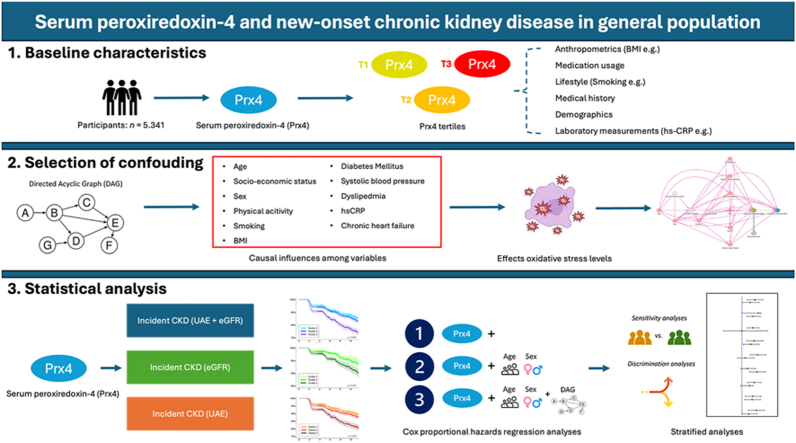
Abstract
Background
Chronic Kidney Disease (CKD), is often detected late due to its asymptomatic nature in the early stage of the disease. Overproduction of reactive oxygen species contributes to various pathological processes through oxidative stress (OS), impacting on cellular structures and functions with previous studies suggesting a link between OS and CKD progression. This study investigated the association between serum peroxiredoxin-4 (Prx4), a biomarker of oxidative stress, and the development of CKD in the general population.
Methods
This study featured data from the Prevention of REnal and Vascular ENd-stage Disease (PREVEND) cohort, involving 5341 participants without CKD at baseline who underwent extensive prospective health evaluations. Serum Prx4 levels were quantified using an immunoluminometric assay. The primary outcome was new-onset CKD as defined by the composite of urinary albumin excretion (UAE) > 30 mg/24-h, an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2, or both.
Results
Baseline median Prx4 level was 0.65 [interquartile range (IQR): 0.42–1.04] U/L, median eGFR was 98 [IQR: 87–108] mL/min/1.73 m2, and median UAE was 8.1 [IQR: 6.0–12.1] mg/L. During a median follow-up of 10.4 [IQR: 6.3–11.4] years, 867 (16.2 %) patients developed new-onset CKD. Higher Prx4 levels were significantly associated with an increased risk of CKD (hazard ratio (HR) per doubling: 1.29 [95 % confidence interval (CI): 1.21–1.37], p < 0.001), also after adjustment for risk factors including sex, smoking status, systolic blood pressure, high-sensitive C-reactive protein, chronic heart failure, diabetes mellitus and dyslipidemia (HR per doubling: 1.16 [1.06–1.24], p < 0.001). Sensitivity analyses confirmed the robustness of these findings.
Conclusions
This study supports the hypothesis that systemic oxidative stress, reflected by higher serum Prx4 levels, is significantly associated with the risk of developing CKD in the general population. These findings suggest that Prx4 could be a valuable biomarker for early risk stratification and prevention strategies in CKD management.
Keywords: Oxidative stress, Chronic kidney disease, Peroxiredoxin-4, Prx4, CKD biomarker
Graphical abstract
Highlights
-
•
Prx4 is an circulating antioxidant biomarker and is reflective of oxidative stress.
-
•
Circulating Prx4 levels are associated with increased risk of developing CKD.
-
•
Prx4 could serve as a biomarker for early risk stratification of CKD management.
1. Introduction
Chronic Kidney Disease (CKD) is a global health challenge with profound implications for individuals and healthcare systems [1]. Defined by a progressive loss of kidney function over time, CKD is usually diagnosed through declining glomerular filtration rates (eGFR) and signs of kidney damage [2]. Its stages range from mild dysfunction to complete kidney failure, necessitating dialysis or transplantation. Patients with progressive loss of kidney function remain asymptomatic most of the time, presenting complications typically associated with kidney dysfunction only in more advanced stages [1]. Given its insidious onset and progression, early detection and prevention of CKD are crucial, underscoring the urgent need for effective prognostic biomarkers.
Reactive oxygen species (ROS), unstable and reactive molecules that are natural byproducts of various cellular processes, including mitochondrial aerobic respiration and peroxisome activity, have functions as regulators of cellular signaling and immune responses [3]. However, large quantities of ROS can be produced in specific circumstances, which can extensively damage cellular structures leading to inflammation and tissue destruction [4]. This process is also referred to as oxidative stress (OS): an imbalance between oxidants and antioxidants, favoring oxidants and causing disruptions in redox signaling and molecular damage [5]. Prior studies established a correlation between oxidative stress and an increased risk or worse disease outcome of various conditions including neurodegenerative diseases, cardiovascular diseases (CVD), inflammatory bowel diseases (IBD), and several types of cancers [[6], [7], [8], [9]]. The role of oxidative stress in the development of chronic kidney disease (CKD) is well-established [10]. However, the role of oxidative stress in disease onset has been relatively underexplored.
Living organisms have developed a variety of both enzymatic and non-enzymatic antioxidants, such as glutathione, thioredoxin, vitamin C, free thiols, catalase, and peroxiredoxins, and some of these biomarkers can be used to measure systemic oxidative stress [3,11]. Peroxiredoxins are antioxidant proteins that actively break down endogenously generated peroxide radicals in a thiol-dependent manner [12]. To date, six isoforms have been identified, but only peroxiredoxin-4 (Prx4) is detectable in the circulation [13]. Generally, increased peroxiredoxin levels are representative of higher oxidative stress burden, serving as a compensatory response [14]. Previous studies have shown that elevated serum Prx4 levels are linked to the development of nephropathy in diabetic patients and cardiovascular disease (CVD) in the general population, including those with microalbuminuria [[15], [16], [17]]. Additionally, higher serum Prx4 levels are associated with cardiovascular and all-cause mortality in individuals with type 2 diabetes [18].
While prior research indicates the potential use of serum peroxiredoxin-4 as a biomarker for incident cardiometabolic diseases, its potential utility as a prognostic indicator for CKD has not been investigated. In this study, we hypothesized that serum concentrations of peroxiredoxin-4, representing a biomarker of systemic oxidative stress, are associated with the development of CKD in the general population. Therefore, we aimed to explore the association between serum Prx4 levels and the incidence of newly developed CKD.
2. Materials and methods
2.1. Study population and study design
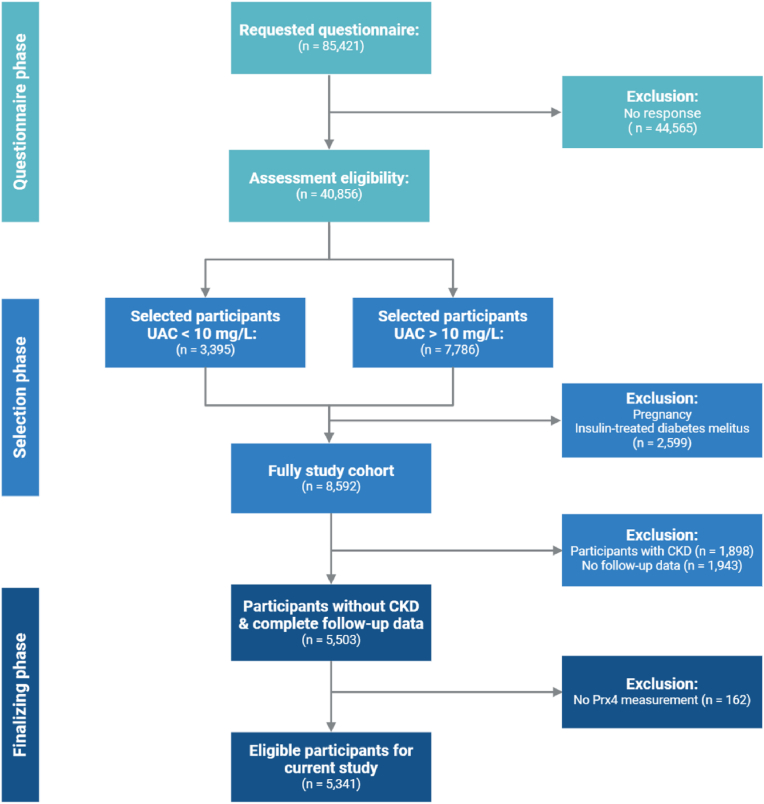
This research incorporated individuals without CKD at baseline, drawn from the Prevention of REnal and Vascular ENd-stage Disease (PREVEND) study. PREVEND is a prospective, population-based cohort study executed in the Netherlands, aimed at examining the link between urinary albumin excretion and the development of kidney and cardiovascular diseases [19,20]. The study features data on many variables that are relevant to cardiovascular and kidney disease collected from residents of the city of Groningen aged 28–75 years (Fig. 1) (see Supplementary Materials for additional information).
