Abstract
Insomnia (sleeplessness) is a potential symptom of stress-induced depression/anxiety (DA), which induces TNF-α expression. Therefore, this study aimed to examine the effect of Lactobacillus (Lactiplantibacillus) plantarum P72, isolated as a strain suppressing lipopolysaccharide-induced expression of TNF-α in Caco2 cells, on DA and insomnia in immobilization stress (IS)- or cultured fecal microbiota (cFM)-treated mice. Oral administration of live or heat-killed P72 (hP72) reduced IS- or cFM-induced DA-like behaviors. They also reduced sleep latency time (SLT) and enhanced sleep duration (SLD). Additionally, P72 upregulated γ-aminobutyric acid (GABA), GABAA receptor α1, serotonin, and 5-HT1A receptor expression, which were downregulated by IS or cFM. Hempseed oil (HO) alone was ineffective against IS-induced DA- and insomnia-like behaviors, but its combination with P72 (PH) or hP72 (hPH) showed enhanced efficacy, reducing DA- and insomnia-like behaviors more strongly than P72 or HO alone. These also reduced the number of NF-κB-positive cells and the expression of TNF-α in the prefrontal cortex and colon. These results imply that P72 and its combination with HO can alleviate DA and insomnia by upregulating serotonergic and GABAergic systems through the suppression of NF-κB signaling.
Keywords: stress, depression, sleep disturbance, Lactobacillus plantarum, hempseed oil, neuroinflammation
1. Introduction
Chronic exposure to stress, including social defeat and immobilization, triggers the release of adrenal hormones like noradrenaline, adrenaline, and glucocorticoids, as well as proinflammatory cytokines like TNF-α and IL-6, leading to depression and anxiety (DA) [1,2]. Stress-induced DA fluctuates the release of serotonin (5-HT) and γ-aminobutyric acid (GABA) from enterochromaffin and neural cells [3,4]. DA triggers sleep disturbance (SD): the majority of patients with DA suffer from insomnia [5]. Therefore, antidepressants and serotonin inducers are often used to treat both DA and insomnia [3,6].
Many lactobacilli and bifidobacteria are known to exhibit beneficial activities against immune and psychiatric disorders by regulating the immune, endocrine, and neural systems [7,8,9]. Lactobacillus plantarum D-9, Lactobacillus reuteri NK33, Bifidobacterium longum 1714, B. longum CCFM687, and Bifidobacterium adolescentis NK98 alleviate DA-like behavior in stress-induced rodents [10,11,12,13]. L. plantarum JYLP-326 and Bifidobacterium breve CCFM1025 improve stress-induced sleep disorder in mice [14,15]. L. plantarum P8 alleviates anxiety in stressed adults [16]. B. longum reduces the psychological stress score in healthy adults [17]. B. longum NCC3001 decreases DA scores in patients with irritable bowel syndrome [18]. L. plantarum PS128 attenuates DA-related symptoms in volunteers with self-reported insomnia [19]. NVP1704 (an NK33 and NK98 mix) also alleviates stress-induced DA in mice and depressive symptoms and sleeplessness in healthy adults [20]. Nevertheless, the action mechanism of probiotics against sleep disorders including insomnia is not fully understood.
Therefore, we isolated Lactobacillus (Lactiplantibacillus) plantarum P72, which increased the secretion of serotonin in vitro, from the bacterial collection of healthy human feces and examined the effect of P72 (live), heat-killed P72 (hP72), and their combination with hempseed oil (HO) on immobilization stress (IS)- or cultured fecal microbiota (cFM)-induced depression and SD (insomnia) in mice.
2. Materials and Methods
2.1. Culture of P72
P72 (KCCM13445P, deposited in the Korean Culture Center for Microorganisms) was cultured in general media such as De Man—Rogosa—Sharpe (MRS) broth and centrifuged (5000× g, 4 °C, and 20 min). The precipitate was suspended in distilled water (for the in vitro experiment) or 0.1% trehalose (for the in vivo experiment) and freeze-dried. The number of live P72 was determined using plate counting on MRS agar plates. Heat-killed P72 (hP72) was prepared by heating P72 suspended in distilled water (for the in vitro experiment) or 1% trehalose (for the in vivo experiment) at 80 °C for 15 min and freeze-drying.
2.2. Culture of SH-SY5Y and Caco-2 Cells
SH-SY5Y or Caco-2 cells were cultured as previously reported [20]. SH-SY5Y cells (1 × 106 cells/mL) were incubated with corticosterone (300 μM, Sigma [20]) in the absence or presence of probiotics (1 × 105 CFU/mL) for 24 h. In the supernatant of the culture, the serotonin level was assayed using its assay kit (DLD Diagnostika GmbH, Hamburg, Germany). Caco-2 cells (1 × 106 cells/mL) were incubated with lipopolysaccharide (LPS, 100 ng/mL, Sigma [20]) in the presence or absence of probiotics (1 × 105 CFU/mL) for 24 h. The TNF-α concentration was determined using its enzyme-linked immunosorbent assay (ELISA) kit (R&D system).
2.3. Animals
C57BL/6 mice (male, 18–21 g, 6 weeks old) were obtained from Koatech Co., Ltd. (PyungTaek-shi, Republic of Korea) and kept in plastic cages with a 5 cm raised wire floor under controlled conditions, as previously reported [20]. All animal experiments were ethically approved by the Committee for the Care and Use of Laboratory Animals in Kyung Hee University (IACC, KHUASP(SE)-23545, 14 March 2023) and were conducted according to the Ethical Policies and Guidelines of the University for Laboratory Animals Care and Use, and the Use of Laboratory Animals and ARRIVE guidelines [21].
2.4. Induction of DA- and Insomina-like Symptom (DILS) in Mice
Experiment 1—Mice were divided into 3 groups (NC, IS, and P72) consisting of 8 mice. IS and P72 groups were exposed to IS daily for 5 days [13]. Test agents (IS, saline; P72, 1 × 109 CFU/mouse/day of P72) were orally administered daily for 5 days from next day after the final IS treatment. The IS-untreated normal control (NC) group was gavaged with saline.
Experiment 2—Mice were divided into 3 groups (NC, cFM, and P72) consisting of 6 mice. The cultured fecal microbiota (2 × 108 colony-forming unit [CFU]/mouse, suspended in 0.1 mL of saline; the number of cultured fecal microbiota of patients with depression and inflammatory bowel disease was counted using general anaerobic medium (Nissui Pharm. Co., Tokyo, Japan)). The culture fecal microbiota, which were prepared as previously reported [22], were orally gavaged in cFM and P72 groups daily for 5 days. Thereafter, test agents (cFM, saline; P72, 1 × 109 CFU/mouse/day of P72) were orally administered daily for 7 days. NC was treated with saline.
Experiment 3—Mice were divided into 7 groups (NC, IS, P72L, P72H, HOH, PH, and DP) consisting of 8 mice. Mice (IS, P72L, P72H, HOH, PH, and DP) were treated with IS daily for 5 days. Thereafter, test agents (IS, saline; P72L, 0.4 × 109 CFU/mouse/day of P72; P72H, 1 × 109 CFU/mouse/day of P72; HOH, 0.24 g/kg of HO; PH, 0.4 × 109 CFU/mouse/day of P72 and 0.12 g/kg of HO; and DP, 20 mg/kg of diphenhydramine) were orally administered (for P72 and HO) or intraperitoneally (for diphenhydramine) daily for 7 days. NC was treated with saline.
Experiment 4—Mice were separated into 3 groups (NC, IS, hP72, hPH) consisting of 6 mice. Mice (IS, hP72, and hPH) were treated with IS daily for 5 days. Thereafter, test agents (IS, vehicle alone; hP72, 1 × 109 CFU/mouse/day of hP72; hPH, 1 × 109 CFU/mouse/day of hP72 and 0.12 g/kg of HO) were orally administered daily for 7 days. NC was treated with saline alone.
DA-like behaviors (total distance moved (TD), distance moved in the center area (DC) and time moved in the center area (TC) in the open field test (OFT), time moved in open arms (OT) and entries in open arms (OE) in the elevated plus maze task (EPMT), and immobility time (IT) in the tail suspension test (TST)) were measured (between 1:00 and 5:00 p.m.), as previously reported [20]. On next day (between 1:00 and 5:00 p.m.), sleep latency time (SLT) and sleep duration (SLD) were measured after the peritoneal injection of pentobarbital sodium (PS) or exposure to isoflurane (IF) saturated in the box [23]. Detailed protocols are described in the Supplementary Methods.
