Abstract
In this pilot study, we assessed the role of autophagy in Crohn’s Disease (CD), particularly in patients with a stenosing phenotype. Through the analysis of biopsied specimens from 36 patients, including 11 controls and 25 CD patients, categorized into inflammatory and stenosing groups, we identified a significant reduction in the autophagosomal marker Lc3b-II in patients with active inflammation and stenosis. This was paralleled by an increase in oxidative stress markers, including sNOX2-dp and H2O2, and a decrease in the antioxidant capacity measured by HBA, suggesting an imbalance in autophagy and oxidative stress mechanisms. Additionally, our findings show a correlation between autophagy markers and oxidative stress levels, indicating that autophagy dysfunction may play a pivotal role in CD and in the progression of a stenosing disease phenotype, by failing to eliminate detrimental molecules and pathogenic bacteria, thereby promoting fibrosis. This study is the first to demonstrate in vivo autophagy inhibition in stenosing CD patients and suggests that stimulating autophagic processes could offer a new avenue for the prevention and treatment of intestinal fibrosis in CD. Our results highlight the importance of exploring the interactions between autophagy, the fibrotic process, and the inflammatory cascade, opening avenues for potential therapeutic interventions in CD management.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79308-z.
Keywords: Inflammatory Bowel Disease (IBD), Autophagic Dysregulation, Reactive Oxygen Species (ROS), Fibrostenotic Complications, Redox Homeostasis
Subject terms: Gastrointestinal diseases, Molecular biology
Introduction
Crohn’s Disease (CD) is a complex and disabling condition where several patients can develop fibrostenotic complications. Despite progress in the field of CD therapy, lessening patients’ gut inflammation is the only effective strategy to date and no medical therapy exists for fibrosis prevention/treatment1.
Autophagy is a cellular degradation process essential for maintaining cellular homeostasis by removing damaged organelles and protein aggregates. Microtubule-associated protein 1 light chain 3 beta (LC3b) is a key component in the formation of autophagosomes, with its lipidated form, LC3b-II, serving as a widely recognized marker for autophagic activity. Decreased levels of LC3b-II indicate impaired autophagy, which may contribute to the pathogenesis of various diseases, including CD2.
Oxidative stress plays a pivotal role in the inflammatory processes of CD. NADPH oxidase 2 (NOX2) is an enzyme complex responsible for generating reactive oxygen species (ROS), particularly superoxide anions (O2·−), which contribute to tissue damage and inflammation. The soluble NOX2-derived peptide (sNOX2-dp) is a plasma marker resulting from NOX2 activation that reflects overall oxidative stress levels. Elevated sNOX2-dp levels have been associated with increased oxidative damage in several inflammatory conditions3.
Increased levels of oxidized circulating biomarkers have been implicated in the pathogenesis of CD, particularly in the occurrence of tissue damage and fibrosis. Among the physiological molecular pathways implicated in the management and regulation of inflammation and oxidative stress, autophagy plays a relevant role. In inflammatory bowel disease (IBD), autophagy-related genes have been linked to disease occurrence, particularly in CD with a fibrostricturing phenotype, thus dysfunction in the process may lead to IBD onset and development. In fact, autophagy has been related to dysfunction of the epithelial barrier, such as defects in the secretion of antimicrobial peptides, alterations in the endoplasmic reticulum stress response, dysbiosis, and altered immune responses4.
In the present study, we evaluated in vivo the role of autophagy in the context of CD and tested the novel hypothesis that autophagy/oxidative stress imbalance can be a relevant feature in CD patients, in particular in those with stenosing phenotype.