Fig. 1.
CONSORT Flowchart of participant selection within the PREVEND cohort study.
2.2. Data collection
This study involved participants completing a comprehensive questionnaire designed to gather information on their demographic details, medical history (including cardiovascular and kidney diseases, and diabetes mellitus), lifestyle practices (such as smoking habits), and medication intake. As a known predictor for CKD, data concerning Race was collected via self-reporting. Anthropometric measurements were taken, blood pressure was measured and blood and urine were taken for several analyses (e.g. high-sensitivity C-reactive protein (hs-CRP), cystatin C, and creatinine) (see Supplementary Materials for detailed descriptions of these measurements). Missing values for each variable were recorded and documented accordingly (see Supplementary Materials).
2.3. Study outcomes and definitions
The study's follow-up period concluded on January 1, 2011. We estimated GFR with the combined creatinine cystatin C-based Chronic Kidney Disease Epidemiology (CKD-EPI) Collaboration equation from 2012, taking into account age, sex, and race [21]. The primary outcome for this study was CKD as a composite outcome of: 1. Urinary albumin excretion (UAE) > 30 mg/24-h; 2. an estimated GFR <60 mL/min/1.73 m2. Secondary outcomes were based on UAE of estimated GFR separately. At the study's onset, dyslipidemia was identified through any of the following criteria: serum cholesterol levels exceeding 6.5 mmol/L, the utilization of lipid-lowering medications, or serum HDL-cholesterol levels <0.9 mmol/L. Participants were considered to have diabetes if they met one or more of the following conditions: a fasting glucose concentration of 7.0 mmol/l or higher, a non-fasting glucose level of ≥11.1 mmol/l, or if they were taking antidiabetic medication following the guidelines established by the American Diabetes Association (ADA) [22]. Prx4 levels were determined using a validated immunoluminometric sandwich assay [23] (see Supplementary Materials for details).
2.4. Statistical analysis
Data are presented as median [interquartile range] or n (%). Data distributions were visually inspected through histograms and normal probability plots (Q-Q plots) for assessment of normality. For analysis, serum Prx4 levels were 2log-transformed, enabling the reporting of hazard ratios (HRs) in relation to each doubling of Prx4 levels. In addition, three groups (tertiles T1, T2 and T3) were created based on Prx4 levels across the entire cohort. Differences in baseline characteristics across these tertiles were examined using one-way analysis of variance (ANOVA) or Kruskal-Wallis tests for continuous variables, and Chi-square tests for categorical variables. To evaluate survival distributions among tertiles of serum Prx4 concentrations, Kaplan-Meier survival analysis was conducted, and comparisons were drawn using log-rank tests. Survival duration was measured from the baseline to the date of the last follow-up visit, occurrence of CKD, death, or until January 1st, 2011, which marked the end of study follow-up. More specifically, participants attended two outpatient visits separated by three weeks during a second (2001–2003), third (2003–2006), fourth (2006–2008), and fifth (2009–2011) round of study investigations. At all these visits, eGFR and urine albumin was measured. Univariable and multivariable Cox proportional hazards regression analyses were performed to explore the relationship between serum Prx4 levels and the incidence of CKD. This was done by using four models based on a priori selected confounders (see section 2.7).
Findings from Cox regression analyses were presented as hazard ratios (HRs) along with their respective 95 % confidence intervals (95%CIs). Stratified analyses were then conducted to investigate the association of serum Prx4 levels and new-onset CKD across various subgroups. Possible interactions with particular variables were examined by fitting models including interaction terms, where a Pinteraction value of <0.05 was considered to indicate significant effect modification. Cutoffs for variables were obtained by splitting the cohort based on the median of the event group. Multiple sensitivity analyses were performed by running the fully adjusted model (model 4) after; adjusting CKD definition at baseline (eGFR from <60 to <65 mL/min/1.73 m2 and UAE from >30 to >25 mg/24-h), excluding the first and 99th percentile of serum Prx4 outcomes, excluding participants with a short duration of follow-up (<2 years), and adding baseline eGFR to the fully adjusted model.
Data analysis was performed using R version 4.2.2 (Vienna, Austria) and data visualization was performed using RStudio v2023.12.1–402.
2.5. Ethical considerations
This study was approved by the Institutional Review Board (IRB) (full name in Dutch: “Medisch Ethische Toetsingscommissie”, IRB no. 01/139) of the University Medical Centre Groningen. All participants provided written informed consent, and the study was conducted in accordance with the principles of the Declaration of Helsinki (2013).
2.6. Selection of confounding factors: directed acyclic graph (DAG)
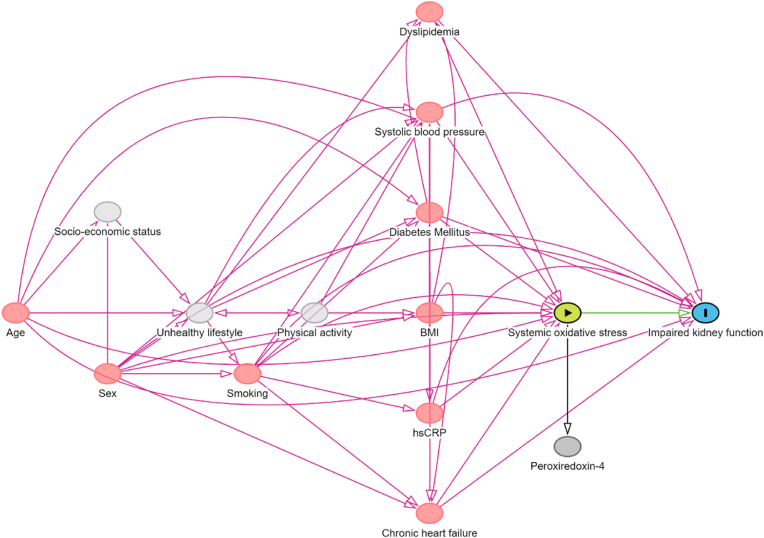
Aligning with established guidelines for confounder adjustment, we utilized causal models, referred to directed acyclic graphs (DAGs), and their underpinning theoretical concepts, to select confounding variables for estimating the outcome of interest [[24], [25], [26]]. The DAG visually represents hypothesized causal mechanisms underlying the variables in question (Fig. 2). Within these graphs, arrows represent the assumed direct causal influences among variables, whereas the lack of an arrow between any two variables implies no such direct effect. Our focus was on examining the association between serum Prx4 levels, representative of systemic oxidative stress, and new-onset CKD, with a particular emphasis on identifying and conditioning on a precise group of confounders to obtain a statistically unconfounded estimate of the effect [[27], [28], [29], [30], [31]]. Using this approach, the following variables were selected for incorporating into statistical analysis: age, sex, smoking status, systolic blood pressure, chronic heart failure, diabetes mellitus, total cholesterol and use of lipid-lowering drugs (dyslipidemia), and high-sensitive C-reactive protein.
Fig. 2.
A Directed Acyclic Graph (DAG) illustrates the hypothesized causal relationships that underlie the connection between systemic oxidative stress (indicated by serum Prx4 levels) and the likelihood of developing chronic kidney disease (CKD) in the general population. Following the DAG, a distinct set of confounding factors were accounted for through conditioning to ensure the derivation of an unconfounded effect estimate.
