Abstract
Introduction
Men with Klinefelter Syndrome (KS) have been previously reported to have an increased risk of Male Breast Cancer (MBC). This systematic review provides the latest information regarding the incidence of MBC in the KS population compared to the standard male population and identifies mechanisms by which MBC may develop in KS.
Material and methods
Several databases were searched including PubMed/MEDLINE and EMBASE between October 2023 and March 2024. The review was conducted in accordance with the latest Preferred Reporting Items for Systematic Reviews and Meta-analyses-guidelines and was registered in PROSPERO (CRD42024551110). Overall, 332 papers were identified for screening. Standardised incidence ratios (SIRs) were calculated in comparison to national incidence figures. Additionally, a literature review was conducted looking at potential MBC mechanisms in KS.
Results
Across Danish and British cohorts, incidence of MBC in KS was significantly higher than the general population: SIR 18.1 (95 % CI: 13.53 to 24.74), p<0.001. Breast cancer rates in women are still far higher (68.50 per 100,000 woman-years). MBC mechanism in KS may involve decreased micro-RNA (MIR-3648 and MIR3647) expression, increased oestrogen/progesterone receptor expression and exogenous androgen use.
Conclusions
Rates of MBC are significantly raised in KS and a higher clinical suspicion of breast cancer should be considered when assessing men with KS. The true aetiology of MBC in KS, however, requires further research. There is a need for an accurate and up to date study of MBC incidence in KS to define the current risk.
Keywords: Klinefelter syndrome, Klinefelter's syndrome, Male breast cancer, Incidence, Mechanism
Highlights
-
•
People with Klinefelter syndrome are at an increased risk of male breast cancer.
-
•
Rates among people with Klinefelter syndrome are still below that of women.
-
•
The mechanism behind this is likely both hormonal and genetic in origin.
-
•
Changes to X-chromosome and MiRNA expression likely plays some role.
-
•
TRT use may also be related to male breast cancer oncogenesis.
1. Introduction
Klinefelter syndrome (KS) is a sex chromosome disorder defined as a phenotypical male having additional X chromosomes (47, XXY) [1]. KS was first described by Klinefelter et al., in 1942, with nine men reported to have large breasts, small testis, and an inability to produce sperm (azoospermia) [2]. This was later attributed to the 47, XXY karyotype in 1959 [3]. KS is believed to affect anywhere between one in 500–1000 new-born males, with specific forms of the disease (i.e. supernumerary X chromosomes i.e. XXXY, XXXXY) being much rarer [4]. People with KS can also demonstrate genetic mosaicism: a phenomenon where two or more cell lineages arise from a single zygote. In the case of KS, their genetic makeup may look like 50 % of cell having XXY, and 50 % of cells having XY sex chromosomes [5].
Metabolic and hormonal manifestations of the disease have shown to be risk factors for several diseases, including cancer [6]. KS has been previously linked to an increased risk of male breast cancer (MBC) – over a ten-fold increase, compared to the standard male population [7]. This literature review identified numerous cohort studies and case reports, concluding that while rates appear significantly higher than the normal population, the true risk was unknown. Case studies mentioned took this further, concluding that rates were higher than even that of women – this remains controversial. A study by the same author identified a relative risk (RR) of 29.64 (95 % CI: 12.26–71.68) across 5 observed cases from the U.S. Veterans Affairs Medical Care System Database [8].
This systematic review provides the latest information regarding the incidence of MBC in the KS population compared to the standard male population. Additionally, a literature review was conducted that identifies proposed mechanisms by which MBC may develop in KS.
