Abstract
9-Aminoacridine structures hold much potential for accessing small molecule therapeutics. This core is present in a range of pharmaceuticals for the treatment of ailments such as malaria, inflammation, viral and bacterial infections, and cancer. For the latter, there remains a need to develop and/or improve chemotherapeutics to counteract issues of uptake, drug resistance, and selectivity for cancer cells over healthy cells. In the design of molecules to address these issues, identifying structural units that present as promising leads for drug developments is key. In this study, four 9-aminoacridine derivatives under consideration as precursors for a drug design project are assessed for their cytotoxicity with representative cell lines PC3 and A549, and for their leadlikeness with SwissADME. Together, the cytotoxicity and in silico investigations coalesce around the same derivative as the most promising lead.
Keywords: 9-aminoacridine, structure-activity relationship, cancer, chemotherapeutic, leadlikeness
Graphical Abstract:

Four 9-aminoacridine derivatives are assessed for their cytotoxicity, and for their leadlikeness. Cytotoxicity and in silico investigations coalesce around the same derivative as the most promising lead.
Introduction
Cancer remains a leading cause of death worldwide, and the number of newly diagnosed cancer cases and fatality rate is expected to significantly increase by 2040.1–3 The most common are prostate, lung, breast, and colon/rectum cancers. Current treatments have exhibited varying levels of success, depending on the type of cancer and how the disease progresses. In the case of drug therapy, there can be problems with selectivity, uptake, and resistance that develops with time and recurrence. Furthermore, a major roadblock in drug development is the inability of many small molecules to penetrate tumours. As such, investigations focused on the design of drug targets that can be seamlessly modified to enhance uptake and permeability have the potential to meet an important chemotherapeutic need.
Acridine is a planar triaryl structure (Figure 1). Acridines are present in compounds known for a wide range of pharmaceutical applications; for example, anticancer, antimalarial, anti-inflammatory, antiviral, antibacterial, and imaging.4–8 Many acridinyl drugs are exploited for their ability to intercalate DNA and RNA. In some cases, the intercalation leads to the formation of DNA-topoisomerase II ternary complexes which trigger DNA strand cleavage and eventually, apoptosis.9 With respect to cancer therapy, other known mechanisms of action are the activation and stabilization of the p53 tumour suppressor protein10, 11 and kinase inhibition.12
Figure 1.

Examples of Acridine Compounds
A few examples of biologically important acridinyl derivatives include the imaging agent acridine orange,6 the multi-purpose quinacrine (antimalarial, antibiotic and anti-inflammatory),13 and the cancer therapeutic Amsacrine (m-AMSA) (Figure 1).9 m-AMSA is approved for the treatment of leukemia and Hodgkin’s and non-Hodgkin’s lymphoma in several countries. It was the first drug that was shown to poison topoisomerase II in vitro14 and in vivo.15 Structure-activity relationship (SAR) studies revealed that the acridine moiety contributed to the drug’s efficacy by “anchoring” the molecule to DNA. In addition, other investigations have also shown that m-AMSA works independent of DNA damage to induce p53 protein transcription and inhibit the protein’s ubiquitination.10 The activity of m-AMSA is significantly diminished with solid tumors.16 Quinacrine, an antimalarial drug, is the focus of drug “repurposing” studies for cancer treatment as a therapeutic and as an imaging agent.17 Significant potency has been exhibited with lung, breast, renal, ovarian and gastrointestinal cancers, to name a few. For the treatment of prostate cancer, investigation have looked at quinacrine in combination with other drugs.
The anti-cancer activity of the 9-aminoacridine (9-AA) core itself presents much potential for accessing small molecule therapeutics via straightforward synthetic modifications to the core skeleton.7, 8, 18 Although there could be inherent nonspecificity with cancer cells versus healthy cells, it is possible to modify the structures of small molecules such as 9-AA to tune them for selectivity. For example, selective uptake could be achieved with the exploitation of the unique nutrient needs of cancer cells by converting the small molecules to amino acid-drug conjugates.19–21
In this study we are exploring the structure-activity relationship (SAR) for 1, 2 and 3a-c to compare their potential as leads for the development of novel cancer therapeutics through further structural optimization (Figure 2). Molecules 2 and 3a-c were selected because they contain the 6-chloro-2-methoxyacrdine cores, also present in the multi-purpose quinacrine. In addition, modifications at the hydroxy group in structures 2 and 3a-c and/or at the 9-position in any of the compounds could be used to attach units to enhance biologically relevant properties such as potency, cell and tumor permeability, and selectivity for cancer cells over healthy counterparts. Compounds 2 and 3a-c are known,12, 22, 23. 9-Aminoacridine hydrochloride 1 is commercially available and serves as the “baseline” (parent) structure for these investigations.
Figure 2.

9-Aminoacridine (9-AA) Derivatives
In this work, for the chosen molecules, the variations between 2 and 3a-c provide valuable SAR data on the implications of the alkyl versus aryl N-substituents. Specifically, 1, 2 and 3a-c were assessed and compared for their drug- and lead-likeness in silico, and for their relative cytotoxicity with cancer cell lines, A549 (lung carcinoma epithelial cells)24 and PC3 (prostate cancer).25, 26 The results reported below show that the in silico and cytotoxicity investigations coalesce around the same compound as the most promising lead. For this lead, a representative IC50 study (figure 4) along with confocal microscopy imaging of changes in cell morphology (figure 5 and 6) was carried out.
