Abstract
Background/Objectives: S-adenosylmethionine (SAMe) is a natural compound implicated in the treatment of liver dysfunction. In this systematic review, our objective was to determine the efficacy, safety, and optimal dose of SAMe in liver diseases. Methods: Using the PRISMA methodology, we searched PubMed, CINAHL, and Web of Science using key MeSH search terms. For title/abstract screening, full-text review, and data extraction, two independent researchers reviewed articles, and a third researcher resolved conflicts. Data extraction also included a quality assessment of included articles. Results: Of the 1881 non-duplicated studies, 15 articles focusing on SAMe use in the liver were included. All included studies (n = 15) scored a 4 or 5 out of 5 points on the quality assessment, which indicated high study quality. Overall, SAMe was effective in improving liver-related parameters with few adverse events, which were primarily mild, transient gastrointestinal complaints. Conclusions: The most common doses were SAMe 1000 mg or 1200 mg per day with or without another treatment or natural supplement. Future studies are needed to assess long-term efficacy and safety data of SAMe and the optimal route of administration in liver diseases.
Keywords: S-adenosylmethionine, SAMe, liver, nutraceutical
1. Introduction
The negative impact of liver disease has increased worldwide, ranking as the eleventh leading cause of death globally and the tenth in the United States [1,2]. Of these liver-related deaths, half are attributed to cirrhosis and the other half to both viral hepatitis and hepatocellular carcinoma (HCC) [1]. Death specifically due to liver cancer, such as HCC, has risen at drastic rates, increasing by 43% in U.S. adults 25 years and older between 2000 and 2016 [3]. Furthermore, the prevalence of non-alcoholic fatty liver disease (NAFLD), a leading contributor of chronic liver disease, has also increased alarmingly over the last couple decades, affecting 32% across the world and up to 47.8% in the United States [4,5].
The pharmacological treatment of liver diseases may include antivirals, immunosuppressants, and/or medications for symptom management [6]. However, challenges with these drug therapies can arise due to complications such as increased risk of infection, metabolic abnormalities, and even development of antiviral resistance. Therefore, with the increasing burden of liver disease, there is a need for treatment options that are both safe and effective in managing their complications.
S-adenosylmethionine (SAMe) is a compound that can be delivered in a supplement that is recognized for its positive effects across many physiological systems [7,8]. SAMe is synthesized in the liver from L-methionine and adenosine triphosphate (ATP), playing a crucial role as a primary methyl donor required for numerous biological functions [7]. In addition, it is a known precursor to glutathione, which establishes the antioxidant potential of SAMe in liver injury and disease [9]. The beneficial effects of SAMe in specific liver diseases has not been fully established in humans. However, preliminary studies of SAMe are promising, especially in improving liver parameters in fatty liver disease, hepatitis, and HCC [10]. In the liver, a reduction in SAMe levels affects lipid metabolism, contributing to the development of hepatic steatosis, injury, and even cancer [11]. Furthermore, SAMe has been shown to be reduced in chronic liver diseases, such as hepatic cirrhosis and HCC [9]. However, many available studies of SAMe use for liver diseases in humans are small or have suboptimal methodology, making an updated review of the literature necessary to assess our current understanding of SAMe in the liver. Therefore, we conducted a systematic review to evaluate the safety, efficacy, and optimal dose of SAMe in liver-related diseases.
2. Methods
For this systematic review, the PRISMA methodology was used and complied with. Details of the PRISMA checklist can be found in the Supplementary Material [12]. Aligning with the research objective, an initial search strategy was identified and a research librarian was consulted to refine the search strategy. The following MeSH search terms were identified and utilized: “S-Adenosylmethionine AND Liver”. The research librarian refined and used these terms in PubMed, CINAHL, and Web of Science to determine the breadth and accuracy of the search. The final search terms were reviewed by the research team, and the research librarian performed the search for 1 January 2004–17 April 2024. Zotero (v 7.0, Fairfax, VA, USA) was used to clean the search results (removing 2 retracted articles) before uploading them into a systematic review management software, Covidence (Melbourne, VIC, Australia). Covidence automatically removed any duplicates.
Before beginning the review process, the senior investigator (AC) trained the research team on the software platform (Covidence) and the protocol. Covidence was utilized for the entire systematic review process. At each phase of the systematic review, two individuals independently conducted this step, with the senior investigator serving in the role of resolving conflicts and checking for consistency in protocol application. Two reviewers had to select “yes” for a study to proceed to the next phase or “no” for a study to be excluded. In the first phase, reviewers could select “maybe”, which would allow for a study to proceed forward to full-text review for further review.
Articles were included if they were research studies, written in English, and contained human subjects. Alternatively, articles were excluded if they were not research articles (ex: review articles, expert opinion, commentaries, or guidelines). Key information that articles had to contain related to the study aims were the use of SAMe in the study and the examination of a liver-related condition.
In Phase I, the screening of titles and abstracts was performed in terms of their adherence to the inclusion criteria and study aims. In Phase II, full-text articles were uploaded into Covidence and evaluated. If a study was excluded in this phase, reviewers had to select a reason for exclusion. In Phase III, the included articles underwent data extraction. A template was pre-built in Covidence to collect data. Given the diversity of study designs, quality assessment was performed using the MMAT (Mixed Methods Appraisal Tool) [13]. If data were missing, this was noted in the final tables. Before finalizing the work, the research team reviewed and approved the final data extraction tables.
