Abstract
BACKGROUND:
Sex-based differences are important in the development and progression of pulmonary arterial hypertension. However, it is not established whether these differences are generalizable to all forms of pulmonary hypertension (PH).
RESEARCH QUESTION:
What are the sex-based differences in right ventricle (RV) function and transplant-free survival in patients with PH from the Redefining Pulmonary Hypertension Through Pulmonary Vascular Disease Phenomics (PVDOMICS) cohort?
STUDY DESIGN AND METHODS:
Patients with PH enrolled in the PVDOMICS cohort study underwent right heart catheterization, cardiac MRI, and echocardiography. A multivariable linear regression model was used to investigate the interactive effect between sex and pulmonary vascular resistance (PVR) on RV ejection fraction (RVEF). Effects of sex, RVEF, and PVR on transplant-free survival were assessed using a Cox proportional hazards model.
RESULTS:
Seven hundred fifty patients with PH (62.8% female) were enrolled, including 397 patients with groups 2 through 5 PH. Patients with group 1 PH were predominantly female (73.4%). Male patients showed multiple markers of worse RV function with significantly lower RVEF (adjusted difference, 5.5%; 95% CI, 3.2%–7.8%; P < .001) on cardiac MRI and lower RV fractional shortening (adjusted difference, 4.0%; 95% CI, 2.3%–5.8%; P < .001) and worse RV free-wall longitudinal strain (adjusted difference, 2.4%; 95% CI, 1.2%–3.6%; P < .001) on echocardiography. Significant interaction was noted between PVR and sex on RVEF, with the largest sex-based differences in RVEF noted at mild to moderate PVR elevation. Male sex was associated with decreased transplant-free survival (adjusted hazard ratio, 1.46; 95% CI, 1.07–1.98; P = .02), partially mediated by differences in RVEF (P = .003).
INTERPRETATION:
In patients with PH in the PVDOMICS study, female sex was more common, whereas male sex was associated with worse RV function and decreased transplant-free survival, most notably at mild to moderate elevation of PVR.
Keywords: pulmonary hypertension, right ventricle, sex differences
Pulmonary hypertension (PH) is a disease characterized by increased pressure in the pulmonary circulation, which leads to right ventricle (RV) strain and failure, resulting in significant morbidity and mortality for those affected. Sex-based differences are important in the development and progression of pulmonary arterial hypertension (PAH), a rare and incurable form of PH.1–3 However, little is known about sex-based differences in other more common forms of PH and whether sex influences RV response to load stress in PH broadly.
Modern registries consistently have shown a female predominance of World Symposium on Pulmonary Hypertension (WSPH) group 1 PAH, with female individuals making up 70% to 80% of cases.3–5 However, less is known about the impact of sex in WSPH groups 2 through 5 PH, and female sex has not been shown previously to be a risk factor for other forms of PH.1 In what has been termed the estrogen paradox,1 despite women having increased risk of PAH developing, men with PAH have worse outcomes compared with women.2,6,7 However, the mechanism by which female sex is both a powerful risk factor for PAH and a protective factor after the disease is present has not been established fully, and it is unknown whether these sex-based differences extend to other forms of PH. One hypothesis is that differences in outcomes are at least partially the result of sex-based differences in RV response to load stress.8,9 In healthy individuals, female sex is associated with higher RV ejection fraction (RVEF), lower RV mass, and smaller RV volumes.10,11 Similarly, male sex is associated with worse RV function in PAH,12,13 as well as in small studies of patients with heart failure with preserved ejection fraction (EF)14 and PH associated with chronic lung disease.15 However, sex differences in RV function across the spectrum of pulmonary vascular disease (PVD) and the impact of sex and gender on RV adaptation to varying degrees of afterload have not been described previously.
The Redefining Pulmonary Hypertension Through Pulmonary Vascular Disease Phenomics (PVDOMICS) study is a precision medicine initiative to characterize PVD intensively using deep clinical phenotyping and comprehensive so-called -omics analysis in a cohort of patients with PH, disease comparators, and healthy control participants.16 The PVDOMICS study provides a unique opportunity to study sex-based differences in disease cause, characteristics, and outcomes across the spectrum of PVD. Using the PVDOMICS cohort, we sought to understand differences in clinical characteristics between men and women in all forms of PH. We further sought to test the hypothesis that male sex is associated with worse RV function across the spectrum of PVD and that sex-based differences in RV function contribute to differences in survival between male and female patients.
Study Design and Methods
Patients were enrolled at seven PVD centers across the United States in a prospective, longitudinal cohort study (ClinicalTrials.gov Identifier: NCT02980887). Phenotyping protocol was approved by local institutional review boards, and patients were enrolled after informed consent. Detailed enrollment, inclusion, and exclusion criteria were described previously.16,17 We include clinical data available as of May 19, 2021, and survival data as of November 22, 2021. In this article, according to the definitions published by the Institute of Medicine, we use the term sex when biologic concepts are described and the term gender when cultural or behavioral influences are thought to play a role.1,18,19
Phenotyping Protocol
Details of the protocol have been published previously.16,17,20,21 Briefly, patients aged ≥ 18 years with known or suspected PVD were recruited from the enrolling centers. Demographic features including sex were self-reported by patients at enrollment. For female participants, menstrual status and last menstrual cycle also were self-reported, with menopause defined as > 12 months since last menses. Phrasing used in enrollment forms is available in e-Appendix 1. Patients underwent clinical phenotyping, including the 6-min walk test, pulmonary function testing, transthoracic echocardiography (TTE), and cardiac MRI within 6 weeks of enrollment. Enrollees with known or suspected PVD underwent protocolized right heart catheterization (RHC).20 Cores at the data coordinating center performed central reading of diagnostic studies. Vital status and heart and lung transplantation status were assessed annually after enrollment.
PVD and RV Function Assessment
Participants were classified according to 2013 WSPH guidelines.22 Hemodynamic definitions used in the cohort were published previously and are presented in e-Table 1.16,17,20 Patients were classified by primary cause of PH at the discretion of the site principal investigator with review by an expert adjudication committee for all patients with a mixed cause of PVD.17,20
For this article, we studied the influence of sex on measures of RV function assessed by TTE,21 cardiac MRI, and RHC in patients with PH.16 RVEF on cardiac MRI was used as the primary measure of RV function, given its prognostic usefulness in PAH23 and its status as the reference standard for measurement of RV function.24 Cardiac MRI was carried out according to a dedicated protocol developed by the PVDOMICS Imaging Group, which was described previously.16 Images were interpreted by a central core masked to participant phenotype.
Statistical Analysis
Between-group comparisons were performed using the Wilcoxon rank-sum test for continuous variables and the Pearson χ2 test or Fisher exact test (if expected counts of fewer than 10) for categorical variables. Multivariable regression was performed to assess the effect of sex on cardiac and hemodynamic variables of interest adjusting for age, BMI, World Health Organization functional class, WSPH group, time since PH diagnosis, exercise frequency and duration, smoking status, and presence of comorbidities including coronary artery disease, diabetes mellitus, chronic kidney disease, and atrial arrhythmias. To assess the effect of sex on the relationship between pulmonary vascular resistance (PVR) and RVEF, a multivariable linear regression model was fitted for RVEF on PVR and sex as well as the interaction between PVR and sex. The covariates described previously also were included. In all the models, smooth relationships between continuous independent variables and response variables were assumed using restricted cubic splines.
Time from enrollment date to lung or heart transplantation, or both, or death is summarized by Kaplan-Meier survival curves, and differences in the transplant-free survival by group were tested using the log-rank test. We also considered lung or heart transplantation, or both, as a competing event for death and estimated cumulative incidences of death for both groups. A multivariable Cox proportional hazards model was fitted for sex, age, PVR, time since PH diagnosis, and WSPH group to determine adjusted effects of sex on transplant-free survival. Interactions between sex and PVR and between sex and age were included in the described model in secondary analyses. The effect of sex on survival mediated by RVEF was estimated in terms of total natural indirect effect using a regression-based causal mediation analysis method.25
All analyses were performed using R version 4.1.0 software (R Foundation for Statistical Computing).26 A detailed statistical analysis plan is available in e-Appendix 1. P values for sex-based differences in cardiac parameters were adjusted for multiple comparisons by controlling the false discovery rate (FDR) at 0.05.27 P values for other comparisons were not adjusted for multiple comparisons, given the exploratory nature of those analyses.
