Abstract
Background
Organic phosphate flame retardants (OPFRs) and phthalate acid esters (PAEs) are common endocrine-disrupting chemicals that cause metabolic disorders. This study aimed to assess the association between joint exposure to OPFRs and PAEs during early pregnancy in women with gestational diabetes mellitus (GDM).
Methods
Seven OPFRs and five PAEs were detected in the urine of 65 GDM patients and 100 controls using gas chromatography-tandem triple quadrupole mass spectrometry (GC-MS). The association of OPFRs and PAEs with GDM was assessed using logistic regression, weighted quantile sum (WQS) regression, and Bayesian kernel machine regression (BKMR) models.
Results
Levels of dibutyl phthalate (DBP), di-2-ethylhexyl phthalate (DEHP), diethyl phthalate (DEP), dimethyl phthalate (DMP), tris (2-butoxyethyl) phosphate (TBEP), tributyl phosphate (TBP), tris (2-chloroethyl) phosphate (TCEP), tris (1,3-dichloro-2-propyl) phosphate (TDCPP), tri-ortho-cresyl phosphate (TOCP), and triphenyl phosphate (TPHP) increased in the GDM group, and the OPFRs and PAEs, except for BBP and TMCP, were associated with GDM in the logistic regression analysis. In the WQS model, the mixture of OPFRs and PAEs was significantly positively associated with GDM (OR = 3.29, 95%CI = 1.27–8.51, P = 0.014), with TDCPP having the highest WQS index weight. BKMR analysis reinforced these results, showing that the overall association of joint exposure to the OPFRs and PAEs with GDM increased at exposure levels of the 55th to 75th percentiles. Independent exposure to TDCPP (OR = 1.42, 95%CI = 1.09–1.86, P = 0.011) and TBEP (OR = 1.29, 95%CI = 1.04–1.60, P = 0.023) were associated with an increased risk of GDM.
Conclusions
Environmental exposure to OPFRs and PAEs is significantly associated with GDM. These findings provide evidence for the adverse effects of exposure to OPFRs and PAEs on the health of pregnant women.
Keywords: Gestational diabetes mellitus, Organic phosphate flame retardants, Phthalate acid esters, Nested case-control study
Introduction
Gestational diabetes mellitus (GDM), defined as glucose intolerance of any degree that develops or is first recognized during pregnancy, is the most common endocrine disease during pregnancy [1]. Women with GDM have a significantly higher susceptibility to pregnancy-induced hypertension, premature delivery, premature rupture of membranes, fetal malformation, dystocia, cesarean section, macrosomia, neonatal respiratory distress syndrome, and jaundice [2–4]. Affected mothers and children are more susceptible to chronic diseases, such as obesity, hyperglycemia, type 2 diabetes mellitus (T2DM), and cardiovascular diseases in their later lives [1, 2]. The global prevalence of GDM ranges from 1 to 28% [5]. In China, the annual incidence increased from 4% in 2010 to 21% in 2020 [6]. The detailed etiology of GDM is still unclear, but the pathophysiological processes are thought to be similar to those of T2DM with insulin resistance and pancreatic β-cell dysfunction, which may be related to oxidative stress, inflammation, and exposure to environmental hazards, such as organic pollutants [7–14].
Organophosphorus flame retardants (OPFRs) and phthalate acid esters (PAEs) are ubiquitous organic pollutants found in a variety of environmental matrices [15, 16]. Although the use of some PAEs has been restricted since 1999, both OPFRs and PAEs have been widely used as flame retardants, plasticizers, lubricants, defoamers, and additives in plastic manufacturing, industrial products, and daily necessities, including cosmetics, food packaging, medical supplies, polyurethane foam, building materials, furniture, electronic appliances, and textiles [16–23]. Because OPFRs and PAEs are usually physically mixed rather than covalently bound to the polymer matrix, they can be easily released into the environment during production and manufacturing through abrasion, leaching, and volatilization. Consequently, they can often be found in the atmosphere, water in lakes and oceans, urban and suburban soils, dust, and sediments [24–26]. Moreover, OPFRs and PAEs have been extensively detected in biological specimens, such as blood, urine, hair, nails, cerebrospinal fluid, amniotic fluid, and various tissues [27–31]. Therefore, their co-exposure to populations is envisaged.
Based on animal and cell models, cumulative evidence has shown that exposure to OPFRs and PAEs may cause reproductive and metabolic toxicity, immunotoxicity, and endocrine-disrupting effects, posing a potential threat to human health [32–39]. Six PAEs have been listed as priority pollutants by the United States Environmental Protection Agency (EPA) and the European Union (EU): benzylbutyl phthalate (BBP), di-n-butyl phthalate (DnBP), di-n-octyl phthalate (DnOP), di-2-ethylhexyl phthalate (DEHP), diethyl phthalate (DEP), and dimethyl phthalate (DMP) [16]. As emerging environmental pollutants, OPFRs are receiving increasing attention because of their adverse health effects. Some OPFRs, such as tris (2-butoxyethyl) phosphate (TBEP), tributyl phosphate (TBP), tris (2-chloroethyl) phosphate (TCEP), tris (1,3-dichloro-2-propyl) phosphate (TDCPP), tri-m-cresyl phosphate (TMCP), and triphenyl phosphate (TPHP), have been categorized as priority substances that require further toxicological studies or regulatory measures [40].
Studies have been conducted to explore the association between PAEs and GDM, but the results have been inconsistent and contradictory [41–44]. Compared with studies on PAEs, there has only been insufficient data for OPFRs, in which exposure to tri-n-butyl phosphate (TNBP), TBEP, and TPHP during pregnancy was shown to be associated with GDM and increased glucose levels in a preliminary report [45]. To date, there have been no reports regarding the association of joint exposure to OPFRs and PAEs with GDM. In the present study, urinary OPFRs including TBEP, TBP, TCEP, TDCPP, TMCP, tri-o-cresyl phosphate (TOCP), and TPHP, and PAEs including BBP, dibutyl phthalate (DBP), DEHP, DEP, and DMP, during the first trimester were determined in 65 GDM cases and 100 controls. The association of joint exposure to the OPFRs and PAEs with GDM was examined using logistic regression, weighted quantile sum (WQS) regression, and Bayesian kernel machine regression (BKMR) analyses.
Materials and methods
Study populations
A birth cohort (2042 mother-child pairs) was developed in Liuzhou Maternity and Child Healthcare Hospital for women and children health research with follow-up via linkage to the medical records of the hospital from September 2016 to December 2018. This study was nested in the cohort study that included 82 GDM cases among 609 pregnant women from July to December 2018. The inclusion criteria were as follows: (1) permanent residents of Liuzhou City aged 20–45 years; (2) gestational age between 10 and 14 weeks as enrolled; (3) complete questionnaires; (4) available plasma and urinary samples; and (5) singleton pregnancy. Women with diabetes before pregnancy, thyroid diseases, liver and kidney diseases, infectious diseases, mental disorders, or communication barriers were excluded. Finally, 65 GDM cases were recruited and matched with 100 controls at a ratio of approximately 1:1.5. The diagnostic criteria for GDM were fasting blood glucose ≥ 5.1 mmol/L during pregnancy, one-hour blood glucose ≥ 10.0 mmol/L, or two-hour blood glucose ≥ 8.5 mmol/L in 75 g oral glucose tolerance test (OGTT) [46]. All participants signed an informed consent form, and the study was approved by the Ethics Committee of Guilin Medical University (No: GLMC20131205), which coincided with the World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects.