3. Results
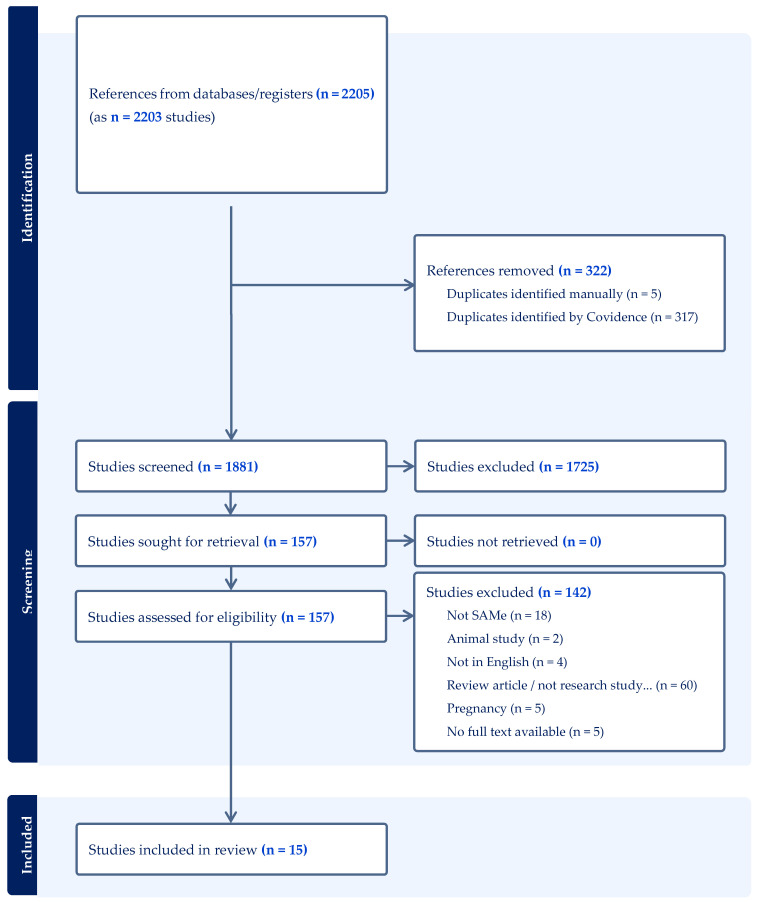
From our search, 2207 articles were identified across several databases (PubMed = 521, CINAHL = 173, Web of Science = 1513). Following the elimination of duplicates, 1881 articles were included in this review. Figure 1 shows the PRISMA flow diagram outlining the study methodology, which led to data extraction from 15 articles.
Figure 1.
PRISMA [12] diagram overviewing study inclusion and exclusion process.
Study Characteristics
Across the 15 articles reviewed [14,15,16,17,18,19,20,21,22,23,24,25,26,27,28], there were 1799 participants, excluding systematic reviews. In studies that reported gender, 69.7% of participants were male. Regarding the duration of the studies, the median study length was 8 weeks, while the average study length was 21.2 weeks.
Liver-related diseases included in the studies were fatty liver disease (both alcoholic and non-alcoholic), neonatal jaundice, cholestatic liver disease, chronic liver disease, chemotherapy-induced liver disease, hepatitis B virus (HBV)-related HCC, hepatitis C, viral hepatitis, and primary biliary cholangitis. Efficacy was assessed using various measures, including liver function [aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), total bilirubin (TBil), gamma-glutamyl-transpeptidase (GGT), alkaline phosphatase (ALP), albumin, etc.], liver fat content, liver morphology, and varied inflammatory mediators/indicators.
Table 1 provides an overview and summary of the outcomes. Overall, positive changes in liver markers were found in all 15 studies with minimal to no adverse effects. SAMe dosing in liver diseases ranged from 200 mg to 2400 mg per day, with the most common doses being 1000 mg to 1200 mg per day. Three studies reported titration up to the target dose.
Table 1.
Overview of study findings related to safety and efficacy.
| Condition | Efficacy Summary | Safety Summary | Dosing Ranges |
|---|---|---|---|
| Liver-Related |
|
|
|
A more comprehensive overview of study characteristics can be found in Table 2. This includes the specific liver disease, interventions, and measurements used to assess liver function. Comparators included placebo, a different nutraceutical (e.g., Si Mo Tang), chemotherapy, or conventional symptomatic treatment.
Table 2.
Characteristics of included studies examining the role of SAMe in liver diseases.
