Abstract
Background
Hypertension is the most common cardiovascular disease in Peru despite the availability of cost-effective, evidence-based treatment. Here we describe the rationale and study design for a hybrid type 2 randomized controlled trial to test the implementation and effectiveness of a community health worker (CHW)-led hypertension control program within the national primary care system in Puno, Peru.
Methods
We will recruit 1068 adult participants with hypertension aged ≥ 18 years in Puno, Peru, via facility-based enrollment and community health fairs. Participants will be individually randomized (1:1) to either continue with usual care or participate in a 12-month CHW-led home-based hypertension control program consisting of blood pressure monitoring, medication adherence support, and healthy lifestyle counseling. Outcome development and reporting are guided by the Consolidated Framework for Implementation Research (CFIR), the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework, and the Proctor et al. framework. Clinical effectiveness outcomes include mean change in systolic blood pressure (primary outcome), diastolic blood pressure, and HbA1C. Implementation outcomes include fidelity (i.e., CHW protocol adherence and dose), reach, adoption, sustainability, acceptability, and cost-effectiveness.
Discussion
The ANDES trial is testing the first CHW-led multicomponent strategy for hypertension and type 2 diabetes management in Peru. This type 2 hybrid trial will provide critical insights into the individual, community, and system-level factors necessary for successful implementation and effectiveness. These data can inform the future adaptation and scaling of the ANDES strategy in Peru and other LMICs, as well as influence policies at the system level to support this transition. Furthermore, by addressing both hypertension and diabetes, the ANDES strategy supports integrated care approaches advocated by the WHO HEARTS technical package, ultimately enhancing health outcomes and reducing morbidity and mortality in the region.
Trial registration
ClinicalTrials.gov, ID: NCT05524987, Addressing Hypertension and Diabetes through Community-Engaged Systems in Puno, Peru (ANDES study), prospectively registered on September 1, 2021.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13063-024-08586-9.
Keywords: Hypertension, Type 2 diabetes, Community health workers, Task-shifting, WHO HEARTS, Implementation, LMICs, Peru
Contributions to the literature
• The Addressing Hypertension and Diabetes through Community-Engaged Systems (ANDES) trial represents one of the few large-scale implementation and effectiveness trials for a hypertension control strategy in Latin America, and the first in Peru.
• The trial aims to provide vital evidence on adapting the World Health Organization’s (WHO) HEARTS strategy for hypertension control in resource-limited settings, addressing a significant gap in research on implementing hypertension control interventions in Latin America.
• The trial will provide new perspectives on adapting community health worker-led approaches for managing hypertension in Latin America, with a focus on measuring the fidelity and acceptability of strategies.
Background
Hypertension remains the leading cause of global cardiovascular disease and premature mortality despite the wide availability of cost-effective, evidence-based treatment, such as medication and lifestyle modifications [1]. The WHO estimates that only 42% of hypertensive individuals are diagnosed and 21% achieve control worldwide [2]. This is largely due to low hypertension awareness and health services accessibility, particularly in low- and middle-income countries (LMICs), where two-thirds of the 1.3 billion adults with hypertension live. Additional barriers include a lack of sufficient health system resources and competing communicable disease management priorities [3, 4]. Furthermore, approximately one-third of hypertensive adults in LMICs also have type 2 diabetes, a comorbidity that increases the risk of severe disease complications and mortality compared to either condition alone [5, 6]. With a hypertension prevalence of 22% and control rate of 5%, Peru is emblematic of these disparities—there are only 16 physicians per 10,000 persons [7] and most are concentrated in urban centers, contributing to low accessibility in rural regions. Additionally, type 2 diabetes prevalence is 7.2% and accounts for 25% of strokes and 32% of myocardial infarctions in Peru [8]. Addressing these challenges and expanding the reach of hypertension interventions in LMICs, including Peru, represents a major public health gap.
Public health is increasingly adopting data-driven, context-specific strategies to enhance the use of evidence-based health interventions. This shift aims to improve healthcare effectiveness, especially in areas with limited resources and high demands. In line with this effort, the World Health Organization (WHO) spearheaded the Global HEARTS initiative in 2016 to strengthen the management of cardiovascular disease, and particularly hypertension, in low-resource global settings [9]. The HEARTS technical package promotes several interventions and strategies, including Healthy lifestyle counseling, Evidence-based treatment protocols, Access to essential medicines and technology, Risk-based CVD management, Team-based care, and Systems for hypertension monitoring systems [9]. As a team-based care strategy, task shifting patient care to community health workers (CHWs) is a strategy to increase the capacity of overburdened health systems and facilitate the introduction and uptake of hypertension interventions [10]. While numerous trials have successfully demonstrated that CHW-based strategies coupling healthy lifestyle counseling and home blood pressure monitoring can significantly reduce blood pressure in high-income countries [10, 11], only few have been conducted in LMICs [12, 13].
The Peruvian Ministry of Health (Ministerio de Salud, or MINSA) has endorsed the adaptation and implementation of HEARTS; however, implementation has been delayed by several factors, including the COVID-19 pandemic and the need for additional research on the local adaptation and implementation of HEARTS-recommended interventions in resource-poor regions, such as Puno, Peru. In response to this gap, the Addressing Hypertension and Diabetes through Community-Engaged Systems (ANDES) trial aims to evaluate the implementation and effectiveness of a HEARTS-based hypertension control strategy to improve medication adherence, promote heart-healthy lifestyles, and reduce hypertension and diabetes in a historically underserved Andean population in Puno, Peru. The trial is a two-arm, individually randomized (1:1) superiority trial with a parallel-group design. Participants in the intervention group follow the ANDES strategy, which includes monthly home visits by CHWs who provide lifestyle coaching, medication adherence support, and blood pressure monitoring. Participants in the control group follow their usual care routine for hypertension and are not offered ANDES strategy services. This trial is expected to generate valuable data on the implementation and effectiveness of the ANDES hypertension control strategy, offering key insights for policies in Peru and other LMICs aiming to enhance the reach and impact of HEARTS-based interventions through CHW delivery.
Methods
Study design
The ANDES study is funded by the National Heart, Lung, and Blood Institute (NHLBI) as a bi-phasic (UG3/UH3) milestone-driven, cooperative agreement with the Global Alliance for Chronic Diseases (GACD). The study’s formative (UG3) phase took place from September 2020 to August 2022 and included mixed-methods research to inform the selection and adaptation of WHO HEARTS technical package components to make up the ANDES hypertension control strategy. Additionally, a pilot study was conducted to assess the feasibility of chosen strategy components. The primary (UG3) phase began in March 2023 and consists of a type 2 hybrid randomized controlled trial (RCT) of the adapted ANDES hypertension control strategy. Research was guided by the Consolidated Framework for Implementation Research (CFIR), the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework, and the Proctor et al. framework for outcomes and reporting [14–16]. The ANDES UH3 trial methods, including the intervention and implementation strategies, are described here according to the following guidelines: the Standardized Reporting Items: Recommendations for Intervention Trials (SPIRIT) (Additional file 1) and the Standards for Reporting Implementation Studies (StaRI) (Additional file 2).
