Abstract
Patient: Female, 54-year-old
Final Diagnosis: Takotsubo cardiomyopathy
Symptoms: Cardiac decompenstion • cardiac insufficiency
Clinical Procedure: Lung transplantation
Specialty: Anesthesiology • Transplantology
Objective:
Challenging differential diagnosis
Background:
Takotsubo syndrome, or stress-induced cardiomyopathy, is a rare but serious condition that mimics myocardial infarction and can cause temporary cardiac dysfunction in the absence of coronary artery disease. General anesthesia can make diagnosis more challenging. Although it has already been described in a context of solid organ transplantation, takotsubo syndrome remains under-reported in lung transplantation, necessitating awareness to avoid diagnostic and management delays.
Case Report:
We report a case of takotsubo syndrome in a 54-year-old woman undergoing pulmonary transplantation for end-stage chronic obstructive pulmonary disease. Preoperative evaluations showed no cardiac pathology. During surgery, she developed severe left ventricular failure with ST-segment elevations and diffuse hypokinesia, leading to cardiogenic shock and multiorgan dysfunction. Delayed diagnosis of takotsubo syndrome and late initiation of veno-arterial extracorporeal membrane oxygenation worsened her condition. Postoperatively, she developed lung abscesses, broncho-cutaneous fistula, and hemorrhagic shock, resulting in a prolonged intensive care unit stay. Two years after the transplant, left ventricular dysfunction was persistent, significantly affecting her quality of life.
Conclusions:
This case report highlights the importance of awareness of takotsubo syndrome associated with lung transplantation, particularly in at-risk patients. Indeed, early diagnosis and management of this cardiomyopathy are crucial for improving outcomes. Multimodal monitoring, including transesophageal echocardiography and continuous ST-segment monitoring, is essential for timely diagnosis. Although rare, this complex clinical condition should be considered in lung transplant recipients with sudden heart failure to ensure prompt and effective treatment. Further research is needed to understand this stress cardiomyopathy in this specific setting and to develop effective management strategies.
Key words: Extracorporeal Membrane Oxygenation; Lung Transplantation; Shock, Cardiogenic; Takotsubo Cardiomyopathy
Introduction
Takotsubo syndrome, also known as stress-induced cardiomyopathy or “broken heart syndrome,” is a rare but life-threatening cardiac condition that was first recognized in the 1990s [1]. It presents clinically as myocardial infarction-like symptoms and is characterized by chest pain, dyspnea, transient ST-segment elevation on the electrocardiogram (ECG), moderate increase in troponins, and temporary cardiac muscle dysfunction, in the absence of coronary artery obstruction [2–5]. Because of its improved clinical characterization, takotsubo syndrome appears to be more common than previously recognized. It is currently estimated that approximately 2% of all patients undergoing emergency coronary angiography for suspicion of acute coronary syndrome present this cardiomyopathy. Its annual incidence is approximately 100 new cases per 1 million population, 90% of whom are postmenopausal women [5–8]. Although the prognosis is generally favorable, serious complications such as cardiac arrest, cardiogenic shock, and severe ventricular arrhythmias can occur [8]. Early diagnosis allows for appropriate and accurate management, thereby avoiding these potential lethal complications.
The etiology of takotsubo syndrome appears to be related to either intense emotional stress or physical stress leading to a cascade of biochemical reactions [4,5]. However, rare cases of takotsubo syndrome developing after liver [9,10], kidney [11,12], heart [13], and lung transplantation [14–17] have been reported, suggesting a potential association between organ transplantation and takotsubo syndrome. However, the low incidence of this entity means that this stress cardiomyopathy is likely to be under-reported in the context of transplantation, and more specifically in lung transplantation, which explains the gaps in current knowledge and the paucity of literature on the subject. General anesthesia can make diagnosis more challenging. The aim of this case report is to increase physicians’ knowledge, understanding, and alertness to the occurrence of this stress cardiopathy in this specific population to avoid delays in diagnosis and management, which can result in poor postoperative outcomes. We present here the second case of takotsubo syndrome occurring during a bilateral lung transplantation, in a recipient with no prior cardiac or coronary pathology. Severe left ventricular failure required the urgent initiation of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) to support cardiac function. However, the delayed recognition of this syndrome by the transplant team resulted in delayed management.