Results and Discussion
Synthesis
The synthesis and structural characterization of 2 and 3a-c were accomplished using procedures and data previously reported in the literature (Scheme 1).27, 28 The compounds were accessed through a nucleophilic aromatic substitution reaction with 6,9-dichloro-2-methoxyacridine 4 in yields of 60% or higher. The aminoethanol derivative 2 was made under basic conditions in the presence of triethylamine. Aminophenols 3a-c required a catalytic amount of hydrochloric acid to facilitate the reactions and were isolated as the hydrochloride salts. Notably, for 3a-c, distinguishing features in the 1H-NMR (Hydrogen Nuclear Magnetic Resonance) spectra are the signal at ~11ppm for the anilinyl N-H unit and a broad signal at ~14ppm for the protonated acridine nitrogen (+N-H). For 2, triplets at ~3.65ppm and ~3.75ppm for the aminoethanol unit’s CH2 protons could be used as key indicators for successful formation of the compound.
Scheme 1.
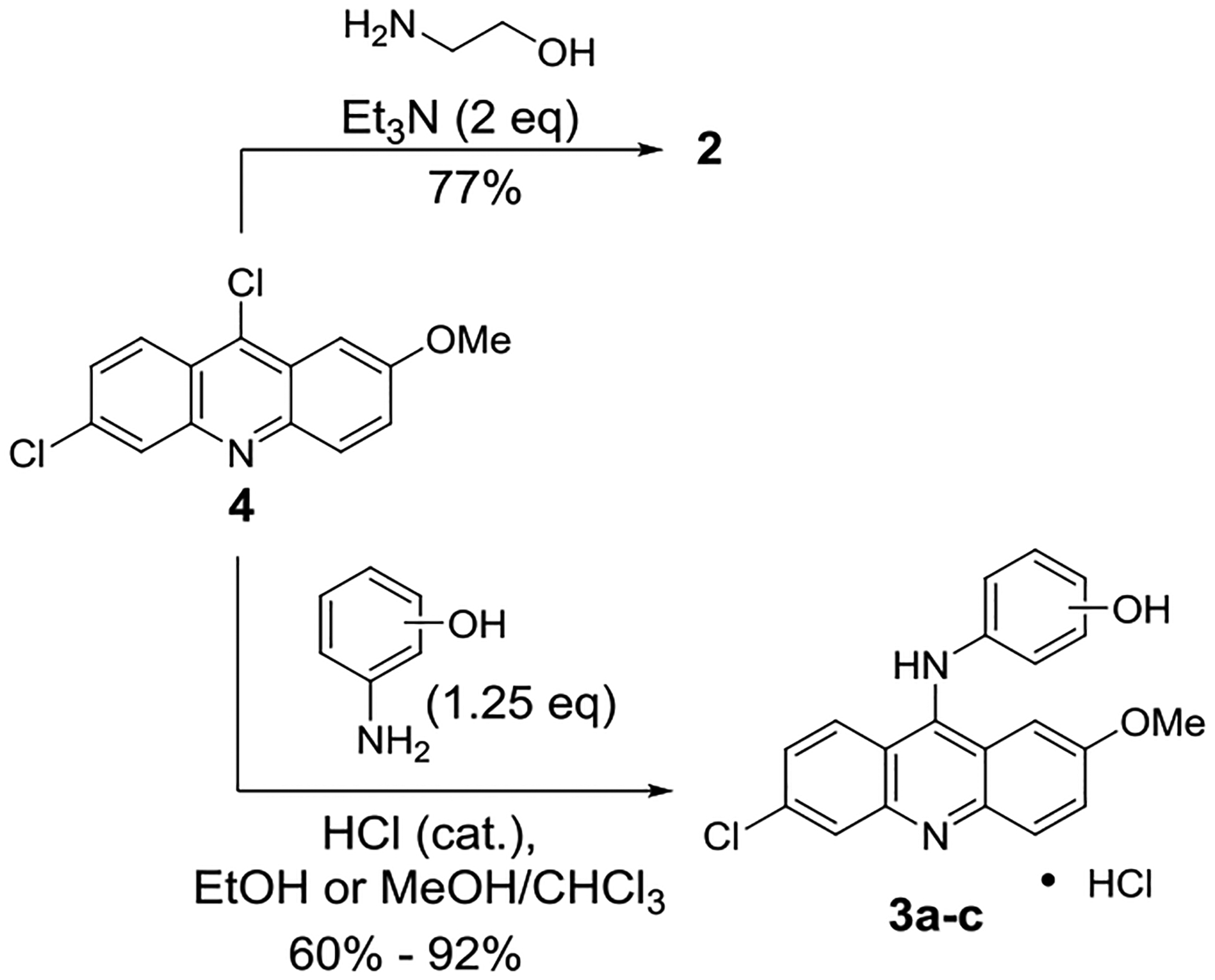
Synthesis of compounds 2 and 3a-c
Drug- and lead-likeness predictions
In silico SwissADME, the freely available tool for evaluating pharmokinetics, was used to assess the drug- and lead-likeness of 1, 2 and 3a-c (Table S1).29, 30 The results for the aminophenol derivatives 3a-c were essentially identical. However, there was a significant difference in the predicted properties for 2.