3. Results
3.1. Baseline characteristics of the study population
In total, 5341 participants were included in the study, of which baseline demographic, clinical and laboratory characteristics are presented in Table 1. Age, body mass index (BMI), systolic blood pressure (SBP), hs-CRP, cystatin C, percentage of participants with chronic heart failure (CHF) and diabetes mellitus were all significantly higher in the highest tertile (T3) of serum Prx4 levels compared with the lower two tertiles (all p < 0.001). Conversely, percentage of current smokers was significantly lower in T3 compared to the lower tertiles (p < 0.001). Median eGFR was significantly lower in T3 compared to T2 and T1 (96 vs 98 vs 100 mL/min/1.73 m2, p < 0 0.001), with the reverse pattern visible in median UAE with T3 having the highest UAE (8.4 vs 8.1 vs 7.9 mg/24-h, p < 0.001).
Table 1.
Baseline demographic, clinical characteristics of the study population and study outcomes, stratified by tertiles of serum Prx4 levels.
| Total | T1 | T2 | T3 | P-value | |
|---|---|---|---|---|---|
| <0.50 U/L | 0.50–0.87 U/L | >0.87 U/L | |||
| n=1781 | n=1780 | n=1780 | |||
| Prx4 (U/L) | 0.65 [0.42–1.04] | 0.37 [0.37–0.42] | 0.65 [0.57–0.75] | 1.30 [1.04–1.81] | <0.001 |
| Prx4 (U/L) (Females) | 0.64 [0.41–1.01] | 0.37 [0.37–0.42] | 0.65 [0.57–0.74] | 1.29 [1.03–1.78] | <0.001 |
| Prx4 (U/L) (Males) | 0.67 [0.43–1.07] | 0.37 [0.37–0.42] | 0.66 [0.57–0.75] | 1.32 [1.04–1.84] | <0.001 |
| Demographics | |||||
| Age (years) | 47.0 [38.8–56.4] | 45.6 [37.7–53.8] | 47.2 [38.7–55.9] | 48.3 [40.2–60.2] | <0.001 |
| Female, n (%) | 2820 (53) | 969 (54) | 945 (53) | 906 (51) | 0.106 |
| Race, n (%) | <0.001 | ||||
| Asian, n (%) | 104 (2) | 16 (0.9) | 29 (2) | 59 (3) | |
| Black, n (%) | 53 (10) | 6 (0.3) | 15 (0.8) | 32 (2) | |
| Other, n (%) | 61 (1) | 21 (1) | 19 (1) | 21 (1) | |
| White, n (%) | 5086 (96) | 1723 (98) | 1707 (96) | 1656 (94) | |
| Anthropometrics | |||||
| BMI (kg/m2) | 25.2 [23.0–27.8] | 24.6 [22.6–27.0] | 25.3 [22.9–27.8] | 25.8 [23.3–28.7] | <0.001 |
| Waist circumference (cm) | 86.5 [77.5–95.0] | 84.5 [77.0–92.5] | 86.5 [77.0–95.0] | 89.0 [80.0–98.0] | <0.001 |
| Risk factors | |||||
| SBP (mmHg) | 123 [112–136] | 121 [111–133] | 123 [113–136] | 126 [114–140] | <0.001 |
| DBP (mmHg) | 72 [66–79] | 71 [65–77] | 72 [66–79] | 74 [68–80] | <0.001 |
| Heart rate (bpm) | 68 [62–75] | 68 [61–74] | 68 [62–74] | 69 [63–76] | <0.001 |
| Smoking | <0.001 | ||||
| Never, n (%) | 1691 (32) | 523 (30) | 587 (33) | 581 (33) | |
| Current, n (%) | 1687 (32) | 662 (37) | 541 (31) | 491 (28) | |
| Former (<1 year), n (%) | 200 (4) | 79 (5) | 58 (3) | 63 (4) | |
| Former (>1 year), n (%) | 1735 (33) | 511 (29) | 585 (33) | 639 (36) | |
| History of CHF, n (%) | 146 (3) | 23 (1) | 46 (3) | 77 (4) | <0.001 |
| History of diabetes, n (%) | 77 (1) | 10 (0.6) | 25 (1) | 42 (2) | <0.001 |
| Medication use | |||||
| Antihypertensive drugs, n (%) | 532 (12) | 122 (9) | 174 (12) | 236 (16) | <0.001 |
| Lipid-lowering drugs, n (%) | 162 (4) | 46 (3) | 46 (3) | 70 (5) | 0.055 |
| Antidiabetic drugs, n (%) | 50 (0.9) | 9 (0.5) | 15 (0.9) | 26 (2) | 0.010 |
| Laboratory measurements | |||||
| Total cholesterol (mmol/L) | 5.50 [4.80–6.30] | 5.50 [4.80–6.30] | 5.40 [4.76–6.20] | 5.55 [4.80–6.30] | 0.019 |
| hs-CRP (mg/L) | 1.07 [0.49–2.56] | 0.84 [0.39–1.84] | 1.05 [0.48–2.45] | 1.42 [0.63–3.38] | <0.001 |
| Cystatin C (mg/L) | 0.86 [0.77–0.95] | 0.85 [0.76–0.93] | 0.85 [0.77–0.94] | 0.88 [0.79–0.98] | <0.001 |
| Creatinine (mmol/L) | 69.6 [61.0–79.3] | 69.6 [61.0–79.3] | 69.6 [61.0–79.3] | 69.6 [61.0–78.3] | 0.866 |
| eGFR (mL/min/1.73 m2) | 98 [87–108] | 100 [89–109] | 98 [88–103] | 96 [84–107] | <0.001 |
| UAE (mg/L) | 8.1 [6.0–12.1] | 7.9 [5.8–11.4] | 8.1 [6.0–12.0] | 8.4 [6.1–13.0] | 0.001 |
| Urine creatinine (mmol/24-h) | 8.2 [6.0–11.1] | 8.1 [5.9–10.9] | 8.2 [6.0–11.2] | 8.2 [6.1–11.1] | 0.428 |
| Urine urea (mmol/24-h) | 223 [165–308] | 223 [165–307] | 225 [162–309] | 224 [166–310] | 0.866 |
| Study outcomes | |||||
| CKD (eGFR <60 mL/min/1.73 m2), n (%) | 289 (5) | 65 (4) | 86 (5) | 138 (8) | <0.001 |
| CKD (UAE >30 mg/24-h), n (%) | 667 (13) | 166 (9) | 199 (11) | 302 (17) | <0.001 |
| CKD (combination), n (%) | 867 (16) | 218 (12) | 257 (14) | 389 (22) | <0.001 |
Data are presented as median [interquartile range] or n (%). Abbreviations: Prx4, peroxiredoxin 4; U/L, units per liter; T1, tertile 1; T2, tertile 2; T3, tertile 3; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; bpm, beats per minute; CHF, congestive heart failure; hs-CRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate; UAE, urinaryalbumin excretion; CKD, chronic kidney disease.
