Abstract
Background: The biological mechanisms leading some tobacco-exposed individuals to develop early-stage chronic obstructive pulmonary disease (COPD) are poorly understood. This knowledge gap hampers development of disease-modifying agents for this prevalent condition.
Objectives: Accordingly, with National Heart, Lung and Blood Institute support, we initiated the SubPopulations and InteRmediate Outcome Measures In COPD Study (SPIROMICS) Study of Early COPD Progression (SOURCE), a multicenter observational cohort study of younger individuals with a history of cigarette smoking and thus at-risk for, or with, early-stage COPD. Our overall objectives are to identify those who will develop COPD earlier in life, characterize them thoroughly, and by contrasting them to those not developing COPD, define mechanisms of disease progression.
Methods/Discussion: SOURCE utilizes the established SPIROMICS clinical network. Its goal is to enroll n=649 participants, ages 30–55 years, all races/ethnicities, with ≥10 pack-years cigarette smoking, in either Global initiative for chronic Obstructive Lung Disease (GOLD) groups 0–2 or with preserved ratio-impaired spirometry; and an additional n=40 never-smoker controls. Participants undergo baseline and 3-year follow-up visits, each including high-resolution computed tomography, respiratory oscillometry and spirometry (pre- and postbronchodilator administration), exhaled breath condensate (baseline only), and extensive biospecimen collection, including sputum induction. Symptoms, interim health care utilization, and exacerbations are captured every 6 months via follow-up phone calls. An embedded bronchoscopy substudy involving n=100 participants (including all never-smokers) will allow collection of lower airway samples for genetic, epigenetic, genomic, immunological, microbiome, mucin analyses, and basal cell culture.
Conclusion: SOURCE should provide novel insights into the natural history of lung disease in younger individuals with a smoking history, and its biological basis.
Keywords: health-related quality of life, preserved ratio-impaired spirometry, smoking-related lung disease, SPIROMICS, imaging phenotypes
Introduction
This article contains supplemental material.
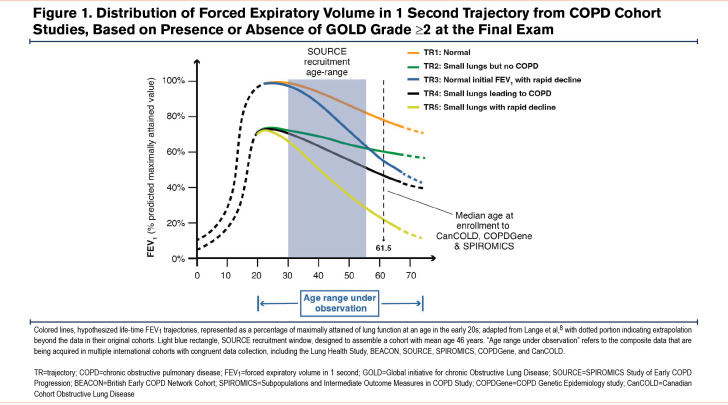
Chronic obstructive pulmonary disease (COPD) is a highly prevalent, heterogeneous disorder with rising morbidity and mortality.1 Available COPD pharmacotherapy neither reliably alters lung function decline nor prolongs survival,2-4 necessitating radically new therapies. The insights on early disease mechanisms required to develop such therapies cannot come from existing COPD cohorts (including: the COPD Genetic Epidemiology [COPDGene®] study,5 the SubPopulations and InteRmediate Outcome Measures In COPD Study [SPIROMICS],6 or the Canadian Cohort Obstructive Lung Disease7); despite eligibility in those studies at younger ages (Figure 1),8 the mean age at enrollment of their Global initiative for chronic Obstructive Lung Disease1 (GOLD) 0 participants was ~60 years, and very few participants entered before age 55 years. Thus, it is essential to investigate younger individuals at-risk for or with documented features of COPD, focusing on discovering reliable biomarkers of early progressive disease.9,10 To guide such future studies, we led an international group that proposed a working definition for research on early changes leading to COPD (“early COPD”).11
Based on those principles, we designed the SPIROMICS Study of Early COPD Progression (SOURCE), described here, which is funded primarily by the National Institutes of Health’s National Heart, Lung and Blood Institute (NHLBI). The goal of SOURCE is to define the clinical characteristics and biological underpinnings of COPD in younger individuals.9,11 While acknowledging the role of other inhalational exposures in driving worldwide COPD prevalence,12 SOURCE focuses on direct tobacco smoke inhalation (the principal cause of COPD in industrialized nations), to have a single etiological exposure. Enrollment criteria were informed by both pathological13,14 and epidemiological data.15-20 Central to our design were data suggesting that spirometric abnormalities in at-risk individuals were noted by age 43 and that symptoms at ages 36 and 43 predicted subsequent spirometrically-confirmed COPD at age 60–64, whereas symptoms at an earlier age did not.19 The importance of this critical age period has also been recently highlighted by analyses of the Tasmanian Longitudinal Health Study.21 Collectively, these previous studies suggest that airway-centered changes, termed small airway abnormality (SAA), are both an early smoking-induced pathology22 and can lead directly to subsequent emphysema.23,24
Importantly, lung pathology can be identified, COPD heterogeneity characterized, and disease progression predicted and quantified using high-resolution computed tomography (HRCT). Via the analytical technique of parametric response mapping (PRM),25-27 HRCT performed at inhalation and exhalation can distinguish emphysema from nonemphysematous air-trapping due to SAA. HRCT can additionally divide those with COPD into clusters with differing disease progression,28 and can identify the following quantifiable imaging abnormalities associated with accelerated deterioration of lung function: airway wall thickening,29 lower total airway count,30 and airway-to-lung ratio31 (often called “dysanapsis,” a term originally based solely on analysis of maximal expiratory flows,32 but now believed at least partially assessable by imaging33). HRCT imaging can also detect airway mucus plugs, which are associated with multiple adverse outcomes.34-36 Further, more rapid lung function decline in middle-aged individuals is associated with visually-assessed emphysema of moderate or greater severity37 and with >10% low attenuation area.38,39 Accordingly, the SOURCE cohort is powered to detect an imaging-based primary outcome. This approach allows for a smaller sample size than would be the case using spirometric outcomes, which are inherently more variable.
Methods and Materials
The full protocol of the SOURCE cohort is available in Appendix A of the online supplement.
Objectives and Specific Hypotheses
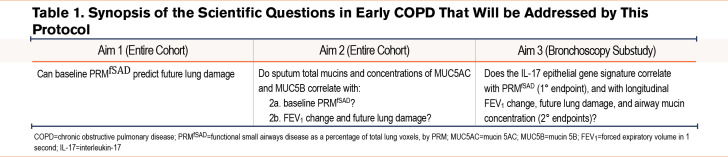
Aim 1 is to determine the relationship in early COPD between initial HRCT-defined SAA and imaging evidence of disease progression. Our primary outcome is a 3-year change in emphysema, as a percentage of total lung voxels determined by PRM (PRMEmph) (Table 1). We postulate that such progression will occur, and that it will correlate with baseline determination of functional small airways disease by PRM (PRMfSAD), in 2 groups of young ever-smokers: those with mild airflow obstruction (GOLD1 stages 1 and 2) and individuals with tobacco exposure and preserved spirometry (TEPS) who have significant respiratory symptoms, determined by a Chronic Airway Assessment Test (CAAT)40 score ≥10. Among older participants in SPIROMICS, we identified symptomatic TEPS as a persistent condition characterized by activity limitation and respiratory exacerbations,41,42 although without airflow obstruction consistent with current COPD diagnostic criteria.
