Highlights
-
•
This Clinical Practice Guideline provides key recommendations for managing rare endocrine tumours.
-
•
Neuroendocrine neoplasms of different origins, parathyroid carcinoma and intrathyroid thymic neoplasms are included.
-
•
The guideline covers clinical imaging and pathological diagnosis, staging and risk assessment, treatment and follow-up.
-
•
The authors comprise a multidisciplinary group of experts from different institutions and countries in Europe.
-
•
Recommendations are based on available scientific data and the authors’ collective expert opinion.
Key words: diagnosis, ESMO guideline, intrathyroid carcinoma, management, parathyroid carcinoma, rare endocrine neoplasms
Neuroendocrine neoplasms
Incidence and epidemiology
Neuroendocrine neoplasms (NENs) encompass well-differentiated neuroendocrine tumours (NETs) and poorly differentiated neuroendocrine carcinomas (NECs). The primary tumour site for NENs remains unidentified in 13%-20% of cases.1 NENs account for ∼8% of unknown primary (UKP) malignancies and UKP-NENs represent the fourth most common NEN with an increasing incidence of 8.4 per million, representing 10%-15% of NETs and NECs.1,2
Genitourinary and gynaecological (GUGy)-NENs account for 1%-2% of GUGy malignancies, 12% of NECs and 4% of NETs.3 Their estimated incidence in Europe is 0.5-1 per million.4 GUGy-NENs arise from the bladder, kidney, ureter, ovary, cervix, endometrium, prostate, testis or presacral space5, 6, 7 (see Section 1 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664).
NENs arising from the head and neck (HN) account for <1% of HN malignancies, 2% of NECs and 0.6% of NETs.4 Their estimated incidence in Europe is 0.1-0.15 per million.4 HN-NENs arise most frequently from the larynx8 followed by the middle ear9 and sinonasal tract10 (see Section 1 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664).
Diagnosis and pathological classification
Pathological classification
Classification of NENs relies on differentiation (well or poorly differentiated), presence or absence of necrosis and grade (G) assessed by Ki-67 index and/or mitotic count (see Table 1 and Section 2 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664).11 Immunohistochemistry (IHC) biomarkers may help to identify primary sites12,13 (see Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103664). For accurate diagnosis, a comprehensive pathology report should include unequivocal grading with morphological differentiation, mitotic count and Ki-67 index. The International Agency for Research on Cancer (IARC)–World Health Organization (WHO) 2018 consensus terminology is used when referring to NENs (irrespective of their primary site), together with the organ-specific WHO classification (see Table 1).14 In addition, a general template for reporting biopsies has been developed.15
Table 1.
Current pathological classification of UKP-, GUGy- and HN-NENs and corresponding IARC–WHO 2018 consensus classification14,68,106, 107, 108
| Common classification for UKP-, HN- and GUGy-NENs used in these guidelines14 | HN-NEN classification108 | GU-NEN classification106 | Gy-NEN classification107 |
|---|---|---|---|
| NET | |||
| G1 NET | G1 NET or middle-ear NET | Well-differentiated NET (no grading) | G1 NET (Ovary: carcinoid tumour, no grading) |
| G2 NET | G2 NET or middle-ear NET | G2 NET | |
| G3 NET | No G3 NET in the classification | No G3 NET in the classification | |
| NEC | |||
| Small-cell NEC | Small-cell NEC | Small-cell NEC | Small-cell NEC |
| Large-cell NEC | Large-cell NEC | Large-cell NEC | Large-cell NEC |
| MiNEN | Mixed NENs | Carcinoma admixed with NEC | |
G, grade; GU, genitourinary; GUGy, genitourinary or gynaecological; Gy, gynaecological; HN, head and neck; IARC, International Agency for Research on Cancer; MiNEN, mixed neuroendocrine–non-neuroendocrine neoplasm; NA, not applicable; NEC, neuroendocrine carcinoma; NEN, neuroendocrine neoplasm; NET, neuroendocrine tumour; UKP, unknown primary; WHO, World Health Organization.
Clinical diagnosis
The objective of the initial diagnostic work-up of UKP-NENs is to identify the occult primary tumour. Metastatic patterns can be indicative of primary tumour location. The most common site for the occult primary is the ileum followed by the pancreas.16 Ileal NETs often present as a mesenteric mass on computed tomography (CT).16 Somatostatin receptor (SSTR) imaging (SRI) with gallium-68-1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid-labelled somatostatin analogue ([68Ga]Ga-DOTA-SSA)–positron emission tomography (PET)–CT is the most sensitive technique for NETs, detecting an occult primary in 61% of cases.17 If SRI is uninformative, [18F]F-dihydroxyphenylalanine (DOPA)–PET–CT and endoscopy techniques can be useful (see Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103664, and Section 3 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664). For NECs, [18F]2-fluoro-2-deoxy-D-glucose (FDG)–PET–CT may be used. The presence of secretory syndromes and elevated hormone levels may also facilitate identification of the primary NET. A minimum panel of peptide hormones should be measured in patients with UKP-NETs [calcitonin, metanephrines and 5-hydroxyindoleacetic acid (5-HIAA)]. Other hormones may be measured depending on symptoms (see Supplementary Table S1, available at https://doi.org/10.1016/j.esmoop.2024.103664, and Section 3 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664). Chromogranin A has demonstrated a sensitivity of 71% and specificity of 84%-85% for the detection of gastroenteropancreatic (GEP) NETs.18
It is important to distinguish between primary GUGy-NETs and metastasis in the GUGy system from primary tumours in another site. Indeed, ovarian, testicular and renal metastases occur in patients with GEP or lung NETs. Symptoms result from local infiltration in patients with more aggressive GUGy-NENs and performance status (PS) deterioration. Less than 15% of GUGy-NETs present with hormone-related symptoms (mostly carcinoid syndrome).1
HN-NENs induce non-specific symptoms such as hoarseness, dysphagia, dyspnoea, nasal obstruction, epistaxis and conductive hearing loss.19,20 Laryngeal NENs arise from the supraglottic region in 85% of cases.8 Middle-ear NENs appear as a retrotympanic mass extending into the ossicles, mastoid, auditory canal and Eustachian tube.9,19 Sinonasal NENs occur in the ethmoid (64% of cases), nasal cavity (32%) and maxillary sinus (14%). The most common metastatic sites are the lymph nodes, lungs, liver and bones.21 Skin metastases can be extremely painful and suggestive of laryngeal NETs.22 Functional syndromes are rare and include carcinoid syndrome, syndrome of inappropriate secretion of antidiuretic hormone, ectopic adrenocorticotropic hormone syndrome and Lambert–Eaton myasthenic syndrome.23
Recommendations
-
•
Diagnostic and therapeutic recommendations for patients with UKP-, GUGy- or HN-NENs should be discussed in a NEN multidisciplinary tumour board (MTB) [V, A].
-
•
UKP-, GUGy- and HN-NENs should be classified and graded according to the common IARC–WHO 2018 consensus classification together with the organ-specific WHO classification [V, A].
-
•
For UKP-NETs, IHC should be carried out to exclude paragangliomas (cytokeratin) and to identify the primary [as a minimum: thyroid transcription factor-1 (TTF-1) and caudal-type homeobox 2 (CDX-2)]. Calcitonin, serotonin and other IHC markers may be used [IV, B].
-
•
In patients with UKP-, GUGy- or HN-NETs, secretory syndromes should be diagnosed based on hormone-related symptoms. As a minimum, metanephrines, 5-HIAA and calcitonin should be measured in those with UKP-NETs [V, A].
-
•
For UKP-NETs, cross-sectional imaging may identify metastatic patterns suggestive of a primary site (e.g. a mesenteric mass indicative of an ileal NET) [IV, A]. Endoscopy and endoscopic ultrasound (US) are recommended to identify pancreatic primaries [V, A].
-
•
SRI–PET is recommended for G1-2 UKP-NETs to visualise the primary tumour [IV, A]. FDG–PET–CT may be helpful in NENs with a higher proliferation index (G3 NETs and NECs) [V, B]. [18F]F-DOPA–PET–CT may also detect an ileum primary, pheochromocytoma or paraganglioma in patients with UKP-NETs [V, B].
-
•
In patients with UKP-NECs, searches for the primary should not delay treatment intervention, apart from cutaneous examination for Merkel-cell carcinoma and IHC for cytokeratin 20 and Merkel-cell polyomavirus, if available [V, A].
Staging and risk assessment
Adequate staging of UKP-, GUGy- and HN-NENs relies on cross-sectional imaging and nuclear medicine techniques. CT scans are effective for the detection of lung metastases whereas magnetic resonance imaging (MRI) is better for liver and bone metastases. MRI is also preferred in young patients as radiation exposure is lower. SRI has a high sensitivity for NETs and should be part of the initial work-up. SSTR scintigraphy is less sensitive and should only be carried out when SRI–PET–CT is not available. Whole body CT, or FDG–PET–CT in case of limited tumour burden, are recommended for the staging of G2-3 NETs and NECs.24
Staging of middle-ear HN-NENs is based on the system proposed by Marinelli et al.19 while staging of GUGy- and HN-NENs is based on the eighth edition of the Union for International Cancer Control (UICC) TNM (tumour–node–metastasis) classification25 (see Supplementary Tables S2-S10, available at https://doi.org/10.1016/j.esmoop.2024.103664). There is no staging classification for UKP-NENs unless an occult primary is identified by the initial diagnostic work-up.
Prognosis for all NENs relies on pathological grading and staging. In patients with metastases, differentiation, WHO grade, age, PS, tumour burden, SRI and/or FDG uptake, the presence of a functional syndrome at baseline and tumour growth rate should be considered for optimal risk assessment.
Recommendations
-
•
CT and/or MRI are recommended for staging. MRI should be mainly considered in young patients to reduce irradiation and—using late arterial phase imaging—improve detection of hepatic, pancreatic, brain and bone NETs [III, A].
-
•
SRI–PET is recommended for initial and preoperative staging, and when indicated for restaging [V, A].
-
•
FDG–PET–CT can be used for staging G2-3 NETs and NECs [IV, B].
-
•
Major independent prognostic factors are disease stage (TNM) and pathological features including differentiation and WHO grade [IV, A]. Age, PS, tumour burden, the presence of functional syndromes and tumour growth rate are additional prognostic factors [IV, B].
Management of local and locoregional disease
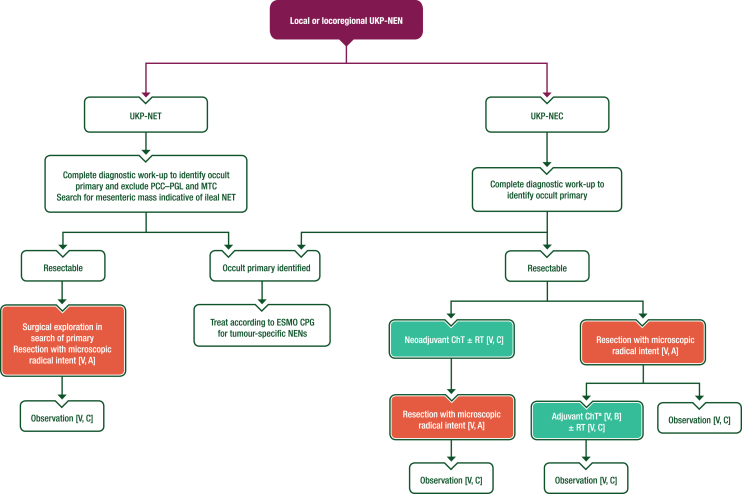
Patients with UKP-NENs may present with lymph node metastases without the detection of a primary tumour or distant metastases. For G1-3 UKP-NETs, radical resection of locoregional disease is the preferred treatment (see Figure 1). While searching for an occult primary, the site of lymph node metastasis may guide surgical exploration (e.g. an involved mesenteric node is suggestive of an ileal primary). There is no evidence to support adjuvant therapy following curative resection of NETs. For UKP-NECs, downstaging of locoregional disease with chemotherapy (ChT) and/or radiotherapy (RT) may be considered before surgery. Some patients with NECs, particularly with isolated inguinal or neck nodes, may have prolonged disease control and survival following multimodal treatment including surgery.26 In this setting, Merkel-cell carcinoma should be ruled out. It is unclear whether post-operative treatment of NECs improves outcomes, but four cycles of carboplatin–etoposide may be considered, particularly if neoadjuvant ChT has not been used.27
Figure 1.
