Abstract
A breast cancer risk assessment tool for Asian populations, incorporating Polygenic Risk Score and Gail Model algorithm, has been established and validated. However, effective methods for delivering personalized risk information remain underexplored. This study aims to identify and develop effective methods for conveying breast cancer risk information to Asian women. Through ten focus group discussions with 32 women in Indonesia and Singapore, we explored preferences for the presentation of risk information. Participants favored comprehensive reports featuring actionable steps, simplified language, non-intimidating visuals, and personalized risk reduction recommendations. Singaporean participants, more aware of breast cancer prevention, showed a lower likelihood of seeking follow-ups upon receiving low-risk results compared to Indonesians. Overall, participants found the reports useful and advocated for similar approaches in other disease assessments. Balancing content and complexity in reports is crucial, highlighting the need for improved patient understanding and engagement with healthcare providers. Future studies could explore physicians’ roles in delivering personalized risk assessments for breast cancer prevention.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12687-024-00735-6.
Keywords: Breast Cancer, Polygenic risk score, Genetic testing, Genetic counselling, Focus group discussion, Predictive genetic testing
Introduction
Breast cancer (BC) is the most common cancer and the leading cause of cancer-related deaths among women. In 2020, there were 2.3 million new cases of women diagnosed with BC and 685 000 deaths worldwide (World Health Organization 2023). In the past few years, there has been a rise in popularity of studies using polygenic risk scores (PRSs) to determine a person’s risk of BC (Hughes et al. 2022; Ohbe et al. 2023; Yanes et al. 2020) PRS, which sums the impact of multiple common genetic variants to estimate disease risk, has demonstrated the potential to improve the identification of individuals at high risk of developing BC (Zeinomar and Chung 2021).
Early diagnosis of BC through mammography screening has been shown to reduce deaths from the disease (Duffy et al. 2020; Lim et al. 2022b). However, mammography does not come without risks. False positive tests and overdiagnosis of tumors that may never advance into a clinical stage that endangers life are commonly cited downsides of mammography screening (Kowalski 2021; Løberg et al. 2015). The benefits of mammography screening are further impeded by the lack of organized screening programs, and the fear of mammogram procedural pain (Fayanju et al. 2014; Lim et al. 2022a; Rajendram et al. 2022).
Numerous risk predictors for BC, such as family history, classical BC risk factors, mammographic density, and PRS, thus play a pivotal role in determining which women are most likely to benefit from surveillance (Ho et al. 2022a, 2023a, b; Pettersson et al. 2014). A BC risk assessment tool using PRS assessment of 313 single nucleotide polymorphisms and a risk prediction algorithm from the Gail Model had previously been established (Gail et al. 1989; Mavaddat et al. 2019), and validated (Chay et al. 2012; Ho et al. 2020, 2022b) for Asian populations. The generated risk report provides information on BC risk stratification and personalized risk-reducing recommendations, which will help doctors in delivering tailored care plans. To improve the communication and delivery of genetic based screening test reports, for example, studies were conducted in the United States (Haga et al. 2014) and the United Kingdom (Farmer et al. 2020) to provide recommendations on the report format. However, the findings reported in the two studies may or may not be applicable to all clinical settings, especially in the Asian context and for BC in particular.
Beyond healthcare providers, the potential impact of disclosing the BC risk assessment report to its targeted customers, namely healthy women with no history of BC, is still not well understood. Almost all published studies are sourced from non-Asian countries where acceptance of BC risk assessment test could be different from the Indonesian and Singaporean populations. Hence, effective ways of returning personal risk information comprising predictions from various genetic and non-genetic risk factors remain yet to be explored. The objective of the study is to identify and develop effective methods for conveying personalized BC risk information to Asian women.
Methods
This was a qualitative study involving focus groups. Focus group discussions (FGDs) was deemed most suitable as it allowed for a deeper discussion on the perception of healthy women in Indonesia and Singapore in relation to BC risk assessment testing. Indonesia and Singapore were chosen because the countries have different policies and practices of BC screening programs. The clinical BC screening program in Indonesia is opportunistically designed as a response to symptoms reported by women. Meanwhile, Singapore has adopted a nation-wide BC clinical screening program referred to as BreastScreen Singapore since 2002 (Loy et al. 2015; Yuniar et al. 2022).
The study was conducted in Indonesia from September to October 2022 and in Singapore from July to August 2022. The Standards for Reporting Qualitative Research (SRQR) was utilized as reporting guidelines. The checklist is shown in S1 File.
Ethical approval for the study was obtained from the Parkway Independent Ethics Committee of Singapore under approval number PIEC/2022/013 and Ethical Committee of Atma Jaya Catholic University of Indonesia under approval number 0007 W/III/PPPE.PM.10.05/09/2022. Written informed consent was sought from all the participants before they were enrolled in the study.
Eligibility criteria and recruitment
The inclusion criteria included women aged above 25 without a prior diagnosis of BC, regardless of their family history of the disease, who were able to use the Zoom application. The mandatory use of the Zoom application was implemented to reduce physical contact during data collection, as a precautionary measure due to the study being conducted amidst the COVID-19 pandemic. Participants were excluded from the study if they did not meet the predefined inclusion criteria.
Convenience sampling method was used both in Indonesia and Singapore. The study was promoted via social media platforms, such as Instagram and LinkedIn, online newsletters, and direct promotion to potential participants by the study team members. Online study promotion was selected because online marketing would be primarily used to advertise the product in the future. Interested individuals were asked to fill out the Microsoft Forms so that the study team members could contact them to check their eligibility and time availability. This form allowed the study team members to identify the individual participants’ information, including name, age, and an explanation of the study procedures was then provided to the eligible individuals, and informed consent was obtained via HelloSign. The participants were offered electronic money credits valued at Rp 50.000 in Indonesia or SGD 20 in Singapore as an incentive to participate. Participation and withdrawal were fully voluntary.
Sample size calculation
Researchers aimed to arrange four different sessions of FGD in each country. Based on existing literature, data saturation of 90% or higher could be achieved by conducting at least four sessions of FGD (Hennink and Kaiser 2022; Hennink et al. 2019).