2. Material and methods
2.1. Incidence
2.1.1. Search strategy
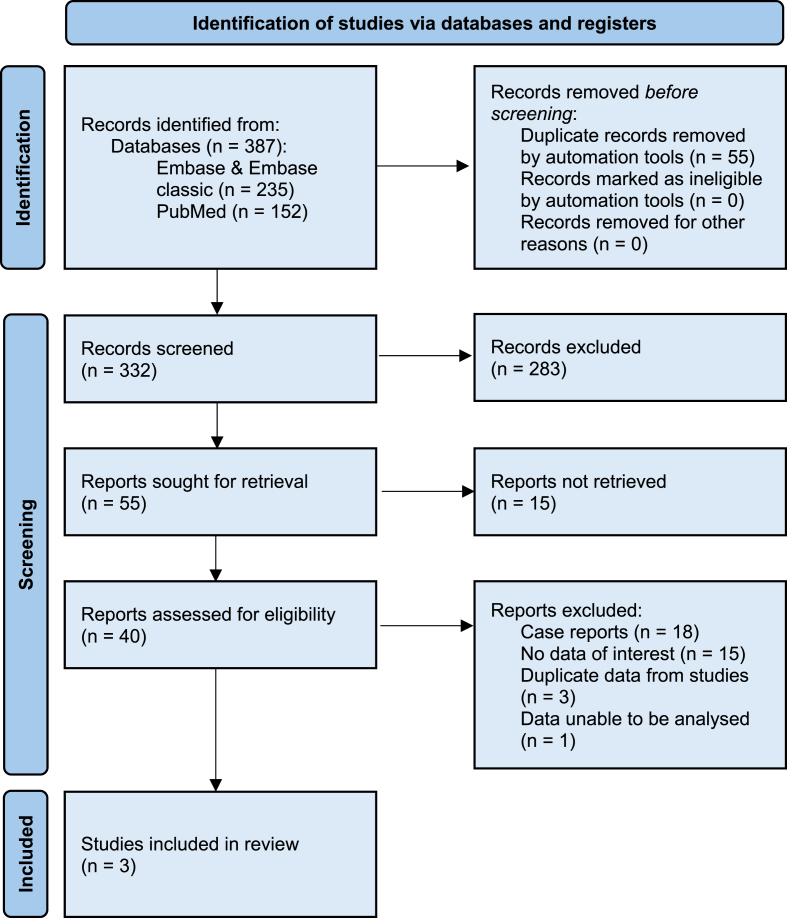
This review followed the Preferred Reporting Items for Systematic Reviews and Meta- Analysis (PRISMA) methodology [9], and the review protocol was registered on PROSPERO (CRD42024551110). An outline of the strategy is included as Fig. 1. PubMed/MEDLINE and Embase/Embase classic were searched including the following search criteria: (“Klinefelter Syndrome” OR “Klinefelter's syndrome” OR “Klinefelter” OR “Klinefelter's”) AND (“male breast cancer” OR “breast cancer”) to identify publications from the past 100 years in English. Articles were filtered to those who's abstract was available. No other search filters were applied at this time. The search was conducted between October 2023 and March 2024.
Fig. 1.
Incidence PRISMA flow diagram. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses.
2.1.2. Review of studies
The initial search yielded 387 results across all databases. Of these, 332 were selected for screening. Two reviewers (B.C and S.N) individually screened, retrieved and reviewed the manuscripts identified, facilitated by the software EndNote. Due to the sparsity of studies, abstract only articles were included. Articles were excluded for the following reasons: (1) Duplicate data/reports, (2) no data of relevance i.e. did not present incidence, (3) not being a cohort study, (4) too few participants i.e. <100. (5) Data unable to be analysed i.e. Standardised Incidence Ratios (SIRs) could not be calculated with the reported data. Three cohort studies were eventually identified and included in the report. Study references were also reviewed, but no further data of relevance was found. Disagreements between reviewers were dealt with at the discretion of the supervising author (T.Y).
