Abstract
Background and objectives:
Intermittent androgen deprivation therapy (iADT) may result in measurable improvements in quality of life over continuous ADT in patients with advanced prostate cancer (aPC). Here, we studied time to castration and testosterone recovery in real-world patients with aPC undergoing iADT with relugolix.
Methods and design:
Eligibility criteria for this retrospective study were histologically confirmed through the diagnosis of aPC and initiation of iADT with relugolix. Primary endpoints were time to castrate level of testosterone after relugolix initiation and time to recovery to noncastrate levels after relugolix discontinuation.
Results:
Overall, 25 patients with aPC were treated with iADT and with relugolix. Median time to serum testosterone <50 ng/dL was 1.13 months [range 0.67–2.5 months]. The median time to recovery >50 ng/dL was 1.4 months [range 0.83–6.57 months] from holding treatment with relugolix.
Conclusion:
iADT with relugolix is associated with a rapid time to testosterone suppression and recovery. These results may guide patients’ counseling and monitoring of serum testosterone and PSA levels in patients wishing to pursue iADT for aPC.
Keywords: advanced prostate cancer, intermittent ADT, Regulolix
Introduction
Androgen deprivation therapy (ADT) has long been the backbone of the treatment of prostate cancer. Adverse effects of androgen deprivation include bone density loss, weight gain, fatigue, anemia, hot flashes, depression, and sexual dysfunction, resulting in a significant impact on patients’ quality of life. 1 The concept of intermittent ADT (iADT) was first published and popularized by Klotz et al, who treated patients with symptomatic bony metastases with estrogen until resolution of pain and resumed therapy once the pain recurred. 2 The benefit of such an approach was a break from treatment-related toxicity. This strategy was later tested using intermittent ADT in men with biochemical recurrence and was shown to have comparable survival outcomes with some improvement in quality of life.3–5 As such, iADT remains one acceptable standard of care for many men with nonmetastatic prostate cancer with biochemical recurrence.3,4,6
Despite the theoretical benefit, overall limited data support quality of life benefits for patients on iADT relative to continuous ADT. One potential reason for this is the prolonged time to testosterone recovery with gonadotropin-releasing hormone (GnRH) agonist therapy. In one large phase III clinical trial of iADT for nonmetastatic prostate cancer with biochemical recurrence after adjuvant/salvage radiation therapy only 35% of men had testosterone recovery to baseline within 2 years after finishing the initial treatment period. 4 This prolonged time to testosterone recovery potentially explains the persistent adverse impact on quality of life even with iADT.
It is possible that alternative methods of ADT therapy might lead to improved quality of life due to a more rapid testosterone recovery. This can be achieved by using a GnRH antagonist instead of a GnRH agonist. In the pivotal phase III HERO trial of relugolix (GnRH antagonist) versus leuprolide (GnRH agonist), there was a faster time to testosterone recovery with relugolix. After discontinuation of ADT, approximately 54% of patients treated with relugolix and only 3% treated with leuprolide had testosterone recovery to > 280 ng/dL by day 90 (p = 0.002) with mean testosterone levels of 288.4 and 58.5 ng/dL, respectively. 7 The drug half-life might also be a significant factor in determining the time to testosterone recovery and, therefore, faster improvements in quality of life. Dearnaley et al. 8 compared relugolix versus degarelix (injectable GnRH agonist with a longer half-life) in men receiving definitive radiation therapy for localized prostate cancer. 8 In total, 65 patients were randomized to relugolix, and 38 patients were randomized to degarelix. Castration, defined as < 50 ng/dL, was achieved in most men for both groups, 95% and 89%, respectively. Profound castration, defined as < 20 ng/dL, was more common with relugolix at 82% as compared to 68% with degarelix. Testosterone recovery was relatively rapid for both groups but was shorter with relugolix compared with degarelix. The median testosterone level by week 36 after drug discontinuation was 256.9 ng/dL for relugolix and 30.0 ng/dL for degarelix. This correlated with improvements in sexual health, global health status, and castration-related symptoms as measured by multiple validated patient-reported outcomes tools (European Organization for Research and Treatment of Cancer core questionnaire (EORTC QLQ-C30), EORTC prostate cancer module (EORTC QLQ-PR25), and the Aging Males’ Symptoms scale).
