Abstract
Mycobacterium smegmatis is a rapidly growing nontuberculous mycobacterium that is rarely isolated from clinical specimens and is frequently considered to be a contaminant. We conducted a retrospective review of mycobacterial cultures positive for M. smegmatis from 1998 to 2023 at our institution to evaluate the clinical significance of recovering this mycobacterium. Antimicrobial susceptibility patterns were also determined. Twenty-two M. smegmatis isolates were identified from 17 patients, 12 of whom met criteria for clinical chart review. M. smegmatis was deemed a cause of infection in 5/5 isolates from skin or soft tissue, 3/3 from bone, 1/1 from blood, and 0/3 from respiratory specimens. All cases thought to be significant were treated with at least 2 active agents for periods varying from 2 weeks up to 8 months. 18 isolates had antimicrobial susceptibility testing performed and all were susceptible to doxycycline, imipenem, linezolid, moxifloxacin, trimethoprim/sulfamethoxazole, and tobramycin while all isolates were resistant to clarithromycin. When recovered in culture, the presence of M. smegmatis should be correlated with clinical presentation as it may represent a true infection.
Keywords: Rapid growing mycobacterium, Mycobacterium, Joint infections, Antibiotic resistance
1. Introduction
Mycobacterium smegmatis is a rapidly growing, late-pigmenting nontuberculous mycobacterium (NTM) [1]. It was first isolated in 1884 from a syphilitic chancre, hence deriving its name [2]. M. smegmatis is commonly found in soil and environmental samples and thus, it is often not considered a true pathogen. However, it has also been isolated from pulmonary and soft tissue samples where it was determined to cause disease [3]. Additionally, M. smegmatis has been reported alongside other rapidly growing mycobacteria (RGM) as a cause of prosthetic joint infection [4].
M. smegmatis has intrinsic resistance to macrolides, driven by an inducible erm gene as previously described [5]. It has also been reported to be resistant to isoniazid in biofilms [6]. Susceptibility patterns from clinical isolates have not been reported for several decades [3]. In fact, while the most recent guidelines for treatment of M. smegmatis disease are from 2007, treatment options are based on data from a 1988 case series [3], [7]. In this article, we identified the clinical impact of M. smegmatis using isolates recovered at our institution over the past 20 years, describe host factors for infection, and provided updated susceptibility findings using clinical isolates.
2. Methods
A retrospective review of all mycobacterial cultures positive for M. smegmatis from patients at the Mayo Clinic Rochester from 1/1/1998 to 12/1/2023 was conducted. Medical charts from Mayo Clinic patients who were older than 18 years were reviewed to determine the significance of recovery of M. smegmatis. Demographics including age at the time of culture and gender were recorded. Charts were also reviewed for immunosuppressive conditions, defined as uncontrolled HIV, cancer receiving chemotherapy, solid organ transplant, or autoimmune disease requiring immunosuppressive therapy. Other medical comorbidities were noted as well.
The clinical relevance of M. smegmatis isolates was defined by clinical and microbiologic criteria including clinical signs and symptoms of mycobacterial infection, appropriate exclusion of other diagnoses, expert consultation supporting the clinical significance of the M. smegmatis isolate, and either positive culture results for M. smegmatis from at least two separate specimens or biopsy with mycobacterial histopathologic features (granulomatous inflammation or acid-fast positive bacillus (AFB) stain) and a positive culture for M. smegmatis. Two physician authors independently assessed charts for this information (PC and JV). Discordance was discussed and resolved by consensus.
Other data assessed included the total number of mycobacterial cultures obtained, including those positive for M. smegmatis, those positive for other organisms, and those that were negative together with AFB stain results. Surgical procedures, antibiotic therapy, and clinical response were also abstracted.
M. smegmatis was identified from AFB positive cultures using 500 bp 16S rRNA gene Sanger sequencing as described previously [8]. Beginning in 2016, matrix-assisted laser desorption, time-of-flight mass spectrometry (MALDI-TOF MS) was used as the primary method to identify isolates [9]. 500 bp 16S rRNA gene Sanger sequencing continued to be used on those isolates unable to be identified.