2.1.3. Data extraction
Data extraction was carried out by the same review authors (B.C and S.N). The following data was extracted: (1) Number of KS patients included in the study, (2) total number of patient-years of KS patients, (3) number of cases of MBC in the KS population, (4) the country of study. Where patient-years totalled a value other than 100,000, cases were scaled accordingly, and incidence was calculated (cases/patient-years x 100,000). Meta-analysis was NOT carried out. Incidence was compared to national data found from a study comparing the rates of breast cancer in men and women published in the International Journal of Cancer [10]. SIRs were calculated and used to compare incidence between the two populations on a national level. Further analysis and comparison with different population characteristics was not possible due to the absence of relevant information reported in the studies. P-values and 95 % confidence intervals (95 % CI) were calculated alongside using an online statistical calculator [11].
2.2. Proposed mechanisms
2.2.1. Search strategy
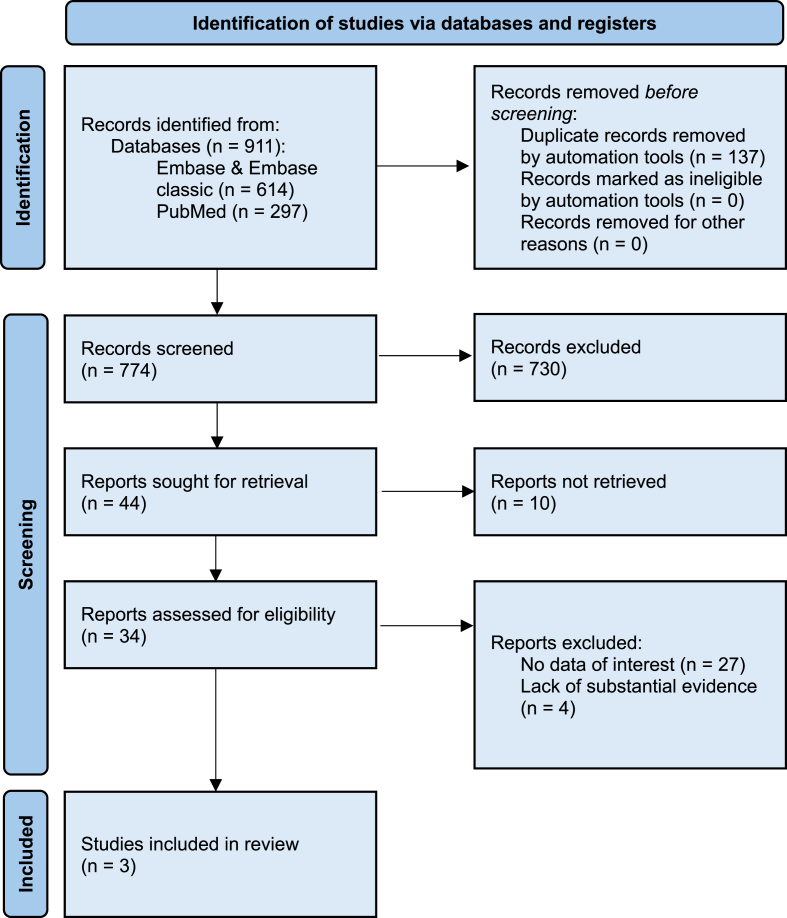
A literature review was conducted looking at proposed mechanism behind MBC oncogenesis in KS patients. To ensure thorough article retrieval, the PRISMA protocol was again utilised to identify articles for inclusion in the review. An outline of the strategy is included as Fig. 2. PubMed/MEDLINE and Embase/Embase Classic were searched using the following search string: (“Klinefelter syndrome” OR “Klinefelter's syndrome” OR “Klinefelter” OR “Klinefelter's” OR “hypogonadism”) AND (“Male breast cancer” OR “Breast cancer”). Hypogonadism was included to identify a broader range of studies of men with a similar hormonal profile to KS, due to the sparsity of mechanistic manuscripts identified in the previous review. Articles were filtered to include those written in English (or where a translated version was available) from the past 100 years. Abstract only articles were included at this stage. No further search filters were applied at this time. The search was conducted between October 2023 and March 2024.
