Abstract
Background: Studies on the association between serum vitamin B6 status and colorectal cancer prognosis are limited and have yielded inconsistent results. This study investigated the association of pyridoxal 5′-phosphate (PLP) and pyridoxic acid ratio (PAr) index with colorectal cancer survival. Methods: A total of 1286 colorectal cancer patients diagnosed since 2010 were selected from the Guangdong Colorectal Cancer Cohort study. Serum levels of PLP, pyridoxal, and 4-pyridoxic acid were measured using ultra-high-performance liquid chromatography–tandem mass spectrometry. The study followed overall mortality and colorectal cancer-specific mortality until December 2023. Multivariable Cox proportional hazards regression models were applied to calculate hazard ratios (HRs) and 95% confidence intervals (95% CIs). Restricted cubic spline and stratified analysis were performed. Results: During a median follow-up of 77.36 months, 331 deaths were recorded, with 293 specifically attributed to colorectal cancer. Higher PLP levels were associated with a longer overall survival (HRQ4 vs. Q1, 0.63; 95% CI: 0.46, 0.87; p for trend = 0.008) and colorectal cancer-specific survival (HRQ4 vs. Q1, 0.62; 95% CI: 0.44, 0.87; p for trend = 0.006). Non-linear associations were observed between serum PLP and overall and colorectal cancer-specific survival (p for non-linear < 0.05). However, PAr was not significantly associated with either overall survival (HRQ4 vs. Q1, 1.03; 95% CI: 0.75, 1.41) or colorectal cancer-specific survival (HRQ4 vs. Q1, 1.01; 95% CI: 0.72, 1.42). The association between serum PLP and both overall survival and colorectal cancer-specific survival (p for interaction < 0.05) varied by alcohol drinking status. Conclusions: Higher serum PLP levels, but not PAr, may be associated with improved overall and colorectal cancer-specific survival.
Keywords: pyridoxal 5′-phosphate, PAr, colorectal cancer, survival, cohort study
1. Introduction
Colorectal cancer is a primary cause of cancer-related morbidity and mortality worldwide [1,2]. The mortality rates for colorectal cancer are on the rise globally [3,4]. From 1990 to 2019, the number of deaths from colorectal cancer increased from 518,126 to 1.09 million [2]. The 5-year survival rate for metastatic colorectal cancer is 14% [5]. Improving long-term outcomes for colorectal cancer patients remains a significant challenge. As cancer cells rely on metabolites from the patient’s diet [6], identifying modifiable factors, such as diet, is essential to prevent mortality in individuals with colorectal cancer [7,8,9,10].
Vitamin B6, mainly obtained through the diet, plays a crucial role in regulating and participating in significant processes such as carbohydrate metabolism, DNA synthesis, and one-carbon cycling, acting as a cofactor and essential nutrient [6,11,12,13,14,15,16]. Blood measurements of B vitamins can overcome the limitation of relying on self-reported dietary intake and may provide a more accurate assessment of exposure, as the bioavailability of serum B vitamins may be compromised [17]. Pyridoxal 5′-phosphate (PLP), the biologically active form of vitamin B6, is commonly utilized as the primary indicator of overall vitamin B6 status in the body [18]. The pyridoxic acid ratio (PAr) index, defined as the ratio of 4-pyridoxic acid (PA) over the sum of PLP and pyridoxal (PL), serves as a marker of increased vitamin B6 catabolism during inflammation and related cellular immune activation [19]. The PAr is significantly associated with inflammatory markers such as C-reactive protein (CRP), an acute phase marker, and kynurenine/tryptophan ratio (KYN/TRP), which is linked to cellular immunity [20]. Inflammation-related processes may lead to an imbalance in the plasma concentrations of B6 vitamers, causing an increase in PA relative to PL + PLP and thus resulting in an elevated PAr [21]. Albumin, the primary carrier of PLP, is often found at reduced levels during inflammation, while increased activity of membrane-bound phosphatases, such as alkaline phosphatase, facilitates the uptake of PLP into tissue. Moreover, oxidative stress can upregulate enzymes involved in aldehyde metabolism, such as aldehyde oxidase (AOX) and aldehyde dehydrogenases, which convert PL to PA. Progressive kidney damage due to chronic inflammation may also increase plasma PA levels in comparison to PLP and PL [21]. Additionally, the relationship between the acute inflammatory response—evidenced by markers like CRP—and the redistribution of PLP from plasma to other tissues suggests a connection between elevated PAr and various inflammatory processes [22]. Therefore, PAr serves as a marker for increased vitamin B6 catabolism during inflammation and related cellular immune activation.
Previous studies have specifically focused on PLP and PAr in relation to colorectal cancer risk [23,24,25,26] and, to a lesser extent, survival [27,28,29,30,31]. The findings regarding the association between PLP levels and colorectal cancer survival have been inconsistent [28,30,31]. One study indicated that elevated plasma PLP levels were associated with a better prognosis for colorectal cancer patients, while two other studies found no significant association. To date, only one study has investigated the relationship between PAr and colorectal cancer survival, reporting a positive association [30]. Therefore, the epidemiological evidence on the protective role of serum PLP against colorectal cancer is inconclusive, and research on PAr and colorectal cancer survival is limited to a single study conducted on European populations.
Studies examining the relationship between circulating vitamin B6 concentrations and colorectal cancer prognosis are limited in China. This study aims to prospectively investigate the association of serum PLP and PAr with survival in Chinese colorectal cancer patients. The hypothesis was that higher serum PLP levels and lower PAr are associated with improved colorectal cancer survival.
2. Materials and Methods
2.1. Study Population
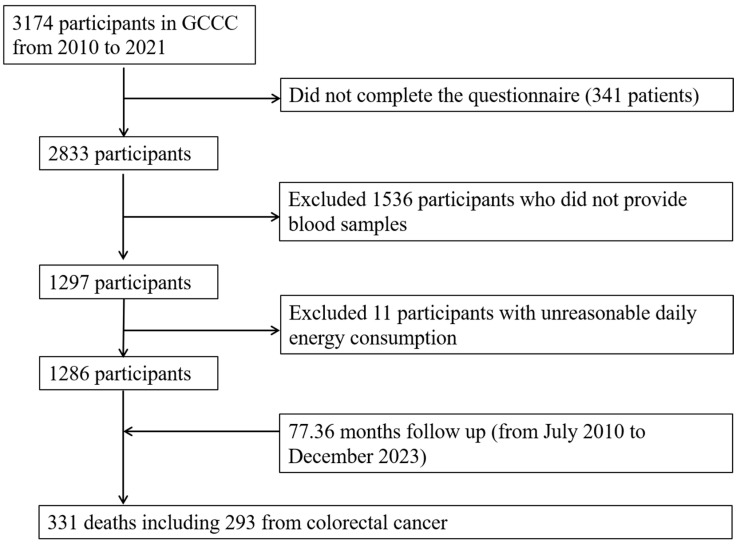
This study utilized data from the Guangdong Colorectal Cancer Cohort (GCCC), an ongoing prospective cohort study aimed at evaluating genetic and environmental factors influencing the survival of individuals diagnosed with colorectal cancer. The cohort was established in July 2010 at the Sun Yat-sen University Cancer Center in Guangzhou, China [32,33]. Potential colorectal cancer patients were screened for eligibility, and subsequent enrollment in the cohort was conducted according to the following inclusion criteria: aged 30–75 years, with a histological diagnosis of primary colorectal cancer (International Classification of Diseases, version 10 [ICD10/C18-C20]) within 3 months of diagnosis, and either being native to Guangdong or having maintained residence in Guangdong for a minimum of 5 years. Patients were not eligible to participate if they had a diagnosis of another type of cancer or if they were unable to communicate or understand Mandarin or Cantonese effectively. A total of 3174 cases were identified for the period between July 2010 and May 2021. Of the aforementioned cases, 2833 were eligible and thus underwent an interview, resulting in a response rate of 89.26%. A total of 1297 blood samples were available and detected from among eligible cases (Figure 1). After excluding 11 cases with unreasonable daily energy consumption information, the final analysis encompassed 1286 cases.
Figure 1.
Flow chart of study participants. Abbreviations: GCCC, Guangdong Colorectal Cancer Cohort study.
2.2. Blood Collection and Biochemical Measurements
On the second day of their hospitalization, each participant provided a fasting venous blood sample of approximately 5 mL. All participants had not received any treatment or surgical operation before the blood collection. Upon arrival at the laboratory, the blood samples were subjected to centrifugation at 3000 rpm for a period of 10 min at a temperature of 4 °C. Subsequently, an aliquot of each serum sample was transferred into eight 200 μL tubes and immediately frozen at −80 °C until analysis was initiated.
Serum vitamin B6 species, comprising PLP, PL, and PA, were measured using the ultra-high-performance liquid chromatography–tandem mass spectrometry (UHPLC-MS/MS) method, as exemplified in previous studies [34,35]. The detection process was performed with an Agilent 1290 UHPLC system in conjunction with an Agilent 6495 triple quadrupole instrument (Agilent, Santa Clara, CA, USA). The precise methodology employed has been previously described in detail [36,37]. The recovery rates of serum vitamin B6 ranged between 85% and 118%, with mean inter-batch coefficients of variation at 11.4% for PLP, 8.1% for PL, and 10.1% for PA, respectively.