Results
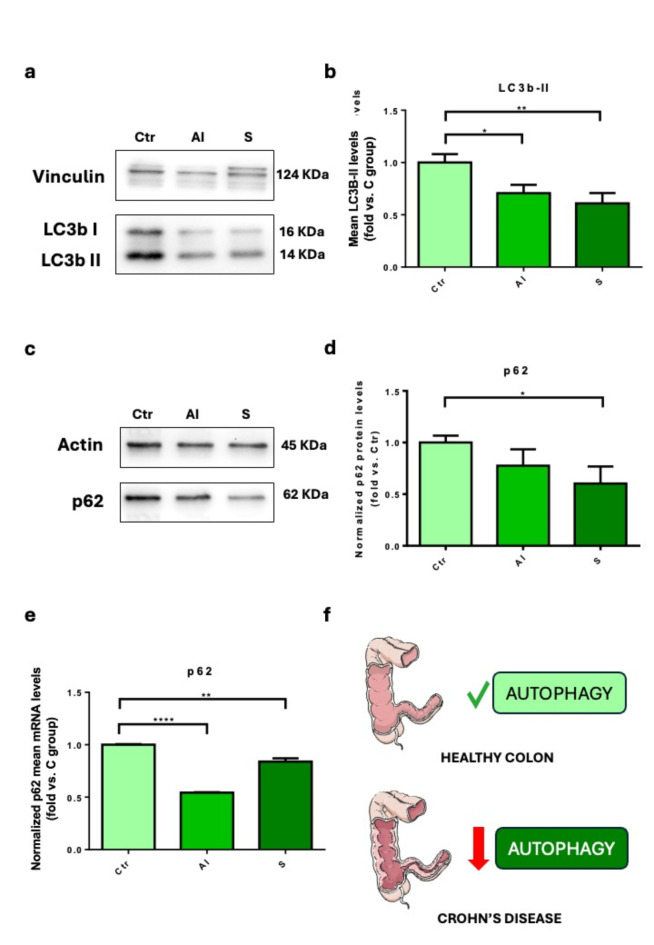
Autophagy was evaluated in bioptic specimens harvested from 11 control and 25 CD patients’ guts. Patients were divided into three groups based on their clinical history and colonoscopy findings: (A) Healthy subjects (Ctr); (B) Inflammatory phenotype (AI = active inflammation) with active disease (B1, with endoscopic findings of inflamed mucosa); and (C) stenosing (S) (B2) (Table 1). The autophagosomal marker Lc3b-II was significantly reduced in samples with active inflammation (P < 0.05) and a stenosing phenotype (P < 0.01) (Fig. 1a–b). We also evaluated p62 production in the intestinal mucosa to assess autophagy inhibition and found a significant difference (P < 0.05) between controls and stenosing patients (Fig. 1c–d). We confirmed this trend also at the transcriptional level, observing a reduction of p62 mRNA in patients with active inflammation (P < 0.0001) and stenosis (P < 0.01) (Fig. 1e). This data shows a consistent pattern of autophagy inhibition in bowel specimens of patients suffering from Crohn’s disease (Fig. 1f).
Table 1.
Characteristics of CD patients included in the study.
| Characteristics | Control group (n = 11) |
Inflammatory CD (n = 15) |
Stenosing CD (n = 10) |
Total CD (n = 25) |
|---|---|---|---|---|
| Age (mean years ± SD) | 69 ± 9** | 44 ± 17 | 51 ± 15 | 47 ± 16 |
| Gender (F/M) | 4/7 | 10/5 | 4/6 | 14/11 |
| Disease duration (mean years ± SD) | n.a | 6.6 ± 7.6 | 15.5 ± 8.7* | 10.2 ± 9.1 |
| Location | ||||
| L1 | n.a | 5 (33%) | 7 (70%) | 12 (46%) |
| L2 | n.a | 7 (47%) | 1 (10%) | 8 (31%) |
| L3 | n.a | 3 (20%) | 2 (20%) | 6 (23%) |
| Therapy | ||||
| Mesalamine | n.a | 10 (67%) | 6 (60%) | 16 (64%) |
| Prednisone < 30 mg/die | n.a | 4 (27%) | – | 4 (16%) |
| None | n.a | 1 (6%) | 4 (40%) | 5 (20%) |
L1, ileum; L2, colon; L3, ileocolon; n.a., not applicable.
* = p < 0.01 vs. Inflammatory CD.
**p < 0.0001 vs. total CD.
Fig. 1.
The autophagic flux is reduced in CD patients. (a–b) We measured the mean levels of Lc3b-II expression in gut samples derived from control (Ctr), active inflammation (AI), stenosing (S) patients. Data show mean ± the standard error mean (SEM) (n = 9–12 independent samples); (c–d) P62 protein levels from Ctr, AI, and S pateints’ gut samples. Data show mean ± the standard error mean (SEM) (n = 5–7 independent samples); (e) p62 mRNA levels from Ctr, AI, and S pateints’ gut samples. Data show mean ± the standard error mean (SEM) (n = 3 independent samples); (f) graphic illustration of Crohn’s disease-associated inhibition of autophagy. *P < 0.05; **P < 0.01; ****P < 0.0001.
