Abstract
Background: Many clinical practice guidelines recommend dietary pulses for the prevention and management of cardiovascular disease and diabetes. The impact of extracted pulse proteins remains unclear. We therefore conducted a systematic review and meta-analysis of randomized controlled trials of the effect of extracted pulse proteins on therapeutic lipid targets. Methods and Findings: MEDLINE, Embase, and the Cochrane Library were searched through April 2024 for trials of ≥3-weeks. The primary outcome was low-density lipoprotein-cholesterol (LDL-C). The secondary outcomes were other lipid targets. Independent reviewers extracted data and assessed the risk of bias. Subgroup analyses included by pulse type and the certainty of evidence was assessed using GRADE. Results: Seven included trials (14 trial comparisons, n = 453) with a median of 4-weeks duration and dose of 35 g/day showed that extracted pulse proteins decreased LDL-C by −0.23 mmol/L (95% confidence interval: −0.36 to −0.10 mmol/L, p < 0.001). Similar effects were observed for non-high-density lipoprotein-cholesterol and apolipoprotein B. No interactions were found by pulse type. Subgroup analyses revealed effect modification by sex, with greater proportions of females seeing greater reductions. GRADE was generally moderate. Conclusions: Extracted pulse proteins likely result in moderate reductions in LDL-C and other lipid targets. Future studies on various types of extracted pulse proteins including assessments by sex are warranted.
Keywords: dietary pulses, extracted proteins, cardiovascular, blood lipids, sex, systematic review, meta-analysis
1. Introduction
Cardiovascular disease (CVD) is a leading cause of death globally, accounting for 32% of deaths in 2019 [1], costing the healthcare system approximately 30 billion annually in Canada [2,3]. A major risk factor for CVD is low-density lipoprotein-cholesterol (LDL-C) [4,5,6]. Despite advances in drug therapies, those at high CVD risk often have elevated LDL-C due to insufficient lowering with statins, statin-related side effects, poor medication adherence, and treatment inertia [7,8]. National dietary guidelines [9] and cardiovascular, diabetes, and obesity clinical practice guidelines for nutrition therapy [10,11,12,13] recommend dietary patterns with an emphasis on plant-based foods and plant-based protein sources. As a result, the food landscape has rapidly evolved in response to the growing consumer demand for environmentally friendly, plant-based products, stimulating food innovation in the plant-based protein sector. Recent food innovations include an increase in the use of extracted proteins from dietary pulses [14], which are non-oil seeds in the legume family, such as dry peas, chickpeas, lentils, and dry beans. Extracted pulse proteins are often used to manufacture protein supplements or to bolster the nutritional profile of foods. A practical translation of guidelines includes healthcare providers providing actionable dietary advice to their clients about good sources of plant proteins that have evidence of supporting health. However, with the emergence of new products, it is unknown whether there is sufficient evidence for healthcare providers to recommend these products.
Previous studies, which are predominantly based on whole food sources of dietary pulses, have demonstrated beneficial effects on lipid targets of CVD. A previous systematic review and meta-analysis revealed a significant reduction in LDL-C with a median of 130 g, or half a cup, of dietary pulses per day compared to a non-pulse-containing control [15]. With the increased interest in plant-protein products, there has been an emergence of randomized controlled trials investigating the potential cardiovascular benefits of extracted pulse proteins. While the primary component of extracted pulse proteins is protein with modest amounts of starch and fiber, whole pulses also contain higher concentrations of dietary fiber, complex carbohydrates, vitamins, minerals, bioactive compounds, and phytochemicals [16]. Although guidelines recommend consuming dietary pulses, there is a notable gap in understanding whether sources of extracted components of pulses, including protein, can yield similar lipid benefits. To address this knowledge gap and support the development of cardiovascular guidelines, as well as clear and effective recommendations for industry and healthcare professionals and their clients, we conducted a systematic review and meta-analysis of randomized controlled trials to investigate the effect of extracted pulse proteins on established lipid targets for cardiovascular and metabolic syndrome risk reduction [10,17] with an assessment of the certainty of evidence using grading of recommendations, assessment, development, and evaluation (GRADE).
2. Materials and Methods
The study followed the Cochrane Handbook for Systematic Reviews of Interventions [18] and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 (Supplemental Table S1) [19]. The study protocol was registered on PROSPERO (CRD42023432826).
2.1. Data Sources and Search Strategy
A systemic search was conducted in MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials databases through 30 April 2024. Supplemental Tables S2 and S3 present the search strategy. There were no language restrictions. Manual searches of references in the included trials were performed to supplement the database searches.
2.2. Study Selection
We included randomized controlled trials in adults of all health backgrounds with intervention periods of at least 3 weeks that investigated the effect of dietary pulse consumption in the form of extracted pulse proteins compared with a non-pulse containing control on LDL-C and other lipids, including non-high-density lipoprotein-cholesterol (non-HDL-C), apolipoprotein B (apoB), high-density lipoprotein-cholesterol (HDL-C), and triglycerides (TGs). We required the follow-up period to be ≥3 weeks, a duration that aligns with the US Food and Drug Administration (FDA) framework for the scientific evaluation of lipid-lowering health claims [20].
Randomized trials in pregnant females or children were excluded. Reports were initially excluded based on a review of their titles and abstracts. The full texts of those reports that remained were reviewed by at least two of the reviewers (SY, SB, VC, SAC, EJL, and YTC) to determine eligibility. In reports containing more than one eligible trial comparison, we included each available trial comparison separately. Reviewer discrepancies were resolved by consensus or arbitration by a senior investigator (LC).
2.3. Data Collection and Quality Assessment
Data were extracted using a standardized electronic form by at least two reviewers independently (SY, SB, VC, SAC, EJL, YTC, and SMG). Information included the pulse source (beans, lentils, chickpeas, dried peas, and legume intervention), the number of participants, the participant health status, sex, gender, and mean age, the study design, the energy balance (relative to the background diet), the energy level (intervention relative to the control), setting, comparator, the feeding control, pulse processing, the food form, the macronutrient profile of the diets, the saturated fat and fiber content of the intervention and control groups, the follow-up duration, cholesterol-lowering medication use, the funding source, and the outcome data. All lipid data that were available in publications that indicated lipid outcomes were measured; thus, no authors were contacted for missing outcome data. Graphically presented data were extracted from figures using the Plot Digitizer [21]. Reviewer discrepancies in data extractions were resolved by consensus or arbitration by a senior investigator (LC).
2.4. Risk of Bias Assessment
The included studies were assessed for risk of bias (ROB) independently by two investigators using the Cochrane Risk of Bias V.2.0 tool [22]. The assessment was performed across six domains of bias (randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported result, and overall bias. Crossover studies were assessed for an additional domain of risk of bias arising from period or carryover effects). The ROB for each domain was assessed as “low” (a plausible bias unlikely to seriously alter the results), “high” (a plausible bias that seriously weakens confidence in results), or “some concern” (a plausible bias that raises some doubt about the results). An overall risk of bias was determined based on judgments from each domain. Reviewer discrepancies were resolved by consensus or arbitration by a senior investigator (LC).
2.5. Outcomes
The primary outcome was LDL-C determination as the primary lipid target for CVD [10]. Secondary outcomes included the determination of other established lipid targets for CVD (non-HDL-C and apoB) and metabolic syndrome (HDL-C and TG) [17]. Mean differences (MDs) between the intervention and control arm and their standard errors (SEs) were extracted for each eligible trial comparison. If unavailable, they were derived from the available data using published formulas [18]. Mean pairwise differences in change-from-baseline values were preferred over end values, when available. When median data were provided, they were converted to mean data with corresponding variances using methods developed by Luo et al. [23] and Wan et al. [24]. When no variance data were available, the standard deviation was borrowed from a trial similar in size, number of participants, and nature of the intervention, including the food source and dose [25]. When an outcome was not reported, but the variables to calculate that variable were, the outcome was calculated using a standard formula. Non-HDL-C was determined using studies that reported both the total cholesterol and HDL-C levels by calculating the difference between the means. The SDs for non-HDL-C were calculated using the inverse variance law using the SDs of total cholesterol and HDL-C levels [26].
2.6. Data Synthesis and Analysis
We used STATA version 17 (StataCorp) for all analyses. Mean pair-wise differences in change from baseline (or end difference) between the extracted pulse proteins group and the non-pulse-containing control group were used as principal effect measurements (significance at p < 0.05). The results were expressed as the MDs with 95% confidence intervals (CI). The generic inverse variance method with the DerSimonian and Laird random-effects model was used for data analyses [18,27]. When the number of trials was ≤5, a fixed effects model was used [28]. Paired analyses were applied to all crossover trials with the use of a within-individual correlation coefficient between the treatments of 0.5, as described by Elbourne et al., to calculate the SEs [29,30,31]. To mitigate a unit-of-analysis error, when arms of trials with multiple intervention or control arms were used more than once, the corresponding sample size was divided by the number of times it was used for the calculation of the standard error of the pooled effect [32].
The Cochran Q statistic and the I2 statistic were used to assess and quantify heterogeneity, where I2 ≥ 50% and PQ < 0.10 represent substantial heterogeneity [18]. Sources of heterogeneity were explored by sensitivity analyses, including individual trial influence, altering the pairwise comparison correlation coefficient, and subgroup analyses. The individual trial influence analysis systematically removed each trial comparison from the meta-analysis with recalculation of the summary effect estimate. A trial whose removal explained the heterogeneity or changed the significance, direction, or magnitude of the effect by more than the minimally important difference (MID) for each outcome (prespecified as 0.1 mmol/L (5%) for LDL-C, non-HDL-C, HDL-C, TG, and 0.04 g/L for apoB) was considered an influential trial [33,34,35,36]. To determine whether the overall results were robust to the use of different correlation coefficients in crossover trials, we also conducted sensitivity analyses using correlation coefficients of 0.25 and 0.75. If ≥10 trials were available, we conducted a priori subgroup analysis to further investigate source heterogeneity using meta-regression (significance at PQ < 0.05) [37,38]. A priori subgroup analyses were conducted by the pulse type, the dose of dietary pulse, the pulse processing method (isolates, concentrates), the food form (food, beverage, mixed, or tablet), the participant health status, sex, gender, and age, cholesterol-lowering medication use, the baseline outcome, the comparator, the duration of follow-up (≤12-weeks, >12-weeks), the study design (crossover, parallel), the energy balance of the intervention relative to the basal diet (positive, neutral, or negative), the feeding control (metabolic, supplemented, or ad libitum), the difference in saturated fat and fiber content between the intervention and control, the type of mean difference (change from baseline, end differences), funding, and the risk of bias domains. Meta-regression analyses were used to assess the significance of each subgroup categorically and, when applicable, continuously. If ≥6 trials are available, generalized least squares trend (GLST) estimation models and spline curve modeling (MKSPLINE procedure) were used to assess linear and nonlinear dose–response relationships [39]. If ≥10 trials are available, then we assessed for the presence of small-study effects (publication bias) by visual inspection of contour-enhanced funnel plots and formal testing with Egger’s and Begg’s tests (significance at p < 0.10) [40,41,42]. If there was evidence of small-study effects (publication bias), then we quantified the size of the potential publication bias or other causes of asymmetry by adjusting for the funnel plot asymmetry and assessing the effect of small-study effects using the trim-and-fill method of Duval and Tweedie [43].
