Abstract
Background and aim
The COVID-19 pandemic has globally impacted all sectors. Early vaccine development was crucial to curb the spread of the virus. However, concerns about vaccine safety and side effects have led to hesitancy. This study aims to examine and compare side effects associated with Pfizer/BioNTech, Oxford/AstraZeneca, and Sinopharm vaccines in Iraqi Kurdistan.
Materials and methods
A population-based study was conducted in the Kurdistan Region of Iraq from September 2022 to April 2023, involving 1,340 participants recruited through face-to-face interviews and online forms. The questionnaire collected demographic data and information on COVID-19 infection and vaccination status.
Results
Among the participants, 52.76% were females, with a mean age of 29.21 years (±13.09 SD). Of these, 67.84% received the Pfizer/BioNTech vaccine, and 60.9% had a prior COVID-19 infection. About 76.94% experienced post-vaccination side effects, lasting an average of 2.8 days (±1.92 SD). Notably, 60% reported no or mild side effects. Common side effects across all vaccines included injection site pain, fever, headache, and fatigue. Side effects were more frequent after the first dose and were highest with Oxford/AstraZeneca, followed by Pfizer/BioNTech and Sinopharm (p = 0.001). Higher rates of side effects were observed in participants aged 36-60, females, married individuals, those with chronic conditions, previously infected individuals, and those who contracted COVID-19 post-vaccination (p = 0.001).
Conclusions
This study reveals that most of the participants experienced either no side effects or only mild reactions following vaccination, with none of the side effects being serious. These findings are expected to boost public confidence and increase vaccine uptake, especially with booster doses now available.
Keywords: covid-19 pandemic, covid-19 vaccines, infectious disease control, kurdistan region of iraq, side effects
Introduction
The COVID-19 pandemic spread rapidly across the globe, placing immense strain on healthcare systems worldwide, including those in the Kurdistan Region of Iraq [1,2]. In response to the escalating crisis, governments around the world implemented a range of preventive measures to curb the transmission of the virus [3,4]. Despite these efforts, the virus continued to proliferate, manifesting in diverse clinical presentations across different regions, reflecting global patterns [5,6]. Achieving herd immunity, a crucial goal for controlling and eventually ending the pandemic hinges on widespread and effective vaccination. In a remarkable display of global scientific collaboration, multiple COVID-19 vaccines were developed and approved in a relatively short period [7,8]. The rapid distribution and uptake of these vaccines became a critical focus globally, as countries sought to decrease the infection rates and restore normalcy.
The World Health Organization (WHO) has granted emergency use authorization to several COVID-19 vaccines. However, only three vaccines were approved and administered in the Kurdistan Region of Iraq including Pfizer/BioNTech mRNA-based vaccine (BNT162b2), Oxford/AstraZeneca vaccine (ChAdOx1 nCoV-19), and Sinopharm (BBIBP-CorV) [9]. Unfortunately, numerous rumors and misconceptions regarding various aspects of the pandemic and vaccine safety have alarmingly spread among the general population [10]. Therefore, several concerns about the vaccine's safety and side effects have been raised and were the main obstacles associated with unwillingness to accept the COVID-19 vaccine [11].
Although the COVID-19 vaccine represents the primary hope for preventing the spread of the infection, it is important to note that no vaccine is entirely devoid of side effects. Individuals have reported a spectrum of responses to vaccines, ranging from minimal to severe adverse effects. Regardless of any side effects, vaccination provides immunity against COVID-19 disease [12]. The potential side effects following vaccination significantly contribute to the population's vaccine hesitancy. These concerns can be addressed by enhancing public awareness of vaccine safety and efficacy, including the disclosure of potential adverse effects [13,14].
This study sought to assess the factors influencing the severity and occurrence of post-vaccination side effects among various demographic groups in Iraqi Kurdistan. Specifically, it examined the impact of pre-existing health conditions, prior COVID-19 infections, and the type of vaccine administered on the development of side effects. By expanding the analysis to include a wider range of demographic and clinical variables, this research aimed to overcome the limitations of earlier studies and offer targeted public health recommendations to improve vaccine safety and acceptance in the region.
Materials and methods
Study design and participants
A population-based study took place in Duhok province, Kurdistan Region of Iraq, from September 2022 to April 2023. Data acquisition occurred through two methods: face-to-face interviews conducted by one of the authors in public places, hospitals, and universities or electronically via the Google Forms platform (Google LLC, Mountain View, California, USA).
The study’s sample size was determined using an online calculator (http://www.raosoft.com/samplesize.html) with a confidence interval of 99%, a 5% margin of error, a population size of 1.5 million, and a response distribution of 50%. The calculator recommended a minimum sample size of 664 for the survey. However, the study enrolled a total of 1340 individuals, which represents twice the recommended sample size. The sample size was increased to strengthen the statistical analysis and ensure the reliability of the study’s results.
Study questionnaire
The study questionnaire was designed based on a previously validated questionnaire with slight modifications made by the author team to align with the objectives of the study [15]. This questionnaire encompassed 21 items that were categorized into two distinct sections: The initial section consisted of 10 items designed to collect data regarding the demographical characteristics of the participants. These included age, gender, marital status, occupation, adherence to a healthy lifestyle, smoking habits, any history of chronic illnesses, any type of allergy, blood group, and the sources from which participants obtained information about COVID-19 vaccines.
