Abstract
Division of labor, the specialization of sometimes phenotypically divergent cell types or group members, is often associated with ecological success in eukaryotic colonial organisms. Despite its many independent evolutionary origins, how division of labor emerges remains unclear. Conventional hypotheses tend toward an “economic” model, so that biological division of labor may reflect a partitioning of preexisting tasks and morphologies into specialized colony members. Here, we present an alternative model of the origin of division of labor, which can explain the evolution of new functions within a colony. We show that in colonies of the Cretaceous aged (103–96 Ma) fossil bryozoan of the genus Wilbertopora, the first cheilostome bryozoan to evolve polymorphism, preexisting morphologies were not simply partitioned among new members, but instead expanded into novel morphospace as they lost functions, specifically feeding. This expansion occurred primarily during two pulses of heightened morphological disparity, suggesting that the evolution of polymorphism corresponded to relaxed constraints on morphology and perhaps to the exploration of novel functions. Using a simple model of physiological connections, we show that regardless of the functionality of these new colony members, all nonfeeding members could have been supported by neighboring feeding members. This suggests that geometric constraints and physiological connectedness could be prerequisites for evolving both polymorphism and division of labor in modular organisms, and that a classic partitioning model of specialization cannot be broadly applied to biological systems.
Significance Statement.
Division of labor and polymorphism, where phenotypically distinct members of a group specialize on tasks, are found in some ants and other colonial invertebrates, but their evolutionary sequence has never been observed. Here, we used the fossil record to uncover the early evolutionary stages in the division of labor of the bryozoan Wilbertopora. The morphological and functional changes in the earliest Wilbertopora colonies show that new polymorphism originated outside ancestral ranges of variation, and their divergence increased as one set lost ancestral functions, including the ability to feed. We found that physiological connectedness of these colonies could have accommodated nonfeeding, functionless members, thus bridging the gap between the evolution of polymorphic modules and the development of division of labor.
Introduction
The separation of tasks and morphologies among individuals within colonial eukaryotic life (termed “division of labor”) is widespread today, suggesting that this way of life confers many ecological advantages. Division of labor has evolved repeatedly in both marine and terrestrial organisms, and has been tied to the ecological and evolutionary successes of disparate clades, including ants, bees, siphonophores, hydrozoans, and bryozoans (1–4). Specialization of tasks among colony members is thought to increase the energetic efficiency of the entire colony, and thus becomes an emergent feature upon which selection acts at evolutionary scales (5, 6). Conventional biological hypotheses for the evolution of division of labor parallel certain principles of economics, where preexisting tasks once performed by generalists become partitioned among specialists (7, 8). This model of partitioning does not explain how the many emergent features of colonial and eusocial division of labor evolved from their ancestral states: bridge-making in ants (9) and the antipredatory bird's head avicularia of the bryozoan Bugula (10) are both examples of behaviors or functions that are not present in their monomorphic ancestors. More recent theoretical evidence suggests that the division of labor can evolve without total specialization, instead persisting through the maintenance of functional generalists (11). Here, we build and test a model of functional and morphological expansion against the classic partitioning model to show that there are multiple, viable pathways for the evolution of division of labor.
There is mixed evidence for the order in which functional and morphological changes occur in the evolution of division labor, as the evolutionary sequence has never been directly observed. Modern eusocial insects are thought to undergo functional change before morphological differentiation (2, 12), while at molecular levels, genetic variation typically precedes functional innovation (13). For example, in gene duplication, one copy maintains ancestral functions while the other can evidently accommodate mutations (and thus eventually accrue new functions) (13). Modules in colonial animals may show similar dynamics, where some colony members maintain ancestral functions (14) and others see increased variation while maintaining viability owing to physiological connections with the rest of the colony (direct gut-to-gut connections in bryozoans or communal feeding in ants) (10, 15). To test the relative timing and roles of functional and morphological change to the evolution of division of labor, we couple direct observations on the functional and morphological variation in an evolutionary time series of a fossil bryozoan lineage with a model of energetic flux. We show that division of labor is not simply a pattern of specialization on preexisting functional or morphological variation, and that physiological connections in ancestral colonies may be a prerequisite for this evolutionary pathway, as they permit colonies to share surplus energy with nonfeeding members.
Testing evolutionary models for the division of labor in bryozoans
Many cheilostome bryozoans today have evolved division of labor, where their “zooids”, or colony members, perform specialized tasks within the colony and express different morphologies, termed polymorphism (3, 15–18). The most common polymorphs include: autozooids that feed and produce gametes, ovicells that brood embryos (19), and avicularia that perform varied tasks from defense to hygiene ((15), Fig. 1). The evolutionary success of cheilostomes through the Cenozoic has been tied to the development of these polymorphs in the Cretaceous genus Wilbertopora, 103 Million years ago (Mya) (20, 21), when avicularia emerged from autozooids, and ovicells formed as composite structures from spine-like zooids (22–24). Thus, fossil colonies of Wilbertopora offer critical empirical evidence for these early evolutionary steps toward division of labor in cheilostomes.
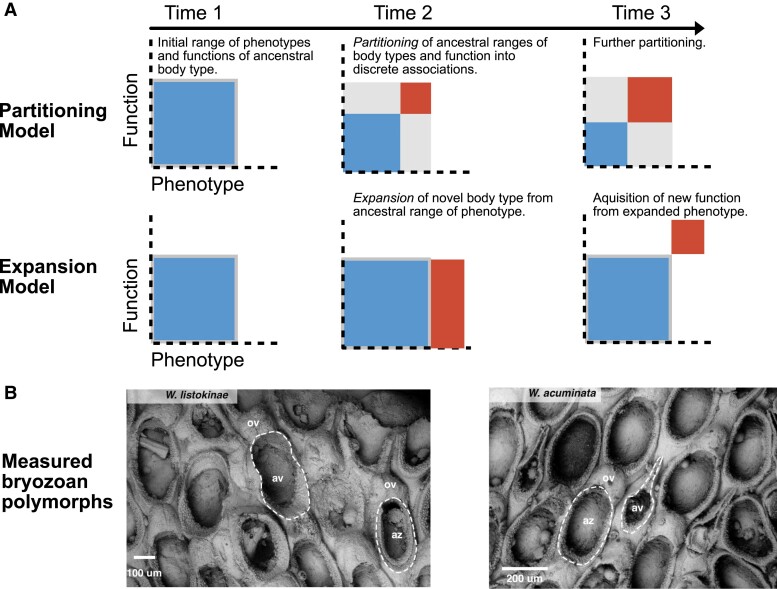
Fig. 1.