Development of focus groups and interview guide
The FGDs were designed to evaluate the preferences regarding the presentation of risk results, the level of detail provided for explanations, recommendations for follow-up actions, the psychological impact of receiving risk results, and participants’ expressed interest in future testing. Prior to the focus groups, all participants received either a detailed high-risk or low-risk mock report that is currently under development by NalaGenetics (S2 File). Apart from the detailed mock risk report, participants in Singapore also received a simplified version of the mock risk report (S4 File), which was adapted from an ongoing research program (Liu et al. 2022). This supplementary report, available only in English, was provided due to the ongoing similar research program in Singapore, making it more relevant for the Singaporean population compared to Indonesia. Both reports contained recommendations that help patients manage their risks and a personalized screening plan to identify the risk of BC early, improving the effectiveness of treatment. All participants were then assigned to either the low-risk result or high-risk result focus group based on the report they had randomly received. The decision to separate the participants into two cohorts was based on the assumption that people who receive high-risk results may have different opinions from those receiving low-risk results.
All FGDs were conducted via Zoom application. FGDs in Indonesia were conducted in Bahasa Indonesia by FA, while FGDs in Singapore were conducted in English by FN. Both facilitators were part of the investigation team, and they had both undergone training for FGD. FA also had previous experience moderating a few sessions of FGD.
Based on the FGD simulations that had been previously conducted, the maximum number of participants was capped at five per FGD session to give each participant enough time to answer a question before moving on to the next question. Participants were reminded that there were no right or wrong answers, allowing them to freely express their opinions. The questions asked during FGD covered aspects that were related to room for improvement in the report prototype. (S3 File). Confirmatory questions were asked following the closed-ended questions to avoid bias.
Data collection
During the FGD, the facilitator began each session by introducing all members of the study team. Next, participants were reminded of the background and objectives of the study. An explanation and interpretation of the report were also provided. Given that the genetic testing is a clinical service, the actual testing process would include comprehensive genetic counseling, covering both pre-test and post-test counseling sessions. All discussions were digitally recorded using the recording feature via the Zoom application.
To ensure that the participants were actively engaged in the discussion, all participants were requested to turn on their web camera during their sessions. The facilitator aimed to finish each session within 90 min to prevent exhausting the participants, while ensuring that sufficient time was allocated to them before moving on to the next question.
Data analysis
All audio recordings were manually transcribed verbatim and reviewed by two independent study team members for assurance. Participants’ identities were anonymized by assigning them a unique code. Language translation to English was performed manually for all FGD responses collected in Bahasa Indonesia.
Through an iterative comparison, inductive thematic analysis was performed. This analysis was performed by coding the FGD participants’ responses; similar codes were then subsequently grouped together to obtain sub-themes. Finally, relevant sub-themes were grouped together into broader themes. Any differences in opinions were resolved through a discussion among the study team members. No specific framework was used to assist with the analysis as the nature of the study was fully exploratory.
Results
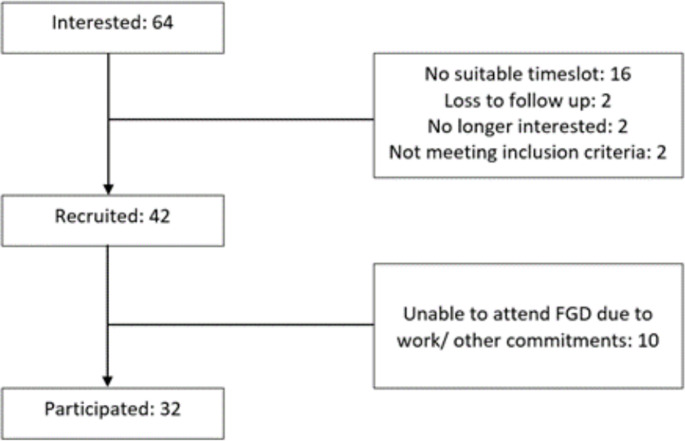
Initially, a total of 64 individuals expressed their interest in participating in this study. Out of the 22 participants that were excluded from the recruitment, 2 were lost to follow up, 2 did not meet the inclusion criteria, 2 decided not to continue with the recruitment process, and 16 could not make it for the allocated time slot. This reduced the number of participants that were recruited to 42. Out of the 42 participants recruited, 10 participants did not manage to attend the FGD due to sudden work or other personal commitments. The final number of participants recruited from both countries was 32, with recruitment flow as shown in Fig. 1.
Fig. 1.
Flowchart of participant recruitment
The demographics of the participants involved in the FGD in Indonesia and Singapore are summarized in Table 1. In Indonesia, participants were between 25 and 30 years old with a mean age of 27.9 years. Out of 15 total participants, 14 disclosed their ethnicity background. 80% of the participants were Indonesian (n = 12), and nine of them were from Jawa ethnic group. In Singapore, the age range of the participants involved was 25 to 49 years old with a mean age of 37.2 years. 82% of the participants were Chinese (n = 14) while the rest were Indian (n = 2) or Burmese (n = 1).
Table 1.
Participant demographics
| Indonesia (n = 15) | Singapore (n = 17) | |
|---|---|---|
| Mean Age (years, range) | 27.9 (25–30) | 37.2 (25–49) |
| Ethnicity | ||
| Indonesia* | 12 (80.0%) | - |
| Chinese | 1 (6.7%) | 14 (82.3%) |
| Indian | - | 2 (11.8%) |
| Others/undisclosed | 2 (13.3%) | 1 (5.9%) |
*Specific ethnic groups included: Jawa (n = 9), Lampung (n = 1), Minangkabau (n = 1), Mixed (n = 1)
A total of ten sessions of FGD were conducted for this study. Each session consisted of between 2 and 5 participants. The discussions with the low-risk result cohort were attended by a total of 18 participants divided into five different sessions. Similarly, the discussions with the high-risk result cohort were attended by a total of 14 participants split into five different sessions. After conducting 5 sessions for each high and low risk cohort, there was no new information obtained, indicating data saturation.
Four key themes were identified from the FGD sessions carried out. The first key theme gave insight on patients’ preference towards receiving a genetic risk report. The second key theme provided information around the areas of improvement for the report prototype (S2 File), and this included subthemes such as the content of the report, the design of the report, and the overall communication of the report. The third key theme provided insight into the potential impact of the BC risk assessment report on users. Meanwhile, the last key theme informed healthy women’s perceptions and expectations of BC risk assessment testing.