Case Report
The patient provided written informed consent, and the publication was approved by the local ethics committee (Faculty Hospital Ethics Committee Erasme-ULB, Brussels, Belgium; N° P2023/260). A 54-year-old woman was referred to our center for bilateral lung transplantation due to end-stage respiratory failure caused by chronic obstructive pulmonary disease GOLD IV, primarily characterized by diffuse emphysema, chronic hypoxemia requiring oxygen therapy (1.5 L/min), and chronic hypercapnia. Pre-transplant arterial blood gas analysis showed a pH of 7.41, pCO2 of 65 mmHg, HCO3− of 33.2 mmol/L, and pO2 of 59 mmHg under 1.5 L/min of supplemental oxygen. Respiratory function testing revealed a severe obstructive syndrome and drastically reduced diffusing capacity of the lungs for carbon monoxide (DLCO) at 15% of predicted values. A chest CT scan confirmed the presence of homogenous pulmonary emphysema. The 6-minute walk test was significantly limited by severe dyspnea, with a covered distance of 60 meters and a drop in pulse oxygen saturation (SpO2) from 98% to 86%. The calculated BODE index (Body Mass Index, Airflow Obstruction, Dyspnea, and Exercise Capacity) was 10, representing an 80% mortality rate at 52 months. The patient’s American Society of Anesthesiologists Physical Status Score (ASA score) was of IV, representing a severe systemic disease that is a constant threat to life.
Pre-transplant cardiac assessment revealed a regular sinus rhythm at 89 bpm with narrow QRS complexes on the electrocardiogram. Transthoracic echocardiography demonstrated preserved left ventricular ejection fraction (LVEF), homogeneous myocardial wall motion, preserved right ventricular (RV) systolic and diastolic functions, and no valvular abnormalities. Right heart catheterization showed moderate post-capillary pulmonary arterial hypertension, with a mean pulmonary artery pressure of 28 mmHg and a transcapillary gradient of 10 mmHg. Coronary angiography revealed normal coronary arteries with right dominance.
The patient had no other significant medical conditions, except for severe malnutrition, characterized by a body mass index (BMI) of 15.2 kg/m2 and osteoporosis. She underwent preoperative rehabilitation, which included enteral nutrition through gastrostomy placement, increasing her BMI to 16.8 kg/m2, and treatment for osteoporosis with oral calcium and vitamin D supplementation. Medication for chronic obstructive pulmonary disease (COPD) was optimized and included the use of inhaled short-acting (fenoterol hydrobromide) and long-acting (fenoterol fumarate) beta2-mimetics, short-acting (ipratropium bromide) and long-acting (glycopyrronium) anti-cholinergics, and corticosteroids (beclometasone dipropionate). The patient had quitted smoking 6 years ago. Three months later than scheduled, due to a positive COVID-19 test result, she was listed for a lung transplant in April 2021. Bilateral lung transplantation was performed in August 2021, with the lungs procured from a brain-dead donor.
Upon arrival in the operating room, she had a waiting time of 140 minutes before the induction of general anesthesia due to delays in organizing retrieval of the donor organs. She did not report any concerns during this waiting period. Anxiolysis was achieved by providing a musical playlist according to the patient’s preference. The surgery started 90 minutes after general anesthesia induction and lasted 400 minutes. The ischemia time was 230 minutes for the right lung and 360 minutes for the left lung.
Upon arrival in the operating room, she had a mean arterial pressure (MAP) of 73 mmHg, a heart rate (HR) of 102 bpm, and a SpO2 of 100% while receiving 2 liters of oxygen. These hemodynamic parameters remained stable during the waiting period, with MAP at 85 mmHg, HR at 99 bpm, and SpO2 at 100% under 2 liters of oxygen in a seated position just before induction of general anesthesia. Cerebral oximetry, measured by near-infrared spectroscopy (NIRS), indicated right and left cerebral oxygenation levels of 68% and 76%, respectively. General anesthesia was induced using remifentanil via target-controlled infusion (TCI), etomidate, and rocuronium. After orotracheal intubation using a 35-F double-lumen tube, general anesthesia was maintained using propofol with TCI titrated based on the depth of anesthesia. Subsequently, a 3-lumen central venous catheter was placed in the right internal jugular vein, and the measured central venous pressure was 20 mmHg.
