Abstract
We present an updated analysis on the incidence of primary brain tumors in the Metropolitan Area of Genova, the capital of the northwestern Italian region Liguria.
The number of cases and incidence rates for all malignant brain tumors, glioblastoma, malignant brain tumors other than glioblastoma, and brain tumors not otherwise specified were calculated for each of seven three-year and one-eighth four-year periods in which the quarter century 1993–2017 was divided. The rates were age-adjusted (AAR) using the 2013 European standard population, presented per 100,000 person-years and the average percentage change over the three-year period was calculated.
The number of cases of all malignant brain tumors and glioblastoma was higher in males than in females in each three-year period and in the entire quarter century 1993–2017. During the latter, the average three-year percentage change in AARs for all brain tumors was minimal [0.6 (95% C.I. = -1.0/2.1) %] while for glioblastoma there was a change of 5.3 (95% C.I. = -0.4/11.3) %. The partially concurrent decline in the incidence rates of malignant brain tumors other than glioblastoma or not otherwise specified suggests that the observed increase in the incidence rate of glioblastoma during 1993–2017 may have been at least partially linked to the improvement during the same period in sensitivity and specificity of the diagnosis of glioblastoma, depleting the reservoirs of other malignant or unspecified brain tumors. Research into possibly increased environmental risk factors (e.g., population exposure to ionizing radiation) for glioblastoma in Genova remains warranted.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79170-z.
Keywords: Brain cancer, Incidence, Age-adjusted rate, Glioblastoma, Glioma, Non-otherwise specified
Subject terms: Cancer, Neuroscience, Diseases, Health care, Medical research, Neurology, Oncology, Pathogenesis, Risk factors
Introduction
A group of tumors with low incidence but high disabling and killing capacity affects the brain both in the glia and in other cellular components and causes important hospitalization burdens1. In the 27-country Europe (EU27), around 28,000 brain tumors are diagnosed every year. The mortality/incidence ratio is higher in low/middle income than in high-income countries, suggesting more difficult access to therapies and lower quality of care in the former23 .
Brain tumors comprise 85–90% of the central nervous system (CNS) tumors https://www.cancer.net/cancer-types/brain-tumor/statistics. Exposure to ionizing radiation at a young age is the only proven environmental risk factor4. Some hereditary conditions, such as HERC2 gene variants, can also predispose to some forms of these tumors5.
Most malignant tumors arise in the glia (gliomas), neurons’ supporting and trophic tissue. Gliomas are more frequent in males and white individuals 6–8. Different mutational profiles have been observed in men and women during the progression of gliomas, suggesting that patient sex may impact the prognosis and efficacy of treatments9. In both sexes, younger age at diagnosis is associated with a better prognosis10.
Among the cell types that make up glia, astrocytes are the ones in which the tumor (astrocytoma) occurs most frequently, with an incidence rate of 4.9 cases per 100,000 person-years in Italy11. Since the latter value remains lower than the European threshold level for the definition of rare cancer (6 cases per 100,000 person-years12, astrocytomas are considered rare. However, they are the most frequent, aggressive and lethal of all brain tumors, with average 5-year relative survival rates of 59.0-89.1% for low-grade tumors [World Health Organization (WHO) grade I, II]; 39.6–55.4% for anaplastic astrocytoma (WHO grade III) and 14.2–23.1% for glioblastoma (GB; WHO grade IV)13.
Among astrocytomas, GB is by far the most common and aggressive. With an incidence of 2 to 5 cases per 100,000 person-years in the EU27 and the United States (US), GB accounts for over 50% of primary malignant brain tumors7. Current treatment guidelines provide the broadest possible surgical resection, but this can only be performed in 50–70% of patients, depending on the brain area affected by the tumor. When resection of the tumor is not possible, a biopsy can sometimes be performed for diagnostic purposes. Subsequent therapies (the so-called “Stupp” protocol from the name of the scientist who developed it) consist of radiotherapy concomitant or followed by the alkylating drug temozolomide14. The effectiveness of this treatment varies depending on the biological status (methylated/mutated) of the tumor genes O6 methylguanine DNA methyltransferase (MGMT) and isocitrate dehydrogenase (IDH) 1/2, respectively, the age of the patients and their general conditions. These treatments rarely prevent the recurrence of the tumor and its subsequent progression. In the last twenty years, no further significant progress has been made in the prevention and treatment of GB, and the therapeutic protocol used at present is essentially the Stupp’s one15. Over the last forty years, the probability of 5-year survival has increased by only 4 − 7%, primarily linked to the introduction of temozolomide, and median survival currently remains limited to 14–16 months after diagnosis7. Every year in the world, approximately 200,000 people die from GB, of which almost 16,000 in Europe and 10,000 in the US. Italy is no exception in terms of incidence and survival rates of patients with malignant brain tumors, including GB11,16.
The main aim of this work was to identify any changes in the incidence of malignant brain tumors with special reference to GB in the metropolitan area of Genova (Northwestern Italy) during the quarter of century between 1993 and 2017 using incidence cases registered by the Liguria Cancer Registry. This analysis period was selected based on the availability of complete relevant data for it.
Materials & methods
Study area
The present is a descriptive epidemiology study conducted in the Metropolitan Area of Genova. The population of the entire Area (67 Municipalities) at its administrative introduction (1 January 2015) was 853,201 inhabitants. Approximately 68% of the population (584,649 inhabitants) lives in the urban area of Genova, and 28% are over 65 years old.
Cancer registration
The Liguria Cancer Registry (LCR) is a property of the Health Councillorship of the Liguria Region and is hosted at the IRCCS Ospedale Policlinico San Martino, Genova. LCR has been active since 1986 and covers the entire population of the Genova Metropolitan Area. LCR is affiliated with the Italian Association of Cancer Registries (AIRTUM), which defines the standard rules for collecting and classifying tumor data according to the WHO-International Agency for Research on Cancer (IARC) recommendations and guidelines. The overall cancer cases of residents in the Genova Metropolitan Area are collected primarily through active consultation of the hospital discharge records provided by the Ligurian Health Information System and the pathological archive departments in the registry area. Topography and morphology of the tumors are coded according to the Third Edition of the International Classification of Oncological Diseases (ICD-O-3) https://apps.who.int/iris/bitstream/handle/10665/96612/9789241548496_eng.pdf. LCR also obtains official mortality data from the Italian National Institute of Statistics (ISTAT) and calculates mortality/incidence ratios. In the present study, the Cancer Registry data were provided in aggregate and completely anonymous form. In line with Italian legislation, since these are epidemiological surveillance evaluations that concern public health, no ethical-administrative approval from the ethics committees was necessary.
Tumor coding
All incident cases of primary brain tumors diagnosed during 1993–2017 and classified according to ICD-O-3, were considered for analysis. Pilocytic astrocytoma (9421/1) was not included in the analysis since classified as non-malignant tumor during the study period. Gliosarcoma (9442/3) is currently categorized by the WHO as a variant of GB and often grouped with GB in clinical trials. However, its peculiar histological and molecular characteristics are established and differences in clinical behavior and response to therapies with respect to GB are probably dependent on the entity of the sarcomatous component 17–20. Since these differences might influence disease classification as well as guide targeted therapy, 9442/3 was not grouped with GB in the present study (Table 1). The inclusion or exclusion of rare gliosarcoma cases (19 total cases in 1993–2017) in the GB group did not significantly affect the results presented in the present study (Tables S2,S3 - Discussion). A few additional ICD-O-3 codes present in the Central Brain Tumor Registry of the U.S. classification table of malignant brain and other CNS tumors (Table 2 of the annual report - https://urlsand.esvalabs.com/?u=https%3 A%2 F%2Facademic.oup.com%2Fneuro-oncology%2Farticle%2F25%2FSupplement_4%2Fiv1%2F7289107%23supplementary-data&e=d1becced&h=b56b3d56&f=n&p=y) which is also based on WHO classification (ICD-O-3 third edition) are missing in our Table 1 and S1. These differences may be justified as following : (i) we have only considered the ICD-O-3 site codes C70 and C71; (ii) some morphologies have been introduced since 2018 (es. 9445/3 IDH-mutated GB) while our study period ended in 2017; (iii) some histological types are not present in our incident cases.