Data and biospecimen collection
Information including maternal age, race, occupation, household income, education, history of diabetes and GDM, and family history of diabetes was collected through face-to-face interviews for filling out a questionnaire. Blood and urine samples were collected during the first antenatal examination in the first trimester (10–14 weeks of pregnancy), and maternal blood pressure, body height, and body weight were measured and recorded. Maternal body mass index (BMI) was calculated by dividing weight (kg) by the square of height (m2). Pregnancy complication information was obtained from the hospital medical record system.
Determination of urinary OPFRs and PAEs
OPFRs and PAEs in urine were determined using high-performance gas chromatography-tandem triple quadrupole mass spectrometry (GC-MS) (Agilent 7000D, USA) at the Changchun Institute of Applied Chemistry, Chinese Academy of Sciences. The standardized compounds of seven OPFRs and five PAEs including TBEP (CAS: 78-51-3, purity 95.00%), TBP (CAS: 126-73-8, purity 98.00%), TCEP (CAS: 115-96-8, purity 98.00%), TDCPP (CAS: 13674-87-8, purity 96.00%), TMCP (CAS: 13674-84-5, purity 98.00%), TOCP (CAS: 78-30-8. purity 98.00%), TPHP (CAS: 513-08-6, purity 98.00%), BBP (CAS:85-68-7, purity 98.00%), DBP (CAS:84-74-2, purity 98.00%), DEHP (CAS:117-81-7, purity 98.00%), DEP (CAS:84-66-2, purity 98.00%), and DMP (CAS:131-11-3, purity 98.00%) were purchased from the China National Standard Center. The detailed analysis procedures for the OPFRs can be found elsewhere [47], and similar procedures were used in the PAEs analysis. In brief, 100 µL of standard solution containing the OPFRs and PAEs of each species at 10 mg/mL in anhydrous ether was placed in a 10 mL conical test tube and dried with nitrogen at room temperature. After re-dissolving in 10 mL of n-hexane, a series of concentrations (0, 1, 2, 5, 10, 20, 50, 100, and 200 ng/mL) were prepared to establish standard curves. The urine samples frozen at -80 °C were thawed at 4 °C, and then purified using a SampliQ OPT (3 mL, 60 mg) solid-phase extraction column by activation with 3 mL methanol for 30 min. After the column was dried by adding 1.68 g of Na2SO4, a 1 mL urine sample was loaded and balanced for 5 min, and then drained at a rate of 10–20 drops/min. The nonpolar impurities in the sample were removed with 10 mL n-hexane, and 6 mL of ether-n-hexane (9:1, v/v) was used to elute the column. The eluent was collected and dried with nitrogen, and re-dissolved in 10 mL of n-hexane for GC/MS analysis with a 122-3832E capillary column (30 m × 250 μm × 0.25 μm, Agilent). The initial temperature of the oven was 80°C, and then increased to 280°C at 12°C/min and maintained for 13 min. High-purity helium (purity ≥ 99.999%) was used as the carrier gas at a flow rate of 2.25 mL/min. The injection volume was 1.0 µL without a shunt. Electron ionization (EI) was used for MS analysis with an ionization voltage of 70 eV, an ion source temperature of 230°C, and a four-stage rod temperature of 150°C. The selected ion monitoring (SIM) model was used to detect SIM masses and retention times as follows: TBEP (299/125, 17.60 min), TBP (155/99, 11.30 min), TCEP (251/249, 13.20 min), TDCPP (380/191, 18.10 min), TMCP (277/125, 13.20 min), TOCP (367/165, 21.50 min), TPHP (325/170, 19.20 min), BBP (206/149, 18.10 min), DBP (223/149, 14.60 min), DEP (177/149, 11.30 min), DEHP (279/149, 18.70 min), and DMP (194/163, 8.0 min). The OPFRs and PAEs were quantified according to standard curves with linear correlation coefficients (squares) greater than 0.997. The limits of quantitation calculated with a signal-to-noise ratio (S/N) of 10/1 for TBEP, TBP, TCEP, TDCPP, TMCP, TOCP, TPHP, BBP, DBP, DEHP, DEP, and DMP were 0.627, 0.342, 0.423, 0.933, 0.387, 0.586, 0.298, 1.453, 4.547, 1.317, 0.670, and 0.510 ng/mL, respectively. Samples showing an exceeded concentration of the standard curve were diluted and re-analyzed, and those with values below the detection limits were replaced with the detection limit divided by the square root of 2 [48]. In the recovery study, six randomly selected samples were fortified with the seven OPFRs and five PAEs at three concentrations (1, 5, and 20 ng/mL) that covered most of the sample analyses. The recovery rates and relative standard deviations were in the range of 85–115% and 2.3–10.2%, respectively. Creatinine levels in the urine samples were measured using the creatine oxidase method (Nanjing Jiancheng, Cat. No.C011-2-1), and the OPFRs and PAEs concentrations were corrected with creatinine.
Statistical analysis
The information from the questionnaire was imported into EpiData. Statistical analyses were performed using R, version 4.2.1. Continuous data with a normal distribution were shown as mean ± standard deviation (x̄ ± SD) and were tested using Student’s t-test, while categorical data were represented as frequencies (n (%)) and were tested using the chi-square test. A log2 transformation was applied to the levels of OPFRs and PAEs to address skewed data that were represented as medians (interquartile range). Spearman’s correlation analysis was used to assess the correlations between OPFRs and PAEs in the control group. Based on the correlation coefficient (r), correlations were classified as very strong (> 0.80), strong (0.60–0.79), moderate (0.40–0.59), weak (0.20–0.39), and very weak (< 0.20) [49].
Binary logistic regression was used to investigate the association of OPFRs and PAEs with GDM. Maternal age, history of GDM, systolic blood pressure (SBP), and household income were adjusted in Model 1. In Model 2, the relationship between a single OPFR or PAE and GDM was estimated, as the other OPFRs and PAEs were further adjusted based on Model 1.
The WQS index was computed to annotate the overall effect of mixtures of the OPFRs and PAEs on the association with GDM, and the index weights of discrete compounds were concurrently assessed in the WQS model [50]. For the analysis, 30% of the data was used as the test dataset, 40% for validation, and 30% for prediction. The β1 coefficient was set as positive or negative, and 10,000 iterations were performed to explore the positive or negative correlation of the overall OPFRs and PAEs with GDM [51]. Log2-tranfored concentrations of the OPFRs and PAEs were used as independent variables, and maternal age, GDM history, SBP, and household income were adjusted in the WQS analysis.
