Abstract
Background
The prediction of maximal heart rate (MHR) and anaerobic threshold heart rate (HRAT) in patients with coronary heart disease (CHD), particularly among the Chinese population, remains a significant challenge. Existing equations for MHR prediction are primarily designed for healthy individuals not on medication for optimized β-blocker (BB) therapy, showing limited efficacy for individuals on various drug regimens. Moreover, the prediction of HRAT lacks established formulas. This study aims to develop equations for MHR and HRAT, assess the accuracy of historical MHR formulas, and examine their correlation with HR measurements at the anaerobic threshold (AT).
Methods
Among 2021 to 2023, 170 CHD patients were recruited. Patients were categorized into groups based on BB usage. BB dose was transformed into carvedilol dose. Multiple linear stepwise regression analysis was employed to identify predictors of MHR and HRAT, incorporating key patient variables according to prior studies (age, sex, height, weight, carvedilol dose, HRrest). The mean absolute percentage errors (MAPEs) were calculated and compared among abovementioned MHR and HRAT prediction formulas. Besides, the percentages of MHR in predicting HRAT among different formulas were calculated.
Results
For the patients with BB medication, the simplified equations derived for MHR and HRAT were 176 − 1.2*age + 0.7*HRrest − 0.4*weight and 98 − 0.6*age + 0.7*HRrest − 0.3*weight, respectively. For those without BB medication, the derived equations for MHR and HRAT were 200 − 1.1*age and 91 − 0.5*age + 0.5*HRrest, respectively. There are significant differences between the results predicted by the new formula and the prior formulas. The new formulas are helpful for predicting the MHR of patients during exercise more accurately and guiding exercise training more scientifically.
Conclusions
The new equations for estimating MHR and HRAT in CHD patients enhance the accuracy of prior formulas. Given the BB impact on sympathetic nerve activity, the predictive formulas for MHR and HRAT were significantly improved.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12872-024-04307-x.
Keywords: Heart rate prediction formulas, Cardiac rehabilitation, Chinese/East Asian population, Coronary heart disease, Cardiopulmonary exercise test, β-blocker
Background
In 2019, cardiovascular diseases (CVD) accounted for 32% of global mortality, culminating in approximately 17.9 million deaths worldwide [1]. In China, the prevalence of CVD reached an alarming 330 million cases, including 11.39 million instances of coronary heart disease (CHD), which contributed to over one million deaths annually [2]. To mitigate the mortality and hospitalization risks associated with CHD, the 2021 European Society of Cardiology (ESC) guidelines advocated individuals with CHD to participate in 75–150 min of exercise weekly in moderate-to-vigorous intensity [3]. Physiologically, the determination of exercise intensity hinges on two critical metrics: the maximum heart rate (MHR) and the anaerobic threshold heart rate (HRAT). Consequently, assessing MHR and HRAT is pivotal in devising tailored exercise protocols for individuals with CHD.
Currently, the measurement of MHR and HRAT predominantly relies on cardiopulmonary exercise test (CPET) which may be time-consuming and less accessible for the individuals. These limitations underscore the clinical importance of predicting MHR and HRAT based on individual characteristics. Existing prediction models, such as the FOX [4], Tanaka [5], and Fairbarn [6] equations, estimate MHR primarily through age. However, these formulas were developed based on data from healthy, medication-free individuals and have shown limited efficacy in the context of exercise-based cardiac rehabilitation. The formulas proposed by Keteyian et al. [7] and Damiano et al. [8] for heart failure (HF) patients under β-blocker (BB) treatment do not encompass the East Asian demographic. To date, CHD is considered a prior stage of HF, but predicting MHR for individuals with CHD was still understudied, especially in East Asian population. Furthermore, HRAT is commonly estimated as a percentage of the predicted MHR, a method that potentially amplifies the margin of error in HRAT prediction.
Therefore, this study was to develop equations for predicting MHR and HRAT among individuals with CHD in East Asian population and compare the accuracy of the new formulas against the existing models in predicting MHR and HRAT.
Methods
Study population
We retrospectively analyzed a total of 210 patients with CHD of Cardiac Rehabilitation Department of First Affiliated Hospital of Sun Yat-sen University in our study from 2022.01 to 2023.08. The inclusion criteria were: (1) confirmed diagnosis of CHD by coronary computed tomography angiography (CTA) or coronary arteriography (CAG) whatever the treatment of percutaneous coronary intervention, (2) age not younger than 40 years old with no limit of gender, (3) NYHA class I-III, (4) optimal medical therapy recommended by guidelines, (5) CPET with ergometer. Exclusion criteria included, (1) complication with sick sinus syndrome or II-degree or higher atrioventricular block or uncontrolled arrhythmia, (2) scheduled preoperative assessment for left ventricular assisted device implantation or surgery, (3) peak respiratory exchange ratio (RER)<1.1, (4) unidentified HRAT, (5) other drugs (e.g. ivabradine, digoxin) affecting HR. 170 people met the above criteria. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Medical Ethical Committee of Clinical Research and Animal Trials of the First Affiliated Hospital of Sun Yat-sen University. (Application ID: [2023]004).