The calculations indicated that all compounds possess druglikeness properties fulfilling each set of requirements used by the program (Table S1). For example, per Veber et al.,31 all four compounds fall within the window of 10 or less rotatable bonds and a polar surface area of less than 140 Å2; and per the “rule of 5”,32 all have molecular weights of less than 500 D, calculated logs for their water-octanol partition coefficients (clog P) of less than 5, fewer than 5 hydrogen-bond donors, and no more than 10 oxygen/nitrogen hydrogen bond acceptors.
To possess leadlikeness, as defined by Teague and colleagues,33 the molecular weight should be between 250 and 350 D, there should be seven or less rotatable bonds, and the logs of the water-octanol partition coefficient (XlogGP3) must be 3.5 or lower. Of the five molecules, 2 was the only structure with leadlikeness characteristics and therefore appears to be the most promising for structural optimization for drug development studies (Table S1).
Cytotoxicity assays NCI-60 cancer cell lines
Investigations probed the anticancer activity of the compounds with PC3 and A549 cell lines. Compounds were screened at a 50 μM dose each and compared to two controls, a 0.2% final concentration of the solvent dimethyl sulfoxide (DMSO) as well as media with no solvent or compound (Figure 3). The cells underwent treatment for 48 hours and percent cell viability was assessed with a colorimetric 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay. Each cell line was analyzed in two independent trials composed of 6 replicates in each trial, with a total of 12 replicates.
Figure 3.

Cell viability studies with A549 and PC3 cancer cell lines with media only, 0.2% of DMSO, and 50 μM each of 1, 2, and 3a-c. The percent difference in cell viability of all compounds compared to the 0.2% DMSO solvent control is indicated in this graph with the corresponding standard deviation.
These cell viability results provide valuable insight into the SAR for the molecules (Figure 3). Of the five compounds, 2 and 3a provoked the greatest cytotoxic response with the PC3 and A549 cancer cells; both were more potent than the parent 9-aminoacridine 1. Among the anilino-acridines 3a-c, results in both cell lines showed that the para-location for the OH, 3a, elicited greater activity than the meta- or ortho-locations, 3b and 3c. In looking at the influence of aromatic versus aliphatic 9-amino substituents on the acridine scaffold, particularly 3a versus 2, molecule 2 emerged as the more potent compound (Figure 3).
For compound 2, the average cell viability with PC3 cells was 53% compared to 76% with 1, and with the A549 line the average viability was 21% versus 67% with 1 (Figure 3). For 3a, the average cell viability was 59% and 25% for PC3 and A549, respectively. Notably, both the SwissADME predictions and cell viability assays converged around 2 as the most robust candidate for drug development.
IC50 evaluation with PC3
Based on the potency of 2 with the 50 μM screen, serial dilution investigations were carried out with one of the cell lines, PC3. There were 21 serial dilutions spanning 100 μM to 0.32 μM (Figures 4 and S1–S5).
Figure 4.

IC50 analysis of compound 2 with PC3 cell lines—The dose response plot with regression line for three independent trials, and the average of those trials were graphed using % cell viability versus concentration (μM). The corresponding IC50 avlues from the three trials are 35.222, 21.468, 25.363, respectively and 27.314 for the average.
Upon 48 hours of treatment, cell viability was quantitated with MTS assays. Three independent serial dilution studies were performed with four replicates per study, and with a control containing 0.2% DMSO only. An average half-maximal inhibitory concentration (IC50) value based on percent cell viability with 2 was determined. The fit of the linear regressions of percent viability on ln(Conc) was good as evidenced by r2 of 0.937, 0.943, 0.928 for the three experiments respectively, and 0.977 for average percent viability (Figures 4 and S2–S5). The corresponding IC50s from the three experiments are 35.22 μM, 21.47 μM, 25.36 μM with 27.31 μM for the average.
Confocal Studies in A549 cells
Morphological changes were tracked with confocal microscopy using A549 cells. The cytotoxicity assays discussed previously helped to determine a timepoint and dose that would not result in complete cell death so that cellular morphological changes could be tracked. Images at 40X magnification showed a distinct difference in the appearance of the cells treated with 2 relative to those of untreated cells (media only) (Figure 5). Imaging was performed using 4´,6-diamidino-2-phenylindole (DAPI) for nuclear staining. Compared to untreated cells, measurements at 20X magnification recorded a wider nuclei width and a longer nuclei length after 24 hours of exposure to 25 μM of 2. On average, nuclei showed an increase in length from 17.4 μm for untreated cells to 19.4 μm with compound 2, and a corresponding increase in width from 13.9 μm for untreated cells to 15.6 μm with compound 2 (Figure 6).
Figure 5.