Theme 1: more participants favor more information regarding their risk profiles
In Singapore, participants were asked to compare the detailed report against the simplified report (S4 File). 14 out of 17 participants (n = 82%) would like to see reports that are more comprehensive in reporting their risk results and providing information on the follow-up steps to manage their BC risk.
I prefer the one with more information … I feel that the other report is too short … [from the detailed report], you can find out which percentile you are [at] as compared to the general population … and I think there is more information with regards to the lifestyle changes [that] you can make (Singapore FGD Participant 5, High-risk result report recipient).
I think the brief report is really short … I will prefer the report to be very detailed … especially if it’s high risk, I want to know how high … (Singapore FGD Participant 13, Low-risk result report recipient).
Nonetheless, two of the Singapore FGD participants preferred the simplified report stated that a detailed report was not needed and would rather consult with their physicians if they needed additional information.
I prefer the [shorter report] that has not too much of wording, the key point is to tell me the results and some basic explanation on what is the risk like … if you need further information, then you can consult with doctor (Singapore FGD Participant, Low-risk result report recipient).
Theme 2: areas of improvement
Subtheme 2.1 the design of the report
In general, participants stated that the report exhibited an aesthetically pleasing and comprehensive visual presentation, characterized by an attractive design. Some participants also expressed a positive view of the report’s colorful and user-friendly format. However, a common observation among many participants was the difficulty in discerning important information, attributed to the use of light font color and a small font size. Additionally, a minority of participants raised concerns regarding the use of alarming color in a risk report, suggesting it might convey an unintended sense of alarm. Furthermore, there were suggestions to enhance the visibility of the test results in the report, with a preference for a concise summary of results on the first page of the report.
In my opinion, the report [visual] is already good, [it is] also user-friendly in [providing] medical information … Yet, the colors [of the background and text] are not contrasting (Indonesia FGD Participant 6, High-risk result report recipient).
And also … like . people normally focus on [text presented] in a larger font size, maybe that is from me … so that [the information] can be more visible (Indonesia FGD Participant 12, Low-risk result report recipient).
Report is comprehensive enough that it can give reassurance, but I am just wondering will there be another color that you all can choose other than pink? … where I am coming from is in terms of colors, it also brings across a message. If I give you a report that is red, of course you will be scared right? (Singapore FGD Participant 11, High-risk result report recipient).
Subtheme 2.2 The content of the report
Summary Risk Group
The majority of participants (n = 24, 75%) found the summary of risk groups to be clear and were able to discern their combined risk group, as well as their categorization based on genetic and non-genetic risks. However, it is worth noting that a subset of participants (n = 6, 19%) encountered challenges in comprehending their risk classification despite the presence of explanatory information.
… [the information around risk group is] already clear, [the report shows] that the genetic risk [group] is low, the clinical risk [group] is also low … so there is overall elevated risk (Indonesia FGD Participant 7, High-risk result report recipient).
I think the risk score is quite clear because they actually put a highlight for that. I can tell whether I am in the high [risk] zone or the low [risk] zone … (Singapore FGD Participant 16, High-risk result report recipient).
Non-genetic risk
Twelve out of eighteen recipients of low-risk reports (67%) found the explanation of non-genetic risk calculation lacked clarity, especially concerning the use of medical terminology and the methodology employed for calculating the risk score. Furthermore, some participants sought clarification regarding the population data used in deriving the risk score. On the other hand, most participants (n = 12, 86%) who received high-risk reports found the explanation to be sufficiently clear.
So … there were only two [risk groups] for the non-genetic risk, right? Any score above 1.3 [%] would be classified into elevated [risk], right? (Indonesia FGD Participant 3, Low-risk result report recipient).
The bottom part about this SEER Registry… I think as a lay person, I wouldn’t know what this is (Singapore FGD Participant 7, Low-risk result report recipient).
Genetic risk
Most participants (n = 20, 63%) felt that the explanation of genetic risk calculation was clear, except for participants from the high-risk group in Indonesia. Overall, the provision of the graph was found to be useful as it aided participants in comprehending their respective risk groups.
The genetic risk score is quite clear because it has indicated the percentage clearly as well as there is a graph … from the graph, I can differentiate what are the risk groups… and 5 years down the road, what will be the risk… (Singapore FGD Participant 4, High-risk result report recipient).
On the contrary, participants who felt that the genetic risk calculation was unclear found the supporting graph confusing, and they did not understand the technical language used.
… since not all people understand statistics, the use of word ‘percentile’ would be confusing …next, for the graph [of genetic risk] … I understand [what the graph is about] but it took a while for me to discern … (Indonesia FGD Participant 10, Low-risk result report recipient).
… next, for the graph [of genetic risk] … I do not really understand … (Indonesia FGD Participant 14, High-risk result report recipient).
Recommendations
Eighteen participants (56%) generally considered the recommendations provided in the report to be adequate and beneficial, while the remaining participants expressed concerns that these recommendations lacked specificity and were overly generic. Several participants (n = 16, 50%) expressed a preference for more personalized recommendations. Additionally, there were suggestions to include easily understandable and reputable websites as supporting evidence for these recommendations.
For myself, the lifestyle [recommendations] section is mostly clear, but it is too general so patients may be questioning what foods should be eaten less or more … (Indonesia FGD Participant 15, High-risk result report recipient).
I feel that this is too generic, it’s not personalized for me … I feel that this is something I can just get from the internet (Singapore FGD Participant 3, Low-risk result report recipient).
Instead of having link to evidence, you could direct it to where I can find more information and where I can read more about it … because if I have a risk [of a certain condition] or I have this condition, I will immediately go and google … and you will read all the scary things [on the internet] which might not even be credible or applicable to you (Singapore FGD Participant 9, Low-risk result report recipient).
Subtheme 2.3 overall communication of the report
Study participants provided feedback aimed at enhancing the overall comprehensibility of the report. Suggestions included the incorporation of a glossary of medical terminology, which could prove beneficial for individuals without a background in medicine or science. Furthermore, participants recommended thorough proofreading to ensure the clarity and accuracy of the information presented in the report.
