Abstract
The incidence of insulin resistance (IR) and hepatic steatosis is increasing, with dietary fiber playing a protective role against these disorders. Ocimum basilicum L., widely used in food, pharmaceutical, and cosmetic industries, but their health-promoting properties remain underexplored. This study evaluated the effects of a fiber-rich fraction of partially defatted basil seeds (BSF) on IR, hepatic steatosis, and polyunsaturated fatty acid and short-chain fatty acid (SCFA) profiles in high-fat diet (HFD)-fed C57BL/6 J male mice. Mice were assigned to four groups and fed either a control diet or HFD, supplemented with BSF or oat flour for 4 weeks. HFD induced IR, hepatic steatosis, proinflammatory state, and a significant decreased in SCFA production. In contrast, supplementation with BSF attenuated IR, steatosis, liver damage, oxidative stress, and inflammation, while increasing n-3 polyunsaturated fatty acids in liver, adipocytes, and erythrocytes, and enhancing SCFA production, suggesting potential therapeutic benefits in managing these conditions.
Subject terms: Obesity, Risk factors, Diagnostic markers
Introduction
During the last 30 years, a significant increase in the prevalence of insulin resistance (IR) has been observed1. Currently, the estimated prevalence ranges from 10% and 30% of the population and is the main cause of type 2 diabetes mellitus (T2DM), which is associated with obesity and metabolic syndrome (MetS)2,3. The frequency of IR depends on various factors such as age, body weight, sex, genetic predisposition, physical activity and diet, among others4. The pathophysiological mechanism of IR has not been fully established, but the pathogenesis of IR is related to free fatty acids accumulation, increase liver glucose production and decreased glucose uptake in insulin-sensitive tissues5. In this context, it has been proposed that the accumulation of ectopic lipids in different tissues as a result of an excessive consumption of simple carbohydrates (glucose, fructose) and saturated fat, trigger the production of adipocytokines exerting deleterious effects on the metabolism of glucose and lipids at distant sites (muscle, liver, arterial tissue), as well as in the vascular function6. The incidence of IR can be estimated by the homeostatic model for insulin resistance (HOMA-IR) proposed by Matthews et al. 7, a feature associated to non-alcoholic fatty liver disease6, one of the most common liver disorders worldwide6. Non-alcoholic fatty liver disease (NAFLD) consists in two clinicopathological entities, i.e., simple steatosis and non-alcoholic steatohepatitis (NASH), which may progress to cirrhosis and hepatocellular carcinoma8. It is estimated that the global prevalence of this pathology is approximately 25% and it has been observed to increase with age9. The diagnosis of NAFLD is based on two main criteria (i) detection of steatosis in alcohol abstinence, either by imaging or by histology and appropriate exclusion of other liver diseases6; and (ii) recognition of the presence of more than 5% of the hepatocytes loaded with fat in the absence of secondary etiology’s (medications, excessive alcohol consumption or hereditary conditions)8,10. Previously, the two-hit hypothesis, based on exposure of the liver to a first hit hepatic steatosis and the second hit oxidative stress were parallel events leading to the pathogenesis of NAFLD6,11. Currently, the multiple or continuous impact model was proposed, suggesting the existence of different, simultaneous, and continuous aggressions in individuals with metabolic disorders such as T2DM or MetS12.
Dietary fiber is a carbohydrate of plant origin that is not digestible by human enzymes13. Some types of fiber can be considered as prebiotics as they are an ingredient selectively fermented by the intestinal microbiota that results in specific changes in its composition and/or activity, thus providing benefits to the host’s health14. Dietary fiber intake is inversely associated with the risk of obesity, as well as with other pathologies related to MetS, T2DM, cardiovascular disease (CVD), and cancer15,16. It is suggested that dietary fiber intake improves insulin sensitivity, lipid profile, chronic inflammation, and favors weight reduction2,15,16. The current recommendation for dietary fiber intake in adults from the World Health Organization (WHO)17 is ≥25 g per day18. Dietary fibers are not digested in the upper gastrointestinal tract but are instead fermented by bacteria in the colon. Depending of the degree of polymerization, particle size, solubility, and viscosity, the fermentability capacity, type of bacteria and metabolites production can be affected19. Dietary fiber intake is linked to overall metabolic health, particularly through key pathways involving insulin sensitivity. It also impacts various other health conditions, including cardiovascular disease, colon health, gut motility, and the risk of colorectal cancer15.
Additionally, polyunsaturated fatty acids (PUFAs) are also beneficial for human health, and include a series of n-6 and n-3 fatty acids, α-linolenic acid (C18:3n − 3, ALA) being a n-3 PUFA that is present in various oils and oilseeds such as: linseed, chia, perilla, basil, hip, rapeseed, soybean, walnuts, avocado, among others20–22. ALA is known as the essential precursor of the longer chain n-3 PUFAs eicosapentaenoic acid (C20:5n-3, EPA) and docosahexaenoic fatty acid (C22:6n-3, DHA)23. The increase in n-3 PUFAs consumption, has been related with the reduction in both steatosis and NASH, by decreasing the content of triglycerides (TG), plasmatic low-density lipoprotein (LDL), and IR11,24. The Food and Agriculture Organization of the United Nations25 recommends an intake of ALA > 0.5% of total caloric energy/day for the adult population and EPA plus DHA 0.250 – 2 g/ day25.
Basil (Ocimum basilicum L.) is a medicinal plant cultivated throughout the world that has been associated with multiple health benefits, and its use has become widespread in the pharmaceutical, food, and cosmetic industries. However, basil seeds have been poorly studied, presenting nutritional and functional attractive characteristics that make them an excellent source of bioactive compounds26. Regarding its nutritional composition, basil seeds stand out for its high content of dietary fiber (43.9–63.8%), which contains ~8% cellulose, ~10% hemicellulose and ~35% lignin27. Its protein content ranges from 10% to 22.5% and its lipid content can reach 33%, with ALA being its main fatty acid28. In this sense, in Chile some companies have recently sought to enter the market to produce oils from seeds rich in n-3 PUFAs in a sustainable manner, including basil seeds among others. During the oil extraction process by cold pressing, an expeller with a high content of dietary fiber and proteins is generated, which can be used for the production of functional ingredients29. The main objective of this experimental work was to evaluate the effects of the intake of fiber-rich fraction of partially defatted basil seeds on IR and hepatic steatosis induced by an HFD. As secondary aims, the assessment of the contribution of this intervention on n-3 PUFAs content in tissues and SCFAs in stools from mice were also evaluated. We hypothesize that the dietary fiber and polyunsaturated fatty acids present in the fiber-rich fraction of partially defatted basil seeds ameliorates high-fat diet-induced insulin resistance and hepatic steatosis through multiple synergistic mechanisms.
Results
Nutritional composition and fatty acid profile of supplements
The results of the nutritional composition and fatty acid profile are showed in Tables 1, 2, respectively. BSF contained ∼65 g/100 g dietary fiber, being mostly insoluble fiber (58.48 g/100 g), values that were significantly higher than those of OAF. The protein content reached 20.24 g/100 g for BSF and 12.56 g/100 g for OAF, while the amount of fat present in the meals were 6.35 and 8,77 g/100 g, respectively. The energy content was 267.5 kcal/100 g for BSF and 457.25 kcal/100 g, for OAF. According to the results presented in Table 2, BSF shows that the 51% (3.24 g/100 g) of the fatty acids corresponding to PUFAs of which, 2.31 g/100 g corresponds to α-linolenic acid and 1.81 g/100 g linoleic acid, followed by 2.70 g/100 g saturated fatty acids (SAFAs) and to a lesser extent monounsaturated fatty acids (MUFAs) with ∼0.41 g/100 g (Table 2). Nevertheless, the OAF presented 12.56 g/100 g protein, 11.72 g/100 g total dietary fiber and 8.77 g/100 g lipids which correspond to ~30% of PUFAs, 3.50 g/100 g SAFAs and 2.67 g/100 g MUFAs. While there are differences in total dietary fiber content between BSF and OAF in this study, it has been described in the literature that the main physiological activities exerted are mainly related to soluble fiber. In this case, the soluble fiber (mucilage) of basil seed is composed of two major fractions of glucomannans, xylans and a minor part of beta-glucans, while oats have mainly beta-glucans. Both have an overall impact on key health outcomes that appears to be parallel30.
Table 1.
Nutritional composition of fiber-rich flours
| Composition | BSF (g/100 g) | OAF (g/100 g) |
|---|---|---|
| Proteins | 20.24 ± 0.39a | 12,56 ± 0,10b |
| Fat | 6.35 ± 0.27a | 8,77 ± 0,58b |
| Total carbohydrates | 68.58a | 76,16b |
| Total dietary Fiber | 64.69 ± 2.86a | 11,72 ± 0,06b |
| Soluble | 6.20a | 4,07b |
| Insoluble | 58.48 ± 0.48a | 7,64 ± 0,17b |
| Ashes | 9.00 ± 0.05a | 1,51 ± 0,02b |
| Energy (kcal) | 267.5a | 457.25b |
Data presented as mean ± standard deviation on a dry basis. *Calculated by difference. T test, (a, b) different letters in the same row represent a significant difference (p < 0.05).
Table 2.
Fatty acid profile of fiber-rich flours
| Fatty acids | BSF | OAF |
|---|---|---|
| (g/100 g) | (g/100 g) | |
| Total Fatty acids | 6.35 ± 0.02a | 8.77 ± 0.05b |
| Total SFAs | 2.70 ± 0.03a | 3.50 ± 0.09b |
| Total MUFAs | 0.41 ± 0.01a | 2.67 ± 0.00b |
| Total PUFAs | 3.24 ± 0.01a | 2.61 ± 0.04b |
| C18:2n-6 (LA) | 1.81 ± 0.01a | 2.45 ± 0.02b |
| C18:3n-3 (ALA) | 2.31 ± 0.02a | 0.15 ± 0.01b |
Values are expressed as media ± SD. Fatty acids correspond to C4:0, C14:0, C16:0, C16:1, C18:0, C18:1n-9, C18:2n-6, C18:3n-6, C18:3n-3, C20:2, C20:3n-6, C22:0, C22:1n-9, C22:2, C24:0. Saturated fatty acids (SFAs) correspond to C4:0, C14:0, C16:0, C18:0, C22:0 and C24:0. Monounsaturated fatty acids (MUFAs) correspond to C16:1, C18:1n-9 and C22: 1n-9. Polyunsaturated fatty acids (PUFAs) correspond to C18:2n-6, C18:3n-6, 3C18:3n-3, C20:2, C20:3n-6 and C22:2. T test, (a, b) different letters in the same row represent a significant difference (p < 0.05)
Body composition changes and food intake
Table 3 shows the daily dietary intake in the different groups. The food intake did not show significant differences, whereas energy intake showed statistically lower consumption in those fed a CD compared to the groups with a HFD and supplemented with BSF or OAF. Similarly with protein and lipid intakes, where the CD group had a significantly lower intake than the other groups. On the contrary, carbohydrate intake was significantly higher in the CD group than the other groups. For water consumption, the OAF group had a significantly lower consumption compared to the other groups. Regarding the general characteristics of the mice, only BSF group-supplemented HFD showed attenuated body weight gain compared to HFD mice (Table 3; Supplementary Fig. 1). For liver weight, the CD group presented a significantly lower weight than the HFD group and the OAF-supplemented group. Regarding adipose tissue, the HFD groups supplemented with BSF and OAF showed a significantly greater increase in adipose tissue than the CD groups. In addition, no differences were observed in the percentage of fat in some epididymal adipose, erythrocytes, and brain tissues. While in stools, significant differences were found between CD v/s HFD and CD v/s HFD and supplemented with OAF of 4.47, 19.25 and 18.33 g/100 g.
Table 3.