Management of local or locoregional UKP-NENs. Purple: algorithm title; orange: surgery; turquoise: non-systemic anticancer therapies or combination of treatment modalities; white: other aspects of management and non-treatment aspects. ChT, chemotherapy; CPG, Clinical Practice Guideline; MTC, medullary thyroid cancer; NEC, neuroendocrine carcinoma; NEN, neuroendocrine neoplasm; NET, neuroendocrine tumour; PCC–PGL, pheochromocytoma–paraganglioma; RT, radiotherapy; UKP, unknown primary. aFour cycles of platinum–etoposide.
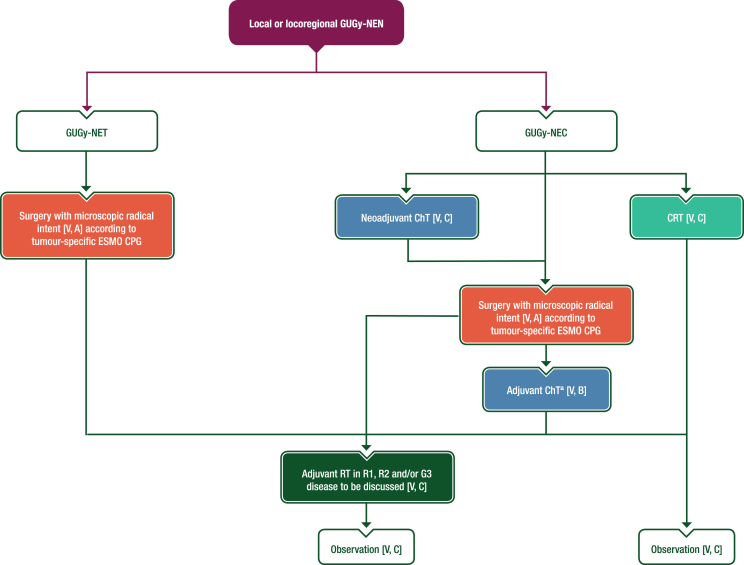
There are no prospective data on the management of local and locoregional disease in patients with GUGy-NENs. Recommendations are therefore extrapolated from site-specific ESMO Clinical Practice Guidelines (CPGs).28 For GUGy-NETs, upfront radical surgery without adjuvant treatment is recommended when feasible (see Figure 2); however, a conservative surgical approach should be discussed whenever possible, particularly for low-grade localised NETs. The role of adjuvant therapy for G3 GUGy-NETs is also unknown. For GUGy-NECs, multimodal therapy should always be discussed for localised disease. Further details regarding the management of local and locoregional GUGy-NENs are available in Section 4 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664.
Figure 2.
Management of local or locoregional GUGy-NENs. Purple: algorithm title; orange, surgery; dark green: RT; blue: systemic anticancer therapy or their combination; turquoise: non-systemic anticancer therapies or combination of treatment modalities; white: other aspects of management and non-treatment aspects. ChT, chemotherapy; CPG, Clinical Practice Guideline; CRT, chemoradiotherapy; G, grade; GUGy, genitourinary or gynaecological; NEC, neuroendocrine carcinoma; NEN, neuroendocrine neoplasm; NET, neuroendocrine tumour; R1, resection with microscopic tumour at the margin; R2, resection with macroscopic tumour at the margin; RT, radiotherapy. aFour cycles of platinum–etoposide.
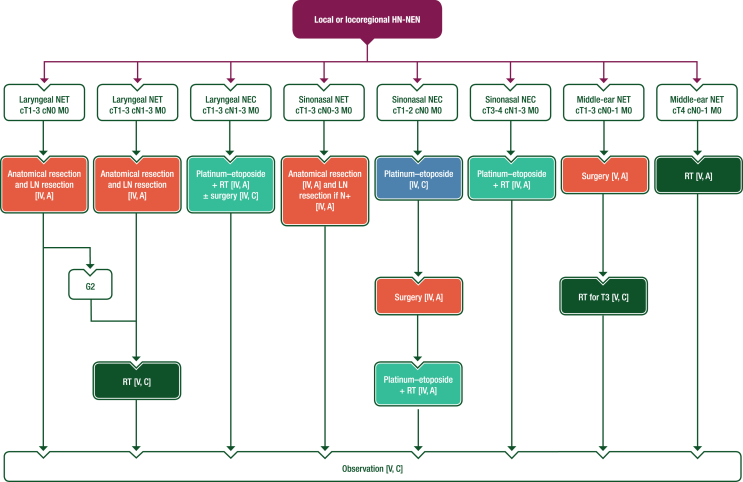
For HN-NENs, locoregional treatment depends on the primary site (see Figure 3). In the larynx, NETs are mainly supraglottic and their sensitivity to ChT and RT may be low. Based on retrospective data, radical resection after completion of whole body imaging (including PET–CT) is recommended whenever possible with homolateral neck dissection, as it may lower the risk of regional lymph node recurrence.8 Post-operative RT should be discussed in a multidisciplinary meeting, particularly for G2 NETs.8 For laryngeal NECs, treatment relies mainly on ChT and RT8; surgery may be discussed in selected cases.29 For sinonasal NETs, surgery was associated with longer survival in retrospective series.30,31 The role of prophylactic neck dissection is unknown. Post-operative RT should be discussed but there is no evidence that it prevents locoregional relapse. For sinonasal NECs, treatment should be multimodal. Retrospective data suggest that induction ChT is beneficial,10 in combination with RT in most cases and surgery in selected cases.21,31 Most middle-ear tumours are NETs. Surgery is the mainstay of treatment,19,32 but complete resection with no tumour at the margin (R0) is challenging and there is a risk of locoregional relapse when ossicles are involved. There are no data on post-operative RT; however, RT may be discussed for patients with a higher risk of relapse due to adherence to important neurovascular structures. RT might prevent extension to the skull base and subsequent debilitating complications. This must be balanced against the risk of long-term RT-induced toxicity.
Figure 3.
Management of local or locoregional HN-NENs. Purple: algorithm title; orange: surgery; dark green: RT; blue: systemic anticancer therapy or their combination; turquoise: non-systemic anticancer therapies or combination of treatment modalities; white: other aspects of management and non-treatment aspects. c, clinical; G, grade; HN, head and neck; LN, lymph node; M, metastasis; N, node; N+, lymph node metastasis; NEC, neuroendocrine carcinoma; NEN, neuroendocrine neoplasm; NET, neuroendocrine tumour; RT, radiotherapy; T, tumour.
Recommendations
-
•
For patients with UKP-NETs with locoregional disease, the aim of surgical resection with exploration is to identify and treat the primary tumour with microscopic radical intent [V, A].
-
•
For GUGy-NETs, surgery of the primary tumour and nodes with microscopic radical intent (according to site-specific ESMO CPGs) is the treatment of choice for local and locoregional disease, irrespective of grade [V, A]. A conservative surgical approach should be discussed whenever feasible, particularly in low-grade, early-stage NETs [V, B].
-
•
For laryngeal and sinonasal NETs, surgery of the primary tumour with microscopic radical intent and homolateral therapeutic neck dissection is recommended whenever possible [IV, A].
-
•
Adjuvant treatment cannot be recommended for UKP-NETs [V, D] but adjuvant RT may be discussed for GUGy-NETs in patients with microscopic (R1) or macroscopic (R2) tumour at the margin and/or G3 disease [V, C].
-
•
For HN-NETs, adjuvant RT may be discussed after radical resection, particularly in case of locoregional spread, non-R0 resection and G2 disease; however, the benefit in terms of local control is unknown [V, C].
-
•
For locoregional sinonasal NECs, neoadjuvant ChT with platinum–etoposide may be considered before surgical resection [IV, C].
-
•
For NECs treated with radical surgery, adjuvant therapy with four cycles of platinum–etoposide may be considered [V, B].
-
•
Neoadjuvant ChT may be an option for UKP- and GUGy-NECs [V, C].
-
•
Combining RT with ChT is recommended for HN-NECs [IV, A] and could be discussed for UKP- and GUGy-NECs as definitive or (neo)adjuvant treatment [V, C].
Management of advanced and metastatic disease
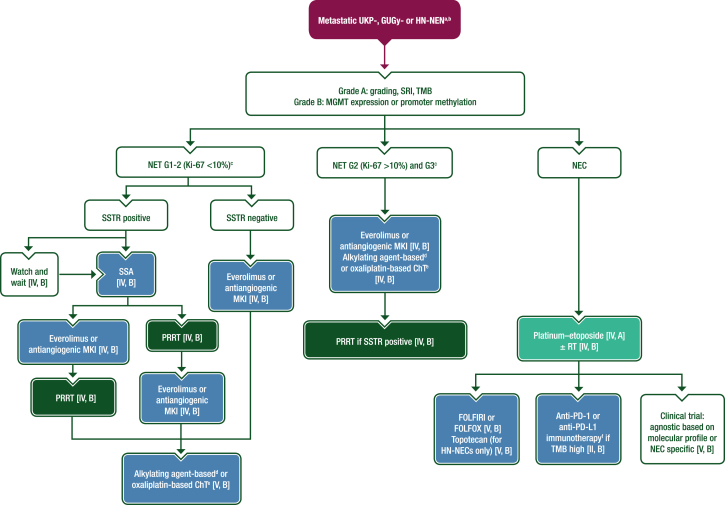
For UKP-NETs there are few clinical trials with low patient numbers and for UKP-NECs there are only retrospective studies; available data are summarised in Section 5 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664. For advanced or metastatic GUGy- and HN-NENs, neither retrospective nor prospective studies are available. Thus, recommendations for the management of UKP-, GUGy- and HN-NENs are based on the management of NENs of GEP or lung origin and so systemic treatments approved for GEP-NETs are used off-label in this setting.28 When an occult primary is identified or suspected (such as a mesenteric mass from an ileal NET), its management should follow the dedicated guideline.
Treatment decisions (see Figure 4) should be based on comprehensive multidisciplinary characterisation of the tumour (see ‘Staging and risk assessment’). In particular, WHO grade, differentiation (well versus poor),33 SSTR status based on SRI–PET–CT24 and presence of a functional syndrome should be considered.
Figure 4.
Management of metastatic UKP-, GUGy- and HN-NENs. Purple: algorithm title; dark green: RT; blue: systemic anticancer therapy or their combination; turquoise: non-systemic anticancer therapies or combination of treatment modalities; white: other aspects of management and non-treatment aspects. 5-FU, 5-fluorouracil; ChT, chemotherapy; EMA, European Medicines Agency; FDA, Food and Drug Administration; FOLFIRI, leucovorin–5-fluorouracil–irinotecan; FOLFOX, leucovorin–5-fluorouracil–oxaliplatin; G, grade; GUGy, genitourinary or gynaecological; HN, head and neck; MGMT, O-6-methylguanine-DNA methyltransferase; MKI, multikinase inhibitor; NEC, neuroendocrine carcinoma; NEN, neuroendocrine neoplasm; NET, neuroendocrine tumour; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PRRT, peptide receptor radionuclide therapy; R0, resection with no tumour at the margin; RT, radiotherapy; SRI, somatostatin receptor imaging; SSA, somatostatin analogue; SSTR, somatostatin receptor; TMB, tumour mutational burden; UKP, unknown primary. aAlways consider enrolment in a clinical trial [V, A]. bRecommendations for the management of metastatic UKP-, GUGy- and HN-NENs are based on the management of NENs of GEP or lung origin and therefore systemic treatments approved for GEP-NETs are used off-label in these settings. cSurgery is recommended if disease is amenable to R0 resection [V, A]. dDacarbazine, temozolomide or streptozocin plus 5-FU or capecitabine. eOxaliplatin plus 5-FU or capecitabine. fPembrolizumab is FDA approved (but not EMA approved) for the treatment of patients with unresectable or metastatic TMB high solid tumours that have progressed following prior treatment and have no alternative treatment options. No other anti-PD-1 or anti-PD-L1 agents are approved for use in patients with TMB high solid tumours.