Aim 2 is to determine whether sputum can serve as a less-invasive biomarker of early COPD. We postulate that hyperconcentration of airway mucins, identifiable in specimens collected by sputum induction, contributes centrally both to the development of airflow obstruction and to SAA43-49 (Table 1).
Aim 3 is to define the biological basis of SAA in early COPD. We postulate that SAA is driven by airway inflammation,11 particularly related to interleukin-17-driven inflammation.50,51 We will test this hypothesis via multiple lung-derived biospecimens collected during bronchoscopy (Table 1Tables 1 and 2).
Study Design and Organization
SOURCE is an ongoing (first participant enrolled October 8, 2021) observational cohort study (ClinicalTrials.gov Identifier: NCT05033990), involving 2 study visits, 3 years apart. The cohort is designed to comprise individuals (n=649) 30–55 years old at enrollment, of both sexes, and all races/ethnic backgrounds, with ≥10 pack-years smoking history. An identical study visit will be performed on additional (n=40) never-smokers in the same age range, half of whom must consent to participate in the bronchoscopy substudy, described below. As of April 22, 2024, a total of n=689 participants have been enrolled, with a median age of 48.0 (interquartile range 38.0, 58.0) years.
SOURCE is not a population-based study, but to attempt to minimize bias, neither does it direct recruitment efforts to primary care or specialty clinics, electronic medical records, smoking cessation programs, or other institutional venues designed to enrich for those with respiratory symptoms. Instead, recruitment relies on a strategy described below in “recruitment and retention.” SOURCE is conducted according to the principles of the Declaration of Helsinki; all participants sign written informed consents. Clinical outcomes, including deaths, will be adjudicated centrally.
SOURCE is recruiting participants at 14 clinical centers utilizing the infrastructure of SPIROMICS,6 another ongoing NHLBI-funded observational study designed to identify biomarkers, heterogeneity, and intermediate endpoints of established COPD. Many aspects of data collection are identical, and are also closely related to those of 2 other cohort studies: (1) the British Early COPD Network Cohort (BEACON)52 which between February 2018 and February 2020 enrolled 30–45 year-old individuals (n=431 current tobacco users and 67 never-smokers); and (2) the ongoing Lung Health Study (LHS),53 in which, via NHLBI funding, the American Lung Association is enrolling (n=4000) healthy individuals ages 25–35. Such congruence will permit data from all these cohorts to be combined, enabling future analyses across the entire adult age range of individuals with or at risk of COPD.
Funding is provided by the NHLBI (R01HL144718) and the COPD Foundation, which are involved in study oversight. The protocol has been approved by a single institutional review board (IRB) at the University of North Carolina at Chapel Hill (UNC) and at all clinical sites. An observational studies monitoring board (OSMB) provides semi-annual and ad hoc evaluations with recommendations to the NHLBI. The OSMB monitors the informed consent document and any modifications, data on adverse events (whether anticipated or not), participant safety, progress in enrollment, and on the quality and completeness of study data. The OSMB also reviews ancillary studies to assess possible increase in participant burden. Additional details on OSMB responsibilities are described in the online supplement.
Additional involved agencies are the Collaborative Studies Coordinating Center (CSCC) (UNC), which functions as the data management, clinical, and statistical coordinating center, and biospecimen repository core; an Imaging Reading Center (University of Iowa), a Pulmonary Function Testing Reading Center (University of California, Los Angeles); a Sputum Slide Reading Center (UNC); a Sequencing Center (Weill Cornell); and Immunophenotyping and Microbiome Cores (University of Michigan). Further details about the responsibilities and procedures of these cores is provided in Appendices B-E in the online supplement.
Inclusion and Exclusion Criteria
Before scheduling the in-person visit, via a Health Insurance Portability and Accountability Act waiver, clinical site coordinators contact participants by phone or email to conduct an initial screening questionnaire assessing potential eligibility.
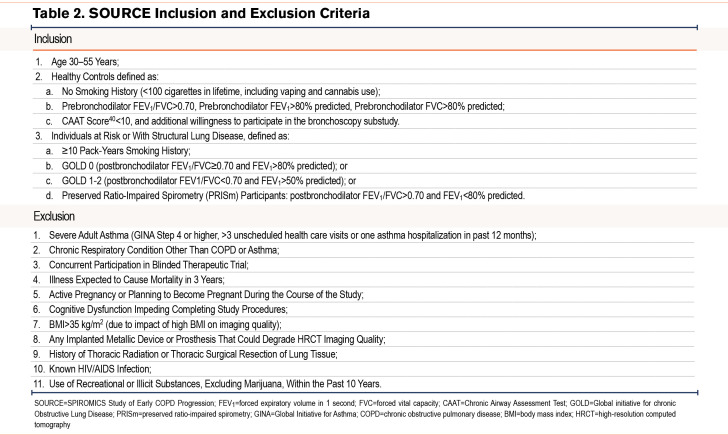
Inclusion criteria for ever-smoker participants align with those proposed to investigate COPD in younger participants10,11 (Table 2). We assess self-reported smoking status at baseline, at the 3-year follow-up visit, and at bronchoscopy, if applicable. Exclusion criteria are designed for participant safety and to minimize confounding causes of lung function decline, interference with the imaging endpoint, or loss to follow-up10,11(Table 2). Individuals with documented immunosuppression, including people living with HIV (even if treated) were excluded based on the postulated central role of lung immunity and inflammation in COPD pathogenesis. Note that a history of asthma or respiratory medication use only during childhood (<18 years old) are not exclusions for the ever-smoker group, but they are for never-smoker control participants, who must also be free of known respiratory disease or symptoms.40 A history of respiratory symptoms compatible with an exacerbation (either solely participant-identified or treated clinically with acute antibiotics or steroids) within the last 30 days is a temporary exclusion, but they can be rescreened for the study ≥30 days after discontinuation of drug therapy.
Power Analysis
The primary analysis will use linear mixed effects models to estimate and perform inference on trajectories of PRMEmph, based on PRMfSAD and PRMEmph at baseline. The power analyses are based on simulations in SAS using Proc Power that assume variability in effect sizes, on a subset of SPIROMICS I participants, age <55 years, GOLD1 stage 0–2, who had imaging data at baseline and at 3 years. Results suggested that for every 10% higher baseline PRMfSAD, there will be ~1% added increase in PRMEmph at 3 years. The simulated power to detect this increase in PRMEmph attributed to baseline PRMfSAD additionally adjusts for baseline PRMEmph. Assuming a Type 1 error of 5% and loss to follow-up of 10%, the simulations indicate >99% power to detect a 1% further increase in emphysema for every 10% higher PRMfSAD at baseline; this result is well within the range of data seen in our preliminary data.