Dietary intake parameters
| CD | HFD | HFD + BSF | HFD + OAF | |
|---|---|---|---|---|
| Dietary intake (g/day) | 2.63a (2.45–2.97) | 2.65a (2.5–2.90) | 2.53a (2.35–2.80) | 2.56a (2.4–2.98) |
| Energy intake (kcal/ day) | 10.39a (9.68–11.73) | 14.15b (13.35–15.49) | 13.51b (12.39–14.24) | 13.67b (12.82–15.06) |
| Protein intake (g/day) | 0.50a (0.47–0.57) | 0.69b (0.66–0.76) | 0.66b (0.62–0.70) | 0.67b (0.63–0.70) |
| Fat intake (g/day) | 0.11a (0.11–0.13) | 1.16b (1.10–1.27) | 1.05c (0.95–1.12) | 1.05c (0.91–1.12) |
| Carbohydrates intake (g/day) | 1.77a (1.65–2.00) | 0.70b (0.66–0.76) | 0.65c (0.60–0.68) | 0.67b (0.63–0.98) |
| Water intake (ml/day) | 3.75a (3.48–4.0) | 3.87a (3.39–4.40) | 3.64a (3.39–4.0) | 3.18b (2.93–3.52) |
| Starting body weight (g) | 22.00a (20.4–22.70) | 23.00a (22.6–24.30) | 23.00a (22–23.60) | 23.20a (22.2–23.20) |
| Final body weight (g) | 27.10a (25–28.9) | 46.80b (44.8– 50.3) | 44.30a (43.2– 45) | 46.50b (45.3–47.80) |
| Final body weight gain (g) | 6.30a (5–6.70) | 23.30b (22.4–25.30) | 21.30a (20.4–21.90) | 23.40b (23.2–24.60) |
| Liver weight (g) | 1.06a (0.94–1.27) b,d | 2.72b (2.38–3.52) a | 2.14a (1.52–2.26) | 2.59b (2.34–2.85) |
| Epididymal adipose tissue weight (g) | 0.57a (0.51–0.67) | 2.06b (1.71–2.23) | 2.14b(1.78–2.22) | 2.11b(1.95–2.62) |
| Epididymal adipose fat (g/100 g) | 76.79a (66.93–77.86) | 77.4a (75.15–79.31) | 75.17a (69.59–84.97) | 74.23a (63.49–65.42) |
| Erythrocytes fat (g/100 g) | 1.60a (1.24–1.89) | 2.09a (1.3–2.33) | 1.33a (1.28–1.5) | 2.06a (1.37–2.09) |
| Brain fat (g/100 g) | 11.88a (10.37–14.11) | 9.26a (1.3–2.33) | 9.80a (9.3–16.27) | 9.35a (7.68–10.68) |
| Stool fat (g/100 g) | 4.47a(2.63–4.83) | 19.25b (18.25–21.38) | 11.5a (10.25–12) | 18.33b (18.3–19.17) |
Values are expressed as median (interquartile range), n = 7. Kruskal Wallis test, followed by Dunn’s test for multiple comparisons; (a, b) different letters in the same row represent a significant difference (p < 0.05).
CD control diet, HFD high fat diet, BSF basil seed flour, OAF oat flour.
Effect of interventions on parameters related to IR and inflammation
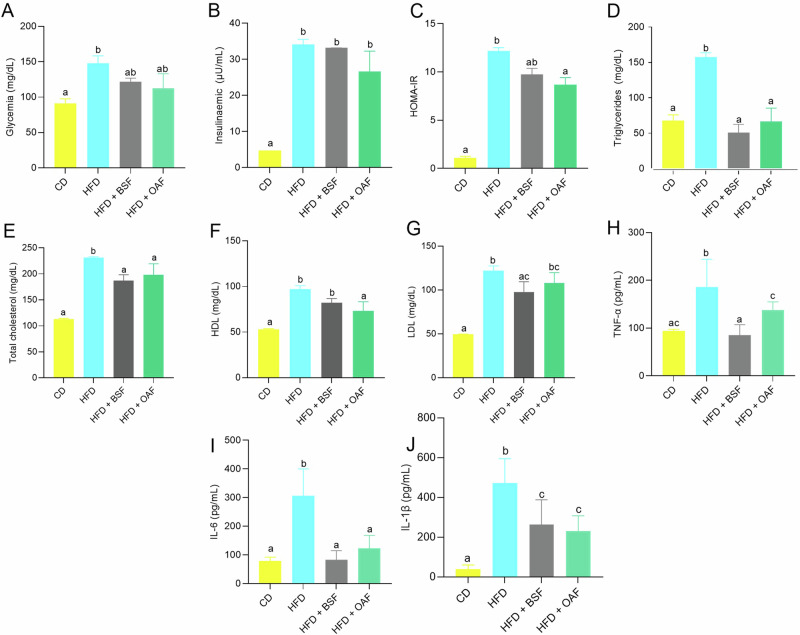
Figure 1 shows the biochemical parameters related to IR by experimental group. The most effective intervention in improving plasma glucose levels was the group supplemented with OAF followed by BSF (Fig. 1A). Coincident with insulin and, consequently, with HOMA-IR compared to the HFD group (Fig. 1B, C). In TG measurements, it was observed that the BSF group like OAF managed to attenuate this parameter compared to the HFD group (Fig. 1D). As well as total cholesterol levels, only significant differences were found between the CD v/s HFD groups. On the other hand, HDL and LDL cholesterol were lower in the supplemented v/s HFD groups, confirming the potential efficacy of BSF and OAF as hypolipidemic effectors, with BSF standing out for its greater effect in reducing these parameters (Fig. 1E–G). The evaluation of inflammation parameters revealed that BSF supplementation caused a statistically significant decrease in plasma TNF-α and IL-6, resembling the results obtained with CD, while those supplemented with OAF showed a higher efficacy in downward control of IL-1β compared to the HFD group (Fig. 1H–J).
Fig. 1. Biochemical parameters related to insulin resistance by experimental group.
A Glycemia (mg/dL); (B): insulinemia (µU/mL); (C): HOMA-IR; (D): triglycerides (mg/dL); (E): total cholesterol (mg/dL); (F): HDL (mg/dL); (G): LDL (mg/dL); (H): TNF-α (pg/mL); (I): IL-6 (pg/mL); (J): IL-1β (pg/mL). Values are represented as medians and interquartile ranges. n = 6–7. CD control diet, HFD high-fat diet, BSF high-fat diet plus fiber-rich basil seed meal, OAF high-fat diet plus oatmeal. Kruskal Wallis test, followed by Dunn’s test for multiple comparisons; (a, b) different letters represent a significant difference (p < 0.05).
Evaluation of the effect of fiber-rich fraction intake on liver damage and steatosis
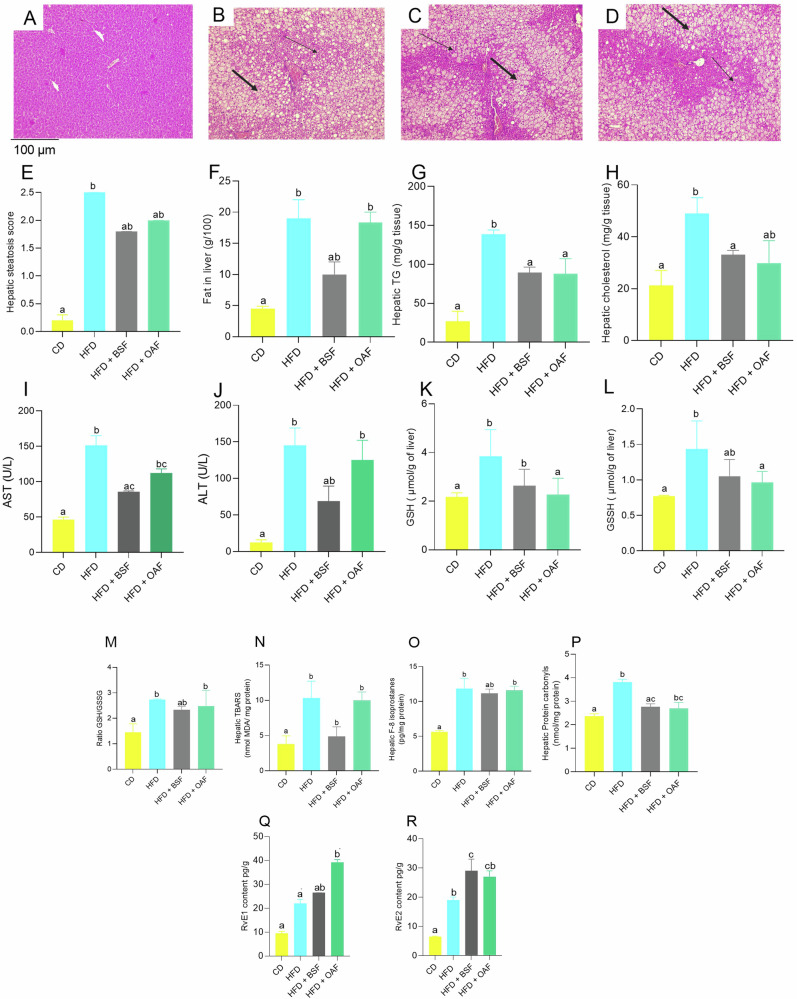
The analysis of the hepatic histological plaques showed that the group fed with CD presented a normal histoarchitecture, without the presence of foci of hepatic steatosis (Fig. 2A). While those fed with HFD for 14 weeks induced microvesicular and macrovesicular hepatic steatosis with migration of nuclei towards the periphery of the hepatocytes, presenting significant foci of steatosis throughout the tissue (Fig. 2B). In the groups supplemented with BSF and OAF, attenuation of steatosis was observed. Likewise, the steatosis decreased in the groups supplemented with BSF or OAF compared to the HFD group (Fig. 2C, D). In addition, the BSF group presented a lower total hepatic fat content (p < 0.05) compared to the HFD and OAT groups (Fig. 2E, F). As well as hepatic TG and cholesterol concentrations were significantly lower in the BSF group compared to the HFD group (Fig. 2G, H). Finally, significant differences were detected between the CD group and the HFD and OAF groups in the plasma levels of transaminases (AST, ALT) in the treated mice, and BSF and CD group were similar, demonstrating the potential of BSF in reversing liver damage (Fig. 2 I–J). Table 4 shows the liver fatty acids composition. As expected, significantly higher SAFAs, MUFAs and PUFAs were observed in all groups fed with HFD (supplemented or not) compared to CD. The group supplemented with BSF had a higher content (p < 0.05) of DHA and EPA compared to the HFD group (Table 4).
Fig. 2. Effect of fiber-rich fraction intake on liver damage and steatosis.
Liver histology by experimental group. (A): CD; (B): HFD; (C): HFD + BSF, (D): HFD + OAF; (E): Hepatic steatosis score; (F): Fat in liver (g/100 g); (G): Hepatic TG (mg/g tissue); (H): Hepatic cholesterol (mg/g tissue); (I): AST (U/L); (J): ALT (U/L); (K): GSH (µmol/g liver); (L): GSSH (µmol/g liver); (M): Ratio GSH/GSSH; (N): Hepatic TBARS (nmol MDA/mg protein); (O): Hepatic F-8 Isoprostanes (pg/mg protein); (P): Hepatic Protein carbonyl (nmol/mg protein); (Q): RvE1 content (pg/g); (R): RvE2 content (pg/g); n = 6–7. Sections were fixed in formaldehyde and stained with hematoxylin eosin (×10 magnification), thick arrows (→) indicate macrovesicular steatosis and thin arrows (→) indicate microvesicular steatosis. Hepatic steatosis score, <1 point absent steatosis, 1- 2 points mild steatosis, 2–3 points moderate steatosis, >3 points severe steatosis. Significant differences according to Kruskal Wallis test, followed by Dunn’s test for multiple comparisons; (a, b) different letters represent a significant difference (p < 0.05).
Table 4.
