Abstract
Acute kidney injury (AKI) has been noticed after both COVID-19 vaccination and infection, affecting risk-benefit evaluations and vaccine hesitancy. We conducted a large-scale N3C cohort study to compare AKI incidence following COVID-19 vaccination and infection. Participants from December 2020 to August 2023 were divided into two groups based on their initially observed COVID-19 antigen exposure: COVID-19 vaccination group (n = 2,953,219) and COVID-19 infection group (n = 3,616,802). AKI was defined by diagnostic codes and serum creatinine changes within a 30 day follow-up window after exposure. The absolute risk of AKI was 0.66% in the vaccination group versus 4.88% in the infection group. After adjusting for various confounders, COVID-19 infection was associated with a significantly higher risk of AKI than COVID-19 vaccination (aHR = 10.31, P < 0.001). Our study reveals that COVID-19 vaccination is associated with a significant lower AKI risk compared to COVID-19 infection.
Subject terms: Public health, Risk factors
Introduction
While vaccination continues to be considered as a critical and highly recommended strategy for preventing COVID-19 infection and its complications1,2, as of December 2023, approximately 30% of the global population3,4, representing over 2 billion of individuals, remains unvaccinated without at least one dose of the COVID-19 vaccines. A significant barrier to achieving widespread immunity through vaccination is the concerns about vaccine safety and adverse events (AEs), constituting an important subset of vaccine hesitancy characterized by reluctance to receive the vaccine among those eligible5,6. Some rare AEs may not have been adequately tested in limited-sized clinical trials or small-scale studies, especially considering the expedited process based on emergency use authorization (EUA)7.
Given above concerns and the expanding portion of the population experiencing COVID-19 infection and its complications, it is crucial to thoroughly evaluate risk-benefit of vaccination during a pandemic context, especially when certain diseases can be both associated with vaccination and natural infection8. Notably, acute kidney injury (AKI) as a rare AE is detected through pharmacovigilance platforms like the Vaccine Adverse Event System (VAERS)9,10, VigiBase since its introduction11,12, as well as case reports and series of AKI after COVID-19 vaccination administration consistently appear13–21. Conversely, AKI is a recognized complication of natural COVID-19 infection, particularly in hospitalized patients with high mortality rates22–24. For now, vaccination is deemed acceptable under the hypothesis that an individual faces a higher risk of developing diseases, such as AKI, from COVID-19 infection compared to that from COVID-19 vaccination. However, this hypothesis has not been extensively tested on a large scale with accurate calculation.
In this study, we used the National COVID Cohort Collaborative (N3C)25,26, the largest U.S. electronic health record (EHR) dataset of patients related to COVID-19. We conducted a retrospective cohort study focusing on AKI as an AE of interest, comparing its incidence following the COVID-19 vaccination and infection. We aimed to validate the hypothesis that the risk of AKI incidence is significantly lower in individuals exposed to COVID-19 vaccination compared to those exposed to COVID-19 infection. Furthermore, we considered both COVID-19 infection and COVID-19 vaccination as different forms of COVID-19 antigens that can induce immune responses27, and explored the association between AKI incidence and two types of COVID-19 antigen and other predefined risk factors.
Methods
Dataset
The N3C dataset serves as a de-identified repository of EHR sourced from over 98 medical institutions across the United States. It aggregates demographic and clinical data from over 20 million individuals, incorporating a cohort of >7 million confirmed COVID-19 patients as of the latest update in November 2023. The dataset provides records of medical information dating back to January 1, 2018. Noteworthy features encompass a diverse range of variables, including diagnoses, laboratory results, medication history, demographics and comorbidities, etc. To ensure standardized integration, storage, and representation of data, the Observational Medical Outcomes Partnership Common Data Model (OMOP CDM)28 is employed by N3C enclave. Further details regarding the dataset’s structure and methodology can be found in previous publications25 and on the official N3C website26.
Ethics approval
The N3C Data Enclave is managed under the authority of the NIH. Data transferred to the National Center for Advancing Translational Sciences (NCATS) from N3C is conducted under a Johns Hopkins University Reliance Protocol (IRB00249128) or individual site agreements with NIH. Data usage of this study was authorized by N3C (DUR-22361BD) and had been reviewed and approved by the Medical School Institutional Review Board (IRB) at the University of Michigan (HUM00192962).
Study design
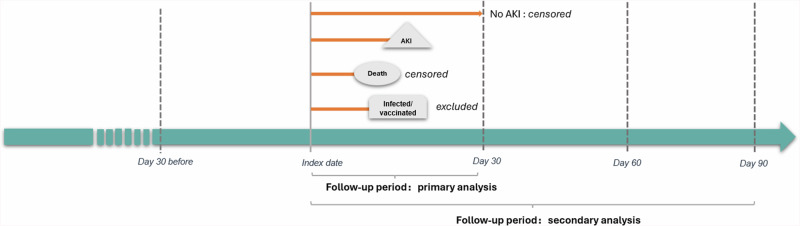
Our study adopted a comparative retrospective observational cohort design29, leveraging de-identified data of N3C from December 11, 2020, the time of COVID-19 vaccine introduction, to August 1, 2023 (Fig. 1). Two cohorts were defined as the vaccination group and the infection group based on patients’ index events, either COVID-19 vaccination or infection, with specific inclusion and exclusion criteria. The follow-up periods started at the index date and extended for a duration of 30 days to capture outcomes, with secondary analyses conducted using follow-up periods of 60 days and 90 days.
Fig. 1. Study design for the vaccination group and the infection group (December 2020 to August 2023).
Notes: Adults included in the study were categorized in two groups based on their initial exposure to COVID-19 antigens in the period over December 2020 – August 2023. The vaccination group: those whose first recorded COVID-19 vaccination preceded any infection formed the vaccine group; The infection group: those whose first documented COVID-19 infection occurred prior to any vaccination. The index event established as the initial instance of either vaccination or infection. Follow-up period: Patients were followed from the index date to 30 days (primary analysis) and 60/90 days (secondary analysis). Censored at the end of 30 days without AKI outcomes or death within 30 days (primary analysis). Selection period: 1 year before index date, assess for demographics and comorbidities. Patients who had AKI within 30 days before the index date were excluded to eliminate suspected non-onset events.Abbreviation: AKI acute kidney injury.
Exposure: COVID-19 vaccination and infection
We defined the index events as the first observed exposure to COVID-19 vaccination or COVID-19 infection. For the vaccination group, we defined the index date as the date of receiving the first dose of the vaccine. The selection of the first dose date as the index date allows us to capture the initial immune response to the COVID-19 antigen, which may result in AKI13–15. Additionally, this helps to avoid selection bias that may occur if patients who experienced adverse events after the first dose did not complete the vaccination schedule. This study includes COVID-19 vaccination recorded in the N3C database, encompassing two types of mRNA vaccines (i.e., BNT162b2 from Pfizer-BioNTech and mRNA-1273 from Moderna) and one recombinant viral vector vaccine (i.e., Ad26.COV2.S from Johnson & Johnson). The sources of N3C vaccination records included EHR, claims data, written prescriptions, and self-reported medication information. For the infection group, diagnosis codes and laboratory tests, including SARS-CoV-2 culture and nucleic acid amplification tests, were utilized to identify COVID-19 infections. Specific concept IDs are detailed in Supplementary Table 1.