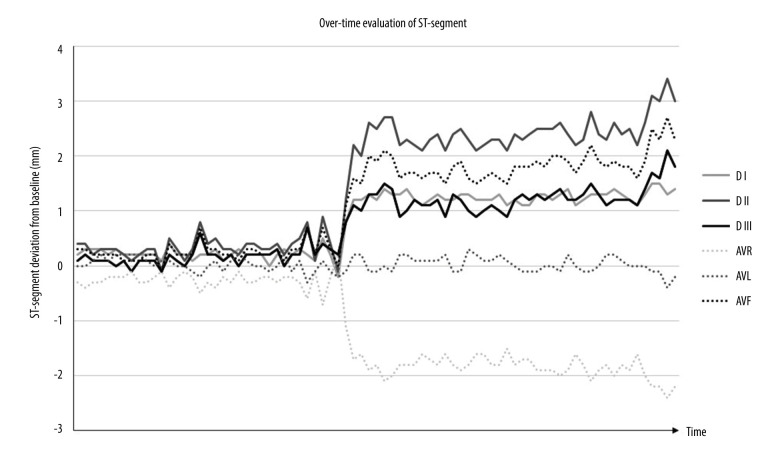
Norepinephrine infusion had to be initiated simultaneously with the induction of general anesthesia to maintain a mean arterial pressure (MAP) of 65 mmHg at a rate of 0.05 µg/kg/min. Changes were observed on the electrocardiogram: shortly after induction of general anesthesia, minor ST-segments deviations (<0.3 mm) were observed, worsening rapidly after the first arterial clamping of the right pulmonary artery, characterized by ST-segment elevation of more than 1 mm in leads I, II, III, and aVF, and an inferior shift of more than 1 mm in aVR. Overtime evolution of the ST-segments is shown in Figure 1, but unfortunately, the capture of the full QRS morphology was not possible with our recording system. The patient quickly exhibited signs of hemodynamic deterioration, with escalating noradrenaline requirements. Transesophageal echocardiography (TEE) revealed diffuse hypokinesia of the left ventricle (LV) associated with a reduced LVEF, while RV systolic function appeared preserved. Inotropic support with dobutamine at 5 µg/kg/min was initiated. Myocardial dysfunction was diagnosed and takotsubo syndrome was suggested and discussed at this stage. Due to ST-segment changes and considering the differential diagnosis of myocardial ischemia, special care was taken to avoid obstruction of the coronary ostia by surgical instruments. However, some members of the transplant team were reluctant to make the diagnosis of acute myocardial dysfunction because the hemodynamic parameters visible on the monitor appeared to be preserved, although this prevented use of vasopressors and cardiotropics.
Figure 1.
Evolution over time of the ST-segment deviation from the isoelectric line for the frontal derivations registered in operating room. Soon after the beginning of surgery, ST-segment elevation of more than 1 mm was observed in leads I, II, III, and aVF, and an inferior shift of more than 1 mm in aVR.
The lung transplantation was performed sequentially through an anterior bithoracotomy as follows: dissection of the hilum; exclusion test followed by pneumonectomy; simultaneously, the donor lungs were prepared by a second team; then reimplantation involving vascular and bronchial sutures, followed by reperfusion. No intraoperative surgical complications were observed. However, the patient’s cardiac and hemodynamic status continued to deteriorate during implantation of the first lung, and the situation worsened after the unclamping right pulmonary artery. The patient subsequently developed severe LV failure with signs of cardiogenic shock, characterized by increasing noradrenaline requirements, reaching the maximum dose of 4.25 µg/kg/min, elevated central venous pressures up to 36 mmHg indicating increased end-diastolic ventricular pressures, an increase in lactate levels up to 7.2 mmol/L, mixed venous oxygen saturation (SvO2) of 57%, a decline in NIRS values by more than 50% and 70% from baseline values for the left and right sides respectively, and oliguria to anuria suggesting low cardiac output. TEE revealed LV ballooning with near-total akinesia and LVEF <10%, while RV systolic function still appeared preserved. In consultation with the intensive care physicians, VA-ECMO support for the failing ventricle was proposed. The suspicion of takotsobu syndrome was strengthened by the ultrasound images showing the severe cardiac failure associated with this LV ballooning. However, due to lack of knowledge about this syndrome and its adverse outcomes on the one hand, and fear of heparin administration associated with this extracorporeal cardiac function support on the other, ECMO placement was deferred until after unclamping of the second lung. Peripheral VA-ECMO was surgically implanted via femoral vessel cannulation. Following the initiation of ECMO, NIRS values showed a significant improvement, norepinephrine requirements dropped drastically from 4.25 to 0.6 µg/kg/min, diuresis resumed, and lactate levels began to decrease. After closure of the chest, the patient was transferred to the intensive care unit (ICU).
