Abstract
Hearing disorders pose significant challenges to individuals experiencing them and their overall quality of life, emphasizing the critical need for advanced pharmacological approaches to address these conditions. Current treatment options often focus on amplification devices, cochlear implants, or other rehabilitative therapies, leaving a substantial gap regarding effective pharmacological interventions. Advancements in our understanding of the molecular and cellular mechanisms involved in hearing disorders induced by noise, aging, and ototoxicity have opened new avenues for drug development, some of which have led to numerous clinical trials, with promising results. The development of optimal drug delivery solutions in animals and humans can also enhance the targeted delivery of medications to the ear. Moreover, large genome studies contributing to a genetic understanding of hearing loss in humans combined with advanced molecular technologies in animal studies have shown a great potential to increase our understanding of the etiologies of hearing loss. The auditory system exhibits circadian rhythms and temporal variations in its physiology, its vulnerability to auditory insults, and its responsiveness to drug treatments. The cochlear clock rhythms are under the control of the glucocorticoid system, and preclinical evidence suggests that the risk/benefit profile of hearing disorder treatments using chronopharmacological approaches would be beneficial. If translatable to the bedside, such approaches may improve the outcome of clinical trials. Ongoing research into the molecular and genetic basis of auditory disorders, coupled with advancements in drug formulation and delivery as well as optimized timing of drug administration, holds great promise of more effective treatments.
Significance Statement
Hearing disorders pose significant challenges to individuals and their overall quality of life, emphasizing the critical need for advanced pharmacological approaches to address these conditions. Ongoing research into the molecular and genetic basis of auditory disorders, coupled with advancements in drug delivery procedures and optimized timing of drug administration, holds the promise of more effective treatments.
I. Introduction
A recent report published by the Global Burden of Disease study predicted that by 2050 an estimated 2.4 billion individuals worldwide will suffer from hearing loss (Global Burden of Disease Hearing Loss Collaborators, 2021). This is nearly twofold more than the numbers predicted for diabetes (Global Burden of Disease Diabetes Collaborators, 2023). Thus, it is not surprising that hearing loss ranks in the top three most common causes of years living with disability, after low back pain and migraine (Global Burden of Disease Hearing Loss Collaborators, 2021). Hearing loss in younger children impedes the development of spoken language and is a major risk factor for dementia in older adults (Thomson et al., 2017; Livingston et al., 2020). The emotional consequences of hearing loss include depression and anxiety, loneliness, and isolation. Hearing loss can range from mild to profound and has multiple origins (e.g., childhood illnesses, pregnancy-related illnesses, injury, genetics, age, ototoxicity, and excessive or prolonged exposure to noise). Hearing aids and cochlear implants have dominated the rehabilitation landscape for hearing loss, albeit by principally providing an amplification of sound intensity for hearing aids or a restored stimulation of the auditory nerve for cochlear implants, without completely addressing the complexity of temporal and spectral sound decoding. With regards to cochlear implants, only 5% of those who qualify receive the intervention (De Raeve, 2016; Raine et al., 2016). Despite these global unmet needs, hearing loss has received substantially less governmental funding to address knowledge gaps than diabetes has (Cederroth et al., 2013). This financing gap is more evident in Europe than in the United States. This is possibly due to the Americans with Disabilities Act, which includes hearing loss as a disability, whereas Europe lacks such broad and detailed definitions of disability and leaves it up to each country to determine their own legislation and its interpretation (Vanhala, 2015). The last decade has witnessed increasing efforts to develop the potential use of therapeutic interventions, other than hearing aids and cochlear implants, such as gene therapy, brainstem or cortical implants, and pharmacological treatments. In this review, we seek to provide a snapshot of the current knowledge on the pharmacology of hearing loss, both the causes and treatments, with the intent to establish a basis from which new opportunities may emerge.
II. A Primer on Cochlear Anatomy, Structure, and Function
The cochlea is a spiral-shaped coiled structure located in the inner ear and is responsible for converting sound vibrations into electrical signals that can be processed by the brain. The cochlea is divided into three fluid-filled chambers: the scala vestibuli, the scala media, and the scala tympani. The scala vestibuli and the scala tympani contain perilymph, which has a composition similar to cerebrospinal fluid, with a high concentration of sodium (140 mM) and a low level of potassium (5 mM). The scala media contains endolymph, which is high in potassium (150 mM) and low in sodium (1 mM). The scala vestibuli and scala tympani are sealed to the oval window and the round window, respectively. The altered ability of the external ear canal or middle ear to transfer sound waves from the ear canal to the inner ear is called conductive hearing loss.
The process of hearing begins when sound waves enter the ear and travel through the ear canal. These sound waves cause the eardrum to vibrate, which, in turn, sets the ossicles (malleus, incus, and stapes) in the middle ear into motion. The movement of the ossicles transmits sound vibrations to the cochlea through the oval window. There are several dysfunctions that can cause conductive hearing loss such as otitis media (middle ear inflammation), otosclerosis (a heritable condition that leads to an extensive ossification of the middle ear bones), and tympanic membrane rupture (e.g., either by noise blasts or mechanical damage).
Inside the cochlea, the mechanical vibrations are converted into electrical signals by the hair cells. These signals are then transmitted to the brain along the cochlear nerve for processing, ultimately allowing us to perceive and interpret sound. There are two types of hair cells: a single row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs). IHCs are sensory cells that transmit sound information to the spiral ganglion neurons (SGNs) and then to the brain. OHCs contain prestin, which is a voltage-sensitive membrane motor protein that is responsible for amplification of sound-induced vibrations within the cochlea (Zheng et al., 2000). Compared with the IHCs, the OHCs are more sensitive to noise trauma, ototoxic drugs, and age-induced hearing loss. Loss of OHCs results in reduced hearing sensitivity and frequency discrimination. The IHC overall appears to be more resistant to cell death than the OHC is. However, the presynaptic region of the IHCs is particularly sensitive to damage induced by noise overstimulation and aging. The basal pole of the IHC contains synaptic ribbons that are responsible for the release of the neurotransmitter glutamate and the activation of the auditory nerve (Glowatzki and Fuchs, 2002). Cochlear synaptopathy induced by noise trauma or aging results in a reduction of synaptic ribbons, causing acute and irreversible hearing loss (Kujawa and Liberman, 2009; Sergeyenko et al., 2013) without any morphological alteration to the IHCs. Cochlear synaptopathy is thought to lead to difficulties in understanding speech in noisy environments, without showing alterations in hearing thresholds (Bakay et al., 2018; Monaghan et al., 2020).
A. The Mechanotransduction Machinery
The stereocilia are located on the apical pole of the hair cells. On the IHCs, they are arranged in a near linear array, while on the OHCs the array is W-formed. The rigid stereocilia are composed primarily of actin and are crosslinked, stimulated by nanometer displacements and graded in height from the shortest to the longest (Flock and Cheung, 1977; Tilney et al., 1992). There are three different types of crosslinks: 1) those that run laterally along each row joining the stereocilia of the same row, allowing the stereocilia of a row move when some have been deflected; 2) those that run laterally between the rows that hold the tips of the shorter stereocilia in toward the taller neighbor; and 3) one per shorter stereocilium running upward toward its taller neighbor that is involved in mechanotransdution (MET) (Pickles et al., 1984). The tip links become stretched when the stereocilia bundle is deflected. In bullfrog hair cells, the movement of the stereocilia toward the kinocilium has been shown to result in depolarization, whereas deflection in the opposite direction causes hyperpolarization (Hudspeth and Corey, 1977). Similar findings were found in mammalian species where deflections toward the longest stereocilium resulted in depolarization (Géléoc et al., 1997; Kennedy et al., 2003; He et al., 2004). The stereocilia pivot at their insertion points at the level of the cuticular plate, causing mechanical forces to open the MET channel (Strelioff and Flock, 1984; Crawford and Fettiplace, 1985; Howard and Ashmore, 1986; Howard and Hudspeth, 1988). It is estimated that there is one (Géléoc et al., 1997) to two (Beurg et al., 2009; Fettiplace et al., 2022) transduction channel for each tip link.
Knowledge of the molecular composition of the MET channel assembly largely stems from genetic studies on monogenic forms of deafness, dominant or recessive, that have provided a number of candidate genes (Richardson et al., 2011), that have then been back-translated and validated in animal models. The tip links are formed from heterodimers of the transmembrane proteins protocadherin-15, forming the lower one-third of the tip link, and cadherin-23, forming the upper two-thirds of the tip link (Kazmierczak et al., 2007). Also found at the upper end of the tip link are myosin motors (myosins IC and VIIA) (Pan and Holt, 2015; Zhao and Müller, 2015), and other proteins such as USH1G, USH, and harmonin (Grati and Kachar, 2011). The channel complex at the lower end of the tip link includes the molecules TMC1, TMC2, LHFPL5, TMIE, TOMT, and CIB2 (Kurima et al., 2002; Santos et al., 2006; Du et al., 2008; Pan et al., 2013; Giese et al., 2017; Cunningham et al., 2020; Jia et al., 2020; Zheng and Holt, 2021).
B. Cochlear Innervation
Cochlear innervation by SGNs is composed of 95% type I auditory afferent nerve fibers and 5% type II nerve fibers. Type I nerve fibers are myelinated and thick; they are mainly connected to IHCs and send sound information to the brain (Spoendlin, 1969, 1972). Type II afferent fibers are unipolar, unmyelinated, and relatively thin, and each one extends to more than 10 OHCs (Ginzberg and Morest, 1983; Berglund and Ryugo, 1987). In contrast, OHCs receive only 5% of the afferent innervation. Single-cell RNA sequencing (scRNAseq) studies show that the types I and type II afferent fibers are also distinguishable molecularly with more than 1700 differentially expressed genes, 350 of which are clearly binary in their expression, with type Is expressing very specific markers such as Epha4, Kcna1, Calb2, and Pvalb and type IIs expressing markers such as Prph, Plk5, Th, and Cacna1g, among others (Sun et al., 2018; Petitpré et al., 2018; Shrestha et al., 2018).
Each IHC is innervated by approximately 20 nerve fibers, depending on cochlear location (Liberman, 1980). Upon stimulation, the IHCs use the synchronized release of hundreds of synaptic vesicles in a manner graded with sound intensity (Glowatzki and Fuchs, 2002). Electrophysiological studies have attempted to record from the type II fibers, but this has proven difficult because of the low numbers and small diameters of their axons (Brown, 1994; Robertson et al., 1999). Compared with the type I synapse, the OHCs have fewer vesicles and a reduced vesicle release probability (Weisz et al., 2014). A study by Robertson et al. in a guinea pig demonstrated that the type II fibers responded to loud sound (Robertson, 1984). Type II neurons also respond weakly to glutamate release from OHCs and more strongly to ATP release (Weisz et al., 2009), most likely from surrounding supporting cells (Lahne and Gale, 2008). The function of the type II SGNs is not well understood, but one current hypothesis is that type II afferent activation by loud sound and modulation by ATP from supporting cells is similar to nociception (thus referred to as auditory nocioception) to protect the hair cells from high sound levels (Flores et al., 2015; Liu et al., 2015).
Type 1 SGNs have three subtypes, and these are classified according to morphological and physiological traits such as threshold and spontaneous firing rate (SR) (Liberman, 1978, 1982; Taberner and Liberman, 2005). The type with low thresholds and high spontaneous firing rates (high SR) respond to low-intensity sounds. Conversely, the subtype combining high thresholds and low spontaneous rates (low SR) can detect high-intensity stimuli. The third subtype displays a combination of low and high SR fibers. Further molecular characterization of SGN using scRNAseq confirmed three transcriptionally unique type 1 subtypes (Sun et al., 2018; Petitpré et al., 2018; Shrestha et al., 2018). The molecular identity of type I neuron subtypes also displays unique profiles but more often a combination or gradients of markers. It is believed that these three subtypes correspond to the thresholds and spontaneous activity of the nerve fibers described previously. Type 1 A would correspond to the high SR expressing high levels of Calb2 and Pcdh20, type 1B the medium SR expressing high levels of Calb1 and Lrrc52, and type 1C the low SR expressing Pou4f1 and Lypd1 (Sun et al., 2018; Petitpré et al., 2018; Shrestha et al., 2018). These findings illustrate the complexity of SGN molecular signatures and their correlation with their functional characterization but inform on potential markers that can be used for the selective pharmacological targeting or development of neurons implicated in specific forms of hearing loss.
Both the IHCs and OHCs receive efferent innervation originating in the superior olivary complex (Guinan et al., 1983, 1984). The base of the OHCs receives innervation from the medial olivocochlear efferent neurons (Warr and Guinan, 1979) that release acetylcholine to activate α9/α10 ACh receptors that allow Ca2+ to enter the OHCs, which activates nearby Ca2+-dependent K+ channels that allow K+ to flow out of the cell and causes hyperpolarization. The functional consequence of medial olivocochlear activation is to hyperpolarize the OHCs that shunt depolarization from MET, resulting in reduced somatic motility and amplification. The IHCs have lateral olivocochlear (LOC) efferent fibers that synapse on the unmyelinated dendrites of type I SGNs. The LOC originates in the region surrounding the lateral superior olive. The LOC releases several transmitters, including dopamine, GABA, acetylcholine, and peptides (Niu and Canlon, 2006; Ruel et al., 2007). Darrow et al. (2006) demonstrated two primary types of LOC synapses based on firing patterns that release acetylcholine and dopamine, respectively (Darrow et al., 2006). However, it is unknown whether these two synapses have opposing actions on the afferent auditory nerve fibers. One primary function of the LOC is to reduce damage to the afferent auditory nerve fibers from excessive sound stimulation (Kujawa and Liberman, 2009).
C. The Cochlear Powerhouse—The Stria Vascularis
The lateral wall of the scala media contains three structures: the outer spiral sulcus, the stria vascularis (SV), and the spiral ligament. The outer spiral sulcus has two cell types, the Claudius cells and the outer sulcus cells, or root cells. Both cell types are believed to be involved in maintaining cochlear fluid balance by recycling potassium for the homeostasis of the high K+ levels (∼157 mM) in endolymph (Kikuchi et al., 1995). The functions may differ between root cells in the apical and basal portions of the cochlea, as root cells in the apex express the water channel protein, aquaporin 5, while aquaporin 4 is expressed at the basal portion of the outer sulcus (Eckhard et al., 2015). The cells in the outer sulus are essential to the recycling of K+ ions that maintain the ion content in the endolymph as well as the endocochlear potential (EP) (Kikuchi et al., 2000; Wangemann, 2006). Potassium ions are then released through basolateral K+ channels and subsequently taken up by the supporting cells.
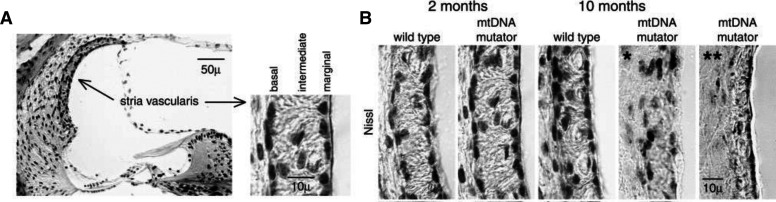
The SV is a highly vascular tissue in the lateral wall and has three major cell types (marginal, intermediate, and basal cells) along with a minor number of spindle cells, pericytes, and endothelial cells (Fig. 1). Together, these cells maintain the ionic composition of the endolymph and produce the EP (Salt et al., 1987; Wangemann, 2006; Nin et al., 2016). The EP has a direct current potential of +80 mV that drives the K+ ions of the endolymph through the transduction channel at the apical pole of the hair cells. For this reason, the SV is often referred to as the cochlear battery, or the powerhouse.
Fig. 1.
Histology of the stria vascularis and effects of aging. (A) Light microscopic image showing a cross-section through the OC illustrating the lateral wall that includes the SV and the spiral ligament. The SV contains three cell layers: the basal, the intermediate, and the marginal cells. The marginal cells face the endolymph. (B) Sections stained for Nissl (cresyl-violet) illustrate the SV from wild-type and a model for aging, the mitochondrial DNA mutator mice at 2 and 10 months of age. All sections are obtained from the middle turn of the cochlea. The SV from the 10-month-old mitochondrial DNA mutator mice demonstrate a disorganized (*) and a thinning (**) of the SV (Niu et al., 2007).
A number of genetic mutations have been associated with dysfunctions of the SV that cause hearing loss (Zhang et al., 2020). These mutations can target proteins located in one or more of the three cell types (i.e., basal, intermediate, or marginal cells) and affect one key step in the transport of K+ from the blood to the scala media. The marginal cells are in contact with the endolymph and are involved in the transport of K+ ions (Wangemann, 1995; Kim and Ricci, 2022). In particular, the K+ channels in the intermediate cells are essential to K+ transport, which regulates the level of the EP. The intermediate cells, which are melanocytes, lie between the marginal and basal cells. Pathology changes of the intermediate cells result in a diminished EP (Steel and Barkway, 1989), since the intermediate cells regulate the K+ concentration and transport through the SV (Chen and Zhao, 2014). The basal cells are connected via gap junctions to the fibrocytes of the spiral ligament and recycle K+ from the perilymph to maintain the EP. Claudin 11 is one of the proteins that is highly specific to a single cell type and for which animal mutants exist. Claudin 11 is located in basal cells, and its dysfunction causes profound hearing loss in mice, associated with a loss of the EP (Gow et al., 2004). Evidently, maintenance of the EP is complex and involves the contribution of the different SV cell types, which appear to play an important role in normal hearing. One mouse model of aging, the mitochondrial mutator mouse (mitochondrial DNA) (Niu et al., 2007), shows degeneration of the different SV cell types, with aging correlating with increased auditory thresholds (Fig. 1).
Due to the difficulty in accessing the human cochlea, most of our knowledge stems from experimental research on animals. ATP6V0A4 causes distal renal tubular acidosis and sensorineural hearing loss in humans. Mice that lack ATP6V0A4 expressed in intermediate cells are completely deaf and also lack the EP (Lorente-Cánovas et al., 2012). Marginal cells, by contrast, have several more mouse models deriving from human syndromes. For example, loss of function of the KCNQ1 or the KCNE1 subunit of the apical K+ channel of marginal cells causes deafness in mice and humans (Vetter et al., 1996), as does the disruption of their basolateral NaK2Cl cotransporter in mice (Delpire et al., 1999). Likewise, human Bartter syndrome IV is an autosomal recessive disorder characterized by congenital deafness and severe renal salt and fluid loss. It is caused by mutations in BSND, which encodes Barttin, a β-subunit of ClC-Ka and ClC-Kb chloride channels. Inner-ear-specific disruption of Bsnd in mice reveals that the endocochlear potential, but not the high potassium concentration, of the scala media depends on the presence of these channels in the epithelium of the SV (Rickheit et al., 2008).
III. Delivery Routes to the Cochlea
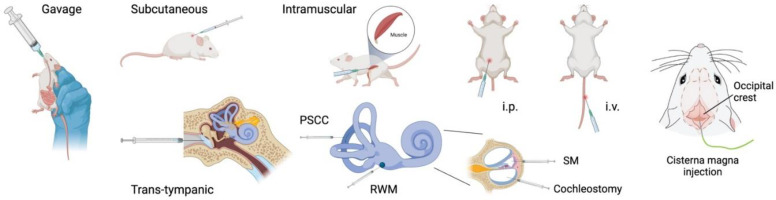
When pharmacological agents are being delivered, it is imperative to maximize their therapeutic efficacy. This can be achieved by ensuring that target exposure is sufficient to obtain the desired benefit while also minimizing risk of adverse events from off-target effects, depending on the properties of the pharmacological agent. The many delivery procedures that are commonly used in the preclinical auditory field include standard systemic routes (intravenous, intraperitoneal, subcutaneous, intramuscular oral, and nasal administration) as well as approaches that leverage more direct delivery into the fluid compartments of the inner ear (Fig. 2).
Fig. 2.
Drug delivery routes in preclinical hearing research. Illustrating the most common procedures for delivering drugs to the inner ear. ip, intraperitoneal; iv, intravenous; PSCC, posterior semicircular canal; RWM, round window membrane; SM, scala media.
A. Systemic Approaches and the Blood-Labyrinth Barrier
With systemic delivery, a critical question arises concerning the distribution of a drug to the inner ear compared with other compartments in the body. Some studies have used induced-coupled plasma mass spectrometry to measure ototoxic drug levels in the cochlea after intraperitoneal injections (Breglio et al., 2017; Tserga et al., 2020) or determined the distribution of dexamethasone to the perilymph after intravenous or intraperitoneal injection (Wang et al., 2018; Ke et al., 2023) or the dose-dependent target exposure of novel otoprotective drug candidates after oral administration (Petremann et al., 2017) using both liquid chromatography and tandem mass spectrometry. However, such approaches are not routinely included in most auditory research efforts involving systemic drug administration. Although systemic delivery is clearly capable of achieving inner ear drug exposure for compounds with appropriate individual characteristics, it typically leads to general exposures in other compartments and may thus increase the risk of side effects, depending on the drug candidate profile.
