Abstract
Objectives:
To investigate the cognitive performance of Saudi children and adolescents with type 1 diabetes mellitus (T1DM) compared to matched control groups.
Methods:
A total of 64 participants (32 T1DM patients and 32 control), matched with age, gender, ethnicity, education, and body mass index were recruited. Cognitive functions were investigated using a highly reliable and valid “Cambridge neuropsychological test automated battery”. The attention switching task (AST) and choice reaction time (CRT) test were carried out to evaluate the cognitive functions.
Results:
The cognitive test parameters AST mean correct latency, AST mean correct latency (congruent), AST mean correct latency (incongruent), and CRT mean correct latency were significantly delayed in the T1DM group compared to their control group (p<0.0001). Moreover, the control group did significantly better on the cognitive test parameters AST and CRT than the T1DM children and adolescents with hemoglobin A1c (HbA1C) ≥8% group (p<0.01).
Conclusion:
The findings demonstrate a delay in cognitive performance among children and adolescents with T1DM. Importantly, elevated HbA1C levels and duration of disease were associated with reduced cognitive functions. The present study findings highlight the importance of stringent glycemic management in children and adolescents with T1DM. The findings can support physicians and policymakers not only to prevent microvascular complications but also to mitigate potential cognitive deficits among children and adolescents with T1DM.
Keywords: type 1 diabetes, diabetes mellitus, cognitive function, HbA1C
Diabetes mellitus (DM) is the most common chronic debilitating disease of all age groups, both genders, urban and rural regions, and developing and developed nations. The epidemiological literature highlights the global rising trends in the incidence of DM.1 Worldwide approximately 537 million, 10.5% of the population are suffering from diabetes and almost half are unaware that they are living with this condition. The International Diabetes Federation (IDF) highlights that the epidemiology of diabetes will rise by approximately 643 million in the year 2030, and 783 million by the year 2045, of these diabetic patients 3 in 4 live in low and middle-income countries.2
Approximately 90% of these diabetic patients have type 2 diabetes mellitus (T2DM) and 9% have type 1 diabetes mellitus (T1DM). Type 1 diabetes mellitus is the most frequent type of diabetes in children and youth in recent years. It is an autoimmune disease due to the destruction of pancreatic beta cells. Its patients require life-long insulin therapy.3
There are multiple factors which can affect the incidence of T1DM such as age, gender, ethnicity, geographic, and seasonal characteristics. Every year, there is an increase of approximately 3-5% in the incidence, the younger children experiencing the greatest rise in the incidence of T1DM.4,5 The epidemiological trends of T1DM are variable at national, regional, and global levels. However, in some countries, the incidence of T1DM is rapidly rising. The top 10 countries for the incidence rate per 100,000 population per annum of T1DM are Finland (52.2%), Sweden (44.1%), Kuwait (41.7%), Qatar (38.1%), Canada (37.9%), Algeria (34.8%), Norway (33.6%), Saudi Arabia (31.4%), United Kingdom (28.1%), and Ireland (27.5%).2
Worldwide, people have great concerns regarding the rising prevalence and complications of DM. The most recent literature highlights the impact of DM on the nervous system, including cognitive function impairment.6-8 The cognitive functions understanding among children is highly essential for the parents and educators to support the children for their development across the multiple domains. By recognizing the intricate processes underlying perception, attention, memory, executive functions, critical thinking, and social cognition, parents and educators can create enriching environments that nurture children’s intellectual, emotional, and social growth. This study aims to investigate the cognitive performance of children and adolescents with T1DM compared to matched control groups and the impact of hemoglobin A1c (HbA1C) and duration of diabetes.
Methods
The present matched, case-control, and cross-sectional study was carried out in the Physiology Department and University Diabetes Centre, College of Medicine, King Saud University, Riyadh, Saudi Arabia, during the period November 2023 to March 2024.
In this study, we employed convenience sampling, for recruiting the children and adolescents with T1DM, who had visited the Diabetes Unit at King Saud University Hospitals, Riyadh, Saudi Arabia. School-going Saudi children and adolescents with an age range between or equal to 11-18 years, clinically diagnosed with T1DM were recruited. The patients were younger than 11 or older than 18; any clinical history of mental illness, dizziness, sleep deprivation, visual problems, physical disability, type 2 diabetic patients; individuals diagnosed with psychiatric disorders, heart diseases, known neuropathy, retinopathy, cerebrovascular diseases; and patients who smoked cigarettes or shisha were excluded from the study.9 The T1DM group was matched with the control group for the same age, gender, ethnicity, weight, height, body mass index (BMI), and level of education, to achieve the appropriate study outcomes for cognitive functions and minimize the study bias factors. All the children in the control group were free from any chronic illnesses such as DM and cardiovascular or neurological disorders.