2.7. Certainty of the Evidence
The certainty of the evidence was assessed using the GRADE approach [44]. The assessments were conducted by 2 independent reviewers (QY, SB), and discrepancies were resolved by consensus or arbitration by the senior author (LC). The evidence was rated as having high, moderate, low, or very low certainty. The included randomized controlled trials were initially rated as high certainty by default and then downgraded or upgraded based on prespecified criteria. The reasons for downgrading the evidence included ROB (assessed by the Cochrane ROB Tool [45]), inconsistency (substantial unexplained interstudy heterogeneity: I2 > 50% and PQ < 0.10), indirectness (the presence of factors that limit the generalizability of the results), imprecision (the 95% CI for effect estimates overlap the MID for benefit or harm or lack of robustness from sensitivity analyses), and publication bias (significant evidence of small-study effects). The reason for upgrading the evidence was the presence of a significant dose–response gradient that supports the direction of the pooled effect estimate [46,47,48,49,50,51]. The importance of the magnitude of the pooled estimates was assessed using our prespecified MIDs and the effect size categories according to the GRADE guidance [52,53,54] as follows: a large effect (≥5× MID); moderate effect (≥2× MID); small important effect (≥1× MID); and trivial/unimportant effect (<1 MID).
3. Results
3.1. Search Results
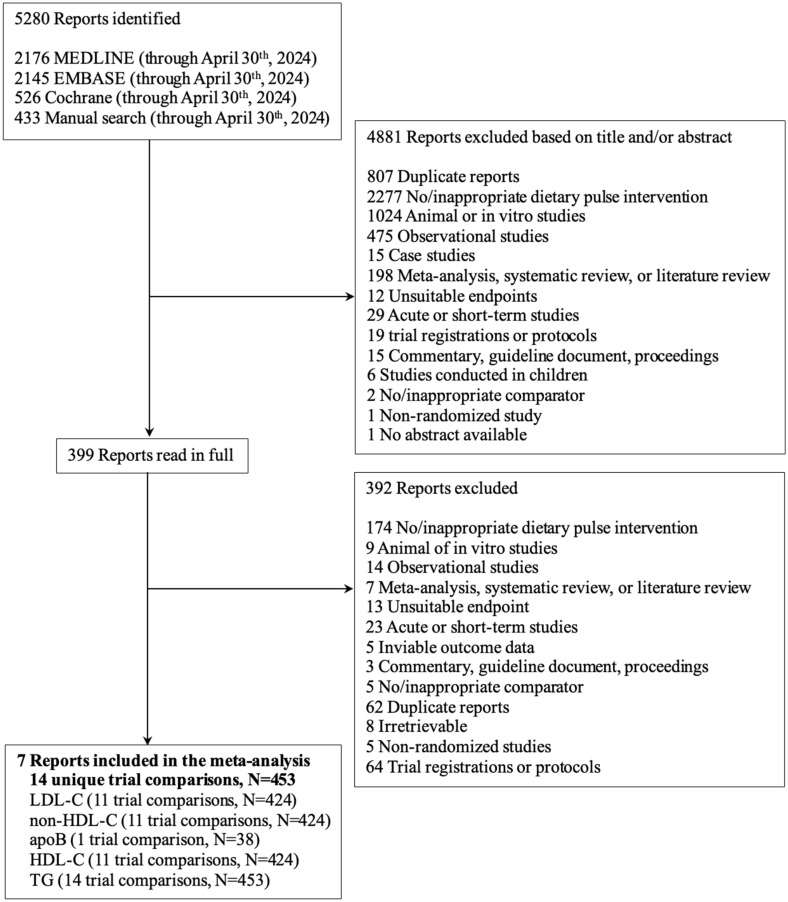
Figure 1 outlines our systematic search results. We identified 5280 reports from our systematic search, 4881 of which were excluded based on the title or abstract. Of the 399 reports reviewed in full, seven trials met our eligibility criteria. The seven trials provided data on 14 trial comparisons (11 trial comparisons on LDL-C, non-HDL-C, HDL-C; 14 trial comparisons on TG; one trial comparison on apoB) involving 453 participants [55,56,57,58,59,60,61,62].
Figure 1.
The flow of the literature on the effect of extracted pulse proteins on blood lipids. apoB, apolipoprotein B; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; non-HDL-C, non-high-density lipoprotein cholesterol; TG, triglyceride.
3.2. Trial Characteristics
Table 1 and Supplemental Table S4 describe the characteristics of the included trials. The trial size had a median of 37 participants (ranging from 24 to 45) for trials of LDL-C, non-HDL-C, and HDL-C; a median of 35 participants (ranging from 14 to 45) for trials of TG; and 38 participants for the trial of apoB. Participants included adults with or without type 2 diabetes or hypercholesterolemia. There were approximately equal ratios of male and female adults. Gender was not reported in any trial. Participants had a median age of 55 y (ranging from 42 to 64 y) for LDL-C, non-HDL-C, and HDL-C, a median age of 54 y (ranging from 42 to 64 y) for TG, and a median age of 57 y (ranging from 54 to 60 y) for apoB. All the trials were conducted in outpatient settings and were performed in Canada (4), the USA (4), Germany (4), Italy (4), Brazil (1), and Australia (1). Most of the trials followed a parallel study design (64% in LDL-C, non-HDL-C, and HDL-C; 71% in TG), except for the apoB outcome, and the feeding control was supplemented. The median dose of extracted pulse proteins was 35 g/day (ranging from 5 to 122 g/day) for LDL-C, non-HDL-C, and HDL-C; 30 g/day (ranging from 1 to 122 g/day) for TG; and 25 g/day for apoB. For most trials, the extracted pulse proteins were produced using a wet extraction method, except for Weiße et al. (2010), Sucher et al. (2017), and Crimarco et al. (2020), where the extraction method was unclear. The extracted pulse proteins were provided in the form of foods (73% in LDL-C, non-HDL-C, and HDL-C; 57% in TG), except for the apoB outcome, where the pulse protein was provided in the form of a beverage. The types of pulses included in trials were beans (55% in LDL-C, non-HDL-C, and HDL-C; 64% in TG, and 100% in apoB), dried peas (36% in LDL-C, non-HDL-C, and HDL-C; 29% in TG; 0% in apoB), and a mix of legumes (9% in LDL-C, non-HDL-C, and HDL-C; 7% in TG; 0% in apoB). The comparators were casein/milk protein (82% in LDL-C, non-HDL-C, and HDL-C; 86% in TG; 100% in apoB) and animal protein (18% in LDL-C, non-HDL-C, and HDL-C; 14% in TG; 0% in apoB), where most indicated that the delivery form and caloric contribution of the control matched that of the intervention. The median follow-up duration was 4 weeks (ranging from 4 to 8 weeks) for LDL-C, non-HDL-C, and HDL-C; and 6 weeks for apoB. Trials were funded by agency sources (64% in LDL-C, non-HDL-C, and HDL-C; 50% in TG; 0% in apoB), followed by industry (27% in LDL-C, non-HDL-C, and HDL-C; 21% in TG; 100% in apoB), and a mix of agency and industry (9% in LDL-C, non-HDL-C, and HDL-C; 29% in TG; 0% in apoB).
Table 1.
A summary of the characteristics of included trial comparisons assessing the effect of extracted pulse proteins on blood lipids *.