Perhaps for a layperson, you need to add footnotes for unfamiliar terminologies not everyone would know. (Indonesia FGD Participant 1, Low-risk result report recipient)
… [the report] may need a glossary [summarizing medical terminologies], I think, as mentioned by Indonesia FGD Participant 1 (Indonesia FGD Participant 2, Low-risk result report recipient).
Theme 3: potential impacts of the BC risk assessment report
Subtheme 3.1 emotional impact
There were polarized reactions on how the participants would feel if the report prototype shown to them was really theirs. Negative emotions were shown by those receiving high-risk reports. Almost all participants (n = 13, 92%) receiving high-risk reports said they would feel afraid, shocked, surprised, sad, worried, and anxious. However, there was one participant who would not feel shocked because she was aware that she had a family history of BC. On the contrary, most of the participants (n = 13, 72%) who received low-risk reports mentioned that they were relieved. Some of them also reported that they would not be negligent in maintaining a healthy lifestyle because the risk score may change over time.
[If this report was really mine], I would feel afraid, for sure. Because I have past experience of losing my grandmother (to breast cancer). But, regardless, I will focus on the treatment [to reduce the risk] and the steps I could take to address this (Indonesia FGD Participant 7, High-risk result report recipient).
If I were the one who receives the report, I would automatically feel relieved. Even though the risk in the future will still exist and may change [to become higher] … (Indonesia FGD Participant 4, Low-risk result report recipient).
I think I will be very worried if I receive a report that says my risk is high … because one of my aunties has breast cancer … (Singapore FGD Participant 5, High-risk result report recipient).
Subtheme 3.2 relationship with the physicians
Overall, most participants (n = 12, 86%) receiving high-risk results reported that there would be a positive change in the relationship with their physicians as they would consult and follow-up with their physicians more often. There were also participants (n = 4, 28.6%) who highlighted that the change in relationship with their physicians would depend on the understanding of the physician towards the report and the suggested interventions.
Meanwhile, among all study participants in Indonesia who received low-risk results, there was a consensus to engage in more frequent consultations with qualified physicians to ensure their risk remained low. Conversely, in Singapore, all study participants receiving low-risk results reported that there would be no changes in the relationship with their physicians.
… [I will be] consulting [with physician] more often; consulting [with physician] what kind of lifestyle is recommended, what would happen if I skipped [healthy eating and exercise] and cheated [from the recommendations] … whether those [nonadherent] things could increase the risk or not. I will ask more questions [to the physician] (Indonesia FGD Participant 15, High-risk result report recipient).
Since we are in the low-risk group, there is no need to go and see my doctor asking about the report … (Singapore FGD Participant 9, Low-risk result report recipient).
Subtheme 3.3 health concerns and inquiries
Participants from Singapore and Indonesia had diverse questions for their physicians. When it came to monitoring and screening for BC, participants from Indonesia were curious about the advantages and potential drawbacks of mammography, whereas individuals from Singapore were inclined to compare it to the guidelines established by the Ministry of Health (MOH) in Singapore. In terms of lifestyle changes, participants were keen to inquire about dietary adjustments, including which foods to increase or decrease, as well as recommended physical activities. Questions also arose about prescribed medications and the significance of surgical procedures. Additionally, a small number of participants expressed interest in learning the correct method for conducting breast self-examinations.
… what I would ask to my physician is on the type of mammography [examination], whether it has [negative] effect to me [or not]? (Indonesia FGD Participant 4, Low-risk result report recipient).
I don’t know if your report is tailored to 40–49 years old … Compared to MOH recommendations, people over 50 [years old] don’t have to go [for mammography] every year … but this is not on your report … (Singapore FGD Participant 7, Low-risk result report recipient).
Next, for the surgical [recommendation], [I may discuss] this later with a genetic counselor, whether we who are at the [high] risk should undergo surgery or not, maybe something like that, [so the question would be more on taking] a surgery [as an] option (Indonesia FGD Participant 7, High-risk result report recipient).
For therapy, [I want to know] more details about the risks… What should be done to reduce the risk? … and more details about the therapy… (Singapore FGD Participant 4, High-risk result report recipient).
Next, second, maybe about the self-breast exam, I may ask more or confirm [with my physician] about the exam, and how frequent I should do that (Indonesia FGD Participant 12, Low-risk result report recipient).
Subtheme 3.4 sharing genetic test results
Besides their physician, the participants would want to share and discuss the result of the genetic test with other parties. Most participants preferred sharing and discussing the report with family members or friends, especially those with medical backgrounds or who have been previously diagnosed with BC.
With whom I would talk to other than my physician if get this report? Perhaps, to my close friends or whoever [I know] who has ever been diagnosed with the same disease, if there are any. If not, I would only discuss this with my physician or the medical professionals [who take care of me] (Indonesia FGD Participant 4, Low-risk result report recipient).
I will probably want to look at this report myself [and] do some research on my own … then probably talk to a family member then a doctor … (Singapore FGD Participant 6, High-risk result report recipient).
Subtheme 3.5 self-awareness
The report also had the potential to change the participants’ outlook on their personal health. All the participants receiving high-risk results felt that the report would help increase their awareness of BC. They were also likely to change their lifestyle by following the recommendations given. Similarly, most of the participants (n = 17, 94%) receiving low-risk results stated that the report would change the way they view their health, their awareness of BC would also increase, and they would be more mindful of taking preventive actions.
Yes, perhaps, [I would] be more aware, for example, that I would need to do self-breast exam more regularly. Generally, I would be more aware [of the breast cancer risk] due to the breast cancer risk prediction report I receive (Indonesia FGD Participant 12, Low-risk result report recipient).
Since I know I am in the high-risk group, I have to [know] what are the preventive methods I can take and moving forward … how do I minimize the risk to myself as much as I can (Singapore FGD Participant 6, High-risk result report recipient).
Theme 4: Future Disease Risk Assessment
The majority of the study participants (n = 31, 97%) found the report useful and expressed enthusiasm about receiving risk assessment reports for other diseases in the future. Suggestions from participants included exploring diseases such as cervical cancer, diabetes, and cardiovascular conditions.