2.3. PAr Definition
The calculation of PAr involves the division of PA by the sum of the PLP and PL. The procedures for quantifying PA, PL, and PLP concentrations are delineated in the preceding section.
2.4. Outcome Ascertainment and Follow-Up
In this study, two primary outcomes were evaluated: overall survival and colorectal cancer-specific survival. Overall survival considered death from any cause, while colorectal cancer-specific survival focused on deaths due to colorectal cancer. Overall survival was defined as the time elapsed between the pathological diagnosis of colorectal cancer and the occurrence of any cause of death. Colorectal cancer-specific survival was defined as the time elapsed between the pathological diagnosis of deaths specifically attributed to colorectal cancer. Participants who did not experience an event of interest within the study period were considered to have been censored on the date of the final follow-up evaluation. Follow-up commenced at the time of colorectal cancer diagnosis and continued until a cancer-related event occurred or the last outcome ascertainment on 1 December 2023. The data pertaining to mortality among participants was gathered on a regular basis through a multifaceted approach, encompassing the reporting system overseen by the Guangzhou Center for Disease Control and Prevention, medical records, and active follow-up through telephone interviews with patients or their next-of-kin. Physicians identified colorectal cancer-specific causes of death based on ICD-10 codes.
2.5. Covariate Ascertainment
At baseline, patient information on demographics, lifestyle factors, dietary habits, and clinical data was obtained through in-person interviews or extracted from medical records [38]. Demographic data included sex, age, marital status, and average monthly income. Lifestyle habits covered smoking status, alcohol consumption, and physical activity.
A validated food frequency questionnaire (FFQ) was employed to ascertain dietary information [38]. Trained interviewers posed inquiries to the participants in a personal face-to-face interview. They requested that the participants estimate the frequency (never, per year, per month, per week, per day) and amount of each food item consumed. The average daily nutrient intake was calculated by multiplying the frequency and portion size of each food item by its nutrient content based on the China Food Composition Table 2002 [39]. Participants were provided with photographs of standard food portions to aid in estimating their intake. The residual method was employed for the purpose of adjusting the dietary intake of nutrients in consideration of energy intake (in kcal/d) [40]. Clinical factors, such as age at diagnosis, cancer stage (I, II, III, and IV), tumor site (colon, rectum), degree of differentiation (well-differentiated, moderately differentiated, poorly differentiated), surgical operation (yes or no), and administration of radiotherapy or chemotherapy (yes or no), were extracted from hospital medical records.
A smoker was defined as someone who had smoked at least one cigarette per day for more than 6 months consecutively or cumulatively in their lifetime. The patient’s smoking status was classified as either “never-smoking” or “ever-smoking” based on the criteria that they had smoked continuously or cumulatively for a period of more than six months. In this categorization, ever-smoking included both current and former smoking. Regular drinking was defined as consuming alcohol at least once per week during the past year. Physical activity levels were assessed based on self-reported occupational, household, and leisure-time activities. The mean metabolic equivalent task (MET)-hours value for each activity was obtained by averaging all comparable activities in the Compendium of Physical Activities [41,42]. The MET-h/week for each subject was calculated according to the following formula: number of days/week × hours per day × MET for a specific activity. Body mass index (BMI) was calculated using the following formula: weight (kg)/height squared (m2).
2.6. Statistical Analysis
In cases where data exhibited abnormal detection patterns, such as values exceeding the upper limit of the quantitative standard curve, the median value was substituted. Baseline characteristics of the study population were presented according to sex-specific quartiles of serum PLP. Continuous variables were expressed as mean (standard deviation) or median (P25, P75), while categorical variables were presented as frequencies (percentages). Differences between groups were assessed using one-way ANOVA or Kruskal-Wallis tests for continuous variables and chi-square tests for categorical variables.
Cox proportional hazards regression models were fitted to evaluate hazard ratios (HRs) and 95% confidence intervals (CIs) to examine the association of serum PLP and PAr with survival outcomes. No violations of the proportional hazard assumption were detected. In the multivariable analyses, adjustments were made for potential confounding factors assessed at study recruitment. Model 1 adjusted for age (continuous), sex (male, female), BMI (continuous), smoking (ever or never), drinking (regular or never), MET (continuous), total energy intake (continuous), and protein intake (continuous). Model 2 included additional adjustments for differentiation (well-differentiated, moderately differentiated, poorly differentiated), surgery (yes or no), radiotherapy or chemotherapy (yes or no), and history of cancer in first-degree relatives (yes or no). A linear trend was tested using the median value for each categorical variable (Q1-Q4) as a continuous variable in Cox proportional hazards modes. Restricted cubic spline (RCS) analysis with 4 knots was used to evaluate dose–response relationships and potential non-linear associations between serum vitamin B6 and survival outcomes. The likelihood ratio test was employed to examine non-linearity.
Stratified analysis was performed by sex, age, BMI, smoking status, cancer stage, and tumor site. Additionally, given the potential influence of alcohol on vitamin B6 absorption and one-carbon metabolism [43], stratified analyses were also conducted according to alcohol drinking status. Interaction tests were performed by comparing multivariate models with and without interaction terms using cross-product terms. The p-value for interaction was derived from the log-likelihood statistic. To examine the robustness of the result, two sensitivity analyses were performed: (1) additional adjustment for dietary vitamin B6 intake based on model 2; (2) additional adjustment for the timing of blood-sample collection based on model 2.
All statistical analyses were performed using R software, version 4.4.0 (R Project for Statistical Computing). p-values < 0.05 were considered statistically significant based on two-sided tests.
3. Results
3.1. Characteristics of Participants in GCCC Study
The median (P25, P75) serum PLP concentration and PAr for these 1286 colorectal cancer patients was 6.03 (3.21–11.10) nmol/L and 0.69 (0.49–1.00), respectively. A median follow-up period of 77.36 months yielded a total of 331 deaths among the 1286 colorectal cancer patients, with 293 of these deaths attributed specifically to colorectal cancer. Among the patients who died, 218 (30.24% out of 721) were male and 113 (20.00% out of 565) were female. The median (P25, P75) age at enrollment was 58.53 (49.98–65.03) years, and 721 (56.07%) of the patients were men. Table 1 presents the baseline characteristics of the 1286 colorectal cancer patients, categorized by quartile of serum PLP. A statistically significant difference was observed in drinking status among patients with varying serum PLP levels (p = 0.017). There was also a significant difference in cancer stage based on serum PLP levels (p < 0.001). Additionally, significant differences were noted in other serum vitamin levels across varying serum PLP quartiles (all p < 0.05). Significant differences were observed in total energy (kcal/day) and protein (g/day) intake across the various groups (both p < 0.05), while significant differences were not noted in dietary B vitamin intake levels across varying serum PLP quartiles (all p > 0.05).
Table 1.
Baseline characteristics of patients with colorectal cancer by the quartiles of serum pyridoxal 5’-phosphate in the Guangdong Colorectal Cancer Cohort.