Based on these data, we hypothesize that iADT therapy using relugolix may result in rapid testosterone recovery in a real-world patient population. The objective of this study is to measure the time to testosterone suppression to castrate levels and time to testosterone recovery to noncastrate levels in a real-world population of patients with advanced prostate cancer treated with relugolix.
Materials and methods
This IRB-approved, single-center retrospective study evaluated a sequential series of patients with a pathological confirmed diagnosis of advanced prostate cancer (metastatic hormone-sensitive prostate cancer or nonmetastatic hormone-sensitive prostate cancer with biochemical recurrence) who were treated with relugolix with an iADT approach between April 2021 (date of FDA approval of relugolix) and August 2023. Patients were excluded if they were treated with GnRH agonists or continuous ADT. This retrospective study was approved by the institutional review board at the University of Utah. For the study, informed consent was waived due to the use of retrospective data. The study fully complied with the US patient confidentiality regulations, including adherence to the Health Insurance Portability and Accountability Act of 1996.
Nonmetastatic disease was defined as a lack of visible metastasis on standard imaging (Computed tomography and nuclear medicine bone scan). If patients had available molecular imaging (ex PSMA-PET/CT), it was not factored in evaluating patient eligibility. iADT was defined as initiating ADT until Prostate-specific antigen (PSA) ⩽4 ng/mL, then stopping ADT until PSA ⩾10 at which point ADT with relugolix was resumed. The frequency of testosterone and PSA testing was performed at the discretion of the treating physician.
The primary endpoints were the time to castrate level of testosterone after relugolix initiation and the time to recovery to noncastrate level after discontinuation of relugolix. Castrate level of serum testosterone was defined as serum testosterone level ⩽50 ng/dL. Secondary endpoints included time to PSA suppression, depth of PSA nadir, and time to PSA relapse. The reporting of this study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement (Supplemental Figure). The retrospective nature of this study exposes it to certain potential biases. For example, patients who opted to undergo iADT at our center may be materially different than those who did not, creating potential for selection bias.
Results
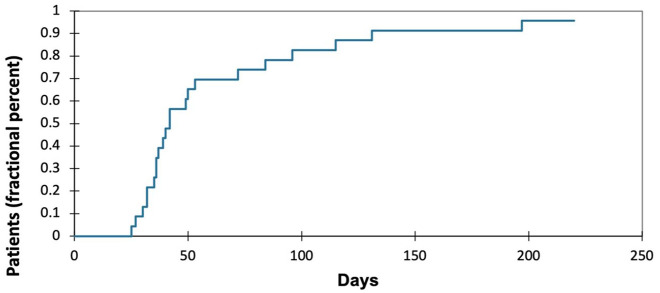
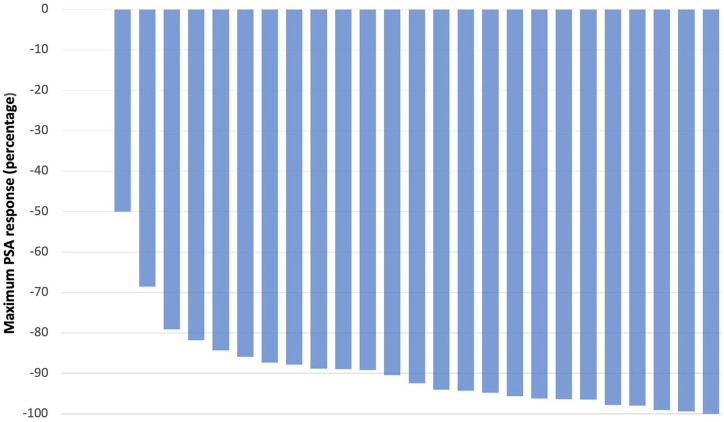
Overall, 25 consecutive patients with advanced prostate cancer treated with iADT with relugolix were eligible and included. The median age was 75 years (range 68–88). Patients with a Gleason score of ⩾8 comprised 52% of the cohort, while 56% had radiographic metastases at the time of treatment initiation. A total of 20 percent of patients had no prior curative intent treatments. Of the 20 patients treated previously with local therapy, 50% of patients had radiation alone, 40% had prostatectomy, 10% had both. The median PSA at the start of therapy was 18.2 ng/mL (range 5.1–99.1 ng/mL). Most patients (n = 20) had PSA and testosterone levels tested monthly. Two patients had tests performed 2 months after discontinuation, one patient at 3 months, and two patients had testing performed greater than 3 months after treatment discontinuation. Median time to testosterone ⩽50 ng/dL was 1.13 months (range 0.67–2.5 months), with recovery >50 ng/dL achieved at a median of 1.4 months after stopping relugolix (range 0.83–6.57 months) (Figure 