Antimicrobial susceptibility testing was performed for isolates using the reference broth microdilution method recommended by Clinical and Laboratory Standards Institute guidelines (CLSI, M24 ED3) and susceptibility patterns were assessed using current CLSI M24S breakpoints [10], [11]. For antimicrobials without breakpoints, the minimal inhibitory concentration was reported. In cases where multiple isolates were obtained from the same patient, MICs from the first isolate were used.
All data from microbiologic reports and chart review were collected and reviewed using REDCap (Research Electronic Data Capture) tools hosted at Mayo Clinic [12], [13]. Data analysis and visualization was performed in Excel (Microsoft Corporation, Redmond, Washington) [14]. Clinical charts were only reviewed if there was a documented research consent. The study was reviewed and approved by an Institutional Review Board of the Mayo Clinic (IRB #23–000626).
3. Results
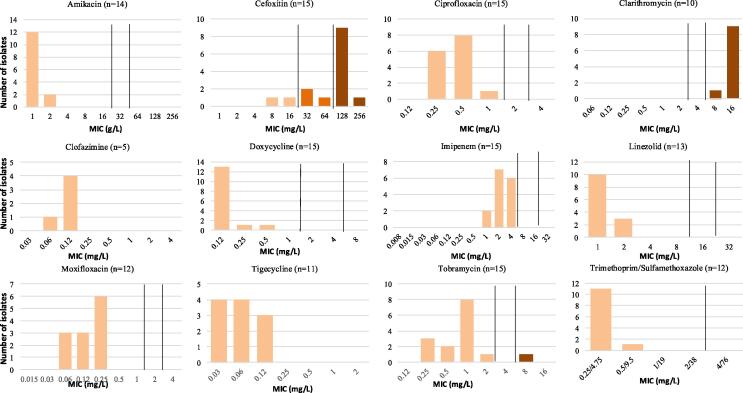
Twenty-two M. smegmatis isolates associated with 17 unique patients were identified from Mayo Clinic patients over the study period. Of these isolates, 14 were from skin or soft tissue (64 %), 3 from bone (14 %), 4 from respiratory tract (18 %), and 1 from blood (5 %). 18 isolates from 15 patients had antimicrobial susceptibility testing performed. Based on CLSI M24S breakpoints, all tested isolates were susceptible to doxycycline, imipenem, linezolid, moxifloxacin, trimethoprim/sulfamethoxazole, and tobramycin (Fig. 1). All isolates were resistant to clarithromycin and most (87 %) were resistant or intermediate susceptibility to cefoxitin.
Fig. 1.
MIC distributions of 22 clinical isolates of M. smegmatis tested by broth microdilution. Clinical breakpoints are indicated with black vertical lines, and isolates are color coded by interpretive categories (tan = susceptible, orange = intermediate, brown = resistant). There are no clinical break points for clofazimine or tigecycline.