To explore the relationship of exposure-response and the potential interaction between species of the OPFRs and PAEs, the BKMR model was used to assess the association of joint exposure to the OPFRs and PAEs with GDM [52]. The following questions were addressed. (1) The overall effect of the OPFRs and PAEs on GDM using their median exposure levels as reference; (2) the effect of individual OPFRs and PAEs on GDM, in which the potential sequential outcome (GDM) at the 25th to 75th percentiles of a single OPFR and PAE was calculated while the other OPFRs and PAEs were fixed at their 25th, 50th, and 75th percentiles; (3) the relationship between an OPFR or PAE and GDM using the univariate expose-response function when other OPFRs and PAEs were at their median levels; (4) the interaction between OPFRs and PAEs using a bivariate exposure-response function with the response of one OPFR or PAE to GDM when the exposure of another OPFR and PAE at the 10th, 50th, and 90th percentiles, respectively, and the other OPFRs and PAEs were fixed at their median. In addition, the interaction was indicated by estimating the modified GDM risk of exposure to one OPFR or PAE when the other OPFRs and PAEs increased from 10th to 90th percentiles.
Because there was a high correlation between species of the OPFRs and PAEs, the Markov chain Monte Carlo (MCMC) algorithm was used to implement probit regression. After 10,000 iterations for hierarchical variable selection, DBP, DEHP, DEP, DMP, TBP, TCEP, TDCPP, and TOCP were classified as Group 1, whereas TBEP and TPHP were classified as Group 2. The BKMR formula was Yi* = h (Group1 [DBP, DEHP, DEP, DMP, TBP, TCEP, TDCPP, TOCP], Group2 [TBEP, TPHP]) + βxi + εi. Yi* is a binary variable (1 = GDM; 0 = control). h () is the exposure-response function of the exposure and outcome, and xi, β, and εi are the covariate, coefficient, and residual terms, respectively. The group posterior inclusion probability (groupPIP) was estimated, and the conditional posterior inclusion probability (condPIP) was computed, which represented the probability that a particular OPFR and PAE within a group was included in the model. A PIP threshold of 0.50 is usually used to determine if it is important [53]. Log2-tranfored concentrations of OPFRs and PAEs were used as independent variables, and maternal age, GDM history, SBP, and household income were adjusted in the BKMR analysis.
Results
Demographic characteristics of study populations
The demographic characteristics of the study population (65 GDM cases and 100 controls), including maternal age, BMI, SBP, diastolic blood pressure (DBP), ethnicity, education level, household income, occupation, history of diabetes and GDM, and family history of diabetes, were presented in Table 1. There were no significant differences between the two groups in terms of BMI, DBP, ethnicity, education level, occupation, history of diabetes, or family history of diabetes (P > 0.05). However, maternal age, rate of GDM history, and SBP were higher, while household income was lower in the GDM group than in the control group (P < 0.05, P < 0.01) (Table 1). These differential characteristics were adjusted for confounders in logistic regression, WQS, and BKMR analyses.
Table 1.
Demographic characteristics of the study populations
| Characteristic | Control (n = 100) Mean ± SD or N (%) |
GDM (n = 65) Mean ± SD or N (%) |
P-value |
|---|---|---|---|
| Maternal age (year) | 31.24 ± 4.88 | 33.65 ± 4.72 | 0.002 |
| Body mass index (BMI, kg/m2) | 21.44 ± 2.61 | 22.15 ± 3.36 | 0.130 |
| SBP (mmHg) | 105.75 ± 9.20 | 108.92 ± 9.76 | 0.036 |
| DBP (mmHg) | 69.65 ± 7.76 | 70.47 ± 8.78 | 0.531 |
| Nationality | 0.116 | ||
| Han | 54 (54.0) | 42 (64.62) | |
| Zhuang | 35 (35.0) | 20 (30.77) | |
| Others | 11 (11.0) | 3 (4.62) | |
| Education level | 0.682 | ||
| High school and below | 26 (26.0) | 22 (33.85) | |
| University and below | 69 (69.0) | 37 (56.92) | |
| Above university | 5 (5.0) | 6 (9.23) | |
| Household income (yuan) | 0.014 | ||
| 0- | 7 (7.0) | 19 (29.23) | |
| 50,000- | 65 (65.0) | 30 (46.15) | |
| 100,000- | 28 (28.0) | 16 (24.62) | |
| Occupation | 0.778 | ||
| Office clerk | 76 (76.0) | 53 (81.54) | |
| Industrial worker | 1 (1.0) | 2 (3.08) | |
| Agricultural worker | 3 (3.0) | 2 (3.08) | |
| Others | 20 (20.0) | 8 (12.31) | |
| History of diabetes | 0.156 | ||
| No | 100 (100.0) | 63 (96.92) | |
| Yes | 0 (0) | 2 (3.08) | |
| History of gestational diabetes | 0.004 | ||
| No | 99 (99.0) | 58 (89.23) | |
| Yes | 1 (1.0) | 7 (10.77) | |
| Family history of diabetes | 0.309 | ||
| No | 96 (96.0) | 60 (92.31) | |
| Yes | 4 (4.0) | 5 (7.69) |
Note: P-values were derived using Student’s t-test or chi-square test. Bold numbers represented statistical significance (P < 0.05)
Levels of urinary OPFRs and PAEs in GDM and control groups
The detection rates of all the OPFRs and PAEs in the urinary samples were above 90%, except for TPHP (83.0%). Compared to the control group, the concentrations of TBEP, TBP, TCEP, TDCPP, TOCP, TPHP, DBP, DEHP, DEP, and DMP in the GDM group increased (P < 0.01). There was no significant difference in the BBP and TMCP levels between the two groups (P > 0.05) (Table 2).
Table 2.