A) Two potential patterns of phenotypic and functional evolution in colonial organisms. In the case of Wilbertopora, squares labeled with “Az” represent the ancestral body type (autozooids); squares labeled with “Av” represent the novel polymorph (avicularia). In the partitioning scenario, avicularia evolve through the partitioning of preexisting functions and variation in Wilbertopora. The range of autozooid functions and morphologies decreases as avicularia take on particular functions that used to be performed by autozooids. The total amount of variation in a colony remains similar to that of the ancestral state, but this variation becomes split between autozooids and avicularia. In the expansion scenario, avicularia evolve as an extension of autozooidal variation, expanding the range of variation in a colony. Avicularia then lose autozooid functions and become adapted to completely new functions in the colony. B) Polymorphs within two Cretaceous species of Wilbertopora. Wilbertopora listokinae, Cheetham et al. (22) (USNM PAL 216175), showing an autozooid (az) and avicularium (av) with ovicells (ov). Wilbertopora acuminata, Cheetham et al. (USNM PAL 216143).
Polymorphs in extant bryozoans can have discrete and continuous differences in both the types and ranges of their morphologies, which reflect differences in the mode and range of their functions (15, 25). In extant colonies and lineages, autozooids and ovicells have constrained morphologies, corresponding to their functional requirements—avicularia show greater morphological variation, perhaps reflecting the many tasks they can perform (26). Therefore, we measured differences in the morphologies of Wilbertopora polymorphs to test whether the evolution of colonies more closely followed a model of partitioning or expansion. Under the partitioning model, avicularia would have expressed morphologies within the range of autozooids, and thus would have decreased in disparity (Fig. 1A). Under the expansion model, avicularian morphology would have evolved outside of the autozooidal range (Fig. 1A), which may or may not have also involved changes to the disparities of either polymorph. We can further constrain the effect of shared functions on the evolutionary morphology in these early colonies by comparing the morphologies of avicularia that lacked the ability to support ovicells to those that had functioning ovicells—these avicularia would have had a functional lophophore and therefore the ability to feed and produce gametes (23) (See Discussion in Supplementary Material, Text Section S7). As avicularia lost these functional ovicells within Wilbertopora, we would expect colonies to show increasing morphological differences between avicularia and autozooids.
Beyond the potential morphological consequences of functional release, the loss of functional ovicells on avicularia would have presented a new metabolic challenge to the later colonies of Wilbertopora. The inability for certain avicularia to feed would have required energetic support from the rest of the colony. To test whether an expansion model for the division of labor could have accommodated an initial loss of function for some colony members, we modeled the energy flux through a set of different colony arrangements to determine if, in theory, observed geometries and physiological connections could have supported a colony‘s total energy budget. We expect that colonies with a lower fraction of feeding members will show a decline in their potential energy acquisition, but that the contribution of each feeding member to the energetic output of the colony should increase. We discuss the implications of the new, multiserial network of zooids on the energy flux for colonies of Wilbertora compared with uniserial forms. In combining these models of energy flux with the analyses of morphological and functional change, we present the fossil record of Wilbertopora bryozoans as one of the first empirical tests (27, 28) of different models for the evolution of division of labor.
Materials and methods
Specimen sampling
All specimens used in this study are housed at the National Museum of Natural History in Washington, D.C. Specimens were collected primarily between 1937 and 1946, with some additional specimens collected in the 1990s, from outcrops of the Washita Group located in North-central Texas (29–31). The Washita group is made up of eight formations (Kiamichi, Duck Creek, Fort Worth, Denton, Weno, Paw Paw, Main Street, and Grayson), with a ninth formation, the Georgetown Formation, replacing the upper half of the Main Street Formation at localities in the southern portions of the sample area (Travis and McLennan Counties) (23). The Washita group is located in north-central Texas and eastern Oklahoma and formed as a result of transgressive–regressive cycles of the epicontinental sea that made up the Western Interior Seaway. We use specimens from these nine conformable units, ranging in age from ∼103 to 96 Ma (31–33).
While species identification was recorded for the dataset, our analysis considered colony-level, and not species-level, variation, so that occurrences across the formations in the Washita group can be considered as a single time series. Species are largely defined by their avicularian morphology, meaning that any cladistic analysis would need to use the same traits to build a phylogeny and to conduct comparative analysis. This makes it difficult to independently consider cladistic evolution and morphological differentiation of avicularia. Additionally, Cheetham et al. (23) hypothesized that there may be ghost lineages in Wilbertopora, making it difficult to estimate rates of trait evolution. Therefore,, for this study, we focused on trait differences between successive geologic formations (time) rather than rates of evolutionary change, allowing us to analyze a greater number of colonies than used in Cheetham et al. All species considered in this analysis were verifiably descended from a recent common ancestor (23, 34, 35). More information on species categorization can be found in the Supplementary Material, Text Sections S1 and S5. Museum registration numbers can be found in Dataset S1.
Digitization and quantification of zooid orifice and opesia morphology
Bryozoan colonies grow on irregular surfaces, making repeatable capture of the orifice and opesia shape in the orificial plane difficult in two dimensions. Therefore, two approaches were taken to minimize the effect of parallax on the orifice and opesia shape: (ⅰ) micro-CT scanning to create three-dimensional (3D) models for colonies growing on curved surfaces, and (ⅱ) microscope photography of colonies growing on flat surfaces. Specific descriptions of the steps followed for the acquisition of both micro-CT scans and photographs can be found in the Supplementary Material, Text Section S2. We collected outlines of the orifice and opesia of autozooids and avicularia within colonies using “Pick Points” in Meshlab (36). Each outline was collected in a clockwise direction with two landmarks placed at the most proximal and distal part of the orifice and opesia. Landmarks were placed on photographs using FIJI (37) and scaled from pixels to millimeters to match units of the 3D mesh data.
Outlines from the 3D mesh data were projected onto the orificial plane to match the 2D outlines derived from photographs. Fifty equally spaced semilandmarks were placed along the curve defined by the initial outline points using a spline. Semilandmarks were then slid to minimize bending energy and reduce artifactual differences in shape driven by their initial, equidistant placement (38–40). The semilandmark configurations were centered on their respective centroids and aligned using the distal and proximal landmarks defining the zooid growth axis. The dimensionality of these aligned semilandmark configurations was reduced using principal components analysis (PCA, Fig. S2), retaining the first nine principal components (PCs) explaining 95% of the total variance in shape data for downstream analyses. The differences in avicularia and autozooid morphology among colonies through time was tested via a residual randomization permutation procedure (using the R function RRPP::lm.rrpp (41)) on a linear model of PC scores against zooid type interacting with time (midpoint in Mya per formation).
Estimation of morphological divergence between zooid types
To test for differences in the shapes of zooid types through time, Euclidean distances were calculated between the predicted mean autozooid shape in the first time interval to the predicted mean shapes for each zooid types per time interval across the 999 coefficients fit via the RRPP procedure (uncertainty expressed as the 95% CI). The differences in avicularia and autozooid morphology within colonies through time was tested by first finding the Euclidean distance between the mean shapes of zooid types within colonies (distances based on mean and median morphologies were similar, Fig. S4), and then regressing that distance against time (model 4 in the Supplementary Material, Text, Fig. S5, Table S4).
Estimation of disparity
Shape disparity for each zooid type across colonies was estimated using the R function geomorph::morphol.disparity (38), and temporal trends were evaluated by regressing disparity across each time interval using RRPP:lm.rrpp (model 5, Fig. S7, Table S5). Shape disparity for each zooid type within colonies was estimated and regressed against time for each interval (model 6, Fig. S8, Table S6). The regression model (model 6) calculates colony-level disparity across zooid types within colonies, and regresses those disparities against time (Fig. S8, Table S6).