Study setting
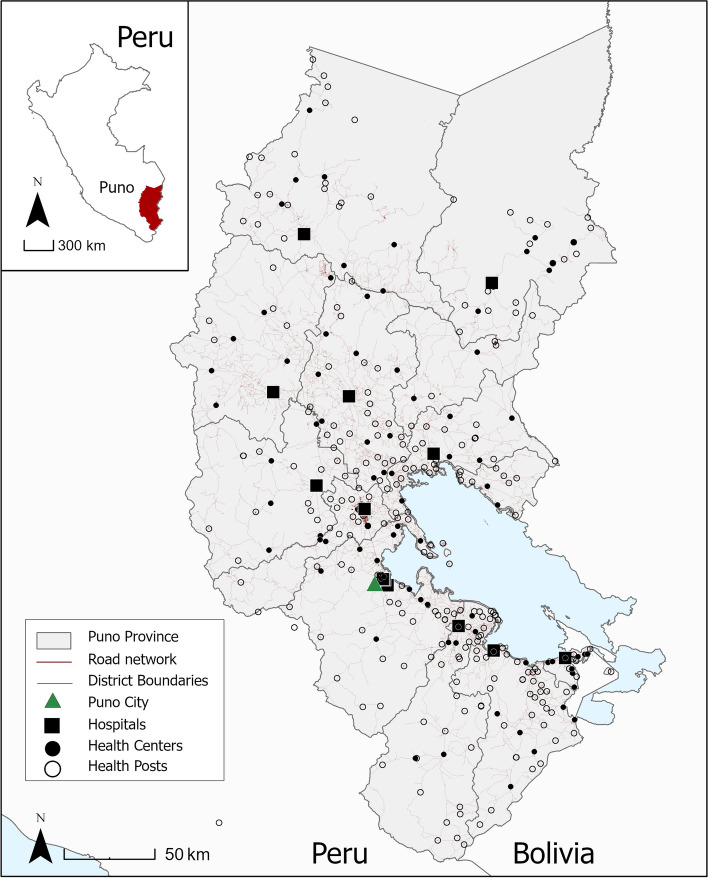
The ANDES trial is being conducted in Puno, Peru, a southeastern province situated at an altitude of 3825 m along the border between Peru and Bolivia (Fig. 1). As of 2017, Puno had a total population of 1.17 million, three-quarters of whom were indigenous Quechua and Aymara [17]. The healthcare system in Peru is divided into several subsystems. Most people in Puno (65%) are either unemployed or informally employed and receive health insurance through the national government insurance scheme, Seguro Integral de Salud (SIS). This scheme is managed by the Regional Health Administration (Dirección Regional de Salud, or DIRESA), which operates under the national Ministry of Health (MINSA). The social security system (EsSalud) covers about 12% of the population insured through formal sector employment. Additionally, 0.7% are covered by the armed forces or police, 0.4% through private insurance, 0.4% through another scheme, and the rest (~ 20%) remain uninsured [17].
Fig. 1.
Study site location and health facilities in Puno, Peru
ANDES intervention
As part of the ANDES hypertension control strategy, a package of evidence-based interventions recommended by the WHO HEARTS technical package are delivered to participants by CHWs [9] (Table 1). Intervention components include healthy lifestyle coaching (i.e., patient-centered health education and motivation delivered with the goal of facilitating behavioral change); blood pressure monitoring; and medication adherence counseling for hypertension participants.
Table 1.
ANDES strategy aligned with WHO HEARTS technical package components
| WHO HEARTS technical package | ANDES program components |
|---|---|
| Healthy lifestyle counseling. Counseling on CVD risk factors and healthy lifestyle interventions. |
Home-based health coaching ▪ Healthy lifestyle counseling (e.g., diet, exercise, alcohol reduction, smoking cessation) provided by CHWs during participant home visits ▪ Motivational interviewing to address individual-level challenges and encourage healthy lifestyle behaviors ▪ Educational materials on hypertension and type 2 diabetes |
| Evidence-based treatment protocols. Standard treatment protocols to improve quality of clinical care, reduce clinical variability, and simplify treatment options. |
Home-based blood pressure monitoring ▪ Blood pressure monitoring by CHWs in participant homes according to WHO HEARTS and national guidelines |
| Access to essential medicines and technology. Improve information on CVD medicine and technology procurement, quantification, distribution, management, and handling of supplies at facility level. |
Medication adherence support ▪ Personalized medication accessibility and adherence counseling led by CHWs in participant homes ▪ Behavior-change tools provided to participants to improve medication adherence (e.g., calendar, pill boxes) |
| Team-based care. Encourage and develop team-based care and task shifting related to the care of CVD. |
Health facility and CHW team-based hypertension care ▪ CHW capacity building to serve as front line hypertension management workers ▪ Motivational text messages to re-enforce education and training for CHWs |
| Systems for monitoring. Monitor and report on the prevention and management of CVD using standardized indicators and data collection tools. |
Performance and quality reporting ▪ Monitoring and reporting of hypertension care linkage and treatment generated by CHW and healthcare facility |
ANDES implementation strategies
The primary strategy for delivering the multi-component hypertension control intervention—i.e., healthy lifestyle coaching, medication adherence support, and blood pressure monitoring—is via monthly CHW visits to participant homes. In Peru, CHWs are frontline public health workers who provide basic healthcare, health education, and advocacy services in communities where they generally live or to which they belong as trusted members. Typically, they do not have formal medical training or certification. For comparison, within the MINSA and EsSalud usual care systems, patients typically receive these services from licensed providers at health facilities; however, the frequency, comprehensiveness, and quality of care vary greatly by numerous factors related to patient characteristics, health insurance, and health facility resources.
During the inaugural CHW home visit, participants receive educational materials on cardiovascular risk factors for hypertension and type 2 diabetes, a calendar for logging daily medication use, medical appointments, and prescription refill schedules, and a pill organizer to facilitate medication adherence. During all monthly visits, CHWs monitor participant blood pressure and deliver health coaching (e.g., on diet, exercise, alcohol reduction, smoking cessation) using a pictorial-based flipchart and motivational interviewing techniques. Upon the request of the participant, the visit frequency can be reduced or discontinued (e.g., due to travel or work). Additionally, capacity building will be conducted to train the local CHWs hired by ANDES, thus contributing to the development of a workforce in frontline hypertension management.
The implementation of the ANDES hypertension control program is further enhanced by additional strategies, guided by the Expert Recommendations for Implementing Change (ERIC) framework taxonomy (Table 2). Evaluative and iterative strategies are used, such as assessing readiness, conducting local needs assessments, and auditing and providing feedback to CHWs. Adaptation and tailoring are integral, with strategies specifically designed to meet the local needs and address barriers identified during research. Stakeholder interrelationships are cultivated through the recruitment of champions, building local coalitions, and training for leadership. Regular training and support for CHWs, dynamic educational material development, and clinical team creation have been designed to bolster the delivery of the interventions. The strategies are based on barriers and facilitators identified during the formative phase, previous literature, and ongoing stakeholder and field staff feedback.
Table 2.