Fig. 2.
Mechanism PRISMA flow diagram. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses.
2.2.2. Review of studies
Initially, 911 papers were identified, with 774 proceeding to screening. Once again, two reviewers (B.C and S.N) individually screened, retrieved and reviewed the manuscripts identified, facilitated by the software EndNote. Papers were excluded for the following reasons: (1) No data of relevance i.e. mechanism was not investigated, (2) Lack of substantial evidence to suggest a mechanistic correlation. Papers were included if they attempted to provide a biological explanation for elevated MBC risk in KS and provided reasonable evidence to support their hypothesis. Subsequently, three papers were included in the report. Study references were also reviewed and provided some genetic and hormonal basis to the proposed hypotheses of the included studies. Disagreements between reviewers were dealt with at the discretion of the supervising author (T.Y).
2.2.3. Data extraction
No formal data analysis was carried out due to the lack of numerical data available. Instead, mechanisms providing the greatest evidence were displayed graphically for clear understanding. Population size was analysed during the article review process to estimate the power of the study – larger populations studied were favoured. Key data was also extracted where available, e.g. Length of testosterone replacement therapy (TRT) treatment, number of MBC diagnoses.
3. Results
3.1. Normal male population
Breast cancer rates in men remain low worldwide in comparison to women – SIR:122 [10]. Registers were analysed across numerous countries worldwide to establish the incidence rates on MBC in men and women. Incidence between countries vary but consistently show rates under 1.00 in 100,000 patient-years (one notable exception being Israel: 1.24 per 100,000). Across Denmark and the United Kingdom, rates of 0.54 and 0.5 were calculated respectively, and later used as comparators in this review.
3.2. Incidence in Klinefelter Syndrome
Three studies were analysed, and their data extracted. MBC incidence was significantly raised compared to the normal male population, both nationally and globally: SIR 18.1 (95 % CI: 13.53 to 24.74), p<0.001. Raw data values were collected and analysed (Table 1) and SIRs were calculated by nation, as well as across all studies and compared globally (Table 2). Breast cancer rates in women still far exceeded men with KS, with rates over seven-fold higher globally – 68.50 per 100,000 woman-years [10].
Table 1.
Raw incidence data.
| Study | Nation | KS patients | Patient-years at risk | MBC cases |
|---|---|---|---|---|
| Swerdlow et al. | United Kingdom | 3518 | 39,574 | 4 |
| Bojesen et al. | Denmark | 832 | 17,101 | 3 |
| Hasle et al. | Denmark | 696 | 23,857 | 0 |
Table 2.
Incidence and SIRs of MBC in KS compared to the non-KS population.
| Study | Incidence∗ (KS) | Incidence∗ (non-KS) | SIR (95 % CI) | P-Value |
|---|---|---|---|---|
| Swerdlow et al. | 10.11 | 0.50 | 20.22 (5.30–55.068) | <0.001 |
| Bojesen et al. | 17.54 | 0.54 | 32.48 (6.50–100.13) | <0.001 |
| Hasle et al. | 0.00 | 0.54 | 0 (N/A) | (N/A) |
| Total | 8.69 | 0.48 (world) | 18.1 (13.53 to 24.74) | < 0.01 |
3.2.1. United Kingdom
A 2005 study [6] analysed 25 UK cytogenetic registers, with 4806 KS patients identified. Of which, 3518 patients were entered into the cohort study. A total of 39,574 patient-years of follow up were included and four patients were subsequently diagnosed with breast cancer (10.11 per 100,000 patient-years). Compared to the national male UK average [10] (0.50 per 100,000 patient-years), incidence is significantly elevated – SIR 20.22 (95 % CI: 5.30 to 55.07), p<0.001. Those with 47, XXY mosaicism were also noted to have a higher incidence still than those without – SIR 33.7 (95 CI:0.90 to 187.70), p = 0.06.