Avicularian ovicells and their relationship to divergence and disparity
Ovicells that grow on avicularia suggest that some avicularia retain ancestral autozooidal functions. For specific discussion of the significance of avicularian ovicells, see Supplementary Material, Text Section S7. Colonies with ovicells were checked for the presence of ovicells on avicularia using meshes and photographs. Colonies that lack ovicells or fall below a certain size (<30 zooids) were excluded from this portion of the analysis because smaller colonies may not have reached sexual maturity yet. For each colony, all zooids were counted to estimate colony size, and ovicells were counted to estimate ovicell frequency.
Linear modeling was used to determine whether there is a significant relationship between divergence within colonies and the presence or absence of avicularian ovicells in colonies (model 7, Table S7). Linear modeling was also used to determine whether there is a significant relationship between avicularian disparity within colonies and the presence of avicularian ovicells within colonies (model 8, Table S8). To estimate whether ovicell frequency differs between colonies that possess ovicells on avicularia and those that lack them, ovicell frequency was regressed against the status of avicularian ovicells (present, absent) in colonies (model 9, Table S9).
Modeling energetic flux through a colony
To model energy flux through a colony, we created an incidence matrix (A) where each zooid in a colony is a node (n), connected to each other as edges (m). Each element of A is assigned based on whether each edge is pointing toward (+1) or away (−1) from each respective node, reflecting the directional flow of energy through the network of the colony. We define a source vector (s) of length n, and a flow vector (f) of length m, which we can use to create an equation for flow conservation:
We also define a potential vector (v) of length n, and we create an resistance matrix (R). We fix the potential of each node depending on whether the corresponding zooid can feed. For nonfeeding zooids, the potential is set to 0, and for feeding zooids it is set to 1. R is a diagonal matrix (), for a vector r, where is the resistance along edge i. We can thus model diffusion through the equation:
Finally, we specify the potential with the matrix–vector equation:
In this equation, I is the identity matrix, and w collects the values above (+1 or 0 for feeders and nonfeeders, respectively).
Taking these three equations together, we can solve for the source vector (s) and the flow vector (f). For full details and the code of our model, see Supplementary Material, Text Section S8.
We interpret the source vector s biologically as describing the pattern of the dearth or excess of energy for each zooid within the colony. Negative elements of s represent the energy sinks of nonfeeding members. Elements that are either positive or zero represents feeding members of the colony. We interpret the positive elements of s as the excess energy required to support nonfeeding members. Conditioned on the assumption that all zooids in the colonies we quantify were alive, we can estimate three quantities: (A) the excess energy acquired by the colony, (B) the average energetic benefit per zooid, and (C) the average energetic cost of nonfeeding members. We define A to be the sum of all nonnegative elements of s, B to be the average of all nonnegative elements of s, and C to be the average of all negative elements of s.
The specific values of the potential vector v and the resistance matrix R can be modified with this approach. We fixed them here to make all colonies comparable. For resistance we set for all zooids, which represent a biological assumption that all zooids have equal ability to move or receive energy from neighbors. This assumption is based on prior empirical study of pore chamber connections between zooids, which determined that polymorphic colonies have similar connections between all individuals as in monomorphic colonies (42). For the potentials, we set all feeders to equal 1 and all nonfeeders to equal 0. One can envision alternative schemes, where perhaps vicarious avicularia have larger potentials than do adventitious avicularia because vicarious avicularia can bud asexually while the latter cannot.
Results and discussion
Evolutionary morphology of avicularia
Consistent with the expectations of the expansion model, zooid polymorphs of Wilbertopora evolved increasingly distinct morphologies through time, with avicularia entering new regions of morphospace relative to autozooids both for the genus as a whole and within individual colonies (Fig. 2A and B, Tables S3 and S4; modeled estimates of shape divergence with 95% CI shown in Fig. S5). Changes in zooid shape, not size, primarily drove this morphological divergence (Tables S1 and S2). Differences in polymorph shapes were mostly derived from changes in avicularian morphology, given that autozooids maintained a similar position and volume of morphospace through the study interval (Fig. 2 and Fig.S3). The increased shape divergence between autozooids and avicularia may be underlain by the evolution of new species, as many of the colonies occupying distinct regions of the morphospace are described as distinct species (inferring evolutionary transitions is a reasonable hypothesis, but note that fossil bryozoan species are delimited by the shapes of their polymorphs) (23) (Figs. S6 and S9). Regardless of whether these shape divergences resulted from species-level evolutionary events, the net result is an expansion of the earliest expressions of zooid shapes, not a simple partitioning of early variation.
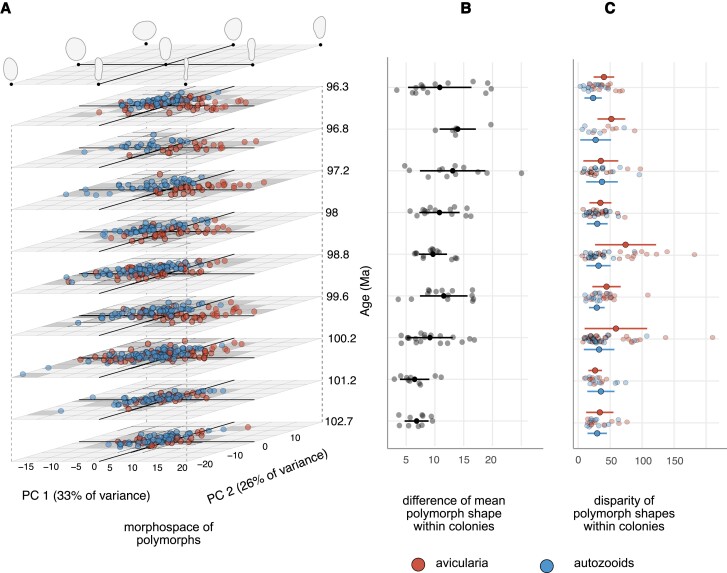
Fig. 2.
Morphological evolution of polymorph shape (as outlines of a zooid's orifice and opesia, which together comprise the frontal area of a zooid) in colonies of Wilbertopora. A) Morphospace of polymorphs defined by first two principal components (PCs). Each point represents a single zooid. B) Difference in mean shapes of autozooids and avicularia within colonies. Points and bars show mean and SD of values, respectively, within time intervals. C) Shape disparity of autozooids and avicularia within colonies; solid points with bars show mean and SD among colonies per time interval. Time slices show the midpoint of stratigraphic formations, but their relative spacing is uniform for visualization purposes.