Results
Sex Differences in PH Cause
Seven hundred fifty patients with PH were enrolled and included in this analysis. Overall, a female predominance (62.8% female) was found (Table 1). Patients (n = 353) with group 1 disease were most predominantly female at 73.4%. Additionally, patients with WSPH group 2 (n = 136 [57.4% female]) and group 4 (n = 57 [61.4%]) disease also showed a female predominance, whereas patients with group 3 disease were nearly balanced by sex (n = 172 [51.2% female]). Group 5 (n = 32) was 65.6% male (P < .001 for consistency of sex proportions among WSPH groups). Female patients in WSPH groups 2 and 3 were nearly all postmenopausal at 96.2% and 92.0%, respectively, whereas group 1 showed a larger percentage of premenopausal participants at 35.1%.
TABLE 1 ].
Patient Characteristics by WSPH Group
| Variable | Group |
Total (N = 750) | ||||
|---|---|---|---|---|---|---|
| 1 (n = 353) | 2 (n = 136) | 3 (n = 172) | 4 (n = 57) | 5 (n = 32) | ||
| Female sex | 259 (73.4) | 78 (57.4) | 88 (51.2) | 35 (61.4) | 11 (34.4) | 471 (62.8) |
| Postmenopausal | 168 (64.9) | 75 (96.2) | 81 (92.0) | 35 (71.4) | 7 (63.6) | 356 (75.6) |
| Age at enrollment, y | ||||||
| Female sex | 52 ± 14 | 68 ± 11 | 63 ± 12 | 56 ± 15 | 57 ± 14 | 61 ± 15 |
| Male sex | 53 ± 14 | 68 ± 13 | 65 ± 10 | 63 ± 12 | 57 ± 13 | 57 ± 15 |
Data are presented as No. (%) or mean ± SD. The n values for group are the number of nonmissing values. Percentages for postmenopausal are in relationship to the number of female participants for each specific WSPH grouping. Menopause was defined as > 12 mo from last menses. WSPH = World Symposium on Pulmonary Hypertension.
The subtype of PH also varied significantly by sex (Table 2). Among patients with WSPH group 1 disease, a significantly higher proportion of female participants demonstrated PAH associated with connective tissue disease than male participants (29.3% vs 18.1%; P = .03). Among patients with WSPH group 2 disease, men were significantly more likely to have systolic heart failure (32.8% vs 9.0%; P < .001), whereas female participants were more likely to have diastolic heart failure (84.6% vs 58.6%; P < .001). Among patients with WSPH group 3 disease, female participants were more likely to demonstrate other interstitial lung disease, most commonly associated with connective tissue disease (47.7% vs 21.4%; P < .001).
TABLE 2 ].
Sex-Based Differences in Pulmonary Hypertension Subtypes
| WSPH Group Subtype | No. | Male Sex | Female Sex | % Femalea | P Value |
|---|---|---|---|---|---|
| Group 1 | |||||
| Total No. | 353 | 94 | 259 | 73.4 | … |
| Idiopathic PAH | 158 | 48 (51.1) | 110 (42.5) | 69.6 | .15b |
| Familial PAH | 27 | 6 (6.4) | 21 (8.1) | 77.8 | .66c |
| HHT | 4 | 0 (0.0) | 4 (1.5) | 100.0 | .58c |
| Drug- and toxin-induced PAH | 16 | 2 (2.1) | 14 (5.4) | 87.5 | .25c |
| Connective tissue disease | 93 | 17 (18.1) | 76 (29.3) | 81.7 | .03b |
| Systemic lupus erythematosus | 16 | 0 (0.0) | 16 (6.2) | 100.0 | .01c |
| Sjögren’s disease | 9 | 1 (1.1) | 8 (3.1) | 88.9 | .45c |
| Rheumatoid arthritis | 7 | 3 (3.2) | 4 (1.5) | 57.1 | .39b |
| Mixed connective tissue disease | 16 | 1 (1.1) | 15 (5.8) | 93.8 | .08c |
| Systemic sclerosis | 42 | 10 (10.6) | 32 (12.4) | 76.2 | .66b |
| HIV | 10 | 4 (4.3) | 6 (2.3) | 60.0 | .47c |
| Congenital heart disease | 36 | 10 (10.6) | 26 (10.0) | 72.2 | .87b |
| Shunt repaired | 15 | 5 (5.3) | 10 (3.9) | 66.7 | .56c |
| Portopulmonary | 18 | 9 (9.6) | 9 (3.5) | 50.0 | .03c |
| Group 2 | |||||
| Total No. | 136 | 58 | 78 | 57.4 | … |
| Valvular heart disease | 35 | 17 (29.3) | 18 (23.1) | 51.4 | .41b |
| Valvular stenosis | 19 | 9 (15.5) | 10 (12.8) | 52.6 | .80c |
| Valvular regurgitation | 29 | 15 (25.9) | 14 (17.9) | 48.3 | .27b |
| Systolic heart failure | 26 | 19 (32.8) | 7 (9.0) | 26.9 | < .001c |
| Diastolic heart failure | 100 | 34 (58.6) | 66 (84.6) | 66.0 | < .001b |
| Cardiomyopathy | 14 | 7 (12.1) | 7 (9) | 50.0 | .58c |
| Hypertrophic | 7 | 3 (5.2) | 4 (5.1) | 57.1 | 1.0c |
| Restrictive | 6 | 4 (6.9) | 2 (2.6) | 33.3 | .40c |
| Group 3 | |||||
| Total No. | 172 | 84 | 88 | 51.2 | … |
| COPD | 66 | 35 (41.7) | 31 (35.2) | 47.0 | .39b |
| Idiopathic pulmonary fibrosis | 15 | 9 (10.7) | 6 (6.8) | 40.0 | .43c |
| Other interstitial lung disease | 60 | 18 (21.4) | 42 (47.7) | 70.0 | < .001b |
| CPFE | 9 | 7 (8.3) | 2 (2.3) | 22.2 | .09c |
| Hypersensitivity pneumonitis | 7 | 6 (7.1) | 1 (1.1) | 14.3 | .06c |
| OSA | 57 | 32 (38.1) | 25 (28.4) | 43.9 | .18b |
| Restrictive lung disease or thoracic cage abnormality | 10 | 7 (8.3) | 3 (3.4) | 30.0 | .32c |
| Cystic fibrosis | 3 | 1 (1.2) | 2 (2.3) | 66.7 | 1.0c |
| Group 4 | |||||
| Total No. | 57 | 22 | 35 | 61.4 | … |
| Prior PTE | 18 | 4 (18.2) | 14 (40.0) | 77.8 | .14c |
| Prior BPA | 4 | 1 (4.5) | 3 (8.6) | 75.0 | 1.0c |
| Group 5 | |||||
| Total No. | 32 | 21 | 11 | 34.4 | … |
| Sarcoidosis | 19 | 13 (61.9) | 6 (54.5) | 31.6 | .72c |
| Hemoglobinopathy | 8 | 5 (23.8) | 3 (27.3) | 37.5 | 1.0c |
Data are presented as No. (%) unless otherwise indicated. Patients could be included in multiple subtypes, so percentages do not add to 100%. BPA = balloon pulmonary angioplasty; CPFE = combined pulmonary fibrosis and emphysema; HHT = hereditary hemorrhagic telangiectasia; PAH = pulmonary arterial hypertension; PTE = pulmonary thromboendarterectomy; WSPH = World Symposium on Pulmonary Hypertension.
Percentage of patients with given subtype of pulmonary hypertension who are female.
Pearson χ2 test.
Fisher exact test.