The systemic delivery of substances aimed at targeting the inner ear needs to bypass the blood-labyrinth barrier (BLB), which divides the vasculature and the perilymph and endolymph. The BLB is fully developed by postnatal day 14 (Suzuki and Kaga, 1999). Its primary function is to maintain the ionic homeostasis of the inner ear fluids and protect the inner ear from substances that have potential adverse effects (Shi, 2011). It is composed of tightly coupled vascular endothelial cells, perivascular resident macrophage-like melanocytes, and pericytes and fibrocytes in the lateral wall (Zhang et al., 2012; Neng et al., 2013). When treating the inner ear with pharmacological substances, it is important to know whether the drug in question can pass this barrier and achieve target exposure. The tight junctions making up the BLB limit the entry of molecules or drugs to the inner ear.
The BLB may limit the accessibility of specific systemically delivered drugs to the inner ear and reduce their therapeutic efficacy, specifically in the case of repurposed drugs that were not initially developed to target the inner ear. Formulation efforts have been made to improve the passage through the BLB and thus enhance distribution into the inner ear. This includes the addition of lipid molecules to increase the hydrophilic nature of the substance. Juhn and Rybak suggested that intravenously injected substances are transported into the cerebrospinal fluid (CSF) and the perilymph via different mechanisms (Juhn and Rybak, 1981; Juhn et al., 1981). The permeability of the BLB can be disrupted by osmotic agents (including diuretics), inflammation, and traumatic noise exposure (Shi, 2016). For instance, the diuretic furosemide can increase the entry of cisplatin and aminoglycosides into the cochlea by disrupting BLB function (Mulders et al., 2014; Li and Steyger, 2011). Acoustic trauma can also increase the permeability of the BLB by damaging the tight junctions (Wu et al., 2017; Ke et al., 2023).
The brain is protected by systemically delivered drugs and toxins by the blood-brain barrier (BBB), which has certain similarities to the BLB. The main function of both the BLB and the BBB is to separate the blood from interstitial fluid to maintain homeostasis. After an intravenous injection of a radioactive tracer, the BLB has been shown to be less permeable than the blood-CSF barrier. After 90 minutes, more of the tracer was in the CSF compared with in the perilymph (Inamura and Salt, 1992). However, the aminoglycoside antibiotic gentamicin can cross the BLB but not the BBB, indicating that these differences could be due to the tightness of the barrier or transport mechanisms over the barrier (Neuwelt et al., 1984). For more information on the differences between the BLB and the BBB see Nyberg et al., (2019).
B. Local Delivery of Drugs
To overcome target exposure difficulties or safety concerns for specific drug candidates, investigators have sought more controlled and reliable routes of delivery to the inner ear. Several procedures can be used to locally deliver drugs to the inner ear, with varying degrees of risks for causing damage to the inner ear. The first approach is to inject the drugs through the tympanic membrane. This is commonly used in the clinic. It is also used on experimental animals where, after being infused into the middle ear, the drug is expected to diffuse across the round window membrane (RWM) and into the perilymph. The RWM is a barrier between the middle ear and the scala tympani. It is semipermeable and allows the passage of numerous substances, depending on their molecular size, concentration, and electrical charge (Goycoolea, 2001). Adding excipients such as DMSO, saponin, or benzyl alcohol can improve penetration through the RWM (Li et al., 2018). Likewise, the use of biodegradable hydrogels placed near the RWM improves cochlear exposure (Endo et al., 2005). Another approach includes intratympanic injection through the otic bone into the middle ear which does not compromise the tympanic membrane (Oishi et al., 2013).
The second approach for improving the control and reliability of drug delivery to the inner ear includes intracochlear procedures. Despite requiring invasive surgery and increasing the risk of damaging the cochlea, these procedures are commonly used in experimental animals. For instance, delivery through the RWM is known to increase drug concentration in the inner ear when compared with systemic injections. However, this procedure can cause damage to the membrane itself and decrease perilymphatic pressure (Plontke et al., 2016; Szeto et al., 2020). There is also the risk of inserting the injection needle too far in and damaging the basal turn of the cochlea. New techniques such as microperforations to the RWM can improve permeability and reduce the risk of damage (Kelso et al., 2015). A third approach is to injection into the semicircular canal (Salt et al., 2012). Using the semicircular canal has the advantage of efficiently introducing drugs or viral vectors into the inner ear without compromising hearing thresholds.
C. Cerebrospinal Fluids Linked to the Cochlear Perilymph
A more recent approach, possibly an intermediary method between systemic and local injections, has been to use the cisternae magna (CM) as an injection site (Fig. 2). The cisterna magna is located in the posterior fossa, dorsal to the medulla and caudal to the cerebellum. It contains CSF, which is transported along the perivascular spaces in what has been termed the glymphatic system (Iliff et al., 2012). Glymphatic fluid transport plays an important homeostatic role, as fluid efflux clears metabolic waste products from the brain (Lohela et al., 2022). The CM freely communicates with the subarachnoid space, which connects the cerebrospinal fluid to the perilymph of the scala tympani via the cochlear aqueduct. Large-particle tracers (e.g., gadobutrol 0.6 kDa) injected into the CM reach the inner ear through dispersive transport via the cochlear aqueduct in adult mice within minutes (Mathiesen et al., 2023). Amine-modified polystyrene microspheres (0.2 and 1 μm in diameter), which could be used for drug delivery, can also reach the cochlea through this route (Mathiesen et al., 2023).
Connections between the inner ear fluids and the CSF have previously been suspected, particularly because the delivery of adeno-associated viruses (AAVs) through the RWM can reach the contralateral ear (Stöver et al., 2000). Recently, AAVs injected in the CM were shown to reach and transduce both left and right cochleae (Blanc et al., 2021). The therapeutic potential of this route was demonstrated by Koch-Mathiesen et al., who showed that in adult deaf Slc17A8 -/- mice, a single CM injection of a vesicular glutamate transporter-3 (VGLUT3) expressing AAV can effectively transduce IHCs and fully rescue auditory brainstem responses to levels equivalent to wild-type mice (Mathiesen et al., 2023). A similar connection between CSF and the cochlea has been suggested in the rhesus macaque (Macaca mulatta), where intracerebroventricular injections of AAV9.EGFP lead to cochlear IHC transduction together with cells of the spiral ligament, and cells of the spiral limbus (Ranum et al., 2023), supporting the translational potential of the CM route for gene therapy.
The application of this approach in humans has been debated, particularly due to the possibly reduced patency of the cochlea aqueduct, which is thought to decline with age (Włodyka, 1978; Gopen et al., 1997). Indirect measures of intracochlear pressure changes during postural changes revealed that the aqueduct was functionally patent in 89% of young adults and in 70% of older adults (Phillips and Marchbanks, 1989; Wagner and Walsted, 2000). Several studies suggest that the cochlear aqueduct in humans can transfer intracranial pressure changes and that enlarged intracranial pressure can be detected by measuring intratympanic pressure (Shimbles et al., 2005; Gwer et al., 2013; Evensen et al., 2018). Interestingly, when assessing magnetic resonance imaging data from cisternograms performed on individuals to assess CSF leaks at the base of the skull, Totten et al. observed the progressive diffusion of gadolinium contrast into the human cochleae and vestibule (Totten et al., 2023). Several ongoing trials are using cisternae magna injections for neurodegenerative diseases (Taghian et al., 2020; Marchi et al., 2022). Overall, these findings strongly support that cerebrospinal fluid transport serves as an effective and accessible route for gene delivery or other otoprotective agents to repair the adult inner ear and thus represents a crucial step toward restoring hearing in rodents and humans.
IV. Structural Damage and Molecular Pathways in the Cochlea: From Noise and Aging to Ototoxic Drugs
Damage to the cochlea has various causes including noise trauma, aging, and ototoxic drugs. These insults can affect the sensory cells and neurons and the nonsensory structures in the cochlea, which can result in reduced hearing sensitivity of various degrees (Fig. 3).
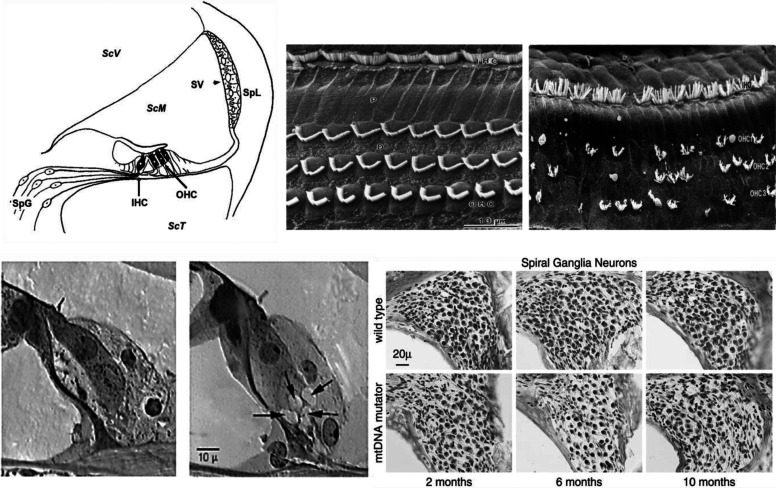
Fig. 3.
Anatomical and histological alterations upon cochlear damage. Examples of the alterations that can occur after damage to the cochlea. (A) Schematic cross-section of the OC showing the inner hair cells (IHCs) and outer hair cells (OHCs), the stria vascularis (SV), the spiral ligament (Spl), scala media (ScM), SGNs (SpG), and the two scala containing perilymph Scala vestibuli (ScV) and Scala tympani (ScT). (B) Scanning electron microscopy images of the apical surface of the OC showing the normal (left) array of stereocilia on the IHCs (upper portion of image) and the three rows of OHCs; (right) an example after noise trauma showing disarrayed IHC stereocilia and missing and disarrayed OHC stereocilia. (C) Section showing the IHCs with intact afferent dendrites at the basal end of the cell and on the right, an example of excitotoxicity after trauma. The arrows are pointing to swollen afferent dendrites. (D) Progressive loss of SGNs in a mouse model of aging, the mitochondrial DNA mutator with increasing age. Representative micrographs showing bundles of SGNs from the middle turn of the wild-type and the mitochondrial DNA mutator mice at 2, 6, and 10 months of age.
A. Noise Trauma
Noise exposure can cause either temporary or permanent hearing loss. In general, temporary hearing loss occurs when the intensity is low to moderate, whereas a permanent hearing loss results from higher intensities. When the intensity of the exposure is high, a combination of mechanical damage and metabolic stress occurs in the cochlea, causing either OHC loss, OHC and IHC loss, or complete loss of the organ of Corti (OC). The damage is irreversible since sensory cells do not spontaneously regenerate. OHCs are more susceptible to cell death than are IHCs. When an OHC dies and degenerates, the hole is then replaced by a scar formed by neighboring phalangeal cells (Raphael et al., 1993). Damage to the stereocilia on the apical portion of the hair cells can include detachments from the tectorial membrane, breaks in the tip links, and loss of rigidity, making them unable to transduce mechanical energy to the cell body (Liberman and Dodds, 1987; Jia et al., 2009). The stereocilia, however, have the potential to repair themselves (Wagner and Shin, 2019), a potential mechanism underlying temporary noise-induced hearing loss. Noise trauma can permanently affect the afferent synapse of the IHCs due to an excessive release of glutamate, which causes excitotoxicity (Puel et al., 1998). Different types of supporting cells in the OC can also show pathology after noise trauma; however, these changes are typically found after impulse noise or very high-intensity sounds (Nordmann et al., 2000) and can depend on genetic background (Herranen et al., 2018).
Noise overstimulation can produce hearing loss in which OHCs, particularly those at the high-frequency region of the cochlea, are affected more than at the low frequency region. It has been suggested that the differential vulnerability can be due to differences in the Ca2+ balance among cochlear locations. Intracellular Ca2+ homeostasis is determined by Ca2+ influx through the mechanotransducer channels and efflux by the plasma membrane CaATPase pump. As a result of noise overstimulation, a Ca2+ overload is thought to make the OHCs in the high-frequency region more vulnerable to noise overstimulation (Fettiplace and Nam, 2019). A third type of hearing loss induced by noise trauma is referred to as hidden hearing loss. This type of hearing loss is distinguished by a loss of the synaptic connections from the hair cell to the neurons, without any hair cell loss (Furman et al., 2013). In mice, a permanent reduction in the number of ribbon synapses after noise trauma is a feature of hidden hearing loss reflected by a decrease in the amplitude from the wave 1 of the auditory brainstem response (ABR) without evidence of a permanent threshold shift (Lin et al., 2011). The ABR wave I amplitude is a measure of auditory nerve activity and noise trauma can cause cochlear deafferentation and reductions in wave 1 amplitude (Kujawa and Liberman, 2009). The functional consequence of this selective loss would lead to difficulties in understanding speech, particularly in noisy environments (Oxenham, 2016; Bakay et al., 2018). These noise-induced changes can eventually lead to a loss of SGNs (Kujawa and Liberman, 2006).
As mentioned above, the SV is also a generator of the endolymphatic potential, which is essential for sensory hair cell transduction and contains marginal, intermediate, and basal cells. The intrastrial fluid-blood barrier contains perivascular resident macrophages that are required for maintaining the EP potential and keeping the tight junctions in the barrier. Acoustic trauma has been shown to break down the tight junctions, resulting in the leakage of serum proteins into the endolymph (Zhang et al., 2013). These changes will affect the endolymphatic potential and result in reduced hearing sensitivity.
B. Aging
Presbycusis or age-induced hearing loss is a complex phenomenon due to the multitude of factors that can influence the hearing organ. Such factors include prior noise exposure or exposure to ototoxic drugs or solvents, genetic factors, vascular pathology, infection, or other hearing related disorders (Keithley, 2020; Eckert et al., 2021). These different factors that cause cell degeneration and hearing loss result from oxidative stress, mitochondrial DNA damage, inflammatory processes, and impaired vascular supply to the inner ear (Seidman et al., 2002; Watson et al., 2017).
Through aging, the OHCs are more susceptible to cell death compared with the IHCs in both human and rodent cochlea (Bredberg, 1968; Wu et al., 2019). In humans, the loss occurs in the apical and basal regions and progresses to the other regions of the cochlea. In rodents, the loss of OHCs starts at the basal region of the cochlea and proceeds toward the apex. Spiral ganglion loss is also apparent in the human and rodent inner ear. During aging, neuronal loss has been demonstrated to be greater than IHC loss (Viana et al., 2015). This is an interesting observation and suggests that, during aging, the SGNs are disconnected from the IHCs.
Over the years, the SV has been indicated as a significant contributor to age-induced hearing loss—a finding that has also been recently supported by genetic studies in humans (see Section VI.). Damage to the lateral wall will alter cochlear homeostasis, potassium recycling, and the maintenance of the EP that drives current through the hair cells and maintains normal hearing. Raising gerbils in a quiet environment revealed that aged gerbils display a significant degree of degeneration of the lateral wall and reductions in the EP (Gratton et al., 1996). The mitochondrial DNA mutator mouse also shows increasing degeneration of the cells in the SV with age (Fig. 1).
In the spiral ligament, type IV fibrocytes show pathological changes with increasing age in the human cochlea, a phenomenon that has been also found in aged gerbils and mice (Spicer and Schulte, 2002; Wu and Marcus, 2003). Surprisingly, spiral ligament fibrocytes degenerate before any loss of hair cells occurs. In animal studies, it appears that age-related hearing loss (ARHL) is the result of strial atrophy and fibrocyte degeneration in the spiral ligament. Earlier studies of human presbycusis suggested that strial atrophy or degeneration of auditory nerve fibers rather than hair loss was the cause of the age-induced hearing (Schuknecht, 1964; Schuknecht and Gacek, 1993). In a more recent study, an analysis of autopsy specimens showed that hair cell degeneration outweighed the effects of strial atrophy (Kaur et al., 2023). These distinctions may be a reflection of species differences as well as the histological procedures that were used in the early human studies.
Another morphological feature that can explain part of the hearing loss induced by age is the myelination of the type 1 SGNs. Myelination of the auditory nerve is required for normal auditory nerve function and the transmission of electrical impulses from the cochlea to the auditory brain (Wan and Corfas, 2017). Degradation of the myelin on nerve fibers and reductions in myelin basic protein have been found in aged animals (Cohen et al., 1990; Xing et al., 2012) and in older human samples. These findings suggest that the degeneration of the myelin sheath around nerve fibers can alter hearing sensitivity in aged subjects.
C. Ototoxicity
Ototoxicity is the process by which drugs and medication negatively impact the function of the inner ear. More than 100 different types of drugs have been found to be ototoxic, including anticancer drugs, aminoglycoside and nonaminoglycoside antibiotics, and antimalarial drugs, leading to an estimated 20 million new cases of hearing loss worldwide per year (Prasad et al., 2024). This section will focus only on the impact of cisplatin and aminoglycosides on hearing.
1. Cisplatin
Cisplatin (cis-diammine dichloroplatinum II) is a highly effective chemotherapeutic agent commonly used in the treatment of solid tumors and several types of malignant tumors, such as those of the head and the neck, in adults and children (Wang and Lippard, 2005; Dasari and Tchounwou, 2014a). Cisplatin is known to have numerous debilitating side effects such as permanent hearing loss, neurotoxicity, and nephrotoxicity (Dasari and Tchounwou, 2014b; Sheth et al., 2017). Carboplatin is also a platinum-based drug used against cancer but has less toxicity than cisplatin. The prevalence of hearing loss following cisplatin treatment is dependent upon the cumulative dose and can range from 4% to 90% depending on a variety of factors such as the age and gender of the patient, which combination of other ototoxic agent(s) or irradiation are used, exposure to concomitant noise, duration of the treatment, and treatment administration methods (Karasawa and Steyger, 2015; Landier, 2016). A recent systematic review of 66 studies has estimated that the global prevalence of ototoxic-induced hearing loss is 43.2% (Dillard et al., 2022). This study revealed that the prevalence of hearing loss for cisplatin alone was 49.2%, whereas that of carboplatin alone was 13.5%. Treatment regimens involving both cisplatin and carboplatin increased the prevalence to 73.2% in children under 5 years of age. One major obstacle to estimating the true impact of platinum-based therapy in humans is the method used to assess hearing loss, which ranges from audiometry to OAEs and other ototoxicity grading scales (e.g., ASHA, CTCAE, Brock, Chang), some of which are exclusively designed for pediatric use. Nonetheless, the prevalence estimates are relatively similar across these different diagnostic methods and imply that nearly half a million cases of hearing loss per year occur based on ototoxicity.
Cisplatin-induced hearing loss typically affects the high-frequency regions at the base of the cochlea and eventually affects the lower frequencies with continued use. In experimental animals, the key structures affected by cisplatin resulting in hearing loss include the marginal cells of the SV, the spiral ligament, and the OHCs; and with extended use, the IHCs, supporting cells, and the SGNs are also affected (Breglio et al., 2017). Similar to the actions of aminoglycosides, cisplatin also enters the inner ear through the bloodstream and must pass the BLB. Cisplatin enters the inner ear fluids via the SV and then enters the hair cells (Prayuenyong et al., 2021). One entry route of cisplatin to the hair cells is via the mechanosensitive transduction channels. Use of fluorescently tagged cisplatin revealed that cisplatin enters the hair cells through mechanoelectrical transduction channels at the tip of the stereocilia (Waissbluth and Daniel, 2013; Kros and Steyger, 2019; Thomas et al., 2013). Moreover, the prolonged effects of cisplatin in the cochlear tissues cause further pathology to the different cell types. This is because platinum remains in the cochlea for long periods of time (Breglio et al., 2017).
Molecular pathways that involve cisplatin-induced ototoxicity include reactive oxygen species (ROS) generation, inflammation, and autophagy, ultimately resulting in cell death. The underlying basis of hearing loss due to ototoxic medications is multifactorial since the drugs can impact the production of the EP, the function of OHCs and their survival, and the coupling between the IHC-afferent neuron synapse. Cisplatin can also enter hair cells by both passive diffusion and active uptake. IHC uptake involves copper transporters such as CTR1 and organic cation transporters such as OCT2. Once in the cells, cisplatin can be hydrolyzed by water to generate aqua-cisplatin complexes that are toxic and can damage DNA. This damage leads to the upregulation of ataxia telangiectasia mutated, which activates the tumor suppressor p53 and leads to the release of the proapoptotic protein Bcl-associated X, ultimately increasing mitochondrial membrane permeability and releasing cytochrome c via caspase 3 activation (Wang et al., 2004; Benkafadar et al., 2017). Entry into hair cells may also occur through the MET, since the blockade of MET channels can reduce cisplatin ototoxicity (Thomas et al., 2013). Hair cells have a high metabolic rate since they have to process information with high spectral and temporal precision of up to 20 kHz in humans and 60 kHz in rodents. Such systems require an active antioxidant defense mechanism to regulate ROS, when subjected to noise or ototoxicity. Some NADPH oxidases (NOX), such as NOX3, are highly abundant in the inner ear and are strongly induced by cisplatin (Mukherjea et al., 2010). Their blockade using small interfering RNA administration in the middle ear cavity can attenuate cisplatin-induced hearing loss.