Table 1.
Data quality indicators of intracranial tumours diagnosed 1993–2017 and archived in the a LCR.
| Entity | Cases (No). | Data quality indicator | ICD-O-3 codes | |||
|---|---|---|---|---|---|---|
|
Death certificate (%) |
Radiological examination (%) |
Microscopic verification (%) |
Topography | Morphology | ||
| All Malignancies brain tumors | 2102 | 1.7 | 38.2 | 56 | ||
| GB | 667 | - | 15.9 | 83.8 | C71. | 9440/3;9441/3 |
| NGB | 882 | - | 29.1 | 70.1 | C71. |
8802/3;9064/3;9085/3;9364/3; 9380/3;9381/3;9382/3;9390/3; 9391/3;9392/3;9400/3;9401/3; 9410/3;9411/3;9420/3;9424/3; 9425/3;9430/3;9442/3;9450/3; 9451/3;9470/3;9471/3;9472/3; 9473/3;9500/3;9503/3;9505/3; 9508/3;9590/3;9591/3;9652/3; 9671/3;9680/3;9684/3;9687/3; 9698/3;9751/3 |
| NOS | 553 | 6.5 | 79.7 | - | C71. | 8000/3 |
| MEN | 3057 | 0.2 | 58 | 38.3 | C70. | 9530–9537/0 |
a LCR, Liguria Cancer Registry; ICD-O-3, International Classification of Diseases for Oncology; GB, glioblastoma; NGB, brain malignancies other than GB; No., observed incident cases; NOS, non-otherwise specified malignancies. The following tumors were excluded: metastases.
Table 2.
Time trend in aAARs of all brain malignancies over the period 1993–2017 by sex (Standard: Europe 2013).
| Period | Male | Female | Both sexes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | CR | AAR | 95%CI | No. | CR | AAR | 95%CI | No. | CR | AAR | 95%CI | |
| 1993–1995 | 121 | 9.17 | 8.81 | 7.22–10.40 | 110 | 7.43 | 6.60 | 5.32–7.88 | 231 | 8.25 | 7.57 | 6.58–8.57 |
| 1996–1998 | 131 | 10.18 | 9.95 | 8.22–11.68 | 116 | 8.03 | 6.90 | 5.59–8.21 | 247 | 9.05 | 8.18 | 7.14–9.22 |
| 1999–2001 | 145 | 11.55 | 10.96 | 9.15–12.77 | 116 | 8.21 | 6.59 | 5.35–7.83 | 261 | 9.78 | 8.58 | 7.52–9.64 |
| 2002–2004 | 130 | 10.53 | 9.72 | 8.03–11.41 | 121 | 8.70 | 6.54 | 5.32–7.75 | 251 | 9.56 | 8.09 | 7.07–9.11 |
| 2005–2007 | 129 | 10.44 | 9.40 | 7.76–11.04 | 116 | 8.35 | 6.55 | 5.30–7.79 | 245 | 9.33 | 7.81 | 6.81–8.81 |
| 2008–2010 | 133 | 10.83 | 9.68 | 8.01–11.33 | 129 | 9.34 | 7.27 | 5.95–8.59 | 262 | 10.04 | 8.40 | 7.35–9.44 |
| 2011–2013 | 135 | 11.01 | 9.66 | 8.01–11.31 | 115 | 8.36 | 6.41 | 5.16–7.65 | 250 | 9.61 | 7.95 | 6.94–8.97 |
| 2014–2017 | 189 | 11.74 | 10.01 | 8.56–11.46 | 166 | 9.25 | 7.15 | 5.99–8.31 | 355 | 10.43 | 8.39 | 7.49–9.30 |
| Total | 1113 | 10.71 | - | - | 989 | 8.48 | - | - | 2102 | 9.53 | - | - |
| 3YMPV | - | - | 0.3 | –2.0/2.8 | - | - | 0.7 | –1.0/2.5 | - | - | 0.6 | –1.0/2.1 |
aAAR, age-adjusted incidence rate per 100,000 person-years (standard: Europe 2013); CI, confidence intervals; CR, crude incidence rate per 100,000 person-years; No., observed incident cases; 3YMPV, 3-year mean percent variation.
Table 1 reports the data quality indicators of brain tumors diagnosed in 1993–2017 and archived in the LCR. In particular, the focus has been on GB, microscopically verified GB (MVGB), other non-GB brain neoplasms (NGB), and brain tumors not otherwise specified (NOS), as well as malignant brain tumors in their entirety. 1.7% of all malignant brain tumors analyzed were known from the death certificate; 38.2% from radiological examination and 56% were subjected to microscopic verification, with a relevant percentage ranging from 83.8% of GBs to 70.1% of malignant tumors other than GB.
The age-adjusted incidence rate (AAR) trends of the benign brain tumors meningioma (MEN; 58% radiologically examined and 38.3% microscopically verified), were analyzed for comparison.
Statistical analysis
Cancer cases and population data (https://encr.eu/node/226) were stratified by sex (male, female, both sexes), age (18 five-year classes from 0 to 90 and more years) and the survey period (7 three-year periods from 1993 to 1995 to 2011–2013 plus 1 four-year period 2014–2017). The AARs per 100,000 person-years were obtained through the direct standardization method21 using the 2013 European Standard Population as reference https://ec.europa.eu/eurostat/web/products-manuals-and-guidelines/-/ks-ra-13-028. Furthermore, changes in tumor incidence over the quarter century 1993–2017 were assessed by calculating the three-year mean percentage change (3YMPV), assuming linearity. Specifically, 3YMPV values were obtained using a weighted log-normal linear regression model applied to the log-transformed AAR. All three-year AAR and 3YMPV values were accompanied by 95% confidence intervals (95% CI). As aforementioned, the present work is essentially a descriptive epidemiological study aimed at identifying temporal trends in the incidence of malignant brain tumors in a small-to-medium sized population. In this context, statistical tests (significant vs. nonsignificant) may be poorly informative in most analyses. Rather than deciding between two alternative hypotheses, our aim was to estimate exploratory epidemiological measures of what had happened during the period under study. Brain tumors, in particular GB, are very rare health events capable of generating comparative indices (3YMPV) with very high sampling variability even in populations larger than ours. In this study this variability was taken into account through 95% CIs which can also be used to evaluate the reliability/stability of our time-trend estimates. Statistical analyses were performed using Stata software, version 16.1 (StataCorp. 2020. Stata Statistical Software: Release 16.1. College Station, TX) and Prism software, version 5.0 (GraphPad Software Inc., San Diego, CA).
