CAPS concentration or treatment with CAPS. The concentration of CAPS used for MSTO-211H (a), NCI-H2052 (b) and NCI-H2452 cells (c,e) was 200 µM (CAPS 200), while NCI-H28 cells (d,f) were treated with CAPS at the concentration of 250 μM (CAPS 250) and 300 μM (CAPS 300). Data show the impact of CAPS treatments on various cell cycle phases: G0-G1; S phase; G2-M and the percentage of apoptotic cells (Apop).
Consistent with the results obtained in MSTO-211H cells, CAPS promoted S-phase cell cycle arrest in NCI-H2052 cells (22.1% ± 3.68 in CAPS 200 versus 8.15% ± 1.91 in DMSO, * p-value < 0.05) (Figure 3b).
In NCI-H2452 cells, we detected an increase, although not significant, in the S-phase cell population following 48 h treatment with CAPS compared to vehicle-treated cells (Figure 3c).
Furthermore, 48 h treatment with CAPS at concentrations of 250 µM and 300 µM did not significantly impair the cell cycle of NCI-H28 cells. On the other hand, the number of apoptotic NCI-H28 cells was significantly increased following 48 h treatment with CAPS 300 µM (5.3% ± 2.69 in CAPS 300 versus 0.37% ± 0.55 in DMSO, * p-value < 0.05) (Figure 3d).
Since we did not observe significant differences in cell cycle distribution following treatment with CAPS for 48 h in both NCI-H2452 and NCI-H28 cells, we further investigated the effects of CAPS on cell cycle in these cell lines after 72 h of treatment (Figure 3e,f).
In NCI-H2452 cells, we detected a significant decrease in G0/G1-phase cell population following 72 h of treatment with CAPS at a concentration of 200 µM (46.10% ± 4.53 in CAPS-treated cells versus 71.3% ± 2.55 in DMSO-treated cells, * p-value < 0.05) (Figure 3e).
In contrast, in NCI-H28 cells, 72 h treatment with CAPS at a concentration of 250 µM did not impair cell cycle, while at a concentration of 300 µM, it resulted in significant cell accumulation in the S phase (21.45% ± 1.34 in CAPS 300-treated cells versus 10.57% ± 1.93 in DMSO-treated cells, * p-value < 0.05) (Figure 3f). In addition, the percentage of apoptotic NCI-H28 cells significantly increased in a dose-dependent manner following 72 h CAPS treatment (Figure 3f).
Overall, these results suggest that the effects of CAPS on the cell cycle may differ in cells derived from different mesothelioma subtypes. In sarcomatoid NCI-H2052 and biphasic MSTO-211H mesothelioma cells, CAPS significantly arrested cells at the S phase following 48 h of treatment. In contrast, in epithelioid NCI-H28 cells, a significant increase in S-phase cell population was achieved only after 72 h of treatment. An enhanced percentage of cells in S phase could indicate an S-phase cell cycle arrest promoted by CAPS in MM cells. Indeed, the results of cytofluorimetric cell cycle analysis in association with those obtained by the MTS assay suggest that CAPS exerts an antiproliferative action by arresting MM cells in the S phase of the cell cycle.
3.3. CAPS Reduces Migration of Various Mesothelioma Lines
Increased migratory capacity represents an important property of cancer cells [60,61]. Thus, to evaluate whether CAPS affected the migration of mesothelioma cells, we utilized two approaches: wound-healing assays to test lateral cell motility in monolayer growth, and Transwell migration assays. We used a CAPS concentration of 100 µM for MSTO-211H, NCI-H2052 and NCI-H2452 cells, and 200 µM for NCI-H28 cells, concentrations that did not inhibit MM cell growth to rule out the possibility that the effect of CAPS on lateral motility, which is assessed in monolayer, could be affected by CAPS action on cell growth.
3.3.1. CAPS Impairs Lateral Motility of Various Mesothelioma Lines
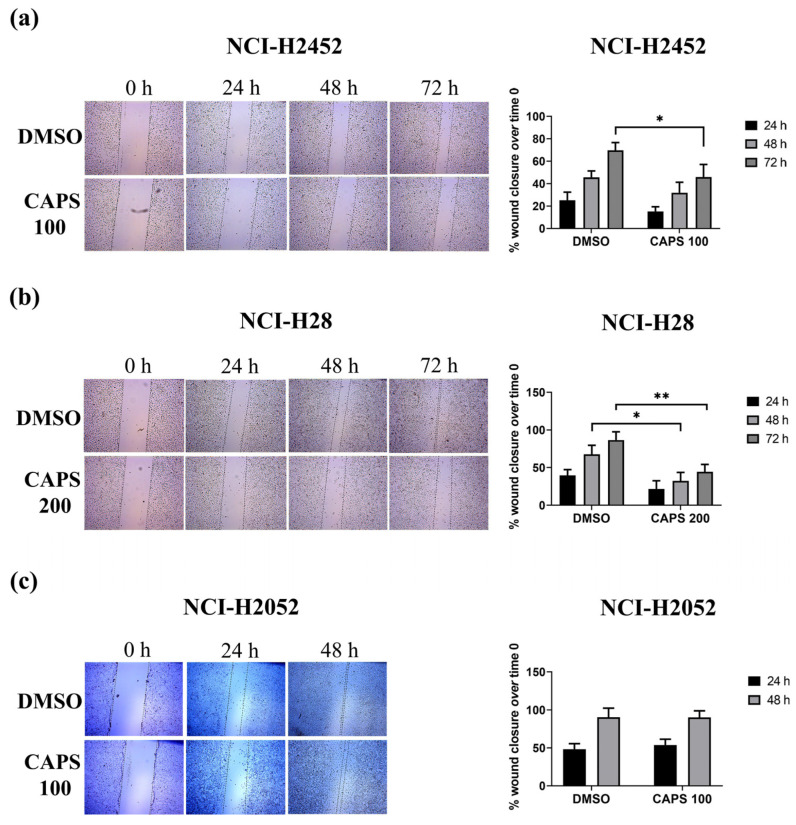
We conducted wound-healing assay on NCI-H2452, NCI-H28 and NCI-H2052 (Figure 4) cells, but not on MSTO-211H cells, as the latter tend to detach when they reach confluence.
Figure 4.
Capsaicin (CAPS) decreases lateral motility of NCI-H2452 and NCI-H28, but not of NCI-H2052 cells. Wound-healing assay was performed in NCI-H2452 (a), NCI-H28 (b) and NCI-H2052 (c) MM cells treated with medium containing either vehicle (DMSO) or CAPS at a concentration of 100 µM (CAPS 100) or 200 µM (CAPS 200) for 24, 48 and 72 h. Representative images of wound-healing assays in MM cells. Quantitative analysis of % wound closure. Data show the percentage of wound area closure compared with time 0. The results are expressed as mean ± standard deviation of three independent experiments. Statistical differences between DMSO and CAPS 100 µM or CAPS 200 µM were assessed by an unpaired t-test for each time of treatment (* p-value < 0.05 versus DMSO; ** p-value < 0.01 versus DMSO).
In NCI-H2452 cells, 72 h of treatment with CAPS significantly reduced lateral motility compared to control cells treated with DMSO alone. Indeed, the mean value of % wound area closure was 69.62% in DMSO alone versus 45.90% in CAPS 100 µM after 72 h of treatment (* p-value < 0.05) (Figure 4a).
In NCI-H28 cells, treatment with CAPS 200 µM for 48 h significantly impaired lateral motility, as the percentage of wound area closure was 32.34% compared to 67.56% in control cells (* p-value < 0.05) (Figure 4b). In addition, the mean value of wound area closure percentage was 44.44% in cells treated with CAPS 200 µM for 72 h compared to 86.62% in the vehicle-treated control group (** p-value ≤ 0.001) (Figure 4b).
Unlike the other MM cell lines, lateral motility of NCI-H2052 was not affected by treatment with CAPS at a concentration of 100 µM, as shown in Figure 4c, suggesting that CAPS did not significantly decrease lateral motility of sarcomatoid-type cells.
Thus, CAPS significantly reduced lateral motility in the NCI-H28 and NCI-H2452 epithelioid MM lines, but not in NCI-H2052 cells, representative of the sarcomatoid type commonly associated with a more aggressive phenotype in patients. These results therefore indicate that the MM subtype could be important in modulating the response to CAPS and highlight the inhibitory action of CAPS on the lateral motility of epithelioid MM cells.