Upon admission to the ICU, the patient’s condition continued to improve under VA-ECMO support, with decreasing vasopressor requirements, characterized by a norepinephrine infusion rate of 0.3 µg/kg/min. SvO2 was 61%, and lactate levels continued to decrease. High-sensitivity troponins were at 500 ng/L, reaching a peak of 735 ng/L on the first postoperative day. Cardiac ultrasound revealed a ballooned LV with near-total akinesia, except for a small lateral hypokinesia (Figure 2). This severe LV failure was associated with the presence of intraventricular sludge. The RV, on the other hand, appeared flattened, with preserved function. The patient received inotropic support, initially with dobutamine, followed by the administration of milrinone and levosimendan. Cardiac function improved rapidly, allowing removal of VA-ECMO support on the second postoperative day and weaning off norepinephrine on the third postoperative day. The perioperative time-line is presented in Figure 3.
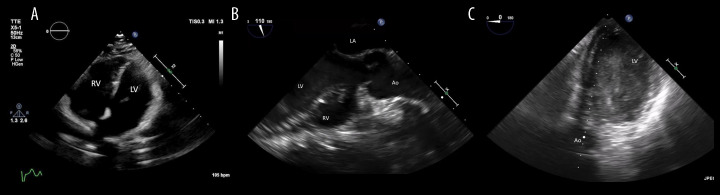
Figure 2.
(A) Preoperative apical 4-chamber view on transthoracic echocardiography showing normalsized ventricles. Postoperative transesophageal echocardiography performed upon arrival in the Intensive Care Unit; (B) Mid-esophageal ascending aortic long-axis view showing a ballooned left ventricle with near-total akinesia and severe dysfunction but with preserved right ventricular function; (C) Trans-gastric view, showing dilated left ventricle associated with intraventricular sludge. LV – left ventricle; RV – right ventricle; LA – left atrium; Ao – aorta.
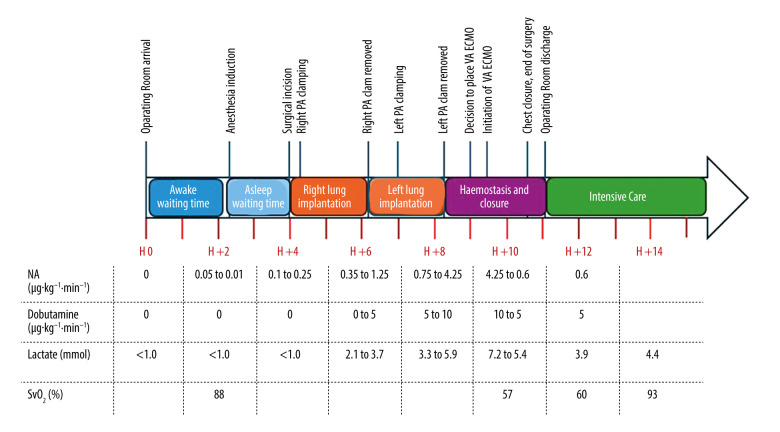
Figure 3.
Timeline representing intraoperative and immediate postoperative events, including evolution of lactatemia and central venous saturation, and required doses of vasopressors (norepinephrine) and cardiotropic agents (dobutamine). PA – pulmonary artery; VA-ECMO – veno-arterial extracorporeal membrane oxygenation; NA – noradrenaline; SvO2 – central venous oxygen saturation.
The patient’s postoperative course was complicated, leading to an extended stay in the ICU. The early postoperative period was marked by rapid development of lung abscesses in the right upper lobes, as well as the occurrence of a broncho-cutaneous fistula, possibly related to lung hypoperfusion during per-operative cardiogenic shock and the delayed support of cardiac function by VA-ECMO. The fistula was covered by a latissimus dorsi muscle flap. Subsequently, the patient presented a stenosis of the right main bronchus and a dehiscence of the bronchial flap, which was managed through an endobronchial approach. Respiratory progression was also characterized by persistent bronchial superinfection with Pseudomonas aeruginosa and the development of subsegmental pulmonary embolism. On the 57th postoperative day, the patient had hemorrhagic shock due to spontaneous rupture of the right pulmonary artery, requiring emergency surgery. The postoperative period was also marked by severe malnutrition and ICU-acquired quadriparesis. Weaning from mechanical ventilation was extremely slow, requiring tracheostomy. The patient was discharged from the ICU on the 149th postoperative day.