| Author (Year) Study Design | Location N of Patients | Study Length |
Intervention (with Dose) and Comparator | Disease | Measurement of Liver Function |
|---|---|---|---|
| Benic (2022) [14] SR n = 28 articles (3 articles on SAMe used for analysis) N/A Varying lengths |
SAMe: 1200 mg/day in each study | Metastatic colorectal cancer (1 study) Cancer chemotherapy-induced liver toxicity (2 studies) |
AST, ALT, LDH, TBil, GGT, and ALP |
| Ferro (2022) [23] RCT n = 140 (127 completed) Europe 12 weeks |
Nutraceutical capsule daily (curcumin complex, ω-3 PUFAs, BPF, artichoke leaf extract, black seed oil, pricoliv, GHS, SAMe 200 mg and other natural ingredients) Comparator: placebo |
Non-alcoholic fatty liver disease | Liver fat content (CAP score) |
| Guo (2015) [16] SR/MA n = 705 participants across 11 studies N/A Varying lengths |
SAMe: 20–30 mg/kg/day (400–1200 mg/day) | Chronic liver diseases | Liver function |
| Guo (2016) [17] Non-randomized experimental n = 697 China 24 months |
All: magnesium isoglycyrrhizinate 100 mg/day Group A and C received: SAMe 1000 mg IV (3 days pre-surgery to 7 days post-surgery) then 1500 mg/day Groups B and D received: placebo |
HBV-Related HCC Group A, B: Early stage Group C, D: Advanced stage |
Liver function |
| Le (2013) [20] RCT n = 14 United States 24 weeks |
SAMe: 400 mg/day Comparator: placebo |
Alcoholic liver disease | Liver morphology |
| Li (2022) [19] Non-randomized experimental n = 149 China 10 days |
Group A: 500 mg SAMe/day Group B: Si Mo Tang Group C: 500 mg SAMe + Si Mo Tang/day |
Neonatal jaundice | Liver function, cardiac enzymes, immune function, serum transferrin (TRF), and C-reactive protein (CRP) levels |
| Liu (2014) [18] RCT n = 81 China Up to 5 days |
Pre-Treatment: SAMe 1000 mg 2 h before surgery and 5 post-op days Post-Treatment: 1000 mg 6 h after surgery for 5 days Comparator: placebo |
HBV-Related HCC requiring resection | ALT, AST, TBil, DBIL |
| Lu (2020) [26] Non-randomized experimental study n = 177 China 1 month |
Group A: ursodeoxycholic acid 15 mg/day + SAMe 1200 mg IV per day Group B: ursodeoxycholic acid 15 mg/day + SAMe 800 mg IV per day Group C (Comparator): ursodeoxycholic acid 15 mg/day |
Cholestatic liver disease | ALT AST TBil |
| Medici (2011) [21] RCT n = 37 United States 24 weeks |
SAMe: 1200 mg/day Comparator: placebo |
Alcoholic liver disease | AST, ALT, bilirubin |
| Morgan (2015) [25] Phase II RCT n = 110 United States 24 weeks |
SAMe: 800 mg/day (4 weeks) increased to 1600 mg/d (4 weeks) increased to 2400 mg/day (16 weeks) Comparator: placebo |
Hepatitis C | Liver: AFP Well-being: SF-36 |
| Qiao (2021) [24] n = 137 Non-randomized experimental study China 14 days |
SAMe: 1000 mg/day injection Comparator: conventional symptomatic treatment |
Viral hepatitis | Albumin ALT AST TBil Liver fiber indicators IL-6 TNF-α |
| Tkachenko (2016) [22] RCT n = 40 Russia 28 days |
Prednisolone + SAMe: 800 mg/day IV for 7 days, then 1200 mg/day oral for 8 weeks Outcomes assessed at 28 days Comparator: prednisolone + placebo |
Severe alcoholic hepatitis | Response rate Liver enzymes: ALT, AST, GGT, ALP and bilirubin |
| Vincenzi (2012) [15] Retrospective analysis n = 78 Europe Varied length due to length of chemotherapy |
Chemotherapy + SAMe: 800 mg/day Comparator: chemotherapy |
Metastatic colorectal cancer: oxaliplatin-induced liver toxicity | Course Delays LFTs: AST, ALT, LDH, TBil, GGT |
| Wunsch (2018) [27] 12 months Non-randomized experimental study n = 24 (18 completed) Europe |
UDCA + SAMe: 1200 mg/day Comparator: UDCA |
Primary biliary cholangitis | ALT AST ALP GGT TBil Albumin INR Total cholesterol |
| Yang (2021) [28] RCT n = 115 China 2 weeks |
UDCA + SAMe: 2000 mg/day IV Comparator: UDCA + SAMe 1000 mg/day IV |
Cholestatic liver disease | Itching improvement ALT AST TBil TBA IL-12 IL-18 |
Acronyms: systematic review (SR), meta-analysis (MA), randomized controlled trial (RCT), hepatitis B virus (HBV), hepatocellular carcinoma (HCC), controlled attenuation parameter (CAP), total bile acid (TBA), alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), a-fetoprotein (AFP), lactate dehydrogenase (LDH), total bilirubin (TBil), direct bilirubin (DBIL), gamma-glutamyl-transpeptidase (GGT), international normalized ratio (INR), tumor necrosis factor alpha (TNF-α), interleukin (IL), polyunsaturated fatty acids (PUFAs), bergamot polyphenol fraction (BPF), glutathione (GHS), ursodeoxycholic acid (UDCA).
Of our 15 included studies, there were 3 articles that evaluated alcoholic liver disease, 3 for cholestatic liver disease, 2 for HCC, 2 for viral hepatitis, 2 for chemotherapy-induced liver toxicity, 1 for non-alcoholic liver disease, 1 for neonatal jaundice, and 1 for chronic liver diseases.
For dosing, strategies differed based on the specific liver disease. For alcoholic liver diseases, doses were between 400 mg and 1200 mg per day, with higher doses in severe alcoholic hepatitis. Higher doses of 800 to 2000 mg per day were seen in cholestatic liver disease. HCC was treated with doses of 1000 mg per day with one study increasing to 1500 mg per day after 10 days. In viral hepatitis, initial doses of 800 mg to 1000 mg per day were given, which increased up to 2400 mg per day after 8 weeks in hepatitis C. Chemotherapy-induced liver toxicity was treated with doses of 1200 mg to 1800 mg per day. The smallest doses were used for non-alcoholic liver disease and neonatal jaundice at 200 mg per day and 500 mg per day, respectively. In the article looking at seven different chronic liver diseases, doses between 400 mg and 1200 mg per day (20–30 mg/kg/day) were seen. Five of our included articles evaluated intravenous formulations of SAMe.
The most common measurements of liver function used in the studies were AST and ALT (n = 12 studies) and TBil or bilirubin (n = 12). Among the other liver measurements, immune function and inflammatory markers were also used.