Results
All malignancies
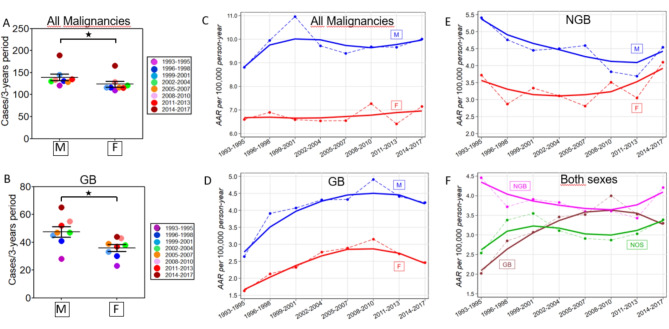
The global number of new cancer cases in Italy in 2022 was 232,150 for males and 204,092 for females, with a male/female ratio of 1.14 https://gco.iarc.who.int/media/globocan/factsheets/populations/380-italy-fact-sheet.pdf. The age-standardized global incidence rate of all cancers in Italy was higher in males (312.1 cases per 100,000 person-years) than in females (264.1 cases), with a male-to-female ratio of 1.18. Similarly, the total number of malignant brain tumors (2102 cases) diagnosed in Genova in the overall time period 1993–2017, whose morphologies are identified by the ICD-O-3 codes (Table 1), was significantly higher in males (1113 cases) than in females (989 cases) (male to female ratio: 1.12; Table 2; Fig. 1A). Such imbalances between men and women have been observed in every three-year period, albeit not always statistically significant. The AARs for all malignant brain tumors in Genova ranged from 7.57 (95%CI = 6.58–8.57) in 1993–1995 to 8.39 (95%CI = 7.49–9.30) in 2014–2017 with a 3YMPV of 0.6 [(95% CI = -1.0/2.1); Table 2; Fig. 1C]. AARs were higher in males [M, blue color; range 8.81 (95% CI = 7.22–10.40) in 1993–1995–10.01 (95% CI = 8.56–11.46) in 2014–2017] compared to females [F, red colour; range 6.60 (95% CI = 5.32–7.88 in 1993–1995) to 7.15 (95% CI = 5.99–8.31 in 2014–2017)] in accordance with sex differences reported worldwide 6,22. The 3YMPV in Genova were 0.3 (95% CI = -2.0/2.8) in males and 0.7 (95% CI = -1.0/2.5) in females. These rises are not significant, but their trend agrees with the data reported for Italy by Miranda-Filho et al. for the period 1993–2007, indicating that the incidence of brain tumors increased more rapidly in females than in males22.
Fig. 1.
Malignant brain tumors in Genova, Italy − 1993–2017. (A) The number of brain malignancies in male (M, left) and female (F, right) in each 3-years – period is shown. Brain malignancies were more common in M vs. F over each 3-year period and the whole 1993–2017 century quarter (two-tailed Mann-Whitney P value < 0.05). (B) The number of GB cases in M (left) and F (right) in each 3-years – period is shown. GB was more common in M vs. F over each 3-year period and the whole 1993–2017 century quarter (two-tailed Mann-Whitney P value < 0.05). (C) Time trend in AARs of all brain malignancies over 1993–2017 by sex. (D) Time trend in AARs of GB over 1993–2017 by sex. (E) Time trend in AARs of brain malignancies other than GB over 1993–2017 by sex. (F) Time trends in AARs of GB, NGB and NOS malignancies in both sexes.
GB
Total (diagnosed both by imaging and under the microscope) GBs (ICD-O-3 codes 9440/3-9441/3 - Table 1, S1) in Genova in the period 1993–2017 were significantly more numerous in males (380 cases) than in females (287 cases) (Table 3; Fig. 1B). Similar sex imbalances were observed for every three years. The AARs for GBs in Genova in both sexes ranged from 2.02 (95% CI = 1.45–2.59) in 1993–1995 to 3.30 (95% CI = 2.67–3.92) in 2014–2017 with a 3YMPV of 5.3 (95% CI = -0.4/11.3) indicating an increasing incidence of GB in Genova over the 25 years examined (Table 3; Fig. 1D). AARs were higher for males [range 2.64 (95% CI = 1.54–3.75) in 1993–1995 to 4.23 (95% CI = 3.19–5.28) in 2014–2017] than females [range 1.63 (95% CI = 0.96–2.31) in 1993–1995–2.46 (95% CI = 1.73–3.20) in 2014–2017]. Like all malignant brain tumor morphologies, 3YMPV in Genova for GB was higher in females [5.1 (95% CI = -1.2 /11.9)] than in males [4.2 (95% CI = -0.9 /9.5)].
Table 3.
Time trend in aAARs of GB over the period 1993–2017 by sex (Standard: Europe 2013).
| Period | Male | Female | Both sexes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | CR | AAR | 95%CI | No. | CR | AAR | 95%CI | No. | CR | AAR | 95%CI | |
| 1993–1995 | 28 | 2.12 | 2.64 | 1.54–3.75 | 23 | 1.55 | 1.63 | 0.96–2.31 | 51 | 1.82 | 2.02 | 1.45–2.59 |
| 1996–1998 | 41 | 3.19 | 3.91 | 2.60–5.22 | 30 | 2.08 | 2.13 | 1.36–2.90 | 71 | 2.60 | 2.85 | 2.17–3.53 |
| 1999–2001 | 45 | 3.59 | 4.07 | 2.81–5.33 | 33 | 2.34 | 2.32 | 1.53–3.11 | 78 | 2.92 | 3.07 | 2.38–3.76 |
| 2002–2004 | 47 | 3.81 | 4.31 | 3.03–5.59 | 38 | 2.73 | 2.77 | 1.87–3.66 | 85 | 3.24 | 3.46 | 2.71–4.21 |
| 2005–2007 | 47 | 3.80 | 4.32 | 3.03–5.61 | 39 | 2.81 | 2.89 | 1.98–3.80 | 86 | 3.28 | 3.52 | 2.76–4.28 |
| 2008–2010 | 55 | 4.48 | 4.91 | 3.59–6.23 | 43 | 3.11 | 3.15 | 2.21–4.10 | 98 | 3.76 | 4.00 | 3.20–4.79 |
| 2011–2013 | 52 | 4.24 | 4.41 | 3.20–5.63 | 37 | 2.69 | 2.72 | 1.84–3.61 | 89 | 3.42 | 3.53 | 2.79–4.26 |
| 2014–2017 | 65 | 4.04 | 4.23 | 3.19–5.28 | 44 | 2.45 | 2.46 | 1.73–3.20 | 109 | 3.20 | 3.30 | 2.67–3.92 |
| Total | 380 | 3.65 | - | - | 287 | 2.46 | - | - | 667 | 3.02 | - | - |
| 3YMPV | - | - | 4.2 | –0.9/9.5 | - | - | 5.1 | –1.2/11.9 | - | - | 5.3 | –0.4/11.3 |
aAAR, age-adjusted incidence rate per 100,000 person-years (standard: Europe 2013); GB, glioblastoma; CI, confidence intervals; CR, crude incidence rate per 100,000 person-years; No., observed incident cases; 3YMPV, 3-year mean percent variation.
Similar results were obtained by expressing GB as a percentage of all neoplasms, which in both sexes increased from 22.1% in 1993–1995 to 30.7% in 2014–2017, with an average value of 31.7% over the entire 1993–2017 study period (Table S4; Fig. S1 C). Again, the increase was more pronounced in males (range 23.1% in 1993–1995 to 34.4% in 2014–2017; 34.1% of all cancers in the entire study period 1993–2017) compared to females (range 20.9% in 1993–1995 to 26.5% in 2014–2017; 29.0% of all malignancies in the entire study period 1993–2017) (Table S4; Fig. S1 A-B).