Mice were euthanatized by exposure to CO2 in a plastic box 24 h after the end of the final experiment, and cervically dislocated. Brain and colon tissues were collected and kept in the freezer (−80 °C) for the measurement of biomarkers. For the immunofluorescence staining, mice were transcardially perfused, as previously reported [13].
2.5. ELISA, Quantitative Polymerase Chain Reaction (qPCR), and Immunofluorescence Staining
The expression levels of inflammatory biomarkers in the brain (prefrontal cortex) and colon tissues were assayed using ELISA [20]. Serotonin 1A receptor (5-HT1AR), 5-HT1BR, melatonin receptor type 1 (MT1R), MT2R, GABA type A receptor subunit alpha1 (GABAARα1), GABAARα2, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels were assayed using qPCR. Primers are indicated in Supplementary Table S1. Their tissue sections were immunofluorescence-stained, as previously reported [13,20]. Detailed methods are indicated in the Supplementary Methods.
2.6. Whole Genome Analysis
The sequencing libraries for the P72 whole genome analysis were prepared, as previously reported [24], and the P72 genome sequence (6 contigs) was obtained.
2.7. Statistical Analysis
Experimental data are described as mean ± standard deviation (SD) and the significance was analyzed by a one-way ANOVA followed by Tukey’s multiple comparison test (p < 0.05), using a GraphPad Prism 9. The resulting F-value and degrees of freedom for datasets of each experiment were discussed.
3. Results
3.1. P72 Increased the Secretion of Serotonin in Corticosterone-Treated SH-SY5Y Cells
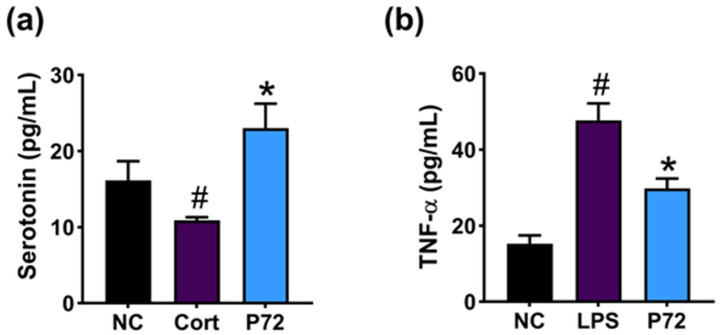
First, we screened probiotics inducing serotonin secretion in corticosterone-treated SH-SY5Y cells from the bacterial collection of healthy human feces. Among tested lactobacilli, P72 potently enhanced corticosterone-suppressed serotonin release (Figure 1a). P72 also suppressed TNF-α expression in LPS-stimulated Caco-2 cells (Figure 1b). P72 was identified as Lactiplantibacillus plantarum, based on Gram staining, API kit, and 16S rDNA and whole genome sequencing. The genome of P72 was 3,350,919 base pairs (contigs 6) with a GC content of 44.4%. The total number of CDS was 3135 (Supplementary Figure S1). The tRNA gene number was 70. The rRNA gene number was 16. The P72 genome sequence showed a phylogenetic similarity to Lactiplantibacillus plantarum NCTC13644 (99.15%) using OrthoANI.
Figure 1.
Effect of P72 on the release of corticosterone-suppressed serotonin in SH-SY5Y cells (a) and the expression of LPS-induced TNF-α in Caco-2 cells (b). In SH-SY5Y cells, NC, saline; Cort, 300 μM corticosterone; P72, 1 × 105 CFU/mL of P72 + corticosterone. In Caco2 cells, NC, saline; LPS, 100 ng/mL of LPS; P72, 1 × 105 CFU/mL of P72 + LPS. Each group represents the mean ± SD (n = 4). # p < 0.05 vs. NC. * p < 0.05 vs. Cort/LPS.
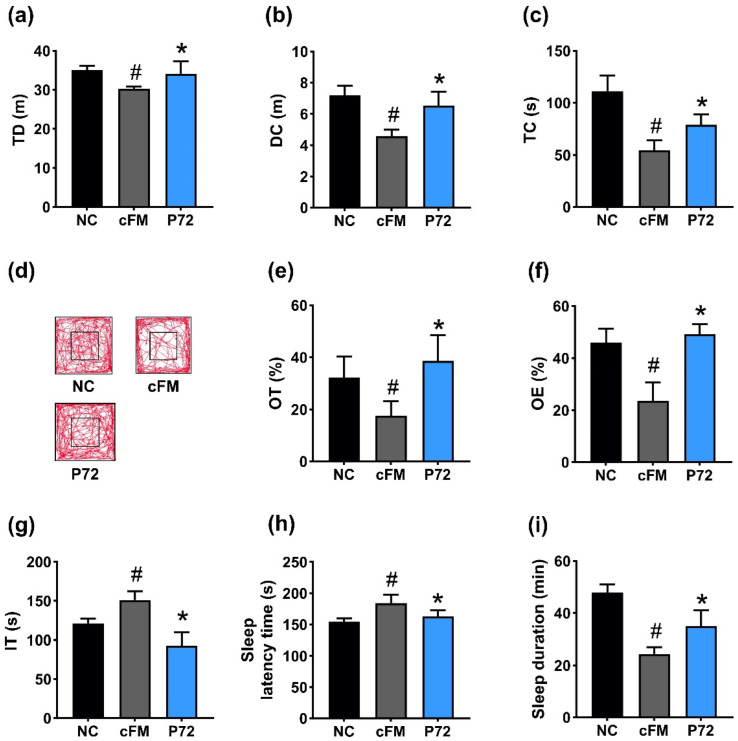
3.2. P72 Mitigated DILS in IS-Exposed Mice
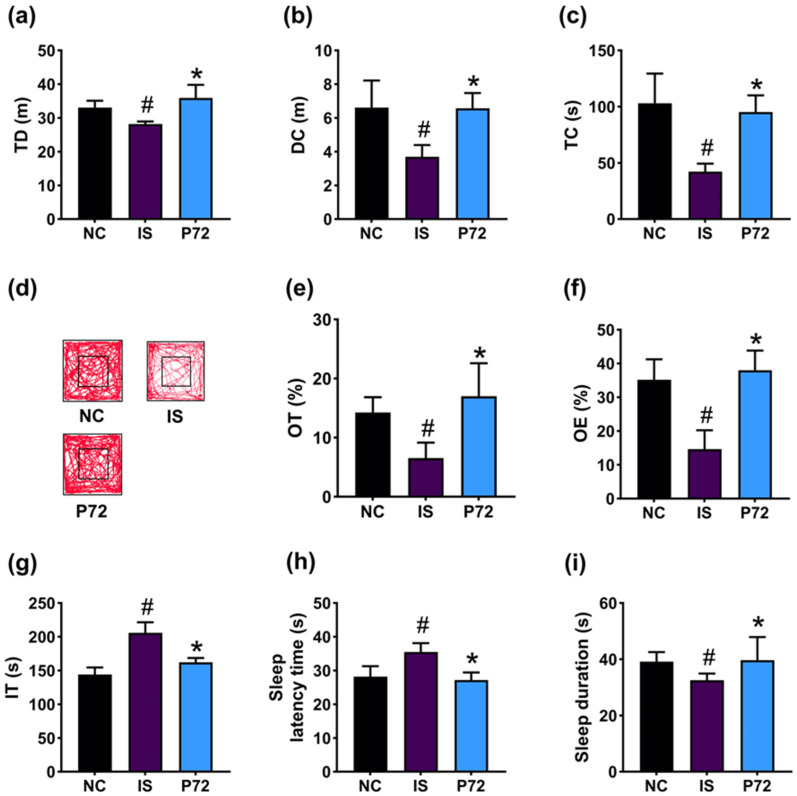
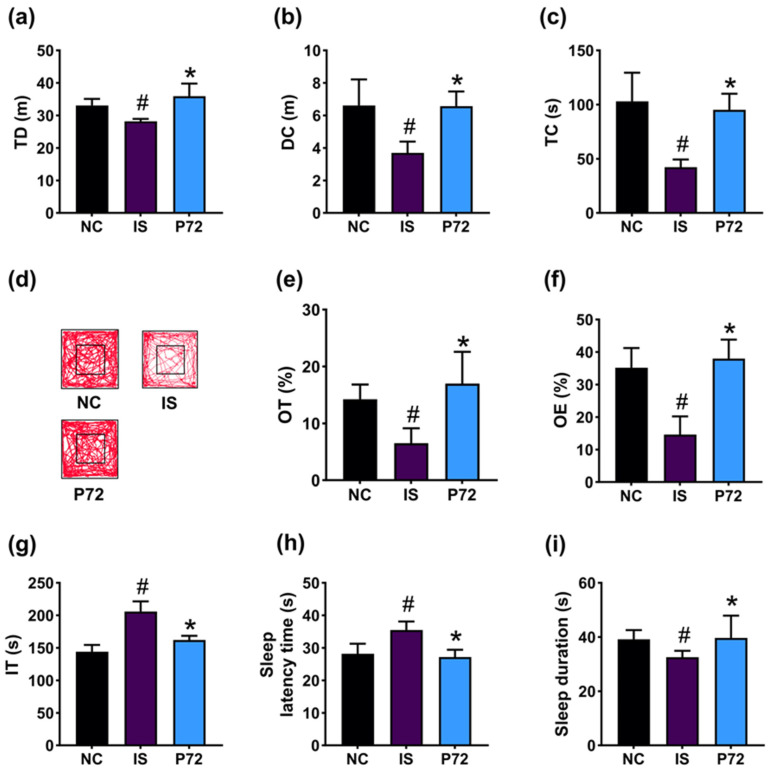