1). The median time on relugolix prior to holding was 1.13 months (range 0.67–4.8 months) and the median time of holding relugolix was 2.9 months (range 0.9–7.9 months). The median PSA nadir achieved with relugolix was 2.02 ng/mL (range 0.1–4.8), at a median of 1.35 months after initiation of therapy (range 0.67–4.8 months) (Figure 2). Median time to PSA relapse >10 ng/mL after holding relugolix was 3.4 months [range 0.9–8.23 months]. No new cardiovascular events defined by new hypertensive urgency or emergency, a new diagnosis of heart failure, coronary artery disease, new myocardial infarction, or percutaneous coronary intervention were observed.
Figure 1.
Cumulative percentage of patients with testosterone recovery >50 ng/dL after stopping relugolix.
Figure 2.
Waterfall plot demonstrating maximum PSA change from baseline during relugolix therapy.
Discussion
This is the first real-world study to show that iADT with relugolix is associated with a rapid time to testosterone suppression. We also show in our cohort that testosterone recovery to noncastrate levels is rapid. These results may guide patients’ counseling and monitoring of serum testosterone and PSA levels in patients wishing to pursue iADT with relugolix for advanced prostate cancer. However, external validation in larger cohorts is needed to better define the expected recovery time and recovery level of testosterone for a broader range of patient demographics. Subsequent investigations are essential to delineate the optimal utilization of this unique agent regarding iADT. This approach might be particularly meaningful for patients who have significant cardiac comorbidities, older age, are frail, or have significant osteoporosis. 9
The adverse effects of ADT seem to be directly related to lowering testosterone levels. Data strongly correlates quality of life measures with absolute testosterone levels in men previously treated with ADT.10,11 However, testosterone recovery time is variable and often delayed in patients treated with GnRH agonist therapy and also in patients treated with degarelix.12–15 Additionally degarelix may not always lead to faster testosterone recovery. For instance, in a randomized phase II trial of degarelix versus GnRH agonist therapy, the time to testosterone recovery above castration levels (defined as >50 ng/dL) was longer with degarelix than GnRH agonist, median 27.3 versus 4.8 weeks respectively (p < 0.001). 16 This is different than what was seen in the HERO trial using relugolix, so the formulation might account for this difference and not the exact mechanism of action.
Because most quality-of-life consequences from ADT are due to suppressed testosterone, it seems reasonable to infer that a more rapid testosterone recovery will lead to greater improvement in quality of life compared to a slower testosterone recovery. Some published data suggest that the rate of recovery is directly proportional to quality of life.17,18 Perhaps the strongest evidence is from the HERO trial. Patients treated in the HERO study with relugolix had faster testosterone recovery than those treated with leuprolide. All patients had similar quality of life, as measured by validated questionnaires, during the castration period. However, after treatment discontinuation, quality-of-life scores were improved for patients treated with relugolix compared with leuprolide. 19 Overall, these data support the hypothesis that relugolix may be particularly useful for iADT use due to a more rapid testosterone recovery leading to the potential for improved quality of life more quickly once therapy is stopped.