[I want to see a similar report for] types of diseases that are related to cancer. For example, cervical cancer, and maybe [risk assessment testing] related to fertility (Indonesia FGD Participant 14, High-risk result report recipient).
I am just thinking about the most common fields … people usually get diabetes, cancers… (Singapore FGD Participant 11, High-risk result report recipient).
Discussion
This FGD was conducted in Indonesia and Singapore, exploring healthy female participants’ perceptions of personalized BC risk assessment results. Our study adds valuable insights to the existing literature in the context of these two countries. Through the FGD, three areas of improvement for the detailed mock risk report were identified, which included the design, content, and communication styles of the report. These three areas coincide with recommendations by Farmer et al. (Farmer et al. 2020) to improve understanding of patients and physicians to genetic test reports.
First, regarding the design of the report, there was feedback from FGD participants to avoid alarming font color to make the result less intimidating. Some sections of the report were also reported to be overly crowded with text. Similar findings were reported by Farmer et al. (Farmer et al. 2020), where the patients recommended avoiding dense blocks of text and using different colors to differentiate ‘positive’ or ‘negative’ results.
Next, on the report content, this FGD found out that participants also demanded more information on recommended actions they could take to reduce their risk of getting BC other than improving the clarity of genetic and non-genetic risk information in the report. These correspond with a study by Huang et al. (Huang et al. 2014) that found out that one of the three things that affects people’s decision to undergo predictive genetic testing is the availability of treatment and prevention for the disease. The presence of recommended actions patients should take and resources that enable them to access further information and support for the disease are what patients are looking forward to in genetic testing reports (Farmer et al. 2020).
Lastly, the report should be communicated in a style that is understandable to patients from non-healthcare professions. Not all patients understand medical or statistical jargon. Therefore, the addition of a glossary could be very helpful for patients. Even though the report is not meant to be self-interpreted by patients themselves without consulting with clinicians, making sure that patients understand the results of their risk assessment test is still imperative. Furthermore, having a user-friendly report could help to improve communications between the patients and their clinicians, thus allowing the counselling session to be more effective (Haga et al. 2014). Patients who do not clearly understand their test results tend to show lower engagement in their personalized care (Elder and Barney 2012) and are unable to adhere to recommended actions (Dean Wantland 2013). As a result, they are more likely to fail in reducing or maintaining their risk level.
Our study found a key difference between Indonesian and Singaporean participants in our FGD. With greater knowledge and awareness towards BC prevention among the Singaporeans, it was observed that Singaporean participants receiving low-risk results would not want to have additional follow-ups with their physicians, compared to the Indonesian participants. Singaporean women may have a higher awareness level of BC screening due to the presence of clinical BC screening subsidies that allow women over 50 years to undergo mammography every two years at a more affordable cost (Health Promotion Board, n.d.). However, the same initiative is absent in Indonesia, and high cost has been reported to be one of the main barriers to access BC screening by Indonesians (Choridah et al. 2021). This was also demonstrated by the disparity in understanding expressed towards mammography, with participants in Indonesia being unfamiliar with the procedure. With the inclusion of perspectives from two different countries of diverse ethnic groups that allowed for a more comprehensive study, we are confident that the results of this study could be broadly applicable to other Asian populations that share sociocultural similarities with Indonesia and Singapore.
The insights obtained from our study are useful to optimize the delivery of personalized risk assessment for BC. Most participants prefer a report that gives comprehensive information, rather than only informing them of which risk group they belong to. This is evident by the fact that the majority of FGD participants in Singapore preferred the detailed report. Patients’ preference over comprehensive genetic testing reports is supported by a study by Brewer et al. (Brewer et al. 2012). In addition to risk group, participants of the study could better understand their risk of getting BC recurrence when they were given a report that clearly showed the risk score, brief information to interpret the report as well as the graph depicting their position in the risk continuum.
The FGD identified the potential impacts of disclosing BC risk reports to patients. Patients would feel anxious upon receiving high-risk results and relieved upon receiving low-risk results from the testing. These findings correspond to the Singapore study conducted by Liow et al. (2022), where the participants reported to be living in fear if they learned that their risk of acquiring BC was high. Given the nature of the testing as a diagnostic prediction, a negative psychological impact could occur following the risk assessment testing regardless of the test result. However, the anxiety level could be modest if patients understood what their report said (Woods et al. 2013) and the testing was performed by expert teams (Bordet et al. 2020). This emphasizes the importance of providing proactive personalized, pre- and post-test counselling conducted by counsellor who has sufficient understanding in personalized risk assessment for BC. Although many testing providers are now offering the option of delivering predictive genetic testing results via telephone or letter for convenience, participants may value the opportunity of having an individualized counselling session with the counsellor (O’Shea et al. 2016).
There is no universally accepted guideline for test providers to ensure that their risk assessment report is understandable by patients. Results from the Gail Model may not be easily understood by patients (Komen n.d.; Stevanato et al. 2022). Moreover, the format of reporting may impact the translation of genetic test results into clinical recommendations, the interpretation of test results, and ultimately, patients’ adherence to the recommended actions (Hao et al. 2022; Lewis et al. 2021). Accordingly, it is recommended that test providers that are planning to market their risk assessment services conduct studies to gain feedback from potential customers and identify potential impacts resulting from service that is still under development. Patients or healthcare service customers could be involved in the planning, development, and improvement of healthcare services (Bergerum et al. 2020; Crawford 2002). The World Health Organization highly recommends patient engagement (World Health Organization 2016) to ensure that the delivered healthcare services result in maximum benefits and minimum risks for targeted populations.
Studies aiming to seek feedback on the report from physicians are also warranted. Participants, particularly those receiving high-risk results, would also consult more frequently with their physician in the hope of reducing their risk level. They might consider seeing another physician if the physician they are seeing is unable to give appropriate recommendations to act on their risk assessment report. This is imperative to make sure that the physicians, be it specialists or non-specialists, can communicate the report clearly and discuss a personalized care plan with their patients more comfortably. In addition, the FGD informed that the majority of the study participants found the risk assessment report to be useful and were interested in seeing similar reports for other diseases, including cancer, diabetes, and cardiovascular conditions. These findings shed light on the other diseases patients demanded which test providers could focus on in the future.