Patient Characteristics
Clinical characteristics for male and female participants with PH are shown in Table 3. No difference was found in the proportion of incident PH by sex, with most patients (72.0% of males and 74.4% of females) having prevalent PH at the time of enrollment. At enrollment, male participants with PH were older (mean ± SD age, 60.7 ± 14.5 years vs 57.1 14.7 years; P = .001), but demonstrated a shorter duration of PH in prevalent cases (2.2 years [interquartile range (IQR), 0.8–5.5 years] vs 3.5 years [IQR, 1.1–8.2 years]; P = .02). No significant differences were found in age between sexes when patients were divided by WSPH group (Table 1). A lower percentage of male participants were receiving PH-specific therapy at enrollment (49.3% vs 61.3%; P = .001), although differences may be related to a lower proportion of male participants with WSPH group 1 PAH and a higher proportion of incident PH within WSPH group 1 specifically. Regarding bedside markers of disease severity, no significant differences were found in World Health Organization functional class or 6-min walk distance by sex. Male participants were significantly more likely to have a history of coronary artery disease (18.0% vs 4.1%; P < .001) and systemic hypertension (48.2% vs 40.7%; P = .045) and to have currently or formerly smoked (60.1% vs 43.3%; P < .001). On pulmonary function tests, male participants showed significantly lower diffusing capacity of the lungs for carbon monoxide (45.7 ± 20.8% predicted vs 55.6 ± 21.6% predicted; P < .001). Level of N-terminal fragment of the prohormone brain-type natriuretic peptide also was significantly higher in male participants (464 pg/mL [IQR, 119–1,441 pg/mL] vs 280 pg/mL [IQR, 102–1,231 pg/mL]; P = .04).
TABLE 3 ].
Descriptive Statistics for Patients With Pulmonary Hypertension
| Variable | No. | Male (n = 279) | Female (n = 471) | P Value |
|---|---|---|---|---|
| Age, y | ||||
| At enrollment | 750 | 60.7 ± 14.5 | 57.1 ± 14.7 | .001a |
| At diagnosis | 748 | 57.7 ± 16.6 (n = 279) | 53.1 ± 16.8 (n = 469) | < .001a |
| Prevalent cases | 748 | 201 (72.0) | 349 (74.4) | .48b |
| Duration of PH in prevalent cases, y | 748 | 2.2 (0.8–5.5) (n = 201) | 3.5 (1.1–8.2) (n = 349) | .02a |
| BMI, kg/m2 | 750 | 29.9 ± 6.8 | 30.2 ± 8.6 | .43a |
| Measured body fat, % | 497 | 28.1 ± 9.9 (n = 176) | 38.0 ± 10.7 (n = 321) | < .001a |
| WHO functional class | 723 | … | … | .99b |
| I | … | 20 (7.4) | 32 (7.0) | … |
| II | … | 93 (34.6) | 155 (34.1) | … |
| III | … | 143 (53.2) | 243 (53.5) | … |
| IV | … | 13 (4.8) | 24 (5.3) | … |
| PH treatments | 746 | … | … | … |
| Any | … | 136 (49.3) | 288 (61.3) | .001b |
| PDE-5 inhibitor | … | 109 (39.5) | 221 (47.0) | .046b |
| sGC stimulator | … | 13 (4.7) | 27 (5.7) | .55b |
| ERA | … | 63 (22.8) | 170 (36.2) | < .001b |
| Prostacyclin | … | 46 (16.7) | 134 (28.5) | < .001b |
| Calcium channel blocker | … | 2 (0.7) | 18 (3.8%) | .01c |
| Diuretic therapy | … | 165 (59.8) | 288 (63.4) | .33b |
| History of coronary disease (MI or coronary artery bypass) | 746 | 50 (18.0) | 19 (4.1) | < .001b |
| Diabetes mellitus | 746 | 75 (27.0) | 105 (22.4) | .16b |
| Chronic kidney disease | 745 | 28 (10.1) | 33 (7.1) | .15b |
| Hypertension | 745 | 134 (48.2) | 190 (40.7) | .045b |
| History of atrial arrhythmias | 746 | 66 (23.7) | 87 (18.6) | .09b |
| Current smoking or history of smoking | 745 | 166 (60.1) | 203 (43.3) | < .001b |
| DLCO, % predicted | 657 | 45.7 ± 20.8 (n = 242) | 55.6 ± 21.6 (n = 415) | < .001a |
| FEV1, % predicted | 678 | 69.4 ± 22.3 (n = 246) | 72.3 ± 20.1 (n = 432 | .13a |
| FVC, % predicted | 678 | 78.3 ± 20.7 (n = 246) | 78.7 ± 19.6 (n = 432) | .77a |
| 6-min walk distance, m | 621 | 350 ± 135 (n = 232) | 347 ± 135 (n = 389) | .67a |
| NT-proBNP, pg/mL | 722 | 464 (119–1,441) (n = 269) | 280 (102–1,231) (n = 453) | .04a |
Data presented as No. (%), mean ± SD, or median (interquartile range) unless otherwise indicated. The n values are the number of nonmissing values. Numbers after proportions are frequencies. DLCO = diffusing capacity of the lungs for carbon monoxide; ERA = endothelin receptor antagonist; MI myocardial infarction; NT-proBNP = N-terminal fragment of the prohormone brain-type natriuretic peptide; PDE-5 = phosphodiesterase-5; PH = pulmonary hypertension; sGC = soluble guanylate cyclase stimulator; WHO = World Health Organization.
Wilcoxon rank-sum test.
Pearson χ2 test.
Fisher exact test.
Of the 750 patients with PH, 449 patients (63.7% female) underwent evaluation of RVEF with cardiac MRI. Characteristics of patients with measures for RVEF on cardiac MRI vs those without RVEF are presented in e-Table 2. No significant differences were found in rates of cardiac MRI by sex. Compared with those who underwent cardiac MRI, those without cardiac MRI were more likely to have comorbidities, including coronary artery disease, chronic kidney disease, and diabetes mellitus, and had evidence of lower FEV1 and FVC and higher N-terminal fragment of the prohormone brain-type natriuretic peptide levels.
Sex-Based Differences in Cardiovascular Function
Adjusted and unadjusted sex-based differences on cardiac MRI, TTE, and RHC are presented in Table 4. Male patients with PH showed multiple markers of worse RV function, with significantly lower RVEF on cardiac MRI (37.3 ± 10.8% vs 41.6 ± 12.3%; adjusted difference, 5.5%; 95% CI, 3.2%–7.8%; P < .001, FDR adjusted) and lower RV fractional shortening by 4.0% (95% CI, 2.3%–5.8%; P < .001, FDR adjusted) and worse RV free-wall longitudinal strain by 2.4% (95% CI, 1.2%–3.6%; P < .001, FDR adjusted) on TTE. Tricuspid annular plane systolic excursion also was lower numerically in male participants (adjusted difference, −0.08 cm; 95% CI, −0.16 to 0.005 cm; P = .12, FDR adjusted), although not significantly. Left ventricle ejection fraction was significantly lower in male participants as measured by both cardiac MRI and TTE (P < .001, FDR adjusted for both). Structural right heart differences also were observed. RV end diastolic volume index and RV mass index on cardiac MRI and right atrial volume index on TTE all were significantly larger in male participants (P < .001, FDR adjusted for all).
TABLE 4 ].