Fatty acid composition of liver
| Fatty acid | CD | HFD | HFD + BSF | HFD + OAF |
|---|---|---|---|---|
| C16:0 | 27.25a (26.46–30) | 27.18a (25.41–27.67) | 26.16a (25.77–28.17) | 26.35a (26.07–27.55) |
| C18:0 | 8.95a (8.27–9.45) | 3.17ab (3.08–3.25) | 3.14ab (2.87–3.51) | 1.81b (0.98–2.84) |
| C18:1n-9 | 31.99a (29.84–33.62) | 45.46a (44.9–45.8) | 41.73a (36.93–42.76) | 44.47a (43.64–46.49) |
| C18:2n-6 (LA) | 10.94a (10.47–12.03) | 13.11b (12.14–14.04) | 14.3b (13.93–15.85) | 13.91b (11.92–15.41) |
| C18:3n-3 (ALA) | 0.00a (0.00–0.00) | 0.31b (0.25–0.36) | 0.70b (0.56–0.84) | 0.36b (0.3–0.39) |
| C20:4n-6 (AA) | 8.44a (3.51–9.66) | 2.99a (2.68–3.43) | 3.08a (2.74–3.47) | 2.96a (2.62–3.02) |
| C20:5n-3 (EPA) | 0.35a (0.07–1.26) | 0.38a (0.34–0.44) | 0.71a (0.45–0.79) | 0.34a (0.28–0.36) |
| C:22:5 n-6 (DPA n-6) | 0.06ac (0.02–0.4) | 0.07ac (0.05–0.09) | 0.12a (0.12–0.13) | 0.05bc (0.04–0.05) |
| C22:5 n-3 (DPA n-3) | 0.16a (0.02–0.69) | 0.15a (0.07–0.18) | 0.21a (0.17–0.32) | 0.01a (0.06–0.09) |
| C22:6n-3 (DHA) | 4.98a (4.16–5.25) | 1.98ab (1.85–2.28) | 3.05ab (2.73–3.32) | 1.79b (1.47–1.83) |
| Total SFAs | 38.27a (36.63–38.76) | 31.77b (30.06–32.12) | 30.95ab (30.23–32.83) | 29.78b (29.36–31.34) |
| Total MUFAs | 36.35a (33.81–37.44) | 49.13b (48.47–49.67) | 46.62ab (42.23–48.14) | 50.83b (49.9–51.54) |
| Total PUFAs | 27.33a (23.83–33.33) | 19.8a (18.64–21.1) | 22.66a (21.6–24.94) | 20.44a (17.58–22.16) |
| Total n-6 PUFAs | 21.11a (17.93–22.92) | 16.17a (15.09–17.32) | 17.56a (16.63–19.31) | 16.93a (14.61–18.48) |
| Total n-3 PUFAs | 5.63a (4.95–6.38) | 2.75ab (2.5–3.18) | 4.27ab (4.15–5.05) | 2.58b (2.19–2.65) |
| n-6/ n-3 PUFAs ratio | 3.47a (2.42–3.8) | 5.81a (5.32–6.13) | 4.12a (3.92–4.32) | 6.60b (6.57–6.76) |
Values are expressed as median (interquartile range) in % mmol the fatty acid, n = 7. Saturated fatty acids (SFAs) correspond to C13:0, C14:0, C16:0, C17:0, C18:0, C20:0, C22:0 and C22:2. Monounsaturated fatty acids (MUFAs) correspond to C14:1, C15:1, C16:1, C17:1, C18:1n-9, C20:1n-9 and C22:1n-9. Polyunsaturated fatty acids (PUFAs) correspond to C18:2 n-6, C18:3 n-6, C18:3 n-3, C20:1 n-9, C20:3n-3, C20:4n-6, C20:5 n-3, C:22:5 n-6, C:22:5 n-3, C24:0 and C22:6n-3; n-6 PUFAs are C18:2n-6 and C20:4n-6; n-3 PUFAs are C18:3n-3, C20:5n-3 and C22:6n-3; n-6/n-3 PUFAs ratio: (C20:4n-6 + C:22:5 n-6)/ (18:3n-3 + C20:5n-3 + C:22:5 n-3 + C22:6n-3). Kruskal Wallis test, followed by Dunn’s test for multiple comparisons; (a, b) different letters in the same row represent a significant difference (p < 0.05).
CD control diet, HFD high fat diet, BSF basil seed flour, OAF oat flour.
Evaluation of inflammatory, oxidative stress and antioxidant liver capacity
As shown in Fig. 2, hepatic TBARS levels exhibited higher levels (p < 0.05) in the HFD and HFD group supplemented with OAF compared to the CD group, while supplementation with BSF successfully normalized liver TBARS (Fig. 2N). Furthermore, F8-isoprostanes levels also decreased with BSF supplementation, unlike the HFD and OAF-supplemented HFD groups (Fig. 2O). Furthermore, as shown in Fig. 2P, HFD mice had significantly higher hepatic levels of protein carbonyls compared with the CD group. However, the magnitude of the increase was smaller in the supplemented groups (BSF and OAF). Regarding the parameters that decreased HFD-induced injury at the hepatic level, it was observed that resolvins (RvD1-RvD2) were up-regulated in supplemented mice (Fig. 2Q–R). With respect to antioxidant defense, HFD mice showed a significant increase in GSH and GSSG levels compared to CD. While supplemented mice exhibited a decrease being significant for OAF compared to HFD group. As for GSH/GSSG ratio it was higher in HFD and supplemented with OAF compared to CD group (Fig. 2K–M).
Fatty acid profile adipose tissue, erythrocytes, brain, and stool
The results of fatty acid composition of epididymal adipose tissue, erythrocytes, brain and stool are presented in Tables 5, 6, 7, 8, respectively. As can be seen, in the adipose tissue from treated mice, a statistically higher amount of ALA was observed in the CD and BSF groups in contrast to the HFD group (Table 5). Meanwhile, in erythrocytes, the fatty acid profile showed similar data since ALA, EPA and DHA were found to be increased in the CD and BSF groups in contrast to the HFD group (Table 6, Supplementary Table 1). In the brain, there was only a significant increase in ALA in the BSF mice compared with the other groups (Table 7). On the other hand, it is highlighted in the stool that supplementation with BSF or OAF increased the content of SFAs compared to the HFD group (Table 8).
Table 5.
Fatty acid composition of epididymal adipose tissue
| AG | CD | HFD | HFD + BSF | HFD + OAF |
|---|---|---|---|---|
| C16:0 | 20.60a (19.61–20.71) | 20.51a (20.3–22.32) | 19.78a (18.20–20.24) | 19.61a (18.67–20.56) |
| C18:0 | 1.66a (1.61–1.72) | 3.66b (3.51–3.89) | 3.39b (3.22–3.59) | 3.58b (3.41–3.74) |
| C18:1n-9 | 47.08a (45.38–47.82) | 49.37ab (48.24–50.65) | 48.46ab (48.37–50.92) | 50.42b (48.99–51.29) |
| C18:2n-6 (LA) | 17.56a (16.57–17.98) | 18.68a (18.5–18.88) | 18.89b (18.78–19.17) | 18.6c (18.54–18.83) |
| C18:3n-3 (ALA) | 0.84a (0.81–0.87) | 0.55b (0.23–0.65) | 1.08a (0.92–1.15) | 0.54b (0.46–0.56) |
| C20:4n-6 (AA) | 0.35a (0.33–0.45) | 0.29a (0.24–0.4) | 0.28a (0.26–0.31) | 0.28a (0.24–0.33) |
| C20:5n-3 (EPA) | 0.07a (0.06–0.07) | 0.07a (0.03–0.11) | 0.06a (0.05–0.07) | 0.03a (0.02–0.05) |
| C:22:5 n-6 (DPA n-6) | 0.01a (0.01–0.01) | 0.01a (0–0.01) | 0.01a (0.01–0.01) | 0.01a (0–0.01) |
| C22:5 n-3 (DPA n-3) | 0.03a (0.03–0.04) | 0.02a (0.01–0.04) | 0.02a (0.01–0.02) | 0.01a (0.01–0.02) |
| C22:6n-3 (DHA) | 0.13a (0.11–0.16) | 0.14a (0.06–0.57) | 0.11a (0.09–0.12) | 0.07a (0.04–0.1) |
| Total SFAs | 24.09a (23.08–24.95) | 25.75a (25.21–27.2) | 24.33a (23.57–25.06) | 25.58a (24.15–26.87) |
| Total MUFAs | 56.74a (55.45–57.72) | 53.61b (52.62–54.5) | 54.14ab (52.9–55.2) | 54.56ab (53.28–55.58) |
| Total PUFAs | 19.85a (18.61–20.14) | 20.42ab (20.18–20.7) | 21.35b (20.83–21.45) | 20.07ab (19.83–20.23) |
| Total n-6 PUFAs | 18.05a (16.89–18.37) | 18.94ab (18.83–19.44) | 19.33b (19.06–19.47) | 19.05ab (18.8–19.09) |
| Total n-3 PUFAs | 1.07a (1.04–1.13) | 0.74ab (0.7–0.84) | 1.31a (1.11–1.4) | 0.67b (0.6–0.73) |
| n-6/ n-3 PUFAs ratio | 16.58a (15.72–17.04) | 25.38ab (22.38–28.06) | 14.96a (13.73–17.91) | 28.73b (26.07–31.64) |
Values are expressed as median (interquartile range) in % mmol the fatty acid, n = 7. Saturated fatty acids (SFAs) correspond to C13:0, C14:0, C16:0, C17:0, C18:0, C20:0, C22:0 and C22:2. Monounsaturated fatty acids (MUFAs) correspond to C14:1, C15:1, C16:1, C17:1, C18:1n-9, C20:1n-9 and C22:1n-9. Polyunsaturated fatty acids (PUFAs) correspond to C18:2 n-6, C18:3 n-6, C18:3 n-3, C20:1 n-9, C20:3n-3, C20:4n-6, C20:5 n-3, C:22:5 n-6, C:22:5 n-3, C24:0 and C22:6n-3; n-6 PUFAs are C18:2n-6 and C20:4n-6; n-3 PUFAs are C18:3n-3, C20:5n-3 and C22:6n-3; n-6/n-3 PUFA ratio: (C20:4n-6 + C:22:5 n-6)/ (18:3n-3 + C20:5n-3 + C:22:5 n-3 + C22:6n-3). Kruskal Wallis test, followed by Dunn’s test for multiple comparisons; (a, b) different letters in the same row represent a significant difference (p < 0.05).
CD control diet, HFD high fat diet, BSF basil seed flour, OAF oat flour.
Table 6.
Fatty acid composition of erythrocytes
| AG | CD | HFD | HFD + BSF | HFD + OAF |
|---|---|---|---|---|
| C16:0 | 38a (36.6–38.7) | 46.19b (44.44–48.01) | 41.31ab (38.13–42.39) | 49.11b (45.3–49.65) |
| C18:0 | 15.32a (14.92–15.93) | 17.33a (16.54–18.79) | 16.73a (15.68–17.13) | 14.61a (13.94–16.11) |
| C18:1n-9 | 15.37a (14.75–15.91) | 15.57a (15.22–15.95) | 17.08a (16.32–18.09) | 16.03a (14.75–16.32) |
| C18:2n-6 (LA) | 1.99a (1.68–2.35) | 0.00b (0.00–0.00) | 0.97a (0.85–1.06) | 0.33b (0.00–0.46) |
| C18:3n-3 (ALA) | 1.50a (1.39–1.6) | 0.61b (0–0.74) | 0.85ab (0.71–1.11) | 0.81b (0.39–1.03) |
| C20:4n-6 (AA) | 19.43a (18.26–20.22) | 16.42b (14.62–17.61) | 16.85ab (16.73–17.66) | 15.74b (15.06–17.32) |
| C20:5n-3 (EPA) | 3.00a (2.11–4.23) | 0.71ab (0.57–1.22) | 1.23ab (0.49–1.51) | 0.51b (0.49–0.59) |
| C:22:5 n-6 (DPA n-6) | 0.54a (0.15–0.7) | 0.08ab (0.05–0.14) | 0.06b (0.02–0.11) | 0.06b (0.05–0.08) |
| C22:5 n-3 (DPA n-3) | 0.9a (0.68–1.21) | 0.28ab (0.23–0.64) | 0.38ab (0.21–0.48) | 0.26b (0.13–0.28) |
| C22:6n-3 (DHA) | 3.11a (2.23–3.62) | 1.95a (1.72–2.21) | 3.97a (3.24–5.43) | 1.26a (0.96–2.59) |
| Total SFAs | 53.32a (52.81–54.36) | 64.84b (62.34–65.27) | 58.19ab (56.26–59.11) | 63.58b (62.66–65.3) |
| Total MUFAs | 15.37a (14.75–15.91) | 15.57a (15.22–15.95) | 17.08a (16.32–18.09) | 16.03a (14.75–16.32) |
| Total PUFAs | 31.31a (30.1–32.06) | 19.32b (18.27–21.88) | 23.55ab (22.8–27.32) | 19.4b (18.08–21.17) |
| Total n-6 PUFAs | 22.15a (20.72–22.68) | 16.48b (14.76–17.67) | 17.97ab (17.61–18.96) | 16.11b(15.31–17.77) |
| Total n-3 PUFAs | 8.11a (6.7–10.19) | 3.45ab (2.84–4.57) | 6.06ab (4.95–7.86) | 2.98b (2.38–3.94) |
| n-6/ n-3 PUFAs ratio | 2.73a (2.1–3.66) | 4.6ab (3.49–5.43) | 2.89ab (2.52–3.67) | 5.32b (4.29–6.62) |
Values are expressed as median (interquartile range) in % mmol the fatty acid, n = 7. Saturated fatty acids (SFAs) correspond to C13:0, C14:0, C16:0, C17:0, C18:0, C20:0, C22:0 and C22:2. Monounsaturated fatty acids (MUFAs) correspond to C14:1, C15:1, C16:1, C17:1, C18:1n-9, C20:1n-9 and C22:1n-9. Polyunsaturated fatty acids (PUFAs) correspond to C18:2 n-6, C18:3 n-6, C18:3 n-3, C20:1 n-9, C20:3n-3, C20:4n-6, C20:5 n-3, C:22:5 n-6, C:22:5 n-3, C24:0 and C22:6n-3; n-6 PUFAs are C18:2n-6 and C20:4n-6; n-3 PUFAs are C18:3n-3, C20:5n-3 and C22:6n-3; n-6/n-3 PUFA ratio: (C20:4n-6 + C:22:5 n-6)/ (18:3n-3 + C20:5n-3 + C:22:5 n-3 + C22:6n-3). Kruskal Wallis test, followed by Dunn’s test for multiple comparisons; (a, b) different letters in the same row represent a significant difference (p < 0.05).