Oxidative stress occurring in hair cells exposed to cisplatin decreases the levels of antioxidant enzymes such as superoxide dismutase, glutathione reductase, glutathione S-transferase, and glutathione peroxidase (Rybak et al., 2000; Campbell et al., 2003). This ultimately leads to the release of cytochrome c and apoptosis. Cisplatin-mediated hair cell death involves not only apoptosis but also ferroptosis and necroptosis. Ferroptosis involves lipid peroxidation, iron accumulation, and the reduction of the mitochondrial membrane potential, all of which precede autophagy. Necroptosis is a passive form of necrosis mediated by receptor interacting protein kinases. Intraperitoneal injection of Necrostatin-1 s, an inhibitor of receptor interacting protein kinase 1, protects against cisplatin-mediated hearing loss in rats (Choi et al., 2019). Downstream of lipid peroxidation is the generation of toxic reactive aldehydes. These reactive aldehydes can activate nonselective cation permeable channels such as the transient receptor potential (TRP) channels, which are involved in taste, touch, smell, and pain. TRPV1 is one type of TRP channel identified in the cochlea that is activated by NOX3-mediated release of ROS. Transtympanic administration of TRPV1 small interfering RNA protects against cisplatin-mediated hearing loss (Mukherjea et al., 2008).
2. Aminoglycosides
Aminoglycoside antibiotics (e.g., kanamycin, neomycin, amikacin, capreomycin, or streptomycin) are used against multidrug-resistant tuberculosis. They are therefore likely to be the most commonly used antibiotics worldwide. A meta-analysis of 18 studies from 10 countries has estimated a global prevalence of aminoglycoside-mediated hearing loss of 40.6% for all drugs (Dillard et al., 2021). Individually, kanamycin reached the highest prevalence (49.7%), followed by amikacin (38.9%) and capreomycin (10.2%). Similar to cisplatin-induced ototoxicity, aminoglycoside ototoxicity can potentially result in the hearing loss of nearly half a million individuals with drug-resistant tuberculosis. The application of World Health Organization guidelines may prevent 50 million cases of hearing loss per year (Dillard et al., 2021).
Aminoglycosides enter through the bloodstream and through the BLB, a specialized structure consisting of tight junction-coupled inner ear endothelial cells, which separates the inner ear tissues from the bloodstream (Li and Steyger, 2011). Aminoglycosides then pass through the different cell types in the SV—first the basal, then the intermediate, and finally the marginal cells. The aminoglycosides primarily enter the endolymph, which is the source of uptake into the hair cells (Li and Steyger, 2011). Electrophysiological studies show that aminoglycoside uptake into the hair cells occurs through mechanosensitive transduction channels (Marcotti et al., 2016) and that functional mechanotransduction channels are required for ototoxicity (Alharazneh et al., 2011). The OHCs are more susceptible to aminoglycosides than are the IHCs. However, with increasing administration, the IHCs and apical OHCs become damaged. The OHCs die and are replaced by a scar formed by neighboring phalangeal cells. Synaptopathy is also evident in cochleae exposed to aminoglycoside antibiotics, where loss of IHC synapses appears before threshold shifts are apparent (Ruan et al., 2014). Ototoxicity starts in the high-frequency region and with extended use the damage progresses to the lower-frequency range (Zettner and Gleser, 2018). The hearing loss induced by aminoglycosides starts in the basal, high-frequency region and with prolonged use will spread to the more apical regions of the cochlea.
Mechanisms regulating the vulnerability to aminoglycoside ototoxicity appear to be centered around the mitochondria. A number of variants at the 12S rRNA of the mitochondrial genome are associated with an increased risk of aminoglycoside ototoxicity (e.g., A1555G, 745 A > G, 792C > T, 839 A > G, 856 A > G, 1310C > T, and 1452T > C), making the 12S tertiary structure more similar to the bacterial 16S rRNA and thus a more likely target of aminoglycosides (Prezant et al., 1993; Lu et al., 2010). Similar to cisplatin ototoxicity, aminoglycoside antibiotics have been reported to generate free radicals in the inner ear and to damage sensory cells and neurons. Mice overexpressing superoxide dismutase 1 display less aminoglycoside-induced ototoxicity compared with the wild-type controls (Sha et al., 2001). Indeed, a number of studies have evidenced the protective effects of antioxidants against ototoxicity (Sha et al., 2001; Feldman et al., 2007; Naeem et al., 2009; Kocyigit et al., 2015). Downstream of ROS signaling, aminoglycosides activate the c-Jun N-terminal kinase (JNK), which triggers apoptosis. Delivery of JNK peptide inhibitors via cochleostomy is able to protect guinea pigs from neomycin-mediated ototoxicity (Wang et al., 2003). The mechanisms of aminoglycoside and cisplatin-mediated ototoxicity appear to differ, whereby ROS-JNK pathways causing apoptosis are recruited during aminoglycoside damage but not during cisplatin (Wang et al., 2004), and ROS-Caspase3-p53 signaling pathways are involved in cisplatin-mediated ototoxicity (Wang et al., 2004; Benkafadar et al., 2017).
Seldom are different aminoglycosides and cisplatin compared in terms of molecular mechanisms within a single experimental study. A comprehensive review of the molecular overlaps and points of divergence for the various forms of cochlear damage is beyond the scope of this review, and some aspects have been covered previously (Yang et al., 2015). Since then, there have been multiple advances showing for instance the involvement of the mTOR pathway in noise and cisplatin-induced hearing loss (Fu et al., 2022), as well as epigenetic modifications, which modulation with the inhibitor of the histone H3 lysine 9 dimethylation (H3K9me2) enzyme G9a (BIX01294) can prevent cisplatin-mediated ototoxicity via a miRNA-dependent induction of authophagy (Mu et al., 2023). Mitophagy is the selective degradation of mitochondria (Lemasters, 2005), which includes mitochondrial fission, the marking of specific mitochondria with ubiquitin-dependent or independent receptors, the recruitment of phagophores, and their expansion. The engulfment of mitochondria by autophagosomes and their fusion with lysosomes form the autolysosome for the final degradation of the cell (Montava-Garriga and Ganley, 2020). Although some proteins involved in mitophagy contribute to ARHL in rodents, their role in ototoxicity remains to be established. Recent advances in scRNAseq, which have been used, for instance, to investigate pathways implicated in noise injury in the cochlea (Milon et al., 2021), may increase our knowledge of the molecular mechanisms underlying noise and aminoglycoside- and cisplatin-induced hearing loss.
V. Drug Treatment in Humans: Evidence from the Ototoxicity Pipeline
Because it is ethically debatable when evaluating the efficacy of a drug to intentionally expose humans to noise in a clinically controlled experimental design, as was the case for ebselen (Kil et al., 2017; Maison and Rauch, 2017), most of the current clinical evidence for protective drugs against hearing loss has emerged from studies on ototoxicity. Only a handful of drugs are currently being tested in humans to reduce cisplatin- or aminoglycoside-induced hearing loss (Lee et al., 2024). Four of these are Food and Drug Administration-approved or repurposed drugs (e.g., sodium thiosulfate, statin modulators, cimetidine, and N-acetylcysteine), and the others are novel developments. The limited pharmaceutical pipeline may stem from the limited translational validation of the preclinical models, the complex clinical development path, or a lack of well-established clinical endpoints and comparators (see Section VII).
Animal studies have shown that sodium thiosulfate (STS) has the ability to inactivate cisplatin by forming platinum thiosulfate complexes that reduce the extracellular levels of free platinum as well as by preventing its cellular uptake. However, since this effect of STS can also impact cisplatin’s antitumor activity when administered simultaneously, timing strategies to administer STS after cisplatin have proven to be beneficial in protecting mice from cisplatin-induced hearing loss without impacting the antineogenic effects of cisplatin (Harned et al., 2008), a beneficial effect on hearing that was replicated in rats and guinea pigs (Muldoon et al., 2000; Dickey et al., 2005). Two completed phase III randomized controlled trials have tested the benefits of intravenous STS in reducing cisplatin-induced hearing loss in pediatric patients (NCT00716976; NCT00652132), both of which showed more than a twofold reduction in the incidence of hearing loss in cisplatin-treated patients when they were given STS (Freyer et al., 2017; Brock et al., 2018). However, the possibility that STS may reduce the survival of pediatric patients with metastatic cancer is currently being evaluated in two other phase III trials (NCT04478292; NCT05382338). Additionally, in two phase II trials (NCT05129748; NCT04541355) are currently evaluating whether STS is also applicable to adults.
Statins have also been suggested as potential protectors to drug-mediated ototoxicity in mice and rats (Fernandez et al., 2020; Lee et al., 2022a). Among patients, statin users have a two- to threefold decrease in the incidence of cisplatin-mediated hearing loss (Fernandez et al., 2021). Two randomized controlled trials are currently testing rosuvastatin (phase II, NCT04817904) or atorvastatin (phase III, NCT04915183) against cisplatin-mediated hearing loss. The low interference of statins with the antitumorigenic actions of cisplatin makes these drugs attractive candidates (Lebo et al., 2018; Gupta et al., 2019).
Knowing the involvement of the antioxidant pathway in both aminoglycoside and cisplatin ototoxicity makes it not so surprising to see Food and Drug Administration drugs, such as N-acetylcysteine (NAC), being repurposed in several preclinical studies against the two ototoxic chemical branches (Somdaş et al., 2015, 2020; Chen et al., 2022). One phase II study (NCT01131468) showed outstanding benefits of NAC treatment in protecting against aminoglycoside-induced toxicity in patients with peritonitis resulting from continuous ambulatory peritoneal dialysis (Tokgoz et al., 2011). Regarding cisplatin, there are still uncertainties as to how effective NAC can be, with results from a first trial with oral NAC (NCT02241876) being inconclusive (Visacri et al., 2019). Other trials with either intravenous or intratympanic applications are ongoing, including a phase IV (NCT04226456).
New developments include SPI-1005 from Sound Pharmaceuticals, which is an oral formulation of ebselen that mimics the activity of glutathione peroxidase. Two animal studies have suggested some potential protective effects (Lynch et al., 2005; Kim et al., 2009), which prompted its testing in a phase II trial evaluating oral SPI-1005 against tobramycin in patients with cystic fibrosis (NCT02819856). A promising candidate against aminoglycoside ototoxicity is ORC-13661, which was developed by Oricula Therapeutics based on an initial small-molecule screen in zebrafish with aminoglycosides identifying ORC-001/PROTO-1 (Owens et al., 2008; Chowdhury et al., 2018). ORC-13661 is a chemical optimization of ORC-001, which has demonstrated full protection of the zebrafish hair cells exposed to neomycin and highly effective protection in rats treated with amikacin (Chowdhury et al., 2018). While ORC-13661 will soon be tested in a phase II study (NCT05730283) on patients with nontuberculous mycobacterial infections treated with amikacin, its benefit against cisplatin remains to be demonstrated. Sensorion Pharmaceuticals developed SENS-401 (R-azasetron besylate), a 5-HT3 (serotonin) receptor antagonist and calcineurin inhibitor, to block apoptosis during ototoxic damage. Oral administration of SENS-401 protected rats from a single cisplatin intravenous infusion (Petremann et al., 2017). This led to the currently ongoing phase II trial (NCT05628233), which is evaluating the efficacy of SENS-401 against cisplatin ototoxicity in adult patients with cancer. ACOU085, developed by Acousia Therapeutics, is a voltage-gated potassium channel subfamily Q member 4 channel (Kv7.4) activator. Kv7.4 is an important ion channel for OHC survival (Nouvian et al., 2003; Kharkovets et al., 2006), and ACOU085 was shown to protect against cisplatin-mediated OHC loss (Dyhrfjeld-Johnsen, personal communication). A phase IIa clinical trial was recently begun in Europe (EudraCT 2023-503696-15-00).
VI. The Emerging Translational Evidence: Linking Rodent Molecular Biology to Human Genetics
Various scRNAseq technologies have indeed allowed for a greater resolution in the molecular understanding of the complex anatomical and cellular landscape that characterizes the cochlea. The use of such technologies in animal experiments such as noise trauma have helped identifying metformin, a drug against several symptoms of the metabolic syndrome, as a potential repurposed drug against hearing loss (Milon et al., 2021). In research fields other than auditory, single cell deep sequencing and analytical tools such as RNA velocity, BRIE2, Cell2Cell communication tools (e.g., Cellphone DB; CellChat), and bifurcation analysis (e.g., scVelo and scFates) have allowed an unprecedented understanding of neural development and neurologic disease progression, which is of high value for the discovery of highly specific and effective drugs (Faure et al., 2022). As a consequence, benefits for the pharmacological R&D pipeline in the auditory field may soon emerge from the growing knowledge that has been acquired in the last decade.
A. Mapping the Cochlear Cellular Landscape with Single-Cell RNA Sequencing
Historically, the complex architecture of the cochlea, given its multiple compartments and dense bony structure, and the low survival rate of murine hair cells after sorting have made it challenging to obtain a comprehensive cellular picture of the cochlea at different stages of its maturation. Table 1 presents, to the best of our knowledge, a comprehensive picture of the published single-cell RNA sequencing scRNAseq studies in rodents. It evidences the wide range of developmental stages, cochlear compartments collected, and strains and backgrounds but also sequencing methods used, the latter of which are key in revealing not only top cellular markers but also more complex mechanisms in cellular function. For instance, the four neuronal subtypes described in Section II and identified by two different laboratories, which used an advanced and full-read RNA sequencing platform called Smart-seq2 on fewer than 500 cells (Petitpré et al., 2018), have never been captured in the commonly used 10X Genomics, even when more than 5000 sorted cells were used (Rai et al., 2020; Sanders and Kelley, 2022; Jean et al., 2023). Out of 24 studies, only 2 used the CBA/CaJ (Milon et al., 2021; Shrestha et al., 2018); the majority of the other studies have used CD-1 or C57BL/6 J mice, both prone to ARHL, which might explain why these focused mainly on the developmental aspects of the cochlea (Table 1). Possibly, one of the most comprehensive analyses ever performed on the adult cochlea is the study of Jean et al., who analyzed 88,006 cells at various stages of postnatal and adult development in C57BL/6 J mice (Jean et al., 2023). However, as commented earlier, not all SGN subtypes were captured, and batch-to-batch effects occurred as well (Jean et al., 2023). New technologies such as the Smart-seq3xpress (Hagemann-Jensen et al., 2022) may provide novel insights into the cellular biology within the cochlea that go beyond the numerous cell types already identified (Jean et al., 2023). These technologies may even provide some strong translational value on organoids and human fetal cochlear tissue that have recently been brought to single-cell technologies (Van Der Valk et al., 2023).
TABLE 1.
Single-cell RNA sequencing studies on the cochlea Studies are listed in chronological order, displaying the cochlear tissue samples, the species and strain, the age at sample collection, the total number of cells used (c = cells; n = nuclei), and the sequencing method used.
| Author | Year | Tissue | Species (Strain) | Age | # Tot Cells | Method | Ref |
|---|---|---|---|---|---|---|---|
| Burns et al. | 2015 | Cochlear epithelium | Mice (CD1) | Neonatal | 97c | Burns et al., 2015 | |
| Petitpré et al. | 2018 | SGN | Mice (C57BL/6 J) | P3 | 478c | Smart-seq2 | Petitpré et al., 2018 |
| Shrest5/11/24 9:54:00AMha et al. | 2018 | SGN | Mice (C57BL/6 J; CBA/CaJ) | P25-27 | 186c | Smart-seq2 | Shrestha et al., 2018 |
| Ranum et al. | 2019 | isolated IHC, OHC, DC | Mice (C3HeB/FeJ) | P15 | 132c | Smart-seq2 | Ranum et al., 2019 |
| Gu et al. | 2020 | Stria | Mice (CBA/J) | P30 | 5,681n | 10 × Genomics | Gu et al., 2020 |
| Kalra et al. | 2020 | Cochlea | Mice (CD1) | P2 | 3,411c | 10 × Genomics | Kalra et al., 2020 |
| Kolla et al. | 2020 | Cochlear duct | Mice (CD1) | E14, E16, P1, P7 | 30,670c | 10 × Genomics | Kolla et al., 2020 |
| Li et al. | 2020 | SGN, HC, glial | Mice (mutant ns) | P1, P8, P14, P30 | ? | Ovation RNA-Seq V2 | Li et al., 2020 |
| Rai et al. | 2020 | Cochlea | Mice (mutants*) | P12, P26, P33 | 5,470c | 10 × Genomics | Rai et al., 2020 |
| Chen et al. | 2021 | Cochlear duct | Rats (Sprague–Dawley) | P1, P7 | 28,557c | 10 × Genomics | Chen et al., 2021 |
| Milon et al. | 2021 | SGN, stria vascularis | Mice (CBA/CaJ; CBA/Ca/Sca) | 2-4 m | 69,117c | 10 × Genomics | Milon et al., 2021 |
| Wang et al. | 2021 | HC, SC | Mice (mutant ns) | P2 | 695c | 10 × Genomics | Wang et al., 2021 |
| Xue et al. | 2021 | Cochlea | Mice (C57) | P20 | 4,527c | 10 × Genomics | Xue et al., 2021 |
| Petitpré et al. | 2022 | SGN | Mice (mutant ns) | E14.5 - E18.5, P3 | 2,308c | Smart-seq2 | Petitpré et al., 2022 |
| Xu et al. | 2022 | Cochlea (upper half) | Mice (C57BL/6 J) | P14, P28 | 7,786c | 10 × Genomics | Xu et al., 2022 |
| Sanders et al. | 2022 | SGN | Mice (CD1) | E14, E16, E18, P1 | 5,441c | 10 × Genomics | Sanders and Kelley, 2022 |
| Iyer et al. | 2022 | Cochlea | Mice (mutant ns) | P8, P15 | 9,693c | 10 × Genomics | Iyer et al., 2022 |
| Piekna-Przybylska et al. | 2023 | SC | Mice (CBA/CaJxC57BL/6 J) | P2 | ∼300c | Smart-Seq Single + | Piekna-Przybylska et al., 2022 |
| E. C. Boussaty et al., preprint, DOI: 10.1101/2023.02.15.528661 | 2023 | Cochlea | Mice (CFW) | 2, 6, 10 m | 36,000n | 10 × Genomics | Boussaty et al., 2023 |
| Jean et al. | 2023 | Cochlea | Mice (C57BL6/J) | P8, P12, P20 | 88,006c | 10 × Genomics | Jean et al., 2023 |
| Jean et al. | 2023 | Cochlea | Mice (C57BL6/J) | P8, P12, P20 | 28,822n | 10 × Genomics | Jean et al., 2023 |
| Sun et al. | 2023 | Cochlea | Mice (C57BL/6 J) | 1, 2, 5, 12, 15 m | 45,972c | 10 × Genomics | Sun et al., 2022 |
| Koh et al. | 2023 | Stria vascularis | Mice (Slc26a4+/+) | P22, P42 | 138c | Smart-seq2 | Koh et al., 2023 |
| Liu et al. | 2023 | Organ of Corti | Mice (C57BL/6 J) | P7 | 9845c | 10 × Genomics | Liu et al., 2023 |
DC, deiter’s cells; IHC, inner hair cells; OHC, outer hair cells; SC, support cells; SGN, spiral ganglion neuron.
* mutant strain background = 129xFVBxC57BL/6J
B. Identifying Cellular Targets in Humans: The Emerging Benefits of Population Genetics
Preclinical incentives have been mainly focused on the regeneration of hair cells. However, the relative contribution of each cochlear region (e.g., HC, SGN, SV) in humans remains debated. This is of utmost translational relevance for defining R&D strategies tailored to patients’ needs. Due to the very difficult access to fresh cochlear material in humans, research has been limited to histological assessments, complexified by the artifacts caused by the nonimmediate preservation of postmortem tissue. With more recent refinements in histological preservation, the role of SV degeneration on ARHL, established by Schuknecht in the 1970s (Schuknecht et al., 1974), was challenged by a study from Wu et al. showing of 120 human cases a severe loss of hair cells at high frequencies with increasing age but also unexpectedly at low frequencies (Wu et al., 2020). A follow-up study from Kaur et al. using a larger number of human cases (n = 160) including more “flat-audiograms” confirmed the hypothesis that a flatter audiometric shape with a high degree of low-frequency hearing loss correlates with greater strial atrophy whereas a down-sloping “sensory” pattern correlated more with hair cell loss (Kaur et al., 2023). As not all forms of hearing loss are alike, predictions of the cellular source of hearing loss based on the audiogram are essential for improving the efficacy of future pharmacological interventions.