β-blockers medication
According to the intake of BB, those who met the criteria above were divided into two groups: with BB group and without BB group regardless of age, sex and complications. To avoid the possible heterogeneous outcomes because of different types of BB, the doses were converted to an equivalent dose of carvedilol. The daily dosages in those who took atenolol, metoprolol, or metoprolol sustained release tablets were divided by four, whereas the doses for bisoprolol were multiplied by five [9].
Cardiopulmonary exercise testing
A maximal, symptom-limited CPET was performed on an ergometer (Ergoline) with a facemask (COSMED K4B2, Italy) collecting and measuring the cardiopulmonary metabolic variables breath by breath. A personalized Raise, Activate, Mobilise, Potentiate (Ramp) exercise protocol was chosen, aiming at a test duration of 10 ± 2 min [10, 11]. For patients with RER ≥ 1.1 included in our study, the exercise duration was not regarded as an exclusion criterion.
The scheme of CPET included four phases: (1) resting for 1 min to relieve the patient’s tension; (2) warming up for 3 min with load-free cycling (no resistance on the pedals); (3) exercising for 5–12 min with increasing resistance of 8–25 W/min increment on the pedals until maximal exertion or symptom limitation; (4) recovering for 6 min which included a load-free cycling for the first 3 min and sitting still for the rest 3 min [12].
A 12-lead electrocardiogram (ECG), heart rate (HR), and blood pressure (BP) were also recorded. Exercise duration, peak oxygen uptake (VO2peak), oxygen uptake at anaerobic threshold (VO2AT), oxygen uptake at rest (VO2rest), metabolic equivalent (METs), RER and other indicators were calculated. AT was determined using the V-slope method by an advanced cardiologist with over 10-year working experience [13, 14].
Classification of the exercise-induced HR response
Baseline HR (HRrest), peak HR (HRpeak), and HRAT were collected during CPETs. HRrest was measured at 1 min sitting on the ergometer after a 15-minute rest. Different MHR predicting formulas were included in the study. HRpeak were analyzed as the percentage of MHR based on different formulas as followed:
Statistical analysis
The Shapiro–Wilk test was used to test normality. Comparisons were conducted according to BB medication (with BB and without BB groups). Unless otherwise indicated, all continuous variables were expressed as mean ± standard deviation (SD), and compared by the independent t-test. Data with skewed distribution were given as median and interquartile range, and compared by Mann-Whitney U test. Categorical variables were expressed in numbers and percentages, and compared by Chi-square test.
Pearson correlation was employed to assess the correlation between variables. Multiple linear stepwise regression analysis was performed to evaluate the predictors for MHR and HRAT by including the main variables of the patients (age, gender, height, weight, carvedilol dose, HRrest). The higher correlation variables (P < 0.05) and their coefficients were obtained to fit the possible predicting formula. All independent variables included in the new MHR and HRAT predicting formula had a partial R2 ≥ 0.01. A Subgroup analysis was conducted by stratifying NYHA class.
The mean absolute percentage errors (MAPEs) = (average absolute percent error for each time period − actual values) / actual values were calculated and compared among abovementioned MHR and HRAT prediction formulas. Besides, the percentages of MHR in predicting HRAT among different formulas were calculated. Statistical analyses were performed using IBM SPSS 19.0 and R v4.2.3 [9].
Results
Demographic and clinical characteristics
The baseline clinical patients’ characteristics were shown in Table 1. A total of 170 CHD who completed CPET were enrolled, including 145 (85.3%) males, mean (SD) aged at 57.6 (9.2) years. Compared to the non-BB group, patients in the BB group had lower HRrest (71.7 versus 78.3 bpm), lower HRpeak (122.9 versus 140.3 bpm), lower VO2peak (17.0 versus 19.2), shorter exercise duration (386.7 versus 425.2) seconds and higher proportion of hypertension of (26.2% versus 17.9%). No significant differences were observed between BB and non-BB groups in other variables.
Table 1.