The ethical committee, institutional review board, College of Medicine, King Saud University, Riyadh, Saudi Arabia, approved the study (Ref# 23/0854/IRB). The parents of the children were fully briefed regarding the test and upon their acceptance to participate in the study, an informed written consent was obtained from the children as well. After administering the test, the patient’s hospital file number was recorded to extract relevant information from the hospital records later. However, this information was kept completely confidential and only the individuals extracting the data had access to it. No identifiable information was recorded or presented in any shared files.
All the data (age, gender, height, weight, BMI, HbA1C, and duration of diabetes) regarding the T1DM patient was collected from patient electronic records. Each patient was assigned a code, and no identifying information was disclosed. For HbA1C, we extracted the previous 2 years’ values and got an average for everyone. Hemoglobin A1c was further categorized into 2 groups: HbA1C <8% and HbA1C ≥8%; duration of diabetes was categorized into 3 groups less than 5 years, 5-10 years, and more than 10 years.
The cognitive functions test was carried out using the Cambridge neuropsychological test automated battery (CANTAB). The CANTAB is a computer-based tool used for assessing cognitive functions and has a wide range of tests for different cognitive domains. The tasks are often non-verbal and typically involve simple interactions with a touchscreen computer. All study participants were seated comfortably in a chair with the computer in front of them on the table. They were informed the tests will take 15-20 minutes. Everyone was provided with an initial explanation of the test procedure. The script provided in the administrative CANTAB guide was used for this test.10 The instructions were repeated if needed, and once the individual was ready, they were asked to begin the test. The following tests were chosen for our study: I) attention switching task (AST, this test assesses cognitive flexibility). Response time and errors in cued switching trials were measured providing robust outcome measures.10 The test parameters, including “AST mean correct latency, AST mean correct latency (congruent), and AST mean correct latency (incongruent), were recorded; II) choice reaction time (CRT, this test measures the speed of response in a simple 2-choice model). The test parameter CRT mean correct latency was recorded.
Statistical analysis
All statistical tests were carried out on the Statistical Package for the Social Sciences, version 29 (IBM Corp., Armonk, NY, USA). Descriptive analysis was carried out and continuous variables were reported as mean ± standard deviation (SD) while categorical variables were expressed in frequency and percentages (%). We then employed the 2-independent sample t-test to compare the variances between diabetic and control groups with cognitive functions. The same test was also used in the stratified analysis, namely, duration of diabetes and HbA1c levels with the cognitive tests. A p-value of <0.05 was considered significant.
Results
In our study, we had a total of 64 participants 32 T1DM patients (17 males and 15 females) and 32 control (17 males and 15 females). Each diabetic patient was matched to an individual in the control group according to age, height, weight, and BMI (Table 1). The mean age of our cases was 14.77 years and the mean BMI was 23.68kg/m2. Approximately 53.3% of the group was male and 46.7% were females. The mean HbA1C of the diabetic group was 9.6%. Most of our patients (62.5%) had diabetes for 5-10 years (Table 1).
Table 1.
- Descriptive analysis of children and adolescents with type 1 diabetes mellitus and control group.
| Variables | Control group (n=32) | T1DM group (n=32) | P-values |
|---|---|---|---|
| Age (years) | 15.23±1.46 | 14.77±1.14 | 0.17 |
| Height (cm) | 163.85±8.47 | 163.37±8.66 | 0.83 |
| Weight (kg) | 64.04±15.64 | 63.52±13.76 | 0.89 |
| BMI (kg/m2) | 23.64±4.26 | 23.68±4.24 | 0.98 |
| HbA1C (%) | -- | 9.6±2.20 | -- |
| Gender | |||
| Male | 17 (53.3) | 17 (53.3) | -- |
| Female | 15 (46.7) | 15 (46.7) | -- |
| Duration of T1DM (years) | |||
| >5 years | -- | 9 (28.1) | -- |
| 5-10 years | -- | 20 (62.5) | -- |
| >10 years | -- | 3 (9.4) | -- |
Values are presented as mean ± standard deviation (SD) or numbers and percentages (%).