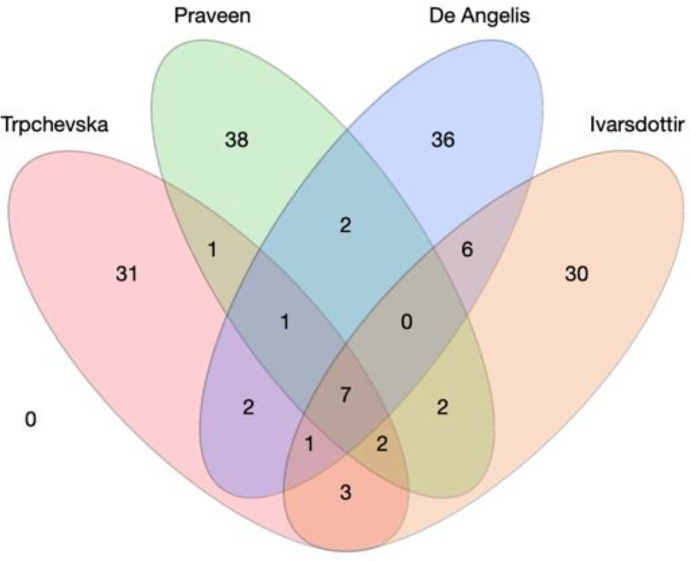
Defining the contribution of a cochlear region based on histology has its own limitation: the functional changes of the region in question may precede the histological evidence. To provide molecular evidence of the cellular origins of ARHL, recent research combined large genome-wide association studies (GWAS) in humans (Table 2) with single-cell cochlear transcriptomics derived from some of the animal work described earlier. The assumption is that the most relevant cells for a given trait would robustly express the disease risk genes, as has been demonstrated in schizophrenia (Skene et al., 2018). Thanks to the availability of UK Biobank, pioneering work by Kalra et al. suggested a primary role for cochlear epithelial cells in human ARHL using scRNAseq data from postnatal day 2 CD-1 mouse cochlear ducts (Kalra et al., 2020). However, this study combined four definitions of hearing abnormality (i.e., hearing difficulty, the use of hearing aids, speech in noise difficulties, and tinnitus) in the GWAS analysis, and the scRNAseq analysis included the SV. A follow-up study from Trpchevska et al. (2022) increased the sample size to 723,266 individuals and used the largest 10X-Genomics scRNAseq of adult mouse SGN and SV available at the time (>50,000 cells) (Milon et al., 2021), complementing it with another dataset using of mature mouse cochlea sample (∼ 100 cells from P15 cochleae) (Ranum et al., 2019). The analysis revealed an enrichment in spindle and root cells, suggesting a role for the SV in ARHL. With cochlear epithelial cells on the one hand and cells from the SV on the other, a recent study from Eshel et al. leveraged new GWAS data (Ivarsdottir et al., 2021; Praveen et al., 2022; De Angelis et al., 2023) and performed enrichment analyses using other scRNAseq datasets to propose that cells from the sensory epithelium, rather than the SV are the cell types involved in ARHL (Eshel et al., 2024). Although Eshel et al.’s study does not systematically compare all scRNAseq datasets with all available highly powered GWAS, it does suggest overall that the results from the enrichment analysis strongly depend on 1) the data source for the GWAS; 2) the enrichment method used [e.g., LDSC (Finucane et al., 2015); MAGMA (de Leeuw et al., 2015); scDRS (Zhang et al., 2022)]; 3) the scRNAseq technology used; and 4) the age of the sample. Indeed, among the genome-wide significant loci that were identified across the four GWAS, only seven were found common (Fig. 4), also indicating that the associations with ARHL may depend on 1) the studied populations, 2) the statistical software used, 3) the statistical method used to adjust for individual cohorts, and 4) the stringency of the meta-analysis (Table 2).
TABLE 2.
GWAS on hearing lossStudies are listed in chronological order, displaying the trait, sample size, cohorts used, number of loci identified, and software used.
| Author | Year | Trait | n (Case/Controls) | Source | # Loci | Software for GWAS | Reference |
|---|---|---|---|---|---|---|---|
| Hoffmann et al. | 2016 | ARHI | 6,527/45,882 | GERA | 1 | PLINK (logistic regression) | Hoffmann et al., 2016 |
| Wells et al. | 2019 | Hearing difficulty | 87,056/163,333 | UKBB | 41 | BOLT-LMM (LMM) | Wells et al., 2019 |
| Wells et al. | 2019 | Hearing aid | 13,178/240,740 | UKBB | 7 | BOLT-LMM (LMM) | Wells et al., 2019 |
| Nagtegaal et al. | 2019 | Audiometry | 9,675/356,141 | CHARGE consortium (6) | 7 | ProbABE, PLINK, GeneABEL, GENESIS, EMMAX (LMM and linear regression) | Nagtegaal et al., 2019 |
| Kalra et al. | 2020 | Hearing difficulty, hearing aid, speech-in-noise, tinnitus | x/330,759 | UKBB | 31 | Hail (linear regression) | Kalra et al., 2020 |
| Liu et al. | 2021 | Hearing difficulty, hearing aid, speech-in-noise, tinnitus | x/362,396 | UKBB | 35/22/2/11 | PLINK (logistic regression) | Liu et al., 2021 |
| Ivarsdottir et al. | 2021 | ARHI | 121,934/591,699 | Iceland/UKBB | 45 | Not-reported (logistic regression) | Ivarsdottir et al., 2021 |
| Trpchevska et al. | 2022 | ARHI self-report and ICD10 | 147,997/575,269 | UKBB, AGES, DTR, EGCUT, FinnGen, FHS, HABC, INGI-FVG, RS, SA, SALT(Y), STAGE, TwinsUK, WGHS | 48 | PLINK, MLMA, SAIGE, GEMMA, Rvtests, Mach2Dat, BOLT-LMM (linear regression, LMM) | Trpchevska et al., 2022 |
| Praveen et al. | 2022 | Hearing problems (hearing difficulty, hearing aid, speech-in-noise) and ICD10 | 125,749/469,497 | UKBB, FinnGen, MALMO, SINAI, GHS | 53 | Regenie (LMM) | Praveen et al., 2022 |
| De Angelis et al. | 2023 | Hearing problems (Hearing difficulty, hearing aid, speech-in-noise) | 145,529/356,296 | UKBB, NHS I, NHS II, HPFS, MVP | 54 | PLINK (logistic regression) | De Angelis et al., 2023 |
ARHI, age-related hearing impairment; GWAS, genome-wide association studies; ICD10, International Classification of Diseases, Tenth Revision.
Fig. 4.
Current large genome-wide association studies on age-related hearing loss. (A) Four-dimensional Wenn Diagram with the number of overlapping loci reported in Trpchevska et al., 2022; Praveen et al., 2022; De Angelis et al., 2023; and Ivarsdottir et al., 2021. Seven loci appear common to the four studies (rs10901863, rs11238325, rs143282422, rs36062310, rs6545432, rs67307131, rs9493627).
These studies highlight the potential of merging human genetics with mouse scRNAseq to better understand the cellular origins of ARHL in humans. However, the debate remains open. We believe that the next logical step would be a coordinated effort to expand the sample size with an optimized phenotypic definition of ARHL and a statistically comprehensive and harmonized GWAS meta-analysis of all cohorts. The improved methods for the isolation and sorting of cells from the whole adult mouse cochlea using deep-sequencing technologies are encouraging and could increase the power of the statistical analyses. The implementation of such tools to human cochlear material will be a necessary step to determine the cellular targets for ARHL.
VII. Future Considerations in Preclinical Research Designs to Enhance Translational Validity
A number of clinical failures have occurred in some of the earliest incentives against hearing loss, including AUT00063, AM-101, SENS-401, OTO-311, and FX-322. Can any lessons be learned from other fields to increase the likelihood of success in the auditory field? A major analysis for the attrition of drug candidates from four big pharmaceutical companies (AstraZeneca, Eli Lilly, GlaxoSmithKline, and Pfizer) (Waring et al., 2015) led to the establishment of the five-dimensional framework from AstraZeneca (Morgan et al., 2018). Briefly, the key features for successful drug development are 1) the right target, 2) the right tissue, 3) the right safety, 4) the right patient, and 5) the right commercial potential. This is where programs containing uncertainty at one of these levels were ended to minimize the risk of failures in larger and more costly clinical trials. Consequently, of the 287 programs aimed at the discovery of small molecules, only 76 were continued. In 10 years, the field has witnessed a fantastic rise in clinical programs for hearing loss, with 8 programs reported in 2013 (Sheridan, 2013) versus 60 programs solely on pharmacology in early 2024 (Table 3). The recent successful restoration of hearing using gene therapy in children with mutations on the OTOF gene has set the bar high (Lv et al., 2024) and illustrates what can be achieved, at least for some specific, well-defined monogenic disorders. As most hearing pathologies will not benefit from such homogenous and monogenic phenotype, more fundamental, translational and applied research will be needed for optimizing treatment of the global population.
TABLE 3.
Active clinical development programs for the pharmacological treatment of hearing loss Current development programs of drug candidates for the treatment of hearing loss (where “in development” is defined as, at minimum, a disclosed, identified target in active development) were identified in the drugs database of global data (January 2024 cutoff) and cross-referenced with company websites. Gene therapy is excluded.
| Company | Hearing Loss Indication | Brand or Drug Name (Active Principle) |
Highest Active Development Stage |
|---|---|---|---|
| Fennec Pharmaceuticals Inc. | Cisplatin-induced ototoxicity (pediatric) | Pedmarky (sodium thiosulfate) |
Marketed |
| Sound Pharmaceuticals Inc. | Ménière’s disease/aminoglycoside-induced ototoxicity | SPI-1005/SPI-3005 (ebselen) |
Phase III/II |
| Acousia Therapeutics GmbH | Cisplatin-induced ototoxicity | ACOU085 | Phase II |
| AudioCure Pharma GmbH | Idiopathic sudden SNHL | AC-102 | Phase II |
| Gateway Biotechnology Inc. | NIHL | GW-HP1 (zonisamide) |
Phase II |
| Nobelpharma Co Ltd | Pendred syndrome/DFNB4 (hearing loss) | NPC-12 (sirolimus) |
Phase II |
| Sensorion SA | Sudden SNHL/cisplatin-induced ototoxicity/hearing preservation after cochlear implantation | SENS-401 (arazasetron) |
Phase II |
| Spiral Therapeutics Inc. | Ménière’s disease | SPT-2101 (dexamethasone) |
Phase I |
| Audion Therapeutics BV | SNHL | AUD-1001 (LY-3056480) |
Phase I |
| IMD Farm Co Ltd | SNHL | IMDSST-03 | Phase I |
| Otologic Pharmaceuticals Inc. | SNHL | NHPN-1010 (acetylcysteine + disufenton sodium) |
Phase I |
| Regeneron Pharmaceuticals Inc. | Cisplatin-induced ototoxicity | DB-020 (sodium thiosulfate) |
Phase I |
NIH, noise-induced hearing loss; SNHL, sensorineural hearing loss.
In this last section, we emphasize the sixth R, “the right time” of treatment—something that is known as chronopharmacology, which should become an element in preclinical research. In this regard, several circadian researchers joined efforts to emphasize the need to control for the timing of drug delivery to evidence the benefits and minimize the side effects (Cederroth et al., 2019a). While treatment at different times of the day has been explored in humans, it has less commonly been explored in preclinical work. This could be a reason for the low correspondence between preclinical data and clinical trials: rodents are nocturnal, and humans are diurnal. An increased awareness about circadian biology is needed for the translation of preclinical data.
A. The Circadian System: Entering the Fourth Dimension
The circadian system has been highly conserved throughout evolution and is a process that dictates phases of activity and rest across the entire body, most often in response to the daily cycles of light and darkness. In mammals it is organized hierarchically, with the bilateral suprachiasmatic nuclei (SCN) of the hypothalamus being the master clock regulating nearly all bodily functions (Kalsbeek et al., 2006; Mohawk et al., 2012). The SCN, the master clock, orchestrates the rhythmicity of peripheral organ function through both sympathetic and parasympathetic pathways and, when the SCN is ablated, peripheral tissues become asynchronous (Yoo et al., 2004). Light is the main entrainment cue for SCN circadian rhythms, but other cues like feeding, locomotor activity, temperature, and hormonal factors can also synchronize peripheral organs. Molecularly, cells contain an autoregulatory transcriptional/translational feedback loop, namely the core clock genes Per, Cry, Clock, Bmal1, Rev-erβ, and Ror (Bass and Takahashi, 2010). The tight coordination of the positive and negative elements of transcription, as well as post-transcriptional and post-translational modifications, impose time delays that produce an accurate and robust cellular oscillator with a 24-h periodicity (Reppert and Weaver, 2002). A disruption of clock genes (Per, Cry, Clock, Bmal1, Rev-erβ, and Ror) in mice is known to generate a variety of phenotypes (Ko and Takahashi, 2006). Disruption of Bmal1 has the greatest impact on clock rhythms and triggers a wide array of disorders including arrhythmic locomotor activity in constant darkness, arthropathy (Bunger et al., 2005), infertility (Alvarez et al., 2008), symptoms of the metabolic syndrome (Lamia et al., 2008), reduced B-cell production (Sun et al., 2006), and decreased lifespan (Kondratov et al., 2006), overall highlighting its important role in maintaining homeostasis. Thus, it is no surprise that some diseases peak in their symptom severity at different times of the day (Table 4).
TABLE 4.
Timing of symptom severity for a selected set of diseases There are several diseases and disorders that exhibit symptoms based on the time of day.
| Disease | Reference |
|---|---|
| Morning | |
| Rheumatoid arthritis | Gibbs and Ray, 2013 |
| Heart attacks and myocardial infarction | Muller, 1999 |
| Migraine headaches | Fox and Davis, 1998 |
| Allergy | Gelfand, 2004 |
| Infection | Long et al., 2016 |
| Evening | |
| Peptic ulcers | Jamali et al., 1995 |
| Hypercholesterolemia | Saito et al., 1991 |
| Temporomandibular joint pain | van Grootel et al., 2005 |
| Asthma | Bohadana et al., 2002 |
| Epilepsy | Pavlova et al., 2004 |
1. The Time of the Day When Glucocorticoids and Circadian Rhythms Meet
Glucocorticoids (GCs) are steroid hormones that are secreted from the adrenal glands and regulated by the stress-responsive hypothalamic-pituitary-adrenal axis (Chrousos, 1995). GCs play an important role in regulating glucose formation in the liver, maintaining homeostasis, and regulating the immune system and stress-related physiology (Munck et al., 1984). They are released in a circadian manner, with a peak concentration found just prior to the onset of the active phase in humans and rodents (daytime for humans and nighttime for rodents when the activity is high) and reaching a minimum during the inactive state. When the SCN is ablated, the secretion of the hormone becomes arrhythmic, demonstrating that GC secretion is under circadian control (Moore and Eichler, 1972; Stephan and Zucker, 1972; Ishida et al., 2005; Radziuk, 2013). GCs, in turn, also entrain the rhythmicity of peripheral organs (Mohawk et al., 2012; Challet, 2015). Indeed, exposing fibroblasts to dexamethasone results in a robust circadian induction of Per1 gene expression, and a phase shift is observed when the drug is applied during the descending phase of corticosterone secretion (Balsalobre et al., 2000). Conversely, clock genes also regulate glucocorticoid homeostasis. Mice with either Bmal1 or Clock mutations show hypercortisolism at the onset of darkness (Turek et al., 2005; Leliavski et al., 2014), and Per2 mutant mice display arrhythmic glucocorticoid release (Zhang et al., 2011). Overall, a close bidirectional interaction occurs between the clock and the glucocorticoid systems.
B. Glucocorticoid Receptors in the Cochlea
Glucocorticoids exert their diverse effects through a specific intracellular receptor, the glucocorticoid receptor (GR), which belongs to the nuclear receptor superfamily and is ubiquitously expressed in almost all human tissues and organs. Unliganded GR is sequestered in the cytoplasm within a complex of chaperones, and glucocorticoid binding induces a conformational change within the receptor, allowing its release from the chaperone complex, dimerization, and translocation to the nucleus where it modulates the expression of target genes. Once in the nucleus, the GR homodimers bind to the glucocorticoid response elements to regulate gene transcription. Therapeutic doses of glucocorticoids are successfully used for treating inflammatory and autoimmune diseases (Boumpas et al., 1993), and the influence of glucocorticoids on auditory function was first reported in the late 1970s, when a substantial improvement of hearing was obtained after therapy with two synthetic analogs of the glucocorticoid hormone (cyclophosphamide and dexamethasone) in patients with autoimmune hearing loss. GRs are widely distributed in the CNS and other organs (Herman et al., 2003; Androutsellis-Theotokis et al., 2013), but they have also been detected in the inner ear of animals and humans in both the cochlear and vestibular systems (Furuta et al., 1994). In the cochlea, GR is found in the hair cells, supporting cells, spiral ligament, and SV, indicating a possible role in the regulation of both sensory and nonsensory tissues within the cochlea. Cochlear GR expression is modulated in response to acoustic trauma or restraint stress (Rarey et al., 1995; Tahera et al., 2006), and glucocorticoid analogs (i.e., dexamethasone) protect against acoustic trauma, triggering a GR-dependent activation of NFκB and MAPK signaling pathways (Tahera et al., 2006).
C. Cochlear Clock Rhythms and Their Modulation by Dexamethasone
Since GCs are potent synchronizers of peripheral clocks (Dickmeis, 2009; Cuesta et al., 2015) due to their interactions with core clock proteins (Balsalobre et al., 2000; Lamia et al., 2011), it has been hypothesized that the cochlea would also display features of the circadian system. Pioneering work from Meltser et al. using the Nanostring technology (Vikhe Patil et al., 2015) showed that the cochlea expresses elements of the autoregulatory transcriptional/translational feedback loop, namely the core clock genes Per1, Per2, Bmal1, and Rev-erbα (Meltser et al., 2014). PER2 is abundantly expressed in hair cells and SGNs, with a tonotopic gradient of circadian activity starting at the apical region and progressing toward the middle turn (Park et al., 2017). Using Period2-Luciferase (PER2::LUC reporter mice, Meltser et al. showed that cochlear explants display robust circadian oscillations that persist over 6 days ex vivo (Meltser et al., 2014). The cochlear clock is also under the control of the SCN, since bilateral SCN electrolytic lesions abolish rhythmicity in the cochlea (Cederroth et al., 2019). Dexamethasone (DEX), a synthetic GR agonist, increases the amplitude of PER2::LUC oscillations ex vivo when administered at trough of PER2 expression and has an opposite effect when given at the peak of the oscillations (Cederroth et al., 2019b). GR antagonists (RU486), but not mineralocorticoid receptor antagonists (spironolactone), blocked the effects of DEX. These findings indicate that the cochlea possesses a circadian system that is responsive to the modulation of GR activity.
D. The Time of the Day Determines the Degree of Trauma in the Cochlea
Delivering the same noise trauma to CBA/Ca/Sca mice during the inactive (daytime) or active (nighttime) phase results in different outcomes. Exposure to a noise trauma during the inactive phase causes a temporary threshold shift that fully recovers after 2 weeks, whereas an exposure during the active phase causes a permanent threshold shift (Meltser et al., 2014). This circadian vulnerability to auditory damage is also seen with aminoglycosides (Yonovitz and Fisch, 1991) and cisplatin (Tserga et al., 2020), where in the latter administration during the active phase leads to greater afferent synapse loss. Loss of the glutamate aspartate transporter function, which causes cochlear synaptopathy (Tserga et al., 2021), exacerbates the vulnerability to cisplatin administered during the active phase (Tserga et al., 2020). The circadian sensitivity to cochlear insults may, however, be dependent on the level of noise (or ototoxic drug) and also on the species, strain, and even substrain (Versteegh et al., 2022). Removal of the adrenal glands abolishes this differential day/night noise sensitivity (Cederroth et al., 2019), strongly supporting the notion that the increased vulnerability to noise seen during the active phase is related to the glucocorticoid system.
Concerning ototoxicity, it is rather surprising to see the low correspondence between preclinical data and the clinical trials. Most studies using mice show small changes in ABR threshold shifts (∼10 dB) after a single cisplatin injection (Kim et al., 2009), which leaves a small dynamic range for assessing the protective effects of drugs. Indeed, studies have demonstrated a greater vulnerability in rats and guinea pigs to aminoglycosides and cisplatin than in mice (Poirrier et al., 2010), which may explain, at least in part, why these larger rodents have been the preferred model in hearing loss preclinical development. However, a clear limitation to a single-bolus paradigm is that it does not mimic the multiple insults that humans receive in serial administrations. In this regard, the Cunningham laboratory developed a highly effective multicycle model of a single cisplatin administration (3.5 mg/kg) in CBA/CaJ mice, three times interspaced with a 1-week recovery period, leading to between 40 and 60 dB ABR threshold shifts at all frequencies, and a near complete loss of distortion products of otoacoustic emissions that persisted 4 months after treatment (Fernandez et al., 2019). Eventually, this model could become a preferred choice for R&D in the pharmaceutical industry, with prior validation in rats or guinea pigs, since mice are amenable to genetic manipulations and enable the validation of drug candidates by inactivating the target protein. It is still necessary, however, to incorporate a multicycle administration at nighttime, during the active phase, to optimize the translational value of this model and more closely mimic the exposure situation occurring in humans.