Cardiac, Echocardiographic, and Hemodynamic Measures by Sex in Patients With Pulmonary Hypertension
| Variable | No. | Male (n = 279) | Female (n = 471) | Adjusted Differencea | P Valueb |
|---|---|---|---|---|---|
| Cardiac MRI | |||||
| RV ejection fraction, % | 449 | 37.3 ± 10.8 (n = 163) | 41.6 ± 12.3 (n = 286) | −5.5 (−7.8 to −3.2) | < .001 |
| RV ejection fraction ≤ 35% | 149 | 67 (41.1) | 82 (28.7) | … | .001c |
| RV stroke volume index, mL/m2 | 449 | 37.1 ± 10.3 (n = 163) | 37.4 ± 11.4 (n = 286) | 0.14 (−2.1 to 2.4) | .90 |
| RV EDV index, mL/m2 | 449 | 106.4 ± 41.8 (n = 163) | 96.2 ± 35.2 (n = 286) | 15.6 (8.2–23.1) | < .001 |
| RV mass index, g/m2 | 448 | 21.5 ± 8.7 (n = 162) | 18.9 ± 9.1 (n = 286) | 4.0 (2.2–5.8) | < .001 |
| LV ejection fraction, % | 451 | 52.9 ± 9.5 (n = 165) | 57.5 ± 8.5 (n = 286) | −4.3 (−6.2 to −2.4) | < .001 |
| RV EDV to LV EDV | 448 | 1.54 ± 0.5 (n = 163) | 1.55 ± 0.67 (n = 285) | 0.07 (−0.06 to 0.19) | .41 |
| RV mass to RV EDV, g/mL | 448 | 0.21 ± 0.06 (n = 162) | 0.20 ± 0.06 (n = 286) | 0.01 (0.00–0.03) | .11 |
| TTE | |||||
| RV fractional shortening, % | 603 | 29.5 ± 9.5 (n = 220) | 32.4 ± 9.9 (n = 383) | −4.0 (−5.8 to −2.3) | < .001 |
| RV free-wall longitudinal strain, % | 508 | −17.6 ± 5.6 (n = 175) | −19.5 ± 6.1 (n = 333) | 2.4 (1.2–3.6) | < .001 |
| TAPSE, cm | 593 | 1.79 ± 0.47 (n = 221) | 1.87 ± 0.48 (n = 372) | −0.08 (−0.16 to 0.01) | .12 |
| RV systolic pressure, mm Hg | 612 | 59.3 ± 19.6 (n = 219) | 61.8 ± 23.4 (n = 393) | 1.6 (−2.2 to 5.4) | .51 |
| TAPSE, mm to RVSP, mm Hg | 513 | 0.34 ± 0.16 (n = 186) | 0.36 ± 0.19 (n = 327) | −0.005 (−0.008 to −0.001) | .02 |
| RV ED basal diameter index, cm/m2 | 644 | 2.31 ± 0.48 (n = 240) | 2.34 ± 0.50 (n = 404) | 0.04 (−0.03 to 0.12) | .35 |
| RA volume index, mL/m2 | 689 | 37.4 ± 20.1 (n = 251) | 31.6 ± 17.5 (n = 438) | 8.7 (5.4–11.9) | < .001 |
| LA volume index, mL/m2 | 627 | 28.5 ± 14.1 (n = 228) | 26.0 ± 12.0 (n = 399) | 0.8 (−1.3 to 3.0) | .53 |
| LV ejection fraction, % | 514 | 57.4 ± 11.3 (n = 193 | 62.6 ± 8.8 (n = 321) | −4.5 (−6.0 to −3.0) | < .001 |
| Global LV strain, % | 449 | −16.8 ± 3.8 (n = 158) | −17.9 ± 3.3 (n = 291) | 0.9 (0.1–1.6) | .04 |
| Tricuspid valve regurgitation | 703 | … | … | … | .56c |
| None or trace | … | 81 (31.2) | 123 (27.8) | … | … |
| Mild | … | 99 (38.1) | 177 (40.0) | … | … |
| Moderate | … | 68 (26.2) | 112 (25.3) | … | … |
| Severe | … | 12 (4.6) | 31 (7.0) | … | … |
| Right heart catheterization | |||||
| PVR, WU | 714 | 5.72 ± 4.13 (n = 261) | 6.31 ± 4.26 (n = 453) | 0.30 (−0.36 to 0.95) | .49 |
| PVR > 5 WU | … | 118 (45.2) | 237 (52.3) | … | .62c |
| Mean PA pressure, mm Hg | 736 | 40.2 ± 12.5 (n = 270) | 41.0 ± 13.9 (n = 466) | 2.7 (0.6–4.8) | .02 |
| Mean RA pressure, mm Hg | 736 | 8.8 ± 4.9 (n = 271) | 7.7 ± 5.1 (n = 465) | 1.40 (0.64–2.15) | .001 |
| Mean PCWP, mm Hg | 730 | 13.2 ± 6.3 (n = 268) | 12.6 ± 6.5 (n = 462) | 0.3 (−0.6 to 1.1) | .58 |
| Cardiac index, L/min/m2 | 724 | 2.63 ± 1.01 (n = 265) | 2.76 ± 0.86 (n = 459) | −0.13 (−0.28 to 0.02) | .15 |
| PA compliance, mL/mm Hg | 722 | 2.45 ± 1.43 (n = 265) | 2.14 ± 1.46 (n = 457) | 0.17 (−0.06 to 0.40) | .23 |
Data presented as No. (%), mean ± SD, or median (interquartile range) unless otherwise indicated. The n values are the number of nonmissing values. Numbers after proportions are frequencies. ED = end diastolic; EDV = end diastolic volume; LA = left atrium; LV = left ventricle; PA = pulmonary artery; PCWP = pulmonary capillary wedge pressure; PVR = pulmonary vascular resistance; RA = right atrium; RV = right ventricle; RVSP = right ventricular systolic pressure; TAPSE = tricuspid annular plane systolic excursion; TTE = transthoracic echocardiography; WU = Wood unit.
Adjusted difference, estimated regression coefficient, presents the effect of sex (male vs female) estimated via multivariable regression model adjusted for age, BMI, World Health Organization functional class, World Symposium on Pulmonary Hypertension group, years of pulmonary hypertension at time of enrollment, current exercise frequency and duration, smoking status, history of atrial arrhythmia, diabetes, kidney disease, and coronary disease.
Adjusted for multiple comparisons by controlling false discovery rate at 0.05. Unless otherwise indicated, P values are from multivariable linear regression model.
Pearson χ2 test.
Sex-based differences in RVEF were consistent across WSPH groups 1 through 5 and remained statistically significant in WSPH groups 1 and 3 (e-Table 3). Among all patients with PH, no significant interaction was noted between sex and age on RVEF, with male sex associated with lower RVEF across the age range of patients. When left ventricle ejection fraction was included in the model, RVEF remained significantly lower in men by 3.1% (95% CI, 1.0%–5.3%; P = .004).
On RHC, mean right atrial pressure was significantly higher in male participants by 1.4 mm Hg (95% CI, 0.6–2.2 mm Hg; P = .001, FDR adjusted). Despite evidence of worse RV function, no evidence was found of worsened RV afterload or disproportionate PVD in male participants; PVR, pulmonary artery (PA) compliance, and pulmonary capillary wedge pressure were similar in male and female participants. Unadjusted mean PA pressures were similar, but men showed higher adjusted mean pulmonary arterial pressure by 2.7 mm Hg (95% CI, 0.6–4.8 mm Hg; P = .02, FDR adjusted). Among female participants, no significant differences were found by menopause status in cardiac parameters on cardiac MRI, TTE, or RHC by multivariable regression (e-Table 4).
Association of PVR and RVEF
To investigate sex-based differences in RV response to afterload, the relationship between PVR and RVEF was assessed in male participants (n = 156) and female participants (n = 279) with PH who had nonmissing values for each (median time between RHC and cardiac MRI, 4 days [IQR, 19–1 days]). In both male and female participants, RVEF was correlated inversely with PVR (rmale = −0.45; rfemale = −0.52) (Fig 1A). Multivariable linear regression demonstrated that RVEF was associated with both PVR and sex, with a significant interaction between PVR and sex on RVEF, the largest sex-based differences in RVEF noted at mild to moderate PVR elevation, and no significant differences seen at more severe elevations (> 10 Wood unit [WU]) (Fig 1B). Estimated sex-based differences in RVEF at different levels of PVR by multivariable regression are shown in Table 5.