| Characteristics | Quartiles of Serum PLP (nmol/L) a | p-Value b | |||
|---|---|---|---|---|---|
| Quartile 1 (n = 323) | Quartile 2 (n = 321) | Quartile 3 (n = 321) | Quartile 4 (n = 321) | ||
| Sex (n, %) | 1.000 | ||||
| Male | 181 (56.04) | 180 (56.07) | 180 (56.07) | 180 (56.07) | |
| Female | 142 (43.96) | 141 (43.93) | 141 (43.93) | 141 (43.93) | |
| Marital status (n, %) | 0.094 | ||||
| Married | 311 (96.28) | 300 (93.46) | 309 (96.26) | 298 (92.83) | |
| Unmarried/divorced/widowed | 12 (3.72) | 21 (6.54) | 12 (3.74) | 23 (7.17) | |
| Residence (n, %) | 0.279 | ||||
| Urban | 211 (65.33) | 192 (59.81) | 211 (65.73) | 213 (66.36) | |
| Rural | 112 (34.67) | 129 (40.19) | 110 (34.27) | 108 (33.64) | |
| Income, Yuan/month, (n, %) | 0.995 | ||||
| less than 2000 | 49 (15.17) | 47 (14.64) | 46 (14.33) | 43 (13.40) | |
| 2001–5000 | 104 (32.20) | 102 (31.78) | 103 (32.09) | 99 (30.84) | |
| 5001–8000 | 90 (27.86) | 99 (30.84) | 97 (30.22) | 97 (30.22) | |
| more than 8001 | 80 (24.77) | 73 (22.74) | 75 (23.36) | 82 (25.55) | |
| Smoking status (n, %) | 0.515 | ||||
| Never | 187 (57.89) | 196 (61.06) | 189 (58.88) | 203 (63.24) | |
| Ever | 136 (42.11) | 125 (38.94) | 132 (41.12) | 118 (36.76) | |
| Drinking, (n, %) | 0.017 | ||||
| Never | 280 (86.69) | 271 (84.42) | 250 (77.88) | 272 (84.74) | |
| Regular | 43 (13.31) | 50 (15.58) | 71 (22.12) | 49 (15.26) | |
| BMI, kg/m2, (n, %) | 0.211 | ||||
| <23.9 | 203 (62.85) | 190 (59.19) | 194 (60.44) | 176 (54.83) | |
| ≥23.9 | 120 (37.15) | 131 (40.81) | 127 (39.56) | 145 (45.17) | |
| Physical activity, median (P25, P75), MET-h per week c | 28.88 (6.17, 52.50) | 28.88 (10.31, 52.50) | 26.25 (13.13, 52.50) | 28.88 (11.25, 52.50) | 0.940 |
| Age at diagnosis in years, y, (n, %) | 0.183 | ||||
| <50 | 78 (24.15) | 85 (26.48) | 69 (21.50) | 92 (28.66) | |
| ≥50 | 245 (75.85) | 236 (73.52) | 252 (78.50) | 229 (71.34) | |
| Cancer stage (n, %) d | <0.001 | ||||
| I | 34 (10.53) | 46 (14.33) | 61 (19.00) | 52 (16.20) | |
| II | 112 (34.67) | 113 (35.20) | 111 (34.58) | 104 (32.40) | |
| III | 98 (30.34) | 106 (33.02) | 96 (29.91) | 130 (40.50) | |
| IV | 70 (21.67) | 49 (15.26) | 52 (16.20) | 34 (10.59) | |
| Tumor site (n, %) | 0.068 | ||||
| Colon | 216 (66.87) | 208 (64.80) | 184 (57.32) | 198 (61.68) | |
| Rectum | 107 (33.13) | 113 (35.20) | 137 (42.68) | 123 (38.32) | |
| Differentiation (n, %) e | 0.297 | ||||
| Well-differentiated | 1 (0.31) | 1 (0.31) | 4 (1.25) | 4 (1.25) | |
| Moderately differentiated | 223 (69.04) | 238 (74.14) | 237 (73.83) | 230 (71.65) | |
| Poorly differentiated | 77 (23.84) | 68 (21.18) | 58 (18.07) | 73 (22.74) | |
| Radiotherapy or chemotherapy (n, %) | 0.656 | ||||
| No | 114 (35.29) | 108 (33.64) | 120 (37.38) | 106 (33.02) | |
| Yes | 209 (64.71) | 213 (66.36) | 201 (62.62) | 215 (66.98) | |
| Surgery (n, %) | 0.239 | ||||
| No | 22 (6.81) | 14 (4.36) | 12 (3.74) | 13 (4.05) | |
| Yes | 301 (93.19) | 307 (95.64) | 309 (96.26) | 308 (95.95) | |
| History of cancer in first-degree relatives (n, %) | 0.753 | ||||
| No | 279 (86.38) | 274 (85.36) | 283 (88.16) | 280 (87.23) | |
| Yes | 44 (13.62) | 47 (14.64) | 38 (11.84) | 41 (12.77) | |
| PL (nmol/L), median (P25, P75) | 10.78 (6.70, 15.62) | 10.92 (7.62, 15.19) | 10.97 (7.78, 16.84) | 16.00 (9.31, 28.70) | <0.001 |
| PA (nmol/L), median (P25, P75) | 11.22 (7.62, 15.73) | 12.68 (9.63, 18.19) | 12.99 (9.24, 18.71) | 15.61 (11.15, 28.60) | <0.001 |
| PAr, median (P25, P75) | 0.84 (0.58, 1.23) | 0.83 (0.58, 1.14) | 0.67 (0.47, 0.94) | 0.49 (0.33, 0.67) | <0.001 |
| Dietary intake, median (P25, P75) f | |||||
| Total energy (kcal/day) | 1544.15 (1263.22, 1817.55) | 1524.82 (1278.14, 1864.33) | 1519.02 (1250.05, 1858.67) | 1424.64 (1152.41, 1752.29) | 0.011 |
| Protein (g/day) | 65.67 (55.35, 79.31) | 66.90 (53.58, 78.52) | 63.30 (52.42, 77.32) | 60.05 (49.28, 75.61) | 0.004 |
| Vitamin B2 (mg/day) | 0.83 (0.71, 0.98) | 0.83 (0.69, 0.98) | 0.82 (0.68, 0.97) | 0.82 (0.70, 1.00) | 0.456 |
| Vitamin B6 (mg/day) | 0.83 (0.71, 0.97) | 0.83 (0.71, 0.93) | 0.80 (0.67, 0.94) | 0.80 (0.69, 0.92) | 0.062 |
| Folate (μg/day) | 208.59 (180.26, 240.70) | 200.84 (178.58, 237.52) | 205.38 (172.86, 242.00) | 208.70 (176.69, 247.09) | 0.397 |
| Vitamin B12 (mg/day) | 1.67 (1.20, 2.15) | 1.65 (1.16, 2.36) | 1.62 (1.16, 2.25) | 1.65 (1.10, 2.37) | 0.939 |
Abbreviations: PLP, pyridoxal 5′-phosphate; BMI, body mass index; MET, metabolic equivalent task; PL, pyridoxal; PA, 4-pyridoxic acid; PAr, the ratio of PA over the combined concentrations of PLP and PL. a Quartile ranges of serum PLP and PL were <3.11, 3.11 to 5.86, 5.86 to 10.78, and ≥10.78 nmol/L in men and <3.33, 3.33 to 6.27, 6.27 to 11.59, and ≥11.59 nmol/L in women. b Chi-square tests or Kruskal-Wallis tests, p-value < 0.05 were considered statistically significant. c Six patients had unknown information on MET. d Eighteen patients had unknown information on cancer stage. e Seventy-two patients had other or unknown degree of differentiation. f Consumption was adjusted for total energy intake using the regression residual method.
3.2. Association of Serum PLP and PAr with Survival
The concentration of serum PLP was found to be significantly associated with improved overall and colorectal cancer-specific survival after adjusting for various covariates. Higher PLP levels, particularly in the highest quartile group, were found to be associated with a 37% longer overall survival (HRQ4 vs. Q1, 0.63; 95% CI: 0.46, 0.87; p for trend = 0.008) and a 38% longer colorectal cancer-specific survival (HRQ4 vs. Q1, 0.62; 95% CI: 0.44, 0.87; p for trend = 0.006). In contrast, PAr was not significantly associated with overall survival (HRQ4 vs. Q1, 1.03; 95% CI: 0.75, 1.41; p for trend = 0.964) or colorectal cancer-specific survival (HRQ4 vs. Q1, 1.01; 95% CI: 0.72, 1.42; p for trend = 0.964) in the adjusted models (Table 2).
Table 2.
Hazard ratio (95% confidence interval) for overall survival and overall survival and colorectal cancer-specific survival according to quartiles of serum pyridoxal 5’-phosphate and PAr in the Guangdong Colorectal Cancer Cohort.
| Death/Number | Crude Model | Model 1 b | Model 2 c | |
|---|---|---|---|---|
| Overall survival | ||||
| PLP, nmol/L | ||||
| Quartile 1 | 96/323 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 82/321 | 0.80 (0.60–1.08) | 0.79 (0.59–1.06) | 0.81 (0.60–1.09) |
| Quartile 3 | 84/321 | 0.79 (0.59–1.06) | 0.78 (0.58–1.05) | 0.81 (0.60–1.09) |
| Quartile 4 | 69/321 | 0.65 (0.47–0.88) | 0.66 (0.48–0.91) | 0.63 (0.46–0.87) |
| p for trend a | 0.004 | 0.021 | 0.008 | |
| PAr | ||||
| Quartile 1 | 73/313 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 85/321 | 1.14 (0.83–1.56) | 1.10 (0.81–1.51) | 1.07 (0.78–1.46) |
| Quartile 3 | 86/323 | 1.17 (0.85–1.59) | 1.13 (0.83–1.55) | 1.10 (0.80–1.51) |
| Quartile 4 | 87/329 | 1.15 (0.84–1.57) | 1.07 (0.78–1.47) | 1.03 (0.75–1.41) |
| p for trend a | 0.293 | 0.797 | 0.964 | |
| Colorectal cancer-specific survival | ||||
| PLP, nmol/L | ||||
| Quartile 1 | 85/323 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 76/321 | 0.84 (0.62–1.15) | 0.83 (0.61–1.13) | 0.85 (0.62–1.16) |
| Quartile 3 | 72/321 | 0.77 (0.56–1.06) | 0.77 (0.56–1.05) | 0.79 (0.58–1.09) |
| Quartile 4 | 60/321 | 0.64 (0.46–0.89) | 0.65 (0.47–0.91) | 0.62 (0.44–0.87) |
| p for trend a | 0.004 | 0.016 | 0.006 | |
| PAr | ||||
| Quartile 1 | 65/313 | 1.00 | 1.00 | 1.00 |
| Quartile 2 | 75/321 | 1.14 (0.82–1.58) | 1.11 (0.80–1.55) | 1.07 (0.76–1.49) |
| Quartile 3 | 78/323 | 1.19 (0.86–1.66) | 1.17 (0.84–1.63) | 1.12 (0.80–1.56) |
| Quartile 4 | 75/329 | 1.13 (0.81–1.58) | 1.06 (0.75–1.48) | 1.01 (0.72–1.42) |
| p for trend a | 0.398 | 0.892 | 0.964 |
a Test for linear trend was based on the median values for each quartile of serum concentration. b Cox proportional hazards regression model adjusted for age (continuous), sex (male, female), BMI (continuous), smoking (ever or never), drinking (regular or never), MET (continuous), total energy intake (continuous), and protein intake (continuous). c Cox proportional hazards regression model additionally adjusted for differentiation (well-differentiated, moderately differentiated, poorly differentiated), surgery (yes or no), radiotherapy or chemotherapy (yes or no), and history of cancer in first-degree relatives (yes or no). Abbreviations: PLP, pyridoxal 5′-phosphate; PAr, the ratio of PA over the combined concentrations of PLP and PL.