Basic characteristics of the study population, M (SD)
| Overall, n = 170 | BB, n = 103 | Non-BB, n = 67 | P-value | |
|---|---|---|---|---|
| Age, years | 57.6 (9.2) | 58.3 (8.7) | 56.5 (9.8) | 0.231 |
| Male (%) | 145(85.3) | 91 (88.3) | 55 (82.1) | 0.380 |
| Height, cm | 167.3 (6.6) | 166.9 (6.8) | 167.8 (6.3) | 0.420 |
| Weight, kg | 68.9 (6.6) | 69.3 (11.9) | 68.2 (8.2) | 0.525 |
| BMI, kg/m2 | 24.5 (2.9) | 24.7 (3.2) | 24.2 (2.4) | 0.248 |
| SBP rest, mmHg | 111.6 (16.3) | 109.1 (16.1) | 115.4 (16.1) | 0.014 |
| DBP rest, mmHg | 70.6 (10.1) | 68.6 (9.5) | 73.7 (10.3) | 0.001 |
| HRrest, bpm | 74.3 (10.7) | 71.7 (10.8) | 78.3 (9.3) | < 0.001 |
| HRAT, bpm | 96.1 (13.3) | 92.3 (12.6) | 101.9 (7.7) | < 0.001 |
| HRpeak, bpm | 129.8 (19.6) | 122.9 (17.8) | 140.3 (17.6) | < 0.001 |
| VO2AT, ml/min/kg | 11.5 (3.0) | 11.2 (2.9) | 12.0 (3.1) | 0.079 |
| VO2peak, ml/min/kg | 17.8 (4.0) | 17.0 (3.6) | 19.2 (4.3) | < 0.001 |
| Metabolic equivalent, MET | 5.1 (1.2) | 4.8 (1.1) | 5.5 (1.2) | < 0.001 |
| Duration, s | 401.7 (84.5) | 386.7 (74.6) | 425.2 (93.8) | 0.004 |
| RER | 1.2 (0.10) | 1.2 (0.08) | 1.2 (0.12) | 0.108 |
| Carvedilol dose equivalent, mg, MD [IQR] | 6.25 [0-12.5] | 12.5 [6.25–12.50] | 0 [0] | < 0.001 |
| Diabetes mellitus, n (%) | 25 (14.7) | 16 (15.5) | 9 (13.4) | 0.826 |
| Hypertension, n (%) | 39 (22.9) | 27 (26.2) | 12 (17.9) | 0.263 |
| NYHA, n (%) | 0.001 | |||
| I | 147 (86.5) | 82 (79.6) | 65 (97.0) | |
| II/III | 23 (13.5) | 21 (20.4) | 2 (3.0) | |
BMI Body mass index, SBP rest Systolic blood pressure at rest, DBP rest Diastolic blood pressure at rest, HRrest Heart rate at rest, HRAT Heart rate at anaerobic threshold, HRpeak Heart rate at peak excercise, VO2AT Oxygen uptake at anaerobic threshold, VO2peak Peak oxygen uptake, MET Metabolic equivalent, RER Respiratory exchange ratio, BB β-blocker, NYHA New York Heart Association
In another way, statistical analysis with basic characteristics stratified by NYHA status was conducted (Table S1). Compared to the NYHA I group, patients in NYHA II/II group were older, had lower HRrest, lowe HRAT, lower HRpeak, lower VO2peak, lower exercise capacity, shorter exercise duration seconds and higher proportion of BB.
For BB group, the independent influencing factors of MHR and HRAT are all including age, HRrest and weight. The new formula for MHR was 176 − 1.2*age + 0.7*HRrest-0.4*weight. And the new formula for HRAT was 98 − 0.6*age + 0.7*HRrest-0.3*weight (Table 2).
Table 2.
Main clinical variables independently associated at HRpeak and HRAT in the patients stratified by BB medication
| With BB | Without BB | |||||
|---|---|---|---|---|---|---|
| Beta | P values | Partial R2 | Beta | P values | Partial R2 | |
| HRpeak | ||||||
| Intercept | 175.67 | < 0.001 | 200.12 | < 0.001 | ||
| Age | -1.24 | < 0.001 | 0.332 | -1.06 | < 0.001 | 0.349 |
| Weight | -0.39 | 0.003 | 0.088 | - | - | - |
| HRrest | 0.65 | < 0.001 | 0.228 | - | - | - |
| HRAT | ||||||
| Intercept | 97.91 | 91.38 | < 0.001 | |||
| Age | -0.60 | < 0.001 | 0.193 | -0.48 | 0.001 | 0.168 |
| Weight | -0.27 | 0.004 | 0.083 | - | - | - |
| HRrest | 0.67 | < 0.001 | 0.389 | 0.48 | 0.001 | 0.156 |
BB β-blocker, HRpeak Heart rate at peak excercise, HRrest Heart rate at rest, HRAT Heart rate at anaerobic threshold
The MAPEs of new MHR and HRAT formulas are 9.4% and 7.3% respectively. Compared with the Fox/Tanaka/Fairbarn formulas, the new MHR formula showed a significantly lower MAPE. And it showed an approximate equality performance with the Keteyian formula (9.4 vs. 8.7). The new HRAT formula showed a satisfactory effect with the MAPE of 7.3% (Table 3).