To comprehend whether P72 could improve DA and insomnia, we investigated its impact in mice exposed to IS. IS treatment increased DA-like behavior: it decreased TD, DC, and TC in the OFT to 85.3% (F2,21 =18.3, p < 0.001), 55.8% (F2,21 = 17.6, p < 0.001), and 41.0% (F2,21 = 27.2, p < 0.001) of NC, respectively, decreased OT and OE in the EPMT to 45.6% (F2,21 = 15.7, p < 0.001) and 41.4% (F2,21 = 38.4, p < 0.001) of NC, respectively, and increased IT in the TST to 142.8% (F2,21 = 60.4, p < 0.001) of NC (Figure 2a–g). However, orally administered P72 (1 × 109 CFU/mouse/day) significantly recovered IS-decreased TD, DC, and TC to 108.6%, 99.4%, and 92.4% of NC, respectively, IS-suppressed OT and OE to 119.2% and 104.2% of NC, respectively, and IS-increased IT to 112.5% of NC.
Figure 2.
Effect of P72 on IS-induced DA- and insomnia-like behaviors in mice. Effect of P72 on TD (a), DC (b), TC (c), and travel pathway (d) in OFT. Effect on OT (e) and OE (f) in the EPMT and IT in TST (g). Effect on SLT (h) and SLD (i). IS, saline; P72, 1 × 109 CFU/mouse/day of P72 in IS/IF-treated mice; NC, saline in mice (not exposed IS/IF). Data values indicate mean ± SD (n = 8). # p < 0.05 vs. NC. * p < 0.05 vs. IS.
Exposed IS caused insomnia in mice, increasing SLT to 126.0% (F2,21 = 23.0, p < 0.001) of NC and decreasing SLD to 83.0% (F2,21 = 4.5, p = 0.023) of NC (Figure 2h,i). However, P72 significantly improved insomnia: it decreased IS-increased SLT to 96.4% of NC and enhanced IS-shortened SLD to 101.3% of NC.
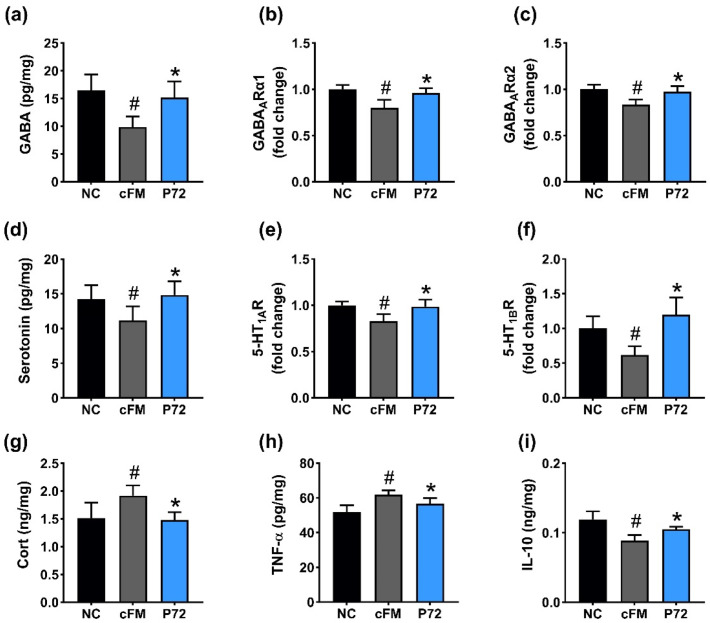
IS exposure significantly downregulated the expression of GABA, its receptors GABAARα1 and GABAARα2, serotonin, and its receptors 5-HT1AR and 5-HT1BR in the prefrontal cortex (Figure 3a–f). Furthermore, IS increased corticosterone and TNF-α levels, while decreasing IL-10 expression (Figure 3g–i). Orally administered P72 upregulated IS-decreased GABA, GABAARα1, GABAARα2, serotonin, 5-HT1AR, and 5-HT1BR expression levels. P72 also decreased IS-induced corticosterone and TNF-α levels, while increasing IL-10 levels.
Figure 3.
Effect of P72 on the expression of DA- and insomnia-related markers in the prefrontal cortex. Effect on GABA (a), GABAARα1 (b), and GABAARα2 (c) expression levels. Effect on serotonin (d), 5-HT1AR (e), and 5-HT1BR (f) expression levels. Effect on corticosterone (Cort, (g)), TNF-α (h), and IL-10 (i) levels. IS, vehicle; P72, 1 × 109 CFU/mouse/day of P72 in IS/IF-treated mice; NC, saline in IS/IF-nontreated mice. Data are mean ± SD (n = 8). # p < 0.05 vs. NC. * p < 0.05 vs. IS.
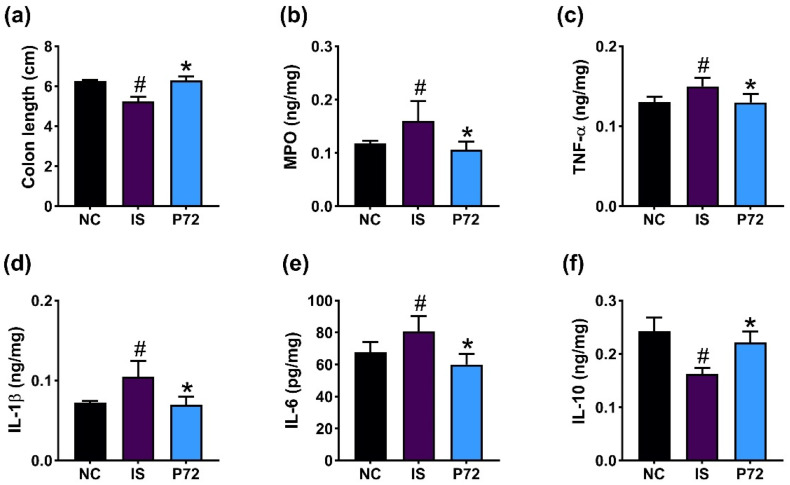
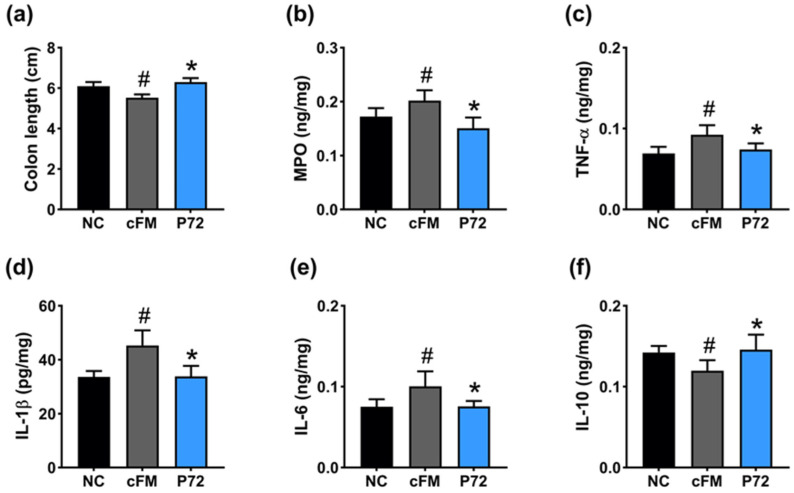
3.3. P72 Alleviated Colitis in IS-Exposed Mice
Exposed IS caused colitis in mice, decreasing colon length and IL-10 levels and increasing myeloperoxidase, TNF-α, IL-1β, and IL-6 levels in the colon (Figure 4). Orally administered P72 (1 × 109 CFU/mouse/day) significantly increased IS-shortened colon length and downregulated IS-induced myeloperoxidase, TNF-α, IL-1β, and IL-6 levels, while upregulating IL-10 levels.