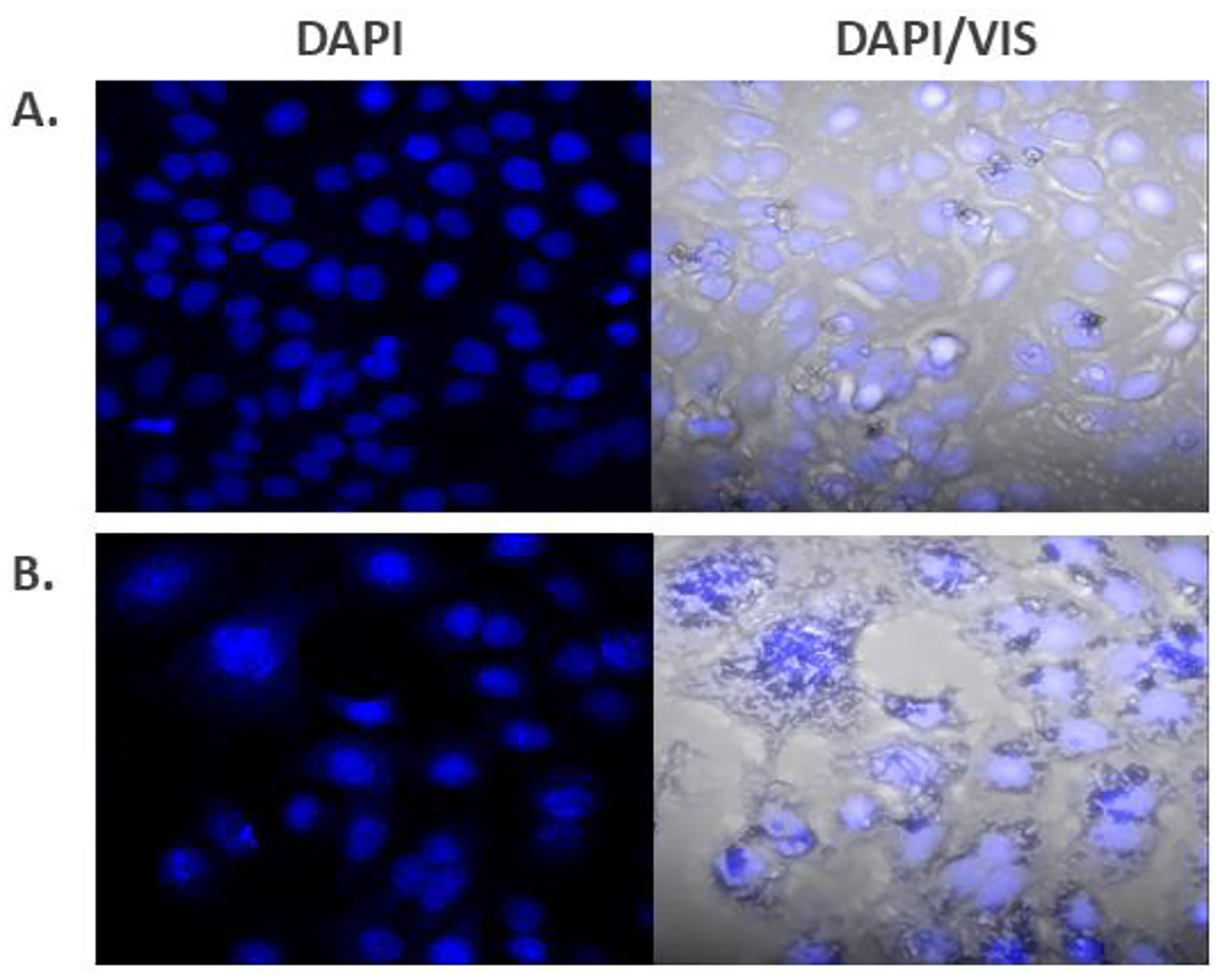
Confocal microscopy images of A549 cells after 24 hours at 40x with DAPI stain (left) and DAPI stained overlaid with visible light (DAPI/VIS) (right)—(A) untreated cells (media only); (B) cells treated with 25 μM of compound 2.
Figure 6.
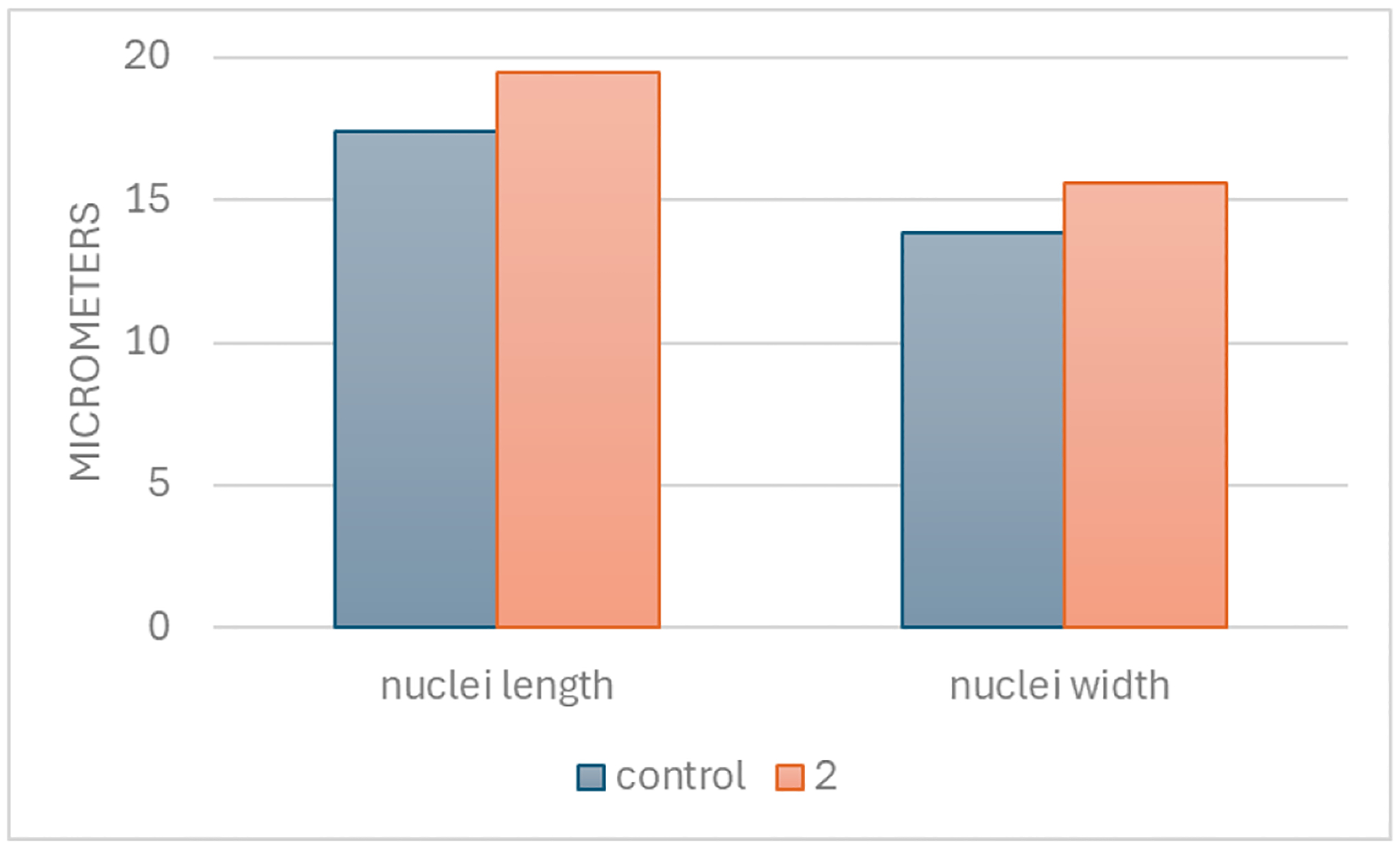
Measurements of A549 cell nuclei with confocal microscopy, a comparison of untreated cells (media only) with cells treated with 25 μM of compound. Nuclei length and nuclei width were quantitated in units of micrometers (μm), with ten replicates per group.
Conclusion
9-Aminoacridine derivatives 2 and 3a-c under consideration as precursors for cancer therapeutics drug development were probed for druglikeness and leadlikeness in silico with SwissADME, and for anticancer properties with cytotoxicity studies. SwissADME calculations indicated that all compounds had druglikeness characteristics; however, only 2 was flagged as a lead. Moreover, the cytotoxicity studies with representative cell lines PC3 and A549 revealed 2 as the most potent. Thus, the cell assays with corresponding confocal microscopy imaging and the in silico analysis collectively suggest 2 as the most promising candidate for moving forward with drug development work targeting cancer cell selectivity.
Experimental Section
General
All reagents and chemicals obtained through commercial sources were used without further purification unless noted. HPLC grade solvents and de-ionized (DI) water were used in the syntheses.