Table 3.
Historical and new equations for estimating MHR and new equations for estimating HRAT and related accuracy data for patients with BB
| Formula | Equations | R2 | SSE, bpm | MAPE, % | ||
|---|---|---|---|---|---|---|
| Overall | NYHA I | NYHA II/III | ||||
| MHR, Historical equations | ||||||
| FOX | 220–age | 0.303 | 14.83 | 33.8 | 32.2 | 40.2 |
| TANAKA | 208–0.7*age | 0.303 | 14.83 | 38.5 | 36.5 | 46.3 |
| Fairbarn | 201 − 0.63*age | 0.303 | 14.83 | 36.1 | 34.1 | 44.0 |
| KETEYIAN | 114 + (0.5*HRrest) – (0.5*age) | 0.437 | 13.32 | 8.7 | 8.5 | 9.2 |
| MHR and HRAT New equation | ||||||
| MHR | 176 − 1.2*age + 0.7*HRrest − 0.4*weight | 0.495 | 12.62 | 9.4 | 9.1 | 10.3 |
| HRAT | 98 − 0.6*age + 0.7*HRrest − 0.3*weight | 0.510 | 8.80 | 7.3 | 10.5 | 6.9 |
MHR Maximal heart rate, HRAT Heart rate at anaerobic threshold, HRrest Heart rate at rest, BB β-blocker
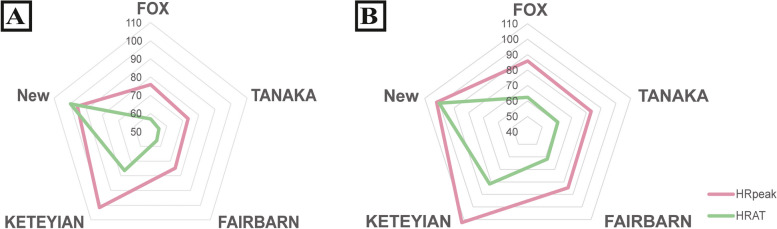
Similar predictive values for MHR and HRAT were observed when stratifying the individuals by NYHA. HRpeak and HRAT in the test are expressed as percentages of MHR according different equations. For HRpeak, the new MHR formula all show nearly 20% higher %MHR compared with the Fox/Tanaka/Fairbarn formulas respectively, and a little lower %MHR compared with the Keteyian formula (95.6 vs. 101.6). The same result could be observed for HRAT (Fig. 1A / Table S3).
Fig. 1.
HRpeak and HRAT are expressed as percentages of MHR
Figure 1A is for BB group. For HRAT, %MHRs are respectively 57.0%, 55.1%, 56.1% and 76.3% to the Fox/Tanaka/Fairbarn/Keteyian formulas and 71.8% for the new formula. For HRpeak, %MHRs are respectively 75.9%, 73.4%, 74.7% and 101.6% to the Fox/Tanaka/Fairbarn/Keteyian formulas and 95.6% to the new formula.
Figure 1B is for Non-BB group. For HRAT, %MHRs are respectively 62.4%, 60.5%, 61.6% and 81.6% to the Fox/Tanaka/Fairbarn/Keteyian formulas and 74.1% for the new formula. For HRpeak, %MHRs are respectively 85.8%, 83.2%, 74.7% and 112.4% to the Fox/Tanaka/Fairbarn/Keteyian formulas and 101.9% to the new formula.
The new formula also shows a 15% higher cut-off value to identify AT intensity domain than the Fox/Tanaka/Fairbarn formulas and a 5% lower cut-off value than the Keteyian formula (Table 4).
Table 4.