3.2. Serum Prx4 levels and the risk of new-onset CKD
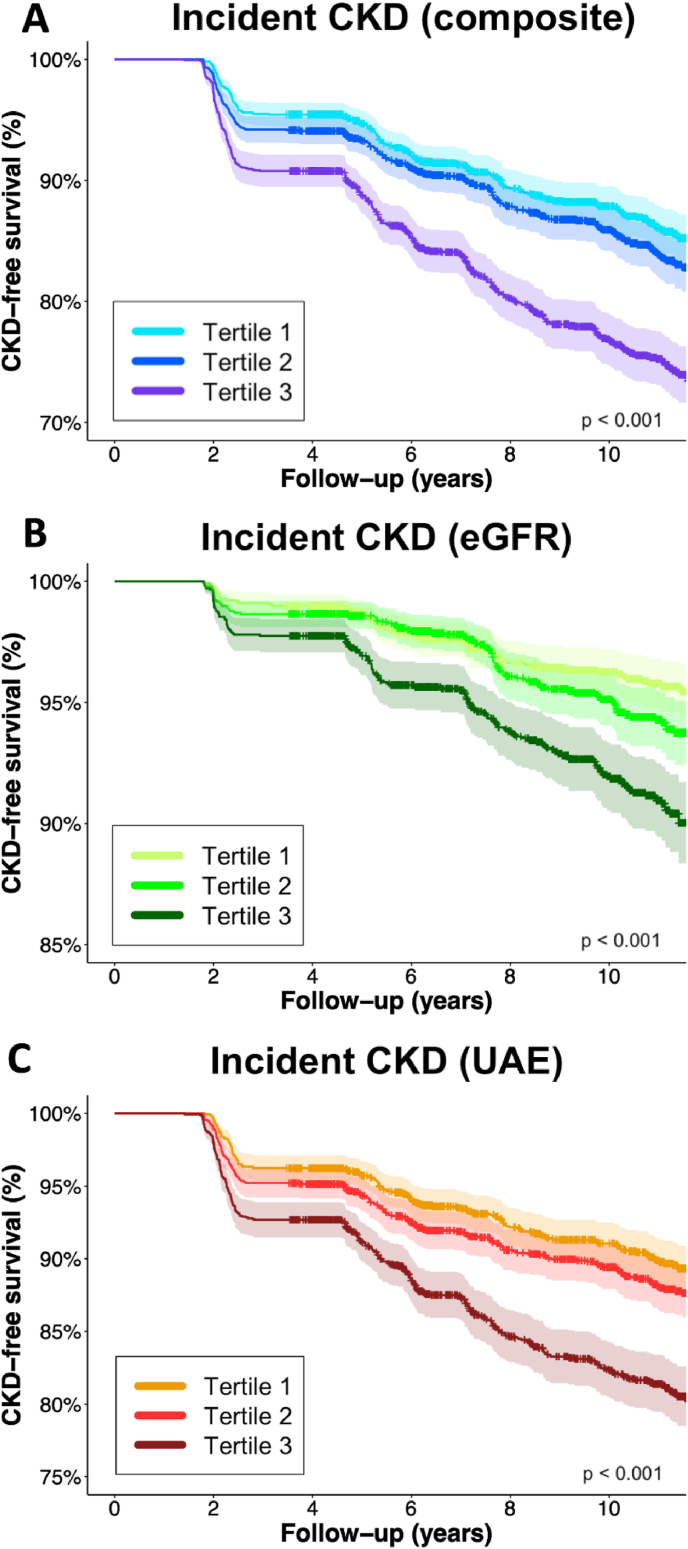
During a median follow-up of 10.4 [IQR: 6.3–11.4] years, 867 (16.2 %) patients developed new-onset CKD. While handling serum Prx4 as continuous (2log-transformed) variable, a significant association between serum Prx4 and the risk of new-onset CKD was found for all three outcomes (HR per doubling: 1.29 [95 % CI: 1.21–1.37] (composite outcome) vs. 1.33 [95 % CI: 1.20–1.47] (eGFR) vs 1.29 [95 % CI: 1.20–1.38] (UAE), all p < 0.001) (Table 2). When adjusting for confounding variables (Model 4), the association between serum Prx4 levels and the composite and UAE outcome for CKD were still significant (HR per doubling: 1.16 [95 % CI: 1.08–1.24], p < 0.001) (HR per doubling: 1.19 [95 % CI: 1.10–1.29], p < 0.001). The fully adjusted model (Model 4) for new-onset CKD based on solely eGFR, however, did not show a significant association (HR per doubling: 1.10 [0.97–1.25], p = 0.127). Across all measures, CKD-free survival rates decline over time (Fig. 3). The survival curves show that the steepest decline is observed in the composite measure, where the CKD-free survival rate drops to around 70 % at 10 years for the third tertile group.
Table 2.
Serum Prx4 levels and the risk of new-onset CKD in the general population.
| HR per doubling of serum Prx4 | T1 |
T2 |
T3 |
|
|---|---|---|---|---|
| <0.50 U/L | 0.50–0.87 U/L | >0.87 U/L | ||
| CKD (composite outcome) (n=867) | ||||
| Model 1 | 1.29 [1.21–1.37], p < 0.001 | 1.00 (reference) | 1.19 [0.99–1.43], p = 0.060 | 1.94 [1.64–2.29], p < 0.001 |
| Model 2 | 1.20 [1.12–1.28], p < 0.001 | 1.00 (reference) | 1.10 [0.92–1.32], p = 0.037 | 1.60 [1.36–1.90], p < 0.001 |
| Model 3 | 1.16 [1.09–1.24], p < 0.001 | 1.00 (reference) | 1.09 [0.91–1.31], p = 0.353 | 1.48 [1.25–1.76], p < 0.001 |
| Model 4 | 1.16 [1.08–1.24], p < 0.001 | 1.00 (reference) | 1.09 [0.91–1.32], p = 0.355 | 1.46 [1.22–1.74], p < 0.001 |
| CKD (eGFR <60 mL/min/1.73 m2) (n=229) | ||||
| Model 1 | 1.33 [1.20–1.47], p < 0.001 | 1.00 (reference) | 1.33 [0.96–1.84], p = 0.082 | 2.21 [1.64–2.96], p < 0.001 |
| Model 2 | 1.19 [1.06–1.33], p=0.003 | 1.00 (reference) | 1.18 [0.85–1.63], p = 0.317 | 1.56 [1.16–2.11], p=0.003 |
| Model 3 | 1.12 [1.00–1.27], p = 0.057 | 1.00 (reference) | 1.17 [0.84–1.61], p = 0.352 | 1.39 [1.02–1.88], p=0.036 |
| Model 4 | 1.10 [0.97–1.25], p = 0.127 | 1.00 (reference) | 1.12 [0.81–1.56], p = 0.500 | 1.33 [0.97–1.81], p = 0.077 |
| CKD (UAE >30 mg/24-h) (n=667) | ||||
| Model 1 | 1.29 [1.20–1.38], p < 0.001 | 1.00 (reference) | 1.21 [0.99–1.49], p = 0.068 | 1.95 [1.61–2.35], p < 0.001 |
| Model 2 | 1.21 [1.13–1.31], p < 0.001 | 1.00 (reference) | 1.13 [0.92–1.39], p = 0.235 | 1.67 [1.38–2.03], p < 0.001 |
| Model 3 | 1.20 [1.11–1.30], p < 0.001 | 1.00 (reference) | 1.12 [0.91–1.39], p = 0.269 | 1.60 [1.32–1.95], p < 0.001 |
| Model 4 | 1.19 [1.10–1.29], p < 0.001 | 1.00 (reference) | 1.24 [0.91–1.40], p = 0.268 | 1.58 [1.29–1.93], p < 0.001 |
Model 1, crude model. Model 2, model 1 with adjustment for age and sex. Model 3, model 2 with adjustment for current smoking, systolic blood pressure, chronic heart failure, diabetes, total cholesterol, use of lipid-lowering drugs. Model 4, model 3 with adjustment for high-sensitive C-reactive protein. Bold P-values indicate statistical significance. Abbreviations: HR, hazard ratio; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; UAE, urinary albumin excretion.
Fig. 3.
Kaplan-Meier survival curves for tertiles of Prx4, representing CKD-free survival based on the composite outcome of incident CKD (eGFR, UAE or both (A), as well as the individual determinants of eGFR (<60 mL/min/1.73 m2) (B) and UAE (>30 mg/24-h) (C). Highest rates of incident CKD were observed in the highest tertile (T3) of Prx4 (log-rank tests, all p < 0.001).
When tertiles of serum Prx4 levels were used instead of serum Prx4 levels as continuous variable, the highest tertile (T3) of Prx4 levels was consistently significantly associated with the risk of new-onset CKD as defined through the composite outcome (HR per doubling 1.94 (95%CI: [1.64–2.29], p < 0.001) (Table 2), also after adjustment for confounding variables as described (Model 4) (HR per doubling:1.46 (95%CI: [1.22–1.74], p < 0.001). When these analyses were performed for the individual components of the composite outcome (eGFR <60 ml/min per 1.73 m2 and UAE >30 mg/24h), a stronger association was found for UAE as individual outcome (Model 4) (HR per doubling:1.58 (95%CI: [1.29–1.94], p < 0.001) compared to eGFR decline as individual outcome (Model 4) (HR per doubling: 1.33 (95%CI: [0.97–1.74], p = 0.077).