E. The Time of the Day and the Effectiveness of Specific Drugs on the Cochlea
There is emerging evidence showing that some drugs are more effective at nighttime than at daytime, whereas the opposite is true for other drugs (Cederroth et al., 2019). An example of how the timing of treatment can improve the overall treatment outcomes in humans is provided by oxaliplatin. The first demonstration of its clinical efficacy in colorectal cancer was provided in a large phase II clinical trial using chronomodulated delivery (Lévi et al., 1992), a finding that was later confirmed in a randomized phase III trial (Lévi et al., 1997).
In the cochlea, the dependency of the differential sensitivity to day/night noise trauma and the different responses of the cochlear clock to DEX depending on day or night treatments ex vivo led to the hypothesis that DEX could also have differing outcomes with regards to the vulnerability to noise trauma depending on when the drug is administered. DEX was protective against acute noise trauma only when administered during the inactive phase, when circulating glucocorticoids are low, but not when administered during the active phase (Cederroth et al., 2019a). Conversely, the improved recovery from the noise exposure during the inactive phase was associated with the ability of the cochlea to increase Bdnf expression in response to noise, a phenomenon that did not occur during the active phase. Treatment with di-hydroxyflavone, an agonist of the BDNF receptor TrkB, effectively protected mice from noise trauma when delivered during the active phase but not during the inactive phase (Meltser et al., 2014). Had the compound not been tested at nighttime, the efficacy of this molecule would have been missed, and the fundamental relevance of circadian mechanisms and TrkB signaling in the treatment of hearing disorders would have been ignored. Table 5 provides some examples of cochlear circadian genes, which are the targets of drugs used in the auditory field. This concept, referred to as chronopharmacology, considers the dependencies between the time of drug administration and the overall biological effect, including side effects. Since identifying the appropriate time for drug administration is intimately related to endogenous circadian rhythms, the presence of a circadian clock in the cochlea could have important implications for drug discovery.
TABLE 5.
Examples of genes that are circadian in the cochlea and are targets of drugs used in the auditory system “Reference” refers to the drugs acting on the target gene.
| Drug | Target Gene | Function | Reference |
|---|---|---|---|
| Cisplatin | Xpc | Nucleotide excision repair | Kang et al., 2010 |
| Gentamicin | Lrp2 | Scavenger receptor, belonging to the low-density lipoprotein receptor family | Dagil et al., 2013; Kim and Ricci, 2022 |
| Salicylate | Cox-2 | Inducible enzyme (e.g., at sites of inflammation and cancer) | Turini and DuBois, 2002; Chen et al., 2015 |
| Dexamethasone | Nos2 | Induced by cytokines and lipopolysaccharide | Tunçtan et al., 2002 |
| 7,8-dihydroxyflavone | Ho-1 | Member of the heat shock protein family and is stressed-induced | Henrich et al., 2023 |
A recent report by Plontke et al. reveals in a large three-arm, parallel-group, randomized, triple-blind clinical trial that even a high dose glucocorticoids does not show benefits against idiopathic sudden sensorineural hearing loss when compared with a low dose (Plontke et al., 2024). But is timing critical for drugs that have long half-lives in humans like dexamethasone? Some researchers argue yes (Zhang et al., 2014), but while we believe that this could apply for the majority of drugs, specific drugs may escape the rule. In our hands, DEX was shown to be more beneficial in protecting mice against noise trauma after administration during the inactive phase. Likewise, cisplatin—which is known to persist in the cochlea over months after a single injection—was revealed to be more harmful to the auditory synapse when administered during the active phase in mice (Tserga et al., 2020). Thus, even for dexamethasone, the quest for an optimal therapeutic intervention may not be over until timing is considered. Auditory researchers should take advantage of the favorable winds of change to include a crucial dimension in preclinical and clinical research: timing of the insult, timing of the drug intervention, and timing of the outcome measure.
F. A New Science with In Vitro and In Vivo Models
In vitro and in vivo models may provide new resources for improving our understanding of the translational relevance of specific drugs targeting the auditory system. PER2::LUC mice are knock-in mice in which a Luc gene is fused in-frame to the 3′ end of the endogenous mPer2 gene. Any organ from these mice can be collected and cultured ex vivo for the real-time monitoring of PER2 oscillations. This system has been used on cochlear explants to show the differential effects of di-hydroxyflavone or DEX treatment when applied during peak or trough PER2 expression (Meltser et al., 2014; Cederroth et al., 2019b), as well as to show the reversible blockade of cochlear rhythms by TEA (a K+ channel antagonist) and 1,2-Bis(2-aminophenoxy)ethane-N,N,N0,N0-tetraacetic acid (an extracellular calcium chelator), providing additional insights in the mechanisms controlling cochlear clock rhythms. This system can thus be used on the cochlea as well as on other organs with more abundant tissue such as the liver, although extrapolations should be done with caution [see examples in Cederroth et al. (2019b)] and allow for the assessment of the direct impact of a drug on the clock system (amplitude, period, and phase).
While there are a large number of cellular in vitro systems, nearly equivalent to all organs of the body, the hearing field has suffered from a lack of such tools not only to facilitate fundamental research but also to allow validation in drug screens on cell systems closer to the target organ. Historically, the HEI-OC1 cells from the Kalinec group at the House Ear Institute have pioneered this research area by immortalizing cells from the OC using the immortomouse (Jat et al., 1991). These cells would proliferate at 33°C and 10% CO2 (the so-called permissive conditions), but when moved to 39°C and 5% CO2 (nonpermissive), they would differentiate into more advanced/mature cell types (Kalinec et al., 2003). However, due to the large amount of cell death occurring during the culture transition to nonpermissive conditions, the toxicity to aminoglycosides or cisplatin could not be demonstrated as easily. As a consequence, this cell line has mostly been used in a permissive condition, during more immature stages and high proliferation. While initially these cells showed high sensitivity to aminoglycosides when compared with NIH3T3 fibroblast cells (Kalinec et al., 2003), they progressively lost this sensitivity while maintaining the sensitivity to cisplatin (Cederroth, 2012). Nonetheless, HEI-OC1 cells have been used in several studies to identify the signaling pathways involved in cisplatin-mediated cell death. However, we found that ATP luminescence-based viability assays and Caspase-3 assays—the most commonly used tools for assessing cisplatin ototoxicity in HEI-OC1 cells—do not always reflect proper cell survival (Cederroth, unpublished observations). Indeed, protective drugs that are involved in mitochondrial function may boost ATP metabolism and lead to the false interpretation that viability has been improved. Likewise, a lower activation of Caspase-3 using protective drugs against cisplatin ototoxicity does not necessarily lead to lower apoptosis. Indeed, flow cytometry combined with ImageStream has revealed that Annexin V positive and Propidium Iodide positive cells (AnxV+/PI+) harbor not only apoptotic but also necrotic cells after cisplatin treatment, which may trigger confounding effects in the viability and Caspase-3 assays. Therefore, it is a combination of assessment tools that should be supportive of a protective mechanism in drug screening (Cederroth, 2012). The Kalinec laboratory published recommendations on how to culture these cells in optimal conditions, depending on the research questions (Kalinec et al., 2016).
Complementing the use of in vitro cell lines is use of the zebrafish (Danio rerio), which has become a replacement model for rodents, allowing for broad screens in a living system (Patton et al., 2021). Moreover, its potential use in the context of ototoxicity has gained attention in the past decades (Chiu et al., 2008). As mentioned, it was at the origin of the identification of PROTO-1 (Owens et al., 2008) and a deeper characterization of the improved chemical variant ORC-13661 showing protective effects (Kitcher et al., 2019). The benefits of the zebrafish as a model of cisplatin ototoxicity have been also demonstrated (Ou et al., 2007; Lee et al., 2022b; Thomas et al., 2013), with some initial drug screens performed (Wertman et al., 2020). However, to our knowledge, the findings related to cisplatin-mediated ototoxicity have not yet been pursued beyond these findings.
Exciting endeavors have recently arisen to develop organoids derived from induced pluripotent stem cells (iPSCs) from humans (Pianigiani and Roccio, 2024). Through a timed series of small molecule guided differentiation, it has been possible to generate three-dimensional cell aggregates (the so-called organoids) that have a remarkable resemblance to inner ear organs. This is a major breakthrough in the field since it enables researchers to bypass the inaccessibility of adult human cochlear tissue for primary cultures. Recent studies have performed in depth-characterizations using single-cell RNA sequencing technologies and compared these human iPSC-derived organoids with human inner ear embryonic tissue (Doda et al., 2023; Van Der Valk et al., 2023). A small limitation persists, however, in that the hair cells from the organoids have a closer identity to vestibular hair cells than to cochlear hair cells (Liu et al., 2016), and hair cell maturation can reach functionality after 150 to 200 days in culture, corresponding to week 18 to 20 of fetal inner ear development (Moore et al., 2023). This is consistent with the notion that iPSC-derived organoids in general maintain a fetal identity (Kim et al., 2020; Corsini and Knoblich, 2022), something that is less seldom seen when using primary cultures from either fetal or adult tissue (Park et al., 2022; Hendriks et al., 2023). As a consequence, while iPSC-derived inner ear organoids may not yet be suitable for high throughput screening (HTS), they are powerful tools for demonstrating target engagement in human tissue, assessing toxicity, or to validate the translational relevance of specific gene therapies (M. V. Ivanchenko et al., preprint, DOI: 10.1101/2023.11.09.566447). Recent advances in retinal organoids provide evidence of the large potential of inner ear organoids in drug R&D (Cowan et al., 2020; Spirig and Renner, 2024).
VIII. Conclusions
The recent fundamental and translational advances in the auditory field are paving the way for some exciting years to come. These advances will not, however, come without hurdles. The difficulty of accessing the cochlear structure whether it be for collecting biopsies or samples or for the precise/quantitative drug delivery will remain a major technical challenge. Likewise, the many structures that the cochlea has and the many cells that comprise each of them illustrate the large heterogeneity in hearing loss phenotypes. The evidence collected in this review is a call for otolaryngology, head and neck surgery clinics to start biobanking temporal bones for histological and molecular analysis, collecting IPSCs for developing patient-derived organoids, obtaining DNA, and joining large consortiums to increase genetic knowledge at a global scale. The current biobanks do not have the required phenotypic resolution to address the existing knowledge gaps. There, hearing loss is often self-reported, and audiograms > 4 kHz are seldom available. We strongly believe that therapeutic success can only be achieved through large collaborations and converged efforts to method and statistical standardization, consensus, and data sharing. If gene therapy for hearing loss has the advantage of specifically targeting a cell type for a given monogenic disorder, pharmacological drugs have the benefits of having a broader action likely more suitable for more common forms of hearing loss such as those caused by aging, noise trauma, or ototoxicity. Their prevention has been a research quest for the past decades, but it is now time to focus on posttrauma therapy, as well as the regeneration of HCs and SGNs and the long-time ignored SV atrophy. The optimization of in vitro models will undoubtedly contribute to accelerating these advancements. Lastly, all preclinical work will have limited translational value unless it considers the time of the day for drug delivery, and the full potency of specific drugs may be underestimated if delivered at the wrong time of the day.
Data Availability
This review article contains no generated or analyzed datasets.
Abbreviations
- ABR
auditory brainstem response
- AAV
adeno-associated virus
- ARHL
age-related hearing loss
- BBB
blood-brain barrier
- BLB
blood-labyrinth barrier
- JNK
c-Jun N-terminal kinase
- CM
cisterna magna
- CSF
cerebrospinal fluid
- DEX
dexamethasone
- EP
endocochlear potential
- GWAS
genome wide association study
- GC
glucocorticoid
- GR
glucocorticoid receptor
- IHC
inner hair cell
- iPSC
induced-pluripotent stem cell
- LOC
lateral olivocochlear
- MET
mechanotransduction
- NAC
N-acetylcysteine
- NOX
NAPDH oxidase
- OC
organ of Corti
- OHC
outer hair cell
- PER2::LUC
Period2 Luciferase
- ROS
reactive oxygen species
- RWM
round window membrane
- SCN
suprachiasmatic nucleus
- scRNAseq
single-cell RNA sequencing
- SGN
spiral ganglion neuron
- STS
sodium thiosulfate
- SR
spontaneous rate
- SV
stria vascularis
- TRP
transient receptor potential.
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Cederroth, Dyhrfjeld-Johnsen, Canlon.
Footnotes
C.R.C. received funding from the Swedish Research Council (2023-02326) and the Rainwater Charitable Foundation. B.C. received funding from the Swedish Research Council (2019-01280), National Institutes of Health National Institute on Deafness and Other Communication Disorders (1R21DC021730-01), Tysta Skolan, Fondation pour l’Audition, and the Karolinska Institute. J.D.J. declares that he is an employee of Acousia. C.R.C. and B.C. have a pending patent application for the delivery of therapeutics to the inner ear via the cisterna magna (international patent application no. PCT/US22/82225, title: “CSF Transport Pathway for Delivery of Agents to the Inner Ear”). All other authors have no competing interests to declare.
An earlier version of this paper appears in The Impact of Circadian Regulation and Chronotherapy in Disease under the doi PHARMREV-AR-2023-001061.
References
- Alharazneh A, Luk L, Huth M, Monfared A, Steyger PS, Cheng AG, Ricci AJ (2011) Functional hair cell mechanotransducer channels are required for aminoglycoside ototoxicity. PLoS One 6:e22347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez JD, Hansen A, Ord T, Bebas P, Chappell PE, Giebultowicz JM, Williams C, Moss S, Sehgal A (2008) The circadian clock protein BMAL1 is necessary for fertility and proper testosterone production in mice. J Biol Rhythms 23:26–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Chrousos GP, McKay RD, DeCherney AH, Kino T (2013) Expression profiles of the nuclear receptors and their transcriptional coregulators during differentiation of neural stem cells. Horm Metab Res 45:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakay WMH, Anderson LA, Garcia-Lazaro JA, McAlpine D, Schaette R (2018) Hidden hearing loss selectively impairs neural adaptation to loud sound environments. Nat Commun 9:4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schütz G, Schibler U (2000) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289:2344–2347. [DOI] [PubMed] [Google Scholar]
- Bass J, Takahashi JS (2010) Circadian integration of metabolism and energetics. Science 330:1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benkafadar N, Menardo J, Bourien J, Nouvian R, François F, Decaudin D, Maiorano D, Puel J-L, Wang J (2017) Reversible p53 inhibition prevents cisplatin ototoxicity without blocking chemotherapeutic efficacy. EMBO Mol Med 9:7–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund AM, Ryugo DK (1987) Hair cell innervation by spiral ganglion neurons in the mouse. J Comp Neurol 255:560–570. [DOI] [PubMed] [Google Scholar]
- Beurg M, Fettiplace R, Nam J-H, Ricci AJ (2009) Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci 12:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc F, Bemelmans A-P, Affortit C, Joséphine C, Puel J-L, Mondain M, Wang J (2021) A single cisterna magna injection of AAV leads to binaural transduction in mice. Front Cell Dev Biol 9:783504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohadana AB, Hannhart B, Teculescu DB (2002) Nocturnal worsening of asthma and sleep-disordered breathing. J Asthma 39:85–100. [DOI] [PubMed] [Google Scholar]
- Boumpas DT, Chrousos GP, Wilder RL, Cupps TR, Balow JE (1993) Glucocorticoid therapy for immune-mediated diseases: basic and clinical correlates. Ann Intern Med 119:1198–1208. [DOI] [PubMed] [Google Scholar]
- Bredberg G (1968) Cellular pattern and nerve supply of the human organ of Corti. Acta Otolaryngol Suppl 236:1+. [PubMed] [Google Scholar]
- Breglio AM, Rusheen AE, Shide ED, Fernandez KA, Spielbauer KK, McLachlin KM, Hall MD, Amable L, Cunningham LL (2017) Cisplatin is retained in the cochlea indefinitely following chemotherapy. Nat Commun 8:1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock PRMaibach RChilds MRajput KRoebuck DSullivan MJLaithier VRonghe MDall'Igna PHiyama E, et al. (2018) Sodium thiosulfate for protection from cisplatin-induced hearing loss. N Engl J Med 378:2376–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC (1994) Antidromic responses of single units from the spiral ganglion. J Neurophysiol 71:1835–1847. [DOI] [PubMed] [Google Scholar]
- Bunger MK, Walisser JA, Sullivan R, Manley PA, Moran SM, Kalscheur VL, Colman RJ, Bradfield CA (2005) Progressive arthropathy in mice with a targeted disruption of the Mop3/Bmal-1 locus. Genesis 41:122–132. [DOI] [PubMed] [Google Scholar]
- Burns JC, Kelly MC, Hoa M, Morell RJ, Kelley MW (2015) Single-cell RNA-Seq resolves cellular complexity in sensory organs from the neonatal inner ear. Nat Commun 6:8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell KCM, Meech RP, Rybak LP, Hughes LF (2003) The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J Am Acad Audiol 14:144–156. [PubMed] [Google Scholar]
- Cederroth CR (2012) Loss of aminoglycoside sensitivity in HEI-OC1 cells? Hear Res 292:83–85; author response, 86. [DOI] [PubMed] [Google Scholar]
- Cederroth CRAlbrecht UBass JBrown SADyhrfjeld-Johnsen JGachon FGreen CBHastings MHHelfrich-Förster CHogenesch JB, et al. (2019a) Medicine in the fourth dimension. Cell Metab 30:238–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cederroth CR, Canlon B, Langguth B (2013) Hearing loss and tinnitus—are funders and industry listening? Nat Biotechnol 31:972–974. [DOI] [PubMed] [Google Scholar]
- Cederroth CR, Park J-S, Basinou V, Weger BD, Tserga E, Sarlus H, Magnusson AK, Kadri N, Gachon F, Canlon B (2019b) Circadian regulation of cochlear sensitivity to noise by circulating glucocorticoids. Curr Biol 29:2477–2487 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E (2015) Keeping circadian time with hormones. Diabetes Obes Metab 17(Suppl 1):76–83. [DOI] [PubMed] [Google Scholar]
- Chen B-C, Lin L-J, Lin Y-C, Lee C-F, Hsu W-C (2022) Optimal N-acetylcysteine concentration for intratympanic injection to prevent cisplatin-induced ototoxicity in guinea pigs. Acta Otolaryngol 142:127–131. [DOI] [PubMed] [Google Scholar]
- Chen JGao DChen JHou SHe BLi YLi SZhang FSun XMammano F, et al. (2021) Single-cell RNA sequencing analysis reveals greater epithelial ridge cells degeneration during postnatal development of cochlea in rats. Front Cell Dev Biol 9:719491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhao H-B (2014) The role of an inwardly rectifying K(+) channel (Kir4.1) in the inner ear and hearing loss. Neuroscience 265:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-DYeh J-KPeng M-TShie S-SLin S-LYang C-HChen T-HHung K-CWang C-CHsieh I-C, et al. (2015) Circadian CLOCK mediates activation of transforming growth factor-β signaling and renal fibrosis through cyclooxygenase 2. Am J Pathol 185:3152–3163. [DOI] [PubMed] [Google Scholar]
- Chiu LL, Cunningham LL, Raible DW, Rubel EW, Ou HC (2008) Using the zebrafish lateral line to screen for ototoxicity. J Assoc Res Otolaryngol 9:178–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M-J, Kang H, Lee YY, Choo O-S, Jang JH, Park S-H, Moon J-S, Choi SJ, Choung Y-H (2019) Cisplatin-induced ototoxicity in rats is driven by RIP3-dependent necroptosis. Cells 8:409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Owens KN, Herr RJ, Jiang Q, Chen X, Johnson G, Groppi VE, Raible DW, Rubel EW, Simon JA (2018) Phenotypic optimization of urea–thiophene carboxamides to yield potent, well tolerated, and orally active protective agents against aminoglycoside-induced hearing loss. J Med Chem 61:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP (1995) The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N Engl J Med 332:1351–1362. [DOI] [PubMed] [Google Scholar]