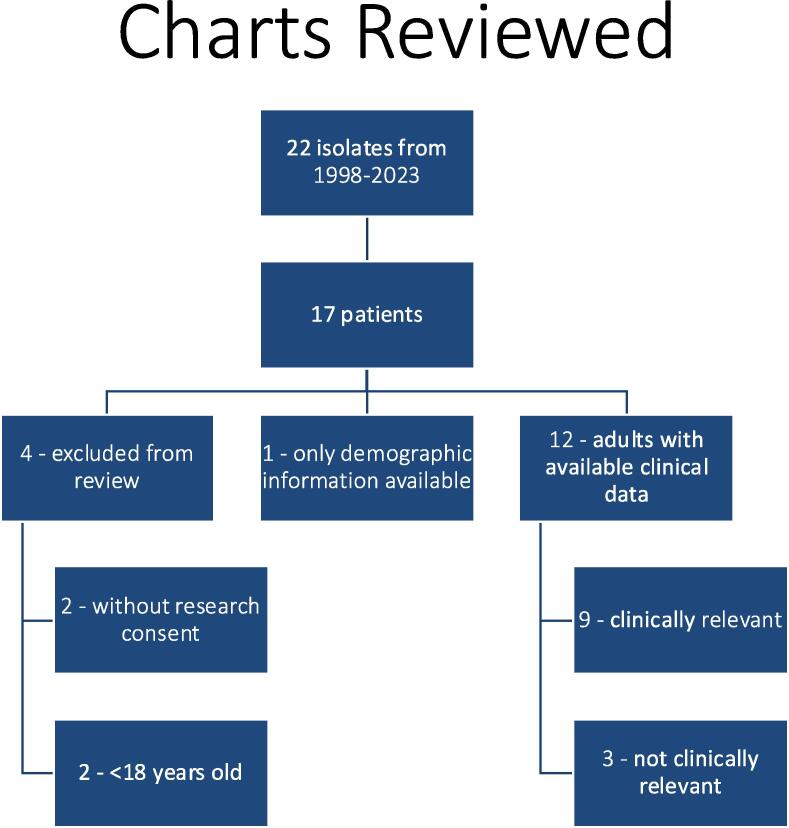
Of the 17 patients identified, 13 met criteria for clinical chart review (Fig. 2). Two patients were younger than 18 years at the time of isolates and another two patients lacked documented research consent to allow chart review. These four patients were excluded from clinical chart review. Of the 13 patients that underwent chart review, the median age was 60 years and 11/13 (85 %) were female. None had any of the defined immunosuppressive conditions, nor were any patients on active treatment with chemotherapy or immunosuppression. One patient was receiving hemodialysis. Other potentially relevant conditions recorded included IgM deficiency, systemic lupus erythematosus (not on treatment), breast cancer (not on active chemotherapy), and dyshidrotic eczema.
Fig. 2.
Identification of cases for chart review.
Of the 13 patients reviewed, one had insufficient documentation at the time of recovery of their isolate to determine clinical significance and thus we can only report demographic and microbiologic information (Table 1). M. smegmatis was considered clinically significant in 9/12 cases (75 %) with sufficient documentation available. The most common type of infection was skin and soft tissue or bone in 8 patients. This included sternum, upper extremity, and lower extremity (Table 2). Of these 8 patients, 6 underwent surgical intervention, including debridement and removal of a breast spacer, sternal debridement in two patients, soft tissue debridement, arthroplasty resection of a knee, and elbow resection with placement of antibiotic spacer. A patient with line-associated infection had their central catheter removed.
Table 1.
Demographics of patients with M. smegmatis infection.
| Characteristics |
| Median age (range) 60 (22–82) |
| Gender (%) |
| Female 11 (85 %) |
| Male 2 (15 %) |
| Source (by isolate) |
| Skin/soft tissue 14 |
| Pulmonary 4 |
| Bone 3 |
| Blood 1 |
Table 2.
Clinical characteristics of M. smegmatis cases.