ANDES implementation strategies based on the Expert Recommendations for Implementing Change (ERIC) taxonomy
| Implementation category | ANDES strategy description |
|---|---|
| Use evaluative and iterative strategies | |
| Assess for readiness and identify barriers and facilitators | Conduct and evaluate formative research to identify health system readiness to incorporate WHO HEARTS-based strategies into Puno health system, including barriers and facilitators, health system gaps, and preferences of providers and community members |
| Audit and provide feedback | Create performance measures for CHWs; conduct quarterly assessments and provide retraining |
| Purposefully re-examine the implementation | Monitor intervention strategies over time according to strategy and intervention-specific schedule |
| Adapt and tailor to context | |
| Tailor strategies | Tailor WHO HEARTS-based intervention components and strategies to address barriers and facilitators identified during formative research and piloting |
| Promote adaptability | Clarify intervention elements that must be maintained to preserve fidelity and ways in which they can be tailored to meet local needs (e.g., CHWs trained to adapt the delivery of educational programming to the unique needs and preferences of participants) |
| Develop stakeholder interrelationships | |
| Identify and prepare champions | Formally engage stakeholders to serve as champions: (1) Director of non-communicable disease in Puno; (2) DIRESA representatives; (3) Peruvian MDs working within Puno health system |
| Build a local coalition; use advisory boards and workgroups | Recruit and cultivate relationships with partners in the implementation effort and define roles: MINSA, DIRESA, EsSalud, CHWs, community members, academic institutions (UPCH, WUSTL, JHU) |
| Recruit, designate, and train for leadership | Hire CHW manager to coordinate CHW schedules and provide ongoing training and audit and feedback assessments |
| Inform local opinion leaders | Keep clinicians and others that refer patients for HTN services or initiate the connection to services informed from pre-implementation through maintenance stage, ensuring that they do not serve as obstacles |
| Conduct local consensus discussions | Conduct formative stakeholder workshops and quarterly meetings with MINSA, DIRESA, and health facility representatives to both characterize and build consensus throughout the intervention period |
| Capture and share local knowledge | WhatsApp group and weekly meetings with CHWs to discuss ongoing challenges and solutions. Quarterly meetings with MINSA and EsSalud representatives to discuss challenges related to facility-based enrollment |
| Train and educate stakeholders | |
| Conduct ongoing training | CHW onboarding, weekly group meetings, refresher trainings, and regular evaluations |
| Develop educational materials | Develop CHW manual and other training materials that facilitate the provision of the ANDES program to participants. Ensure that materials are easy-to-use, translate complex topics into actionable target messages. Evaluate materials using formative evaluation feedback |
| Make training dynamic | Supplemented the standard onboarding CHW training package with small group, interactive breakout sessions to cater to the variable skillsets and backgrounds. Conduct weekly meetings to interactively discuss challenges and adjust as necessary |
| Use train-the-trainer strategies | CHW supervisor trained by ANDES investigators (cardiologist, Puno-based general physician, and Peru-based dietician) to provide ongoing management and training to team of CHWs who conduct home-based health coaching and health monitoring |
| Support clinicians | |
| Develop resource sharing agreements | Partnership with MINSA and DIRESA to identify hypertensive patients |
| Develop partnerships with organizations (DIRESA, MINSA, EsSalud) that have resources needed to implement the ANDES program components (e.g., data sharing agreements, agreements to share necessary equipment such as telemedicine equipment, or sharing the cost of bringing in experts who provide training and consultation) | |
| Create new clinical teams | Provide training and capacity for CHWs to serve as part of the hypertension care team. Promote a task-shifting approach in which CHWs facilitate the diagnosis and retention in clinical care for hypertensive patients through home-based health coaching and monitoring |
ANDES CHW training
The intensive CHW training program consists of an onboarding phase, weekly workshops, quarterly refresher sessions, and regular evaluations. A key component of this program is a comprehensive CHW training manual, which was developed using formative research and in collaboration with local health system partners and a visual education specialist. The manual features illustrations of people and food typical of Puno and covers (1) key competencies in hypertension management, such as hypertension pathophysiology, Peruvian diagnosis and treatment guidelines, and the WHO HEARTS technical package components; (2) practical skills for effective participant interaction, including interpersonal communication, rapport building, adherence to research ethics, and ensuring personal and participant safety; and (3) education on all aspects of the ANDES strategy and hands-on training in CHW-specific components, such as health coaching and blood pressure monitoring in participant homes. During the onboarding phase, all CHWs receive a copy of the manual, which is used as a training guide for in-person onboarding training sessions as well as a reference guide for CHWs during the planning of the in-home participant visits. After the onboarding phase, weekly workshops consist of group discussions with the CHW supervisor, all CHWs, and research staff to review implementation challenges and successes from the prior week, brainstorm solutions, and adjust approaches as needed. Quarterly refresher sessions and evaluations will be conducted by research staff and the CHW supervisor to provide continual knowledge reinforcement and feedback.
Intervention tailoring
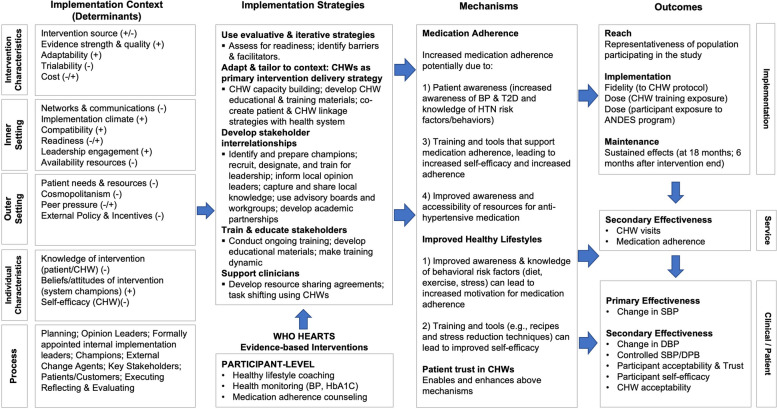
To select and adapt HEARTS components to the local Puno context, several formative research activities were conducted during the UG3 phase, including a rapid health assessment, in-depth interviews, a discrete choice experiment survey, and human-centered design workshops (Table 3). Overall, the goals of these activities were to characterize the current health system gaps in hypertension care; identify potential barriers and facilitators to implementing a CHW-led program based on WHO HEARTS; and co-develop a formative ANDES hypertension control program and implementation strategies with key local stakeholders. Guided by the CFIR framework, key implementation barriers and facilitators were mapped to proposed intervention components and implementation strategies using an Implementation Research Logic Model (IRLM) (Fig. 2). Additionally, potential mechanisms by which the intervention and implementation strategies could influence study outcomes, according to the RE-AIM [15] and Proctor et al. [14] implementation frameworks, were outlined in the IRLM.
Table 3.
ANDES formative research components, details, and findings
| Formative research | Participants and aims | Key findings |
|---|---|---|
| Rapid health assessment |
Participants: health system representatives (MINSA, DIRESA, clinicians, CHWs) Aims: identify gaps in hypertension care and policy; co-develop conceptual framework for proposed ANDES implementation strategy |
▪ No existing CHW-led hypertension programs ▪ Lack of prioritization of chronic disease care |
| In-depth interviews |
Participants: health system representatives, clinicians, CHWs, and hypertension patients Aims: identify potential challenges and solutions related to the implementation of the proposed ANDES strategy components |
▪ Barriers: health awareness and education, geographic accessibility, facility resources, lack of medications ▪ Facilitators: readiness for change, free medication at MINSA facilities |
| Discrete choice experiment survey |
Participants: community members with and without hypertension Aims: assess the relative preferences for CHW-managed health care |
▪ Preference for in-home vs. facility CHW visits ▪ Monthly frequency |
| Human-centered design workshops |
Participants: health system representatives, clinicians, CHWs, and hypertension patients Aims: identify potential challenges and solutions related to the implementation of the proposed ANDES strategy components |
▪ Willingness to engage in inter-stakeholder collaboration for co-creation of protocols |
Fig. 2.
ANDES Logic model. Adaptation of Implementation Research Logic Model template from Smith et al. [18]
To test feasibility of the proposed ANDES multicomponent program, a pilot study was conducted from May 2022 to August 2022. A total of 146 adult participants with hypertension were identified through a combination of health fairs and facility-based screening. Participants were followed for an average of 2 months and received home visits by CHWs (contracted by the ANDES study) that included blood pressure monitoring and health coaching (i.e., education on hypertension, medication adherence, diet, and exercise). Implementation feasibility and early effectiveness blood pressure outcomes will be published separately.
Randomized control trial: UH3 phase
As part of the UH3 phase, a type 2 hybrid effectiveness and implementation RCT is currently underway to evaluate the effect of the ANDES multicomponent hypertension control strategy on systolic blood pressure and other secondary outcomes in a cohort of 1068 adults with hypertension in Puno, Peru. The trial is a two-arm, individually randomized superiority trial with parallel-group design. Participants are allocated 1:1 to intervention and control groups. Participants in the intervention group follow the ANDES hypertension control strategy, which includes monthly home visits by CHWs who provide lifestyle coaching, medication adherence support, and blood pressure monitoring. Participants in the control group follow their usual care routine for hypertension and are not offered ANDES strategy services.