T1DM: type 1 diabetes mellitus, BMI: body mass index, HbA1C: hemoglobin A1C
In this study, we compared the different parameters of the 2 tests between the diabetic and the control groups (Table 2). The mean values for the AST parameters (mean correct latency, mean correct latency-congruent, and mean correct latency-incongruent) and CRT (mean correct latency) were significantly delayed in the T1DM group compared to the control group (p<0.001).
Table 2.
- Cognitive function tests comparison between type 1 diabetic patients and matched control.
| Tests | T1DM group | Control group | P-values |
|---|---|---|---|
| AST mean correct latency (ms) | 1007.70±174.79 | 491.10±148.67 | <0.001 |
| AST mean correct latency, congruent (ms) | 971.09±182.41 | 483.60±115.93 | <0.001 |
| AST mean correct latency, incongruent (ms) | 1046.85±169.06 | 467.30±141.73 | <0.001 |
| CRT mean correct latency (ms) | 515.24±111.30 | 411.77±82.78 | <0.001 |
Values are presented as mean ± standard deviation (SD).
T1DM: type 1 diabetes mellitus, AST: attention seeking test, CRT: choice reaction time, ms: milliseconds
In this study, we categorized the T1DM patients based on the HbA1C levels into 2 groups with one group having HbA1C levels less than 8% and the other group having equal to or more than 8% HbA1C. We compared each group to their matched control (Table 3). For HbA1C <8%, all AST parameters were significantly delayed in the T1DM group compared to their control group (p<0.01). However, the CRT test was insignificant (p=0.287), showing that the test results were not different between the diabetic and control groups for CRT. For HbA1C ≥8%, for all the parameters of both tests (AST and CRT), the control group did significantly better on the tests compared to the diabetic group (p<0.01) as seen in Table 3. The results revealed that the cognitive functions significantly declined among patients with T1DM compared to their matched control group.
Table 3.
- Cognitive function test with type 1 diabetic patients categorized by hemoglobin A1c level and their matched control.
| Tests | T1DM group | Control group | P-values |
|---|---|---|---|
| HbA1C <8% (n=9) | |||
| AST mean correct latency (ms) | 985.69±186.47 | 501.71±170.85 | <0.001 |
| AST mean correct latency, congruent (ms) | 944.37±197.07 | 390.71±89.92 | <0.001 |
| AST mean correct latency, incongruent (ms) | 1028.81±176.39 | 528.86±179.23 | <0.001 |
| CRT mean correct latency (ms) | 476.90±70.97 | 446.86±118.04 | 0.287 |
| HbA1C ≥8% (n=23) | |||
| AST mean correct latency (ms) | 1014.01±179.00 | 485.14±148.17 | <0.001 |
| AST mean correct latency, congruent (ms) | 978.10±185.78 | 509.18±111.03 | <0.001 |
| AST mean correct latency, incongruent (ms) | 1052.83±174.45 | 445.09±128.93 | <0.001 |
| CRT mean correct latency (ms) | 533.63±120.82 | 398.86±69.48 | <0.001 |
Values are presented as mean ± standard deviation (SD).
T1DM: type 1 diabetes mellitus, HbA1C: hemoglobin A1C, AST: attention seeking test, CRT: choice reaction time, ms: milliseconds
For the duration of the T1DM disease, we had 3 categories. For each category, the results for diabetic patients and their matched controls were analyzed (Table 4). Patients who had T1DM for <5 years had a significantly decreased performance in all parameters of the AST test compared to their peers in the control group (p<0.01). Their CRT test performance was not significantly changed (Table 4).
Table 4.