Within the expansion model, it is possible for both the old and new polymorphs to show reduced morphological variation, possibly reflecting specialization on fewer tasks. However, the Wilbertopora fossil series shows a general similarity and stability in the morphological variation of zooids within colonies through time, but with two notable exceptions (at 100.2 Ma and 98.8 Mya; Fig. 2A and C, Figs. S7 and S8). In the first half of the time series, two pulses in avicularian disparity correspond to the acquisition of novel elongate forms and the retention of the ovate autozooidal-like forms among colonies (Fig. 2A and C). Neither of these pulses is likely to be an aberration within the dataset, as they do not coincide with known environmental changes (i.e. ocean anoxic events (43, 44)) nor dramatic changes in depositional environment (32). The subsequent decline in avicularian disparity to prepulse levels corresponds to the decrease in frequency of autozooidal-like forms, and consequently the shift in shape divergence between these two polymorphs (Fig. 3). Thus, Wilbertopora evidently fits the expansion model for the evolution of its polymorphs, with avicularia expressing novel morphologies and higher levels of within-colony morphological variation than autozooids, suggesting that changes in the function of avicularia may have also been underway through the study interval.
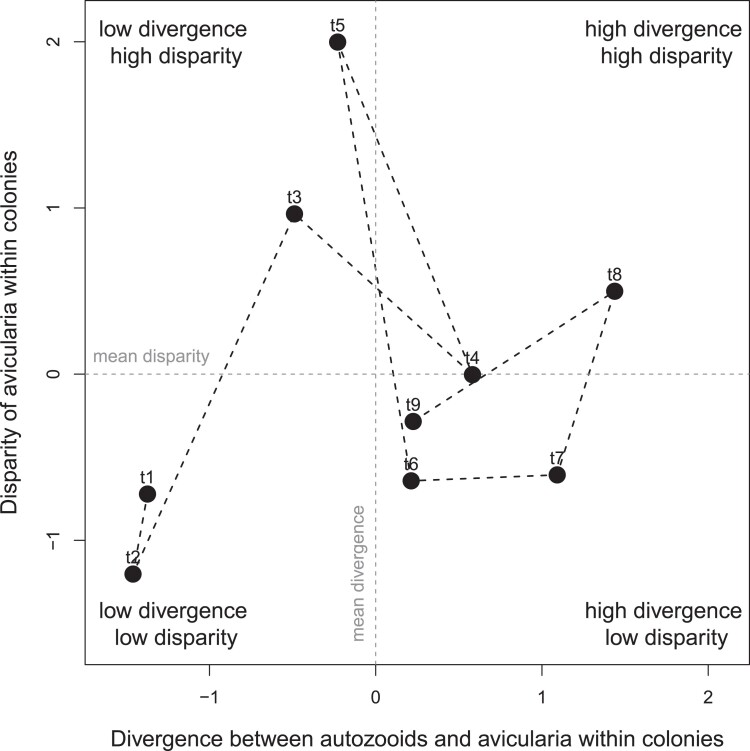
Fig. 3.
Mean-centered and scaled (Z-scores) divergence of autozooidal and avicularian morphologies compared to avicularian disparity within colonies over time. The time series progresses from time t1 to t9, with each labeled point representing a subsequent formation in the Washita group (see midpoints of time intervals in Fig. 2). The intervals with high avicularian disparity tend to precede intervals with high divergence between autozooids and avicularia within colonies.
The greatest shape divergence between polymorphs occurred within colonies that contained avicularia lacking autozooidal functions, as evidenced from the lack of avicularian ovicells (Fig. 4A, Table S7, following the interpretations of functional avicularian ovicells laid out by Cheetham et al. (23)). Even in colonies where avicularia could have supported ovicells, avicularia lacking ovicells were morphologically distinct from those associated with ovicells and from autozooids (model 10, Table S10). This shape divergence suggests that avicularia, which were derived from autozooids, were apparently released from phenotypic constraints tied to autozooidal functions. Furthermore, the disparity of avicularian shapes tended to be higher within colonies that lacked avicularian ovicells than in those that possessed them (Fig. 4B, Table S8), consistent with the idea that early avicularia might be vestigial autozooids derived from functional release (26, 45–47).
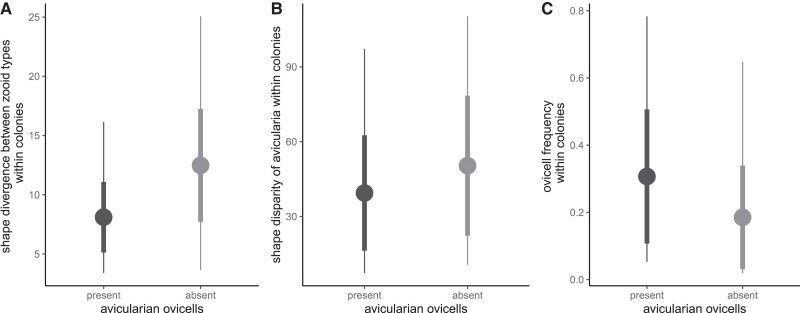
Fig. 4.
Comparing A) divergence, B) disparity, and C) ovicell frequency between Wilbertopora colonies with and without avicularian ovicells. Points show the mean value, thick line segments show standard deviation (SD), and thin line segments show the range of values. Divergence is significantly different between groups (Table S7, P < 0.01), disparity is marginally different between groups (Table S8, P ∼ 0.1), and ovicell frequency is significantly different between groups (Table S9, P < 0.01). Results here show colonies with more than 30 zooids and have at least one ovicell.
Modular developmental decoupling of autozooids and avicularia (48) may have allowed for selection to act independently on each polymorph, ultimately reenforcing morphological and functional differences. The loss of ovicells on avicularia in certain colonies also corresponded to a reduction in the overall frequency of ovicells across the colony (Fig. 4A and C, Tables S7 and S9). Such shifts in the frequency of reproductive individuals in a colony are seen in other colonial, social, and multicellular organisms in the form of germline sequestration as evolutionary potential emerges at new hierarchical levels of organization (49–51).
Taken together, these results show three key features of Wilbertopora evolution: (ⅰ) avicularia evolved novel morphologies and were not a simple partitioning of preexisting autozooid morphologies, (ⅱ) high pulses of disparity among early avicularia suggest an initial relaxation of their functional constraints compared with autozooids, and (ⅲ) the divergent morphologies of avicularia potentially reflected selection on avicularia for multiple novel functions rather than specialization on preexisting ones. We propose that avicularia may have first appeared as a developmental aberration of autozooids in colonies. Then, the pulses of heightened disparity in avicularian morphology represented a transitional phase away from their initially shared functions with autozooids, which remained constrained as the sole reproductive members of the colony. During this interval, avicularia may have begun exploring a wide range of novel functions, serving as a type of generalist polymorph (11), rather than a specialized one. As an analog, gene duplications often result in similar transitional phases where initial variation in one gene copy is neutral but is later exapted to serve novel functions (52). Early avicularia may have expressed similarly neutral variation, but this scenario requires an additional mechanism to explain the persistence of potentially nonfunctional colony members. Thus, we consider an evolutionary pathway where even nonfunctional avicularia could have evolved in colonies despite their apparent energetic cost. This pathway may explain why avicularia had different morphologies among colonies as well as relatively high disparity within them.