Considering only MVGB diagnoses, the AARs ranged from 1.49 (95% CI = 1.00-1.98) in the three years 1993–1995 to 3.09 (95% CI = 2.49–3.70) in the three years 2014–2017 with a 3YMPV of 8.9 (95% CI = 3.0 /15.2) (Table S5). The AARs were higher for males [range 1.77 (95% CI = 0.95–2.59) in the three years 1993–1995 to 4.24 (95% CI = 3.19–5.28) in the three years 2014–2017] than for females [range 1.29 (95% CI = 0.69–1.89) in the three years 1993–1995 to 2.11 (1.43–2.79) in the three years 2014–2017] (Table S5, Fig. S2). The 3YMPV for MVGB was 9.2 (95% CI = 3.2 /15.6)] in males and 7.3 (95% CI = -1.0 /16.1)] in females.
Similar increasing trends were observed when MVGBs were expressed as a percentage of all GBs (Table S4). MVGB always constituted > 50% of all GB diagnoses for any three years and sex (Table S4).
NGB
Thirty-eight non-GB malignant brain tumor morphologies, whose ICD-O-3 codes are listed in Table 1 and S1, were included in this entity. The AARs for this group of multiple pathologies ranged from 4.46 (95% CI = 3.61–5.30) in 1993–1995 to 4.21 (95% CI = 3.51–4.92) in 2014–2017 with a 3YMPV of − 1.0 [95% CI = -4.2 /2.3 (Table 4; Fig. 1E)]. Stratifying by sex, NGB AARs were higher in males [range 5.41 (95% CI = 3.96–6.86) in 1993–1995 to 4.54 (95% CI = 3.43–5.64) in 2014–2017] than in females [range 3.72 (95% CI = 2.71–4.73) in 1993–1995 to 4.10 (95% CI = 3.07–4.95) in 2014–2017]. This sex difference progressively decreased over time due to the higher 3YMPV in females [1.6 (95% CI = -3.3/6.9)] compared to males [-3.1 (95% CI = -6.3/ 0.2)].
Table 4.
Time trend in aAARs of NGB over the period 1993–2017 by sex (Standard: Europe 2013).
| Period | Male | Female | Both sexes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | CR | AAR | 95%CI | No. | CR | AAR | 95%CI | No. | CR | AAR | 95%CI | ||
| 1993–1995 | 66 | 5.00 | 5.41 | 3.96–6.86 | 60 | 4.05 | 3.72 | 2.71–4.73 | 126 | 4.50 | 4.46 | 3.61–5.30 | |
| 1996–1998 | 61 | 4.74 | 4.76 | 3.43–6.09 | 47 | 3.26 | 2.87 | 2.00-3.75 | 108 | 3.96 | 3.72 | 2.96–4.48 | |
| 1999–2001 | 64 | 5.10 | 4.45 | 3.26–5.64 | 47 | 3.33 | 3.34 | 2.35–4.33 | 111 | 4.16 | 3.91 | 3.13–4.69 | |
| 2002–2004 | 59 | 4.78 | 4.50 | 3.25–5.76 | 44 | 3.16 | 3.11 | 2.17–4.05 | 103 | 3.92 | 3.83 | 3.05–4.61 | |
| 2005–2007 | 55 | 4.45 | 4.59 | 3.31–5.87 | 41 | 2.95 | 2.81 | 1.94–3.69 | 96 | 3.66 | 3.60 | 2.86–4.33 | |
| 2008–2010 | 48 | 3.91 | 3.82 | 2.63–5.01 | 50 | 3.62 | 3.51 | 2.51–4.50 | 98 | 3.76 | 3.61 | 2.86–4.36 | |
| 2011–2013 | 50 | 4.08 | 3.69 | 2.61–4.77 | 44 | 3.20 | 3.05 | 2.12–3.99 | 94 | 3.61 | 3.43 | 2.70–4.15 | |
| 2014–2017 | 72 | 4.48 | 4.54 | 3.43–5.64 | 74 | 4.13 | 4.10 | 3.07–4.95 | 146 | 4.29 | 4.21 | 3.51–4.92 | |
| Total | 475 | 4.57 | - | - | 407 | 3.49 | - | - | 882 | 4.00 | - | - | |
| 3YMPV | - | - | –3.1 | –6.3/0.2 | - | - | 1.6 | –3.3/6.9 | - | - | –1.0 | –4.2/2.3 | |
aAAR, age-adjusted incidence rate per 100,000 person-years (standard: Europe 2013); NGB, brain malignancies other than GB; CI, confidence intervals; CR, crude incidence rate per 100,000 person-years; No., observed incident cases; 3YMPV, 3-year mean percent variation.
NOS
Despite the constant progress of diagnostic techniques for brain tumors between 1993 and 2017, evidenced by the periodic publication of updated editions of the WHO classification of CNS tumors23, the classification of a significant number of neoplastic lesions remained not otherwise specified (NOS). In the period 1993–2017, the AARs for these lesions in Genova were between 2.54 (95% CI = 1.83/3.24) in 1993–1995 and 3.39 (95% CI = 2.73/4.05) in 2014–2017 with a 3YMPV equal to 0.8 (95% CI = -3.3/5.1) (Table 5). After stratification by sex, the AARs for NOS tumors ranged from 2.76 (95% CI = 1.63–3.90) in 1993–1995 to 4.58 (95% CI = 3.31–5.85) in 2014–2017 for males and from 2.26 (95% CI = 1.40–3.13) in 1993–1995 to 2.67 (95% CI = 1.91–3.43) in 2014–2017 for females (Table 5; Fig. S5). 3YMPV was 3.0 (95%CI = -4.1/10.6) in males and − 0.7 (95%CI = -4.6/3.3) in females.
Table 5.
Time trend in aAARs of NOS over the period 1993–2017 by sex (Standard: Europe 2013).