This study has several limitations apart from its retrospective nature and small sample size. We only included patients on iADT monotherapy. Recently, the EMBARK clinical trial demonstrated improved clinical outcomes in patients with biochemically recurrent prostate cancer when treated with intensified therapy. 20 Most of the patients in this study were treated using the iADT approach, with therapy suspended at 37 weeks of treatment if PSA was undetectable. It is possible that relugolix, when used as iADT as part of combination therapy may not lead to as significant of improvement in quality of life compared to relugolix use in the monotherapy setting. Similar considerations apply to relugolix use when combined with surgery or radiation therapy. Additional studies need to be conducted in these settings to understand the testosterone recovery with relugolix when used as an intensified therapy.
Since this was a real-world, noncomparative study, no conclusions regarding differences in efficacy or quality of life versus alternative treatment strategies can be drawn. This study explored a single on/off cycle of iADT. Estimating the time to castration resistance was outside the scope of this study. No quality-of-life instruments were recorded for these patients, so formal quality-of-life comparisons cannot be performed. Moreover, the single-center retrospective nature of this study exposes it to certain potential biases. For example, patients who opted to undergo iADT at our center may be materially different than those who did not, creating the potential for selection bias.
However, even with these limitations, our study is the first to demonstrate rapid testosterone suppression and recovery with iADT with relugolix in patients with advanced prostate cancer. This study also provides further data on time to testosterone suppression and recovery to noncastrate level with intermittent relugolix therapy.
Conclusion
Intermittent ADT with relugolix in real-world patients with advanced prostate cancer is associated with rapid testosterone suppression and recovery. Larger studies in real-world patients are needed to validate these findings.
Supplemental Material
Supplemental material, sj-docx-1-tau-10.1177_17562872241293779 for Testosterone suppression and recovery in patients with advanced prostate cancer treated with intermittent androgen deprivation therapy with relugolix by Patrick Campbell, Georges Gebrael, Arshit Narang, Chadi Hage Chehade, Vinay Mathew Thomas, Gliceida Galarza Fortuna, Nicolas Sayegh, Nishita Tripathi, Clara Tandar, Emre Dal, Haoran Li, Umang Swami, Neeraj Agarwal and Benjamin L. Maughan in Therapeutic Advances in Urology
Acknowledgments
None.
Footnotes
ORCID iD: Patrick Campbell  https://orcid.org/0009-0005-2256-6974
https://orcid.org/0009-0005-2256-6974
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Patrick Campbell, Division of Medical Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Georges Gebrael, Division of Medical Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Arshit Narang, Division of Medical Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Chadi Hage Chehade, Division of Medical Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Vinay Mathew Thomas, Division of Medical Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Gliceida Galarza Fortuna, Division of Medical Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Nicolas Sayegh, Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, USA.
Nishita Tripathi, Department of Internal Medicine, Detroit Medical Center Sinai Grace Hospital, Detroit, MI, USA.
Clara Tandar, Division of Medical Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Emre Dal, Division of Medical Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Haoran Li, Division of Medical Oncology, Department of Internal Medicine, University of Kansas Cancer Center, Westwood, KS, USA.
Umang Swami, Division of Medical Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Neeraj Agarwal, Division of Medical Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT, USA.
Benjamin L. Maughan, Division of Medical Oncology, Department of Internal Medicine, Huntsman Cancer Institute, University of Utah, Salt Lake City, UT 84112, USA.
Declarations
Ethics approval and consent to participate: IRB Approved (00067518) – Huntsman Cancer Institute, University of Utah; this is an IRB-approved retrospective study, all patient information was de-identified and patient consent was not required. Patient data will not be shared with third parties.
Consent for publication: Not applicable.
Author contributions: Patrick Campbell: Data curation; Formal analysis; Writing – original draft; Writing – review & editing.
Georges Gebrael: Data curation; Writing – review & editing.
Arshit Narang: Writing – review & editing.
Chadi Hage Chehade: Writing – review & editing.