Surgery and locoregional therapies
Surgery should be discussed for slowly proliferating (oligo)metastatic tumours in patients with G1-2 UKP-, GUGy- or HN-NETs; this includes surgical exploration for occult primary identification in case of UKP-NETs.34 Metastatic spread in HN-NETs frequently involves the skin, particularly in those arising from the larynx.20,22 Skin metastases are usually multiple and small in size, causing hard-to-treat pain and discomfort, which affects patients’ quality of life. In most cases, they are resistant to systemic treatment. Repeated locoregional therapies may be used, including surgical resection, electrochemotherapy35 and RT for the largest tumours.22
Somatostatin analogues
Two phase III trials [CLARINET (lanreotide) and PROMID (octreotide)] have reported longer progression-free survival (PFS) or time to progression with somatostatin analogue (SSA) treatment compared with placebo in patients with metastatic GEP-NETs. Both studies included some patients with UKP-NETs but none with GUGy- or HN-NETs.36,37 Extrapolating the results from CLARINET and PROMID, SSAs may be recommended for tumour control in advanced SSTR-positive, G1-2 NETs (Ki-67 <10%) or slowly progressive NETs.38
Management of functional syndromes
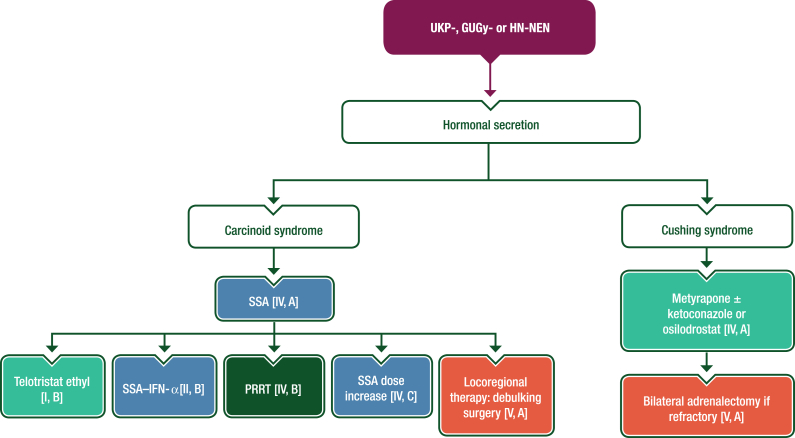
SSAs are recommended as first-line therapy for carcinoid syndrome and other functional syndromes (Figure 5). Beyond SSAs, studies exploring the role of telotristat ethyl39 or interferon-α (IFN-α)40 for carcinoid syndrome control included patients with any primary tumour site so their use is also applicable to UKP-NETs. No data are available on the treatment of carcinoid syndrome associated with GUGy- or HN-NETs. Management of carcinoid syndrome should follow the European Neuroendocrine Tumor Society 2022 guidance paper on carcinoid syndrome and carcinoid heart disease41; management of Cushing syndrome should follow the ESMO CPG on lung and thymic carcinoids.28
Figure 5.
Management of carcinoid syndrome and Cushing syndrome induced by UKP-, GUGy- or HN-NENs. Purple: algorithm title; orange: surgery; dark green: RT; blue: systemic anticancer therapy or their combination; turquoise: non-systemic anticancer therapies or combination of treatment modalities; white: other aspects of management and non-treatment aspects. GUGy, genitourinary or gynaecological; HN, head and neck; IFN-α, interferon-α; NEN, neuroendocrine neoplasm; PRRT, peptide receptor radionuclide therapy; SSA, somatostatin analogue; UKP, unknown primary.
Targeted therapy
Based on data from the RADIANT-2,42 RADIANT-343 and RADIANT-444 trials (NETs of various primaries), everolimus monotherapy may be considered as first-line treatment for SSTR-negative G1-2 UKP-, GUGy- and HN-NETs, or as second-line treatment after SSAs in patients with progressive disease. The RADIANT studies, however, included only 36 patients with UKP-NETs and no patients with GUGy- or HN-NETs. Based on the randomised CABINET,45 AXINET46 and SANET-ep47 trials, antiangiogenic multikinase inhibitors (MKIs) may be considered as alternatives to everolimus for first-line treatment of SSTR-negative G1-2 UKP-, GUGy- and HN-NETs, or for second-line treatment after SSAs in patients with progressive disease. These studies included 34-49 patients with UKP-NETs or NETs of other origin not otherwise specified (see Section 5 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664).
ChT
In patients with progressive G2 (Ki-67 >10%) and G3 NETs, alkylating agent-based and oxaliplatin-based ChT regimens may be considered. Most data for management of G3 NETs with these regimens are from patients with pancreatic NETs, with response rates of up to 30% and median PFS of 8-16 months.48 Loss of O-6-methylguanine-DNA methyltransferase (MGMT) expression or promoter hypermethylation may prompt use of alkylating agent-based ChT (see Section 5 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664).49,50 For the management of NECs, first-line carboplatin–etoposide or cisplatin–etoposide is recommended based on retrospective series showing response rates of 40%-60%, median PFS of 4-6 months and median overall survival (OS) of 12 months.2,10,21,29,51 Carboplatin–etoposide or cisplatin–etoposide should not be used for well-differentiated NETs, regardless of grade.33,51
Peptide receptor radionuclide therapy
Patients with UKP-, GUGy- and HN-NETs were excluded from the randomised phase III NETTER-1 study, which evaluated peptide receptor radionuclide therapy (PRRT) with [177Lu]Lutetium-DOTA-Tyr3-octreotate in patients with mid-gut NETs.52 Retrospective studies have reported equivalent efficacy with PRRT in patients with UKP-NETs versus other primaries (∼30% response rate and PFS of 20-25 months), thereby supporting its use.53 There are no retrospective studies on the use of PRRT in GUGy- or HN-NETs. PRRT may be an option for patients with SSTR-positive UKP-, GUGy- or HN-NETs who have progressed under SSA treatment.
Precision medicine
The ESMO Precision Medicine Working Group recommends determination of tumour mutational burden (TMB) in NENs,54 following results from the TMB-high cohort of the KEYNOTE-158 basket trial of pembrolizumab.55 In this study, two of five patients with TMB-high NENs had a response (40% response rate compared with 1% in those with non-TMB-high NENs); however, no immunotherapy is approved in Europe for TMB-high tumours and the availability of next-generation sequencing for patients with NENs is limited. No recurrent molecular alterations have yet been reported in UKP-, GUGy- or HN-NENs. Molecular profiling, when available, should be offered and may provide information to guide agnostic clinical trials and/or compassionate treatment requests.
Recommendations
-
•
Comprehensive characterisation, including WHO grade, differentiation (well versus poor), SSTR status and the presence of a functional syndrome, is recommended before making treatment decisions [V, A].
-
•
For metastatic UKP-, GUGy- and HN-NETs, surgery is recommended if the disease is amenable to R0 resection and/or for debulking to control hormonal syndromes in functional NETs [V, A].
-
•
SSAs are the first-line treatment for patients with carcinoid syndrome [IV, A]. In case of uncontrolled carcinoid syndrome, telotristat ethyl (for syndrome control) [I, B], the combination of an SSA with IFN-α [II, B], PRRT [IV, B], SSA dose increase [IV, C] or locoregional treatment (debulking) of metastatic disease [V, A] can be used.
-
•
For patients with Cushing syndrome, metyrapone ± ketoconazole or osilodrostat are recommended [IV, A]. Bilateral adrenalectomy is recommended in refractory cases [V, A].
-
•
Given the rarity of UKP-, GUGy- and HN-NETs, enrolment of patients in clinical trials is recommended whenever possible [V, A].
-
•
SSAs are recommended as first-line therapy for tumour growth control in advanced, SSTR-positive UKP-, GUGy- and HN-NETs with Ki-67 <10% [IV, B].
-
•
A watch-and-wait strategy may be followed in patients with low Ki-67, low tumour burden and stable disease [IV, B].
-
•
Everolimus is recommended first line in SSTR-negative G1-2 NETs and second line (after SSA treatment) in patients with SSTR-positive progressive disease [IV, B; European Medicines Agency (EMA) and Food and Drug Administration (FDA) approval is for NETs of pancreatic, gastrointestinal or lung origin only]. Antiangiogenic MKIs may be an alternative in these settings [IV, B].
-
•
PRRT may be an option for patients with SSTR-positive (on all evaluable targets) G1-2 UKP-, GUGy- and HN-NETs progressing on SSAs [IV, B].
-
•
For progressive G1-2 UKP-, GUGy- and HN-NETs, alkylating agent-based ChT [dacarbazine, temozolomide or streptozocin combined with 5-fluorouracil (5-FU) or capecitabine] or oxaliplatin-based ChT (oxaliplatin combined with 5-FU or capecitabine) may be used despite limited data [V, B]; MGMT status may guide ChT regimen choice.
-
•
Everolimus [IV, B] or antiangiogenic MKIs [IV, B] can be recommended for first-line treatment of G2 (Ki-67 >10%) and G3 UKP-, GUGy- and HN-NETs. Alkylating agent- or oxaliplatin-based ChT can also be recommended in this setting [IV, B]. PPRT may be recommended for patients with SSTR-positive progressive disease [IV, B].
-
•
Platinum–etoposide is recommended as first-line treatment for UKP-, GUGy- and HN-NECs [IV, A]. For HN-NECs with isolated lymph node metastases and no other metastatic sites, RT may be discussed after ChT [IV, B]. The second-line ChT regimens leucovorin–5-FU–irinotecan (FOLFIRI) and leucovorin–5-FU–oxaliplatin (FOLFOX) can be used [V, B] and topotecan is also an option for HN-NECs [V, B].
-
•
Molecular profiling with TMB determination should be offered when available to guide agnostic clinical trial inclusion [V, B] and/or compassionate programmed cell death protein 1 or programmed death-ligand 1 immunotherapy requests, particularly in high-grade NEC [II, B].
Follow-up, long-term implications and survivorship
Follow-up of patients with metastatic UKP-, GUGy- and HN-NENs should be personalised [based on morphology (well versus poorly differentiated), WHO grade, staging, presence of residual disease or metastases, and SRI or FDG–PET positivity] and include imaging and monitoring of clinical symptoms and biochemical parameters. With the exception of UKP-NENs (see Section 6 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664), there are no data on long-term follow-up. Follow-up carries the risk of excessive radiation exposure, so low-dose CT (for lung metastases) or MRI should be used whenever possible, especially in patients for whom prolonged follow-up is anticipated. There is a consensus for the use of molecular imaging for SSTR-positive NETs, especially SRI with [68Ga]Ga-DOTA-SSA–PET–CT, at initial staging and for post-surgical restaging, but not for routine follow-up.24 Molecular imaging may play a role, however, in the follow-up of secondary lesions that are hard to evaluate with conventional imaging, such as peritoneal carcinomatosis and bone metastases.
Recommendations
-
•
For patients with completely resected G1 and low G2 (Ki-67 <10%) UKP-, GUGy- or HN-NETs, follow-up using low-dose CT or MRI is recommended every 6 months for 2 years, annually for years 3-5 and every second year thereafter [V, C].
-
•
For patients with completely resected G2 (Ki-67 ≥10%) and G3 UKP-, GUGy- or HN-NETs, follow-up using low-dose CT or MRI is recommended every 3 months for 2 years, every 6 months in year 3, annually for years 4-5 and every second year thereafter [V, C].
-
•
For patients with completely resected UKP-, GUGy- or HN-NECs, follow-up should be carried out every 2-3 months for 2 years, every 6 months in year 3 and then discussed on a case-by-case basis thereafter [V, C].