Cohort building, inclusion and exclusion criteria
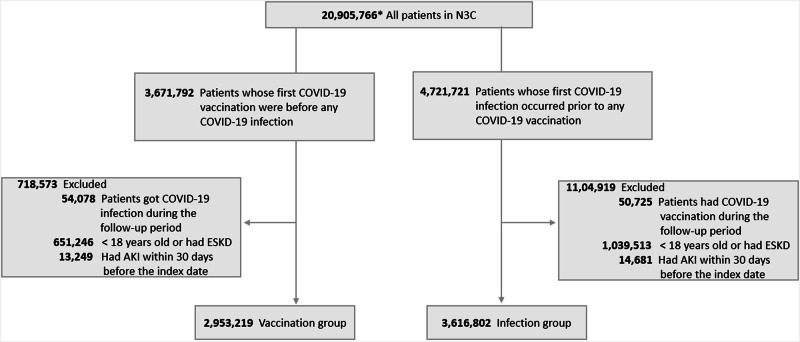
Two patient groups were identified based on their initially observed COVID-19 antigen exposure: the vaccination group, with the documented first vaccination preceding any infection, and the infection group, with the first documented infection occurring before any vaccination. For the vaccination group, the inclusion criteria included: (1) patients receiving the first dose of COVID-19 vaccines between December 11, 2020, and August 1, 2023. Only the first dose was considered for patients who received multiple doses, with the index date being the day of the first vaccination; (2) patients having never been diagnosed with COVID-19 infection or tested positive for COVID-19 before the index date or during the follow-up period. Accordingly, patients remained in the group if they experienced the first COVID-19 infection after the specific follow-up period. For the infection group, the inclusion criteria included: (1) patients who were first diagnosed with COVID-19 infection or testing positive for COVID-19 between December 11, 2020, and August 1, 2023; (2) patients who had never received COVID-19 vaccines before the first infection or during the follow-up period. Patients were classified as not vaccinated if no documented vaccination records were found in any of the N3C data sources. Similarly, patients remained in the group if their first COVID-19 vaccination was initiated after the specific follow-up period. Patients were censored at death or the end of the follow-up period. Patients who were (1) ≤18 years old, (2) diagnosed with end-stage kidney disease, and (3) with diagnostic AKI codes within the 30 days before the index date were excluded to eliminate suspected non-onset AKI. Figure 2 illustrates the patient selection process. All concept IDs used in this study are detailed in Supplementary Table 1.
Fig. 2. Flowchart of patient selection for the vaccination group and the infection group in N3C.
Notes: Adults included in the study were categorized into two groups based on their initial exposure to COVID-19 antigens (vaccines or pathogens) over December 2020 – August 2023. The vaccine group: Firstly those who received COVID-19 vaccines were included, after excluding any preceded infection or follow-up period infection, people younger than 18 years old or had ESKD history, the vaccine group formed; The infection group: Those who were confirmed withCOVID-19 infection after 2020-12-11 were included, after excluding any preceded vaccination or follow-up period vaccination, people younger than 18 years old or had ESKD history, the infection group formed. Abbreviation: ESKD end stage kidney disease, AKI acute kidney injury.
Outcome
The primary outcome was the incidence of AKI within 30 days after the index date. AKI was defined by both diagnostic codes and/or serum creatinine changes, according to the Kidney Disease Improving Global Outcomes (KDIGO) criteria23,30, which include: (1) A serum creatinine change of >0.3 mg/dL within any 48-h period during the follow-up; (2) The lowest serum creatinine value rising by >1.5 times within any 7 day period during the follow-up. (3) The maximum serum creatinine during the follow-up period being at least 1.5 times higher than the patient’s baseline creatinine value. A patient’s baseline serum creatinine was determined by the following criteria30,31: (1) the average serum creatinine value within 2 years prior to the index date; (2) If no records were available within the 2 year period, the lowest serum creatinine value during the follow-up period was considered as the baseline value.
Primary analysis
We assessed each patient’s history of previous AKI and other comorbidities within 1 year prior to the index date using the N3C data. The demographic characteristics and AKI incidence in both groups were compared using Chi-square tests for categorical variables and t-tests for continuous variables. Absolute risk and relative risk of AKI, along with their 95% confidence intervals, were calculated. Time-to-event data were analyzed using the Kaplan-Meier (KM) method and Cox proportional hazards model. The Cox model, adjusted for demographic factors and comorbidity status, was utilized to evaluate the hazard ratio of AKI incidence following COVID-19 infection compared to COVID-19 vaccination.
Secondary analysis
We conducted a secondary analysis encompassing three dimensions:(1) Temporal phases: We divided the study period into three phases corresponding to the predominant strains during the pandemic era: Alpha (Phase 1, P1) from December 11, 2020, to April 1, 2021; Delta (Phase 2, P2) from April 1, 2021, to November 30, 2021; and Omicron and its subvariants (Phase 3, P3) from December 1, 2021, until the study’s end date32. (2) Extended follow-up period: We extended the follow-up period from 30 days to 60 and 90 days after the index date. (3) AKI measurement methods: We included different AKI measurements, defined by either diagnostic codes only, changes in serum creatinine only, or both. These dimensions aimed to assess the consistency of results between two groups across different temporal phases, extended time-to-event periods and AKI measurement methods. We further assessed AKI incidence by different strains (Alpha, Delta and Omicron) in the infection group and by different types of vaccines (mRNA vaccines and viral vector vaccines) in the vaccination group.
All analyses were conducted within the N3C enclave using SQL, Python, and R programming languages. Various packages, including ggplot2, survival, and survminer, were employed for analysis.
Results
Of the 6,570,021 adults included in the study, 2,953,219 individuals received COVID-19 vaccines (mean age 52.60 [SD 18.19], 60.3% female, 66.4% white), while 3,616,802 individuals contracted COVID-19 infection (mean age 47.99 [SD 17.79], 56.3% female, 68.6% white). Vaccination distribution peaked in P2 (54.8%), then P1 (34.0%). Conversely, most initial infections occurred in P3 (44.9%), followed by P2 (35.4%) (Table 1).
Table 1.