3.2.2. Denmark
An early study [12] analysed the Danish Cytogenetic Register to identify 707 men with KS, of which, 696 patients were entered into the cohort study. A total of 23,857 patient-years of follow up were included and no cases of breast cancer were recorded. A later Danish study [13] investigated 4865 patients (859 with KS), of which, 832 KS patients were included in the cohort study, amounting to 17,101 patient-years of follow up. Three cases of MBC were reported (17.54 per 100,000 patient-years). Both studies were compared to the national Danish male average [10] – SIR 0, p<0.001 and 32.48 (95 % CI: 6.50 to 100.13), p<0.001 respectively.
3.3. Mortality
Data on mortality from MBC in KS was included in one study [6] (Swerdlow et al.) and showed significantly raised mortality compared to the normal male population – Standard Mortality ratio (SMR) 57.8 (95 % CI:18.80 to 135.00), p<0.001. It was also found that those with 47, XXY mosaicism had strikingly high mortality rates, even when compared to normal 47, XXY males – SMR 222.8 (95 % CI: 45.90 to 651.00), p<0.001. The reason for this is unknown although the increase in incidence in this group may correlate with the observed increase in mortality.
3.4. Proposed mechanisms
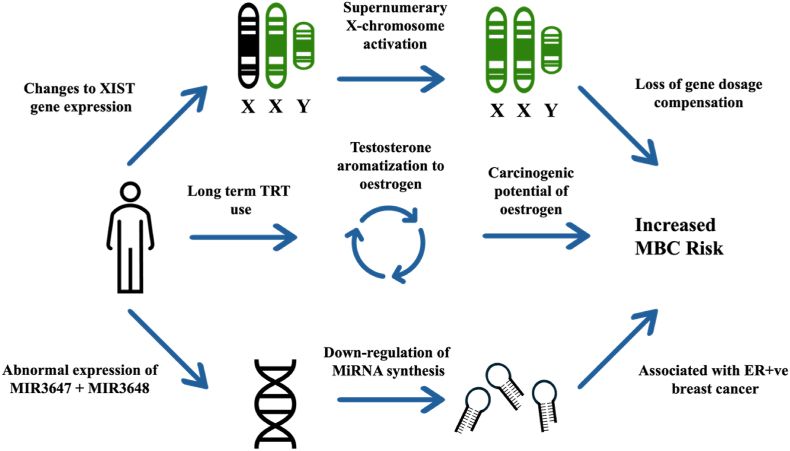
Mechanistic studies into MBC are sparse, particularly those looking concurrently at KS patients. Genetic and hormonal manifestations of KS are likely to play the biggest role in MBC development – details of proposed mechanisms are outlined below (Fig. 3).
Fig. 3.
Proposed mechanisms of MBC development in KS.
3.4.1. Hormonal
Hormonally, androgens are thought to play some role in cancer development. An early 2006 study [14] investigated 45 men with hypergonadotropic hypogonadism treated with 250 mg IM testosterone, every three to four weeks for upwards of five years (some much longer). Two men (5.6 %) were subsequently diagnosed with MBC. Notably, both cases of MBC were in men treated for over ten years and developed ER-positive tumours.
3.4.2. Genetic
Evidence suggests that patients with KS may have a genetic predisposition to developing MBC. Transcriptome analysis of cells from ten patients with KS found dysregulated expression of 73 genetic transcripts compared to controls [15]. Profiling analysis (qRT-PCR) revealed the downregulation of two micro-RNA (miRNA) precursors (MIR3648 and MIR3647), the former being associated with oestrogen receptor (ER)-positive breast cancer [16].
X-chromosome activation is also thought to play a role in oncogenesis. A study [17] demonstrated cancer cells (PKS-1) from a KS patient showed activation of additional X-chromosomes. The FMR-1 gene was used to measure chromosome inactivation. A further 13 female cancer cell lines were investigated and 100 % of breast cancer cells showed the loss of their inactive X-chromosome. It is thought that the activation of normally inactive X-chromosomes may lead to an increased susceptibility for cancer in KS.