Recruitment and Retention
As a cohort analyzing a disease-state and not a genuine epidemiological cohort, SOURCE was not authorized by NHLBI to employ measures to obtain a truly random, population-based sample. Moreover, the study was designed to maximize recruitment and retention of younger individuals, many of whom are employed or caring for children or elderly relatives. Hence, we have focused self-referral via public-facing social media, rather than on print, radio, or television ads. This approach was informed by the previous experience of our investigative team in recruiting a similar population to the Redefining Therapy in Early COPD Study.54,55
We initially developed a central SOURCE website,56 which included a map of our clinical sites (Figure 2) linking to webpages hosted by them. Besides the hope that brand recognition of those major regional medical centers would enhance participant trust, this process allows immediate capture by the sites of contact and screening information. Study coordinators then rapidly follow up by email, text, or telephone per the preference of interested individuals (at least 5 days weekly, in many cases including in the evening if initial calls during working hours failed).
To direct potential participants to those websites, we developed an intensive social media campaign designed by a company that specializes in recruitment of tobacco users for clinical research studies (BUMP Digital Marketing, Toronto, Ontario). We specifically target the desired age range within geographic extents tailored to each clinical center. The collaboration with BUMP has been essential to recover from delayed enrollment due to the SARS-CoV2 pandemic, which coincided with the funding of SOURCE in May 2020. Moreover, their expertise allowed our campaign to evolve with changes in the privacy policy of major social media platforms that essentially precluded targeting of those who use tobacco products. Although this intensive social media approach necessarily is limited to those with some computer literacy, efforts were made to avoid biasing towards higher levels of education, social class, or health literacy.
Retention strategies include continued engagement via phone call, text message, and/or email every 6 months. IRB-approved newsletters are being sent roughly annually. Participants are compensated for the baseline visit (including a reduced rate for screen-failure) and follow-up visit at 3 years. Bronchoscopy substudy participants receive additional compensation.
Baseline and Follow-Up Assessments
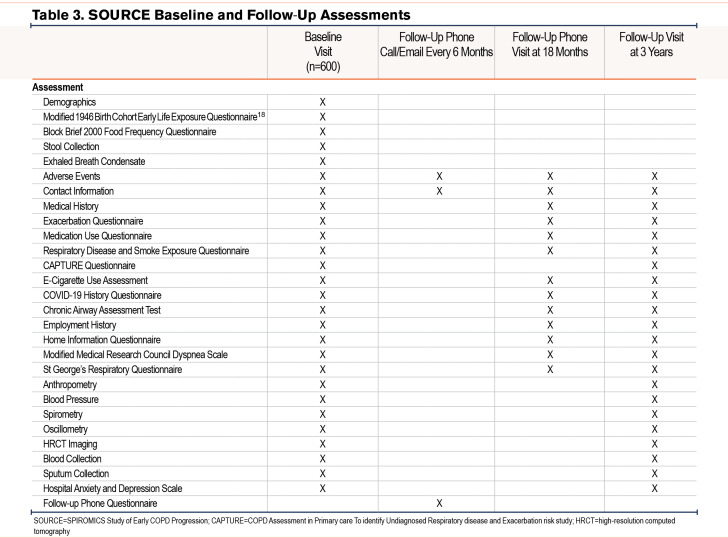
At baseline and follow-up visits, all participants provide detailed information on demographics, exposures, medical history focusing on respiratory symptoms, and medication usage (Table 3). They also undergo biospecimen collection, and the procedures outlined below.
Lung Function Testing
All participants undergo 2 tests of lung function, both pre- and postbronchodilator administration, at baseline and follow-up. The first is respiratory oscillometry, an effort-independent assessment of peripheral, central, or heterogeneous airway abnormalities with reported sensitivity similar or superior to spirometry.57-59 Oscillometry measurements are performed, as described in greater detail in the online supplement, using the Resmon Pro™ (MGC Diagnostics, St. Paul, Minnesota), following technical standards published by the European Respiratory Society.60,61 The second is spirometry, using the Easy One PC spirometer ® (ndd Medical Technologies, Andover, Massachusetts) according to 2019 American Thoracic Society/European Respiratory Society guidelines.62-65 As in SPIROMICS, bronchodilation is induced by 4 puffs each of ipratropium bromide HFA and albuterol sulfate HFA, followed by a 30-minute wait before postbronchodilator testing. The SOURCE protocol was developed using Hankinson predicted values.66 We are aware of recent controversy over use of race- and ethnicity-based adjustments, in which SPIROMICS investigators have taken leading roles.67,68 For consistency during recruitment to the cohort, inclusion criteria were not altered but we have calculated and can make available results using the Global Lung Function Initiative (GLI), “Other,” and “Global” predictions.69 See Appendix B in the online supplement for additional details.
Imaging
Thoracic imaging in SOURCE builds on the extensive experience of the SPIROMICS investigators,70 but uses a novel protocol (developed initially for Precision Intervention for Severe asthma71) designed to minimize radiation exposure in this younger group of participants. At baseline and at the 3-year follow-up, all SOURCE participants undergo 2 series of low-dose lung HRCT scans using iterative reconstruction and dose modulation to adjust for body size on a slice-by-slice basis. Due to dose modulation, radiation dose fluctuates slightly by design between participants of differing builds. Scanners are calibrated using a phantom standard to several U.S. COPD cohorts.72,73 Protocols are specific to scanner make and model, to keep all scanners within comparable levels of noise and image spatial resolution. Hence, target milliampere-seconds and iterative reconstruction parameters differ between participants. We estimate computed tomography (CT) dose index-volume and effective dose for an average-size person with a 30cm scan length. Inspiratory and expiratory scans are performed with breath held at total lung capacity and residual volume, respectively. HRCTs are transmitted to the Imaging Reading Center following a published workflow,70 which includes an automated web portal system.
HRCT generates a very high number of imaging variables, both for whole lung at both total lung capacity and residual volume, and for individual lobes and lung regions. The SPIROMICS investigators have chosen a subset of salient variables considered to be broadly applicable to analyses that use imaging as predictors or outcomes (Supplemental Table 1 in the online supplement). See Appendix C for additional details.
Biological Sample Collection
SOURCE collects a robust set of biospecimens from all participants at both enrollment and at the 3-year follow-up visit (Table 3). To date, the vast majority of participants have agreed to subsequent use by investigators outside SOURCE, including from industry. The SOURCE investigators welcome collaboration and biospecimens can be obtained via approval of an ancillary study.
Blood, Urine, and Nasal Swabs
We collect 7 tubes of blood for future serum, plasma, DNA, and RNA analyses. Additionally, the laboratories at each clinical center perform a complete blood count with differential, capturing variables such as eosinophil counts that are recommended to guide COPD therapy.74,75
Urine is banked for future analyses.76 For participants of childbearing potential, a pregnancy test is performed, and the results received before HRCT imaging.
Because the nasal mucosal transcriptome may reflect pathobiology occurring in the lower respiratory tract,77-82 a nasal swab is collected from the midturbinate region for potential future gene expression studies and microbiome analysis. See Appendix D in the online supplement for details on collection and storage of these sample types.