Baseline Characteristics of Patients Included in COVID-19 Vaccination Group and Infection Group
| Total (N = 6,570,021) no.(%) | Vaccination Group (N = 2,953,219) no.(%) | Infection Group (N = 3,616,802) no.(%) | P-valueb | |
|---|---|---|---|---|
| Age, mean(SD), y | 50.06 (18.22) | 52.60 (18.19) | 47.99 (17.97) | <0.001 |
| Age category, y | ||||
| <30 | 1,072,333 (16.3) | 390,224 (13.2) | 682,109 (18.9) | <0.001 |
| 30-49 | 2,183,346 (33.2) | 894,977 (30.3) | 1,288,369 (35.6) | |
| 50-64 | 1,651,881 (25.1) | 774,464 (26.2) | 877,417 (24.3) | |
| 65-90 | 1,662,461 (25.3) | 893,554 (30.3) | 768,907 (21.3) | |
| Race | ||||
| White | 4,442,098 (67.6) | 1,961,718 (66.4) | 2,480,380 (68.6) | <0.001 |
| Black | 895,130 (13.6) | 424,031 (14.4) | 471,099 (13.0) | |
| Asian | 212,645 (3.2) | 126,783 (4.3) | 85,862 (2.4) | |
| Other | 289,348 (4.4) | 92,820 (3.1) | 196,528 (5.4) | |
| Unknown | 730,800 (11.1) | 347,867 (11.8) | 382,933 (10.6) | |
| Gender | ||||
| Female | 3,820,272 (58.1) | 1,781,799 (60.3) | 2,035,693 (56.3) | <0.001 |
| Male | 2,756,048 (41.9) | 1,171,420 (39.7) | 1,581,109 (43.7) | |
| Ethnicity | ||||
| Hispanic or Latino | 693,295 (10.6) | 326,377 (11.1) | 366,918 (10.1) | <0.001 |
| Not Hispanic or Latino | 5,265,056 (80.1) | 2,444,651 (82.8) | 2,820,405 (78.0) | |
| Unknown | 611,670 (9.3) | 182,191 (6.2) | 429,479 (11.9) | |
| AKI defined by | ||||
| Only diagnostic codes | 26,466 (0.4) | 3,518 (0.1) | 22,948 (0.6) | <0.001 |
| Only Scra changes | 96,724 (1.5) | 11,351 (0.4) | 85,373 (2.4) | |
| Both methods | 73,162 (1.1) | 4,752 (0.2) | 68,410 (1.9) | |
| Previous AKI history | 91,699 (1.4) | 48,000 (1.6) | 43,699 (1.2) | <0.001 |
| Hypertension | 1,297,616 (19.8) | 707,056 (23.9) | 590,560 (16.3) | <0.001 |
| Diabetes mellitus | 271,745 (4.1) | 144,695 (4.9) | 127,050 (3.5) | <0.001 |
| Heart failure | 189,004 (2.9) | 102,316 (3.5) | 86,688 (2.4) | <0.001 |
| Cardiovascular disease | 711,533 (10.8) | 379,434 (12.8) | 332,099 (9.2) | <0.001 |
| Obesity | 574,859 (8.7) | 300,759 (10.2) | 274,100 (7.6) | <0.001 |
| Period of strains | ||||
| P1: Alpha | - | - | 713,347 (19.7) | <0.001 |
| P2: Delta | - | - | 1,280,905 (35.4) | <0.001 |
| P3: Omicron | - | - | 1,622,550 (44.9) | <0.001 |
| Vaccine type | ||||
| mRNA | - | 2,645,093 (89.6) | - | <0.001 |
| Viral vector | - | 172,269 (5.8) | - | <0.001 |
| Unknownc | - | 135,857 (4) | - | <0.001 |
a Abbreviation for Serum Creatinine.
b Comparisons are made between the COVID-19 group and COVID-19 vaccination group using t-tests for continuous variables and Chi-square tests for categorical variables.
c Types of COVID-19 vaccine were not reported.
The absolute 30 day risk of AKI in the vaccination group was 0.66% (95% CI, 0.64% – 0.68%), contrasting with 4.88% (95% CI, 4.81% – 4.95%) in the infection group, resulting in a relative risk of 7.35 (95% CI, 7.25 – 7.46, P < 0.001). Among the 19,621 patients experiencing AKI in the vaccination group (mean age 63.91 [SD 15.22] years, 48.9% female, and 62.8% white), the onset occurred after a mean of 13.16 [SD 9.50] days. In comparison, the 176,731 patients with AKI in the infection group (mean age 63.95 [SD 15.87] years, 42.7% female, and 64.0% white) had an onset after a mean of 3.46 [SD 6.09] days. Overall, serum creatinine changes were the predominant indicators of AKI events, capturing 57.9% for the vaccination group and 48.3% for the infection group. Diagnostic codes accounted for the smallest proportion, at 17.9% and 13.0%, respectively. Our study found that 3.1% of AKI patients (n = 610) of the vaccination group and 14.3% of AKI patients (n = 25,366) of the infection group deceased within the 30 day observed period (Supplementary Table 2).
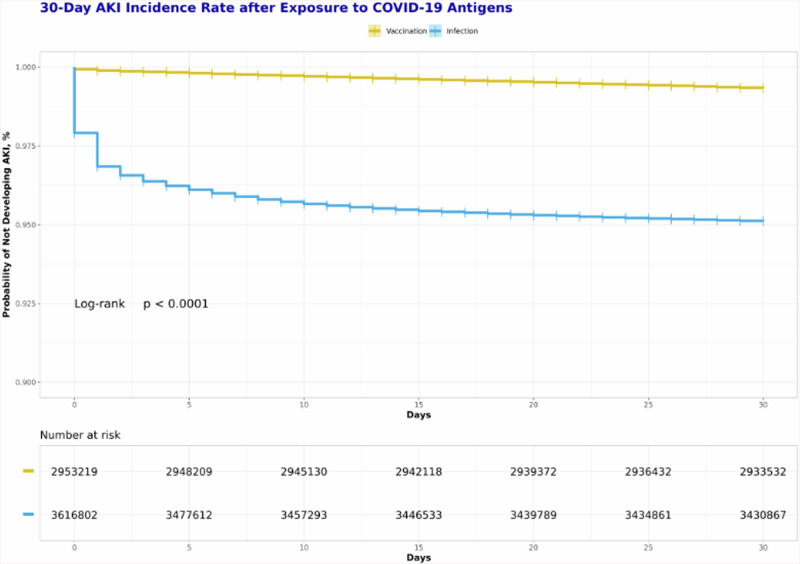
The time-to-event analysis revealed that the probability of developing AKI in the infection group was significantly higher than that in the vaccination group from day 0 to day 30(Log-Rank test, P < 0.001)(Fig. 3). Notably, the KM curve for the infection group exhibited a substantial drop from day 0 to day 3, followed by a smoothing out of the curve after 10 days. In contrast, the curve for the vaccination group showed a consistently smooth trend throughout the entire follow-up period.
Fig. 3. Comparative Kaplan-Meier analysis of AKI incidence within 30 days after exposure to COVID-19 antigens.
Notes: Adults included in the study were categorized in two groups based on their initial exposure to COVID-19 antigens in the period over December 2020 to August 2023. The vaccination group: those whose first recorded COVID-19 vaccination preceded any infection formed the vaccination group; The infection group: those whose first documented COVID-19 infection occurred prior to any vaccination. Patients were followed from the index date to 30 days to observe AKI incidence. The probability of developing AKI in the infection group was significantly higher than that in the vaccination group from day 0 to day 30(Log-Rank test, P < 0.001). Abbreviation: AKI acute kidney injury.
In the univariate analysis of AKI incidence using the Cox proportional hazards model(Table 2), COVID-19 infection demonstrated a 7.55-fold higher hazard of AKI incidence compared to COVID-19 vaccination (HR, 7.55; 95% CI, 7.43-7.66, P < 0.001). In the multivariable analysis adjusting for demographics, previous AKI history, and comorbidities, the COVID-19 infection remained a significantly higher risk factor with an adjusted hazard ratio (aHR) of 10.31 (95% CI, 10.16–10.47, P < 0.001) compared to the COVID-19 vaccination.