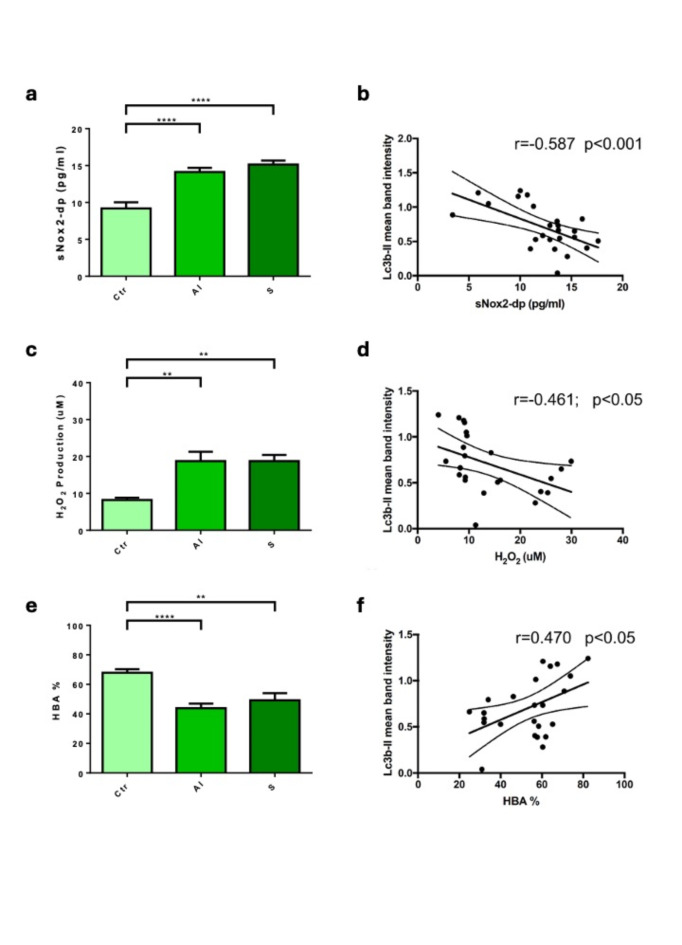
Compared to the control, sNOX2-dp, a marker of NOX2 activation, significantly increased in the active inflammation group (P < 0.0001) and the stenosis group (P < 0.0001) (Fig. 2a). H2O2, a reactive and diffusible molecule formed as a product of the NOX2-derived superoxide dismutation, significantly increased in the active inflammation group (P < 0.01), and in the stenosis group (P < 0.01) compared to the control (Fig. 2c).
Fig. 2.
CD patients show increased circulating markers of oxidative stress. (a, c, e) sNOX2-dp (A), H2O2 (C) and HBA (E) oxidative stress markers were measured in serum samples from Ctr (n = 11), AI (n = 15) and S (n = 10) groups. Data show mean ± the standard error mean (SEM); (b, d, f) simple linear correlation between Lc3b-II values and sNOX2-dp (b), H2O2 (d) and HBA (f) serum levels (r = Spearman’s correlation coefficient). **P < 0.01; ****P < 0.0001.
Lastly, the antioxidant power was evaluated by HBA, a measure of the percentage of H2O2 that is neutralized from serum by activating antioxidant systems. Our results showed that HBA is significantly reduced in the active inflammation group (P < 0.0001) and in the stenosis group (P < 0.01) (Fig. 2e).
We also studied the association between autophagy and oxidative stress by performing linear regression. We found that Lc3b-II correlated with sNOX2-dp (r = − 0.587; p < 0.01) (Fig. 2b), H2O2 (r = -0.461; p < 0.05) (Fig. 2d), and with HBA (r = 0.640; p < 0.001) (Fig. 2f).
Discussion
This study is the first to demonstrate a defect in autophagic machinery in stenosing CD patients in vivo. To date, many reports indicate that autophagy impairment may have a relevant role in CD onset and development, but evidence comes prevalently from experimental models and genetic studies, while real-life studies are virtually lacking. In fact, previous genome-wide association studies (GWAS) identified strong associations between polymorphisms of autophagy-related genes, such as Atg16L1 and immunity-related GTPase M (IRGM), and CD pathogenesis. NOD2, the first gene identified in association with CD, activates Atg16L1 and autophagy. This paper demonstrates for the first time that defective autophagy is a distinctive pattern in CD patients in vivo, particularly relevant in those with a stenosing phenotype, where the lack of elimination of detrimental molecules (i.e. reactive oxygen species) and/or pathogenic bacteria could lead to activating pro-fibrotic mediators, via molecular pathways yet to be fully characterized5. Autophagy stimulation is anti-fibrotic, while autophagy inhibition worsened fibrosis in a heterotopic transplant model of intestinal fibrosis and human fibroblast cultures6. Despite a rigorous dissection of the autophagic flux using inhibitors of the autolysosome formation was impossible to perform in this kind of human specimens, the consistent reduction of LC3b-II protein and p62 protein and mRNA is a robust indicator of autophagy inhibition according to international guidelines2.