Nuclear Magnetic Resonance (NMR) spectra, used to characterize the structures, were recorded on JEOL 300MHz and JEOL 400MHz spectrometers; each has a multinuclear probe with multiple RF channels and variable temperature capability. The solvent used was deuterated dimethyl sulfoxide ((CD3)2SO, DMSO-D6). NMR signals were recorded in parts per million (ppm) relative to the residual solvent. 1H-NMR signals are described with singlet (s), doublet (d), doublet of doublet (dd), triplet (t), multiplet (m); coupling constants (J; Hz) and integration. Melting points were measured with Vernier Melt Station using Vernier LabQuest 2 and are uncorrected.
Confocal microscopy was performed at the Core Research Laboratory (CCRL) at Georgia Southern University with a Zeiss Confocal Laser Scanning Microscope, LSM-710 and Inverted Axio Observer Z1. Airyscan detector and 405 diode laser allowed for resolution of around 158 nm with 40x lens.
AccuSkan FC plate reader at 492 nm filter and SkanIT Software were used for the cell viability assays in the cytotoxicity investigations.
The commercial supplier for 9-aminoacridine hydrochloride was TCI America (monohydrate, purity > 98%), and for dimethyl sulfone, the commercial supplier was Ambeed (purity 99%). Both compounds were used directly.
Purity for the synthesized compounds was determined using quantitative NMR (qNMR) experiments with dimethyl sulfone ((CH3)2SO2,) (supplier: Ambeed, 99% purity) as the internal standard, and DMSO-D6 as the solvent.34 Calculations were performed using the MNova 15 Purity Calculator tool.