Exhaled Breath Condensate
Exhaled breath condensate (EBC) is collected only at baseline using the RTube (Respiratory Research, Inc., Austin, Texas), a handheld single-use device. Participants are asked to refrain from eating or drinking for at least one hour before the EBC collection. Consistent with American Thoracic Society/European Respiratory Society recommendations,83 participants wear a nose clip, are cautioned not to touch the chilled sleeve, and are coached to breathe naturally without hyperventilation for approximately 10 minutes, which results in up to 2mL of vapors, aerosols, and moisture collected. Samples will be analyzed as described in the online supplement.
Sputum
See Appendix E in the online supplement for details on sputum induction and sample collection and storage. Briefly, after tongue scraping and gargling in the seated position and without eating for ≥1 hour, participants undergo sputum induction using 0.9%–5% saline (depending on baseline forced expiratory volume in 1 second [FEV1]) via an ultrasonic nebulizer. We monitor drop in FEV1 and use the “whole sputum” method, defined as the raw, unaltered, total expectorated secretions collected. Following removal of raw sample for subsequent mucin and microbiome analysis, at least 75mg of total nonsaliva sputum material must be available for cell-free supernatant recovery and cytospin generation. Cytospins are prepared for differential cell analysis. Supernatant samples are banked for future nucleotide and cytokine analyses, although the absence of protease inhibitors in those samples must be considered. Sputum total mucin concentrations are measured by size exclusion chromatography with multi-angle light scattering analysis and differential refractive index detection (SEC/MALS/dRI) and individual mucin Mucin 5AC and Mucin 5B concentrations are measured by labeled mass spectrometry.84
Stool
Stool samples are banked for future analyses of the gut microbiome as a mediator of respiratory disease. Samples are collected at home using the DNA/RNA Shield Fecal Collection Tube (Zymo Research, Irvine, California), which stabilizes degradation of nucleic acids at room temperature. Participants are given a prepackaged collection kit with barcode label, instruction forms, and mailers permitting return directly to the Microbiome Core via the U.S. Postal Service.
Bronchoscopy Substudy
The bronchoscopy substudy, modeled closely on our previous SPIROMICS substudy,85 will enroll a subgroup of participants at 8 clinical centers. Any ever-smoking participant is eligible (target sample size n=80); agreement to bronchoscopy is an inclusion criterion for never-smokers (n=20). Participants undergo 2 substudy visits. At the first, they are examined by the site bronchoscopist and undergo safety testing (complete blood count, prothrombin/partial thromboplastin time, basic chemistry panel, and pregnancy testing in anyone who could become pregnant). Between 1–12 weeks later, the participant returns for the bronchoscopy procedure.
Details of the SOURCE bronchoscopy substudy will be published elsewhere. Briefly, differences from our published protocol in SPIROMICS I85 include deletion of endobronchial biopsies (not performed in SPIROMICS II or SOURCE), and addition of distal epithelial brushings (performed only at Weill Cornell in SPIROMICS II) at the 7 clinical sites participating in the SOURCE bronchoscopy substudy.86 Epithelial brushes and adherence-purified alveolar macrophages will undergo RNA isolation and sequencing. Using RNA sequence data from the distal and proximal brushes, we will calculate gene signature of type 1, type 2, and type 17 inflammation.51,87 We will establish basal airway cell cultures from distal epithelial brushes, which will be expanded in vitro and cryopreserved for future functional and molecular analyses.86 Specific bronchoscopic brushes will collect airway mucins to compare to sputum results.
From bronchoalveolar lavage (BAL), we will generate cytospin slides for analysis of differential cell counts at the Sputum Slide Reading Center. Viable BAL leukocytes will be analyzed at the Immunophenotyping Core 6 and 24 hours after ex vivo stimulation using agonists recognized by specific host toll-like receptors (TLR4, TLR7/8) to activate innate responses, or by direct T cell activation (anti-CD3 plus anti-CD28). We will reserve supernatants for multiplexed measurement of inflammatory mediators tailored to the cell panel and the stimulus. After staining using panels of phenotypic markers for alveolar macrophages, and subsets of monocytes, dendritic cells, and T cells, and for intracellular production of cytokines (3 per cell type), flow cytometry will be performed and analyzed as described.88,89
Quality Assurance and Control Plan
The CSCC will follow Good Clinical Practice and Quality Guidelines, as standardized and described in the International Council for Harmonisation, for all clinical research study conduct, data management, statistical computing, and biostatistical analysis activities.
To assure data quality and participant privacy, the CSCC follows detailed standard operating procedures. All participant data are entered via electronic case report forms into the Carolina Data Acquisition and Reporting Tool (CDART). This secure, web-based application minimizes the chance of incorrect data entry via extensive cross-checks and automatic calculation of derived variables and total scores from questionnaires. CDART also facilitates completeness of data collection. It has extensive capacity to generate reports with SAS output (including eligibility, windows for follow-up calls, and medication tracking) and to produce mailing labels for sample shipment. Erroneous data entry is minimized via range checks, but when encountered, trigger a protocolized corrective and preventive action process that not only corrects the specific discrepancy but also attempts to prevent recurrence. The corrective action plan is developed and implemented jointly by the data management programmer, software developer, and research staff. Details on the data management plan are provided in the online supplement.
Statistical Analyses
Presentation of results will focus on estimating parameters, such as regression coefficients and correlation coefficients, with corresponding 95% confidence intervals. When reporting p-values, we will give actual values rather than dichotomizing by significance. We will not adjust for data missingness involving few participants (generally <10%), but when the proportion is larger or if potential bias is suspected, we will use multiple imputation by chained equations. Before analyses for a manuscript, additional details, particularly sensitivity analyses and handling of missing data, will be provided in a specific statistical analysis plan.
The primary analysis in Aim 1 will use linear mixed effects models to estimate and perform inference on trajectories of PRMEmph over 3 years, based on baseline PRMfSAD and PRMEmph. In secondary analysis, we will similarly explore the relationship between baseline PRMfSAD and: (1) further increases in PRMfSAD; (2) FEV1 decline over 3 years; (3) changes in oscillometry indices; (4) changes in patient-reported outcomes such as CAAT score40; and (5) respiratory exacerbations.
Aim 1 will also examine the relationship between baseline PRMEmph and further emphysema at 3 years, as supported by preliminary analysis of the subset of participants in SPIROMICS who were in the age range of the SOURCE cohort and who have longitudinal data. Using multivariable linear mixed models, additional analyses will be performed to adjust for age, sex, early life exposures, smoking history, and ongoing smoking status (as a time-dependent covariate where appropriate), baseline FEV1, and concomitant medications. We will perform subgroup analysis of the possible effects of racial and ethnic groups,67 along with status of social determinants of health (SDOH). We will also investigate potential interactions between any of these variables and PRM metrics with the primary outcome, to detect whether any modify relationships between PRM metrics and emphysema development.
The primary analysis of Aim 2 will investigate associations between baseline total sputum mucin concentrations and baseline PRMfSAD. We will perform further analyses using multivariable models to adjust for age, sex, racial/ethnic groups, early life exposures, smoking history and smoking status, concomitant medications, and baseline FEV1% predicted. Potential interactions/effect modification will be explored. We will next investigate the association between baseline mucin concentration and 3-year change in mucin concentrations with progression of PRM-defined HRCT abnormalities and change in FEV1.