The second section of the questionnaire consisted of 11 items to gather information about participants' COVID-19 infection and vaccination status. Vaccination status questions included the type of vaccine received, number of doses, development of post-vaccination side effects, duration and severity of the side effects, the specific dose at which side effects occurred, and whether they had contracted COVID-19 after vaccination. Post-vaccination side effects were further categorized into two groups: local and systemic side effects. The local side effects included pain, edema, itching, redness, and hotness at the injection site. Systemic side effects included a list of symptoms such as headache, fatigue, fever, chills, joint pain, muscle pain, diarrhea, nausea, and hair loss. In addition, participants had the opportunity to report any other symptoms not mentioned in the provided list. To assess the severity of side effects, a Likert scale from 1 to 10 was used, with scores of 1 to 3 considered mild, 4 to 7 classified as moderate, and 8 to 10 categorized as severe. The complete study questionnaire is provided in the appendix.
Participant eligibility criteria
The eligibility criteria encompassed individuals aged 18 years or older, living in Duhok province, who had received at least one dose of COVID-19 vaccines distributed by the Iraqi Kurdistan Ministry of Health, and who consented to participate in the study.
Ethical approval
The final study protocol and design received formal approval from the Ethics and Scientific Committee of the College of Medicine, University of Zakho in Kurdistan Region, Iraq on July 5, 2022, with reference number (JUL2022/E04). Written informed consent was obtained from all individuals.
Statistical analysis
GraphPad Prism version 8.0 (GraphPad Software, Boston, Massachusetts USA) was used to perform statistical analysis. Descriptive statistics were calculated as frequencies and percentages. The mean and standard deviation were calculated for the numerical variables. The association between demographic characteristics and the development and severity of post-vaccination side effects was studied by the Chi-square test. Logistic regression analysis was employed to investigate factors associated with the likelihood of developing post-vaccination side effects. A p-value less than 0.05 was considered statistically significant.
Results
Demographic characteristics and health profile
The study recruited a total of 1340 participants who received COVID-19 vaccine. The mean age of the participants was (29.21 ± 13.09) years old. Females accounted for 52.76% of the study participants, and roughly two-thirds of the study participants were single. Half of the study participants were students, and 57.76% maintained a healthy lifestyle. One-fifth of the participants reported being smokers, and only 15.52% had chronic health problems. Approximately one-fifth of the participants reported having some form of allergy. Blood groups O and A were the most prevalent among the study participants. Table 1 summarizes demographic and health characteristics of the study respondents.
Table 1. Demographic characteristics of the study’s participants.
SD: standard deviation
| Variables | Frequency | % |
| Age (year) | ||
| 18-25 | 811 | 60.52 |
| 26-35 | 224 | 16.72 |
| 36-50 | 171 | 12.76 |
| 51-60 | 77 | 5.75 |
| >60 | 57 | 4.25 |
| Mean ± SD | 29.21±13.09 | |
| Gender | ||
| Male | 633 | 47.24 |
| Female | 707 | 52.76 |
| Marital status | ||
| Single | 899 | 67.09 |
| Married | 441 | 32.91 |
| Occupation | ||
| Healthcare worker | 197 | 14.7 |
| Non-healthcare worker | 427 | 31.87 |
| Student | 716 | 53.43 |
| Do you maintain a healthy lifestyle? | ||
| Yes | 774 | 57.76 |
| No | 566 | 42.24 |
| Smoking | ||
| Yes | 300 | 22.39 |
| No | 1040 | 77.61 |
| Chronic health problems | ||
| Yes | 208 | 15.52 |
| No | 1132 | 84.48 |
| Had any type of allergy | ||
| Yes | 290 | 21.64 |
| No | 1050 | 78.36 |
| Blood group | ||
| A | 463 | 34.55 |
| B | 257 | 18.18 |
| O | 485 | 36.19 |
| AB | 135 | 10.07 |
Source of knowledge about COVID-19 vaccines
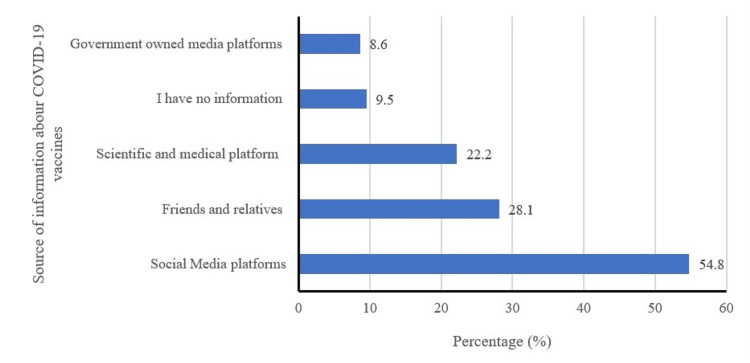
Figure 1 illustrates the primary sources of information on COVID-19 vaccines. Interestingly, it was observed that over half of the participants obtained information from social media platforms, while approximately one-third acquired information from friends and relatives. By contrast, only 22.2% accessed information from scientific and medical platforms, and a mere (8.6%) relied on government-owned media platforms.
Figure 1. Source of information about COVID-19 vaccines (can choose multiple answers) .
COVID-19 infection and vaccination data
Table 2 highlights data regarding participants' past COVID-19 infections and vaccination data. Three-fifths of the study participants had experienced a prior COVID-19 infection. Two-thirds of the participants received the Pfizer/BioNTech vaccine, and a significant majority (76.87%) completed the two-dose vaccination regimen. Notably, around one-third (32.4%) reported contracting COVID-19 after vaccination, with a majority indicating that the infection occurred following the second dose of vaccination. The majority of the participants, accounting for 76.94% experienced side effects following administration of the COVID-19 vaccine with the mean duration of the side effects (2.8 ± 1.92 days). Approximately one-third of the participants encountered mild symptoms after vaccination, while 11.12% reported severe symptoms.
Table 2. History of COVID-19 infection and vaccination data among vaccinated population.