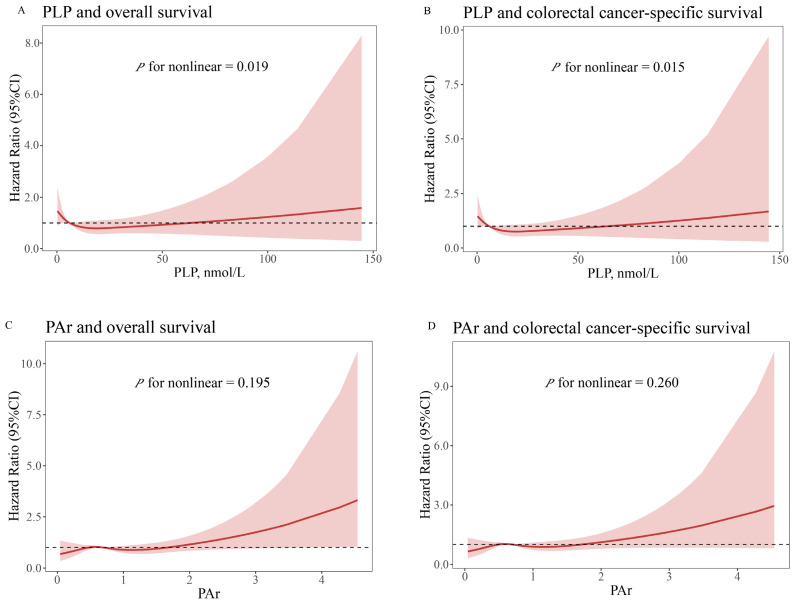
Further analyses, detailed in Figure 2, revealed a non-linear, reverse J-shaped association between serum PLP and both overall survival (p for non-linear = 0.019) and colorectal cancer-specific survival (p for non-linear = 0.015), as identified by the RCS analysis. Nevertheless, no statistically significant non-linear relationship was identified between PAr and either overall survival or colorectal cancer-specific survival (p for non-linear > 0.050).
Figure 2.
Multivariable-adjusted relationships between serum pyridoxal 5′-phosphate and PAr and survival outcome by the restricted cubic spline model. The solid red line represents the hazard ratio value, the shaded area represents the confidence interval, and the dashed line represents a hazard ratio value of 1. (A) PLP and overall survival. (B) PLP and colorectal cancer-specific survival. (C) PAr and overall survival. (D) PAr and colorectal cancer-specific survival. Cox proportional hazards regression model adjusted for age (continuous), sex (male, female), BMI (continuous), smoking (ever or never), drinking (regular or never), MET (continuous), total energy intake (continuous), protein intake (continuous), degree of differentiation (well-differentiated, moderately differentiated, poorly differentiated), surgery (yes or no), radiotherapy or chemotherapy (yes or no), and history of cancer in first-degree relatives (yes or no). Abbreviations: CI, confidence interval; PLP, pyridoxal 5′-phosphate; PAr, the ratio of PA over the combined concentrations of PLP and PL.
3.3. Stratified and Sensitivity Analyses
Table 3 shows the association between serum vitamin B6 levels and survival outcomes in colorectal cancer patients stratified by factors such as sex, age, BMI, smoking status, alcohol drinking status, cancer stage, and tumor site. Interaction tests revealed that alcohol consumption significantly modified the relationship between serum PLP levels and colorectal cancer survival. Specifically, the association between serum PLP and both overall survival (p for interaction = 0.030) and colorectal cancer-specific survival (p for interaction = 0.031) varied by alcohol consumption. However, no significant interactions were found between PLP levels and survival outcomes across other factors such as sex, age, BMI, smoking status, cancer stage, or tumor site (all p for interaction > 0.05). Similarly, no significant interactions were found between PAr and survival outcomes across any of the examined variables (all p for interaction > 0.05). Specifically, the associations of serum PLP or PAr with overall survival and colorectal cancer-specific survival were consistent across subgroups such as sex, age, BMI, smoking status, cancer stage, and tumor site.
Table 3.
Hazard ratios (95% CI) for survival outcomes by pyridoxal 5’-phosphate and PAr stratified by selected covariates in the Guangdong Colorectal Cancer Cohort Study.
| Overall Survival | Colorectal Cancer-Specific Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| Quartile 1 | Quartile 4 a | p for Trend b | p for Interaction c | Quartile 1 | Quartile 4 a | p for Trend b | p for Interaction c | |
| PLP, nmol/L | ||||||||
| Sex | 0.468 | 0.581 | ||||||
| Male | 1.00 | 0.64 (0.43–0.94) | 0.062 | 1.00 | 0.66 (0.44–1.01) | 0.088 | ||
| Female | 1.00 | 0.66 (0.38–1.15) | 0.077 | 1.00 | 0.61 (0.34–1.08) | 0.044 | ||
| Age | 0.674 | 0.705 | ||||||
| <50 | 1.00 | 0.68 (0.37–1.24) | 0.436 | 1.00 | 0.56 (0.29–1.07) | 0.196 | ||
| ≥50 | 1.00 | 0.62 (0.43–0.89) | 0.011 | 1.00 | 0.65 (0.44–0.97) | 0.023 | ||
| BMI | 0.611 | 0.888 | ||||||
| <23.9 | 1.00 | 0.72 (0.48–1.08) | 0.106 | 1.00 | 0.65 (0.42–1.01) | 0.044 | ||
| ≥23.9 | 1.00 | 0.50 (0.30–0.83) | 0.026 | 1.00 | 0.58 (0.34–0.99) | 0.080 | ||
| Smoking status | 0.098 | 0.118 | ||||||
| Ever | 1.00 | 0.84 (0.54–1.31) | 0.748 | 1.00 | 0.9 (0.55–1.460) | 0.855 | ||
| Never | 1.00 | 0.51 (0.32–0.80) | 0.002 | 1.00 | 0.48 (0.29–0.77) | 0.001 | ||
| Drinking status | 0.030 | 0.031 | ||||||
| Regular | 1.00 | 0.57 (0.24–1.32) | 0.181 | 1.00 | 0.72 (0.27–1.90) | 0.261 | ||
| Never | 1.00 | 0.67 (0.48–0.94) | 0.029 | 1.00 | 0.64 (0.45–0.92) | 0.020 | ||
| Cancer stage | 0.717 | 0.581 | ||||||
| I–III | 1.00 | 0.80 (0.51–1.26) | 0.323 | 1.00 | 0.74 (0.45–1.22) | 0.169 | ||
| IV | 1.00 | 1.08 (0.67–1.74) | 0.637 | 1.00 | 0.95 (0.60–1.50) | 0.433 | ||
| Tumor site | 0.340 | 0.501 | ||||||
| Colon | 1.00 | 0.71 (0.47–1.07) | 0.174 | 1.00 | 0.68 (0.44–1.06) | 0.126 | ||
| Rectum | 1.00 | 0.70 (0.41–1.16) | 0.132 | 1.00 | 0.74 (0.42–1.28) | 0.177 | ||
| PAr | ||||||||
| Sex | 0.811 | 0.769 | ||||||
| Male | 1.00 | 0.95 (0.65–1.40) | 0.790 | 1.00 | 0.92 (0.61–1.39) | 0.627 | ||
| Female | 1.00 | 1.11 (0.62–2.00) | 0.936 | 1.00 | 1.14 (0.62–2.11) | 0.782 | ||
| Age | 0.889 | 0.797 | ||||||
| <50 | 1.00 | 1.08 (0.53–2.20) | 0.742 | 1.00 | 1.37 (0.65–2.90) | 0.427 | ||
| ≥50 | 1.00 | 1.06 (0.74–1.52) | 0.890 | 1.00 | 0.97 (0.66–1.42) | 0.812 | ||
| BMI | 0.838 | 0.943 | ||||||
| <23.9 | 1.00 | 1.08 (0.72–1.62) | 0.913 | 1.00 | 1.12 (0.74–1.71) | 0.705 | ||
| ≥23.9 | 1.00 | 1.00 (0.59–1.69) | 0.769 | 1.00 | 0.88 (0.49–1.58) | 0.751 | ||
| Smoking status | 0.452 | 0.425 | ||||||
| Ever | 1.00 | 0.94 (0.61–1.44) | 0.995 | 1.00 | 0.85 (0.54–1.36) | 0.681 | ||
| Never | 1.00 | 1.03 (0.64–1.68) | 0.645 | 1.00 | 1.11 (0.66–1.85) | 0.840 | ||
| Drinking status | 0.882 | 0.945 | ||||||
| Regular | 1.00 | 0.93 (0.49–1.80) | 0.989 | 1.00 | 0.84 (0.42–1.71) | 0.695 | ||
| Never | 1.00 | 1.04 (0.72–1.50) | 0.971 | 1.00 | e | e | ||
| Cancer stage | 0.717 | 0.593 | ||||||
| I–III | 1.00 | 0.89 (0.57–1.41) | 0.492 | 1.00 | 0.88 (0.52–1.47) | 0.418 | ||
| IV | 1.00 | 0.89 (0.55–1.42) | 0.911 | 1.00 | e | 0.825 | ||
| Tumor site | 0.819 | 0.770 | ||||||
| Colon | 1.00 | 1.04 (0.68–1.59) | 0.800 | 1.00 | 0.93 (0.59–1.47) | 0.455 | ||
| Rectum | 1.00 | 0.88 (0.53–1.47) | 0.699 | 1.00 | 0.98 (0.58–1.68) | 0.972 | ||
a Cox proportional hazards regression model adjusted for age (continuous), sex (male, female), BMI (continuous), smoking (ever or never), drinking (regular or never), MET (continuous), total energy intake (continuous), protein intake (continuous), differentiation (well-differentiated, moderately differentiated, poorly differentiated), surgery (yes or no), radiotherapy or chemotherapy (yes or no), and history of cancer in first-degree relatives (yes or no). b The linear trend was conducted using the median values for each quartile of serum concentration. c The interaction term’s significance was assessed using the Wald method to calculate the p-value for interaction, which tested the multiplicative interaction between serum vitamin B6 levels and the respective stratified variable. Abbreviations: PLP, pyridoxal 5′-phosphate; PAr, the ratio of PA over the combined concentrations of PLP and PL.