Linking energetic controls to polymorphism and division of labor
Wilbertopora is one of the first cheilostome bryozoans to exhibit exclusively multiserial growth (3, 17), which unlocked many potential arrangements of members within colonies. Prior to Wilbertopora, cheilostome colonies were primarily uniserial or pluriserial and formed a diffuse network of branches (22) (but see one possible exception in Conopeum (53)). Zooids in modern uniserial colonies connect to two or three other zooids at most, and commonly die off after sexual and asexual reproduction, making the colony susceptible to overgrowth by competitors (17). In contrast, the zooids of Wilbertopora colonies form a two-dimensional sheet through their multiserial growth, which presents a different suite of geometric and physiological challenges (Fig. 1).
Cheilostome zooids stop growing after asexual budding, becoming locked in the interior of the colony, and have with few further energetic outlets, unlike cyclostome zooids, which can continue to grow via calcification after asexual budding (54). Thus, multiserial growth leads to tightly packed zooids, increasing feeding efficiency by multiplying the per-zooid acquisition of food over that seen in uniserial colonies (55, 56). As a multiserial colony grows, its number of zooids scales with its surface area, while the growing edge scales with its circumference. Given that the area of a circle increases faster than its circumference, like the ratio of a sphere's volume to its surface area (57), this nonlinear relationship has metabolic consequences. When all zooids in a colony can feed, the energy required by the growing edge of a colony is exceeded quadratically by the number of feeders in its interior. Nonfeeding zooids can be supported by the colony once it reaches a certain size, and may serve as a sink for otherwise unutilized energetic resources. This would allow colonies to utilize more of the nutrients taken up by autozooids, and would increase the flux of energy through the colony.
We quantified the potential energy flux through a colony by modeling the patterns of nutrient diffusion that are expected to occur given the observed network of zooids (Fig. 5, see Supplementary Material, Text Section S8). In our model, zooids are networked in what is essentially a bucket brigade, where energy is acquired by feeding zooids, used locally for maintenance, and the excess is passed diffusively from zooid to zooid toward nonfeeding polymorphic zooids and the growing edge, which both act as energy sinks. We ask if the network structure is sufficient to nourish nonfeeding members and if the presence of nonfeeding members throughout the network increases the energy flux within the colony. As with a bucket brigade, this requires the smooth hand-off of energy from zooid to zooid.
Fig. 5.
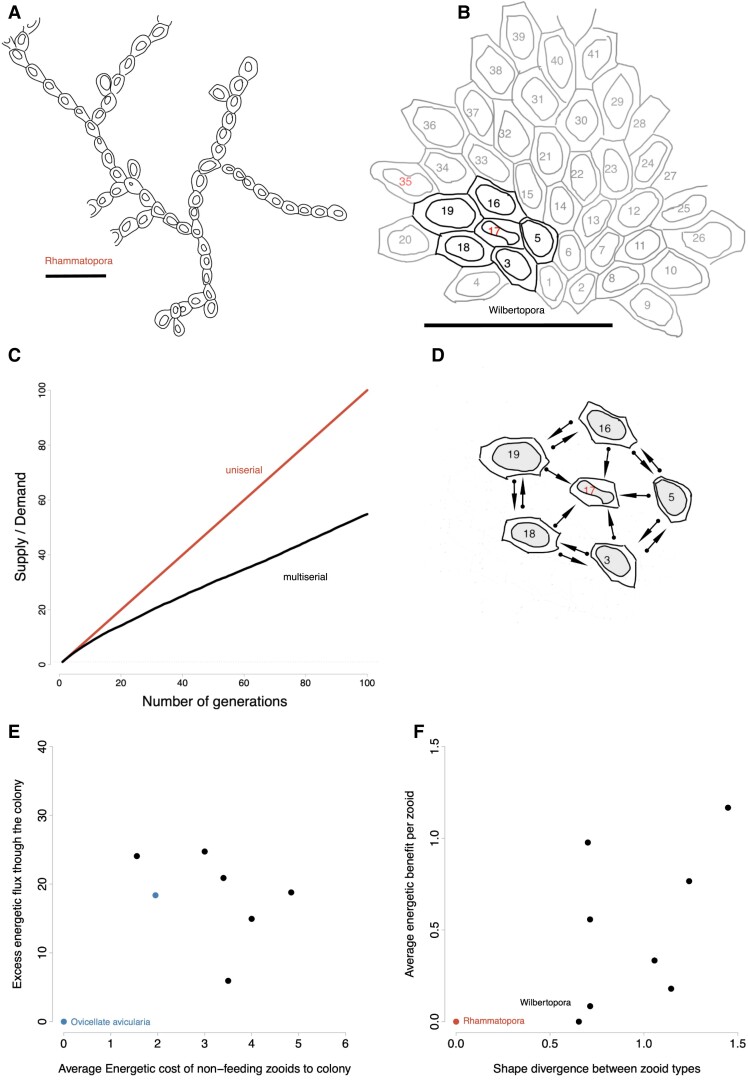
Bryozoan colonies vary in growth form and therefore in their network of physiological connections. Growth forms illustrated for A) the uniserial Rhammatopora (PI BZ 8149) and B) multiserial Wilbertopora (USNMPAL 186572). The different budding geometries of each genus lead to different ratios of feeding zooids to growing-edge zooids. Autozooids (feeding zooids) are denoted with “Az” and avicularia (non-feeding zooids) are denoted with “Av”. C) The supply/demand ratio within a colony is a function of the number of feeding zooids and the number of newly budded zooids. Multiserial growth, exhibited in Wilbertopora, generates a smaller energy surplus than uniserial growth, indicating higher colony efficiency. The presence of more nonfeeding polymorphs reduces the energy surplus in colonies and works to equilibrate energetic supply with demand. D) An example of an incidence matrix showing physiological connections for a colony of Wilbertopora. Zooids with red labels in B and D are nonfeeding avicularia, zooids with black labels denote feeding autozooids. The funicular system allows the transport of nutrients between zooids. Feeding zooids can transport nutrients between each other, but nonfeeding avicularia only receive nutrients from neighbors. Arrows indicate directions of energy exchange. We model diffusion of nutrients along the graph of the colony to evaluate if the colony can support extra nonfeeding zooids. We find that adding polymorphic nonfeeders within a colony increases the E) total colony flux of energy and F) average per zooid energy benefit increases with the presence of nonfeeding zooids and their divergence. The contemporaneous genus Rhammatopora only has feeding zooids and is plotted as a point of comparison.
We scored incidence matrices (an n × m matrix with n nodes and m edges) for exemplar colonies of Wilbertopora species, in order to quantify these patterns of energy flow through a colony. We find that the cost of nonfeeding members is easily met by preexisting feeding members. As a result, older zooids in the colony serve to increase supply and substantially increase the total energy flux through the colony: up to 100 times the flux per colony (Fig. 5C) and up to 1.25 times the flux per autozooid in a colony (Fig. 5D).