The hybrid design enables the evaluation of the ANDES blood pressure control program’s effectiveness in a real-world context, including the processes and factors that influence its successful implementation. A parallel (1:1) individual-level randomization was selected to maximize power for the analysis of a multi-component individual-level strategy in a population that frequently seeks medical care at multiple levels in the health system, which would limit the use of a cluster-level randomization.
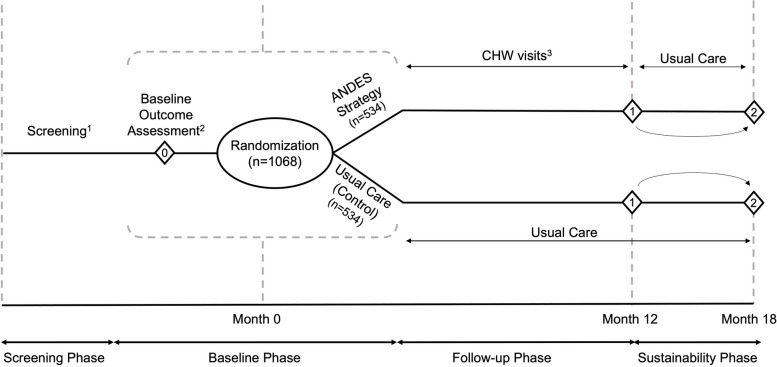
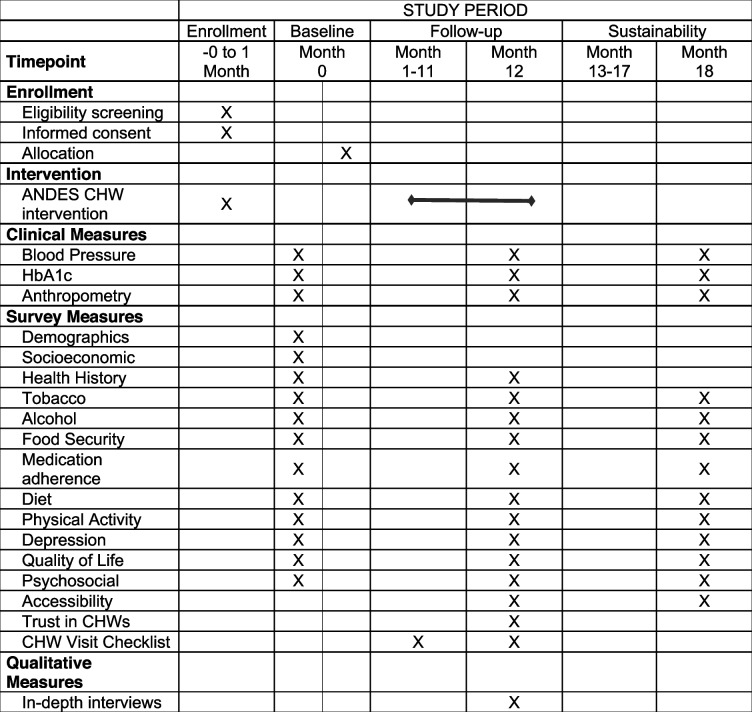
The main UH3 trial consists of 4 phases (Fig. 3; Table 4). In the screening phase, participant screening and recruitment occurs. Next, during a baseline evaluation phase, participants receive baseline assessments and are then randomized into a usual care (“control”) arm or the ANDES implementation program (“intervention”) arm. During the 12 months following randomization, intervention participants follow the ANDES hypertension control program including monthly visits by CHWs. Meanwhile, control participants follow a usual care program and have no contact with ANDES CHWs or study staff. Study implementation and effectiveness endpoints are measured in both groups at the end of the 12 months. Following these assessments, participants begin a 6-month sustainability phase, during which both arms follow a usual care program. The final follow-up visit occurs at the end of this 6-month period, approximately 18 months post-randomization.
Fig. 3.
Overview of ANDES trial design. Schedule of enrollment, intervention, and follow-up assessments. 1Screening will be conducted at the health facilities and health fairs; 2ANDES study team will conduct all study outcome assessments, including the baseline outcome assessment and the follow-up assessments (represented by diamond shapes). 3CHW workers will conduct all participant home visits
Table 4.
Schedule for enrollment, intervention, and outcome data assessments for the ANDES trial according to the SPIRT 2013 guidelines
The primary objectives of the ANDES trial are to (1) evaluate the effect of the ANDES implementation strategy on systolic blood pressure and other secondary clinical outcomes (diastolic blood pressure and HbA1c); (2) measure implementation and service outcomes, including fidelity (dose and adherence to CHW protocol), reach, acceptability, medication adherence, and sustainability; and (3) asses the cost-effectiveness of the ANDES implementation strategy in reducing blood pressure compared to usual care.
Participant eligibility criteria
Participants are eligible if they are ≥ 18 years old, have a diagnosis of hypertension and are currently experiencing uncontrolled hypertension, are served by the MINSA or EsSalud health sector, and are willing to receive monthly CHW home visits for the next year. Hypertension is defined as having an SBP ≥ 140 mmHg or DBP ≥ 90 mmHg documented on two or more separate occasions by a physician, according to HEARTS criteria [9]. Hypertension is considered uncontrolled if SBP ≥ 140 mmHg or DBP ≥ 90 mmHg if the patient is not on antihypertensive medication, or SBP ≥ 130 mmHg or DBP ≥ 80 mmHg if the patient is currently taking antihypertensive medications. Exclusion criteria: (a) unwilling or unable to provide informed consent (e.g., due to cognitive impairment); (b) pregnant or plan to become pregnant in the ensuing 18 months; (c) plan to move out of the study area in the ensuing 18 months; (d) currently receives home medical visits; (e) lives more than 1 hour from Puno City Center; (f) plans to travel for more than 3 months in the next year; (g) another member of the household is already participating in ANDES; (h) has severe chronic kidney disease, or is on or planning to start dialysis; (i) is bedridden and unable to attend regular medical appointments; or has liver failure (meeting Child-Pugh B or C criteria) [19].
Recruitment
Participant recruitment for the ANDES trial began in March 2023 and will continue for 24 months with a target enrollment of 1068 participants (45 participants per month). Recruitment strategies include health facility-based recruitment and community health fairs across the study area. Health facility-based recruitment occurs via regular (daily to weekly) screening fairs at MINSA and EsSalud facilities run by ANDES field staff. This effort is coordinated through an inter-institutional agreement between the local study team institutions (UPCH, Prisma), the Ministry of Health, EsSalud, and DIRESA-Puno. The screening fairs are open to any person walking by and are also advertised via hospital flyers. Additionally, ANDES screening fair staff actively invite participants as they walk by or wait for appointments. Lastly, facility personnel identify hypertensive patients from their records on a weekly basis. During routine phone scheduling for follow-up care, they conduct an initial screening and invite interested patients to the ANDES screening fairs. If a potential participant is deemed eligible, they are provided with detailed information about the ANDES study and invited to join. Informed consent is administered by trained ANDES field staff. The selected MINSA and EsSalud health facilities, all within a 1 h travel distance from Puno City Center, were chosen to encompass a mix of urban and rural participants. Additionally, health fairs are organized as 1-day events offering hypertension screening, in coordination with local health facility administrators from DIRESA and EsSalud. Outreach campaigns and advertisements, such as local radio announcements, posters, and flyers, are utilized to attract community members.