To address the potential impact of dietary intake and blood sample storage time on biomarker concentrations, we repeated our analyses, further adjusting for these factors in the fully adjusted model. Our results remained consistent. Sensitivity analyses confirmed that the associations of serum PLP levels with both overall and colorectal cancer-specific survival remained significant, even after adjusting for dietary vitamin B6 intake or the timing of blood-sample collection.
4. Discussion
This study demonstrated that colorectal cancer patients with elevated serum PLP levels experienced improved overall and colorectal cancer-specific survival. Additionally, the associations between serum PLP and survival were influenced by the drinking status of patients. No significant association was ascertained between serum PAr and either overall or colorectal cancer-specific survival. Sensitivity analyses confirmed the robustness of these findings.
To date, the existing research on this topic is relatively sparse, with only a limited number of studies having examined the relationship between circulating vitamin B6 concentrations and survival among colorectal cancer patients, with inconsistent findings reported [28,30,31]. Two of these studies, each involving fewer than 500 colorectal cancer cases, found no association between blood PLP levels and overall colorectal cancer survival [28,31], likely due to their small sample sizes. In contrast, a study involving 2031 patients with stage I-III colorectal cancer reported that elevated blood PLP levels were associated with improved overall survival [30], a finding consistent with our own results. Several factors influence PLP concentrations, including dietary intake, use of nutritional supplements, inflammatory status, serum levels of albumin and alkaline phosphatase, lifestyle habits, and exposure to food additives [44,45]. The variation in the estimated associations between PLP and cancer survival across different studies may be attributed to a number of factors, such as differences in study populations, methods of PLP measurement, sample sizes, follow-up periods, and vitamin B6 composition. Additionally, we found a non-linear relationship between serum PLP and survival in colorectal cancer patients, indicating there may be an optimal range for cancer patients. The findings imply that maintaining a specific concentration range of vitamins in the human body may be essential for ensuring optimal performance of the body’s normal physiological function [46,47]. In the process of DNA synthesis, 5,10-methylene tetrahydrofolate plays a role in the conversion of uracil to thymidylate. The regeneration of this enzyme is dependent on the presence of vitamin B6 [48]. There is a paucity of research examining the dose–response relationship between PLP and survival in colorectal cancer patients, and this study offers valuable insights in that area.
Our analysis did not reveal a significant association between low PAr and improved survival in colorectal cancer patients. To date, only one cohort study has investigated the role of PAr in colorectal cancer prognosis [30]. In contrast to our findings, the aforementioned study reported that lower PAr levels were associated with a 55% longer survival in adjusted models [30]. PA is a metabolite of vitamin B6 that is synthesized in the liver by PL and excreted primarily by the kidneys [45]. A study has demonstrated a correlation between elevated PA levels in elderly males and females and impaired kidney function [49]. This finding sheds light on the underlying mechanism contributing to elevated PAr levels in this population. As a result, stronger correlations between PAr and colorectal cancer survival, as reported by Holowatyj et al., were more likely to be observed. The discrepancy between our findings and the aforementioned study could be due to the timing of sample collection. Their study collected samples at least two weeks after treatment, whereas our samples were taken before diagnosis. Post-treatment vitamin B6 concentrations may have been influenced by medical interventions such as medications or surgery. In addition, differences in inflammatory status may explain the conflicting results, with the potential for collinearity between variables in the model that were adjusted for inflammation-related variables. This resulted in a reduction in the predictive value of vitamin B6 for the risk of mortality [50]. PAr, which is negatively correlated with PLP levels, serves as a reliable biomarker of systemic inflammation. It is useful for identifying individuals with elevated inflammatory status, as evidenced by markers like CRP and other inflammatory biomarkers [19,22,23,45,51,52]. Both PAr and inflammation may influence the progression of undiagnosed colorectal cancer, and the observed lack of associations in our study could be partially attributed to unmeasured inflammatory factors. The results of a nested case–control study indicated that PAr may be positively associated with tumor progression in colorectal cancer rather than with its initiation [23]. However, the role of PAr in colorectal cancer prognosis remains unclear, primarily due to limited data. Further study is required to gain a deeper understanding of the potential role of PAr in colorectal cancer prognosis.
Although the exact mechanism by which lower PLP levels contribute to poorer survival outcomes in patients with colorectal cancer is not fully understood, several possible mechanisms have been proposed. Deficiency of PLP may compromise DNA synthesis [53], alter methylation patterns [54], promote angiogenesis [55], increase oxidative stress [56], drive inflammation [57], and impair anti-tumor immunity [58]. It is also possible that the increased recruitment of the PLP cofactor for enzymes in the kynurenine pathway, the synthesis and catabolism of immunomodulatory sphingolipids, and serine hydroxymethylase supporting immune cell proliferation play a role in this process. This is supported by evidence from studies [59,60]. An additional cause of low plasma PLP is increased catabolism of vitamin B6 during inflammation [19]. Chronic inflammation can cause cellular DNA mutation and promote cancer development [61,62,63]. Pyridoxine supplementation significantly enhances the immune response in elderly individuals [64] or patients [65,66]. Therefore, it can be surmised that the association between vitamin B6 levels and mortality risk is mediated by its involvement in immune and inflammatory processes.
The present study revealed a significant interaction between serum PLP levels and alcohol consumption in relation to colorectal cancer survival. The findings suggest that alcohol consumption may elevate mortality in colorectal cancer patients, particularly those with low serum PLP levels. This interaction is consistent with the known biological mechanisms. First, alcohol consumption decreases vitamin B6 levels by interfering with methionine synthase [67], which plays a crucial role in vitamin B6 synthesis and absorption [68]. Second, chronic alcohol consumption decreases glutathione levels, which are synthesized from homocysteine through a process facilitated by two vitamin B6-dependent enzymes [67]. A reduction in glutathione levels might contribute to an increased vulnerability to DNA damage. It is also noteworthy that among patients over 50 years old or those who had never smoked, higher serum PLP levels were associated with longer overall and cancer-specific survival, although the interaction was not statistically significant. Additionally, our findings revealed that elevated serum PLP levels were linked to improved overall survival in males and in patients with overweight or obesity. Although the associations observed in some subgroups did not reach statistical significance due to the limited sample size, the results of the stratified analyses generally aligned with the main analysis.
A substantial amount of research has been conducted to determine the prognostic significance of various factors in colorectal cancer, aiming to identify effective prognostic markers such as epigenetic biomarkers, micro RNAs, and elements of the tumor microenvironment [69,70,71,72]. However, the transfer of these new biomarkers and targets from research to clinical practice requires larger-scale, multicenter trials to confirm their efficacy [70]. The importance of nutrition in cancer prognosis is gaining recognition in current cancer research [73], with nutrient supplementation emerging as a potential adjunctive treatment for colorectal cancer [74,75]. The underlying causes for the low circulating levels of PLP observed in colorectal cancer patients in our study are unclear; however, they may be associated with changes in intestinal microbiota and compromised metabolic function [76,77]. If colorectal cancer patients indeed have reduced absorption or metabolism of B vitamins, then oral supplementation or direct intravenous/intramuscular injection of B vitamins may effectively enhance their vitamin B6 levels [20,78]. In addition, non-pharmacological treatments for colorectal cancer often involve modifications in dietary habits and lifestyle changes [79,80]. Given the high prevalence of vitamin B6 deficiency among this patient group in China and the correlation between high PLP levels and improved survival rates, adjusting nutritional practices to address vitamin B6 deficiency could have significant implications for improving patient outcomes. Thus, the findings from this study provide valuable insights for developing effective nutritional strategies tailored for colorectal cancer patients [81].