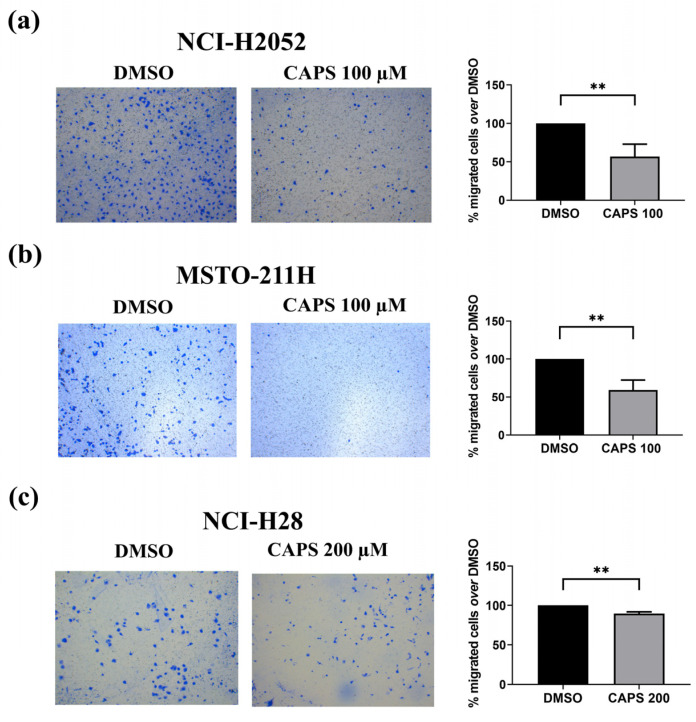
3.3.2. CAPS Reduces Transwell Migration of Various Mesothelioma Cells
We then carried out a Transwell migration assay in NCI-H2052, MSTO-211H and NCI-H28 cells to evaluate the effect of CAPS on the anchorage-independent migratory ability of MM cells.
CAPS exerted an antimigratory action on NCI-H2052 and MSTO-211H cells at a concentration of 100 µM, which did not affect cell proliferation. Indeed, CAPS treatment led to a reduction in the percentage of migrated NCI-H2052 and MSTO-211H cells compared to cells treated with medium containing DMSO alone (Figure 5a, Figure 5b, respectively).
Figure 5.
Capsaicin (CAPS) inhibits Transwell migration in malignant mesothelioma (MM) cells. Migration of NCI-H2052 (a), MSTO-211H (b) and NCI-H28 (c) cells, treated with medium containing DMSO alone (DMSO) or CAPS at a concentration of 100 µM (CAPS 100) or 200 µM (CAPS 200), was conducted by Transwell migration assays, as described in Materials and Methods. Representative images of MM cells. Quantitative analysis of percentage of migrated cells versus DMSO-treated cells. Data are expressed as mean ± standard deviation of three independent experiments. Statistical differences between DMSO and CAPS 100 µM or CAPS 200 µM were evaluated by an unpaired t-test (** p-value < 0.01 compared to DMSO).
For NCI-H28 cell migration, we used a CAPS concentration of 200 µM as this concentration did not affect the proliferation of this cell line. CAPS exposure at 200 µM significantly inhibited the migratory capacity of NCI-H28 cells as compared to DMSO-treated control cells (Figure 5c).
Collectively, these results demonstrate that CAPS inhibited the migration of MM cells, highlighting an additional important anticancer property of CAPS on MM cells. Thus, CAPS can reduce the aggressive phenotype of MM cells by suppressing the migration of MM cells.
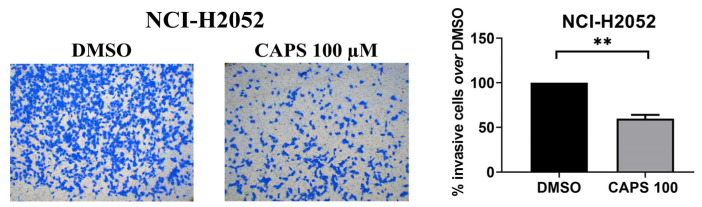
3.4. CAPS Reduces Invasive Ability of Mesothelioma Cells
The ability of cancer cells to migrate and invade through tissues is a feature underlying the process of tumor metastasis [62,63]. Thus, we evaluated the effect of CAPS on the invasion of mesothelioma cells. We focused on NCI-H2052 sarcomatoid-type mesothelioma cells since several studies have revealed the presence of distant metastases originating from sarcomatoid-type mesothelioma [64,65,66,67]. Thus, we treated NCI-H2052 mesothelioma cells with medium containing either the vehicle alone or CAPS at a concentration of 100 µM that did not impair cell growth, and we found a reduction in the ability of CAPS-treated NCI-H2052 MM cells to invade through Matrigel-coated Transwell chambers (Figure 6).
Figure 6.
Capsaicin (CAPS) reduced the invasive capacity of NCI-H2052 cells. Invasion of NCI-H2052 cells, treated with medium containing DMSO alone (DMSO) or CAPS 100 µM (CAPS 100), was performed by Transwell invasion assays using Matrigel-coated chambers. Representative images of Transwell invasion assay on NCI-H052 cells. Quantitative analysis of percentage of invasive cells versus DMSO-treated cells. Statistical differences between DMSO and CAPS 100 µM were evaluated by an unpaired t-test (** p-value < 0.01 compared to DMSO).
This result evidenced the anti-invasive action of CAPS in MM cells, specifically the sarcomatoid MM subtype. Given the highly aggressive nature and the ability of MM cells to metastasize, the anti-invasive action exerted by CAPS on sarcomatoid-type MM cells could represent a valid pharmacological approach for future studies.
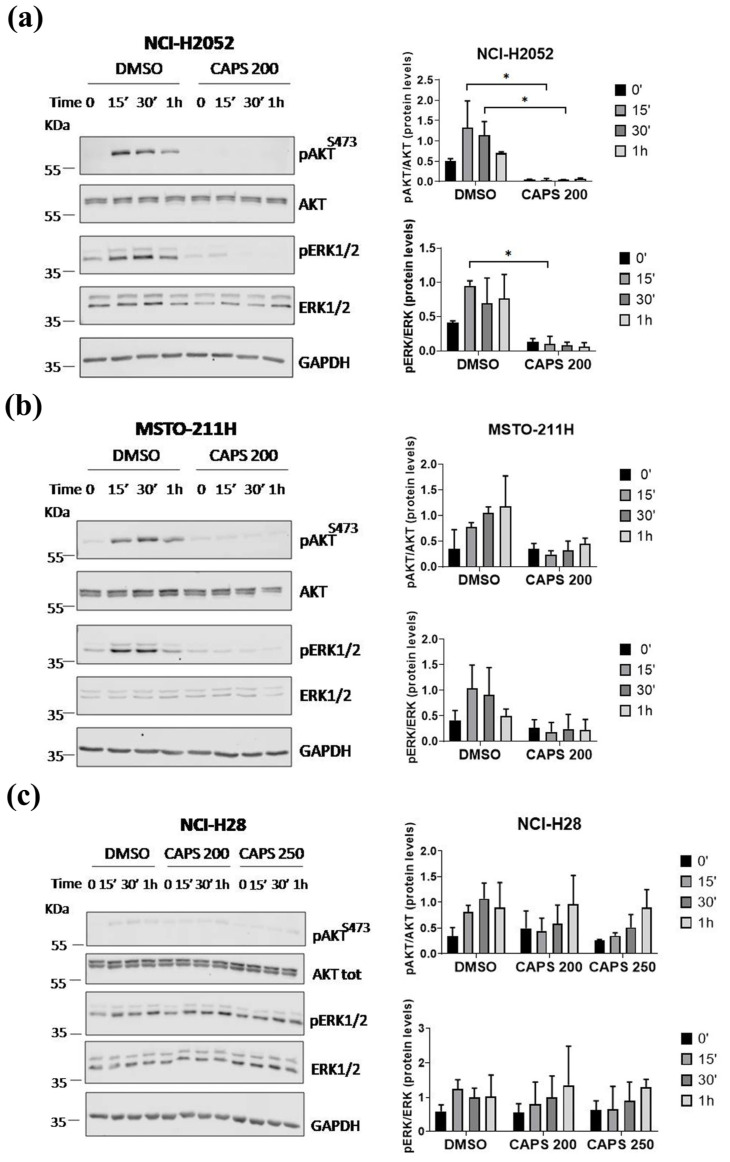
3.5. CAPS Inhibits the Activation of the AKT and ERK1/2 Signaling Pathways in MM Cells
AKT and ERK1/2 are serine-threonine kinases with a critical role in the modulation of proliferation and migration of cancer cells [41,42].
Thus, to initially characterize the mechanisms of CAPS action in inhibiting the proliferation, cell cycle progression and motility of MM cells, we tested the level of AKT and ERK1/2 activation by immunoblot in NCI-H2052 (Figure 7a), MSTO-211H (Figure 7b) and NCI-H28 (Figure 7c) cells with and without CAPS treatment.
Figure 7.