| Trial Characteristics | LDL-C | Non-HDL-C | apoB | HDL-C | TG |
|---|---|---|---|---|---|
| Trial comparisons (n) | 11 | 11 | 1 | 11 | 14 |
| Study size, median (range) a | 37 (24–45) | 37 (24–45) | 38 | 37 (24–45) | 35 (14–45) |
| Age (y), median (range) | 55 (42–64) | 55 (42–64) | 57 (54–60) | 55 (42–64) | 54 (42–64) |
| Health status (n) | Absence of disease = 2, T2D = 1, Hypercholesterolemia = 8 | Absence of disease = 2, T2D = 1, Hypercholesterolemia = 8 | Hypercholesterolemia = 1 | Absence of disease = 2, T2D = 1, Hypercholesterolemia = 8 | Absence of disease = 5, T2D = 1, Hypercholesterolemia = 8 |
| Male:female ratio (%) b | 44:59 | 44:59 | 16:84 | 44:59 | 45:58 |
| Country (No. of comparisons) | Australia = 1, Brazil = 1, Canada = 1, Germany = 4, Italy = 4, USA = 1 | Australia = 1, Brazil = 1, Canada = 1, Germany = 4, Italy = 4, USA = 1 | Brazil = 1 | Australia = 1, Brazil = 1, Canada = 1, Germany = 4, Italy = 4, USA = 1 | Australia = 1, Brazil = 1, Canada = 4, Germany = 4, Italy = 4, USA = 4 |
| Study design (%), crossover:parallel | 36:64 | 36:64 | 100:0 | 36:64 | 29:71 |
| Feeding control (%), met:sup:DA:met,sup | 0:100:0:0 | 0:100:0:0 | 0:100:0:0 | 0:100:0:0 | 0:100:0:0 |
| Lipid medication use ratio (%), yes:no:mixed:unclear | 0:82:0:8 | 0:82:0:18 | 0:100:0:0 | 0:82:0:18 | 0:86:0:14 |
| Settings (%), inpatients:outpatients:inpatient,outpatient | 0:100:0 | 0:100:0 | 0:100:0 | 0:100:0 | 0:100:0 |
| Baseline BW (kg), median (range) c | 77.4 (66.7–89.5) | 77.4 (66.7–89.5) | 66.7 (62.2–71.2) | 77.4 (66.7–89.5) | 81.1 (66.7–89.5) |
| Baseline BMI (kg/m2), median (range) | 26.0 (24.7–30.6) | 26.0 (24.7–30.6) | 27.3 (26.1–28.5) | 26.0 (24.7–30.6) | 27.3 (24.7–31.5) |
| Baseline outcome d, median (range) | 4.1 (3.1–4.9) | 4.9 (3.6–5.6) | 1.3 (1.3–1.4) | 1.5 (1.1–1.7) | 1.5 (1.1–1.8) |
| Follow-up duration (week), median (range) | 4 (4–8) | 4 (4–8) | 6 | 4 (4–8) | 4 (4–8) |
| Pulse protein dose (g/day), median (range) | 35 (5–122) | 35 (5–122) | 25 | 35 (5–122) | 30 (1–122) |
| Intervention and food source (%), extracted and make into a food:beverage:food & beverage:tablet | 73:9:9:9 | 73:9:9:9 | 0:100:0:0 | 73:9:9:9 | 57:7:7:29 |
| Comparator (No. of comparisons) | Animal protein = 2; Casein, milk protein = 9 | Animal protein = 2; Casein, milk protein = 9 | Casein, milk protein = 1 | Animal protein = 2; Casein, milk protein = 9 | Animal protein = 2; Casein, milk protein = 12 |
| Energy balance (%), neutral:positive:negative e | 91:9:0 | 91:9:0 | 100:0:0 | 91:9:0 | 71:29:0 |
| Energy control (%), substitution:addition:subtraction f | 100:0:0 | 100:0:0 | 100:0:0 | 100:0:0 | 100:0:0 |
| Funding sources (%), A:I:A,I:NR g | 64:27:9 | 64:27:9 | 0:100:0:0 | 64:27:9 | 50:21:29 |
A, agency; apoB, apolipoprotein B; BMI, body mass index; BW, body weight; DA, dietary advice; HDL-C, high-density lipoprotein cholesterol; I, industry; LDL-C, low-density lipoprotein cholesterol; met, metabolic; NR, not reported; non-HDL-C, non-high-density lipoprotein cholesterol; sup, supplement; T2D, type 2 diabetes; TG, triglyceride. * All numbers with the exception of baseline values were rounded to the nearest whole number to improve readability. a All sample sizes reflect participants included in the data analyzed. b Not all studies reported females and males analyzed. Sitori et al. (2012) [56] reported the number of females and males recruited. c Not all trials reported baseline values. Baseline values were not reported for baseline BW (n = 5). d units for LDL-C, non-HDL-C, HDL-C, and TG are in mmol/L and for apoB, g/L. e Neutral energy balance refers to the maintenance of usual energy intake. A positive energy balance refers to a greater-than-normal energy intake. A negative energy balance refers to a deficit in normal energy intake. f Energy control refers to the energy intake of the intervention group compared to the control group where substitution refers to energy matched between intervention and comparator, addition refers to excess energy between the intervention and the comparator, and subtraction refers to a deficit in energy between the intervention and the comparator. g Agency funding is from government, university, or not-for-profit sources. The majority of industry funding is from trade organizations that obtain revenue from the sale of products.
3.3. Risk of Bias
Supplemental Figures S1 and S2 show the risk of bias assessment for individual trials using the Cochrane Risk of Bias Tool 2.0. Across outcomes, most trials were assessed as having a low ROB in outcome measurement (100%), selection domains (100%), and missing outcome domains (91–93%); and some concerns in randomization domains (64–71%) and deviation from the intended intervention (45–57%). Only one trial was assessed as having a high ROB in the missing outcome domain (7–9%) and deviation from the intended intervention (7–9%). Most trials were judged overall as low (50–64%), some trials were assessed as some concerns (27–43%), and one trial was assessed as high (7–9%).
3.4. Primary Outcome
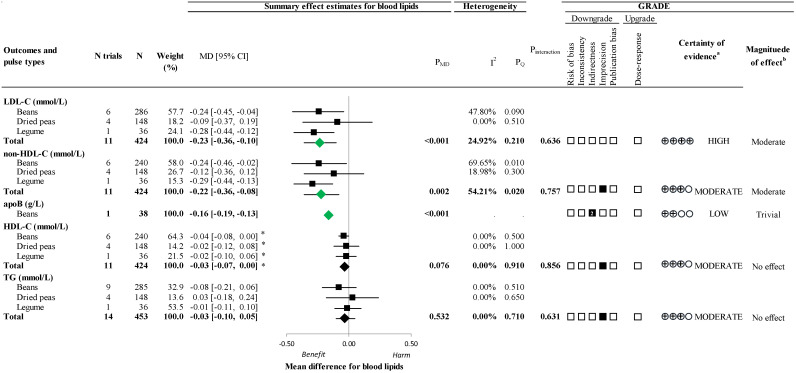
Figure 2 and Supplemental Figure S3 show the effect of extracted pulse proteins on LDL-C. Extracted pulse protein consumption resulted in a significant reduction in LDL-C (11 trials; MD −0.23 mmol/L; 95% CI: −0.36 to −0.10; p < 0.001) with no substantial heterogeneity (I2 = 24.92%; PQ = 0.21)
Figure 2.
A summary plot of the effect of extracted pulse proteins on blood lipids in randomized controlled trials. Data are expressed as weighted mean differences with 95% confidence intervals of the summary effect estimates using the generic inverse variance method modeled by random effect (≥5 trial comparisons) or fixed effect (<5 trial comparisons) meta-analyses. The between-study heterogeneity was assessed using the Cochran Q statistic, where PQ < 0.100 was considered statistically significant, and quantified by the I2 statistic, where I2 ≥ 50% was considered evidence of substantial heterogeneity. The effect estimates of total extracted pulse proteins from different sources are denoted as diamonds. The effect estimates of individual extracted pulse protein types are denoted as squares. Any statistically significant reductions are highlighted in green. The grading of recommendations, assessment, development, and evaluation (GRADE) of randomized controlled trials are rated as having a “high” certainty of evidence and can be downgraded by 5 domains and upgraded by 1 domain. The white squares represent no downgrades, filled black squares indicate a single downgrade or upgrade for each outcome, and the black square with a white “2” indicates a double downgrade for each outcome. a Because all included trials were randomized controlled trials, the certainty of the evidence was graded as high for all outcomes by default and then downgraded or upgraded based on prespecified criteria. Criteria for downgrades included risk of bias (ROB) (downgraded if most trials were considered to be at high ROB); inconsistency (downgraded if there was substantial unexplained heterogeneity: I2 ≥ 50%; PQ < 0.10); indirectness (downgraded if there were factors absent or present relating to the participants, interventions, or outcomes that limited the generalizability of the results); imprecision (downgraded if the 95% confidence intervals crossed the minimally important difference (MID) for harm or benefit set at 0.1 mmol/L (5%) for LDL-C, non-HDL-C, HDL-C, and TG and ± 0.04 g/L for apoB [32,33,34,35], or there was a concern with the robustness of the estimate resulting from sensitivity analyses); and publication bias (downgraded if there was evidence of publication bias based on the funnel plot asymmetry and/or significant Egger’s or Begg’s test (p < 0.10) with the confirmation of evidence of small study effects by adjustment using the trim-and-fill analysis of Duval and Tweedie [42]). The criteria for upgrades included a significant dose–response gradient that supports the direction of the pooled effect estimate. Please see Supplemental Table S7 for details on the GRADE assessment. b For the interpretation of the magnitude, we used the MIDs (see a) to assess the importance of the magnitude of our point estimate using the effect size categories according to the new GRADE guidance [51,52,53] as follows: a large effect (≥5× MID); moderate effect (≥2× MID); small important effect (≥1× MID); and trivial/unimportant effect (<1 MID). Please see Supplemental Table S7 for details on the GRADE assessment. * Owing to the difference in the directionality of HDL-C compared with the other outcomes with regards to signal for benefit or harm, the sign for the MD was changed. apoB, apolipoprotein B; CI, confidence interval; GRADE, grading of recommendations, assessment, development, and evaluation; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; MD, mean difference; N, number; non-HDL-C, non-high-density lipoprotein cholesterol; PMD, p-value of the mean difference; PQ, p-value of the heterogeneity; ROB, risk of bias; TG, triglycerides.
3.5. Secondary Outcomes
Figure 2 and Supplemental Figures S4–S6 present the effect of extracted pulse proteins on non-HDL-C, apoB, HDL-C, and TG. Extracted pulse protein consumption resulted in a significant reduction in non-HDL-C (11 trials; MD = −0.22 mmol/L; 95% CI: −0.36 to −0.08; p = 0.002) with substantial heterogeneity (I2 = 54.21%; PQ = 0.02) and a significant reduction in apoB (1 trial; MD = −0.16 g/L; 95% CI: −0.19 to −0.13; p < 0.001). There was no effect in HDL-C (11 trials; MD = 0.03 mmol/L; 95% CI: −0.00 to 0.07; p = 0.076) with no substantial heterogeneity (I2 = 0.00%; PQ = 0.91), and no effect in TG (14 trials; MD = −0.03 mmol/L; 95% CI: −0.10, 0.05; p = 0.532) with no substantial heterogeneity (I2 = 0.00%; PQ = 0.71).
3.6. Adverse Events and Acceptability
Supplemental Table S5 presents the data reported in five trials [55,56,57,58,62] on acceptability and five trials [56,57,58,59,62] on adverse events. Of the five trials reporting on acceptability, participants mainly reported good acceptability of the extracted pulse protein product, except Sirtori et al. (2012) [56] which reported low satisfaction with the consumption of the pulse protein bar. Among the five trials reporting on adverse events, minor gastrointestinal side effects, flatulence, and obstipation were the most reported symptoms, experienced similarly in both the intervention and control groups.