Vinay Thomas: Writing – review & editing.
Gliceida Galarza Fortuna: Writing – review & editing.
Nicolas Sayegh: Writing – review & editing.
Nishita Tripathi: Writing – review & editing.
Clara Tandar: Writing – review & editing.
Emre Dal: Writing – review & editing.
Haoran Li: Writing – review & editing.
Umang Swami: Writing – review & editing.
Neeraj Agarwal: Conceptualization; Methodology; Resources; Supervision; Writing – original draft; Writing – review & editing.
Benjamin L. Maughan: Conceptualization; Methodology; Supervision; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Neeraj Agarwal does not report any personal conflicts of interest since April 15, 2021. However, the following are his lifetime personal conflicts of interest: consulting fees from Astellas, Astra Zeneca, Aveo, Bayer, Bristol-Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics; and his institution has received research funding from Arnivas, Astellas, Astra Zeneca, Bavarian Nordic, Bayer, Bristol-Myers Squibb, Calithera, Celldex, Clovis, Crispr, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Gilead, Glaxo Smith Kline, Immunomedics, Janssen, Lava, Medivation, Merck, Nektar, Neoleukin, New Link Genetics, Novartis, Oric, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon. Umang Swami has been paid for a consulting or advisory role by Astellas, AstraZeneca, Adaptimmune, Exelixis, Gilead, Imvax, Janssen, Pfizer, Seattle Genetics and Sanofi and his institution has received research funding from Janssen, Exelixis and Astellas/Seattle Genetics. Benjamin L. Maughan is a paid consultant/advisor to AbbVie, Pfizer, AVEO oncology, Janssen, Astellas, Bristol-Myers Squibb, Clovis, Tempus, Merck, Exelixis, Bayer Oncology and Peloton Therapeutics; and Huntsman Cancer Institute has received research funding from Exelixis, Bavarian-Nordic, Clovis, Genentech, and Bristol-Myers Squibb on his behalf. The other authors declare no conflicts of interest.
Data availability statement: Uploaded with manuscript.
References
- 1. Nguyen PL, Alibhai SMH, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. Eur Urol 2015; 67(5): 825–836. [DOI] [PubMed] [Google Scholar]
- 2. Klotz LH, Herr HW, Morse MJ, et al. Intermittent endocrine therapy for advanced prostate cancer. Cancer 1986; 58(11): 2546–2550. [DOI] [PubMed] [Google Scholar]
- 3. Salonen AJ, Taari K, Ala-Opas M, et al. The FinnProstate Study VII: intermittent versus continuous androgen deprivation in patients with advanced prostate cancer. J Urol 2012; 187(6): 2074–2081. [DOI] [PubMed] [Google Scholar]
- 4. Crook JM, O’Callaghan CJ, Duncan G, et al. Intermittent androgen suppression for rising PSA level after radiotherapy. New England J Med 2012; 367(10): 895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Salonen AJ, Taari K, Ala-Opas M, et al. Advanced prostate cancer treated with intermittent or continuous androgen deprivation in the randomised FinnProstate Study VII: quality of life and adverse effects. Eur Urol 2013; 63(1): 111–120. [DOI] [PubMed] [Google Scholar]
- 6. Perera M, Roberts MJ, Klotz L, et al. Intermittent versus continuous androgen deprivation therapy for advanced prostate cancer. Nat Rev Urol 2020; 17(8): 469–481. [DOI] [PubMed] [Google Scholar]
- 7. Shore ND, Saad F, Cookson MS, et al. Oral relugolix for androgen-deprivation therapy in advanced prostate cancer. New England J Med 2020; 382(23): 2187–2196. [DOI] [PubMed] [Google Scholar]
- 8. Dearnaley DP, Saltzstein DR, Sylvester JE, et al. The oral gonadotropin-releasing hormone receptor antagonist relugolix as neoadjuvant/adjuvant androgen deprivation therapy to external beam radiotherapy in patients with localised intermediate-risk prostate cancer: a randomised, open-label, parallel-group phase 2 trial. Eur Urol 2020; 78(2): 184–192. [DOI] [PubMed] [Google Scholar]