4. Discussion
MBC risk in KS has not been fully understood, with evidence being sparse and contradictory. This review demonstrates that KS significantly increases the risk of MBC compared to the standard male population. While the increased risk is clear, the mechanism through which MBC develops in KS is still yet to be fully established, although it is likely to be hormonal and genetic in origin.
MBC incidence was raised by a significant degree in those with KS across both Danish and UK populations [6,12,13]. Data collected across three cohort studies totalling over 5000 patients demonstrated this, with incidence being on the order of an 18-fold increase compared to non-KS males. Compared globally, both nations demonstrated that KS is a strong risk factor for MBC and highlights the importance of further cohort studies to accurately define the current risk. Notably, incidence is still far below that of the average female population [10]. ER-positive MBC seemed a more common subtype of cancer in KS. The exact cause of this is unknown, although MiRNA precursors mentioned in this review were shown to increase ER-positive carcinogenesis. In addition, both recorded cases of MBC linked to long term TRT use were ER-positive, possibly suggesting an androgenic relationship. Further studies may do well to investigate the histological type and invasive nature of these tumours as well as long term patient outcomes.
Allowing for the rarity of KS, the cohort studies discussed were of sufficient size. However, study heterogeneity in their population characteristics limited the strength of conclusions. Factors that are known to influence cancer e.g. smoking, BMI and age [18], were not controlled between studies. While traditionally incidence may be age-adjusted, raw data from these studies was not available, instead only age ranges for the populations studied. The use of TRT was also not controlled in the studies. Despite the study limitations, the risk KS plays in MBC development is clear, and should influence clinical suspicion in those with KS. A more robust study should ensure population characteristics are controlled, or at least explicitly reported on a patient-by-patient basis.
The mechanism behind MBC oncogenesis in KS is not clear, although likely is both androgenic and genetic in nature. Understanding mechanisms of MBC in KS is challenging due to the insufficiency of dedicated studies. Producing powerful mechanistic studies is equally hard - part of the difficulty arises from MBC being exceedingly rare in comparison to female breast cancer, accounting for <1 % of breast cancer diagnoses [19]. Not only do studies require sufficient participants, but they also require sufficient incentive to study. Clearly, incentive diminishes the fewer people effected.
The hypergonadotropic hypogonadal state of KS patients isn't thought to increase cancer risk, rather the use of testosterone replacement therapy (TRT) to counter this is. Medras et al. [14] investigated the relationship between MBC and TRT. While testosterone IM dosage was controlled, treatment time varied from 5 to 26 years. Serum testosterone levels varied between 3 and 5 ng/ml (max 11 ng/ml). It is not known how the effects of TRT on MBC change with length of treatment, but it is reasonable to assume a longer time taking TRT increases the chances of cancer developing. Conversion of testosterone to oestrogen by aromatase is believed to play a crucial role in breast carcinogenesis, with several studies identifying certain forms of oestrogen to be carcinogenic [[20], [21], [22]]. The exact mechanism behind oestrogen-mediated DNA damage is not known. Additionally, these oestrogens may disrupt DNA replication through an upregulation of oestrogen-mediated transcription and undergo transformation to catechol oestrogens believed to cause oxidative damage and proliferation of breast tissue [14].
Genetically, the regulation of MIR3648 and MIR3687 appears to play some role. Next-generation sequencing (NGS) analysis and qRT-PCR were used to measure expression of these miRNAs. Emmadi et al. [16] previously found dysregulation of MIR3647 was positively correlated with ER-positive breast carcinoma. The role of MIR3648 in breast cancer is still not known, although it has been described in solid tumours. Emmadi et al. also states that expression of different miRNAs could also act as prognostic and predictive biomarkers for breast cancer. Biomarkers aside, the role miRNAs play in cancer is still not well understood. Studies looking at the specific expression of miRNAs or their precursors in MBC in KS are needed.