Capsaicin (CAPS) impairs AKT and ERK1/2 activation in malignant mesothelioma (MM) cells. Levels of total and phosphorylated AKT and ERK1/2 were evaluated by Western blotting in NCI-H2052 (a), MSTO-211H (b) and NCI-H28 (c) cells serum-starved for 5 h and then treated with 1% fetal bovine serum medium containing DMSO alone (DMSO) or CAPS at concentrations of 200 µM (CAPS 200) or 250 µM (CAPS 250) for 15′, 30′ and 1 h. Representative Western blotting images of two independent experiments (n = 2) for phosphoAKT (pAKT), total AKT (AKT), phosphoERK1/2 (pERK1/2), total ERK1/2 (ERK1/2) and GAPDH are shown. Densitometric analysis of phospho AKT/total AKT ratio and phospho ERK1/2/total ERK1/2 ratio. The values are expressed as mean ± standard deviation. Statistical differences were evaluated by one-way analysis of variance (ANOVA) (* p-value < 0.05).
The cells were serum-starved in SFM for 5 h and then stimulated with 1% FBS-containing media supplemented with either DMSO as control or CAPS (200 µM or 250 µM) for 15 min, 30 min and 1 h.
As shown in Figure 7a,b, while serum induced strong and sustained activation of AKT and ERK1/2 in both NCI-H2052 and MSTO-211H DMSO-treated control MM cells, CAPS significantly inhibited the activation of both signaling pathways.
The results were slightly different in NCI-H28 cells. Notably, NCI-H28 cells showed rapid activation of AKT, which was not as strong as demonstrated in NCI-H2052 and MSTO-211H cells, and strong ERK1/2 activation. Significantly, treatment with CAPS at concentrations of 200 µM or 250 µM only resulted in delayed activation of AKT in NCI-H28 cells, which was detectable at 1 h of serum stimulation (Figure 7c). In contrast, CAPS at a concentration of 200 µM did not markedly reduce ERK1/2 activation, whereas at a concentration of 250 µM, it resulted in delayed ERK1/2 activation (Figure 7c).
Collectively, these results suggest that CAPS significantly inhibited the activation of AKT and ERK1/2 in MSTO-211H and NCI-H2052 MM cells. Conversely, in NCI-H28 cells, treatment with CAPS 250 µM only resulted in delayed AKT and ERK1/2 activation, suggesting that additional pathways targeted by CAPS can contribute to sustaining proliferation and motility of NCI-H28 MM cells.
4. Discussion
MM is a very aggressive cancer mostly associated with asbestos exposure. MM has poor prognosis despite the use of surgery and chemotherapy drugs. Therefore, developing and discovering new pharmacological therapeutic approaches is crucial to improve the therapeutic strategies for this disease [20,68].
Capsaicin (CAPS) is a capsaicinoid with several beneficial health effects [33]. In vivo and in vitro studies demonstrated antitumor activity of CAPS in various tumor models [44,69]. However, its effect on various MM cells was not previously established. Thus, this study aimed at the characterization of the anticancer properties of CAPS on MM cells. Specifically, we investigated the effect of CAPS on proliferation, migration and cell cycle in various MM lines, representative of MM subtypes.
Here, we demonstrated that (a) CAPS inhibits cell growth of MM cells; (b) CAPS induces S-phase cell cycle arrest in various MM cells; (c) CAPS reduces lateral motility, migration in Transwell and invasion of MM cells; and (d) CAPS delays or inhibits AKT and ERK1/2 activation in MM cells. Our results prove a strong antitumor action of CAPS on MM cells.
Specifically, we demonstrated the anti-proliferative action of CAPS on various MM cell lines at CAPS concentrations that are in line with those reported in the literature showing anticancer action against other cancer cells [44].
FDA-approved pharmacological treatment for pleural mesothelioma patients includes either the use of cisplatin plus pemetrexed or the combination nivolumab plus ipilimumab [19]. Unfortunately, resistance to conventional chemotherapy drugs, such as cisplatin, affects response to MM therapy [24,58,59]. Thus, in this study, we investigated whether CAPS had an effect on growth of cisplatin-resistant cells.
We demonstrated that CAPS significantly inhibited cell proliferation of cisplatin-resistant MSTO-211H and NCI-H2052 MM cells. Our in vitro results support scientific research towards new pharmacological strategies for MM since CAPS treatment could overcome cisplatin resistance of MM cells and have possible future implications in the clinical arena. Accordingly, other studies proved the antitumor action of CAPS on other cisplatin-resistant tumor cells. In this regard, Catanzaro et al. demonstrated that CAPS exerted a cytotoxic action and promoted cell cycle arrest in cisplatin-resistant ovarian and cervical squamous carcinoma cells. The action of CAPS on these cisplatin-resistant tumor cells was even stronger than on cisplatin-sensitive tumor cells [50]. Indeed, CAPS sensitizes various tumor cells to chemotherapeutics or radiotherapy. Indeed, CAPS in combination with conventional therapeutic approaches increases their efficacy, as observed by in vitro and in vivo studies on various tumor models [48,49]. CAPS sensitizes multidrug-resistant cells to doxorubicin [70]. This suggests the importance of studying CAPS in combination with other therapeutic approaches in MM therapy to improve the efficacy of conventional treatments, especially considering the occurrence of drug resistance. Chemotherapeutic agents are commonly associated with various adverse effects. There is a growing interest in discovering the beneficial properties, such as antitumor properties, of natural compounds in the scientific community [71]. Furthermore, natural compounds can exhibit an interesting ability to synergize, significantly improving the efficacy of currently available treatments [70,72]. Since we have proven the antitumor action of CAPS on cisplatin-resistant MM cells, future studies could evaluate the potential synergistic effects of CAPS in combination with other natural compounds in MM therapy.
Several studies have shown that the antiproliferative action of CAPS on various cancer cells is associated with cell cycle arrest at various phases depending on cell line [45,47,73]. Specifically, CAPS promotes G0/G1-phase cell cycle arrest in leukemia, osteosarcoma, colon cancer and bladder cancer cells [38,39,73], as well as G2/M-phase arrest in KB squamous cell carcinoma and MDA-MB-231 breast cancer cells [43,47].
Here, we investigated the effect of CAPS on MM cell cycle and demonstrated that CAPS induced S-phase cell cycle arrest in various MM cell lines. Our results are consistent with those reported by Chang et al. [45] and Yoon et al. [74], proving CAPS-promoted S-phase cell cycle arrest in MCF-7 and BT-20 human breast cancer cells. Similarly, CAPS analogues and derivatives are well known to cause S-phase arrest in various cancer lines [75,76].
In this work, we demonstrated for the first time that CAPS inhibited the migration of several MM cell lines at concentrations that did not affect cell growth. Indeed, CAPS exerted its anti-migratory action on NCI-H28, NCI-H2452 and MSTO-211H cells, consistently with other studies proving anti-migratory action of CAPS on several cancer cell lines [34,35,77,78,79].
Although CAPS did not affect lateral motility of NCI-H2052 cells, it inhibited Transwell migration in this cell line. In this regard, it is worth pointing out that wound-healing and Transwell migration assays measure two different types of cells motility [80]. While the first assay assesses collective migration of confluent cell monolayers characterized by cell–cell interactions, the second one enables the analysis of migration of single cells capable of crossing a membrane [81,82]. Furthermore, we prove that CAPS treatment reduces the invasive capacity of NCI-H2052 cells. This result highlights another interesting antitumor property of CAPS against MM cells. Indeed, the process of metastasis formation is related to the ability of cancer cells to migrate and invade tissues [62,63]. Distant metastasis formation has been found in patients with MM [64,83,84], and therefore the identification of new substances or drugs that can reduce the migratory and invasive ability is crucial. Our results on sarcomatoid MM cell invasion following CAPS treatment are in line with other studies that demonstrated the anti-invasive action of CAPS on renal and breast cancer cells [35,77]. Here, we provide the first evidence of the anti-invasive effect of CAPS on sarcomatoid-type MM cells. These results are very promising considering that the prognosis of patients with sarcomatoid-type MM is poor, with a lower median survival than patients with biphasic and epithelioid-type MM [14,85,86,87].