- Cohen GM, Park JC, Grasso JS (1990) Comparison of demyelination and neural degeneration in spiral and Scarpa’s ganglia of C57BL/6 mice. J Electron Microsc Tech 15:165–172. [DOI] [PubMed] [Google Scholar]
- Corsini NS, Knoblich JA (2022) Human organoids: new strategies and methods for analyzing human development and disease. Cell 185:2756–2769. [DOI] [PubMed] [Google Scholar]
- Cowan CSRenner MDe Gennaro MGross-Scherf BGoldblum DHou YMunz MRodrigues TMKrol JSzikra T, et al. (2020) Cell types of the human retina and its organoids at single-cell resolution. Cell 182:1623–1640.e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Fettiplace R (1985) The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol 364:359–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta M, Cermakian N, Boivin DB (2015) Glucocorticoids entrain molecular clock components in human peripheral cells. FASEB J 29:1360–1370. [DOI] [PubMed] [Google Scholar]
- Cunningham CL, Qiu X, Wu Z, Zhao B, Peng G, Kim Y-H, Lauer A, Müller U (2020) TMIE defines pore and gating properties of the mechanotransduction channel of mammalian cochlear hair cells. Neuron 107:126–143.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagil R, O’Shea C, Nykjær A, Bonvin AMJJ, Kragelund BB (2013) Gentamicin binds to the megalin receptor as a competitive inhibitor using the common ligand binding motif of complement type repeats: insight from the nmr structure of the 10th complement type repeat domain alone and in complex with gentamicin. J Biol Chem 288:4424–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow KN, Simons EJ, Dodds L, Liberman MC (2006) Dopaminergic innervation of the mouse inner ear: evidence for a separate cytochemical group of cochlear efferent fibers. J Comp Neurol 498:403–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB (2014a) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasari S, Tchounwou PB (2014b) Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 740:364–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis FZeleznik OAWendt FRPathak GATylee DSDe Lillo AKoller DCabrera-Mendoza BClifford REMaihofer AX, et al. (2023) Sex differences in the polygenic architecture of hearing problems in adults. Genome Med 15:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw CA, Mooij JM, Heskes T, Posthuma D (2015) MAGMA: generalized gene-set analysis of GWAS data. PLoS Comput Biol 11:e1004219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Raeve L (2016) Cochlear implants in Belgium: prevalence in paediatric and adult cochlear implantation. Eur Ann Otorhinolaryngol Head Neck Dis 133(Suppl 1):S57–S60. [DOI] [PubMed] [Google Scholar]
- Delpire E, Lu J, England R, Dull C, Thorne T (1999) Deafness and imbalance associated with inactivation of the secretory Na-K-2Cl co-transporter. Nat Genet 22:192–195. [DOI] [PubMed] [Google Scholar]
- Dickey DT, Wu YJ, Muldoon LL, Neuwelt EA (2005) Protection against cisplatin-induced toxicities by N-acetylcysteine and sodium thiosulfate as assessed at the molecular, cellular, and in vivo levels. J Pharmacol Exp Ther 314:1052–1058. [DOI] [PubMed] [Google Scholar]
- Dickmeis T (2009) Glucocorticoids and the circadian clock. J Endocrinol 200:3–22. [DOI] [PubMed] [Google Scholar]
- Dillard LK, Lopez-Perez L, Martinez RX, Fullerton AM, Chadha S, McMahon CM (2022) Global burden of ototoxic hearing loss associated with platinum-based cancer treatment: a systematic review and meta-analysis. Cancer Epidemiol 79:102203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dillard LK, Martinez RX, Perez LL, Fullerton AM, Chadha S, McMahon CM (2021) Prevalence of aminoglycoside-induced hearing loss in drug-resistant tuberculosis patients: a systematic review. J Infect 83:27–36. [DOI] [PubMed] [Google Scholar]
- Doda DAlonso Jimenez SRehrauer HCarreño JFValsamides VDi Santo SWidmer HREdge ALocher Hvan der Valk WH, et al. (2023) Human pluripotent stem cell-derived inner ear organoids recapitulate otic development in vitro. Development 150:dev201865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XSchwander MMoresco EMYViviani PHaller CHildebrand MSPak KTarantino LRoberts ARichardson H, et al. (2008) A catechol-O-methyltransferase that is essential for auditory function in mice and humans. Proc Natl Acad Sci USA 105:14609–14614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Harris KC, Lang H, Lewis MA, Schmiedt RA, Schulte BA, Steel KP, Vaden KI, Dubno JR (2021) Translational and interdisciplinary insights into presbyacusis: a multidimensional disease. Hear Res 402:108109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckhard ADos Santos ALiu WBassiouni MArnold HGleiser CHirt BHarteneck CMüller MRask-Andersen H, et al. (2015) Regulation of the perilymphatic-endolymphatic water shunt in the cochlea by membrane translocation of aquaporin-5. Pflugers Arch 467:2571–2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T, Nakagawa T, Kita T, Iguchi F, Kim T-S, Tamura T, Iwai K, Tabata Y, Ito J (2005) Novel strategy for treatment of inner ears using a biodegradable gel. Laryngoscope 115:2016–2020. [DOI] [PubMed] [Google Scholar]
- Eshel M, Milon B, Hertzano R, Elkon R (2024) The cells of the sensory epithelium, and not the stria vascularis, are the main cochlear cells related to the genetic pathogenesis of age-related hearing loss. Am J Hum Genet 111:614–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evensen KB, O’Rourke M, Prieur F, Holm S, Eide PK (2018) Non-invasive estimation of the intracranial pressure waveform from the central arterial blood pressure waveform in idiopathic normal pressure hydrocephalus patients. Sci Rep 8:4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure L, Techameena P, Hadjab S (2022) Emergence of neuron types. Curr Opin Cell Biol 79:102133. [DOI] [PubMed] [Google Scholar]
- Feldman L, Efrati S, Eviatar E, Abramsohn R, Yarovoy I, Gersch E, Averbukh Z, Weissgarten J (2007) Gentamicin-induced ototoxicity in hemodialysis patients is ameliorated by N-acetylcysteine. Kidney Int 72:359–363. [DOI] [PubMed] [Google Scholar]
- Fernandez K, Spielbauer KK, Rusheen A, Wang L, Baker TG, Eyles S, Cunningham LL (2020) Lovastatin protects against cisplatin-induced hearing loss in mice. Hear Res 389:107905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez K, Wafa T, Fitzgerald TS, Cunningham LL (2019) An optimized, clinically relevant mouse model of cisplatin-induced ototoxicity. Hear Res 375:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez KAAllen PCampbell MPage BTownes TLi C-MCheng HGarrett JMulquin MClements A, et al. (2021) Atorvastatin is associated with reduced cisplatin-induced hearing loss. J Clin Invest 131:e142616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R, Nam J-H (2019) Tonotopy in calcium homeostasis and vulnerability of cochlear hair cells. Hear Res 376:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R, Furness DN, Beurg M (2022) The conductance and organization of the TMC1-containing mechanotransducer channel complex in auditory hair cells. Proc Natl Acad Sci USA 119:e2210849119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane HKBulik-Sullivan BGusev ATrynka GReshef YLoh P-RAnttila VXu HZang CFarh K, et al. RACI Consortium. (2015) Partitioning heritability by functional annotation using genome-wide association summary statistics. Nat Genet 47:1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flock A, Cheung HC (1977) Actin filaments in sensory hairs of inner ear receptor cells. J Cell Biol 75:339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores EN, Duggan A, Madathany T, Hogan AK, Márquez FG, Kumar G, Seal RP, Edwards RH, Liberman MC, García-Añoveros J (2015) A non-canonical pathway from cochlea to brain signals tissue-damaging noise. Curr Biol 25:606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AW, Davis RL (1998) Migraine chronobiology. Headache 38:436–441. [DOI] [PubMed] [Google Scholar]
- Freyer DRChen LKrailo MDKnight KVillaluna DBliss BPollock BHRamdas JLange BVan Hoff D, et al. (2017) Effects of sodium thiosulfate versus observation on development of cisplatin-induced hearing loss in children with cancer (ACCL0431): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 18:63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu XLi PZhang LSong YAn YZhang ALiu WYe CZhang YYue R, et al. (2022) Activation of Rictor/mTORC2 signaling acts as a pivotal strategy to protect against sensorineural hearing loss. Proc Natl Acad Sci USA 119:e2107357119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman AC, Kujawa SG, Liberman MC (2013) Noise-induced cochlear neuropathy is selective for fibers with low spontaneous rates. J Neurophysiol 110:577–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta H, Mori N, Sato C, Hoshikawa H, Sakai S, Iwakura S, Doi K (1994) Mineralocorticoid type I receptor in the rat cochlea: mRNA identification by polymerase chain reaction (PCR) and in situ hybridization. Hear Res 78:175–180. [DOI] [PubMed] [Google Scholar]
- Géléoc GS, Lennan GW, Richardson GP, Kros CJ (1997) A quantitative comparison of mechanoelectrical transduction in vestibular and auditory hair cells of neonatal mice. Proc Biol Sci 264:611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand EW (2004) Inflammatory mediators in allergic rhinitis. J Allergy Clin Immunol 114:S135–S138. [DOI] [PubMed] [Google Scholar]
- Gibbs JE, Ray DW (2013) The role of the circadian clock in rheumatoid arthritis. Arthritis Res Ther 15:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese APJTang Y-QSinha GPBowl MRGoldring ACParker AFreeman MJBrown SDMRiazuddin SFettiplace R, et al. (2017) CIB2 interacts with TMC1 and TMC2 and is essential for mechanotransduction in auditory hair cells. Nat Commun 8:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzberg RD, Morest DK (1983) A study of cochlear innervation in the young cat with the Golgi method. Hear Res 10:227–246. [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Diabetes Collaborators (2023) Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402:203–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Hearing Loss Collaborators (2021) Hearing loss prevalence and years lived with disability, 1990-2019: findings from the Global Burden of Disease Study 2019. Lancet 397:996–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowatzki E, Fuchs PA (2002) Transmitter release at the hair cell ribbon synapse. Nat Neurosci 5:147–154. [DOI] [PubMed] [Google Scholar]
- Gopen Q, Rosowski JJ, Merchant SN (1997) Anatomy of the normal human cochlear aqueduct with functional implications. Hear Res 107:9–22. [DOI] [PubMed] [Google Scholar]
- Gow A, Davies C, Southwood CM, Frolenkov G, Chrustowski M, Ng L, Yamauchi D, Marcus DC, Kachar B (2004) Deafness in Claudin 11-null mice reveals the critical contribution of basal cell tight junctions to stria vascularis function. J Neurosci 24:7051–7062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goycoolea MV (2001) Clinical aspects of round window membrane permeability under normal and pathological conditions. Acta Otolaryngol 121:437–447. [DOI] [PubMed] [Google Scholar]
- Grati M, Kachar B (2011) Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc Natl Acad Sci USA 108:11476–11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton MA, Schmiedt RA, Schulte BA (1996) Age-related decreases in endocochlear potential are associated with vascular abnormalities in the stria vascularis. Hear Res 102:181–190. [DOI] [PubMed] [Google Scholar]
- Gu S, Olszewski R, Taukulis I, Wei Z, Martin D, Morell RJ, Hoa M (2020) Characterization of rare spindle and root cell transcriptional profiles in the stria vascularis of the adult mouse cochlea. Sci Rep 10:18100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinan JJ, Warr WB, Norris BE (1983) Differential olivocochlear projections from lateral versus medial zones of the superior olivary complex. J Comp Neurol 221:358–370. [DOI] [PubMed] [Google Scholar]
- Guinan JJ, Warr WB, Norris BE (1984) Topographic organization of the olivocochlear projections from the lateral and medial zones of the superior olivary complex. J Comp Neurol 226:21–27. [DOI] [PubMed] [Google Scholar]
- Gupta AStokes WEguchi MHararah MAmini AMueller AMorgan RBradley CRaben DMcDermott J, et al. (2019) Statin use associated with improved overall and cancer specific survival in patients with head and neck cancer. Oral Oncol 90:54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwer S, Sheward V, Birch A, Marchbanks R, Idro R, Newton CR, Kirkham FJ, Lin J-P, Lim M (2013) The tympanic membrane displacement analyser for monitoring intracranial pressure in children. Childs Nerv Syst 29:927–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagemann-Jensen M, Ziegenhain C, Sandberg R (2022) Scalable single-cell RNA sequencing from full transcripts with Smart-seq3xpress. Nat Biotechnol 40:1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harned TM, Kalous O, Neuwelt A, Loera J, Ji L, Iovine P, Sposto R, Neuwelt EA, Reynolds CP (2008) Sodium thiosulfate administered six hours after cisplatin does not compromise antineuroblastoma activity. Clin Cancer Res 14:533–540. [DOI] [PubMed] [Google Scholar]
- He DZZ, Jia S, Dallos P (2004) Mechanoelectrical transduction of adult outer hair cells studied in a gerbil hemicochlea. Nature 429:766–770. [DOI] [PubMed] [Google Scholar]
- Hendriks DBrouwers JFHamer KGeurts MHLuciana LMassalini SLópez-Iglesias CPeters PJRodríguez-Colman MJChuva de Sousa Lopes S, et al. (2023) Engineered human hepatocyte organoids enable CRISPR-based target discovery and drug screening for steatosis. Nat Biotechnol 41:1567–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich L, Kiessling I, Steimer M, Frase S, Kaiser S, Schallner N (2023) Circadian dependency of microglial heme oxygenase-1 expression and inflammation determine neuronal injury in hemorrhagic stroke. J Inflamm (Lond) 20:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE (2003) Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol 24:151–180. [DOI] [PubMed] [Google Scholar]
- Herranen A, Ikäheimo K, Virkkala J, Pirvola U (2018) The stress response in the non-sensory cells of the cochlea under pathological conditions-possible role in mediating noise vulnerability. J Assoc Res Otolaryngol 19:637–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann TJ, Keats BJ, Yoshikawa N, Schaefer C, Risch N, Lustig LR (2016) A Large genome-wide association study of age-related hearing impairment using electronic health records. PLoS Genet 12:e1006371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard J, Ashmore JF (1986) Stiffness of sensory hair bundles in the sacculus of the frog. Hear Res 23:93–104. [DOI] [PubMed] [Google Scholar]
- Howard J, Hudspeth AJ (1988) Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog’s saccular hair cell. Neuron 1:189–199. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ, Corey DP (1977) Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci USA 74:2407–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJWang MLiao YPlogg BAPeng WGundersen GABenveniste HVates GEDeane RGoldman SA, et al. (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura N, Salt AN (1992) Permeability changes of the blood-labyrinth barrier measured in vivo during experimental treatments. Hear Res 61:12–18. [DOI] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, Tsujimoto G, Okamura H (2005) Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab 2:297–307. [DOI] [PubMed] [Google Scholar]
- Ivarsdottir EVHolm HBenonisdottir SOlafsdottir TSveinbjornsson GThorleifsson GEggertsson HPHalldorsson GHHjorleifsson KEMelsted P, et al. (2021) The genetic architecture of age-related hearing impairment revealed by genome-wide association analysis. Commun Biol 4:706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer AAHosamani INguyen JDCai TSingh SMcGovern MMBeyer LZhang HJen H-IYousaf R, et al. (2022) Cellular reprogramming with ATOH1, GFI1, and POU4F3 implicate epigenetic changes and cell-cell signaling as obstacles to hair cell regeneration in mature mammals. Elife 11:e79712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamali F, Thomson AB, Kirdeikis P, Tavernini M, Zuk L, Marriage B, Simpson I, Mahachai V (1995) Diurnal variation in the pharmacokinetics of nizatidine in healthy volunteers and in patients with peptic ulcer disease. J Clin Pharmacol 35:1071–1075. [DOI] [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D (1991) Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci USA 88:5096–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean PWong Jun Tai FSingh-Estivalet ALelli AScandola CMegharba SSchmutz SRoux SMechaussier SSudres M, et al. (2023) Single-cell transcriptomic profiling of the mouse cochlea: an atlas for targeted therapies. Proc Natl Acad Sci USA 120:e2221744120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Yang S, Guo W, He DZZ (2009) Fate of mammalian cochlear hair cells and stereocilia after loss of the stereocilia. J Neurosci 29:15277–15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Zhao Y, Kusakizako T, Wang Y, Pan C, Zhang Y, Nureki O, Hattori M, Yan Z (2020) TMC1 and TMC2 proteins are pore-forming subunits of mechanosensitive ion channels. Neuron 105:310–321.e3. [DOI] [PubMed] [Google Scholar]
- Juhn SK, Rybak LP (1981) Labyrinthine barriers and cochlear homeostasis. Acta Otolaryngol 91:529–534. [DOI] [PubMed] [Google Scholar]
- Juhn SK, Rybak LP, Prado S (1981) Nature of blood-labyrinth barrier in experimental conditions. Ann Otol Rhinol Laryngol 90:135–141. [DOI] [PubMed] [Google Scholar]
- Kalinec GM, Park C, Thein P, Kalinec F (2016) Working with auditory HEI-OC1 cells. J Vis Exp 115:54425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinec GM, Webster P, Lim DJ, Kalinec F (2003) A cochlear cell line as an in vitro system for drug ototoxicity screening. Audiol Neurootol 8:177–189. [DOI] [PubMed] [Google Scholar]
- Kalra G, Milon B, Casella AM, Herb BR, Humphries E, Song Y, Rose KP, Hertzano R, Ament SA (2020) Biological insights from multi-omic analysis of 31 genomic risk loci for adult hearing difficulty. PLoS Genet 16:e1009025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Perreau-Lenz S, Buijs RM (2006) A network of (autonomic) clock outputs. Chronobiol Int 23:521–535. [DOI] [PubMed] [Google Scholar]
- Kang T-H, Lindsey-Boltz LA, Reardon JT, Sancar A (2010) Circadian control of XPA and excision repair of cisplatin-DNA damage by cryptochrome and HERC2 ubiquitin ligase. Proc Natl Acad Sci USA 107:4890–4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasawa T, Steyger PS (2015) An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol Lett 237:219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur C, Wu P-Z, O’Malley JT, Liberman MC (2023) Predicting atrophy of the cochlear stria vascularis from the shape of the threshold audiogram. J Neurosci 43:8801–8811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Müller U, Kachar B (2007) Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449:87–91. [DOI] [PubMed] [Google Scholar]
- Ke Y, Ma X, Jing Y, Diao T, Yu L (2023) The breakdown of blood-labyrinth barrier makes it easier for drugs to enter the inner ear. Laryngoscope 134:2377–2386. [DOI] [PubMed] [Google Scholar]
- Keithley EM (2020) Pathology and mechanisms of cochlear aging. J Neurosci Res 98:1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso CM, Watanabe H, Wazen JM, Bucher T, Qian ZJ, Olson ES, Kysar JW, Lalwani AK (2015) Microperforations significantly enhance diffusion across round window membrane. Otol Neurotol 36:694–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy HJ, Evans MG, Crawford AC, Fettiplace R (2003) Fast adaptation of mechanoelectrical transducer channels in mammalian cochlear hair cells. Nat Neurosci 6:832–836. [DOI] [PubMed] [Google Scholar]
- Kharkovets T, Dedek K, Maier H, Schweizer M, Khimich D, Nouvian R, Vardanyan V, Leuwer R, Moser T, Jentsch TJ (2006) Mice with altered KCNQ4 K+ channels implicate sensory outer hair cells in human progressive deafness. EMBO J 25:642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi T, Adams JC, Miyabe Y, So E, Kobayashi T (2000) Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med Electron Microsc 33:51–56. [DOI] [PubMed] [Google Scholar]
- Kikuchi T, Kimura RS, Paul DL, Adams JC (1995) Gap junctions in the rat cochlea: immunohistochemical and ultrastructural analysis. Anat Embryol (Berl) 191:101–118. [DOI] [PubMed] [Google Scholar]
- Kil J, Lobarinas E, Spankovich C, Griffiths SK, Antonelli PJ, Lynch ED, Le Prell CG (2017) Safety and efficacy of ebselen for the prevention of noise-induced hearing loss: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 390:969–979. [DOI] [PubMed] [Google Scholar]
- Kim J, Koo B-K, Knoblich JA (2020) Human organoids: model systems for human biology and medicine. Nat Rev Mol Cell Biol 21:571–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ricci AJ (2022) In vivo real-time imaging reveals megalin as the aminoglycoside gentamicin transporter into cochlea whose inhibition is otoprotective. Proc Natl Acad Sci USA 119:e2117946119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S-JPark CHan ALYoun M-JLee J-HKim YKim E-SKim H-JKim J-KLee H-K, et al. (2009) Ebselen attenuates cisplatin-induced ROS generation through Nrf2 activation in auditory cells. Hear Res 251:70–82. [DOI] [PubMed] [Google Scholar]