Local CHWs are recruited on a rolling basis to serve as full-time CHWs for the ANDES project. They are paid approximately 1500 PEN (USD 400) per month. For comparison, most CHWs in Peru are unpaid, some are paid per visit, and others are paid a monthly salary similar to the cited ANDES salary. To meet ANDES CHW visit demand, we anticipate hiring 24 CHWs. All current ANDES CHWs have at least 1 year of prior experience as a CHW in Peru. Some have prior experience delivering health coaching interventions, but none has prior experience with hypertension management.
Randomization
The allocation sequence was generated by the Data Coordinating Center at Washington University using computer-generated random numbers with stratum-stratified permuted block randomization to ensure balance across study groups within each healthcare network. Blinded study staff are responsible for enrolling participants, and after enrollment, an unblinded data manager manually initiates the randomization process in REDCap. Following randomization, participants and CHWs are aware of study assignment, but the rest of the research team, including field staff collecting study outcome data and conducting analyses, are blinded to group assignment.
Study outcomes
In accordance with type 2 hybrid design [16], our study is framed to evaluate both implementation and effectiveness outcomes. Data collection and outcome assessment is guided by the RE-AIM and Proctor et al. implementation science frameworks, as detailed in Table 5 [14, 15]. Our primary effectiveness outcome, upon which our trial is powered, is systolic blood pressure, specifically the between-group difference in mean systolic blood pressure between baseline and 12 months. Secondary effectiveness outcomes include between-group differences in the mean change in diastolic blood pressure and HbA1c. Trial implementation will be assessed by fidelity across two domains: protocol adherence by CHWs and intervention dose received by participants. Other implementation outcomes include intervention reach, i.e., the representativeness of individuals who participate in the ANDES intervention; CHW and participant acceptability of ANDES strategy components; self-reported medication adherence; and sustainability, i.e., the extent to which service and patient outcomes are sustained 6 months following the end of intervention delivery (~ 18 months post-randomization). Additionally, costs associated with the delivery of the ANDES multicomponent strategy (excluding costs specific to research) will be determined.
Table 5.
Implementation, service, and patient outcomes according to the RE-AIM and Proctor frameworks
| Domains | Outcome measures |
|---|---|
| Reach: representativeness of population participating in the ANDES multicomponent intervention | |
| Participant-level | Differences between ANDES participants and general population within study area (demographics, including age, gender, SES indicators, language) |
| Facility-level | Differences between participating health centers and all health centers in study area (% clinic classification, physician availability, resources) |
| Effectiveness: the effect of the ANDES strategy on patient outcomes (at 12 months) | |
| Individual-level |
• Systolic blood pressure (mmHg) • Diastolic blood pressure (mmHg) • Percentage of participants with controlled hypertension (BP < 140/ < 90 mmHg) • Percentage of participants with HbA1c ≥ 6.5% |
| Implementation: fidelity to the protocol, including consistency of delivery as intended, and dose (at 12 months) | |
| CHW-level |
• Percentage of total possible visits by CHWs that were (1) attempted and (2) conducted • Percentage of visits in which a CHW adhered to health coaching protocol |
| Sustainability: extent to which service and patient outcomes are sustained (at 18 months post-randomization) | |
| Participant-level |
• % participants with controlled hypertension (BP < 140/ < 90 mmHg) • % participants with HbA1c ≥ 6.5% • % patients who take medication as prescribed • % participants who implement recommended lifestyle/behavioral changes • % participants (at 18 months) who have had at least one health facility visit for their hypertension within the preceding 3 months |
| Acceptability | |
| Participant-level |
• Participant acceptability of the ANDES implementation strategy as assessed via in-depth interviews • Participant trust of CHW score |
| CHW-level | • CHW acceptability of the ANDES implementation strategy as assessed via in-depth interviews with all CHWs |
| Service outcomes | |
| Patient-level |
• % participants who take medication as prescribed • % participants who implement recommended lifestyle/behavioral changes • % participants who visit a health facility following referral by CHW • % participants who were undiagnosed at enrollment who receive diagnosis by 12 months |
| Cost-effectiveness | |
| Facility-level | • Predicted change in system-level costs following a system-wide implementation of the ANDES strategy (based on predicted differences in healthcare utilization) |
SES socioeconomic status, SBP systolic blood pressure, DBP diastolic blood pressure, HbA1c hemoglobin A1C, BMI body mass index, CHW community health workers, HTN hypertension, T2D type 2 diabetes
Study outcome assessments
The timing of all clinical assessments and other measures used for outcome analysis are outlined in Table 4. All assessments are conducted by trained ANDES research staff except CHW visit checklists, which are conducted by CHWs and participants.
Blood pressure measurements
A trained member of the research team will measure blood pressure and heart rate at the participant’s home after 5 min of seated rest, in the right arm and in triplicate, with at least 1 min between measurements, using a validated, automatic oscillometric sphygmomanometer (Omron, model HEM-907XL, Kyoto, Japan). Briefly, the participant will refrain from exercise and use of tobacco, alcohol, or caffeinated beverages and will be asked to empty their bladder. No cooking using biomass fuels in the 30 min prior to measurement will be allowed. Clothing will be removed to expose the right upper-arm and an appropriately sized cuff will be positioned on the upper arm. Before starting the measurement, the participant will be instructed to sit on a chair in a quiet room for 5 min with legs uncrossed, back supported by the chair, and arm supported on a table at the level of the heart (mid-sternum). Participant will refrain from talking or engaging in other activities during the 5 min prior to and during the acquisition of the blood pressure measurements. The final measurement will be the average of all three SBP/DBP measurements. Hypertension is defined as an average SBP/DBP reading of ≥ 140/90 mmHg, respectively.
Diabetes assessment
Type 2 diabetes will be determined using an FDA-approved laboratory-based HbA1c test system (A1CNow + HbA1c Point-of-Care Test, PTS Diagnostics, Whitestown, IN, USA) as a secondary aim to assess the extent to which the multicomponent intervention affects diabetes control. A measurement will be taken from each participant at baseline, 12-, and 18-month visits. A trained member of the research staff will perform a minimally invasive finger stick to collect two drops of whole blood. The diagnosis of type 2 diabetes will be defined as ongoing treatment with a hypoglycemic drug or insulin (documentation to be obtained from the health facility) or a HbA1c ≥ 7%. For those participants who are newly diagnosed with diabetes, a referral to the health care clinic will be facilitated by the ANDES research staff.
Anthropometry
Height, weight, and waist circumference will be assessed using a stadiometer (SECA 213, SECA, Hamburg, Germany), measuring tape (SECA circumference tape 203, Hamburg, Germany), and digital balance (SECA 803, SECA, Hamburg, Germany) at baseline, 12, and 18 months. All anthropometric measurements will be measured on a flat surface in triplicate with the mean value used as the final anthropometric measurement.
Lifestyle habits
Information about diet, alcohol intake, tobacco use, and physical activity is collected at baseline, 12-month, and 18-month follow-up visits using validated, standardized questionnaires, including the STEPwise approach to NCD risk factor surveillance (STEPS) [20], the Alcohol Use Disorders Identification Test (AUDIT) [21], the Global Adult Tobacco Survey (GATS) [22], and the International Physical Activity Questionnaire (IPAQ) [23].
Medication adherence
Participant adherence to antihypertensive medications is assessed using the Hill-Bone Compliance Scale [24]. The scale consists of 14 questions and 3 subscales assessing 3 key components of treatment compliance: reducing sodium intake, appointment keeping, and mediation adherence. The medication subscale assesses prescription filling habits, forgetfulness, and intentionally deciding not to take antihypertensive medications.