Table 2.
Association Between COVID-19 Vaccine and Infection and AKI Incidence: Results from Univariable and Multivariable-Adjusted Cox Models
| HR | Adjusted HR | |
|---|---|---|
| COVID-19 vaccination | Reference | Reference |
| COVID-19 infection | 7.55 (7.43-7.66)a | 10.31 (10.16-10.47)a |
| Previous AKI history | 8.90 (8.77-9.03)a | 3.17 (3.12-3.23)a |
| Age <30 | Reference | Reference |
| Age:30-49 | 2.17 (2.11-2.22)a | 2.26 (2.20-2.32)a |
| Age:50-64 | 5.26 (5.13-5.40)a | 5.55 (5.41-5.69)a |
| Age:65-90 | 10.78 (10.52-11.06)a | 11.43 (11.14-11.72)a |
| White race | Reference | Reference |
| Black race | 1.62 (1.60-1.64)a | 2.09 (2.06-2.11)a |
| Asian race | 0.73 (0.71-0.76)a | 1.10 (1.06-1.13)a |
| Other race | 0.91 (0.89-0.93)a | 1.17 (1.14-1.20)a |
| No race info | 0.90 (0.89-0.92)a | 1.28 (1.25-1.30)a |
| Female | Reference | Reference |
| Male | 1.83 (1.80-1.85)a | 1.59 (1.58-1.60)a |
| Hispanic or Latino | Reference | Reference |
| Not Hispanic or Latino | 1.27 (1.25-1.29)a | 0.93 (0.91-0.95)a |
| Unknown ethnicity | 0.89 (0.87-0.91)a | 0.59 (0.57-0.60)a |
| Hypertension | 2.55 (2.53-2.57)a | 0.98 (0.97-0.99)a |
| Diabetes mellitus | 4.02 (3.97-4.07)a | 1.67 (1.65-1.70)a |
| Heart failure | 5.79 (5.71-5.86)a | 1.70 (1.67-1.72)a |
| Cardiovascular disease | 3.58 (3.54-3.61)a | 1.38 (1.36-1.40)a |
| Obesity | 1.57 (1.52-1.56)a | 0.97 (0.96-0.98) a |
Abbreviation: HR hazard ratio, CI confidence interval.
ap < 0.001.
The multivariable analysis also demonstrated significantly higher risks of AKI in older age groups (age group of 65-90: aHR, 11.43; 95% CI, 11.14–11.72, P < 0.001) and among individuals with a previous AKI history (aHR, 3.17; 95% CI, 3.12-3.23, P < 0.001) (Table 2). Additionally, male gender (aHR, 1.59; 95% CI, 1.58–1.60, P < 0.001), diabetes mellitus (aHR, 1.67; 95% CI, 1.65–1.70, P < 0.001), a history of heart failure (aHR, 1.70; 95% CI, 1.67–1.72, P < 0.001) and cardiovascular disease (aHR, 1.38; 95% CI, 1.36–1.40, P < 0.001) were associated with an increased hazard of developing AKI. Individuals of black race (aHR, 2.09; 95% CI, 2.06–2.11, P < 0.001), asian race (aHR, 1.10; 95% CI, 1.06–1.13, P < 0.001), other races (aHR, 1.17; 95% CI, 1.14–1.20, P < 0.001) and unknown race (aHR, 1.28; 95% CI, 1.25–1.30, P < 0.001) exhibited higher hazards of AKI. Individuals who did not identify themselves as Hispanic or Latino had a lower hazard (aHR, 0.93; 95% CI, 0.91–0.95, P < 0.001), as did those with no ethnicity information (aHR, 0.59; 95% CI, 0.57-0.60, P < 0.001), compared to individuals who identified themselves as Hispanic or Latino.
The secondary analysis results were consistent with primary analysis results. The risk of AKI was lower in the vaccination group compared to the infection group across all pandemic periods (Supplementary Fig. 1). Extending the observation period to 60 and 90 days showed a similar trend of the K-M curve to the initial 30 day period (Supplementary Fig. 2). Different methods of measuring AKI consistently showed lower risk with vaccination compared to infection. In all outcomes, COVID-19 infection posed a higher hazard of AKI than COVID-19 vaccination (Supplementary Fig. 3).
In the vaccination group, 2,645,093 patients received mRNA vaccines, with 16,455 (0.6%) experiencing AKI events, and 172,269 patients received recombinant viral vector vaccines, with 1,533 (0.9%) experiencing AKI events (p < 0.001). In the infection group, 1,622,550 patients were infected in the Omicron phase, with 77,635 (4.8%) developing AKI, compared to 1,280,905 patients in the Delta phase, with 65,215 (5.1%) developing AKI, and 713,347 patients in the Alpha phase, with 33,881 (4.7%) developing AKI (p < 0.001) (Supplementary Table 2).
Discussion
In a retrospective observational cohort study with a large-scale population, we observed a substantial disparity in AKI incidence between the COVID-19 vaccination group and the COVID-19 infection group. The infection group exhibited a significantly higher risk of AKI compared to the vaccination group.
Our findings contribute to addressing vaccine hesitancy arising from concerns about vaccine safety, particularly underscoring a significantly lower risk of AKI following COVID-19 vaccination. With the ongoing emergence of variants and a growing population affected by COVID-19, regular vaccination stands as a crucial preventive measure against the disease. Consequently, our results offer insights for the risk-benefit evaluation of vaccination in a post-pandemic context, where patients face over 10 times higher risk of AKI after COVID-19 infection compared to vaccination after adjustment. Another important aspect of the risk-benefit evaluation is to examine the natural background rate of the specific AE. Previous studies on the background rate of AKI generally varied considerably due to differences in the targeted population, diagnostic criteria, and whether the AKI is hospital or community-acquired, ranging from 1% to 60%33; our finding of the AKI incidence rate after COVID-19 vaccination is 0.66%, but direct comparisons cannot be made. Further investigation into this aspect is crucial.
To our knowledge, our study is the first to use EHR data to investigate the risk of AKI after COVID-19 vaccination and compare it with the risk of AKI after COVID-19 infection in a large-scale general population. Previous case studies documenting AKI following COVID-19 vaccination lacked generalizability due to limited population sizes; Other studies relied data from pharmacovigilance databases: one study identified 1,113 AKI cases after COVID-19 vaccination from VAERS, however the fundamental incidence rate could not be calculated due to a lack of data on the total vaccinated population10. Another study reported an AKI incidence rate of 3.03 per million administered COVID-19 vaccine doses using EudraVigilance and VAERS11. This rate was much lower than our finding, as they considered the number of administered COVID-19 vaccine doses across countries instead of analyzing the number of vaccinated patients individually, and data from pharmacovigilance may be subject to reporting bias34. While our study leveraged over 70 multi-center integrated EHR datasets, ensuring a representative and comprehensive population for robust results. We positively identified AKI cases through clinical diagnosis and laboratory results, rather than relying on self-reporting. So our findings offer a supplementary and positive method for vaccine safety surveillance35, further helping to address vaccine hesitancy.
