Abstract
Background
Stroke in young needs an individualized approach before considering thrombolysis. Here we present a case of undiagnosed mitral valve prolapse presenting with stroke due to associated infective endocarditis. Young stroke patients presenting with fever need a panoramic approach.
Case summary
This 39-year-old female with a background history of fever and loss of weight for two months presented to the emergency department with a history of altered sensorium and aphasia. The Magnetic Resonance Imaging (MRI) showed a hyperacute infarct. Hence, thrombolysis with alteplase was considered. Post thrombolysis, the patient had a parenchymal bleed. The diagnostic evaluation yielded stroke secondary to infective endocarditis because of undiagnosed mitral valve prolapse.
Conclusion
Mitral valve prolapse is not associated with stroke in young patients. However, the undiagnosed infective endocarditis and subsequent septic emboli led to an increased risk. Emergency physicians and intensivists should anticipate infective causes before considering thrombolysis, as the results could be counterproductive.
Keywords: Mitral valve prolapse, Infective endocarditis, Acute ischemic stroke
Background
According to community-based data, the incidence rate of first stroke in Indian patients under the age of 40 years is 8.8% [1]. In hospital-based data, the incidence rate soared up to 31.4% [2]. Mitral valve prolapse (MVP) is not associated with an increased incidence of stroke in young patients [3]. The risk of stroke in mitral valve prolapse rises with age more than 50 years, presence of atrial fibrillation, and left atrial dilation due to significant mitral regurgitation [4]. MVP is classified as an intermediate risk factor for infective endocarditis by the European Society of Cardiology [5]. However, mitral valve disease is associated with an increased risk of infective endocarditis among the intermediate-risk group, equating to the high-risk group [6]. Stroke is an associated complication with infective endocarditis with a frequency of 20% [7]. Infective endocarditis is an absolute contraindication for intravenous tissue plasminogen activator (tPA) in acute ischemic stroke. There is a risk of hemorrhagic conversion of subclinical infarcts [8]. Here, we present a case of a 39-year-old female with the first presentation of mitral valve prolapse as acute ischemic stroke secondary to infective endocarditis.
Case summary
This 39-year-old lady with a history of fever for two months associated with weight loss presented to the emergency department with acute onset of right-sided weakness, altered sensorium, and aphasia. She presented within 30 min of symptoms and was last seen normal 2 h before. On neurological examination, her Glasgow Coma Score (GCS) was E4V1M5 with National Institutes of Health Stroke Score of 16. The motor assessment showed right upper limb 2/5, right lower limb 2/5, left upper limb 4/5, and left lower limb 4/5. Deep tendon reflexes on the right side were exaggerated along with planter in withdrawal response. Systemic examination was non-contributory. The Magentic Resonance Imaging (MRI) of the brain was suggestive of hyperacute non-hemorrhagic infarcts in the left frontal cortex, left precentral gyrus, and perisylvian