Levels of the urinary OPFRs and PAEs in GDM and control groups
| OPFRs and PAEs |
Detection rate (%) | Control (n = 100) ng/mg creatinine |
GDM (n = 65) ng/mg creatinine |
P-value | ||
|---|---|---|---|---|---|---|
| P 50 | P25 - P75 | P 50 | P25 - P75 | |||
| TBP | 98.30 | 1.10 | 0.78–2.81 | 2.38 | 1.23–4.31 | 0.001 |
| TBEP | 92.00 | 14.83 | 5.21–46.61 | 33.08 | 13.23–66.40 | 0.002 |
| TCEP | 96.60 | 1.49 | 0.92–3.15 | 2.69 | 1.32–4.11 | 0.009 |
| TDCPP | 98.30 | 17.21 | 8.11–40.02 | 44.22 | 18.94–68.90 | < 0.001 |
| TMCP | 93.10 | 4.511 | 1.82–9.06 | 4.37 | 2.80–8.93 | 0.761 |
| TOCP | 95.40 | 2.037 | 1.09–4.03 | 4.25 | 1.73–9.19 | < 0.001 |
| TPHP | 83.00 | 1.305 | 0.84–3.29 | 2.47 | 1.15–3.40 | 0.007 |
| BBP | 98.90 | 24.86 | 12.13–67.70 | 25.67 | 9.18–81.12 | 0.881 |
| DBP | 98.90 | 50.40 | 20.85–81.48 | 80.71 | 35.37–178.06 | 0.005 |
| DEHP | 98.90 | 9.02 | 4.71–20.56 | 15.92 | 8.01–26.26 | 0.003 |
| DEP | 99.00 | 1.99 | 1.27–4.45 | 3.82 | 1.62–5.54 | 0.004 |
| DMP | 98.30 | 1.76 | 1.10–3.91 | 3.11 | 1.64–4.76 | 0.006 |
Note: Concentrations of urinary OPFRs and PAEs were presented as median (interquartile range). P-values were derived using Student’s t-test (log2-transformed data). Bold numbers represented statistical significance (P < 0.05)
Correlation between OPFRs and PAEs in control population
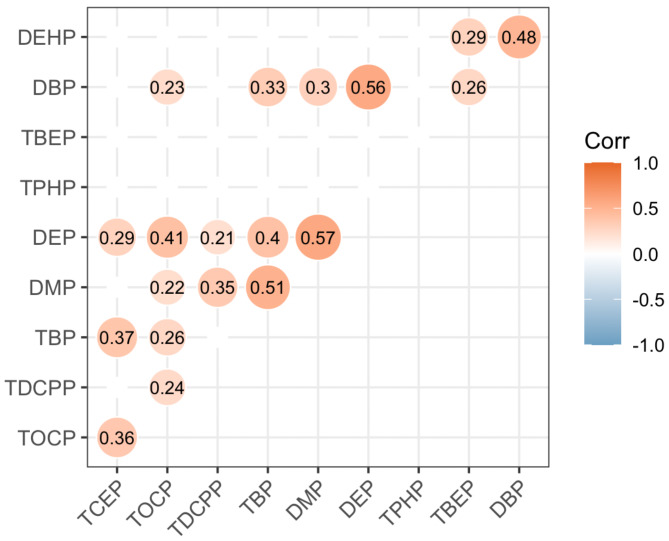
Correlation analysis revealed that DEP was moderately correlated with TOCP, TBP, DMP, and DBP, TBP with DMP, and DBP with DEHP (0.59 ≥ r ≥ 0.40) (Fig. 1). TOCP was weakly correlated with TDCPP, TBP, DMP, DBP, and TCEP, as well as DBP with TBP, DMP, and TBEP, TDCPP with DMP and DEP, TCEP with TBP and DEP, and DEHP with TBEP (0.39 ≥ r ≥ 0.20) (Fig. 1).
Fig. 1.
Heat map of the correlation between OPFRs and PAEs in the control population. The numbers in the figure were Spearman correlation coefficients (r). Correlations without statistical significance were hidden in the figure
Association of OPFRs and PAEs with GDM in logistic regression model
In logistic regression analysis, TBP, TBEP, TCEP, TDCPP, TOCP, TPHP, DBP, DEHP, DEP, and DMP (except for BBP and TMCP) were associated with GDM (P < 0.05, P < 0.01), in which the maternal age, history of GDM, household income, and SBP were adjusted, showing that for every 2-fold increase in TBP, TBEP, TCEP, TDCPP, TOCP, TPHP, DBP, DEHP, DEP, and DMP concentrations, the risk of GDM increased by 51%, 31%, 46%, 58%, 48%, 47%, 34%, 52%, 43%, and 51%, respectively (Table 3). However, only TDCPP and TBEP were significantly associated with GDM, while the other OPFRs and PAEs were adjusted as covariates, in addition to maternal age, history of GDM, household income, and SBP. For every two-fold increase in TDCPP and TPHP concentrations, GDM risk increased by 42% and 29%, respectively (P < 0.05) (Table 3).
Table 3.
Association of exposure to OPFRs and PAEs with GDM in logistic regress analysis
| OPFRs | Model 1a | P-value | Model 2b | P-value |
|---|---|---|---|---|
| TBP | 1.51 (1.18–1.93) | 0.001 | 1.29 (0.96–1.73) | 0.094 |
| TBEP | 1.31 (1.09–1.58) | 0.005 | 1.29 (1.04–1.60) | 0.023 |
| TCEP | 1.46 (1.11–1.94) | 0.007 | 1.17 (0.81–1.67) | 0.408 |
| TDCPP | 1.58 (1.26–1.99) | < 0.001 | 1.42 (1.09–1.86) | 0.011 |
| TMCP | 1.06 (0.83–1.34) | 0.664 | 0.94 (0.70–1.25) | 0.649 |
| TOCP | 1.48 (1.17–1.86) | 0.001 | 1.33 (1.00–1.78) | 0.051 |
| TPHP | 1.47 (1.11–1.95) | 0.008 | 1.31 (0.94–1.81) | 0.111 |
| BBP | 1.03 (0.86–1.22) | 0.765 | 0.92 (0.74–1.14) | 0.450 |
| DBP | 1.34 (1.08–1.65) | 0.008 | 1.11 (0.85–1.45) | 0.442 |
| DEHP | 1.52 (1.18–1.95) | 0.001 | 1.20 (0.88–1.64) | 0.243 |
| DEP | 1.43 (1.08–1.88) | 0.012 | 0.90 (0.61–1.34) | 0.603 |
| DMP | 1.51 (1.13–2.01) | 0.005 | 0.94 (0.63–1.40) | 0.750 |
Note: aMaternal age, history of GDM, household income, SBP were adjusted. bMaternal age, history of GDM, household income, SBP, the other OPFRs and PAEs were adjusted
Total effects of OPFRs and PAEs on association with GDM in WQS model
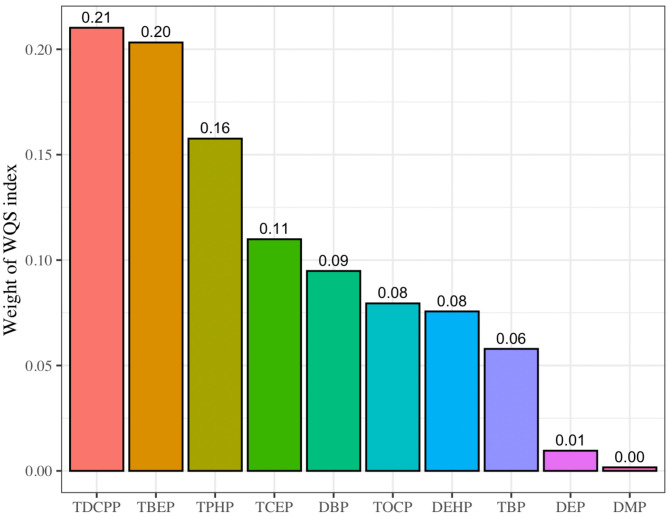
After adjusting for maternal age, history of GDM, SBP, and household income, the mixture of OPFRs and PAEs was significantly positively associated with GDM (OR = 3.29, 95%CI = 1.27–8.51, P = 0.014) in the WQS regression model, indicating that GDM risk increased by 229% for every two-fold increase in exposure to the OPFRs and PAEs. TDCPP had the highest WQS index weight, accounting for 21.4% of the overall effect on GDM, followed by TBEP, TPHP, TCEP, DBP, TOCP, DEHP, TBP, DEP, and DMP, with the weights of 20.2%, 15.6%, 10.7%, 9.3%, 8.3%, 7.7%, 5.9%, 1.0%, and 0.1%, respectively (Fig. 2). No significant association between the β1 coefficient and GDM was observed in the negative direction of analysis.