Possible cut-off values to identify the AT intensity domain and related accuracy data, with BB
| Formula | Percentage | MAPE, % | ||
|---|---|---|---|---|
| Overall | NYHA grade I | NYHA grade II/III | ||
| FOX | 55% | 13.3 | 13.5 | 12.5 |
| 60% | 9.7 | 9.8 | 9.6 | |
| 65% | 11.3 | 11.3 | 11.4 | |
| TANAKA | 55% | 11.5 | 11.8 | 10.4 |
| 60% | 9.7 | 9.8 | 9.3 | |
| 65% | 13.6 | 13.3 | 14.7 | |
| Fairbarn | 55% | 12.3 | 12.7 | 11.0 |
| 60% | 9.6 | 9.7 | 9.1 | |
| 65% | 12.5 | 12.3 | 13.2 | |
| KETEYIAN | 75% | 9.8 | 9.9 | 9.3 |
| 80% | 7.7 | 7.7 | 7.9 | |
| 85% | 9.6 | 9.7 | 9.4 | |
| New formula | 70% | 10.6 | 10.5 | 11.0 |
| 75% | 7.9 | 7.7 | 8.6 | |
| 80% | 9.1 | 9.1 | 9.0 |
AT Anaerobic threshold, BB β-blocker
For non-BB group, the independent influencing factor of MHR only includes age. And the new formula of MHR was 200 − 1.1*age. Whereas for HRAT, despite age, HRrest is another influence factor. The new formula for HRAT was 91 − 0.5*age + 0.5*HRrest (Table 2). The MAPEs of new MHR and HRAT formulas are 8.2% and 7.6% respectively. Compared with the Fox/Tanaka/Fairbarn formulas, the new MHR formula showed a significantly lower MAPE. And it also showed a much lower MAPE compared with the Keteyian formula (8.2 vs. 12.5). The new HRAT formula showed a satisfactory effect with the MAPR of 7.6% (Table S2). Also in non-BB group, HRpeak and HRAT in the test are expressed as percentages of MHR according to different equations. For HRpeak, the new MHR formula shows 16.1%, 18.7% and 17.2% higher %MHR compared with the Fox/Tanaka/Fairbarn formulas respectively and show a more satisfactory %MHR compared with the Keteyian formula (101.9 vs. 112.4). For HRAT, the new MHR formula shows 11.7%, 13.6% and 12.5% higher %MHR compared with the Fox/Tanaka/Fairbarn formulas respectively, and shows 7.5% lower %MHR compared with the Keteyian formula. The new HRAT formula shows a satisfactory %HRAT of 100.1% (Fig. 1B / Table S4). The new formula also shows a 15% higher cut-off value to identify the anaerobic threshold (AT) intensity domain than the Fox/Tanaka/Fairbarn formulas and a 5% lower cut-off value than the Keteyian formula (Table S5).
Discussion
This study was a retrospective study on MHR and HRAT predicting for CHD patients. For the patients with BB, the main influencing factors of MHR include age, HRrest and weight, whereas for those without BB, the influencing factor of MHR only include age. For the subgroup analysis of NYHA grade, no new formula was established for the small sample size. The MHR and HRAT formulas established in the present study may be more accuracy than prior formula which included weight only.
Demographic characteristics displayed variation among different ethnicities (i.e., height, weight, heart rate recovery and body mass index) [15–17]. Most studies recruited Americans or Europeans for developing and/or validating MHR formulas, so developed universal formulas may be difficult to predict the MHR in Asians [18]. Park et al., cross validated MHR prediction formulas among healthy Koreans of 7–55 years old and found that there were significant differences between the formula predicted age and the actual MHR [18]. There is a lack of research on East Asian population especially in CHD.
Recent research has challenged traditional views of heart rate dynamics during incremental exercise by revealing that the heart rate performance curve (HRPC) is non-linear and non-uniform [19]. This finding underscores the importance of considering HRPC deflection when setting %MHR targets for exercise prescription [19]. The progression of HR with increasing exercise intensity is best described as an S-shaped curve, a concept previously introduced by Brooke and Hamley [20]. The formula proposed by Fox et al. in 1971, HRmax = 220-age, remains the most prevalently utilized method for predicting MHR. However, this equation has been critiqued for its broad standard deviation, and for its tendency to overestimate MHR in younger adults while underestimating it in older individuals [5, 21, 22]. Alternative formulas, such as those by Tanaka et al. and Fairbarn et al., have demonstrated similar levels of accuracy. Both FOX and Tanaka formulas tended to overestimate the MHR for males and females over 15 years old, and the two universal equations were not suitable to predict the MHR of Koreans except for children aged from 7 to 14 [18]. Yet, these models were primarily developed from data involving healthy, non-medicated individuals and exhibit limited applicability for patients in exercise-based cardiac rehabilitation, particularly those on BB medication. Existing formulas, including those tailored for HF patients on BB by Keteyian et al. and Damiano et al., also do not accommodate the East Asian demographics, highlighting a significant gap in current HR prediction models.
Our study highlights the relationship between MHR, HRAT, age, body weight, and HRrest in CHD patients, with a distinction between those with BB and those without. For CHD patients with BB, MHR and HRAT correlate with age, HRrest, and weight, suggesting the effects of BB dosage may be indirectly accounted for by these variables. Conversely, for those without BB, MHR is only age-dependent, while HRAT also depends on HRrest. This indicates the need for personalized HR predictions in CHD patients, emphasizing the interplay between medication and physiological factors in exercise prescription. Our findings advocate for a nuanced approach to cardiac rehabilitation, underscoring the differential impact of BB therapy on HR predictions.