CD control diet, HFD high fat diet, BSF basil seed flour, OAF oat flour.
Table 7.
Fatty acid composition of brain
| AG | CD | HFD | HFD + BSF | HFD + OAF |
|---|---|---|---|---|
| C16:0 | 24.09ab (24.02–24.14) | 26.61a (24.86–27.92) | 23.79b (23.6–24.46) | 23.98a (23.54–24.73) |
| C18:0 | 21.58ab (21.38–21.8) | 21.03a (19.39–21.21) | 21.6b (21.5–21.77) | 21.44ab (21.34–21.7) |
| C18:1n-9 | 23.2a (22.85–23.39) | 21.23a (21–21.59) | 22.06a (21.63–23.06) | 22.34a (21.44–23.41) |
| C18:2n-6 (LA) | 0.56a (0.53–0.87) | 0.99a (0.9–1.01) | 0.98a (0.95–1.24) | 1.11a (0.9–1.31) |
| C18:3n-3 (ALA) | 0.00a (0.00–0.00) | 0.00a (0.00–0.00) | 0.09b (0.07–0.15) | 0.00a (0.00–0.00) |
| C20:4n-6 (AA) | 9.62a (9.44–9.97) | 9.82a (9.65–10.62) | 9.54a (9.25–9.79) | 9.82a (9.34–9.99) |
| C20:5n-3 (EPA) | 2.6a (2.57–2.64) | 2.64a (2.52–2.75) | 2.59a (2.54–2.79) | 2.70a (2.59–2.76) |
| C:22:5 n-6 (DPA n-6) | 0.26a (0.17–0.27) | 0.21a (0.15–1.66) | 0.2a (0.15–0.26) | 0.17a (0.13–0.2) |
| C22:5 n-3 (DPA n-3) | 0.31a (0.29–0.47) | 0.27a (0.25–0.29) | 0.27a (0.23–0.31) | 0.20a (0.18–0.23) |
| C22:6n-3 (DHA) | 13.46a (13.34–13.63) | 13.32a (13.01–13.98) | 13.72a (13.5–14.22) | 13.42a (13.15–13.66) |
| Total SFAs | 46.91a (46.69–47.22) | 48.28a (46.53–48.77) | 47.05a (46.66–47.4) | 46.77a (46.15–48.12) |
| Total MUFAs | 25.68a (25.17–25.95) | 23.64a (23–24.04) | 24.59a (23.99–25.62) | 24.61a (23.32–25.99) |
| Total PUFAs | 27.76a (27.3–27.93) | 28.27a (27.61–30.43) | 28.25a (27.95–28.46) | 27.79a (27.35–28.02) |
| Total n-6 PUFAs | 10.82a (10.53–10.99) | 12.42a (11.03–13.05) | 10.88a (10.41–11.21) | 11.22a (10.42–11.3) |
| Total n-3 PUFAs | 16.39a (16.34–16.7) | 16.33a (15.81–16.98) | 16.85a (16.62–17.2) | 16.42a (16.02–16.64) |
| n-6/ n-3 PUFAs ratio | 0.64a (0.62–0.66) | 0.72a (0.69–0.8) | 0.66a (0.63–0.68) | 0.70a (0.67–0.71) |
Values are expressed as median (interquartile range) in % mmol the fatty acid, n = 7. Saturated fatty acids (SFAs) correspond to C13:0, C14:0, C16:0, C17:0, C18:0, C20:0, C22:0 and C22:2. Monounsaturated fatty acids (MUFAs) correspond to C14:1, C15:1, C16:1, C17:1, C18:1n-9, C20:1n-9 and C22:1n-9. Polyunsaturated fatty acids (PUFAs) correspond to C18:2 n-6, C18:3 n-6, C18:3 n-3, C18:3n-6, C20:1 n-9, C20:3n-3, C20:4n-6, C20:5 n-3, C:22:5 n-6, C:22:5 n-3, C24:0 and C22:6n-3; n-6 PUFAs are C18:2n-6 and C20:4n-6; n-3 PUFAs are C18:3n-3, C20:5n-3 and C22:6n-3; n-6/n-3 PUFA ratio: (C20:4n-6 + C:22:5 n-6)/ (18:3n-3 + C20:5n-3 + C:22:5 n-3 + C22:6n-3). Kruskal Wallis test, followed by Dunn’s test for multiple comparisons; (a, b) different letters in the same row represent a significant difference (p < 0.05).
CD control diet, HFD high fat diet, BSF basil seed flour, OAF oat flour.
Table 8.
Fatty acid composition of stool
| AG | CD | HFD | HFD + BSF | HFD + OAF |
|---|---|---|---|---|
| C16:0 | 32.53a (29.31–34.74) | 20.09b (20.06–20.33) | 28.58ab (27.78–29.73) | 24.32b (24.27–24.93) |
| C18:0 | 37.02a (33.06–37.8) | 41.96a (37.37–43.56) | 37.57a (35.25–41.25) | 37.31a (36.9–40.35) |
| C18:1n-9 | 15.42a (15.38–18.22) | 27.2b (25.9–28.99) | 20.65ab (18.6–23.48) | 20.55ab (19.69–21.19) |
| C18:2n-6 (LA) | 5.93a (5.7–6.82) | 5.10a (5.04–6.27) | 8.48ab (6.7–9.27) | 11.09b (10.52–11.87) |
| C18:3n-3 (ALA) | 0.00a (0.00–0.00) | 0.00a (0.00–0.00) | 2.36b (1.83–2.4) | 0.00a (0.00–0.00) |
| Total SFAs | 75.01a (73.47–77.17) | 65.81b (60.89–66.6) | 68.44ab (64.85–72.86) | 68.36ab (66.56–69.45) |
| Total MUFAs | 19.06a (16.76–19.18) | 27.51b (25.9–29.41) | 20.65ab (18.6–23.48) | 20.55ab (19.69–21.19) |
| Total PUFAs | 5.93a (5.70–7.72) | 6.79ab (6.74–7.48) | 10.92b (8.53–11.67) | 11.09b (10.86–11.87) |
Values are expressed as median (interquartile range) in % mmol the fatty acid, n = 7. Saturated fatty acids (SFAs) correspond to C13:0, C14:0, C16:0, C17:0, C18:0, C20:0, C22:0 and C22:2. Monounsaturated fatty acids (MUFAs) correspond to C14:1, C15:1, C16:1, C17:1, C18:1n-9, and C22:1n-9. Polyunsaturated fatty acids (PUFAs) correspond to C18:2 n-6, C18:3 n-6, C18:3 n-3, C18:3n-6, C20:1 n-9, C20:3n-3, C20:4n-6, C24:0 and C22:6n-3. Kruskal Wallis test, followed by Dunn’s test for multiple comparisons; (a, b) different letters in the same row represent a significant differences (p < 0.05).
CD control diet, HFD high fat diet, BSF basil seed flour, OAF oat flour.
Mice liver gene expression of enzymes related to lipid metabolism and inflammatory response
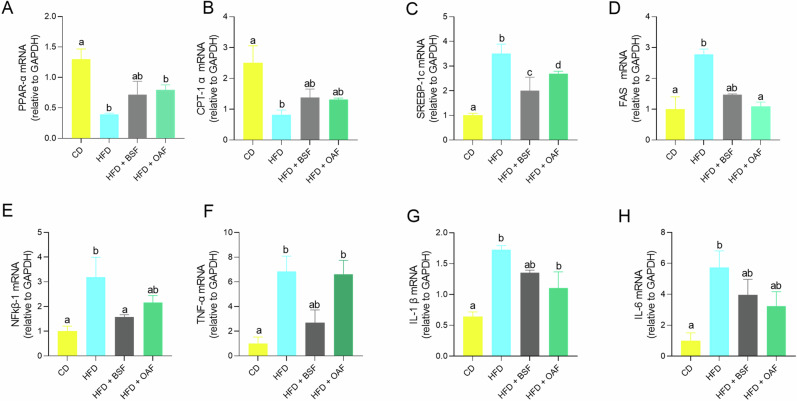
Figure 3 shows hepatic gene expression by experimental group. PPAR-α mRNA expression required to stimulate fatty acid β-oxidation enzymes (Fig. 3A) was significantly reduced in HFD-fed and OAF-supplemented mice relative to CD and BSF-supplemented mice. PPAR-α-mediated CPT1α was significantly decreased only in the HFD group, and to a lesser extent in the supplemented groups, relative to the CD group (Fig. 3B). On the other hand, mRNA expression of SREBP-1c, FAS required for de novo lipogenesis and cytokines related to inflammatory response (Fig. 3C–F) were significantly increased in the HFD group compared to CD group. However, they were significantly attenuated in the BSF-supplemented groups, except for SREBP-1c and IL-1β (Fig. 3C, G).
Fig. 3. Mice liver gene expression of enzymes related to lipid metabolism and inflammatory response.
A PPAR-α mRNA (relative to GAPDH); (B): CPT-1α mRNA (relative to GAPDH); (C): SREBP-1c mRNA (relative to GAPDH); (D): FAS mRNA (relative to GAPDH); (E): NFkβ-1 mRNA (relative to GAPDH); (F): TNF-α mRNA (relative to GAPDH); (G): IL-1β mRNA (relative to GAPDH); (H): IL-6 mRNA (relative to GAPDH). Values are represented as medians and interquartile ranges. CD control diet, HFD high-fat diet, BSF basil seed meal rich in dietary fiber, OAF oat flour. Kruskal Wallis test, followed by Dunn’s test for multiple comparisons; (a, b) different letters represent a significant difference (p < 0.05).
Influence of dietary fiber-rich fraction of partially defatted basil seeds on SCFAs content and other parameters
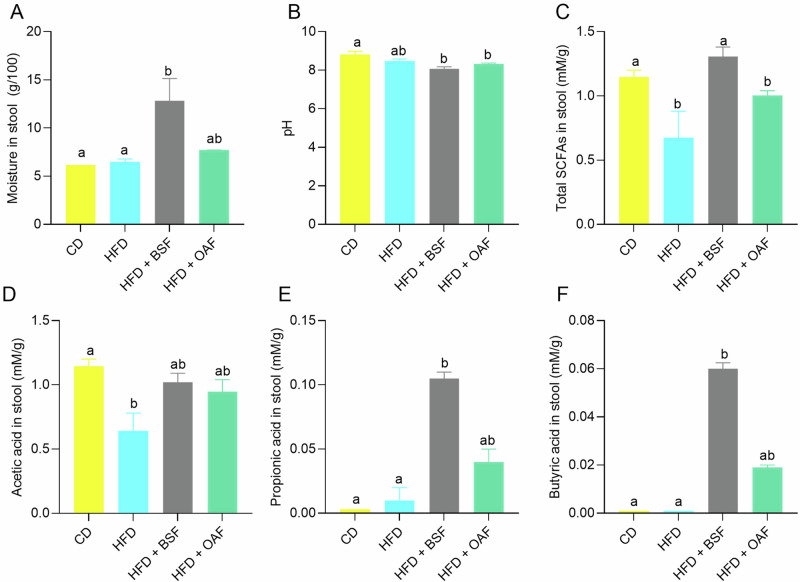
The general characteristics stool and SCFAs are shown in Fig. 4. Post-supplementation, stool moisture (BSF or OAF) showed a considerable increase in the water holding capacity, both presenting significant differences with the CD group, and in the case of BSF there was significantly higher stool moisture compared to the HFD group (Fig. 4A). In terms of pH of stool, HFD groups with or without supplementation showed statistically lower differences than CD group (Fig. 4B). In the post-supplementation treatment mice, the CD and BSF groups presented a statistically higher increase of total SCFAs than the HFD group (Fig. 4C). The SCFAs analysis revealed that acetic acid was the most abundant in all groups, followed by propionic and butyric acids, in the case of BSF-supplemented mice (Fig. 4D–F). In general terms, the following order was found, acetic acid > propionic acid > butyric acid > isobutyric acid > valeric acid > isovaleric acid. Due to their minimal contribution, the last three fatty acids are not presented in separate graphs; however, they are included in the total SCFAs in Fig. 4C.