Aim 3 is an exploratory analysis designed to generate hypotheses and to provide biospecimens for future ancillary studies. We will correlate baseline PRMfSAD with the interleukin-17 gene signature in airway epithelium51 in both segmental and more distal airways. Secondary analyses will explore correlations of baseline PRMfSAD with: (1) genomic signatures of type 1 and type 2 airway inflammation, (2) functional capacity of ex vivo BAL leukocytes following in vitro stimulation in bulk cultures, and (3) total airway mucin concentration, directly measured from bronchoscopically-collected samples. We will also examine the association of gene expression in the upper (nasal) and lower airways.
Discussion
The SOURCE study is designed with 3 goals: (1) to test whether an imaging biomarker, PRMfSAD, can identify which younger individuals with a smoking history are at-risk for accelerated SAA and hence, early emphysema; (2) to determine whether sputum mucin analyses can noninvasively detect such individuals; and (3) to provide lung-derived biospecimens for mechanistic studies of airway inflammation in early COPD. This cohort will also provide a multitude of biospecimens available for future multiomics analysis (including respiratory and gut microbiome) and correlation with the dense array of physiological, imaging, and exposure data.
Several innovative features of the SOURCE cohort merit mention. Chief, relative to previous large COPD cohorts, is the exclusive recruitment of younger individuals, many without airflow obstruction at enrollment. Due to the high congruence of the SOURCE and SPIROMICS protocols, we can leverage data from those within the appropriate age range when enrolled in SPIROMICS, and extend them by combination with the even younger BEACON and LHS cohorts. Like SPIROMICS, SOURCE focuses on SDOH.67,90 We are aided by the evolution of CT scanners, with improved spatial resolution at significantly lower radiation dose, while still attaining equivalent or improved quantitative measures.70,91-94 This sensitive imaged-based approach enhances the power to identify significant pathways in our biospecimen assessments involving blood, exhaled breath condensate, sputum, and bronchoscopy samples. We will also be able to compare imaging results to noninvasive measurement of small airway function by respiratory oscillometry. The principal limitation of SOURCE is that it is not population-based.
Abbreviations
Abbreviations: BAL=bronchoalveolar lavage; BEACON=British Early COPD Network Cohort; BMI=body mass index; CAAT=Chronic Airway Assessment Test; CanCOLD=Canadian Cohort Obstructive Lung Disease; CAPTURE=COPD Assessment in Primary care To identify Undiagnosed Respiratory disease and Exacerbation risk study; CDART=Carolina Data Acquisition and Reporting Tool; COPD=chronic obstructive pulmonary disease; COPDGene=COPD Genetic Epidemiology study; CSCC=Collaborative Studies Coordinating Center; CT=computed tomography; EBC=exhaled breath condensate; FEV1=forced expiratory volume in 1 second; FTP=file transfer protocol; FVC=forced vital capacity; GINA=Global Initiative for Asthma; GLI=Global Lung Function Initiative; GOLD=Global initiative for chronic Obstructive Lung Disease; HRCT=high-resolution computed tomography; IL-17=interleukin-17; IRB=institutional review board; IT=information technology; LHS=Lung Health Study; MUC5AC=mucin 5AC; MUC5B=mucin 5B; NHLBI=National Heart, Lung and Blood Institute; OSMB=Observational Study Monitoring Board; PRISm=preserved ratio-impaired spirometry; PRM=parametric response mapping; PRMEmph=emphysema as a percentage of total lung vcoxels, determined PRM; PRMfSAD=functional small airways disease as a percentage of total lung voxels, by PRM; SAA=small airway abnormality; SDOH=social determinants of health; SEC/MALS/dRI=size exclusion chromatography with multi-angle light scattering analysis and differential refractive index detection; SOURCE=SPIROMICS Study of Early COPD Progression; SPIROMICS=Subpopulations and Intermediate Outcome Measures in COPD Study; TEPS=tobacco exposure and preserved spirometry; TR=trajectory; UNC=University of North Carolina at Chapel Hill
Funding Statement
SOURCE is supported by a National Institutes of Health’s NHLBI grant, R01 HL144718, supplemented by contributions made through the COPD Foundation from Amgen; AstraZeneca/MedImmune; Bayer; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc.; Chiesi Farmaceutici S.p.A.; Forest Research Institute, Inc.; Genentech; GlaxoSmithKline; Grifols Therapeutics, Inc.; Ikaria, Inc.; MGC Diagnostics; Novartis Pharmaceuticals Corporation; Nycomed GmbH; Polarean; ProterixBio; Regeneron Pharmaceuticals, Inc.; Sanofi; Sunovion; Takeda Pharmaceutical Company; Theravance Biopharma; and Mylan/Viatris.
References
- 1.Vogelmeier CF,Criner GJ,Martinez FJ,et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report. GOLD executive summary. Am J Respir Crit Care Med. 2017;195(5):557-582. doi: https://doi.org/10.1164/rccm.201701-0218PP [DOI] [PubMed] [Google Scholar]
- 2.Calverley PMA,Anderson JA,Celli B,et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775-789. doi: https://doi.org/10.1056/NEJMoa063070 [DOI] [PubMed] [Google Scholar]
- 3.Tashkin DP,Celli B,Senn S,et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543-1554. doi: https://doi.org/10.1056/NEJMoa0805800 [DOI] [PubMed] [Google Scholar]
- 4.Han MK,Martinez CH,Au DH,et al. Meeting the challenge of COPD care delivery in the USA: a multiprovider perspective. Lancet Respir Med. 2016;4(6):473-526. doi: https://doi.org/10.1016/S2213-2600(16)00094-1 [DOI] [PubMed] [Google Scholar]
- 5.Regan EA,Hokanson JE,Murphy JR,et al. Genetic epidemiology of COPD (COPDGene) study design. COPD. 2010;7(1):32-43. doi: https://doi.org/10.3109/15412550903499522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Couper D,LaVange LM,Han M,et al. Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax. 2014;69(5):492-495. doi: https://doi.org/10.1136/thoraxjnl-2013-203897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bourbeau J,Tan WC,Benedetti A,et al. Canadian Cohort Obstructive Lung Disease (CanCOLD): fulfilling the need for longitudinal observational studies in COPD. COPD. 2014;11(2):125-132. doi: https://doi.org/10.3109/15412555.2012.665520 [DOI] [PubMed] [Google Scholar]
- 8.Lange P,Celli B,Agusti A,et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111-122. doi: https://doi.org/10.1056/NEJMoa1411532 [DOI] [PubMed] [Google Scholar]
- 9.Ritchie AI,Martinez FJ. The challenges of defining early chronic obstructive pulmonary disease in the general population. Am J Respir Crit Care Med. 2021;203(10):1209-1210. doi: https://doi.org/10.1164/rccm.202011-4176ED [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez FJ,Agusti A,Celli BR,et al. Treatment trials in young patients with chronic obstructive pulmonary disease and pre-chronic obstructive pulmonary disease patients: time to move forward. Am J Respir Crit Care Med. 2022;205(3):275-287. doi: https://doi.org/10.1164/rccm.202107-1663SO [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez FJ,Han MK,Allinson JP,et al. At the root: defining and halting progression of early chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;197(12):1540-1551. doi: https://doi.org/10.1164/rccm.201710-2028PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su X,Gu H,Li F,Shi D,Wang Z. Global, regional, and national burden of COPD attributable to occupational particulate matter, gases, and fumes, 1990-2019: findings from the Global Burden of Disease Study 2019. Int J Chron Obstruct Pulmon Dis. 2023;18:2971-2983. doi: https://doi.org/10.2147/COPD.S436879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auerbach O,Stout AP,Hammond EC,Garfinkel L. Changes in bronchial epithelium in relation to cigarette smoking and in relation to lung cancer. N Engl J Med. 1961;265:253-267. doi: https://doi.org/10.1056/NEJM196108102650601 [DOI] [PubMed] [Google Scholar]