Given the antiproliferative and antimigratory properties of CAPS on different MM lines, we investigated the effect of CAPS on the activation of AKT and ERK1/2, serine-threonine kinases involved in various cellular mechanisms such as cell proliferation, survival and migration [41,42]. In detail, we proved that CAPS treatment significantly inhibited both AKT and ERK1/2 activation in MSTO-211H and NCI-H2052 MM cells. Thus, we speculate that CAPS exerts its anti-proliferative and anti-migratory action by inhibiting AKT and ERK1/2 signaling pathways, which we, along with other authors, demonstrated are important for MM motility [57,88,89,90]. Our results are in line with other studies proving that the anti-proliferative, anti-migrative, anti-invasive and pro-apoptotic action of CAPS is related to AKT and ERK pathways inactivation on various tumor lines [40,78,91,92]. The inactivation of these pathways has also been discovered in combinatorial treatments of CAPS with other anticancer drugs. Indeed, CAPS enhances the cytotoxicity of chemotherapeutics or compounds with well-known antitumor action, such as sorafenib and erlotinib, through mechanisms involving AKT inhibition [93,94] in various tumor cells. In addition, CAPS in combination with cisplatin suppresses transforming growth factor-β1-induced epithelial–mesenchymal transition (EMT) through the activation of claudin 1 and inhibition of the PI3K/AKT/mTOR pathway in squamous cell carcinoma of the tongue [95]. In contrast, ERK inactivation was associated with EMT inhibition in nasopharyngeal carcinoma cells co-treated with CAPS and cisplatin. In these cells, CAPS synergizes with cisplatin, exhibiting higher efficacy than treatment with cisplatin alone [92]. Taken together, these data support the evidence that CAPS can inhibit AKT and MAPK action in various cancer models, including MM. Conversely, low concentrations of CAPS can lead to AKT and ERK1/2 activation, as well as stimulate the invasion and migration of colorectal cancer cells [96,97,98]. Some natural compounds with a known anticancer action can exhibit different biological action depending on concentrations used, as, in fact, low CAPS concentrations can promote cancer cell proliferation [97,99]. Similarly, prolonged exposure to chemotherapeutic agents such as cisplatin or gemcitabine has been associated with increased tumor mass in cancer models [100]. However, the CAPS concentrations we used are within the range of concentrations with proven antitumor action in various cancer lines and therefore far from 0.1 to 10 µM CAPS range for which prolonged exposure promotes colorectal cancer cell proliferation and migration [44,97]. However, understanding which concentrations might be toxic or not beneficial requires further investigation in animal models. The evaluation of the effect of CAPS as a promoter or inhibitor of tumor cell growth in murine models is quite complex. Oral administration of CAPS at a concentration of 2.5 mg/Kg body weight was effective in inhibiting tumor formation in murine models of pancreatic cancer [101]. On the contrary, other studies proved a carcinogenic effect of CAPS used at different concentration [102]. Future studies are therefore required to further define the impact of low or high concentrations of CAPS, using various routes of administration, in murine models of mesothelioma.
In addition, our cytofluorimetric analysis revealed an enhanced number of apoptotic MSTO-211H and NCI-H28 cells following CAPS treatment. Since CAPS promotes apoptosis in different cancer cell lines through caspase-3 activation [36,103,104], we investigated the effect of CAPS on the levels of the total and cleaved form of caspase-3 in various MM lines and found that CAPS did not promote the cleavage of caspase-3 in MM cells but this preliminary evidence needs further verification. Our results are in line with Cömertpay et al., who found no significant differences in caspase-3 activation in CRL-5946 MM cells following treatment with CAPS or vehicle alone, proving that CAPS did not induce caspase-3-dependent cell death in these cells [105]. In this regard, other caspase-3-independent mechanisms may be involved in CAPS-induced apoptosis. Indeed, some studies performed on breast cancer cells detected increased intracellular calcium levels and subsequent translocation of apoptosis-inducing factor (AIF) from the mitochondria to the nucleus following CAPS treatment, thus also providing evidence for CAPS-induced caspase-independent apoptotic mechanisms [106,107]. However, the effect of CAPS on MM cells apoptosis remains to be fully elucidated.
Although further studies are necessary to investigate the mechanism underlying the antitumor action of CAPS on MM cells, our results demonstrate both antiproliferative and antimigratory properties of CAPS in various MM and its potential use in MM therapy.
CAPS inhibits the growth of cisplatin-resistant MM cells supporting the feasibility and possible clinical validity of combinatorial treatments with CAPS and current therapeutic regimens for MM therapy. Furthermore, developing anticancer drugs with fewer adverse effects is crucial for cancer patients. Chemotherapeutic agents, including cisplatin, are often associated with nausea, neuropathy and other adverse effects [108,109]. Natural products could represent a winning choice to counteract these negative effects. Topical CAPS administration exhibits beneficial effects in reducing episodes of nausea and vomiting (ClinicalTrials.govNCT04918069) [110], as well as neuropathic pain in patients undergoing chemotherapy treatments [111].
Despite its beneficial properties, CAPS is a substance that can cause irritation and burning of mucosa [112]. However, CAPS exhibits low bioavailability and half-life regardless of the route of administration [113]. For this reason, current studies are investigating possible strategies to deliver CAPS, such as liposomes and microemulsions [112,114]. To evaluate the potential use of CAPS in the clinics, it is necessary to consider the pharmacokinetics and metabolism of CAPS. Following oral administration, CAPS is mostly metabolized in the liver. In human liver microsomes, CAPS is converted into three major metabolites: 16-hydroxycapsaicin, 17-hydroxycapsaicin and 16,17-dehydrocapsaicin [113,115]. In contrast, following topical CAPS administration, vanillylamine and vanillic acid represent the main metabolic products in the skin [113]. A study conducted on volunteers proved that oral administration of a CAPS-rich extract via gel capsules reduced glucose levels [116]. Pharmacokinetic studies of CAPS were performed from blood of these volunteers. The highest observed CAPS concentration was 2.47 ng/mL at 45 min, but CAPS levels were detectable up to 90 min, suggesting a potentially rapid CAPS absorption [116]. In addition, another study demonstrated that oral administration of CAPS and isoflavone promoted hair growth in subjects with alopecia [117], but no pharmacokinetic parameter of CAPS was reported.
This limited number of studies on patients or volunteers highlights the use of CAPS as a promising approach. Notably, in this work, we proved the anti-proliferative, anti-migratory and anti-invasive properties of CAPS on various MM cell lines and its growth inhibitory action on cisplatin-resistant MM cells, thereby supporting a possible use of CAPS for MM therapy.
5. Conclusions
Mesothelioma is associated with poor prognosis. The development of new therapeutic strategies is an essential goal in the fight against mesothelioma. Considering the intriguing antitumor properties of CAPS in other tumor models, this work investigated the effect of CAPS on proliferation, migration and invasion of various MM cells, representative of MM subtypes. Specifically, CAPS inhibits the proliferation of both parental and cisplatin-resistant cells. These in vitro results strongly support the possible use of CAPS in clinical practice. However, further validation in 3D models or animal xenograft models is required before evaluating the use of CAPS in clinical therapy.