- 9. Nam W, Choi SY, Yoo SJ, et al. Factors associated with testosterone recovery after androgen deprivation therapy in patients with prostate cancer. Invest Clin Urol 2018; 59(1): 18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klotz L. Intermittent androgen deprivation therapy: clarity from confusion. Eur Urol 2013; 64: 731–733. [DOI] [PubMed] [Google Scholar]
- 11. Tzortzis V, Samarinas M, Zachos I, et al. Adverse effects of androgen deprivation therapy in patients with prostate cancer: focus on metabolic complications. Hormones 2017; 16(2): 115–123. [DOI] [PubMed] [Google Scholar]
- 12. Tunn U, Canepa G, Kochanowsky A, et al. Testosterone recovery in the off-treatment time in prostate cancer patients undergoing intermittent androgen deprivation therapy. Prostate Cancer Prostatic Dis 2012; 15(3): 296–302. [DOI] [PubMed] [Google Scholar]
- 13. Ng E, Woo HH, Turner S, et al. The influence of testosterone suppression and recovery on sexual function in men with prostate cancer: observations from a prospective study in men undergoing intermittent androgen suppression. J Urol 2012; 187(6): 2162–2167. [DOI] [PubMed] [Google Scholar]
- 14. Bruchovsky N, Klotz L, Crook J, et al. Locally advanced prostate cancer—biochemical results from a prospective phase II study of intermittent androgen suppression for men with evidence of prostate-specific antigen recurrence after radiotherapy. Cancer 2007; 109(5): 858–867. [DOI] [PubMed] [Google Scholar]
- 15. Klotz L, Loblaw A, Siemens R, et al. A phase II, randomized, multicenter study comparing 10 months versus 4 months of degarelix therapy in prolonging the off treatment interval in men with localized prostate cancer receiving intermittent androgen deprivation therapy for biochemical recurrence following radical local therapy. J Urol 2018; 200(2): 335–343. [DOI] [PubMed] [Google Scholar]
- 16. Sasaki H, Miki K, Tashiro K, et al. Differences in sex hormone recovery profile after cessation of 12-week gonadotropin-releasing hormone antagonist versus agonist therapy. Andrology 2022; 10(2): 270–278. [DOI] [PubMed] [Google Scholar]
- 17. Nabid A, Carrier N, Martin A-G, et al. Quality of life comparison based on testosterone recovery after androgen deprivation therapy in patients cured from high-risk prostate cancer: long term data from a phase III trial. JCO Oncol Pract 2023; 19(11): 333–333. [Google Scholar]
- 18. Shah S, Pepin A, Forsthoefel M, et al. Testosterone as a biomarker for quality of life (QOL) following androgen deprivation therapy (ADT) and stereotactic body radiotherapy (SBRT). Cureus 2023; 15(8): e44440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tombal B, Collins S, Morgans AK, et al. Impact of relugolix versus leuprolide on the quality of life of men with advanced prostate cancer: results from the phase 3 HERO study. Eur Urol 2023; 84: 579–587. [DOI] [PubMed] [Google Scholar]
- 20. Shore ND, et al. LBA02-09 EMBARK: a phase 3 randomized study of enzalutamide or placebo plus leuprolide acetate and enzalutamide monotherapy in high-risk biochemically recurrent prostate cancer. J Urol 2023; 209 (Suppl. 4): e1190. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tau-10.1177_17562872241293779 for Testosterone suppression and recovery in patients with advanced prostate cancer treated with intermittent androgen deprivation therapy with relugolix by Patrick Campbell, Georges Gebrael, Arshit Narang, Chadi Hage Chehade, Vinay Mathew Thomas, Gliceida Galarza Fortuna, Nicolas Sayegh, Nishita Tripathi, Clara Tandar, Emre Dal, Haoran Li, Umang Swami, Neeraj Agarwal and Benjamin L. Maughan in Therapeutic Advances in Urology