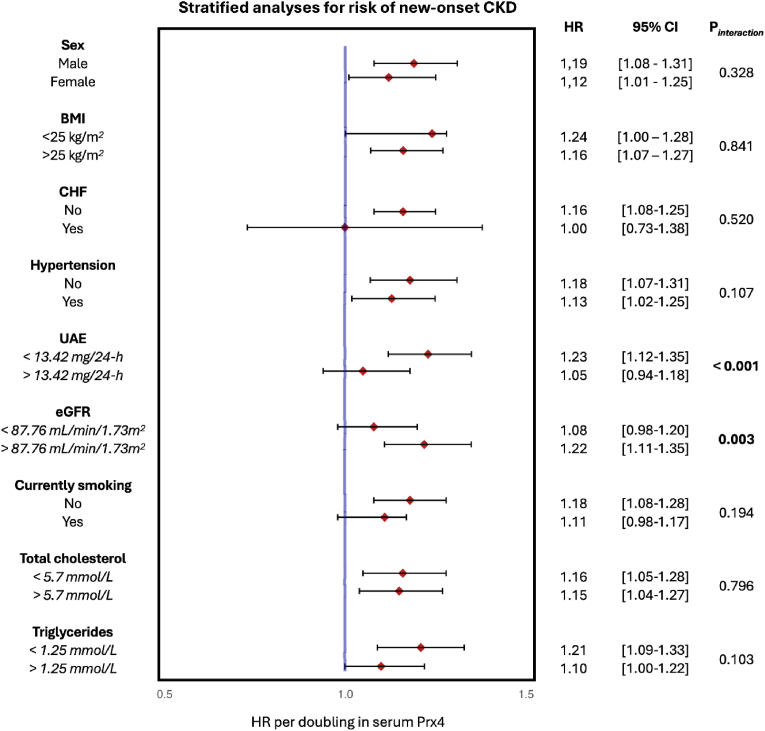
3.3. Stratified analyses
Following these observations, we conducted additional investigations to determine the link between Prx4 serum levels and the incidence of CKD within different subgroups (see Fig. 4). Two variables, baseline eGFR (p = 0.003) and baseline UAE (p < 0.001), showed significant interactions for the association between serum Prx4 and the risk of new-onset CKD. Participants with a relatively lower eGFR (<87.76 mL/min/1.73 m2) showed lower HR per doubling (HR: 1.08) compared to participants with a higher eGFR (>87.76 mL/min/1.73 m2) (HR: 1.22). Participants with a lower UAE (<13.42 mg/24-h) demonstrated an overall higher HR per doubling (HR: 1.23) compared with participants with a higher UAE (>13.42 mg/24-h) (HR: 1.05).
Fig. 4.
Stratified analyses for the association between serum Prx4 levels and the risk of incident CKD across various subgroups. Hazard ratios (HRs) are shown with corresponding 95 % confidence intervals (95%CIs).
3.4. Sensitivity analyses
Four different sensitivity analyses were performed to evaluate the robustness of the observed association (Table 3). Firstly, the cutoff values for CKD at baseline were adjusted by changing the definition of baseline CKD to eGFR <65 mL/min/1.73 m2 (<60 mL/min/1.73 m2 initially) and UAE to >25 mg/24-h (>30 mg/24-h initially). This did not materially change the association, showing a significant association and comparable HR (HR per doubling 1.14, 95%CI: [1.06–1.22], p < 0.001). Secondly, the first and 99th percentile of serum Prx4 values were excluded, which again did not substantially change the association (HR per doubling 1.17, 95%CI: [1.08–1.27, p < 0.001). Thirdly, all participants with a follow-up less than two years were excluded, resulting once again in a similar HR, and maintained statistical significance (HR per doubling 1.14, 95%CI: [1.05–1.22], p < 0.001). Lastly, baseline eGFR was added to the Cox regression model of Model 4, resulting in a similar HR per doubling (1.14, 95%CI: [1.06–1.22, p < 0.001).
Table 3.
Sensitivity analysis of serum Prx4 levels and the risk of new-onset CKD in the general population.
| HR per doubling of serum Prx4 (Model 4, composite outcome) | |
|---|---|
| Cutoff adjustments for CKD | 1.14 [1.06–1.22], p < 0.001 |
| Exclusion of participants within the first and 99th percentile | 1.17 [1.08–1.27]. p < 0.001 |
| Exclusion of participants with <2 years follow-up time | 1.14 [1.06–1.22], p < 0.001 |
| Addition of baseline eGFR to the Cox regression model | 1.14 [1.06–1.22], p < 0.001 |
Cutoff adjustments for CKD were eGFR <65 mL/min/1.73 m2 & UAE to >25 mg/24-h. Bold P-values indicate statistical significance. Abbreviations: HR, hazard ratio; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate.
4. Discussion
This study provides support to the hypothesis that systemic oxidative stress is associated with the development of CKD in the general population, by demonstrating a robust association between serum Prx4 levels and the risk of new-onset CKD. The observation that higher Prx4 values corresponded to an increased risk of CKD, irrespective of traditional risk factors such as age, sex, and other comorbidities, highlights the potential of serum Prx4 levels as a novel biomarker for early CKD risk stratification. Further, when we additionally adjusted for baseline eGFR in our models, the association between serum Prx4 levels and incident CKD remained statistically significant, affirming the robustness of our findings in associating Prx4 levels with CKD risk. Moreover, stratified analyses revealed that UAE (>13.42 mg/24-h) and eGFR (<87.76 mL/min/1.73 m2) (Pinteraction <0.001, Pinteraction = 0.003, respectively) were the only variables with a significant difference in HR across subgroups. This observation suggests that the role of serum Prx4 as a biomarker for new-onset chronic kidney disease (CKD) may be less pronounced in individuals already exhibiting signs of kidney function decline. The performed sensitivity analyses also consistently demonstrated a robust association with the risk of CKD progression. Collectively, these findings indicate that oxidative stress may play an important role in the development of CKD.
One plausible mechanism pertaining to the observed association between serum Prx4 levels and the risk of developing CKD involves endothelial dysfunction, which is a well-documented consequence of oxidative stress, and impairs renal blood flow regulation and filtration [[32], [33], [34]]. Furthermore, higher oxidative stress and an impaired renal blood flow can cause a hypoxia and low-grade inflammation, which exacerbating oxidative stress and kidney injury even more [[33], [34], [35]]. Prior studies on peroxiredoxins and kidney diseases, including those in the context of diabetes, provide a foundation for understanding the role of oxidative stress in kidney pathology [36,37]. Oxidative stress triggers mitochondrial destabilization, resulting in reduced cellular energy production and increased cell death through apoptosis, which can lead to loss of renal tubular epithelial cells [37]. By drawing parallels with these studies, our findings may suggest a potential role for Prx4 in signaling early oxidative damage within the kidneys and the connection with oxidative stress.
Prx4 is a key player in the body's antioxidant defense system, serving as a biomarker of oxidative stress by neutralizing peroxides and protecting cells from oxidative damage [12,13,15]. While our study focused on Prx4 concentrations, it is important to note that we do not distinguish between the oxidized and reduced forms of Prx4, which is a limitation given the significance of the redox state in Prx4 activity. Prx4 undergoes redox-dependent conformational changes that influence its enzymatic activity, as described in previous studies [38,39]. Reduced Prx4 plays a role in scavenging ROS by forming disulfide bonds between cysteine residues, while the oxidized form may lose its ROS-neutralizing capabilities due to overoxidation [39]. Understanding these distinct redox states is critical because the reduced and oxidized forms of Prx4 contribute differently to cellular protection against oxidative stress. Similarly, the distinction between free thiols (FTs) and total thiols (reduced plus oxidized) is crucial when evaluating the thiol redox metabolome [40]. Systemic free thiols primarily reflect the antioxidant capacity of the extracellular space, while total thiols would provide a broader view, including oxidized forms, which indicate oxidative stress burden and which is referred to as the ‘redox reserve’ [40]. Both thiol states offer valuable insight into the redox balance, and incorporating measurements of reduced and oxidized forms of Prx4 or thiols could provide a more detailed understanding of the redox landscape in chronic kidney disease (CKD). While these measurements can be challenging due to sample handling requirements, they are meaningful for assessing enzyme activity and redox status [40].