- 14.Anderson JA,Dunnill MS,Ryder RC. Dependence of the incidence of emphysema on smoking history, age, and sex. Thorax. 1972;27(5):547-551. doi: https://doi.org/10.1136/thx.27.5.547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson DO,Ferris BG Jr.. Role of tobacco smoking in the causation of chronic respiratory disease. N Engl J Med. 1962;267:787-794. doi: https://doi.org/10.1056/NEJM196210182671601 [DOI] [PubMed] [Google Scholar]
- 16.de Marco R,Accordini S,Cerveri I,et al. Incidence of chronic obstructive pulmonary disease in a cohort of young adults according to the presence of chronic cough and phlegm. Am J Respir Crit Care Med. 2007;175(1):32-39. doi: https://doi.org/10.1164/rccm.200603-381OC [DOI] [PubMed] [Google Scholar]
- 17.Guerra S,Sherrill DL,Venker C,Ceccato CM,Halonen M,Martinez FD. Chronic bronchitis before age 50 years predicts incident airflow limitation and mortality risk. Thorax. 2009;64(10):894-900. doi: https://doi.org/10.1136/thx.2008.110619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allinson JP,Hardy R,Donaldson GC,Shaheen SO,Kuh D,Wedzicha JA. The presence of chronic mucus hypersecretion across adult life in relation to chronic obstructive pulmonary disease development. Am J Respir Crit Care Med. 2016;193(6):662-672. doi: https://doi.org/10.1164/rccm.201511-2210OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allinson JP,Hardy R,Donaldson GC,Shaheen SO,Kuh D,Wedzicha JA. Combined impact of smoking and early-life exposures on adult lung function trajectories. Am J Respir Crit Care Med. 2017;196(8):1021-1030. doi: https://doi.org/10.1164/rccm.201703-0506OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen SJ,Wang CH,Li B,et al. Risk factors for FEV1 decline in mild COPD and high-risk populations. Int J Chron Obstruct Pulmon Dis. 2017;12:435-442. doi: https://doi.org/10.2147/COPD.S118106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan DJ,Lodge CJ,Walters EH,et al. Can we use lung function thresholds and respiratory symptoms to identify pre-chronic obstructive pulmonary disease? A prospective, population-based cohort study. Am J Respir Crit Care Med. 2024;209(12):1431-1440. doi: https://doi.org/10.1164/rccm.202212-2330OC [DOI] [PubMed] [Google Scholar]
- 22.Auerbach O,Gere JB,Forman JB,et al. Changes in the bronchial epithelium in relation to smoking and cancer of the lung; a report of progress. N Engl J Med. 1957;256(3):97-104. doi: https://doi.org/10.1056/NEJM195701172560301 [DOI] [PubMed] [Google Scholar]
- 23.Leopold JG,Gough J. The centrilobular form of hypertrophic emphysema and its relation to chronic bronchitis. Thorax. 1957;12(3):219-235. doi: https://doi.org/10.1136/thx.12.3.219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koo HK,Vasilescu DM,Booth S,et al. Small airways disease in mild and moderate chronic obstructive pulmonary disease: a cross-sectional study. Lancet Respir Med. 2018;6(8):591-602. doi: https://doi.org/10.1016/S2213-2600(18)30196-6 [DOI] [PubMed] [Google Scholar]
- 25.Galbàn CJ,Han MK,Boes JL,et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711-1715. doi: https://doi.org/10.1038/nm.2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Labaki WW,Gu T,Murray S,et al. Voxel-wise longitudinal parametric response mapping analysis of chest computed tomography in smokers. Acad Radiol. 2019;26(3):306-312. doi: https://doi.org/10.1016/j.acra.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vasilescu DM,Martinez FJ,Marchetti D,et al. Noninvasive imaging biomarker identifies small airway damage in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2019;200(5):575-581. doi: https://doi.org/10.1164/rccm.201811-2083OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haghighi B,Choi S,Choi J,et al. Imaging-based clusters in former smokers of the COPD cohort associate with clinical characteristics: the SubPopulations and intermediate outcome measures in COPD study (SPIROMICS). Respir Res. 2019;20:153. doi: https://doi.org/10.1186/s12931-019-1121-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed Hoesein FAA,de Jong PA,Lammers JWJ,et al. Airway wall thickness associated with forced expiratory volume in 1 second decline and development of airflow limitation. Eur Respir J. 2015;45(3):644-651. doi: https://doi.org/10.1183/09031936.00020714 [DOI] [PubMed] [Google Scholar]
- 30.Kirby M,Tanabe N,Tan WC,et al. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression. Findings from a population-based study. Am J Respir Crit Care Med. 2018;197(1):56-65. doi: https://doi.org/10.1164/rccm.201704-0692OC [DOI] [PubMed] [Google Scholar]
- 31.Smith BM,Kirby M,Hoffman EA,et al. Association of dysanapsis with chronic obstructive pulmonary disease among older adults. JAMA. 2020;323(22):2268-2280. doi: https://doi.org/10.1001/jama.2020.6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis. 1980;121(2):339-342. https://www.atsjournals.org/doi/abs/10.1164/arrd.1980.121.2.339?journalCode=arrd [DOI] [PubMed] [Google Scholar]
- 33.Vameghestahbanati M,Hiura GT,Barr RG,Sieren JC,Smith BM,Hoffman EA. CT-assessed dysanapsis and airflow obstruction in early and mid adulthood. Chest. 2022;161(2):389-391. doi: https://doi.org/10.1016/j.chest.2021.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Okajima Y,Come CE,Nardelli P,et al. Luminal plugging on chest ct scan: association with lung function, quality of life, and COPD clinical phenotypes. Chest. 2020;158(1):121-130. doi: https://doi.org/10.1016/j.chest.2019.12.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dunican EM,Elicker BM,Henry T,et al. Mucus plugs and emphysema in the pathophysiology of airflow obstruction and hypoxemia in smokers. Am J Respir Crit Care Med. 2021;203(8):957-968. doi: https://doi.org/10.1164/rccm.202006-2248OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz AA,Orejas JL,Grumley S,et al. Airway-occluding mucus plugs and mortality in patients with chronic obstructive pulmonary disease. JAMA. 2023;329(21):1832-1839. doi: https://doi.org/10.1001/jama.2023.2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McAllister DA,Ahmed FS,Austin JHM,et al. Emphysema predicts hospitalisation and incident airflow obstruction among older smokers: a prospective cohort study. PLoS One. 2014;9(4):e93221. doi: https://doi.org/10.1371/journal.pone.0093221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vestbo J,Edwards LD,Scanlon PD,et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184-1192. doi: https://doi.org/10.1056/NEJMoa1105482 [DOI] [PubMed] [Google Scholar]