Limitations
A limitation in the study pertained to the utilization of mock reports, potentially leading to participants’ responses not accurately reflecting their genuine emotions. Participants may have reacted differently if real reports had been used, especially for those assigned to high-risk groups. However, the use of mock reports is a common practice in the field, employed to effectively survey a large number of participants. In addition, the low mean age of participants in our study poses a notable limitation that warrants discussion. With the majority of participants aged between 25 and 40, our findings may not fully capture the experiences and perspectives of older individuals who are a higher risk of developing BC. Furthermore, the implications of BC risk, screening behaviours, and healthcare decision-making may vary significantly across different age groups. Hence, future research efforts should aim to include a more diverse age range to ensure a comprehensive understanding of the factors influencing BC perceptions and behaviours. Another limitation within the study was the potential for selection bias due to the inclusion of only participants who were able to use Zoom Application. This introduces an increased technological access factor that may potentially influence the psychological impact and subsequent responses to receiving a risk report. The ability to access information and resources through technology may contribute to enhanced health literacy, potentially influencing individuals’ understanding and reactions. Existing research indicates that individuals with lower health literacy may face challenges in comprehending information related to genomics (Kaphingst et al. 2016). Therefore, future research should consider extending the study to a more diverse sample to address the identified limitation.
Conclusion
This study summarized how a BC risk assessment report can be personalized for each individual to facilitate effective delivery of care plans for patients with different genetic and non-genetic risk profiles. With the increase in accessibility and availability of BC risk assessment service, many patients are interested in finding out more about their test results. Overall, most patients would prefer a test report to be well-balanced between content and complexity. The study also highlighted the importance of the psychological impact of patients receiving their test report, which is greatly influenced by the patients’ degree of understanding and interpretation of the reports. In our study, we discovered that Singaporean participants, who had higher awareness of BC prevention, were less inclined to seek additional physician follow-ups after receiving low-risk results compared to Indonesian participants. Finally, as most patients would likely increase their engagement with their physicians upon receiving their test results, future studies could be extended to physicians who are directly involved in the patient care delivery of BC prevention.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to thank Professor Irwanto of Universitas Katolik Indonesia Atma Jaya for his guidance and training in the conduct and data analysis of focus group discussions.
Author contributions
FA and FN contributed equally to project administration, investigation, formal analysis, and manuscript writing. JA, SGT, ST and LL assisted in data curation and analysis. EA assisted in project administration. FAA, MW, STBS, AC, LS and AI conceptualized study design and supervised execution. SJH, JL and MH conceptualized study design and methodology. All authors read and approved the final manuscript.
Funding
This study was funded by Nalagenetics Pte Ltd.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled accessed data storage at Nalagenetics Pte Ltd.
Declarations
Ethics approval
Ethics committee/IRB of Parkway Independent Ethics Committee of Singapore gave ethical approval for this work. Ethics committee/IRB of Atma Jaya Catholic University of Indonesia gave ethical approval for this work. I confirm that all necessary informed consent was obtained from all subjects and the appropriate institutional forms have been archived, and that any patient/participant/sample identifiers included were not known to anyone outside the research group so cannot be used to identify individuals.
Consent for publication
Not applicable.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fatma Aldila and Fiona Ng FJ contributed equally to this work.
Faustina Audrey Agatha, Marco Wijaya and Stevany Tiurma Br Sormin also contributed equally to this work.
References
- Bergerum C, Engström AK, Thor J, Wolmesjö M (2020) Patient involvement in quality improvement – a ‘tug of war’ or a dialogue in a learning process to improve healthcare? BMC Health Serv Res 20(1):1115. 10.1186/s12913-020-05970-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordet C, Brice S, Maupain C, Gandjbakhch E, Isidor B, Palmyre A, Moerman A, Toutain A, Akloul L, Brehin A-C, Sawka C, Rooryck C, Schaefer E, Nguyen K, Dupin Deguine D, Rouzier C, Billy G, Séné K, Denjoy I, Charron P (2020) Psychosocial impact of Predictive Genetic Testing in Hereditary Heart diseases: the PREDICT Study. J Clin Med 9(5):1365. 10.3390/jcm9051365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer NT, Richman AR, DeFrank JT, Reyna VF, Carey LA (2012) Improving communication of breast cancer recurrence risk. Breast Cancer Res Treat 133(2):553–561. 10.1007/s10549-011-1791-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chay WY, Ong WS, Tan PH, Leo J, Ho NQ, Wong GH, Chia CS, Chow KS, Tan KY, M., Ang P (2012) Validation of the Gail model for predicting individual breast cancer risk in a prospective nationwide study of 28,104 Singapore women. Breast Cancer Res 14(1):R19. 10.1186/bcr3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choridah L, Icanervilia AV, De Wit MJM, Van Asselt ADI, Kurniawan WT, Fahmi YI, Rengganis AA (2021) Knowledge and Acceptance towards Mammography as breast Cancer Screening Tool among Yogyakarta women and Health Care Providers (Mammography Screening in Indonesia). J Cancer Educ 36(3):532–537. 10.1007/s13187-019-01659-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford MJ (2002) Systematic review of involving patients in the planning and development of health care. BMJ 325(7375):1263–1263. 10.1136/bmj.325.7375.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean Wantland W-TC (2013) Engagement with Health Care Providers affects Self- Efficacy, Self-Esteem, Medication Adherence and Quality of Life in people living with HIV. J AIDS Clin Res 04(01). 10.4172/2155-6113.1000256 [DOI] [PMC free article] [PubMed]