Given the established benefits of COVID-19 vaccination36–38, comprehensive comparisons of AE risks between infection and vaccination facilitates the risk-benefit balance of COVID immunization strategies. Previous studies have highlighted substantially higher risks of thrombotic events after COVID-19 infection compared to COVID-19 vaccination in both self-controlled and cohort studies39–41. Similarly, a self-controlled study revealed the increased risk of immune-mediated neurological events after infection, whereas no such increase was found after vaccination41. In addition to the aforementioned AEs, AKI incidence following vaccination warrants investigation, considering the theoretical possibility of vaccine-induced podocyte injury leading to kidney injury42,43. Therefore, our study contributes to a comprehensive safety profile of COVID-19 vaccines.
Two aforementioned studies utilized a self-controlled study design, which might introduce time-varying confounders44. While our study adopted an observational cohort study for comparison. Furthermore, our research included secondary analysis and time-to-event analysis. We observed a consistently stable time-to-event curve within 30 days after COVID-19 vaccination, indicating a low risk of AKI. It is possible for events to occur beyond the 30 day follow-up period or vary with different strains and AKI measurement methods. However, extended studies over 60 and 90 days follow-up periods, various strains and AKI measurement consistently demonstrated this low-risk pattern in line with our primary findings.
We calculated the crude rates and found statistical differences between vaccine types, suggesting that the viral vector vaccine has a higher crude incidence rate of AKI than the mRNA vaccine. Our results are consistent with a previous study that found higher AKI reporting rates for viral vector vaccines compared to mRNA vaccines after COVID-19 vaccination11. However, our findings show associations instead of causality. Further studies are needed to investigate the biological link between AKI and vaccine types.
Note that the etiology of AKI following COVID-19 vaccination was not investigated in this study. While the etiology has not not been determined yet, recent studies hypothesize that tissue antigens like transglutaminase, antiextraction nuclear antigen, and thyroid peroxidase can react with SARS-CoV-2 antibodies from vaccination45,46. The process of COVID-19 vaccines may activate antigen-presenting cells (APCs), leading to strong CD4+ and CD8 + T-cell responses and significant inflammatory cytokine release. This may trigger a cytokine storm and future lead to AKI.
We observed that the crude incidence of AKI was the highest in the Delta phase, followed by the Omicron and Alpha phases. A study with smaller sample size found a statistically significant difference of AKI incidence, indicating a higher incidence rate in the Omicron group compared to the Delta group47, but the conclusion was made among critically ill patients, which was a totally different study population from ours. Therefore, further studies are needed in this regard.
The possible pathophysiology of COVID-19 infection-related AKI involves several complex mechanisms48. These include the potential direct viral infection of renal cells, which can damage the kidney tissue directly. Additionally, the inflammatory and immune responses triggered by the virus can lead to further renal injury through cytokine release and immune cell infiltration49. Activation of the coagulation system can result in microthrombi formation, impairing renal blood flow and causing ischemic damage. Moreover, the renin-angiotensin system may become dysregulated, exacerbating renal injury through altered blood pressure regulation and inflammatory pathways. Together, these factors contribute to the multifaceted pathogenesis of COVID-19 related AKI.
Our results identified many predefined risk factors that contributed to AKI incidence after exposure to COVID-19 antigens. We found that age was highly associated with the risk of AKI, which was consistent with recent reports on AKI after COVID-19 vaccination and infection10,11. This susceptibility might be attributed to physiological alterations associated with aging, a gradual decline in renal function, compromised immune responses, comorbidities and undiagnosed chronic diseases among the elderly50. We also found that the factor of previous AKI history increased the risk of AKI. This could be attributed to potential lingering effects on renal resilience, the persistence or progression of underlying conditions, and the sensitization to subsequent insults. We observed that minority races and ethnicities had lower risk of AKI. But this might be underestimated by residual confounding factors such as socioeconomic factors, cultural influences, lifestyle differences, or healthcare-seeking behaviors, et al. To draw more robust conclusions, further investigations are imperative.
Our study has some limitations. Firstly, the inherent weaknesses of EHR data pose challenges, such as potential inaccuracies, missing or incomplete records about COVID-19 vaccination and infection in N3C, and variations in data quality. Despite the extensive data available in N3C, it represents only a portion of the country rather than its entirety, limiting the generalizability of our findings. Our study design is retrospective and observational, introducing potential biases due to unmeasured confounders and lacking the ability to establish causal relationships between outcomes and exposures. Given these limitations, our findings need to be interpreted with caution. Meanwhile, the novelty of our research is also embedded in the above limitations. The N3C dataset provides the largest ever EHR dataset related to COVID-19 for an unparalleled opportunity to investigate the vaccine adverse events and compare them with the occurrences of the diseases or symptoms following infections. COVID-19 pandemic made the majority of people worldwide infected; however, the massive COVID-19 vaccination campaign significantly reduced the severity of the disease. The simultaneous comparison of an adverse event such as the AKI provides us a unique angle to investigate the risk of a health outcome such as AKI following vaccination or natural infection. This work significantly supports our addressing of the vaccine hesitancy issue, promoting the wider usage of vaccination for the benefit of public health.
In conclusion, our retrospective cohort study reveals a significant difference in AKI incidence between individuals who received the COVID-19 vaccination and those who had COVID-19 infection. Specifically, individuals who received the COVID-19 vaccination experienced a significantly lower risk of AKI incidence compared to those who had COVID-19 infection.
Disclaimer
The N3C Publication committee confirmed that this manuscript msid:1834.309 is in accordance with N3C data use and attribution policies; however, this content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the N3C program.