| Period | Male | Female | Both sexes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | CR | AAR | 95%CI | No. | CR | AAR | 95%CI | No. | CR | AAR | 95%CI | |
| 1993–1995 | 27 | 2.05 | 2.76 | 1.63–3.90 | 27 | 1.82 | 2.26 | 1.40–3.13 | 54 | 1.93 | 2.54 | 1.83–3.24 |
| 1996–1998 | 29 | 2.25 | 4.02 | 2.43–5.63 | 39 | 2.70 | 3.04 | 2.06–4.02 | 68 | 2.49 | 3.38 | 2.54–4.22 |
| 1999–2001 | 36 | 2.87 | 4.65 | 2.97–6.34 | 36 | 2.55 | 2.87 | 1.91–3.84 | 72 | 2.70 | 3.55 | 2.70–4.41 |
| 2002–2004 | 24 | 1.95 | 3.01 | 1.71–4.32 | 39 | 2.80 | 3.13 | 2.14–4.12 | 63 | 2.40 | 3.11 | 2.32–3.90 |
| 2005–2007 | 27 | 2.19 | 3.24 | 1.88–4.60 | 36 | 2.59 | 2.75 | 1.84–3.66 | 63 | 2.40 | 2.91 | 2.17–3.66 |
| 2008–2010 | 30 | 2.44 | 3.23 | 1.99–4.47 | 36 | 2.61 | 2.59 | 1.72–3.45 | 66 | 2.53 | 2.87 | 2.15–3.58 |
| 2011–2013 | 33 | 2.69 | 3.78 | 2.43–5.12 | 34 | 2.47 | 2.52 | 1.67–3.37 | 67 | 2.58 | 3.03 | 2.30–3.76 |
| 2014–2017 | 52 | 3.23 | 4.58 | 3.31–5.85 | 48 | 2.68 | 2.67 | 1.91–3.43 | 100 | 2.94 | 3.39 | 2.73–4.05 |
| Total | 258 | 2.48 | - | - | 295 | 2.53 | - | - | 553 | 2.51 | - | - |
| 3YMPV | - | - | 3.0 | –4.1/10.6 | - | - | –0.7 | –4.6/3.3 | - | - | 0.8 | –3.3/5.1 |
aAAR, age-adjusted incidence rate per 100,000 person-years (standard: Europe 2013); NOS, non-otherwise specified malignancies; CI, confidence intervals; CR, crude incidence rate per 100,000 person-years; No., observed incident cases; 3YMPV, 3-year mean percent variation
Comparative analysis of neoplasms
Plots of AAR trends per 100,000 person-years of GB, NGB, and NOS in both sexes have been overlaid in Fig. 1F for ease of comparison. From the analysis of both the AARs (Tables 3, 4 and 5; Fig. 1D, E, F, S3 A, B) and the percentages of GB on all tumors (Table S4; Figs. S1, S4) it can be seen that the increase in values of GB incidence between 1993 and 2010 were partially reflected by corresponding decreases in NGB and NOS (the latter limited to 2004–2010), with greater evidence in males than females (Fig. 1F, S1, S3). In 2011–2017 the incidence of GB reached a plateau and reversed the trend. Complementary trends occurred for NGB (magenta) and NOS (green) as their incidence progressively increased in the same period 2011–2017.
Effect of patients’ age at diagnosis
The percentages of GB, NGB, and NOS in all tumors were stratified into patients aged ≤ 49 years, 50–69 years, and ≥ 70 years (Tables S6, S7, S8; Fig. S4). These age groups were chosen considering that the threshold-values (50 and 69 years) permitted defining categories discernibly different by age and at the same time with reasonable sample sizes. Other choices would have strongly reduced the number of cases in the younger group (for instance, 40 instead of 50 years) or attenuated the age differences between adjacent groups (for instance, 79 instead of 69 years).
In younger patients (≤ 49 years) of both sexes, NGBs represented the most frequent neoplasms with a mean value of 75.8% over the entire study period 1993– 2017 (Table S6, Fig. S4 C). Stratification by sex led to similar results, with 76.4% and 75.0% NGB in male and female patients, respectively, during the entire study period 1993–2017 (Table S6, Fig. S4 A-B). The morphologies that most frequently affected those young patients were: 9380/3 Glioma, malignant (19.65% of cases); 9400/3 Astrocytoma, NOS (17.19% of cases); 9401/3 Astrocytoma, anaplastic (13.68% of cases) (Table S1).
In patients aged between 50 and 69 years of both sexes, GB represented the most frequent neoplasm, with an average value of 44.3% over the entire study period 1993– 2017 (Table S7, Fig. S4 F). Stratification by sex showed a higher frequency in males than females, with 47.4% and 39.9% of GBs, respectively, over the entire study period 1993–2017 (Table S7, Fig. S4 D-E). NGB closely followed GB with an average value of 41.6% over the entire study period 1993–2017 (Table S7, Fig. S4 F). Stratification by sex showed similar percentages of NGB in males and females, with 40.4% and 43.4%, respectively, over the entire study period 1993–2017 (Table S7, Fig. S4 D-E).
In older patients (≥ 70 years) of both sexes, NOS represented the most frequent neoplasms, with a mean value of 43.9% in the entire study period 1993– 2017 (Table S8, Fig. S4 I). Stratification by sex showed similar percentages in males and females, with 41.9% and 45.6% NOS over the entire study period 1993–2017, respectively (Table S8, Fig. S4 G-H).
Therefore, different tumors characterized patients with different ages at diagnosis, with NGB, GB/NGB, and NOS representing the most frequent entities in patients aged ≤ 49 years, 50–69 years, and ≥ 70 years, respectively.
MEN
MENs are the most common benign intracranial tumors https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Meningiomas. Their morphologies are identified by the ICD-O-3 codes listed in Table 1 and S1. We wished to compare the epidemiology of MEN with that of malignant brain tumors in Genova. Contrary to the malignant brain tumors, the total number of MEN (3057 cases) diagnosed in Genova in the entire study period 1993–2017 was significantly more numerous in females (2206 cases) than in males (851 cases) (Table S9). Such sex imbalances between females and males were observed in every three-year period. The AARs for MEN in patients of both sexes in Genova ranged from 9.11 (95% CI = 7.78–10.44) in 1993–1995 to 14.70 (95% CI = 13.35–16.05) in 2014–2017 with a 3YMPV of 1.9 [(95% CI = -7.4/12.3); Table S9, Fig. S6]. AARs were higher in females [range 11.08 (95% CI = 9.25–12.91) in 1993–1995 to 17.83 (95% CI = 15.88–19.79) in 2014–2017] than in males [range 6.87 (95% CI = 4.68–9.06) in 1993–1995 to 10.93 (95% CI = 9.05–12.80) in 2014–2017] in accordance with sex differences reported globally 22,6. The 3YMPV in Genova were 3.2 (-3.7/10.6) in males and 1.6 (-8.5/12.9) in females (Table S9).
Discussion
We queried the Liguria Cancer Registry database to analyze changes in the incidence of primary malignant brain tumors in the metropolitan area of Genova (northwestern Italy) between 1993 and 2017. To our knowledge, this type of analysis has never been carried out over such a long period on the Genoese population which has specific demographic properties such as a high average age24.
Two factors that are likely to impact the overall incidence of brain tumors are ionizing radiation (which may increase the risk)25–27 and a history of allergies (which may decrease the risk)28,29. An association between ageing and immunosuppression in the CNS has been reported, particularly in older age30. However, the role of the immunological system in the control of brain cancer is far from clear31. Likewise, the potential effect of cell phone use has been extensively studied, but the results remain inconclusive32–34. The socio-demographic index (SDI) is a composite indicator of per capita income, average education level and fertility rate of a population6,35. An inversely proportional relationship between SDI and brain cancer incidence in the population has been suggested36,37. The origin of most malignant tumors of the brain, as well as most other organs, remains undetermined as multiple mechanisms are likely involved.
The AARs of all malignant brain tumors examined in Genova without distinction of sex [range 7.57 (95% CI = 6.58–8.57) in 1993–1995 to 8.39 (95% CI = 7.49–9.30) in 2014–2017; Table 2; Fig. 1C] are in line with those reported in 2019 by Fan and collaborators for the Western Europe population [7.73 (95% C.I. = 5.05–9.52)]37 and slightly higher than those estimated by Leece et al. in Southern Europe [6.89 (95% CI = 6.78–6.99) cases/105 people]38. The latter difference, although not significant, could be favored by a higher SDI in Genova compared to some of the Southern Europe countries examined by Leece et al. (Malta, Portugal, Spain, Italy, Croatia, Serbia, and Slovenia). Although the socioeconomic conditions of large portions of the Genova population have progressively worsened during 1993–2017 39, the Gross Value Added (GVA) recorded in Genova remained relatively high compared to several Southern Europe countries (including Italy) examined by Leece et al. and may have contributed to a higher incidence of tumors24,36,37. The global incidence of brain tumors in Genova remained essentially stable between 1993 and 2017 with a 3YMPV of 0.6 (95% CI = -1.0/2.1) (Table 2). These estimates are consistent with previous estimates of temporal trends in brain cancer incidence worldwide. Miranda-Filho et al. reported limited changes in the AARs of both male and female brain tumors in Italy during the period 1993–2007 22. Additionally, zero change worldwide (95% CI = -5.6 to 5.3) in age-standardized life years lost between 2007 and 2017 due to CNS tumors has been reported6.