Avicularia can be supported by autozooids that produce more energy than required by the growing edge of the colony and maintenance of existing zooids, even when avicularia serve no distinct functional role. Thus, losses of function can precede any gains, disconnecting the emergence of polymorphs from division of labor. The energetic surplus due to multiserial growth releases nonfeeding members from these particular functional constraints and permits them to vary, much as one copy of a duplicated gene is free to vary. After colonies pass through this phase of functional loss, evidenced by the decline in avicularia having ovicells (Fig. 4C), we observe distinct regions of morphospace occupied by different species of Wilbertopora (Fig. 2A). The avicularian phenotypes of those species are diverse and already resemble phenotypes of modern avicularia with disparate functions (23, 58). As with gene duplication, independent lineages may follow different evolutionary paths. This result explains why it is so difficult to pinpoint a single ancestral function of avicularia, because the later species of Wilbertopora already possess avicularian forms similar to the general classes of avicularian types observed today (15).
Energy sinks also promote increased uptake of nutrients from the water column, which along with increased feeding efficiency (55), may serve to deplete food resources for competing encrusting animals (59, 60). Furthermore, this energetic surplus may explain how brooding behavior, a costly reproductive strategy (61), has become the dominant mode of reproduction in cheilostomes, as well as how subsequent evolutionary novelties in cheilostomes have evolved, such as multizooidal budding and frontal avicularia. Thus, the energetics afforded to cheilostomes from their multiserial growth may underlie their ecologic and taxonomic diversification from the Cretaceous to today (20, 21), adding energy flux to their ecological strategies that include varieties of flow patterns (62), feeding efficiencies (55), budding patterns (17), and polymorph frequencies (56, 63).
Conclusions
Multiple pathways for the evolution of division of labor
Colonial organisms consisting of a single member-type are limited to fewer life-history strategies than polymorphic colonies. Despite this scaling of polymorph frequencies with the variety of life-history strategies (63), corresponding tradeoffs between energetic flux through a colony and its investment in growth and reproduction have resulted in a range of ecological strategies. Together, these two factors may have contributed to heightened speciation rates and extinction rates as cheiliostome lineages established their complex competitive networks, including their nontransitive overgrowth interactions (64).
The energetic underpinnings of polymorphism in cheilostomes may offer insight into the polymorphic expression, or the lack thereof, in other colonial groups. In ants, foraging/hunting worker castes acquire surplus energetic resources that can sustain defensive and reproductive caste types (2); siphonophores and hydrozoans share energy and nutrients through physiological connections between colony members, supporting polymorphs that specialize in mobility and defense (4, 65). In these connected modular systems, the evolutionary origin of polymorphism may be similar to the process we observed in cheilostomes, where developmental aberrations did not compromise fitness, permitting novel modules to arise and serve new functions. This process might explain why some hydrozoan and siphonophore polymorphs, like tentaculozooids (65, 66), serve functions not present in monomorphic or solitary species, making them likely candidates for the expansion model.
Some colonial or subcolonial organisms, such as photosymbiotic corals and endosymbiotic arthropods, generate surplus energy but lack polymorphism (67–69). Such sustained monomorphism may be related to a limited repertoire of reproductive strategies that generally excludes extended parental care (69, 70), which is often considered a precursor to division of labor (71). The brooding and feeding of offspring through most larval and juvenile stages increases energetic flux through a colony, and this habit appears simultaneously with polymorphism in cheilostome bryozoans (17, 22, 49). Thus, the strength of selection on reproductive strategies, coupled with the capacity to acquire surplus energy, may modulate the development of polymorphs and division of labor in colonial organisms.
Polymorphism has long held an air of mystery. It is simultaneously associated with highly successful clades of colonial organisms, but it is also relatively rare across taxa (49). Economic ideas of specialization have strongly influenced evolutionary thinking (72) on division of labor, namely that functional loss, such as altruism, is difficult to evolve (2, 73, 74), but equating morphological polymorphism with division of labor has led to little progress in understanding either. Darwin's discussion of polymorphism reads as remarkably modern (75) because it fixates on the functional gains rather than any potential for intermediate losses, underscoring how little progress we have made toward understanding the dynamics of this evolutionary phenomenon. Equating polymorphism with economic division of labor has confounded our insight into how functions can evolve (76), hiding how the developmental origins of novel features can be distinct from their role in ecological innovation (77). We find that phenotypic polymorphism within colonies is best thought of as any other biological novelty: how new forms developmentally arise and what ecological advantages they initially may, or may not, provide.
Supplementary Material
Acknowledgments
We thank J. Sanner and J.B.C. Jackson for their collection and maintenance of the Smithsonian Institution bryozoan collection; J.J. Hill and S. Whitaker at the NMNH Scientific Imaging Facility; D. Erwin, G. Hunt, and M. Fau for discussion; two anonymous reviewers and editor Z. Zhou for their constructive feedback.
Contributor Information
Sarah Leventhal, Department of Geological Sciences and University of Colorado Museum of Natural History, University of Colorado Boulder, Boulder, UCB 265, CO 80309, USA.
Stewart M Edie, Department of Paleobiology, National Museum of Natural History, Smithsonian Institution, Washington, DC 20013, USA.
Rebecca Morrison, Department of Computer Science, University of Colorado Boulder, UCB 430 UCB, Boulder, CO 80309, USA.
Carl Simpson, Department of Geological Sciences and University of Colorado Museum of Natural History, University of Colorado Boulder, Boulder, UCB 265, CO 80309, USA.
Supplementary Material
Supplementary material is available at PNAS Nexus online.
Funding
This research was supported by the Smithsonian Institution Fellowship Program and the Paleontological Society student research grant. Publication of this article was funded by the University of Colorado Boulder Libraries Open Access Fund.
Author Contributions
SL: conceptualization, data curation, formal analysis, writing original draft, writing—review and editing; SME: conceptualization, data curation, formal analysis, writing—original draft, writing—review and editing; RM: formal analysis; CS: conceptualization, formal analysis, writing—original draft, writing—review and editing.
Preprints
This manuscript was posted on a preprint: DOI 10.1101/2024.07.05.602267.
Data Availability
Data and code to reproduce analysis are included in the supplementary materials.