Acceptability
Participant acceptability of the ANDES implementation strategy will be assessed at 12 months post-randomization in all participants using a previously validated “Trust in CHWs” scale [25] that was translated into Spanish by our field and investigative team. The survey comprises 10 questions and was designed to assess trust across 2 domains: health care competence and respectful communication. Participant acceptability will also be assessed via in-depth interviews in a subset of participants after they complete their 12-month follow-up visit. This subset will be recruited using maximum variability sampling to ensure a representative sample across selected characteristics (e.g., urbanicity, hypertension control, age, and self-reported gender). CHW acceptability of the ANDES implementation strategy will be assessed via in-depth interviews with all CHWs.
Fidelity
Implementation fidelity is determined by assessing two key domains: CHW protocol adherence and the intervention dose received by participants. CHW protocol adherence is measured through CHW visit records, tracking the percentage of scheduled visits that were attempted and completed, as well as CHW visit checklists that document the specific intervention activities completed during the visits. CHWs are instructed to fill out the checklist with the participant to enhance the reliability of visit completeness reporting. Intervention dose is defined as the amount of the intervention that participants are exposed to and is measured by the number of completed CHW visits and specific activities conducted during those visits (e.g., blood pressure monitoring, counseling). The dose is influenced by both CHW fidelity to the protocol and participant adherence to the intervention, defined as the willingness to accept CHW visits and actively participate in the visit activities.
Plans for participant retention
The ANDES study uses various strategies to promote participant retention and ensure the completion of follow-up assessments. CHWs are trained to develop relationships of trust with participants, including using respectful communication, providing tailored counseling on health benefits, and facilitating study participation (i.e., offering flexible scheduling and assisting with healthcare appointment scheduling and medication acquisition). Participant engagement is tracked using CHW visit records and checklists; these are reported as part of the fidelity outcomes. To promote retention and participation in study outcome visits (baseline, 12, and 18 months), the ANDES field staff ensures that all participants are well-informed about study timelines and importance of each follow-up visit. Regardless of participant engagement in CHW-delivered interventions, we will continue to collect key outcome data at all follow-up intervals.
Statistical analysis
We will report all participants enrolled, eligible, and lost to follow-up in accordance with the Consolidated Standards for Reporting Trials (CONSORT) statement. Baseline characteristics, including age, gender, anthropometry, blood pressure, HbA1c, self-reported medication adherence, comorbidities, and healthcare accessibility, will be reported. Continuous data will be summarized as means with standard deviations or as medians with minimum and maximum values, contingent on their distribution. Categorical data will be summarized as proportions.
The primary and secondary effectiveness outcomes (Table 5) will be assessed using baseline and 12-month visits in accordance with the intention-to-treat (ITT) principle, in which all participants are accounted for regardless of loss to follow-up or level of protocol adherence, and intervention exposure is determined solely by randomized allocation. ITT facilitates an unbiased assessment of effectiveness in a real-world context. In the ITT analysis for the primary effectiveness outcome, we will use linear regression modeling. In this model, the dependent variable will be the change in SBP, and the primary independent variable will be group assignment. Additionally, the model will include the randomization strata by health network as a covariate. The primary and secondary effectiveness outcomes will utilize a two-sided test at an α-level of 0.05. We will use 95% confidence intervals for the purposes of estimation of health effects.
The primary analysis will be a mixed model analysis of variance without imputation. We use this approach both because it is more powerful when missing data rates are substantial and because imputation is highly uncertain in a study that includes no post-randomization assessments prior to the primary time point (12 months in ANDES). Subjects will be analyzed as part of the group to which they were assigned without consideration of whether they adhered to the intervention.
Within the intervention arm, the association between the proportion of blood pressure control at 12 months and secondary outcomes—such as implementation fidelity (adherence and dose) and participant-reported acceptability (trust in CHWs)—will be assessed.
The approach to calculating expected costs is based on the likelihood that individuals receiving treatment will control their conditions, thereby incurring lower costs, or fail to control their conditions, leading to higher costs through referrals and more complex care. A probability tree will be constructed using study data to compare the expected total costs for study participants versus non-participants. This comparison will help assess whether the ANDES model of care is potentially cost-saving from a societal perspective, or alternatively, to determine the net cost to the Peruvian healthcare system per unit of improvement in hypertension outcomes.
No interim analyses will be conducted.
Sample size
To detect a between-group difference in a mean change in systolic blood pressure of 2.5 mmHg between intervention and control arms with 95% confidence, 90% power, and a standard deviation of 12 mmHg, and assuming a conservative loss-to-follow-up of 10% at 12 months, we will randomize 1068 participants (534 participants per arm). We selected a target mean change of 2.5 mmHg as a minimal clinically important difference (MCID) based on prior research showing that even a 2.0 mmHg reduction in SBP can significantly reduce cardiovascular disease incidence and mortality [26, 27]. A recent CHW hypertension control trial in Argentina also demonstrated a 4 mmHg difference between groups, suggesting a potential for larger effects [12]. Our chosen MCID of 2.5 mmHg lies between the well-evidenced lower bound of 2 mmHg for clinically meaningful reductions in SBP and the 4 mmHg change in SBP observed in the Argentina trial, reflecting our decision to favor a more conservative effect size. The standard deviation of 12 mmHg was based on data from low-income participants in a household air pollution intervention trial conducted in the ANDES study area in Puno [28].
All patient effectiveness outcomes will be assessed using an intention-to-treat analysis.
Monitoring
The ANDES Data and Safety Monitoring Board (DSMB) comprises investigators with expertise in cardiovascular health, global RCTs, and biostatistics. To oversee study development, the DSMB has met yearly with the principal investigators. Central data management is conducted at WUSTL by the data management core (DMC). The DMC will monitor the data entered into REDCap on a daily basis to ensure the security of the de-identified information and to identify any quality issues. All adverse events and protocol violations will be reported to UPCH and Washington University in St. Louis. All protocol amendments will be approved by study team leadership and their respective Institutional Review Board Committees.
Discussion
The objectives of the ANDES trial are to evaluate the implementation and effectiveness of a CHW-led multicomponent strategy to reduce hypertension and type 2 diabetes in an underserved Andean population in Puno, Peru. The ANDES strategy adapts several components of the WHO HEARTS technical package and is designed to lower blood pressure by targeting improvements in antihypertensive medication adherence and healthy lifestyle behaviors. The trial is also designed to assess the sustained effects of the ANDES program on behaviors and health outcomes 6 months after the end of the intervention period. Overall, this trial is expected to provide valuable information for subsequent adaptation and scaling-up of the ANDES strategy across the Puno region and similar settings in Peru.
The ANDES trial is timely and represents an innovative approach within the Latin American context, particularly in Peru. While several studies have demonstrated that CHW-led strategies can improve hypertension management and related outcomes [10–12, 29], few have been conducted in Latin America [12]. This gap is significant because the effectiveness of CHW-based strategies to deliver hypertension care is influenced by various implementation factors (e.g., reach, fidelity) as well as unique local contextual factors at multiple levels, including individual, community, and system levels [see review [30]]. For example, socioeconomic disparities, healthcare infrastructure, and cultural dynamics in the region may affect the success of CHW-led strategies. Therefore, further research on CHW-led hypertension strategies in Latin America is essential. Recent findings from Argentina suggest that CHW-delivered hypertension management, including blood pressure monitoring and counseling in participant homes, is effective in improving outcomes for hypertensive patients and their families [4, 12]. The ANDES trial has adopted and tailored several essential components advocated by HEARTS and utilized in the Argentina study. ANDES represents Peru’s first CHW-managed hypertension control program.