3.7. Sensitivity Analyses
Supplemental Figures S7–S10 show the individual trial influence analyses for the effect of extracted pulse proteins. The removal of Bahr et al. 2015 [57] (milk protein) partially explained the substantial heterogeneity (original: I2 = 54.21%, PQ < 0.02; after study removed: I2 = 49%, PQ = 0.041) and the removal of Frota et al., 2015 [58] fully explained the substantial heterogeneity (original: I2 = 54.21%, PQ < 0.02; after study removed: I2 = 0%, PQ = 0.503) for non-HDL-C without affecting the magnitude or direction of the effect. The removal of Bahr et al. 2015 [57] (milk protein) resulted in a gain of significance for an increase in HDL-C.
Supplemental Table S6 shows sensitivity analyses for the different correlation coefficients (0.25 and 0.75) used in paired analyses of crossover trials for each outcome. The use of these different correlation coefficients did not alter the direction, magnitude, or significance of the effect or evidence of substantial heterogeneity.
3.8. Subgroup Analyses
Supplemental Figures S11–S24 show categorical and continuous meta-regression analyses for the effect of extracted pulse proteins, where there were at least 10 trial comparisons. There was significant effect modification for the effect of extracted pulse proteins on LDL-C and non-HDL-C by food form, where the one trial that included extracted pulse proteins in beverages showed greater reductions. There was significant effect modification for the effect of extracted pulse proteins on TG by missing outcome reporting, where trials assessed as low ROB showed greater reductions. There was significant effect modification by the proportion of females and males on LDL-C and non-HDL-C, where trials with a greater proportion of females saw a greater reduction. Continuous subgroup analyses across outcomes by proportion of females are summarized in Table 2. In a sensitivity analysis where the one trial which included extracted pulse proteins in beverages, which had the greatest proportion of females (84%) of all included trials (Frota et al., 2015) [58], was removed from the subgroup analyses of sex, there was an attenuation and loss of significant effect modification by sex for LDL-C; however, the effect modification remained significant for non-HDL-C.
Table 2.
Continuous meta-regression analysis for the effect of extracted pulse proteins by the proportion of females *.
| Outcome | Female Proportion Range | Trials | Beta [95% CI] | p | Residual I2 (%) | PQ |
|---|---|---|---|---|---|---|
| LDL-C | 0.35–0.84 | 11 | −1.53 [−2.47 to −0.59] | 0.001 | 0 | 0.959 |
| non-HDL-C | 0.35–0.84 | 11 | −1.60 [−2.36 to −0.84] | <0.001 | 0 | 0.851 |
| HDL-C | 0.35–0.84 | 11 | 0.15 [−0.12 to 0.43] | 0.277 | 0 | 0.940 |
| TG | 0.35–0.84 | 14 | −0.16 [−0.87 to 0.55] | 0.657 | 0 | 0.646 |
Data are presented as the between-group mean difference (95% CI) for a 1-unit change in the predictor variable. β-coefficients were estimated using continuous meta-regression analyses. A positive β-coefficient implies an increase in outcome in the isoflavone intervention as the subgroup variable increases, and a negative β-coefficient implies a decrease in outcome. Residual I2 reports inter-study heterogeneity not explained by the subgroup and was estimated using the Cochran Q statistic. CI, confidence interval; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; non-HDL-C, non-high-density lipoprotein cholesterol; TG, triglyceride. * continuous meta-regression analyses could not be performed for apolipoprotein B (apoB) as there was only one trial comparison.
3.9. Dose–Response Analyses
Supplemental Figures S25–S28 show linear and non-linear dose–response analyses. There was no dose–response for the effect of extracted pulse proteins on LDL-C, non-HDL-C, HDL-C, or TG.
4. Small-Study Effects
Supplemental Figures S29–S32 present the contour-enhanced funnel plots and publication bias assessments for all outcomes with ≥10 trials available. There was no evidence of funnel plot asymmetry or publication bias for any outcome.
GRADE Assessment
Figure 2 and Supplemental Table S7 show the certainty of evidence assessments by GRADE. The certainty of evidence for the effect of extracted pulse proteins was high for LDL-C (moderate effect); moderate for non-HDL-C (moderate effect), HDL-C (no effect), and TG (no effect), owing to downgrades for imprecision; and low for apoB (trivial effect) owing to a double downgrade for very serious indirectness.
5. Discussion
We conducted a systematic review and meta-analysis including 14 trial comparisons providing data on 453 middle-aged adults with or without type 2 diabetes or hypercholesterolemia, assessing the effect of extracted pulse proteins predominantly from beans and dried peas with a median dose of 25–35 g per day over a median follow-up of 4–6 weeks. We showed that extracted pulse proteins resulted in a moderate reduction in LDL-C (−0.23 mmol/L) and non-HDL-C (−0.22 mmol/L), a trivial reduction in apoB (−0.16 mmol/L), and no significant effect on HDL-C and TG. Subgroup analyses of LDL-C and non-HDL-C revealed effect modification by food form, where the one trial using extracted pulse proteins as beverages saw a greater reduction, and by sex, where trials with a greater proportion of females saw a greater reduction. We did not observe a dose–response across outcomes, with most trials providing a narrow dose range between 25 and 35 g/d of extracted pulse proteins, limiting our ability to assess the dose–response gradient.
5.1. Findings in Relation to the Literature
Our systematic review and meta-analysis is the first to assess the effect of extracted pulse proteins on established lipid targets. However, a previous systematic review and meta-analysis of whole dietary pulses in 26 trials [15] similarly showed a reduction in LDL-C of 0.17 mmol/L (95% CI: −0.25 to −0.09 mmol/L) with a median dose of 130 g of whole pulses (~12 g protein) per day. However, where we did observe a significant moderate reduction in non-HDL-C of 0.22 mmol/L (95% CI: −0.36 to −0.08); their results were non-significant, yet did tend to show a reduction (−0.09 mmol/L, 95% CI: −0.19 to 0.00 mmol/L). In their subgroup analyses, like our analyses, they did not see significant interaction by pulse types, indicating that all types of dietary pulses behave similarly. Another systematic review and meta-analysis which examined extracted plant proteins which included soy, nuts, and pulses in substitution for animal protein showed a significant reduction in LDL-C of 0.16 mmol/L (95% CI −0.20 to −0.12 mmol/L), non-HDL-C of 0.18 mmol/L (95% CI −0.22 to −0.14 mmol/L), and apoB of 0.05 g/L (95% CI −0.06 to −0.03 g/L), with no subgroup difference by protein type [63]. A further systematic review and meta-analysis on the consumption of extracted soy protein at a median dose of 25 g per day also showed a reduction in LDL-C of 0.12 mmol/L (95% CI −0.17 to −0.072 mmol/L) [64].
Our finding of significant effect modification in the analyses for LDL-C and non-HDL-C by sex is in opposition to that observed in the previous systematic reviews and meta-analyses on whole dietary pulses [15]. Where we saw that trials with a greater proportion of females had greater reductions in LDL-C and non-HDL-C, the previous study found that trials with more males had a greater reduction in LDL-C. In this previous study, the analysis of LDL-C had substantial heterogeneity which was not explained by the subgroup of sex (residual I2 = 53%, p = 0.01), with no significant effect modification for non-HDL-C where there was substantial heterogeneity (I2 = 98%). In contrast, our study had no substantial heterogeneity in the analysis of LDL-C, and for non-HDL-C, the heterogeneity was fully explained by sex (I2 = 54% to I2 = 0%). The previous study had no other significant subgroups; however, we found that the food form also significantly modified the effect on LDL-C and non-HDL-C. The greater reduction in LDL-C and non-HDL-C observed in the one trial using beverages [58] could partially be explained by the fact that this trial had the greatest proportion of females (84%) among all included trials. As a sensitivity analysis, we removed Frota et al., 2015 [58] from the continuous subgroup analysis by sex and found attenuation and a loss in the significance of effect modification by sex for LDL-C (p = 0.101); however, significance was retained for non-HDL-C (p = 0.048). The greater reduction in LDL-C and non-HDL-C observed in trials with greater proportions of females could be related to greater adherence to dietary intervention in females and a difference in food preferences between females and males, with females tending to consume more foods including dietary pulses [65]. The sex effect could also be partially explained by females receiving a higher proportion of their protein requirement through the intervention compared to men since the same absolute amount of extracted pulse protein was given to both females and males across all trials. Additionally, the previous study included trials with a median age of 51 y, a baseline LDL-C of 3.5 mmol/L, and a median dose of 130 g whole pulses which provides approximately 12 g protein/day, whereas our trials had a median age of 55 y, baseline LDL-C of 4.1 mmol/L, and a median dose of 35 g/day of extracted pulse protein provided mainly within foods. Future investigations into sex effects in response to pulse proteins should consider factors that may influence sex effects, such as menopausal status for females and baseline LDL-C levels.
There are several mechanisms that may explain the observed effect of extracted pulse proteins on blood lipids. Pulse proteins may alter the gut microbiota composition in hosts, which can affect cholesterol metabolism. Tong et al. showed that mice that were fed pea protein for 30 days had an increased abundance of Muribaculaceae and changes in metabolites correlating with reduced LDL-C levels when compared to those that were fed pork protein [66]. Additionally, human studies demonstrate that bioactive peptides from soy proteins, which, like dietary pulses, are considered legumes, may increase hepatic LDL-C receptor expression [67], resulting in an increased clearance of apoB-containing particles from circulation [68]. Furthermore, the mechanism may not be related to changes in body weight since of the nine trials in the present analysis which reported changes in body weight, eight showed reductions in lipid targets independent of body weight.
5.2. Strengths and Limitations
Our systematic review and meta-analysis have several strengths. First, we conducted a comprehensive and reproducible search examining the effect of dietary pulses on blood lipids, allowing us to identify effects on extracted pulse proteins. Second, we only included randomized controlled trials which provided evidence that is less susceptible to bias. Third, our meta-analysis had a comprehensive exploration of possible sources of heterogeneity. Fourth, we investigated the shape and strength of dose–response relationships. Fifth, we applied the GRADE approach to assess the certainty of evidence.