- Cognitive function test with type 1 diabetic patients categorized by duration of diabetes and their matched controls.
| Duration of T1DM | T1DM group | Control group | P-values |
|---|---|---|---|
| <5 years (n=9) | |||
| AST mean correct latency (ms) | 991.45±125.25 | 558.67±171.32 | <0.001 |
| AST mean correct latency, congruent (ms) | 944.71±122.36 | 440.56±141.21 | <0.001 |
| AST mean correct latency, incongruent (ms) | 1039.03±127.84 | 496.22±177.89 | <0.001 |
| CRT mean correct latency (ms) | 462.04±76.06 | 418.44±105.34 | 0.373 |
| 5-10 years (n=20) | |||
| AST mean correct latency (ms) | 983.96±201.96 | 454.59±121.28 | 0.001 |
| AST mean correct latency, congruent (ms) | 947.24±208.97 | 516.18±90.70 | 0.001 |
| AST mean correct latency, incongruent (ms) | 1024.32±197.29 | 468.00±135.78 | 0.001 |
| CRT mean correct latency (ms) | 532.17±120.07 | 418.12±78.50 | 0.03 |
| >10 years (n=3) | |||
| AST mean correct latency (ms) | 1135.60±98.174 | 448.00±201.70 | 0.006 |
| AST mean correct latency, congruent (ms) | 1126.26±116.06 | 459.67±163.11 | 0.004 |
| AST mean correct latency, incongruent (ms) | 1146.00±78.70 | 379.00±41.24 | 0.001 |
| CRT mean correct latency (ms) | 546.46±143.91 | 364.00±37.51 | 0.101 |
Values are presented as mean ± standard deviation (SD).
T1DM: type 1 diabetes mellitus, AST: attention seeking test, CRT: choice reaction time, ms: milliseconds
Most of the patients had diabetes between 5-10 years. All these patients’ cognitive performance was significantly decreased than the control group in all parameters of both tests (AST [p<0.01], CRT [p=0.03]). There were only 3 patients in our study who had T1DM for more than 10 years. Compared to the control group, they all significantly scored lower in all the components of the AST test, but their CRT score no significant difference was observed in the CRT scores (p=0.101, Table 4).
Discussion
Worldwide, DM has high ranking importance in global healthcare organizations due to its rising prevalence and adopting the shape of pandemic-like conditions. Over the decades, the scientific literature has been highlighting the various complications of DM, however, the cognitive functions among school-going children and adolescents were not properly investigated. Cognitive dysfunctions have been considered as a major comorbidity of DM, which may result in a higher risk of hyper and hypoglycemic events. The present study established the impact of T1DM on cognitive function impairment among school-going children and adolescents. The results revealed that cognitive functions were reduced among children and adolescents with T1DM. Furthermore, high HbA1C and duration of diseases also impaired the cognitive functions among T1DM compared to their matched control. The cognitive performance of children with T1DM is reduced compared to their healthy peers. This is most probably due to poor metabolic control in children with T1DM Which adversely affects cognitive functions.11
Similarly, Kálcza-Jánosi et al12 established a link that T1DM was associated with delayed performance in cognitive domains. Tonoli et al13 reported that T1DM causes a decrease in cognitive performance related to non-diabetic controls. The findings suggest that cognitive function decline was more severe in adults, indicating that age and diabetes duration contribute to cognitive function impairment. Similarly, in the present study, we found that AST mean correct latency, AST mean correct latency (congruent), AST mean correct latency (incongruent), and CRT mean correct latency, were significantly delayed in the T1DM group.
The literature demonstrates a great harmony from the various cross-sectional studies that children and adolescents, with T1DM, have cognitive deficits compared to normal control subjects.14-16
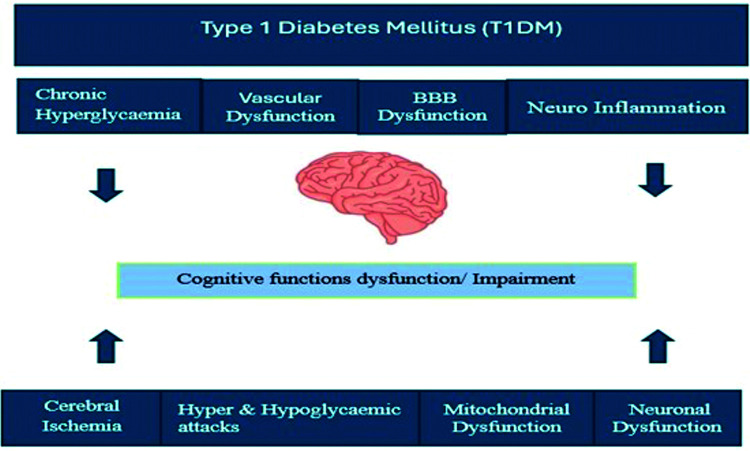
Over the last decade, the incidence of cognitive dysfunction in T1DM patients has been increasing and has been recognized as a major comorbidity of DM.17 Apart from classical vascular risk factors (namely, hypertension, atherosclerosis, micro and macroangiopathy), other factors have been involved in the pathogenesis of diabetes-related cognitive dysfunction. These factors include decreased beta-cell function, chronic hyperglycemia, repeated hyper and hypoglycemic attacks, vascular dysfunction, mitochondrial dysfunction, cerebral ischemia, neuro-inflammation, and neuronal dysfunction. All these factors are involved in the pathogenesis of cognitive dysfunction in diabetic patients (Figure 1).17-23
Figure 1.