Similarly, gene expression from X-chromosome activation may also be involved. 46, XX females have one active and one inactive X-chromosome (Barr body) [23]. Chromosome inactivation is crucial to ensure genes are expressed appropriately and not overexpressed (gene-dosage compensation) [24]. The presence of the Barr body in KS patients’ cells [25] and XIST gene expression [26] (involved in the inactivation process) point to the usual inactivation of the additional X-chromosome [3]. As reported, abnormal X-chromosome activation was seen in KS derived prostate cancer cells (PKS-1) and subsequently in female derived breast cancer cells [17]. Expression of the XIST gene was found to be lost in PKS-1 cells. It is reasonable to theorise that a similar defect in the XIST gene may be responsible for MBC in KS.
5. Conclusions
In conclusion, evidence persists that KS is a risk factor for MBC and prompt diagnosis should be sought in those presenting with concerning symptoms. Incidence is still far below that of the female population. Reasonable and logical mechanisms have been theorised and it is likely that the underlying mechanism is both hormonal and genetic. Larger and more robust studies are needed to ascertain its true aetiology. Likewise, further cohort studies will continue to help quantify the risk of MBC in KS and may provide guidance on its diagnosis and management.
CRediT authorship contribution statement
Benjamin Cook: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Sasha Nayar: Writing – original draft, Visualization, Methodology, Investigation, Data curation, Conceptualization. Simon Filson: Writing – review & editing, Conceptualization. Tet Yap: Writing – review & editing, Supervision, Methodology, Conceptualization.
References
- 1.Los E., Leslie S.W., Ford G.A. StatPearls Publishing; 2024 Jan. Klinefelter syndrome. [PubMed] [Google Scholar]
- 2.Klinefelter H.F., Reifenstein E.C., Albright F. Syndrome characterized by gynecomastia, aspermatogenesis without A-leydigism, and increased excretion of follicle-stimulating hormone. J Clin Endocrinol Metabol. 1942;2(11):615–627. doi: 10.1210/jcem-2-11-615. [DOI] [Google Scholar]
- 3.Bonomi M., Rochira V., Pasquali D., Balercia G., Jannini E.A., Ferlin A. Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. J Endocrinol Invest. 2017;40(2):123–134. doi: 10.1007/s40618-016-0541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bojesen A., Juul S., Gravholt C.H. Prenatal and postnatal prevalence of klinefelter syndrome: a national registry study. J Clin Endocrinol Metabol. 2003;88(2):622–626. doi: 10.1210/jc.2002-021491. [DOI] [PubMed] [Google Scholar]
- 5.Queremel Milani D.A., Chauhan P.R. StatPearls Publishing; 2024 Jan. Genetics, mosaicism. [PubMed] [Google Scholar]
- 6.Swerdlow A.J., Schoemaker M.J., Higgins C.D., Wright A.F., Jacobs P.A. Cancer incidence and mortality in men with klinefelter syndrome: a cohort study. JNCI: Journal of the National Cancer Institute. 2005;97(16):1204–1210. doi: 10.1093/jnci/dji240. [DOI] [PubMed] [Google Scholar]
- 7.Brinton L.A. Breast cancer risk among patients with Klinefelter syndrome. Acta Paediatr. 2011;100(6):814–818. doi: 10.1111/j.1651-2227.2010.02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinton L.A. Etiologic factors for male breast cancer in the U.S. Veterans Affairs medical Care System database. Breast Cancer Res Treat. 2010;119(1):185–192. doi: 10.1007/s10549-009-0379-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PRISMA flow diagram. www.prisma-statement.org.
- 10.Ly D., Forman D., Ferlay J., Brinton L.A., Cook M.B. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2012;132(8):1918–1926. doi: 10.1002/ijc.27841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Comparison of Two Rates. https://www.medcalc.org/calc/rate_comparison.php.