Autophagy activation may also play an antioxidant role in protecting cells from oxidative stress by mediating the clearance of protein aggregates, damaged intracellular organelles, and excessive ROS in cells7,8. Previous studies indicate that O2•- produced by NOX2 can lead to cell damage and tissue injury in many autoimmune diseases and that CD patients have a significantly higher expression level of NOX2 than healthy individuals9. Here we confirmed that CD patients showed increased activation of NOX2, with increased levels of H2O2. Impairment of a balanced redox status was also supported by a significant decrease in the percentage of HBA, as an index of reduced detoxifying mechanisms of ROS. In addition, autophagy defects may lead to intestinal inflammation due to the loss of inhibition of inflammasome formation2.
Interestingly, the present study preliminary suggests that autophagy reduction may play a key role in the fibrotic complications of CD patients. Drugs commonly used to treat CD may interact with the autophagic pathway and autophagy regulators may represent a potential therapeutic target10. This could provide a novel approach to the prevention and treatment of intestinal inflammation and fibrosis by stimulating autophagic processes. Examining the interactions of autophagy with the fibrotic process, inflammatory cascade, and microbiota composition may deepen our understanding of the intricate network that contributes to CD onset and development, potentially leading to future groundbreaking clinical applications.
However, our study has certain limitations that should be acknowledged. We did not characterize the degree of fibrosis versus inflammation in the stenotic lesions, which makes it challenging to directly link the observed anomalies in autophagy and oxidative stress markers specifically to the fibrotic process.
Additionally, the cross-sectional design of our study limits the ability to establish causality between autophagy impairment and fibrotic complications in CD. Our sample size was relatively small and future studies with larger cohorts, further analyses and longitudinal designs are necessary to validate our findings. This will help mitigate the variability due to subjective factors (e.g. age) and elucidate the precise mechanisms by which autophagy impairment contributes to phlogosis-driven derangements in CD.
Methods
Population
This study included 36 patients with a confirmed diagnosis of CD who were followed at S. Giovanni Addolorata Hospital IBD Center and referred for a colonoscopy. To be included in the study, patients had to meet certain criteria, such as having a firm diagnosis of CD (according to clinical, endoscopic, histological and radiological records) for at least one year and providing informed consent for the study. Patients who were undergoing therapy with immunomodulators, high doses of steroids (prednisone > 25mg/day), had an incomplete endoscopic examination, or were unable to obtain biopsies were excluded from the study. Patients were divided into groups according to Montreal Classification (Table 1).
Bioptic samples were collected according to disease location (terminal ileum or cecum): in Group C (stenosing) they were obtained at the site of an endoscopic visible stenosis, in Group B (inflammatory) in sites with active inflammation (mucosa with hyperemia or erosions, or in the area around ulcers) and in Group A (controls) in normal mucosa. Bioptic samples were stored at -20°C until used. For each patient, 20ml of a blood sample was collected, plasma was separated by centrifugation, and stored at −20°.
This study was approved by the “Lazio2” ethics committee (prot.n. 0169810). All participants provided written informed consent, and the study was conducted in full compliance with the Helsinki Declaration and other relevant community standards.
Autophagy evaluation
In brief, protein lysates were obtained from intestinal biopsies. Then western blot was performed using the following antibodies diluted in 5% skimmed milk in TBST: LC3 (MBL (Japan) M186-3), p62 (Cell Signalling) and Vinculin (Santa Cruz sc-25336). P62 was also studied at a transcriptional level by RT-qPCR. The mRNA was extracted from samples using a commercial kit (Qiagen) and following the manifacturer’s instructions.
H2O2 production
The Hydrogen Peroxide (H2O2) was measured by using a colorimetric assay as previously described11. A standard curve of H2O2 (0–200 μM) was performed for each assay. Briefly, 50 μL of cell supernatant was mixed with 3,3′,5,5′ tetramethylbenzidine (50 μL, Sigma-Aldrich, St. Louis, Missouri, USA) in 0.42 mol/L citrate buffer, pH 3.8, and 10 μL of horseradish peroxidase (52.5 U/mL, Sigma-Aldrich, St. Louis, Missouri, USA). The samples were incubated at room temperature for 20 min, and the reaction was stopped by adding 10 μL 18 N sulphuric acid. The reaction product was measured spectrophotometrically at 450 nm and expressed as μM.