Figure 1 –
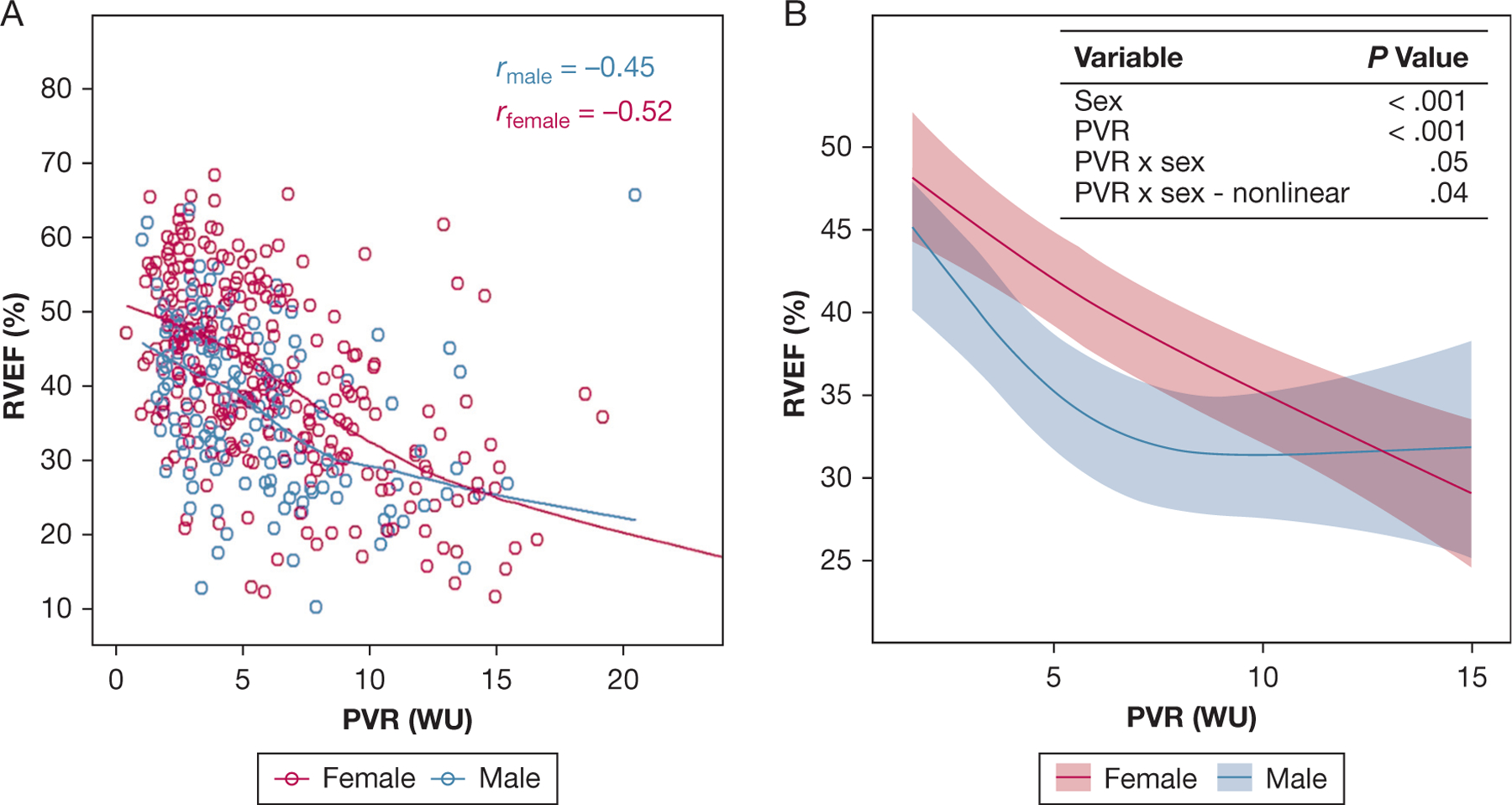
A, Scatterplot showing RVEF and PVR in male and female patients with pulmonary hypertension (PH). Locally weighted scatterplot smoothing lines and Spearman correlation coefficients for linear correlations are presented. RVEF is associated inversely with PVR in both male and female patients. B, Multivariable linear regression plot showing RVEF with PVR and sex. Model is adjusted for age, time since PH diagnosis, World Symposium on Pulmonary Hypertension group, BMI, World Health Organization functional class, smoking status, current exercise frequency and duration, and presence of comorbidities including coronary artery disease, diabetes, chronic kidney disease, and atrial arrhythmias. P values reported for effect of the variables of interest on RVEF based on the multivariable linear regression model. Significant nonlinear interaction is present between PVR and sex on RVEF (P = .05 for interaction, P = .04 for nonlinearity). The largest sex-based differences in RVEF are noted at mild to moderate elevations of PVR. Mean differences in RVEF by PVR are presented in Table 5. Largest sex-based differences in RVEF are noted at mild to moderate PVR elevations and are not significant at significant PVR elevations (> 10 WU). PVR = pulmonary vascular resistance; RVEF = right ventricle ejection fraction; WU = Wood unit.
TABLE 5 ].
Sex-Based Differences in RVEF by PVR Percentile
| PVR, WU | Estimated Mean Difference in RVEF, % |
|---|---|
| 2.2 | −3.8 (−7.7 to 0.1) |
| 3.0 | −4.9 (−7.8 to −2.0) |
| 4.9 | −6.7 (−9.4 to −4.1) |
| 7.5 | −6.4 (−9.5 to −3.2) |
| 10.9 | −2.6 (−6.2 to 1.1) |
Data presented as point estimate (95% CI) at each listed at 10th, 25th, 50th, 75th, and 90th percentile for PVR. Estimated mean difference in RVEF between male patients compared with female patients by multivariable linear regression. Model includes age, time since pulmonary hypertension diagnosis, World Symposium on Pulmonary Hypertension group, BMI, World Health Organization functional class, smoking status, presence of coronary artery disease, diabetes, chronic kidney disease, atrial arrhythmias, exercise frequency and duration, and an interaction term between PVR and sex. RVEF = right ventrcle ejection fraction; PVR = pulmonary vascular resistance; WU = Wood unit.
Impact of Sex on Survival
We next sought to understand if differences in RV function translated into clinically important differences in survival. Patients were followed up for 31 ± 13 months, with a median of 36 months and IQR of 24 to 37 months, and 10% of the patients were followed up for > 48 months. During the entire follow-up period, 45 patients underwent lung or heart transplantation, or both, and 137 patients died; the 4-year event rate was estimated to be 30.9% (95% CI, 26.4%–35.2%). Among the 471 female patients, 25 patients underwent transplantation and 73 died, for a 4-year event rate of 26.2% (95% CI, 20.9%–31.2%). Among the 279 male patients, 20 patients underwent transplantation and 64 died, for an overall 4-year event rate of 39.8% (95% CI, 30.9%–47.6%) (e-Table 5). After adjusting for the competing risk of transplantation, the cumulative incidence of death was estimated to be 17.6% (95% CI, 13.4%–21.7%) for female patients and 27.2% (95% CI, 20.4%–33.9%) for male patients (e- Fig 1).
Among all patients with PH, male sex was associated with decreased transplant-free survival, as shown in Figure 2 (hazard ratio, 1.63 [95% CI, 1.22–2.19; P = .001]; adjusted hazard ratio, 1.46 [95% CI, 1.07–1.98; P = .02]). The effect of sex on survival, with higher risk of transplantation or death in male patients, was not significantly different across the age spectrum (e-Fig 2). Among female patients, postmenopausal participants showed decreased transplant-free survival (e-Fig 3), although this difference was not significant after adjustment for age, PVR, time since PH diagnosis, and WSPH group (P = .70). When the interaction between PVR and sex was included in the model, the effect of sex on survival was most notable at lower values of PVR (e-Fig 4). Adjusted effects of sex on transplant-free survival by PVR percentile are presented in Table 6. Male sex was associated with significantly increased risk of death or transplant at PVR of < 5 WU, whereas no significant difference was found in transplant-free survival by sex at higher PVR levels. The regression-based causal mediation analysis demonstrated that RVEF significantly mediated the sex-based differences in transplant-free survival (total natural indirect effect, 0.20; 95% CI, 0.07–0.33; P = .003), with an estimated proportion mediated of 68% (e-Fig 5).