Table 3 provides the study outcomes results for efficacy, while Table 4 shows safety results. Table 5 shows the quality assessments. All 15 studies included efficacy data and 11 studies included safety data. Improvements in liver function were seen in all 15 studies. SAMe significantly improved liver function in all studies evaluating chemotherapy-induced liver toxicity, HCC, and viral hepatitis. It also demonstrated significant beneficial effects on liver fat content in non-alcoholic fatty liver disease. For alcoholic liver disease, significant improvements were seen in two of the three studies, with one study showing significant improvement only in smooth muscle actin. The combination of SAMe and Si Mo Tang in neonatal jaundice demonstrated an effective rate of 96%, which was significantly higher than either agent alone. For cholestatic liver diseases, significant improvements were seen in both liver function and symptoms of itching.
Table 3.
Study efficacy outcomes for studies examining the use of SAMe in liver diseases.
| Author (Year) | Efficacy |
|---|---|
| Benic (2022) [14] | Positive improvement in liver function with SAMe treatment in all three studies (p at least ≤ 0.04) |
| Ferro (2022) [23] | Greater CAP score reduction in the nutraceutical group vs. placebo (−34 ± 5 dB/m vs. −20 ± 5 dB/m, respectively; p = 0.045) More improvements seen in the following:
|
| Guo (2015) [16] |
|
| Guo (2016) [17] | Positive impact of SAMe on Group C vs. D:
|
| Le (2013) [20] | SAMe vs. placebo:
|
| Li (2022) [19] |
|
| Liu (2014) [18] | Pre-treatment (SAMe) vs. post-treatment placebo:
|
| Lu (2020) [26] |
|
| Medici (2011) [21] | All patients:
|
| Morgan (2015) [25] | Liver:
|
| Qiao (2021) [24] | SAMe vs. comparator:
|
| Tkachenko (2016) [22] | Liver:
|
| Vincenzi (2012) [15] | SAMe:
|
| Wunsch (2018) [27] | SAMe:
|
| Yang (2021) [28] |
|
Acronyms: mean difference (MD), liver function tests (LFTs), alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), direct bilirubin (DBIL), gammaglutamyl-transpeptidase (GGT), interleukin (IL).
Table 4.
Study safety outcomes for studies examining the use of SAMe in liver diseases.
| Author (Year) | Safety |
|---|---|
| Benic (2022) [14] | Not discussed |
| Ferro (2022) [23] | One person dropped out within 12 weeks due to allergy, one to diarrhea, and three to abdominal discomfort (bloating, pain or cramps) in the nutraceutical group |
| Guo (2015) [16] | SAMe did not increase the number of adverse events or the death rate compared with the placebo: RR [95% CI] = 0.94 [0.59, 1.52], p = 0.81 |
| Guo (2016) [17] | Less postoperative complications in SAMe Group C vs. D (63/235 vs. 79/206, p < 0.01) |
| Le (2013) [20] | Not discussed |
| Li (2022) [19] | Not discussed |
| Liu (2014) [18] | No significant differences between groups |
| Lu (2020) [26] |
|
| Medici (2011) [21] |
|
| Morgan (2015) [25] |
|
| Qiao (2021) [24] | Total incidence of adverse effects:
|
| Tkachenko (2016) [22] | Hepatorenal syndrome (HRS) occurred in 20% in the prednisolone group (4 of 20 patients) while no HRS cases were registered in the prednisolone plus SAMe group (p = 0.035) |
| Vincenzi (2012) [15] | Not discussed |
| Wunsch (2018) [27] | Well-tolerated No severe adverse effects |
| Yang (2021) [28] | No significant differences in safety outcomes (p > 0.05) |
Regarding study quality assessments, all studies (n = 15) achieved a 4 or 5 out of 5 points using the MMAT Score. This indicates a high study quality for all our included articles.
Table 5.
Quality assessment of liver studies (n = 15 studies).
| Author Year | Clear Research Questions | Data Address Question | Total MMAT Score (Out of 5) |
|---|---|---|---|
| Benic (2022) [14] | Yes | Yes | 5 |
| Ferro (2022) [23] | Yes | Yes | 5 |
| Guo (2015) [16] | Yes | Yes | 5 |
| Guo (2016) [17] | Yes | Yes | 5 |
| Le (2013) [20] | Yes | Yes | 5 |
| Li (2022) [19] | Yes | Yes | 5 |
| Liu (2014) [18] | Yes | Yes | 5 |
| Lu (2020) [26] | Yes | Yes | 5 |
| Medici (2011) [21] | Yes | Yes | 4 |
| Morgan (2015) [25] | Yes | Yes | 5 |
| Qiao (2021) [24] | Yes | Yes | 5 |
| Tkachenko (2016) [22] | Yes | Yes | 4 |
| Vincenzi (2012) [15] | Yes | Yes | 5 |
| Wunsch (2018) [27] | Yes | Yes | 5 |
| Yang (2021) [28] | Yes | Yes | 4 |
In the 11 studies assessing safety, gastrointestinal symptoms had the most reported complaints. Five of these studies reported that adverse events were not significantly different between groups and three reported that adverse events were less in SAMe-treated groups.