Figure 4.
Effect of P72 on IS-induced colitis in mice. Effect on colon length (a) and myeloperoxidase (MPO, (b)), TNF-α (c), IL-1β (d), IL-6 (e), and IL-10 levels (f) in the colon. IS, vehicle; P72, 1 × 109 CFU/mouse/day of P72 in IS/IF-treated mice; NC, saline in IS/IF-untreated mice. Data are mean ± SD (n = 8). # p < 0.05 vs. NC. * p < 0.05 vs. IS.
3.4. P72 Alleviated cFM Transplantation-Induced DILS and Gut Inflammation in Mice
Next, we investigated the effect of P72 on cFM transplantation-induced DA- and insomnia-like behavior in mice. cFM transplantation reduced TD, DC, and TC in OFT to 86.3% (F2,21 = 13.5, p < 0.01), 63.3% (F2,21 = 33.8, p < 0.01), and 49.0% (F2,21 = 48.0, p < 0.01) of NC, respectively, and OT and OE in EPMT to 54.3% (F2,21 = 14.4, p = 0.01) and 51.3% (F2,21 = 49.1, p < 0.01) of NC, respectively (Figure 5a–f). cFM transplantation also increased IT in TST to 124.7% (F2,21 = 44.7, p < 0.01) of NC (Figure 5g). However, orally administered P72 (1 × 109 CFU/mouse/day) significantly recovered cFM-decreased TD, DC, and TC to 97.2%, 90.7%, and 70.8% of NC, respectively, and OT and OE to 120.0% and 112.2% of NC, respectively. P72 also restored cFM-increased IT to 77.5% of NC.
Figure 5.
Effect of P72 on DA- and insomnia-like behavior in cFM-transplanted mice. Effect of P72 on TD (a), DC (b), TC (c), and travel pathway (d) in OFT. Effect on OT (e) and OE (f) in EPMT and IT in TST (g). Effect on SLT (h) and SLD (i). IS, vehicle; P72, 1 × 109 CFU/mouse/day of P72 in cFM/IF-treated mice; NC, vehicle (saline) in cFM/IF-nontreated mice. Data values indicate mean ± SD (n = 6). # p < 0.05 vs. NC. * p < 0.05 vs. cFM.
cFM transplantation increased SLT to 119.5% (F2,21 = 18.1, p < 0.001) of NC and reduced SLD to 50.9% (F2,21 = 61.2, p < 0.001) of NC (Figure 5h,i). However, orally administered P72 significantly recovered cFM-increased SLT to 105.5% of NC and raised cFM-decreased SLD to 73.2% of NC.
cFM transplantation decreased GABA, GABAARα1, GABAARα2, serotonin, 5-HT1AR, and 5-HT1BR expression in the prefrontal cortex (Figure 6a–f). cFM transplantation increased corticosterone and TNF-α levels, while decreasing IL-10 levels (Figure 6g–i). Orally administered P72 upregulated cFM transplantation-suppressed GABA, GABAARα1, GABAARα2, serotonin, 5-HT1AR, and 5-HT1BR expression. P72 also decreased cFM transplantation-induced corticosterone and TNF-α expression, while increasing cFM transplantation-suppressed IL-10 expression.
Figure 6.
Effect of P72 on cFM transplantation-induced DA- and insomnia-related markers in the prefrontal cortex. Effect of P72 on GABA (a), GABAARα1 (b), and GABAARα1 (c) levels. Effect on serotonin (d), 5-HT1AR (e), and 5-HT1BR (f) levels. Effect on corticosterone (g), TNF-α (h), and IL-10 (i) levels. cFM, vehicle; P72, 1 × 109 CFU/mouse/day of P72 in cFM/IF-treated mice; NC, vehicle (saline) in cFM/IF-untreated mice. Data are mean ± SD (n = 6). # p < 0.05 vs. NC. * p < 0.05 vs. cFM.
cFM transplantation caused inflammation in the colon of mice: it shortened colon length, increased myeloperoxidase, TNF-α, IL-1β, and IL-6 levels, and decreased IL-10 levels (Figure 7). Orally administered P72 significantly recovered suppressed cFM transplantation-increased myeloperoxidase, TNF-α, IL-1β, and IL-6 levels.
Figure 7.
Effect of P72 on cFM transplantation-induced colitis in mice. Effect on colon length (a), myeloperoxidase (MPO, (b)), TNF-α (c), IL-1β (d), IL-6 (e), and IL-10 expression (f) in the colon. cFM, vehicle; P72, 1 × 109 CFU/mouse/day of P72 in cFM/IF-treated mice; NC, vehicle (saline) in cFM/IF-nontreated mice. Data are mean ± SD (n = 6). # p < 0.05 vs. NC. * p < 0.05 vs. IS.
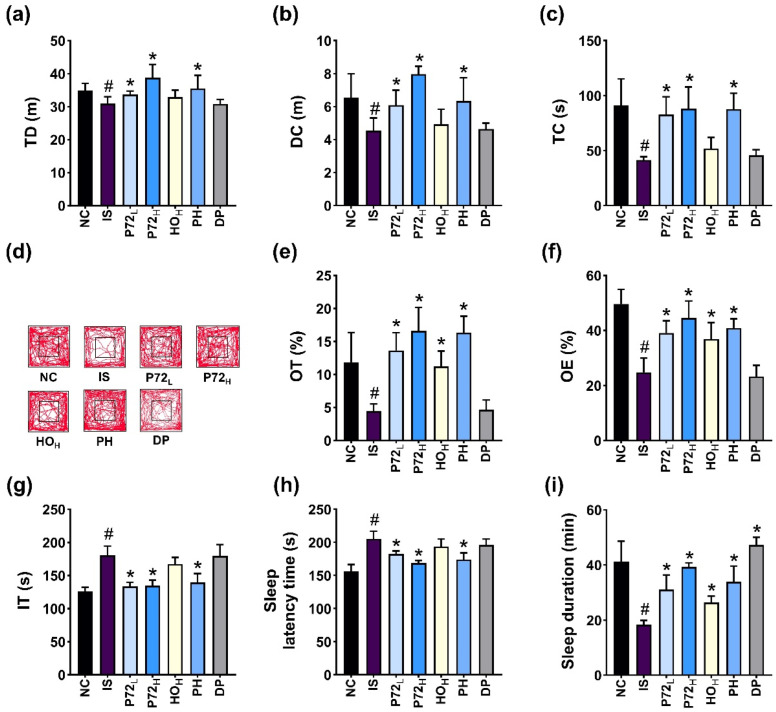
3.5. P72 and Its Combination with HO (PH) Alleviated DILS and Gut Inflammation in IS-Exposed Mice
The effect of P72 combined with HO (PH) on DA- and insomnia-like behavior was investigated in IS-exposed mice (Figure 8). Exposure to IS significantly reduced TD, DC, and TC in OFT to 88.8% (F6,49 = 9.0, p < 0.01), 69.5% (F6,49 = 13.0, p < 0.01), and 46.8% (F6,49 = 17.7, p < 0.01) of NC, respectively, and OT and OE in EPMT to 37.5% (F6,49 = 25.6, p < 0.01) and 49.9% (F6,49 = 30.4, p < 0.01) of NC, respectively (Figure 8a–f). IS increased IT in TST to 143.1% (F6,49 = 33.1, p < 0.01) of NC (Figure 8g). However, orally administered P72 and PH (0.4 × 109 and 1 × 109 CFU/mouse/day) significantly increased IS-suppressed TD, DC, and TC: P72 at 0.4 × 109 CFU/mouse (P72L) and PH (0.4 × 109 CFU/mouse of P72 + 0.12 g/kg of HO [HOL]) increased TD to 94.8% and 103.2%, respectively, DC to 93.3% and 97.0% of NC, respectively, and TC to 91.8% and 95.3%, respectively. However, HO at 0.24 g/kg (HOH) did not affect TD, DC, or TC. P72L, PH, and HOH increased IS-induced OT in EPMT to 114.9%, 137.8%, and 94.9% of NC, respectively, and OT to 79.2%, 73.42%, and 85.4% of NC, respectively. P72L and PH reduced IT to 105.8% and 110.5% of NC. However, HO did not affect IT.