In related studies, higher circulating Prx4 levels have been associated with an increased risk of developing nephropathy in patients with type 2 diabetes and with end-stage kidney disease [15,17]. The results of our study underscore those findings, with serum Prx4 fulfilling its role as a potential biomarker for detecting early kidney damage through oxidative stress. However, like other biomarkers such as uric acid, homocysteine and FTs, the use of a single biomarker has limitations in accurately diagnosing disease and clarifying pathophysiological mechanisms [11,22,41,42]. The human redox system encompasses various complex and intertwined mechanisms, including the oxidative stress response, redox signaling, and metabolic balance, which are integral to cellular homeostasis [43]. To effectively map the redox landscape in diseases such as CKD, combining individual biomarkers that represent different components of these mechanisms could be a promising alternative. When comparing Prx4 to other redox biomarkers tested for CKD, it is also important to consider the specificity and sensitivity of these markers in reflecting oxidative stress and their relationship with kidney damage. For example, FT have also shown associations with the development of CKD, but represent another level of the redox balance, and provide an indication of the presence of antioxidants that can neutralize ROS [11,40]. FTs, representing free sulfhydryl groups (R–SH), provide a read-out of the systemic in vivo redox status and are considered reflective of the body's ability to combat oxidative stress. These FTs, largely harbored by blood proteins such as albumin, reflect not only the antioxidant capacity, but also play a central role in the dynamic regulation of the extracellular redox environment as transducers of redox exchange reactions [44,45]. While both Prx4 and FTs may be considered indicative of systemic oxidative stress, Prx4 specifically reflects the oxidative burden that has reached a level requiring enzymatic neutralization. In contrast, FTs are deemed to more generally reflect the antioxidant capacity, and are essential in counteracting overproduction of reactive species, thereby indicating a more favorable redox status when present in high concentrations. Combining these two biomarkers, and preferably complemented by others, would provide a more comprehensive characterization of the redox system alterations involved in CKD development. To achieve this, high-throughput measurement platforms such as mass spectrometry-based techniques should be considered to map the entire redox metabolome. This approach would allow for the simultaneous quantification and identification of multiple redox-sensitive molecules, providing a broader coverage of the redox state and the potential for yielding more accurate biomarkers for disease progression [40].
One of the main strengths of this study includes its embedding in a large, prospective cohort from the PREVEND study. By using this well-characterized dataset, our statistical models could be complemented with known risk factors of CKD, allowing a comprehensively adjusted analysis while preserving sufficient statistical power. Further, by conducting additional stratified- and sensitivity analyses, we have validated the consistency and robustness of the association between serum Prx4 levels and CKD risk. Even after correcting for baseline eGFR, the association between serum Prx4 levels and incident CKD did not drastically change. Likewise, when excluding participants who had less than two years of follow-up, the association between serum Prx4 levels and the risk of new-onset CD was sustained, reducing the likelihood of reverse causation. Interestingly, in this dataset, smoking did not appear to correlate with higher Prx4 levels. In a previous study linking FTs to new-onset CKD, current smokers had significantly increased FT levels compared to non-smokers [35]. This might suggest that smoking may lead to an overcompensation of extracellular antioxidant capacity. However, these findings contrast with another study where Prx4 was increased in patients with lung cancer, suggesting that the relationship between smoking and Prx4 may be more complex or influenced by other factors [46]. The integration of serum Prx4 measurement into clinical practice could be a valuable addition to the management of CKD, shifting the focus toward prevention and early intervention. Prx4 levels in our cohort align closely with another Dutch cohort studying cardiovascular mortality in type 2 diabetes mellitus (median 0.79 U/L; IQR 0.53–1.25 U/L) but are significantly lower than levels reported in studies involving sepsis and SIRS, which is expected given the pathophysiological differences and the healthy nature of our cohort [23,47,48]. However, there are several study limitations that need consideration. First, the observational nature of the study does not allow to infer causality with regard to the association between serum Prx4 levels and CKD development. Although causality is very likely based on our finding, we would not conclude that elevated Prx4 is a direct contributor to kidney damage. We theorise, based on the function of Prx4 as an antioxidant protein that actively break down endogenously generated peroxide radicals in a thiol-dependent manner, it is part of an oxidative stress defense pathway that influences possible kidney damage [12,14]. Secondly, the most significant differences in HR in the tertile analysis were found between T1 and T3, suggesting that only the highest thirds of serum Prx4 levels could predict CKD incidence. Although this is not a pure limitation by itself, further studies also need to be conducted to determine specific cutoff values to strike a balance between sensitivity and specificity. Lastly, the PREVEND cohort study encompasses predominantly Caucasian participants living in the northern parts of the Netherlands, which may limit the external generalizability of our findings to ethnically and geographically distinct populations. This aspect warrants cautious interpretation of our results, since CKD prevalence and risk factors may significantly vary across different ethnicities and geographies [49,50].
Our study lays the foundation for future longitudinal and interventional studies aimed at further elucidating the role of oxidative stress in kidney disease and exploring Prx4-targeted strategies for CKD detection, prevention, and management. Prx4 possibly has the potential to detect CKD in an early stage of the disease, wherein kidney function is still preserved and treatment could be started to prevent further deterioration. In addition, CKD might be prevented through management of hypertension, which has been linked to and is at least partially mediated by oxidative stress [51]. Moreover, interventional studies looking at the impact of antioxidant therapy on serum Prx4 levels and CKD risk could provide insight into potential therapeutic strategies. These strategies could also involve lifestyle- and dietary interventions [52]. Prx4 is involved in cellular antioxidant systems and provides a direct measure of peroxide activity, which other markers may not capture so specifically. This specificity makes Prx4 an excellent candidate for inclusion in a broader panel of biomarkers that assess various aspects of redox biology, potentially yielding a more comprehensive understanding of the oxidative state and its impact on renal health [12,13].
In conclusion, this study contributes to the emerging evidence of the role of oxidative stress, here reflected by serum Prx4 levels, in the development of CKD. While promising, these findings call for further investigation to fully understand the utility of serum Prx4 as a biomarker associated with CKD and its potential in guiding preventive and therapeutic interventions.
CRediT authorship contribution statement
Sem Geertsema: Writing – review & editing, Writing – original draft, Visualization, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Paul Geertsema: Writing – review & editing, Writing – original draft, Validation, Software, Methodology, Investigation, Formal analysis, Conceptualization. Lyanne M. Kieneker: Writing – review & editing, Validation, Methodology, Investigation, Data curation. Amaal E. Abdulle: Writing – review & editing, Software, Methodology, Conceptualization. Sacha la Bastide-van Gemert: Writing – review & editing, Validation, Software, Conceptualization. Stephan J.L. Bakker: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. Robin P.F. Dullaart: Writing – review & editing, Validation, Conceptualization. Gerard Dijkstra: Writing – review & editing, Validation, Supervision, Conceptualization. Ron T. Gansevoort: Writing – review & editing, Validation, Supervision, Resources, Project administration, Methodology, Funding acquisition, Data curation, Conceptualization. Klaas Nico Faber: Writing – review & editing, Validation, Supervision, Conceptualization. Harry van Goor: Writing – review & editing, Validation, Supervision, Project administration, Methodology, Investigation, Conceptualization. Arno R. Bourgonje: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Methodology, Investigation, Data curation, Conceptualization.
Data sharing statement
The datasets utilized and analyzed in the present study are accessible from the corresponding author upon reasonable request.
Funding
The PREVEND study was supported by the Dutch Kidney Foundation (Nierstichting) (grant no. E.013). The funders were not involved in the design of the study, data collection and analysis, preparation of the manuscript, or decision to publish.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
All authors would like to express their gratitude towards all participants of the PREVEND study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2024.103408.