-
•
For patients with completely resected UKP-, GUGy- or HN-NENs, the best molecular imaging technique (according to SRI–PET and/or FDG–PET–CT positivity at initial staging) is recommended 6 months after resection and then in case of unclear or abnormal imaging results [V, C].
-
•
For patients with UKP-, GUGy- or HN-NENs and residual or metastatic disease, restaging is recommended every 4-6 months [G1 and low G2 (Ki-67 <10%) NETs] or every 3-4 months [G2 (Ki-67 ≥10%) and G3 NETs]; the interval can be increased in case of stable disease [V, C].
-
•
For patients with UKP-, GUGy- or HN-NENs and residual or metastatic disease, systematic follow-up using PET imaging is not recommended but may be considered in case of doubtful or abnormal imaging results, increasing biomarkers, clinical suspicion of new metastasis (particularly in the bone) and/or depending on the metastatic sites and their measurability using conventional imaging, especially when locoregional therapies are discussed [V, C].
-
•
Patients with disseminated disease may benefit from referral to specialised palliative care units for appropriate pain treatment, psychosocial support and rehabilitation [II, B].
Parathyroid carcinoma
Incidence and epidemiology
Parathyroid carcinoma (PC) is a rare neoplasm, accounting for <1% of primary hyperparathyroidism cases.56 PC affects male and female patients with a mean age at diagnosis of 50 years. PC occurs as a sporadic form, or less frequently as familial forms such as hyperparathyroidism-jaw tumour syndrome (HPT-JT; ∼15% of cases) linked to a germline mutation in the CDC73/HRPT2 gene.57 Rarely, it can occur as multiple endocrine neoplasia (MEN) type 1 (MEN1) or type 2A (MEN2A).58 Even in the absence of a family history, 20%-40% of patients with apparently sporadic PC have a germline CDC73 mutation.58,59
Diagnosis, pathology and molecular biology
Diagnosis
Clinical presentation
PC can be suspected in patients who present with severe primary hyperparathyroidism characterised by severe hypercalcaemia-related symptoms and markedly elevated parathyroid hormone (PTH) levels. Less than 10% of patients have normal serum calcium and PTH levels and present with a non-functional PC with only local symptoms, such as a palpable cervical mass, hoarseness, dysphagia or dyspnoea.60
Laboratory studies
Most patients with PC have markedly high levels of calcium (>14 mg/dl or 3.5 mmol/l) and non-suppressed intact PTH 1-84 molecule (3-10 times above the upper limit of normal). The combined presence of high serum calcium (>3 mmol/l) and a parathyroid lesion >3 cm (the so-called ‘>3, >3 rule’), should raise suspicion of PC. Some patients with PC may overproduce N-terminal PTH fragment and have elevated serum and urinary levels of human chorionic gonadotropin; their determination could be useful for differential diagnosis with benign adenoma, although this has only been tested in a small series.61
Imaging
Neck US is the first-line imaging technique for the diagnosis of parathyroid lesions. Its sensitivity varies from 67% to 96% with a positive predictive value of 89.7%-97%62; however, there are no specific patterns of malignancy (see Section 7 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664). Fine-needle aspiration (FNA) with cytology and washout PTH measurement can help to differentiate from lesions of other origins (e.g. thyroid, distant metastases from other tumours).63 Complications associated with the FNA procedure (including occasional seeding) are rare.64
Contrast-enhanced CT and MRI can be used in highly suspicious cases to evaluate infiltration of surrounding tissue but are not routinely carried out for differential diagnosis.65
Parathyroid scintigraphy with [99mTc]Tc-sesta-methoxyisobutylisonitrile (MIBI) single-photon emission CT (SPECT) or SPECT–CT, in combination with neck US, is the procedure of choice for preoperative identification of hyperfunctioning parathyroids. During initial PC evaluation, the sensitivity and diagnostic accuracy of [99mTc]Tc-sesta-MIBI scintigraphy are ≥80% when used with neck US or CT. Nevertheless, there are no clear scintigraphic parameters that allow a real preoperative differential diagnosis between PC and adenoma. PET procedures such as [11C]-methionine–PET–CT and [18F]-choline–PET–CT are effective for the diagnosis of primary hyperparathyroidism but their added value in the differential diagnosis between PC and adenoma is unknown.66
Pathology
Histological diagnosis of PC is based on the 2022 WHO classification criteria: (i) vascular invasion; (ii) lymphatic invasion; (iii) perineural (intraneural) invasion; (iv) local malignant invasion into adjacent anatomical structures; or (v) histologically or cytologically documented metastatic disease. The morphology of PC is usually reminiscent of parathyroid adenoma or hyperplasia.67,68 Features suggestive of malignancy and warranting a diagnosis of atypical adenoma [also called parathyroid neoplasm of uncertain malignant potential or atypical parathyroid tumour (APT)] are macroscopically evident necrosis, size >3 cm, weight >500 mg, mitotic index >5 mitoses/2 mm2 and the presence of atypical mitosis67,69 (see Section 7 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664). Some rare variants have been described, including oncocytic carcinomas and carcinosarcomas with more aggressive behaviour.70,71 Although PC generally shows a higher proliferation index (Ki-67 6%-9% compared with <4% in adenoma), there is no predefined Ki-67 cut-off to support or exclude a PC diagnosis. The International Collaboration on Cancer Reporting (ICCR) has issued a dataset on PC and APT that covers PC grading, considering it high grade in the presence of sheets of pleomorphic enlarged nuclei, coagulative necrosis, abnormal mitosis and/or increased proliferation rate.69
Molecular biology
The most frequent molecular alteration in PC is on the CDC73 gene (40%-50% of cases). Phosphatidylinositol-3-kinase (PI3K)–protein kinase b (AKT)–mammalian target of rapamycin (mTOR) is the most frequently altered pathway (40%-80% of cases).65,72,73 IHC evaluation of parafibromin loss of expression to identify a double-hit inactivation of the CDC73 gene is a recognised ancillary diagnostic method for malignancy, as is evaluation of loss of p27, other types of cyclins or cell cycle regulators, galectin-3 and protein gene product 9.5.69,74
Recommendations
-
•
Genetic testing for germline CDC73 mutation should be carried out to rule out HPT-JT in all patients with PC, including those without a family history [IV, A].
-
•
The combined presence of markedly elevated serum calcium [>12 mg/dl (>3 mmol/l)] and parathyroid lesions >3 cm should raise suspicion of PC [IV, B].
-
•
US of the neck should be carried out to assess lesions suspicious for PC [IV, A].
-
•
Contrast-enhanced CT and MRI are used to evaluate locoregional invasion before planning surgery [V, A].
-
•
FNA cytology and washout PTH measurements can be used to differentiate PC from other neck or thyroid nodules but cannot differentiate it from adenoma [V, C].
-
•
[99mTc]Tc-sesta-MIBI–SPECT may be useful for the diagnosis of PC but cannot differentiate it from adenoma [IV, C].
-
•
PC pathology reporting should follow the 2022 WHO classification (fifth edition) and ICCR template [IV, A].
Staging and risk assessment
The prognosis for patients with PC is variable, with 5- and 10-year OS rates after surgery of ∼80% and 40%-80%, respectively. Good prognosis is associated with early diagnosis and radicality of initial surgery, whereas poor prognosis is mainly related to older age and the presence of lymph node metastases.75, 76, 77, 78, 79
Staging is based on the eighth edition of the American Joint Committee on Cancer (AJCC) TNM system (see Supplementary Table S11, available at https://doi.org/10.1016/j.esmoop.2024.103664).80 Only 15%-30% of patients have level VI lymph node metastases and one-third have distant metastases, most commonly in the lung, liver or bone. The detection of locoregional or distant metastases is based on neck US, thoracic and abdominal CT, or MRI.
Among nuclear medicine techniques, total body acquisition with [99mTc]Tc-sesta-MIBI can identify possible extra-cervical uptake and unexpected lesions. FDG–PET–CT can also be applied as PC generally shows high FDG uptake. It can be used to complement conventional imaging in the initial staging and for residual or relapsing disease, albeit based on limited data.81 Other PET tracers such as [11C]-methionine–PET–CT and [18F]-choline–PET–CT have not been validated for routine staging of PC.66,81,82
Recommendations
-
•
PC staging should follow the eighth edition of the AJCC TNM system [IV, A].
-
•
Cross-sectional imaging is the reference method [V, A] but additional FDG–PET–CT may improve staging accuracy [IV, C].
Management of local and locoregional disease
Management of hypercalcaemia
Medical management of hypercalcaemia is the main treatment for patients awaiting surgery and those with inoperable PC. Cinacalcet, a potent second-generation calcium mimetic, is effective for controlling hypercalcaemia in patients with inoperable or persistent disease.83,84 The greatest reductions in calcium were reported in patients with the highest serum calcium levels before therapy. The cornerstones of treatment for severe or symptomatic hypercalcaemia are intravenous saline hydration and bisphosphonate administration (see Section 7 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664).
Surgery
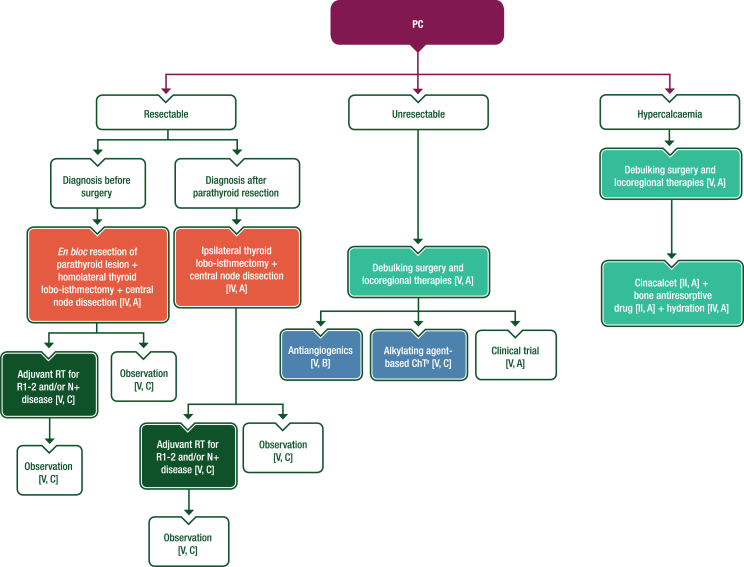
Surgery is the recommended first-line treatment for PC (Figure 6).85 Inadequate and non-radical surgery represent the strongest prognostic factors for recurrence and mortality. In case of high suspicion of PC, en bloc removal of the parathyroid lesion avoiding capsule rupture and local seeding, homolateral thyroid lobo-isthmectomy and central node dissection are recommended.86
Figure 6.
Management of PC. Purple: algorithm title; orange: surgery; dark green: RT; blue: systemic anticancer therapy or their combination; turquoise: non-systemic anticancer therapies or combination of treatment modalities; white: other aspects of management and non-treatment aspects. 5-FU, 5-fluorouracil; ChT, chemotherapy; N+, lymph node metastasis; PC, parathyroid carcinoma; R1, resection with microscopic tumour at the margin; R2, resection with macroscopic tumour at the margin; RT, radiotherapy. aDacarbazine, temozolomide or streptozocin plus 5-FU or capecitabine.
RT
Analysis of data from the National Cancer Database identified 885 patients with PC who underwent surgery between 2004 and 2016, including 126 (14.2%) who also received external beam RT. The latter group had a higher frequency of extensive regional disease, nodal metastases and residual microscopic disease. The 5-year OS rate was 85.3% with a median follow-up of 60.8 months. RT was not associated with a difference in OS based on multivariate analysis87; however, small series from highly specialised tertiary centres have reported lower locoregional relapse rates in patients undergoing adjuvant RT,88, 89, 90 particularly in patients with R1 or R2 resection.90 Although not recommended for all patients, adjuvant RT should be discussed in the case of R1 or R2 resection and/or lymph node metastasis (N+) and/or persistent hypercalcaemia.