- 12.Hasle H., Mellemgaard A., Nielsen J., Hansen J. Cancer incidence in men with Klinefelter syndrome. Br J Cancer. 1995;71(2):416–420. doi: 10.1038/bjc.1995.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bojesen A., Juul S., Birkebæk N.H., Gravholt C.H. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metabol. 2006;91(4):1254–1260. doi: 10.1210/jc.2005-0697. [DOI] [PubMed] [Google Scholar]
- 14.Medras M., Filus A., Jozkow P., Winowski J., Sicinska-Werner T., Sicinska Werner T. Breast cancer and long-term hormonal treatment of male hypogonadism. Breast Cancer Res Treat. 2006;96(3):263–265. doi: 10.1007/s10549-005-9074-y. [DOI] [PubMed] [Google Scholar]
- 15.Cimino L., Salemi M., Cannarella R., et al. Decreased miRNA expression in klinefelter syndrome. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-16892-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emmadi R., Canestrari E., Arbieva Z.H., et al. Correlative analysis of miRNA expression and oncotype dx recurrence score in estrogen receptor positive breast carcinomas. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0145346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawakami T., Zhang C., Taniguchi T., et al. Characterization of loss-of-inactive X in Klinefelter syndrome and female-derived cancer cells. Oncogene. 2004;23(36):6163–6169. doi: 10.1038/sj.onc.1207808. [DOI] [PubMed] [Google Scholar]
- 18.Patel A.V., Deubler E., Teras L.R., et al. Key risk factors for the relative and absolute 5‐year risk of cancer to enhance cancer screening and prevention. Cancer. 2022;128(19):3502–3515. doi: 10.1002/cncr.34396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konduri S., Singh M., Bobustuc G., Rovin R., Kassam A. Epidemiology of male breast cancer. Breast. 2020;54:8–14. doi: 10.1016/j.breast.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mobley J.A., Brueggemeier R.W. Estrogen receptor-mediated regulation of oxidative stress and DNA damage in breast cancer. Carcinogenesis. 2003;25(1):3–9. doi: 10.1093/carcin/bgg175. [DOI] [PubMed] [Google Scholar]
- 21.Russo J., Russo I.H. Genotoxicity of steroidal estrogens. Trends Endocrinol Metabol. 2004;15(5):211–214. doi: 10.1016/j.tem.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Turan V.K., Sanchez R.I., Li J.J., et al. The effects of steroidal estrogens in ACI rat mammary carcinogenesis: 17β-estradiol, 2-hydroxyestradiol, 4-hydroxyestradiol, 16α-hydroxyestradiol, and 4-hydroxyestrone. J Endocrinol. 2004;183(1):91–99. doi: 10.1677/joe.1.05802. [DOI] [PubMed] [Google Scholar]
- 23.Anoop U.R., Ramesh V., Balamurali P.D., Nirima O., Premalatha B., Karhikshree V.P. Role of Barr bodies obtained from oral smears in the determination of sex. Indian J Dent Res. 2004;15(1):5–7. [PubMed] [Google Scholar]
- 24.Patrat C., Ouimette J.F., Rougeulle C. X chromosome inactivation in human development. Development. 2020;147(1):dev183095. doi: 10.1242/dev.183095. [DOI] [PubMed] [Google Scholar]
- 25.Shamsuddin A.K., Tang C.K. Barr bodies in testis with Klinefelter syndrome. Urology. 1980;15(1):74–76. doi: 10.1016/0090-4295(80)90548-8. [DOI] [PubMed] [Google Scholar]
- 26.Brown C.J., Ballabio A., Rupert J.L., et al. A gene from the region of the human X inactivation centre is expressed exclusively from the inactive X chromosome. Nature. 1991;349(6304):38–44. doi: 10.1038/349038a0. [DOI] [PubMed] [Google Scholar]