Fig. 4. General characteristics stool and SCFAs.
SCFA present in the stool of the treated mice. A Moisture in stool (g/100 g); (B): pH; (C): Total SCFAs (mM/g); (D): Acetic acid in stool (mM/g); (E): Propionic acid in stool (mM/g); (F): Butyric acid in stool (mM/g). Values are represented as medians and interquartile ranges. CD control diet, HFD high-fat diet, BSF basil seed meal rich in dietary fiber, OAF oat flour, SCFA short-chain fatty acids, FA fatty acids. Kruskal Wallis test, followed by Dunn’s test for multiple comparisons; (a, b) different letters represent a significant difference (p < 0.05).
Discussion
The by-products of the basil seed oil industry have not been explored, and the nutritional composition of the fiber-rich fraction of partially defatted BSF has not been reported. The analysis revealed that BSF has 59.09 ± 2.62 g of dietary fiber per 100 g of flour, a value that is comparable to that previously reported by Capitani et al. 31 for chia seeds pressing fiber-rich fraction (51.98 g/100 g), and higher than those fiber-rich fractions from cereals such as wheat (20.93 g/100 g), pearl millet (21.92 g/100 g) and sorghum (23.4 g/100 g)32. According to some authors, the total dietary fiber of whole basil seed ranged between 22.6 to 36.30 g/100 g, which implies that the fraction could possess 1.6–2.6 times more dietary fiber than the seed28,33–35. In this context, less than 50 g of BSF could meet the daily recommendations of European Food Safety Authority (EFSA) and American Dietetic Association (ADA), which recommend an average daily intake > 25 g of dietary fiber36,37. The protein content (20.24 g/100 g) is also high compared with some pseudo cereals such as quinoa (14.5 g/100 g) and amaranth (16.5 g/100 g). In addition, the amino acid profile illustrates the high nutritional quality of the protein, including all the essential amino acids except S-containing types and tryptophan28. Conversely, despite cold pressing to extract and obtain basil seed oil, the resulting pellet, which is subsequently crushed and sieved to make BSF, still retains 6.35 g/100 g lipids, of which 2.31 g/100 g correspond to ALA and 1.8 g/100 g to linoleic acid (C18:2n-6, LA), amounts that could be enough to provide a health effect28. In general terms, the fiber-rich by products can increase the dietary fiber contents in the food matrix resulting in healthy food products, as previously reported by Elleuch et al. 38.
In the recent decades, the association between dietary fiber intake and health benefits (body weight, lipid regulation and insulin sensitivity), have been extensively studied15,39–41. This association has sparked interest in the search for new sources of fibers with different characteristics that can be used in the development of functional foods. In this context, BSF is presented as a new alternative of dietary fiber source. In our study, after 4 weeks of supplementation with 20% BSF or OAF (as control) in adult male rats with hepatic steatosis without changes in food consumption and energy intake, did present significant changes when comparing the HFD and supplemented CD groups. Fiber supplementation (BSF or OAF) resulted in lower body weight compared to their HFD controls; in particular, the inclusion of BSF, after exhibiting a greater effect on body weight, resulted in a lower body weight compared to the HFD control. Similar behavior was observed by Palou et al. (2015) where pectin supplementation for one month resulted in decreased body-fat content and ameliorate insulin and leptin resistance16. Additional studies have reported that the intake of dietary fiber can ameliorate adiposity and IR in mice fed a HFD16,42. In contrast, epididymal adipose tissue weight did not differ in HFD mice with or without supplementation16,43, presumably because both studies acted from prevention and not from reversion, as in the present investigation.
As a critical component, IR strongly promotes hepatic steatosis and there is evidence to support an association between dietary fiber intake and improved IR2,6,15,42. The present investigation showed that the supplementation with BSF and/or OAF attenuate IR by presenting a reduction in insulin and glycemic parameters compared to HFD. In addition, when comparing blood lipid parameters (TG, total cholesterol, HDL, and LDL), the BSF group showed a significant attenuation in TG, LDL and total cholesterol compared to HFD. Similar results were observed by Jangra et al. 42 where the two fermentable dietary fiber (gum acacia and inulin) analyzed resisted the enhancement in serum TG, total cholesterol, LDL and VLDL compared to high fat and sucrose diet used by Villanueva-Suárez et al. 44 where the HFD was supplemented with 20% artichoke fiber with a significant reduction of TG, LDL and total cholesterol. Also, it could be assumed that ALA provided by BSF supplementation (33 mg) could partly explain the results whose effect is possibly due to the BSF capacity to increase the excretion of bile salts45, and appetite regulation; reported in the group supplemented with BSF, in which a downward trend in food intake was observed, although it was not significant compared to the other groups, since excess calories are determinant in the development of IR43,46. In this sense, a study by Kim et al, showed that rats fed 10% perilla oil, characterized by a high content of ALA (60%) for 4 weeks, is able to reduce TG and cholesterol levels in plasma47. In addition, the mechanism by which dietary fiber intake diminishes TG in rats has been describe as decrease in de novo fatty acid synthesis in the liver through the inhibition of lipogenic enzymes16. Moreover, the presence of β-glucans, contained in oats and basil seeds, is also linked to lowering total and LDL cholesterol levels by influencing the absorption of carbohydrates and cholesterol in the intestine26,45. Furthermore, a study identified that basil seeds contained bioactive peptides with antioxidant activity and α-amylase inhibitory activities, by participating as analogs in the hydrogen bonding network in the active site of the amylase/substrate complex, thus inhibiting the hydrolysis process26,27. In this context, the synergistic effects of dietary fiber, ALA and other bioactives compounds could be attributed to the fact that fiber can trap bile acids, hindering the absorption of cholesterol, increasing the use of circulating cholesterol for its synthesis, thus affecting plasma lipids; reducing the systemic inflammation by lowering the levels of proinflammatory cytokines, improving the SCFAs profile which affect the insulin sensitivity by improving gut barrier function; activating PPARs by PUFAs enhancing insulin sensitivity and promoting fatty acid oxidation, between others14,48.
As previously reported, high-fat feeding in rodents causes marked hepatic steatosis and insulin resistance due to a mismatch between hepatic lipid uptake and lipid export13. In the histology, particularly in those from mice supplemented with BSF and OAF, a decrease in hepatic steatosis was observed, which was corroborated by the steatosis score, the amount of fat in the tissue, hepatic TG, and cholesterol. These results suggest that basil by-products might diminish fat accumulation preventing triglyceridemic and hepatic steatosis. These findings are in agreement with those previously reported by Villanueva-Suárez et al. 44 who studied the artichoke dietary fiber composed by a mix of low and large degree of polymerized fructans, which are in synergy with other polysaccharides producing a protective effect against hepatic steatosis.
Besides, the fatty acid profile of the liver tissue was identified and quantified resulting in a higher amount of ALA, EPA and DHA in mice fed with BSF v/s CD. This is presumably due to the amount of ALA contained in the BSF supplementation and because ALA is efficiently converted to DHA in the liver47. DHA increases the expression of PPAR-α genes, capable of inhibiting the nuclear transcription factor enhancer of activated NF-κB, restoring β-oxidation of fatty acids, and mediating oxidative, inflammatory (TNF-α, IL-1β, IL-6) and peroxisomal processes6,49. Proven in this study given that BSF supplementation enhanced the expression of PPAR-α mRNA and increased CPT1α. Consequently, it favors the reversal of NAFLD and hepatocellular damage11. In the process of inflammation and adipocyte dysfunction, M1-type macrophages release IL-6, IL-1β, to enhance phagocytosis and promote an inflammatory response. Additionally, through NLRP3 in the liver stimulated by NF-kβ and oxidative stress, gene expressions of the proinflammatory factors IL-6 and TNF-α have also been demonstrated, which could clarify the origin of the plasma proinflammatory cytokines found in HFD mice50–52. In addition, n-3 PUFAs exert effects on gene expression by regulating two other nuclear lipogenic factors, the carbohydrate response element binding protein ChREBP-1c, and FAS, thereby inhibiting de novo fatty acid synthesis25. Additionally, it has been shown that NAFLD in obese patients may predict a worse long-term prognosis denoted by the higher transaminase levels, relative to non-obese NAFLD11. Concordant with this, animals fed with HFD exhibited significantly higher levels of transaminases (AST, ALT and GGT) compared to those fed with CD, while only the BSF group was able to attenuate liver damage, showing no significant changes compared to CD.
Furthermore, in interventional mice, plasma levels of the inflammatory cytokines TNF-α, IL-6 and IL-1β were significantly increased after 14 weeks with HFD, whereas in mice supplemented with BSF or with OAF these values were attenuated compared to HFD. HFD increases the risk of metabolic complications, and because in humans, excess lipid tissue would be susceptible to mononuclear cell infiltration and secretion of proinflammatory cytokines such as TNF-α, IL1-β, IL-6 and resistin6,53,54. In this context, it was proposed that these cytokines can induce IR by activating several serine-threonine kinase pathways (such as the transcription factor NF-κB) which, in turn, phosphorylate insulin receptor substrate 1 (IRS-1) at serine or threonine leading to IRS degradation, affecting the downstream signaling cascade of insulin and consequently suppresses GLUT4 localization in cell membranes, favoring a reduced capacity for glucose uptake and utilization by cells6,53,54.
Another key event in the pathogenesis of NALFD is the formation of reactive oxygen species (ROS) during inflammation, which, in addition to mitochondrial damage, perpetuates the accumulation of metabolic intermediates, with induction of endoplasmic reticulum stress, that over time, triggers exacerbated ROS production11. As expected, the HFD group exhibited an increase (p < 0.05) in TBARS and hepatic isoprostanes, markers of lipid peroxidation; curiously, even though the BSF group was associated with the highest amount of DHA, a fatty acid sensitive to oxidation due to the elevated number of double bonds, it was the group with the lowest levels of TBARS, presenting significant differences compared to HFD. This protection could be attributed to the action of PPAR-α activity and the peptides with antioxidant activity previously mentioned26. In addition, lipid peroxidation by-products have been shown to be highly reactive and capable of modifying nucleophilic lysine, cysteine, and histidine residues in proteins, predominantly in later stages of NALFD55. In this sense, BSF group presented a significantly lower amount of protein carbonyls in contrast to the HFD group, indicating that supplementation attenuated ROS production and therefore lipid and protein oxidation, making lipid/protein peroxidation a validated marker of oxidative stress.
In contrast to this feature, in all living organisms, ROS levels are controlled by a complex network of antioxidant defenses, which reduce (but do not completely prevent) oxidative damage to biomolecules56 Glutathione peroxidase (GSH-Px) is one of the main cellular antioxidant enzymes, which reduces complex hydroperoxides into their respective alcohols using GSH as a reducing agent57. This may explain why, in this study, it was observed that although HFD mice showed a significant increase in GSH and GSSG levels (possibly as a compensatory mechanism) compared to CD and supplemented mice, their oxidative stress parameters were exacerbated.
There are limited studies addressing the impact of the amount of dietary ALA on its own accumulation and conversion to longer chain n-3 PUFAs, an aspect being relevant to study because (i) its storage (adipose tissue) may represent a reservoir of slow-release ALA that other tissues use over time (e.g., brain)23; and (ii) the generation of lipid mediators that can be produced in the conversion of ALA to DHA, namely, the specific pro-resolving mediators (SPMs) with dual anti-inflammatory and proresolving bioactivity, including resolvins, protectins and maresins, which modulate the function of endothelial cells and the immune system58. Along with this, it is essential to counteract the harmful effects of high intakes of carbohydrates and palmitic acid (C:16:0), given that they induce vascular inflammation by activating the immune system (Toll-like receptors (TLR) 2 and 4). Consequently, enhancement in the formation of precursors of lipid intermediates such as ceramides, IL-6, and NF-κB activation, represents a mechanism by which NAFLD promotes the development of vascular damage and atherosclerosis12,59,60. In this context, hamsters fed different amounts of ALA for 5 weeks (between 1 and 40 g ALA/100 g total fatty acids), showed a dramatic ALA content increase in epididymal adipose tissue, but to a much lesser extent than in red blood cells, although DHA content did not change47. The results of the present investigation corroborated that the ALA (2.6 g/100 g) contained in the supplementation with BSF, increased the levels of this fatty acid in epidemic adipose tissue and in erythrocytes. However, in these mice there was unexpectedly a significant increase of DHA in erythrocyte phospholipids compared to the HFD group. In addition, HFD diet has the ability to alter the fatty acid composition of tissues which can be corrected with dietary intervention, as they contribute to preserve the antioxidant capacity61, as demonstrated in our study. Regarding stool composition, supplementation reduced the absorption of saturated fats, leading to a higher amount of palmitic acid in the feces compared to the HFD group, as well as increased levels of n-6 and n-3 polyunsaturated fatty acids. This effect may be attributed to the fiber content mentioned earlier (64). These findings may partially explain the observed changes in the fatty acid profiles of various tissues and the overall lipid profile.