Recommendations
-
•
Medical treatment of hypercalcaemia is required before surgery [V, A].
-
•
Cinacalcet is recommended to control hypercalcaemia [II, A].
-
•
Hydration with saline infusion, at a starting dose of 200-500 ml/h, is the first step in the treatment of severe or symptomatic hypercalcaemia [IV, A].
-
•
The bone antiresorptive drug zoledronic acid is recommended as first-line treatment and should be preferred over pamidronate for severe or symptomatic hypercalcaemia [II, A].
-
•
Denosumab is recommended in patients with resistant hypercalcaemia [III, A; FDA approved, not EMA approved].
-
•
When a preoperative diagnosis of PC is made, upfront surgery with en bloc removal of the parathyroid lesion (avoiding capsule rupture and local seeding), homolateral thyroid lobo-isthmectomy and central node dissection is recommended [IV, A].
-
•
In case of pathological diagnosis of PC after parathyroidectomy, reoperation with ipsilateral thyroid lobo-isthectomy and central node dissection should be discussed within 1 month of initial surgery [IV, A].
-
•
RT may be discussed in an MTB, particularly for patients with R1 or R2 resection and/or N+ and/or persistent hypercalcaemia [V, C].
Management of advanced and metastatic disease
PC is often locally advanced and distant metastases can occur in the lung (40%), bone (30%) and liver (10%). Brown tumours (osteolytic lesions of the bone caused by hyperparathyroidism) should be differentiated from bone metastases, but this may be difficult as they can show FDG uptake; MRI can be useful to better characterise bone lesions.91 In case of known distant metastases, the probability of achieving a complete response is low and frequently only locoregional treatments can control the disease.92 Cinacalcet should be continued in case of persistent severe hypercalcaemia.
Data on the efficacy of systemic treatments in PC are scarce with only 79 patients reported in the literature between 1898 and 2018.93 A recent pooled analysis of all metastatic PC cases reported that the most frequent treatments were dacarbazine or anthracycline-containing ChT and antiangiogenic drugs.93 The most frequent ChT protocols included dacarbazine monotherapy, dacarbazine–5-FU, cyclophosphamide monotherapy or methotrexate–doxorubicin–cyclophosphamide–lomustine, but response rates were low.93 Most cases in which antiangiogenics were used involved sorafenib.94,95 PC is usually radioresistant; despite limited data showing that RT can be applied as adjuvant therapy to reduce local recurrence, its role is mainly palliative.85
Recommendations
-
•
The efficacy of systemic treatment in advanced and metastatic disease is limited; thus, whenever possible, debulking surgery and/or locoregional treatments should be discussed to control tumour burden and hormonal secretion [V, A].
-
•
First-line systemic treatment may involve antiangiogenic drugs [V, B] or alkylating agent-based ChT (dacarbazine, temozolomide or streptozocin combined with 5-FU or capecitabine) [V, C].
-
•
Inclusion in clinical trials should be prioritised whenever possible [V, A].
Follow-up, long-term implications and survivorship
The reported risk of recurrence is ∼50% at 2-5 years (based mainly on cases in which non-radical surgery was used).56,75, 76, 77,79 Laboratory follow-up includes lifelong measurement of calcium and PTH every 3 months, although longer intervals (up to 4-6 months) are an option.79 Progressive and repeated increases in PTH and calcium levels are suggestive of PC recurrence and cross-sectional imaging (neck US, CT and/or MRI) should be carried out to identify the site of recurrence. There is no validated role for scintigraphy with [99mTc]Tc-sesta-MIBI in the follow-up of PC.
Recurrences and distant metastases can be small or below the resolution of imaging (<1 cm), and occasionally PC can evolve towards aggressive and rapidly progressive forms and lose the ability for significant uptake of [99mTc]Tc-sesta-MIBI. In these cases, FDG–PET can complement conventional imaging.
The identification of a germline CDC73 mutation should prompt periodic screening for tumours associated with HPT-JT (ossifying fibromas of the mandible or maxilla, benign and malignant uterine involvement, renal cysts).58 Genetic testing should be carried out in all family members of affected individuals. In first-degree relatives, measurement of serum calcium levels and US monitoring are recommended.
Recommendations
-
•
Lifelong laboratory follow-up, including measurement of calcium and PTH, is recommended every 3-4 months during the first 2 years, every 6 months during the subsequent 3 years and annually thereafter [V, C].
-
•
In case of calcium and/or PTH elevation, neck US and CT scan of the neck, chest and abdomen together with a neck MRI are recommended [V, A]. FDG–PET can be proposed to complete work-up, especially in rapidly progressing cases [V, C].
Intrathyroid thymic carcinoma
Incidence and epidemiology
Previously known as carcinoma showing thymus-like differentiation (CASTLE), intrathyroid thymic carcinoma (ITC) is rare and believed to arise from the thymic remnants or branchial pouch96 and, more rarely, from major salivary glands.97 ITC accounts for <0.15% of thyroid neoplasms.98 Females are affected slightly more frequently than males and the median age at diagnosis is 50 years.99
Diagnosis, pathology and molecular biology
ITC presents as a slow-growing neck mass with hard consistency and poor mobility, located at the lower poles of the thyroid gland. It appears as a cold nodule on thyroid scintigraphy and as a solid, heterogenous and hypoechoic mass on US. Most patients have locally advanced disease with invasion of surrounding structures, including the recurrent laryngeal nerve, strap muscle, trachea, oesophagus, soft tissues and skin. Cervical lymph nodes are frequently involved but few patients have distant metastases.99 At diagnosis, ∼20% of patients have dyspnoea, dysphagia, right laryngeal nerve paralysis and pain. FNA does not provide a definitive diagnosis; histological confirmation is warranted.
ITC exhibits histological and immunophenotypic resemblances to eutopic thymic carcinoma. The tumour cells generally grow in a solid or nested pattern, identical to squamous-cell carcinoma arising in the thymus. Positive IHC results for cluster of differentiation (CD)5, CD117 and p63 suggest a diagnosis of ITC (see Section 8 of the Supplementary Material, available at https://doi.org/10.1016/j.esmoop.2024.103664). Similar to thymomas and thymic carcinoma, on comparative genomic hybridisation ITC is characterised by chromosomal imbalances such as gains on chromosomal arm 1q and losses on 6p, 6q and 16q.100 The most frequently altered gene is the telomerase reverse transcriptase promoter mutation C228T, identified in 22% of cases.101 EGFR mutations have been documented102 but their prevalence cannot be estimated due to the limited number of cases analysed.
Recommendations
-
•
ITC should be suspected in the presence of a cold nodule in the lower lobes of the thyroid gland with a cytological diagnosis of poorly differentiated carcinoma [V, A].
-
•
Positive IHC for CD5, CD117 and p63 may allow for a preoperative diagnosis [V, B].
-
•
Histological features that allow for a post-operative diagnosis of ITC are lymphoid stroma, squamous-cell differentiation, infrequent mitoses and rare necrosis, positive IHC for CD5, CD117 and p63, negative IHC for thyroglobulin, TTF-1 and calcitonin, and Ki-67 <20% [IV, A].
Staging and risk assessment
Disease stage is the main prognostic factor. Application of the eighth edition of the UICC staging classification25 for thyroid tumours is recommended, even if not specific for ITC (see Supplementary Table S12, available at https://doi.org/10.1016/j.esmoop.2024.103664). Neck US, CT of the neck, thorax and abdomen, and neck MRI can be used for staging. On CT, ITC appears as a well-defined soft tissue density without calcification, whereas on MRI, it appears as an isointense and hyperintense mass on T1/T2-weighted images.103 Similar to thymoma, ITC can show intense uptake on FDG–PET–CT; however, data on the use of FDG–PET–CT in patients with ITC are very limited.104
Recommendations
-
•
Accurate assessment of local extension with US or MRI is crucial for planning appropriate surgical intervention [IV, A].
-
•
CT of the chest and abdomen should be carried out to rule out the rare possibility of distant synchronous metastasis [V, A]. Complementary FDG–PET–CT may be useful in some cases [V, C].
Management of local and locoregional disease
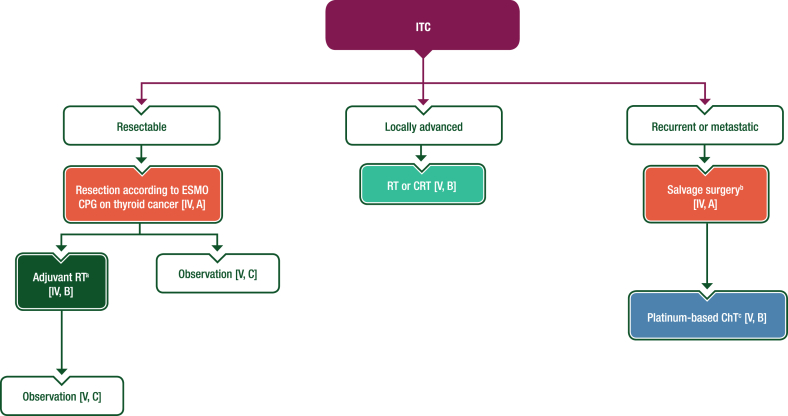
Surgery is the treatment of choice (Figure 7). A pooled analysis of published cases99 showed that thyroidectomy (total, subtotal or near total) was the most frequent surgical procedure. Due to frequent nodal involvement at diagnosis, neck lymph node dissection [i.e. modified radical neck dissection (levels I-V), selective neck dissection of central compartment (level VI) or lateral selective neck dissection (levels II-IV)] was carried out in >55% of cases. Among patients with a small ITC, 40% underwent thyroid lobectomy without lymph node dissection. RT was used in only two cases of locally advanced ITC not amenable to surgery with radical intent.
Figure 7.
Management of ITC. Purple: algorithm title; orange: surgery; dark green: RT; blue: systemic anticancer therapy or their combination; turquoise: non-systemic anticancer therapies or combination of treatment modalities; white: other aspects of management and non-treatment aspects. ChT, chemotherapy; CPG, clinical practice guideline; CRT, chemoradiotherapy; ITC, intrathyroid thymic carcinoma; MTB, multidisciplinary tumour board; RT, radiotherapy. aIn selected cases after careful MTB discussion. bAdjuvant RT may be discussed for patients who have not received prior RT [V, B]. cCisplatin-based regimens with either doxorubicin and cyclophosphamide or etoposide.
The prognosis for patients with local or locoregional disease is very good. In the pooled analysis,99 estimated median disease-free survival was 144 months (range 91-197 months), median OS was not reached and ∼85% of patients were alive after 5 years. Based on these data, surgery is recommended as the first-line therapeutic option in patients with ITC with locoregional disease. The type of surgery (i.e. lobectomy or thyroidectomy) should be planned according to the available guideline for thyroid cancers,28 using conservative surgical treatment whenever possible. Adjuvant RT does not improve prognosis and can be avoided in most patients, although it may be discussed for more advanced or aggressive cases. There is no role for adjuvant ChT. Patients with locoregional disease not amenable to surgery with radical intent could be offered RT or chemoradiotherapy (CRT).
Recommendations
-
•
Surgery is the mainstay of therapy. The type of surgery (i.e. lobectomy or thyroidectomy) should be planned according to the available guidelines for thyroid cancers [IV, A].
-
•
Due to the indolent course of the disease and its good prognosis, conservative surgical treatment is preferred whenever possible [V, B].
-
•
Neck dissection should be carried out according to guidelines for thyroid cancers [V, A].
-
•
RT or CRT are reasonable options in case of locally advanced disease not suitable for radical surgery [V, B].
-
•
If possible, adjuvant RT should be avoided due to the indolent disease course of ITC [IV, D]. It is an option in selected cases after careful multidisciplinary discussion [IV, B].