SD: standard deviation
| COVID-19 infection and vaccination data | Frequency | % |
| Previously had a positive test for COVID-19 | ||
| Yes | 816 | 60.9 |
| No | 524 | 39.1 |
| Type of vaccine | ||
| Pfizer/BioNTech | 909 | 67.84 |
| Oxford/AstraZeneca | 261 | 19.48 |
| Sinopharm | 170 | 12.69 |
| Number of doses of COVID-19 vaccine received | ||
| One dose | 181 | 13.51 |
| Two doses | 1030 | 76.87 |
| Three doses | 129 | 9.63 |
| Infected with COVID-19 after vaccination | ||
| Yes | 434 | 32.39 |
| No | 906 | 67.61 |
| Infected with COVID-19 after which dose | ||
| 1st dose | 142 | 32.72 |
| 2nd dose | 255 | 58.76 |
| 3rd dose | 37 | 8.52 |
| Post-vaccination side effects | ||
| Yes | 1031 | 76.94 |
| No | 309 | 23.06 |
| Duration of symptoms after vaccination (Day Mean±SD) | 2.8±1.92 | |
| Severity of side effects after vaccination | ||
| Mild | 493 | 36.79 |
| Moderate | 389 | 29.03 |
| Severe | 149 | 11.12 |
| No symptoms | 309 | 23.06 |
Comparison of post-vaccination side effects based on the type of vaccine
Table 3 provides a summary of post-vaccination side effects associated with various COVID-19 vaccines. When comparing these side effects based on the vaccine type received, it was observed that pain and hotness at the injection site were the most common local reactions. These were more prevalent after the first dose of the vaccine and in participants who received Oxford/AstraZeneca or Pfizer/BioNTech vaccines compared to participants who received the Sinopharm vaccine. In addition, other local side effects after the first dose were more pronounced in participants who received the Oxford/AstraZeneca vaccine.
Table 3. Prevalence of post-vaccination side effects based on the type of vaccine administered.
| Type of vaccine | Side effects | After the first dose | After the second dose | After both doses | None |
| n (%) | n (%) | n (%) | n (%) | ||
| Pfizer-BioNTech | Systemic side effects | ||||
| (n = 909) | Headache | 182 (20.02) | 99 (10.89) | 48 (5.28) | 580 (63.81) |
| Fatigue | 174 (19.14) | 92 (10.12) | 53 (5.83) | 590 (64.91) | |
| Fever | 251 (27.61) | 131 (14.41) | 60 (6.61) | 467 (51.38) | |
| Chills and tremors | 57 (6.27) | 34 (3.74) | 16 (1.76) | 802 (88.22) | |
| Joint pain | 71 (7.81) | 47 (5.17) | 35 (3.85) | 756 (83.17) | |
| Myalgia | 114 (12.54) | 54 (5.94) | 33 (3.63) | 708 (77.89) | |
| Diarrhea | 16 (1.76) | 11 (1.21) | 11 (1.21) | 871 (95.82) | |
| Nausea | 27 (2.97) | 14 (1.54) | 13 (1.43) | 855 (94.06) | |
| Local side effect | |||||
| Local pain | 267 (29.37) | 128 (14.08) | 72 (7.92) | 442 (48.62) | |
| Local edema | 46 (5.06) | 25 (2.75) | 14 (1.54) | 824 (90.65) | |
| Itching | 21 (2.31) | 15 (1.65) | 6 (0.66) | 867 (95.38) | |
| Local redness | 42 (4.62) | 25 (2.75) | 9 (0.99) | 833 (91.74) | |
| Local hotness | 77 (8.47) | 49 (5.39) | 25 (2.75) | 758 (83.39) | |
| Oxford/AstraZeneca | Systemic side effects | ||||
| (n = 261) | Headache | 68 (26.05) | 68 (26.05) | 5 (1.92) | 120 (45.98) |
| Fatigue | 71 (27.20) | 19 (7.28) | 10 (3.83) | 161 (61.69) | |
| Fever | 95 (36.39) | 33 (12.64) | 16 (6.13) | 117 (44.83) | |
| Chills and tremors | 41 (15.71) | 8 (3.07) | 3 (1.15) | 209 (80.08) | |
| Joint pain | 10 (3.83) | 19 (7.28) | 6 (2.29) | 226 (86.59) | |
| Myalgia | 36 (13.79) | 20 (7.66) | 12 (4.60) | 193 (73.95) | |
| Diarrhea | 11 (4.21) | 2 (0.77) | 0 (0.0) | 248 (95.02) | |
| Nausea | 24 (9.20) | 6 (2.30) | 4 (1.53) | 227 (86.97) | |
| Local side effect | |||||
| Local pain | 87 (33.33) | 35 (14.41) | 21 (8.05) | 118 (45.21) | |
| Local oedema | 16 (6.13) | 7 (2.68) | 6 (2.30) | 232 (88.89) | |
| Itching | 17 (6.51) | 5 (1.92) | 4 (1.53) | 235 (90.04) | |
| Local redness | 18 (6.90) | 8 (3.07) | 4 (1.53) | 231 (88.51) | |
| Local hotness | 30 (11.49) | 7 (2.68) | 4 (1.53) | 220 (84.29) | |
| Sinopharm | Systemic side effects | ||||
| (n = 170) | Headache | 23 (13.53) | 13 (7.65) | 15 (8.82) | 119 (70.0) |
| Fatigue | 18 (10.59) | 14 (8.24) | 23 (13.53) | 115 (67.65) | |
| Fever | 22 (12.94) | 25 (14.71) | 17 (10.0) | 106 (62.35) | |
| Chills and tremors | 4 (2.35) | 4 (2.35) | 8 (4.71) | 154 (90.59) | |
| Joint pain | 10 (5.88) | 12 (7.06) | 10 (5.88) | 138 (81.18) | |
| Myalgia | 11 (6.47) | 9 (5.29) | 12 (7.06) | 138 (81.18) | |
| Diarrhea | 5 (2.94) | 2 (1.18) | 2 (1.18) | 161 (94.71) | |
| Nausea | 5 (2.94) | 6 (3.53) | 3 (1.76) | 156 (91.76) | |
| Local side effect | |||||
| Local pain | 28 (16.47) | 20 (11.76) | 18 (10.58) | 104 (61.18) | |
| Local edema | 9 (5.29) | 5 (2.94) | 11 (6.47) | 145 (85.29) | |
| Itching | 2 (1.18) | 7 (4.12) | 3 (1.76) | 158 (92.94) | |
| Local redness | 7 (4.12) | 3 (1.76) | 4 (2.35) | 156 (91.76) | |
| Local hotness | 13 (7.65) | 5 (2.94) | 11 (6.47) | 141 (82.94) |
The most common systemic side effects included fever, headache, and fatigue. Interestingly, systemic side effects reported after the first dose of Sinopharm were lower than those observed with either Oxford/AstraZeneca or Pfizer/BioNTech vaccines. However, all systemic side effects were more prominent with the Oxford/AstraZeneca vaccine compared to the Pfizer/BioNTech vaccine, except joint pain.