Second, the ANDES trial is aligned with MINSA priorities to address the ongoing cardiovascular disease epidemic in Peru, and particularly to improve hypertension care. Prior research from the PERU MIGRANT study estimated hypertension awareness, treatment, and control rates of 48%, 40%, and 30%, respectively, with only 5% of hypertensives being both treated and controlled [31–33]. Furthermore, type 2 diabetes prevalence in Peru has doubled to 7.2% over the last 10 years, and is rising, with undiagnosed/pre-diabetes prevalence estimated at 35%. Identification of type 2 diabetes in people with hypertension is important because approximately 33% of people with hypertension also have type 2 diabetes, a combination that markedly increases morbidity and mortality. To address this escalating burden of hypertension and type 2 diabetes, MINSA and the local Puno Ministry of Health (DIRESA) have agreed to adopt the ANDES strategy as part of their efforts to implement the WHO HEARTS technical package in the Puno region. Third, by addressing both hypertension and diabetes, the ANDES strategy is also in line with recent appeals to investigate ways in which hypertension and type 2 diabetes management can be integrated as part of the HEARTS technical package, given the substantial overlap in risk factors, prognosis, and treatment [34].
The study design has many strengths. First, the ANDES CHW-led implementation strategy aims to be a pragmatic, cost-effective, sustainable, and culturally appropriate approach to increased awareness, treatment, and control of hypertension and type 2 diabetes. Currently, CHWs are utilized in Peru to primarily support the provision of communicable disease and maternal and child health care. Adapting a task-shifting model, the multicomponent ANDES hypertension control program aims to train and deploy CHWs to share the burden of hypertension education and medication adherence counseling, thereby potentially saving the existing health system and providers time and money. Furthermore, as members of the community, CHWs share many cultural, educational, and language characteristics with local participants, potentially reducing communication and trust barriers that can contribute to a lack of medication adherence [3, 4]. In the ANDES trial, CHWs are financially incentivized as paid, full-time staff, whereas most CHW work in Peru has been traditionally unpaid or minimally paid. This monetary benefit complements the often-reported intrinsic motivation of Peruvian CHWs to positively impact health outcomes within their local communities through community engagement (based on unpublished formative ANDES results).
Second, the biphasic design approach allowed for a comprehensive formative stage during which qualitative and quantitative research was conducted to collect data on barriers and facilitators to hypertension care in Puno, community preferences for CHW-led care, and other stakeholder preferences for ANDES strategy components. Third, the trial will provide information on implementation costs, which is crucial for local and national scale-up and sustainability. Lastly, beyond providing technical and policy-relevant information on the effectiveness and implementation of CHW-led hypertension care, the ANDES study has provided an impetus for diverse stakeholder communication, collaboration, and capacity building. For example, local CHW training and capacity building through expert-informed training programs that are co-created with local leaders will provide a model for future implementation efforts in the region.
Potential limitations of the study
While an individual randomization scheme was chosen to maximize efficiency and power, there is the potential for contamination, in which control participants may inadvertently receive the benefits of the implementation strategy due to social or geographical proximity [35]. Additionally, several other unmeasured factors may contribute to hypertension in Puno, such as environmental factors (e.g., altitude and indoor air pollution exposures from cooking with open fire stoves), individual factors (e.g., age, genetics, traditional risk factors), and limited access to health care. However, we anticipate that randomization will mitigate any significant confounding from these variables. Lastly, there is the potential for differentially higher attrition in the control arm since they are not receiving CHW or research visits during the first 12-month follow-up period; despite this risk, it is important that mid-study outcome visits are not conducted to avoid influencing the health care behaviors in the control arm and potentially biasing results towards the null.
Implications
The ANDES trial is testing the first CHW-led multicomponent strategy for hypertension and type 2 diabetes management in Peru. Importantly, this hybrid implementation and effectiveness trial will provide critical insights into the individual, community, and system-level factors that influence the implementation and effectiveness of the ANDES strategy. These data can inform the future adaptation and scaling of the ANDES strategy in Peru and other LMICs, as well as influence policies at the system level to support this transition. Furthermore, by addressing both hypertension and diabetes, the ANDES strategy supports integrated care approaches advocated by the WHO HEARTS technical package, ultimately enhancing health outcomes and reducing morbidity and mortality in the region.
Trial status
ANDES protocol version 2.0 (April 26, 2024). The recruitment process began on March 1, 2023 and is expected to end by March 31, 2025.
Supplementary Information
Additional file 1. Standardized Reporting Items: Recommendations for Intervention Trials (SPIRIT).
Additional file 2. The Standards for Reporting Implementation Studies (StaRI).
Acknowledgements
The authors wish to acknowledge formative research participants, health system stakeholders (MINSA and DIRESA), teams at the participating public health centers, and the A.B. PRISMA field staff. We also thank the members of the Data and Safety Monitoring Board who are overseeing the conduct of this study, including Vilma Irazola, MD (chair), Lawrence Appel, MD, and Leonardo Epstein, PhD.
Disclaimer
The content is solely the responsibility of the authors and does not represent the policy of the National Heart, Lung, and Blood Institute, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, United States (U.S.) Department of Health and Human Services, or the U.S. Government.
Trial sponsor
The trial sponsor is Universidad Peruana Cayetano Heredia (UPCH). Investigators from UPCH contributed to study design, data collection, management, analysis and interpretation, writing of reports, and the decision to submit this publication (as detailed in Author Acknowledgements). The primary contact for UPCH is below:
Stella M. Hartinger-Peña
Associate Professor
School of Public Health and Administration
Universidad Peruana Cayetano Heredia
Av. Honorio Delgado 430
San Martin de Porras
Lima, Peru
Email: stella.hartinger.p@upch.pe
Abbreviations
- ANDES
Addressing Hypertension and Diabetes through Community-Engaged Systems in Puno, Peru
- CHW
Community health worker
- LMIC
Low- and middle-income country
- MINSA
Peruvian Ministry of Health (Ministerio de Salud)
- PAHO
Pan American Health Organization
- REDCap
Research Electronic Data Capture
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
Authors’ contributions
SH, VG, WC, and EG conceptualized and designed the study; LU, KS, LCA, KC, KW, JG, PA, LB, ZV, and AB made substantial contributions to study design; RH, JM, and GC provided local expertise and adaptive recommendations for study design. LU and VG drafted primary manuscript; LF, KW, MH, JG, CM, AM, NR, MW, and VT made substantial contributions to manuscript drafting. All authors read and approved the final manuscript.
Funding
The study is supported by the National Heart, Lung, and Blood Institute (UG3 HL152371), NIH, Bethesda, MD, USA (multiple principal investigators: Hartinger-Peña, Dávila-Román, Checkley, Geng). The grant funding mechanism is a cooperative agreement (U-grant) that requires substantial involvement from NIH program or scientific staff. Accordingly, the NHLBI program representatives participate regularly in research team meetings, review protocols and documents, participate in Data and Safety Monitoring Board activities, and contribute to the writing and approval of manuscripts, including the current one.
Data availability
The datasets generated and/or analyzed will be available in the NHLBI BioLINCC repository (https://biolincc.nhlbi.nih.gov/home/) after completion of the study.
Declarations
Ethics approval and consent to participate
Ethical oversight is provided by the Universidad Peruana Cayetano Heredia in Lima, Peru Research Ethics Committee (104372). The study was also reviewed and approved by Washington University in St. Louis Human Research Protections Office (202108158) and registered at Johns Hopkins University School of Medicine institutional review board (IRB).
Consent for publication
Not applicable. No identifying images or personal or clinical details of participants are included here or will be in the trial reports. Participant information materials and the informed consent form are available from the corresponding author upon request.
Competing interests
MDH has received travel support from the American Heart Association and World Heart Federation. MDH has an appointment at The George Institute for Global Health, which has a patent, license, and has received investment funding with intent to commercialize fixed-dose combination therapy through its social enterprise business, George Medicines. MDH has pending patents for heart failure polypills.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
William Checkley, Victor G. Dávila-Román, and Stella M. Hartinger-Peña are co-last authors.
References
- 1.Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Fact sheets: hypertension. https://www.who.int/news-room/fact-sheets/detail/hypertension. Accessed 13 Mar 2024.