In this study, the analysis of the SCFAs composition in the stool indicates that mice fed with BSF had a significantly higher amount of total SCFAs than the CD and HFD groups. This aspect has been little studied in the literature so it would be important to further assess the SCFA profile in the future.
In addition, the stool of the BSF mice had higher moisture and fat compared to the control groups (CD and HFD) due to the water absorption/retention capacity produced mainly by the soluble fiber and the action of SCFAs that regulate the absorption of water, minerals and fat62,14. Furthermore, SCFAs also contribute to decrease the pH which inhibit potential pathogens and promote growth of beneficial bacteria14. Conversely, the pH analysis reflected a slight decrease in the supplemented groups compared to CD and HFD, presumably due to the production of SCFAs, since, being weak acids, they can decrease the colonic pH and, as a result the pH of stool. This could be a potential advantage because it has been observed that a low pH can inhibit the growth of sensitive pathogenic bacteria and favor the growth of beneficial bacteria14. This latter aspect is relevant in the pathogenesis of NALFD because intestinal dysbiosis could alter the production of bacteria and metabolites such as tryptophan, bacterial lipopolysaccharides (LPS) and thus deregulate the inflammatory activation of Kupffer cells, change the enterohepatic circulation of bile acids, causing inflammation and finally hepatic steatosis2. Evidence further highlighted the beneficial role of gut microbiota-derived SCFAs in managing energy homeostasis by regulating PPAR-α and enzymes involved in mitochondrial lipid oxidation such as acetyl-CoA carboxylase and carnitine-palmitoyl transferase 113.
The analysis of the SCFA profile in mice stool, identified that the BSF group contributed significantly to the production of SCFAs followed by those fed with OAF, acetic acid > propionic acid > butyric acid. Similar findings were reported by several authors, which detected significant increase of these SCFAs, both in vitro, mice and human models14,43,63.
On the other hand, the dietary fiber contained in supplements could be considered as potential prebiotic, since it is known that bacteria belonging to the phylum Bacteroidetes make up a large proportion of the intestinal microbiota and mainly produce propionate together with acetate14. In addition, Wongputtisin and Khanongnuch64 investigated the prebiotic properties crude basil oligosaccharide. The results revealed an increase in total lactic acid bacteria and decrease in Salmonella-Shigella spp group, suggesting the prebiotic potential of this fiber.
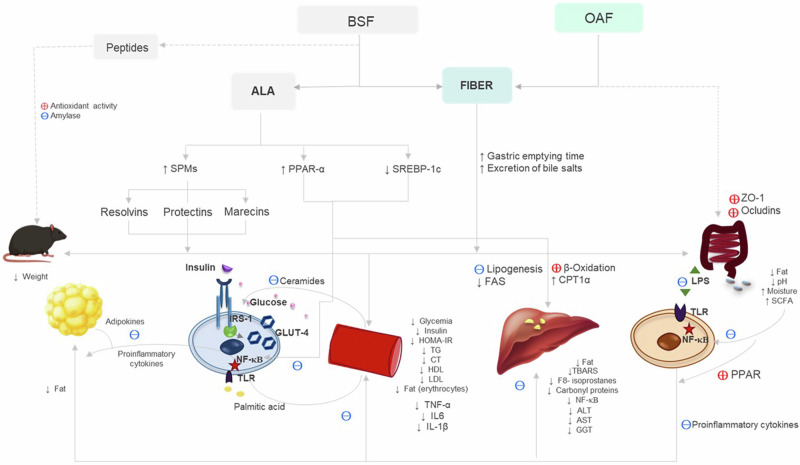
Also, the specific effect of oat on the previously mentioned results could be based on what was recently published by Gao et al. (2022), who reported that oat fiber supplementation is able to block the TLR4 signaling pathway and decrease the expression of NF-κB p65 in intestinal tissues of male mice. In addition, they indicated that oat fiber increases the expression of tight junction proteins, including zonula occludens-1 (ZO-1) and occludin, thus contributing to maintaining the integrity of the intestinal barrier65. The mechanisms by which BSF and OAF can reduce IR and steatosis, mentioned in the discussion, are summarized in Fig. 5.
Fig. 5. Summary of the effects of supplementation with BSF and OAF and the possible metabolic pathways or mechanisms involved in the reversal of IR and hepatic steatosis.
Fiber, present in BSF and OAF, plays a crucial role in improving metabolic parameters through several potential mechanisms. At the digestive level, fiber increases gastric emptying time, decreases lipid absorption (such as palmitic acid), and promotes the excretion of bile salts. These effects help reduce body weight by decreasing nutrient absorption and promoting fat elimination. This results in lower fat accumulation in adipocytes, improved homeostasis, and decreased secretion of proinflammatory cytokines (such as TNF-α, IL-1β, and IL-6) that alter insulin signaling, thus contributing to the improvement of IR. At the vascular level, reduced palmitic acid presence decreases vascular inflammation by decreasing the activation of TLR2 and TLR4, NF-κB, and consequently proinflammatory cytokines, thereby preventing the phosphorylation IRIS-1. This also restores lipid metabolism by preventing the accumulation of lipid intermediates such as ceramides and enhancing insulin sensitivity. At the hepatic level, fiber reduces caloric intake and the amount of circulating nutrients (TG, cholesterol), inhibits de novo fatty acid synthesis in the liver, and reduces mitochondrial damage. This leads to less nutrient oxidation and improved hepatocyte integrity, reflected in decreased lipid peroxidation and reduced hepatic enzyme levels. At the intestinal level, soluble fiber increases water retention and reduces intestinal pH by favoring the production of SCFAs. This inhibits the growth of pathogenic bacteria and prevents dysbiosis, which could otherwise deregulate inflammation. On the other hand, oats could improve the integrity of the intestinal barrier by increasing the expression of tight junction proteins such as ZO-1 and occludin, preventing the passage of LPS and the activation of TLR4. Meanwhile, BSF, rich in ALA, upon conversion to DHA, activates PPAR-α, regulating β-oxidation and fatty acid synthesis, and produces anti-inflammatory lipid mediators such as resolvins and protectins, which modulate endothelial and immune system function, improving metabolic health. Additionally, it contains peptides with antioxidant activity and amylase inhibitory capabilities. ↑, increases; ↓ decreases, ( + ) stimulates, (-) inhibits.
In conclusion, this research aimed to determine the effects of fiber-rich fraction of partially defatted basil seeds against IR and hepatic steatosis induced by a high-fat diet and its contribution on the tissue content of n-3 PUFAs and SCFAs in mice. The HFD diet induced IR, hepatic steatosis, proinflammatory status, oxidative status and a significant decrease in SCFA production. In contrast, the HFD diet supplemented with BSF and to a lesser extent with OAF achieved protective effects by exhibiting an improvement in IR-related parameters, attenuation of steatosis, liver damage and oxidative stress, as well as, decreased inflammatory status, increased n-3 PUFAs in liver, adipocytes and erythrocyte and increased SCFAs. Taking this into account, supplementation with BSF exerted protection, so this product could be considered as a potential therapeutic line for the management of IR and reversal of hepatic steatosis in humans. In addition, this study allowed the revaluation of industrial residues from the production of basil seed oil emerging in the market, by proving that the expeller obtained can become an excellent source of dietary fiber, insoluble fiber and ALA. It remains for future research to determine and quantify the polyphenols that may remain in the product since there is a possibility that they could contribute to or explain the decrease in the metabolic complications studied.
Methods
Materials
The basil seeds were cultivated in the Central Zone of Chile, specifically in O´Higgins and Maule Regions and provided by the company SPS Food (South Pacific Seeds), Chile.
By-product obtention and characterization
Firstly, basil seeds expeller was obtained after the cold pressing process using a Komet vegetable oil extractor (model DD85G, IBG Monforte, Germany). Subsequently, the partially defatted pellets were subjected to grinding in a micropulverizer model ZM-200, RETSCH mill for 10 min at 13,440 × g. Afterward, the fiber-rich fraction was obtained by dry fractionation using the methodology proposed by Vázquez-Ovando et al. 66 modified by using an analytical sieve model RS-200, RETSCH with a sieve of 212 µm pore size for 10 min. The portion that remains at the top of the sieve corresponds to the fiber-rich fraction of partially defatted basil seeds.
After that, the nutritional composition was determined by using AACC methods and expressed in g/100 g on dry basis67. In brief, the ash content was performed in a muffle furnace by incineration according to Official Methods 08-0368; the protein content was determined by using the Kjeldahl method and the moisture in an oven (Biobase, China) at 105 °C until constant weight. The total, soluble and insoluble dietary fiber contents were determined by the total dietary fiber assay procedure of AOAC Method 991.43 based on an enzymatic and gravimetric method by using a K-TDFR kit, Megazyme, Ireland69.
The total fat was determined according to Bligh and Dyer70, and quantified gravimetrically (g fat/100 g sample). Prior to chromatographic analysis for fatty acid determination, lipid samples were derivatized to fatty acid methyl esters (FAMEs) with BF3 (20% methanol solution) according to the methodology of Morrison and Smith71. The FAMEs were analyzed by gas-liquid chromatography (GC) on an Agilent GC unit (7890 A), using a capillary column (Agilent HP-88.60m × 0.25 mm; ID. 0.25 mm) and a flame ionization detector using C23:0 as internal standard (Nu-Check Prep, USA).
Animal model and diets
The control diet (CD) with 10% of calories as fat, and HFD with 60% calories as fat, were obtained from the Research Diets, Inc. laboratory (USA). The dietary fiber supplementation consisting of fiber-rich fraction of partially defatted basil seeds (BSF), or oat flour (OAF) used as control, were calculated based on the WHO recommendations: 25 g/day considering a 2000-calorie diet, in conjunction with estimates reported in the literature (Supplementary Table S1)11,15,19. The sample size was calculated using a spreadsheet from the Clinical Epidemiology and Biostatistics Unit of the Complejo Hospitalario Universitario A Coruña, considering the change in hepatic lipid content as a critical variable; with a reduction of 7.32% ± 0.162 according to data reported in the literature16 and considering the statistical significance parameters of p < 0.05, power of 0.9 and a margin of loss of 20%, it was estimated that 6 animals per group were required. Male C57BL/6 J mice (n = 28) obtained from the University of Chile biotherium were randomly assigned to 4 groups (n = 7), one group was given CD and 3 groups received HFD. After 10 weeks, two HFD groups were supplemented with fiber-rich fraction of partially defatted basil seeds (BSF) or oat flour (OAF) for 4 weeks. The mice had free access to diet and water, with ambient temperature control on a light-dark cycle of 12 hours each. The animals belonging to each group were kept in separate cages. After 14 weeks of intervention and a 12-h fasting, the animals were anesthetized with inhaled isoflurane (Lunan Better Pharmaceutical Co., Ltd., China). The procedure was performed in the Biotherium of the Department of Nutrition, Faculty of Medicine, University of Chile with the support of a veterinarian. The procedure started with the control groups (CD and HFD) and then continued with the supplemented groups (BSF and OAF). Blood samples were extracted by cardiac puncture and then centrifuged at 1646 × g for 15 min. In addition, samples from liver, brain and epididymal adipose tissue were collected and weighed, prior to being introduced into liquid nitrogen. The collected samples were stored at –80 °C until subsequent analysis, except for a liver section destined for histology that was stored in formaldehyde.
Ethics statement
Experimental animal protocols and animal’s procedures complied with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences, NIH Publication 86-23, revised 1985) and were approved by the Ethics Committee of the Faculty of Medicine, University of Chile (protocol CBA #1118 FMUCH).
Body weight and dietary and water intake
Body weight, dietary and water intake were measured daily by means of a scale and a graduated test tube, respectively and expressed in grams or ml as appropriate.