References
- 1. Wilson EO, Hölldobler B. 2005. Eusociality: origin and consequences. Proc Natl Acad Sci U S A. 102(38):13367–13371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilson EO. 1953. The origin and evolution of polymorphism in ants. Q Rev Biol. 28(2):136–156. [DOI] [PubMed] [Google Scholar]
- 3. Taylor PD. Bryozoan paleobiology Wiley, 2020. [Google Scholar]
- 4. Dunn C. 2009. Siphonophores. Curr Biol. 19(6):R233–R234. [DOI] [PubMed] [Google Scholar]
- 5. Goldsby HJ, Dornhaus A, Kerr B, Ofria C. 2012. Task-switching costs promote the evolution of division of labor and shifts in individuality. Proc Natl Acad Sci U S A. 109(34):13686–13691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bonabeau E, Sobkowski A, Theraulaz G, Deneubourg JL. 1997. Adaptive task allocation inspired by a model of division of labor in social insects. In: BCEC. Santa Fe, New Mexico: Santa Fe Institute Working Papers. p. 36–45. [Google Scholar]
- 7. Duarte A, Pen I, Keller L, Weissing FJ. 2012. Evolution of self-organized division of labor in a response threshold model. Behav Ecol Sociobiol. 66(6):947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rueffler C, Hermisson J, Wagner GP. 2012. Evolution of functional specialization and division of labor. Proc Natl Acad Sci U S A. 109(6):E326–E335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reid CR, et al. 2015. Army ants dynamically adjust living bridges in response to a cost-benefit trade-off. Proc Natl Acad Sci U S A. 112(49):15113–15118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kaufmann KW. 1971. The form and functions of the avicularia of Bugula (Phylum Ectoprocta). New Haven (CT): Peabody Museum of Natural History, Yale University. [Google Scholar]
- 11. D’Orazio AE, Waite TA. 2008. Incomplete division of labor: error-prone multitaskers coexist with specialists. J Theor Biol. 250(3):449–460. [DOI] [PubMed] [Google Scholar]
- 12. Jeanson R, Kukuk PF, Fewell JH. 2005. Emergence of division of labour in halictine bees: contributions of social interactions and behavioural variance. Anim Behav. 70(5):1183–1193. [Google Scholar]
- 13. Taylor JS, Raes J. 2004. Duplication and divergence: the evolution of new genes and old ideas. Annu Rev Genet. 38(1):615–643. [DOI] [PubMed] [Google Scholar]
- 14. Harmer SF. 1900. Memoirs: a revision of the genus Steganoporella. J Cell Sci. S2-43(170):225–297. [Google Scholar]
- 15. Carter MC. 2008. The functional morphology of avicularia in cheilostome bryozoans. Open Access Te Herenga Waka-Victoria University of Wellington
- 16. Lidgard S, Carter MC, Dick MH, Gordon DP, Ostrovsky AN. 2012. Division of labor and recurrent evolution of polymorphisms in a group of colonial animals. Evol Ecol. 26(2):233–257. [Google Scholar]
- 17. McKinney FK, Jackson JBC. Bryozoan evolution University of Chicago Press, 1991. [Google Scholar]
- 18. Ryland JS. Bryozoans Hutchinson Univ. Libr., 1970 [Google Scholar]
- 19. Ostrovsky AN. 2004. Brood chambers (ovicells) of cheilostome bryozoans (Bryozoa: Gymnolaemata): structure, research history, and modern problematics. Russ J Mar Biol. 30(S1):S43–S55. [Google Scholar]
- 20. Taylor PD. 1988. Major radiation of cheilostome bryozoans: triggered by the evolution of a new larval type? Hist Biol. 1(1):45–64. [Google Scholar]
- 21. Jablonski D, Lidgard S, Taylor PD. 1997. Comparative ecology of bryozoan radiations; origin of novelties in cyclostomes and cheilostomes. Palaios. 12(6):505–523. [Google Scholar]
- 22. Ostrovsky AN, Taylor PD, Dick MH, Mawatari SF. 2008. Pre-Cenomanian cheilostome Bryozoa: current state of knowledge. Origin and Evolution of Natural Diversity: Proceedings of the International Symposium, The Origin and Evolution of Natural Diversity, Held from 1-5 October 2007 In Sapporo, Japan. 21st Century COE for Neo-Science of Natural History, Hokkaido University. p. 69–74.
- 23. Cheetham AH, Sanner J, Taylor PD, Ostrovsky AN. 2006. Morphological differentiation of avicularia and the proliferation of species in the mid-Cretaceous Wilbertopora Cheetham, 1954 (Bryozoa: Cheilostomata). J Paleontol. 80(1):49–71. [Google Scholar]
- 24. Cheetham AH. 1954. A new early Cretaceous cheilostome bryozoan from Texas. J Paleontol. 28(2):177–184. [Google Scholar]
- 25. Di Martino E, Liow LH. 2022. Changing allometric relationships among fossil and recent populations in two colonial species. Evolution. 76(10):2424–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carter MC, Lidgard S, Gordon DP, Gardner JP. 2011. Functional innovation through vestigialization in a modular marine invertebrate. Biol J Linn Soc. 104(1):63–74. [Google Scholar]
- 27. Cheetham AH, Larwood G. Study of cheilostome polymorphism using principal components analysis. Living and Fossil Bryozoa Academic Press, London. 1973. p. 385–409. [Google Scholar]
- 28. Harvell CD, Padilla DK. 1990. Inducible morphology, heterochrony, and size hierarchies in a colonial invertebrate monoculture. Proc Natl Acad Sci U S A. 87(2):508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bailey TL, Evans FG, Adkins WS. 1945. Revision of stratigraphy of part of cretaceous in Tyler Basin, Northeast Texas. AAPG Bull. 29(2):170–186. [Google Scholar]
- 30. Brown TE. Stratigraphy of the Washita Group in central Texas Baylor University, Department of Geology, 1971. [Google Scholar]
- 31. Scott RW, Benson DG, Morin RW, Shaffer BL, Oboh-Ikuenobe FE. Integrated Albian-Lower Cenomanian chronostratigraphy standard, Trinity River section, Texas. 2003. In: S Gulf Coast Cretaceous Stratigraphy and Paleoecology: Gulf Coast Section, Society of Economic Paleontologists and Mineralogists, Bob F. Perkins Memorial Conference, Vol. 23. p. 277–334.