Fig. 2.
WQS index weights of the OPFRs and PAEs associated with GDM. The. analysis was based on the WQS regression modeled in the positive direction with respect to the outcome (GDM)
Association of exposures to OPFRs and PAEs with GDM in BKMR analysis
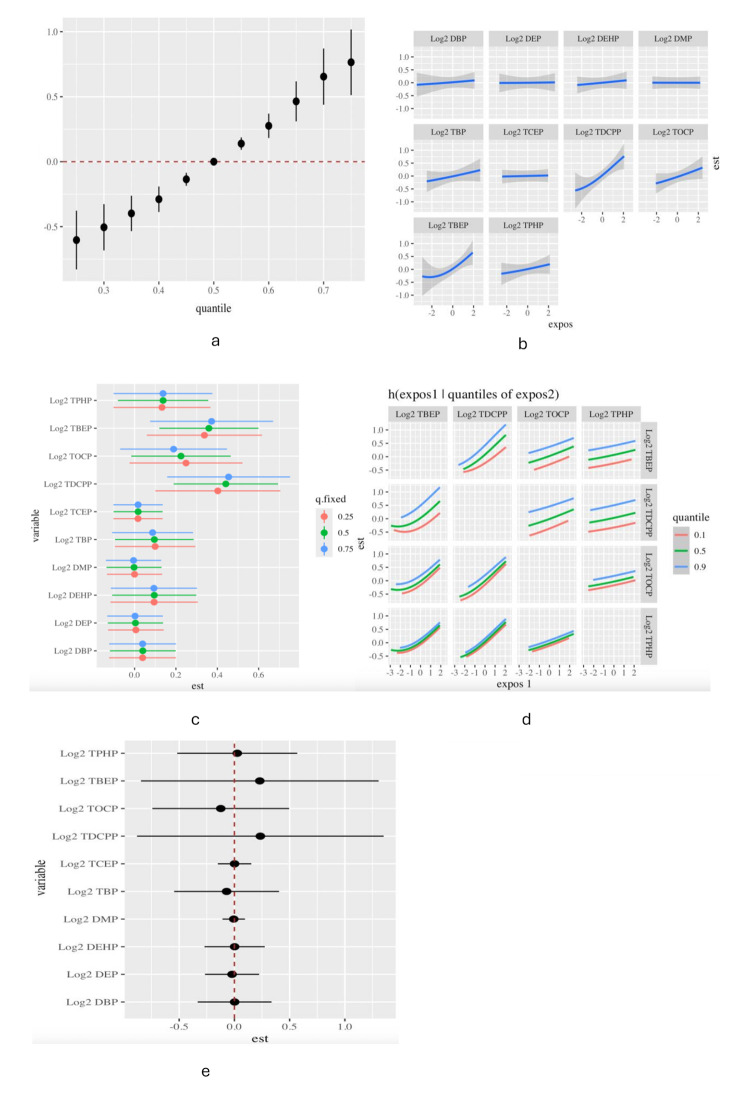
The PIPs derived from the BKMR model for the two groups (groupPIP) and each of the OPFRs and PAEs (condPIP) were listed, and two OPFRs (TDCPP and TBEP) were identified as important (PIPs > 0.50) (Table 4). The overall association of joint exposure to the OPFRs and PAEs with potential sequential outcomes was determined to be a significant increase in GDM when all the OPFRs and PAEs were at their 55th to 75th percentiles compared to their 50th percentiles, and the increasing trend remained at the 75th percentile (Fig. 3a). When other OPFRs and PAEs were fixed at median exposure levels, DBP, DEHP, TBEP, TBP, TDCPP, TOCP, and TPHP were positively associated with GDM (Fig. 3b). When the exposure levels of the other OPFRs and PAEs were fixed at the 25th, 50th, and 75th percentiles, only TDCPP and TBEP were significantly positively associated with GDM (Fig. 3c). Based on the estimation of bivariate exposure-response functions, a potential interaction was observed between the slope of the curve for TDCPP at the 10th, 50th, and 90th modifications of TBEP (with the other OPFRs fixed at the median). Similarly, TOCP potentially interacted with TDCPP and TBEP (Fig. 3d). However, the GDM risk of exposure to TDCPP, TBEP, and TOCP was not significantly modified by only 0.24-, 0.23-, and 0.12-unit changes, respectively, when the remaining OPFRs and PAEs increased from the 10th to 90th percentiles (Fig. 3e), indicating no interaction between them.
Table 4.
PIPs of the OPFRs and PAEs
| OPERs and PAEs | Groups | GroupPIP | CondPIP |
|---|---|---|---|
| Log2 DBP | 1 | 0.994 | 0.017 |
| Log2 DEHP | 1 | 0.994 | 0.014 |
| Log2 DEP | 1 | 0.994 | 0.019 |
| Log2 DMP | 1 | 0.994 | 0.004 |
| Log2 TBP | 1 | 0.994 | 0.054 |
| Log2 TCEP | 1 | 0.994 | 0.007 |
| Log2 TDCPP | 1 | 0.994 | 0.897 |
| Log2 TOCP | 1 | 0.994 | 0.118 |
| Log2 TBEP | 2 | 0.882 | 0.767 |
| Log2 TPHP | 2 | 0.882 | 0.103 |
Note. The bold numbers represented “important” as PIP > 0.50
Fig. 3.
Association of exposure to the OPFRs and PAEs with GDM in BKMR analysis. (a) Overall effects of OPFRs and PAEs exposure on GDM. (b) The univariate exposure-response effect of individual OPFRs and PAEs on GDM, while the exposure levels of the other OPFRs and PAEs were fixed at the median levels. (c) Association of the OPFRs and PAEs with GDM when the other OPFRs and PAEs were at the 25th, 50th, and 75th percentiles, respectively. (d) The bivariate exposure-response function for one OPFR with another OPFR that was fixed at the 10th, 50th, and 90th percentiles, and the other OPFRs were fixed at their medians. (e) GDM risk for exposure to one OPFRs and PAEs when the other OPFRs and PAEs increased from the 10th to 90th percentiles
Discussion
As emerging environmental pollutants and persistent organic pollutants (POPs), OPFRs and PAEs have attracted increasing attention from the international community because of their adverse effects on reproductive, maternal, and child health. Based on a nested case-control study, we found that the levels of urinary TBEP, TBP, TCEP, TDCPP, TOCP, TPHP, DBP, DEHP, DEP, and DMP remarkably increased in the GDM population. Mixtures of the OPFRs and PAEs, and individual exposure to TDCPP and TBEP were associated with GDM. Furthermore, TDCPP had the highest WQS index weight for GDM, followed by TBEP, which accounted for nearly 40% of the total weight. This study revealed the adverse effects of joint exposure to OPFRs and PAEs on the health of pregnant women.