- Duffy SW, Tabár L, Yen AM, Dean PB, Smith RA, Jonsson H, Törnberg S, Chen SL, Chiu SY, Fann JC, Ku MM, Wu WY, Hsu C, Chen Y, Svane G, Azavedo E, Grundström H, Sundén P, Leifland K, Chen TH (2020) Mammography screening reduces rates of advanced and fatal breast cancers: results in 549,091 women. Cancer 126(13):2971–2979. 10.1002/cncr.32859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder NC, Barney K (2012) But what does it Mean for me? Primary care patients’ communication preferences for test results notification. Joint Comm J Qual Patient Saf 38(4):168–AP1. 10.1016/S1553-7250(12)38022-7 [DOI] [PubMed] [Google Scholar]
- Farmer GD, Gray H, Chandratillake G, Raymond FL, Freeman ALJ (2020) Recommendations for designing genetic test reports to be understood by patients and non-specialists. Eur J Hum Genet 28(7):885–895. 10.1038/s41431-020-0579-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayanju OM, Kraenzle S, Drake BF, Oka M, Goodman MS (2014) Perceived barriers to mammography among underserved women in a Breast Health Center Outreach Program. Am J Surg 208(3):425–434. 10.1016/j.amjsurg.2014.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gail MH, Brinton LA, Byar DP, Corle DK, Green SB, Schairer C, Mulvihill JJ (1989) Projecting individualized probabilities of developing breast Cancer for White females who are being examined annually. JNCI J Natl Cancer Inst 81(24):1879–1886. 10.1093/jnci/81.24.1879 [DOI] [PubMed] [Google Scholar]
- Haga SB, Mills R, Pollak KI, Rehder C, Buchanan AH, Lipkus IM, Crow JH, Datto M (2014) Developing patient-friendly genetic and genomic test reports: formats to promote patient engagement and understanding. Genome Med 6(7):58. 10.1186/s13073-014-0058-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao L, Kraft P, Berriz GF, Hynes ED, Koch C, Korategere V, Kumar P, Parpattedar SS, Steeves M, Yu W, Antwi AA, Brunette CA, Danowski M, Gala MK, Green RC, Jones NE, Lewis ACF, Lubitz SA, Natarajan P, Vassy JL, Lebo MS (2022) Development of a clinical polygenic risk score assay and reporting workflow. Nat Med 28(5):1006–1013. 10.1038/s41591-022-01767-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Promotion Board (n.d.) Breast cancer subsidies in Singapore. https://www.healthhub.sg/a-z/costs-and-financing/30/breast-cancer-screening-subsidies. Accessed 30 May 2023
- Hennink M, Kaiser BN (2022) Sample sizes for saturation in qualitative research: a systematic review of empirical tests. Soc Sci Med 292:114523. 10.1016/j.socscimed.2021.114523 [DOI] [PubMed] [Google Scholar]
- Hennink MM, Kaiser BN, Weber MB (2019) What influences saturation? Estimating sample sizes in Focus Group Research. Qual Health Res 29(10):1483–1496. 10.1177/1049732318821692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W-K, Tan M-M, Mavaddat N, Tai M-C, Mariapun S, Li J, Ho P-J, Dennis J, Tyrer JP, Bolla MK, Michailidou K, Wang Q, Kang D, Choi J-Y, Jamaris S, Shu X-O, Yoon S-Y, Park SK, Kim S-W, Antoniou AC (2020) European polygenic risk score for prediction of breast cancer shows similar performance in Asian women. Nat Commun 11(1):3833. 10.1038/s41467-020-17680-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PJ, Ho WK, Khng AJ, Yeoh YS, Tan BK-T, Tan EY, Lim GH, Tan S-M, Tan VKM, Yip C-H, Mohd-Taib N-A, Wong FY, Lim EH, Ngeow J, Chay WY, Leong LCH, Yong WS, Seah CM, Tang SW, Hartman M (2022a) Overlap of high-risk individuals predicted by family history, and genetic and non-genetic breast cancer risk prediction models: implications for risk stratification. BMC Med 20(1):150. 10.1186/s12916-022-02334-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho W-K, Tai M-C, Dennis J, Shu X, Li J, Ho PJ, Millwood IY, Lin K, Jee Y-H, Lee S-H, Mavaddat N, Bolla MK, Wang Q, Michailidou K, Long J, Wijaya EA, Hassan T, Rahmat K, Tan VKM, Teo S-H (2022b) Polygenic risk scores for prediction of breast cancer risk in Asian populations. Genet Sci 24(3):586–600. 10.1016/j.gim.2021.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho PJ, Lim EH, Hartman M, Wong FY, Li J (2023a) Breast cancer risk stratification using genetic and non-genetic risk assessment tools for 246,142 women in the UK Biobank. Genet Sci 25(10):100917. 10.1016/j.gim.2023.100917 [DOI] [PubMed] [Google Scholar]
- Ho PJ, Tan IB, Chong DQ, Khor CC, Yuan J-M, Koh W-P, Dorajoo R, Li J (2023b) Polygenic risk scores for the prediction of common cancers in East asians: a population-based prospective cohort study. eLife 12:e82608. 10.7554/eLife.82608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M-Y, Huston SA, Perri M (2014) Consumer preferences for the predictive genetic test for Alzheimer disease. J Genet Couns 23(2):172–178. 10.1007/s10897-013-9627-x [DOI] [PubMed] [Google Scholar]
- Hughes E, Wagner S, Pruss D, Bernhisel R, Probst B, Abkevich V, Simmons T, Hullinger B, Judkins T, Rosenthal E, Roa B, Domchek SM, Eng C, Garber J, Gary M, Klemp J, Mukherjee S, Offit K, Olopade OI, Lanchbury JS (2022) Development and validation of a breast Cancer polygenic risk score on the basis of genetic ancestry composition. JCO Precision Oncol 6:e2200084. 10.1200/PO.22.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaphingst KA, Blanchard M, Milam L, Pokharel M, Elrick A, Goodman MS (2016) Relationships between Health Literacy and Genomics-Related Knowledge, Self-Efficacy, Perceived Importance, and communication in a medically Underserved Population. J Health Communication 21(Suppl 1):58–68. 10.1080/10810730.2016.1144661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komen SG (n.d.) Estimating breast cancer risk (GAIL model). https://www.komen.org/breast-cancer/risk-factor/understanding-risks/gail-method/. Accessed 29 May 2023