- Kitcher SRKirkwood NKCamci EDWu PGibson RMRedila VASimon JARubel EWRaible DWRichardson GP, et al. (2019) ORC-13661 protects sensory hair cells from aminoglycoside and cisplatin ototoxicity. JCI Insight 4:e126764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS (2006) Molecular components of the mammalian circadian clock. Hum Mol Genet 15(Spec No 2):R271–R277. [DOI] [PubMed] [Google Scholar]
- Kocyigit I, Vural A, Unal A, Sipahioglu MH, Yucel HE, Aydemir S, Yazici C, İlhan Sahin M, Oymak O, Tokgoz B (2015) Preventing amikacin related ototoxicity with N-acetylcysteine in patients undergoing peritoneal dialysis. Eur Arch Otorhinolaryngol 272:2611–2620. [DOI] [PubMed] [Google Scholar]
- Koh J-Y, Affortit C, Ranum PT, West C, Walls WD, Yoshimura H, Shao JQ, Mostaert B, Smith RJH (2023) Single-cell RNA-sequencing of stria vascularis cells in the adult Slc26a4(−/−) mouse. BMC Med Genomics 16:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolla LKelly MCMann ZFAnaya-Rocha AEllis KLemons APalermo ATSo KSMays JCOrvis J, et al. (2020) Characterization of the development of the mouse cochlear epithelium at the single cell level. Nat Commun 11:2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP (2006) Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev 20:1868–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kros CJ, Steyger PS (2019) Aminoglycoside- and cisplatin-induced ototoxicity: mechanisms and otoprotective strategies. Cold Spring Harb Perspect Med 9:a033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC (2006) Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci 26:2115–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC (2009) Adding insult to injury: cochlear nerve degeneration after “temporary” noise-induced hearing loss. J Neurosci 29:14077–14085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurima KPeters LMYang YRiazuddin SAhmed ZMNaz SArnaud DDrury SMo JMakishima T, et al. (2002) Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet 30:277–284. [DOI] [PubMed] [Google Scholar]
- Lahne M, Gale JE (2008) Damage-induced activation of ERK1/2 in cochlear supporting cells is a hair cell death-promoting signal that depends on extracellular ATP and calcium. J Neurosci 28:4918–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM (2011) Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480:552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamia KA, Storch K-F, Weitz CJ (2008) Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci USA 105:15172–15177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landier W (2016) Ototoxicity and cancer therapy. Cancer 122:1647–1658. [DOI] [PubMed] [Google Scholar]
- Lebo NL, Griffiths R, Hall S, Dimitroulakos J, Johnson-Obaseki S (2018) Effect of statin use on oncologic outcomes in head and neck squamous cell carcinoma. Head Neck 40:1697–1706. [DOI] [PubMed] [Google Scholar]
- Lee CH, Jeon J, Lee SM, Kim SY (2022a) Pravastatin administration alleviates kanamycin-induced cochlear injury and hearing loss. IJMS 23:4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DS, Schrader A, Warchol M, Sheets L (2022b) Cisplatin exposure acutely disrupts mitochondrial bioenergetics in the zebrafish lateral-line organ. Hear Res 426:108513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Fernandez K, Cunningham LL (2024) Hear and now: ongoing clinical trials to prevent drug-induced hearing loss. Annu Rev Pharmacol Toxicol 64:211–230. [DOI] [PubMed] [Google Scholar]
- Leliavski A, Shostak A, Husse J, Oster H (2014) Impaired glucocorticoid production and response to stress in Arntl-deficient male mice. Endocrinology 155:133–142. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ (2005) Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res 8:3–5. [DOI] [PubMed] [Google Scholar]
- Lévi FMisset J-LBrienza SAdam RMetzger GItzakhi MCaussanel J-PKunstlinger FLecouturier SDescorps-Declère A, et al. (1992) A chronopharmacologic phase II clinical trial with 5-fluorouracil, folinic acid, and oxaliplatin using an ambulatory multichannel programmable pump. High antitumor effectiveness against metastatic colorectal cancer. Cancer 69:893–900. [DOI] [PubMed] [Google Scholar]
- Lévi F, Zidani R, Misset JL (1997) Randomised multicentre trial of chronotherapy with oxaliplatin, fluorouracil, and folinic acid in metastatic colorectal cancer. International Organization for Cancer Chronotherapy. Lancet 350:681–686. [DOI] [PubMed] [Google Scholar]
- Li C, Li X, Bi Z, Sugino K, Wang G, Zhu T, Liu Z (2020) Comprehensive transcriptome analysis of cochlear spiral ganglion neurons at multiple ages. Elife 9:e50491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Steyger PS (2011) Systemic aminoglycosides are trafficked via endolymph into cochlear hair cells. Sci Rep 1:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Hartsock JJ, Dai C, Salt AN (2018) Permeation enhancers for intratympanically applied drugs studied using fluorescent dexamethasone as a marker. Otol Neurotol 39:639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC (1978) Auditory-nerve response from cats raised in a low-noise chamber. J Acoust Soc Am 63:442–455. [DOI] [PubMed] [Google Scholar]
- Liberman MC (1980) Morphological differences among radial afferent fibers in the cat cochlea: an electron-microscopic study of serial sections. Hear Res 3:45–63. [DOI] [PubMed] [Google Scholar]
- Liberman MC (1982) Single-neuron labeling in the cat auditory nerve. Science 216:1239–1241. [DOI] [PubMed] [Google Scholar]
- Liberman MC, Dodds LW (1987) Acute ultrastructural changes in acoustic trauma: serial-section reconstruction of stereocilia and cuticular plates. Hear Res 26:45–64. [DOI] [PubMed] [Google Scholar]
- Lin HW, Furman AC, Kujawa SG, Liberman MC (2011) Primary neural degeneration in the Guinea pig cochlea after reversible noise-induced threshold shift. J Assoc Res Otolaryngol 12:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Glowatzki E, Fuchs PA (2015) Unmyelinated type II afferent neurons report cochlear damage. Proc Natl Acad Sci USA 112:14723–14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HLiu HWang LSong LJiang GLu QYang TPeng HCai RZhao X, et al. (2023) Cochlear transcript diversity and its role in auditory functions implied by an otoferlin short isoform. Nat Commun 14:3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Johansson Å, Rask-Andersen H, Rask-Andersen M (2021) A combined genome-wide association and molecular study of age-related hearing loss in H. sapiens. BMC Med 19:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X-P, Koehler KR, Mikosz AM, Hashino E, Holt JR (2016) Functional development of mechanosensitive hair cells in stem cell-derived organoids parallels native vestibular hair cells. Nat Commun 7:11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston GHuntley JSommerlad AAmes DBallard CBanerjee SBrayne CBurns ACohen-Mansfield JCooper C, et al. (2020) Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396:413–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohela TJ, Lilius TO, Nedergaard M (2022) The glymphatic system: implications for drugs for central nervous system diseases. Nat Rev Drug Discov 21:763–779. [DOI] [PubMed] [Google Scholar]
- Long JE, Drayson MT, Taylor AE, Toellner KM, Lord JM, Phillips AC (2016) Morning vaccination enhances antibody response over afternoon vaccination: a cluster-randomised trial. Vaccine 34:2679–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente-Cánovas B, Ingham N, Norgett EE, Golder ZJ, Karet Frankl FE, Steel KP (2012) Mice deficient in the H+-ATPase a4 subunit have severe hearing impairment associated with enlarged endolymphatic compartments within the inner ear. Dis Model Mech 6:434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu JLi ZZhu YYang ALi RZheng JCai QPeng GZheng WTang X, et al. (2010) Mitochondrial 12S rRNA variants in 1642 Han Chinese pediatric subjects with aminoglycoside-induced and nonsyndromic hearing loss. Mitochondrion 10:380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv JWang HCheng XChen YWang DZhang LCao QTang HHu SGao K, et al. (2024) AAV1-hOTOF gene therapy for autosomal recessive deafness 9: a single-arm trial. Lancet 403:2317–2325. [DOI] [PubMed] [Google Scholar]
- Lynch ED, Gu R, Pierce C, Kil J (2005) Reduction of acute cisplatin ototoxicity and nephrotoxicity in rats by oral administration of allopurinol and ebselen. Hear Res 201:81–89. [DOI] [PubMed] [Google Scholar]
- Maison SF, Rauch SD (2017) Ethical considerations in noise-induced hearing loss research. Lancet 390:920–922. [DOI] [PubMed] [Google Scholar]
- Marchi PM, Marrone L, Azzouz M (2022) Delivery of therapeutic AAV9 vectors via cisterna magna to treat neurological disorders. Trends Mol Med 28:79–80. [DOI] [PubMed] [Google Scholar]
- Marcotti W, Corns LF, Goodyear RJ, Rzadzinska AK, Avraham KB, Steel KP, Richardson GP, Kros CJ (2016) The acquisition of mechano-electrical transducer current adaptation in auditory hair cells requires myosin VI. J Physiol 594:3667–3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen BKMiyakoshi LMCederroth CRTserga EVersteegh CBork PARHauglund NLGomolka RSMori YEdvall NK, et al. (2023) Delivery of gene therapy through a cerebrospinal fluid conduit to rescue hearing in adult mice. Sci Transl Med 15:eabq3916. [DOI] [PubMed] [Google Scholar]
- Meltser I, Cederroth CR, Basinou V, Savelyev S, Lundkvist GS, Canlon B (2014) TrkB-mediated protection against circadian sensitivity to noise trauma in the murine cochlea. Curr Biol 24:658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milon BShulman EDSo KSCederroth CRLipford ELSperber MSellon JBSarlus HPregernig GShuster B, et al. (2021) A cell-type-specific atlas of the inner ear transcriptional response to acoustic trauma. Cell Rep 36:109758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS (2012) Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 35:445–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan JJM, Garcia-Lazaro JA, McAlpine D, Schaette R (2020) Hidden hearing loss impacts the neural representation of speech in background noise. Curr Biol 30:4710–4721 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montava-Garriga L, Ganley IG (2020) outstanding questions in mitophagy: what we do and do not know. J Mol Biol 432:206–230. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB (1972) Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res 42:201–206. [DOI] [PubMed] [Google Scholar]
- Moore STNakamura TNie JSolivais AJAristizábal-Ramírez IUeda YManikandan MReddy VSRomano DRHoffman JR, et al. (2023) Generating high-fidelity cochlear organoids from human pluripotent stem cells. Cell Stem Cell 30:950–961.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PBrown DGLennard SAnderton MJBarrett JCEriksson UFidock MHamrén BJohnson AMarch RE, et al. (2018) Impact of a five-dimensional framework on R&D productivity at AstraZeneca. Nat Rev Drug Discov 17:167–181. [DOI] [PubMed] [Google Scholar]
- Mu Y-R, Zou S-Y, Li M, Ding Y-Y, Huang X, He Z-H, Kong W-J (2023) Role and mechanism of FOXG1-related epigenetic modifications in cisplatin-induced hair cell damage. Front Mol Neurosci 16:1064579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea D, Jajoo S, Kaur T, Sheehan KE, Ramkumar V, Rybak LP (2010) Transtympanic administration of short interfering (si)RNA for the NOX3 isoform of NADPH oxidase protects against cisplatin-induced hearing loss in the rat. Antioxid Redox Signal 13:589–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjea D, Jajoo S, Whitworth C, Bunch JR, Turner JG, Rybak LP, Ramkumar V (2008) Short interfering RNA against transient receptor potential vanilloid 1 attenuates cisplatin-induced hearing loss in the rat. J Neurosci 28:13056–13065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulders WHAM, McMahen C, Robertson D (2014) Effects of chronic furosemide on central neural hyperactivity and cochlear thresholds after cochlear trauma in Guinea pig. Front Neurol 5:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muldoon LL, Pagel MA, Kroll RA, Brummett RE, Doolittle ND, Zuhowski EG, Egorin MJ, Neuwelt EA (2000) Delayed administration of sodium thiosulfate in animal models reduces platinum ototoxicity without reduction of antitumor activity. Clin Cancer Res 6:309–315. [PubMed] [Google Scholar]
- Muller JE (1999) Circadian variation and triggering of acute coronary events. Am Heart J 137:S1–S8. [DOI] [PubMed] [Google Scholar]
- Munck A, Guyre PM, Holbrook NJ (1984) Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev 5:25–44. [DOI] [PubMed] [Google Scholar]
- Naeem M, Rahimnajjad NA, Behnoud F (2009) Can aspirin protect or at least attenuate gentamicin ototoxicity in humans? Saudi Med J 31:587; author reply 587–587; author reply 588. [PubMed] [Google Scholar]
- Nagtegaal APBroer LZilhao NRJakobsdottir JBishop CEBrumat MChristiansen MWCocca MGao YHeard-Costa NL, et al. (2019) Genome-wide association meta-analysis identifies five novel loci for age-related hearing impairment. Sci Rep 9:15192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neng L, Zhang F, Kachelmeier A, Shi X (2013) Endothelial cell, pericyte, and perivascular resident macrophage-type melanocyte interactions regulate cochlear intrastrial fluid-blood barrier permeability. J Assoc Res Otolaryngol 14:175–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuwelt EA, Baker DE, Pagel MA, Blank NK (1984) Cerebrovascular permeability and delivery of gentamicin to normal brain and experimental brain abscess in rats. J Neurosurg 61:430–439. [DOI] [PubMed] [Google Scholar]
- Nin F, Yoshida T, Sawamura S, Ogata G, Ota T, Higuchi T, Murakami S, Doi K, Kurachi Y, Hibino H (2016) The unique electrical properties in an extracellular fluid of the mammalian cochlea; their functional roles, homeostatic processes, and pathological significance. Pflugers Arch 468:1637–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Canlon B (2006) The signal transduction pathway for the dopamine D1 receptor in the guinea-pig cochlea. Neuroscience 137:981–990. [DOI] [PubMed] [Google Scholar]
- Niu X, Trifunovic A, Larsson N-G, Canlon B (2007) Somatic mtDNA mutations cause progressive hearing loss in the mouse. Exp Cell Res 313:3924–3934. [DOI] [PubMed] [Google Scholar]
- Nordmann AS, Bohne BA, Harding GW (2000) Histopathological differences between temporary and permanent threshold shift. Hear Res 139:13–30. [DOI] [PubMed] [Google Scholar]
- Nouvian R, Ruel J, Wang J, Guitton MJ, Pujol R, Puel J-L (2003) Degeneration of sensory outer hair cells following pharmacological blockade of cochlear KCNQ channels in the adult guinea pig. Eur J Neurosci 17:2553–2562. [DOI] [PubMed] [Google Scholar]
- Nyberg S, Abbott NJ, Shi X, Steyger PS, Dabdoub A (2019) Delivery of therapeutics to the inner ear: the challenge of the blood-labyrinth barrier. Sci Transl Med 11:eaao0935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi N, Chen F-Q, Zheng H-W, Sha S-H (2013) Intra-tympanic delivery of short interfering RNA into the adult mouse cochlea. Hear Res 296:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou HC, Raible DW, Rubel EW (2007) Cisplatin-induced hair cell loss in zebrafish (Danio rerio) lateral line. Hear Res 233:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens KN, Santos F, Roberts B, Linbo T, Coffin AB, Knisely AJ, Simon JA, Rubel EW, Raible DW (2008) Identification of genetic and chemical modulators of zebrafish mechanosensory hair cell death. PLoS Genet 4:e1000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxenham AJ (2016) Predicting the perceptual consequences of hidden hearing loss. Trends Hear 20:2331216516686768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Géléoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, Holt JR (2013) TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79:504–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Holt JR (2015) The molecules that mediate sensory transduction in the mammalian inner ear. Curr Opin Neurobiol 34:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J-S, Cederroth CR, Basinou V, Sweetapple L, Buijink R, Lundkvist GB, Michel S, Canlon B (2017) Differential phase arrangement of cellular clocks along the tonotopic axis of the mouse cochlea ex vivo. Curr Biol 27:2623–2629.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SE, Kang S, Paek J, Georgescu A, Chang J, Yi AY, Wilkins BJ, Karakasheva TA, Hamilton KE, Huh DD (2022) Geometric engineering of organoid culture for enhanced organogenesis in a dish. Nat Methods 19:1449–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton EE, Zon LI, Langenau DM (2021) Zebrafish disease models in drug discovery: from preclinical modelling to clinical trials. Nat Rev Drug Discov 20:611–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlova MK, Shea SA, Bromfield EB (2004) Day/night patterns of focal seizures. Epilepsy Behav 5:44–49. [DOI] [PubMed] [Google Scholar]
- Petitpré C, Faure L, Uhl P, Fontanet P, Filova I, Pavlinkova G, Adameyko I, Hadjab S, Lallemend F (2022) Single-cell RNA-sequencing analysis of the developing mouse inner ear identifies molecular logic of auditory neuron diversification. Nat Commun 13:3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitpré CWu HSharma ATokarska AFontanet PWang YHelmbacher FYackle KSilberberg GHadjab S, et al. (2018) Neuronal heterogeneity and stereotyped connectivity in the auditory afferent system. Nat Commun 9:3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petremann M, Tran Van Ba C, Broussy A, Romanet C, Dyhrfjeld-Johnsen J (2017) Oral administration of clinical stage drug candidate SENS-401 effectively reduces cisplatin-induced hearing loss in rats. Otol Neurotol 38:1355–1361. [DOI] [PubMed] [Google Scholar]
- Phillips AJ, Marchbanks RJ (1989) Effects of posture and age on tympanic membrane displacement measurements. Br J Audiol 23:279–284. [DOI] [PubMed] [Google Scholar]
- Pianigiani G, Roccio M (2024) Inner ear organoids: strengths and limitations. J Assoc Res Otolaryngol 25:5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO, Comis SD, Osborne MP (1984) Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res 15:103–112. [DOI] [PubMed] [Google Scholar]
- Piekna-Przybylska D, Na D, Zhang J, Baker C, Ashton JM, White PM (2022) Single cell RNA sequencing analysis of mouse cochlear supporting cell transcriptomes with activated ERBB2 receptor indicates a cell-specific response that promotes CD44 activation. Front Cell Neurosci 16:1096872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plontke SKGirndt MMeisner CFischer IBöselt ILöhler JLudwig-Kraus BRichter MSteighardt JReuter B, et al. HODOKORT Trial Investigators. (2024) High-dose glucocorticoids for the treatment of sudden hearing loss. NEJM Evid 3:EVIDoa2300172. [DOI] [PubMed] [Google Scholar]
- Plontke SK, Hartsock JJ, Gill RM, Salt AN (2016) Intracochlear drug injections through the round window membrane: measures to improve drug retention. Audiol Neurootol 21:72–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirrier AL, Van den Ackerveken P, Kim TS, Vandenbosch R, Nguyen L, Lefebvre PP, Malgrange B (2010) Ototoxic drugs: difference in sensitivity between mice and guinea pigs. Toxicol Lett 193:41–49. [DOI] [PubMed] [Google Scholar]
- Prasad KBorre EDDillard LKAyer ADer CBainbridge KEMcMahon CMTucci DLWilson BSSchmidler GDS, et al. (2024) Priorities for hearing loss prevention and estimates of global cause-specific burdens of hearing loss: a systematic rapid review. Lancet Glob Health 12:e217–e225. [DOI] [PubMed] [Google Scholar]
- Praveen KDobbyn LGurski LAyer AHStaples JMishra SBai YKaufman AMoscati ABenner C, et al. Decibel-REGN collaboration. (2022) Population-scale analysis of common and rare genetic variation associated with hearing loss in adults. Commun Biol 5:540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prayuenyong P, Baguley DM, Kros CJ, Steyger PS (2021) Preferential cochleotoxicity of cisplatin. Front Neurosci 15:695268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezant TRAgapian JVBohlman MCBu XOztas SQiu WQArnos KSCortopassi GAJaber LRotter JI, et al. (1993) Mitochondrial ribosomal RNA mutation associated with both antibiotic-induced and non-syndromic deafness. Nat Genet 4:289–294. [DOI] [PubMed] [Google Scholar]
- Puel JL, Ruel J, Gervais d'Aldin C, Pujol R (1998) Excitotoxicity and repair of cochlear synapses after noise-trauma induced hearing loss. Neuroreport 9:2109–2114. [DOI] [PubMed] [Google Scholar]
- Radziuk JM (2013) The suprachiasmatic nucleus, circadian clocks, and the liver. Diabetes 62:1017–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai V, Wood MB, Feng H, Schabla NM, Tu S, Zuo J (2020) The immune response after noise damage in the cochlea is characterized by a heterogeneous mix of adaptive and innate immune cells. Sci Rep 10:15167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine C, Atkinson H, Strachan DR, Martin JM (2016) Access to cochlear implants: time to reflect. Cochlear Implants Int 17(Suppl 1):42–46. [DOI] [PubMed] [Google Scholar]
- Ranum PT, Goodwin AT, Yoshimura H, Kolbe DL, Walls WD, Koh J-Y, He DZZ, Smith RJH (2019) Insights into the biology of hearing and deafness revealed by single-cell RNA sequencing. Cell Rep 26:3160–3171 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranum PT, Tecedor L, Keiser MS, Chen YH, Leib DE, Liu X, Davidson BL (2023) Cochlear transduction via cerebrospinal fluid delivery of AAV in non-human primates. Mol Ther 31:609–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael Y, Athey BD, Wang Y, Hawkins JE (1993) Structure of the reticular lamina and repair after noise injury. Rev Laryngol Otol Rhinol (Bord) 114:171–175. [PubMed] [Google Scholar]