The cardiac function did not return to its initial state, and the cardiac evolution was characterized by persistent presence of systolic heart failure, with an LVEF of 35 to 40% at discharge from the ICU. A beta-blocker (bisoprolol 2.25 mg 2) and an angiotensin-converting enzyme inhibitor (lisinopril 2.5 mg) were initially employed to treat this persistent heart failure. Six months postoperatively, LVEF was still 35% and treatment with a digitalis glycoside complex (sacubitril)/angiotensin II receptor antagonist (valsartan) was initiated but had to be temporarily discontinued due to renal deterioration. Additionally, an SGLT2 inhibitor (empagliflozin 10 mg) and a potassium-sparing diuretic (spironolactone 25 mg) were introduced. Two years after the takotsubo event, the patient still had heart failure characterized by an LVEF of 40%, diffuse hypokinesia, and septal dyskinesia, with right heart function within normal limits. The persistence of heart failure significantly affected our patient’s quality of life.
Discussion
We describe here a case of takotsubo syndrome occurring during a bilateral lung transplant. In the present case, despite adequate monitoring, the late diagnosis, and lack of awareness of takotsubo syndrome by the whole team, combined with surgical imperatives, did not allow for earlier placement of VAECMO. The subsequent low cardiac output during the surgical period could potentially explain the postoperative complications observed in the first transplanted lung, which included pulmonary abscess and broncho-cutaneous fistula.
Takotsubo syndrome, although increasingly recognized, remains underestimated and often misdiagnosed. Our patient met the diagnostic criteria proposed by the Heart Failure Association of the European Society of Cardiology [5] – transient LV dysfunction characterized by near-total hypokinesia of the LV wall and apical ballooning, intraoperative ST-segment elevation, moderate elevation of troponin levels, and the absence of coronary artery disease. The age and sex of our patient also supported a diagnosis of takotsubo syndrome. Indeed, nearly 90% of takotsubo patients are middle-aged women, with an average age of 67–70 years [5,7]. Although the pathophysiological mechanisms of takotsubo syndrome remain incompletely understood, it is often triggered by a stressful event, either emotional or physical. Excessive sympathetic stimulation and the resulting increase in catecholamines appear to play a key role. The contribution of coronary vasoconstriction and/or micro-vascular spasm has also been suggested [4,8]. In addition, severe respiratory disease and/or lung procedure may increase the risk of developing takotsubo syndrome [18]. Organ transplants, because of the intense physical and emotional stress involved, and especially lung transplants, because of the precarious respiratory condition of the patients, pose a particular risk of developing this cardiomyopathy and require the highest level of vigilance in these patients.
Our patient, predisposed by her age, postmenopausal status, and end-stage respiratory failure, experienced intense emotional stress related to the need for transplantation and the long wait in the operating room. This may have contributed to development of the initial signs of takotsubo syndrome, including the need for vasopressors and mild ST-segment changes, which manifested shortly after induction of anesthesia. However, this syndrome may have been exacerbated by the significant physical stress of the major surgical procedure itself. Indeed, ST-segment changes increased significantly shortly after arterial clamping of the first lung. However, it is not possible to definitively determine the relative contribution of each of these etiologies. These factors, which should have attracted the full attention of the medical team, probably triggered an intense release of catecholamines, leading to an early left ventricular dysfunction during bilateral lung transplantation.