- Pathophysiological mechanisms of type 1 diabetes mellitus and cognitive function impairment.
Study strengths & limitations
This study has some strengths. First, the study participants were matched based on their age, gender, weight, height, BMI, ethnicity, and educational and socioeconomic status. Second, the study findings are consistent with other studies on T1DM. Third, the results support the hypothesis that the duration of diseases and poor glycemic control was also related to cognitive function impairment in individuals with T1DM. There are a few limitations of this study. First, it was difficult to exclude some confounding factors such as genetics, environmental pollution, and more, which may influence cognitive function. Second, due to the cross-sectional design, causality could not be established.
In conclusion, the study findings demonstrate a delay in cognitive performance among children and adolescents with T1DM. The uncontrolled HbA1C and duration of disease were associated with reduced cognitive functions. The present study findings highlight the importance of stringent glycemic management in T1DM. The findings can support physicians and policymakers not only to prevent known microvascular or macrovascular complications but also to mitigate potential cognitive deficits among children and adolescents with T1DM. The clinicians must advise for cognitive functions assessment with other routine examinations, it will support the early diagnosis of any abnormal cognitive functions in children and young adults with T1DM. Moreover, understanding the cognitive functions of school-aged children and adolescents is instrumental in fostering comprehensive, efficient, and supportive educational environments in various cultural contexts. By understanding the school-aged children and adolescent’s cognitive profile, parents, and educators can implement targeted interventions, tailor instruction, and promote academic success while nurturing their overall well-being. Investing in cognitive function assessments is not merely on identifying deficits but on unlocking the potential of school-going children and adolescents ensuring they succeed academically, socially, and emotionally. Addressing the underlying cognitive concerns, parents, educators, and health professionals can support T1DM children and adolescents in developing confidence and a positive attitude towards learning. The prevention strategies and disease-modifying therapies can improve the long-term cognitive outcomes for children and adolescents with T1DM. A better understanding of the pathophysiological and molecular mechanisms underlying diabetes-related cognitive dysfunction could be critical for establishing effective prevention and intervention strategies. Further sizeable sample-sized studies would be carried out to further investigate the association between T1DM, duration of disease, glycemic control, and cognitive functions.
Acknowledgment
The authors gratefully acknowledge the grant from University Diabetes Centre, King Saud University, Riyadh, Saudi Arabia, for supporting this study. The authors also would like to thank Mrs. Hanan G. Al Hajji, Department of Physiology, College of Medicine, King Saud University, Riyadh, Saudi Arabia, for her help in the preparation of the figure and typing the Abstract, and Sofia Fields Author Services (www.sofiafields.com) for the English language editing.