When examining side effects associated with the second dose of COVID-19 vaccines, there was a significant reduction in the incidence of side effects compared to the first dose. Nevertheless, itching at the injection site notably increased with the second dose of Sinopharm. Furthermore, fever after the second dose of Sinopharm was more prevalent than with Oxford/AstraZeneca and Pfizer/BioNTech.
Severity of post-vaccination side effects
Table 4 presents an analysis of the severity of adverse effects based on participants' demographic characteristics, health profiles, and vaccination data. The majority of participants in the age groups of 36-50 and 51-60 years experienced side effects following vaccination (p-value < 0.001). Married participants, by contrast, tended to experience more severe side effects compared to unmarried participants. Occupational differences were also associated with side effects severity (p-value < 0.001), with approximately two-thirds of students reporting either no or mild side effects.
Table 4. Severity of post-vaccination side effects according to demographic characteristics, health profile, and vaccination data .
The p-value was calculated using the chi-square test.
| Variables | Mild n (%) | Moderate n (%) | Severe n (%) | No side effect n (%) | p-value |
| Age (year) | |||||
| 18-25 | 318 (39.21) | 217 (26.76) | 68 (8.38) | 208 (25.65) | <0.001 |
| 26-35 | 68 (30.36) | 79 (35.27) | 32 (14.29) | 45 (20.09) | |
| 36-50 | 63 (36.84) | 51 (29.83) | 28 (16.37) | 29 (16.96) | |
| 51-60 | 22 (28.57) | 30 (38.96) | 12 (15.58) | 13 (16.88) | |
| >60 | 22 (38.60) | 12 (21.05) | 9 (15.79) | 14 (24.56) | |
| Gender | |||||
| Male | 233 (36.81) | 178 (28.12) | 63 (9.95) | 159 (25.12) | 0.26 |
| Female | 260 (36.78) | 211 (29.84) | 86 (12.16) | 150 (21.22) | |
| Marital status | |||||
| Single | 335 (37.26) | 247 (27.47) | 85 (9.45) | 232 (25.81) | < 0.001 |
| Married | 158 (35.83) | 142 (32.20) | 64 (14.51) | 77 (17.46) | |
| Occupation | |||||
| Healthcare worker | 71 (36.04) | 67 (34.01) | 25 (12.69) | 34 (17.26) | <0.001 |
| Non-healthcare worker | 143 (33.49) | 140 (32.79) | 66 (15.46) | 78 (18.27) | |
| Student | 279 (38.97) | 182 (25.42) | 58 (8.10) | 197 (27.51) | |
| Do you maintain a healthy lifestyle? | |||||
| Yes | 291 (37.60) | 226 (29.20) | 81 (10.47) | 176 (22.74) | 0.78 |
| No | 202 (35.69) | 163 (28.79) | 68 (12.01) | 133 (23.49) | |
| Smoking | |||||
| Yes | 113 (37.67) | 87 (29.0) | 28 (9.33) | 72 (24.0) | 0.72 |
| No | 380 (36.54) | 302 (29.04) | 121 (11.63) | 237 (22.79) | |
| Chronic health problems | |||||
| Yes | 62 (29.81) | 76 (36.54) | 30 (14.42) | 40 (19.23) | 0.007 |
| No | 431 (38.07) | 313 (27.65) | 119 (10.51) | 269 (23.76) | |
| Had any type of allergy | |||||
| Yes | 94 (32.41) | 83 (28.62) | 42 (14.48) | 71 (24.48) | 0.07 |
| No | 399 (38.0) | 306 (29.14) | 107 (10.19) | 238 (22.67) | |
| Blood group | |||||
| A | 197 (42.55) | 101 (21.81) | 46 (9.94) | 119 (25.70) | <0.001 |
| B | 88 (34.24) | 73 (28.40) | 29 (11.28) | 67 (26.07) | |
| O | 172 (35.46) | 163 (33.61) | 55 (11.34) | 95 (19.59) | |
| AB | 36 (26.67) | 52 (38.51) | 19 (14.07) | 28 (20.74) | |
| Infected with COVID-19 before vaccination | |||||
| Yes | 304 (37.25) | 262 (32.11) | 106 (12.99) | 144 (17.65) | <0.001 |
| No | 189 (36.07) | 127 (24.24) | 43(8.21) | 165 (31.49) | |
| Type of vaccine received | |||||
| Pfizer-BioNTech | 344 (37.84) | 280 (30.80) | 83 (9.13) | 202 (22.22) | |
| Oxford/AstraZeneca | 78 (29.88) | 84 (32.18) | 44 (16.86) | 55 (21.07) | <0.001 |
| Sinopharm | 71 (41.76) | 25 (14.71) | 22 (12.94) | 52 (30.59) | |
| Infected with COVID-19 after vaccination | |||||
| Yes | 163 (37.56) | 157 (36.18) | 55 (12.67) | 59 (13.59) | <0.001 |
| No | 330 (36.42) | 232 (25.61) | 94 (10.38) | 250 (27.59) | |
| Total | 493 (36.79) | 389 (29.33) | 149 (10.82) | 309 (33.33) | |
The severity of side effects was significantly influenced by chronic health problems (p-value = 0.007). Participants with chronic health issues were more likely to experience moderate and severe side effects compared to those without such problems. A noteworthy association was found between the blood group and side effects severity (p-value < 0.001). Individuals with blood group A tended to have less severe symptoms, with 25.7% experiencing no side effects and 42.55% developing mild side effects. Conversely, those with blood group AB had more severe symptoms, with 38.51% and 14.07% experiencing moderate and severe side effects, respectively.