Several cases of takotsubo syndrome have been reported in the setting of kidney and liver transplantation [9–12], and more rarely in heart or lung transplantation [13–17,19], highlighting the importance of stress in this cardiomyopathy. Indeed, only 4 cases of takotsubo syndrome have been documented in the perioperative period of lung transplantation, and to our knowledge, this is only the second case described during lung transplantation. The first case, reported by Duclos et al, described the history of a 50-year-old man who experienced takotsubo syndrome leading to cardiac arrest after induction of anesthesia for lung transplantation [16]. The resuscitation was successful, and the team decided to proceed with the transplant. They supported the patient’s cardiovascular status with norepinephrine and dobutamine. Proceeding with transplantation was considered best for several reasons. First, the risk of ischemia was considered low in the absence of coronary artery disease, and the diagnosis of takotsubo seemed clear from the ultrasound characterization. Second, they considered it unacceptable to lose the graft, or worse, the patient, without a new offer of rapid transplantation, given the transient nature of this cardiomyopathy. Finally, their therapeutic strategy was clearly discussed and planned. It included the use of vasopressors and catecholamines initially, and the possibility of circulatory support by ECMO if necessary. The postoperative course was uneventful and allowed the patient to be discharged from the intensive care unit on the 4th postoperative day. This case differs from ours. First, although the patient had suffered cardiac arrest, the cardiac dysfunction appeared less severe than in our patient. Myocardial akinesia was less extensive, with LVEF of 25%, and hemodynamics improved with vasopressors and catecholamines without requiring ECMO. Second, and most importantly, the team was aware of takotsubo syndrome, its consequences, and how to manage it. Kassegne et al reported the case of 58-year-old women who developed takotsubo syndrome following the phone call announcing the lung transplantation, resulting in postponement of the transplant [19]. The patient was not admitted to the operating room or anesthetized. This confirms that the call for a transplant should be considered as a major emotional stress factor that is sufficient to induce takotsubo syndrome on its own. Takotsubo syndrome has also been reported in the early postoperative period following lung transplantation in 2 patients in their sixties. Both developed acute graft dysfunction, leading to the death of one of them. These cases confirm that the stress induced by lung transplantation surgery is a triggering factor capable of inducing takotsubo syndrome, with potentially fatal consequences [15,17].
Differentiating takotsubo syndrome from acute myocardial ischemic injury is not easy because these 2 conditions present very similar clinical features. This diagnosis is particularly challenging in patients undergoing general anesthesia. Indeed, the lack of verbal communication prevents patients from expressing the typical symptoms of takotsubo syndrome, such as chest pain and dyspnea. In our clinical practice, the administration of noradrenaline at a low dose concomitant with anesthesia induction is a standard protocol for frail patients to maintain blood pressure as close to basal levels as possible. In this case, the dose of noradrenaline had to be rapidly increased, but this sign alone was not sufficient to suspect a cardiac origin, as anesthetic agents can cause vasoplegia. The diagnosis of heart failure was made on the basis of a combination of signs, including the use of increasing doses of nor-adrenaline, ST-segment changes, signs of low cardiac output, and TEE ultrasound images. In our patient, preoperative coronary angiography showed healthy coronary arteries without any atheromatosis. For this reason, the cardiology team deemed a postoperative coronary angiography unnecessary. They attributed the initial cardiac dysfunction to takotsubo syndrome, which was likely exacerbated by delayed management and a prolonged period of low cardiac output during surgery due to the late placement of ECMO, leading to coronary hypoperfusion. However, we cannot completely exclude the possibility of coronary trunk obstruction during surgical manipulations. As soon as the diagnosis of LV failure associated with ST-segment elevation was made, the surgeons were notified, and all precautions were taken to avoid obstruction of blood flow in the coronary arteries. However, these measures did not improve the patient’s cardiac and hemodynamic condition, which continued to deteriorate. Additionally, the troponin values, dosed in the immediate postoperative period, were too low to be indicative of massive LV ischemia. These 2 later observations confirmed the diagnosis of takotsubo syndrome.
Our patient presented a severe form of takotsubo syndrome, complicated by cardiogenic shock, which is an unfavorable prognostic factor. Approximately 10% of patients with takotsubo syndrome develop cardiogenic shock requiring mechanical support to maintain cardiac function [5,20]. VA-ECMO is recommended to ensure adequate cardiac output and support cardiac function as a bridge until ventricular function improves, as takotsubo syndrome is transient and reversible. The use of an intra-aortic balloon pump in this condition is generally avoided as it can worsen one of the possible complications of takotsubo syndrome – LV outflow tract obstruction [5]. Given the multiple organ dysfunction exhibited by our patient, characterized by renal and cerebral involvement, cellular hypoxia, associated with low cardiac output, we, after collegial discussion between anesthesiologists, intensivists, and surgeons, implemented hemodynamic support by placing a peripheral VA-ECMO, as recommended. However, this support therapy was initiated late, when both lungs were already transplanted.