Synthetic Procedures
2-((6-Chloro-2-methoxyacridin-9-yl)amino)ethan-1-ol (2):
The synthetic procedure for compound 2 was adapted from a method reported by Lourenço et al.27 6,9-Dichloro-2-methoxyacridine 4 (1.02 g, 3.68 mmol.) and triethylamine (1.00 mL, 7.17 mmol.) were added to 2-aminoethanol (12 mL, 199 mmol.) in an oven dried round bottom flask fitted with a stir bar and reflux condenser and drying tube. The reaction mixture was heated until all of the solid dissolved, no higher, and left at that temperature overnight. Upon cooling, an orange-yellow precipitate formed. The resulting suspension was placed in an ice bath and diluted with cold DI water (0°C). The crude solid was collected by vacuum filtration and washed sequentially with cold DI water (0°C) and cold diethyl ether (0°C). Each wash was done at least three times. Compound 2 was collected as an orange powdery solid (855 mg, 77% yield). Compound 2 is known.22 M.p.: 188.2–189.4 °C. 1H-NMR (free base) (400MHz, DMSO-D6) δ 8.36 (d, J = 9.4 Hz, 1H), 7.85 (d, J = 2.1 Hz, 1H), 7.81 (d, J = 9.3 Hz, 1H), 7.63 (d, J = 2.3 Hz, 1H), 7.38 (dd, J = 9.3, 2.6 Hz, 1H), 7.30 (J = 9.3, 2.2 Hz, 1H), 6.72 (t, J = 6.2 Hz, 1H (NH)), 4.89 (t, J = 5.2 Hz, 1H (OH)), 3.89 (s, 3H), 3.74 (m, 2H), 3.65 (m, 2H). 13C-NMR (free base) (101 MHz, DMSO-D6) δ 155.1, 150.8, 148.0, 146.2, 133.5, 130.7, 127.1, 126.5, 124.2, 122.7, 117.4, 114.9, 100.8, 60.9, 55.6, 52.0.
Example of synthesis for anilino compounds 3a-b
4-((6-chloro-2-methoxyacridin-9-yl)amino)phenol hydrochloride salt (3a): Compound 3a was made according to the procedure described by Go et al.28 with slight modifications. 4-Aminophenol (246 mg, 2.25 mmol.) and 6,9-dichloro-2-methoxyacridine 4 (501mg, 1.81 mmol.) were added to an ethanol-chloroform co-solvent mixture (30 mL, v : v, 4 : 1, ethanol : chloroform), followed by approximately 4 drops of concentrated hydrochloric acid (pH ~5). The reaction mixture was stirred vigorously at room temperature under ambient conditions for 18 hours. The resulting precipitate was collected by vacuum filtration and washed sequentially with chloroform and acetone; each wash was done ~5 times. Compound 3a was isolated as the hydrochloride salt in as a bright orange powdery solid (639 mg, 92%). Compound 3a is known.23 Purity: 98% (qNMR). Decompose: 250.0 °C. 1H-NMR (HCl salt) (400MHz, DMSO- D6) δ 14.29 (bs, Acridine +N-H, 1H), 11.27 (bs, NH), 9.95 (bs, OH), 8.02–7.97 (m, 2H), 7.94 (d, J = 9.7 Hz, 1H), 7.63–7.74 (m, 2H), 7.39 (d, J = 9.3 Hz, 1H), 7.25 (d, J = 8.6 Hz, 2H), 6.90 (d, J = 8.7 Hz, 2H), 3.70 (s, 3H). 13C NMR (HCl salt) (101 MHz, DMSO-D6) δ 157.6, 156.0, 154.1, 140.3, 139.3, 135.9, 131.9, 128.6, 128.4, 127.1, 124.2, 121.3, 118.2, 117.0, 114.9, 111.6, 104.1, 56.3. See the Supporting Information for the procedures for 3b and 3c.
Cytotoxicity Studies
To assess the compounds for anti-cancer activity, lung cancer (A549) and prostate cancer (PC3) cells from American Type Culture Company (ATCC) were cultured according to standard recommended protocol in F12K base media supplemented with 10% Fetal Bovine Serum (FBS) and 1% Pen-Strep. For experimentation, 10,000 cells per well were seeded in a 96-well plate format and incubated for 24 hours prior to treatment with 50 μM dose for compounds 1 and 2, and 3a-c with a 0.2 % (final) concentration of DMSO solvent. Results were compared with two different control groups: 1) cells treated with media with no compound and 2) cells treated with a 0.2% final concentration of DMSO. Cell viability was quantitated using the colorimetric MTS Assay, which requires NAD(P)H-dependent enzymes released from viable cells to convert the MTS tetrazolium compound to a formazan dye. The formazan dye was detected with an AccuSkan FC plate reader at 492 nm filter and SkanIT Software.
IC50 analysis
Compound 2 was further assessed with calculation of its IC50 value with PC3 cell lines. The compound was serially diluted from 100 μM to 0.32 μM with a final concentration of 0.2% DMSO in a 96-well format. After 48-hour incubation with the serial dilutions of the compound, cell viability was quantitated with MTS assay as described above. The IC50 study was independently replicated 3 times for analysis.
For the statistical analysis, percent viability was linearly regressed on the natural log of concentration and the slope and intercept of the regression line were determined. The estimated regression equation was then solved for log(conc) corresponding to 50% viability. This value was then exponentiated to get the IC50 in terms of concentration. The average percent for each concentration over the three experiments was then regressed on ln(Conc), and the IC50 determined from the regression line. The assumptions (normality and homoscedasticity) were tested and found tenable. Analyses were performed using SAS software.