Participants who had previously contracted COVID-19 before vaccination reported more severe symptoms compared to those without prior infection, and this association was statistically significant (p-value < 0.001). The type of vaccine administered also played a crucial role in side effects severity (p-value < 0.001). Individuals who received the Oxford/AstraZeneca vaccine were more prone to developing moderate to severe side effects compared to their counterparts. Conversely, over two-thirds of those who received the Sinopharm vaccine reported either no side effects or mild ones. Notably, no significant associations were found between the severity of post-vaccination side effects and gender, maintenance of a healthy lifestyle, smoking status, or any type of allergy.
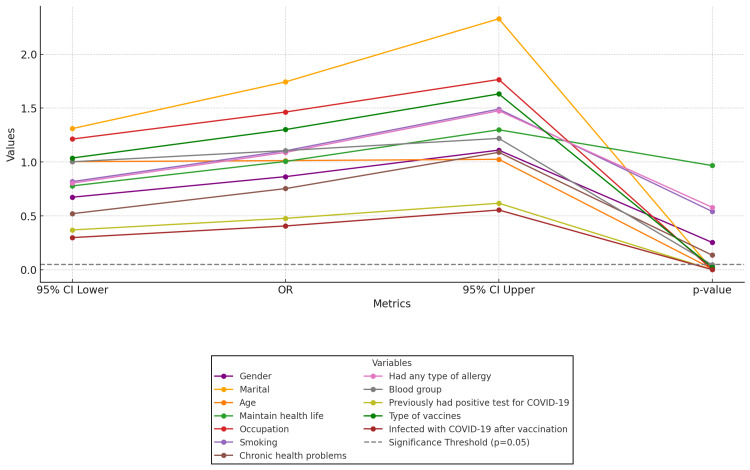
Figure 2 presents the results of multiple logistic analyses for the study variables, highlighting significant factors associated with post-vaccination side effects. Marital status, age, occupation (particularly healthcare workers), blood group O, a previous positive COVID-19 test, and receiving the Oxford/AstraZeneca vaccine were all linked to an increased likelihood of experiencing side effects. Conversely, gender, maintaining a healthy lifestyle, smoking, chronic health conditions, and allergies did not show a significant association with the likelihood of side effects.
Figure 2. Parallel coordinate plot of logistic regression for post-vaccination side effects.
Incidence of infection before and after COVID-19 vaccine
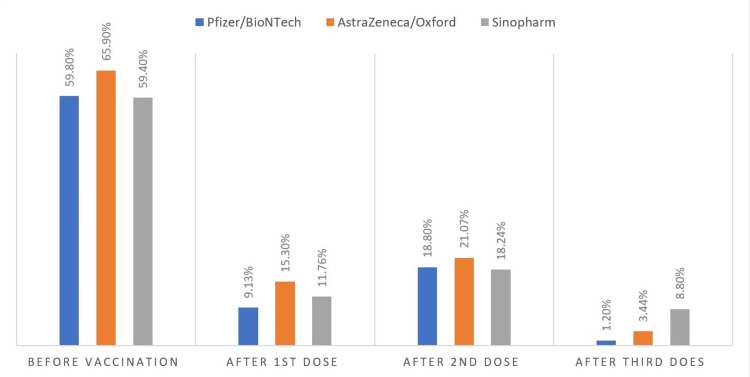
Figure 3 illustrates the prevalence of COVID-19 infection among the study participants before and after vaccination. Approximately three-fifths of the total participants who received the Pfizer/BioNTech vaccine had encountered COVID-19 infection before vaccination. Following the administration of the Pfizer/BioNTech vaccine, there was a marked reduction in the incidence of COVID-19 infection, with only 9.13% and 18.8% developing infection after the first and second doses of the vaccine, respectively. Importantly, the incidence of infection significantly dropped to just 1.2% after the third (booster) dose.
Figure 3. COVID-19 infection before and after vaccination .
For individuals who received the Oxford/AstraZeneca vaccine, two-thirds had experienced COVID-19 infection before vaccination. The administration of the following vaccine led to a reduction in the incidence of COVID-19 infection to 15.3%, 21.7%, and 3.44% after the first, second, and third (booster) doses, respectively. The Sinopharm vaccine also contributed to a decline in the rate of COVID-19 infection, although the highest incidence after the third (booster) dose was reported among individuals who received this vaccine. Notably, recipients of the Pfizer/BioNTech vaccine exhibited a lower incidence of COVID-19 infection after vaccination compared to those who received the Oxford/AstraZeneca or Sinopharm vaccines.