Supplementary information
Acknowledgements
This study was done under the auspices of two NIH U24 grants (U24AI171008 and R01AI158543), a grant from the Centers for Disease Control and Prevention (NU58DP006956), a grant from National Natural Science Foundation of China (Grant No. 82460015) and a non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (2019PT320003). The funders played no role in the study design, data collection, analysis, and interpretation of data, or the writing of this manuscript. The analyses described in this publication were conducted with data or tools accessed through the NCATS N3C Data Enclave and N3C Attribution & Publication Policy v 1.2-2020-08-25b supported by NCATS U24 TR002306. This research was possible because of the patients whose information is included within the data and the organizations (covid.cd2h.org/dtas) and scientists (covid.cd2h.org/dtas) who have contributed to the on-going development of this community resource (cite this 10.1093/jamia/ocaa196). We gratefully acknowledge the following core contributors to N3C: Adam B. Wilcox, Adam M. Lee, Alexis Graves, Alfred (Jerrod) Anzalone, Amin Manna, Amit Saha, Amy Olex, Andrea Zhou, Andrew E. Williams, Andrew Southerland, Andrew T. Girvin, Anita Walden, Anjali A. Sharathkumar, Benjamin Amor, Benjamin Bates, Brian Hendricks, Brijesh Patel, Caleb Alexander, Carolyn Bramante, Cavin Ward-Caviness, Charisse Madlock-Brown, Christine Suver, Christopher Chute, Christopher Dillon, Chunlei Wu, Clare Schmitt, Cliff Takemoto, Dan Housman, Davera Gabriel, David A. Eichmann, Diego Mazzotti, Don Brown, Eilis Boudreau, Elaine Hill, Elizabeth Zampino, Emily Carlson Marti, Emily R. Pfaff, Evan French, Farrukh M Koraishy, Federico Mariona, Fred Prior, George Sokos, Greg Martin, Harold Lehmann, Heidi Spratt, Hemalkumar Mehta, Hongfang Liu, Hythem Sidky, J.W. Awori Hayanga, Jami Pincavitch, Jaylyn Clark, Jeremy Richard Harper, Jessica Islam, Jin Ge, Joel Gagnier, Joel H. Saltz, Joel Saltz, Johanna Loomba, John Buse, Jomol Mathew, Joni L. Rutter, Julie A. McMurry, Justin Guinney, Justin Starren, Karen Crowley, Katie Rebecca Bradwell, Kellie M. Walters, Ken Wilkins, Kenneth R. Gersing, Kenrick Dwain Cato, Kimberly Murray, Kristin Kostka, Lavance Northington, Lee Allan Pyles, Leonie Misquitta, Lesley Cottrell, Lili Portilla, Mariam Deacy, Mark M. Bissell, Marshall Clark, Mary Emmett, Mary Morrison Saltz, Matvey B. Palchuk, Melissa A. Haendel, Meredith Adams, Meredith Temple-O’Connor, Michael G. Kurilla, Michele Morris, Nabeel Qureshi, Nasia Safdar, Nicole Garbarini, Noha Sharafeldin, Ofer Sadan, Patricia A. Francis, Penny Wung Burgoon, Peter Robinson, Philip R.O. Payne, Rafael Fuentes, Randeep Jawa, Rebecca Erwin-Cohen, Rena Patel, Richard A. Moffitt, Richard L. Zhu, Rishi Kamaleswaran, Robert Hurley, Robert T. Miller, Saiju Pyarajan, Sam G. Michael, Samuel Bozzette, Sandeep Mallipattu, Satyanarayana Vedula, Scott Chapman, Shawn T. O’Neil, Soko Setoguchi, Stephanie S. Hong, Steve Johnson, Tellen D. Bennett, Tiffany Callahan, Umit Topaloglu, Usman Sheikh, Valery Gordon, Vignesh Subbian, Warren A. Kibbe, Wenndy Hernandez, Will Beasley, Will Cooper, William Hillegass, Xiaohan Tanner Zhang. Details of contributions available at covid.cd2h.org/core-contributors. We also appreciate the Unit for Laboratory Animal Medicine of the University of Michigan for hosting YP’s visiting research.N3C Consortium Data partners of N3C consortium: Advocate Health Care Network — UL1TR002389: The Institute for Translational Medicine (ITM) • Aurora Health Care Inc — UL1TR002373: Wisconsin Network For Health Research • Boston University Medical Campus — UL1TR001430: Boston University Clinical and Translational Science Institute • Brown University — U54GM115677: Advance Clinical Translational Research (Advance-CTR) • Carilion Clinic — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • Case Western Reserve University — UL1TR002548: The Clinical & Translational Science Collaborative of Cleveland (CTSC) • Charleston Area Medical Center — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) • Children’s Hospital Colorado — UL1TR002535: Colorado Clinical and Translational Sciences Institute • Columbia University Irving Medical Center — UL1TR001873: Irving Institute for Clinical and Translational Research • Dartmouth College — None (Voluntary) Duke University — UL1TR002553: Duke Clinical and Translational Science Institute • George Washington Children’s Research Institute — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • George Washington University — UL1TR001876: Clinical and Translational Science Institute at Children’s National (CTSA-CN) • Harvard Medical School — UL1TR002541: Harvard Catalyst • Indiana University School of Medicine — UL1TR002529: Indiana Clinical and Translational Science Institute • Johns Hopkins University — UL1TR003098: Johns Hopkins Institute for Clinical and Translational Research • Louisiana Public Health Institute — None (Voluntary) • Loyola Medicine — Loyola University Medical Center • Loyola University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Maine Medical Center — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • Mary Hitchcock Memorial Hospital & Dartmouth Hitchcock Clinic — None (Voluntary) • Massachusetts General Brigham — UL1TR002541: Harvard Catalyst • Mayo Clinic Rochester — UL1TR002377: Mayo Clinic Center for Clinical and Translational Science (CCaTS) • Medical University of South Carolina — UL1TR001450: South Carolina Clinical & Translational Research Institute (SCTR) • MITRE Corporation — None (Voluntary) • Montefiore Medical Center — UL1TR002556: Institute for Clinical and Translational Research at Einstein and Montefiore • Nemours — U54GM104941: Delaware CTR ACCEL Program • NorthShore University HealthSystem — UL1TR002389: The Institute for Translational Medicine (ITM) • Northwestern University at Chicago — UL1TR001422: Northwestern University Clinical and Translational Science Institute (NUCATS) • OCHIN — INV-018455: Bill and Melinda Gates Foundation grant to Sage Bionetworks • Oregon Health & Science University — UL1TR002369: Oregon Clinical and Translational Research Institute • Penn State Health Milton S. Hershey Medical Center — UL1TR002014: Penn State Clinical and Translational Science Institute • Rush University Medical Center — UL1TR002389: The Institute for Translational Medicine (ITM) • Rutgers, The State University of New Jersey — UL1TR003017: New Jersey Alliance for Clinical and Translational Science • Stony Brook University — U24TR002306 • The Alliance at the University of Puerto Rico, Medical Sciences Campus — U54GM133807: Hispanic Alliance for Clinical and Translational Research (The Alliance) • The Ohio State University — UL1TR002733: Center for Clinical and Translational Science • The State University of New York at Buffalo — UL1TR001412: Clinical and Translational Science Institute • The University of Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • The University of Iowa — UL1TR002537: Institute for Clinical and Translational Science • The University of Miami Leonard M. Miller School of Medicine — UL1TR002736: University of Miami Clinical and Translational Science Institute • The University of Michigan at Ann Arbor — UL1TR002240: Michigan Institute for Clinical and Health Research • The University of Texas Health Science Center at Houston — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • The University of Texas Medical Branch at Galveston — UL1TR001439: The Institute for Translational Sciences • The University of Utah — UL1TR002538: Uhealth Center for Clinical and Translational Science • Tufts Medical Center — UL1TR002544: Tufts Clinical and Translational Science Institute • Tulane University — UL1TR003096: Center for Clinical and Translational Science • The Queens Medical Center — None (Voluntary) • University Medical Center New Orleans — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • University of Alabama at Birmingham — UL1TR003096: Center for Clinical and Translational Science • University of Arkansas for Medical Sciences — UL1TR003107: UAMS Translational Research Institute • University of Cincinnati — UL1TR001425: Center for Clinical and Translational Science and Training • University of Colorado Denver, Anschutz Medical Campus — UL1TR002535: Colorado Clinical and Translational Sciences Institute • University of Illinois at Chicago — UL1TR002003: UIC Center for Clinical and Translational Science • University of Kansas Medical Center — UL1TR002366: Frontiers: University of Kansas Clinical and Translational Science Institute • University of Kentucky — UL1TR001998: UK Center for Clinical and Translational Science • University of Massachusetts Medical School Worcester — UL1TR001453: The UMass Center for Clinical and Translational Science (UMCCTS) • University Medical Center of Southern Nevada — None (voluntary) • University of Minnesota — UL1TR002494: Clinical and Translational Science Institute • University of Mississippi Medical Center — U54GM115428: Mississippi Center for Clinical and Translational Research (CCTR) • University of Nebraska Medical Center — U54GM115458: Great Plains IDeA-Clinical & Translational Research • University of North Carolina at Chapel Hill — UL1TR002489: North Carolina Translational and Clinical Science Institute • University of Oklahoma Health Sciences Center — U54GM104938: Oklahoma Clinical and Translational Science Institute (OCTSI) • University of Pittsburgh — UL1TR001857: The Clinical and Translational Science Institute (CTSI) • University of Pennsylvania — UL1TR001878: Institute for Translational Medicine and Therapeutics • University of Rochester — UL1TR002001: UR Clinical & Translational Science Institute • University of Southern California — UL1TR001855: The Southern California Clinical and Translational Science Institute (SC CTSI) • University of Vermont — U54GM115516: Northern New England Clinical & Translational Research (NNE-CTR) Network • University of Virginia — UL1TR003015: iTHRIV Integrated Translational health Research Institute of Virginia • University of Washington — UL1TR002319: Institute of Translational Health Sciences • University of Wisconsin-Madison — UL1TR002373: UW Institute for Clinical and Translational Research • Vanderbilt University Medical Center — UL1TR002243: Vanderbilt Institute for Clinical and Translational Research • Virginia Commonwealth University — UL1TR002649: C. Kenneth and Dianne Wright Center for Clinical and Translational Research • Wake Forest University Health Sciences — UL1TR001420: Wake Forest Clinical and Translational Science Institute • Washington University in St. Louis — UL1TR002345: Institute of Clinical and Translational Sciences • Weill Medical College of Cornell University — UL1TR002384: Weill Cornell Medicine Clinical and Translational Science Center • West Virginia University — U54GM104942: West Virginia Clinical and Translational Science Institute (WVCTSI) Submitted: Icahn School of Medicine at Mount Sinai — UL1TR001433: ConduITS Institute for Translational Sciences • The University of Texas Health Science Center at Tyler — UL1TR003167: Center for Clinical and Translational Sciences (CCTS) • University of California, Davis — UL1TR001860: UCDavis Health Clinical and Translational Science Center • University of California, Irvine — UL1TR001414: The UC Irvine Institute for Clinical and Translational Science (ICTS) • University of California, Los Angeles — UL1TR001881: UCLA Clinical Translational Science Institute • University of California, San Diego — UL1TR001442: Altman Clinical and Translational Research Institute • University of California, San Francisco — UL1TR001872: UCSF Clinical and Translational Science Institute NYU Langone Health Clinical Science Core, Data Resource Core, and PASC Biorepository Core — OTA-21-015A: Post-Acute Sequelae of SARS-CoV-2 Infection Initiative (RECOVER) Pending: Arkansas Children’s Hospital — UL1TR003107: UAMS Translational Research Institute • Baylor College of Medicine — None (Voluntary) • Children’s Hospital of Philadelphia — UL1TR001878: Institute for Translational Medicine and Therapeutics • Cincinnati Children’s Hospital Medical Center — UL1TR001425: Center for Clinical and Translational Science and Training • Emory University — UL1TR002378: Georgia Clinical and Translational Science Alliance • HonorHealth — None (Voluntary) • Loyola University Chicago — UL1TR002389: The Institute for Translational Medicine (ITM) • Medical College of Wisconsin — UL1TR001436: Clinical and Translational Science Institute of Southeast Wisconsin • MedStar Health Research Institute — None (Voluntary) • Georgetown University — UL1TR001409: The Georgetown-Howard Universities Center for Clinical and Translational Science (GHUCCTS) • MetroHealth — None (Voluntary) • Montana State University — U54GM115371: American Indian/Alaska Native CTR • NYU Langone Medical Center — UL1TR001445: Langone Health’s Clinical and Translational Science Institute • Ochsner Medical Center — U54GM104940: Louisiana Clinical and Translational Science (LA CaTS) Center • Regenstrief Institute — UL1TR002529: Indiana Clinical and Translational Science Institute • Sanford Research — None (Voluntary) • Stanford University — UL1TR003142: Spectrum: The Stanford Center for Clinical and Translational Research and Education • The Rockefeller University — UL1TR001866: Center for Clinical and Translational Science • The Scripps Research Institute — UL1TR002550: Scripps Research Translational Institute • University of Florida — UL1TR001427: UF Clinical and Translational Science Institute • University of New Mexico Health Sciences Center — UL1TR001449: University of New Mexico Clinical and Translational Science Center • University of Texas Health Science Center at San Antonio — UL1TR002645: Institute for Integration of Medicine and Science • Yale New Haven Hospital — UL1TR001863: Yale Center for Clinical Investigation.
Author contributions
Y.P. was responsible for cohort data generation. Y.P. and Y.Han were responsible for data analysis and writing the first version of the manuscript. Y.P., X.Y., and Y.He initiated the project and provided the original project design. Y.P., Y.Han, C.Z., J.Z., Y.He, X.Y. played roles in developing research questions and ways to address the questions. C.Z. and L.Z. served as statistics experts. YHe served as the vaccine adverse event domain expert. X.Y. served as clinical domain expert. All authors participated in result interpretation, discussion, and paper editing. The corresponding authors are X.Y. and Y.He.
Data availability
All data of this study is available in the N3C Data Enclave for researchers with an approved protocol and data use request from an institutional review board. Data access is governed by the National Institutes of Health. More information on the enclave and instructions for data access can be found at https://covid.cd2h.org/for-researchers.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A list of authors and their affiliations appears at the end of the paper.
Contributor Information
Xianwei Ye, Email: yxw1205@163.com.
Yongqun He, Email: yongqunh@med.umich.edu.
N3C Consortium:
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-024-00964-3.
References
- 1.Guo, J. et al. Real-world effectiveness of seasonal influenza vaccination and age as effect modifier: a systematic review, meta-analysis and meta-regression of test-negative design studies. Vaccine42, 1883–1891 (2024). [DOI] [PubMed] [Google Scholar]
- 2.Bollyky, T. J., Nuzzo, J., Huhn, N., Kiernan, S. & Pond, E. Global vaccination must be swifter. Nature603, 788–792 (2022). [DOI] [PubMed] [Google Scholar]
- 3.Our World in Data. Coronavirus (COVID-19) Vaccinations. https://ourworldindata.org/covid-vaccinations (2023).
- 4.Mathieu, E. et al. A global database of COVID-19 vaccinations. Nat. Hum. Behav.5, 947–953 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Dubé, E. & MacDonald, N. E. COVID-19 vaccine hesitancy. Nat. Rev. Nephrol.18, 409–410 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Attwell, K., Hannah, A. & Leask, J. COVID-19: talk of ‘vaccine hesitancy’ lets governments off the hook. Nature602, 574–577 (2022). [DOI] [PubMed] [Google Scholar]
- 7.FDA. Emergency Use Authorization. https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/emergency-use-authorization#vaccines (2020).