Figure 8.
Effects of P72, HO, and PH on IS-induced DA- and insomnia-like behavior in mice. Effects on OT (a) and OE (b) in EPMT and IT in TST (c). Effects on TD (d), CD (e), TC (f), and travel pathway (g) in OFT. Effects on SLT (h) and SLD (i). IS, vehicle; P72L, 4 × 108 CFU/mL of P72; P72H, 1 × 109 CFU/mouse of P72; HOH, 0.24 g/kg of HO; PH, 0.4 × 109 CFU/mouse/day of P72 and 0.12 g/kg of HO; DP, 20 mg/kg DPH, in IS/PS-exposed mice; NC, vehicle, in IS/PS-nontreated mice. Data are mean ± SD (n = 8). # p < 0.05 vs. NC. * p < 0.05 vs. IS.
Exposed IS enhanced insomnia-like behavior in mice: it increased SLT to 131.3% (F6,49 = 28.7, p < 0.01) of NC and reduced SLD to 44.5% (F6,49 = 39.0, p < 0.01) of NC (Figure 8h,i). However, P72L and PH significantly reduced IS-increased SLT to 116.7% and 111.2% of NC, respectively. However, HOH did not significantly affect IS-increased SLT. P72L, PH, and HOH recovered IS-decreased SLD to 75.3%, 95.2% and 64.0% of NC, respectively.
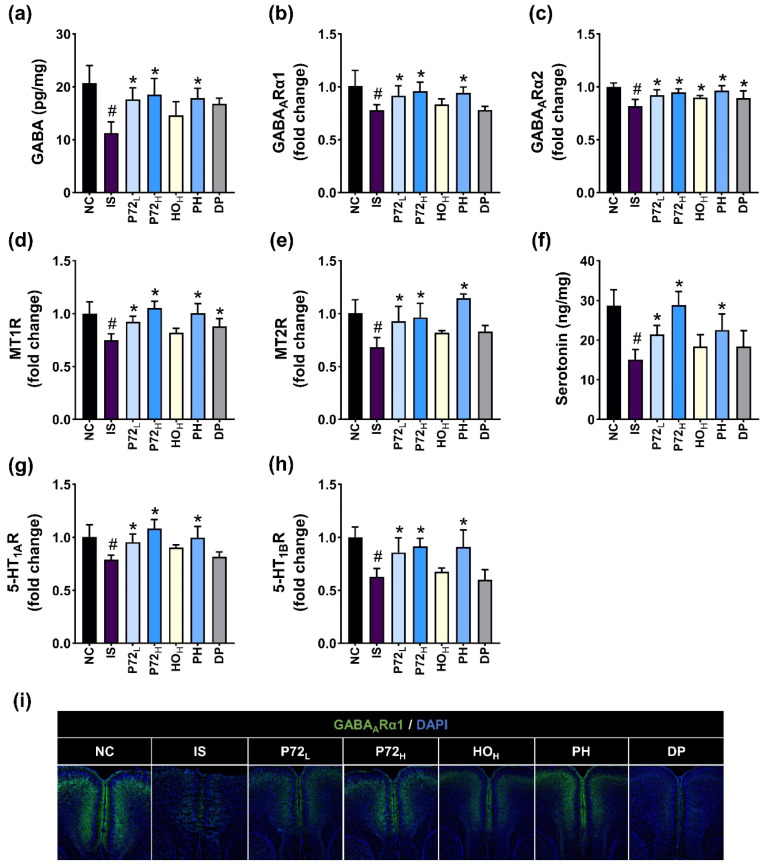
IS exposure downregulated the expression of GABA, GABAARα1, GABAARα2, MT1R, MT2R, serotonin, 5-HT1AR, 5-HT1BR, and the number of GABA1ARα1-positive cells (Figure 9). Orally administered P72L, P72H, or PH upregulated IS-suppressed GABA, serotonin, GABAARα1 and GABAARα2, MT1R, MT2R, 5-HT1AR, and 5-HT1BR expression and GABA1ARα1-positive cell numbers. Diphenhydramine did not induce IS-decreased levels of GABA, serotonin, and their receptors except GABA1ARα2 and MT1R.
Figure 9.
Effects of P72, HO, and PH on IS-suppressed DA- and insomnia-related biomarker levels in the prefrontal cortex. Their effects on GABA (a), GABAARα1 (b), and GABAARα2 (c) levels. Their effects on MT1R (d) and MT2R (e) levels. Their effects on serotonin (f), 5-HT1AR (g), and 5-HT1BR (h) levels. (i) Their effects on GABAARα1-positive cell populations. IS, vehicle; P72L, 4 × 108 CFU/mL of P72; P72H, 1 × 109 CFU/mouse of P72; HOH, 0.24 g/kg of HO; PH, 0.4 × 109 CFU/mouse/day of P72 and 0.12 g/kg of HO; DP, 20 mg/kg DP, in IS/PS-exposed mice; NC, vehicle, in IS/PS-nontreated mice. Data are mean ± SD (n = 6). # p < 0.05 vs. NC. * p < 0.05 vs. IS.
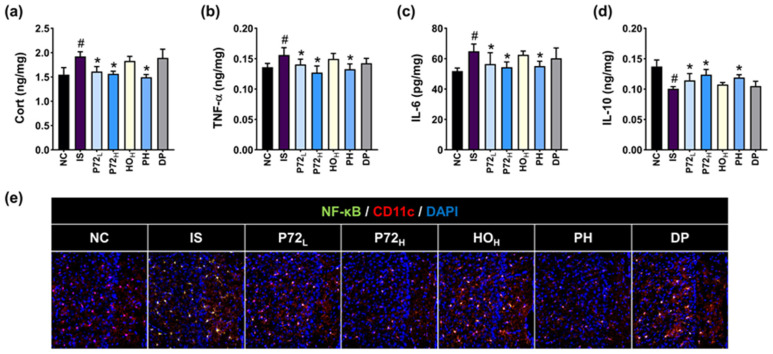
Exposed IS upregulated corticosterone, TNF-α, and IL-6 levels and NF-κB+ Iba1+ cell numbers, and decreased IL-10 levels in the prefrontal cortex (Figure 10). Orally administered P72L, P72H, or PH significantly reduced IS-increased biomarker levels, while increasing IS-decreased IL-10 levels.
Figure 10.
Effects of P72, HO, and PH on IS-induced inflammatory biomarker levels in the prefrontal cortex. Their effects on Cort (a), TNF-α (b), IL-6 (c), and IL-10 (d) levels and NF-κB+ Iba1+ cell numbers (e). IS, vehicle; P72L, 4 × 108 CFU/mL of P72; P72H, 1 × 109 CFU/mouse of P72; HOH, 0.24 g/kg of HO; PH, 0.4 × 109 CFU/mouse/day of P72 and 0.12 g/kg of HO; DP, 20 mg/kg DP, in IS/PS-exposed mice; NC, vehicle, in IS/PS-nontreated mice. Data are mean ± SD (n = 8). # p < 0.05 vs. NC. * p < 0.05 vs. IS.