The main interest of this case presentation lies in the late diagnosis and recognition of it by the entire team. However, part of the surgical team, focused on the complexity of the lung transplant procedure, underestimated the severity of our patient’s cardiac dysfunction, because of blood pressure maintained with increasing doses of norepinephrine. This explains the delayed placement of VA-ECMO. Nevertheless, the entire team agreed to proceed with the bilateral lung transplant for clear ethical reasons, including the unacceptability of graft loss, and because cardiac function could be supported by ECMO, given that this cardiomyopathy is known to be reversible. There is no measure to prevent takotsubo syndrome. Nevertheless, transplant teams must be aware of the inherent risk of the intense emotional stress of the phone conversation and the surgical stress itself, particularly in the context of lung transplantation, in susceptible patients. Practitioners should consider this syndrome when a patient presents with sudden heart failure despite recent satisfactory cardiovascular evaluation. Lack of awareness of takotsubo syndrome and its challenging diagnosis, especially in a patient under general anesthesia, can lead to delayed diagnosis. To prevent this, a multimodal monitoring approach is essential. First, TEE should be used routinely during lung transplantation. It can assess LVEF, myocardial segment mobility and contractility, and can detect the ballooning typical of takotsubo syndrome. In addition, it provides dynamic indices for the assessment of filling pressures and cardiac output. Continuous ST-segment monitoring on the anesthesia monitor is also essential for the detection of ECG changes. Finally, basic monitoring of a lung transplant patient should include other indirect indicators of cardiac function and cardiac output, such as cerebral oximetry monitoring, regular central venous saturation measures, and hourly diuresis.
Reported cases of takotsubo syndrome in lung transplant recipients remain rare, leaving a notable gap in the literature. The low reported incidence that this stress cardiomyopathy is likely under-reported in this setting, contributing to the current lack of knowledge. Therefore, documentation of these rare cases, such as ours, is crucial to raise awareness among medical teams involved in lung transplantation. This will improve the understanding of the pathophysiology of this syndrome, the exploration of diagnostic challenges, and the discussion of appropriate management strategies. However, the inherent limitations of case reports preclude drawing robust conclusions. Further studies are essential to investigate the prevalence and outcomes of takotsubo syndrome in these patients, taking into account the unique stressors associated with lung transplantation. Better understanding and vigilance for this stress cardiomyopathy may prevent delays in diagnosis and treatment, thereby improving postoperative outcomes.
Conclusions
In summary, we present a case of takotsubo syndrome which occurred during a bilateral lung transplantation, and whose late diagnosis unfortunately led to delayed management. It is crucial to raise awareness among clinicians about the potential association between takotsubo syndrome, respiratory failure, and bilateral lung transplantation. Such awareness, combined with appropriate monitoring, would promote early diagnosis and effective management of this complex clinical situation. Despite its rarity, takotsubo syndrome should be considered in lung transplant recipients who present with sudden heart failure to facilitate timely and appropriate treatment. Additional research is essential to understand this stress cardiomyopathy in the context of lung transplantation and to develop effective management strategies.
Acknowledgments
We would like to express our gratitude to Pr. Grimaldi David for his insightful comments on this manuscript and Pr. Turgay Tuna for his general support.
Footnotes
Publisher’s note: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher
Department and Institution Where Work Was Done
Departments of Anesthesiology, Intensive Care, and Thoracic Surgery of H.U.B Erasme, Brussels, Belgium.
Ethics Statement
This case report was approved by or local ethics committee (P2023/260).