Our analysis also has limitations that should be considered when interpreting the results. First, we double downgraded for indirectness for apoB since there was only one trial comparison in predominantly females with hyperlipidemia, which lacks reproducibility and leads to poor applicability of the results to the general adult population. Second, we downgraded for serious imprecision for non-HDL-C, HDL-C, and TG since the 95% CIs of the pooled effect estimate crossed the prespecified MIDs for non-HDL-C and TG, which means that results may not be clinically relevant, and there was a lack of robustness for HDL-C in sensitivity analyses.
Weighing the strengths and limitations, the certainty of evidence was high for LDL-C, moderate for non-HDL-C, HDL-C, and TG, and low for apoB.
5.3. Implications
National dietary guidelines [69] and clinical practice guidelines on nutrition therapy for dyslipidemia, CVD, and diabetes [10,70,71] have a focus on plant-based dietary patterns, including plant protein-based foods. Good sources of protein from plants include soy, dietary pulses, nuts, and seeds. Soy and nuts have health claims for cholesterol and coronary heart disease risk reduction [33,72,73], while dietary pulses do not. The observed reduction of 0.23 mmol in LDL-C from fairly high doses (25–35 g/d) of extracted pulse proteins across a variety of food forms and supplements is similar to reductions observed with 25 g/d soy protein [33] and 45 g/d nuts [72,73], as well as with 3 g/day of beta-glucan oat fiber [6,34,74], 7 g/d psyllium [34,74] and 2 g/d plant sterols [5,75], which also carry health claims. The global consumption of dietary pulses is low, at 21 g per capita per day, without change over the past three decades, according to the Food and Agriculture Organization [76]. There is thus an opportunity to increase the population’s intake of dietary pulses for cardiovascular health. The present findings support the use of extracted pulse proteins in nutrient-dense food products in alignment with dietary recommendations. These findings also support healthcare providers in translating guidelines by providing evidence to endorse dietary advice for consuming plant-based protein foods containing extracted pulse proteins for cholesterol reduction, such as plant-based burgers, ground rounds, or plant protein-enriched beverages. However, extracted pulse protein products may not be complete proteins and should be recommended within the context of a dietary pattern that includes a variety of protein foods. This issue may be particularly important in vegetarian and vegan diets for complementarity to ensure the adequate intake of all indispensable amino acids. Traditional sources of pulse proteins, such as whole cooked pulses which are associated with cardiovascular benefit [15,77], not only increase plant protein but also dietary fiber, polyphenols, and other phytonutrients. Thus, ensuring the use of extracted pulse protein products within a dietary pattern high in foods providing other beneficial nutrients, such as dietary fiber, will leverage additive cardiovascular benefits [78].
Additionally, systematic review and meta-analysis assessing the effect of substituting animal for plant protein have demonstrated benefits on blood lipids [63]. Therefore, the use of plant-based protein food sources as substitutes for animal protein foods can support the management of dyslipidemia and reduce the risk of CVD. Moreover, the demonstration of effect modification by sex reinforces the urgent need for the application of sex and gender-based analyses in cardiovascular research to provide evidence to equitably address heart disease, which is driven by underrepresented groups including women [79,80]. Further to our results, emerging evidence underscoring disparities in nutritional behaviors across sexes [65] and the gender spectrum [81,82], including preferences for the intake of dietary pulses [65], drive the call for studies to investigate sex and gender differences in the cardiovascular benefits of pulse intake to inform the tailoring of recommendations in future guideline development.
6. Conclusions
In conclusion, our systematic review and meta-analysis identified 14 trial comparisons providing data on 453 middle-aged adults with or without type 2 diabetes or hypercholesterolemia, investigating the effect of extracted pulse proteins on therapeutic lipid targets. The synthesis of evidence from available randomized controlled trials provided a reliable indication that consuming extracted pulse proteins at a mean dose of 35 g per day results in a moderate reduction in LDL-C (−0.23 mmol/L), as well as a good indication for a moderate reduction in non-HDL-C (−0.22 mmol/L) and a trivial reduction in apoB (−0.16 mmol/L), with no effect in HDL-C or TG. The main sources of uncertainty in secondary outcomes were imprecision, as well as indirectness due to only one trial reporting on apoB. To address these uncertainties, there remains a need for larger, high-quality randomized trials assessing a broader variety of pulse types, including chickpeas and lentils, as there were no studies identified on extracted pulse proteins from these pulse types, as well as further exploration of effects by sex and gender. This evidence may direct future policy and guideline updates regarding the use of extracted pulse proteins in food products for the management of cholesterol and the prevention of CVD.
Acknowledgments
Megan Churchill, RD Candidate, Research Coordinator, provided research administration support to the team during study development and data analysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu16213765/s1, Table S1: PRISMA Checklist; Table S2: Search strategy for randomized controlled trials assessing the effect of extracted pulse proteins on blood lipids; Table S3: PICOTS framework of the search strategy; Table S4: Table of characteristics of the randomized controlled trial assessing the effect of extracted pulse proteins on blood lipids; Table S5: Assessment of study product acceptability and adverse events*; Table S6: Sensitivity analyses of the use of correlation coefficients of 0.25 and 0.75 for crossover trials in the primary analysis of the effect of extracted pulse proteins on blood lipids; Table S7: GRADE assessment of the certainty of evidence; Figure S1: Risk of bias proportion graph for the effect of extracted pulse proteins on blood lipids in parallel trials; Figure S2: Risk of bias proportion graph for the effect of extracted pulse proteins on blood lipids in crossover trials; Figure S3: Forest plot of randomized controlled trials of the effect of extracted pulse proteins on LDL-C; Figure S4: Forest plot of randomized controlled trials of the effect of extracted pulse proteins on non-HDL-C; Figure S5: Forest plot of randomized controlled trials of the effect of extracted pulse proteins on HDL-C; Figure S6: Forest plot of randomized controlled trials of the effect of extracted pulse proteins on TG; Figure S7: Sensitivity analysis of the systematic removal of each trial for the effect of extracted pulse proteins on LDL-C; Figure S8: Sensitivity analysis of the systematic removal of each trial for the effect of extracted pulse proteins on non-HDL-C; Figure S9: Sensitivity analysis of the systematic removal of each trial for the effect of extracted pulse proteins on HDL-C; Figure S10: Sensitivity analysis of the systematic removal of each trial for the effect of extracted pulse proteins on TG; Figure S11 (1 of 3): Subgroup analyses for the effect of extracted pulse proteins on LDL-C; Figure S11 (2 of 3): Subgroup analyses for the effect of extracted pulse proteins on LDL-C; Figure S11 (3 of 3): Subgroup analyses for the effect of extracted pulse proteins on LDL-C; Figure S12 (1 of 3): Subgroup analyses for the effect of extracted pulse proteins on non-HDL-C; Figure S12 (2 of 3): Subgroup analyses for the effect of extracted pulse proteins on non-HDL-C; Figure S12 (3 of 3): Subgroup analyses for the effect of extracted pulse proteins on non-HDL-C; Figure S13 (1 of 3): Subgroup analyses for the effect of extracted pulse proteins on HDL-C; Figure S13 (2 of 3): Subgroup analyses for the effect of extracted pulse proteins on HDL-C; Figure S13 (3 of 3): Subgroup analyses for the effect of extracted pulse proteins on HDL-C; Figure S14 (1 of 3): Subgroup analyses for the effect of extracted pulse proteins on TG; Figure S14 (2 of 3): Subgroup analyses for the effect of extracted pulse proteins on TG; Figure S14 (3 of 3): Subgroup analyses for the effect of extracted pulse proteins on TG; Figure S15: Risk of bias subgroup analyses for the effect of extracted pulse proteins on LDL-C; Figure S16: Risk of bias subgroup analyses for the effect of extracted pulse proteins on non-HDL-C; Figure S17: Risk of bias subgroup analyses for the effect of extracted pulse proteins on HDL-C; Figure S18: Risk of bias subgroup analyses for the effect of extracted pulse proteins on TG; Figure S19: Continuous meta-regression analysis for the effect of extracted pulse proteins on LDL-C*; Figure S20: Continuous meta-regression analysis for the effect of extracted pulse proteins on non-HDL-C*; Figure S21: Continuous meta-regression analysis for the effect of extracted pulse proteins on HDL-C*; Figure S22: Continuous meta-regression analysis for the effect of extracted pulse proteins on TG*; Figure S23: Continuous meta-regression analysis for the effect of extracted pulse proteins on LDL-C* (with Frota et al., 2015 [58] removed); Figure S24: Continuous meta-regression analysis for the effect of extracted pulse proteins on non-HDL-C* (with Frota et al., 2015 [58] removed); Figure S25: Linear and non-linear meta-regression analysis for the effect of extracted pulse proteins on LDL-C; Figure S26: Linear and non-linear meta-regression analysis for the effect of extracted pulse proteins on non-HDL-C; Figure S27: Linear and non-linear meta-regression analysis for the effect of extracted pulse proteins on HDL-C; Figure S28: Linear and non-linear meta-regression analysis for the effect of extracted pulse proteins on TG; Figure S29: Publication bias funnel plots for the effect of extracted pulse proteins on LDL-Cl; Figure S30: Publication bias funnel plots for the effect of extracted pulse proteins on non-HDL-C; Figure S31: Publication bias funnel plots for the effect of extracted pulse proteins on HDL-C; Figure S32: Publication bias funnel plots for the effect of extracted pulse proteins on TG.
Author Contributions
The authors’ responsibilities were as follows: L.C. and S.M.G. engaged in project conception and designed the research (the development of the overall research plan and study oversight); S.Y., S.B., S.M.G., S.A.-C., V.C., E.J.L., L.H. and Y.-T.C. conducted the research (hands-on conduct of the experiments and data collection); S.Y., S.A.-C., S.B. and L.C. analyzed the data or performed the statistical analysis; All authors engaged in the interpretation of results. Although S.Y. and L.C. drafted the manuscript, all authors contributed. L.C. and S.M.G. oversaw the research and had the primary responsibility for the final content; L.C. took responsibility for the integrity of the data and the accuracy of the data analysis; All authors contributed to the critical revision of the manuscript for important intellectual content. The corresponding author (L.C.) attests that all listed authors meet the authorship criteria and that no others meeting the criteria have been omitted. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data described in the manuscript, code book, and analytic code will be made available on request.