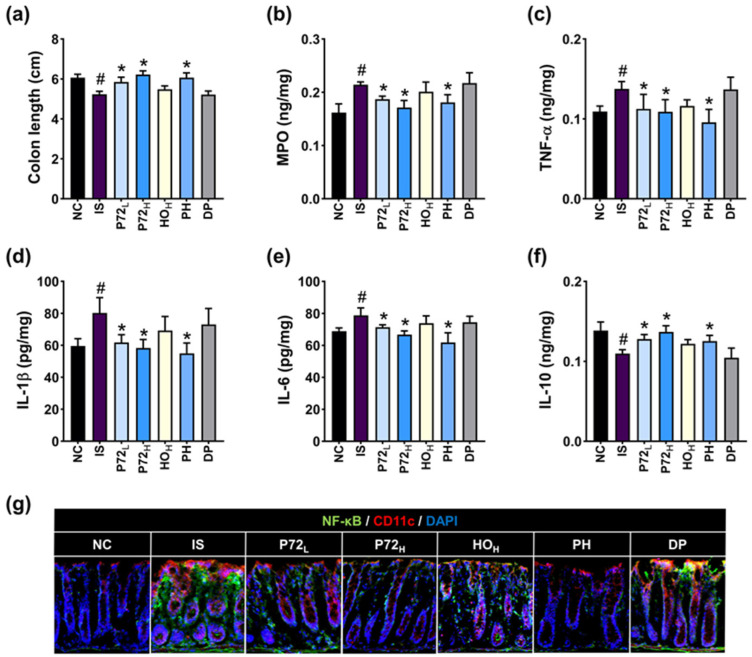
Exposure to IS caused inflammation in the colon of mice: it decreased colon length and IL-10 levels and increased myeloperoxidase, TNF-α, IL-1β, and IL-6 levels and NF-κB+ CD11c+ cell populations (Figure 11). Oral administration of PH and P72 significantly recovered IS-increased myeloperoxidase, TNF-α, IL-1β, and IL-6 levels and NF-κB+ CD11c+ cell populations and IS-decreased IL-10 levels.
Figure 11.
Effects of P72, HO, and PH on IS-induced colitis in mice. Their effects on colon length (a), myeloperoxidase (MPO, (b)), TNF-α (c), IL-1β (d), IL-6 (e), and IL-10 expression (f), and NF-κB+ CD11c+ cell populations (g) in the colon. IS, vehicle; P72L, 4 × 108 CFU/mL of P72; P72H, 1 × 109 CFU/mouse of P72; HOH, 0.24 g/kg of HO; PH, 0.4 × 109 CFU/mouse/day of P72 and 0.12 g/kg of HO; DP, 20 mg/kg DP, in IS/PS-exposed mice; NC, vehicle, in IS/PS-nontreated mice. Data are mean ± SD (n = 8). # p < 0.05 vs. NC. * p < 0.05 vs. IS.
3.6. hP72 and Its Combination with HO (hPH) Alleviated IS-Induced DILS in Mice
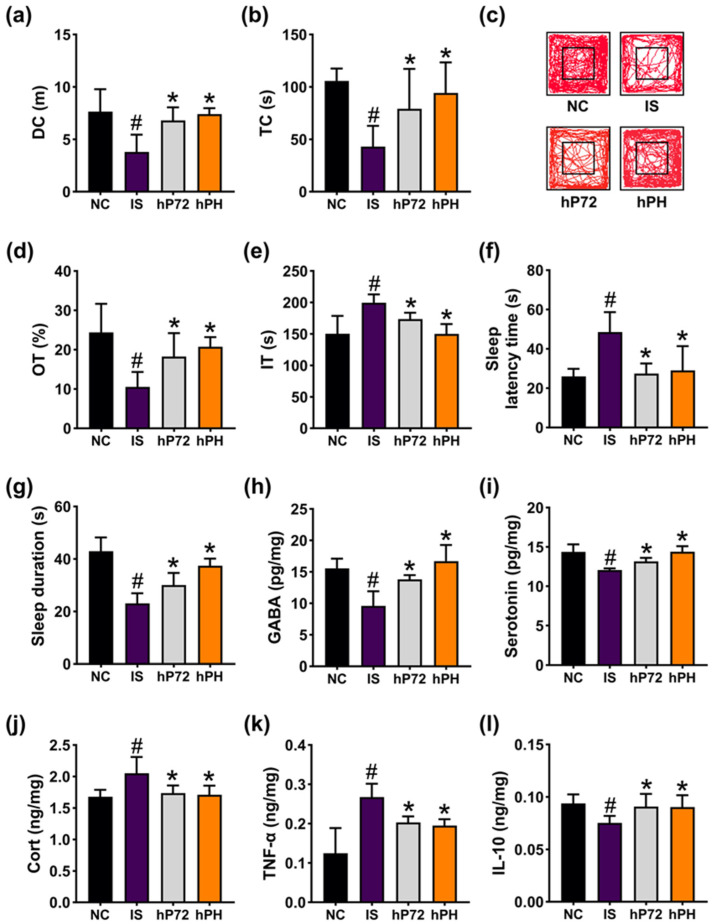
The effects of hP72 and hPH against DILS was investigated in mice exposed to IS. Exposed IS reduced DC and TC in OFT to 49.5% (F3,28 = 11.6, p < 0.001) and 40.7% (F3,28 = 9.2, p < 0.001) of NC, respectively, and OT in EPMT to 43.21% (F3,28 = 10.4, p < 0.001) of NC, while increasing IT in TST to 132.8% (F3,28 = 12.22, p < 0.001) of NC (Figure 12a–e). Orally administered hP72 or hPH significantly increased IS-suppressed DC to 88.9% and 96.9% of NC, respectively, TC to 74.8% and 89.1% of NC, respectively, and OT to 74.7% and 85.1% of NC, respectively. They also decreased IT to 115.6% and 100.2% of NC, respectively.
Figure 12.
Effects of hP72 and hPH on DILS in IS-treated mice. Their effects on DC (a), TC (b), and travel pathway (c) in OFT. Their effects on OT (d) in EPMT and IT in TST (e). Their effects on SLT (f) and SLD (g). Their effects on GABA (h), serotonin (i), Cort (j), TNF-α (k), and IL-10 (l) levels in the prefrontal cortex. IS, vehicle; hP72, 1 × 109 CFU/mouse of hP72; hPH, 1 × 109 CFU/mouse of hP72 and 0.12 g/kg of HO in IS/IF-treated mice; NC, IS/IF-nontreated mice. Data are mean ± SD (n = 8). # p < 0.05 vs. NC. * p < 0.05 vs. IS.
Exposed IS enhanced insomnia-like behavior in mice: it increased SLT to 186.5% (F3,28 = 12.58, p < 0.001) of NC and reduced SLD to 53.6% (F3,28 = 32.75, p < 0.001) of NC (Figure 12f,g). Orally administered hP72 or hPH significantly reduced IS-increased SLT to 105.5% and 111.5% of NC, respectively, and enhanced IS-decreased SLD to 69.9% and 87.2% of NC, respectively. hP72 and hPH significantly increased IS-suppressed GABA and serotonin levels in the prefrontal cortex. They downregulated IS-increased corticosterone and TNF-α levels, while increasing IS-decreased IL-10 expression.
4. Discussion
We found that IS exposure and cFM transplantation increased DA- and sleep disturbance-like behaviors in mice, suppressing GABA and serotonin levels and inducing TNF-α levels and NF-κB-positive cell populations in the brain. Stress-induced DA triggers sleep disturbance, including insomnia and systemic inflammation [1,25,26]. Systemic inflammation including gut inflammation induces DA [27,28]. Depression has been suggested to be closely associated with reduced serotonin and GABA concentrations and increased TNF-α concentrations, which suppresses the release of serotonin and GABA, although there are many conflicting results [29,30,31]. Stress also causes gut dysbiosis, which is strongly involved with the outbreak of DA and systemic inflammation [32]. Fecal microbiota transplantation from DA patients induces DA-like behavior and neuroinflammation in transplanted mice [33]. These observations imply that depression-induced gut dysbiosis can induce sleep disturbance, including insomnia, by decreasing GABA and serotonin levels and increasing NF-κB-mediated TNF-α expression.