Author Contributions
Conceptualization, E.A. and A.M.; methodology, E.A. and A.C.; investigation, E.A.; resources, E.A. and A.M.; data curation, E.A., A.M. and R.C.; writing—original draft preparation, E.A. and A.M.; writing—review and editing, E.A., A.M. and R.C.; visualization, E.A., A.C., A.M., M.Q., E.V., S.D., A.G. and R.C.; supervision, A.M., R.C., E.V., S.D. and A.G.; project administration, A.M. and R.C.; funding acquisition, A.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This work was funded by the Sbarro Health Research Organization (www.SHRO.org).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tipu S.A., Ahmed I., Ishtiaq S. Malignant mesothelioma. Pak. J. Med. Sci. 2013;29:1433–1438. doi: 10.12669/pjms.296.3938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hajj G., Cavarson C., Pinto C., Venturi G., Navarro J., Lima V. Malignant pleural mesothelioma: An update. J. Bras. Pneumol. 2021;47:e20210129. doi: 10.36416/1806-3756/e20210129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stevenson J., Ettinger D., Wood D., Aisner D., Akerley W., Bauman J., Bharat A., Bruno D., Chang J., Chirieac L., et al. NCCN Guidelines® Insights: Mesothelioma: Pleural, Version 1.2024. J. Natl. Compr. Canc. Netw. 2024;22:72–81. doi: 10.6004/jnccn.2024.0014. [DOI] [PubMed] [Google Scholar]
- 4.Han J., Park S., Yon D.K., Lee S.W., Woo W., Dragioti E., Koyanagi A., Jacob L., Kostev K., Radua J., et al. Global, Regional, and National Burden of Mesothelioma 1990–2019 A Systematic Analysis of the Global Burden of Disease Study 2019. Ann. Am. Thorac. Soc. 2023;20:976–983. doi: 10.1513/AnnalsATS.202209-802OC. [DOI] [PubMed] [Google Scholar]
- 5.Wadowski B., De Rienzo A., Bueno R. The Molecular Basis of Malignant Pleural Mesothelioma. Thorac. Surg. Clin. 2020;30:383–393. doi: 10.1016/j.thorsurg.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mangone L., Storchi C., Pinto C., Giorgi Rossi P., Bisceglia I., Romanelli A. Incidence of malignant mesothelioma and asbestos exposure in the Emilia-Romagna region, Italy. Med. Lav. 2022;113:e2022047. doi: 10.23749/mdl.v113i5.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu L., Dell’Anno I., Lapidot M., Sekido Y., Chan M.-L., Kohno M., Serre-Beinier V., Felley-Bosco E., de Perrot M. Progress of malignant mesothelioma research in basic science: A review of the 14th international conference of the international mesothelioma interest group (iMig2018) Lung Cancer. 2019;127:138–145. doi: 10.1016/j.lungcan.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 8.International Agency for Research on Cancer . Arsenic, Metals, Fibres and Dusts. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, No. 100C. International Agency for Research on Cancer; Lyon, France: 2012. ASBESTOS (CHRYSOTILE, AMOSITE, CROCIDOLITE, TREMOLITE, ACTINOLITE AND ANTHOPHYLLITE) pp. 219–309. [Google Scholar]
- 9.Aryal A., Morley C. Call for a global ban policy on and scientific management of asbestos to eliminate asbestos-related diseases. J. Public Health Policy. 2020;41:279–285. doi: 10.1057/s41271-020-00223-4. [DOI] [PubMed] [Google Scholar]
- 10.Carbone M., Pass H., Ak G., Alexander H.J., Baas P., Baumann F., Blakely A., Bueno R., Bzura A., Cardillo G., et al. Medical and Surgical Care of Patients With Mesothelioma and Their Relatives Carrying Germline BAP1 Mutations. J. Thorac. Oncol. 2022;17:873–889. doi: 10.1016/j.jtho.2022.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carbone M., Adusumilli P.S., Alexander H.R., Baas P., Bardelli F., Bononi A., Bueno R., Felley-Bosco E., Galateau-Salle F., Jablons D., et al. Mesothelioma: Scientific clues for prevention, diagnosis, and therapy. CA Cancer J. Clin. 2019;69:402–429. doi: 10.3322/caac.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asciak R., George V., Rahman N.M. Update on biology and management of mesothelioma. Eur. Respir. Rev. 2021;30:200226. doi: 10.1183/16000617.0226-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brcic L., Kern I. Clinical significance of histologic subtyping of malignant pleural mesothelioma. Transl. Lung Cancer Res. 2020;9:924–933. doi: 10.21037/tlcr.2020.03.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dacic S. Pleural mesothelioma classification—Update and challenges. Mod. Pathol. 2022;35:51–56. doi: 10.1038/s41379-021-00895-7. [DOI] [PubMed] [Google Scholar]
- 15.Roca E., Aujayeb A., Astoul P. Diagnosis of Pleural Mesothelioma: Is Everything Solved at the Present Time? Curr. Oncol. 2024;31:4968–4983. doi: 10.3390/curroncol31090368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karpes J.B., Shamavonian R., Dewhurst S., Cheng E., Wijayawardana R., Ahmadi N., Morris D.L. Malignant Peritoneal Mesothelioma: An In-Depth and Up-to-Date Review of Pathogenesis, Diagnosis, Management and Future Directions. Cancers. 2023;15:4704. doi: 10.3390/cancers15194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun H.H., Vaynblat A., Pass H.I. Diagnosis and prognosis—Review of biomarkers for mesothelioma. Ann. Transl. Med. 2017;5:244. doi: 10.21037/atm.2017.06.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazarika M., White R.M., Johnson J.R., Pazdur R. FDA Drug Approval Summaries: Pemetrexed (Alimta®) Oncologist. 2004;9:482–488. doi: 10.1634/theoncologist.9-5-482. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Food and Drug Administration FDA Approves Drug Combination for Treating Mesothelioma. First Approval in 16 Years for Mesothelioma, a Type of Cancer Caused by Inhaling Asbestos Fibers. [(accessed on 26 April 2024)];2020 Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-drug-combination-treating-mesothelioma.
- 20.Sinn K., Mosleh B., Hoda M.A. Malignant pleural mesothelioma: Recent developments. Curr. Opin. Oncol. 2021;33:80–86. doi: 10.1097/CCO.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 21.Hu Z.I., Ghafoor A., Sengupta M., Hassan R. Malignant mesothelioma: Advances in immune checkpoint inhibitor and mesothelin-targeted therapies. Cancer. 2021;127:1010–1020. doi: 10.1002/cncr.33433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogelzang N.J., Rusthoven J.J., Symanowski J., Denham C., Kaukel E., Ruffie P., Gatzemeier U., Boyer M., Emri S., Manegold C., et al. Phase III Study of Pemetrexed in Combination With Cisplatin Versus Cisplatin Alone in Patients With Malignant Pleural Mesothelioma. J. Clin. Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 23.Santoro A., O’Brien M.E., Stahel R.A., Nackaerts K., Baas P., Karthaus M., Eberhardt W., Paz-Ares L., Sundstrom S., Liu Y., et al. Pemetrexed Plus Cisplatin or Pemetrexed Plus Carboplatin for Chemonaïve Patients with Malignant Pleural Mesothelioma: Results of the International Expanded Access Program. J. Thorac. Oncol. 2008;3:756–763. doi: 10.1097/JTO.0b013e31817c73d6. [DOI] [PubMed] [Google Scholar]
- 24.Oehl K., Vrugt B., Wagner U., Kirschner M.B., Meerang M., Weder W., Felley-Bosco E., Wollscheid B., Bankov K., Demes M.C., et al. Alterations in BAP1 Are Associated with Cisplatin Resistance through Inhibition of Apoptosis in Malignant Pleural Mesothelioma. Clin. Cancer Res. 2021;27:2277–2291. doi: 10.1158/1078-0432.CCR-20-4037. [DOI] [PubMed] [Google Scholar]
- 25.Baas P., Scherpereel A., Nowak A.K., Fujimoto N., Peters S., Tsao A.S., Mansfield A.S., Popat S., Jahan T., Antonia S., et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375–386. doi: 10.1016/S0140-6736(20)32714-8. [DOI] [PubMed] [Google Scholar]