Figure 2 –
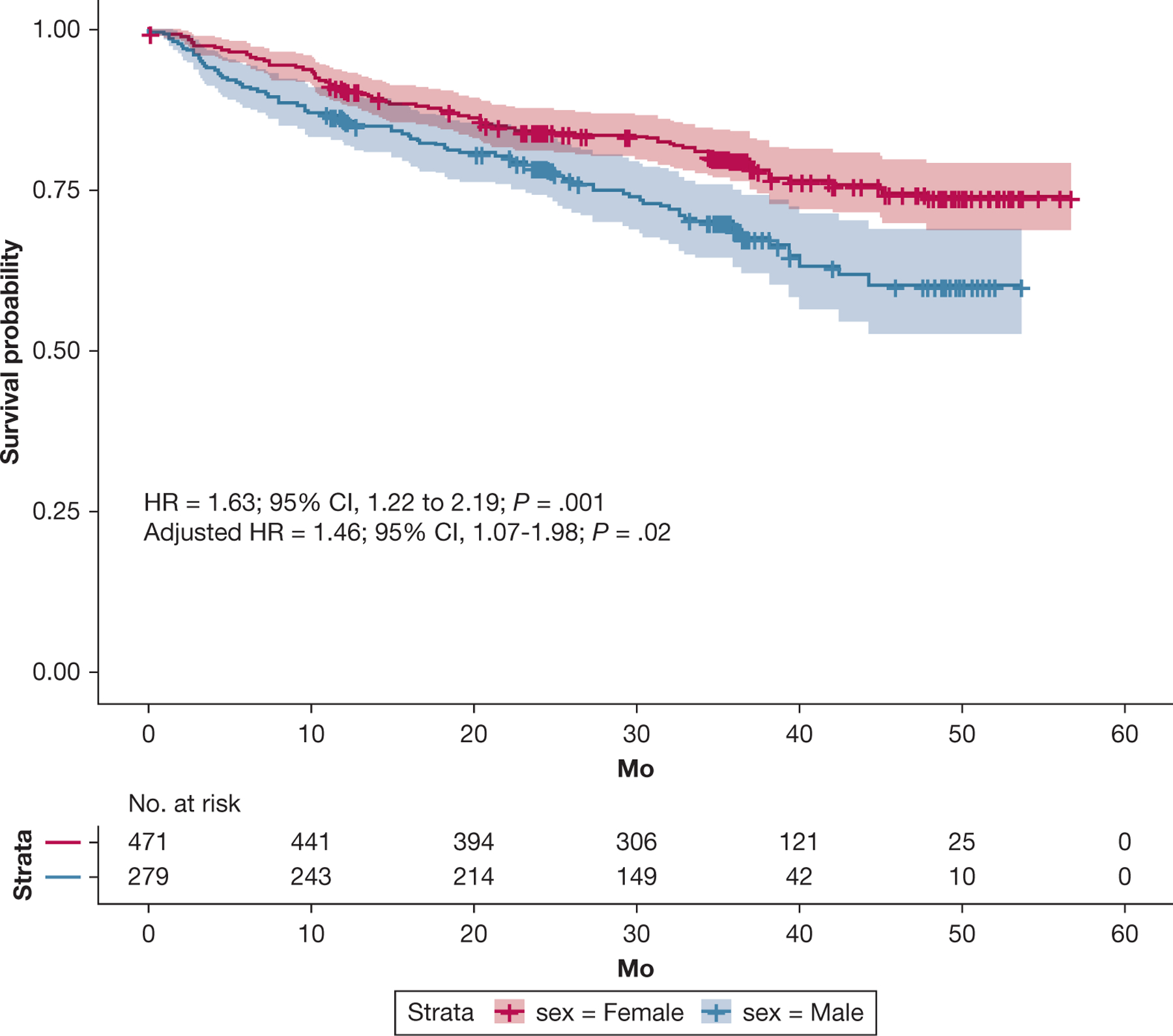
Kaplan-Meier curve showing transplant-free survival by sex in patients with pulmonary hypertension (PH). Data are presented with confidence bands. Among all patients with PH, male sex was associated with decreased transplant-free survival (HR, 1.63 [95% CI, 1.22–2.19; P = .001]; adjusteda HR, 1.46 [95% CI, 1.07–1.98; P = .02]). aAdjusted for pulmonary vascular resistance, age, time since PH diagnosis, and World Symposium on Pulmonary Hypertension group. HR = hazard ratio.
TABLE 6 ].
Adjusted Effects of Sex on Transplant-Free Survival by PVR Percentile
| PVR, WU | HR, male vs female |
|---|---|
| 2.2 | 2.23 (1.14–4.37) |
| 3.1 | 1.98 (1.22–3.22) |
| 5.0 | 1.60 (1.12–2.27) |
| 7.7 | 1.27 (0.82–1.96) |
| 11.6 | 1.04 (0.64–1.68) |
Data presented as point estimate HR (95% CI) at 10th, 25th, 50th, 75th, and 90th percentile of PVR. HRs determined by Cox proportional hazards model. Age, time since pulmonary hypertension diagnosis, World Symposium on Pulmonary Hypertension group, and sex-PVR interaction were included in the model. HR = hazard ratio; PVR = pulmonary vascular resistance; WU = Wood unit.
Discussion
In this multicenter, prospective cohort study, deep phenotyping of patients allowed for the evaluation of sex-based differences across the spectrum of PVD. As expected, we found that WSPH group 1 was most predominantly female. In this cohort, WSPH groups 2 and 4 also showed female predominance. Although patients in the PVDOMICS study with PH were more likely to be female, male participants with PH showed worse RV function as measured by echocardiography, cardiac MRI, and RHC. As expected, RVEF was correlated negatively with PVR in both male and female participants,28 but we found a novel, significant interaction between sex and PVR on RVEF, with sex-based differences in RVEF most apparent at mild to moderate elevations of PVR. Male sex also was associated with decreased transplant-free survival across the spectrum of PVD, which was explained partially by differences in RVEF. Similar to sex-based differences in RV function, sex-based differences in transplant-free survival were most notable at mild PVR elevations (< 5 WU).
The PVDOMICS study provides a unique opportunity to evaluate sex-based differences across the spectrum of PVD as a multicenter, prospective cohort study that enrolled patients with all forms of PH. Further, it allows for investigation into the potential interaction of multiple assessments of cardiopulmonary physiologic features such as RVEF and PVR because of the deep phenotyping performed on large proportions of the patients enrolled. The study also is strengthened by the reliability of the study measures, given the protocolized collection of images and hemodynamic measurements with a close temporal relationship, central core interpretation of studies, and rigorous adjudication of PH grouping assigned to enrolled patients.16,17,20,21 The multimodal assessment of RV function and the inclusion of participants across the spectrum of PH strengthen the validity and generalizability of our findings beyond WPSH group 1 PAH.
Although male sex is associated with worse outcomes in PAH partially related to disproportionate RV dysfunction, it is unknown if this is a unique feature of PAH or if it extends to male patients with PH regardless of cause. Our results show that male sex is associated with worse RV function across the spectrum of PVD, which expands on prior studies that have shown similar findings in healthy cohorts,10,11 patients with PAH,12 patients with heart failure with preserved ejection fraction,14 and patients with PH associated with chronic lung disease.15 Although the magnitude of difference in RVEF between male and female participants in our study is similar to what was seen in healthy cohorts,10,11 our data show the PH-specific impact of these differences in RV function, because RV systolic dysfunction was significantly more common in male participants with PH, and differences in RVEF mediated a significant proportion of the sex-based differences in transplant-free survival. Further, we observed a novel interaction between sex and PVR on RVEF, with sex-based differences in RVEF most notable at mild to moderate elevations of PVR. Our study suggested that sex-based differences in transplant-free survival largely may be driven by patients with mild elevations of PVR (< 5 WU), where sex-based differences in RV function are most notable.
Overall, our study suggested that the RV in male patients is less able to compensate for mild increases in afterload seen in what might be considered hemodynamically less severe forms of PH and that this steep decline in RVEF with small increases in PVR contributes to the observed differences in mortality between male and female participants with PH. This is consistent with prior data that have demonstrated protective effects of estrogen hormones on RV contractility and fibrosis in human and experimental PH.29–31 Taken together, these data suggest that hormonal factors may influence RV response to load stress in PH, but the mechanisms by which this occurs in human disease need to be investigated further. Because our study did not include data on reproductive factors such as number of pregnancies or use of hormonal contraceptives or other hormonal therapies, we are unable to identify the impact of these factors on the sex-based differences observed.