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Sato H. Tako-tsubo-like left ventricular dysfunction due to multivessel coronary spasm. In: Kodama K, Haze K, Hori M, editors. Clinical aspect of myocardial injury: From ischemia to heart failure. Tokyo: Kagakuhyoronsha Publishing Co; 1990. pp. 56–64. [Google Scholar]
- 2.Summers MR, Prasad A. Takotsubo cardiomyopathy: Definition and clinical profile. Heart Fail Clin. 2013;9:111–22. vii. doi: 10.1016/j.hfc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 3.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–38. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 4.Ghadri JR, Wittstein IS, Prasad A, et al. International expert consensus document on takotsubo syndrome (Part I): Clinical characteristics, diagnostic criteria, and pathophysiology. Eur Heart J. 2018;39:2032–46. doi: 10.1093/eurheartj/ehy076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyon AR, Bossone E, Schneider B, et al. Current state of knowledge on takotsubo syndrome: A position statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8–27. doi: 10.1002/ejhf.424. [DOI] [PubMed] [Google Scholar]
- 6.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J. 2008;155:408–17. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Y-Hassan S, Tornvall P. Epidemiology, pathogenesis, and management of takotsubo syndrome. Clin Auton Res. 2018;28:53–65. doi: 10.1007/s10286-017-0465-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pelliccia F, Kaski JC, Crea F, Camici PG. Pathophysiology of takotsubo syndrome. Circulation. 2017;135:2426–41. doi: 10.1161/CIRCULATIONAHA.116.027121. [DOI] [PubMed] [Google Scholar]
- 9.Tachotti Pires LJ, Cardoso Curiati MN, Vissoci Reiche F, et al. Stress-induced cardiomyopathy (takotsubo cardiomyopathy) after liver transplantation – report of two cases. Transplant Proc. 2012;44:2497–500. doi: 10.1016/j.transproceed.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 10.Vitin AA, Pennington MW, Bowdle TA, et al. Stress (takotsubo) cardiomyopathy during liver transplantation: Case study and literature review. Transplant Proc. 2018;50:211–16. doi: 10.1016/j.transproceed.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 11.Gołębiewska J, Stopczyńska I, Dębska-Ślizień A, et al. Tako-tsubo cardiomyopathy on the first day after renal transplantation – case report and literature review. Transplant Proc. 2014;46:2920–22. doi: 10.1016/j.transproceed.2014.09.075. [DOI] [PubMed] [Google Scholar]
- 12.Vailas MG, Vernadakis S, Kakavia K, et al. A heartbreaking renal transplantation: Is norepinephrine the culprit to blame? Transplant Proc. 2016;48:3088–91. doi: 10.1016/j.transproceed.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Behnes M, Baumann S, Borggrefe M, Haghi D. Biventricular takotsubo cardiomyopathy in a heart transplant recipient. Circulation. 2013;128:e62–63. doi: 10.1161/CIRCULATIONAHA.113.001519. [DOI] [PubMed] [Google Scholar]
- 14.Michel-Cherqui M, Felten ML, Liu N, et al. Management of takotsubo cardiomyopathy in a lung transplant recipient. Transplantation. 2010;90:692–94. doi: 10.1097/TP.0b013e3181ebf76a. [DOI] [PubMed] [Google Scholar]
- 15.Yazıcıoğlu A, Subaşı M, Türkkan S, et al. An uncommon cause for grade 3 primary graft dysfunction after lung transplantation: Takotsubo cardiomyopathy. Turk Gogus Kalp Damar Cerrahisi Derg. 2018;26(3):487–91. doi: 10.5606/tgkdc.dergisi.2018.14905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duclos G, Mignon A, Zieleskiewicz L, et al. Takotsubo cardiomyopathy following induction of anesthesia for lung transplantation, an unexpected complication. J Cardiothorac Vasc Anesth. 2018;32:1855–57. doi: 10.1053/j.jvca.2017.10.022. [DOI] [PubMed] [Google Scholar]
- 17.Omosule A, Malik MF, Cisneros L, Guruswamy J. Takotsubo cardiomyopathy after double-lung transplantation: Role of early extracorporeal membrane oxygenation support. J Cardiothorac Vasc Anesth. 2019;33:2503–7. doi: 10.1053/j.jvca.2018.10.042. [DOI] [PubMed] [Google Scholar]
- 18.Manfredini R, Fabbian F, Giorgi AD, et al. Heart and lung, a dangerous liaison-Tako-tsubo cardiomyopathy and respiratory diseases: A systematic review. World J Cardiol. 2014;6:338–44. doi: 10.4330/wjc.v6.i5.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kassegne L, Degot T, Morel O, et al. Acute cardiac failure due to takotsubo cardiomyopathy secondary to a phone call for lung transplantation: A case report. Transplant Proc. 2019;51:3167–70. doi: 10.1016/j.transproceed.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 20.Ghadri JR, Wittstein IS, Prasad A, et al. International expert consensus document on takotsubo syndrome (part II): Diagnostic workup, outcome, and management. Eur Heart J. 2018;39:2047–62. doi: 10.1093/eurheartj/ehy077. [DOI] [PMC free article] [PubMed] [Google Scholar]