Metabolic parameters
Serum glucose (mg/dL), and insulin (μUI/mL) were measured using specific diagnostic kits (Wiener Lab, Argentina). The IR was determined following the Eq. (1) according to Matthews et al. 7 :
| 1 |
Total cholesterol, LDL, high density lipoproteins (HDL), and TG levels (mg/dL) were measured by using test strips (KENSHIN-2, Japan). LDL was determined by applying the Eq. (2) by Friedewald 197272.
| 2 |
Evaluation of hepatic steatosis and damage
The hepatic accumulation of lipids was evaluated quantitatively by determining the percentage of cells with infiltration of lipid vesicles in liver sections stained with hematoxylin-eosin. The qualitative evaluation considered the presence of macro- and micro-vesicular hepatic steatosis according to Brunt et al. 73. TG and total cholesterol were determined using a specific enzyme kit (Wiener Lab, Argentina) and expressed in mg/g liver. Serum aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyl transpeptidase (GGT) levels were measured using test strips (KENSHIN-2, Japan) and expressed in U/L.
Oxidative stress and inflammatory parameters
Hepatic levels of F8-isoprostanes and carbonylated proteins were measured by ELISA and colorimetric kits (Cayman Chemical, USA) and expressed in pg/mg tissue and mmol/mg tissue, respectively. Hepatic thiobarbituric acid reactive substances (TBARS) levels were determined by using a colorimetric kit (R&D Systems, USA) and expressed in μM MDA/mg tissue. Serum levels of tumor necrosis factor alpha (TNF-α), interleukin 6 (IL-6), and interleukin 1 beta (IL-1β) were used using ELISA kits (R&D Systems, USA).
Evaluation of antioxidant defense in liver
The evaluation of reduced glutathione (GSH), oxidized glutathione (GSSG) and GSH/GSSG ratio was measured using an enzymatic recycling method, as previously described by Rahman et al. (2006) and expressed in μmol/g liver74. In short, the amount of total glutathione was determined by analyzing the formation of 5-thio-2-nitrobenzoic acid (TBA) from 5,5-dithiobis 2-nitrobenzoic acid (DTNB). GSSG was measured by adding 2-vinylpyridine, which inhibits TBA formation when DTNB and glutathione react. Total GSH and GSSG levels were defined by the changes in the optical density at 412 nm for 2 min at every 30 s interval.
Analysis of fat content and fatty acid profile in liver, adipose, erythrocytes, brain tissue and stool
The determination of total fat and fatty acid profile in the liver and stool was performed according to previously described in “By-product obtention and characterization” section, quantified gravimetrically and expressed as g fat/100 g tissue or stool.
Quantification of resolvins
Resolvins D1 (RvD1) and resolvins D2 (RvD2) were determined by using specific ELISA kits (pg/mg tissue), with sensitivities of 5.0 and 2.0 pg/mL, respectively (MyBioSource, USA), according to manufacturer’s instructions.
Gene expression assays
Total RNA was isolated from liver samples using Trizol (Ambion, Carlsbad, CA, USA), according to the supplier’s protocols. Purified RNA was treated with DNAase I (DNA-freeTM Kit; Invitrogen, Vilnus, Lithuania). Then 1 µg of total RNA was used to generate single-stranded cDNA (Peroxisome proliferator-activated receptor alpha; PPAR-α, Sterol response element binding protein; SREBP-1c, Nuclear factor kappa-light-chain-enhancer of activated B cells; NF-kβ-1, Carnitine palmitoyl transferase 1α; CPT1α), Fatty acid synthase; FAS, TNF-α, IL-1β, IL-6, using High-Capacity cDNA Reverse Transcription Kit (Appliedbiosystems, Vilnus, Lithuania). The cDNA was amplified using the TaqMan® Gene Expression Assays (Appliedbiosystems, Pleasanton, CA, USA) in a total volume of 20 µL. Real time PCR was performed in an AriaMx qPCR System (Agilent Technologies, Penang, Malaysia) following the manufacturer’s recommendations (Appliedbiosystems, Pleasanton, CA, USA). Expression levels of target genes studied were normalized by the expression of GAPDH as internal control. Fold change between groups was calculated by the 2-(ΔΔCt) method, as established by Pfaffl75
Determination of pH, moisture and SCFAs in stools
The total fat of the stool samples was extracted according to Bligh and Dyer, 195976, quantified gravimetrically and expressed as g of fat/100 g stool. Stool moisture was determined by placing the samples in an oven at 105 °C until they reached a constant weight. Samples for SCFAs stool analysis were performed according to methodology of Tirado et al. 77. The pH of fecal samples was taken once a week and frozen at –20 °C for subsequent analysis. Prior to the analysis, the samples were prepared according to the methodology proposed by García et al. 63. In short, 200 mg of each sample was extracted in Eppendorf tubes and mechanically shaking with 1 ml deionized water for 1 min. Then, the pH of each sample was adjusted to 2–3 and subsequently centrifuged for 10 min at 26.342 × g. Finally, 195 μl supernatant was transferred to a vial and 10 μl of internal control (2 ethyl butyric acid 40 mM) was added. The samples were stored at –20 °C until analysis. SCFAs were determined according to Sasaki et al. 14 by using gas chromatography (GC) on Agilent GC unit (7890 A), by means of a capillary column (Agilent HP-88.60m × 0.25 mm; ID. 0.25 mm) and a flame ionization detector with C6H12O2 as internal standard (Sigma-Aldrich, USA).
Statistical analysis
All the analysis was performed in triplicate. The results of proximate analysis were reported as means ± standard deviation (SD). One-way ANOVA was used to compare means values, and significant differences (p < 0.05) were calculated with the Tukey´s post-hoc test. Since the data obtained from the animal model did not exhibit normality of variances, the results were analyzed using non-parametric statistical methods and are presented as median and interquartile ranges. The normal distribution of the data was evaluated using the Shapiro Wilk test, and the Kruskal Wallis test was applied, followed by the Dunn’s test for multiple comparisons, values with different letters represent significant difference (p < 0.05).
Supplementary information
Acknowledgements
We are grateful to National Agency for Research and Development (ANID, Chile) FONDECYT regular grant 1201489.
Abbreviations
- ADA
American Dietetic Association
- ALA
α-linolenic acid
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BSF
fiber-rich fraction of partially defatted basil seeds
- CD
control diet
- CPT1α
carnitine palmitoyl transferase 1α
- CVD
cardiovascular disease
- DHA
docosahexaenoic fatty acid
- DNTB
5,5-dithiobis 2-nitrobenzoic acid
- EFSA
European Food Safety Authority
- EPA
eicosapentaenoic acid
- FAMEs
fatty acid methyl esters
- FAS
fatty acid synthase
- GC
gas chromatography
- GGT
gamma-glutamyl transpeptidase
- GSH
reduced glutathione
- GSH-Px
Glutathione peroxidase
- GSSG
oxidized glutathione
- HDL
high density lipoproteins
- HFD
high-fat diet
- HOMA-IR
homeostatic model for insulin resistance
- IL-1β
interleukin 1 beta
- IL-6
interleukin 6
- IR
insulin resistance
- IRS-1
phosphorylate insulin receptor substrate 1
- LA
linoleic acid
- LDL
low-density lipoprotein
- MetS
metabolic syndrome
- MUFAs
monounsaturated fatty acids
- NAFLD
Non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- OAF
oat flour
- PPAR-α
peroxisome proliferator-activated receptor alpha
- PUFAs
polyunsaturated fatty acids
- Resolvins D1
RvD1
- Resolvins D2
RvD2
- ROS
reactive oxygen species
- SAFAs
saturated fatty acids
- SCFAs
short-chain fatty acids
- SD
standard deviation
- SPMs
pro-resolving mediator
- SREBP-1c
sterol response element binding protein
- NF-kβ-1
nuclear factor kappa-light-chain-enhancer of activated B cells
- T2DM
type 2 diabetes mellitus
- TBA
5-thio-2-nitrobenzoic acid
- TBARS
thiobarbituric acid reactive substances
- TG
triglycerides
- TLR
toll-like receptors
- TNF-α
Tumor necrosis factor alpha
- WHO
World Health Organization
- ZO-1
zonula occludens-1
Author contributions
Conceptualization: F.C., C.C., C.A., M.L.,V.R., C.H., E.A., V.L.A., M.L.; Data curation: F.C., C.C., C.A., M.L.,V.R., C.H., E.A., V.L.A., M.L.; Formal analysis: F.C., C.C., C.A., M.L., V.R., C.H., E.A., V.L.A., M.L.; Investigation: F.C., C.C., C.A., M.L., V.R., C.H., E.A., V.L.A., M.L.; Methodology: F.C., C.C., C.A., M.L., V.R., C.H., E.A., V.L.A., M.L.; Validation: F.C., C.C., C.A., M.L., V.R., C.H., E.A., V.L.A., M.L.; Writing: F.C., C.C., C.A., M.L., V.R., C.H., E.A., V.L.A., M.L.; Review & editing: V.R., V.L.A., M.L.
Data availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41538-024-00329-z.
References
- 1.Lee, S. H., Park, S. Y. & Choi, C. S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. J.46, 15–37 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanase, D. M. et al. The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J. Diabetes Res2020, 3920196 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yudhani, R. D. et al. In vitro insulin resistance model: A recent update. J. Obes.2023, 1–13 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gołąbek, K. D. & Regulska-Ilow, B. Dietary support in insulin resistance: An overview of current scientific reports. Adv. Clin. Exp. Med.28, 1577–1585 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Liu, L., Han, J., Li, H., Liu, M. & Zeng, B. The establishment of insulin resistance model in FL83B and L6 cell. AIP Conference Proceedings189010.1063/1.5005194 (2017).
- 6.Fujii, H., Kawada, N. & Japan Study Group Of Nafld, J.-N. The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2110.3390/ijms21113863 (2020). [DOI] [PMC free article] [PubMed]
- 7.Matthews, D. R. et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia28, 412–419 (1985). [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto, E., Taniai, M. & Tokushige, K. Characteristics and diagnosis of NAFLD/NASH. J. Gastroenterol. Hepatol.28, 64–70 (2013). [DOI] [PubMed] [Google Scholar]
- 9.Cotter, T. G. & Rinella, M. Nonalcoholic fatty liver disease 2020: The state of the disease. Gastroenterology158, 1851–1864 (2020). [DOI] [PubMed] [Google Scholar]
- 10.Sheka, A. C. et al. Nonalcoholic steatohepatitis: A review. JAMA323, 1175–1183 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Cariou, B., Byrne, C. D., Loomba, R. & Sanyal, A. J. Nonalcoholic fatty liver disease as a metabolic disease in humans: A literature review. Diabetes Obes. Metab.23, 1069–1083 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dewidar, B., Kahl, S., Pafili, K. & Roden, M. Metabolic liver disease in diabetes - From mechanisms to clinical trials. Metabolism111s, 154299 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moszak, M., Szulińska, M. & Bogdański, P. You are what you eat-the relationship between diet, microbiota, and metabolic disorders-A review. Nutrients1210.3390/nu12041096 (2020). [DOI] [PMC free article] [PubMed]
- 14.Sasaki, D. et al. Low amounts of dietary fibre increase in vitro production of short-chain fatty acids without changing human colonic microbiota structure. Sci. Rep.8, 435 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barber, T. M., Kabisch, S., Pfeiffer, A. F. H. & Weickert, M. O. The health benefits of dietary fibre. Nutrients12, 3209 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palou, M., Sánchez, J., García-Carrizo, F., Palou, A. & Picó, C. Pectin supplementation in rats mitigates age-related impairment in insulin and leptin sensitivity independently of reducing food intake. Mol. Nutr. Food Res59, 2022–2033 (2015). [DOI] [PubMed] [Google Scholar]
- 17.(WHO) World Health Organization, Guideline: Sugars Intake for Adults and Children (2015). [PubMed]
- 18.Samarasinghe, K. et al. The basics of Dietary Fibers in Dietary Fibers 94 IntechOpen, Available from: 10.5772/intechopen.101468 (2022).
- 19.Fu J, Zheng Y, Gao Y. & Xu W. Dietary fiber intake and gut microbiota in human health. Microorganisms10, 2507. 10.3390/microorganisms10122507(2022). [DOI] [PMC free article] [PubMed]
- 20.Food Sanitary Regulations, Ministry of Health, Government of Chile (2022).