Our study emphasized the importance of RV function, specifically RVEF on cardiac MRI, in predicting outcomes in patients with PH, but also highlighted that RVEF should be evaluated in the context of the pulmonary circulation and degree of RV afterload present. This may be especially relevant in the care of patients with WSPH group 3 PH associated with chronic lung disease, because these patients showed the highest rates of death and transplantation despite a median PVR of 4.7 WU in the cohort. These data suggest that factors other than RV afterload are contributing to RV failure in patients with PH, and the mechanisms behind RV failure and maladaptation in these patients warrant further investigation. Possible explanations include sex differences in intrinsic RV disease related to fibrosis, ischemia, or metabolic dysfunction and cardiomyocyte lipotoxicity, which previously were shown to be important in PAH.32,33
Our study has several limitations. Enrollment bias may obscure the true male and female prevalences because gender-specific differences may persist in patient willingness to participate in trials such as this. We are also limited by our inability to assess trajectory of change in RV function or PVR because follow-up data presently are limited to vital status and organ transplantation status. It is possible that sex-related differences exist in the rate of change of RVEF and PVR that contribute to the difference in observed survival. Although similar proportions of prevalent PH exist between male and female patients, male patients with prevalent PH showed a shorter median duration of PH at enrollment that could reflect the presence of more severe disease. This difference is unlikely to explain the differences in RV function or survival because these analyses were adjusted for time since diagnosis. The heterogeneity of the cohort also may have impacted our ability to identify factors that are associated independently with sex. We attempted to adjust for as many factors as possible given the sample size of the study; however, given the small number of patients in the various subgroups of PH, we were unable to adjust for specific subtypes of PH such as PAH associated with systemic sclerosis or systemic lupus erythematosus, so we cannot identify how these specific disease subtypes impact the interaction of sex with RV function in PAH. PH treatment status was not included in the multivariable analysis because we were unable to account for changes in treatment during the follow-up period, although differences in PH treatment between men and women may impact the results. These results do not allow us to identify mechanisms behind the sex-based differences in RV function observed. Although the consistency of these results implies a biological basis for the difference, it is possible that gender differences in behavioral patterns, such as diet, exercise, or tobacco use; occupational history; family roles; and timing and frequency of seeking medical care impact the observed results. Despite the prospective nature of this cohort, some data are missing that could impact the results. Cardiac MRI was not performed in 40% of patients, and patients without cardiac MRI data seemed to be more likely to have comorbidities including coronary disease, chronic kidney disease, and diabetes. No significant differences were found in missingness of cardiac MRI by sex, although it is possible that reasons for not undergoing cardiac MRI were different between male and female participants and could influence the results. Although sex-based differences in cardiac parameters were adjusted for multiple comparisons, many secondary analyses were performed, increasing the likelihood of false discovery, and these analyses should be considered exploratory. Further, although we performed noninvasive assessments of RV-PA coupling through assessment of TASPE to right ventricular systolic pressure ratio and by comparison of RVEF with PVR, pressure-volume loops were not available to assess RV-PA coupling, which previously was shown to be more sensitive in detecting sex-based differences in PH and may have amplified our findings.29
Interpretation
In conclusion, a female predominance exists in the PVDOMICS cohort of PH enrollees that is most pronounced in groups 1, 2, and 4 PH. Among all patients with PH studied, male sex was associated with worsened RV function and decreased transplant-free survival, which is attributable partially to sex-based differences in RVEF. Sex-based difference in both RVEF and transplant-free survival were most notable in patients with mild to moderate elevations in PVR.
Supplementary Material
Take-home Points.
Study Question:
What sex-based differences exist in right ventricular (RV) function and transplant-free survival in patients with pulmonary hypertension (PH) from the Redefining Pulmonary Hypertension Through Pulmonary Vascular Disease Phenomics cohort?
Results:
Male patients showed significantly lower right ventricle ejection fraction (RVEF; adjusted difference, 5.5%; 95% CI, 3.2%–7.8%; P < .001) on cardiac MRI and decreased adjusted transplant-free survival (HR, 1.46; 95% CI, 1.07–1.98; P = .02), partially mediated by differences in RVEF (P = .003).
Interpretation:
Among all patients with PH, male sex was associated with worsened right ventricle function and decreased transplant-free survival, which is attributable partially to sex-based differences in RVEF and was most notable in patients with mild to moderate elevations of PVR.
Funding/Support
The study was supported by the National Institutes of Health [Grants U01 HL125218 [E. B. R.]), U01 HL125205 (R. P. F.), U01 HL125212 (A. R. H.), U01 HL125208 (F.P. R.), U01 HL125175 (P. M. H.), U01 HL125215 (J. A. L.), and U01 HL125177 (G. J. B.)] and the Pulmonary Hypertension Association.
Role of sponsors:
Study sponsors were not involved in the drafting or approval of this manuscript.
ABBREVIATIONS:
- EF
ejection fraction
- FDR
false discovery rate
- IQR
interquartile range
- PA
pulmonary artery
- PAH
pulmonary arterial hypertension
- PH
pulmonary hypertension
- PVD
pulmonary vascular disease
- PVDOMICS
Redefining Pulmonary Hypertension Through Pulmonary Vascular Disease Phenomics
- PVR
pulmonary vascular resistance
- RHC
right heart catheterization
- RV
right ventricle
- RVEF
right ventricle ejection fraction
- TTE
transthoracic echocardiography
- WSPH
World Symposium on Pulmonary Hypertension
- WU
Wood unit
Footnotes
TRIAL REGISTRY: ClinicalTrials.gov; No.: NCT02980887; URL: www.clinicaltrials.gov
Financial/Nonfinancial Disclosures
The authors have reported to CHEST Pulmonary the following: N. J. S. reports grant funding from Bayer. A. R. H. reports associate editor for pulmonary vascular disease at CHEST and consulting fees from Bayer, United Therapeutics, Merck, GossamerBio, Tenax, and Janssen. H. M. D. reports grant funding from Bayer and consulting fees from Janssen. E. M. H. reports grant funding from Insmed, Acceleron (Merck), and Cereno and consulting fees from Bioventrix. F. P. R. reports grant funding from Merck, Janssen, Acceleron, Insmed, United Therapeutics, Bayer, and Aerovate. M. M. P. reports grant funding from Janssen/Actellion, Insmed, and Bayer and serving on the speakers bureau of Lantheus Medical Imaging. R. F. P. reports grant funding from Bayer, United Therapeutics, Medtronic, Acceleron, and Gossamer Bio and honoraria for lectures and presentations from Janssen and Gossamer Bio. P. M. H. serves on the Merck Scientific Steering Committee (unrelated to the current work). J. A. L. reports grant funding from Astellas and Aria CV and honoraria for lectures and presentations from Abbott Vascular, United Therapeutics, and NATF. None declared (H. N., G. J. B., N. G. C., A. A. D., S. E., N. S. H., M. S. J., C. L. J., E. J., D. H. K., A. B. L., E. B. R., R. R. V., C. Y.).
Additional information: The e-Appendix, e-Figures, and e-Tables are available online under “Supplementary Data.”
Contributor Information
Nicholas J. Shelburne, Division of Allergy, Pulmonary and Critical Care Medicine, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN; INTEGRIS Advanced Cardiopulmonary Care, INTEGRIS Baptist Medical Center, Oklahoma City, OK.
Hui Nian, Department of Biostatistics, Vanderbilt University Medical Center, Nashville, TN.
Gerald J. Beck, Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland.
Nancy G. Casanova, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Department of Medicine, University of Arizona, Tucson, AZ.
Ankit A. Desai, Department of Medicine, Indiana University, Indianapolis, IN.
Hilary M. DuBrock, Division of Pulmonary and Critical Care Medicine, Mayo Clinic, Rochester, MN.
Serpil Erzurum, Lerner Research Institute, Cleveland Clinic, Cleveland; Respiratory Institute, Cleveland Clinic, Cleveland.