Footnotes
References
- 1.Meo SA, Sheikh SA, Sattar K, Akram A, Hassan A, Meo AS, et al. . Prevalence of type 2 diabetes mellitus among men in the Middle East: a retrospective study. Am J Mens Health 2019; 13: 1557988319848577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.IDF. Facts and figures. [Updated 2021; accessed 2024 Apr 25]. Available from: https://idf.org/about-diabetes/diabetes-facts-figures/
- 3.Maahs DM, West NA, Lawrence JM, Mayer-Davis EJ. Epidemiology of type 1 diabetes. Endocrinol Metab Clin North Am 2010; 39: 481-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patterson CC, Dahlquist GG, Gyürüs E, Green A, Soltész G. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005-20: a multicentre prospective registration study. Lancet 2009; 373: 2027-2033. [DOI] [PubMed] [Google Scholar]
- 5.Kandemir N, Vuralli D, Ozon A, Gonc N, Ardicli D, Jalilova L, et al. . Epidemiology of type 1 diabetes mellitus in children and adolescents: a 50-year, single-center experience. J Diabetes 2024; 16: e13562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Li Y, Zhao K, Qin C, Chen Y, Liu Y, et al. . Abnormal cerebral blood flow and brain function in type 2 diabetes mellitus. Endocrine 2024; 85: 433-442. [DOI] [PubMed] [Google Scholar]
- 7.de Souza Stork S, Hübner M, Biehl E, Danielski LG, Bonfante S, Joaquim L, et al. . Diabetes exacerbates sepsis-induced neuroinflammation and brain mitochondrial dysfunction. Inflammation 2022; 45: 2352-2367. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Li X, Wang W, Shi G, Wu R, Guo L, et al. . Longitudinal associations of newly diagnosed prediabetes and diabetes with cognitive function among Chinese adults aged 45 years and older. J Diabetes Res 2022; 2022: 9458646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meo SA, Aldeghaither M, Alnaeem KA, Alabdullatif FS, Alzamil AF, Alshunaifi AI, et al. . Effect of motor vehicle pollution on lung function, fractional exhaled nitric oxide and cognitive function among school adolescents. Eur Rev Med Pharmacol Sci 2019; 23: 8678-8686. [DOI] [PubMed] [Google Scholar]
- 10.Cambridge Cognition Limited. Cantab research suite 6 test administration guide. [Updated 2014; accessed 2024 Apr 26]. Available from: https://cambridgecognition.com/
- 11.Kar Ş, Er E, Ata A, İnal-Kaleli İ, Özcan T, Köse S, et al. . Effect of metabolic control on cognitive functions in children and adolescents with type 1 diabetes mellitus. J Pediatr Endocrinol Metab 2023; 36: 636-642. [DOI] [PubMed] [Google Scholar]
- 12.Kálcza-Jánosi K, Lukács A, Barkai L, Szamosközi I. [Cognitive functions in type 1 and type 2 diabetes. Meta-analysis]. Orv Hetil 2013; 154: 694-699. [In Hungarian] [DOI] [PubMed] [Google Scholar]
- 13.Tonoli C, Heyman E, Roelands B, Pattyn N, Buyse L, Piacentini MF, et al. . Type 1 diabetes-associated cognitive decline: a meta-analysis and update of the current literature. J Diabetes 2014; 6: 499-513. [DOI] [PubMed] [Google Scholar]
- 14.Brands AM, Biessels GJ, de Haan EH, Kappelle LJ, Kessels RP. The effects of type 1 diabetes on cognitive performance: a meta-analysis. Diabetes Care 2005; 28: 726-735. [DOI] [PubMed] [Google Scholar]
- 15.Johnston H, McCrimmon R, Petrie J, Astell A. An estimate of lifetime cognitive change and its relationship with diabetes health in older adults with type 1 diabetes: preliminary results. Behav Neurol 2010; 23: 165-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohmann S, Popow C, Rami B, König M, Blaas S, Fliri C, et al. . Cognitive functions and glycemic control in children and adolescents with type 1 diabetes. Psychol Med 2010; 40: 95-103. [DOI] [PubMed] [Google Scholar]
- 17.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 2018; 14: 591-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold SE, Arvanitakis Z, Macauley-Rambach SL, Koenig AM, Wang HY, Ahima RS, et al. . Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat Rev Neurol 2018; 14: 168-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaspar JM, Baptista FI, Macedo MP, Ambrósio AF. Inside the diabetic brain: role of different players involved in cognitive decline. ACS Chem Neurosci 2016; 7: 131-142. [DOI] [PubMed] [Google Scholar]
- 20.Feinkohl I, Aung PP, Keller M, Robertson CM, Morling JR, McLachlan S, et al. . Severe hypoglycemia and cognitive decline in older people with type 2 diabetes: the Edinburgh type 2 diabetes study. Diabetes Care 2014; 37: 507-515. [DOI] [PubMed] [Google Scholar]
- 21.Geijselaers SLC, Sep SJS, Stehouwer CDA, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol 2015; 3: 75-89. [DOI] [PubMed] [Google Scholar]
- 22.Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, et al. . Glucose levels and risk of dementia. N Engl J Med 2013; 369: 540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rawlings AM, Sharrett AR, Mosley TH, Ballew SH, Deal JA, Selvin E. Glucose peaks and the risk of dementia and 20-year cognitive decline. Diabetes Care 2017; 40: 879-886. [DOI] [PMC free article] [PubMed] [Google Scholar]