Conflicts of Interest
S.M.G. has received research support from the Canadian Institutes of Health Research (CIHR), IWK Health, Mount Saint Vincent University, Medavie, Natural Sciences and Engineering Council of Canada (NSERC), and the Canadian Foundation of Dietetic Research. She has received an honorarium from Dietitians of Canada, Diabetes Canada, and the Joannah & Brian Lawson Centre for Child Nutrition Temerty Faculty of Medicine, University of Toronto. She currently works with clients, including those living with or at risk of cardiovascular disease, as part of Dr. Lee Baggley and Associates. T.A.K. reports receiving grants from Canadian Institutes of Health Research (CIHR), Institute for the Advancement of Food and Nutrition Sciences (IAFNS, formerly ILSI North America), and National Honey Board (USDA Checkoff program) and Toronto 3D Knowledge Synthesis and Clinical Trials foundation. He has received honorariums from Advancement of Food and Nutrition Sciences (IAFNS), the International Food Information Council (IFIC), and the Calorie Control Council (CCC), the International Sweeteners Association (ISA), AmCham Dubai, and Ontario Maple Syrup Producers’ Association (OMSPA). A.Z. is a casual research associate at INQUIS Clinical Research Ltd., a contract research organization, and has received consulting fees from the Glycemic Index Foundation. C.P.F.M. is an employee of Protein Industries Canada and a former employee of Kellogg Canada and Pulse Canada. C.W.C.K. has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri-Foods Canada (AAFC), the Almond Board of California, Barilla, the Canadian Institutes of Health Research (CIHR), the Canola Council of Canada, the International Nut and Dried Fruit Council, the International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd., the Peanut Institute, Pulse Canada, and Unilever. He has received in-kind research support from the Almond Board of California, Barilla, the California Walnut Commission, Kellogg Canada, Loblaw Companies, Nutrartis, Quaker (PepsiCo), the Peanut Institute, Primo, Unico, Unilever, and WhiteWave Foods/Danone. He has received travel support and/or honoraria from the Barilla, the California Walnut Commission, the Canola Council of Canada, General Mills, the International Nut and Dried Fruit Council, the International Pasta Organization, Lantmannen, Loblaw Brands Ltd., the Nutrition Foundation of Italy, the Oldways Preservation Trust, Paramount Farms, the Peanut Institute, Pulse Canada, Sun-Maid, Tate & Lyle, Unilever, and White Wave Foods/Danone. He has served on the scientific advisory board for the International Tree Nut Council, the International Pasta Organization, the McCormick Science Institute, and the Oldways Preservation Trust. He is a founding member of the International Carbohydrate Quality Consortium (ICQC), an Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD, and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. D.J.A.J. has received research grants from Loblaw Companies Ltd., the Almond Board of California, the Soy Nutrition Institute (SNI), and the Canadian Institutes of Health Research (CIHR). He has received in-kind supplies for trials as research support from the Almond Board of California, the Walnut Council of California, the American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, and WhiteWave Foods. He has been on the speaker’s panel, served on the scientific advisory board and/or received travel support and/or honoraria from Lawson Centre Nutrition Digital Series, Nutritional Fundamentals for Health (NFH)-Nutramedica, the Saint Barnabas Medical Center, The University of Chicago, the 2020 China Glycemic Index (GI) International Conference, the Atlantic Pain Conference, the Academy of Life Long Learning, the Almond Board of California, the Canadian Agriculture Policy Institute, the Loblaw Companies Ltd., the Griffin Hospital (for the development of the NuVal scoring system), the Coca-Cola Company, Epicure, Danone, Diet Quality Photo Navigation (DQPN), Better Therapeutics (FareWell), Verywell, the True Health Initiative (THI), Heali AI Corp, the Institute of Food Technologists (IFT), the Soy Nutrition Institute (SNI), the Herbalife Nutrition Institute (HNI), Herbalife International, Pacific Health Laboratories, Nutritional Fundamentals for Health (NFH), the Soy Foods Association of North America, the Nutrition Foundation of Italy (NFI), the Toronto Knowledge Translation Group (St. Michael’s Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society (CNS), and the American Society of Nutrition (ASN). He is a member of the International Carbohydrate Quality Consortium (ICQC). His wife, Alexandra L Jenkins, is a senior scientist for INQUIS Clinical Research Inc. (Clinical Research Organization), and his two daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the low glycemic index plant foods advocated here, The Portfolio Diet for Cardiovascular Risk Reduction (Academic Press/Elsevier 2020 ISBN:978-0-12-810510-8) and his sister, Caroline Brydson, received funding through a grant from the St. Michael’s Hospital Foundation to develop a cookbook for one of his studies. AE-S is the founder and holds shares in Nutrigenomix Inc. J.L.S. has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of Health Research (CIHR), Diabetes Canada, American Society for Nutrition (ASN), National Honey Board (U.S. Department of Agriculture [USDA] honey “Checkoff” program), Institute for the Advancement of Food and Nutrition Sciences (IAFNS), Pulse Canada, Quaker Oats Center of Excellence, INC International Nut and Dried Fruit Council Foundation, The United Soybean Board (USDA soy “Checkoff” program), Protein Industries Canada (a Government of Canada Global Innovation Cluster), Almond Board of California, European Fruit Juice Association, The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), The Plant Protein Fund at the University of Toronto (a fund which has received contributions from IFF among other donors), The Plant Milk Fund at the University of Toronto (a fund established by the Karuna Foundation through Vegan Grants), and The Nutrition Trialists Network Fund at the University of Toronto (a fund established by donations from the Calorie Control Council, Physicians Committee for Responsible Medicine, and Login5 Foundation). He has received food donations to support randomized controlled trials from the Almond Board of California, California Walnut Commission, Danone, Nutrartis, Soylent, and Dairy Farmers of Canada. He has received travel support, speaker fees and/or honoraria from FoodMinds LLC, Nestlé, Abbott, General Mills, Nutrition Communications, International Food Information Council (IFIC), Arab Beverage Association, International Sweeteners Association, Calorie Control Council, and Phynova. He has or has had ad hoc consulting arrangements with Almond Board of California, Perkins Coie LLP, Tate & Lyle, Ingredion, and Brightseed. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada/Canadian Association of Bariatric Physicians and Surgeons. He serves as an unpaid member of the Board of Trustees of IAFNS. He is a Director at Large of the Canadian Nutrition Society (CNS), founding member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His spouse is a former employee of Nestle Health Science and AB InBev.. L.C. has received research support from the Canadian Institutes of Health Research (CIHR), Protein Industries Canada (a Government of Canada Global Innovation Cluster), the United Soybean Board (USDA soy “Checkoff” program), and the Alberta Pulse Growers Association. S.Y., S.B., S.A.-C., V.C., E.J.L., L.H., Y.-T.C., J.G., V.H., T.A.K., S.B.M., R.J.d.S., J.B., M.M.E., V.V., R.J.G., and L.A.L. report no relevant competing interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
S.Y. received research support from a Banting and Best Diabetes Centre Charles Hollenberg Summer Studentship. S.B. received research support from an internal sabbatical research grant awarded to SMG through Mount Saint Vincent University 2022–23, an Undergraduate Research Opportunity Program Award from the Department of Nutritional Sciences, University of Toronto, and a Toronto 3D Knowledge Synthesis Summer Student Top-Up Award. S.A.-C. received research support from a CIHR Canadian Graduate Scholarship Doctoral Award (funding reference number 476251). S.M.G. received support through an internal sabbatical research grant, awarded to SMG through Mount Saint Vincent University, 2022–23, and an internal travel grant, awarded to SMG through Mount Saint Vincent University, 2022. V.C. was funded by a Toronto 3D Research Fellowship Award, CIHR Canada Graduate Scholarship—Masters (CGS-M) Research Award, and a University of Toronto Department of Nutritional Sciences Fellowship. A.Z. was funded by the Toronto 3D Postdoctoral Fellowship Award. Y.-T.C. received research support from the Toronto 3D Knowledge Synthesis Summer Student Scholarship. E.J.L. received research support from an Undergraduate Research Opportunity Program Award from the Department of Nutritional Sciences, University of Toronto. D.J.A.J. was funded by the Government of Canada through the Canada Research Chair Endowment. L.C. was funded by a Toronto 3D New Investigator Award.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Cardiovascular Diseases (CVDs) [(accessed on 10 June 2024)]. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)#:~:text=Key%20facts,to%20heart%20attack%20and%20stroke.
- 2.Canada S. Morality, Summary List of Causes 2008. 2011. [(accessed on 10 June 2024)]. Available online: https://www150.statcan.gc.ca/n1/en/catalogue/84F0209X2008000.
- 3.The Conference Board of Canada The Canadian Heart Health Strategy: Risk Factors and Future Cost Implications. [(accessed on 10 June 2024)]. Available online: https://sencanada.ca/content/sen/committee/412/SOCI/Briefs/2015-05-07ReportCdnCardiovascularSociety-AddInfoConferenceBoardofCanada_e.pdf.