Discussion
Throughout history, vaccination has played an essential role in the prevention and eradication of infectious diseases. In the face of the COVID-19 pandemic, vaccination emerged as a pivotal strategy to curb the spread of the disease, alleviate its impact on public health, and mitigate economic crises. The development of effective vaccines has emerged as a global health priority to mitigate infection rates, mortality, and hospitalizations [16,17]. Studies have demonstrated that vaccinated patients exhibit a lower likelihood of severe chest involvement, oxygen requirement, and mortality compared to their unvaccinated counterparts. In a remarkably short timeframe, several vaccines from multiple different companies have been available worldwide [16,17]. However, vaccines were not free from side effects, and at this point, several rumors regarding vaccine safety were circulating in the communities. These misconceptions acted as a hindrance to achieving high vaccination rates. Understanding the safety and side effects of COVID-19 vaccines is essential worldwide to address public concerns and ensure broad vaccine acceptance. This study aimed to identify potential side effects of COVID-19 vaccines, highlighting the global need for ongoing monitoring and analysis of vaccine safety and efficacy in every country.
In the present study, around half of the participants indicated that their information on COVID-19 vaccines came from social media platforms. Interestingly, this contrasts with a study conducted in Saudi Arabia where half of the participants relied on government-owned media platforms for COVID-19 vaccine information [18]. The study found a relatively high prevalence of COVID-19 infection among participants, standing at 60.9%. This contrasts with previous studies conducted in the Kurdistan Region and Iraq, which reported a lower percentage of individuals with a positive history of COVID-19 infection [19,20]. The high prevalence observed in our study could be attributed to its recency compared to other studies, and potentially being influenced by the emergence of more COVID-19 variants and waves in the region.
In our study, approximately three-fourths of the participants experienced post-vaccination side effects, showing slightly higher rates compared to another study conducted in Iraq [19]. Among those who developed side effects after vaccination, 37% reported mild side effects, while 29% and 11% reported moderate and severe side effects, respectively. This aligns with a study conducted in Saudi Arabia, where mild to moderate side effects were commonly reported after COVID-19 vaccination [18]. Severe side effects were observed in around one-tenth of our study participants, consistent with previous studies indicating that approximately one-tenth of vaccinated individuals may experience severe side effects [18].
The most frequently reported side effects by participants in the current study included injection site pain, fever, headache, and fatigue. These findings align with studies conducted in neighboring countries [21,22]. In our study, individuals who received the Oxford/AstraZeneca vaccine experienced injection site pain more frequently, followed by Pfizer/BioNTech and Sinopharm. This difference arises from the distinct mechanisms of the vaccines; AstraZeneca’s viral vector platform uses adenovirus to deliver the spike protein, often provoking a stronger immune response and more noticeable side effects than mRNA vaccines like Pfizer /BioNTech [23]. However, a study in Bahrain found that pain at injection sites is more commonly observed after the Pfizer/BioNTech vaccine compared to AstraZeneca/Oxford and Sinopharm [24]. An Egyptian study indicated that side effects were more frequently observed after the first dose of vaccination with Pfizer/BioNTech, AstraZeneca/Oxford, and Sinopharm compared to the second dose, a pattern similar to what we observed in our study [25]. Furthermore, individuals who received the Pfizer-BioNTech vaccine in our study reported more side effects compared to those who received other vaccines. These findings are consistent with studies conducted in Iran and Syria [21,26].
Although a statistically significant association was not identified, the percentage of females experiencing side effects was higher than that of males, aligning with similar findings reported in a study from Saudi Arabia [27]. Moreover, a study conducted in Iran found that post-vaccination side effects were more frequently observed in females following the administration of the Sinopharm vaccine [22]. Participants with chronic health conditions were also more prone to experiencing moderate to severe side effects after vaccination, a result consistent with studies conducted in Syria and the UAE [15,21]. However, a study in Iraq reported that side effects were more pronounced among individuals without comorbidities [20]. Having a history of COVID-19 infection before vaccination was identified as another determinant of developing side effects after COVID-19 vaccination, aligning with findings reported in studies from Iraq and Turkey [19,28]. Nonetheless, a study in Saudi Arabia found that side effects were more pronounced in individuals with no previous history of infection [27].
All three vaccine types administered to the study participants have contributed to a reduction in COVID-19 infections. Following the second dose of the Pfizer/BioNTech, AstraZeneca/Oxford, and Sinopharm vaccines, the incidence of infection decreased to 18.8%, 21.07%, and 18.24%, respectively. However, a study in Iraq reported a lower incidence of infection after the second dose of Pfizer/BioNTech, AstraZeneca/Oxford, and Sinopharm to 5.6%, 9.1%, and 13.6%, respectively [29]. The variation in the incidence of COVID-19 infection after vaccination between our studies may be attributed to the timing of this study, conducted after the emergence of the Omicron variant, against which vaccines were found to be less effective, as a study from Iraqi Kurdistan has documented an outbreak of the Omicron variant among vaccinated healthcare workers [30].
Limitations
The study gained strength from its large sample size, which effectively assessed adverse effects linked to the COVID-19 vaccine within the Iraqi Kurdish population. It stands out as one of the limited studies investigating post-vaccination side effects in the Kurdistan Region of Iraq. Notwithstanding these strengths, certain limitations are worth considering. First, the study's confinement to a single province in Iraqi Kurdistan limits the generalizability of the findings to a national level. Second, relying on self-reported side effects introduces the potential for reporting bias and recall bias, as the participants may not accurately recall all the symptoms they experienced or the duration, acknowledging a need for caution in interpreting the results. Lastly, the study predominantly concentrated on short-term side effects. To comprehensively explore long-term adverse effects associated with COVID-19 vaccines, we propose the undertaking of a nationwide cohort study.