P72 downregulated LPS-increased TNF-α levels in Caco-2 cells and upregulated LPS-decreased serotonin secretion in SH-SY5Y cells. P72 also upregulated GABA and serotonin concentrations and downregulated TNF-α and IL-6 levels and NF-κB-positive cell numbers in IS- or cFM-exposed mice. P72 decreased IS-induced DA- and sleep disturbance-like behavior. Lactobacillus mucosae NK41 alleviates depression in mice [34]. Anti-inflammatory Bifidobacterium infantis CCFM687 alleviates depression in mice [12]. Anti-inflammatory Lactobacillus plantarum NK151 also mitigates depression in mice [22]. These observations imply that P72 can mitigate depression and sleep disturbance by inducing GABA and serotonin production and suppressing NF-κB-mediated TNF-α expression.
P72 increased the levels of GABA and serotonin and the expression of GABA receptors GABAARα1 and GABAARα2, 5-HT receptors 5-HT1AR and 5-HT1BR, and melatonin receptors MT1R and MT1R. GABA and its receptor agonists such as benzodiazepine also alleviates DA and insomnia [35]. 5-HT1AR and HT1BR agonists, such as tandospirone, mitigate depression and insomnia [36,37,38]. Diazepam, a positive allosteric modulator of the GABAA receptor, improves sleep disturbance (including insomnia) by increasing GABAAR [39]. Ramelteon, a melatonin receptor agonist, also improves DA and insomnia symptoms [29]. Stress upregulates TNF-α expression in immune cells, which downregulates the expression and actions of serotonin, GABA, and their receptors [40,41]. P72 suppressed TNF-α expression and NF-κB activation in vitro and in vivo. These observations imply that P72 can alleviate DA and sleep disturbance, including insomnia, by regulating serotonergic and GABAAergic systems through the suppression of NF-κB signaling.
hP72, which was heat-killed, also alleviated DA and sleep disturbance in mice, while HO did not. Nevertheless, PH (a mix of P72 and HO) and hPH (a mix of hP72 and HO) alleviated DA and sleep disturbance, including insomnia, more potently than P72 or hP72 alone. They increased GABA, serotonin, and their receptor expression and decreased TNF-α expression. These observations imply that the active components of P72 against DA and sleep disturbance may be heat-stable, and their action mechanism may exhibit a significant difference.
5. Conclusions
P72 (live) and hP72 (heat-killed) upregulated 5-HT, GABA, and their receptor levels, and downregulated corticosterone and TNF-α levels and NF-κB activation in the brain and intestine. P72 decreased DA- and insomnia-like behavior. PH more strongly alleviated DILS than P72 or HO alone. The efficacies of hP72 and hPH were significantly different to those of P72 and PH, respectively. Finally, P72 and its supplement PH may mitigate DA and insomnia by upregulating serotonergic and GABAA-ergic systems through the suppression of NF-κB signaling.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16213711/s1: Analysis Figure S1. Taxonomic classification by genome-wide comparative analysis of P72. Table S1. Primers used in this study. Methods—Preparation of mice with immobilization stress, Behavioral tasks, ELISA, qPCR, immunofluorescence staining. Refs. [13,23,42,43,44] are cited in Supplementary Materials.
Author Contributions
J.-S.B.: Writing—original draft, Supervision, Software, Validation, Supervision, Resources, Methodology, Formal analysis. D.-Y.L.: Writing—original draft, Supervision, Software, Validation, Resources, Methodology, Formal analysis. Y.-J.S.: Validation, Supervision, Resources, Methodology. D.-H.K.: Writing—editing, Project administration, Investigation, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved in 2023-03-14 by the Committee for the Care and Use of Laboratory Animals in the University and registered under the code KHUASP(SE)-23545.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
Kim, D.-H. was employed by PBLbioLab, Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding Statement
This work was supported by the Medical Research Program (2017R1A5A2014768) through the National Research Foundation of Korea (NRF), funded by the Ministry of Science and ICT and the Ministry of Food and Drug Safety (22203MFDS539), and funded by the Korea Healthy Industry Development Institute.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Tsigos C., Chrousos G.P. Hypothalamic-pituitary-adrenal axis, neuroendocrine factors and stress. J. Psychosom. Res. 2002;53:865–871. doi: 10.1016/S0022-3999(02)00429-4. [DOI] [PubMed] [Google Scholar]
- 2.Hassamal S. Chronic stress, neuroinflammation, and depression: An overview of pathophysiological mechanisms and emerging anti-inflammatories. Front. Psychiatry. 2023;14:1130989. doi: 10.3389/fpsyt.2023.1130989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dicks L.M.T. Gut Bacteria and Neurotransmitters. Microorganisms. 2022;10:1838. doi: 10.3390/microorganisms10091838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodo T.W., de Aquino M.T.P., Shimamoto A., Shanker A. Critical Neurotransmitters in the Neuroimmune Network. Front. Immunol. 2020;11:1869. doi: 10.3389/fimmu.2020.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reynolds C.F., 3rd, Kupfer D.J. Sleep research in affective illness: State of the art circa 1987. Sleep. 1987;10:199–215. doi: 10.1093/sleep/10.3.199. [DOI] [PubMed] [Google Scholar]
- 6.Xu F., Xie Q., Kuang W., Dong Z. Interactions Between Antidepressants and Intestinal Microbiota. Neurotherapeutics. 2023;20:359–371. doi: 10.1007/s13311-023-01362-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Plaza-Diaz J., Ruiz-Ojeda F.J., Gil-Campos M., Gil A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019;10:S49–S66. doi: 10.1093/advances/nmy063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams N.T. Probiotics. Am. J. Health Syst. Pharm. 2010;67:449–458. doi: 10.2146/ajhp090168. [DOI] [PubMed] [Google Scholar]
- 9.Borchers A.T., Selmi C., Meyers F.J., Keen C.L., Gershwin M.E. Probiotics and immunity. J. Gastroenterol. 2009;44:26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 10.Jia L., Xiao L., Fu Y., Shao Z., Jing Z., Yuan J., Xie Y., Guo J., Wang Y., Geng W. Neuroprotective effects of probiotics on anxiety- and depression-like disorders in stressed mice by modulating tryptophan metabolism and the gut microbiota. Food Funct. 2024;15:2895–2905. doi: 10.1039/D3FO03897A. [DOI] [PubMed] [Google Scholar]
- 11.Savignac H.M., Kiely B., Dinan T.G., Cryan J.F. Bifidobacteria exert strain-specific effects on stress-related behavior and physiology in BALB/c mice. Neurogastroenterol. Motil. 2014;26:1615–1627. doi: 10.1111/nmo.12427. [DOI] [PubMed] [Google Scholar]
- 12.Tian P., Zou R., Song L., Zhang X., Jiang B., Wang G., Lee Y.K., Zhao J., Zhang H., Chen W. Ingestion of Bifidobacterium longum subspecies infantis strain CCFM687 regulated emotional behavior and the central BDNF pathway in chronic stress-induced depressive mice through reshaping the gut microbiota. Food Funct. 2019;10:7588–7598. doi: 10.1039/C9FO01630A. [DOI] [PubMed] [Google Scholar]