Serum sNox2-dp
Nox2 activity was measured in serum as sNox2-dp with a previously reported ELISA method12. Briefly, the assay is based on: (1) coating reference standards of known concentrations of sNOX2dp and serum samples (1 µg of protein) into ELISA 96 well plate overnight at 4 °C; (2) washing away of unbound materials from samples; (3) addition in each well of anti-sNox2dp-horseradish peroxidase (HRP) monoclonal antibody against the amino acidic sequence (224–268) of the extracellular membrane portion of Nox2, (4) quantification of immobilized antibody-enzyme conjugates by monitoring HRP activities in the presence of the substrate 3,3’,5,5’-tetramethylbenzidine (TMB). The enzyme activity is measured spectrophotometrically by the increased absorbency at 450 nm after acidification of formed products (2 M sulphuric acid). Since the increase in absorbency is directly proportional to the amount of sNox2dp of the unknown sample, the latter can be derived by interpolation from a reference curve generated in the same assay with reference standards of known concentrations of sNox2dp (0–200 pg/ml). Values were expressed as pg/ml; intra-assay and inter-assay coefficients of variation were < 10%. Values were expressed as pg/mL; intra- and inter-assay coefficients of variation (CV) were < 10%.
Serum HBA
Serum hydrogen peroxide (H2O2) breakdown activity (HBA) was measured with an HBA assay kit (Aurogene, code HPSA-50). The % of HBA was calculated using the following formula: % of HBA = Ac − As/Ac * 100 where Ac is the absorbance of H2O2 (1.4 mg/mL), As is the absorbance in the presence of the serum sample.
Statistics
Continuous variables are reported as mean ± standard error. Comparison between groups for continuous variables was carried out by two-tailed Student’s T-test (Ctr vs. AI) or (Ctr vs. S) or by ANOVA with Bonferroni’s correction. Simple linear regression analysis was performed by Spearman’s rank correlation test. A value of P < 0.05 was considered statistically significant. All analyses were performed using GraphPad Software-Prism7.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
L.S., D.V., A.D., and C.N. conducted the experiments. L.S. also wrote the manuscript and analyzed the data, with C.N. assisting in data analysis. G.F. and C.P. conceived and designed the study. S.S. and R.C. provided critical insights into autophagy and oxidative stress analysis, respectively. M.C.D.P., R.U., L.P., G.F., G.V., M.G.G., affiliated with the S. Giovanni-Addolorata Hospital, contributed to sample collection and clinical management of the study. M.P. and E.D.F. provided significant support in writing and critically reviewing the manuscript.
Funding
This work was supported by the Italian Ministry of Health [grant number RF-2021-12375256] and a Research Grant from EU, PNRR, HEAL ITALIA PE 6, PE 00000019 to Giacomo Frati.
Data availability
All data are available upon reasonable request to the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethical considerations
This study was approved by the “Lazio2” ethics committee (prot.n. 0169810). Data were protected according to the EU privacy regulation.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lichtenstein, G. R. et al. ACG clinical guideline: Management of Crohn’s disease in adults. Am. J. Gastroenterol.113(4), 481–517 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)(1). Autophagy17(1), 1–382 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nocella, C. et al. Structure, activation, and regulation of NOX2: At the crossroad between the innate immunity and oxidative stress-mediated pathologies. Antioxidants (Basel)12(2) (2023). [DOI] [PMC free article] [PubMed]
- 4.Shao, B. Z. et al. The role of autophagy in inflammatory bowel disease. Front Physiol.12, 621132 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cadwell, K. et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature456(7219), 259–263 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosin-Roger, J. et al. Autophagy stimulation as a potential strategy against intestinal fibrosis. Cells8(9) (2019). [DOI] [PMC free article] [PubMed]
- 7.Iop, L. et al. The light and shadow of senescence and inflammation in cardiovascular pathology and regenerative medicine. Mediators Inflamm.2017, 7953486 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Irace, F. G. et al. Role of oxidative stress and autophagy in thoracic aortic aneurysms. JACC Basic Transl. Sci.6(9–10), 719–730 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tavassolifar, M. J. et al. Redox imbalance in Crohn’s disease patients is modulated by Azathioprine. Redox Rep.26(1), 80–84 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hooper, K. M. et al. Inflammatory bowel disease drugs: A focus on autophagy. J. Crohns Colitis11(1), 118–127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nocella, C. et al. A novel role of MMP2 in regulating platelet NOX2 activation. Free Radic. Biol. Med.152, 355–362 (2020). [DOI] [PubMed] [Google Scholar]
- 12.Carnevale, R. et al. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol.84(7), 1566–1574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are available upon reasonable request to the corresponding author.