The prospective design and the use of a highly accurate and precise LC-MS/MS-based method for measuring serum vitamin B6 levels are the two principal strengths of this study. Additionally, the study benefits from a considerable sample size, a lengthy follow-up period, detailed questionnaire data, and adjustments for various potential confounding factors. To our knowledge, this research is the first to demonstrate the association between serum vitamin B6 and survival outcomes in colorectal cancer patients in China. However, this study has some limitations. Firstly, the majority of participants in this study had serum PLP concentrations that were lower than those reported in previous studies. Nevertheless, it should be noted that the degree of the detection coefficients of variations in our data was relatively low (less than 15%), indicating that the serum PLP levels detected with the same method likely had minimal impact on the estimates. Secondly, vitamin B6 is present in foods like meat, which has been associated with an increased risk of cancer, while vegetables and fruits contain other nutrients that may have cancer-fighting properties. These food sources or compounds could potentially offset the effects of vitamin B6. Thus, it is a challenge to separate the independent impact of vitamin B6 on colorectal cancer prognosis from that of other foods or nutrients. Thirdly, as this is an observational study, residual confounding may have constrained our ability to establish causality. However, risk estimates remained unvaried following adjustment for diverse dietary and lifestyle factors, thereby suggesting that residual confounding is unlikely to account for our findings. Fourthly, serum vitamin B6 concentrations were only measured at baseline, and dynamic changes during the follow-up were not tracked. However, several cohort studies have shown that serum vitamin B6 levels tend to remain relatively stable over time [26]. Finally, the results of this study are based on the Chinese population, and caution is required when generalizing the findings.
5. Conclusions
This cohort study indicates that higher serum PLP levels may be associated with improved overall survival and colorectal cancer-specific survival. In contrast, PAr did not show a significant association with either overall survival or colorectal cancer-specific survival. Our findings underscore the potential importance of vitamin B6 in colorectal cancer prognosis and provide novel evidence suggesting that increased vitamin B6 intake could be beneficial for colorectal cancer patients.
Acknowledgments
We express our sincere gratitude to all participants of the study for their invaluable participation.
Author Contributions
Conceptualization, Data curation, Formal analysis, Methodology, Visualization, Writing—original draft, X.L.; Data curation, Methodology, Investigation, L.X.; Resources, Investigation, Q.-J.O.; Investigation, H.X.; Investigation, Y.-Y.C.; Conceptualization, Resources, Writing—review and editing, Y.-J.F.; Conceptualization, Data curation, Funding acquisition, Methodology, Project administration, Resources, Supervision, Visualization, Writing—original draft, Writing—review and editing, C.-X.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Participants provided written informed consent upon inclusion in the study. The study was approved by the Ethical Committee of the School of Public Health, Sun Yat-sen University (approval number 2020-116 on 21 August 2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request. The data are not publicly available due to technical limitations.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This study was supported by the Guangdong Basic and Applied Basic Research Foundation (No. 2021A1515011751). The funder was not involved in the design of the study, data collection and analysis, or the writing of the manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2019 Colorectal Cancer Collaborators Global, regional, and national burden of colorectal cancer and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Gastroenterol. Hepatol. 2022;7:627–647. doi: 10.1016/S2468-1253(22)00044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy C.C., Zaki T.A. Changing epidemiology of colorectal cancer—Birth cohort effects and emerging risk factors. Nat. Rev. Gastroenterol. Hepatol. 2024;21:25–34. doi: 10.1038/s41575-023-00841-9. [DOI] [PubMed] [Google Scholar]
- 4.Baidoun F., Elshiwy K., Elkeraie Y., Merjaneh Z., Khoudari G., Sarmini M.T., Gad M., Al-Husseini M., Saad A. Colorectal Cancer Epidemiology: Recent Trends and Impact on Outcomes. Curr. Drug Targets. 2021;22:998–1009. doi: 10.2174/1389450121999201117115717. [DOI] [PubMed] [Google Scholar]
- 5.Biller L.H., Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669–685. doi: 10.1001/jama.2021.0106. [DOI] [PubMed] [Google Scholar]
- 6.Franco C.N., Seabrook L.J., Nguyen S.T., Leonard J.T., Albrecht L.V. Simplifying the B Complex: How Vitamins B6 and B9 Modulate One Carbon Metabolism in Cancer and Beyond. Metabolites. 2022;12:961. doi: 10.3390/metabo12100961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedlak J.C., Yilmaz Ö.H., Roper J. Metabolism and Colorectal Cancer. Annu. Rev. Pathol. 2023;18:467–492. doi: 10.1146/annurev-pathmechdis-031521-041113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeong Y.J., Rogers T.J., Anderson C.E., Lien E.C. Tumor lipid metabolism: A mechanistic link between diet and cancer progression. Curr. Opin. Biotechnol. 2023;84:102993. doi: 10.1016/j.copbio.2023.102993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah U.A., Iyengar N.M. Plant-Based and Ketogenic Diets as Diverging Paths to Address Cancer: A Review. JAMA Oncol. 2022;8:1201–1208. doi: 10.1001/jamaoncol.2022.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zitvogel L., Pietrocola F., Kroemer G. Nutrition, inflammation and cancer. Nat. Immunol. 2017;18:843–850. doi: 10.1038/ni.3754. [DOI] [PubMed] [Google Scholar]
- 11.Nasir A., Bullo M.M.H., Ahmed Z., Imtiaz A., Yaqoob E., Jadoon M., Ahmed H., Afreen A., Yaqoob S. Nutrigenomics: Epigenetics and cancer prevention: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2020;60:1375–1387. doi: 10.1080/10408398.2019.1571480. [DOI] [PubMed] [Google Scholar]
- 12.Stidley C.A., Picchi M.A., Leng S., Willink R., Crowell R.E., Flores K.G., Kang H., Byers T., Gilliland F.D., Belinsky S.A. Multivitamins, folate, and green vegetables protect against gene promoter methylation in the aerodigestive tract of smokers. Cancer Res. 2010;70:568–574. doi: 10.1158/0008-5472.CAN-09-3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardy T.M., Tollefsbol T.O. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics. 2011;3:503–518. doi: 10.2217/epi.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duthie S.J. Folate and cancer: How DNA damage, repair and methylation impact on colon carcinogenesis. J. Inherit. Metab. Dis. 2011;34:101–109. doi: 10.1007/s10545-010-9128-0. [DOI] [PubMed] [Google Scholar]
- 15.Kawakita D., Matsuo K., Sato F., Oze I., Hosono S., Ito H., Watanabe M., Yatabe Y., Hanai N., Hasegawa Y., et al. Association between dietary folate intake and clinical outcome in head and neck squamous cell carcinoma. Ann. Oncol. 2012;23:186–192. doi: 10.1093/annonc/mdr057. [DOI] [PubMed] [Google Scholar]
- 16.Mikkelsen K., Stojanovska L., Apostolopoulos V. The Effects of Vitamin B in Depression. Curr. Med. Chem. 2016;23:4317–4337. doi: 10.2174/0929867323666160920110810. [DOI] [PubMed] [Google Scholar]
- 17.Bassett J.K., Brinkman M.T., Dugué P.A., Ueland P.M., Midttun Ø., Ulvik A., Bolton D., Southey M.C., English D.R., Milne R.L., et al. Circulating concentrations of B group vitamins and urothelial cell carcinoma. Int. J. Cancer. 2019;144:1909–1917. doi: 10.1002/ijc.31927. [DOI] [PubMed] [Google Scholar]
- 18.Leklem J.E. Vitamin B-6: A status report. J. Nutr. 1990;120((Suppl. S11)):1503–1507. doi: 10.1093/jn/120.suppl_11.1503. [DOI] [PubMed] [Google Scholar]
- 19.Ulvik A., Midttun Ø., Pedersen E.R., Eussen S.J., Nygård O., Ueland P.M. Evidence for increased catabolism of vitamin B-6 during systemic inflammation. Am. J. Clin. Nutr. 2014;100:250–255. doi: 10.3945/ajcn.114.083196. [DOI] [PubMed] [Google Scholar]
- 20.Ryan K.M., Allers K.A., Harkin A., McLoughlin D.M. Blood plasma B vitamins in depression and the therapeutic response to electroconvulsive therapy. Brain Behav. Immun. Health. 2020;4:100063. doi: 10.1016/j.bbih.2020.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zuo H., Ueland P.M., Midttun Ø., Vollset S.E., Tell G.S., Theofylaktopoulou D., Travis R.C., Boutron-Ruault M.C., Fournier A., Severi G., et al. Results from the European Prospective Investigation into Cancer and Nutrition Link Vitamin B6 Catabolism and Lung Cancer Risk. Cancer Res. 2018;78:302–308. doi: 10.1158/0008-5472.CAN-17-1923. [DOI] [PubMed] [Google Scholar]