| Patient # (age/gender) | Clinically relevant (# isolates) | Site of infection (#isolates) | Hardware involved | Surgery? | Antibiotics | Treatment Duration | Clinical outcome | Immuno-suppression |
|---|---|---|---|---|---|---|---|---|
| 1 (75F) | No (1) | Pulmonary | NA | NA | NA | NA | NA | IgM deficiency * |
| 2 (60F) | Inad (1) | Pulmonary | NA | NA | NA | NA | NA | No |
| 3 (64F) | Yes (1) | Breast | Breast Tissue expander | Expander removed | TMP/SMX; doxycycline; moxifloxacin | 3 months | Resolved | SLE * |
| 4 (82F) | Yes (3) | Sternum | Sternal wires | Wire removal, debridement | TMP/SMX; doxycycline; moxifloxacin | 6 months | Resolved | No |
| 5 (54F) | Yes (1) | Right leg | No | No | Amikacin; Ciprofloxacin; minocycline 6 mo delay to treatment |
unknown | unknown | No |
| 6 (56F) | No (1) | Pulmonary | NA | NA | Rifampin, Isoniazid, Pyrazinamide, Ethambutol (RIPE therapy) | 6 months (for presumed TB) | NA | No |
| 7 (59 M) | Yes (1) | Right knee | TKA | 2-stage TKA replacement | Doxycycline and amikacin; ciprofloxacin, TMP/SMX; briefly with ethambutol Change from doxycycline and amikacin due to dizziness and vertigo; ethambutol was cost-prohibitive |
6 weeks | Resolved | No |
| 8 (35F) | Yes (1) | Leg | No | No | doxycycline; TMP/SMX | 3 months | Resolved | No |
| 9 (48F) | Yes (1) | Blood | PICC catheter | PICC removed | cefoxitin, ciprofloxacin, clarithromycin; ciprofloxacin, doxycycline, imipenem Change of therapy in response to susceptibilities |
1 month | Resolved | Breast cancer, LVAD * |
| 10 (79F) | No (1) | Pulmonary − BAL | NA | NA | NA | NA | NA | No |
| 11 (69F) | Yes (1) | Right elbow | Total elbow arthroplasty | Removal, spacer | Imipenem, vancomycin, doxycycline, TMP/SMX 4-drug induction for 6 weeks, 6-month consolidation with doxycycline and TMP/SMX |
6 months | Resolved | No |
| 12 (22F) | Yes (1) | Left thigh | No | Debridement | Imipenem, linezolid, doxycycline; imipenem stopped at discharge |
2 weeks | Resolved | No |
| 13 (70 M) | Yes (1) | Sternum | Sternal wires | Debridement | Imipenem, doxycycline, TMP/SMX | 8 months | Resolved | No |
Inad: inadequate clinical information available to determine impact.
LVAD: Left Ventricular Assist Device.
NA: not applicable.
TMP/SMX: Trimethoprim with Sulfamethoxazole.
* Relevant health history, Not prespecified immunocompromising condition.
Four patients had isolates from respiratory sources. Of these, three were deemed not true infection by treating clinicians. Each case had only one positive culture for M smegmatis. One patient had 9 cultures that were positive for another NTM, and the others had 1–3 negative mycobacterial cultures in addition to the singe culture positive for M. smegmatis. The 4th pulmonary case lacked sufficient information in the chart to determine significance by the authors. M. smegmatis was not isolated from genitourinary tract or central nervous system.
All 9 cases considered clinically significant were treated with at least two active antimicrobial agents as outlined in Table 1. Treatment duration varied, ranging from 2 weeks to 8 months, with 5 patients treated for at least three months and four for less than three months (Table 2). All 9 M. smegmatis cases had clinical resolution. There was no relapse or recurrence of any cases in our review.
4. Discussion
The antimicrobial susceptibility patterns of 18 M. smegmatis isolates and details of illness, treatment, and response to therapy in 12 patients are reported. While this organism has been previously described to cause disease, it is also isolated from the environment, and thus is often considered a commensal organism or contaminant when isolated in culture. Overall, 75 % of isolates represented disease. This is higher than 16 % previously reported for Mycobacterium avium and 63 % of isolates for Mycobacterium intracellulare, indicating the need to consider M. smegmatis a true pathogen in the proper clinical context [15]. In this series, all isolates from skin, soft tissue, and bone were felt to represent true infection, while none of the isolates from respiratory sources were considered significant.
Failure to attribute disease to M. smegmatis may delay treatment and has the potential to prolong hospitalization. While some cases occurred in patients with potentially immunosuppressive conditions, none would be classically defined as severely immunosuppressed.
The most recent M. smegmatis case series by Wallace et al was published in 1988 for RGM submitted to the University of Texas in Tyler from 1978 to 1987 [3]. Twenty-one patients were identified from throughout the US, though primarily Florida and Texas, as well as Australia. They reported 7 cases of disease after cardiac surgery, 6 of which were sternal wound infection. In our series, 2 cases also involved the sternum post-operatively.