- Rarey KE, Gerhardt KJ, Curtis LM, ten Cate WJ (1995) Effect of stress on cochlear glucocorticoid protein: acoustic stress. Hear Res 82:135–138. [DOI] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418:935–941. [DOI] [PubMed] [Google Scholar]
- Richardson GP, de Monvel JB, Petit C (2011) How the genetics of deafness illuminates auditory physiology. Annu Rev Physiol 73:311–334. [DOI] [PubMed] [Google Scholar]
- Rickheit G, Maier H, Strenzke N, Andreescu CE, De Zeeuw CI, Muenscher A, Zdebik AA, Jentsch TJ (2008) Endocochlear potential depends on Cl- channels: mechanism underlying deafness in Bartter syndrome IV. EMBO J 27:2907–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson D (1984) Horseradish peroxidase injection of physiologically characterized afferent and efferent neurones in the guinea pig spiral ganglion. Hear Res 15:113–121. [DOI] [PubMed] [Google Scholar]
- Robertson D, Sellick PM, Patuzzi R (1999) The continuing search for outer hair cell afferents in the guinea pig spiral ganglion. Hear Res 136:151–158. [DOI] [PubMed] [Google Scholar]
- Ruan Q, Ao H, He J, Chen Z, Yu Z, Zhang R, Wang J, Yin S (2014) Topographic and quantitative evaluation of gentamicin-induced damage to peripheral innervation of mouse cochleae. Neurotoxicology 40:86–96. [DOI] [PubMed] [Google Scholar]
- Ruel J, Wang J, Rebillard G, Eybalin M, Lloyd R, Pujol R, Puel J-L (2007) Physiology, pharmacology and plasticity at the inner hair cell synaptic complex. Hear Res 227:19–27. [DOI] [PubMed] [Google Scholar]
- Rybak LP, Husain K, Morris C, Whitworth C, Somani S (2000) Effect of protective agents against cisplatin ototoxicity. Am J Otol 21:513–520. [PubMed] [Google Scholar]
- Saito Y, Yoshida S, Nakaya N, Hata Y, Goto Y (1991) Comparison between morning and evening doses of simvastatin in hyperlipidemic subjects. A double-blind comparative study. Arterioscler Thromb 11:816–826. [DOI] [PubMed] [Google Scholar]
- Salt AN, Melichar I, Thalmann R (1987) Mechanisms of endocochlear potential generation by stria vascularis. Laryngoscope 97:984–991. [PubMed] [Google Scholar]
- Salt AN, Hartsock JJ, Gill RM, Piu F, Plontke SK (2012) Perilymph pharmacokinetics of markers and dexamethasone applied and sampled at the lateral semi-circular canal. J Assoc Res Otolaryngol 13:771–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders TR, Kelley MW (2022) Specification of neuronal subtypes in the spiral ganglion begins prior to birth in the mouse. Proc Natl Acad Sci USA 119:e2203935119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos RLPEl-Shanti HSikandar SLee KBhatti AYan KChahrour MHMcArthur NPham TLMahasneh AA, et al. (2006) Novel sequence variants in the TMIE gene in families with autosomal recessive nonsyndromic hearing impairment. J Mol Med (Berl) 84:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht HF (1964) Further observations on the pathology of presbycusis. Arch Otolaryngol 80:369–382. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR (1993) Cochlear pathology in presbycusis. Ann Otol Rhinol Laryngol 102:1–16. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Watanuki K, Takahashi T, Belal AA, Kimura RS, Jones DD, Ota CY (1974) Atrophy of the stria vascularis, a common cause for hearing loss. Laryngoscope 84:1777–1821. [DOI] [PubMed] [Google Scholar]
- Seidman MD, Khan MJ, Tang WX, Quirk WS (2002) Influence of lecithin on mitochondrial DNA and age-related hearing loss. Otolaryngol Head Neck Surg 127:138–144. [DOI] [PubMed] [Google Scholar]
- Sergeyenko Y, Lall K, Liberman MC, Kujawa SG (2013) Age-related cochlear synaptopathy: an early-onset contributor to auditory functional decline. J Neurosci 33:13686–13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha SH, Zajic G, Epstein CJ, Schacht J (2001) Overexpression of copper/zinc-superoxide dismutase protects from kanamycin-induced hearing loss. Audiol Neurootol 6:117–123. [DOI] [PubMed] [Google Scholar]
- Sheridan C (2013) Investors start backing hearing loss treatments. Nat Biotechnol 31:575–576. [DOI] [PubMed] [Google Scholar]
- Sheth S, Mukherjea D, Rybak LP, Ramkumar V (2017) Mechanisms of cisplatin-induced ototoxicity and otoprotection. Front Cell Neurosci 11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X (2016) Pathophysiology of the cochlear intrastrial fluid-blood barrier (review). Hear Res 338:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X (2011) Physiopathology of the cochlear microcirculation. Hear Res 282:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimbles S, Dodd C, Banister K, Mendelow AD, Chambers IR (2005) Clinical comparison of tympanic membrane displacement with invasive ICP measurements. Acta Neurochir Suppl 95:197–199. [DOI] [PubMed] [Google Scholar]
- Shrestha BR, Chia C, Wu L, Kujawa SG, Liberman MC, Goodrich LV (2018) Sensory neuron diversity in the inner ear is shaped by activity. Cell 174:1229–1246 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skene NGBryois JBakken TEBreen GCrowley JJGaspar HAGiusti-Rodriguez PHodge RDMiller JAMuñoz-Manchado AB, et al. Major Depressive Disorder Working Group of the Psychiatric Genomics Consortium. (2018) Genetic identification of brain cell types underlying schizophrenia. Nat Genet 50:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somdaş MA, Güntürk İ, Balcıoğlu E, Avcı D, Yazıcı C, Özdamar S (2020) Protective effect of N-acetylcysteine against cisplatin ototoxicity in rats: a study with hearing tests and scanning electron microscopy. Braz J Otorhinolaryngol 86:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somdaş MA, Korkmaz F, Gürgen SG, Sagit M, Akçadağ A (2015) N-acetylcysteine prevents gentamicin ototoxicity in a rat model. J Int Adv Otol 11:12–18. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA (2002) Spiral ligament pathology in quiet-aged gerbils. Hear Res 172:172–185. [DOI] [PubMed] [Google Scholar]
- Spirig SE, Renner M (2024) Toward retinal organoids in high-throughput. Cold Spring Harb Perspect Med 14:e41275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoendlin H (1972) Innervation densities of the cochlea. Acta Otolaryngol 73:235–248. [DOI] [PubMed] [Google Scholar]
- Spoendlin H (1969) Innervation patterns in the organ of corti of the cat. Acta Otolaryngol 67:239–254. [DOI] [PubMed] [Google Scholar]
- Steel KP, Barkway C (1989) Another role for melanocytes: their importance for normal stria vascularis development in the mammalian inner ear. Development 107:453–463. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I (1972) Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69:1583–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöver T, Yagi M, Raphael Y (2000) Transduction of the contralateral ear after adenovirus-mediated cochlear gene transfer. Gene Ther 7:377–383. [DOI] [PubMed] [Google Scholar]
- Strelioff D, Flock A (1984) Stiffness of sensory-cell hair bundles in the isolated guinea pig cochlea. Hear Res 15:19–28. [DOI] [PubMed] [Google Scholar]
- Sun GZheng YFu XZhang WRen JMa SSun SHe XWang QJi Z, et al. (2022) Single-cell transcriptomic atlas of mouse cochlear aging. Protein Cell 14:180–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Babola T, Pregernig G, So KS, Nguyen M, Su S-SM, Palermo AT, Bergles DE, Burns JC, Müller U (2018) Hair cell mechanotransduction regulates spontaneous activity and spiral ganglion subtype specification in the auditory system. Cell 174:1247–1263.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Yang Z, Niu Z, Peng J, Li Q, Xiong W, Langnas AN, Ma MY, Zhao Y (2006) MOP3, a component of the molecular clock, regulates the development of B cells. Immunology 119:451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Kaga K (1999) Development of blood-labyrinth barrier in the semicircular canal ampulla of the rat. Hear Res 129:27–34. [DOI] [PubMed] [Google Scholar]
- Szeto B, Chiang H, Valentini C, Yu M, Kysar JW, Lalwani AK (2020) Inner ear delivery: challenges and opportunities. Laryngoscope Investig Otolaryngol 5:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taberner AM, Liberman MC (2005) Response properties of single auditory nerve fibers in the mouse. J Neurophysiol 93:557–569. [DOI] [PubMed] [Google Scholar]
- Taghian TMarosfoi MGPuri ASCataltepe OIKing RMDiffie EBMaguire ASMartin DRFernau DBatista AR, et al. (2020) A safe and reliable technique for CNS delivery of AAV vectors in the cisterna magna. Mol Ther 28:411–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Hansson AC, Canlon B (2006) Glucocorticoid receptor and nuclear factor-kappa B interactions in restraint stress-mediated protection against acoustic trauma. Endocrinology 147:4430–4437. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Hailey DW, Stawicki TM, Wu P, Coffin AB, Rubel EW, Raible DW, Simon JA, Ou HC (2013) Functional mechanotransduction is required for cisplatin-induced hair cell death in the zebrafish lateral line. J Neurosci 33:4405–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson RS, Auduong P, Miller AT, Gurgel RK (2017) Hearing loss as a risk factor for dementia: a systematic review. Laryngoscope Investig Otolaryngol 2:69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, DeRosier DJ (1992) Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu Rev Cell Biol 8:257–274. [DOI] [PubMed] [Google Scholar]
- Tokgoz B, Ucar C, Kocyigit I, Somdas M, Unal A, Vural A, Sipahioglu M, Oymak O, Utas C (2011) Protective effect of N-acetylcysteine from drug-induced ototoxicity in uraemic patients with CAPD peritonitis. Nephrol Dial Transplant 26:4073–4078. [DOI] [PubMed] [Google Scholar]
- Totten DJ, Booth KTA, Mosier KM, Cumpston EC, Whitted C, Okechuku V, Koontz NA, Nelson RF (2023) Human cochlear diffusion from the cerebrospinal fluid space with gadolinium contrast. Mol Ther 31:2566–2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trpchevska NFreidin MBBroer LOosterloo BCYao SZhou YVona BBishop CBizaki-Vallaskangas ACanlon B, et al. Estonian Biobank Research Team (2022) Genome-wide association meta-analysis identifies 48 risk variants and highlights the role of the stria vascularis in hearing loss. Am J Hum Genet 109:1077–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserga E, Damberg P, Canlon B, Cederroth CR (2021) Auditory synaptopathy in mice lacking the glutamate transporter GLAST and its impact on brain activity. Prog Brain Res 262:245–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tserga E, Moreno-Paublete R, Sarlus H, Björn E, Guimaraes E, Göritz C, Cederroth CR, Canlon B (2020) Circadian vulnerability of cisplatin-induced ototoxicity in the cochlea. FASEB J 34:13978–13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunçtan B, Weigl Y, Dotan A, Peleg L, Zengil H, Ashkenazi I, Abacioğlu N (2002) Circadian variation of nitric oxide synthase activity in mouse tissue. Chronobiol Int 19:393–404. [DOI] [PubMed] [Google Scholar]
- Turek FWJoshu CKohsaka ALin EIvanova GMcDearmon ELaposky ALosee-Olson SEaston AJensen DR, et al. (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308:1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turini ME, DuBois RN (2002) Cyclooxygenase-2: a therapeutic target. Annu Rev Med 53:35–57. [DOI] [PubMed] [Google Scholar]
- van der Valk WH, van Beelen ESA, Steinhart MR, Nist-Lund C, Osorio D, de Groot JCMJ, Sun L, van Benthem PPG, Koehler KR, Locher H (2023) A single-cell level comparison of human inner ear organoids with the human cochlea and vestibular organs. Cell Rep 42:112623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Grootel RJ, van der Glas HW, Buchner R, de Leeuw JRJ, Passchier J (2005) Patterns of pain variation related to myogenous temporomandibular disorders. Clin J Pain 21:154–165. [DOI] [PubMed] [Google Scholar]
- Vanhala L (2015) The diffusion of disability rights in Europe. Human Rights Quart 37:831–853. [Google Scholar]
- Versteegh CPC, Tserga E, Fontana JM, Moreno-Paublete R, Sarlus H, Zisiadis G-A, Cederroth CR, Canlon B (2022) Differential effects of noise exposure between substrains of CBA mice. Hear Res 415:108395. [DOI] [PubMed] [Google Scholar]
- Vetter DE, Mann JR, Wangemann P, Liu J, McLaughlin KJ, Lesage F, Marcus DC, Lazdunski M, Heinemann SF, Barhanin J (1996) Inner ear defects induced by null mutationof the isk gene. Neuron 17:1251–1264. [DOI] [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CACP, Santos F, Merchant SN, Liberman LD, Liberman MC (2015) Cochlear neuropathy in human presbycusis: confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res 327:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikhe Patil K, Canlon B, Cederroth CR (2015) High quality RNA extraction of the mammalian cochlea for qRT-PCR and transcriptome analyses. Hear Res 325:42–48. [DOI] [PubMed] [Google Scholar]
- Visacri MBQuintanilha JCFde Sousa VMAmaral LSde F L Ambrósio RCalonga LCuri SFBBde T Leme MFChone CTAltemani JMC, et al. (2019) Can acetylcysteine ameliorate cisplatin-induced toxicities and oxidative stress without decreasing antitumor efficacy? A randomized, double-blind, placebo-controlled trial involving patients with head and neck cancer. Cancer Med 8:2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner EL, Shin J-B (2019) Mechanisms of hair cell damage and repair. Trends Neurosci 42:414–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner N, Walsted A (2000) Postural-induced changes in intracranial pressure evaluated non-invasively using the MMS-10 tympanic displacement analyser in healthy volunteers. Acta Otolaryngol Suppl 543:44–47. [PubMed] [Google Scholar]
- Waissbluth S, Daniel SJ (2013) Cisplatin-induced ototoxicity: transporters playing a role in cisplatin toxicity. Hear Res 299:37–45. [DOI] [PubMed] [Google Scholar]
- Wan G, Corfas G (2017) Transient auditory nerve demyelination as a new mechanism for hidden hearing loss. Nat Commun 8:14487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Lippard SJ (2005) Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov 4:307–320. [DOI] [PubMed] [Google Scholar]
- Wang J, Ladrech S, Pujol R, Brabet P, Van De Water TR, Puel J-L (2004) Caspase inhibitors, but not c-Jun NH2-terminal kinase inhibitor treatment, prevent cisplatin-induced hearing loss. Cancer Res 64:9217–9224. [DOI] [PubMed] [Google Scholar]
- Wang J, Van De Water TR, Bonny C, de Ribaupierre F, Puel JL, Zine A (2003) a peptide inhibitor of c-Jun N-terminal kinase protects against both aminoglycoside and acoustic trauma-induced auditory hair cell death and hearing loss. J Neurosci 23:8596–8607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lee MP, Jones S, Liu J, Waldhaus J (2021) Mapping the regulatory landscape of auditory hair cells from single-cell multi-omics data. Genome Res 31:1885–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Han L, Diao T, Jing Y, Wang L, Zheng H, Ma X, Qi J, Yu L (2018) A comparison of systemic and local dexamethasone administration: from perilymph/cochlea concentration to cochlear distribution. Hear Res 370:1–10. [DOI] [PubMed] [Google Scholar]
- Wangemann P (1995) Comparison of ion transport mechanisms between vestibular dark cells and strial marginal cells. Hear Res 90:149–157. [DOI] [PubMed] [Google Scholar]
- Wangemann P (2006) Supporting sensory transduction: cochlear fluid homeostasis and the endocochlear potential. J Physiol 576:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring MJArrowsmith JLeach ARLeeson PDMandrell SOwen RMPairaudeau GPennie WDPickett SDWang J, et al. (2015) An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat Rev Drug Discov 14:475–486. [DOI] [PubMed] [Google Scholar]
- Warr WB, Guinan JJ (1979) Efferent innervation of the organ of corti: two separate systems. Brain Res 173:152–155. [DOI] [PubMed] [Google Scholar]
- Watson N, Ding B, Zhu X, Frisina RD (2017) Chronic inflammation - inflammaging - in the ageing cochlea: A novel target for future presbycusis therapy. Ageing Res Rev 40:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz C, Glowatzki E, Fuchs P (2009) The postsynaptic function of type II cochlear afferents. Nature 461:1126–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz CJC, Glowatzki E, Fuchs PA (2014) Excitability of type II cochlear afferents. J Neurosci 34:2365–2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells HRR, Freidin MB, Zainul Abidin FN, Payton A, Dawes P, Munro KJ, Morton CC, Moore DR, Dawson SJ, Williams FMK (2019) GWAS identifies 44 independent associated genomic loci for self-reported adult hearing difficulty in UK Biobank. Am J Hum Genet 105:788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertman JN, Melong N, Stoyek MR, Piccolo O, Langley S, Orr B, Steele SL, Razaghi B, Berman JN (2020) The identification of dual protective agents against cisplatin-induced oto- and nephrotoxicity using the zebrafish model. Elife 9:e56235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Włodyka J (1978) Studies on cochlear aqueduct patency. Ann Otol Rhinol Laryngol 87:22–28. [DOI] [PubMed] [Google Scholar]
- Wu J, Han W, Chen X, Guo W, Liu K, Wang R, Zhang J, Sai N (2017) Matrix metalloproteinase-2 and -9 contribute to functional integrity and noise-induced damage to the blood-labyrinth-barrier. Mol Med Rep 16:1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P-Z, O’Malley JT, de Gruttola V, Liberman MC (2020) Age-related hearing loss is dominated by damage to inner ear sensory cells, not the cellular battery that powers them. J Neurosci 40:6357–6366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PZ, Liberman LD, Bennett K, de Gruttola V, O’Malley JT, Liberman MC (2019) Primary neural degeneration in the human cochlea: evidence for hidden hearing loss in the aging ear. Neuroscience 407:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Marcus DC (2003) Age-related changes in cochlear endolymphatic potassium and potential in CD-1 and CBA/CaJ mice. J Assoc Res Otolaryngol 4:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Samuvel DJ, Stevens SM, Dubno JR, Schulte BA, Lang H (2012) Age-related changes of myelin basic protein in mouse and human auditory nerve. PLoS One 7:e34500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Tu S, Pass C, Zhang Y, Liu H, Diers J, Fu Y, He DZZ, Zuo J (2022) Profiling mouse cochlear cell maturation using 10× genomics single-cell transcriptomics. Front Cell Neurosci 16:962106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue N, Song L, Song Q, Santos-Sacchi J, Wu H, Navaratnam D (2021) Genes related to SNPs identified by genome-wide association studies of age-related hearing loss show restriction to specific cell types in the adult mouse cochlea. Hear Res 410:108347. [DOI] [PubMed] [Google Scholar]
- Yang C-H, Schrepfer T, Schacht J (2015) Age-related hearing impairment and the triad of acquired hearing loss. Front Cell Neurosci 9:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonovitz A, Fisch JE (1991) Circadian rhythm dependent kanamycin-induced hearing loss in rodents assessed by auditory brainstem responses. Acta Otolaryngol 111:1006–1012. [DOI] [PubMed] [Google Scholar]
- Yoo S-HYamazaki SLowrey PLShimomura KKo CHBuhr EDSiepka SMHong H-KOh WJYoo OJ, et al. (2004) PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci USA 101:5339–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettner EM, Gleser MA (2018) Progressive hearing loss among patients with cystic fibrosis and parenteral aminoglycoside treatment. Otolaryngol Head Neck Surg 159:887–894. [DOI] [PubMed] [Google Scholar]
- Zhang F, Dai M, Neng L, Zhang JH, Zhi Z, Fridberger A, Shi X (2013) Perivascular macrophage-like melanocyte responsiveness to acoustic trauma–a salient feature of strial barrier associated hearing loss. FASEB J 27:3730–3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu Z, Zhou L, Li H, Teng H, Dai W, Wang Y, Sun ZS (2011) Deficiency of antinociception and excessive grooming induced by acute immobilization stress in Per1 mutant mice. PLoS One 6:e16212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wu X, Lin X (2020) Gene therapy for genetic mutations affecting non-sensory cells in the cochlea. Hear Res 394:107858. [DOI] [PubMed] [Google Scholar]
- Zhang MJHou KDey KKSakaue SJagadeesh KAWeinand KTaychameekiatchai ARao PPisco AOZou J, et al. (2022) Polygenic enrichment distinguishes disease associations of individual cells in single-cell RNA-seq data. Nat Genet 54:1572–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB (2014) A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci USA 111:16219–16224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WDai MFridberger AHassan ADegagne JNeng LZhang FHe WRen TTrune D, et al. (2012) Perivascular-resident macrophage-like melanocytes in the inner ear are essential for the integrity of the intrastrial fluid-blood barrier. Proc Natl Acad Sci USA 109:10388–10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Müller U (2015) The elusive mechanotransduction machinery of hair cells. Curr Opin Neurobiol 34:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, Dallos P (2000) Prestin is the motor protein of cochlear outer hair cells. Nature 405:149–155. [DOI] [PubMed] [Google Scholar]
- Zheng W, Holt JR (2021) The mechanosensory transduction machinery in inner ear hair cells. Annu Rev Biophys 50:31–51. [DOI] [PMC free article] [PubMed] [Google Scholar]