In the period 1993–2017 examined here, GBs constituted 667/2102 = 31.7% of all malignant brain tumors in Genova (Tables 2 and 3), a fraction lower than the 48.6% reported in 2021 by Miller and colleagues in the US7. Due to the notable size difference between the populations covered by the LCR in this study (~ 0.8 × 106 people) and the US Central Brain Tumor Registry in the study by Miller and colleagues (the entire US population, i.e., 332 × 106 people), the difference above in the contribution of GB to all brain tumors in Genova compared to the US is statistically significant (z-test; p < 0.001).
The average AAR per 105 person-years for the GBs calculated over the eight three-year periods between 1993 and 2017 in both sexes was 3.22 ± 0.59 in Genova (Table 3). This average value is almost identical to that previously reported for the entire US population by Miller and collaborators (3.23)7. The mean AAR for GB was higher in Genova males (4.10 ± 0.65 cases per 105/person-year) than in females (2.51 ± 0.48 cases per 105/person-year) (Table 3; Fig. 1D)]. These values are similar to those reported for the US population (4.03 vs. 2.54 cases per 105/person-year for males and females, respectively)7. GB AARs increased from 1993 to 2010 in Genova, both in males and females (Table 3; Fig. 1D, F). The corresponding decrease recorded for NGB in 1993–2010 and NOS in 2004–2010 (Fig. 1F) suggests that the increased temporal trend of GB AAR during 1993–2010 may have been at least partially linked to the significant advances in imaging and histopathology carried out in those years for the diagnosis of GB, to the detriment of diagnoses previously left unspecified (NOS-green) or incorrectly classified (NGB-magenta)40. This phenomenon was more evident in males (Table 3; Fig. S3A) than in females (Table 3; Fig. S3B).
Exposure to ionizing radiation during childhood is an established environmental risk factor for brain tumors41 while the effects of exposure during adulthood are less clear 25,42. The possible contribution to the increase in the incidence of GB in the period 1993–2010 linked to an increase in environmental exposure of Genova residents to ionizing radiation is not currently quantifiable. However, data on similar changes in the AARs of MENs (see below) may indicate that such a contribution cannot be ruled out.
Looking in more detail at MVGBs only, their AARs (Table S5; Fig. S2) as well as their percentage of all malignancies (Table S4) grew more linearly over the period 1993–2017 compared to GBs, suggesting a possible increasing use of brain biopsy, likely linked to improving surgical techniques43. The development in molecular diagnostics probably had a role in the observed trends. Traditionally, GBs has been classified based on their morphological features, with a significant variability in patient survival. Over the 1993–2017 years, advances in molecular profiling have gradually changed the diagnostic approach and classification of malignant brain tumors, leading to the development of an integrated morphological and molecular classification endowed with improved diagnostic capacity44. This expanding knowledge of brain tumor genetics and the development of new technologies required a number of updates in diagnosis and prognosis, which were regularly published in subsequent editions of WHO classifications of CNS tumors in 199345, 200046, 200747, 201648 and 202149. Merely to quote an example, in addition to classical morphological and histological analyses, the classification of astrocytoma and GB as subtypes of adult diffuse gliomas is now based on IDH mutation status molecular identification. An open question is whether MVGBs may represent a group of tumors with common characteristics compared to tumors that do not require microscopic confirmation. This critical question is beyond the scope of our study but deserves future attention. Currently, the LCR does not record any information regarding the reasons why a GB is submitted for microscopic confirmation. The analysis of trends by age could, however, suggest some considerations: for example, biopsies increased over time more in young people (≤ 49 years; Table S6) than in older people (50–69 and ≥ 70 years; Tables S7, S8) indicating more in-depth diagnostic investigations in younger and rarer GB patients compared to older and more frequent ones. Whether a more in-depth diagnostic activity may have contributed to the worldwide rising incidence of cancer in people under the age of 50 is beyond the aims of this article but deserves consideration for future studies50.
AARs for NGB showed a limited reduction in 3YMPV of -1.0% (95% CI = -4.2/2.3) (Table 4; Fig. 1E and F). Our data are similar to those reported by Crocetti and collaborators, who demonstrated that the incidence trend in Europe for gliomas during the seven years 1995–2002 was essentially flat51. They are also similar to the results obtained in the US by Lin and colleagues, showing that during the entire study period of more than forty years 1975–2018, the incidence of glioma did not change [3YMPV = 0%]52. Our data, however, differ from the studies reported above due to the temporal trend of AARs: while in Crocetti et al. a substantially flat temporal trend was observed in the period 1995–2002 and in Lin et al. the incidence of glioma increased from 1975 to 1987 (3YMPV = 5.4% [95% CI = 3.9–6.9%]) and decreased from 1987 to 2018 (3YMPV= -1.2% [95% CI = -1.5 / -0.9%]) in the present study pronounced decreases in NGB were observed from 1993 to 2010 with subsequent recovery in the following seven years (Table 4, S4, S6, S7, S8, Fig. 1E and F). After stratification by age (Tables S6, S7, S8; Fig. S4), patients ≤ 49 years appear to be affected by higher percentages of NGB, with predominant morphologies represented by 9380/3 Glioma, malignant; 9400/3 Astrocytoma, NOS; 9401/3 Anaplastic astrocytoma (Table S1).
The above trend differences compared to our results could be linked on the one hand, to the analysis of partially different ICD-O-3 malignant brain tumor morphologies in our study (41 morphologies: Table 1, S1) compared to the studies by Crocetti et al. (120 morphologies) and Lin et al. (20 morphologies) as well as the smaller size of the population analyzed in our study (2,102 total cases of malignant brain tumors registered in the period 1993–2017 compared to the total 44,947 cases registered in 1995–2002 analyzed by Crocetti et al. in 2012 and 62,159 cases registered in the period 1975–2018 and analyzed by Lin et al. in 2021 51,52.
The limited variations in the temporal trend of NGB incidence between Genova, EU27 and the US could be in part due to differences in genetic susceptibility and exposure to environmental factors of the populations. Mousavi and colleagues suggested that adhering to a Mediterranean diet may be associated with a lower likelihood of contracting glioma53. In contrast, a systematic and comprehensive investigation of diet and glioma risk in three large prospective studies conducted in the UK and US found weak or no associations between food groups, specific nutrients or dietary patterns and glioma risk54. Additional factors of variability may be linked to the different standard populations used in the present study (European standard population 2013), Crocetti et al. (European standard population 2008) and Lin et al. (US standard population 2000). In all studies, the male to female rate ratios were similar [1.31 in the present study; 1.40 in51; 1.47 in52).