Our institution previously reported a series of 8 patients who developed prosthetic joint infection (PJI) due to RGM, from 1969 to 2006 [4]. Only those patients who underwent hardware removal had a resolution of their infection. They identified one case of M. smegmatis, which is also included in our series. We identified an additional PJI case due to M. smegmatis after the previous study period. We report an additional three soft tissue infections, one of which required debridement, and all of which resolved without recurrence.
There are few details on the cases of M smegmatis reported from pulmonary sources by Wallace et al. [3]. It is unclear whether these were considered true infection or if they were isolated with additional organisms. Other reports include infection in a patient with lipoid pneumonia, one with prior gastrectomy, and one patient who was otherwise healthy [3], [16], [17]. In our series, none of the three patients with M. smegmatis isolated from a respiratory source had clear disease. Isolation from numerous patients’ respiratory samples in our series indicates the potential for M. smegmatis to colonize the airway.
The majority of our cases (85 %) were in women. Pulmonary disease with Mycobacterium avium and Mycobacterium intracellulare has been reported in a higher percent of women undergoing mycobacterial culture than men in the past [15]. This phenomenon differs in our cohort, however, as we report mostly skin and musculoskeletal disease. Further, M. avium and M. intracellulare are both slow-growing NTM species. Further study is needed to define what biologic or behavioral factors, if any, pre-dispose women to NTM disease.
All M. smegmatis isolates in our series were susceptible to tetracycline antibiotics, fluoroquinolones, imipenem, and TMP/SMX. All cases were treated with doxycycline or minocycline as part of a combination antimicrobial regimen. The American Thoracic Society recommendations for treatment of NTM disease state that treatment should be based on microbiologic susceptibilities [7], although there are no specific treatment guidelines for M. smegmatis. Based on cases reviewed in this study, we recommend treatment with two oral agents from doxycycline, TMP/SMX, moxifloxacin, or linezolid, and the addition of at least one IV agent, either amikacin or imipenem for serious bone, prosthetic joint, or skin and soft-tissue infections.
Based on the cases reviewed, the optimal treatment duration remains unclear, though it seems clear that treatment should extend beyond clinical resolution of infectious symptoms. Cases treated with 2–6 weeks of “induction” therapy including IV antibiotics, followed by 2–6 months of “consolidation” achieved cure, though so did shorter courses. On review, it appears clinicians opted for longer duration in patients who were older or had potential involvement of surgical hardware by the infection. A reasonable approach for serious infections is intensive therapy involving at least three agents, including at least one IV antibiotic until clinical resolution. This can be followed by a course of two oral antibiotics for which susceptibility of the organism is confirmed. Surgical specialists should be involved to determine the benefit of debridement, particularly in cases where retrievable hardware is present.
While it is conceivable that M. smegmatis could occasionally be a true respiratory pathogen, no cases in our series were consistent with pulmonary NTM disease. In cases where numerous NTMs are isolated, it would likely be appropriate to focus treatment on other more well-established pathogens, such as Mycobacterium abscessus complex or Mycobacterium avium complex. It should be noted that M. smegmatis will likely be macrolide resistant. Our isolates were susceptible to amikacin, thus it may respond to combination therapy containing this agent.
Compared to previous case series, we also report susceptibility to amikacin, doxycycline, and the fluoroquinolones, though our testing involved moxifloxacin rather than ciprofloxacin [3]. We also report susceptibility of all isolates to linezolid and resistance to cefoxitin and clarithromycin.
A limitation of our case series is that clinical cure was determined via chart review. If patients had recurrence or relapse in infection but did not seek care, or sought care at another institution, these recurrences may have been missed. As a single center study, we cannot comment on geographic differences of disease incidence or susceptibility.
In our case series, Mycobacterium smegmatis was a true pathogen when isolated from non-respiratory sites. These cases resolved with treatment, including removal of affected hardware and debridement of deep infection sites. The antimicrobial susceptibility pattern was predictable and consideration of this organism as a pathogen in appropriate situations can prevent delays in treatment.