- 26.Barbier M.C., Fengler A., Pardo E., Bhadhuri A., Meier N., Gautschi O. Cost Effectiveness and Budget Impact of Nivolumab Plus Ipilimumab Versus Platinum Plus Pemetrexed (with and Without Bevacizumab) in Patients with Unresectable Malignant Pleural Mesothelioma in Switzerland. Pharmacoeconomics. 2023;41:1641–1655. doi: 10.1007/s40273-023-01305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang L., Song X., Zeng W., Zheng Z., Lin W. First-line nivolumab plus ipilimumab for unresectable MPM in China: A cost-effectiveness analysis. Orphanet J. Rare Dis. 2023;18:326. doi: 10.1186/s13023-023-02925-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ye Z., Tang Z.-Q., Xu Z., Zhou Q., Li H. Cost-effectiveness of nivolumab plus ipilimumab as first-line treatment for American patients with unresectable malignant pleural mesothelioma. Front. Public Health. 2022;10:947375. doi: 10.3389/fpubh.2022.947375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andretta E., Costa C., Longobardi C., Damiano S., Giordano A., Pagnini F., Montagnaro S., Quintiliani M., Lauritano C., Ciarcia R. Potential Approaches Versus Approved or Developing Chronic Myeloid Leukemia Therapy. Front. Oncol. 2021;11:801779. doi: 10.3389/fonc.2021.801779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciarcia R., Longobardi C., Ferrara G., Montagnaro S., Andretta E., Pagnini F., Florio S., Maruccio L., Lauritano C., Damiano S. The Microalga Skeletonema marinoi Induces Apoptosis and DNA Damage in K562 Cell Line by Modulating NADPH Oxidase. Molecules. 2022;27:8270. doi: 10.3390/molecules27238270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rahman M.M., Rahaman M.S., Islam M.R., Rahman F., Mithi F.M., Alqahtani T., Almikhlafi M.A., Alghamdi S.Q., Alruwaili A.S., Hossain M.S., et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules. 2021;27:233. doi: 10.3390/molecules27010233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maiuolo J., Gliozzi M., Carresi C., Musolino V., Oppedisano F., Scarano F., Nucera S., Scicchitano M., Bosco F., Macri R., et al. Nutraceuticals and Cancer: Potential for Natural Polyphenols. Nutrients. 2021;13:3834. doi: 10.3390/nu13113834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adaszek Ł., Gadomska D., Mazurek Ł., Łyp P., Madany J., Winiarczyk S. Properties of capsaicin and its utility in veterinary and human medicine. Res. Vet. Sci. 2019;123:14–19. doi: 10.1016/j.rvsc.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 34.Lee G.-R., Jang S.H., Kim C.J., Kim A.-R., Yoon D.-J., Park N.-H., Han I.-S. Capsaicin suppresses the migration of cholangiocarcinoma cells by down-regulating matrix metalloproteinase-9 expression via the AMPK–NF-κB signaling pathway. Clin. Exp. Metastasis. 2014;31:897–907. doi: 10.1007/s10585-014-9678-x. [DOI] [PubMed] [Google Scholar]
- 35.Que T., Ren B., Fan Y., Liu T., Hou T., Dan W., Liu B., Wei Y., Lei Y., Zeng J., et al. Capsaicin inhibits the migration, invasion and EMT of renal cancer cells by inducing AMPK/mTOR-mediated autophagy. Chem. Biol. Interact. 2022;366:110043. doi: 10.1016/j.cbi.2022.110043. [DOI] [PubMed] [Google Scholar]
- 36.Chen M., Xiao C., Jiang W., Yang W., Qin Q., Tan Q., Lian B., Liang Z., Wei C. Capsaicin Inhibits Proliferation and Induces Apoptosis in Breast Cancer by Down-Regulating FBI-1-Mediated NF-κB Pathway. Drug Des. Dev. Ther. 2021;15:125–140. doi: 10.2147/DDDT.S269901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brown K.C., Witte T.R., Hardman W.E., Luo H., Chen Y.C., Carpenter A.B., Lau J.K., Dasgupta P. Capsaicin Displays Anti-Proliferative Activity against Human Small Cell Lung Cancer in Cell Culture and Nude Mice Models via the E2F Pathway. PLoS ONE. 2010;5:e10243. doi: 10.1371/journal.pone.0010243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Qian K., Wang G., Cao R., Liu T., Qian G., Guan X., Guo Z., Xiao Y., Wang X. Capsaicin Suppresses Cell Proliferation, Induces Cell Cycle Arrest and ROS Production in Bladder Cancer Cells through FOXO3a-Mediated Pathways. Molecules. 2016;21:1406. doi: 10.3390/molecules21101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Deng X., Lei T., Yu C., Wang Y., Zhao G., Luo X., Tang K., Quan Z., Jiang D. Capsaicin inhibits proliferation and induces apoptosis in osteosarcoma cell lines via the mitogen-activated protein kinase pathway. Oncol. Rep. 2017;38:2685–2696. doi: 10.3892/or.2017.5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang J.-H., Lai F.-J., Chen H., Luo J., Zhang R.-Y., Bu H.-Q., Wang Z.-H., Lin H.-H., Lin S.-Z. Involvement of the phosphoinositide 3-kinase/Akt pathway in apoptosis induced by capsaicin in the human pancreatic cancer cell line PANC-1. Oncol. Lett. 2013;5:43–48. doi: 10.3892/ol.2012.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revathidevi S., Munirajan A.K. Akt in cancer: Mediator and more. Semin. Cancer Biol. 2019;59:80–91. doi: 10.1016/j.semcancer.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Roskoski R. ERK1/2 MAP kinases: Structure, function, and regulation. Pharmacol. Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 43.Wu D., Jia H., Zhang Z., Li S. Capsaicin suppresses breast cancer cell viability by regulating the CDK8/PI3K/Akt/Wnt/β-catenin signaling pathway. Mol. Med. Rep. 2020;22:4868–4876. doi: 10.3892/mmr.2020.11585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chapa-Oliver A., Mejía-Teniente L. Capsaicin: From Plants to a Cancer-Suppressing Agent. Molecules. 2016;21:931. doi: 10.3390/molecules21080931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang H., Chen S., Chien S., Kuo S., Tsai H., Chen D. Capsaicin may induce breast cancer cell death through apoptosis-inducing factor involving mitochondrial dysfunction. Hum. Exp. Toxicol. 2011;30:1657–1665. doi: 10.1177/0960327110396530. [DOI] [PubMed] [Google Scholar]
- 46.Thoennissen N.H., O’Kelly J., Lu D., Iwanski G.B., La D.T., Abbassi S., Leiter A., Karlan B., Mehta R., Koeffler H.P. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and -negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene. 2010;29:285–296. doi: 10.1038/onc.2009.335. [DOI] [PubMed] [Google Scholar]
- 47.Lin C.-H., Lu W.-C., Wang C.-W., Chan Y.-C., Chen M.-K. Capsaicin induces cell cycle arrest and apoptosis in human KB cancer cells. BMC Complement. Altern. Med. 2013;13:46. doi: 10.1186/1472-6882-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venier N.A., Colquhoun A.J., Sasaki H., Kiss A., Sugar L., Adomat H., Fleshner N.E., Klotz L.H., Venkateswaran V. Capsaicin: A novel radio-sensitizing agent for prostate cancer. Prostate. 2015;75:113–125. doi: 10.1002/pros.22896. [DOI] [PubMed] [Google Scholar]
- 49.Zhang S., Wang D., Huang J., Hu Y., Xu Y. Application of capsaicin as a potential new therapeutic drug in human cancers. J. Clin. Pharm. Ther. 2020;45:16–28. doi: 10.1111/jcpt.13039. [DOI] [PubMed] [Google Scholar]
- 50.Catanzaro D., Vianello C., Ragazzi E., Caparrotta L., Montopoli M. Cell Cycle Control by Natural Phenols in Cisplatin-Resistant Cell Lines. Nat. Prod. Commun. 2014;9:1934578X1400901. doi: 10.1177/1934578X1400901015. [DOI] [PubMed] [Google Scholar]
- 51.Huh H.-C., Lee S.-Y., Lee S.-K., Park N.H., Han I.-S. Capsaicin Induces Apoptosis of Cisplatin-Resistant Stomach Cancer Cells by Causing Degradation of Cisplatin-Inducible Aurora-A Protein. Nutr. Cancer. 2011;63:1095–1103. doi: 10.1080/01635581.2011.607548. [DOI] [PubMed] [Google Scholar]
- 52.Costa A., Forte I., Pentimalli F., Iannuzzi C., Alfano L., Capone F., Camerlingo R., Calabrese A., von Arx C., Benot Dominguez R., et al. Pharmacological inhibition of CDK4/6 impairs diffuse pleural mesothelioma 3D spheroid growth and reduces viability of cisplatin-resistant cells. Front. Oncol. 2024;14:1418951. doi: 10.3389/fonc.2024.1418951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Di Marzo D., Forte I.M., Indovina P., Di Gennaro E., Rizzo V., Giorgi F., Mattioli E., Iannuzzi C.A., Budillon A., Giordano A., et al. Pharmacological targeting of p53 through RITA is an effective antitumoral strategy for malignant pleural mesothelioma. Cell Cycle. 2014;13:652–665. doi: 10.4161/cc.27546. [DOI] [PubMed] [Google Scholar]
- 54.Ventura E., Iannuzzi C.A., Pentimalli F., Giordano A., Morrione A. RBL1/p107 Expression Levels Are Modulated by Multiple Signaling Pathways. Cancers. 2021;13:5025. doi: 10.3390/cancers13195025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arnedo Alejandra Wound Healing Size Tool. [(accessed on 6 November 2022)]. Available online: https://github.com/AlejandraArnedo/Wound-healing-size-tool/wiki#reference.