- 3.Mills KT, Bundy JD, Kelly TN, Reed JE, Kearney PM, Reynolds K, et al. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mills KT, Rubinstein A, Irazola V, Chen J, Beratarrechea A, Poggio R, et al. Comprehensive approach for hypertension control in low-income populations: rationale and study design for the hypertension control program in Argentina. Am J Med Sci. 2014;348(2):139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hypertension in Diabetes Study (HDS): II. Increased risk of cardiovascular complications in hypertensive type 2 diabetic patients. J Hypertens. 1993;11(3):319–25. [DOI] [PubMed] [Google Scholar]
- 6.American Heart Association. Health threats from high blood pressure. 2022. https://www.heart.org/en/health-topics/high-blood-pressure/health-threats-from-high-blood-pressure. Accessed 1 Nov 2023.
- 7.Medical doctors (per 10 000 population). https://www.who.int/data/gho/data/indicators/indicator-details/GHO/medical-doctors-(per-10-000-population). Accessed 16 Jun 2024.
- 8.Villena JE. Diabetes mellitus in Peru. Ann Glob Health. 2015;81(6):765–75. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. HEARTS technical package for cardiovascular disease management in primary health care: risk based CVD management. 2020. https://www.who.int/publications/i/item/9789240001367. Accessed 4 Mar 2023.
- 10.Brownstein JN, Chowdhury FM, Norris SL, Horsley T, Jack L, Zhang X, et al. Effectiveness of community health workers in the care of people with hypertension. Am J Prev Med. 2007;32(5):435–47. [DOI] [PubMed] [Google Scholar]
- 11.Shah MK, Wyatt LC, Gibbs-Tewary C, Zanowiak J, Mammen S, Mohsin FM, et al. Protocol and baseline characteristics for a community health worker-led hypertension and diabetes management program for South Asians in Atlanta: the DREAM Atlanta study. Contemp Clin Trials. 2022;120:106864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He J, Irazola V, Mills KT, Poggio R, Beratarrechea A, Dolan J, et al. Effect of a community health worker-led multicomponent intervention on blood pressure control in low-income patients in Argentina: a randomized clinical trial. JAMA. 2017;318(11):1016–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cappuccio FP, Kerry SM, Micah FB, Plange-Rhule J, Eastwood JB. A community programme to reduce salt intake and blood pressure in Ghana [ISRCTN88789643]. BMC Public Health. 2006;24(6):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Proctor E, Silmere H, Raghavan R, Hovmand P, Aarons G, Bunger A, et al. Outcomes for implementation research: conceptual distinctions, measurement challenges, and research agenda. Adm Policy Ment Health. 2011;38(2):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glasgow RE, Harden SM, Gaglio B, Rabin B, Smith ML, Porter GC, et al. RE-AIM planning and evaluation framework: adapting to new science and practice with a 20-year review. Front Public Health. 2019;7:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damschroder LJ, Reardon CM, Widerquist MAO, Lowery J. The updated Consolidated Framework for Implementation Research based on user feedback. Implement Sci. 2022;17(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Instituto Nacional de Estadística e Informática. Peru: Perfil Sociodemographico: Informe National. Censos Nacionales 2017: XII de Población, VII de Vivienda y III de Comunidades Indígenas. 2018. https://www.inei.gob.pe/media/MenuRecursivo/publicaciones_digitales/Est/Lib1539/. Accessed 5 Jul 2023.
- 18.Smith JD, Li DH, Rafferty MR. The Implementation Research Logic Model: a method for planning, executing, reporting, and synthesizing implementation projects. Implement Sci. 2020;15(1):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsoris A, Marlar CA. Use of the Child Pugh score in liver disease. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. http://www.ncbi.nlm.nih.gov/books/NBK542308/. Accessed 25 Jun 2024. [PubMed]
- 20.Riley L, Guthold R, Cowan M, Savin S, Bhatti L, Armstrong T, et al. The World Health Organization STEPwise approach to noncommunicable disease risk-factor surveillance: methods, challenges, and opportunities. Am J Public Health. 2016;106(1):74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction. 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Global Tobacco Surveillance System Data (GTSSData). Global Adult Tobacco Survey (GATS) — overview. https://nccd.cdc.gov/GTSSDataSurveyResources/Ancillary/Documentation.aspx?SUID=1&DOCT=1. Accessed 18 Oct 2024.
- 23.Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–95. [DOI] [PubMed] [Google Scholar]
- 24.Kim MT, Hill MN, Bone LR, Levine DM. Development and testing of the Hill-Bone Compliance to High Blood Pressure Therapy Scale. Prog Cardiovasc Nurs. 2000;15(3):90–6. [DOI] [PubMed] [Google Scholar]
- 25.Sripad P, McClair TL, Casseus A, Hossain S, Abuya T, Gottert A. Measuring client trust in community health workers: a multi-country validation study. J Glob Health. 2021;11:07009. [DOI] [PMC free article] [PubMed]
- 26.Hardy ST, Loehr LR, Butler KR, Chakladar S, Chang PP, Folsom AR, et al. Reducing the blood pressure–related burden of cardiovascular disease: impact of achievable improvements in blood pressure prevention and control. J Am Heart Assoc. 2015;4(10):e002276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stamler J, Rose G, Stamler R, Elliott P, Dyer A, Marmot M. INTERSALT study findings. Public health and medical care implications. Hypertension. 1989;14(5):570–7. [DOI] [PubMed] [Google Scholar]
- 28.Checkley W, Williams KN, Kephart JL, Fandiño-Del-Rio M, Steenland NK, Gonzales GF, et al. Effects of a household air pollution intervention with liquefied petroleum gas on cardiopulmonary outcomes in Peru. A randomized controlled trial. Am J Respir Crit Care Med. 2021;203(11):1386–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jafar TH, Gandhi M, de Silva HA, Jehan I, Naheed A, Finkelstein EA, et al. A community-based intervention for managing hypertension in rural South Asia. N Engl J Med. 2020;382(8):717–26. [DOI] [PubMed] [Google Scholar]
- 30.Abdel-All M, Putica B, Praveen D, Abimbola S, Joshi R. Effectiveness of community health worker training programmes for cardiovascular disease management in low-income and middle-income countries: a systematic review. BMJ Open. 2017;7(11):e015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernabe-Ortiz A, Sanchez JF, Carrillo-Larco RM, Gilman RH, Poterico JA, Quispe R, et al. Rural-to-urban migration and risk of hypertension: longitudinal results of the PERU MIGRANT study. J Hum Hypertens. 2017;31(1):22–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lerner AG, Bernabe-Ortiz A, Gilman RH, Smeeth L, Miranda JJ. The ‘rule of halves’ does not apply in Peru: awareness, treatment, and control of hypertension and diabetes in rural, urban and rural-to-urban migrants. Crit Pathw Cardiol. 2013;12(2):53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davies AR, Miranda JJ, Gilman RH, Smeeth L. Hypertension among adults in a deprived urban area of Peru – undiagnosed and uncontrolled? BMC Res Notes. 2008;1(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flood D, Edwards EW, Giovannini D, Ridley E, Rosende A, Herman WH, et al. Integrating hypertension and diabetes management in primary health care settings: HEARTS as a tool. Rev Panam Salud Publica. 2022;2(46):e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hemming K, Taljaard M, Moerbeek M, Forbes A. Contamination: how much can an individually randomized trial tolerate? Stat Med. 2021;40(14):3329–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Standardized Reporting Items: Recommendations for Intervention Trials (SPIRIT).
Additional file 2. The Standards for Reporting Implementation Studies (StaRI).
Data Availability Statement
The datasets generated and/or analyzed will be available in the NHLBI BioLINCC repository (https://biolincc.nhlbi.nih.gov/home/) after completion of the study.