4. Discussion
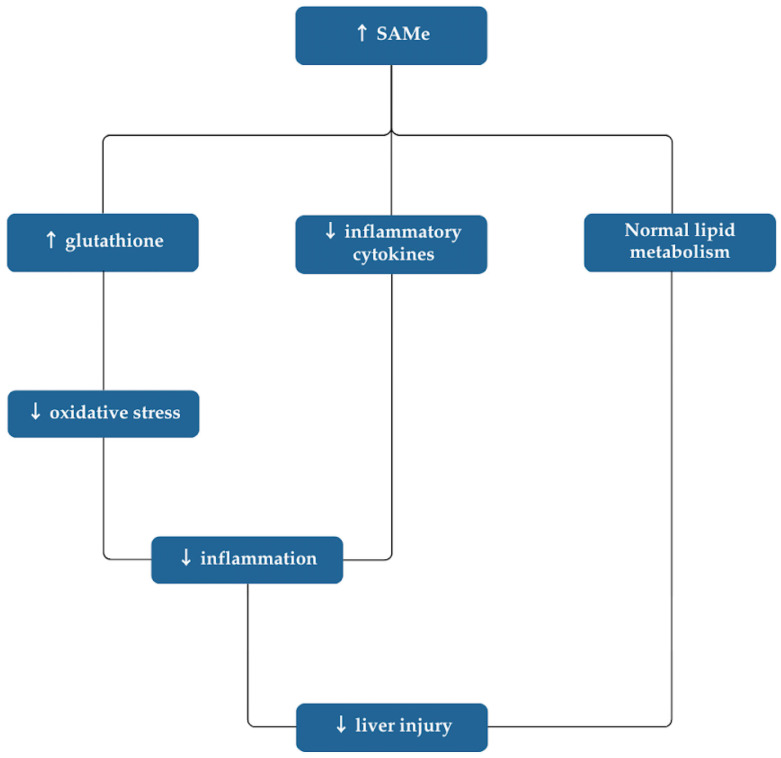
Based on our results, SAMe appears to exhibit therapeutic advantages in the treatment of liver diseases. Figure 2 illustrates the proposed mechanisms by which SAMe impacts the condition of the liver.
Figure 2.
Mechanism of SAMe in liver disease.
SAMe seemed to be particularly helpful after chemotherapy to improve both liver function and patient outcomes in metastatic colorectal cancer [14,15]. Other liver diseases that have benefited from SAMe use include non-alcoholic fatty liver disease, chronic liver diseases, HCC, and neonatal jaundice [14,15,16,17,18,19]. On the other hand, some studies report that SAMe may not be as beneficial in alcoholic liver disease [20,21]. Nevertheless, its beneficial effects on many hepatic measurements make its application widespread among liver diseases. Furthermore, SAMe may have synergistic effects in combination with other therapies, such as with prednisolone in alcoholic hepatitis or with Si Mo Tang in neonatal jaundice [19,22].
While most studies compared SAMe (or a combination) to placebo, a few directly compared SAMe or its combination to other supplements or conventional treatments. In the study comparing SAMe and Si Mo Tang in neonatal jaundice, there was no statistical significance between the two therapies; however, their combined effects had a significantly higher effective rate than either alone [19]. In viral hepatitis, SAMe also significantly outperformed conventional symptomatic treatment in improving liver function tests and response rates [24]. For combination regimens, the addition of SAMe to prednisolone in severe alcoholic hepatitis significantly increased the rate of responders compared to prednisolone alone [22]. Likewise, adding SAMe to chemotherapy significantly improved a number of assessments, including the need for course delay, grade of liver toxicity, and liver parameters (AST, ALT, TBil, and GGT) [15]. Ursodeoxycholic acid (UDCA), a common treatment for patients with cholestatic liver disease, combined with SAMe 1200 mg per day also showed to be significantly better compared to UDCA alone in achieving a total effective rate [26]. This same combination in primary biliary cholangitis showed significant improvement in fatigue and itch symptoms and, in non-cirrhotic patients, in ALP, GGT and cholesterol levels [27]. Despite the promising results, however, further studies are needed to directly assess SAMe as a monotherapy or in combination with the standard of care in more types of liver diseases.
SAMe was dosed in the range of 200 mg to 2400 mg per day, with the most common doses being 1000 mg or 1200 mg per day. The optimal dose for maximizing efficacy and minimizing adverse effects may depend on several factors, including the type of liver disease and patient-specific considerations, such as gender and age [23]. Based on the current evidence, we recommend starting doses of 800 mg or 1000 mg per day for most liver diseases. For patients with neonatal jaundice, a lower dose of SAMe 500 mg (with Si Mo Tang) appears to be effective. On the other hand, patients with cholestatic liver disease may require higher doses of 1200 mg per day to increase efficacy. If weight-based dosing is desired, a range of 20 to 30 mg per kg per day is a good reference for chronic liver diseases [16]. Considering dosing route, intravenous SAMe may be a helpful option in HCC, cholestatic liver disease, viral hepatitis, and alcoholic liver disease. Nevertheless, the oral route is also commonly used and effective, and it may be less expensive [7]. However, bioavailability of different oral formulations must be considered as it may vary and potentially affect treatment outcomes [7].
Among the studies reviewed, SAMe demonstrated a favorable safety profile. The most frequently reported side effects included gastrointestinal disturbances, such as diarrhea, abdominal discomfort, and nausea. Other studies reported no difference compared to placebo or even less adverse events in the SAMe group [8,17,22,24]. These gastrointestinal effects were generally mild and resolved upon discontinuation of therapy. In one study reporting severe adverse events, the highest dose of SAMe (2400 mg per day) seen in our included studies was used [25]. This supports our recommendation to avoid this higher dose in liver disease [29,30,31,32,33].