Conclusions
The study found that fever, headache, and fatigue were the most commonly reported side effects of all three types of vaccines: Pfizer/BioNTech, Oxford/AstraZeneca, and Sinopharm. Encouragingly, three-fifths of the participants experienced either no side effects or mild ones, suggesting a positive trend in promoting COVID-19 vaccine acceptance within our community.
Notably, side effects were more frequently documented after the initial vaccine dose compared to the second. Among the vaccine recipients, Pfizer-BioNTech vaccine recipients were more prone to experiencing side effects, followed by Oxford/AstraZeneca and Sinopharm recipients. Determinants for experiencing side effects after vaccination included participants aged 36-60, females, individuals with chronic health problems, and those previously infected with COVID-19.
Acknowledgments
We would like to express sincere gratitude to all participants who took part in this study.
Appendices
Survey on the side effects of COVID-19 vaccines
By signing below, I hereby confirm my enrollment in this study
Signature _________________
Part 1. Sociodemographic Characteristics
Variables
Age (year)
Gender
Male
Female
Marital status
Single
Married
Occupation
Healthcare worker
Non-healthcare worker
Student
Do you maintain a healthy lifestyle?
Yes
No
Smoking
Yes
No
Chronic health problems
Yes
No
Had any type of allergy
Yes
No
Blood group
A
B
O
AB
Part 2. Source of Information About COVID-19 Vaccination
Social media platforms
Government-owned media platforms
Scientific and medical platform
Friends and relatives
I have no information
Part 3. COVID-19 Infection and Vaccination Data
Previously had a positive test for COVID-19
Yes
No
Type of vaccine
Pfizer/BioNTech
Oxford/AstraZeneca
Sinopharm
Number of doses of COVID-19 vaccine received
One dose
Two doses
Three doses
Infected with COVID-19 after vaccination
Yes
No
Infected with COVID-19 after which dose
Not Infected
1st dose
2nd dose
3rd dose
Post-vaccination side effects
Yes
No
Side effects occurred after which dose
No side effects
1st dose
2nd dose
3rd dose
Local side effects
Local pain
Local oedema
Itching
Local redness
Local hotness
Systemic side effects
Headache
Fatigue
Fever
Chills
Joint pain
Myalgia
Diarrhea
Nausea
Duration of symptoms after vaccination in days
Severity of side effects after vaccination (use the scale from 0 to 10)
Disclosures
Human subjects: Consent was obtained or waived by all participants in this study. Ethics and Scientific Committee of University of Zakho College of Medicine in Kurdistan Region, Iraq issued approval JUL2022/E04 (dated July 05, 2022).
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Acquisition, analysis, or interpretation of data: Ahmed A. Mosa, Lilaz S. Hito, Dania S. Jamil, Israa T. Shukur, Dana S. Abdulkareem
Drafting of the manuscript: Ahmed A. Mosa, Lilaz S. Hito, Dania S. Jamil, Israa T. Shukur, Dana S. Abdulkareem
Critical review of the manuscript for important intellectual content: Ahmed A. Mosa, Ibrahim A. Naqid, Nawfal R. Hussein
Concept and design: Ibrahim A. Naqid, Nawfal R. Hussein
Supervision: Ibrahim A. Naqid, Nawfal R. Hussein
References
- 1.Impact of Covid-19 pandemic on surgical practice in Kurdistan, Iraq: an online cross-sectional survey. Hussein NR, Musa DH, Ibrahim N, Naqid IA, M. Saleem ZS, Jacksi K. 2020;27:47–51. doi: 10.1016/j.ijso.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The impact of COVID-19 pandemic on the care of patients with kidney diseases in Duhok City, Kurdistan Region of Iraq. Hussein NR, M. Saleem ZS, Ibrahim N, Musa DH, Naqid IA. Diabetes Metab Syndr. 2020;14:1551–1553. doi: 10.1016/j.dsx.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strict social distancing measures helped early control of SARS-CoV-2 spread in Duhok city, Iraq. Hussein NR, Naqid I. J Infect Dev Ctries. 2022;16:1370–1371. doi: 10.3855/jidc.12901. [DOI] [PubMed] [Google Scholar]
- 4.The impact of breaching lockdown on the spread of COVID-19 in Kurdistan Region, Iraq. Hussein NR, Naqid IA, Saleem ZS, Musa DH, Ibrahim N. Avicenna J Clin Microbiol Infect. 2020;7 [Google Scholar]
- 5.A sharp increase in the number of COVID-19 cases and case fatality rates after lifting the lockdown in Kurdistan region of Iraq. Hussein NR, Naqid IA, Saleem ZSM, Almizori LA, Musa DH, Ibrahim N. https://journals.lww.com/annals-of-medicine-and-surgery/fulltext/2020/09000/a_sharp_increase_in_the_number_of_covid_19_cases.32.aspx. Ann Med Surg. 2020;27:140–142. doi: 10.1016/j.amsu.2020.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A rare case of absolute thrombocytopaenia in a COVID-19 patient: case report. Mohammad AM, Sgery AS, Hussein NR. Ann Med Surg (Lond) 2021;72:103097. doi: 10.1016/j.amsu.2021.103097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Systematic review and meta-analysis of COVID-19 vaccination acceptance. Norhayati MN, Che Yusof R, Azman YM. Front Med (Lausanne) 2021;8:783982. doi: 10.3389/fmed.2021.783982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Self-reported real-world safety and reactogenicity of COVID-19 vaccines: a vaccine recipient survey. Mathioudakis AG, Ghrew M, Ustianowski A, et al. Life (Basel) 2021;11 doi: 10.3390/life11030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Status of COVID-19 Vaccines within WHO EUL/PQ evaluation process. 2023. https://www.american-club.com/files/files/WHO_COVID-19_EUL_23-March-2021.pdf https://www.american-club.com/files/files/WHO_COVID-19_EUL_23-March-2021.pdf