- 4.National Cholesterol Education Program (US) Expert Panel on Detection. Treatment of High Blood Cholesterol in Adults Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of a health claim related to 3 g/day plant sterols/stanols and lowering blood LDL-cholesterol and reduced risk of (coronary) heart disease pursuant to Article 19 of Regulation (EC) No 1924/2006. EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) EFSA J. 2012;10:2693. doi: 10.2903/j.efsa.2012.2693. [DOI] [Google Scholar]
- 6.EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA) Scientific Opinion on the substantiation of a health claim related to oat beta-glucan and lowering blood cholesterol and reduced risk of (coronary) heart disease pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2010;8:1885. doi: 10.2903/j.efsa.2010.1885. [DOI] [Google Scholar]
- 7.Sud M., Han L., Koh M., Abdel-Qadir H., Austin P.C., Farkouh M.E., Godoy L.C., Lawler P.R., Udell J.A., Wijeysundera H.C., et al. Low-Density Lipoprotein Cholesterol and Adverse Cardiovascular Events After Percutaneous Coronary Intervention. J. Am. Coll. Cardiol. 2020;76:1440–1450. doi: 10.1016/j.jacc.2020.07.033. [DOI] [PubMed] [Google Scholar]
- 8.Sarak B., Savu A., Kaul P., McAlister F.A., Welsh R.C., Yan A.T., Goodman S.G. Lipid Testing, Lipid-Modifying Therapy, and PCSK9 (Proprotein Convertase Subtilisin-Kexin Type 9) Inhibitor Eligibility in 27 979 Patients with Incident Acute Coronary Syndrome. Circ. Cardiovasc. Qual. Outcomes. 2021;14:e006646. doi: 10.1161/CIRCOUTCOMES.120.006646. [DOI] [PubMed] [Google Scholar]
- 9.Health Canada Canada’s Food Guide. [(accessed on 10 June 2024)]; Available online: https://food-guide.canada.ca/en/
- 10.Pearson G.J., Thanassoulis G., Anderson T.J., Barry A.R., Couture P., Dayan N., Francis G.A., Genest J., Gregoire J., Grover S.A., et al. 2021 Canadian Cardiovascular Society Guidelines for the Management of Dyslipidemia for the Prevention of Cardiovascular Disease in Adults. Can. J. Cardiol. 2021;37:1129–1150. doi: 10.1016/j.cjca.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 11.Tobe S.W., Stone J.A., Anderson T., Bacon S., Cheng A.Y.Y., Daskalopoulou S.S., Ezekowitz J.A., Gregoire J.C., Gubitz G., Jain R., et al. Canadian Cardiovascular Harmonized National Guidelines Endeavour (C-CHANGE) guideline for the prevention and management of cardiovascular disease in primary care: 2018 update. CMAJ. 2018;190:E1192–E1206. doi: 10.1503/cmaj.180194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diabetes Canada Clinical Practice Guidelines Expert Committee. Punthakee Z., Goldenberg R., Katz P. Definition, Classification and Diagnosis of Diabetes, Prediabetes and Metabolic Syndrome. Can. J. Diabetes. 2018;42((Suppl. 1)):S10–S15. doi: 10.1016/j.jcjd.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Brown J., Clarke C., Johnson Stoklossa C., Sievenpiper J. Canadian Adult Obesity Clinical Practice Guidelines: Medical Nutrition Therapy in Obesity Management. [(accessed on 10 June 2024)]. Available online: https://obesitycanada.ca/guidelines/nutrition.
- 14.Hertzler S.R., Lieblein-Boff J.C., Weiler M., Allgeier C. Plant Proteins: Assessing Their Nutritional Quality and Effects on Health and Physical Function. Nutrients. 2020;12:3704. doi: 10.3390/nu12123704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha V., Sievenpiper J.L., de Souza R.J., Jayalath V.H., Mirrahimi A., Agarwal A., Chiavaroli L., Mejia S.B., Sacks F.M., Di Buono M., et al. Effect of dietary pulse intake on established therapeutic lipid targets for cardiovascular risk reduction: A systematic review and meta-analysis of randomized controlled trials. CMAJ. 2014;186:E252–E262. doi: 10.1503/cmaj.131727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emily M.T., Padhi D.D.R. A review of the relationship between pulse consumption and reduction of cardiovascular disease risk factors. J. Funct. Foods. 2017;38:635–643. doi: 10.1016/j.jff.2017.03.043. [DOI] [Google Scholar]
- 17.Alberti K.G., Eckel R.H., Grundy S.M., Zimmet P.Z., Cleeman J.I., Donato K.A., Fruchart J.C., James W.P., Loria C.M., Smith S.C., Jr., et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3. [(accessed on 10 June 2022)]. Available online: https://training.cochrane.org/handbook/current.
- 19.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. bmj. 2020;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration . Guidance for Industry: Evidence-Based Review System for The Scientific Evaluation of Health Claims. FDA; Silver Spring, MD, USA: 2009. [Google Scholar]
- 21.SourceForge Plot Digitizer. 2001. [(accessed on 24 October 2015)]. Available online: http://plotdigitizer.sourceforge.net/
- 22.Sterne J.A.C., Savovic J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 23.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 24.Wan X., Wang W., Liu J., Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Furukawa T.A., Barbui C., Cipriani A., Brambilla P., Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. J. Clin. Epidemiol. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Ku H. Notes on the Use of Propagation of Error Formulas. J. Res. Natl. Bur. Stand. 1966;70:263–273. doi: 10.6028/jres.070C.025. [DOI] [Google Scholar]
- 27.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 28.Tufanaru C., Munn Z., Stephenson M., Aromataris E. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. JBI Evid. Implement. 2015;13:196–207. doi: 10.1097/XEB.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 29.Elbourne D.R., Altman D.G., Higgins J.P., Curtin F., Worthington H.V., Vail A. Meta-analyses involving cross-over trials: Methodological issues. Int. J. Epidemiol. 2002;31:140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- 30.Follmann D., Elliott P., Suh I., Cutler J. Variance imputation for overviews of clinical trials with continuous response. J. Clin. Epidemiol. 1992;45:769–773. doi: 10.1016/0895-4356(92)90054-Q. [DOI] [PubMed] [Google Scholar]
- 31.Balk E.M., Earley A., Patel K., Trikalinos T.A., Dahabreh I.J. Empirical Assessment of Within-Arm Correlation Imputation in Trials of Continuous Outcomes. Agency for Healthcare Research and Quality (US); Rockville, MD, USA: 2012. AHRQ Methods for Effective Health Care. [PubMed] [Google Scholar]
- 32.Deeks J.J., Higgins J.P.T. Statistical Algorithms in Review Manager 5. 2010. [(accessed on 30 June 2017)]. Available online: https://training.cochrane.org/handbook/current/statistical-methods-revman5.
- 33.Food Directorate Health Products and Food Branch, Health Canada . Summary of Health Canada’s Assessment of a Health Claim About Soy Protein and Cholesterol Lowering. Bureau of Nutritional Sciences; Ottawa, ON, Canada: 2015. [Google Scholar]
- 34.Food Directorate Health Products and Food Branch, Health Canada . Oat Products and Blood Cholesterol Lowering. Bureau of Nutritional Sciences; Ottawa, ON, Canada: 2010. [Google Scholar]
- 35.Roberts W.C. The rule of 5 and the rule of 7 in lipid-lowering by statin drugs. Am. J. Cardiol. 1997;80:106–107. doi: 10.1016/S0002-9149(97)00298-1. [DOI] [PubMed] [Google Scholar]
- 36.Nicholls S.J., Brandrup-Wognsen G., Palmer M., Barter P.J. Meta-analysis of comparative efficacy of increasing dose of Atorvastatin versus Rosuvastatin versus Simvastatin on lowering levels of atherogenic lipids (from VOYAGER) Am. J. Cardiol. 2010;105:69–76. doi: 10.1016/j.amjcard.2009.08.651. [DOI] [PubMed] [Google Scholar]
- 37.Borenstein M., Hedges L.V., Higgins J.P.T., Rothstein H.R. Introduction to Meta-Analysis. John Wiley & Sons; Chichester, UK: 2009. [Google Scholar]
- 38.Thompson S.G., Higgins J.P. How should meta-regression analyses be undertaken and interpreted? Stat. Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 39.Fu R., Gartlehner G., Grant M., Shamliyan T., Sedrakyan A., Wilt T.J., Griffith L., Oremus M., Raina P., Ismaila A., et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J. Clin. Epidemiol. 2011;64:1187–1197. doi: 10.1016/j.jclinepi.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 40.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Begg C.B., Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 42.Sterne J.A., Gavaghan D., Egger M. Publication and related bias in meta-analysis: Power of statistical tests and prevalence in the literature. J. Clin. Epidemiol. 2000;53:1119–1129. doi: 10.1016/S0895-4356(00)00242-0. [DOI] [PubMed] [Google Scholar]
- 43.Duval S., Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341X.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 44.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64:383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 45.Higgins J.P., Altman D.G., Gotzsche P.C., Juni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A., et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andrews J., Guyatt G., Oxman A.D., Alderson P., Dahm P., Falck-Ytter Y., Nasser M., Meerpohl J., Post P.N., Kunz R., et al. GRADE guidelines: 14. Going from evidence to recommendations: The significance and presentation of recommendations. J. Clin. Epidemiol. 2013;66:719–725. doi: 10.1016/j.jclinepi.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 47.Brunetti M., Shemilt I., Pregno S., Vale L., Oxman A.D., Lord J., Sisk J., Ruiz F., Hill S., Guyatt G.H., et al. GRADE guidelines: 10. Considering resource use and rating the quality of economic evidence. J. Clin. Epidemiol. 2013;66:140–150. doi: 10.1016/j.jclinepi.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 48.Guyatt G.H., Oxman A.D., Schünemann H.J. GRADE guidelines-an introduction to the 10th-13th articles in the series. J. Clin. Epidemiol. 2013;66:121–123. doi: 10.1016/j.jclinepi.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 49.Guyatt G.H., Thorlund K., Oxman A.D., Walter S.D., Patrick D., Furukawa T.A., Johnston B.C., Karanicolas P., Akl E.A., Vist G., et al. GRADE guidelines: 13. Preparing summary of findings tables and evidence profiles-continuous outcomes. J. Clin. Epidemiol. 2013;66:173–183. doi: 10.1016/j.jclinepi.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 50.Guyatt G., Oxman A.D., Sultan S., Brozek J., Glasziou P., Alonso-Coello P., Atkins D., Kunz R., Montori V., Jaeschke R., et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J. Clin. Epidemiol. 2013;66:151–157. doi: 10.1016/j.jclinepi.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 51.Guyatt G.H., Oxman A.D., Santesso N., Helfand M., Vist G., Kunz R., Brozek J., Norris S., Meerpohl J., Djulbegovic B., et al. GRADE guidelines: 12. Preparing summary of findings tables-binary outcomes. J. Clin. Epidemiol. 2013;66:158–172. doi: 10.1016/j.jclinepi.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 52.Schünemann H., Brożek J., Guyatt G., Oxman A. GRADE Handbook. [(accessed on 10 November 2018)]. Available online: https://gdt.gradepro.org/app/handbook/handbook.html.