- 21.Rincón-Cervera, M. et al. Vegetable oils rich in alpha linolenic acid increment hepatic n-3 LCPUFA, modulating the fatty acid metabolism and antioxidant response in rats. Prostaglandins Leukot. Ess. Fat. Acids111, 25–35 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Calderón Bravo, H., Vera Céspedes, N., Zura-Bravo, L. & Muñoz, L. A. Basil seeds as a novel food, source of nutrients and functional ingredients with beneficial properties: A review. Foods1010.3390/foods10071467 (2021). [DOI] [PMC free article] [PubMed]
- 23.Domenichiello, A. F., Kitson, A. P. & Bazinet, R. P. Is docosahexaenoic acid synthesis from α-linolenic acid sufficient to supply the adult brain? Prog. Lipid Res59, 54–66 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Fu, Y. et al. Associations among dietary omega-3 polyunsaturated fatty acids, the gut microbiota, and intestinal immunity. Mediat. Inflamm.2021, 8879227 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.FAO, Fats and fatty acids in human nutrition. 1–204 p, Available in: https://www.fao.org/fileadmin/user_upload/nutrition/docs/requirements/fatsandfattacidsreport.pdf (2010). [PubMed]
- 26.Farías, C., Cisternas, C., Morales, G., Muñoz, L. & Valenzuela, R. Albahaca: Composición química y sus beneficios en salud. Rev. Chil. de. Nutrición49, 502–512 (2022). [Google Scholar]
- 27.Afifah, N. H. & Gan, C.-Y. Antioxidative and amylase inhibitor peptides from basil seeds. Int. J. Peptide Res. Ther.2210.1007/s10989-015-9477-5 (2015).
- 28.Calderón Bravo, H., Vera Céspedes, N., Zura-Bravo, L. & Muñoz, L. A. Basil seeds as a novel food, source of nutrients and functional ingredients with beneficial properties: A review. Foods10, 1467 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fundacion para la Innovacion Agraria, Development of functional ingredients from Chia, Basil and Viborera seeds produced in Chile. Available in: https://bibliotecadigital.fia.cl/handle/20.500.11944/148200 (2018).
- 30.Dybka-Stępień, K., Otlewska, A., Góźdź, P. & Piotrowska, M. The renaissance of plant mucilage in health promotion and industrial applications: A review. Nutrients1310.3390/nu13103354 (2021). [DOI] [PMC free article] [PubMed]
- 31.Capitani, M. I., Spotorno, V., Nolasco, S. M. & Tomás, M. C. Physicochemical and functional characterization of by-products from chia (Salvia hispanica L.) seeds of Argentina. LWT - Food Sci. Technol.45, 94–102 (2012). [Google Scholar]
- 32.Siddiq, A. & Prakash, A. J. Antioxidant properties of digestive enzyme-treated fibre-rich fractions from wheat, finger millet, pearl millet and sorghum: A comparative evaluation. Cogent Food Agric.1, 1073875 (2015). [Google Scholar]
- 33.Nazir, S. & Wani, I. A. Physicochemical characterization of basil (Ocimum basilicum L.) seeds. J. Appl. Res. Med. Aromat. Plants22, 100295 (2021).
- 34.Mathews, S., Singhal, R. S. & Kulkarni, P. R. Ocimum basilicum: A new non-conventional source of fibre. Food Chem.47, 399–401 (1993).
- 35.Rezapour, R., Ghiassi Tarzi, B. & Movahed, S. The effect of adding sweet basil seed powder (Ocimum basilicum L.) on rheological properties and staling of baguette bread. J. Food Biosci. Technol.6, 41–46 (2016).
- 36.EFSA. Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J.8, 1462 (2010). [Google Scholar]
- 37.American Dietetic Association. Position of the American dietetic association: Health implications of dietary fiber. J. Am. Dietetic Assoc.108, 1716–1731 (2008). [DOI] [PubMed] [Google Scholar]
- 38.Elleuch, M. et al. Dietary fibre and fibre-rich by-products of food processing: Characterisation, technological functionality and commercial applications: A review. Food Chem.124, 411–421 (2011). [Google Scholar]
- 39.Bulsiewicz, W. J. The importance of dietary fiber for metabolic health. Am. J. Lifestyle Med. 17, 639–648 (2023). [DOI] [PMC free article] [PubMed]
- 40.Weickert, M. O. & Pfeiffer, A. F. H. Metabolic effects of dietary fiber consumption and prevention of diabetes. J. Nutr.138, 439–442 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Wei, B. et al. Dietary fiber intake and risk of metabolic syndrome: A meta-analysis of observational studies. Clin. Nutr.10.1016/j.clnu.2017.10.019 (2017). [DOI] [PubMed]
- 42.Jangra, S., K, R. S., Sharma, R. K., Pothuraju, R. & Mohanty, A. K. Ameliorative effect of fermentable fibres on adiposity and insulin resistance in C57BL/6 mice fed a high-fat and sucrose diet. Food Funct.10, 3696–3705 (2019). [DOI] [PubMed] [Google Scholar]
- 43.Abernathy, B. E., Schoenfuss, T. C., Bailey, A. S. & Gallaher, D. D. Polylactose exhibits prebiotic activity and reduces adiposity and nonalcoholic fatty liver disease in rats fed a high-fat diet. J. Nutr.151, 352–360 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villanueva-Suárez, M. J., Mateos-Aparicio, I., Pérez-Cózar, M. L., Yokoyama, W. & Redondo-Cuenca, A. Hypolipidemic effects of dietary fibre from an artichoke by-product in Syrian hamsters. J. Funct. Foods56, 156–162 (2019). [Google Scholar]
- 45.Shah, B. R., Li, B., Al Sabbah, H., Xu, W. & Mráz, J. Effects of prebiotic dietary fibers and probiotics on human health: With special focus on recent advancement in their encapsulated formulations. Trends Food Sci. Technol.102, 178–192 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kojta, I., Chacińska, M. & Błachnio-Zabielska, A. Obesity, bioactive lipids, and adipose tissue inflammation in insulin resistance. Nutrients1210.3390/nu12051305 (2020). [DOI] [PMC free article] [PubMed]
- 47.Barceló-Coblijn, G. & Murphy, E. J. Alpha-linolenic acid and its conversion to longer chain n−3 fatty acids: Benefits for human health and a role in maintaining tissue n−3 fatty acid levels. Prog. Lipid Res.48, 355–374 (2009). [DOI] [PubMed] [Google Scholar]
- 48.Ouyang, Y., Zhao, J. & Wang, S. Multifunctional hydrogels based on chitosan, hyaluronic acid and other biological macromolecules for the treatment of inflammatory bowel disease: A review. Int J. Biol. Macromol.227, 505–523 (2023). [DOI] [PubMed] [Google Scholar]
- 49.Shi, Y. et al. Trace elements, PPARs, and metabolic syndrome. Int. J. Mol. Sci. 2110.3390/ijms21072612 (2020). [DOI] [PMC free article] [PubMed]
- 50.Gao, Q. et al. Microbe-derived antioxidants alleviate liver and adipose tissue lipid disorders and metabolic inflammation induced by high fat diet in mice. Int. J. Mol. Sci.2410.3390/ijms24043269 (2023). [DOI] [PMC free article] [PubMed]
- 51.Videla, L. A., Valenzuela, R., Zúñiga-Hernández, J. & Del Campo, A. Relevant aspects of combined protocols for prevention of N(M)AFLD and other non-communicable diseases. Mol. Nutr. Food Res68, e2400062 (2024). [DOI] [PubMed] [Google Scholar]
- 52.Videla, L. A., Valenzuela, R., Del Campo, A. & Zúñiga-Hernández, J. Omega-3 lipid mediators: modulation of the M1/M2 macrophage phenotype and its protective role in chronic liver diseases. Int. J. Mol. Sci.2410.3390/ijms242115528 (2023). [DOI] [PMC free article] [PubMed]
- 53.Yaribeygi, H., Farrokhi, F. R., Butler, A. E. & Sahebkar, A. Insulin resistance: Review of the underlying molecular mechanisms. J. Cell Physiol.234, 8152–8161 (2019). [DOI] [PubMed] [Google Scholar]
- 54.da Silva Rosa, S. C., Nayak, N., Caymo, A. M. & Gordon, J. W. Mechanisms of muscle insulin resistance and the cross-talk with liver and adipose tissue. Physiol. Rep.8, e14607 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shearn, C. T. et al. Differential carbonylation of proteins in end-stage human fatty and nonfatty NASH. Free Radic. Biol. Med.113, 280–290 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Del Campo, A., Valenzuela, R., Videla, L. A. & Zúñiga-Hernandez, J. Cellular functional, protective or damaging responses associated with different redox imbalance intensities: A comprehensive review. Curr. Med. Chem.30, 3927–3939 (2023). [DOI] [PubMed] [Google Scholar]
- 57.Weaver, K. & Skouta, R. The selenoprotein glutathione peroxidase 4: From molecular mechanisms to novel therapeutic opportunities. Biomedicines1010.3390/biomedicines10040891 (2022). [DOI] [PMC free article] [PubMed]
- 58.Markworth, J. F. et al. Divergent shifts in lipid mediator profile following supplementation with n-3 docosapentaenoic acid and eicosapentaenoic acid. FASEB J.30, 3714–3725 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yazıcı, D. & Sezer, H. Insulin resistance, obesity and lipotoxicity. Adv. Exp. Med. Biol.960, 277–304 (2017). [DOI] [PubMed] [Google Scholar]
- 60.Kasper, P. et al. NAFLD and cardiovascular diseases: A clinical review. Clin. Res. Cardiol.110, 921–937 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Valenzuela, R. et al. Hydroxytyrosol prevents reduction in liver activity of Δ-5 and Δ-6 desaturases, oxidative stress, and depletion in long chain polyunsaturated fatty acid content in different tissues of high-fat diet fed mice. Lipids Health Dis.16, 64 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Almeida-Alvarado, S. L., Aguilar-López, T. & Hervert-Hernández, D. La fibra y sus beneficios a la salud. An. Venezolanos de. Nutrición27, 73–76 (2014). [Google Scholar]
- 63.García-Villalba, R. et al. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples. J. Sep Sci.35, 1906–1913 (2012). [DOI] [PubMed] [Google Scholar]
- 64.Wongputtisin, P. & Khanongnuch, C. Prebiotic properties of crude oligosaccharide prepared from enzymatic hydrolysis of basil seed gum. Food Sci. Biotechnol.24, 1767–1773 (2015). [Google Scholar]
- 65.Gao, H., Song, R.-J., Jiang, H., Zhang, W. & Han, S.-F. Oat fiber supplementation alleviates intestinal inflammation and ameliorates intestinal mucosal barrier via acting on gut microbiota-derived metabolites in LDLR–/– mice. Nutrition95, 111558 (2022). [DOI] [PubMed] [Google Scholar]
- 66.Vázquez-Ovando, A., Betancur-Ancona, D. & Chel-Guerrero, L. Physicochemical and functional properties of a protein-rich fraction produced by dry fractionation of chia seeds (Salvia hispanica L.). CyTA. J. Food11, 75–80 (2013). [Google Scholar]
- 67.AACC. Method 30 - 20: Crude fat in grain and stock feeds, method 44 -15A: Moisture, method 46-13: Crude protein-Micro-Kjeldahl method, method 54 - 21: Farinograph method for flour., (AACC, 1995).
- 68.AACC. Method 08-03.01: Ash - Rapid Method. (2000).
- 69.Lee, S. C., Prosky, L. & De Vries, J. W. Determination of total, soluble, and insoluble dietary fiber in foods: enzymatic-gravimetric method, MES-TRIS buffer: collaborative study. J. Assoc. Off. Anal. Chem.75, 395–416 (1992). [Google Scholar]
- 70.Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol.37, 911–917 (1959). [DOI] [PubMed] [Google Scholar]
- 71.Morrison, W. R. & Smith, L. M. Preparation of fatty acid methyl esters and dimethylacetals from lipids with boron fluoride–methanol. J. Lipid Res.5, 600–608 (1964). [PubMed] [Google Scholar]
- 72.Friedewald, W. T., Levy, R. I. & Fredrickson, D. S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem.18, 499–502 (1972). [PubMed] [Google Scholar]
- 73.Brunt, E. M., Janney, C. G., Di Bisceglie, A. M., Neuschwander-Tetri, B. A. & Bacon, B. R. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am. J. Gastroenterol.94, 2467–2474 (1999). [DOI] [PubMed] [Google Scholar]
- 74.Rahman, I., Kode, A. & Biswas, S. K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc.1, 3159–3165 (2006). [DOI] [PubMed] [Google Scholar]
- 75.Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res29, e45 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bligh, E. G. & Dyer, W. J. A rapid method of total lipid extraction and purification. Can. J. Biochem Physiol.37, 911–917 (1959). [DOI] [PubMed] [Google Scholar]
- 77.Tirado, D. F., Montero, P. M. & Acevedo, D. Estudio Comparativo de Métodos Empleados para la Determinación de Humedad de Varias Matrices Alimentarias. Inf.ón tecnol.ógica26, 03–10 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.