Exposures of OPFRs and PAEs on populations
PAEs are highly abundant chemicals primarily used to fabricate soft and flexible plastic materials. Although the restriction of some PAEs, such as DEHP, DnBP, butylbenzyl phthalate (BBzP), diisobutyl phthalate (DiBP), and di-n-pentyl phthalate (DnPeP), was implemented between 1999 and 2020 [54], PAEs are still widely used, with global production estimated at 300–500 million tons by 2050 [16, 55]. Moreover, China has become the world’s largest producer and consumer of plasticizers, accounting for nearly half of global consumption [16]. As a substitute for PAEs, the production and use of OPFRs dramatically increase from 100,000 tons worldwide in 1992 to 1,050,000 tons in 2018 [15, 56]. This significantly increases the risk of exposure to OPFRs and PAEs. Correspondingly, the detectable concentrations of metabolites of PAEs, such as DEHP, DEP, and DBP, in urine were 0.1–1000 µg/L among populations in different countries and areas [57]. In Chinese pregnant women, the average concentration of PAE metabolites in urine was 5.7–28.6 ng/mL [58], while the levels of OPFRs, including TBEP, TBP, TCEP, TCPP, tris (2-ethylhexyl) phosphate (TEHP), and TPHP in the plasma of citizens in Zhejiang, China, ranged from 1.191 to 13.030 ng/mL [59]. In our study, seven OPFRs and five PAEs were detected in most urine samples, and the median concentrations in the study population ranged from 1.104 to 50.397 ng/mg creatinine and 2.383–80.713 ng/mg creatinine, respectively. Except for BBP and TMCP, the other OPFRs and PAEs significantly increased in patients with GDM. It has been reported that environmental exposure to OPFRs is significantly associated with individual behaviors, such as frequency of eating out and hand-washing habits before eating [60]. The high exposure of the population to OPFRs and PAEs is probably related to the environmental pollution in Liuzhou, a heavy industrial city, and the living behaviors of local residents whose health literacy levels are lower than those of other areas in China [61].
Spearman’s correlation analysis indicated significant correlations between the species of OPFRs and PAEs. DEP was moderately correlated with DBP, TBP, DMP, and TOCP, TBP with DMP, and DBP with DEHP. A week correlation was also observed in most of the other OPFRs and PAEs. These correlations not only suggest a common source of exposure to the environmental pollutants in the study populations but also provide information for BKMR analysis and for further studies of the joint exposure and potential adverse effects of OPFRs and PAEs in the future.
Association of exposure to OPFRs and PAEs with GDM
The association of exposure to OPFRs and PAEs with GDM was examined using different statistical models. TDCPP and TBEP were associated with GDM in the logistic regression analysis. These findings were reinforced in the WQS and BKMR models, demonstrating that mixtures of OPFRs and PAEs (WQS indices) were positively associated with GDM, which increased in an exposure-response pattern in the BKMR model. Moreover, accounting for the majority of the WQS indices, TDCPP and TBEP were identified as important (PIPs > 0.50), and individual exposure to TDCPP and TBEP was positively associated with GDM. Although a systematic review and meta-analysis revealed that exposure to PAEs reflected by their metabolites, including DEHP, mono-n-butyl phthalate (MBP), mono-benzyl phthalate (MBzP), mono-3-carboxypropyl phthalate (MCPP), mono-(2-ethylhexyl) phthalate (MEHP), mono-ethyl phthalate (MEP), and mono-isobutyl phthalate (MiBP), was significantly positively associated with the risk of GDM (OR = 1.10; 95% CI = 1.04–1.16; n = 7) [41], some studies found no association between phthalate exposure and GDM [42, 43], or only with GDM risk factors, such as gestational weight gain (GWG) [43], or even with decreased odds of GDM (higher with 1st trimester MCPP, Q4 v. Q1: 0.30; 95% CI: 0.13–0.67) [44]. A case-control study conducted in Hangzhou, China, including 130 and 67 women with and without GDM, respectively, showed that serum TBOEP (OR = 2.63; 95% CI: 1.68–4.11) was positively associated with GDM and increased glucose levels [45]. In 349 adolescents (12-19-year) from the National Health and Nutrition Examination Survey (NHANES), urinary BDCPP was positively associated with prediabetes [62]. However, to date, there have been no reports concerning the association of joint exposure to OPFRs and PAEs with GDM. In the present study, joint exposure was identified, or PAEs were excluded from individual exposure to be associated with GDM, indicating that OPFRs (TDCPP and TBEP) were more harmful to pregnant women’s health. Although OPFRs are less environmentally persistent and have a shorter half-life than PAEs in the human body, it has been proposed that the replacement of PAEs (polybrominated diphenyl ethers) with OPFRs is likely a regrettable substitution because the in vitro activity of OPFRs (TDCPP and TPHP) is comparable to that of some PAEs, and some OPFRs (TCEP, TDCPP, and TCPP) may be associated with an increased risk of cancer and reproductive effects [40].
Joint exposure to environmental pollutants may involve complex interactions between chemicals, leading to adverse health effects of individual compounds below the threshold values for their toxicity. This synergy is often seen as the pollutants can act in the same mechanistic pathway or one influence the clearance of the others [63]. The chemical structure of OPFRs contains oxygen on the phosphate group connected to an alkyl chain or a benzene ring [64], and APEs consist of a planar aromatic hydrocarbon and two fatty side chains [65]. Based on structural characteristics, it is reasonable to speculate that the interaction between the homologous series of OPFRs or APEs will play a role in GDM development. However, we did not observe a significant interaction between these pollutants in this study, indicating their independent effect on GDM. Further research is required to clarify these issues.
Limitations of the study
To our knowledge, this is the first study to report a positive association of joint exposure to OPFRs and PAEs with GDM based on a nested case-control study, and the association was estimated and confirmed using different statistical models. However, we did not determine the metabolites of the OPFRs and PAEs in this study. Consequently, it is difficult to accurately assess the dose of long-term environmental exposures of OPFRs and PAEs on populations, especially for a single determination that is restricted to seven and five species, respectively. In addition, this was a small sample size study including 65 GDM cases and 100 controls, and all participants were residents of Liuzhou, which may have geographical limitations.
Conclusion
Joint exposure to OPFRs and PAEs, and individual exposure to TDCPP and TBEP were significantly positively associated with GDM. This study provides evidence of the adverse effects of environmental exposure to OPFRs and PAEs on the health of pregnant women.