Robert P. Frantz, Department of Internal Medicine, the Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN.
Paul M. Hassoun, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Johns Hopkins University, Baltimore.
Nicholas S. Hill, Division of Pulmonary, Critical Care, and Sleep Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Evelyn M. Horn, Division of Cardiology, Department of Pediatrics and Medicine, Columbia University Medical Center-New York Presbyterian Hospital.
Miriam S. Jacob, Department of Cardiovascular Medicine, Cleveland Clinic, Cleveland.
Christine L. Jellis, Department of Cardiovascular Medicine, Cleveland Clinic, Cleveland.
Elizabeth Joseloff, Pulmonary Hypertension Association, Silver Spring, MD.
Deborah H. Kwon, Department of Cardiovascular Medicine, Cleveland Clinic, Cleveland.
A. Brett Larive, Department of Quantitative Health Sciences, Cleveland Clinic, Cleveland.
Jane A. Leopold, Tufts Medical Center, Division of Cardiovascular Medicine, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA.
Margaret M. Park, Department of Cardiovascular Medicine, Cleveland Clinic, Cleveland.
Franz P. Rischard, Division of Pulmonary, Allergy, Critical Care and Sleep Medicine, Department of Medicine, University of Arizona, Tucson, AZ.
Erika B. Rosenzweig, Perkin Heart Failure Center, Weill Cornell Medical Center, the Division of Pediatric Cardiology, Department of Pediatrics and Medicine, Columbia University Medical Center-New York Presbyterian Hospital.
Rebecca R. Vanderpool, Division of Cardiovascular Medicine, Department of Internal Medicine, The Ohio State University, Columbus, OH.
Chang Yu, Department of Population Health, NYU Grossman School of Medicine, New York, NY.
Anna R. Hemnes, Division of Allergy, Pulmonary and Critical Care Medicine, Department of Medicine, Vanderbilt University Medical Center, Nashville, TN.
References
- 1.Hester J, Ventetuolo C, Lahm T. Sex, gender, and sex hormones in pulmonary hypertension and right ventricular failure. Compr Physiol 2019;10(1):125–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro S, Traiger GL, Turner M, McGoon MD, Wason P, Barst RJ. Sex differences in the diagnosis, treatment, and outcome of patients with pulmonary arterial hypertension enrolled in the registry to evaluate early and long-term pulmonary arterial hypertension disease management. Chest. 2012;141(2):363–373. [DOI] [PubMed] [Google Scholar]
- 3.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–387. [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173(9): 1023–1030. [DOI] [PubMed] [Google Scholar]
- 5.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987;107(2):216–223. [DOI] [PubMed] [Google Scholar]
- 6.Humbert M, Sitbon O, Chaouat A, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2):156–163. [DOI] [PubMed] [Google Scholar]
- 7.Benza RL, Miller DP, Gomberg-Maitland M, et al. Predicting survival in pulmonary arterial hypertension: insights from the Registry to Evaluate Early and Long-Term Pulmonary Arterial Hypertension Disease Management (REVEAL). Circulation. 2010;122(2): 164–172. [DOI] [PubMed] [Google Scholar]
- 8.Vonk-Noordegraaf A, Haddad F, Chin KM, et al. Right heart adaptation to pulmonary arterial hypertension: physiology and pathobiology. J Am Coll Cardiol 2013;62(25 suppl):D22–D33. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs W, van de Veerdonk MC, Trip P, et al. The right ventricle explains sex differences in survival in idiopathic pulmonary arterial hypertension. Chest. 2014;145(6):1230–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kawut SM, Lima JA, Barr RG, et al. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123(22): 2542–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foppa M, Arora G, Gona P, et al. Right ventricular volumes and systolic function by cardiac magnetic resonance and the impact of sex, age, and obesity in a longitudinally followed cohort free of pulmonary and cardiovascular disease: the Framingham Heart Study. Circ Cardiovasc Imaging. 2016;9(3):e003810. [DOI] [PubMed] [Google Scholar]
- 12.Kawut SM, Al-Naamani N, Agerstrand C, et al. Determinants of right ventricular ejection fraction in pulmonary arterial hypertension. Chest. 2009;135(3):752–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swift AJ, Capener D, Hammerton C, et al. Right ventricular sex differences in patients with idiopathic pulmonary arterial hypertension characterised by magnetic resonance imaging: pair-matched case controlled study. PloS One. 2015;10(5):e0127415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35(48): 3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Prins KW, Rose L, Archer SL, et al. Clinical determinants and prognostic implications of right ventricular dysfunction in pulmonary hypertension caused by chronic lung disease. J Am Heart Assoc 2019;8(2):e011464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemnes AR, Beck GJ, Newman JH, et al. PVDOMICS: a multi-center study to improve understanding of pulmonary vascular disease through phenomics. Circ Res 2017;121(10):1136–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hemnes AR, Leopold JA, Radeva MK, et al. Clinical characteristics and transplant-free survival across the spectrum of pulmonary vascular disease. J Am Coll Cardiol 2022;80(7):697–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller VM. In pursuit of scientific excellence: sex matters. Am J Physiol Lung Cell Mol Physiol 2012;302(9):L801–L802. [DOI] [PubMed] [Google Scholar]
- 19.Wizemann TM, Pardue ML; Institute of Medicine Committee on Understanding the Biology of Sex Gender Differences. The National Academies Collection: reports funded by National Institutes of Health, The Future of Research on Biological Sex Differences: Challenges and Opportunities. In: Exploring the Biological Contributions to Human Health: Does Sex Matter? National Academies Press (US); 2001:173–184. [PubMed] [Google Scholar]
- 20.Tang WHW, Wilcox JD, Jacob MS, et al. Comprehensive diagnostic evaluation of cardiovascular physiology in patients with pulmonary vascular disease: insights from the PVDOMICS program. Circ Heart Fail 2020;13(3):e006363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jellis CL, Park MM, Abidov A, et al. Comprehensive echocardiographic evaluation of the right heart in patients with pulmonary vascular diseases: the PVDOMICS experience. Eur Heart J Cardiovasc Imaging. 2022;23(7):958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl):D34–D41. [DOI] [PubMed] [Google Scholar]
- 23.Alabed S, Shahin Y, Garg P, et al. Cardiac-MRI predicts clinical worsening and mortality in pulmonary arterial hypertension: a systematic review and meta-analysis. JACC Cardiovasc Imaging. 2021;14(5):931–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Houston BA, Brittain EL, Tedford RJ. Right ventricular failure. N Engl J Med 2023;388(12):1111–1125. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida K, Li Y. regmedint: Regression-based causal mediation analysis with interaction and effect modification terms. R package version 1.0.0. R Foundation for Statistical Computing website; 2022. Accessed July 19, 2022. https://CRAN.R-project.org/package=regmedint [Google Scholar]
- 26.R Foundation for Statistical Computing. R: a language and environment for statistical computing. R Foundation for Statistical Computing; 2021.. Version 4.1.0.. [Google Scholar]
- 27.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Methodol 1995;57(1):289–300. [Google Scholar]
- 28.Bellofiore A, Wang Z, Chesler NC. What does the time constant of the pulmonary circulation tell us about the progression of right ventricular dysfunction in pulmonary arterial hypertension? Pulm Circ 2015;5(2):291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tello K, Richter MJ, Yogeswaran A, et al. Sex differences in right ventricular-pulmonary arterial coupling in pulmonary arterial hypertension. Am J Respir Crit Care Med 2020;202(7):1042–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frump AL, Albrecht M, Yakubov B, et al. 17b-Estradiol and estrogen receptor a protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J Clin Invest 2021;131(6):e129433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng TC, Tabima DM, Caggiano LR, et al. Sex differences in right ventricular adaptation to pressure overload in a rat model. J Appl Physiol 2022;132(3): 888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brittain EL, Talati M, Fessel JP, et al. Fatty acid metabolic defects and right ventricular lipotoxicity in human pulmonary arterial hypertension. Circulation. 2016;133(20):1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lippmann MR, Maron BA. The right ventricle: from embryologic development to RV failure. Curr Heart Fail Rep 2022;19(5):325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