Confocal Microscopy of A549 cells
A549 cells were cultured according to standard manufacturer protocol by American Type Culture Company (ATCC). Four-well chamber slides were seeded for 24-hours with 10,000 cells per well prior to a 24-hour incubation with media or 25μM of compound 2. Following the incubation period, fixation and permeabilization of the cells was initiated. Each well was fixed with 4% formaldehyde in phosphate-buffered saline (PBS) for 15 minutes at room temperature followed by three washes with dPBS. Next, cells were permeabilized with 0.5% Triton X-100 in dPBS for another 15 minutes for subsequent staining with 4´,6-diamidino-2-phenylindole (DAPI). To block nonspecific binding sites prior to DAPI administration, cells were incubated with 3% bovine serum albumin for 60 minutes. All chamber wells were treated with a final concentration of 1 μg/mL DAPI 5 minutes followed by washes with dPBS prior to imaging with the confocal microscope.
Supplementary Material
Acknowledgements
GB, JM, AS, JK and KSA acknowledge the support of the Georgia Southern (GS) University Faculty Research Committee Seed Grant, and the GS Department of Biochemistry, Chemistry & Physics. DD acknowledges the GS College of Science and Mathematics (COSM) College Office of Undergraduate Research (COUR) award. AS, DD, DW, JK and KSA acknowledge the support of the National Cancer Institute (NCI) of the National Institutes of Health (NIH) under Award # R15CA277652.
AS and KSA thank Dr. Jeff Orvis for technical assistance.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Abbreviations
- 9-AA
9-aminoacridine
- A459
adenocarcinomic human alveolar basal epithelial cells
- DAPI
4´,6-diamidino-2-phenylindole
- DMSO
dimethyl sulfoxide
- FBS
Fetal Bovine Serum
- m-AMSA
Amsacrine
- MTS
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
- PBS
phosphate-buffered saline
- PC3
prostatic adenocarcinoma cells
- qNMR
quantitative Nuclear Magnetic Resonance Spectroscopy
- SAR
structure-activity relationship
- VIS
visible light
Footnotes
((Additional Supporting Information may be found in the online version of this article.))
Reference
- 1.Garner WB, Smith BD, Ludmir EB, Wakefield DV, Shabason J, Williams GR, Martin MY, Wang Y, Ballo MT and VanderWalde NA, Predicting future cancer incidence by age, race, ethnicity, and sex, Journal of Geriatric Oncology, 2023, 14, 101393. [DOI] [PubMed] [Google Scholar]
- 2.Cancer, World Health Organization (WHO), https://www.who.int/news-room/fact-sheets/detail/cancer, (accessed December 16, 2023).
- 3.Rahib L, Wehner MR, Matrisian LM and Nead KT, Estimated Projection of US Cancer Incidence and Death to 2040, JAMA Network Open, 2021, 4, e214708–e214708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fonte M, Tassi N, Gomes P and Teixeira C, Acridine-Based Antimalarials-From the Very First Synthetic Antimalarial to Recent Developments, Molecules, 2021, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wainwright M, Acridine—a neglected antibacterial chromophore, Journal of Antimicrobial Chemotherapy, 2001, 47, 1–13. [DOI] [PubMed] [Google Scholar]
- 6.Byvaltsev VA, Bardonova LA, Onaka NR, Polkin RA, Ochkal SV, Shepelev VV, Aliyev MA and Potapov AA, Acridine Orange: A Review of Novel Applications for Surgical Cancer Imaging and Therapy, Frontiers in Oncology, 2019, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varakumar P, Rajagopal K, Aparna B, Raman K, Byran G, Gonçalves Lima CM, Rashid S, Nafady MH, Emran TB and Wybraniec S, Acridine as an Anti-Tumour Agent: A Critical Review, Molecules, 2023, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bacherikov VA, Chou T-C, Dong H-J, Zhang X, Chen C-H, Lin Y-W, Tsai T-J, Lee R-Z, Liu LF and Su T-L, Potent antitumor 9-anilinoacridines bearing an alkylating N-mustard residue on the anilino ring: synthesis and biological activity, Bioorganic & Medicinal Chemistry, 2005, 13, 3993–4006. [DOI] [PubMed] [Google Scholar]
- 9.Denny WA and Baguley BC, in Molecular Aspects of Anticancer Drug-DNA Interactions: Volume 2, eds. Neidle S and Waring M, Macmillan Education UK, London, 1994, pp. 270–311. [Google Scholar]
- 10.Wang W, Ho WC, Dicker DT, MacKinnon C, Winkler JD, Marmorstein R and El-Deiry WS, Acridine derivatives activate p53 and induce tumor cell death through bax, Cancer Biology & Therapy, 2005, 4, 893–898. [DOI] [PubMed] [Google Scholar]
- 11.Guo C, Gasparian AV, Zhuang Z, Bosykh DA, Komar AA, Gudkov AV and Gurova KV, 9-Aminoacridine-based anticancer drugs target the PI3K/AKT/mTOR, NF-κB and p53 pathways, Oncogene, 2009, 28, 1151–1161. [DOI] [PubMed] [Google Scholar]
- 12.Luan X, Gao C, Zhang N, Chen Y, Sun Q, Tan C, Liu H, Jin Y and Jiang Y, Exploration of acridine scaffold as a potentially interesting scaffold for discovering novel multi-target VEGFR-2 and Src kinase inhibitors, Bioorganic & Medicinal Chemistry, 2011, 19, 3312–3319. [DOI] [PubMed] [Google Scholar]