The specific mechanisms for the result were understudied. Studies have shown that intrinsic pacemaker rate declines linearly from birth at a rate of ~ 0.8 bpm/year in humans and ~ 4 bpm/month in mice [23]. The slowdown of the intrinsic pacemaker rate is the main cause for the accompanying decline in MHR, playing a significant role in the loss of aerobic capacity in older adults [24]. This is consistent with the negative correlation of age. HRAT was related to HRrest which may be explained by the relationship between sympathetic and parasympathetic nervous systems [25]. For CHD patients without BB, HRrest is generally low and sympathetic nerves are at a relatively low level. As exercise increases, sympathetic nerves activate and parasympathetic nerves are suppressed, resulting in increased HR. At AT, the relationship between sympathetic and parasympathetic nerves is shown as HRAT, and the cardiopulmonary function reflects the aerobic exercise capacity of the body. HRrest represents the role of parasympathetic nerve at AT in the HRAT formula. As exercise continues, the sympathetic nerves activate to the top at which HR is almost entirely influenced by the sympathetic nerves, resulting in the MHR formula being only age-dependent. For CHD patients with BB, their HRrest was usually higher than those without BB, which indirectly implied that the sympathetic nerves in this group were relatively higher than the without BB group. The inhibitory effect of BB on sympathetic nerves was reflected in the difference between HRrest and basal HR, which was directly related to drug dose. And due to the fat-solubility characteristics of the drug, the individual effect of BB was related to individual body weight. The effects of BB dosage on HR during exercise are indirectly accounted for by HRrest and weight. Because of the effect of BB on sympathetic nerves [26], the MHR during exercise is also suppressed to varying degrees. The MHR of the patients with BB was not only related to age, but also related to resting HR and body weight.
This study has remarkable clinical implications:
Firstly, the HRAT and MHR prediction formula may be used more scientifically and accurately in clinical work in Chinese population.
Secondly, exercise prescriptions for CHD patients in future clinical trials on remote or home-based cardiac rehabilitation may refer to the present formulas, which may ultimately guiding optimal exercise training in a more effective way.
In addition, the prediction formulas were separately provided according to whether patients were under BB medication or not to help patients or medical staff individualize exercise HR.
This study has several strengths including the subjects of East Asian and subgroup analyses according to whether they were taking BB or not which may indicate more accurate of the formulas.
Nevertheless, there are also some limitations in this study:
Firstly, the study is a single-center retrospective study with a small sample size. The generalization of the results is limited for the lack of external verification.
Secondly, the CHD patients included in this study were able to finish CPET with RER not less than 1.1. As such, the study’s conclusions may not apply to CHD patients with multiple comorbidities, a bedridden status, or a poor exercise capacity who cannot tolerate the exercise with RER over 1.1.
Thirdly, this study is an observational study, and the included variables refer to previous studies. Some unknown confounders may be unadjusted.
Finally, this study only examined the MHR and HRAT using cycling, which may limit the generalization of the formula to treadmill-based CPET. However, previous meta-analysis reported a non-significant difference of age-predicted MHR by treadmill compared to cycling ergometers [6].
Conclusions
Among individuals with CHD, the maximum heart rates were lower than estimated due to the disease natrue and medications. BB could reduce the HR by inhibiting sympathetic activity. The MHR and HRAT could be estimated by comprehensively considering the indicators including age, HRrest, weight and the BB blockers. This may promote the effectiveness in the clincial practice of cardiac rehabilitation.
Supplementary Information
Acknowledgements
We thank the staff and participants of the study for their contributions.
Abbreviations
- MHR
Maximal heart rate
- HRAT
Anaerobic threshold heart rate
- CHD
Coronary heart disease
- AT
Anaerobic threshold
- RER
Respiratory exchange ratio
- MAPEs
the mean absolute percentage errors
- CVD
Cardiovascular diseases
- ESC
European Society of Cardiology
- CPET
Cardiopulmonary exercise test
- BB
β-blockers
- HF
Heart failure
- CTA
Computed tomography angiography
- CAG
Coronary arteriography
- ECG
Electrocardiogram
- HR
Heart rate
- BP
Blood pressure
- VO2peak
Peak oxygen uptake
- VO2AT
Oxygen uptake at anaerobic threshold
- VO2rest
Oxygen uptake at rest
- METs
Metabolic equivalent
- RER
Respiratory exchange rate
- HRPC
Heart rate performance curve
Authors’ contributions
LL, LX and XC contributed to the data collation. LL, ZH, DM contributed to the analysis and interpretation of data for the work. ZH, MM and XY contributed to the design of the paper. LL and ZH drafted the manuscript. MM and XY critically revised the manuscript. All authors reviewed the manuscript. All gave final approval and agreed to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
This study was supported by the Sanming City Science and Technology Joint Funding Project (2022-S-56). Meiming Lin has designed the paper and critically revised the manuscript.