- 22.Zuo H., Tell G.S., Ueland P.M., Nygård O., Vollset S.E., Midttun Ø., Meyer K., Ulvik A. The PAr index, an indicator reflecting altered vitamin B-6 homeostasis, is associated with long-term risk of stroke in the general population: The Hordaland Health Study (HUSK) Am. J. Clin. Nutr. 2018;107:105–112. doi: 10.1093/ajcn/nqx012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gylling B., Myte R., Schneede J., Hallmans G., Häggström J., Johansson I., Ulvik A., Ueland P.M., Van Guelpen B., Palmqvist R. Vitamin B-6 and colorectal cancer risk: A prospective population-based study using 3 distinct plasma markers of vitamin B-6 status. Am. J. Clin. Nutr. 2017;105:897–904. doi: 10.3945/ajcn.116.139337. [DOI] [PubMed] [Google Scholar]
- 24.Neuhouser M.L., Cheng T.Y., Beresford S.A., Brown E., Song X., Miller J.W., Zheng Y., Thomson C.A., Shikany J.M., Vitolins M.Z., et al. Red blood cell folate and plasma folate are not associated with risk of incident colorectal cancer in the Women’s Health Initiative observational study. Int. J. Cancer. 2015;137:930–939. doi: 10.1002/ijc.29453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinstein S.J., Albanes D., Selhub J., Graubard B., Lim U., Taylor P.R., Virtamo J., Stolzenberg-Solomon R. One-carbon metabolism biomarkers and risk of colon and rectal cancers. Cancer Epidemiol. Biomark. Prev. 2008;17:3233–3240. doi: 10.1158/1055-9965.EPI-08-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eussen S.J., Vollset S.E., Hustad S., Midttun Ø., Meyer K., Fredriksen A., Ueland P.M., Jenab M., Slimani N., Boffetta P., et al. Plasma vitamins B2, B6, and B12, and related genetic variants as predictors of colorectal cancer risk. Cancer Epidemiol. Biomark. Prev. 2010;19:2549–2561. doi: 10.1158/1055-9965.EPI-10-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dray X., Boutron-Ruault M.C., Bertrais S., Sapinho D., Benhamiche-Bouvier A.M., Faivre J. Influence of dietary factors on colorectal cancer survival. Gut. 2003;52:868–873. doi: 10.1136/gut.52.6.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung E.Y., Roxburgh C.S., Talwar D., O’Reilly D.S., McKee R.F., Horgan P.G., McMillan D.C. The relationships between plasma and red cell vitamin B2 and B6 concentrations and the systemic and local inflammatory responses in patients with colorectal cancer. Nutr. Cancer. 2012;64:515–520. doi: 10.1080/01635581.2012.661512. [DOI] [PubMed] [Google Scholar]
- 29.Lochhead P., Nishihara R., Qian Z.R., Mima K., Cao Y., Sukawa Y., Kim S.A., Inamura K., Zhang X., Wu K., et al. Postdiagnostic intake of one-carbon nutrients and alcohol in relation to colorectal cancer survival. Am. J. Clin. Nutr. 2015;102:1134–1141. doi: 10.3945/ajcn.115.115162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holowatyj A.N., Ose J., Gigic B., Lin T., Ulvik A., Geijsen A., Brezina S., Kiblawi R., van Roekel E.H., Baierl A., et al. Higher vitamin B6 status is associated with improved survival among patients with stage I-III colorectal cancer. Am. J. Clin. Nutr. 2022;116:303–313. doi: 10.1093/ajcn/nqac090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Je Y., Lee J.E., Ma J., Zhang X., Cho E., Rosner B., Selhub J., Fuchs C.S., Meyerhardt J., Giovannucci E. Prediagnostic plasma vitamin B6 (pyridoxal 5′-phosphate) and survival in patients with colorectal cancer. Cancer Causes Control. 2013;24:719–729. doi: 10.1007/s10552-013-0152-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong X., Fang Y.J., Pan Z.Z., Li B., Wang L., Zheng M.C., Chen Y.M., Zhang C.X. Dietary fat, fatty acid intakes and colorectal cancer risk in Chinese adults: A case-control study. Eur. J. Cancer Prev. 2013;22:438–447. doi: 10.1097/CEJ.0b013e32835e88c4. [DOI] [PubMed] [Google Scholar]
- 33.Ma T., Tu K., Ou Q., Fang Y., Zhang C. Comparing the Associations of Dietary Patterns Identified through Principal Component Analysis and Cluster Analysis with Colorectal Cancer Risk: A Large Case-Control Study in China. Nutrients. 2023;16:147. doi: 10.3390/nu16010147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meisser Redeuil K., Longet K., Bénet S., Munari C., Campos-Giménez E. Simultaneous quantification of 21 water soluble vitamin circulating forms in human plasma by liquid chromatography-mass spectrometry. J. Chromatogr. A. 2015;1422:89–98. doi: 10.1016/j.chroma.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 35.Midttun O., Hustad S., Solheim E., Schneede J., Ueland P.M. Multianalyte quantification of vitamin B6 and B2 species in the nanomolar range in human plasma by liquid chromatography-tandem mass spectrometry. Clin. Chem. 2005;51:1206–1216. doi: 10.1373/clinchem.2005.051169. [DOI] [PubMed] [Google Scholar]
- 36.Xu L., Fang Y.J., Che M.M., Abulimiti A., Huang C.Y., Zhang C.X. Association of Serum Pyridoxal-5′-Phosphate, Pyridoxal, and PAr with Colorectal Cancer Risk: A Large-Scale Case-Control Study. Nutrients. 2022;14:2389. doi: 10.3390/nu14122389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu L., Wu Q.X., Li X., Fang Y.J., Zhou R.L., Che M.M., Ma T., Zhang C.X. Serum flavin mononucleotide but not riboflavin is inversely associated with the risk of colorectal cancer. Food Funct. 2022;13:12246–12257. doi: 10.1039/D2FO02580A. [DOI] [PubMed] [Google Scholar]
- 38.Zhang C.X., Ho S.C. Validity and reproducibility of a food frequency Questionnaire among Chinese women in Guangdong province. Asia Pac. J. Clin. Nutr. 2009;18:240–250. [PubMed] [Google Scholar]
- 39.Yang Y.X., Wang G.Y., Pan X.C. China Food Composition Table. Peking University Medical Press; Beijing, China: 2002. [Google Scholar]
- 40.Willett W., Stampfer M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986;124:17–27. doi: 10.1093/oxfordjournals.aje.a114366. [DOI] [PubMed] [Google Scholar]
- 41.Ainsworth B.E., Haskell W.L., Whitt M.C., Irwin M.L., Swartz A.M., Strath S.J., O’Brien W.L., Bassett D.R., Jr., Schmitz K.H., Emplaincourt P.O., et al. Compendium of physical activities: An update of activity codes and MET intensities. Med. Sci. Sports Exerc. 2000;32:S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 42.Ainsworth B.E., Haskell W.L., Herrmann S.D., Meckes N., Bassett D.R., Jr., Tudor-Locke C., Greer J.L., Vezina J., Whitt-Glover M.C., Leon A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011;43:1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 43.Rumgay H., Murphy N., Ferrari P., Soerjomataram I. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients. 2021;13:3173. doi: 10.3390/nu13093173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Midttun Ø., Theofylaktopoulou D., McCann A., Fanidi A., Muller D.C., Meyer K., Ulvik A., Zheng W., Shu X.O., Xiang Y.B., et al. Circulating concentrations of biomarkers and metabolites related to vitamin status, one-carbon and the kynurenine pathways in US, Nordic, Asian, and Australian populations. Am. J. Clin. Nutr. 2017;105:1314–1326. doi: 10.3945/ajcn.116.151241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueland P.M., Ulvik A., Rios-Avila L., Midttun Ø., Gregory J.F. Direct and Functional Biomarkers of Vitamin B6 Status. Annu. Rev. Nutr. 2015;35:33–70. doi: 10.1146/annurev-nutr-071714-034330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bjelakovic G., Gluud C. Vitamin and mineral supplement use in relation to all-cause mortality in the Iowa Women’s Health Study. Arch. Intern. Med. 2011;171:1633–1634. doi: 10.1001/archinternmed.2011.459. [DOI] [PubMed] [Google Scholar]
- 47.Vrolijk M.F., Opperhuizen A., Jansen E., Hageman G.J., Bast A., Haenen G. The vitamin B6 paradox: Supplementation with high concentrations of pyridoxine leads to decreased vitamin B6 function. Toxicol. Vitr. 2017;44:206–212. doi: 10.1016/j.tiv.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 48.Selhub J. Folate, vitamin B12 and vitamin B6 and one carbon metabolism. J. Nutr. Health Aging. 2002;6:39–42. [PubMed] [Google Scholar]
- 49.Bates C.J., Pentieva K.D., Prentice A. An appraisal of vitamin B6 status indices and associated confounders, in young people aged 4-18 years and in people aged 65 years and over, in two national British surveys. Public Health Nutr. 1999;2:529–535. doi: 10.1017/S1368980099000713. [DOI] [PubMed] [Google Scholar]
- 50.Wang P., Huang J., Xue F., Abuduaini M., Tao Y., Liu H. Associations of serum vitamin B6 status with the risks of cardiovascular, cancer, and all-cause mortality in the elderly. Front. Immunol. 2024;15:1354958. doi: 10.3389/fimmu.2024.1354958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwak H.K., Hansen C.M., Leklem J.E., Hardin K., Shultz T.D. Improved vitamin B-6 status is positively related to lymphocyte proliferation in young women consuming a controlled diet. J. Nutr. 2002;132:3308–3313. doi: 10.1093/jn/132.11.3308. [DOI] [PubMed] [Google Scholar]