Abbreviations (in order of appearance)
- CKD
- Chronic Kidney Disease
- eGFR
- estimated Glomerular Filtration Rate
- ROS
- Reactive Oxygen Species
- OS
- Oxidative Stress
- CVD
- cardiovascular disease
- IBD
- Inflammatory Bowel Disease
- Prx4
- Peroxiredoxin 4
- PREVEND
- Prevention of REnal and Vascular ENd-stage Disease study
- UAC
- Urinary Albumin Concentration
- UAE
- urinary 24-h albumin excretion
- UMCG
- University Medical Center Groningen
- hs-CRP
- high-sensitivity C-Reactive Protein
- CKD-EPI
- Chronic Kidney Disease Epidemiology Collaboration (a formula used to estimate GFR)
- HRs
- Hazard Ratios
- ANOVA
- Analysis of Variance
- 95%CIs
−95 % Confidence Intervals
- DAGs
- Directed Acyclic Graphs
- BMI
- Body Mass Index
- CHF
- Congestive Heart Failure
- SBP
systolic blood pressure
- DBP
diastolic blood pressure
- BPM
- beats per minutes
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.Ammirati A.L. Chronic kidney disease. Rev. Assoc. Med. Bras. 2020;66(1):3–9. doi: 10.1590/1806-9282.66.S1.3. [DOI] [PubMed] [Google Scholar]
- 2.Drawz P., Rhaman M. American College of Physicians; 2015. In the Clinic ® Chronic Kindey Disease. [Google Scholar]
- 3.Thapa P., Ding N., Hao Y., Alshahrani A., Jiang H., Wei Q. Essential roles of peroxiredoxin IV in inflammation and cancer. Molecules. 2022;27(19):6513. doi: 10.3390/molecules27196513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sies H., Jones D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020;21(7):363–383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 5.Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teleanu D.M., Niculescu A.G., Lungu I.I., Radu C.I., Vladâcenco O., Roza E., et al. An overview of oxidative stress, neuroinflammation and neurodegenerative diseases. Int. J. Mol. Sci. 2022;23(11):5938. doi: 10.3390/ijms23115938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steven S., Frenis K., Oelze M., Kalinovic S., Kuntic M., Jimenez M.T.B., et al. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/7092151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geertsema S., Bourgonje A.R., Fagundes R.R., Gacesa R., Weersma R.K., van Goor H., et al. The NRF2/Keap1 pathway as a therapeutic target in inflammatory bowel disease. Trends Mol. Med. 2023;29(10):830–842. doi: 10.1016/j.molmed.2023.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Jelic M.D., Mandic A.D., Maricic S.M., Srdjenovic B.U. Oxidative stress and its role in cancer. J. Cancer Res. Therapeut. 2021;17(1):22–28. doi: 10.4103/jcrt.JCRT_862_16. [DOI] [PubMed] [Google Scholar]
- 10.Dounousi E., Papavasiliou E., Makedou A., Ioannou K., Katopodis K.P., Tselepis A., et al. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. 2006;48(5):752–760. doi: 10.1053/j.ajkd.2006.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Bourgonje A.R., Gabriëls R.Y., De Borst M.H., Bulthuis M.L.C., Faber K.N., Van Goor H., et al. Serum free thiols are superior to fecal calprotectin in reflecting endoscopic disease activity in inflammatory bowel disease. Antioxidants. 2019;8(9):351. doi: 10.3390/antiox8090351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wood Z.A., Schröder E., Harris J.R., Poole L.B. Structure, mechanism and regulation of peroxiredoxins. 2003. http://tibs.trends.com Available from: [DOI] [PubMed]
- 13.Okado-Matsumoto A., Matsumoto A., Fujii J., Taniguchi N. Peroxiredoxin IV is a secretable protein with heparin-binding properties under reduced conditions 1. J. Biochem. 2000;127(3):493–501. doi: 10.1093/oxfordjournals.jbchem.a022632. [DOI] [PubMed] [Google Scholar]
- 14.Rabilloud T., Heller M., Gasnier F., Luche S., Rey C., Aebersold R., et al. Proteomics analysis of cellular response to oxidative stress. Evidence for in vivo overoxidation of peroxiredoxins at their active site. J. Biol. Chem. 2002;277(22):19396–19401. doi: 10.1074/jbc.M106585200. [DOI] [PubMed] [Google Scholar]
- 15.Abbasi A., Corpeleijn E., Gansevoort R.T., Gans R.O.B., Struck J., Schulte J., et al. Circulating peroxiredoxin 4 and type 2 diabetes risk: the prevention of renal and vascular endstage disease (PREVEND) study. Diabetologia. 2014;57(9):1842–1849. doi: 10.1007/s00125-014-3278-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbasi A., Corpeleijn E., Postmus D., Gansevoort R.T., de Jong P.E., Gans R.O.B., et al. Peroxiredoxin 4, a novel circulating biomarker for oxidative stress and the risk of incident cardiovascular disease and all-cause mortality. J. Am. Heart Assoc. 2012;1(5) doi: 10.1161/JAHA.112.002956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bourgonje A.R., van Goor H., Bakker S.J.L., Hillebrands J.L., Bilo H.J.G., Dullaart R.P.F., et al. Serum peroxiredoxin-4, a biomarker of oxidative stress, is associated with the development of nephropathy in patients with type 2 diabetes (Zodiac-65) Free Radic. Biol. Med. 2024;20:186–190. doi: 10.1016/j.freeradbiomed.2023.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Gerrits E.G., Alkhalaf A., Landman G.W.D., Van Hateren K.J.J., Groenier K.H., Struck J., et al. Serum peroxiredoxin 4: a marker of oxidative stress associated with mortality in type 2 diabetes (ZODIAC-28) PLoS One. 2014;9(2) doi: 10.1371/journal.pone.0089719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hillege H.L., Janssen W.M.T., Bak A.A.A., Diercks G.F.H., Grobbee D.E., Crijns H.J.G.M., et al. Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J. Intern. Med. 2001;249(6):519–526. doi: 10.1046/j.1365-2796.2001.00833.x. [DOI] [PubMed] [Google Scholar]
- 20.Bourgonje A.R., Abdulle A.E., Bourgonje M.F., Binnenmars S.H., Gordijn S.J., Bulthuis M.L.C., et al. Serum free sulfhydryl status associates with new-onset chronic kidney disease in the general population. Redox Biol. 2021;48 doi: 10.1016/j.redox.2021.102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inker L.A., Schmid C.H., Tighiouart H., Eckfeldt J.H., Feldman H.I., Greene T., et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. N. Engl. J. Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbasi A., Corpeleijn E., Gansevoort R.T., Gans R.O.B., Hillege H.L., Stolk R.P., et al. Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: the PREVEND study. J. Clin. Endocrinol. Metab. 2013;98(8) doi: 10.1210/jc.2013-1680. [DOI] [PubMed] [Google Scholar]
- 23.Schulte J., Struck J., Bergmann A., Köhrle J. Immunoluminometric assay for quantification of peroxiredoxin 4 in human serum. Clin. Chim. Acta. 2010;411(17–18):1258–1263. doi: 10.1016/j.cca.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 24.Pearl J. Casusality: models, reasoning and inference. Econom. Theor. 2003;19(4):675–685. [Google Scholar]
- 25.La Bastide-Van Gemert S., van den Heuvel E. Exploring causal hypotheses: breaking with long-standing research traditions. Dev. Med. Child Neurol. 2013;55(11):975–976. doi: 10.1111/dmcn.12269. [DOI] [PubMed] [Google Scholar]