- 32. Scott RW, Fee D, Magee R, Laali H. Epeiric depositional models for the lower Cretaceous Washita Group North-Central Texas. Virtual Landscapes of Texas, 1978. [Google Scholar]
- 33. Scott RW, Oboh-Ikuenobe FE, Benson DG, Holbrook JM. 2009. Numerical age calibration of the Albian/Cenomanian boundary. Stratigraphy. 6(1):17–32. [Google Scholar]
- 34. Bapst DW. 2014. Assessing the effect of time-scaling methods on phylogeny-based analyses in the fossil record. Paleobiology. 40(3):331–351. [Google Scholar]
- 35. Hunt G, Slater G. 2016. Integrating paleontological and phylogenetic approaches to macroevolution. Annu Rev Ecol Evol Syst. 47(1):189–213. [Google Scholar]
- 36. Cignoni P, Callieri M, Corsini M, Dellepiane M, Ganovelli F. 2008. MeshLab: an Open-Source Mesh Processing Tool. Sixth Eurographics Italian Chapter Conference. 129–136. [Google Scholar]
- 37. Schindelin J, et al. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods. 9(7):676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Adams DC, Otárola-Castillo E. 2013. Geomorph: an R package for the collection and analysis of geometric morphometric shape data. Methods Ecol Evol. 4(4):393–399. [Google Scholar]
- 39. Gunz P, Mitteroecker P, Bookstein FL. 2005. Semilandmarks in three dimensions. In: Modern morphometrics in physical anthropology. New York (NY): Kluwer Academic/Plenum Publishers. p. 73–98. [Google Scholar]
- 40. Gunz P, Mitteroecker P. 2013. Semilandmarks: a method for quantifying curves and surfaces. Hystrix Ital J Mammal. 24(1):103–109. [Google Scholar]
- 41. Collyer ML, Adams DC. 2019. RRPP: an R package for fitting linear models to high-dimensional data using residual randomization. Methods Ecol Evol. 9(7):1772–1779. 10.1111/2041-210X.13029. [DOI] [Google Scholar]
- 42. Bone EK, Keough MJ. 2010. Does polymorphism predict physiological connectedness? A test using two encrusting bryozoans. Biol Bull. 219(3):220–230. [DOI] [PubMed] [Google Scholar]
- 43. Leckie RM, Bralower TJ, Cashman R. 2002. Oceanic anoxic events and plankton evolution: biotic response to tectonic forcing during the mid-Cretaceous. Paleoceanography. 17(3):13–11. [Google Scholar]
- 44. Jones MM, et al. 2023. Abrupt episode of mid-Cretaceous ocean acidification triggered by massive volcanism. Nat Geosci. 16(2):169–174. [Google Scholar]
- 45. Guthrie RD. 1965. Variability in characters undergoing rapid evolution, an analysis of Microtus molars. Evolution. 19(2):214–233. [Google Scholar]
- 46. Tague RG. 1997. Variability of a vestigial structure: first metacarpal in Colobus guereza and Ateles geoffroyi. Evolution. 51(2):595–605. [DOI] [PubMed] [Google Scholar]
- 47. Carter MC, Gordon DP, Gardner JP. 2010. Polymorphism and vestigiality: comparative anatomy and morphology of bryozoan avicularia. Zoomorphology. 129(3):195–211. [Google Scholar]
- 48. Snell-Rood EC, et al. 2011. Developmental decoupling of alternative phenotypes: insights from the transcriptomes of horn-polyphenic beetles. Evolution. 65(1):231–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Simpson C. 2012. The evolutionary history of division of labour. Proc Biol Sci. 279(1726):116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Buss LW. 1983. Evolution, development, and the units of selection. Proc Natl Acad Sci U S A. 80(5):1387–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Buss LW. The evolution of individuality Princeton University Press, 1987. [Google Scholar]
- 52. Magadum S, Banerjee U, Murugan P, Gangapur D, Ravikesavan R. 2013. Gene duplication as a major force in evolution. J Genet. 92(1):155–161. [DOI] [PubMed] [Google Scholar]
- 53. Dzik J. 1975. The origin and early phylogeny of the cheilostomatous Bryozoa. Acta Palaeontol Pol. 20(3):395–423. [Google Scholar]
- 54. Boardman RS, Cheetham AH. 1969. Skeletal growth, intracolony variation, and evolution in Bryozoa: a review. J Paleontol. 43:205–233. [Google Scholar]
- 55. Eckman JE, Okamura B. 1998. A model of particle capture by bryozoans in turbulent flow: significance of colony form. Am Nat. 152(6):861–880. [DOI] [PubMed] [Google Scholar]
- 56. Okamura B, Harmelin J, Jackson JBC. Refuges revisited: enemies versus flow and feeding as determinants of sessile animal distribution and form. 2001. In: Jackson JBC, Lidgard S, McKinney FK, editors. Evolutionary patterns: growth, form, and tempo in the fossil record. Chicago: University of Chicago Press. p. 61–93. [Google Scholar]
- 57. McMahon T. 1973. Size and shape in biology. Science. 179(4079):1201–1204. [DOI] [PubMed] [Google Scholar]
- 58. Winston JE. 1986. Victims of avicularia. Mar Ecol. 7(2):193–199. [Google Scholar]
- 59. Buss LW. 1979. Bryozoan overgrowth interactions—the interdependence of competition for space and food. Nature. 281(5731):475–477. [Google Scholar]
- 60. Buss LW, Jackson JBC. 1981. Planktonic food availability and suspension-feeder abundance: evidence of in situ depletion. J Exp Mar Biol Ecol. 49(2-3):151–161. [Google Scholar]
- 61. Ginther SC, Cameron H, White CR, Marshall DJ. 2024. Metabolic loads and the costs of metazoan reproduction. Science. 384(6697):763–767. [DOI] [PubMed] [Google Scholar]
- 62. Lidgard S, McKinney FK, Taylor PD. 1993. Competition, clade replacement, and a history of cyclostome and cheilostome bryozoan diversity. Paleobiology. 19(3):352–371. [Google Scholar]
- 63. Simpson C. 2021. An ecological driver for the macroevolution of morphological polymorphism within colonial invertebrates. J Exp Zool B Mol Dev Evol. 336(3):231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Buss LW, Jackson JBC. 1979. Competitive networks: nontransitive competitive relationships in cryptic coral reef environments. Am Nat. 113(2):223–234. [Google Scholar]
- 65. Dunn CW. 2005. Complex colony-level organization of the deep-sea siphonophore Bargmannia elongata (Cnidaria, Hydrozoa) is directionally asymmetric and arises by the subdivision of pro-buds. Dev Dyn. 234(4):835–845. [DOI] [PubMed] [Google Scholar]
- 66. Namikawa H, Mawatari SF, Calder DR. 1992. Role of the tentaculozooids of the polymorphic hydroid Stylactaria conchicola (Yamada) in interactions with some epifaunal space competitors. J Exp Mar Biol Ecol. 162(1):65–75. [Google Scholar]
- 67. Stanley GD, Lipps JH. 2011. Photosymbiosis: the driving force for reef success and failure. Paleontol Soc Pap. 17:33–59. [Google Scholar]
- 68. Muscatine L, Weis V. Productivity of zooxanthellae and biogeochemical cycles. 1992. In: Primary productivity and biogeochemical cycles in the sea. Springer. p. 257–271. [Google Scholar]
- 69. Spanier E, Cobb JS, James MJ. 1993. Why are there no reports of eusocial marine crustaceans? Oikos. 67(3):573–576. [Google Scholar]
- 70. Baird AH, Guest JR, Willis BL. 2009. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu Rev Ecol Evol Syst. 40(1):551–571. [Google Scholar]
- 71. Andersson M. 1984. The evolution of eusociality. Annu Rev Ecol Syst. 15(1):165–189. [Google Scholar]
- 72. Smith A. The wealth of nations W. Strahan and T. Cadell, London, 1776. [Google Scholar]
- 73. Haldane JBS. 1932. The time of action of genes, and its bearing on some evolutionary problems. Am Nat. 66(702):5–24. [Google Scholar]
- 74. Hamilton WD. 1963. The evolution of altruistic behavior. Am Nat. 97(896):354–356. [Google Scholar]
- 75. Darwin C. On the origin of species Routledge, 1859. [Google Scholar]
- 76. Gould SJ, Vrba ES. 1982. Exaptation—a missing term in the science of form. Paleobiology. 8(1):4–15. [Google Scholar]
- 77. Erwin DH. 2021. A conceptual framework of evolutionary novelty and innovation. Biol Rev Camb Philos Soc. 96(1):1–15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code to reproduce analysis are included in the supplementary materials.