Management of advanced and metastatic disease
Among 120 patients with ITC who underwent primary tumour resection, 29 (24%) experienced recurrence at a median of 19 months (range 13-25 months).99 The recurrence was locoregional in 21 patients (72%), and was treated surgically in 90% and using RT in 42% of cases, with excellent prognosis. A minority (∼10%) of patients developed distant metastases; five patients (4%) had metastases at diagnosis and eight (6%) developed metachronous distant metastases. The most common metastatic site was the lung. Data on the efficacy of systemic treatments in patients with metastatic ITC are limited. Similar to the recommendations for locally advanced or metastatic thymoma or thymic carcinoma,28 cisplatin-containing regimens have been used in most ITC cases, with a reported objective remission rate of 58%.99 Immunotherapy with the immune checkpoint inhibitor pembrolizumab was used in one case, resulting in long-lasting disease control. Based on this single experience, it is not possible to recommend immunotherapy for the treatment of metastatic ITC.
Recommendations
-
•
In case of local recurrence, salvage surgery is recommended [IV, A] and adjuvant RT may be discussed by an MTB for patients who have not received prior RT [V, B].
-
•
In case of metastatic disease, cisplatin-based combination ChT regimens with either doxorubicin and cyclophosphamide or etoposide are the recommended options [V, B].
Follow-up, long-term implications and survivorship
Follow-up is aimed at identifying locoregional recurrences and, more rarely, distant recurrences, usually within the first 5 years after initial treatment.
Recommendations
-
•
In localised disease, neck US and CT scan of the neck and thorax are recommended 6-12 months after initial treatment and then annually up to 5 years [V, C].
-
•
In metastatic disease, follow-up by CT and/or MRI (in case of liver, bone or brain metastases) every 3-6 months is recommended, depending on the tumour growth rate [V, A].
Methodology
This CPG was developed in accordance with the ESMO standard operating procedures for CPG development (https://www.esmo.org/Guidelines/ESMO-Guidelines-Methodology). The relevant literature has been selected by the expert authors. The FDA/EMA or other regulatory body approval status of new therapies/indications is reported at the time of writing this CPG. Levels of evidence and grades of recommendation have been applied using the system shown in Supplementary Table S13, available at https://doi.org/10.1016/j.esmoop.2024.103664.105 Statements without grading were considered justified standard clinical practice by the authors. For future updates to this CPG, including eUpdates and Living Guidelines, please see the ESMO Guidelines website: https://www.esmo.org/guidelines/guidelines-by-topic/endocrine-and-neuroendocrine-cancers.
Acknowledgements
Manuscript editing support was provided by Guy Atchinson and Claire Bramley (ESMO Guidelines staff) and Angela Corstorphine, Nicky French and Sian-Marie Lucas of Kstorfin Medical Communications Ltd (KMC); this support was funded by ESMO. Support for managing the reference bibliography was provided by Bruno Potterie from the Gustave Roussy library.
Funding
No external funding has been received for the preparation of this guideline. Production costs have been covered by ESMO from central funds.
Disclosure
JH reports personal financial interests for advisory board membership from Ipsen, Lilly and PharmaMar; institutional financial interests for advisory board membership from Eisai, HRA Pharma and Roche; institutional financial interest as an invited speaker from AAA, ITM Radiopharma and Pfizer; and institutional funding from Lilly. AL reports personal financial interests as an invited speaker from AAA, Advanz Pharma, AstraZeneca, Eisai, GenFit, Incyte, Ipsen, Merck, Pfizer, QED, Roche and Servier; personal financial interests for advisory board membership from Albireo Pharma, AstraZeneca, Boston Scientific, Eisai, GenFit, Ipsen, Nutricia, QED, Roche, Servier and Taiho; personal financial interests for writing engagements from Incyte, QED and Servier; institutional financial interest for an educational event from AstraZeneca and institutional access to FM molecular profiling from Roche; non-financial interests as principal investigator (PI) of trials for AstraZeneca, Boehringer Ingelheim, Camurus, Jazz Therapeutics, Merck, Novocure, Panbela Therapeutics, QED, Roche, Servier and Taiho; and travel and educational support from AAA, Advanz Pharma, Bayer, Delcath, Ipsen, Mylan, Novartis, Pfizer, Roche and Sirtex. EG reports personal financial interests as an invited speaker from Adacap, Adium, AstraZeneca, Bristol Myers Squibb (BMS), Dr. Reddy’s, Eisai, Eusa Pharma, Ipsen, Janssen, Lilly, Merck KGa, Pfizer and Roche; personal financial interests for advisory board membership from Astellas, Bayer, MSD, Novartis and Sanofi-Genzyme; institutional financial interests for advisory board membership from Caris Life Sciences and OncoDNA (Biosequence); and institutional research grants from Astellas, AstraZeneca, Ipsen (as coordinating PI), Lexicon, Merck, MTEM/Threshold, Nanostring Technologies, Pfizer and Roche. DD reports personal financial interests for advisory board membership from Eisai and as an invited speaker from Novartis; and institutional financial interest as coordinating PI for Bayer Healthcare. GK reports personal financial interest from stocks/shares in Lambda Therapeutics; institutional financial interest as an invited speaker for Ipsen; institutional research grants from BIOKOSMOS, Faran and Ipsen; and institutional funding from Pfizer and Sandoz; non-remunerated roles as a member of the advisory board of the European Neuroendocrine Tumor Society (ENETS), member of the Education Committee of ENETS, head of the ENETS task force of MEN1 and member of the Educational Committee of the European Society of Endocrinology. ETJ reports institutional financial interests for chairing a scientific board and as an invited speaker from Ipsen. BT reports personal financial interests as an invited speaker from Accord Healthcare, Amgen, Astellas and Ferring; personal financial interests for advisory board membership from Amgen, Astellas, AstraZeneca, Bayer, Janssen, MSD, Myovant, Novartis AAA, Pfizer and Sanofi; personal financial interests for expert testimony from Astellas; and non-remunerated roles as Past President and member of the Board of Directors of the European Organisation of Research and Treatment of Cancer and as a Member of the Board of Directors of ISSECAM. MP reports personal financial interests as an invited speaker from AAA, Boehringer Ingelheim, Ipsen, Lilly, MSD, Novartis, Recordati, SERB and Sanofi; personal financial interests for advisory board membership from AAA and Reimser; personal financial interest as an advisor for ITM Radiopharma; institutional financial interests as a local PI from AAA, ITM Radiopharma and Novartis, coordinating PI from Ipsen and steering committee member from AAA, Crinetics and Novartis; and non-remunerated roles as a member of the ESMO Faculty and congress scientific committee, Past President and current Vice President of ENETS, and a speaker and advisor for the patient support group Netzwerk NET (Germany) and International Neuroendocrine Cancer Alliance. JT reports personal financial interests for advisory board membership from BMS and Nanobiotix; travel support from Merck; and non-remunerated leadership roles with the Groupe d’Oncologie Radiothérapie Tête Et Cou. MFvV has reported no conflicts of interest. PH has reported no conflicts of interest. CD reports institutional financial interests for advisory board membership from Intrasens and Ipsen. EB reports personal financial interests as project lead and PI from Ipsen and for advisory board membership from Novartis AAA; institutional research grants from HRA Pharma and Novartis; and non-remunerated roles as a PI for Enterome and a leadership role in the ENDOCAN network. AB reports personal financial interests as an invited speaker from Amgen and HRA Pharma; personal financial interests for advisory board membership from Amgen, Astellas, Ferring, Ipsen, Janssen and Novartis AAA; institutional funding from Astellas and Janssen; and receipt of product samples from Novartis and Sanofi.
Supplementary data
References
- 1.Dasari A., Shen C., Halperin D., et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3(10):1335–1342. doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hadoux J., Kanaan C., Durand A., et al. Prognostic factors of metastatic neuroendocrine carcinoma under first-line treatment with platinum etoposide with a focus on NEC score and Rb expression: results from the multicentre RBNEC study of the Groupe d'Etude des Tumeurs Endocrines (GTE) and the ENDOCAN-RENATEN network. Eur J Cancer. 2021;152:100–115. doi: 10.1016/j.ejca.2021.04.030. [DOI] [PubMed] [Google Scholar]
- 3.Pósfai B., Kuthi L., Varga L., et al. The colorful palette of neuroendocrine neoplasms in the genitourinary tract. Anticancer Res. 2018;38(6):3243–3254. doi: 10.21873/anticanres.12589. [DOI] [PubMed] [Google Scholar]
- 4.van der Zwan J.M., Mallone S., van Dijk B., et al. Carcinoma of endocrine organs: results of the RARECARE project. Eur J Cancer. 2012;48(13):1923–1931. doi: 10.1016/j.ejca.2012.01.029. [DOI] [PubMed] [Google Scholar]
- 5.Matrood S., Apostolidis L., Schrader J., et al. Multicenter analysis of presacral neuroendocrine neoplasms-clinicopathological characterization and treatment outcomes of a rare disease. Front Endocrinol (Lausanne) 2021;12 doi: 10.3389/fendo.2021.709256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moch H., Cubilla A.L., Humphrey P.A., et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. doi: 10.1016/j.eururo.2016.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Winer I., Kim C., Gehrig P. Neuroendocrine tumors of the gynecologic tract update. Gynecol Oncol. 2021;162(1):210–219. doi: 10.1016/j.ygyno.2021.04.039. [DOI] [PubMed] [Google Scholar]
- 8.van der Laan T.P., Plaat B.E., van der Laan B.F., et al. Clinical recommendations on the treatment of neuroendocrine carcinoma of the larynx: a meta-analysis of 436 reported cases. Head Neck. 2015;37(5):707–715. doi: 10.1002/hed.23666. [DOI] [PubMed] [Google Scholar]
- 9.Ramsey M.J., Nadol J.B., Jr., Pilch B.Z., et al. Carcinoid tumor of the middle ear: clinical features, recurrences, and metastases. Laryngoscope. 2005;115(9):1660–1666. doi: 10.1097/01.mlg.0000175069.13685.37. [DOI] [PubMed] [Google Scholar]
- 10.Turri-Zanoni M., Maragliano R., Battaglia P., et al. The clinicopathological spectrum of olfactory neuroblastoma and sinonasal neuroendocrine neoplasms: refinements in diagnostic criteria and impact of multimodal treatments on survival. Oral Oncol. 2017;74:21–29. doi: 10.1016/j.oraloncology.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Rindi G., Mete O., Uccella S., et al. Overview of the 2022 WHO classification of neuroendocrine neoplasms. Endocr Pathol. 2022;33(1):115–154. doi: 10.1007/s12022-022-09708-2. [DOI] [PubMed] [Google Scholar]
- 12.Bellizzi A.M. Immunohistochemistry in the diagnosis and classification of neuroendocrine neoplasms: what can brown do for you? Hum Pathol. 2020;96:8–33. doi: 10.1016/j.humpath.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berner A.M., Pipinikas C., Ryan A., et al. Diagnostic approaches to neuroendocrine neoplasms of unknown primary site. Neuroendocrinology. 2020;110(7-8):563–573. doi: 10.1159/000504370. [DOI] [PubMed] [Google Scholar]
- 14.Rindi G., Klimstra D.S., Abedi-Ardekani B., et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Mod Pathol. 2018;31(12):1770–1786. doi: 10.1038/s41379-018-0110-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Velthuysen M.F., Couvelard A., Rindi G., et al. ENETS standardized (synoptic) reporting for neuroendocrine tumour pathology. J Neuroendocrinol. 2022;34(3) doi: 10.1111/jne.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Dosso S., Treglia G., Pascale M., et al. Detection rate of unknown primary tumour by using somatostatin receptor PET/CT in patients with metastatic neuroendocrine tumours: a meta-analysis. Endocrine. 2019;64(3):456–468. doi: 10.1007/s12020-019-01934-9. [DOI] [PubMed] [Google Scholar]
- 17.Ma H., Kan Y., Yang J.G. Clinical value of (68)Ga-DOTA-SSTR PET/CT in the diagnosis and detection of neuroendocrine tumors of unknown primary origin: a systematic review and meta-analysis. Acta Radiol. 2021;62(9):1217–1228. doi: 10.1177/0284185120958412. [DOI] [PubMed] [Google Scholar]
- 18.Zatelli M.C., Torta M., Leon A., et al. Chromogranin A as a marker of neuroendocrine neoplasia: an Italian Multicenter Study. Endocr Relat Cancer. 2007;14(2):473–482. doi: 10.1677/ERC-07-0001. [DOI] [PubMed] [Google Scholar]
- 19.Marinelli J.P., Cass S.P., Mann S.E., et al. Adenomatous neuroendocrine tumors of the middle ear: a multi-institutional investigation of 32 cases and development of a staging system. Otol Neurotol. 2018;39(8):e712–e721. doi: 10.1097/MAO.0000000000001905. [DOI] [PubMed] [Google Scholar]
- 20.Soga J., Osaka M., Yakuwa Y. Laryngeal endocrinomas (carcinoids and relevant neoplasms): analysis of 278 reported cases. J Exp Clin Cancer Res. 2002;21(1):5–13. [PubMed] [Google Scholar]
- 21.Mitchell E.H., Diaz A., Yilmaz T., et al. Multimodality treatment for sinonasal neuroendocrine carcinoma. Head Neck. 2012;34(10):1372–1376. doi: 10.1002/hed.21940. [DOI] [PubMed] [Google Scholar]
- 22.Wang K.R., Jia Y.J., Zhou S.H., et al. Cutaneous and subcutaneous metastases from atypical laryngeal carcinoids: case report and review of the literature. Medicine (Baltimore) 2016;95(7) doi: 10.1097/MD.0000000000002796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferlito A., Rinaldo A., Bishop J.A., et al. Paraneoplastic syndromes in patients with laryngeal neuroendocrine carcinomas: clinical manifestations and prognostic significance. Eur Arch Otorhinolaryngol. 2016;273(3):533–536. doi: 10.1007/s00405-014-3351-5. [DOI] [PubMed] [Google Scholar]
- 24.Ambrosini V., Kunikowska J., Baudin E., et al. Consensus on molecular imaging and theranostics in neuroendocrine neoplasms. Eur J Cancer. 2021;146:56–73. doi: 10.1016/j.ejca.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Union for International Cancer Control . 8th ed. John Wiley & Sons, Inc.; Oxford, UK: 2017. TNM Classification of Malignant Tumours. [Google Scholar]
- 26.Faggiano A., Sabourin J.C., Ducreux M., et al. Pulmonary and extrapulmonary poorly differentiated large cell neuroendocrine carcinomas: diagnostic and prognostic features. Cancer. 2007;110(2):265–274. doi: 10.1002/cncr.22791. [DOI] [PubMed] [Google Scholar]
- 27.Merola E., Rinke A., Partelli S., et al. Surgery with radical intent: is there an indication for G3 neuroendocrine neoplasms? Ann Surg Oncol. 2020;27(5):1348–1355. doi: 10.1245/s10434-019-08049-5. [DOI] [PubMed] [Google Scholar]
- 28.European Society for Medical Oncology Clinical Practice Guidelines: Endocrine and Neuroendocrine Cancers. 2024. https://www.esmo.org/guidelines/guidelines-by-topic/endocrine-and-neuroendocrine-cancers Available at.