- 8.Storrar, J., Kudose, S. & Woywodt, A. Have we missed AINything? acute interstitial nephritis in SARS-CoV-2 infection and vaccination. Clin. Kidney J.15, 1643–1652 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.CDC. Vaccine Adverse Event Reporting System (VAERS). https://vaers.hhs.gov/ (2023).
- 10.Luo, H. et al. Acute kidney injury after COVID-19 vaccines: a real-world study. Ren. Fail.44, 958–965 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anastassopoulou, C. et al. Adverse events of acute nephrotoxicity reported to EudraVigilance and VAERS after COVID-19 vaccination. Vaccine41, 7176–7182 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Kronbichler, A., Jung, S. Y., Kim, M. S. & Shin, J. I. Distinct glomerular disease association after vaccination with BNT162b2 and mRNA-1273: a VigiBase analysis. Kidney Int.101, 415–416 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shakoor, M. T., Birkenbach, M. P. & Lynch, M. ANCA-associated vasculitis following Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis.78, 611–613 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lebedev, L. et al. Minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis78, 142–145 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leclerc, S., Royal, V., Lamarche, C. & Laurin, L. P. Minimal change disease with severe acute kidney injury following the oxford-AstraZeneca COVID-19 Vaccine: a case report. Am. J. Kidney Dis.78, 607–610 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sekar, A., Campbell, R., Tabbara, J. & Rastogi, P. ANCA glomerulonephritis after the moderna COVID-19 vaccination. Kidney Int.100, 473–474 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim, J. H. et al. New-onset nephrotic syndrome after janssen COVID-19 vaccination: a case report and literature review. J. Korean Med. Sci.36, e218 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maas, R. J., Gianotten, S. & van der Meijden, W. A. G. An additional case of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis.78, 312 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komaba, H., Wada, T. & Fukagawa, M. Relapse of minimal change disease following the Pfizer-BioNTech COVID-19 vaccine. Am. J. Kidney Dis.78, 469–470 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holzworth, A., Couchot, P., Cruz-Knight, W. & Brucculeri, M. Minimal change disease following the moderna mRNA-1273 SARS-CoV-2 vaccine. Kidney Int100, 463–464 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aydın, M. F. et al. Relapse of primary membranous nephropathy after inactivated SARS-CoV-2 virus vaccination. Kidney Int.100, 464–465 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan, L. et al. AKI in hospitalized patients with COVID-19. J. Am. Soc. Nephrol.32, 151–160 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoo, Y. J. et al. Geographic and temporal trends in COVID-associated acute kidney injury in the national COVID cohort collaborative. Clin. J. Am. Soc. Nephrol.18, 1006–1018 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin, S. et al. Characterization of acute kidney injury in critically ill patients with severe coronavirus disease 2019. Clin. Kidney J.13, 354–361 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haendel, M. A. et al. The national COVID cohort collaborative (N3C): rationale, design, infrastructure, and deployment. J. Am. Med. Inform. Assoc.28, 427–443 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.NCATS. National COVID Cohort Collaborative.https://covid.cd2h.org (2023).
- 27.Rijkers, G. T. et al. Antigen presentation of mRNA-based and virus-vectored SARS-CoV-2 vaccines. Vaccines (Basel)9, 848 (2021). [DOI] [PMC free article] [PubMed]
- 28.Hripcsak, G. et al. Observational health data sciences and informatics (OHDSI): opportunities for observational researchers. Stud. Health Technol. Inform.216, 574–578 (2015). [PMC free article] [PubMed] [Google Scholar]
- 29.Hernán, M. A. & Robins, J. M. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol.183, 758–764 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grams, M. E. et al. Performance and limitations of administrative data in the identification of AKI. Clin. J. Am. Soc. Nephrol.9, 682–689 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowe, B., Xie, Y., Xu, E. & Al-Aly, Z. Kidney outcomes in long COVID. J. Am. Soc. Nephrol.32, 2851–2862 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC. COVID Data Tracker. https://covid.cdc.gov/covid-data-tracker/#variant-proportions (2023).
- 33.Kellum, J. A. et al. Acute kidney injury. Nat. Rev. Dis. Primers7, 52 (2021). [DOI] [PubMed] [Google Scholar]
- 34.Varricchio, F. et al. Understanding vaccine safety information from the vaccine adverse event reporting system. Pediatr. Infect Dis. J.23, 287–294 (2004). [DOI] [PubMed] [Google Scholar]
- 35.Lo Re, V. et al. Global covid-19 vaccine rollout and safety surveillance-how to keep pace. Bmj373, n1416 (2021). [DOI] [PubMed] [Google Scholar]
- 36.Polack, F. P. et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med.383, 2603–2615 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heath, P. T. et al. Safety and efficacy of NVX-CoV2373 covid-19 vaccine. N. Engl. J. Med.385, 1172–1183 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai, C. et al. A comprehensive analysis of the efficacy and safety of COVID-19 vaccines. Mol. Ther.29, 2794–2805 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hippisley-Cox, J. et al. Risk of thrombocytopenia and thromboembolism after covid-19 vaccination and SARS-CoV-2 positive testing: self-controlled case series study. Bmj374, n1931 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taquet, M., Husain, M., Geddes, J. R., Luciano, S. & Harrison, P. J. Cerebral venous thrombosis and portal vein thrombosis: a retrospective cohort study of 537,913 COVID-19 cases. EClinicalMedicine39, 101061 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li, X. et al. Association between covid-19 vaccination, SARS-CoV-2 infection, and risk of immune mediated neurological events: population based cohort and self-controlled case series analysis. Bmj376, e068373 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arunachalam, P. S. et al. Systems vaccinology of the BNT162b2 mRNA vaccine in humans. Nature596, 410–416 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar, N. et al. Cytotoxic T-lymphocyte elicited vaccine against SARS-CoV-2 employing immunoinformatics framework. Sci. Rep.11, 7653 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen, I., Douglas, I. & Whitaker, H. Self controlled case series methods: an alternative to standard epidemiological study designs. Bmj354, i4515 (2016). [DOI] [PubMed] [Google Scholar]
- 45.Li, Y., Gong, Y. & Xu, G. New insights into kidney disease after COVID-19 infection and vaccination: histopathological and clinical findings. Qjm117, 317–337 (2024). [DOI] [PubMed] [Google Scholar]
- 46.Ma, Y., Huang, Y. & Xu, G. New insights into the mucosal immune pathogenesis of IgA nephropathy from the perspective of COVID-19 vaccination. Qjm116, 181–195 (2023). [DOI] [PubMed] [Google Scholar]
- 47.Corriero, A. et al. COVID-19 variants in critically Ill patients: a comparison of the delta and Omicron variant profiles. Infect Dis. Rep.14, 492–500 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Legrand, M. et al. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol.17, 751–764 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng, S. et al. Early versus late acute kidney injury among patients with COVID-19-a multicenter study from Wuhan, China. Nephrol. Dial Transplant.35, 2095–2102 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Sullivan, E. D., Hughes, J. & Ferenbach, D. A. Renal aging: causes and consequences. J. Am. Soc. Nephrol.28, 407–420 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data of this study is available in the N3C Data Enclave for researchers with an approved protocol and data use request from an institutional review board. Data access is governed by the National Institutes of Health. More information on the enclave and instructions for data access can be found at https://covid.cd2h.org/for-researchers.