CRediT authorship contribution statement
Patrick D. Crowley: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. James J. Vaillant: Writing – review & editing, Visualization, Validation, Investigation, Formal analysis, Data curation, Conceptualization. Joshua D. Shirley: Writing – review & editing, Investigation, Data curation. Nancy L. Wengenack: Writing – review & editing, Visualization, Supervision, Investigation, Data curation. Mary Jo Kasten: Writing – review & editing, Writing – original draft, Supervision, Methodology, Investigation, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jctube.2024.100489.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Brown-Elliott B.A., Philley J.V. Rapidly growing mycobacteria. Microbiol Spectr. 2017;5:1. doi: 10.1128/microbiolspec.TNMI7-0027-2016. [DOI] [PubMed] [Google Scholar]
- 2.Lustgarten S. The bacillus of syphilis. Lancet. 1885;125(3214):609–610. doi: 10.1016/S0140-6736(02)17741-5. [DOI] [Google Scholar]
- 3.Wallace R.J., Nash D.R., Tsukamura M., Blacklock Z.M., Silcox V.A. Human disease due to Mycobacterium smegmatis. J Infect Dis. 1988;158(1):52–59. doi: 10.1093/infdis/158.1.52. [DOI] [PubMed] [Google Scholar]
- 4.Eid A.J., Berbari E.F., Sia I.G., Wengenack N.L., Osmon D.R., Razonable R.R. Prosthetic joint infection due to rapidly growing mycobacteria: report of 8 cases and review of the literature. Clin Infect Dis. 2007;45(6):687–694. doi: 10.1086/520982. [DOI] [PubMed] [Google Scholar]
- 5.Nash K.A. Intrinsic macrolide resistance in Mycobacterium smegmatis is conferred by a novel erm gene, erm(38) Antimicrob Agents Chemother. 2003;47(10):3053–3060. doi: 10.1128/AAC.47.10.3053-3060.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teng R., Dick T. Isoniazid resistance of exponentially growing Mycobacterium smegmatis biofilm culture. FEMS Microbiol Lett. 2003;227(2):171–174. doi: 10.1016/S0378-1097(03)00584-6. [DOI] [PubMed] [Google Scholar]
- 7.Griffith D.E., Aksamit T., Brown-Elliott B.A., et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 8.Hall L., Doerr K.A., Wohlfiel S.L., Roberts G.D. Evaluation of the MicroSeq system for identification of mycobacteria by 16S ribosomal DNA sequencing and its integration into a routine clinical mycobacteriology laboratory. J Clin Microbiol. 2003;41(4):1447–1453. doi: 10.1128/JCM.41.4.1447-1453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckwalter S.P., Olson S.L., Connelly B.J., et al. Evaluation of Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry for Identification of Mycobacterium species, Nocardia species, and Other Aerobic Actinomycetes. J Clin Microbiol. 2016;54(2):376–384. doi: 10.1128/JCM.02128-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CLSI. Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes. 3rd ed. CLSI standard M24. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. [PubMed]
- 11.CLSI. Performance Standards for Susceptibility Testing of Mycobacteria, Nocardia spp., and Other Aerobic Actinomycetes. 2nd edition. CLSI supplement M24S. Clinical and Laboratory Standards Institute; 2023. [PubMed]
- 12.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Microsoft Corporation. (2018). Microsoft Excel. Retrieved from https://office.microsoft.com/excel.
- 15.Han X.Y., Tarrand J.J., Infante R., Jacobson K.L., Truong M. Clinical Significance and Epidemiologic Analyses of Mycobacterium avium and Mycobacterium intracellulare among Patients without AIDS. J Clin Microbiol. 2005;43(9):4407–4412. doi: 10.1128/JCM.43.9.4407-4412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Driks M., Weinhold F., Cokingtin Q. Pneumonia caused by Mycobacterium smegmatis in a patient with a previous gastrectomy. BMJ Case Rep. 2011;2011:3281. doi: 10.1136/bcr.08.2010.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ergan B., Coplu L., Alp A., Artvinli M. Mycobacterium smegmatis pneumonia. Respirology. 2004;9(2):283–285. doi: 10.1111/j.1440-1843.2004.00570.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.