Furthermore, one potential concern that has been raised regarding the safety of SAMe is its potential to increase homocysteine levels. After SAMe is demethylated, it is converted to S-adenosylhomocysteine (SAH) and then further hydrolyzed to homocysteine (Hcy) [34]. Homocysteine can either enter the trans-sulfuration pathway to promote glutathione synthesis or convert back to methionine and, subsequently, SAMe [34,35]. The concern with elevated Hcy is primarily related to its link to endothelial cell dysfunction and atherosclerosis [36]. Besides an increased level of SAMe, Hcy can be elevated for several other reasons, such as genetics, B vitamin and folate deficiencies, increased age, and certain drugs and disease states [36]. While the change in Hcy levels was not a primary focus in our review, no adverse effects related to Hcy levels were reported. Two of our studies assessed Hcy levels over 24-week study periods and found that there were no changes in serum concentration in either treatment or placebo groups [21,25]. Importantly, one of these studies required daily supplementation with a multivitamin with B6, B12, and folic acid [25]. Nevertheless, the impact of aforementioned factors on homocysteine warrants further investigation and research.
Our systematic review has several notable strengths. The application of PRISMA methodology enhances the rigor and reliability of our data and design as a systematic review. Moreover, all included studies were rated as high-quality, which further supports the strength of the evidence. Additionally, this review encompasses a broad range of liver diseases, providing an extensive evaluation of SAMe’s therapeutic potential in this area. Nonetheless, this review is limited by the variability in study designs, populations, and dosages. Though it is difficult to generalize findings, data in a wide variety of contexts can be helpful for different applications of SAMe use. Our review process is also not immune to potential human error. Thus, another limitation is any inconsistencies that may have occurred in applying the inclusion and exclusion criteria in our literature search.
Other than in HBV-related HCC, there is a lack of long-term high-quality studies assessing SAMe in liver diseases. In this area, most of the published data have evaluated SAMe for 12 months or less. Therefore, follow-up studies are needed in these patients to understand long-term impact of SAMe even if only a short treatment duration was used. Other current challenges of SAMe use in liver diseases include the inconsistency of improvements in liver measurements in some studies, specifically LFTs and liver morphology in alcoholic liver disease. In addition, the bioavailability of oral SAMe is reported to be 1–2%, which may limit the impact of treatment when given orally. However, newer types of enteric formulations are being developed to help increase systemic absorption [37,38]. Finally, there is a lack of clear clinical guidelines or consensus on the use of SAMe in the treatment of liver diseases, which limits its potential to benefit patients, as well as provide additional data.
Future high-quality studies are needed to assess long-term safety and efficacy of SAMe in liver diseases. Moreover, none of the studies in our review compared SAMe to standard therapy. More large-scale trials across various types of liver disease and compared to standard therapy would be helpful in establishing clear evidence of SAMe’s efficacy and safety. Furthermore, enhancing the bioavailability of oral formulations and comparing them to alternative routes (e.g., intravenous, intramuscular) is necessary to optimize therapy. Understanding the impact of SAMe on routes of administration, specific liver diseases, and various demographics (including age, gender, homocysteine levels, and nutritional status) is crucial to working toward its integration in clinical guidelines.
5. Conclusions
The current state of the evidence indicates that SAMe can improve liver function parameters and alleviate disease symptoms. Before using SAMe in this context, health care professionals should consider patient-specific factors, such as the specific liver disease being treated, SAMe formulation and dosage, and goals of therapy. SAMe can serve as a valuable alternative to first-line therapies, especially if they are ineffective, inappropriate, or if the patient wants an effective approach without considerable side effects. Future research is essential to evaluate SAMe’s long-term efficacy and safety and to determine the optimal route of administration based on liver disease.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nu16213668/s1, PRISMA checklist.
Author Contributions
Conceptualization, A.M.H.C.; methodology, A.M.H.C. and J.A.D.; software, A.M.H.C. and J.A.D.; formal analysis, A.M.H.C. and K.E.R.B.; investigation, H.M., E.C., N.G., J.A.D. and A.M.H.C.; data curation, A.M.H.C.; writing—original draft preparation, K.E.R.B. and A.M.H.C.; writing—review and editing, K.E.R.B., H.M., E.C., N.G., J.A.D. and A.M.H.C.; supervision, A.M.H.C. and J.A.D.; project administration, A.M.H.C. and J.A.D. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
No new data were created outside of what is published in this article. The study protocol, data collection forms, data extracted from included studies, and all other materials used in this review can be made available upon request. No amendments were made to the study protocol.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Devarbhavi H., Asrani S.K., Arab J.P., Nartey Y.A., Pose E., Kamath P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023;79:516–537. doi: 10.1016/j.jhep.2023.03.017. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Chronic Liver Disease and Cirrhosis. [(accessed on 10 September 2024)]; Available online: https://www.cdc.gov/nchs/fastats/liver-disease.htm.