- 56.Suarez-Arnedo A., Torres Figueroa F., Clavijo C., Arbeláez P., Cruz J.C., Muñoz-Camargo C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE. 2020;15:e0232565. doi: 10.1371/journal.pone.0232565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ventura E., Xie C., Buraschi S., Belfiore A., Iozzo R., Giordano A., Morrione A. Complexity of progranulin mechanisms of action in mesothelioma. J. Exp. Clin. Cancer Res. 2022;41:333. doi: 10.1186/s13046-022-02546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Facchetti G., Petrella F., Spaggiari L., Rimoldi I. Malignant Pleural Mesothelioma: State of the art and advanced cell therapy. Eur. J. Med. Chem. 2017;142:266–270. doi: 10.1016/j.ejmech.2017.07.063. [DOI] [PubMed] [Google Scholar]
- 59.Ocak B. The Importance of Platinum Sensitivity in Metastatic Malignant Pleural Mesothelioma Patients. Eurasian J. Med. Investig. 2021;5:89–94. doi: 10.14744/ejmi.2020.39466. [DOI] [Google Scholar]
- 60.Yamaguchi H., Wyckoff J., Condeelis J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005;17:559–564. doi: 10.1016/j.ceb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 61.Novikov N.M., Zolotaryova S.Y., Gautreau A.M., Denisov E.V. Mutational drivers of cancer cell migration and invasion. Br. J. Cancer. 2021;124:102–114. doi: 10.1038/s41416-020-01149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meirson T., Gil-Henn H., Samson A.O. Invasion and metastasis: The elusive hallmark of cancer. Oncogene. 2020;39:2024–2026. doi: 10.1038/s41388-019-1110-1. [DOI] [PubMed] [Google Scholar]
- 63.Wu J., Jiang J., Chen B., Wang K., Tang Y., Liang X. Plasticity of cancer cell invasion: Patterns and mechanisms. Transl. Oncol. 2021;14:100899. doi: 10.1016/j.tranon.2020.100899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen K.-B., Huang Y.-J., Huang Y., Wu Z.-W., Jin X.-L., Zhang H., Xiang X.-P., Chen L., Chen L. Metastasis of Sarcomatoid Malignant Mesothelioma With p16/CDKN2A Deletion Manifested as a Subcutaneous Mass in the Back: A Case Report and Review of Literature. Int. J. Surg. Pathol. 2021;29:856–863. doi: 10.1177/10668969211005094. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto J., Ueta K., Takenaka M., Takahashi M., Nishizawa S. Sarcomatoid Malignant Mesothelioma Presenting with Intramedullary Spinal Cord Metastasis: A Case Report and Literature Review. Glob. Spine J. 2014;4:115–120. doi: 10.1055/s-0033-1361589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nishikubo M., Kin Y., Tane S., Nakamura K., Miyagi Y., Miura A., Nishio W., Senzaki H., Uchino K. Cellular cannibalism and consequent thrombocytopenia in a patient with bone marrow metastasis of malignant pleural mesothelioma: A case report. Mol. Clin. Oncol. 2021;15:163. doi: 10.3892/mco.2021.2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sternbach D.S., Henderson D.L., Mehrotra D.B., Fantasia D.J. Oral metastasis of pleural sarcomatoid mesothelioma, a rare aggressive variant of mesothelioma and a diagnostic challenge. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2022;133:e153. doi: 10.1016/j.oooo.2021.12.094. [DOI] [Google Scholar]
- 68.van der Bij S., Koffijberg H., Burgers J.A., Baas P., van de Vijver M.J., de Mol B.A.J.M., Moons K.G.M. Prognosis and prognostic factors of patients with mesothelioma: A population-based study. Br. J. Cancer. 2012;107:161–164. doi: 10.1038/bjc.2012.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Clark R., Lee S.-H. Anticancer Properties of Capsaicin Against Human Cancer. Anticancer Res. 2016;36:837–843. [PubMed] [Google Scholar]
- 70.Li H., Krstin S., Wang S., Wink M. Capsaicin and Piperine Can Overcome Multidrug Resistance in Cancer Cells to Doxorubicin. Molecules. 2018;23:557. doi: 10.3390/molecules23030557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang Y., Chen M., Yu H., Yuan G., Luo L., Xu X., Xu Y., Sui X., Leung E.L.-H., Wu Q. The Role and Mechanisms of Action of Natural Compounds in the Prevention and Treatment of Cancer and Cancer Metastasis. Front. Biosci. 2022;27:192. doi: 10.31083/j.fbl2706192. [DOI] [PubMed] [Google Scholar]
- 72.Qi C., Wang D., Gong X., Zhou Q., Yue X., Li C., Li Z., Tian G., Zhang B., Wang Q., et al. Co-Delivery of Curcumin and Capsaicin by Dual-Targeting Liposomes for Inhibition of aHSC-Induced Drug Resistance and Metastasis. ACS Appl. Mater. Interfaces. 2021;13:16019–16035. doi: 10.1021/acsami.0c23137. [DOI] [PubMed] [Google Scholar]
- 73.Jin J., Lin G., Huang H., Xu D., Yu H., Ma X., Zhu L., Ma D., Jiang H. Capsaicin Mediates Cell Cycle Arrest and Apoptosis in Human Colon Cancer Cells via Stabilizing and Activating p53. Int. J. Biol. Sci. 2014;10:285–295. doi: 10.7150/ijbs.7730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yoon J.-H., Ahn S.-G., Lee B.-H., Jung S.-H., Oh S.-H. Role of autophagy in chemoresistance: Regulation of the ATM-mediated DNA-damage signaling pathway through activation of DNA–PKcs and PARP-1. Biochem. Pharmacol. 2012;83:747–757. doi: 10.1016/j.bcp.2011.12.029. [DOI] [PubMed] [Google Scholar]
- 75.Ferreira A.K., Tavares M.T., Pasqualoto K.F.M., de Azevedo R.A., Teixeira S.F., Ferreira-Junior W.A., Bertin A.M., De-Sá-Junior P.L., Barbuto J.A.M., Figueiredo C.R., et al. RPF151, a novel capsaicin-like analogue: In vitro studies and in vivo preclinical antitumor evaluation in a breast cancer model. Tumor Biol. 2015;36:7251–7267. doi: 10.1007/s13277-015-3441-z. [DOI] [PubMed] [Google Scholar]
- 76.Khan A., Naaz F., Basit R., Das D., Bisht P., Shaikh M., Lone B.A., Pokharel Y.R., Ahmed Q.N., Parveen S., et al. 1,2,3-Triazole Tethered Hybrid Capsaicinoids as Antiproliferative Agents Active against Lung Cancer Cells (A549) ACS Omega. 2022;7:32078–32100. doi: 10.1021/acsomega.2c03325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li B.-H., Yuan L. Inhibitory effects of capsaicin on migration and invasion of breast cancer MDA-MB-231 cells and its mechanism. Sheng Li Xue Bao. 2017;69:183–188. [PubMed] [Google Scholar]
- 78.Hwang Y.P., Yun H.J., Choi J.H., Han E.H., Kim H.G., Song G.Y., Kwon K., Jeong T.C., Jeong H.G. Suppression of EGF-induced tumor cell migration and matrix metalloproteinase-9 expression by capsaicin via the inhibition of EGFR-mediated FAK/Akt, PKC/Raf/ERK, p38 MAPK, and AP-1 signaling. Mol. Nutr. Food Res. 2011;55:594–605. doi: 10.1002/mnfr.201000292. [DOI] [PubMed] [Google Scholar]
- 79.Islam A., Yang Y.-T., Wu W.-H., Chueh P.J., Lin M.-H. Capsaicin attenuates cell migration via SIRT1 targeting and inhibition to enhance cortactin and β-catenin acetylation in bladder cancer cells. Am. J. Cancer Res. 2019;9:1172–1182. [PMC free article] [PubMed] [Google Scholar]
- 80.Justus C.R., Leffler N., Ruiz-Echevarria M., Yang L.V. In vitro cell migration and invasion assays. J. Vis. Exp. 2014;88:51046. doi: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kramer N., Walzl A., Unger C., Rosner M., Krupitza G., Hengstschläger M., Dolznig H. In vitro cell migration and invasion assays. Mutat. Res. Mutat. Res. 2013;752:10–24. doi: 10.1016/j.mrrev.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Liang C.-C., Park A.Y., Guan J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 83.Quijano Moreno S.L., García de Lacoba M. Metastasis of malignant pleural mesothelioma to the scalp following chemotherapy: A case report and review of the literature. Rev. Española Patol. 2022;55:S27–S31. doi: 10.1016/j.patol.2020.02.008. [DOI] [PubMed] [Google Scholar]
- 84.Naldi G., Bergomi S., Visca P., Cecere F. Ovarian metastasis from malignant pleural mesothelioma. Tumori J. 2020;106:NP49–NP51. doi: 10.1177/0300891620941610. [DOI] [PubMed] [Google Scholar]
- 85.Meyerhoff R.R., Yang C.F.J., Speicher P.J., Gulack B.C., Hartwig M.G., D’Amico T.A., Harpole D.H., Berry M.F. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J. Surg. Res. 2015;196:23–32. doi: 10.1016/j.jss.2015.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verma V., Ahern C.A., Berlind C.G., Lindsay W.D., Shabason J., Sharma S., Culligan M.J., Grover S., Friedberg J.S., Simone C.B. Survival by Histologic Subtype of Malignant Pleural Mesothelioma and the Impact of Surgical Resection on Overall Survival. Clin. Lung Cancer. 2018;19:e901–e912. doi: 10.1016/j.cllc.2018.08.007. [DOI] [PubMed] [Google Scholar]