- 53.Santesso N., Glenton C., Dahm P., Garner P., Akl E.A., Alper B., Brignardello-Petersen R., Carrasco-Labra A., De Beer H., Hultcrantz M., et al. GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. J. Clin. Epidemiol. 2020;119:126–135. doi: 10.1016/j.jclinepi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 54.Balshem H., Helfand M., Schunemann H.J., Oxman A.D., Kunz R., Brozek J., Vist G.E., Falck-Ytter Y., Meerpohl J., Norris S., et al. GRADE guidelines: 3. Rating the quality of evidence. J. Clin. Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 55.Weisse K., Brandsch C., Zernsdorf B., Nkengfack Nembongwe G.S., Hofmann K., Eder K., Stangl G.I. Lupin protein compared to casein lowers the LDL cholesterol:HDL cholesterol-ratio of hypercholesterolemic adults. Eur. J. Nutr. 2010;49:65–71. doi: 10.1007/s00394-009-0049-3. [DOI] [PubMed] [Google Scholar]
- 56.Sirtori C.R., Triolo M., Bosisio R., Bondioli A., Calabresi L., De Vergori V., Gomaraschi M., Mombelli G., Pazzucconi F., Zacherl C., et al. Hypocholesterolaemic effects of lupin protein and pea protein/fibre combinations in moderately hypercholesterolaemic individuals. Br. J. Nutr. 2012;107:1176–1183. doi: 10.1017/S0007114511004120. [DOI] [PubMed] [Google Scholar]
- 57.Bahr M., Fechner A., Kiehntopf M., Jahreis G. Consuming a mixed diet enriched with lupin protein beneficially affects plasma lipids in hypercholesterolemic subjects: A randomized controlled trial. Clin. Nutr. 2015;34:7–14. doi: 10.1016/j.clnu.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Frota Kde M., dos Santos Filho R.D., Ribeiro V.Q., Areas J.A. Cowpea protein reduces LDL-cholesterol and apolipoprotein B concentrations, but does not improve biomarkers of inflammation or endothelial dysfunction in adults with moderate hypercholesterolemia. Nutr. Hosp. 2015;31:1611–1619. doi: 10.3305/nh.2015.31.4.8457. [DOI] [PubMed] [Google Scholar]
- 59.Kohno M., Sugano H., Shigihara Y., Shiraishi Y., Motoyama T. Improvement of glucose and lipid metabolism via mung bean protein consumption: Clinical trials of GLUCODIA isolated mung bean protein in the USA and Canada. J. Nutr. Sci. 2018;7:e2. doi: 10.1017/jns.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sucher S., Markova M., Hornemann S., Pivovarova O., Rudovich N., Thomann R., Schneeweiss R., Rohn S., Pfeiffer A.F.H. Comparison of the effects of diets high in animal or plant protein on metabolic and cardiovascular markers in type 2 diabetes: A randomized clinical trial. Diabetes Obes. Metab. 2017;19:944–952. doi: 10.1111/dom.12901. [DOI] [PubMed] [Google Scholar]
- 61.Pivovarova-Ramich O., Markova M., Weber D., Sucher S., Hornemann S., Rudovich N., Raila J., Sunaga-Franze D., Sauer S., Rohn S., et al. Effects of diets high in animal or plant protein on oxidative stress in individuals with type 2 diabetes: A randomized clinical trial. Redox Biol. 2020;29:101397. doi: 10.1016/j.redox.2019.101397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crimarco A., Springfield S., Petlura C., Streaty T., Cunanan K., Lee J., Fielding-Singh P., Carter M.M., Topf M.A., Wastyk H.C., et al. A randomized crossover trial on the effect of plant-based compared with animal-based meat on trimethylamine-N-oxide and cardiovascular disease risk factors in generally healthy adults: Study With Appetizing Plantfood-Meat Eating Alternative Trial (SWAP-MEAT) Am. J. Clin. Nutr. 2020;112:1188–1199. doi: 10.1093/ajcn/nqaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li S.S., Blanco Mejia S., Lytvyn L., Stewart S.E., Viguiliouk E., Ha V., de Souza R.J., Leiter L.A., Kendall C.W.C., Jenkins D.J.A., et al. Effect of Plant Protein on Blood Lipids: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2017;6:e006659. doi: 10.1161/JAHA.117.006659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blanco Mejia S., Messina M., Li S.S., Viguiliouk E., Chiavaroli L., Khan T.A., Srichaikul K., Mirrahimi A., Sievenpiper J.L., Kris-Etherton P., et al. A Meta-Analysis of 46 Studies Identified by the FDA Demonstrates that Soy Protein Decreases Circulating LDL and Total Cholesterol Concentrations in Adults. J. Nutr. 2019;149:968–981. doi: 10.1093/jn/nxz020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barrea L., Verde L., Suarez R., Frias-Toral E., Vasquez C.A., Colao A., Savastano S., Muscogiuri G. Sex-differences in Mediterranean diet: A key piece to explain sex-related cardiovascular risk in obesity? A cross-sectional study. J. Transl. Med. 2024;22:44. doi: 10.1186/s12967-023-04814-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tong L.T., Xiao T., Wang L., Lu C., Liu L., Zhou X., Wang A., Qin W., Wang F. Plant protein reduces serum cholesterol levels in hypercholesterolemia hamsters by modulating the compositions of gut microbiota and metabolites. iScience. 2021;24:103435. doi: 10.1016/j.isci.2021.103435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lovati M.R., Manzoni C., Gianazza E., Arnoldi A., Kurowska E., Carroll K.K., Sirtori C.R. Soy protein peptides regulate cholesterol homeostasis in Hep G2 cells. J. Nutr. 2000;130:2543–2549. doi: 10.1093/jn/130.10.2543. [DOI] [PubMed] [Google Scholar]
- 68.Baum J.A., Teng H., Erdman J.W., Jr., Weigel R.M., Klein B.P., Persky V.W., Freels S., Surya P., Bakhit R.M., Ramos E., et al. Long-term intake of soy protein improves blood lipid profiles and increases mononuclear cell low-density-lipoprotein receptor messenger RNA in hypercholesterolemic, postmenopausal women. Am. J. Clin. Nutr. 1998;68:545–551. doi: 10.1093/ajcn/68.3.545. [DOI] [PubMed] [Google Scholar]
- 69.Health Canada Canada’s Food Guide: Healthy Eating Recommendations. [(accessed on 17 December 2019)]; Available online: https://food-guide.canada.ca/en/healthy-eating-recommendations/
- 70.Grundy S.M., Stone N.J., Bailey A.L., Beam C., Birtcher K.K., Blumenthal R.S., Braun L.T., de Ferranti S., Faiella-Tommasino J., Forman D.E., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Diabetes Canada Clinical Practice Guidelines Expert Committee. Sievenpiper J.L., Chan C.B., Dworatzek P.D., Freeze C., Williams S.L. Nutrition Therapy. Can. J. Diabetes. 2018;42((Suppl. 1)):S64–S79. doi: 10.1016/j.jcjd.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 72.Taylor C.L. US Food and Drug Adminstration Qualified Health Claims: Letter of Enforcement Discretion—Nuts and Coronary Heart Disease (Docket No 02P-0505) [(accessed on 10 June 2024)]; Available online: https://wayback.archive-it.org/7993/20171114183724/https://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm072926.htm.
- 73.Tarantino L.M. US Food and Drug Adminstration Qualified Health Claims: Letter of Enforcement Discretion—Walnuts and Coronary Heart Disease (Docket No 02P-0292) [(accessed on 10 June 2024)]; Available online: https://wayback.archive-it.org/7993/20171114183725/https://www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm072910.htm.
- 74.Food and Drug Administration. HHS Food labeling: Health claims; soluble fiber from certain foods and risk of coronary heart disease. Interim final rule. Fed. Regist. 2008;73:9938–9947. [PubMed] [Google Scholar]
- 75.Canada, Bureau of Nutritional Sciences, Food Directorate, Health Products and Food Branch, Health Canada Plant Sterols and Blood Cholesterol Lowering. [(accessed on 10 June 2024)];2010 Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-labelling/health-claims/assessments/plant-sterols-blood-cholesterol-lowering-nutrition-health-claims-food-labelling.html.
- 76.Rawal V., Navarro D.K. The Global Economy of Pulses. Food and Agriculture Organization of the United Nations; Rome, Italy: 2019. [Google Scholar]
- 77.Viguiliouk E., Glenn A.J., Nishi S.K., Chiavaroli L., Seider M., Khan T., Bonaccio M., Iacoviello L., Mejia S.B., Jenkins D.J.A., et al. Associations between Dietary Pulses Alone or with Other Legumes and Cardiometabolic Disease Outcomes: An Umbrella Review and Updated Systematic Review and Meta-analysis of Prospective Cohort Studies. Adv. Nutr. 2019;10((Suppl. 4)):S308–S319. doi: 10.1093/advances/nmz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chiavaroli L., Nishi S.K., Khan T.A., Braunstein C.R., Glenn A.J., Mejia S.B., Rahelic D., Kahleova H., Salas-Salvado J., Jenkins D.J.A., et al. Portfolio Dietary Pattern and Cardiovascular Disease: A Systematic Review and Meta-analysis of Controlled Trials. Prog. Cardiovasc. Dis. 2018;61:43–53. doi: 10.1016/j.pcad.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Khan S.U., Khan M.Z., Raghu Subramanian C., Riaz H., Khan M.U., Lone A.N., Khan M.S., Benson E.M., Alkhouli M., Blaha M.J., et al. Participation of Women and Older Participants in Randomized Clinical Trials of Lipid-Lowering Therapies: A Systematic Review. JAMA Netw. Open. 2020;3:e205202. doi: 10.1001/jamanetworkopen.2020.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fazli G.S., Moineddin R., Bierman A.S., Booth G.L. Ethnic variation in the conversion of prediabetes to diabetes among immigrant populations relative to Canadian-born residents: A population-based cohort study. BMJ Open Diabetes Res. Care. 2020;8:e000907. doi: 10.1136/bmjdrc-2019-000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Howard Wilsher S., Fearne A., Panagiotaki G. “That is an Awful Lot of Fruit and Veg to Be Eating”. Focus Group Study on Motivations for the Consumption of 5 a Day in British Young Men. Nutrients. 2019;11:1893. doi: 10.3390/nu11081893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Raparelli V., Romiti G.F., Spugnardi V., Borgi M., Cangemi R., Basili S., Proietti M., The Eva Collaborative G. Gender-Related Determinants of Adherence to the Mediterranean Diet in Adults with Ischemic Heart Disease. Nutrients. 2020;12:759. doi: 10.3390/nu12030759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data described in the manuscript, code book, and analytic code will be made available on request.