- Kowalski AE (2021) Mammograms and Mortality: how has the evidence evolved? J Economic Perspect 35(2):119–140. 10.1257/jep.35.2.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis ACF, Green RC, Vassy JL (2021) Polygenic risk scores in the clinic: translating risk into action. Hum Genet Genomics Adv 2(4):100047. 10.1016/j.xhgg.2021.100047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YX, Lim ZL, Ho PJ, Li J (2022a) Breast Cancer in Asia: incidence, mortality, early detection, Mammography Programs, and risk-based screening initiatives. Cancers 14(17):4218. 10.3390/cancers14174218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim ZL, Ho PJ, Khng AJ, Yeoh YS, Ong ATW, Tan BKT, Tan EY, Tan S-M, Lim GH, Lee JA, Tan VK-M, Hu J, Li J, Hartman M (2022b) Mammography screening is associated with more favourable breast cancer tumour characteristics and better overall survival: case-only analysis of 3739 Asian breast cancer patients. BMC Med 20(1):239. 10.1186/s12916-022-02440-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liow JJK, Lim ZL, Sim TMY, Ho PJ, Goh S-A, Choy SD, Chew YJ, Tan BK-T, Tan VKM, Hartman M, McCrickerd K, Li J (2022) It will lead you to make better decisions about your Health—A Focus Group and Survey Study on women’s attitudes towards Risk-based breast Cancer screening and personalised risk assessments. Curr Oncol 29(12):9181–9198. 10.3390/curroncol29120719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Ho PJ, Tan THL, Yeoh YS, Chew YJ, Riza M, Khng NK, Goh AJ, Wang S-A, Oh Y, Chin HB, Kwek CH, Zhang SC, Ong ZP, Quek DLS, Tan ST, Wee CC, Li HL, Iau J, P. T. C., Hartman M (2022) BREAst screening tailored for HEr (BREATHE)—A study protocol on personalised risk-based breast cancer screening programme. PLoS ONE 17(3):e0265965. 10.1371/journal.pone.0265965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Løberg M, Lousdal ML, Bretthauer M, Kalager M (2015) Benefits and harms of mammography screening. Breast Cancer Res 17(1):63. 10.1186/s13058-015-0525-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loy EY, Molinar D, Chow KY, Fock C (2015) National breast Cancer Screening Programme, Singapore: evaluation of participation and performance indicators. J Med Screen 22(4):194–200. 10.1177/0969141315589644 [DOI] [PubMed] [Google Scholar]
- Mavaddat N, Michailidou K, Dennis J, Lush M, Fachal L, Lee A, Tyrer JP, Chen T-H, Wang Q, Bolla MK, Yang X, Adank MA, Ahearn T, Aittomäki K, Allen J, Andrulis IL, Anton-Culver H, Antonenkova NN, Arndt V, Easton DF (2019) Polygenic risk scores for prediction of breast Cancer and breast Cancer subtypes. Am J Hum Genet 104(1):21–34. 10.1016/j.ajhg.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea R, Meany M, Carroll C, Cody N, Healy D, Green A, Lynch SA (2016) Predictive genetic testing and Alternatives to face to Face results Disclosure: a retrospective review of patients preference for alternative modes of BRCA 1 and 2 results Disclosure in the Republic of Ireland. J Genet Couns 25(3):422–431. 10.1007/s10897-015-9887-8 [DOI] [PubMed] [Google Scholar]
- Ohbe H, Hachiya T, Yamaji T, Nakano S, Miyamoto Y, Sutoh Y, Otsuka-Yamasaki Y, Shimizu A, Yasunaga H, Sawada N, Inoue M, Tsugane S, Iwasaki M, for the Japan Public Health Center-based Prospective Study Group (2023) Development and validation of genome-wide polygenic risk scores for predicting breast cancer incidence in Japanese females: a population-based case-cohort study. Breast Cancer Res Treat 197(3):661–671. 10.1007/s10549-022-06843-6 [DOI] [PubMed] [Google Scholar]
- Pettersson A, Graff RE, Ursin G, Dos Santos Silva I, McCormack V, Baglietto L, Vachon C, Bakker MF, Giles GG, Chia KS, Czene K, Eriksson L, Hall P, Hartman M, Warren RML, Hislop G, Chiarelli AM, Hopper JL, Krishnan K, Tamimi RM (2014) Mammographic density phenotypes and risk of breast Cancer: a Meta-analysis. JNCI: J Natl Cancer Inst 106(5). 10.1093/jnci/dju078 [DOI] [PMC free article] [PubMed]
- Rajendram P, Singh P, Han KT, Utravathy V, Wee HL, Jha A, Thilagaratnam S, Pathadka S (2022) Barriers to breast cancer screening in Singapore: a literature review. Ann Acad Med Singapore 51(8):493–501. 10.47102/annals-acadmedsg.2021329 [PubMed] [Google Scholar]
- Stevanato K, Pedroso R, Dell Agnolo C, Santos L, Pelloso F, Carvalho M, Pelloso S (2022) Use and Applicability of the Gail Model to calculate breast Cancer risk: a scoping review. Asian Pac J Cancer Prev 23(4):1117–1123. 10.31557/APJCP.2022.23.4.1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SS, Schwartz E, Tuepker A, Press NA, Nazi KM, Turvey CL, Nichol WP (2013) Patient experiences with full Electronic Access to Health Records and Clinical Notes through the My HealtheVet Personal Health Record Pilot: qualitative study. J Med Internet Res 15(3):e65. 10.2196/jmir.2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2016) Patient engagement. World Health Organ. https://iris.who.int/handle/10665/252269
- World Health Organization (2023) Breast Cancer. https://www.who.int/news-room/fact-sheets/detail/breast-cancer. Accessed 30 May 2023
- Yanes T, Young M-A, Meiser B, James PA (2020) Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res 22(1):21. 10.1186/s13058-020-01260-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuniar P, Kardinah K, Sinulingga D, Kartikawati A, Sirait, Lady, Widyastuti W, Utomo B, Moorin R, Norman R, Makate M, Robinson S (2022) Contextualizing policy implementation, challenges, and plans for improving breast cancer early detection programs in Indonesia [Preprint]. Research Square. 10.21203/rs.3.rs-1863931/v1
- Zeinomar N, Chung WK (2021) Cases in Precision Medicine: the role of polygenic risk scores in breast Cancer Risk Assessment. Ann Intern Med 174(3):408–412. 10.7326/M20-5874 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled accessed data storage at Nalagenetics Pte Ltd.