Author contributions
B.H, XY. Z, and Q.L contributed to the conception and design of the study. XY. Y, XY. Z, XF. Q, SD. W, JY. W, CC. Z, and M.Z collected data and organized the database. Q.L performed the statistical analysis and wrote the first draft of the manuscript. J.Z, DY. Z, and B.H organized the birth cohort study and wrote sections of the manuscript. All authors contributed to manuscript revision and approved the submitted version.
Funding
This study was supported by the Key Research and Development Program of Guangxi (Grant Number 2018AB62004) and the National Natural Science Foundation of China (Grant Number 81760614).
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
All participants signed an informed consent form, and the study was approved by the Ethics Committee of Guilin Medical University (No. GLMC20131205). We ensured that the study protocols coincided with the relevant guidelines and regulations, including the ethical principles for medical research involving human subjects declared by the World Medical Association (Helsinki).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qi Lang and Xianfeng Qin contributed equally to this work.
Contributor Information
Xiaoying Zhang, Email: xiaoyingzhang79@163.com.
Bo Huang, Email: Bo.Huang@glmc.edu.cn.
References
- 1.Paschou SA, et al. <ArticleTitle Language=“En”>Screening and management of major endocrinopathies during pregnancy: an update. Endocrine. 2023;80(1):10–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon JH, Jang HC. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab J. 2022;46(1):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Salomon C, et al. Gestational Diabetes Mellitus Is Associated With Changes in the Concentration and Bioactivity of Placenta-Derived Exosomes in Maternal Circulation Across Gestation. Diabetes. 2016;65(3):598–609. [DOI] [PubMed] [Google Scholar]
- 4.Ye W, et al. Gestational diabetes mellitus and adverse pregnancy outcomes: systematic review and meta-analysis. BMJ. 2022;377:e067946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saeedi M, et al. Increasing prevalence of gestational diabetes mellitus when implementing the IADPSG criteria: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2021;172:108642. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H, et al. The prevalence of gestational diabetes mellitus before and after the implementation of the universal two-child policy in China. Front Endocrinol (Lausanne). 2022;13:960877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacNeill S, et al. Rates and risk factors for recurrence of gestational diabetes. Diabetes Care. 2001;24(4):659–62. [DOI] [PubMed] [Google Scholar]
- 8.McIntyre HD, et al. Gestational diabetes mellitus. Nat Rev Dis Primers. 2019;5(1):47. [DOI] [PubMed] [Google Scholar]
- 9.Lappas M, et al. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal. 2011;15(12):3061–100. [DOI] [PubMed] [Google Scholar]
- 10.Orhan H, et al. Circulating biomarkers of oxidative stress in complicated pregnancies. Arch Gynecol Obstet. 2003;267(4):189–95. [DOI] [PubMed] [Google Scholar]
- 11.Karowicz-Bilinska A. [Lipid peroxidation in women with gestational hypertension complicated by asymetric intrauterine growth retardation]. Ginekol Pol. 2006;77(6):435–40. [PubMed] [Google Scholar]
- 12.Pasinski J, et al. [The influence of vitamin C and E use on concentration of endothelin-1 and lipid peroxides in the serum of pregnant women with arterial hypertension]. Ginekol Pol. 2013;84(1):32–7. [DOI] [PubMed] [Google Scholar]
- 13.Jorgensen ME, Borch-Johnsen K, Bjerregaard P. A cross-sectional study of the association between persistent organic pollutants and glucose intolerance among Greenland Inuit. Diabetologia. 2008;51(8):1416–22. [DOI] [PubMed] [Google Scholar]
- 14.Turyk M, et al. Prevalence of diabetes and body burdens of polychlorinated biphenyls, polybrominated diphenyl ethers, and p,p’-diphenyldichloroethene in Great Lakes sport fish consumers. Chemosphere. 2009;75(5):674–9. [DOI] [PubMed] [Google Scholar]
- 15.Tian YX, et al. A critical review on sources and environmental behavior of organophosphorus flame retardants in the soil: Current knowledge and future perspectives. J Hazard Mater. 2023;452:131161. [DOI] [PubMed] [Google Scholar]
- 16.Huang L, et al. Phthalic Acid Esters: Natural Sources and Biological Activities. Toxins (Basel). 2021;13(7):495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X, Zhao X, Shi Z. Organophosphorus flame retardants in breast milk from Beijing, China: Occurrence, nursing infant’s exposure and risk assessment. Sci Total Environ. 2021;771:145404. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, et al. Rapid electrochemical reduction of a typical chlorinated organophosphorus flame retardant on copper foam: degradation kinetics and mechanisms. Chemosphere. 2021;264(Pt 2):128515. [DOI] [PubMed] [Google Scholar]
- 19.Araki A, et al. Phosphorus flame retardants in indoor dust and their relation to asthma and allergies of inhabitants. Indoor Air. 2014;24(1):3–15. [DOI] [PubMed] [Google Scholar]
- 20.Cooper EM, et al. Results from Screening Polyurethane Foam Based Consumer Products for Flame Retardant Chemicals: Assessing Impacts on the Change in the Furniture Flammability Standards. Environ Sci Technol. 2016;50(19):10653–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stapleton HM, et al. Detection of organophosphate flame retardants in furniture foam and U.S. house dust. Environ Sci Technol. 2009;43(19):7490–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stapleton HM, et al. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 2012;46(24):13432–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao DW, Wen ZD. Phthalate esters in the environment: A critical review of their occurrence, biodegradation, and removal during wastewater treatment processes. Sci Total Environ. 2016;541:986–1001. [DOI] [PubMed] [Google Scholar]
- 24.Tang B, et al. Bioconcentration and biotransformation of organophosphorus flame retardants (PFRs) in common carp (Cyprinus carpio). Environ Int. 2019;126:512–22. [DOI] [PubMed] [Google Scholar]
- 25.Hou R, Xu Y, Wang Z. Review of OPFRs in animals and humans: Absorption, bioaccumulation, metabolism, and internal exposure research. Chemosphere. 2016;153:78–90. [DOI] [PubMed] [Google Scholar]
- 26.Fromme H, et al. Occurrence of phthalates and bisphenol A and F in the environment. Water Res. 2002;36(6):1429–38. [DOI] [PubMed] [Google Scholar]
- 27.Butt CM, et al. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol. 2014;48(17):10432–8. [DOI] [PubMed] [Google Scholar]
- 28.Cooper EM, et al. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401(7):2123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao F, et al. Organophosphorus Flame Retardants in Pregnant Women and Their Transfer to Chorionic Villi. Environ Sci Technol. 2017;51(11):6489–97. [DOI] [PubMed] [Google Scholar]
- 30.Zhao F, et al. Urinary biomarkers for assessment of human exposure to monomeric aryl phosphate flame retardants. Environ Int. 2019;124:259–64. [DOI] [PubMed] [Google Scholar]