- 3.Xu J.Q. Trends in Liver Cancer Mortality Among Adults Aged 25 and over in the United States, 2000–2016. National Center for Health Statistics; Hyattsville, MD, USA: 2018. NCHS Data Brief, no 314. [Google Scholar]
- 4.Riazi K., Azhari H., Charette J.H., Underwood F.E., King J.A., Afshar E.E., Swain M.G., Congly S.E., Kaplan G.G., Shaheen A.A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022;7:851–861. doi: 10.1016/S2468-1253(22)00165-0. [DOI] [PubMed] [Google Scholar]
- 5.Teng M.L., Ng C.H., Huang D.Q., Chan K.E., Tan D.J., Lim W.H., Yang J.D., Tan E., Muthiah M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023;29:S32–S42. doi: 10.3350/cmh.2022.0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wazir H., Abid M., Essani B., Saeed H., Ahmad Khan M., Nasrullah F., Qadeer U., Khalid A., Varrassi G., Muzammil M.A., et al. Diagnosis and treatment of liver disease: Current trends and future directions. Cureus. 2023;15:e49920. doi: 10.7759/cureus.49920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limveeraprajak N., Nakhawatchana S., Visukamol A., Siripakkaphant C., Suttajit S., Srisurapanont M. Efficacy and acceptability of S-adenosyl-L-methionine (SAMe) for depressed patients: A systematic review and meta- analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2024;132:110985. doi: 10.1016/j.pnpbp.2024.110985. [DOI] [PubMed] [Google Scholar]
- 8.Di Pierro F., Settembre R. Preliminary results of a randomized controlled trial carried out with a fixed combination of S-adenosyl-L-methionine and betaine versus amitriptyline in patients with mild depression. Int. J. Gen. Med. 2015;8:73–78. doi: 10.2147/IJGM.S79518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anstee Q.M., Day C.P. S-adenosylmethionine (SAMe) therapy in liver disease: A review of current evidence and clinical utility. J. Hepatol. 2012;57:1097–1109. doi: 10.1016/j.jhep.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 10.Pascale R.M., Simile M.M., Calvisi D.F., Feo C.F., Feo F. S-Adenosylmethionine: From the discovery of its inhibition of tumorigenesis to its use as a therapeutic agent. Cells. 2022;11:409. doi: 10.3390/cells11030409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-Ramos D., Lopitz-Otsoa F., Millet O., Alonso C., Lu S.C., Mato J.M. One carbon metabolism and S-Adenosylmethionine in non-alcoholic fatty liver disease pathogenesis and subtypes. Livers. 2022;2:243–257. doi: 10.3390/livers2040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hong Q.N., Pluye P., Fàbregues S., Bartlett G., Boardman F., Cargo M., Dagenais P., Gagnon M.-P., Griffiths F., Nicolau B., et al. Mixed Methods Appraisal Tool (MMAT) Canadian Intellectual Property Office, Industry Canada; Gatineau, QC, Canada: 2018. Version 2018. Registration of Copyright (#1148552) [Google Scholar]
- 14.Benic M.S., Nezic L., Vujic-Aleksic V., Mititelu-Tartau L. Novel therapies for the treatment of drug-induced liver injury: A systematic review. Front. Pharmacol. 2022;12:785790. doi: 10.3389/fphar.2021.785790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vincenzi B., Daniele S., Frezza A.M., Berti P., Vespasiani U., Picardi A., Tonini G., Vincenzi B., Daniele S., Frezza A.M., et al. The role of S-adenosylmethionine in preventing oxaliplatin-induced liver toxicity: A retrospective analysis in metastatic colorectal cancer patients treated with bevacizumab plus oxaliplatin-based regimen. Support. Care Cancer. 2012;20:135–139. doi: 10.1007/s00520-010-1078-4. [DOI] [PubMed] [Google Scholar]
- 16.Guo T., Chang L., Xiao Y.S., Liu Q.Y. S-Adenosyl-L-Methionine for the treatment of chronic liver disease: A systematic review and meta-analysis. PLoS ONE. 2015;10:e0122124. doi: 10.1371/journal.pone.0122124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo T., He Y., Ma W., Liu Z., Liu Q. Feasibility and efficacy of S-Adenosyl-L-methionine in patients with HBV-related HCC with different BCLC stages. Gastroenterol. Res. Pract. 2016;2016:4134053. doi: 10.1155/2016/4134053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu G.Y., Wang W., Jia W.D., Xu G.L., Ma J.L., Ge Y.S., Yu J.H., Sun Q.K., Meng F.L. Protective effect of S-adenosylmethionine on hepatic ischemia-reperfusion injury during hepatectomy in HCC patients with chronic HBV infection. World J. Surg. Oncol. 2014;12:27. doi: 10.1186/1477-7819-12-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L., Wang J.Q., Geng S.X., Liu F., Ping L.L., Gu X.H., Fan X.A., Yang M., Liang L.X., Guo W. Efficacy of adenosylmethionine combined with Si Mo Tang in treatment of neonatal jaundice. Am. J. Transl. Res. 2022;14:3926–3935. [PMC free article] [PubMed] [Google Scholar]
- 20.Le M.D., Enbom E., Traum P.K., Medici V., Halsted C.H., French S.W. Alcoholic liver disease patients treated with S-adenosyl-L-methionine: An in-depth look at liver morphologic data comparing pre and post treatment liver biopsies. Exp. Mol. Pathol. 2013;95:187–191. doi: 10.1016/j.yexmp.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medici V., Virata M.C., Peerson J.M., Stabler S.P., French S.W., Gregory Iii J.F., Albanese A., Bowlus C.L., Devaraj S., Panacek E.A., et al. S-adenosyl-L-methionine Treatment for Alcoholic Liver Disease: A Double-Blinded, Randomized, Placebo-Controlled Trial. Alcohol. Clin. Exp. Res. 2011;35:1960–1965. doi: 10.1111/j.1530-0277.2011.01547.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tkachenko P., Maevskaya M., Pavlov A., Komkova I., Pavlov C., Ivashkin V. Prednisolone plus S-adenosil-L-methionine in severe alcoholic hepatitis. Hepatol. Int. 2016;10:983–987. doi: 10.1007/s12072-016-9751-4. [DOI] [PubMed] [Google Scholar]