fissure (Fig. 1A, B). No vascular abnormality attributing to stroke was identified on the MR angiogram. The patient was managed as acute ischemic stroke in a window period and thrombolysed with alteplase after formal neurological consultation. Unfortunately, the patient had a worsening sensorium post-thrombolysis and required intubation to secure the airway. Repeat computed tomography (CT) was suggestive of acute intra-parenchymal hemorrhage in the right occipital lobe. There were no features of hemorrhagic transformation in the infarct territories or increased intracranial pressure.
Fig. 1.
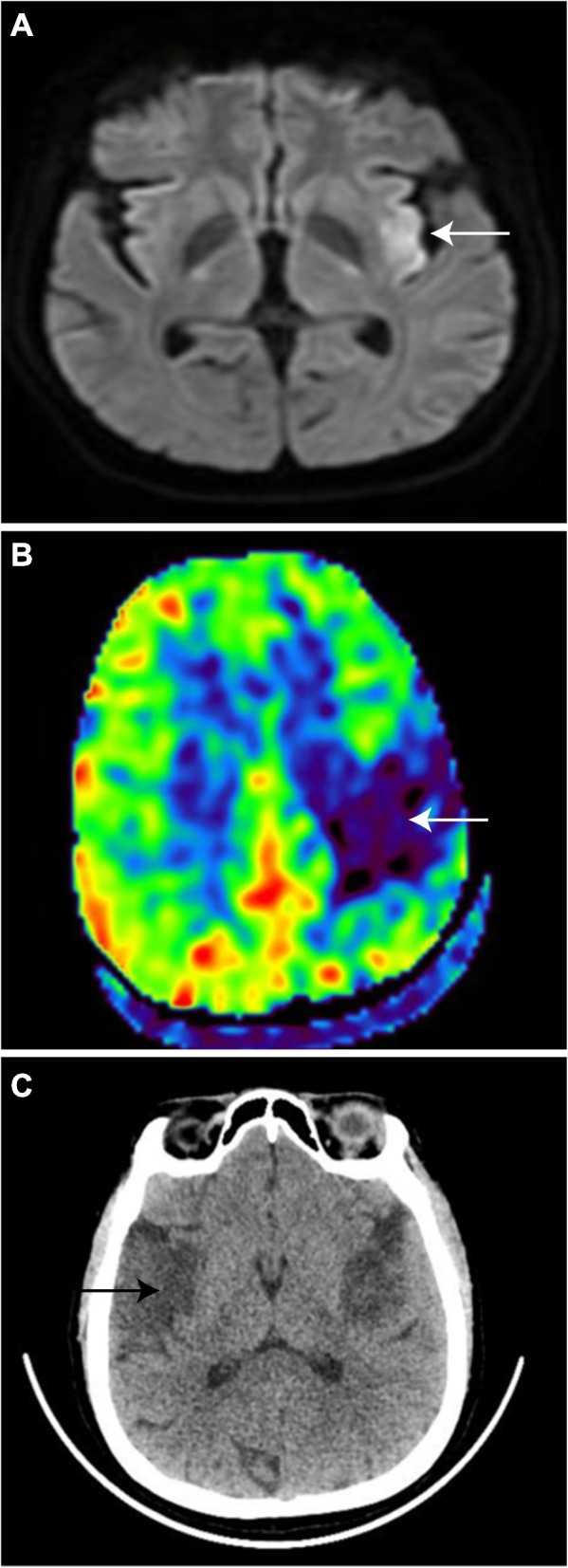
A, B Hyperacute left perisylvian fissure infarct on MRI and CT perfusion imaging respectively. C CT brain with new right MCA territory infarct
The background medical history revealed intermittent episodes of fever along with significant weight loss in the last two months. The patient was evaluated on an outpatient basis for complaints of fever, and diagnostic evaluation, including a Positron Emission Tomography-Computed Tomography (PET-CT) scan and sputum testing for tuberculosis was negative.
The patient was also evaluated for vasculitis, infectious, autoimmune, or paraneoplastic etiologies. The autoimmune profile (antinuclear antibody [ANA], and antineutrophil cytoplasmic antibody [ANCA]), MRI brain and vessel wall for vasculitis, lumbar puncture for tuberculosis, viruses, and India ink for cryptococcal infection and autoimmune and paraneoplastic workup were negative. The workup for underlying immunodeficiency and Venereal Disease Research Laboratory (VDRL) test for syphilis, as well as hepatitis B and C, were negative.
The patient developed a new onset of upper motor neuron features on the left side after 48hrs. Her GCS persistently remained low. Repeat CT brain showed a new right MCA infarct (Fig. 1C).
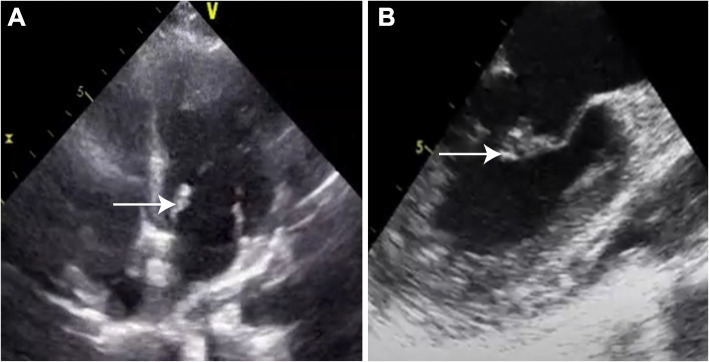
Because of the multi-territorial infarcts on the MRI brain, suspicion of cardioembolic stroke was high on the differentials. The transthoracic echocardiography raised suspicion of vegetation on the mitral valve though the PET-CT done as part of pyrexia of unknown origin was negative (Fig. 2A). The transesophageal echocardiography was suggestive of infective endocarditis with vegetation of size 6 × 6 mm on the anterior mitral valve leaflet (Fig. 2B). There was associated anterior mitral valve thickening suggestive of undiagnosed mitral valve prolapse and moderate mitral regurgitation but no left atrial enlargement. The patient was started on antibiotics for infective endocarditis although the blood cultures and serology for brucella were negative. The patient had a significant functional deficit with right-sided hemiparesis. Given the difficulty in weaning, the patient was tracheostomized and managed with physiotherapy and single-agent antiplatelet therapy. The surgical repair was postponed because of a recent hemorrhagic stroke. During the stay in the rehabilitation center, the patient developed right lower limb deep vein thrombosis and was initiated on a direct oral anticoagulant. Total six-weeks course of antibiotics for infective endocarditis was given. Patient showed clinical improvement with resolution of fever and vegetation echocardiographically.
Fig. 2.