- 13.Ehsanian R, Van Waes C and Feller SM, Beyond DNA binding - a review of the potential mechanisms mediating quinacrine’s therapeutic activities in parasitic infections, inflammation, and cancers, Cell Communication and Signaling, 2011, 9, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nelson EM, Tewey KM and Liu LF, Mechanism of antitumor drug action: poisoning of mammalian DNA topoisomerase II on DNA by 4’-(9-acridinylamino)-methanesulfon-m-anisidide, Proc Natl Acad Sci U S A, 1984, 81, 1361–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Rowe TC, Nelson EM and Liu LF, In vivo mapping of DNA topoisomerase II-specific cleavage sites on SV40 chromatin, Cell, 1985, 41, 127–132. [DOI] [PubMed] [Google Scholar]
- 16.Larsson R and Nygren P, Cytotoxic activity of topoisomerase II inhibitors in primary cultures of tumor cells from patients with human hematologic and solid tumors, Cancer, 1994, 74, 2857–2862. [DOI] [PubMed] [Google Scholar]
- 17.Kumar M and Sarkar A, Repurposing of Anti-Malarial Drug Quinacrine for Cancer Treatment: A Review, Scientia Pharmaceutica, 2022, 90. [Google Scholar]
- 18.Cho E-H, Chung S-G, Lee S-H, Kwon H-S and Kang D-W, 9-Aminoacridine derivatives and process for the preparation thereof, European Patent Office, 2000, 1062207. [Google Scholar]
- 19.Hayashi K and Anzai N, Novel therapeutic approaches targeting L-type amino acid transporters for cancer treatment, World Journal of Gastrointestinal Oncology, 2017, 9, 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nair RN, Mishra JK, Li F, Tortosa M, Yang C, Doherty JR, Cameron M, Cleveland JL, Roush WR and Bannister TD, Exploiting the co-reliance of tumours upon transport of amino acids and lactate: Gln and Tyr conjugates of MCT1 inhibitors, Medchemcomm, 2016, 7, 900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin S-E, Jin H-E and Hong S-S, Targeting L-type amino acid transporter 1 for anticancer therapy: clinical impact from diagnostics to therapeutics, Expert Opinion on Therapeutic Targets, 2015, 19, 1319–1337. [DOI] [PubMed] [Google Scholar]
- 22.Šebestík J, Šafařík M, Stibor I and Hlaváček J, Acridin-9-yl exchange: A proposal for the action of some 9-aminoacridine drugs, Peptide Science, 2006, 84, 605–614. [DOI] [PubMed] [Google Scholar]
- 23.Elmenoufy AH, Gentile F, Jay D, Karimi-Busheri F, Yang X, Soueidan OM, Weilbeer C, Mani RS, Barakat KH, Tuszynski JA, Weinfeld M and West FG, Targeting DNA Repair in Tumor Cells via Inhibition of ERCC1–XPF, Journal of Medicinal Chemistry, 2019, 62, 7684–7696. [DOI] [PubMed] [Google Scholar]
- 24.Lieber M, Smith B, Szakal A, Nelson-Rees W and Todaro G, A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells, Int J Cancer, 1976, 17, 62–70. [DOI] [PubMed] [Google Scholar]
- 25.Tai S, Sun Y, Squires JM, Zhang H, Oh WK, Liang C-Z and Huang J, PC3 is a cell line characteristic of prostatic small cell carcinoma, The Prostate, 2011, 71, 1668–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF and Jones LW, Establishment and characterization of a human prostatic carcinoma cell line (PC-3), Invest Urol, 1979, 17, 16–23. [PubMed] [Google Scholar]
- 27.de Souza MVN, Pais KC, Kaiser CR, Peralta MA, de L. Ferreira M and Lourenço MCS, Synthesis and in vitro antitubercular activity of a series of quinoline derivatives, Bioorganic & Medicinal Chemistry, 2009, 17, 1474–1480. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TH, Lee CY, Teruya K, Ong WY, Doh-ura K and Go ML, Antiprion activity of functionalized 9-aminoacridines related to quinacrine, Bioorg Med Chem, 2008, 16, 6737–6746. [DOI] [PubMed] [Google Scholar]
- 29.SwissADME Tool, http://www.swissadme.ch/index.php, (accessed February 12, 2024).
- 30.Daina A, Michielin O and Zoete V, SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules, Scientific Reports, 2017, 7, 42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW and Kopple KD, Molecular properties that influence the oral bioavailability of drug candidates, J Med Chem, 2002, 45, 2615–2623. [DOI] [PubMed] [Google Scholar]
- 32.Lipinski CA, Lombardo F, Dominy BW and Feeney PJ, Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings1PII of original article: S0169–409X(96)00423–1. The article was originally published in Advanced Drug Delivery Reviews 23 (1997) 3–25.1, Advanced Drug Delivery Reviews, 2001, 46, 3–26. [DOI] [PubMed] [Google Scholar]
- 33.Teague SJ, Davis AM, Leeson PD and Oprea T, The Design of Leadlike Combinatorial Libraries, Angewandte Chemie International Edition, 1999, 38, 3743–3748. [DOI] [PubMed] [Google Scholar]
- 34.Pauli GF, Chen S-N, Simmler C, Lankin DC, Gödecke T, Jaki BU, Friesen JB, McAlpine JB and Napolitano JG, Importance of Purity Evaluation and the Potential of Quantitative 1H NMR as a Purity Assay, Journal of Medicinal Chemistry, 2014, 57, 9220–9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