- 29.Deep N.L., Ekbom D.C., Hinni M.L., et al. High-grade neuroendocrine carcinoma of the larynx: the mayo clinic experience. Ann Otol Rhinol Laryngol. 2016;125(6):464–469. doi: 10.1177/0003489415619179. [DOI] [PubMed] [Google Scholar]
- 30.Likhacheva A., Rosenthal D.I., Hanna E., et al. Sinonasal neuroendocrine carcinoma: impact of differentiation status on response and outcome. Head Neck Oncol. 2011;3:32. doi: 10.1186/1758-3284-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Laan T.P., Iepsma R., Witjes M.J., et al. Meta-analysis of 701 published cases of sinonasal neuroendocrine carcinoma: the importance of differentiation grade in determining treatment strategy. Oral Oncol. 2016;63:1–9. doi: 10.1016/j.oraloncology.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Bell D., El-Naggar A.K., Gidley P.W. Middle ear adenomatous neuroendocrine tumors: a 25-year experience at MD Anderson Cancer Center. Virchows Arch. 2017;471(5):667–672. doi: 10.1007/s00428-017-2155-6. [DOI] [PubMed] [Google Scholar]
- 33.Heetfeld M., Chougnet C.N., Olsen I.H., et al. Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer. 2015;22(4):657–664. doi: 10.1530/ERC-15-0119. [DOI] [PubMed] [Google Scholar]
- 34.Bartlett E.K., Roses R.E., Gupta M., et al. Surgery for metastatic neuroendocrine tumors with occult primaries. J Surg Res. 2013;184(1):221–227. doi: 10.1016/j.jss.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 35.Gehl J., Sersa G., Matthiessen L.W., et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018;57(7):874–882. doi: 10.1080/0284186X.2018.1454602. [DOI] [PubMed] [Google Scholar]
- 36.Caplin M.E., Pavel M., Ćwikła J.B., et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371(3):224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 37.Rinke A., Müller H.H., Schade-Brittinger C., et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27(28):4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 38.Pavel M., Öberg K., Falconi M., et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(7):844–860. doi: 10.1016/j.annonc.2020.03.304. [DOI] [PubMed] [Google Scholar]
- 39.Kulke M.H., Hörsch D., Caplin M.E., et al. Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol. 2017;35(1):14–23. doi: 10.1200/JCO.2016.69.2780. [DOI] [PubMed] [Google Scholar]
- 40.Doberauer C., Mengelkoch B., Kloke O., et al. Treatment of metastatic carcinoid tumors and the carcinoid syndrome with recombinant interferon alpha. Acta Oncol. 1991;30(5):603–605. doi: 10.3109/02841869109092426. [DOI] [PubMed] [Google Scholar]
- 41.Grozinsky-Glasberg S., Davar J., Hofland J., et al. European neuroendocrine tumor society (ENETS) 2022 guidance paper for carcinoid syndrome and carcinoid heart disease. J Neuroendocrinol. 2022;34(7) doi: 10.1111/jne.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pavel M.E., Hainsworth J.D., Baudin E., et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378(9808):2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 43.Yao J.C., Shah M.H., Ito T., et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364(6):514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yao J.C., Fazio N., Singh S., et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet. 2016;387(10022):968–977. doi: 10.1016/S0140-6736(15)00817-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chan J., Geyer S., Ou F.S., et al. LBA53 alliance A021602: phase III, double-blinded study of cabozantinib versus placebo for advanced neuroendocrine tumors (NET) after progression on prior therapy (CABINET) Ann Oncol. 2023;34(suppl 2):S1292. [Google Scholar]
- 46.Garcia-Carbonero R., Benavent M., Jimenez Fonseca P., et al. 1097O The AXINET trial (GETNE1107): axitinib plus octreotide LAR improves PFS by blinded central radiological assessment vs placebo plus octreotide LAR in G1-2 extrapancreatic NETs. Ann Oncol. 2021;32(suppl 5):S907–S908. [Google Scholar]
- 47.Xu J., Shen L., Zhou Z., et al. Surufatinib in advanced extrapancreatic neuroendocrine tumours (SANET-ep): a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(11):1500–1512. doi: 10.1016/S1470-2045(20)30496-4. [DOI] [PubMed] [Google Scholar]
- 48.de Mestier L., Lamarca A., Hernando J., et al. Treatment outcomes of advanced digestive well-differentiated grade 3 NETs. Endocr Relat Cancer. 2021;28(8):549–561. doi: 10.1530/ERC-21-0109. [DOI] [PubMed] [Google Scholar]
- 49.Hegi M.E., Diserens A.C., Gorlia T., et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 50.Walter T., Lecomte T., Hadoux J., et al. LBA54 Alkylating agent-based vs oxaliplatin-based chemotherapy in neuroendocrine tumours according to the O6-methylguanine-DNA methyltransferase (MGMT) status: a randomized phase II study (MGMT-NET) on behalf of the French Group of Endocrine Tumors (GTE) and ENDOCAN-RENATEN network. Ann Oncol. 2023;34(suppl 2):S1292–S1293. [Google Scholar]
- 51.Moertel C.G., Kvols L.K., O'Connell M.J., et al. Treatment of neuroendocrine carcinomas with combined etoposide and cisplatin. Evidence of major therapeutic activity in the anaplastic variants of these neoplasms. Cancer. 1991;68(2):227–232. doi: 10.1002/1097-0142(19910715)68:2<227::aid-cncr2820680202>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 52.Strosberg J.R., Caplin M.E., Kunz P.L., et al. (177)Lu-dotatate plus long-acting octreotide versus high-dose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22(12):1752–1763. doi: 10.1016/S1470-2045(21)00572-6. [DOI] [PubMed] [Google Scholar]
- 53.Brabander T., van der Zwan W.A., Teunissen J.J.M., et al. Long-term efficacy, survival, and safety of [(177)Lu-DOTA(0),Tyr(3)]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res. 2017;23(16):4617–4624. doi: 10.1158/1078-0432.CCR-16-2743. [DOI] [PubMed] [Google Scholar]
- 54.Mosele F., Remon J., Mateo J., et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO Precision Medicine Working Group. Ann Oncol. 2020;31(11):1491–1505. doi: 10.1016/j.annonc.2020.07.014. [DOI] [PubMed] [Google Scholar]
- 55.Marabelle A., Fakih M., Lopez J., et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 2020;21(10):1353–1365. doi: 10.1016/S1470-2045(20)30445-9. [DOI] [PubMed] [Google Scholar]
- 56.Lee P.K., Jarosek S.L., Virnig B.A., et al. Trends in the incidence and treatment of parathyroid cancer in the United States. Cancer. 2007;109(9):1736–1741. doi: 10.1002/cncr.22599. [DOI] [PubMed] [Google Scholar]
- 57.Carpten J.D., Robbins C.M., Villablanca A., et al. HRPT2, encoding parafibromin, is mutated in hyperparathyroidism-jaw tumor syndrome. Nat Genet. 2002;32(4):676–680. doi: 10.1038/ng1048. [DOI] [PubMed] [Google Scholar]
- 58.Cardoso L., Stevenson M., Thakker R.V. Molecular genetics of syndromic and non-syndromic forms of parathyroid carcinoma. Hum Mutat. 2017;38(12):1621–1648. doi: 10.1002/humu.23337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shattuck T.M., Välimäki S., Obara T., et al. Somatic and germ-line mutations of the HRPT2 gene in sporadic parathyroid carcinoma. N Engl J Med. 2003;349(18):1722–1729. doi: 10.1056/NEJMoa031237. [DOI] [PubMed] [Google Scholar]
- 60.Wilkins B.J., Lewis J.S., Jr. Non-functional parathyroid carcinoma: a review of the literature and report of a case requiring extensive surgery. Head Neck Pathol. 2009;3(2):140–149. doi: 10.1007/s12105-009-0115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rubin M.R., Bilezikian J.P., Birken S., et al. Human chorionic gonadotropin measurements in parathyroid carcinoma. Eur J Endocrinol. 2008;159(4):469–474. doi: 10.1530/EJE-08-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Christakis I., Vu T., Chuang H.H., et al. The diagnostic accuracy of neck ultrasound, 4D-computed tomographyand sestamibi imaging in parathyroid carcinoma. Eur J Radiol. 2017;95:82–88. doi: 10.1016/j.ejrad.2017.07.026. [DOI] [PubMed] [Google Scholar]
- 63.Carral F., Jiménez A.I., Tomé M., et al. Safety and diagnostic performance of parathyroid hormone assay in fine-needle aspirate in suspicious parathyroid adenomas. Endocrinol Diabetes Nutr (Engl Ed) 2021;68(7):481–488. doi: 10.1016/j.endien.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 64.Shah K.S., Ethunandan M. Tumour seeding after fine-needle aspiration and core biopsy of the head and neck--a systematic review. Br J Oral Maxillofac Surg. 2016;54(3):260–265. doi: 10.1016/j.bjoms.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Hu Y., Zhang X., Wang O., et al. The genomic profile of parathyroid carcinoma based on whole-genome sequencing. Int J Cancer. 2020;147(9):2446–2457. doi: 10.1002/ijc.33166. [DOI] [PubMed] [Google Scholar]
- 66.Petranović Ovčariček P., Giovanella L., Carrió Gasset I., et al. The EANM practice guidelines for parathyroid imaging. Eur J Nucl Med Mol Imaging. 2021;48(9):2801–2822. doi: 10.1007/s00259-021-05334-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Erickson L.A., Mete O., Juhlin C.C., et al. Overview of the 2022 WHO classification of parathyroid tumors. Endocr Pathol. 2022;33(1):64–89. doi: 10.1007/s12022-022-09709-1. [DOI] [PubMed] [Google Scholar]
- 68.International Agency for Research on Cancer WHO Classification of Tumours. Endocrine tumours. 2022. https://tumourclassification.iarc.who.int/welcome/# Available at.