- 13.Jang H.M., Lee K.E., Kim D.H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients. 2019;11:819. doi: 10.3390/nu11040819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu R., Fang Y., Li H., Liu Y., Wei J., Zhang S., Wang L., Fan R., Wang L., Li S., et al. Psychobiotic Lactobacillus plantarum JYLP-326 relieves anxiety, depression, and insomnia symptoms in test anxious college via modulating the gut microbiota and its metabolism. Front. Immunol. 2023;14:1158137. doi: 10.3389/fimmu.2023.1158137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tian P., O’Riordan K.J., Lee Y.K., Wang G., Zhao J., Zhang H., Cryan J.F., Chen W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress. 2020;12:100216. doi: 10.1016/j.ynstr.2020.100216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lew L.C., Hor Y.Y., Yusoff N.A.A., Choi S.B., Yusoff M.S.B., Roslan N.S., Ahmad A., Mohammad J.A.M., Abdullah M., Zakaria N., et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019;38:2053–2064. doi: 10.1016/j.clnu.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Boehme M., Rémond-Derbez N., Lerond C., Lavalle L., Keddani S., Steinmann M., Rytz A., Dalile B., Verbeke K., Van Oudenhove L., et al. Bifidobacterium longum subsp. longum Reduces Perceived Psychological Stress in Healthy Adults: An Exploratory Clinical Trial. Nutrients. 2023;15:3122. doi: 10.3390/nu15143122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto-Sanchez M.I., Hall G.B., Ghajar K., Nardelli A., Bolino C., Lau J.T., Martin F.P., Cominetti O., Welsh C., Rieder A., et al. Probiotic Bifidobacterium longum NCC3001 Reduces Depression Scores and Alters Brain Activity: A Pilot Study in Patients with Irritable Bowel Syndrome. Gastroenterology. 2017;153:448–459.e448. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Ho Y.T., Tsai Y.C., Kuo T.B.J., Yang C.C.H. Effects of Lactobacillus plantarum PS128 on Depressive Symptoms and Sleep Quality in Self-Reported Insomniacs: A Randomized, Double-Blind, Placebo-Controlled Pilot Trial. Nutrients. 2021;13:2820. doi: 10.3390/nu13082820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baek J.S., Lee D.Y., Han S.W., Kim D.H. A probiotic NVP1704 alleviates stress-induced sleeplessness/depression-like symptoms in mice by upregulating serotonergic and GABAergic systems and downregulating NF-κB activation. Lett. Appl. Microbiol. 2024;77:ovae065. doi: 10.1093/lambio/ovae065. [DOI] [PubMed] [Google Scholar]
- 21.Percie du Sert N., Hurst V., Ahluwalia A., Alam S., Avey M.T., Baker M., Browne W.J., Clark A., Cuthill I.C., Dirnagl U., et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020;18:e3000410. doi: 10.1371/journal.pbio.3000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoo J.W., Shin Y.J., Ma X., Son Y.H., Jang H.M., Lee C.K., Kim D.H. The Alleviation of Gut Microbiota-Induced Depression and Colitis in Mice by Anti-Inflammatory Probiotics NK151, NK173, and NK175. Nutrients. 2022;14:2080. doi: 10.3390/nu14102080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma Y., Ma H., Eun J.S., Nam S.Y., Kim Y.B., Hong J.T., Lee M.K., Oh K.W. Methanol extract of Longanae Arillus augments pentobarbital-induced sleep behaviors through the modification of GABAergic systems. J. Ethnopharmacol. 2009;122:245–250. doi: 10.1016/j.jep.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Lee D.Y., Shin Y.J., Kim J.K., Jang H.M., Joo M.K., Kim D.H. Alleviation of cognitive impairment by gut microbiota lipopolysaccharide production-suppressing Lactobacillus plantarum and Bifidobacterium longum in mice. Food Funct. 2021;12:10750–10763. doi: 10.1039/D1FO02167B. [DOI] [PubMed] [Google Scholar]
- 25.Lach G., Schellekens H., Dinan T.G., Cryan J.F. Anxiety, Depression, and the Microbiome: A Role for Gut Peptides. Neurotherapeutics. 2018;15:36–59. doi: 10.1007/s13311-017-0585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porkka-Heiskanen T., Zitting K.M., Wigren H.K. Sleep, its regulation and possible mechanisms of sleep disturbances. Acta Physiol. 2013;208:311–328. doi: 10.1111/apha.12134. [DOI] [PubMed] [Google Scholar]
- 27.Craig C.F., Filippone R.T., Stavely R., Bornstein J.C., Apostolopoulos V., Nurgali K. Neuroinflammation as an etiological trigger for depression comorbid with inflammatory bowel disease. J. Neuroinflamm. 2022;19:4. doi: 10.1186/s12974-021-02354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irving P., Barrett K., Nijher M., de Lusignan S. Prevalence of depression and anxiety in people with inflammatory bowel disease and associated healthcare use: Population-based cohort study. Evid. Based Ment. Health. 2021;24:102–109. doi: 10.1136/ebmental-2020-300223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krystal A.D. New Developments in Insomnia Medications of Relevance to Mental Health Disorders. Psychiatr. Clin. N. Am. 2015;38:843–860. doi: 10.1016/j.psc.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Riemann D., Krone L.B., Wulff K., Nissen C. Sleep, insomnia, and depression. Neuropsychopharmacology. 2020;45:74–89. doi: 10.1038/s41386-019-0411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amidfar M., Kim Y.K. Recent Developments on Future Antidepressant-related Serotonin Receptors. Curr. Pharm. Des. 2018;24:2541–2548. doi: 10.2174/1381612824666180803111240. [DOI] [PubMed] [Google Scholar]
- 32.Madison A., Kiecolt-Glaser J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019;28:105–110. doi: 10.1016/j.cobeha.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hashimoto K. Neuroinflammation through the vagus nerve-dependent gut-microbiota-brain axis in treatment-resistant depression. Prog. Brain Res. 2023;278:61–77. doi: 10.1016/bs.pbr.2023.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Kim J.K., Lee K.E., Lee S.A., Jang H.M., Kim D.H. Interplay Between Human Gut Bacteria Escherichia coli and Lactobacillus mucosae in the Occurrence of Neuropsychiatric Disorders in Mice. Front. Immunol. 2020;11:273. doi: 10.3389/fimmu.2020.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howland R.H. Sleep interventions for the treatment of depression. J. Psychosoc. Nurs. Ment. Health Serv. 2011;49:17–20. doi: 10.3928/02793695-20101208-01. [DOI] [PubMed] [Google Scholar]
- 36.Chen R., Lin Q., Wu J., Lin Y., Lin T., Wu W., Chen X., Wu S., Zeng G., Lin X., et al. Augmentation therapy with tandospirone citrate in vascular depression patients with mild cognitive impairment: A prospective randomized clinical trial. J. Psychiatr. Res. 2023;159:274–282. doi: 10.1016/j.jpsychires.2022.12.023. [DOI] [PubMed] [Google Scholar]
- 37.Tsutsui R., Shinomiya K., Sendo T., Kitamura Y., Kamei C. Effects of the 5-HT(1A) Receptor Agonist Tandospirone on ACTH-Induced Sleep Disturbance in Rats. Biol. Pharm. Bull. 2015;38:884–888. doi: 10.1248/bpb.b14-00887. [DOI] [PubMed] [Google Scholar]
- 38.Tiger M., Varnäs K., Okubo Y., Lundberg J. The 5-HT(1B) receptor—A potential target for antidepressant treatment. Psychopharmacology. 2018;235:1317–1334. doi: 10.1007/s00213-018-4872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leonard B.E. Sleep disorders and anxiety: Biochemical antecedents and pharmacological consequences. J. Psychosom. Res. 1994;38((Suppl. S1)):69–87. doi: 10.1016/0022-3999(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 40.Nau F., Jr., Yu B., Martin D., Nichols C.D. Serotonin 5-HT2A receptor activation blocks TNF-α mediated inflammation in vivo. PLoS ONE. 2013;8:e75426. doi: 10.1371/journal.pone.0075426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malynn S., Campos-Torres A., Moynagh P., Haase J. The pro-inflammatory cytokine TNF-α regulates the activity and expression of the serotonin transporter (SERT) in astrocytes. Neurochem. Res. 2013;38:694–704. doi: 10.1007/s11064-012-0967-y. [DOI] [PubMed] [Google Scholar]
- 42.Jang H.M., Lee K.E., Lee H.J., Kim D.H. Immobilization stress-induced Escherichia coli causes anxiety by inducing NF-κB activation through gut microbiota disturbance. Sci. Rep. 2018;8:13897. doi: 10.1038/s41598-018-31764-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Joo M.K., Ma X., Yoo J.W., Shin Y.J., Kim H.J., Kim D.H. Patient-derived Enterococcus mundtii and its capsular polysaccharides cause depression through the downregulation of NF-κB-involved serotonin and BDNF expression. Microbes Infect. 2023;25:105116. doi: 10.1016/j.micinf.2023.105116. [DOI] [PubMed] [Google Scholar]
- 44.Lee K.E., Kim J.K., Han S.K., Lee D.Y., Lee H.J., Yim S.V., Kim D.H. The extracellular vesicle of gut microbial Paenalcaligenes hominis is a risk factor for vagus nerve-mediated cognitive impairment. Microbiome. 2020;8:107. doi: 10.1186/s40168-020-00881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during the current study are available from the corresponding author on reasonable request.