- 10.The pandemic of coronavirus: misconceptions from the land of Mesopotamia. Mohammad AM. Int J Surg Glob Health. 2021;27:52. [Google Scholar]
- 11.Flattening the curve of COVID-19 vaccine rejection-an international overview. Feleszko W, Lewulis P, Czarnecki A, Waszkiewicz P. Vaccines (Basel) . 2021;27:1–8. doi: 10.3390/vaccines9010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evaluation of side effects associated with COVID-19 vaccines in Saudi Arabia. Alhazmi A, Alamer E, Daws D, et al. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9060674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Public awareness about coronavirus vaccine, vaccine acceptance, and hesitancy. Elgendy MO, Abdelrahim ME. J Med Virol. 2021;93:6535–6543. doi: 10.1002/jmv.27199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Side effects and perceptions following COVID-19 vaccination in Jordan: a randomized, cross-sectional study implementing machine learning for predicting severity of side effects. Hatmal MM, Al-Hatamleh MA, Olaimat AN, Hatmal M, Alhaj-Qasem DM, Olaimat TM, Mohamud R. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaccine side effects following COVID-19 vaccination among the residents of the UAE-an observational study. Ganesan S, Al Ketbi LM, Al Kaabi N, et al. Front Public Health. 2022;10:876336. doi: 10.3389/fpubh.2022.876336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heterologous COVID-19 vaccination; Sinopharm and Pfizer-BioNTech. Mohammad AM, Sgery ASH. Iraq Med J. 2021;26:139–141. [Google Scholar]
- 17.Mortality rates among vaccinated and unvaccinated covid19 patients in Duhok hospitals, Kurdistan Region, Iraq. . Tahlo WF, Mohammad AM. Adv Med J. 2024;8:75–85. [Google Scholar]
- 18.Side effects and perceptions of COVID-19 vaccination in Saudi Arabia: a cross-sectional study. Al-Hanawi MK, Keetile M, Kadasah NA, Alshareef N, Qattan AM, Alsharqi O. Front Med (Lausanne) 2022;9:899517. doi: 10.3389/fmed.2022.899517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Potential adverse effects of COVID19 vaccines among Iraqi population; a comparison between the three available vaccines in Iraq; a retrospective cross-sectional study. Almufty HB, Mohammed SA, Abdullah AM, Merza MA. Diabetes Metab Syndr. 2021;15:102207. doi: 10.1016/j.dsx.2021.102207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Assessment of acceptance, concerns and side effects towards COVID-19 vaccination among the community: A cross-sectional study from Baghdad, Iraq. Albasry Z, Al-Taie A. Clin Epidemiol Glob Health. 2023;20:101217. doi: 10.1016/j.cegh.2023.101217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Covid-19 vaccination reported side effects and hesitancy among the Syrian population: a cross-sectional study. Najjar M, Albuaini S, Fadel M, et al. Ann Med. 2023;55:2241351. doi: 10.1080/07853890.2023.2241351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adverse effects following COVID-19 vaccination in Iran. Babaee E, Amirkafi A, Tehrani-Banihashemi A, et al. BMC Infect Dis. 2022;22:476. doi: 10.1186/s12879-022-07411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.mRNA and adenoviral vector vaccine platforms utilized in COVID-19 vaccines: technologies, ecosystem, and future directions. Okuyama R. Vaccines (Basel) 2023;11 doi: 10.3390/vaccines11121737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Unfolding the mild to moderate short-term side effects of four COVID-19 vaccines used in Bahrain: a cross-sectional study. Zahid MN. https://www.mdpi.com/2076-393X/9/11/1369/htm. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Side effects and efficacy of COVID-19 vaccines among the Egyptian population. Elgendy MO, El-Gendy AO, Mahmoud S, Mohammed TY, Abdelrahim ME, Sayed AM. Vaccines (Basel) 2022;10 doi: 10.3390/vaccines10010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Study protocol: cohort event monitoring for safety signal detection after vaccination with COVID-19 vaccines in Iran. Aliyari R, Mahdavi S, Enayatrad M, et al. BMC Public Health. 2022;22:1153. doi: 10.1186/s12889-022-13575-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Side-effects of COVID-19 vaccines among the Saudi population: a cross-sectional study. Almughais ES, Alharbi AH, Aldarwish HA, Alshammari AF, Alsuhaymi RS, Almuaili JA, Alanizy AM. Saudi Med J. 2022;43:386–393. doi: 10.15537/smj.2022.43.4.20210905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prevalence and risk factors of CoronaVac side effects: an independent cross-sectional study among healthcare workers in Turkey. Riad A, Sağıroğlu D, Üstün B, Pokorná A, Klugarová J, Attia S, Klugar M. J Clin Med. 2021;10:2629. doi: 10.3390/jcm10122629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assessment of COVID-19 vaccination among healthcare workers in Iraq; adverse effects and hesitancy. Darweesh O, Khatab N, Kheder R, et al. PLoS One. 2022;17:0. doi: 10.1371/journal.pone.0274526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.COVID-19 vaccine breakthrough infection among fully vaccinated healthcare workers in Duhok governorate, Iraqi Kurdistan: a retrospective cohort study. Almufty HB, Mamani MM, Ali AH, Merza MA. J Med Virol. 2022;94:5244–5250. doi: 10.1002/jmv.27985. [DOI] [PMC free article] [PubMed] [Google Scholar]