- 69.Williams M.D., DeLellis R.A., Erickson L.A., et al. Pathology data set for reporting parathyroid carcinoma and atypical parathyroid neoplasm: recommendations from the International Collaboration on Cancer Reporting. Hum Pathol. 2021;110:73–82. doi: 10.1016/j.humpath.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 70.Erickson L.A., Jin L., Papotti M., et al. Oxyphil parathyroid carcinomas: a clinicopathologic and immunohistochemical study of 10 cases. Am J Surg Pathol. 2002;26(3):344–349. doi: 10.1097/00000478-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 71.Nacamuli R., Rumore G.J., Clark G. Parathyroid carcinosarcoma: a previously unreported entity. Am Surg. 2002;68(10):900–903. [PubMed] [Google Scholar]
- 72.Cetani F., Banti C., Pardi E., et al. CDC73 mutational status and loss of parafibromin in the outcome of parathyroid cancer. Endocr Connect. 2013;2(4):186–195. doi: 10.1530/EC-13-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pandya C., Uzilov A.V., Bellizzi J., et al. Genomic profiling reveals mutational landscape in parathyroid carcinomas. JCI Insight. 2017;2(6) doi: 10.1172/jci.insight.92061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Erickson L.A., Mete O. Immunohistochemistry in diagnostic parathyroid pathology. Endocr Pathol. 2018;29(2):113–129. doi: 10.1007/s12022-018-9527-6. [DOI] [PubMed] [Google Scholar]
- 75.Harari A., Waring A., Fernandez-Ranvier G., et al. Parathyroid carcinoma: a 43-year outcome and survival analysis. J Clin Endocrinol Metab. 2011;96(12):3679–3686. doi: 10.1210/jc.2011-1571. [DOI] [PubMed] [Google Scholar]
- 76.Hundahl S.A., Fleming I.D., Fremgen A.M., et al. Two hundred eighty-six cases of parathyroid carcinoma treated in the U.S. between 1985-1995: a national cancer data base report. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1999;86(3):538–544. doi: 10.1002/(sici)1097-0142(19990801)86:3<538::aid-cncr25>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 77.Sadler C., Gow K.W., Beierle E.A., et al. Parathyroid carcinoma in more than 1000 patients: a population-level analysis. Surgery. 2014;156(6):1622–1629. doi: 10.1016/j.surg.2014.08.069. [DOI] [PubMed] [Google Scholar]
- 78.Schaapveld M., Jorna F.H., Aben K.K., et al. Incidence and prognosis of parathyroid gland carcinoma: a population-based study in The Netherlands estimating the preoperative diagnosis. Am J Surg. 2011;202(5):590–597. doi: 10.1016/j.amjsurg.2010.09.025. [DOI] [PubMed] [Google Scholar]
- 79.Villar-del-Moral J., Jiménez-García A., Salvador-Egea P., et al. Prognostic factors and staging systems in parathyroid cancer: a multicenter cohort study. Surgery. 2014;156(5):1132–1144. doi: 10.1016/j.surg.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 80.Landry C.S., Wang T.S., Asare E.A., et al. In: AJCC Cancer Staging Manual. 8th ed. Edge S., Byrd D.R., Compton C.C., et al., editors. Springer International Publishing; New York, NY: 2017. Parathyroid; pp. 911–918. [Google Scholar]
- 81.Hatzl M., Röper-Kelmayr J.C., Fellner F.A., et al. 18F-fluorocholine, 18F-FDG, and 18F-fluoroethyl tyrosine PET/CT in parathyroid cancer. Clin Nucl Med. 2017;42(6):448–450. doi: 10.1097/RLU.0000000000001652. [DOI] [PubMed] [Google Scholar]
- 82.Deandreis D., Terroir M., Al Ghuzlan A., et al. 18Fluorocholine PET/CT in parathyroid carcinoma: a new tool for disease staging? Eur J Nucl Med Mol Imaging. 2015;42(12):1941–1942. doi: 10.1007/s00259-015-3130-6. [DOI] [PubMed] [Google Scholar]
- 83.Silverberg S.J., Rubin M.R., Faiman C., et al. Cinacalcet hydrochloride reduces the serum calcium concentration in inoperable parathyroid carcinoma. J Clin Endocrinol Metab. 2007;92(10):3803–3808. doi: 10.1210/jc.2007-0585. [DOI] [PubMed] [Google Scholar]
- 84.Takeuchi Y., Takahashi S., Miura D., et al. Cinacalcet hydrochloride relieves hypercalcemia in Japanese patients with parathyroid cancer and intractable primary hyperparathyroidism. J Bone Miner Metab. 2017;35(6):616–622. doi: 10.1007/s00774-016-0797-0. [DOI] [PubMed] [Google Scholar]
- 85.Wilhelm S.M., Wang T.S., Ruan D.T., et al. The American Association of Endocrine surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg. 2016;151(10):959–968. doi: 10.1001/jamasurg.2016.2310. [DOI] [PubMed] [Google Scholar]
- 86.Schulte K.M., Talat N., Miell J., et al. Lymph node involvement and surgical approach in parathyroid cancer. World J Surg. 2010;34(11):2611–2620. doi: 10.1007/s00268-010-0722-y. [DOI] [PubMed] [Google Scholar]
- 87.Limberg J., Stefanova D., Ullmann T.M., et al. The use and benefit of adjuvant radiotherapy in parathyroid carcinoma: a national cancer database analysis. Ann Surg Oncol. 2021;28(1):502–511. doi: 10.1245/s10434-020-08825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Busaidy N.L., Jimenez C., Habra M.A., et al. Parathyroid carcinoma: a 22-year experience. Head Neck. 2004;26(8):716–726. doi: 10.1002/hed.20049. [DOI] [PubMed] [Google Scholar]
- 89.Christakis I., Silva A.M., Williams M.D., et al. Postoperative local-regional radiation therapy in the treatment of parathyroid carcinoma: the MD Anderson experience of 35 years. Pract Radiat Oncol. 2017;7(6):e463–e470. doi: 10.1016/j.prro.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 90.Munson N.D., Foote R.L., Northcutt R.C., et al. Parathyroid carcinoma: is there a role for adjuvant radiation therapy? Cancer. 2003;98(11):2378–2384. doi: 10.1002/cncr.11819. [DOI] [PubMed] [Google Scholar]
- 91.Hong W.S., Sung M.S., Chun K.A., et al. Emphasis on the MR imaging findings of brown tumor: a report of five cases. Skeletal Radiol. 2011;40(2):205–213. doi: 10.1007/s00256-010-0979-0. [DOI] [PubMed] [Google Scholar]
- 92.Wang P., Xue S., Wang S., et al. Clinical characteristics and treatment outcomes of parathyroid carcinoma: a retrospective review of 234 cases. Oncol Lett. 2017;14(6):7276–7282. doi: 10.3892/ol.2017.7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alberti A., Smussi D., Zamparini M., et al. Treatment and outcome of metastatic parathyroid carcinoma: a systematic review and pooled analysis of published cases. Front Oncol. 2022;12 doi: 10.3389/fonc.2022.997009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Akirov A., Asa S.L., Larouche V., et al. The clinicopathological spectrum of parathyroid carcinoma. Front Endocrinol (Lausanne) 2019;10:731. doi: 10.3389/fendo.2019.00731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rozhinskaya L., Pigarova E., Sabanova E., et al. Diagnosis and treatment challenges of parathyroid carcinoma in a 27-year-old woman with multiple lung metastases. Endocrinol Diabetes Metab Case Rep. 2017;2017:16. doi: 10.1530/EDM-16-0113. 0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kakudo K., Bai Y., Ozaki T., et al. Intrathyroid epithelial thymoma (ITET) and carcinoma showing thymus-like differentiation (CASTLE): CD5-positive neoplasms mimicking squamous cell carcinoma of the thyroid. Histol Histopathol. 2013;28(5):543–556. doi: 10.14670/HH-28.543. [DOI] [PubMed] [Google Scholar]
- 97.Kunc M., Kamieniecki A., Walczak G., et al. Intrasalivary thymic carcinoma: a case report and literature review. Head Neck Pathol. 2022;16(3):857–864. doi: 10.1007/s12105-021-01394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Roka S., Kornek G., Schüller J., et al. Carcinoma showing thymic-like elements—a rare malignancy of the thyroid gland. Br J Surg. 2004;91(2):142–145. doi: 10.1002/bjs.4510. [DOI] [PubMed] [Google Scholar]
- 99.Gurizzan C., Zamparini M., Volante M., et al. Outcome of patients with intrathyroidal thymic carcinoma: a pooled analysis. Endocr Relat Cancer. 2021;28(8):593–604. doi: 10.1530/ERC-21-0123. [DOI] [PubMed] [Google Scholar]
- 100.Veits L., Mechtersheimer G., Steger C., et al. Chromosomal imbalances in carcinoma showing thymus-like elements (CASTLE) Virchows Arch. 2011;459(2):221–226. doi: 10.1007/s00428-011-1117-7. [DOI] [PubMed] [Google Scholar]
- 101.Tahara I., Oishi N., Mochizuki K., et al. Identification of recurrent TERT promoter mutations in intrathyroid thymic carcinomas. Endocr Pathol. 2020;31(3):274–282. doi: 10.1007/s12022-020-09635-0. [DOI] [PubMed] [Google Scholar]
- 102.Rajeshwari M., Singh V., Nambirajan A., et al. Carcinoma showing thymus like elements: report of a case with EGFR T790M mutation. Diagn Cytopathol. 2018;46(5):413–418. doi: 10.1002/dc.23859. [DOI] [PubMed] [Google Scholar]
- 103.Wu B., Sun T., Gu Y., et al. CT and MR imaging of thyroid carcinoma showing thymus-like differentiation (CASTLE): a report of ten cases. Br J Radiol. 2016;89(1060) doi: 10.1259/bjr.20150726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ardighieri L., Tomasoni M., Battocchio S., et al. Carcinoma showing thymus-like differentiation (CASTLE) arising in the sublingual gland. Int J Surg Pathol. 2021;29(3):301–307. doi: 10.1177/1066896920941604. [DOI] [PubMed] [Google Scholar]
- 105.Dykewicz C.A. Summary of the guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. Clin Infect Dis. 2001;33(2):139–144. doi: 10.1086/321805. [adapted from: Gross PA, Barrett TL, Dellinger EP, et al. Purpose of quality standards for infectious diseases. Clin Infect Dis. 1994;18(3):421] [DOI] [PubMed] [Google Scholar]
- 106.International Agency for Research on Cancer WHO Classification of Urinary and Male Genital Tumours. 2022. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Urinary-And-Male-Genital-Tumours-2022 Available at.
- 107.International Agency for Research on Cancer WHO Classification of Female Genital Tumours. 2020. https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Female-Genital-Tumours-2020 Available at.
- 108.Mete O., Wenig B.M. Update from the 5th edition of the world health organization classification of head and neck tumors: overview of the 2022 WHO classification of head and neck neuroendocrine neoplasms. Head Neck Pathol. 2022;16(1):123–142. doi: 10.1007/s12105-022-01435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.