- 23.Ferro Y., Pujia R., Mazza E., Lascala L., Lodari O., Maurotti S., Pujia A., Montalcini T. A new nutraceutical (Livogen Plus®) improves liver steatosis in adults with non-alcoholic fatty liver disease. J. Transl. Med. 2022;20:377. doi: 10.1186/s12967-022-03579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiao J.G., Zhao C.Y. Therapeutic effect of adenosylmethionine on viral hepatitis and related factors inducing diseas. Am. J. Transl. Res. 2021;13:9485–9494. [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan T.R., Osann K., Bottiglieri T., Pimstone N., Hoefs J.C., Hu K.Q., Hassanein T., Boyer T.D., Kong L., Chen W.P., et al. A phase II randomized, controlled trial of S-Adenosylmethionine in reducing serum α-fetoprotein in patients with Hepatitis C cirrhosis and elevated AFP. Cancer Prev. Res. 2015;8:864–872. doi: 10.1158/1940-6207.CAPR-15-0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu Y., Yang Y., Kang Z., Chen Q., Han R., Lu W., Zhang M., Ma Z., Huang G., Zu H. High-dose S-adenosylmethionine combined with ursodeoxycholic acid is more suitable for the treatment of cholestatic liver disease. Int. J. Clin. Exp. Med. 2020;13:7365–7371. [Google Scholar]
- 27.Wunsch E., Raszeja-Wyszomirska J., Barbier O., Milkiewicz M., Krawczyk M., Milkiewicz P. Effect of S-adenosyl-L-methionine on liver biochemistry and quality of life in patients with primary biliary cholangitis treated with ursodeoxycholic acid. A prospective, open label pilot study. J. Gastrointestin. Liver Dis. 2018;27:273–279. doi: 10.15403/jgld.2014.1121.273.icz. [DOI] [PubMed] [Google Scholar]
- 28.Yang Y., Kang Z., Lu Y., Chen Q., Han R., Lu W., Zhang M., Ma Z., Huang G., Zu H. Comparison of the efficacy and liver function with different doses of S-adenosylmethionine combined with ursodeoxycholic acid for cholestatic liver disease. Int. J. Clin. Exp. Med. 2021;14:1317–1323. [Google Scholar]
- 29.Carpenter D.J. St. John’s wort and S-adenosyl methionine as “natural” alternatives to conventional antidepressants in the era of the suicidality boxed warning: What is the evidence for clinically relevant benefit? Altern. Med. Rev. 2011;16:17–39. [PubMed] [Google Scholar]
- 30.Dolcetta D., Parmigiani P., Salmaso L., Bernardelle R., Cesari U., Andrighetto G., Baschirotto G., Nyhan W.L., Hladnik U. Quantitative evaluation of the clinical effects of S-adenosylmethionine on mood and behavior in Lesch-Nyhan patients. Nucleosides Nucleotides Nucleic Acids. 2013;32:174–188. doi: 10.1080/15257770.2013.774012. [DOI] [PubMed] [Google Scholar]
- 31.Sarris J., Byrne G.J., Bousman C., Stough C., Murphy J., MacDonald P., Adams L., Nazareth S., Oliver G., Cribb L., et al. Adjunctive S-adenosylmethionine (SAMe) in treating non-remittent major depressive disorder: An 8-week double-blind, randomized, controlled trial. Eur. Neuropsychopharmacol. 2018;28:1126–1136. doi: 10.1016/j.euroneuro.2018.07.098. [DOI] [PubMed] [Google Scholar]
- 32.Olsufka W., Abraham M.-A. Treatment-emergent hypomania possibly associated with over-the-counter supplements. Ment. Health Clin. 2017;7:160–163. doi: 10.9740/mhc.2017.07.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chitiva H., Audivert F., Alvarez C. Suicide attempt by self-burning associated with ingestion of S-adenosylmethionine: A review of the literature and case report. J. Nerv. Ment. Dis. 2012;200:99–101. doi: 10.1097/NMD.0b013e31823fafdf. [DOI] [PubMed] [Google Scholar]
- 34.Bravo A.C., Aguilera M.N.L., Marziali N.R., Moritz L., Wingert V., Klotz K., Schumann A., Grünert S.C., Spiekerkoetter U., Berger U., et al. Analysis of S-Adenosylmethionine and S-Adenosylhomocysteine: Method Optimisation and Profiling in Healthy Adults upon Short-Term Dietary Intervention. Metabolites. 2022;12:373. doi: 10.3390/metabo12050373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z., Wang F., Liang B., Su Y., Sun S., Xia S., Shao J., Zhang Z., Hong M., Zhang F., et al. Methionine metabolism in chronic liver diseases: An update on molecular mechanism and therapeutic implication. Signal Transduct. Target Ther. 2020;5:280. doi: 10.1038/s41392-020-00349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan D., Chu J., Lin H., Zhu G., Qian J., Yu Y., Yao T., Ping F., Chen F., Liu X. Mechanism of homocysteine-mediated endothelial injury and its consequences for atherosclerosis. Front. Cardiovasc. Med. 2023;9:1109445. doi: 10.3389/fcvm.2022.1109445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cameron B.R., Ferreira L., MacDonald I.D. Pharmacokinetic study of a novel oral formulation of S-adenosylmethionine (MSI-195) in healthy subjects: Dose escalation, food effect and comparison to a commercial nutritional supplement product. BMC Pharmacol. Toxicol. 2020;21:88. doi: 10.1186/s40360-020-00466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amasya G., Ergin A.D., Erkan Cakirci O., Ozçelikay A.T., Sezgin Bayindir Z., Yuksel N. A study to enhance the oral bioavailability of s-adenosyl-l-methionine (SAMe): SLN and SLN nanocomposite particles. Chem. Phys. Lipids. 2021;237:105086. doi: 10.1016/j.chemphyslip.2021.105086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No new data were created outside of what is published in this article. The study protocol, data collection forms, data extracted from included studies, and all other materials used in this review can be made available upon request. No amendments were made to the study protocol.