- 26.Lederer D.J., Bell S.C., Branson R.D., Chalmers J.D., Marshall R., Maslove D.M., et al. Control of confounding and reporting of results in causal inference studies. Ann. Am. Thorac. Soc. 2019;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 27.Singh-Manoux A., Shipley M.J., Bell J.A., Canonico M., Elbaz A., Kivimaki M. Association between inflammatory biomarkers and all-cause, cardiovascular and cancer-related mortality. CMAJ (Can. Med. Assoc. J.) 2017;189(10):384–390. doi: 10.1503/cmaj.160313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wonisch W., Falk A., Sundl I., Winklhofer-Roob B.M., Lindschinger M. Oxidative stress increases continuously with BMI and age with unfavourable profiles in males. Aging Male. 2012;15(3):159–165. doi: 10.3109/13685538.2012.669436. [DOI] [PubMed] [Google Scholar]
- 29.Pencina M.J., D'Agostino R.B., Larson M.G., Massaro J.M., Vasan R.S. Predicting the 30-year risk of cardiovascular disease: the framingham heart study. Circulation. 2009;119(24):3078–3084. doi: 10.1161/CIRCULATIONAHA.108.816694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzoulaki I., Elliott P., Kontis V., Ezzati M. Worldwide exposures to cardiovascular risk factors and associated health effects: current knowledge and data gaps. Circulation. 2016;133(23):2314–2333. doi: 10.1161/CIRCULATIONAHA.115.008718. [DOI] [PubMed] [Google Scholar]
- 31.Münzel T., Gori T., Bruno R.M., Taddei S. Is oxidative stress a therapeutic target in cardiovascular disease? Eur. Heart J. 2010;31(22):2741–2748. doi: 10.1093/eurheartj/ehq396. European Heart Journal. 2010. pp. 2741–2748. [DOI] [PubMed] [Google Scholar]
- 32.Kao M.P.C., Ang D.S.C., Pall A., Struthers A.D. Oxidative stress in renal dysfunction: mechanisms, clinical sequelae and therapeutic options. J. Hum. Hypertens. 2010;24(1):1–8. doi: 10.1038/jhh.2009.70. [DOI] [PubMed] [Google Scholar]
- 33.Bourgonje A.R., Bourgonje M.F., la Bastide-van Gemert S., Nilsen T., Hidden C., Gansevoort R.T., et al. A prospective study of the association between plasma calprotectin levels and new-onset CKD in the general population. Kidney Int. Rep. 2024;9(5):1265–1275. doi: 10.1016/j.ekir.2024.02.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bourgonje AR, Abdulle AE, Bourgonje MF, Kieneker LM, la Bastide-van Gemert S, Gordijn SJ, et al. Plasma neutrophil gelatinase-associated lipocalin associates with new-onset chronic kidney disease in the general population. Biomolecules. 1;13(2); 338. doi: 10.3390/biom13020338. [DOI] [PMC free article] [PubMed]
- 35.Bourgonje A.R., Abdulle A.E., Bourgonje M.F., Binnenmars S.H., Gordijn S.J., Bulthuis M.L.C., et al. Serum free sulfhydryl status associates with new-onset chronic kidney disease in the general population. Redox Biol. 2021;48 doi: 10.1016/j.redox.2021.102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B.C., Lan H.Y., Lv L.L. Adv. Exp. Med. Biol. 2019;1165 http://www.springer.com/series/5584 [Google Scholar]
- 37.Small D.M., Bennett N.C., Roy S., Gabrielli B.G., Johnson D.W., Gobe G.C. Oxidative stress and cell senescence combine to cause maximal renal tubular epithelial cell dysfunction and loss in an in vitro model of kidney disease. Nephron Exp. Nephrol. 2013;122(3–4):123–130. doi: 10.1159/000350726. [DOI] [PubMed] [Google Scholar]
- 38.Okado-Matsumoto A., Matsumota A., Fujii J., Taniguchi N. Peroxiredoxin IV is a secreatable protein with heparin-binding properties under reduced conditions. J. Biochem. 2000;127(3):493–501. doi: 10.1093/oxfordjournals.jbchem.a022632. [DOI] [PubMed] [Google Scholar]
- 39.Wang X., Wang L., Wang X., Sun F., Wang C.C. Structural insights into the peroxidase activity and inactivation of human peroxiredoxin 4. Biochem. J. 2012;441(1):113–118. doi: 10.1042/BJ20110380. [DOI] [PubMed] [Google Scholar]
- 40.Sutton T.R., Minnion M., Barbarino F., Koster G., Fernandez B.O., Cumpstey A.F., et al. A robust and versatile mass spectrometry platform for comprehensive assessment of the thiol redox metabolome. Redox Biol. 2018;16:359–380. doi: 10.1016/j.redox.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y.N., Ma S.X., Chen Y.Y., Chen L., Liu B.L., Liu Q.Q., et al. Chronic kidney disease: biomarker diagnosis to therapeutic targets. Clin. Chim. Acta. 2019;499:54–63. doi: 10.1016/j.cca.2019.08.030. [DOI] [PubMed] [Google Scholar]
- 42.Krata N., Foroncewicz B., Zagożdżon R., Moszczuk B., Zielenkiewicz M., Pączek L., et al. Peroxiredoxins as markers of oxidative stress in IgA nephropathy, membranous nephropathy and lupus nephritis. Arch. Immunol. Ther. Exp. 2021;70(1):3. doi: 10.1007/s00005-021-00638-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourgonje A.R., Kloska D., Grochot-Przęczek A., Feelisch M., Cuadrado A., van Goor H. Personalized redox medicine in inflammatory bowel diseases: an emerging role for HIF-1α and NRF2 as therapeutic targets. Redox Biol. 2023;60 doi: 10.1016/j.redox.2023.102603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koning A.M., Meijers W.C., Pasch A., Leuvenink H.G.D., Frenay A.R.S., Dekker M.M., et al. Serum free thiols in chronic heart failure. Pharmacol. Res. 2016;111:452–458. doi: 10.1016/j.phrs.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 45.Cortese-Krott M.M., Koning A., Kuhnle G.G.C., Nagy P., Bianco C.L., Pasch A., et al. The reactive species interactome: evolutionary emergence, biological significance, and opportunities for redox metabolomics and personalized medicine. Antioxidants Redox Signal. 2017;27(10):684–712. doi: 10.1089/ars.2017.7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hao Y., Jiang H., Thapa P., Ding N., Alshahrani A., Fujii J., et al. Critical role of the sulfiredoxin-peroxiredoxin IV Axis in urethane-induced non-small cell lung cancer. Antioxidants. 2023;12(2):367. doi: 10.3390/antiox12020367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schulte J., Struck J., Köhrle J., Müller B. Circulating levels of peroxiredoxin 4 as a novel biomarker of oxidative stress in patients with sepsis. Shock. 2011;35(5):460–465. doi: 10.1097/SHK.0b013e3182115f40. [DOI] [PubMed] [Google Scholar]
- 48.Nickel C.H., Ruedinger J., Misch F., Blume K., Maile S., Schulte J., Köhrle J., Hartmann O., Giersdorf S., Bingisser R. Copeptin and peroxiredoxin-4 independently predict mortality in patients with nonspecific complaints presenting to the emergency department. Acad. Emerg. Med. 2011 Aug;18(8):851–859. doi: 10.1111/j.1553-2712.2011.01126.x. [DOI] [PubMed] [Google Scholar]
- 49.Norton J.M., Moxey-Mims M.M., Eggers P.W., Narva A.S., Star R.A., Kimmel P.L., et al. Social determinants of racial disparities in CKD. J. Am. Soc. Nephrol. 2016;27(9):2576–2595. doi: 10.1681/ASN.2016010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fischer M.J., Hsu J.Y., Lora C.M., Ricardo A.C., Anderson A.H., Bazzano L., et al. CKD progression and mortality among hispanics and non-hispanics. J. Am. Soc. Nephrol. 2016;27(11):3488–3497. doi: 10.1681/ASN.2015050570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schulz E., Gori T., Münzel T. Oxidative stress and endothelial dysfunction in hypertension. Hypertens. Res. 2011;34:665–673. doi: 10.1038/hr.2011.39. [DOI] [PubMed] [Google Scholar]
- 52.Ávila-Escalante M.L., Coop-Gamas F., Cervantes-Rodríguez M., Méndez-Iturbide D., Aranda-González I.I. The effect of diet on oxidative stress and metabolic diseases—clinically controlled trials. J. Food Biochem. 2020;44(5) doi: 10.1111/jfbc.13191. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.