In 1993–2017 in Genova, more than one in four malignant brain tumors (553/2102 = 26.3%) remained undiagnosed (NOS; Table 1). In particular, the AAR per 100,000 person-years of NOS increased from 1993 to 2001, probably due to the introduction of MRI, which made it possible to detect subtle or small brain lesions unsuitable for early biopsy techniques and to find patients with only mild symptoms (Table 5; Fig. 1F, S3, S5). Subsequently, from 2002 to 2010, the progressive improvement of biopsy techniques43 and of histopathological and molecular characterization of brain tumor morphologies, evidenced by the regular publication of subsequent editions of the WHO classification of CNS tumors23 may have facilitated a reduction in brain tumors remaining undiagnosed (NOS), with a corresponding increase of diagnosed GBs, the most frequent malignancies (Fig. 1F; Tables 3 and 5). The growing detection activity from 2010 to 2017 of small lesions linked to the ever-increasing availability of MRI devices (the installation of which in Italy in that period was around a hundred new devices/year https://www.statista.com/statistics/557511/magnetic-resonance-imaging-scanners-in-italy/ could explain the increase in the AAR of NGB and NOS in 2010–2017.
Compared to the above malignant tumor types, most MEN are diagnosed incidentally55. The AAR per 100,00 person-years of MENs increased from 1993 to 2004, then reached a plateau in 2004–2007 and decreased thereafter (Table S9; Fig. S6). This “bell-shaped” time trend in the AAR of MEN resembles the time trend in the AAR of GB, except for the well-known difference that MEN, unlike GB, are more common in females than in males7. Similar causes could underlie the similar time courses of GB and MEN. Like that of GBs, the increased detection capacity of MENs in the period 1993–2004 could be explained by the increasing availability of MRI in those years, followed by a plateau and decline in 2004–2017 related to improvements in the interpretative capacity of MRI imaging. In other words, not all lesions diagnosed radiologically in the period 1993–2004 as MEN and not constantly subjected to surgical removal given their benign nature could have been MEN (38.3% f MEN only were microscopically verified – Table 1). MEN classification underwent significant changes in 2004–2007 (e.g. WHO defined the histological features for atypia) and this may have contributed to the trend we report56. Furthermore, exposure to ionizing radiation has been recognized as an environmental risk factor for both GB and MEN25,57. That a fraction of the increase in the incidence of MEN in the period 1993–2004 might be attributed to greater exposure of the Genova population to ionizing radiation (for example, for medical reasons) rather than to improvements in diagnosis cannot presently be ruled out.
The investigation on GB incidence might have been limited by the exclusion of cases with tumors with sarcomatous component (9442/3 gliosarcoma). However, the incidence of such cases was very low (19 cases in Genova in 1993–2017), their exclusion did not affect significantly the results and is suggested by increasing histological and molecular evidences 17–20.
Conclusions
The incidence of total malignant brain tumors in Genova showed a limited increase [0.6 (95% CI = -1.0/2.1) % every three years] in the quarter century 1993–2017. The incidence of GB, the most common and lethal malignant brain tumor, has shown an increase of 5.3 (95% CI = -0.4/11.3) % every three years. The data suggest that a significant, albeit still undetermined, part of that GB increase may be linked to progress in sensitivity and specificity of GB diagnoses in Genova over 1993–2017. However, further research into possibly increased exposure of the Genova population to environmental risk factors for GB (e.g. ionizing radiation for medical reasons) remains warranted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Rosangela Filiberti, Alessandra Rosa (Clinical Epidemiology, IRCCS Ospedale Policlinico San Martino), Paolo Nozza (Hospital Pathologic Anatomy, IRCCS Ospedale Policlinico San Martino), Laura Barletta (Neuro-Radiology, IRCCS Ospedale Policlinico San Martino) and Salvina Barra (Radiation Oncology, IRCCS Ospedale Policlinico San Martino) for valuable discussions.
Abbreviations
- 3YMPV
3-year mean percent variation
- AAR
age-adjusted incidence rate per 100,000 person-years (standard: Europe 2013)
- AIRTUM
Italian Association of Cancer Registries
- CI
confidence intervals
- CNS
central nervous system
- CR
crude incidence rate per 100,000 person-years
- EU27
European Union at 27 countries
- F
female
- GB
glioblastoma
- IARC
International Agency for Research on Cancer
- ICD-O-3
International Classification of Diseases for Oncology
- IDH
isocitrate dehydrogenase
- INAIL
Italian National Institute for Insurance on Accidents at Work
- ISTAT
Italian National Institute of Statistics
- LCR
Liguria Cancer Registry
- M
male
- MGMT
O6 methylguanine DNA methyltransferase
- MVGB
microscopically-verified GB
- NGB
brain malignancies other than GB
- No.
observed incident cases
- NOS
non-otherwise specified malignancies
- SDI
Socio-Demographic Index
- SEER
Surveillance, Epidemiology, and End Results
- US
United States
- WHO
World Health Organization
Author contributions
G.F. conceived the study, analysed data, wrote the paper and supervised the study; C.C., A.P. and E.M. registered and analyzed data, wrote the paper; D.C. analyzed data; L.B. supervised the study; V.F. designed and performed analyses, wrote the paper.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Liguria Cancer Registry.
Declarations
Competing interests
The authors declare no competing interests.
Declaration of generative AI and AI-assisted technologies in the writing process
During the preparation of this work the author(s) did not use generative AI and/or AI-assisted technologies in the writing process.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rosa, A., Fontana, V., Filiberti, R. A., Pronzato, P. & Mannucci, M. Descriptive epidemiology of hospitalization of patients with a rare tumor in an Italian region. Curr. Oncol.29, 9711–9721 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mohammadi, E. et al. A global, regional, and national survey on burden and quality of Care Index (QCI) of brain and other central nervous system cancers; global burden of disease systematic analysis 1990–2017. PLoS One. 16, e0247120 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerstl, J. V. E. et al. A national stratification of the global macroeconomic burden of central nervous system cancer. J. Neurosurg., 1–9 (2022). [DOI] [PubMed]
- 4.Vienne-Jumeau, A., Tafani, C. & Ricard, D. Environmental risk factors of primary brain tumors: a review. Rev. Neurol. (Paris). 175, 664–678 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Choi, D. et al. The genomic landscape of familial glioma. Sci. Adv.9, eade2675 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer incidence, mortality, years of Life Lost, Years lived with disability, and disability-adjusted life-years for 29 Cancer groups, 1990 to 2017: a systematic analysis for the global burden of Disease Study. JAMA Oncol.5, 1749–1768 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller, K. D. et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J. Clin.71, 381–406 (2021). [DOI] [PubMed] [Google Scholar]
- 8.Wang, G. et al. Importance of the intersection of age and sex to understand variation in incidence and survival for primary malignant gliomas. Neuro Oncol.24, 302–310 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, H. et al. Sex difference of mutation clonality in diffuse glioma evolution. Neuro Oncol.21, 201–213 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ostrom, Q. T. et al. Age-specific genome-wide association study in glioblastoma identifies increased proportion of ‘lower grade glioma’-like features associated with younger age. Int. J. Cancer. 143, 2359–2366 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.AIRTUM Working Group. Italian cancer figures–report 2015: the burden of rare cancers in Italy. Epidemiol. Prev.40, 1–120 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Gatta, G. et al. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol.18, 1022–1039 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Girardi, F. et al. Global survival trends for brain tumors, by histology: analysis of individual records for 67,776 children diagnosed in 61 countries during 2000–2014 (CONCORD-3). Neuro Oncol.25, 593–606 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stupp, R. et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl. J. Med.352, 987–996 (2005). [DOI] [PubMed] [Google Scholar]