- 52.Chiang E.P., Smith D.E., Selhub J., Dallal G., Wang Y.C., Roubenoff R. Inflammation causes tissue-specific depletion of vitamin B6. Arthritis Res. Ther. 2005;7:R1254–R1262. doi: 10.1186/ar1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ames B.N. DNA damage from micronutrient deficiencies is likely to be a major cause of cancer. Mutat. Res. 2001;475:7–20. doi: 10.1016/S0027-5107(01)00070-7. [DOI] [PubMed] [Google Scholar]
- 54.Anderson O.S., Sant K.E., Dolinoy D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012;23:853–859. doi: 10.1016/j.jnutbio.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Matsubara K., Komatsu S., Oka T., Kato N. Vitamin B6-mediated suppression of colon tumorigenesis, cell proliferation, and angiogenesis (review) J. Nutr. Biochem. 2003;14:246–250. doi: 10.1016/S0955-2863(03)00009-3. [DOI] [PubMed] [Google Scholar]
- 56.Komatsu S., Watanabe H., Oka T., Tsuge H., Kat N. Dietary vitamin B6 suppresses colon tumorigenesis, 8-hydroxyguanosine, 4-hydroxynonenal, and inducible nitric oxide synthase protein in azoxymethane-treated mice. J. Nutr. Sci. Vitaminol. 2002;48:65–68. doi: 10.3177/jnsv.48.65. [DOI] [PubMed] [Google Scholar]
- 57.Shen J., Lai C.Q., Mattei J., Ordovas J.M., Tucker K.L. Association of vitamin B-6 status with inflammation, oxidative stress, and chronic inflammatory conditions: The Boston Puerto Rican Health Study. Am. J. Clin. Nutr. 2010;91:337–342. doi: 10.3945/ajcn.2009.28571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bargiela D., Cunha P.P., Veliça P., Foskolou I.P., Barbieri L., Rundqvist H., Johnson R.S. Vitamin B6 Metabolism Determines T Cell Anti-Tumor Responses. Front. Immunol. 2022;13:837669. doi: 10.3389/fimmu.2022.837669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakakeeny L., Roubenoff R., Obin M., Fontes J.D., Benjamin E.J., Bujanover Y., Jacques P.F., Selhub J. Plasma pyridoxal-5-phosphate is inversely associated with systemic markers of inflammation in a population of U.S. adults. J. Nutr. 2012;142:1280–1285. doi: 10.3945/jn.111.153056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paul L., Ueland P.M., Selhub J. Mechanistic perspective on the relationship between pyridoxal 5′-phosphate and inflammation. Nutr. Rev. 2013;71:239–244. doi: 10.1111/nure.12014. [DOI] [PubMed] [Google Scholar]
- 61.Elinav E., Nowarski R., Thaiss C.A., Hu B., Jin C., Flavell R.A. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat. Rev. Cancer. 2013;13:759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 62.Colotta F., Allavena P., Sica A., Garlanda C., Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: Links to genetic instability. Carcinogenesis. 2009;30:1073–1081. doi: 10.1093/carcin/bgp127. [DOI] [PubMed] [Google Scholar]
- 63.Federico A., Morgillo F., Tuccillo C., Ciardiello F., Loguercio C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer. 2007;121:2381–2386. doi: 10.1002/ijc.23192. [DOI] [PubMed] [Google Scholar]
- 64.Talbott M.C., Miller L.T., Kerkvliet N.I. Pyridoxine supplementation: Effect on lymphocyte responses in elderly persons. Am. J. Clin. Nutr. 1987;46:659–664. doi: 10.1093/ajcn/46.4.659. [DOI] [PubMed] [Google Scholar]
- 65.Casciato D.A., McAdam L.P., Kopple J.D., Bluestone R., Goldberg L.S., Clements P.J., Knutson D.W. Immunologic abnormalities in hemodialysis patients: Improvement after pyridoxine therapy. Nephron. 1984;38:9–16. doi: 10.1159/000183270. [DOI] [PubMed] [Google Scholar]
- 66.Cheng C.H., Chang S.J., Lee B.J., Lin K.L., Huang Y.C. Vitamin B6 supplementation increases immune responses in critically ill patients. Eur. J. Clin. Nutr. 2006;60:1207–1213. doi: 10.1038/sj.ejcn.1602439. [DOI] [PubMed] [Google Scholar]
- 67.Larsson S.C., Giovannucci E., Wolk A. Vitamin B6 intake, alcohol consumption, and colorectal cancer: A longitudinal population-based cohort of women. Gastroenterology. 2005;128:1830–1837. doi: 10.1053/j.gastro.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 68.Yasuda H., Hatano T., Honda T., Tsutsui M., Hattori N., Ando M., Komatsu N. Vitamin B6 Deficiency Anemia Attributed to Levodopa/Carbidopa Intestinal Gel Therapy for Parkinson’s Disease: A Diagnostic Pitfall for Myelodysplastic Syndrome with Ring Sideroblasts. Intern. Med. 2022;61:3719–3722. doi: 10.2169/internalmedicine.9577-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gallo G., Vescio G., De Paola G., Sammarco G. Therapeutic Targets and Tumor Microenvironment in Colorectal Cancer. J. Clin. Med. 2021;10:2295. doi: 10.3390/jcm10112295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo X.J., Zhao Q., Liu J., Zheng J.B., Qiu M.Z., Ju H.Q., Xu R.H. Novel Genetic and Epigenetic Biomarkers of Prognostic and Predictive Significance in Stage II/III Colorectal Cancer. Mol. Ther. 2021;29:587–596. doi: 10.1016/j.ymthe.2020.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao Q., Tian Y., Deng Z., Yang F., Chen E. Epigenetic Alteration in Colorectal Cancer: Potential Diagnostic and Prognostic Implications. Int. J. Mol. Sci. 2024;25:3358. doi: 10.3390/ijms25063358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giannopoulou N., Constantinou C. Recent Developments in Diagnostic and Prognostic Biomarkers for Colorectal Cancer: A Narrative Review. Oncology. 2023;101:675–684. doi: 10.1159/000531474. [DOI] [PubMed] [Google Scholar]
- 73.Chan D.S.M., Cariolou M., Markozannes G., Balducci K., Vieira R., Kiss S., Becerra-Tomás N., Aune D., Greenwood D.C., González-Gil E.M., et al. Post-diagnosis dietary factors, supplement use and colorectal cancer prognosis: A Global Cancer Update Programme (CUP Global) systematic literature review and meta-analysis. Int. J. Cancer. 2024;155:445–470. doi: 10.1002/ijc.34906. [DOI] [PubMed] [Google Scholar]
- 74.Henriksen H.B., Ræder H., Bøhn S.K., Paur I., Kværner A.S., Billington S., Eriksen M.T., Wiedsvang G., Erlund I., Færden A., et al. The Norwegian dietary guidelines and colorectal cancer survival (CRC-NORDIET) study: A food-based multicentre randomized controlled trial. BMC Cancer. 2017;17:83. doi: 10.1186/s12885-017-3072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ravasco P., Monteiro-Grillo I., Camilo M. Individualized nutrition intervention is of major benefit to colorectal cancer patients: Long-term follow-up of a randomized controlled trial of nutritional therapy. Am. J. Clin. Nutr. 2012;96:1346–1353. doi: 10.3945/ajcn.111.018838. [DOI] [PubMed] [Google Scholar]
- 76.Rosenberg J., Ischebeck T., Commichau F.M. Vitamin B6 metabolism in microbes and approaches for fermentative production. Biotechnol. Adv. 2017;35:31–40. doi: 10.1016/j.biotechadv.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 77.Feng Z., Hua J., Guo F., Liu Z., Zhao Y., Wu W. A retrospective analysis of vitamin B6 deficiency and associated changes of gut microbes in Crohn’s disease. Eur. J. Clin. Nutr. 2023;77:1034–1043. doi: 10.1038/s41430-023-01324-5. [DOI] [PubMed] [Google Scholar]
- 78.Zheng C., Ge Q., Luo C., Hu L., Shen Y., Xue Q. Enteral nutrition improves the prognosis and immune nutritional status of patients in the cardiothoracic surgery recovery unit: A propensity score-matched analysis. Clin. Nutr. 2022;41:2699–2705. doi: 10.1016/j.clnu.2022.10.012. [DOI] [PubMed] [Google Scholar]
- 79.Martínez-Montoro J.I., Martínez-Sánchez M.A., Balaguer-Román A., Gil-Martínez J., Mesa-López M.J., Egea-Valenzuela J., Ruiz-Alcaraz A.J., Queipo-Ortuño M.I., Ferrer M., Fernández-García J.C., et al. Dietary modulation of gut microbiota in patients with colorectal cancer undergoing surgery: A review. Int. J. Surg. 2022;104:106751. doi: 10.1016/j.ijsu.2022.106751. [DOI] [PubMed] [Google Scholar]
- 80.Duncan M., Moschopoulou E., Herrington E., Deane J., Roylance R., Jones L., Bourke L., Morgan A., Chalder T., Thaha M.A., et al. Review of systematic reviews of non-pharmacological interventions to improve quality of life in cancer survivors. BMJ Open. 2017;7:e015860. doi: 10.1136/bmjopen-2017-015860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen Y., Liu B.L., Shang B., Chen A.S., Liu S.Q., Sun W., Yin H.Z., Yin J.Q., Su Q. Nutrition support in surgical patients with colorectal cancer. World J. Gastroenterol. 2011;17:1779–1786. doi: 10.3748/wjg.v17.i13.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of our study are available from the corresponding author upon reasonable request. The data are not publicly available due to technical limitations.