A, B Transthoracic and transesophageal ECHO with mitral valve prolapse and vegetation at anterior mitral leaflet
Discussion
While evaluating a patient aged less than 45 years presenting with prolonged fever and stroke, infectious differentials should take precedence over non-infectious ones [6, 9]. Infectious differentials include endocarditis due to valvular disease, tuberculosis, syphilis, human immunodeficiency virus, hepatitis C-associated cryoglobulinemia, viral infections such as Varicella Zoster, Cytomegalovirus, Epstein Barr virus, and fungal infections such as mucormycosis, aspergillosis, and cryptococcosis [9]. The non-infectious causes include Systemic Lupus Erythematosus (SLE), vasculitis, antiphospholipid antibodies syndrome (APLA), and malignancy [10]. The possible causes of stroke in infective endocarditis are embolic infarction or hemorrhage. The hemorrhage is attributed to septic emboli causing pyogenic arteritis and mycotic aneurysms. Most ischemic events are usually aggregated at the time of uncontrolled infection. The risk of recurrent embolism is low when infection is controlled, negating the role of anticoagulation for secondary prevention [11]. The 2020 systematic literature review showed a fourfold increase in the risk of intracranial hemorrhage with intravenous thrombolysis compared to mechanical thrombectomy [12]. The management of young stroke needs a personalized approach based on history, clinical and radiological findings. In patients with multiple cortical infarcts in multiple territories and bilateral hemispherical involvement should raise suspicion of cardioembolic stroke. In patients with the possibility of cardioembolic stroke, the incorporation of transesophageal echocardiography changed management in 16.7% of the patients [13]. Before considering the decision of thrombolysis in young stroke, due efforts should be made to rule out infective endocarditis as it has a substantial impact on the management.
Conclusion
Time should not be the only factor driving the decision of thrombolysis in young stroke patients. An expeditious approach to rule out differentials like infective endocarditis and other infective etiologies should take pre-eminence over the haste for thrombolysis in patients presenting with fever and stroke.
Acknowledgements
We acknowledge the support from the department of Cardiothoracic and vascular surgery (CTVS), Cardiology and cardiac anaesthesia.
Authors’ contributions
KA-Concept, design and writing a manuscript. AAH- Concept, Design, and approving final draft. All authors have read and approved the final manuscript.
Funding
No funding was received for this study.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Approval from institutional ethical committee of St John’s medical college was obtained (IEC no 94/2024).
Consent for publication
Consent from legally acceptable representative (LAR) was obtained.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Das SK, Banerjee TK, Biswas A, Roy T, Raut DK, Mukherjee CS, et al. A prospective community-based study of stroke in Kolkata, India. Stroke. 2007;38(3):906–10. [DOI] [PubMed] [Google Scholar]
- 2.Hussain M, Sharma SR, Jamil MD. A hospital-based study of stroke in young from North East India. Ann Indian Acad Neurol. 2018;21(3):184–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilon D, Buonanno FS, Joffe MM, Leavitt M, Marshall JE, Kistler JP, et al. Lack of evidence of an association between mitral-valve prolapse and stroke in young patients. N Engl J Med. 1999;341(1):8–13. [DOI] [PubMed] [Google Scholar]
- 4.Avierinos JF, Brown RD, Foley DA, Nkomo V, Petty GW, Scott C, et al. Cerebral ischemic events after diagnosis of mitral valve prolapse: a community-based study of incidence and predictive factors. Stroke. 2003;34(6):1339–44. [DOI] [PubMed] [Google Scholar]
- 5.Delgado V, Ajmone Marsan N, de Waha S, Bonaros N, Brida M, Burri H, et al. 2023 ESC guidelines for the management of endocarditis: developed by the task force on the management of endocarditis of the European Society of Cardiology (ESC) endorsed by the European Association for Cardio-Thoracic Surgery (EACTS) and the European Association of Nuclear Medicine (EANM). Eur Heart J. 2023;44(39):3948–4042. [DOI] [PubMed] [Google Scholar]
- 6.Zegri-Reiriz I, de Alarcón A, Muñoz P, Martínez Sellés M, González-Ramallo V, Miro JM, et al. Infective endocarditis in patients with bicuspid aortic valve or mitral valve prolapse. J Am Coll Cardiol. 2018;71(24):2731–40. [DOI] [PubMed] [Google Scholar]
- 7.Wang A, Gaca JG, Chu VH. Management considerations in infective endocarditis: a review. JAMA. 2018;320(1):72–83. [DOI] [PubMed] [Google Scholar]
- 8.Gopal M, Lakhani S, Lee VH. Intravenous thrombolysis in acute ischemic stroke patients with unsuspected infective endocarditis. J Stroke Cerebrovasc Dis. 2021;30(3): 105502. [DOI] [PubMed] [Google Scholar]
- 9.Murala S, Nagarajan E, Bollu PC. Infectious causes of stroke. J Stroke Cerebrovasc Dis. 2022;31(4): 106274. [DOI] [PubMed] [Google Scholar]
- 10.Griffiths D, Sturm J. Epidemiology and etiology of young stroke. Stroke Res Treat. 2011;2011:209370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hart RG, Foster JW, Luther MF, Kanter MC. Stroke in infective endocarditis. Stroke. 1990;21(5):695–700. [DOI] [PubMed] [Google Scholar]
- 12.Bettencourt S, Ferro JM. Acute ischemic stroke treatment in infective endocarditis: systematic review. J Stroke Cerebrovasc Dis. 2020;29(4): 104598. [DOI] [PubMed] [Google Scholar]
- 13.Khariton Y, House JA, Comer L, Coggins TR, Magalski A, Skolnick DG, et al. Impact of transesophageal echocardiography on management in patients with suspected cardioembolic stroke. Am J Cardiol. 2014;114(12):1912–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.