- 31.He MJ, et al. Phthalate esters in biota, air and water in an agricultural area of western China, with emphasis on bioaccumulation and human exposure. Sci Total Environ. 2020;698:134264. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y, et al. Hazards of phthalates (PAEs) exposure: A review of aquatic animal toxicology studies. Sci Total Environ. 2021;771:145418. [DOI] [PubMed] [Google Scholar]
- 33.Farhat A, et al. Ovo effects of two organophosphate flame retardants–TCPP and TDCPP–on pipping success, development, mRNA expression, and thyroid hormone levels in chicken embryos. Toxicol Sci. 2013;134(1):92–102. [DOI] [PubMed] [Google Scholar]
- 34.Liu X, Ji K, Choi K. Endocrine disruption potentials of organophosphate flame retardants and related mechanisms in H295R and MVLN cell lines and in zebrafish. Aquat Toxicol. 2012;114–115:173–81. [DOI] [PubMed] [Google Scholar]
- 35.Moser VC, et al. Neurotoxicological and thyroid evaluations of rats developmentally exposed to tris(1,3-dichloro-2-propyl)phosphate (TDCIPP) and tris(2-chloro-2-ethyl)phosphate (TCEP). Neurotoxicol Teratol. 2015;52(Pt B):236–47. [DOI] [PubMed] [Google Scholar]
- 36.Wang Q, et al. Exposure of zebrafish embryos/larvae to TDCPP alters concentrations of thyroid hormones and transcriptions of genes involved in the hypothalamic-pituitary-thyroid axis. Aquat Toxicol. 2013;126:207–13. [DOI] [PubMed] [Google Scholar]
- 37.Welsh JJ, et al. Teratogenic potential of triphenyl phosphate in Sprague-Dawley (Spartan) rats. Toxicol Ind Health. 1987;3(3):357–69. [DOI] [PubMed] [Google Scholar]
- 38.Liu YE, et al. Organophosphorus flame retardants in fish from Rivers in the Pearl River Delta, South China. Sci Total Environ. 2019;663:125–32. [DOI] [PubMed] [Google Scholar]
- 39.Yao Y, et al. Exposure to organophosphate ester flame retardants and plasticizers during pregnancy: Thyroid endocrine disruption and mediation role of oxidative stress. Environ Int. 2021;146:106215. [DOI] [PubMed] [Google Scholar]
- 40.Blum A, et al. Organophosphate Ester Flame Retardants: Are They a Regrettable Substitution for Polybrominated Diphenyl Ethers? Environ Sci Technol Lett. 2019;6(11):638–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yan D, et al. Endocrine-disrupting chemicals and the risk of gestational diabetes mellitus: a systematic review and meta-analysis. Environ Health. 2022;21(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shapiro GD, et al. Exposure to phthalates, bisphenol A and metals in pregnancy and the association with impaired glucose tolerance and gestational diabetes mellitus: The MIREC study. Environ Int. 2015;83:63–71. [DOI] [PubMed] [Google Scholar]
- 43.Zukin H, et al. Prenatal exposure to phthalates and maternal metabolic outcomes in a high-risk pregnant Latina population. Environ Res. 2021;194:110712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.James-Todd T, et al. Urinary phthalate and DINCH metabolite concentrations and gradations of maternal glucose intolerance. Environ Int. 2022;161:107099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jin H, et al. A preliminary report on the association between maternal serum organophosphate ester concentrations and gestational diabetes mellitus. Heliyon. 2023;9(3):e14302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mack LR, Tomich PG. Diagnosis, Classification, and Clinical Care. Obstet Gynecol Clin North Am. 2017;44(2):207–17. [DOI] [PubMed] [Google Scholar]
- 47.Lang Q, et al. Environmental exposures to organophosphorus flame retardants in early pregnancy and risks of gestational diabetes mellitus: a nested case-control study. Sci Rep. 2024;14(1):13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee M, et al. Accommodating detection limits of multiple exposures in environmental mixture analyses: an overview of statistical approaches. Environ Health. 2024;23(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Papageorgiou SN. On correlation coefficients and their interpretation. J Orthod. 2022;49(3):359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Araki A, et al. Combined exposure to phthalate esters and phosphate flame retardants and plasticizers and their associations with wheeze and allergy symptoms among school children. Environ Res. 2020;183:109212. [DOI] [PubMed] [Google Scholar]
- 51.Keil AP, et al. A Quantile-Based g-Computation Approach to Addressing the Effects of Exposure Mixtures. Environ Health Perspect. 2020;128(4):47004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bobb JF, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics. 2015;16(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Y, et al. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ Int. 2019;123:325–36. [DOI] [PubMed] [Google Scholar]
- 54.Vogel N, et al. Current exposure to phthalates and DINCH in European children and adolescents - Results from the HBM4EU Aligned Studies 2014 to 2021. Int J Hyg Environ Health. 2023;249:114101. [DOI] [PubMed] [Google Scholar]
- 55.Wang Y, Qian H. Phthalates and Their Impacts on Human Health. Healthc (Basel). 2021;9(5):603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang J et al. A Review of a Class of Emerging Contaminants: The Classification, Distribution, Intensity of Consumption, Synthesis Routes, Environmental Effects and Expectation of Pollution Abatement to Organophosphate Flame Retardants (OPFRs). Int J Mol Sci, 2019. 20(12). [DOI] [PMC free article] [PubMed]
- 57.Wang Y, Zhu H, Kannan K. A Review of Biomonitoring of Phthalate Exposures. Toxics. 2019;7(2):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu Y, et al. Relationship between maternal phthalate exposure and offspring size at birth. Sci Total Environ. 2018;612:1072–8. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Q, et al. Partitioning behavior-oriented health risk assessment on internal organophosphorus flame retardants exposure. Environ Res. 2023;216(Pt 4):114704. [DOI] [PubMed] [Google Scholar]
- 60.Chen FS, et al. Urinary levels of organophosphate flame retardants metabolites in a young population from Southern Taiwan and potential health effects. Front Endocrinol (Lausanne). 2023;14:1173449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li Y, et al. The development and progress of health literacy in China. Front Public Health. 2022;10:1034907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Luo K, et al. Urinary organophosphate esters metabolites, glucose homeostasis and prediabetes in adolescents. Environ Pollut. 2020;267:115607. [DOI] [PubMed] [Google Scholar]
- 63.Lagunas-Rangel FA, et al. Role of the Synergistic Interactions of Environmental Pollutants in the Development of Cancer. Geohealth. 2022;6(4):e2021GH000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang L, et al. A new and systematic review on the efficiency and mechanism of different techniques for OPFRs removal from aqueous environments. J Hazard Mater. 2022;431:128517. [DOI] [PubMed] [Google Scholar]
- 65.Chen X, Li Y. Toxicity remission of PAEs on multireceptors after molecular modification through a 3D-QSAR pharmacophore model coupled with a gray interconnect degree method. Turk J Chem. 2021;45(2):307–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.