- 15.Weller, M. et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol.18, 170–186 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chebil, C. et al. Incidence, survival and geoepidemiological analysis of meningiomas and glioblastomas in the province of Catania during the 2003–2016 period. Environ. Res.200, 111286 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Han, S. J. et al. Clinical characteristics and outcomes for a modern series of primary gliosarcoma patients. Cancer. 116, 1358–1366 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Smith, D. R. et al. Clinical and molecular characteristics of gliosarcoma and modern prognostic significance relative to conventional glioblastoma. J. Neurooncol. 137, 303–311 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frandsen, S. et al. Clinical characteristics of Gliosarcoma and outcomes from standardized treatment relative to Conventional Glioblastoma. Front. Oncol.9, 1425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaki, M. M. et al. Genomic landscape of gliosarcoma: distinguishing features and targetable alterations. Sci. Rep.11, 18009–18006 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naing, N. N. Easy way to learn standardization: direct and indirect methods. Malays J. Med. Sci.7, 10–15 (2000). [PMC free article] [PubMed] [Google Scholar]
- 22.Miranda-Filho, A., Piñeros, M., Soerjomataram, I., Deltour, I. & Bray, F. Cancers of the brain and CNS: global patterns and trends in incidence. Neuro Oncol.19, 270–280 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horbinski, C., Berger, T., Packer, R. J. & Wen, P. Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat. Rev. Neurol.18, 515–529 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Vercelli, M. & Lillini, R. Deindustrialisation, demographic decline, aging, economic crisis and social involution in a metropolitan area analysed by applying Socio-Economic and Health Deprivation Indices. J. Prev. Med. Hyg.62, E709–E717 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braganza, M. Z. et al. Ionizing radiation and the risk of brain and central nervous system tumors: a systematic review. Neuro Oncol.14, 1316–1324 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Berrington, A. et al. Relationship between paediatric CT scans and subsequent risk of leukaemia and brain tumours: assessment of the impact of underlying conditions. Br. J. Cancer. 114, 388–394 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auvinen, A. et al. Diagnostic radiological examinations and risk of intracranial tumours in adults-findings from the Interphone Study. Int. J. Epidemiol.51, 537–546 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Linos, E., Raine, T., Alonso, A. & Michaud, D. Atopy and risk of brain tumors: a meta-analysis. J. Natl. Cancer Inst.99, 1544–1550 (2007). [DOI] [PubMed] [Google Scholar]
- 29.Zhang, C. & Zhu, Q. Allergy is associated with reduced risk of glioma: a meta-analysis. Allergol. Immunopathol. (Madr). 45, 553–559 (2017). [DOI] [PubMed] [Google Scholar]
- 30.Ladomersky, E. et al. The coincidence between increasing Age, Immunosuppression, and the incidence of patients with Glioblastoma. Front. Pharmacol.10, 200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mahajan, S., Schmidt, M. H. H. & Schumann, U. The Glioma Immune Landscape: A Double-Edged Sword for Treatment Regimens. Cancers (Basel) 15, doi: (2024). 10.3390/cancers15072024 (2023). [DOI] [PMC free article] [PubMed]
- 32.Ostrom, Q. T., Gittleman, H., Stetson, L., Virk, S. & Barnholtz-Sloan, J. S. Epidemiology of intracranial gliomas. Prog Neurol. Surg.30, 1–11 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Choi, K. H. et al. Mobile phone Use and Time Trend of Brain Cancer Incidence Rate in Korea. Bioelectromagnetics. 42, 629–648 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Castaño-Vinyals, G. et al. Wireless phone use in childhood and adolescence and neuroepithelial brain tumours: results from the international MOBI-Kids study. Environ. Int.160, 107069 (2022). [DOI] [PubMed] [Google Scholar]
- 35.GBD 2017 Oesophageal Cancer Collaborators. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet Gastroenterol. Hepatol.5, 582–597 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wanner, M. et al. Geographical variation in malignant and benign/borderline brain and CNS tumor incidence: a comparison between a high-income and a middle-income country. J. Neurooncol. 149, 273–282 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan, Y. et al. Burden and trends of brain and central nervous system cancer from 1990 to 2019 at the global, regional, and country levels. Arch. Public. Health. 80, 209–205 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leece, R. et al. Global incidence of malignant brain and other central nervous system tumors by histology, 2003–2007. Neuro Oncol.19, 1553–1564 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vercelli, M. & Lillini, R. Application of Socio-Economic and Health Deprivation indices to study the relationships between socio-economic status and disease onset and outcome in a metropolitan area subjected to aging, demographic fall and socio-economic crisis. J. Prev. Med. Hyg.62, E718–E727 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thenuwara, G., Curtin, J. & Tian, F. Advances in diagnostic tools and therapeutic approaches for gliomas: a Comprehensive Review. Sens. (Basel). 23, 9842. 10.3390/s23249842 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor, A. J. et al. Population-based risks of CNS tumors in survivors of childhood cancer: the British Childhood Cancer Survivor Study. J. Clin. Oncol.28, 5287–5293 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lopes, J., Baudin, C., Leuraud, K., Klokov, D. & Bernier, M. Ionizing radiation exposure during adulthood and risk of developing central nervous system tumors: systematic review and meta-analysis. Sci. Rep.12, 16209–16207 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bex, A. & Mathon, B. Advances, technological innovations, and future prospects in stereotactic brain biopsies. Neurosurg. Rev.46, 5–w (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frosina, G. Recapitulating the key advances in the diagnosis and prognosis of high-Grade gliomas: second half of 2021 Update. Int. J. Mol. Sci.24, 6375. 10.3390/ijms24076375 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kleihues, P., Burger, P. C. & Scheithauer, B. W. The new WHO classification of brain tumours. Brain Pathol.3, 255–268 (1993). [DOI] [PubMed] [Google Scholar]
- 46.Kleihues, P. et al. The WHO classification of tumors of the nervous system. J. Neuropathol. Exp. Neurol.61, 215–219 (2002). [DOI] [PubMed] [Google Scholar]
- 47.Louis, D. N. et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol.114, 97–109 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Louis, D. N. et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol.131, 803–820 (2016). [DOI] [PubMed] [Google Scholar]
- 49.Louis, D. N. et al. The 2021 WHO classification of tumors of the Central Nervous System: a summary. Neuro Oncol.23, 1231–1251 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ledford, H. Why are so many young people getting cancer? What the data say. Nature. 627, 258–260 (2024). [DOI] [PubMed] [Google Scholar]
- 51.Crocetti, E. et al. Epidemiology of glial and non-glial brain tumours in Europe. Eur. J. Cancer. 48, 1532–1542 (2012). [DOI] [PubMed] [Google Scholar]
- 52.Lin, D. et al. Trends in Intracranial Glioma incidence and mortality in the United States, 1975–2018. Front. Oncol.11, 748061 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mousavi, S. M. et al. Adherence to the Mediterranean dietary pattern in relation to glioma: a case-control study. Clin. Nutr.40, 313–319 (2021). [DOI] [PubMed] [Google Scholar]
- 54.Kuan, A. S. et al. Diet and risk of glioma: combined analysis of 3 large prospective studies in the UK and USA. Neuro Oncol.21, 944–952 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Islim, A. I. et al. The management of incidental meningioma: an unresolved clinical conundrum. Neurooncol Adv.5, i26–i34 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trybula, S. J. et al. The evolving classification of meningiomas: Integration of Molecular discoveries to inform patient care. Cancers (Basel). 16, 1753. 10.3390/cancers16091753 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Memon, A., Rogers, I., Paudyal, P. & Sundin, J. Dental X-Rays and the risk of thyroid Cancer and meningioma: a systematic review and Meta-analysis of current epidemiological evidence. Thyroid. 29, 1572–1593 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at Liguria Cancer Registry.