Data availability
Data could be shared by reasonable request to the corresponding authors.
Declarations
Ethics approval and consent to participate
The data in this research was medical record data and informed consent was not required. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and was approved by the Medical Ethical Committee of Clinical Research and Animal Trials of the First Affiliated Hospital of Sun Yat-sen University. (Application ID: [2023]004).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Leilei Wang and Zihao Huang contributed to the manuscript equally.
Contributor Information
Meiming Lin, Email: 13850818587@139.com.
Xiuyu Leng, Email: meirerme@163.com.
References
- 1.World health statistics 2021: monitoring health for the SDGs, sustainable development goals. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- 2.Writing committee of the report on cardiovascular health and diseases in china. Report on Cardiovascular Health and Diseases in China 2021: An Updated Summary. Biomed Environ Sci. 2022;35(7):573–603. doi: 10.3967/bes2022.079. PMID: 35945174. [DOI] [PubMed] [Google Scholar]
- 3.Pelliccia A, Sharma S, Gati S, Bäck M, Börjesson M, Caselli S, ESC Scientific Document Group. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42(1):17–96. 10.1093/eurheartj/ehaa605. Erratum in: Eur Heart J. 2021;42(5):548–549. PMID: 32860412. [DOI] [PubMed]
- 4.Fox SM 3rd, Naughton JP, Haskell WL. Physical activity and the prevention of coronary heart disease. Ann Clin Res. 1971;3(6):404–32. [PubMed]
- 5.Tanaka H, Monahan KD, Seals DR. Age-predicted maximal heart rate revisited. J Am Coll Cardiol. 2001;37:153–6. [DOI] [PubMed] [Google Scholar]
- 6.Fairbarn MS, Blackie SP, McElvaney NG, Wiggs BR, Paré PD, Pardy RL. Prediction of heart rate and oxygen uptake during incremental and maximal exercise in healthy adults. Chest. 1994;105(5):1365–9. [DOI] [PubMed] [Google Scholar]
- 7.Keteyian SJ, Kitzman D, Zannad F, Landzberg J, Arnold JM, Brubaker P, et al. Predicting maximal HR in heart failure patients on β-blockade therapy. Med Sci Sports Exerc. 2012;44(3):371–6. 10.1249/MSS.0b013e318234316f. PMID: 21900844; PMCID: PMC3755356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Magrì D, Piepoli M, Gallo G, Corrà U, Metra M, Paolillo S, et al. Old and new equations for maximal heart rate prediction in patients with heart failure and reduced ejection fraction on beta-blockers treatment: results from the MECKI score data set. Eur J Prev Cardiol. 2022;29(12):1680–8. 10.1093/eurjpc/zwac099. Erratum in: Eur J Prev Cardiol. 2023 Dec 22;: PMID: 35578814. [DOI] [PubMed] [Google Scholar]
- 9.Paolillo S, Mapelli M, Bonomi A, Corrà U, Piepoli M, Veglia F, et al. Prognostic role of β-blocker selectivity and dosage regimens in heart failure patients. Insights from the MECKI score database. Eur J Heart Fail. 2017;19(7):904–14. 10.1002/ejhf.775. Epub 2017 Feb 24. PMID: 28233458. [DOI] [PubMed] [Google Scholar]
- 10.Agostoni P, Dumitrescu D. How to perform and report a cardiopulmonary exercise test in patients with chronic heart failure. Int J Cardiol. 2019;288:107–13. 10.1016/j.ijcard.2019.04.053. Epub 2019 Apr 18. PMID: 31047701. [DOI] [PubMed] [Google Scholar]
- 11.Task Force of the Italian Working Group on Cardiac Rehabilitation Prevention; Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology; Piepoli MF, Corrà U, Agostoni PG, Belardinelli R, Cohen-Solal A, Hambrecht R, et al. Statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction: recommendations for performance and interpretation. Part I: definition of cardiopulmonary exercise testing parameters for appropriate use in chronic heart failure. Eur J Cardiovasc Prev Rehabil. 2006;13(2):150 – 64. 10.1097/01.hjr.0000209812.05573.04. PMID: 16575267. [DOI] [PubMed]