- 39.Koo HK,Jin KN,Kim DK,Chung HS,Lee C. Association of incidental emphysema with annual lung function decline and future development of airflow limitation. Int J Chron Obstruct Pulmon Dis. 2016;11(1):161-166. doi: https://doi.org/10.2147/COPD.S96809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tomaszewski EL,Atkinson MJ,Janson C,et al. Chronic Airways Assessment Test: psychometric properties in patients with asthma and/or COPD. Respir Res. 2023;24:106. doi: https://doi.org/10.1186/s12931-023-02394-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Woodruff PG,Barr RG,Bleecker E,et al. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811-1821. doi: https://doi.org/10.1056/NEJMoa1505971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McKleroy W,Shing T,Anderson WH,et al. Longitudinal follow-up of participants with tobacco exposure and preserved spirometry. JAMA. 2023;330(5):442-453. doi: https://doi.org/10.1001/jama.2023.11676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Esther CR Jr.,Hill DB,Button B,et al. Sialic acid-to-urea ratio as a measure of airway surface hydration. Am J Physiol Lung Cell Mol Physiol. 2017;312(3):L398-L404. doi: https://doi.org/10.1152/ajplung.00398.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Button B,Anderson WH,Boucher RC. Mucus hyperconcentration as a unifying aspect of the chronic bronchitic phenotype. Ann Am Thorac Soc. 2016;13(Suppl 2):S156-S162. https://www.atsjournals.org/doi/10.1513/AnnalsATS.201507-455KV?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kesimer M,Ford AA,Ceppe A,et al. Airway mucin concentration as a marker of chronic bronchitis. N Engl J Med. 2017;377(10):911-922. doi: https://doi.org/10.1056/NEJMoa1701632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kesimer M,Smith BM,Ceppe A,et al. Mucin concentrations and peripheral airways obstruction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(11):1453-1456. doi: https://doi.org/10.1164/rccm.201806-1016LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Esther CR Jr.,O'Neal WK,Anderson WH,et al. Identification of sputum biomarkers predictive of pulmonary exacerbations in COPD. Chest. 2022;161(5):1239-1249. doi: https://doi.org/10.1016/j.chest.2021.10.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singanayagam A,Footitt J,Marczynski M,et al. Airway mucins promote immunopathology in virus-exacerbated chronic obstructive pulmonary disease. J Clin Invest. 2022;132(8):e120901. doi: https://doi.org/10.1172/JCI120901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mikami Y,Grubb BR,Rogers TD,et al. Chronic airway epithelial hypoxia exacerbates injury in muco-obstructive lung disease through mucus hyperconcentration. Sci Transl Med. 2023;15(699):eabo7728. doi: https://doi.org/10.1126/scitranslmed.abo7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vanaudenaerde BM,Verleden SE,Vos R,et al. Innate and adaptive interleukin-17-producing lymphocytes in chronic inflammatory lung disorders. Am J Respir Crit Care Med. 2011;183(8):977-986. doi: https://doi.org/10.1164/rccm.201007-1196PP [DOI] [PubMed] [Google Scholar]
- 51.Christenson SA,van den Berge M,Faiz A,et al. An airway epithelial IL-17A response signature identifies a steroid-unresponsive COPD patient subgroup. J Clin Invest. 2019;129(1):169-181. doi: https://doi.org/10.1172/JCI121087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ritchie AI,Donaldson GC,Hoffman EA,et al. Structural predictors of lung function decline in young smokers with normal spirometry. Am J Respir Crit Care Med. 2024;209(10):1208-1218. doi: https://doi.org/10.1164/rccm.202307-1203OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.American Lung Association (ALA). Lung health cohort study. ALA website. Accessed December 2023. https://www.lung.org/research/lung-health-cohort-study [Google Scholar]
- 54.Han MK,Ye W,Wang D,et al. Bronchodilators in tobacco-exposed persons with symptoms and preserved lung function. N Engl J Med. 2022;387(13):1173-1184. doi: https://doi.org/10.1056/NEJMoa2204752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han MK,Ye W,Kim DY,Woodruff P. Design of the redefining therapy in early COPD study. Chronic Obstr Pulm Dis. 2020;7(4):382-389. doi: https://doi.org/10.15326/jcopdf.7.4.2020.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.SOURCE. SOURCE: SPIROMICS Study of Early COPD Progression. SOURCE website. Accessed December 2023. https://sourcestudy.net/source/ [Google Scholar]
- 57.van Noord JA,Wellens W,Clarysse I,Cauberghs M,Van de Woestijne KP,Demedts M. Total respiratory resistance and reactance in patients with upper airway obstruction. Chest. 1987;92(3):475-480. doi: https://doi.org/10.1378/chest.92.3.475 [DOI] [PubMed] [Google Scholar]
- 58.Zerah F,Lorino AM,Lorino H,Harf A,Macquin-Mavier I. Forced oscillation technique vs spirometry to assess bronchodilatation in patients with asthma and COPD. Chest. 1995;108(1):41-47. doi: https://doi.org/10.1378/chest.108.1.41 [DOI] [PubMed] [Google Scholar]
- 59.Dias Faria AC,de Costa AA,Lopes AJ,Jansen JM,de Melo PL. Forced oscillation technique in the detection of smoking-induced respiratory alterations: diagnostic accuracy and comparison with spirometry. Clinics (Sao Paulo). 2010;65(12):1295-1304. doi: https://doi.org/10.1590/S1807-59322010001200012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oostveen E,MacLeod D,Lorino H,et al. The forced oscillation technique in clinical practice: methodology, recommendations and future developments. Eur Respir J. 2003;22(6):1026-1041. doi: https://doi.org/10.1183/09031936.03.00089403 [DOI] [PubMed] [Google Scholar]
- 61.King GG,Bates J,Berger KI,et al. Technical standards for respiratory oscillometry. Eur Respir J. 2020;55(2):1900753. doi: https://doi.org/10.1183/13993003.00753-2019 [DOI] [PubMed] [Google Scholar]
- 62.Miller MR,Crapo R,Hankinson J,et al. General considerations for lung function testing. Eur Respir J. 2005;26(1):153-161. doi: https://doi.org/10.1183/09031936.05.00034505 [DOI] [PubMed] [Google Scholar]
- 63.Miller MR,Hankinson J,Brusasco V,et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319-338. doi: https://doi.org/10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 64.Pellegrino R,Viegi G,Brusasco V,et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948-968. doi: https://doi.org/10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 65.Wanger J,Clausen JL,Coates A,et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511-522. doi: https://doi.org/10.1183/09031936.05.00035005 [DOI] [PubMed] [Google Scholar]
- 66.Hankinson JL,Odencrantz JR,Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179-187. doi: https://doi.org/10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 67.Baugh AD,Shiboski S,Hansel NN,et al. Reconsidering the utility of race-specific lung function prediction equations. Am J Respir Crit Care Med. 2022;205(7):819-829. doi: https://doi.org/10.1164/rccm.202105-1246OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhakta NR,Bime C,Kaminsky DA,et al. Race and ethnicity in pulmonary function test interpretation: an official American Thoracic Society statement. Am J Respir Crit Care Med. 2023;207(8):978-995. doi: https://doi.org/10.1164/rccm.202302-0310ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bowerman C,Bhakta NR,Brazzale D,et al. A race-neutral approach to the interpretation of lung function measurements. Am J Respir Crit Care Med. 2023;207(6):768-774. doi: https://doi.org/10.1164/rccm.202205-0963OC [DOI] [PubMed] [Google Scholar]