- 87.Yang H., Testa J.R., Carbone M. Mesothelioma epidemiology, carcinogenesis, and pathogenesis. Curr. Treat. Options Oncol. 2008;9:147–157. doi: 10.1007/s11864-008-0067-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li C., Rezov V., Joensuu E., Vartiainen V., Rönty M., Yin M., Myllärniemi M., Koli K. Pirfenidone decreases mesothelioma cell proliferation and migration via inhibition of ERK and AKT and regulates mesothelioma tumor microenvironment in vivo. Sci. Rep. 2018;8:10070. doi: 10.1038/s41598-018-28297-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu W.-F., Liu F., Ma Y.-C., Qian Z.-R., Shi L., Mu H., Ding F., Fu X.-Q., Li X.-H. Baicalin Regulates Proliferation, Apoptosis, Migration, and Invasion in Mesothelioma. Med. Sci. Monit. 2019;25:8172–8180. doi: 10.12659/MSM.919872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Laszlo V., Valko Z., Kovacs I., Ozsvar J., Hoda M.A., Klikovits T., Lakatos D., Czirok A., Garay T., Stiglbauer A., et al. Nintedanib is active in malignant pleural mesothelioma cell models and inhibits angiogenesis and tumor growth in vivo. Clin. Cancer Res. 2018;24:3729–3740. doi: 10.1158/1078-0432.CCR-17-1507. [DOI] [PubMed] [Google Scholar]
- 91.Shin D.-H., Kim O.-H., Jun H.-S., Kang M.-K. Inhibitory effect of capsaicin on B16-F10 melanoma cell migration via the phosphatidylinositol 3-kinase/Akt/Rac1 signal pathway. Exp. Mol. Med. 2008;40:486. doi: 10.3858/emm.2008.40.5.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu Y., Kong W., Zhao S., Xiong D., Wang Y. Capsaicin enhances cisplatin-induced anti-metastasis of nasopharyngeal carcinoma by inhibiting EMT and ERK signaling via serpin family B member 2. Carcinogenesis. 2024;45:556–568. doi: 10.1093/carcin/bgae032. [DOI] [PubMed] [Google Scholar]
- 93.Dai N., Ye R., He Q., Guo P., Chen H., Zhang Q. Capsaicin and sorafenib combination treatment exerts synergistic anti-hepatocellular carcinoma activity by suppressing EGFR and PI3K/Akt/mTOR signaling. Oncol. Rep. 2018;40:3235–3248. doi: 10.3892/or.2018.6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen J.-C., Ko J.-C., Yen T.-C., Chen T.-Y., Lin Y.-C., Ma P.-F., Lin Y.-W. Capsaicin enhances erlotinib-induced cytotoxicity via AKT inactivation and excision repair cross-complementary 1 (ERCC1) down-regulation in human lung cancer cells. Toxicol. Res. 2019;8:459–470. doi: 10.1039/C8TX00346G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li Z., Zhao Q., Liu X., Zhou X., Wang Y., Zhao M., Wu F., Zhao G., Guo X. Capsaicin combined with cisplatin inhibits TGF-β1-induced EMT and TSCC cells migration via the Claudin-1/PI3K/AKT/mTOR signaling pathway. Cancer Cell Int. 2024;24:300. doi: 10.1186/s12935-024-03485-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.YANG J., LI T.Z., XU G.H., LUO B.B., CHEN Y.X., ZHANG T. Low-concentration capsaicin promotes colorectal cancer metastasis by triggering ROS production and modulating Akt/mTOR and STAT-3 pathways. Neoplasma. 2013;60:364–372. doi: 10.4149/neo_2013_048. [DOI] [PubMed] [Google Scholar]
- 97.Luján-Méndez F., Roldán-Padrón O., Castro-Ruíz J., López-Martínez J., García-Gasca T. Capsaicinoids and Their Effects on Cancer: The “Double-Edged Sword” Postulate from the Molecular Scale. Cells. 2023;12:2573. doi: 10.3390/cells12212573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu N.-C., Hsieh P.-F., Hsieh M.-K., Zeng Z.-M., Cheng H.-L., Liao J.-W., Chueh P.J. Capsaicin-Mediated tNOX (ENOX2) Up-regulation Enhances Cell Proliferation and Migration in Vitro and in Vivo. J. Agric. Food Chem. 2012;60:2758–2765. doi: 10.1021/jf204869w. [DOI] [PubMed] [Google Scholar]
- 99.Hatono M., Ikeda H., Suzuki Y., Kajiwara Y., Kawada K., Tsukioki T., Kochi M., Suzawa K., Iwamoto T., Yamamoto H., et al. Effect of isoflavones on breast cancer cell development and their impact on breast cancer treatments. Breast Cancer Res. Treat. 2021;185:307–316. doi: 10.1007/s10549-020-05957-z. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y., Liu H., Zheng Q., Li H., You H., Feng Y., Feng W. Promotion of tumor progression induced by continuous low-dose administration of antineoplastic agent gemcitabine or gemcitabine combined with cisplatin. Life Sci. 2022;306:120826. doi: 10.1016/j.lfs.2022.120826. [DOI] [PubMed] [Google Scholar]
- 101.Zhang R., Humphreys I., Sahu R.P., Shi Y., Srivastava S.K. In vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis. 2008;13:1465–1478. doi: 10.1007/s10495-008-0278-6. [DOI] [PubMed] [Google Scholar]
- 102.Popescu G.D.A., Scheau C., Badarau I.A., Dumitrache M.-D., Caruntu A., Scheau A.-E., Costache D.O., Costache R.S., Constantin C., Neagu M., et al. The Effects of Capsaicin on Gastrointestinal Cancers. Molecules. 2020;26:94. doi: 10.3390/molecules26010094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu T., Wang G., Tao H., Yang Z., Wang Y., Meng Z., Cao R., Xiao Y., Wang X., Zhou J. Capsaicin mediates caspases activation and induces apoptosis through P38 and JNK MAPK pathways in human renal carcinoma. BMC Cancer. 2016;16:790. doi: 10.1186/s12885-016-2831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jung M.-Y., Kang H.-J., Moon A. Capsaicin-induced apoptosis in SK-Hep-1 hepatocarcinoma cells involves Bcl-2 downregulation and caspase-3 activation. Cancer Lett. 2001;165:139–145. doi: 10.1016/S0304-3835(01)00426-8. [DOI] [PubMed] [Google Scholar]
- 105.Cömertpay S., Demirbanka F.G. Lowered Cyclin E levels increase the efficiency and the specificity of capsaicin against cancerous cells of mesothelium. Cell. Mol. Biol. 2020;66:98–104. doi: 10.14715/cmb/2020.66.6.18. [DOI] [PubMed] [Google Scholar]
- 106.Chou C., Wu Y., Wang Y., Chou M., Kuo S., Chen D. Capsaicin-induced apoptosis in human breast cancer MCF-7 cells through caspase-independent pathway. Oncol. Rep. 2009;21:665–671. doi: 10.3892/or_00000269. [DOI] [PubMed] [Google Scholar]
- 107.Lee M.-J., Kee K.-H., Suh C.-H., Lim S.-C., Oh S.-H. Capsaicin-induced apoptosis is regulated by endoplasmic reticulum stress- and calpain-mediated mitochondrial cell death pathways. Toxicology. 2009;264:205–214. doi: 10.1016/j.tox.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 108.Acklin S., Xia F. The Role of Nucleotide Excision Repair in Cisplatin-Induced Peripheral Neuropathy: Mechanism, Prevention, and Treatment. Int. J. Mol. Sci. 2021;22:1975. doi: 10.3390/ijms22041975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Shin Y., Kim B., Kim W. Cisplatin-Induced Nausea and Vomiting: Effect of Herbal Medicines. Plants. 2022;11:3395. doi: 10.3390/plants11233395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bright H., Singh A., Joel A., Georgy J., John A., Rajkumar P., Jiji H., Stehno-Bittel L., Samuel P., Chandy S. Randomized Placebo-Controlled Trial of Topical Capsaicin for Delayed Chemotherapy-Induced Nausea and Vomiting. JCO Glob. Oncol. 2024;10:e2400130. doi: 10.1200/GO.24.00130. [DOI] [PubMed] [Google Scholar]
- 111.Moreno-Alonso D., Llorens-Torromé S., Corcoy de Febrer B., Amandi García M., Serrano-Bermúdez G., Trelis-Navarro J., Mayoral-Rojals V., Serrano-Afonso A. Adhesive capsaicin 8% patch for improved control of pain caused by chemotherapy-induced peripheral neuropathy in patients with multiple myeloma: A single-centre, seven-case series. J. Oncol. Pharm. Pract. 2024;30:752–758. doi: 10.1177/10781552241230887. [DOI] [PubMed] [Google Scholar]
- 112.Al-Samydai A., Alshaer W., Al-Dujaili E.A.S., Azzam H., Aburjai T. Preparation, Characterization, and Anticancer Effects of Capsaicin-Loaded Nanoliposomes. Nutrients. 2021;13:3995. doi: 10.3390/nu13113995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rollyson W.D., Stover C.A., Brown K.C., Perry H.E., Stevenson C.D., McNees C.A., Ball J.G., Valentovic M.A., Dasgupta P. Bioavailability of capsaicin and its implications for drug delivery. J. Control. Release. 2014;196:96–105. doi: 10.1016/j.jconrel.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhu Y., Zhang J., Zheng Q., Wang M., Deng W., Li Q., Firempong C.K., Wang S., Tong S., Xu X., et al. In vitro and in vivo evaluation of capsaicin-loaded microemulsion for enhanced oral bioavailability. J. Sci. Food Agric. 2015;95:2678–2685. doi: 10.1002/jsfa.7002. [DOI] [PubMed] [Google Scholar]
- 115.Chanda S., Bashir M., Babbar S., Koganti A., Bley K. In Vitro Hepatic and Skin Metabolism of Capsaicin. Drug Metab. Dispos. 2008;36:670–675. doi: 10.1124/dmd.107.019240. [DOI] [PubMed] [Google Scholar]
- 116.Chaiyasit K., Khovidhunkit W., Wittayalertpanya S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thai. 2009;92:108–113. [PubMed] [Google Scholar]
- 117.Harada N., Okajima K., Arai M., Kurihara H., Nakagata N. Administration of capsaicin and isoflavone promotes hair growth by increasing insulin-like growth factor-I production in mice and in humans with alopecia. Growth Horm. IGF Res. 2007;17:408–415. doi: 10.1016/j.ghir.2007.04.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.