- 12.Huang F, Leng X, Kasukurthi MV, Huang Y, Li D, Tan S, et al. Utilizing machine learning techniques to predict the efficacy of Aerobic Exercise intervention on young hypertensive patients based on cardiopulmonary Exercise Testing. J Healthc Eng. 2021;2021:6633832. 10.1155/2021/6633832. PMID: 33968353; PMCID: PMC8084649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang H, Song L, Ning X, Ma Y, Xue A, Zhao H, et al. Enhanced external counterpulsation ameliorates endothelial dysfunction and elevates exercise tolerance in patients with coronary artery disease. Front Cardiovasc Med. 2022;9: 997109. 10.3389/fcvm.2022.997109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carriere C, Corrà U, Piepoli M, Bonomi A, Merlo M, Barbieri S, et al. Anaerobic threshold and respiratory compensation point identification during cardiopulmonary exercise tests in chronic heart failure. Chest. 2019;156(2):338–47. 10.1016/j.chest.2019.03.013. Epub 2019 Mar 27. PMID: 30926397. [DOI] [PubMed] [Google Scholar]
- 15.Esco MR, Williford HN. Race influences the relationship between aerobic power and heart rate recovery. J Sports Med Phys Fitness. 2013;53(6):583–7 PMID: 24247181. [PubMed] [Google Scholar]
- 16.O’Neal WT, Alam AB, Sandesara PB, Claxton JS, MacLehose RF, Chen LY, et al. Sex and racial differences in cardiovascular disease risk in patients with atrial fibrillation. PLoS ONE. 2019;14:e0222147. 10.1371/journal.pone.0222147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egan KJ, Knutson KL, Pereira AC, von Schantz M. The role of race and ethnicity in sleep, circadian rhythms and cardiovascular health. Sleep Med Rev. 2017;33:70–8. 10.1016/j.smrv.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park JH, Jung HC, Jung YS, Song JK, Lee JM. Re-visiting maximal heart rate prediction using cross-validation in population aged 7–55 years. Int J Environ Res Public Health. 2022;19(14): 8509. 10.3390/ijerph19148509. PMID: 35886359; PMCID: PMC9320369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofmann P, Pokan R, von Duvillard SP, Seibert FJ, Zweiker R, Schmid P. Heart rate performance curve during incremental cycle ergometer exercise in healthy young male subjects. Med Sci Sports Exerc. 1997;29:762–8. 10.1097/00005768-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Brooke JD, Hamley EJ. The heart-rate–physical work curve analysis for the prediction of exhausting work ability. Med Sci Sports. 1972;4:23–6. [PubMed] [Google Scholar]
- 21.Pollock ML, Franklin BA, Balady GJ, Chaitman BL, Fleg JL, Fletcher B, et al. AHA science advisory. resistance exercise in individuals with and without cardiovascular disease: benefits, rationale, safety, and prescription: an advisory from the committee on exercise, rehabilitation, and prevention, council on clinical cardiology, american heart association; position paper endorsed by the american college of sports medicine. Circulation. 2000;101(7):828–33. 10.1161/01.cir.101.7.828. PMID: 10683360. [DOI] [PubMed] [Google Scholar]
- 22.Gellish RL, Goslin BR, Olson RE, McDonald A, Russi GD, Moudgil VK. Longitudinal modeling of the relationship between age and maximal heart rate. Med Sci Sports Exerc. 2007;39(5):822–9. 10.1097/mss.0b013e31803349c6. PMID: 17468581. [DOI] [PubMed] [Google Scholar]
- 23.Larson ED, St Clair JR, Sumner WA, Bannister RA, Proenza C. Depressed pacemaker activity of sinoatrial node myocytes contributes to the age-dependent decline in maximum heart rate. Proc Natl Acad Sci U S A. 2013;110(44):18011–6. 10.1073/pnas.1308477110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi S, Baudot M, Vivas O, Moreno CM. Slowing down as we age: aging of the cardiac pacemaker’s neural control. Geroscience. 2022;44(1):1–17. 10.1007/s11357-021-00420-3. Epub 2021 Jul 22. PMID: 34292477; PMCID: PMC8811107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawada T, Sugimachi M, Shishido T, Miyano H, Sato T, Yoshimura R, Miyashita H, Nakahara T, Alexander J Jr, Sunagawa K. Simultaneous identification of static and dynamic vagosympathetic interactions in regulating heart rate. Am J Physiol. 1999;276(3):R782–9. 10.1152/ajpregu.1999.276.3.R782. PMID: 10070139. [DOI] [PubMed] [Google Scholar]
- 26.Andersen S, Andersen A, de Man FS, Nielsen-Kudsk JE. Sympathetic nervous system activation and β-adrenoceptor blockade in right heart failure. Eur J Heart Fail. 2015;17(4):358–66. 10.1002/ejhf.253. Epub 2015 Feb 22. PMID: 25704592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data could be shared by reasonable request to the corresponding authors.