- 70.Sieren JP,Newell JD Jr.,Barr RG,et al. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794-806. doi: https://doi.org/10.1164/rccm.201506-1208PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Georas SN,Wright RJ,Ivanova A,et al. The Precision Intervention for Severe and/or Exacerbation-Prone (PrecISE) asthma network: an overview of network organization, procedures, and interventions. [published correction appears in J Allergy Clin Immunol, 2022, 2, 150, 491]. J Allergy Clin Immunol. 2022;149(2):488-516. e9.doi: https://doi.org/10.1016/j.jaci.2021.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rodriguez A,Ranallo FN,Judy PF,Gierada DS,Fain SB. CT reconstruction techniques for improved accuracy of lung CT airway measurement. Med Phys. 2014;41(11):111911. doi: https://doi.org/10.1118/1.4898098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo J,Wang C,Chan KS,et al. A controlled statistical study to assess measurement variability as a function of test object position and configuration for automated surveillance in a multicenter longitudinal COPD study (SPIROMICS). Med Phys. 2016;43(5):2598-2610. doi: https://doi.org/10.1118/1.4947303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Singh D,Agusti A,Anzueto A,et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5):1900164. doi: https://doi.org/10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 75.Singh D,Bafadhel M,Brightling CE,et al. Blood eosinophil counts in clinical trials for chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2020;202(5):660-671. doi: https://doi.org/10.1164/rccm.201912-2384PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang WZ,Rice MC,Hoffman KL,et al. Association of urine mitochondrial DNA with clinical measures of COPD in the SPIROMICS cohort. JCI Insight. 2020;5(3):e133984. doi: https://doi.org/10.1172/jci.insight.133984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sridhar S,Schembri F,Zeskind J,et al. Smoking-induced gene expression changes in the bronchial airway are reflected in nasal and buccal epithelium. BMC Genomics. 2008;9:259. doi: https://doi.org/10.1186/1471-2164-9-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang X,Sebastiani P,Liu G,et al. Similarities and differences between smoking-related gene expression in nasal and bronchial epithelium. Physiol Genomics. 2010;41(1):1-8. doi: https://doi.org/10.1152/physiolgenomics.00167.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Imkamp K,Berg M,Vermeulen CJ,et al. Nasal epithelium as a proxy for bronchial epithelium for smoking-induced gene expression and expression Quantitative Trait Loci. J Allergy Clin Immunol. 2018;142(1):314-317. e15.doi: https://doi.org/10.1016/j.jaci.2018.01.047 [DOI] [PubMed] [Google Scholar]
- 80.Hijazi K,Malyszko B,Steiling K,et al. Tobacco-related alterations in airway gene expression are rapidly reversed within weeks following smoking-cessation. Sci Rep. 2019;9:6978. doi: https://doi.org/10.1038/s41598-019-43295-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ghosh B,Park B,Bhowmik D,et al. Strong correlation between air-liquid interface cultures and in vivo transcriptomics of nasal brush biopsy. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L1056-L1062. doi: https://doi.org/10.1152/ajplung.00050.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qi C,Jiang Y,Yang IV,et al. Nasal DNA methylation profiling of asthma and rhinitis. J Allergy Clin Immunol. 2020;145(6):1655-1663. doi: https://doi.org/10.1016/j.jaci.2019.12.911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horváth I,Hunt J,Barnes PJ,et al. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26(3):523-548. doi: https://doi.org/10.1183/09031936.05.00029705 [DOI] [PubMed] [Google Scholar]
- 84.Radicioni G,Ceppe A,Ford AA,et al. Airway mucin MUC5AC and MUC5B concentrations and the initiation and progression of chronic obstructive pulmonary disease: an analysis of the SPIROMICS cohort. Lancet Respir Med. 2021;9(11):1241-1254. doi: https://doi.org/10.1016/S2213-2600(21)00079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wells JM,Arenberg DA,Barjaktarevic I,et al. Safety and tolerability of comprehensive research bronchoscopy in COPD. Results from the SPIROMICS bronchoscopy substudy. Ann Am Thorac Soc. 2019;16(4):439-446. doi: https://doi.org/10.1513/AnnalsATS.201807-441OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zuo WL,Shenoy SA,Li S,et al. Ontogeny and biology of human small airway epithelial club cells. Am J Respir Crit Care Med. 2018;198(11):1375-1388. doi: https://doi.org/10.1164/rccm.201710-2107OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Christenson SA,Steiling K,van den Berge M,et al. Asthma-COPD overlap. Clinical relevance of genomic signatures of type 2 inflammation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(7):758-766. doi: https://doi.org/10.1164/rccm.201408-1458OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Freeman CM,Crudgington S,Stolberg VR,et al. Design of a multi-center immunophenotyping analysis of peripheral blood, sputum and bronchoalveolar lavage fluid in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS). J Transl Med. 2015;13:19. doi: https://doi.org/10.1186/s12967-014-0374-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Martinez CH,Li SX,Hirzel AJ,et al. Alveolar eosinophilia in current smokers with chronic obstructive pulmonary disease in the SPIROMICS cohort. J Allergy Clin Immunol. 2018;141(1):429-432. doi: https://doi.org/10.1016/j.jaci.2017.07.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ejike CO,Dransfield MT,Hansel NN,et al. Chronic obstructive pulmonary disease in America's Black population. Am J Respir Crit Care Med. 2019;200(4):423-430. doi: https://doi.org/10.1164/rccm.201810-1909PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Newell JD Jr.,Fuld MK,Allmendinger T,et al. Very low-dose (0.15 mGy) chest CT protocols using the COPDGene 2 test object and a third-generation dual-source CT scanner with corresponding third-generation iterative reconstruction software. Invest Radiol. 2015;50(1):40-45. doi: https://doi.org/10.1097/RLI.0000000000000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hammond E,Chan KS,Ames JC,et al. Impact of advanced detector technology and iterative reconstruction on low-dose quantitative assessment of lung computed tomography density in a biological lung model. Med Phys. 2018;45(8):3657-3670. doi: https://doi.org/10.1002/mp.13057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hammond E,Sloan C,Newell JD Jr.,et al. Comparison of low- and ultralow-dose computed tomography protocols for quantitative lung and airway assessment. Med Phys. 2017;44(9):4747-4757. doi: https://doi.org/10.1002/mp.12436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sieren JP,Newell JD Jr.,Judy PF,et al. Reference standard and statistical model for intersite and temporal comparisons of CT attenuation in a multicenter quantitative lung study. Med Phys. 2012;39(9):5757-5767. doi: https://doi.org/10.1118/1.4747342 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplemental material.